Abstract
Purpose:
To determine (i) the relationship between candidate biomarkers of the antiproliferative (Ki67) response to letrozole and palbociclib alone and combined in ER+/HER2− breast cancer; and (ii) the pharmacodynamic effect of the agents on the biomarkers.
Experimental Design:
307 postmenopausal women with ER+/HER2− primary breast cancer were randomly assigned to neoadjuvant treatment with letrozole for 14 weeks; letrozole for 2 weeks, then letrozole+palbociclib to 14 weeks; palbociclib for 2 weeks, then letrozole+palbociclib to 14 weeks; or letrozole+palbociclib for 14 weeks. Biopsies were taken at baseline, 2 and 14 weeks and surgery at varying times after stopping palbociclib. Immunohistochemical analyses were conducted for Ki67, c-PARP, ER, PgR, RB1, CCNE1, and CCND1.
Results:
Higher baselines ER and PgR were significantly associated with a greater chance of complete cell-cycle arrest (CCCA: Ki67 <2.7%) at 14 weeks and higher baseline Ki67, c-PARP, and CCNE1 with a lower chance. The interaction with treatment was significant only for c-PARP. CCND1 levels were decreased c.20% by letrozole at 2 and 14 weeks but showed a tendency to increase with palbociclib. CCNE1 levels fell 82% (median) in tumors showing CCCA but were unchanged in those with no CCCA. Only 2/9 tumors showed CCCA 3–9 days after stopping palbociclib. ESR1 mutations were found in 2/4 tumors for which surgery took place ≥6 months after starting treatment.
Conclusions:
High CCNE1 levels were confirmed as a biomarker of resistance to letrozole+palbociclib. Ki67 recovery within 3–9 days of discontinuing palbociclib indicates incomplete suppression of proliferation during the “off” week of its schedule.
Translational Relevance.
Addition of CDK4/6 inhibitors to endocrine treatments provides substantial improvements in progression-free survival in advanced ER+ breast cancer. Early results from trials in the adjuvant setting show improvement in recurrence-free survival with abemaciclib but so far not with palbociclib. There are no established biomarkers to improve the targeting of CDK4/6 inhibitors. We conducted a neoadjuvant trial of the aromatase inhibitor letrozole ± palbociclib and assessed the relationship of a set of candidate biomarkers with the antiproliferative effect of the treatments on expression of Ki67, an established marker of response to endocrine treatment. We confirmed that tumors with high CCNE1 expression respond less well to letrozole + palbociclib. Ki67 expression recovered within 3–9 days of ceasing palbociclib, indicating that suppression of proliferation is not fully maintained in the 3-week on 1-week off schedule. These data may lead to improved targeting of palbociclib and explain its limited activity in the adjuvant setting.
Introduction
Despite adjuvant endocrine therapy achieving major improvements in clinical outcome of patients with estrogen receptor–positive (ER+) HER2− breast cancer many patients become resistant and die from their disease. CDK4/6 inhibitors have provided the most substantial improvement in the treatment of patients with advanced ER+ disease over the last 20 years. Palbociclib, abemaciclib, and ribociclib have each shown substantial improvements in progression-free survival (PFS), and in some cases overall survival, when combined with an aromatase inhibitor or fulvestrant compared with the endocrine agent alone (1–5). Recently, a trial of abemaciclib + endocrine therapy versus endocrine therapy alone in high-risk ER+/HER2− primary breast cancer was reported at an interim analysis to have shown significant reductions in early relapse with the combination (6). In contrast, a similar trial of palbociclib plus endocrine therapy versus endocrine therapy alone in lower risk patients was stopped after a median follow-up of 23.7 months because of the low likelihood of it showing a significant improvement in outcome (7). There are multiple possible explanations for these different clinical results between the two inhibitors (8).
The neoadjuvant setting is arguably the most direct and informative way to study the interaction of these drugs with the disease. In our randomized neoadjuvant comparison of letrozole with or without palbociclib (PALLET trial), we reported that malignant cell proliferation as assessed by Ki67 staining was suppressed more completely by the combination than by letrozole alone (9). There was, however, no evidence of a significant enhancement in clinical response compared with letrozole alone over the 14-week duration. Apoptosis was also suppressed more by the combination and this may have impeded tumor regression despite the enhanced antiproliferative effect.
No biomarkers of resistance to added CDK4/6 inhibitors in primary breast cancer have been identified with confidence. We therefore assessed in tumor samples from the PALLET trial the relationship between suppression of Ki67 and the pretreatment and on-treatment expression of a set of prespecified candidate molecules involved in endocrine and cell-cycle signaling. We considered the achievement of complete cell-cycle arrest (CCCA, Ki67 < 2.7%) as the end-point of efficacy as reported in the NeoPalAna study of anastrozole ± palbociclib (10). The design of PALLET enabled us to assess the effects of palbociclib alone, of palbociclib when added to letrozole and of letrozole when added palbociclib. We measured cleaved-PARP (c-PARP) as an indicator of apoptosis because induction or suppression of cell death by palbociclib could impact on its overall impact on tumor growth. Levels of ER and PgR were assessed because of their key role in characterizing the endocrine responsiveness of ER+ breast cancer. CCND1 was measured because it is a key determinant of CDK4/6 activity and was known to be markedly downregulated by estrogen deprivation (11). CCND1 amplification status was also assessed because this has been reported to significantly have an impact on the prognostic significance of increased CCND1 expression in ER+ breast cancer (12). CCNE1 was included in the candidate markers because it is downstream on CDK4/6 but can impact on E2F signaling and enhance cell cycling independent of CCND1 and CDK4/6. Total RB1 was also included given its central role in the promotion of the cell cycle and there was clinical evidence that RB1 loss led to much poorer outcome for patients treated with CDK4/6 inhibitors (13).
Schedule of treatment is different between the three CDK4/6 inhibitors: Abemaciclib continuously; palbociclib and ribociclib as 3-week on 1-week off cycles. In the PALLET trial, core-cut biopsies were taken after 14 weeks with most patients subsequently scheduled for surgery at an unspecified but recorded time. This allowed us to assess the degree of recovery of Ki67 in the excised tumor at varying times after the end of their treatment with letrozole + palbociclib and continuation on letrozole alone.
Materials and Methods
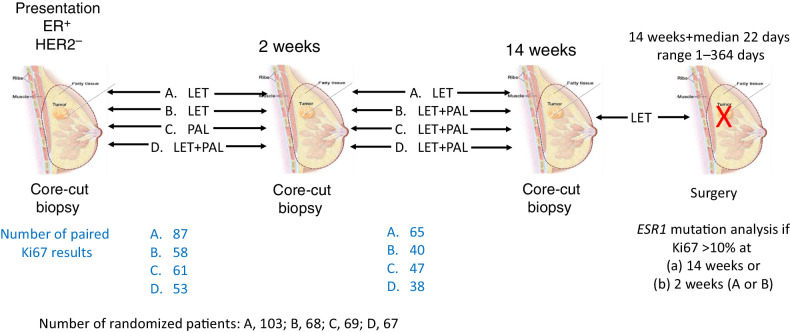
Full details of the clinical trial methodology have been published (9); selected details are provided below as relevant to this biomarker report. International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS). The conduct of the trials and the analyses reported here were approved by relevant ethics committees and all patients provided written informed consent. Figure 1 illustrates the design of the trial and the acquisition of samples for analysis.
Figure 1.
Schema of PALLET study design showing timing of treatment changes and sample acquisition and the number of samples available for paired Ki67 comparisons.
Three hundred and seven postmenopausal women with unilateral, operable ER+/HER2− breast cancer ≥-cm diameter were randomized 3:2:2:2 to treatment with letrozole (2.5 mg/d) for 14 weeks (group A); letrozole for 2 weeks followed by letrozole plus palbociclib (125 mg/d, 3 weeks on 1 week off) to 14 weeks (group B); palbociclib for 2 weeks followed by letrozole plus palbociclib to 14 weeks (group C); letrozole plus palbociclib for 14 weeks (group D). Core-cut biopsies were taken at baseline, 2 and 14 weeks. Treatment with palbociclib was discontinued at the end of second week of the 4th cycle (i.e., at 14 weeks) but letrozole was continued until surgery that was timed at the physician's discretion. Time of surgery relative to the last dose of palbociclib was recorded. For this biomarker study the primary end-point was CCCA.
The following biomarkers were analyzed by IHC: Ki67, cleaved-PARP (c-PARP), ER, progesterone receptor status (PgR), RB1, CCNE1, and CCND1. Methodologic details are provided in the Supplementary Table S1. For Ki67, CCND1, CCNE1, PgR, c-PARP, and RB1 the measure was overall percentage of positive invasive cells. ER was measured using the estimated H-score (score 0–300). CCND1 was also analyzed by FISH; tumors were considered amplified if the CCND1:CEP11 ratio was >2.0 as described previously for HER2 (14). Tumors with Ki67 IHC scores <2.7% after weeks 2 and 14 of treatment were classified as achieving CCCA at the respective timepoint.
ESR1 mutations were assessed by droplet-digital PCR with 7 hotspot mutations in two multiplex reactions and positive results being validated by singleplex reactions as described previously (15).
Statistical analysis
Statistical analyses were performed in Stata version 16.1 and R version 4.1.0. The association of median baseline values of each biomarker with CCCA at weeks 14 and 2 was compared using a Wilcoxon rank sum test and presented in box plots. Letrozole was compared with letrozole+palbociclib by comparing group A versus groups B+C+D for CCCA at week 14 and groups A+B versus C and groups A+B versus D for CCCA at week 2. Geometric mean changes in biomarkers from baseline to either week 2 or 14 or from week 2 to 14 were reported with 95% confidence interval (CI) and t tests of the logarithmic values allowed comparisons between treatment groups. Interactions between biomarkers and treatment groups for CCCA were tested using logistic regression models. Given that the study was mainly explorative uncorrected P values are shown. To make some adjustment for multiple testing a P value of <0.01 was considered statistically significant.
Results
Demographics of the patients treated and a consort diagram of samples available have been published previously (9). In summary, mean age was 64.9 years; 54% were recruited from UK and 46% from North America; nearly three quarters were intermediate histopathologic grade; all were locally determined to be ER+ and 70% were PgR+; 24% of tumors were lobular and 70% ductal. Patient characteristics in the subset of patients with at least 1 biomarker assessed at baseline were similar to those for the whole study (Supplementary Table S2). Change in Ki67 between baseline and 14 weeks was available in 190 patients with the predominant reasons for unavailable results being missing or unevaluable 14-week samples. The number of samples available for each biomarker at each time point is shown in the Tables and Figures.
Association of baseline biomarker levels with CCCA
Letrozole compared with letrozole+palbociclib: CCCA after 14 weeks
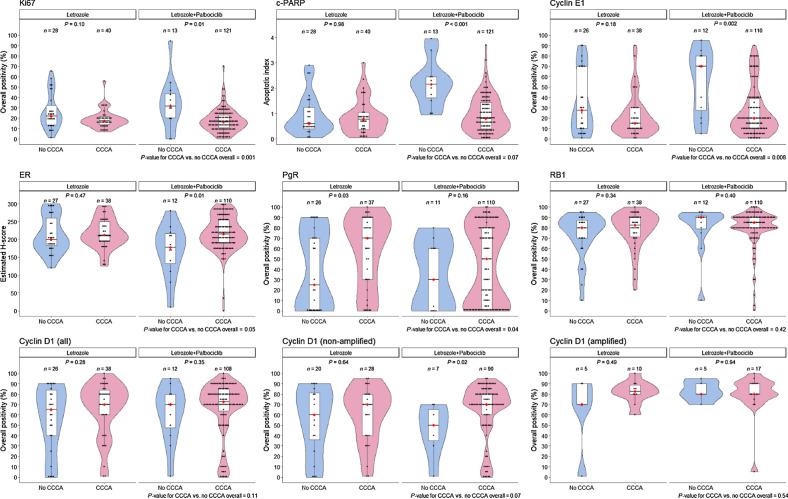
Ki67 at baseline was higher overall in tumors that did not develop CCCA at 14 weeks (P = 0.001). This was statistically significant for letrozole+palbociclib (P = 0.01) but not for letrozole alone (P = 0.10); however, there was no significant interaction with treatment (P = 0.51, Supplementary Table S3). There was a highly significant association between baseline levels of c-PARP and incidence of CCCA at 14 weeks with letrozole+palbociclib (P < 0.001) but no such association with letrozole alone (P = 0.98); interaction P = 0.01. Notably, c-PARP levels correlated significantly with Ki67 levels at baseline (r = 0.55, n = 244, P < 0.001).
Tumors that showed there was a trend for CCCA to be associated with higher baseline levels of ER than those that did not achieve CCCA (P = 0.05). This trend was strong for letrozole+palbociclib (P = 0.01) but not for letrozole alone (P = 0.47). In contrast, although tumors showing CCCA had higher levels of PgR (P = 0.04), the trend was substantial only for letrozole alone (P = 0.03); however, the effect was not statistically significantly different from that with letrozole+palbociclib (Fig. 2). There was no association of RB1 levels with CCCA in either the letrozole alone or letrozole+palbociclib groups.
Figure 2.
Box plots showing the relationship between median biomarker values at baseline in letrozole ± palbociclib according to CCCA or no CCCA at 14 weeks. The black dots represent the values in individual patients. The red dot indicates the median for clarity when the median line is obscured by data points. P values are derived from Wilcoxon tests.
For patients receiving letrozole+palbociclib median levels of CCNE1 at baseline were over 3-times higher in those that did not achieve CCCA compared with those that did (P = 0.002). For patients receiving letrozole alone the levels were about 1.8 times higher (P = 0.18) for non-CCCA versus CCCA. The test for interaction with treatment was not significant (P = 0.27).
There was no association of baseline CCND1 levels as measured by IHC with CCCA at 14 weeks in the overall population. CCND1 amplification status was available in tumors from 293 patients: 217 were non-amplified and 76 (25.9%) showed amplification. In tumors not amplified for CCND1, higher baseline CCND1 expression was associated with a greater chance of CCCA although this only showed a trend to significance for letrozole+palbociclib (P = 0.02; overall population P = 0.07). Sixty-three patients on letrozole alone had both Ki67 values at 14 weeks and FISH status for CCND1 at baseline. Five of the 15 cases with amplified CCND1 (33%) showed no CCCA whereas 20 of the 48 cases without amplification did not achieve CCCA (43%): odds ratio (OR), 1.47 (95% CI, 0.43–4.99; P = 0.53). 119 patients on letrozole+palbociclib had Ki67 at 14 weeks and CCND1 FISH status at baseline. Five of the 22 cases with amplified CCND1 (23%) showed no CCCA compared with 7 of the 97 cases without amplification (7%): OR, 0.28 (95% CI, 0.08–0.97; P = 0.05; test for treatment Pinteraction = 0.06.
A multivariable model for CCCA at 14 weeks showed that individual significant differences in baseline values of Ki67 and CCNE1 remain after adjusting for all other biomarkers (Supplementary Table S3).
Letrozole compared with letrozole+palbociclib: CCCA after 2 weeks
Baseline Ki67 and c-PARP levels were both higher in non-CCCA cases with letrozole alone and letrozole+palbociclib. With each this was statistically significant only for letrozole alone but there was not a statistically significant interaction with treatment. The only other substantial relationship was with CCNE1 levels that at baseline showed a strong trend to association with no achievement of CCCA for letrozole alone (P = 0.02) but not for letrozole+palbociclib (Supplementary Fig. S1).
Palbociclib alone: CCCA after 2 weeks
None of the biomarkers other than Ki67 (P = 0.01) showed a significant relationship at baseline with CCCA at 2 weeks. Of the 15 patients with CCND1 amplified at baseline 5 (33%) did not show CCCA, a very similar proportion to the 12 (28%) of 43 that were not amplified (Supplementary Fig. S1).
Treatment-related changes in biomarkers:
The Ki67 data have been summarized previously (9) but are reported here more extensively. Values shown for all biomarkers are shown in the Table 1 and Fig. 3 as geometric means ± 95% CIs.
Table 1.
Changes in biomarker expression with treatment.
| Ki67 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 81 | −83.0 | −87.8 to −76.2 | <0.001 | 64 | −30.1 | −49.8 to −2.6 | 0.03 | 65 | −88.5 | −92.3 to −82.9 | <0.001 | |
| B: Let/Let+Palbo | 56 | −77.7 | −85.9 to −64.9 | <0.001 | 41 | −88.5 | −93.2 to −80.6 | <0.001 | 40 | −97.5 | −98.6 to −95.8 | <0.001 | |
| C: Palbo/Let+Palbo | 60 | −95.0 | −96.9 to −91.9 | <0.001 | 45 | −54.2 | −74.7 to −17.2 | 0.01 | 47 | −97.5 | −98.5 to −95.7 | <0.001 | |
| D: Let+Palbo/Let+Palbo | 48 | −97.1 | −98.1 to −95.7 | <0.001 | 34 | 8.0 | −29.8–65–9 | 0.72 | 38 | −97.1 | −98.5 to −94.6 | <0.001 | |
| A+B: Let (2 wk) | 137 | −81.0 | −85.5 to −75.2 | <0.001 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 125 | −97.4 | −98.1 to −96.4 | <0.001 | |||||||||
| A+B vs. C | <0.001 | A vs. B | <0.001 | A vs. B+C+D | <0.001 | ||||||||
| A+B vs. D | <0.001 | A vs. C | 0.19 | ||||||||||
| C vs. D | 0.15 | A vs. D | 0.06 | ||||||||||
| c-PARP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 70 | −9.9 | −23.8–6.6 | 0.22 | 48 | −34.3 | −46.6 to −19.1 | <0.001 | 47 | −31.4 | −46.9 to −11.5 | 0.005 | |
| B: Let/Let+Palbo | 51 | −26.4 | −39.4 to −10.7 | 0.003 | 31 | −48.7 | −59.7 to −34.7 | <0.001 | 33 | −59.9 | −69.3 to −47.6 | <0.001 | |
| C: Palbo/Let+Palbo | 50 | −37.4 | −48.3 to −24.2 | <0.001 | 37 | −25.8 | −42.8 to −3.6 | 0.03 | 37 | −56.0 | −66.4 to −42.5 | <0.001 | |
| D: Let+Palbo/Let+Palbo | 39 | −24.4 | −37.4 to −8.8 | 0.005 | 23 | −28.6 | −42.6 to −11.3 | 0.004 | 28 | −54.0 | −65.6 to −38.5 | <0.001 | |
| A+B: Let (2 wk) | 121 | −17.3 | −27.1 to −6.1 | 0.004 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 98 | −56.8 | −63.0 to −49.7 | <0.001 | |||||||||
| A+B vs. C | 0.03 | A vs. B | 0.09 | A vs. B+C+D | 0.001 | ||||||||
| A+B vs. D | 0.45 | A vs. C | 0.51 | ||||||||||
| C vs. D | 0.34 | A vs. D | 0.61 | ||||||||||
| RB1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 79 | −13.4 | −23.7 to −1.8 | 0.03 | 63 | −17.5 | −36.0–6.4 | 0.14 | 65 | −23.8 | −38.9 to −4.9 | 0.02 | |
| B: Let/Let+Palbo | 54 | −25.4 | −40.5 to −6.4 | 0.01 | 39 | 16.3 | −17.8–64.6 | 0.38 | 36 | −22.8 | −34.7 to −8.7 | 0.004 | |
| C: Palbo/Let+Palbo | 56 | 7.9 | −6.5–24.4 | 0.29 | 39 | −25.0 | −42.6 to −2.0 | 0.04 | 42 | −19.1 | −42.8–14.6 | 0.23 | |
| D: Let+Palbo/Let+Palbo | 49 | −16.1 | −31.9–3.4 | 0.1 | 32 | −23.1 | −52.5–24.6 | 0.28 | 38 | −39.9 | −60.4 to −9.0 | 0.02 | |
| A+B: Let (2 wk) | 133 | −18.5 | −27.5 to −8.3 | <0.001 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 116 | −27.7 | −40.1 to −12.6 | <0.001 | |||||||||
| A+B vs. C | 0.005 | A vs. B | 0.16 | A vs. B+C+D | 0.04 | ||||||||
| A+B vs. D | 0.57 | A vs. C | 0.23 | ||||||||||
| C vs. D | 0.004 | A vs. D | 0.99 | ||||||||||
| ER | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 81 | −4.2 | −8.5–0.3 | 0.07 | 67 | −1.9 | −6.3–2.7 | 0.41 | 66 | −4.9 | −9.3 to −0.3 | 0.04 | |
| B: Let/Let+Palbo | 56 | 13.6 | −13.6–49.5 | 0.35 | 37 | −1.9 | −8.3–4.9 | 0.57 | 35 | 1.2 | −9.5–13.1 | 0.83 | |
| C: Palbo/Let+Palbo | 59 | 0.9 | −3.9–5.9 | 0.72 | 42 | −11 | −22.9–2.8 | 0.11 | 43 | −9.8 | −23.4–6.3 | 0.21 | |
| D: Let+Palbo/Let+Palbo | 50 | −3.2 | −6.0 to −0.3 | 0.03 | 32 | −3.1 | −7.9–1.9 | 0.21 | 36 | −8.1 | −13.1 to −2.8 | 0.004 | |
| A+B: Let (2 wk) | 137 | 2.7 | −8.3–15.2 | 0.64 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 114 | −6.0 | −12.5–1.0 | 0.09 | |||||||||
| A+B vs. C | 0.64 | A vs. B | 0.81 | A vs. B+C+D | 0.91 | ||||||||
| A+B vs. D | 0.36 | A vs. C | 0.37 | ||||||||||
| C vs. D | 0.30 | A vs. D | 0.53 | ||||||||||
| PgR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 75 | −87.1 | −92.8 to −76.8 | <0.001 | 63 | −76.1 | −87.0 to −56.2 | <0.001 | 64 | −96.9 | −98.4 to −93.8 | <0.001 | |
| B: Let/Let+Palbo | 55 | −89.0 | −94.2 to −79.2 | <0.001 | 39 | −57.6 | −80.2 to −9.2 | 0.03 | 39 | −95.8 | −98.3 to −89.5 | <0.001 | |
| C: Palbo/Let+Palbo | 56 | −11.5 | −50.1–56.9 | 0.67 | 40 | −87.1 | −94.3 to −70.4 | <0.001 | 41 | −87.1 | −94.9 to −67.7 | <0.001 | |
| D: Let+Palbo/Let+Palbo | 50 | −88.3 | −94.3 to −75.9 | <0.001 | 37 | −75.5 | −87.2 to −53.4 | <0.001 | 40 | −97.4 | −98.9 to −93.7 | <0.001 | |
| A+B: Let (2 wk) | 130 | −87.9 | −92.1 to −81.5 | <0.001 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 120 | −94.8 | −96.9 to −91.2 | <0.001 | |||||||||
| A+B vs. C | <0.001 | A vs. B | 0.76 | A vs. B+C+D | 0.31 | ||||||||
| A+B vs. D | 0.69 | A vs. C | 0.09 | ||||||||||
| C vs. D | <0.001 | A vs. D | 0.30 | ||||||||||
| CCNE1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 75 | −63.6 | −74.0 to −49.0 | <0.001 | 63 | −18.6 | −49.4–30.8 | 0.39 | 64 | −68.2 | −78.5 to −52.9 | <0.001 | |
| B: Let/Let+Palbo | 54 | −61.8 | −76.6 to −37.6 | <0.001 | 39 | −45.7 | −68.3 to −7.0 | 0.03 | 38 | −82.9 | −91.2 to −67.0 | <0.001 | |
| C: Palbo/Let+Palbo | 57 | −47.1 | −63.8 to −22.7 | 0.001 | 42 | −24.2 | −60.0–43.7 | 0.39 | 42 | −64.8 | −78.3 to −42.9 | <0.001 | |
| D: Let+Palbo/Let+Palbo | 45 | −68.1 | −78.5 to −52.6 | <0.001 | 28 | −2.6 | −45.9–75.2 | 0.93 | 33 | −73.9 | −85.9 to −51.7 | <0.001 | |
| A+B: Let (2 wk) | 129 | −62.9 | −71.9 to −50.9 | <0.001 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 113 | −74.7 | −81.8 to −64.7 | <0.001 | |||||||||
| A+B vs. C | 0.06 | A vs. B | 0.90 | A vs. B+C+D | 0.51 | ||||||||
| A+B vs. D | 0.45 | A vs. C | 0.61 | ||||||||||
| C vs. D | 0.04 | A vs. D | 0.50 | ||||||||||
| CCND1 (IHC) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 77 | −66.0 | −77.9 to −47.5 | <0.001 | 62 | −58.2 | −75.8 to −27.6 | 0.002 | 63 | −80.9 | −87.6 to −70.8 | <0.001 | |
| B: Let/Let+Palbo | 54 | −67.8 | −81.7 to −43.3 | <0.001 | 34 | −14.7 | −49.8–44.8 | 0.54 | 35 | −67.6 | −82.6 to −39.7 | 0.001 | |
| C: Palbo/Let+Palbo | 57 | 28.7 | −3.1–71.1 | 0.08 | 40 | −70.1 | −83.7 to −45.1 | <0.001 | 43 | −64.5 | −81.1 to −33.4 | 0.002 | |
| D: Let+Palbo/Let+Palbo | 46 | −58.9 | −74.0 to −35.1 | <0.001 | 35 | −46.2 | −74.4–13.1 | 0.1 | 40 | −77.7 | −88.6 to −56.4 | <0.001 | |
| A+B: Let (2 wk) | 131 | −66.7 | −76.3 to −53.2 | <0.001 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 118 | −70.5 | −79.5 to −57.6 | <0.001 | |||||||||
| A+B vs. C | <0.001 | A vs. B | 0.06 | A vs. B+C+D | 0.01 | ||||||||
| A+B vs. D | 0.63 | A vs. C | 0.21 | ||||||||||
| C vs. D | <0.001 | A vs. D | 0.76 | ||||||||||
| CCND1 (IHC in non-amplified) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 60 | −69.3 | −81.0 to −50.3 | <0.001 | 47 | −54.7 | −76.0 to −14.4 | 0.02 | 48 | −84.7 | −90.7 to −74.7 | <0.001 | |
| B: Let/Let+Palbo | 45 | −70.2 | −84.5 to −42.8 | <0.001 | 26 | −23.1 | −54.7–30.4 | 0.32 | 28 | −71.0 | −86.0 to −40.0 | 0.002 | |
| C: Palbo/Let+Palbo | 43 | 29.1 | −10.1–85.3 | 0.16 | 32 | −73.2 | −87.2 to −43.7 | 0.001 | 35 | −65.7 | −83.4 to −29.2 | 0.005 | |
| D: Let+Palbo/Let+Palbo | 33 | −64.3 | −80.1–36.2 | 0.001 | 23 | −65.8 | −87.8 to −4.4 | 0.04 | 29 | −85.4 | −94.0 to −64.8 | <0.001 | |
| A+B: Let (2 wk) | 105 | −69.7 | −79.4 to −55.4 | <0.001 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 92 | −75.1 | −83.9–61.4 | <0.001 | |||||||||
| A+B vs. C | <0.001 | A vs. B | 0.12 | A vs. B+C+D | 0.01 | ||||||||
| A+B vs. D | 0.86 | A vs. C | 0.32 | ||||||||||
| C vs. D | <0.001 | A vs. D | 0.64 | ||||||||||
| CCND1 (IHC in amplified) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 2 weeks | 2 to 14 weeks | Baseline to 14 weeks | |||||||||||
| Group | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | n | Geomean Δ (%) | 95% CI | P | |
| A: Let/Let | 16 | −51.9 | −84.8–52.1 | 0.2 | 11 | −13.0 | −62.4–101.3 | 0.72 | 14 | −60.1 | −82.8 to −7.4 | 0.03 | |
| B: Let/Let+Palbo | 7 | −56.6 | −91.3–117.3 | 0.25 | 6 | 119.9 | −69.2–1467.9 | 0.35 | 5 | −11.9 | −32.7–15.4 | 0.26 | |
| C: Palbo/Let+Palbo | 14 | 27.8 | −14.6–91.3 | 0.21 | 7 | −58.7 | −85.5–17.4 | 0.08 | 8 | −58.9 | −91.3–94.2 | 0.22 | |
| D: Let+Palbo/Let+Palbo | 12 | −44.3 | −75.0–24.1 | 0.14 | 10 | 46.1 | −39.6–253.3 | 0.36 | 10 | −19.8 | −32.7 to −4.4 | 0.02 | |
| A+B: Let (2 wk) | 23 | −53.4 | −80.4–10.9 | 0.08 | |||||||||
| B+C+D: Let + Palbo (14 wk) | 23 | −35.1 | −60.0–5.2 | 0.08 | |||||||||
| A+B vs. C | 0.001 | A vs. B | 0.52 | A vs. B+C+D | 0.09 | ||||||||
| A+B vs. D | 0.59 | A vs. C | 0.02 | ||||||||||
| C vs. D | 0.01 | A vs. D | 0.39 | ||||||||||
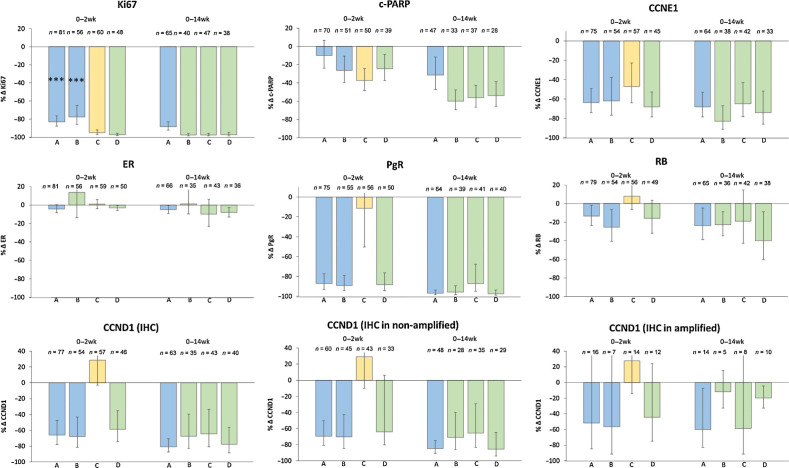
Figure 3.
Geometric mean changes (±95% CI) in biomarkers between baseline and 2 weeks and between baseline and 14 weeks. P values for the degree of change and the comparison between treatments are shown in Table 1.
Effect of letrozole alone (Groups A+B to 2 weeks and group A to 14 weeks):
Changes to 2 weeks are shown for arms A and B combined. Ki67 levels were suppressed by 81.0% and 88.5% at 2 and 14 weeks, respectively. 61/145 (42.1%) and 40/68 (58.8%) patients showed CCCA at 2 and 14 weeks, respectively. C-PARP is a marker of apoptosis. Levels of c-PARP were suppressed by 17.3% and 31.4% at 2 and 14 weeks, respectively. There was no significant effect on ER levels but PgR levels fell by 87.9% by week 2 and 96.9% by week 14. CCND1 levels fell by 66.7% by week 2 and significantly further to 80.9% lower than baseline by week 14. Similar changes were seen in CCND1 levels in cases not amplified for CCND1. In cases amplified for CCND1, levels also fell but to a slightly lesser extent. Letrozole led to a highly significant 18.5% fall in RB1 levels by 2 weeks that was maintained to 14 weeks, although that change was not significantly compared with baseline (P = 0.02). CCNE1 levels were decreased by 62.9% by 2 weeks and by 68.2% by 14 weeks, although the change between weeks 2 and 14 was not significant.
Effect of palbociclib alone (Group C to 2 weeks):
After 2 weeks, palbociclib suppressed Ki67 by 95.0% and c-PARP by 37.4%. 44/61 (72.1%) of patients showed CCCA. By 2 weeks, palbociclib had no significant effect on ER, PgR or RB1 expression by week 2 but CCNE1 levels fell by 47.1%. CCND1 levels were 28.7% higher but this was not statistically significant (P = 0.08).
Effect of letrozole+palbociclib (Group D to 2 weeks and groups B+C+D to 14 weeks):
Changes between baseline and 14 weeks are described previously for arms B, C, and D combined. After 2 and 14 weeks letrozole+palbociclib suppressed Ki67 by 97.1% and 97.4%, respectively, and 47/53 (72.1%) and 113/125 (88.6%) of patients showed CCCA. After 2 and 14 weeks, letrozole+palbociclib suppressed c-PARP by 24.4% and 56.8%, respectively. ER levels were unaffected by the combination at either 2 or 14 weeks. After 2 weeks, RB1 levels were 16.1% lower (P = 0.10) and by 14 weeks were suppressed by 27.7% (P < 0.001). PgR levels were suppressed by 88.3% and 94.8% at 2 and 14 weeks, respectively, and CCND1 by 58.9% and 70.5%, respectively. At 14 weeks CCND1 levels were suppressed to a lesser extent in patients with amplified CCND1 compared with non-amplified status: with letrozole alone by 60.1% versus 84.7%, respectively and by letrozole+palbociclib 35.1% versus 75.1%, respectively.
Effect of adding palbociclib to letrozole (Group B from 2 weeks to 14 weeks):
Ki67 levels fell 88.5% between 2 and 14 weeks (relative to the 2-week value) compared with 30.1% with letrozole alone over that period. C-PARP fell 48.7% between 2 and 14 weeks (relative to the 2-week value) compared with 34.3% with letrozole alone over that period. Between weeks 2 and 14 after palbociclib was added to letrozole there was no substantial effect on ER, PgR or RB1 levels. Adding palbociclib appeared to diminish the additional suppression of CCND1 seen with letrozole alone between 2 and 14 weeks but the difference was not statistically significant (P = 0.06). The apparently greater suppression of CCNE1 by palbociclib added to letrozole compared with continued letrozole alone was also not statistically significant (P = 0.90).
Effect of adding letrozole to palbociclib (Group C from 2 weeks to 14 weeks):
Adding letrozole to palbociclib after 2 weeks led to a further 54.2% fall in Ki67 and 25.8% fall in c-PARP relative to the 2-week value. There was no impact on ER levels after letrozole was added to palbociclib at week 2. PgR and CCND1 levels were both suppressed markedly after adding letrozole to palbociclib to a proportionally similar extent to the effect of continuing letrozole alone between week 2 and 14. CCNE1 levels did not fall significantly further on addition of letrozole to palbociclib at week 2.
Comparison of the effect of letrozole versus letrozole+palbociclib at 14 weeks (Group A vs. groups B+C+D):
Ki67 and c-PARP levels were both suppressed to a significantly greater degree by the combination than by letrozole alone (P < 0.001 and P = 0.01, respectively). Other biomarkers were not statistically different between the treatments after 14 weeks. CCND1 was suppressed to a lesser extent with the combination than with letrozole alone. This difference was borderline statistically significant (P = 0.01) and was consistent with the increase in CCND1 seen with palbociclib alone after 2 weeks and the apparently impeded suppression of CCND1 with letrozole alone when palbociclib was added to letrozole at 2 weeks.
Association of change in CCNE1 levels with CCCA
Given the association of CCNE1 levels at baseline with lack of CCCA and the suppressive effects of both letrozole alone and letrozole+palbociclib on CCNE1 levels, we asked whether this suppression varied according to the acquisition or not of CCCA. In cases treated with letrozole alone the median fall in CCNE1 was 82% (n = 36) in those that achieved CCCA levels at 14 weeks, and 0% (n = 25) in those that did not. In cases treated with letrozole+palbociclib the median falls were 51% (n = 99) and 0% (n = 12), respectively. Thus, CCNE1 levels were higher after 14 weeks as well as at baseline in the poor responders to both letrozole and letrozole+palbociclib.
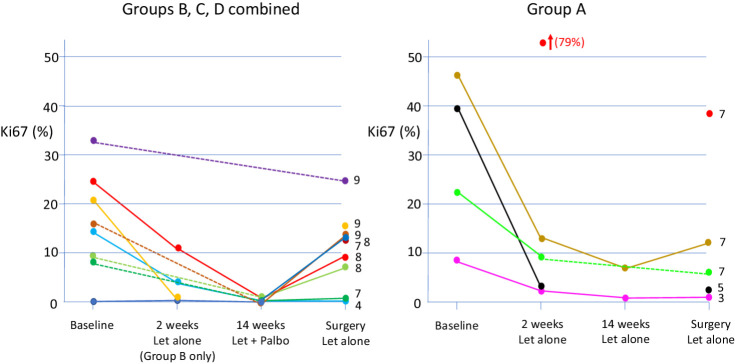
Change in ki67 between end of palbociclib treatment and surgery
Ki67 values and recorded time from end of trial treatment were available in 57 surgical samples from group A and 119 surgical samples from groups B+C+D. The median time from end of treatment to surgery was 22 days (range, 1 to 364 days) in group A and a median 23 days (range, 1 to 278 days) in groups B+C+D. Five and 9 patients from groups A and B+C+D, respectively, had surgical samples taken within a time window of 3–9 days that approximates to the 7 days off palbociclib each cycle. Individual Ki67 values for these patients at all 4 time points are shown in Fig. 4. Six of the 9 patients in group B+C+D also had Ki67 values available at week 14 when still on palbociclib and all showed CCCA whereas just 2 of the 9 continued to show CCCA in the surgical sample. Five of the 9 patients were from group B, including 4 patients in whom Ki67 showed CCCA after 2 weeks of letrozole alone. In 2 of those 4 the surgical levels of Ki67 were much higher (16.5% and 14.0%) than the levels after 2 weeks (both <1.0%). The patients in group A had surgical levels of Ki67 similar to or less than the values after 2 weeks.
Figure 4.
Individual values of Ki67 at baseline, 2 and 14 weeks and at surgery for the cases in which tumors were removed surgically between 3 and 9 days after stopping palbociclib and continuing on letrozole alone. Dotted lines join values where a value was unavailable from an intermediate time point. Numbers on the right hand side of each panel indicate the number of days between ceasing palbociclib and surgery for the respective sample.
ESR1 mutations in surgical samples
ESR1 mutation analysis was conducted in 25 surgical biopsies from tumors that showed Ki67 >10% at surgery after good suppression of Ki67 at either (i) week 14 or (ii) for those without a 14-week value, receiving letrozole at week 2, because this is evidence of acquired resistance to letrozole. Two cases were identified with mutations: One, the D538G mutation (VAF 4.52%; group B) and the other with both a D538G and a Y537N mutation (VAF 0.34% and 0.09%, respectively; group A). These mutations have been described previously as leading to ligand-independent ER and are associated with resistance to aromatase inhibitors and are frequently observed in metastatic disease that has become to aromatase inhibitors but not in primary disease (16, 17). These two tumors were from patients with the longest duration of presurgical treatment (278 and 364 days, respectively), after finishing trial treatment and therefore c.12 and 15 months after starting neoadjuvant AI, respectively. The duration of presurgical treatment of the other 23 ranged from 15 weeks to 9 months with only 2 of the 23 being treated for more than 6 months.
Discussion
The PALLET trial is the largest randomized neoadjuvant study of an aromatase inhibitor and a CDK4/6 inhibitor (9). Its design allowed a comparison of the effects of letrozole and palbociclib alone and in combination at 2 weeks and of the aromatase inhibitor versus the combination at 14 weeks. It is clear that letrozole and palbociclib both substantially suppressed proliferation as measured by Ki67, the primary biomarker end-point and that the effects of letrozole and palbociclib are additive between weeks 2 and 14 (groups B and C). The profound antiproliferative effect of palbociclib alone over a 2-week period was reported in the POP trial (18) and the separate and additive effects of abemaciclib and anastrozole were shown over 2 weeks in NeoMonarch (19). In the latter study, all patients received combined treatment after 2 weeks such that the effects of the combination could not be compared with the aromatase inhibitor alone over a prolonged period. In hormone receptor–positive HER2-negative cases in the POP trial, Ki67 was suppressed by only 70% (geomean) compared with 90% in PALLET. In NeoMonarch after 2 weeks, the Ki67 the suppression by abemaciclib was 91% and therefore similar to the PALLET result. In the NeoPalAna study (10), CCCA 2 weeks after adding palbociclib to anastrozole occurred in 39/45 (87%) patients that was also similar to the 89% CCCA after 2 weeks in PALLET in the combined treatment group. The difference between the data from PALLET and POP may be due to the different Ki67assays used.
A key goal of our correlative science research in PALLET was to determine whether any of the putative markers of resistance to AI or CDK4/6i were correlated with lack of CCCA. The data indicated that high baseline levels of CCNE1 were significantly associated with resistance to letrozole+palbociclib at 14 weeks. There was also a non-significant trend to a similar association with letrozole alone. Given that the test for interaction with treatment was also not significant, we cannot conclude that this relationship was exclusive to the combination treatment. CCNE1 is downstream of CCND1 in the cell cycle and its prediction of poor antiproliferative response may in part be related to high proliferation (Ki67) itself also significantly predicting low chance of achieving CCCA in the letrozole+palbociclib group in PALLET. However, high levels of CCNE1 promote E2F signaling and can promote progression of the cycle independent of effects on CCND1 and CDK4/6. Our findings are consistent with the observation that amplification of CCNE1 occurred in MCF7cells that acquire palbociclib resistance (20). The POP short-term presurgical trial of single-agent palbociclib and the NeoPalAna study of anastrozole+palbociclib both reported poorer Ki67 response in tumors with high baseline levels of CCNE1 mRNA levels (10, 18) and the NeoMONARCH study reported a numerically higher but statistically non-significant relationship with Ki67 resistance to anastrozole+abemaciclib (19). CCNE1 transcript levels above the median were also predictive of poor response to palbociclib added to fulvestrant in patients with advanced breast cancer in PALOMA3 (21). Prat and colleagues (22) recently reported a study of the correlation of intrinsic subtypes with PFS in three trials of the addition of ribociclib to endocrine therapy in advanced ER+/HER2− disease. CCNE1 levels were highest in tumors characterized as basal-like and this was the only subgroup that did not gain an improvement in outcome from added ribociclib. Overall, the consistent findings from these studies provide a strong level of evidence for high CCNE1 be associated with poor response to CDK4/6 inhibition alongside endocrine therapy.
Higher values of ER and PgR were seen in tumors exhibiting CCCA at 14 weeks with no evidence of a treatment interaction. Together with the CCND1 IHC data, these results suggest that CCCA is likely in tumors in which luminal features are more pronounced. There are conflicting data on the relationship between intrinsic subtype and benefit of adding a CDK4/6 inhibitor to endocrine treatment in patients with advanced disease. In the PALOMA2 study, significant improvements in PFS with added palbociclib were confined to cases intrinsically subtyped as luminal A or B (23). However, Prat and colleagues (22) reported that PFS was improved by adding ribociclib in both HER2-enriched and luminal subtypes. In PALLET, data on intrinsic subtype are currently unavailable.
CCND1 amplification was present in about a quarter of the patients in PALLET. In the PALOMA 1 study, a cohort was recruited on the basis of CCND1 amplification±p16 loss with the expectation of greater benefit from the addition of palbociclib to letrozole in this subgroup (2). However, early analysis indicated a trend toward lower benefit so the selection was discontinued. In PALLET 21% and 7% of the amplified and non-amplified cases, respectively, that were treated with letrozole+palbociclib showed a lack of CCCA supporting a lesser degree of response in the amplified subset. A plausible explanation for this observation is that letrozole+palbociclib is not able completely to negate the major drive to the cell cycle that emanates from the amplified gene. A much higher immunohistochemical expression of CCND1 in cases where the CCND1 gene was amplified was apparent in this study and letrozole+palbociclib suppressed CCND1 levels less in amplified cases (by 35.1%) than in non-amplified tumors (75.1%).
A difference in the biological significance of CCND1 expression according to the mutational status of the CCND1 gene is clear from studies of prognosis: high expression associated with amplification is related to poor outcome in patients treated with adjuvant endocrine therapy whereas high expression in non-amplified tumors is associated with better outcome (12). This latter association is likely due to CCND1 being a highly estrogen-dependent gene. In PALLET, this is consistent with a higher expression of CCND1 in non-amplified cases being associated with a greater likelihood of CCCA at 14 weeks in the whole cohort, although this was only statistically significant for the letrozole+palbociclib group.
There is considerable evidence that loss of RB1 is associated with resistance to CDK4/6 inhibition. In the Li and colleagues (13) series of 348 patients receiving a CDK4/6 inhibitor, the 2.5% of patients with RB1 loss had a statistically significant, poorer PFS than those without such loss (median 3.6 months vs. 10.1 months). O'Leary and colleagues (24) reported that 4.8% of patients treated with fulvestrant+palbociclib in PALOMA3 acquired RB1 mutations versus none in the fulvestrant alone arm. Our group reported that in two T47D breast cancer cell lines palbociclib-resistance variants acquired a mutation in the RB1 gene and loss of heterozygocity (25). In addition, continued treatment of a ribociclib-resistant PDX with ribociclib led to enrichment of the RB1 mutant allele fraction (20). It should be noted that the above observations allude to metastatic disease and/or acquired resistance to CDK4/6 inhibition. In the current study of treatment-naïve disease, there was no evidence that RB1 protein levels could identify women at low likelihood of response to added palbociclib. Our on-going genomic analyses will enable us to identify the small number of primary tumors that have loss of RB1 or the somewhat larger group that may have deficient RB1 functionality.
We believe that the observation that letrozole suppressed RB1 levels is novel and has not been reported to occur with other AIs or other endocrine treatments. Notably, this did not occur in association with the antiproliferative effects of palbociclib. We are completing our RNAseq analyses of samples from groups B, C, and D and have specifically reviewed the data on transcript levels of RB1. In parallel with the IHC data, significantly lower RB1 mRNA values than at baseline were found at both 2 and 14 weeks in each set of samples from letrozole-treated patients but not in samples after 2 weeks' palbociclib alone. The decrease in RB1 expression therefore seems to be at least transcriptional. Further study of this observation is merited to determine whether it indicates a novel relationship between estrogen deprivation and cell-cycle control.
Presurgical change in Ki67 has been validated as an intermediate biomarker of clinical benefit from adjuvant endocrine treatment (26, 27). It has not, however, been similarly validated for CDK4/6 inhibition. The NeoMONARCH trial found that suppression of Ki67 with abemaciclib plus anastrozole was greater than that with anastrozole alone. In the adjuvant setting the MonarchE trial showed better recurrence-free survival for the combination (6). Although the combination of letrozole+palbociclib showed greater Ki67 suppression than letrozole alone in PALLET, the PALLAS adjuvant trial showed no benefit from the combination over the AI alone (7). There are a number of potential explanations for the differences between the data from the adjuvant trials, including the different scheduling of the CDK4/6 inhibitor and different proportions completing CDK4/6 inhibitor treatment per protocol. Our data showing speedy recovery in Ki67 levels during the days after moving from combination letrozole+palbociclib to letrozole alone just before surgery may be relevant to this difference in scheduling. Although the number of patients was small, it was sufficient to show that the suppressive effects of palbociclib were rapidly lost over a time scale similar to the “one week off” in the 3-weeks on, 1-week-off scheduling of palbociclib. However, the degree to which this may impact on clinical outcome is unknown.
The changes in biomarker levels with letrozole alone were largely those expected from an aromatase inhibitor with profound reductions in Ki67 and PgR by 2 weeks and somewhat more complete suppression with continued treatment (26, 28, 29). The lack of impact of palbociclib on PgR levels is consistent with its lack of effect on the ER axis. The reduction in the expression of CCND1, an estrogen-dependent gene, at least in CCND1 non-amplified tumors, is also as expected. Given that CCND1 is upstream of CDK4/6 inhibition, no decrease was to be expected with palbociclib. It was interesting to note the increase in CCND1 levels with palbociclib alone and the lesser suppression seen at 14 weeks with the combination compared with letrozole alone. Neither of these were statistically significant but if these results were replicated they would emphasize the value of adding an endocrine agent to the CDK4/6 inhibitor to reduce any build-up of this major stimulant of CDK4/6.
We have previously described the decrease in apoptosis that is seen with the TUNEL method in tumors treated with aromatase inhibitors (30–32). In this study, we used the less complex method of measuring apoptosis with c-PARP with very similar results. Palbociclib alone also showed a decrease in c-PARP and the addition of palbociclib to letrozole enhanced the suppression. We have suggested that these decreases in apoptosis are likely due to the intrinsic linkage of programmed cell death to cell proliferation (29, 31). This linkage is reflected in the correlation seen in the baseline samples from the current study between c-PARP and Ki67. The decrease in apoptosis is consistent with the relatively slow speed of tumor shrinkage with endocrine treatment and CDK4/6 inhibitors whose action is cytostatic rather than cytocidal. The greater decrease in apoptosis with added palbociclib may contribute to the lack of increase in clinical response.
It was particularly notable that although the suppression of Ki67 at 2 weeks by palbociclib alone was greater than that by letrozole alone (95% vs. 81%), the suppression of CCNE1 levels was less (47% vs. 63%). NeoMONARCH had comparative CCNE1 data at 2 weeks between abemaciclib alone and anastrozole alone but the data were of mRNA expression (18). In that case, CCNE1 levels were suppressed more by the CDK4/6 inhibitor than by the aromatase inhibitor. It is unclear for the moment whether this is a drug-specific effect or one which is dependent on means of measuring CCNE1. It was clear that not only were baseline levels of CCNE1 associated with non-CCCA in PALLET but those levels were unaffected by treatment in the non-CCCA cases whereas the levels were markedly suppressed in the CCCA cases. Thus, the relationship between CCNE1 levels and Ki67 response was considerably greater at 14 weeks than before treatment. NeoMONARCH had very small numbers of patients to examine this but CCNE1 mRNA levels were decreased in those that were intrinsically sensitive to abemaciclib+anastrozole but not those that were intrinsically resistant (18).
ESR1 mutations that are constitutively active in the absence of ligand are often acquired during treatment of metastatic disease with an aromatase inhibitor but are much less common in primary breast cancers (16, 17). We recently reported that ESR1 mutations can arise in tumors where patients have been treated for protracted periods of time with neoadjuvant AI. Five of 29 patients that were treated for at least 6 months developed ESR1 mutations and these were associated with higher proliferation than those tumors that maintained wild-type ESR1 (15). In the current study, we therefore tested for mutation in the surgical sample of those cases where Ki67 increased substantially by the time of surgery after showing good evidence of Ki67 suppression during treatment. Only 4 of the 26 patients received >6 months' AI but 2 of those cases developed ESR1 mutations, in one case two separate mutations. This therefore validates the findings in our earlier study that long-term presurgical treatment of tumors with AI can lead to the acquisition of ESR1 mutations in the primary tumor.
Strengths of this study include the randomized design that allowed analyses of predictive factors and pharmacodynamic response to letrozole and palbociclib alone and combined. The primary biomarker and specified secondary end-point analyses were conducted within a centralized highly experienced laboratory. Weaknesses include the small proportion of cases with lack of CCCA that limited the power for identifying predictive factors of resistance. This might be at least partly ameliorated by the use of a proliferative gene expression score, although few of these have the amount of evidence for clinical relevance that Ki67 and CCCA have. The current analysis limited itself to those secondary biomarkers considered most relevant at the beginning of the trial. Genome-wide exploratory analyses are on-going and will provide an assessment of the importance of immune-related factors such as interferon signaling that have recently been reported to be related to resistance to CDK4/6 inhibition in laboratory models and clinical samples from both NeoMONARCH and NeoPalAna (33).
Authors' Disclosures
M. Dowsett reports grants from Pfizer and grants and personal fees from Lilly during the conduct of the study; and personal fees from Radius, G1 Therapeutics, Nanostring, AbbVie, H3 Biomedicine, Zentalis, Agilent, Roche, Astrazeneca, Besins, and ROVI outside the submitted work; as well as a patent for AIR-CIS pending. L.S. Kilburn reports grants from Cancer Research UK during the conduct of the study. M.F. Rimawi reports grants from Pfizer during the conduct of the study and personal fees from Macrogenics, Novartis, Seagen, Daiichi, and Genentech outside the submitted work. C.K. Osborne reports personal fees from Pfizer during the conduct of the study; other support from GeneTex and O'Melveny and Myers, and personal fees from Genentech, Astrazeneca, Tolmar, Eli Lilly, and Wolters Kluwer outside the submitted work. K.L. Pogue-Geile reports grants from NSABP Foundation during the conduct of the study as well as other support from Pfizer outside the submitted work. Y. Liu reports other support from Pfizer Inc., during the conduct of the study. S.L. Puhalla reports grants, personal fees, and other support from Pfizer during the conduct of the study; grants from Astrazeneca, Puma Biotechnology, Medivation, and Eli Lilly, grants and other support from Abbvie and Roche Genentech, and other support from Tesaro and Clovis Oncology outside the submitted work. M.C. Cheang reports other support from Nanostring Technologies and personal fees from Veracyte outside the submitted work; as well as a patent for Breast Cancer Classifier: US Patent No. 9,631,239 licensed and with royalties paid from Nanostring/Veractye. S. Perry reports grants from Pfizer during the conduct of the study and grants from Eli Lilly & Co. and Bayer HealthCare Pharmaceuticals Inc. outside the submitted work. N.C. Turner has received advisory board honoraria from Astrazeneca, Bristol-Myers Squibb, Lilly, Merck Sharpe and Dohme, Novartis, Pfizer, Roche/Genentech, Bicycle Therapeutics, Taiho, Zeno pharmaceuticals, Repare Therapeutics and research funding from Astrazeneca, Bio-Rad, Pfizer, Roche/Genentech, Clovis, Merck Sharpe and Dohme, and Guardant Health. J.M. Bliss reports grants and non-financial support from Pfizer during the conduct of the study and grants from Astrazeneca, Merck Sharpe & Dohme, Puma Biotechnology, Pfizer, Roche, Novartis, Eli Lilly, and Clovis Oncology outside the submitted work. S.R. Johnston reports grants and personal fees from Pfizer and personal fees from Eli Lilly during the conduct of the study as well as personal fees from Novartis and Eisai and grants and personal fees from Puma Biotechnology and Astrazeneca outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
We are grateful to the members of the Ralph Lauren Center for Breast Cancer Research who performed the biomarker analyses. Funded by Pfizer with additional core support to Institute of Cancer Research Clinical Trials and Statistics Unit in the United Kingdom from Cancer Research UK (C1491/A15955) and from the NSABP Foundation in North America. Support also provided by National Institute for Health Research funding to the Royal Marsden and Institute of Cancer Research Biomedical Research Center.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
M. Dowsett: Conceptualization, formal analysis, supervision, funding acquisition, writing–original draft, writing–review and editing. L. Kilburn: Data curation, formal analysis, writing–review and editing. M.F. Rimawi: Conceptualization, investigation, writing–review and editing. C.K. Osborne: Conceptualization, funding acquisition, investigation, writing–review and editing. K. Pogue-Geile: Investigation, writing–review and editing. Y. Liu: Writing-review and editing. S.A. Jacobs: Conceptualization, investigation, project administration, writing–review and editing. M. Finnigan: Investigation, writing–review and editing. S. Puhalla: Investigation, writing–review and editing. A. Dodson: Formal analysis, supervision, investigation, writing–review and editing. V. Martins: Investigation, writing–original draft. M. Cheang: Formal analysis, investigation, writing–review and editing. S. Perry: Data curation, project administration, writing–review and editing. C. Holcombe: Investigation, writing–review and editing. N.C. Turner: Investigation, writing–review and editing. C. Swift: Investigation, writing–review and editing. J.M. Bliss: Conceptualization, formal analysis, funding acquisition, project administration, writing–review and editing. S. Johnston: Conceptualization, supervision, funding acquisition, project administration, writing–review and editing.
References
- 1. Finn RS, Martin M, Rugo HS, Boer K, Bondarenko IM, Kulyket SO, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–36. [DOI] [PubMed] [Google Scholar]
- 2. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 3. Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone receptor–positive advanced breast cancer. N Engl J Med 2015;373:209–19. [DOI] [PubMed] [Google Scholar]
- 4. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018;29:1541–7. [DOI] [PubMed] [Google Scholar]
- 5. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N Yap YS, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–36. [DOI] [PubMed] [Google Scholar]
- 6. Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib Combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020;38:3987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mayer EL, Dueck AC, Martin M, Rubovszky G, Burstein HJ, Bellet-Ezquerra M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2021;22:212–22. [DOI] [PubMed] [Google Scholar]
- 8. Wolff AC. CDK4/6 inhibition in early-stage breast cancer: The New Standard? J Clin Oncol 2020;38:3977–9. [DOI] [PubMed] [Google Scholar]
- 9. Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor–positive early breast cancer: PALLET Trial. J Clin Oncol 2018;37:178–89. [DOI] [PubMed] [Google Scholar]
- 10. Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor–positive breast cancer. Clin Cancer Res 2017;23:4055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Urruticoechea A, Aguilar H, Solé X, Capellà G, Martin L-A, Dowsett M, et al. Preclinical validation of early molecular markers of sensitivity to aromatase inhibitors in a mouse model of post-menopausal hormone-sensitive breast cancer. Breast Cancer Res Treat 2008;109:463–70. [DOI] [PubMed] [Google Scholar]
- 12. Lundgren K, Brown M, Pineda S, Cuzick J, Salter J, Zabaglo L, et al. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res 2012;14:R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor-suppressor promotes resistance to CDK4/6 inhibitors via the Hippo Pathway. Cancer Cell 2018;34:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4014. [DOI] [PubMed] [Google Scholar]
- 15. Leal MF, Haynes BP, Schuster E, Yeo B, Afentakis M, Zabaglo L, et al. Early enrichment of ESR1 mutations and the impact on gene expression in presurgical primary breast cancer treated with aromatase inhibitors. Clin Cancer Res 2019;25:7485–96. [DOI] [PubMed] [Google Scholar]
- 16. Jeselsohn R Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor—a mutations in pretreated advanced estrogen receptor–positive breast cancer. Clin Cancer Res 2014;20:1757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Pearson A, Tarazona N, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 2015;7:313ra182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arnedos M, Bayar MA, Cheaib B, Scott V, Bouakka I, Valent A, et al. Modulation of Rb phosphorylation and antiproliferative response to palbociclib: the preoperative-palbociclib (POP) randomized clinical trial. Annals Oncol 2018;29:1755–62. [DOI] [PubMed] [Google Scholar]
- 19. Hurvitz SA, Martin M, Press MF, Chan D, Fernandez-Abad M, Petru E, et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR+/HER2− breast cancer. Clin Cancer Res 2020;26:566–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor–positive breast cancer. Cancer Res 2016;76:2301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, et al. Cyclin E1 expression and palbociclib efficacy in previously treated Hormone receptor–positive metastatic breast cancer. J Clin Oncol 2019;37:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prat A, Chaudhury A, Solovieff N, Pare L, Martinez D, Chic N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol 2021;39:1458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finn RS, Liu Y, Martin M, Rugo HS, Dieras V, Im S-A, et al. Comprehensive Gene expression biomarker analysis of cyclin-dependent kinases 4/6 and endocrine pathways from the PALOMA-2 study [abstract]. In:Proceedings of the 2017 San Antonio Breast Cancer Symposium; 2017 Dec 5–9; San Antonio, TX. Philadelphia (PA): AACR; 2018. Abstract nr P2–09–10. [Google Scholar]
- 24. O'Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 2018;8:1390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pancholi S, Ribas R, Simigdala N, Schuster E, Nikitorowicz-Buniak J, Ressa A, et al. Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Onogene 2020;39:4781–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A'Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167–70. [DOI] [PubMed] [Google Scholar]
- 27. Smith IE, Robertson J, Kilburn L, Wilcox M, Evans A, Holcombe C, et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone sensitive early breast cancer: (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol 2020;21:1443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Jänicke F, et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res2003;63:6523–31. [PubMed] [Google Scholar]
- 29. Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol 2005;23:2477–92. [DOI] [PubMed] [Google Scholar]
- 30. Harper-Wynne CL, Sacks NPM, Shenton K, MacNeill FA, Sauven P, Laidlaw IJ, et al. Comparison of the systemic and intratumoral effects of tamoxifen and the aromatase inhibitor vorozole in postmenopausal patients with primary breast cancer. J Clin Oncol 2002;20:1026–35. [DOI] [PubMed] [Google Scholar]
- 31. Dowsett M, Smith IE, Ebbs SR, MacNeill FA, Sauven P, Laidlaw IJ, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res 2006;12:1024s–30s. [DOI] [PubMed] [Google Scholar]
- 32. Harrington EA, Fanidi A, Evan GI. Oncogenes and cell death. Curr Opin Genet Dev 1994;4:120–9. [DOI] [PubMed] [Google Scholar]
- 33. De Angelis C, Fu X, Cataldo ML, Nardone A, Pereira R, Veeraraghavan J, et al. Activation of the interferon signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast cancer. Clin Cancer Res 2021;27:4870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.