Abstract
Background:
Essential tremor (ET) is a highly prevalent neurological disease that frequently runs in families. A recent and controversial proposal is to separate ET patients into two distinct groups – ET vs. ET-plus. If this were a valid construct, one would expect in familial aggregation studies to observe that ET-plus would cluster in some families yet be absent in others, rather than being randomly distributed across families. We examined whether there is evidence of familial aggregation of ET-plus.
Methods:
Probands (n=84 [56 ET-plus and 28 ET]) and their first- and second-degree relatives (n=182 and 48) enrolled in a genetics study. Chi-squares and generalized estimating equations (GEE) tested associations between probands’ ET-plus status and the ET-plus status of their relatives.
Results:
Chi-squares analyses revealed that ET-plus was no more prevalent in relatives of probands diagnosed with ET-plus than in relatives of probands diagnosed with ET, p>0.05. Restricting relatives to first-degree relatives similarly did not detect a significant association (p = 0.88). GEE yielded similar results (respective p’s = 0.39 and 0.81).
Conclusion:
The data demonstrate that ET-plus does not seem to aggregate in families. As such, they do not lend support to the notion that ET-plus is a valid biological construct.
Keywords: essential tremor, epidemiology, genetic epidemiology, familial aggregation, clinical, essential tremor-plus
Introduction
Essential tremor (ET) is one of the most prevalent movement disorders, affecting 5% of the population aged ≥65 years [1, 2]. Its hallmark clinical feature is kinetic tremor, although additional motor and non-motor features may occur [2, 3]. ET clusters or aggregates within families [4–8], and greater proportions of ET probands have relatives diagnosed with ET than do non-ET probands [9]. In one analysis, first-degree relatives of ET probands were approximately five times more likely to be diagnosed with ET than were first-degree relatives of control probands [10].
Recently, debate has emerged regarding whether ET is a single disease entity. Some suggest that ET cases who either display non-motor features, or experience motor features in addition to action tremor, should be re-classified as ‘ET-plus’, a distinct entity. Specifically, this proposed new designation, ‘ET-plus’, includes cases who display either (1) impaired tandem gait, (2) questionable dystonic posturing, (3) memory impairment, or (4) mild neurological signs of unknown significance. Such nomenclatural issues have far-reaching consequences, impacting the design and conduct of epidemiological studies, clinical trials and other research [11]. Hence, they require intensive scrutiny.
If ‘ET-plus’ vs. ET differentiation is a valid construct, each entity should have a distinctive biological basis and separable phenotypic characteristics. By extension, underlying susceptibility genes may differ. Importantly, one would expect familial aggregation studies to reveal that ET-plus, as a distinctive entity, clusters in some ET families and is absent in others, rather than being randomly distributed across families. This has yet to be studied.
To test this, we examined whether familial aggregation occurs for ET-plus. Specifically, we compared the prevalence of ET-plus among relatives of probands with and without ET-plus.
Methods
Ascertainment of Probands and Relatives
Probands and their relatives enrolled between 2015 and 2019 in the Family Study of Essential Tremor (FASET) [12, 13], a large genetics initiative whose goal was to identify ET genes. The Columbia and Yale University Institutional Review Boards, the universities of the principal investigators, approved study procedures, and written consent was obtained from participants. Initial contact was made with potential participants via advertisements on several ET websites. We sought potential probands who met the following criteria: 1) diagnosed with ET by a physician; (2) tremor onset by age 50; and (3), at least two relatives reporting a physician-made diagnosis of ET. A senior movement disorders neurologist (E.D.L.) examined four Archimedes spirals provided by each potential proband (two left, two right). If at least one spiral received a Washington Heights-Inwood Genetic Study of Essential Tremor (WHIGET) rating ≥2 (moderate or greater tremor), probands were enrolled [14]. Adult relatives whose contact information was provided by probands were subsequently contacted and enrolled. The sample of probands and relatives enrolled in FASET was geographically diverse, drawing from 46 of the 50 US states, as well as from the District of Columbia.
Evaluation
A trained research assistant visited probands and relatives in their homes. Demographic and clinical questionnaires were administered. A neurological examination, including assessments of postural, kinetic, intention and rest tremors, as well as dystonia and other movement disorders, such as Parkinson’s disease (PD), was videotaped and later evaluated by E.D.L. Procedural details pertinent to the phenotyping of ET and ET-plus appear below [12].
Study Sample
There were 433 enrollees. We subsequently excluded data from probands (1) not meeting criteria for ET, as defined below (n=4), (2) exhibiting “mild dystonia” on examination, as discussed below (n=5), or (3) for whom no first- or second-degree relatives enrolled (n=39). Enrollees (probands or relatives) were additionally excluded if they had a diagnosis of PD (n=11) or were missing pertinent data (n=11). Finally, we excluded relatives who could not be classified as first- or second-degree relatives (n=31), or whose proband was eliminated for reason(s) listed above (n=18). The final sample (n=314) included 84 probands and 230 relatives.
Phenotyping: ET
Participants were defined as having ET if E.D.L. assigned a WHIGET diagnosis of definite, probable, or possible ET; these required at a minimum for possible ET a moderate or greater amplitude kinetic tremor during three or more activities [14]. Of those meeting ET criteria, 19.4%, 50.7% and 29.9% met the definite, probable and possible diagnostic criteria, respectively.
Phenotyping: ET-Plus
Participants designated as having ET-plus met the above-described criteria for ET, and a minimum of one of the following: impaired tandem gait, impaired cognition, questionable dystonic posturing, or mild neurological signs of unknown significance (Table 1).
Table 1.
Criteria for Identification of Cases as ET and ET-Plus
| Classification | Criteria | %a |
|---|---|---|
| ET | No diagnosis of Parkinson’s Disease | 100.0 |
| Meets criteria for one of the following: | ||
| Diagnosis of definite ET | 19.4 | |
| Diagnosis of probable ET | 50.7 | |
| Diagnosis of possible ET | 29.9 | |
| ET-Plusb | Identified as ET | 100.0 |
| No diagnosis of Parkinson’s Disease | 100.0 | |
| Meets criteria for at least one of the following: | ||
| Impaired tandem gait | 51.9 | |
| Impaired cognition | 26.0 | |
| Questionable dystonic posturing | 17.6c | |
| Questionable dystonic posturing | 13.0 | |
| Mild dystonia | 4.6d | |
| Mild neurologic signs of unknown significance | 45.0e | |
| Intention tremor | 31.3 | |
| Rest tremor | 19.8 |
Percent ET or ET-plus cases in final sample meeting each criterion
See text for detailed definitions of criteria for identification of impaired tandem gait, impaired memory (i.e., impaired cognition), questionable dystonic posturing, and mild neurologic signs of unknown significance.
Including both questionable dystonic posturing and mild dystonia.
No probands with mild dystonia were enrolled; thus, all ET-plus cases with mild dystonia were family members.
ET-plus cases meeting criteria for intention tremor, rest tremor, or both.
Impaired tandem gait.
During the videotaped neurological examination, participants walked 10 steps heel-to-toe in a straight line. The number of steps taken off the line (i.e., missteps) served as our assessment of tandem gait impairment. Cut-offs for impaired tandem gait were based on the normative number of missteps reported in the literature for healthy controls drawn from different ages (i.e., 20–29 through 80–89 years)[15, 16]. We classified participants with more missteps than the mean for their age group as impaired tandem gait.
Impaired Cognition.
We assessed cognitive performance, rather than the more limited domain of memory, via scores on the Mini-Mental State Evaluation (MMSE), a 30-item measure of global cognition [17]. We derived age/education specific cut-offs for the identification of impairment from published normative data [18]. Specifically, we identified the mean performance for 14 age groups (e.g., 25–29 years), stratified by four levels of years of education (0–4, 5–8, 9–12, 12+ years), yielding 56 age/education groups. Participants scoring below the mean of their age/education group met our criterion for impaired cognition.
Questionable dystonic posturing.
“Questionable dystonic posturing”, one of the criteria for ET-plus specified in the Consensus paper [19], included individuals with subtle abnormal postures, such as finger pointing or spooning [20–22]. However, a small number of ET cases had dystonic postures that were very mild relative to their kinetic tremor, and that followed their ET diagnosis by many years, but these postures were neither subtle nor of unclear nature. We also included these cases, termed “mild dystonia”, in this category.
Mild neurological signs of unknown significance.
The presence of either intention tremor or rest tremor satisfied this criterion. Intention tremor was assessed in each arm during the finger-nose-finger maneuver, and scored as either 0 (absent), 0.5 (possibly present), or 1 (clearly present). A rating of 1 in at least one arm met the criterion for intention tremor [23]. Rest tremor was assessed while seated with hands in lap, standing with arms at sides, or walking. Rest tremor was categorized as 0 (absent) or 1 (present).
Statistical Analyses
Chi-square statistics compared the distributions of categorical variables (e.g., sex) across or within subject categories (e.g., probands, relatives, age groups). Kolmogorov-Smirnov tests revealed neither age nor years of education to be normally distributed (p’s <0.001); Kruskal-Wallis tests compared these variables across groups.
If ET-plus aggregates in families, one would expect higher prevalence among relatives of probands with ET-plus than among relatives of probands without ET-plus. To test this, we calculated chi-square statistics comparing the prevalence of ET-plus among all relatives (ET and non-ET) of probands with ET-plus versus all relatives of probands without ET-plus. Mann-Whitney and Kruskal-Wallis tests compared the ages of relatives of probands with and without ET-plus.
Finally, we calculated generalized estimating equations (GEEs) in which the presence of ET-plus in probands predicted the presence of ET-plus in their relatives, controlling for age. These provide a more conservative test, accounting for the non-independence of proband-relative pairs within families. These GEEs were conducted separately among first-degree relatives (more genetically similar to their proband) and second-degree relatives (less genetically similar to their proband).
Results
Final Sample
The final sample (n = 314) included 84 probands, 182 first-degree relatives (64 children, 106 siblings, 12 parents), and 48 second-degree relatives (13 grandchildren, 7 aunts/uncles, and 28 niece/nephews). The number of relatives per proband ranged from 1 – 6 (mean = 2.74, median = 3.0).
Probands, first-degree relatives, and second-degree relatives did not differ in sex composition, p = 0.47, or years of education, p = 0.20, but did differ in age, p <0.001. Second-degree relatives were on average younger (mean = 42.2 ± 17.3 years) than were first-degree relatives or probands (means = 60.2 ± 15.0 and 68.1 ± 10.7 years, respectively, p’s <0.05).
Prevalence of ET and ET-Plus
Based on above-described operational definitions of ET and ET-plus, we sorted enrollees into three mutually exclusive categories: ‘normal’, ‘ET’, and ‘ET-plus’ (Table 2). Thirteen enrollees not classified due to missing data (Table 2) were excluded from subsequent analyses. Calculations of the prevalence of ET-plus refers to the proportion of the overall sample (i.e., ET and normal) as opposed to the proportion of ET enrollees satisfying the ET-plus criteria.
Table 2.
Age-Stratified ET and ET-Plus Classifications of Probands, First-degree and Second-degree Relatives
| Probands (n=84) | 1st-degree Relatives (n=182) | 2nd-degree Relatives (n=48) | |
|---|---|---|---|
| All ages | |||
| ET | 21 (27.3) | 24 (13.6) | 12 (25.0) |
| ET-Plus | 56 (72.7) | 66 (37.5) | 9 (18.8) |
| Normal | 0 (00.0) | 86 (48.9) | 27 (56.3) |
| Not classifieda | 7 | 6 | 0 |
| pb | 0.001 | 0.001 | 0.001 |
| 0–40 yrs | |||
| ET | 1 (100.0) | 2 (10.0) | 10 (38.5) |
| ET-Plus | 0 (00.0) | 3 (15.0) | 2 (07.7) |
| Normal | 0 (00.0) | 15 (75.0) | 14 (53.8) |
| Not classifieda | 0 | 0 | 0 |
| 41–50 yrs. | |||
| ET | 1 (33.3) | 8 (27.6) | 0 (00.0) |
| ET-Plus | 2 (66.7) | 5 (17.2) | 0 (00.0) |
| Normal | 0 (00.0) | 16 (55.2) | 7 (100.0) |
| Not classifieda | 0 | 0 | 0 |
| 51–60 yrs. | |||
| ET | 5 (45.5) | 7 (17.1) | 1 (14.3) |
| ET-Plus | 6 (54.5) | 9 (22.0) | 2 (28.6) |
| Normal | 0 (00.0) | 25 (61.0) | 4 (57.1) |
| Not classifieda | 1 | 0 | 0 |
| 61–70 yrs. | |||
| ET | 10 (31.3) | 5 (11.1) | 1 (16.7) |
| ET-Plus | 22 (68.8) | 23 (51.1) | 4 (66.7) |
| Normal | 0 (00.0) | 17 (37.8) | 1 (16.7) |
| Not classifieda | 1 | 1 | 0 |
| 71+ yrs. | |||
| ET | 4 (13.3) | 2 (04.9) | 0 (00.0) |
| ET-Plus | 26 (86.7) | 26 (63.4) | 1 (50.0) |
| Normal | 0 (00.0) | 13 (31.7) | 1 (50.0) |
| Not classifieda | 5 | 5 | 0 |
Enrollees who met criteria for ET, but cannot be classified regarding ET-plus due to missing data. These cases are not included in calculation of percentages.
Chi-square tests comparing ET, ET-plus, and normal frequencies within group. Bolded p values are significant at p < 0.05.
Values are frequencies (column percentages).
Probands were more often identified as ET-plus (72.7%) than ET (27.3%), p <0.001. First-degree relatives were most likely to be classified as normal (48.9%), and least likely to meet the criteria for ET (13.6%), p <0.001.Second-degree relatives were most likely to be classified as normal (56.3%), and least likely to meet the criteria for ET-plus (18.8%), p <0.001 (Table 2)
The relatives of probands with and without ET-plus did not differ in age, all p’s >0.40.
Proband ET-Plus Status as a Predictor of Relative ET-Plus Status
Individual level data are presented in Figure 1. Chi-squares revealed no difference in the overall prevalence of ET-plus in relatives of probands with ET-plus (32.4%) versus that observed for relatives of probands without ET-plus (40.0%), p = 0.29. Similarly, among first-degree relatives, no differences were found between the prevalence of ET-plus in those whose probands had ET-plus versus those whose probands did not have ET-plus (38.5% versus 37.3%), p = 0.88. Contrary to expectations based on familial aggregation, ET-plus was actually more prevalent among second-degree relatives of probands who did not have ET-plus than among those related to probands who did (50.0% versus 7.4%), p = 0.002.
Figure 1: ET vs ET-plus status of enrollees in each family.
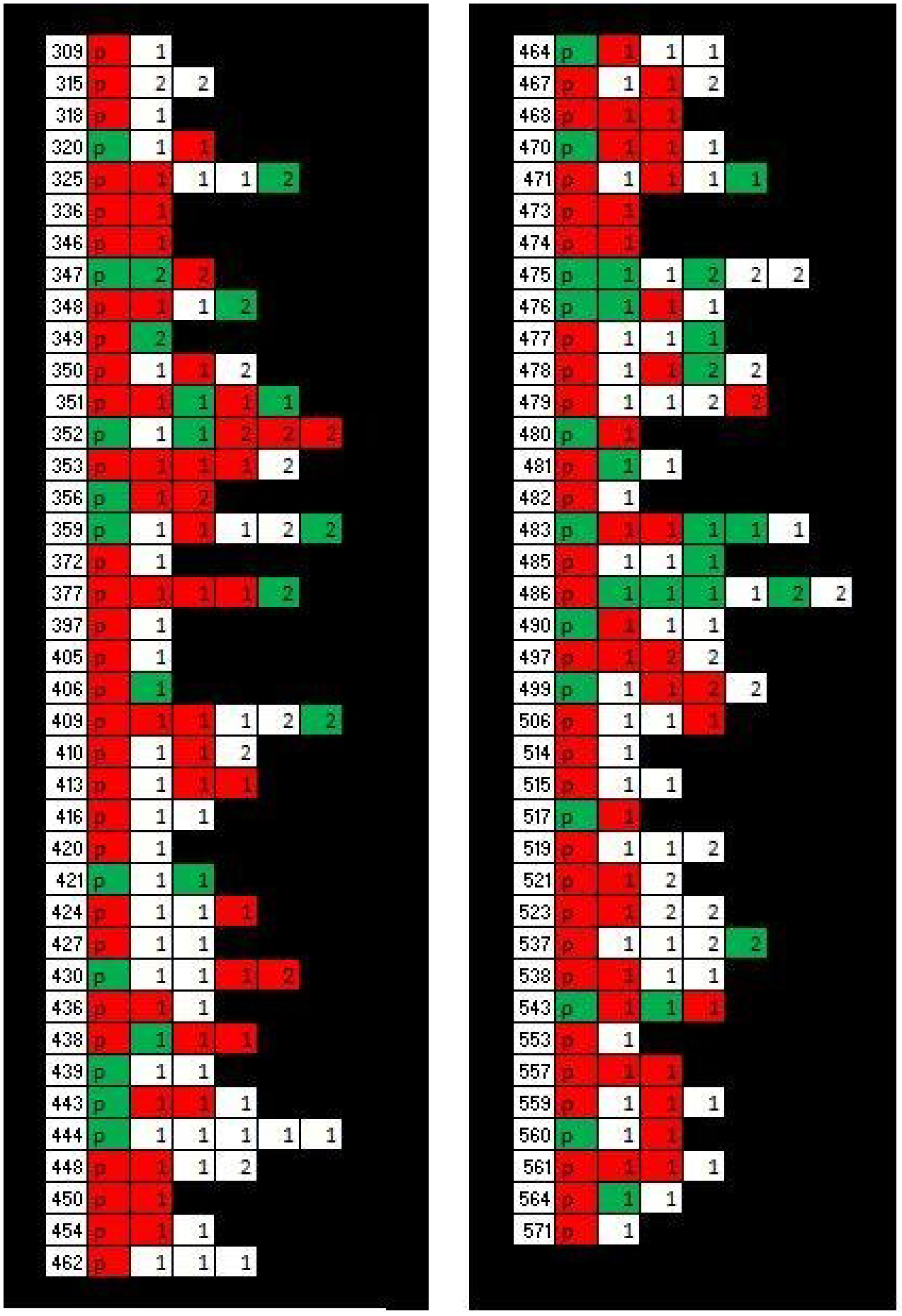
Figure depicts ET/ET-plus status of members of each family included in the final analyses. Left hand column lists family identification code; cells to the right of each code represent members of that family. Status indicated by cell color; red=ET-plus, green=ET, white=normal. P= proband, 1= first-degree relative, 2= second-degree relative. N families/probands = 77 (84 enrolled probands minus seven with unclassified ET/ET-plus status due to missing data), N relatives = 201 (230 enrolled relatives minus 6 with unclassified ET/ET plus status and minus 23 additional relatives of probands with unclassified status).
GEEs yielded parallel results. Specifically, proband ET-plus status was not a significant predictor of relative ET-plus status when controlling for age in equations including either all relatives (odds ratio [OR] = 0.75, 95% confidence interval [CI] = 0.38 – 1.46, p = 0.39), or first-degree relatives (OR=1.09, 95% CI = 0.54 – 2.20, p = 0.81). Although proband status was a significant predictor of second-degree relatives’ ET-plus status (OR = 0.09, 95% CI = 0.01 – 0.60, p = 0.01), this again reflected a higher prevalence of ET-plus among second-degree relatives of probands not diagnosed with ET-plus than among second-degree relatives of probands with ET-plus.
Secondary Analysis
Our analyses revealed no evidence of familial aggregation utilizing an operational definition of ET-plus directly drawn from the previous literature [19]. We also conducted analyses using an alternative set of criteria. Specifically, we no longer included either impaired memory or tandem gait as potential avenues to a diagnosis of ET-plus; these features may inflate the number of enrollees ultimately identified with ET-plus as (1) both are frequently observed at older ages, and (2) our rather broad assessment of memory (i.e., the MMSE) may result in more classifications of impairment than would a more narrowly focused test of memory performance.
Chi-squares revealed no differences in the prevalence of more narrowly defined ET-plus in relatives of probands with ET-plus as opposed to relatives of probands without ET-plus: all relatives (14.0% versus 17.1%), first-degree relatives only (17.3% versus 18.6%), and second-degree relatives only (0% versus 11.5%), p’s = 0.53, 0.83, and 0.13, respectively. Again, GEEs yielded parallel results. Specifically, proband ET-plus status did not predict relative ET-plus status in equations including either all relatives (OR = 0.89, 95% CI= 0.35 – 2.22, p = 0.80) or first-degree relatives (OR = 1.01, 95% CI = 0.40 – 2.53, p = 0.99). (We were unable to compute a parallel equation based on second-degree relatives’ data, given the small number of ET-plus cases in this group).
Discussion
Our data reveal no evidence of familial aggregation of ET-plus. The overall prevalence of ET-plus did not differ between relatives of ET-plus probands (32.4%) and relatives of probands not meeting the criteria for ET-plus (40.0%). Moreover, when stratified by genetic relatedness of family member and proband, the one significant association observed was contrary to the predictions of a familial aggregation model. Specifically, ET-plus was more often observed among second-degree relatives of probands who did not have ET-plus than among those of probands who did have ET-plus.
The lack of familial aggregation of ET-plus, in turn, does not lend credence to the position that the clinical manifestations associated with ET-plus constitute a disease entity with an etiology and course distinct from that of ET. That is, they do not lend support for the notion of ET-plus as a valid biological construct.
Our data contribute to a growing body of work that favors a ‘state’ versus ‘trait’ interpretation of ET-plus [24]. For example, one prospective analysis assigned a diagnosis of ET or ET-plus independently at each of three time intervals [21]. ET-plus diagnoses became more prevalent across time, a finding consistent with the view that the ET-plus reflects an advanced stage of ET. Moreover, ET-plus diagnoses were not particularly stable across time and reverted to an ET diagnosis for a sizable number of cases. Whether such diagnostic instability could contribute to a null finding in a study such as this one is not entirely clear, and we are not able to test this possibility. Another study revealed that the presence of individual elements of the ET-plus designation (e.g., impaired gait, memory impairment, rest tremor) correlated with age and tremor duration [25], again consistent with the suggestion that ET-plus is best described as a stage in the ET disease process.
There were potential limitations to consider. First, in some families, there were few enrolled relatives (Figure 1). Despite this, there were numerous families (n = 46, 54.8%) with three or more enrolled relatives, and many (n = 24, 28.6%) with four or more. Second, if relatives of ET-plus probands and relatives of ET probands had differed by age, this might have affected their risk of ET-plus (i.e., if ET-plus occurs with more advanced age). However, relatives of ET-plus probands and non-ET-plus probands did not differ in age. Furthermore, our GEEs controlled for age. Another issue is that low prevalence of ET or ET-plus among relatives could theoretically have biased results towards the null; however, we selectively enrolled relatives reporting a history of tremor, and more than one-half of enrolled relatives met the criteria for ET or ET-plus (51.0%). Finally, our study is cross-sectional, as is the case for studies of familial aggregation. It would be of additional value to follow probands and their relatives across time and examine the longitudinal course of the prevalence of ET and ET-plus in such groups.
This study also has numerous strengths. First, the sample was drawn from the largest family study of ET to date. Second, it is the only study to examine the prevalence of ET vs. ET-plus in a sample of ET probands as well their first-degree and second-degree relatives (i.e., within the framework of families). Third, all probands and relatives were diagnosed by a movement disorders neurologist based on carefully developed operational procedures.
In sum, the creation of an ET-plus designation would have far-reaching consequences, impacting the design of epidemiological studies, clinical trials and other research [11]. Hence, it deserves intense scrutiny. In this genetic epidemiological study, we assessed whether the newly proposed entity, “ET-plus”, aggregates in ET families. It did not appear to do so. These, along with other published data, do not support the notion that ET-plus is a valid biological construct.
Funding:
This work was supported by National Institutes of Health awards #R01-NS073873 and #R01-NS086736. NIH played no role in the design, collection, analysis or interpretation of these data, or in the writing of this report.
Footnotes
Statement of Ethics: Research complies with internationally accepted standards for research practice and reporting.
Research with Human Subjects: Performed with approval of appropriate ethics committee(s) and appropriate informed consent.
Conflicts of Interest: The authors have no relationship at the time of submission that could reasonably be perceived as a potential conflict of interest.
Data Availability:
The data that support the findings reported here are available from EDL.
References
- 1.Louis ED and McCreary M, How Common is Essential Tremor? Update on the Worldwide Prevalence of Essential Tremor. Tremor Other Hyperkinet Mov (N Y), 2021. 11: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanker V, Essential tremor: diagnosis and management. Bmj, 2019. 366: p. l4485. [DOI] [PubMed] [Google Scholar]
- 3.Reich SG, Essential Tremor. Med Clin North Am, 2019. 103(2): p. 351–356. [DOI] [PubMed] [Google Scholar]
- 4.Tan EK and Schapira AH, Hunting for genes in essential tremor. Eur J Neurol, 2008. 15(9): p. 889–90. [DOI] [PubMed] [Google Scholar]
- 5.Testa CM, Key issues in essential tremor genetics research: Where are we now and how can we move forward? Tremor Other Hyperkinet Mov (N Y), 2013. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwindt G and Rezmovitz J, Essential tremor. Cmaj, 2017. 189(44): p. E1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diez-Fairen M, et al. , Exome-wide rare variant analysis in familial essential tremor. Parkinsonism Relat Disord, 2021. 82: p. 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, et al. , Whole-Genome Study of a Multigenerational Family with Essential Tremor. Can J Neurol Sci, 2021: p. 1–6. [DOI] [PubMed] [Google Scholar]
- 9.Louis ED, Clark L, and Ottman R, Familial Aggregation and Co-Aggregation of Essential Tremor and Parkinson’s Disease. Neuroepidemiology, 2016. 46(1): p. 31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis ED, et al. , Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol, 2001. 49(6): p. 761–9. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, “Essential Tremor Plus”: A Problematic Concept: Implications for Clinical and Epidemiological Studies of Essential Tremor. Neuroepidemiology, 2020. 54(2): p. 180–184. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, et al. , Prevalence and features of unreported dystonia in a family study of “pure” essential tremor. Parkinsonism Relat Disord, 2013. 19(3): p. 359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalec M, et al. , The spiral axis as a clinical tool to distinguish essential tremor from dystonia cases. Parkinsonism Relat Disord, 2014. 20(5): p. 541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis ED, et al. , The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology, 1997. 16(3): p. 124–33. [DOI] [PubMed] [Google Scholar]
- 15.Cohen HS, et al. , Tandem walking as a quick screening test for vestibular disorders. Laryngoscope, 2018. 128(7): p. 1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vereeck L, et al. , Clinical assessment of balance: normative data, and gender and age effects. Int J Audiol, 2008. 47(2): p. 67–75. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, and McHugh PR, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 1975. 12(3): p. 189–98. [DOI] [PubMed] [Google Scholar]
- 18.Crum RM, et al. , Population-based norms for the Mini-Mental State Examination by age and educational level. Jama, 1993. 269(18): p. 2386–91. [PubMed] [Google Scholar]
- 19.Bhatia KP, et al. , Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord, 2018. 33(1): p. 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CY and Louis ED, “Spooning”: A Subtle Sign of Limb Dystonia. Tremor Other Hyperkinet Mov (N Y), 2018. 8: p. 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iglesias-Hernandez D, et al. , “ET Plus”: Instability of the Diagnosis During Prospective Longitudinal Follow-up of Essential Tremor Cases. Front Neurol, 2021. 12: p. 782694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vives-Rodriguez A and Louis ED, Index Finger Pointing (Likely a Subtle Form of Hand Dystonia): Prevalence Across Movement Disorders. Front Neurol, 2018. 9: p. 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sternberg EJ, et al. , Postural and Intention Tremors: A Detailed Clinical Study of Essential Tremor vs. Parkinson’s Disease. Front Neurol, 2013. 4: p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis ED, et al. , Essential tremor-plus: a controversial new concept. Lancet Neurol, 2020. 19(3): p. 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis ED, Huey ED, and Cosentino S, Features of “ET plus” correlate with age and tremor duration: “ET plus” may be a disease stage rather than a subtype of essential tremor. Parkinsonism Relat Disord, 2021. 91: p. 42–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings reported here are available from EDL.


