Abstract
Quantitative electroencephalography (qEEG) refers to the numerical analysis and/or visual transformations of raw electroencephalography (EEG) signals. Evaluation of qEEG in ICUs faces unique challenges that warrant investigation separate from those conducted in other settings. Additionally, the pathophysiology, management, and EEG patterns of critically ill conditions often significantly differ between adults and children. Thus, it is important to distinguish the qEEG literature specifically performed in adult ICUs. The aim of this review is to summarize the studies using qEEG for clinical evaluation of patients in adult ICUs performed over the past decade (since 2010), and to present the state of the art of these techniques. Overall, these studies have reported that qEEG can reveal important information faster than typically possible with traditional methods of reviewing the raw EEG only, with reasonable accuracy. However, it is crucial to emphasize that qEEG must be reviewed in conjunction with raw EEG and in context of understanding the patients’ clinical status. Because each qEEG panel only focuses on a few aspects of the entire EEG, different combinations of qEEG panels may be required for optimal analyses of each medical condition and individual patient. Currently in practical terms, qEEG can serve as a complementary, valuable tool for portions of the EEG that require more detailed review. Further multi-center collaborative studies are needed to ultimately develop standardized methods of employing qEEG that are generalizable across institutions. As qEEG techniques continue to advance, including those involving machine learning, qEEG will further benefit from algorithms specifically suited for ICUs.
Keywords: Quantitative Electroencephalography, Continuous Electroencephalography, Adult Intensive Care
Background
Quantitative electroencephalography (qEEG) refers to the numerical analysis and/or visual transformations of raw electroencephalography (EEG) signals. This field of research and clinical applications have been evolving rapidly, as traditional methods of visually analyzing the raw EEG signals have considerable limitations. These limitations include the lengthy amount of time spent reviewing vast amounts of data, as well as the extremely detailed nature of raw EEG interpretation which may be reduced (but not completely eliminated) by presenting high-level visual qEEG panels. Further, since qEEG essentially provides a “bird’s eye” view of compressed raw data from continuous EEG (cEEG) recordings, there has been interest in its suitability for rapid detection of target events by non-expert EEG readers. In the intensive care units (ICU), the ability to collect time-efficient, objective, and reliable data is crucial, as patients are critically ill, often with life-threatening conditions. As the use of cEEG in ICUs has expanded greatly, various methods of applying qEEG techniques in different clinical scenarios have also been developed [1]. The evaluation of cEEG and qEEG in ICUs is different from that of other inpatient units. First, brain function is affected by metabolic disturbances from systemic illness and/or multi-organ failure, fluctuations in intracranial pressure (ICP), and use of sedatives that include anesthetics and potent analgesics. Diffuse cerebral disturbances therefore result in often widespread alterations of the EEG activity. Second, electrical interference signals from machines, such as ICP monitors, ventilators, continuous veno-venous hemofiltration devices, extracorporeal membrane oxygenation (ECMO) can introduce EEG artifacts. Third, the physical exams, including neurological exams, of ICU patients are often unreliable or unobtainable, making accurate diagnostic tests, including EEGs, all the more necessary and invaluable. Additionally, the pathophysiology, management, and EEG patterns of critically ill conditions are often significantly different between adults and children. Thus, it is important to distinguish the qEEG literature specifically performed in the adult ICU settings, compared to those in non-ICU settings or involving pediatric patients. The aim of this review is to summarize the studies using qEEG for clinical evaluation of patients in the adult ICU setting performed over the past decade (since 2010), and to present the state of the art of these techniques.
Methods
Eligibility Criteria
Inclusion criteria consisted of all manuscripts published in the English language between 2010 and 2022 that evaluate neurophysiologists’ use of qEEG for adult (age ≥18 years) patients hospitalized in the ICU with one of the following medical conditions: seizures, delayed cerebral ischemia, altered mental status, post-cardiac arrest coma, ECMO support, ICP monitoring, and/or EEG suppression monitoring. We included randomized control trials, prospective cohort studies, retrospective cohort studies, and case series, but not editorial or commentary articles. The intervention of interest was the application of qEEG by neurophysiology experts. The outcome of interest was the qEEG results for each aforementioned medical condition. For comparison, the gold standard of EEG interpretation was considered to be the evaluation of the raw EEG.
Exclusion criteria consisted of manuscripts focusing on the pediatric (age <18 years) population, patients who are not hospitalized in the ICU setting, review of the qEEG by non-neurophysiologists, animal studies, and/or basic sciences, published before 2010, and/or written in a language other than English.
Literature Search
The PubMed website, maintained at the United States National Library of Medicine, was initially searched for articles between 2010 and 2022 using the following search terms: ((quantitative EEG) OR (qEEG) OR (quantitative electroencephalography)) AND ((ICU) OR (intensive care) OR (critically ill)), resulting in 238 manuscripts. Titles and abstracts of these manuscripts were reviewed, and those that were not written in the English language, or did not focus on neurophysiologists’ evaluation of qEEG, adult ICU population, or aforementioned medical conditions of interest were removed from review, resulting in 83 manuscripts. The entirety of these manuscripts were then reviewed, of which only 44 met the inclusion criteria. Some of these manuscripts included citations to other relevant articles that also met the inclusion criteria, but were not captured in the initial PubMed search. The most likely reason that these articles were not captured in the initial PubMed search was their titles and abstracts not specifically mentioning the terms “quantitative” EEG, but instead referring to the specific qEEG panels, such as “amplitude EEG” or “burst suppression ratio”. These additional articles were subsequently reviewed as well. Ultimately, 87 relevant manuscripts were included in this review (Figure 1).
Figure 1:
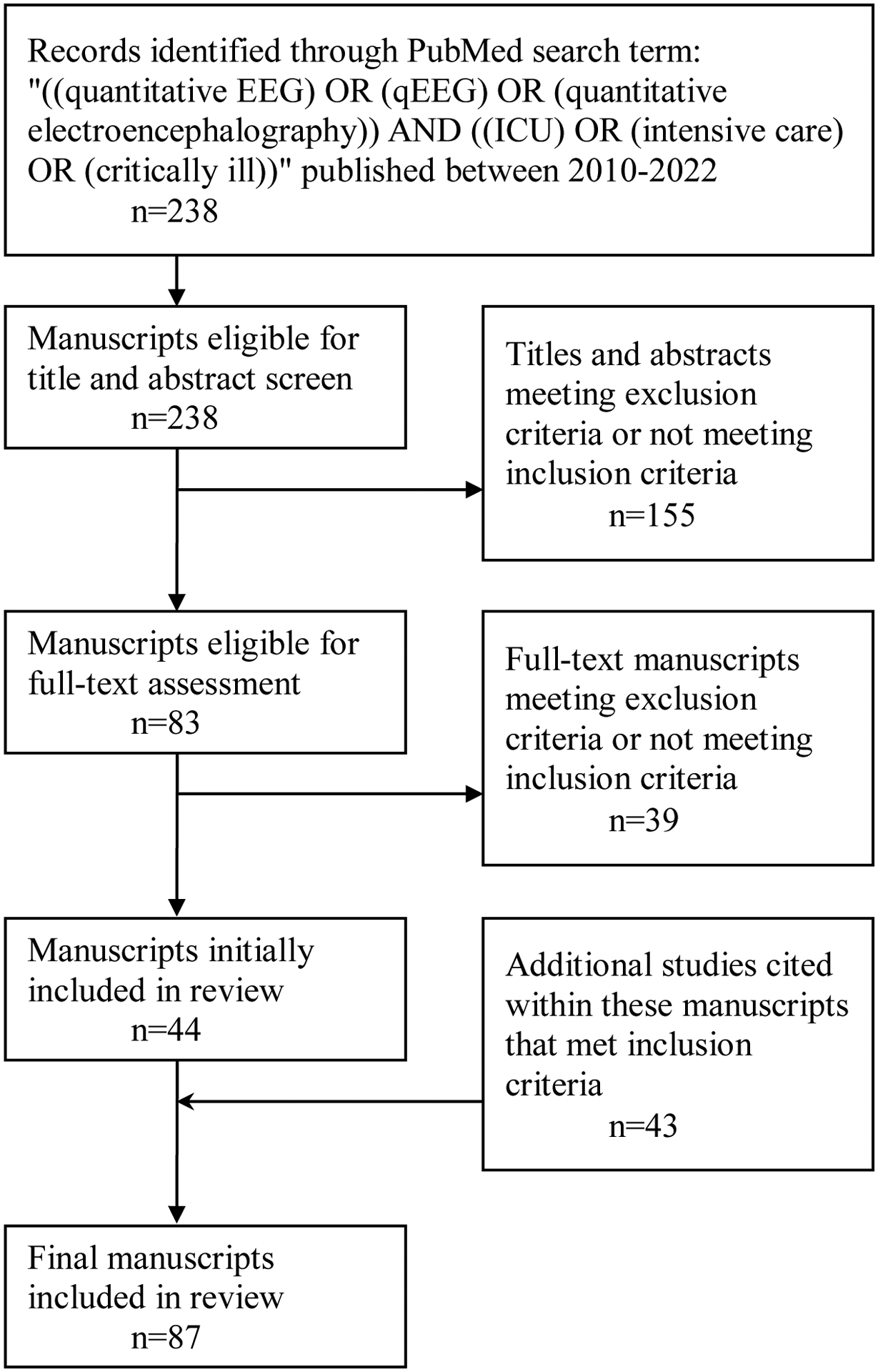
Manuscript Search Method
Brief Summary of qEEG panels
There is no single way to extract and display quantitative EEG data. Instead, qEEG analyses consist of the display of numerous panels, largely divided into 4 categories based on a focus on amplitude, frequency, rhythmicity, or symmetry. Additionally, automated seizure detection algorithms (ASDA) are often employed when cEEG is used for seizure detection. Among the various, commercially available EEG software packages that incorporate qEEG capabilities, Persyst (Persyst Development Corporation) is the most frequently used qEEG software in both the clinical and research settings, and has been approved by the United States Food and Drug Administration (FDA). Table 1 gives an overview of the different panels, and Figures 2–5 show some examples. Each qEEG panel represents a deconstructed component of the EEG and highlights a different aspect of the raw EEG. Thus, each qEEG panel has its own specific benefits and/or shortcomings for the evaluation of various disease processes. While qEEG can generally provide a comprehensive understanding of the raw EEG, this is best achieved when several panels are used in combination with one another. Most importantly, qEEG must be evaluated together with the relevant portions of raw EEG for verification. Certain EEG software packages, including Persyst, allow simultaneous viewing of both the raw EEG and qEEG panels.
Table 1:
Description of Commonly Used Quantitative EEG panels in Intensive Care Units
| qEEG Panel | Purpose | Clinical Use Examples |
|---|---|---|
| Amplitude-focused | ||
| Amplitude integrated EEG (aEEG) | Assess changes in minimum/maximum amplitudes across 1–2 second epochs, filtered to 2–20Hz, for each hemisphere |
|
| Envelope trend | Assess evolutionary changes in median or peak amplitude across 10–20 second epochs for each hemisphere |
|
| Suppression Ratio trend | Assess the average percentage of EEG signals below a specific amplitude threshold |
|
| Frequency-focused | ||
| Fast Fourier Transform (FFT) | Mathematical algorithm that deconstructs waveform data into frequency and amplitude data |
|
| Compressed Spectral Array (CSA)# | Refers to any time-compressed spectrogram produced via FFT |
|
| Color Density Spectral Array (CDSA)* | Deconstructs EEG signal into component frequencies, each of which is plotted with power in color-gradient across 1–10 second epochs, for each hemisphere |
|
| Alpha/Delta ratio (ADR) | Extracts alpha and delta frequency data from EEG signal and plots the trend of their power ratios for each hemisphere |
|
| Relative Alpha Variability (RAV) | Selectively extracts alpha frequency data from EEG signal to plot the trend of percentage of alpha frequency band over the total range of frequencies |
|
| Rhythmicity-focused | ||
| Rhythmicity spectrogram | Proprietary Persyst panel, not available in other qEEG software packages. Deconstructs EEG signal into 4 frequency bins (1–4, 4–9, 9–16, 16–25Hz), and plots the power trend of each frequency bin in color-gradient over time for each hemisphere. |
|
| Symmetry-focused | ||
| Asymmetry spectrogram | Compares power differences between left and right hemispheres across all frequencies |
|
| Automated Seizure Detection Algorithm (ASDA) | Incorporates numerous qEEG parameters to mark the EEG timepoints concerning for seizure |
|
Abbreviations: DCI: delayed cerebral ischemia; EEG: electroencephalography; qEEG: quantitative electroencephalography
Note: The term Compressed Spectral Array is defined and used loosely in the literature. The abbreviation of “CSA” is also sometimes used with a different meaning of “Color Spectral Array” in the literature. Thus, careful examination is required to understand how the authors of each publication defined the term for the purpose of that study.
Note: Also known as Color Spectrogram, Color Spectral Array (CSA), Density Spectral Array (DSA), or FFT Spectrogram, even though CDSA is not the only type of spectrogram produced by Fast Fourier Transform.
Figure 2:
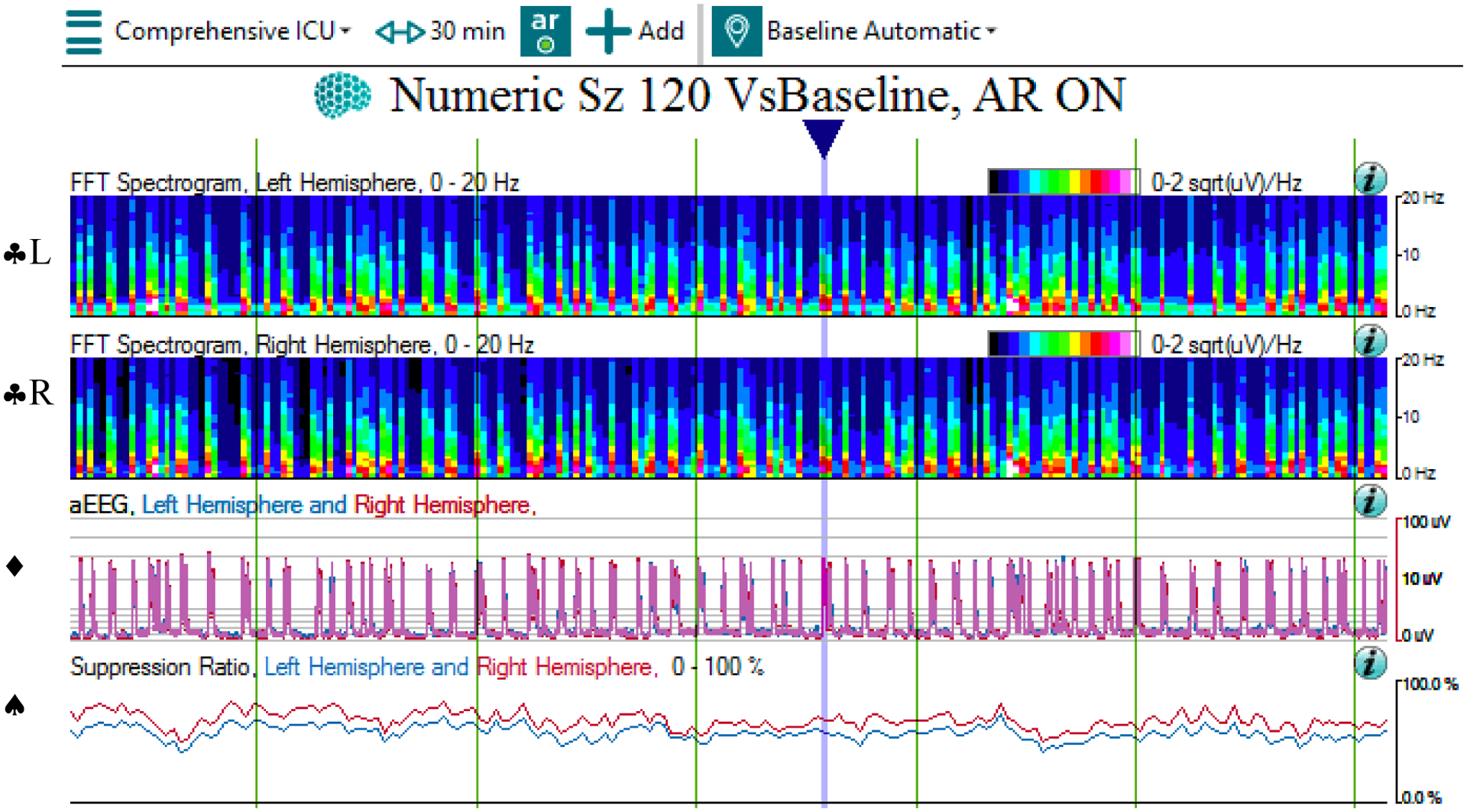
QEEG and raw EEG of a burst suppression pattern
2a: The qEEG panels show transient increase in power and amplitude on the CDSA (♣L, ♣R) and aEEG (♦) panels, as well as a high suppression ratio (♠), corresponding to the bursts (blue arrow), across 30 minutes. Note that the time window of 30 minutes was chosen to better show the individual bursts. Also, note that the CDSA panels for the left (♣L) and right (♣R) hemispheres are separated into two panels. In contrast, the aEEG panel depicts the tracings of left and right hemispheres in the same panel as blue and red colors respectively.
Abbreviations: ♣L: CDSA spectrogram of left hemisphere; ♣R: CDSA spectrogram of right hemisphere; ♦: aEEG spectrogram; ♠: Suppression ratio spectrogram
2b: The corresponding raw EEG shows a burst in an otherwise suppressed background across 10 seconds.
Figure 5:
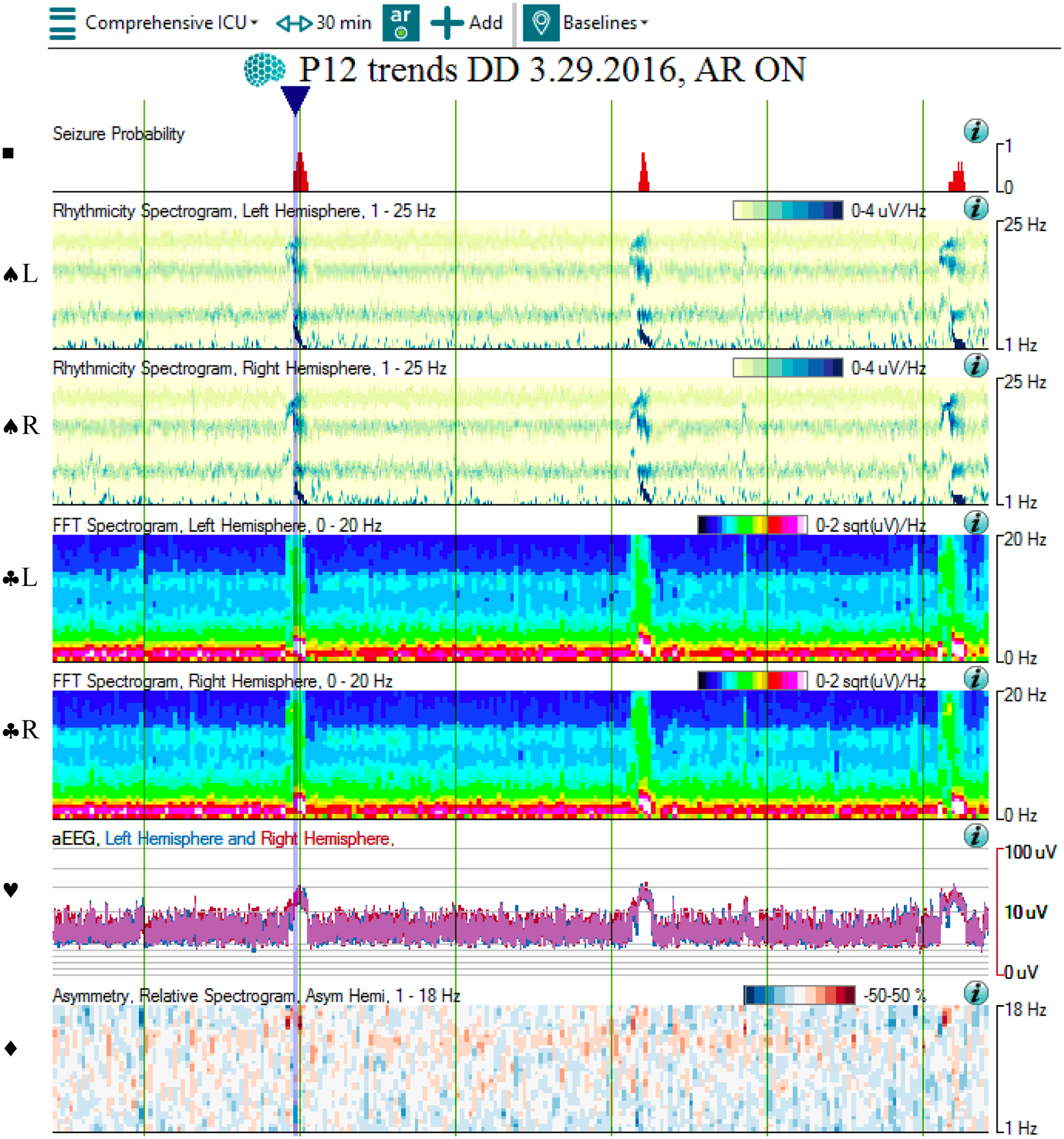
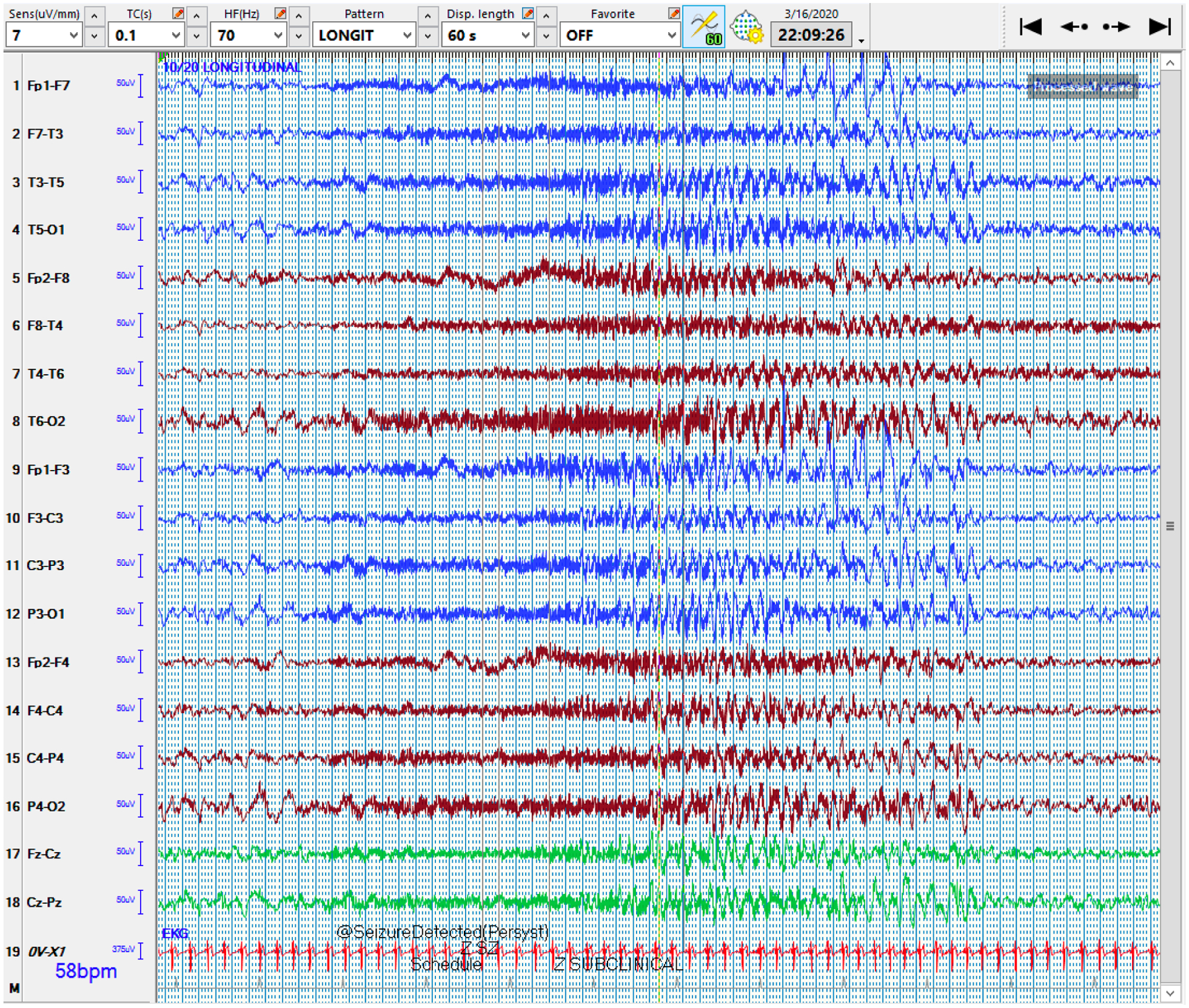
QEEG and raw EEG of a bilateral seizure
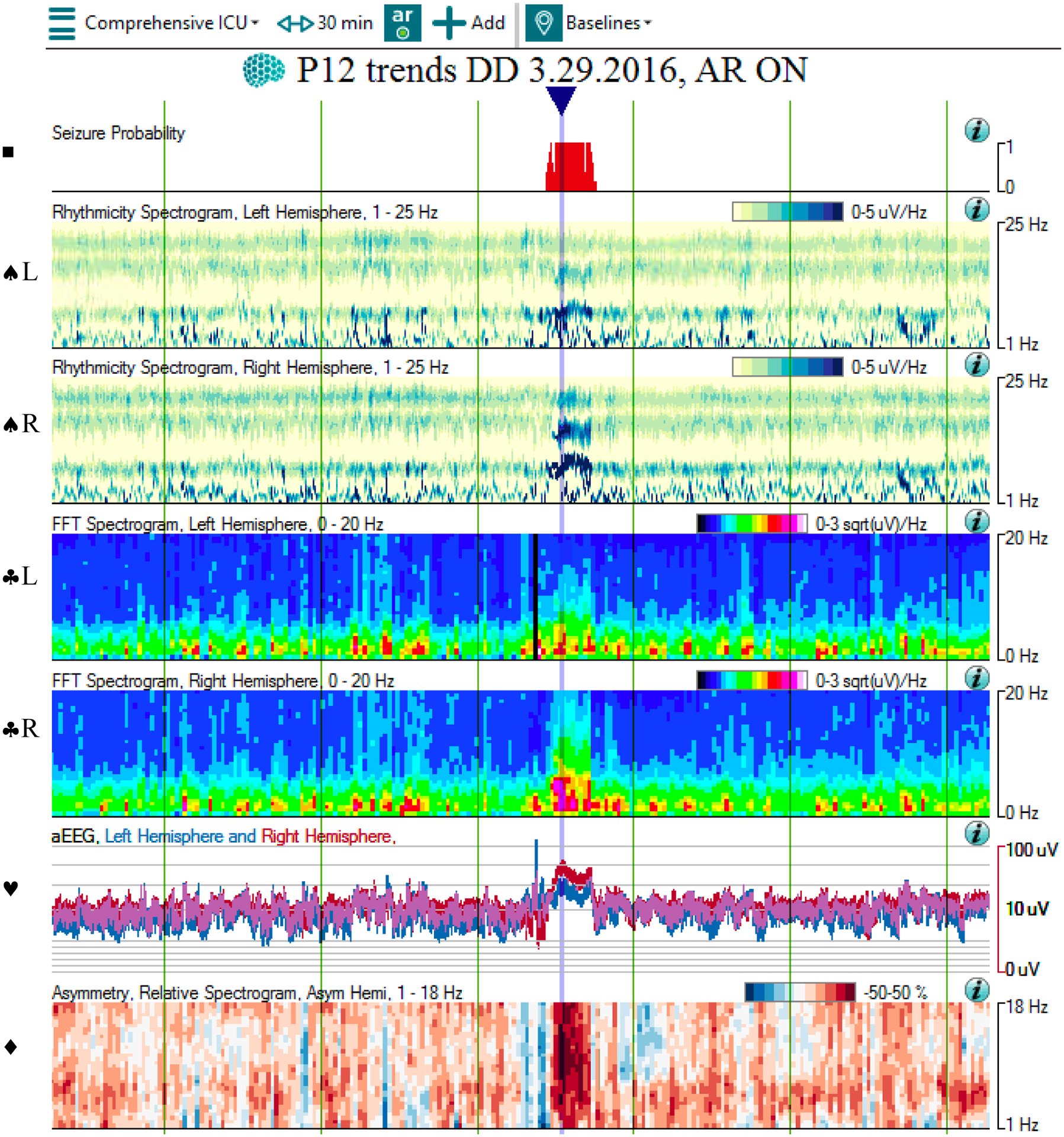
5a: The qEEG shows multiple bilateral seizures occurring over 30 minutes. At the blue arrow, the Seizure Probability panel (◼) identifies one of the seizures. The rhythmicity panel (♠L, ♠R) shows evolution of rhythmicity across frequency bands in bilateral hemispheres. The CDSA (♣L, ♣R) and aEEG (♥) panels show greater increase in amplitude and power in bilateral hemispheres. The asymmetry panel (♦) does not show any laterality of the seizure. Note that the time window of 30 minutes was chosen to better show the evolution of rhythmicity across the frequency bands.
Abbreviations: ◼ Seizure probability panel; ♠L: Rhythmicity spectrogram of left hemisphere; ♠R: Rhythmicity spectrogram of right hemisphere; ♣L: CDSA spectrogram of left hemisphere; ♣R: CDSA spectrogram of right hemisphere; ♥: aEEG spectrogram; ♦: Asymmetry spectrogram
5b: The corresponding raw EEG shows the development and resolution of rhythmic activity in both hemispheres, with evolution in frequency, morphology, and distribution. Note that the time window of 60 seconds was chosen to evolution and devolution of the entire seizure.
Seizure Detection and Management
EEG patterns of convulsive and non-convulsive seizures as well as patterns on the ictal-interictal continuum are common among critically ill patients. In a 2016 survey of members in the American Clinical Neurophysiology Society (ACNS), 92% of neurophysiologists reported using qEEG in the ICU setting for seizure detection [2]. For the purpose of this review, only the studies involving neurophysiologists’ review of qEEG for adult ICU patients are discussed. Specifically, studies of non-neurophysiologists’ use of qEEG and/or pediatric patients are excluded. Compared to reviewing raw cEEGs, much of the enthusiasm for using qEEG has been attributed to the reduced time to review the cEEG data and reasonable accuracy for detecting electrographic epileptiform activity.
Reduced Time of EEG Analysis
Numerous studies have reported that using qEEG reduces the cEEG review time, compared to using raw cEEG alone. One multi-center study performed by experts of raw EEG and qEEG assessed the collective use of 5 qEEG panels, consisting of envelope trend, CDSA, rhythmicity spectrogram, asymmetry spectrogram, and aEEG, for seizure detection in adult ICU patients [3]. This study revealed that the median time to review 6-hour EEG epochs was significantly shorter when using qEEG panels alone or together with the raw cEEG, compared to reviewing the raw cEEG epoch without the help of automated software (6 vs 14.5 vs 19 minutes, p=0.00003). Time-savings by using qEEG in this study were greatest for epochs with no or few seizures, as opposed to epochs with multiple seizures. The most helpful qEEG panels based on a survey in that study were CSA and rhythmicity spectrogram. However, these reductions in analysis time were at the expense of accuracy (51–67% sensitivity and 24 false positive events per 24 hours for qEEG alone vs 63–68% sensitivity and 12 false positive events per 24 hours for qEEG with raw EEG).
In another study involving 3 neurophysiologists with no prior qEEG experience, 4 hours of training on CDSA and aEEG were provided, followed by tasking them with identifying seizures without access to the raw EEG [4]. This study showed that the use of CDSA or aEEG alone reduced the review time for a 24-hour EEG (median time 30.9–55 minutes for CDSA vs 30.5–48.5 minutes for aEEG vs 90–120 minutes for raw EEG alone). The associated measures of accuracy were within reasonable limits as well, as discussed in the next section.
A third study evaluated 3 neurophysiology fellows’ use of CSA, consisting of CDSA and asymmetry spectrogram, for seizure detection [5]. The mean time to review 24 hours of cEEG data was significantly reduced upon reviewing both the CSA and raw cEEG together, compared to the raw cEEG alone (8 minutes vs 38 minutes, p<0.005) without greatly compromising detection accuracy, as discussed in the next section.
While the use of qEEG appears to shorten the review time of EEGs for seizure detection, these results must be interpreted with caution and consideration of two key factors. First, a reduction in the EEG review time is not meaningful if the accuracy of the results is poor. Second, although the initial response to compensate for the low accuracy of individual qEEG panels may be to review numerous panels along with select epochs of raw EEG, the overall review time may then increase, thereby undercutting the time-saving benefit. Thus, a delicate balance must exist among efficiency, accuracy, and practicality in order for qEEG to truly be useful.
Accuracy of qEEG
Individual qEEG panels
Overall, using a single qEEG panel for seizure detection has been shown to not always achieve sufficient accuracy. In a retrospective study by a qEEG expert comparing various Persyst qEEG panels, the sensitivity ranges were as follows: for focal seizures, 29% (rhythmicity spectrogram) to 94% (asymmetry spectrogram); for focal seizures with secondary generalization, 46% (asymmetry spectrogram) to 84% (FFT spectrogram); and for generalized seizures, 16% (asymmetry spectrogram) to 79% (seizure detection trend) [6]. While one limitation of this study was the inclusion of both adults and children in the ICU and epilepsy monitoring units (EMUs), these results suggest that each qEEG panel has its own advantages and disadvantages for identifying different seizure subtypes.
The use of CDSA and aEEG for seizure detection were specifically compared in an aforementioned study involving 3 neurophysiologists without prior qEEG experience [4]. Results were reported as median outcome for each EEG reviewer, giving a range of median outcomes across reviewers, as follows: for CDSA, median values of 80% sensitivity, 4 false positive events per 24 hours, 4 false negative events per 24 hours, and moderate interrater agreement (κ=0.52); For aEEG, median values of 81.3% sensitivity, 2 false positive events per 24 hours, 4 false negative events per 24 hours, and substantial interrater agreement (κ=0.68) [4].
Combination of qEEG panels
Further studies focused on using combinations of numerous qEEG panels together for seizure detection. Most of these studies have shown this approach to be superior to using single qEEG panel alone. One study with 2 qEEG experts explored the use of perhaps the most comprehensive collection of Persyst qEEG panels without the raw EEG, consisting of muscle and eye artifact intensity, seizure and spike detection, seizure probability, rhythmic delta detection, rhythmicity spectrogram, aEEG, FFT spectrogram, asymmetry spectrogram, and suppression ratio [7]. This study reported 93% sensitivity, 61% specificity, 6.5 false positive events per 24 hours, and seizure counts not significantly different from those detected by examining the raw cEEG only. The overall interrater agreement for seizure counts was moderate (κ=0.52), but was better for “definite” seizures (κ=0.64), while poorer for “probable” seizures (κ=0.15). A similar study also evaluated 5 neurophysiologists’ use of 4 Persyst qEEG panels without the raw EEG, consisting of rhythmicity spectrogram, CDSA, asymmetry spectrogram, and aEEG [8]. This study reported 87% sensitivity and 61% specificity, false positive rate of 39%, and moderate interrater agreement (κ=0.48) among neurophysiologists for detecting seizures. Another aforementioned study of 3 neurophysiology fellows using CSA, consisting of CDSA and asymmetry spectrogram, along with the raw EEG reported high sensitivities of 87.3% for seizure detection, 88.5% for epileptiform discharges, and 100% for periodic epileptiform discharges, although it did not report or focus on specificity [5]. Taken together, these studies confirm reasonable sensitivity for seizure detection, but at the expense of specificity, which is only moderate at best. It is also possible that these low specificities are partly due to the study designs that focused on maximizing sensitivity.
In contrast, another study of 9 qEEG experts using 5 Persyst qEEG panels with and without the raw EEG reported less encouraging outcomes [3]. The qEEG tools used in this study included the envelope trend, CSA, rhythmicity spectrogram, asymmetry spectrogram, and aEEG. This study revealed 63–68% mean sensitivities and 12 false positive events per 24 hours when reviewing the qEEG and raw EEG together, and 51–67% mean sensitivities and 24 false positive events per 24 hours when reviewing only the qEEG without the raw EEG. However, this study had notable conditions that stray from the practical use of qEEG in the clinical setting. For example, qEEG reviewers of this study were asked to mark seizures that were only “probable” or “likely”, were not allowed to review the raw EEG unless specifically indicated by the qEEG areas of interest, and could not access the concurrent video recording of the patients. Moreover, a large focus of this study was placed on shortened review time with qEEG.
Overall, the use of qEEG has certainly shown to be promising. However, compared to the gold standard of evaluating the raw EEG, relying solely on qEEG is not currently recommended, given the suboptimal accuracy measures and interrater reliability. Thus, the general consensus in seizure detection is to use qEEG for initial screening and confirmatory purposes that must be reviewed together with the raw EEG [9].
Seizure characteristics influencing qEEG performance
Studies have sought to identify factors that influence the accuracy of qEEG trends for seizure detection. Some of these factors include the “definite” presence of seizures based on the raw EEG; the amplitude, frequency, duration, focality, and recurrence of seizures; rapidity of the seizure evolutions; amount of periodic patterns and/or EEG artifact; number of EEG channels used; and the expertise levels of the evaluating neurophysiologists [3, 4, 6, 10, 11]. The use of qEEG resulted in higher accuracies when reviewed by qEEG experts, when more EEG channels were used as a basis of calculating the qEEG data, and/or when seizures were characterized as “definite”, repetitive, and/or longer in duration. In contrast, the use of qEEG resulted in lower accuracies when the raw EEGs had frequent periodic patterns, and/or seizures characterized as low amplitude, low frequency, slow and/or subtle evolution, short duration, and/or infrequent.
Automated seizure detection
ASDAs incorporate numerous EEG parameters to identify EEG timepoints potentially concerning for seizures, and are included in commercially available EEG software packages, such as Persyst. Studies have reported widely variable abilities of proprietary ASDAs to detect seizures of adult ICU patients, with ranges of 10.1–76.1% sensitivity and 0.3–23 false positive events per 24 hours [3, 6, 11, 12]. The poor accuracy of ASDAs for seizure detection in the ICU setting is likely related to their original development based on EEG data of epilepsy patients in EMUs. Otherwise healthy epilepsy patients typically have fairly well-defined seizures patterns that lend themselves more readily to automated detection. In contrast, critically ill patients often have a significantly abnormal EEG background that confounds the EEG analysis. This is a serious limitation, given that EEG patterns of seizures are often vastly different between patients who are critically ill and those who are not. For example, two commercially available ADSAs, Optima’s IdentEvent and Persyst’s Reveal, were both developed with EEG data of EMU patients, and showed sensitivities of 79.5% and 76% respectively in that patient population [13, 14]. However, when these ADSAs were later applied to adult ICU patients, their sensitivities significantly decreased to 10.1–12.9% [12]. While the manufacturers’ threshold settings of ASDA can be adjusted to increase their sensitivities, the false positive rates rise as a result, rendering them less useful. Additionally, although some studies have reported on developing qEEG algorithms customized for the ICU setting with higher sensitivity and lower false detection rates, their validations across multiple institutions remain to be explored [12, 15].
Standardization of qEEG terms
Any reports on EEG and EEG interpretation were hampered in the past by the absence of a standardized EEG nomenclature. The ACNS developed a standardized terminology for critical care EEG in 2012, which has been highly beneficial for worldwide research efforts [16]. Since then, the ACNS Standardized Critical Care EEG Terminology was updated and expanded upon in 2021 [17]. Similarly, a standardized nomenclature for spectrogram qEEG patterns was proposed in 2020, including new terminology, such as “solid flames”, “broadband-monotonous”, and “low power” [18]. However, further prospective studies involving the use of these terminologies are needed to understand their clinical significance.
Management of Refractory Status Epilepticus
The use of qEEG in the management of refractory status epilepticus is discussed in the “Monitoring of EEG Suppression” section below. Monitoring of EEG suppression is used for several indications and therefore discussed under its own headline.
Detection of Delayed Cerebral Ischemia
Among the various vascular injuries, subarachnoid hemorrhage (SAH) has benefitted the most from the implementation of qEEG. This stems from the potentially dangerous complications of delayed cerebral ischemia (DCI) secondary to cerebral vasospasm, which can develop days after the initial insult and can worsen the outcomes of SAH patients. Thus, timely identification of DCI and timely management is crucial in SAH patients. EEG can be helpful in detecting DCI as the background frequency mix changes in predicable ways during developing ischemia. According to the 2016 ACNS survey, 28.0% of neurophysiologists working in the ICU setting reported using qEEG for detection of vasospasm and/or DCI [2]. The signature qEEG markers for identifying DCI include decrease in power of the alpha frequency band, relative alpha variability (RAV), and alpha/delta ratio (ADR) [19–22]. The definition of the alpha frequency band has varied among studies, ranging from 8–12 to 8–13 Hz. While the exact definition of RAV has also varied, RAV is usually expressed as a ratio of alpha frequencies over the total range of frequencies in the delta, theta, alpha and beta bands. Examples used in different studies include 6–14Hz/1–20Hz or 8–12.5Hz/1–30Hz. ADR is a measure that reflects the decreased alpha power and/or increased delta power observed in patients with DCI. All of these measures are evaluated as trends over time rather than as absolute ratios.
Persistent alpha power reduction by ≥40% for ≥5 hours has been shown to differentiate between patients with and without DCI (78–89% sensitivity, 77–84% specificity) [19, 23]. Notably, alpha power reduction can be suggestive of DCI much earlier than the use of traditional transcranial dopplers by a median of 1–2.3 days [19, 23]. This is likely due to the continuous, automated nature of the EEG recording, compared to the examiner-driven, intermittent doppler studies. Moreover, SAH patients who did not have this reduction in alpha power were found to have a good functional outcome after 6 months [24]. Another study revealed that the lack of increase in alpha power after DCI treatment was suggestive of poor response that require more aggressive spasmolytic management [25]. On the other hand, the reduction in mean daily alpha power was associated with a risk of clinical deterioration after an initial response to DCI treatment that was not attributable to other causes [25].
Similarly, a decrease in RAV or ADR by ≥38% has been shown to detect DCI in SAH patients (100% sensitivity, 83.3% specificity) earlier than diagnoses based on clinical suspicion or new ischemic lesion on computed tomography of the brain (by 7 hours and 44 hours respectively) [21]. The findings regarding the association of decreased ADR and DCI were further corroborated by a meta-analysis of five studies, with a pooled sensitivity and specificity of 83% and 74% respectively [26]. Few smaller studies have further explored the use of the theta frequency band, with reports that ≥40% reduction in theta power for ≥6 hours, or ≥30% decrease in alpha-theta/delta ratio for ≥3.7 hours appears to be a reliable marker for DCI [23, 27].
One major limitation of these studies, however, is the inconsistent standards of diagnosing DCI, ranging from mandatory imaging evidence to clinical suspicion only, and therefore the lack of a true gold standard [28].
Coma and Altered Mental Status
ICU patients often have and/or develop changes in mental status in setting of their critical illnesses, metabolic derangement, and/or medication side effects. The presentation of altered mental status is highly variable, ranging from confusion to a comatose state. The differential diagnosis is also extremely broad, including non-convulsive seizure, posterior circulation stroke, intracranial hemorrhage, intracranial hypertension, cerebral edema, hypoxic ischemic brain injury, toxic/metabolic encephalopathy (delirium), and medication side effects. Due to the wide range of etiologies for altered mental status, various studies are performed as part of the work up, often including cEEG. The initial goal for cEEG in the context of coma and altered mental status is to rule out subclinical seizures as an etiology so that emergent seizure treatment can be administered and its effect monitored.
Encephalopathy can produce a series of non-specific EEG findings that translate into non-specific qEEG patterns. Few small studies reported qEEG changes in adult ICU patients with encephalopathy, including decreased power of higher frequencies in the beta and gamma bands, increased power of delta and theta bands, and reduced functional connectivity [29–32]. While these EEG findings are not unique to encephalopathy, qEEG can be useful in monitoring improvement or worsening of the encephalopathic state, particularly if the alteration of consciousness is significant and subclinical seizures are of ongoing concern.
Sleep quality and arousal patterns in affected patients can also provide clues for the presence of an encephalopathy. These include Stimulus Induced Rhythmic Periodic or Ictal Discharges (SIRPIDs), often associated with offending medications such as Cefepime, which can be identified on qEEG by their sputtering offset pattern [33, 34].
Moreover, there is some evidence that qEEG obtained during the critical illness period may be useful not only in the immediate setting during the patients’ ICU stay, but even after their discharge from the hospital. One very small study reported that qEEG features of adult patients during critical illness correlate with domain-specific cognitive performance at 12-month follow up [35]. ICU survivors with lower relative alpha power in the hospital had worse visuospatial/constructional ability at 12 months after discharge (p=0.008), while those with higher interhemispheric coherence or higher theta-range spectral variability had worse delayed memory performance (p=0.17 and 0.033, respectively) at follow up [35]. Thus, qEEG findings of adult ICU patients may potentially have a role in prognostication related to recovery of mental status, although larger studies are required for further validation.
Post-Cardiac Arrest Coma
Post-cardiac arrest patients are a special subgroup of patients with altered mental status, as they are often comatose. According to the 2016 ACNS survey, 21.3% of neurophysiologists working in the ICU setting reported using qEEG for cardiac arrest patients [2]. The primary use of cEEG for cardiac arrest patients has been for assessment of brain injury severity and seizure detection during the cooling and rewarming stages as well as thereafter. In the comatose post-cardiac arrest patient population, the identification of relevant EEG features with extremely high specificity for poor neurological outcome is crucial, because they may influence decisions related to withdrawal of life sustaining therapies. Nonetheless, EEG should not be used as stand-alone tool for prognostication.
EEG Reactivity
Several studies have reported on the significant predictive value of presence or absence of EEG reactivity for neurological prognostication of comatose post-cardiac arrest patients [36–39]. EEG reactivity is defined as a change in cerebral activity to external stimulation [16]. However, the evaluation of EEG reactivity with the raw cEEG has been shown to be particularly challenging due to poor interrater reliability [40]. In this context, the use of qEEG to assess EEG reactivity has been explored with various methods. A comprehensive study analyzing sixty-two qEEG features pre- and post-stimulus with machine learning techniques identified that spectral power and entropy changes achieved best outcome prediction performances [41]. Other studies have also published on their own unique methodologies for quantifying EEG reactivity, including customized qEEG algorithms and/or machine learning techniques, some of which reported up to 100% specificity or being consistent with the results obtained by conventional analysis of raw cEEG with substantial interrater agreement [42–47]. However, because these analyses were typically research-based and used qEEG markers other than the commercially available panels, most algorithms remain unvalidated. Furthermore, the software threshold for detecting reactivity (e.g. 50%) may be set differently for each patient based on their clinical status, and the evaluation of reactivity is not always uniform, as the number and intensity of physical stimulations can also vary for each patient. Consequently, the use of qEEG for assessing reactivity has not been widely applied to clinical practice.
EEG Background
A suppressed background pattern is another well-known EEG feature highly associated with poor neurological outcome [37, 39]. Numerous studies of post-cardiac arrest patients have explored the identification of EEG background with qEEG methods. aEEG has often been used for this purpose due to its simplicity of interpretation, although it only assesses the changes in amplitude and misses other details related to the background pattern. Studies using aEEG confirmed that recovery of a normal EEG background amplitude following cardiac arrest is associated with favorable neurological outcome [48–53]. In contrast, EEG backgrounds with persistently very low amplitude and/or discontinuous, burst-suppressed, or suppressed patterns are associated with poor neurological outcome [48–54]. Other qEEG panels that more specifically evaluate the suppressed background pattern include the burst suppression ratio, background continuity index, and approximate entropy. Studies using these methods showed that longer inter-burst intervals (IBI), low background continuity index, and/or low approximate entropy are associated with poor neurological outcome [42, 45, 55–60]. These qEEG methods also had better predictive abilities than aEEG, and excellent agreement with the traditional method of visually inspecting the EEG. Another key component in the analysis of EEG background is the temporal trend of qEEG features, as patients’ clinical outcomes appear to differ based on whether the qEEG remains persistently poor or improves over time [50, 52, 61–63]. Thus, it is crucial to serially evaluate the changes in the qEEG background metrics over time.
Other background EEG features not considered to be the traditionally “highly malignant” patterns of post-cardiac arrest patients have also been explored with qEEG. In one study that correlated the outcome of comatose patients after cardiac arrest with various qEEG metrics, including total power and total variability, significant differences were seen between patients with good and poor neurological outcomes [64]. Those with good neurological outcome had higher total power across the frequency spectrum and higher variability across all power bands. Other studies have corroborated these findings as well [57, 66–67]. However, patients whose qEEG analysis initially showed high power that subsequently declined over the next 48 hours post-cardiac arrest ultimately had poor neurological outcome [64]. This finding further supports the notion that the temporal trends of qEEG metrics are important to monitor.
Cerebral Recovery Index and Bispectral Index
Based on the aforementioned findings of numerous EEG variables, other studies focused on developing a comprehensive, single quantitative metric to aid with prognostication of comatose post-cardiac arrest patients. The goal of these indices is to provide objective data extracted from the EEG data in order to provide a simple, numeric tool that is not subject to interrater variability. However, herein lies one of the problems of such indices – they are derived via computation only and therefore subject to EEG artifact.
One example is the cerebral recovery index (CRI), a probability score ranging from 0–1. CRI has undergone several rounds of optimization, the most recent of which occurred in 2018, incorporating 44 qEEG features representing time, frequency, and entropy, as well as machine learning algorithms [68–70]. In that study, CRI at 12 hours after cardiac arrest predicted poor neurological outcome with 100% specificity and 66% sensitivity, and good neurological outcome with 95% specificity and 72% sensitivity. While these results are encouraging, they have not yet been validated in other institutions, and thus require further multi-center studies.
Another combinative qEEG measure that has been studied is the bispectral index (BIS), a dimensionless value ranging 0–100 derived from EEG signals that represents the patients’ level of consciousness. BIS has traditionally been used to monitor the depth of anesthesia, and its use has expanded to other contexts due to its ease of interpretation and set-up. In the cardiac arrest population, higher and lower BIS values have been associated with good and poor neurological outcome respectively [60, 71–76]. However, these studies used varying threshold values of BIS, which causes difficulty implementing BIS in a standardized manner across institutions.
Effects of Sedation on qEEG
Sedatives are known to affect the raw EEG, but some studies have specifically explored the effect of sedatives on qEEG parameters in post-cardiac arrest patients. One study that compared aEEG and suppression ratio values at 10 minutes prior to sedation interruption and at the last 5 minutes of interruption showed that sedation interruption (median 1 hour, range 12–720 minutes) decreased the suppression ratio while increasing the aEEG signals without affecting the prognosticative abilities of these qEEG trends [77]. One of the limitations of this study was that they did not differentiate the individual effects of numerous types of sedatives/anesthetics (i.e. propofol, fentanyl, midazolam, dexmedetomidine, ketamine), which are known to have different EEG signatures [78]. A later study specifically focused on the effects of propofol on qEEG 1 hour before and after cessation, and similarly showed that propofol was associated with significantly decreased EEG amplitude, decreased background continuity and dominant frequency, and increased burst suppression ratio [79]. However, a limitation that both studies have in common is the relatively short sedation interruption period, given that the pharmacokinetics and pharmacodynamics of these medications may vastly differ in post-cardiac arrest patients, who often undergo targeted-temperature management and have concurrent multi-organ failures, compared to the general population.
Special Patient Population: ECMO
ECMO is a type of temporary life support that provides extracorporeal support to patients with refractory cardiac and respiratory failure. This patient population is heterogeneous due to the wide range of indications for ECMO support, including cardiac arrest, post-cardiotomy shock, refractory cardiogenic shock, and hypoxic respiratory failure. The ECMO machinery is also complex and may introduce various EEG artifacts. Studies on qEEG in adult ECMO patients is highly limited. In one small study of post-cardiac arrest ECMO patients, aEEG monitoring provided accurate early prognostic information by predicting patients with continuous normal voltage within the first 24 hours to have good outcome at 6 months with 100% sensitivity and 93% specificity. [80] Another reported lower mean BIS values for post-cardiac arrest ECMO patients who ultimately progressed to brain death (mean BIS 4 during therapeutic hypothermia, 4 post-rewarming) compared to those who did not (mean BIS 39 during therapeutic hypothermia, 57 post-rewarming) [81]. As the use of ECMO is exponentially increasing, further research is needed to better assess the prognostic role of qEEG in ECMO patients.
Intracranial Pressure (ICP) Monitoring
Monitoring ICP in patients with significant brain injury is important, as increased ICP is associated with unfavorable outcome. The limitation of current clinical practice in ICP monitoring is its invasiveness. Etiologies of increased ICP include cerebral edema, intracranial hemorrhage, brain masses, and certain types of meningitis. In patients with refractory intracranial hypertension, one treatment option is to intentionally induce a pharmacologic comatose state (e.g. “pentobarbital coma”) with the aim of burst suppression on EEG. In this context, the use of EEG in this patient population is largely focused on evaluating the treatment effects of sedatives to titrate their doses, and to a lesser degree on identifying signs concerning for intracranial hypertension, although some have explored this.
Detection of Intracranial Hypertension
Few case studies have explored the application of qEEG in the evaluation of increased ICP and brain herniation. One case series included 3 patients with escalating intracranial hypertension and herniation due to hemorrhagic/ischemic stroke [82]. In this study, those 3 patients were found to have progressive asymmetric loss of power of the faster frequencies that was worse and present earlier on the side of the lesions according to CDSA spectrogram, incremental decrease of amplitude on aEEG, and eventual suppression starting on the side of the lesion as indicated on the suppression ratio panel [82]. Upon retrospective analysis, these findings were found to be present 3–24 hours prior to the clinical evidence for herniation [82]. Another case series reported 2 patients with increased ICP that led to herniation, with qEEG findings of increased low-frequency power and subsequent suppression [83]. Notably, the patient with ipsilateral cerebral edema and uncal herniation had asymmetric qEEG findings starting on the ipsilateral side, whereas the other patient with diffuse cerebral edema and transtentorial herniation had symmetric qEEG findings in bilateral hemispheres [83]. These studies were further corroborated by 2 other case reports, one of which further reported the finding of increased delta/alpha ratio that coincided with the timing of the other qEEG findings [84–85]. However, there are clinical scenarios in which applying these results may be extremely dangerous. For example, if a patient with pre-existing pathological EEG spikes were to develop herniation, the decreased power in faster frequencies may result in the disappearance of the EEG spikes. This pseudo-normalization of the EEG may then be mistaken as an improvement in the EEG, which would adversely affect the patient’s outcome. Thus, utmost caution must be taken when interpretation the EEGs of patients who are risk of brain herniation, and further studies are needed to better characterize any other unique EEG markers for these patients.
Management of Refractory Intracranial Hypertension
The use of qEEG in the management of refractory intracranial hypertension is discussed in the “Monitoring of EEG Suppression” and has its own section below. This is due to the fact that monitoring of EEG suppression is not limited to the ICP indications, but also occurs in the same way for the management of status epilepticus using burst suppression.
Monitoring of EEG Suppression
The level of EEG continuity is determined based on the percentage of the EEG record that is suppressed, and is graded as follows: “continuous” (<1%), “nearly continuous” (1–9%), discontinuous (10–49%), “burst suppression” (50–99%), and “suppression” (>99%) [16]. In the 2016 ACNS survey, 59% of neurophysiologists working in the ICU setting reported using qEEG for burst suppression monitoring [2]. Monitoring of EEG continuity is useful for numerous critical illnesses, largely divided into two categories: 1) patients with intentionally induced pharmacological comatose states and burst suppression patterns of EEG for management of refractory status epilepticus and/or intracranial hypertension; and 2) patients with pathological altered mental statuses, including comatose states, due to conditions affecting the brain such as post-cardiac arrest.
The detection of burst suppression with qEEG for the management of refractory status epilepticus is essentially the same process used for post-cardiac arrest comatose patients, as discussed in a prior section. The most commonly used qEEG trend when treating refractory status epilepticus is the burst suppression ratio. Notably, some studies have shown that achieving longer IBI with sedating medications do not necessarily result in improved patient outcome [86–87]. Another study also showed that there were no differences in any of the qEEG spectral power metrics between status epilepticus patients with and without successful weaning of sedating medications [88]. However, this study revealed that qEEG connectivity metrics, including greater network density, characteristic path length, clustering coefficient, size of largest component, and characteristic path length of the largest component, and significantly fewer independent components were associated with successful anesthetic weans [88]. One limitation of relying on qEEG for detecting burst suppression in this patient population has been noted to be the high variation in the amount of burst suppression achieved by anesthetic medications, likely related to significant inter- and intra-individual pharmacokinetic/pharmacodynamic variation [89]. Further qEEG studies are still needed for this patient population, given the lack of clear guidelines for the optimal length and depth of burst suppression with sedating medications.
The use of BIS has also been explored for patients in pharmacologically-induced coma. Overall, these small studies showed strong positive correlations between BIS and EEG burst count, and strong inverse correlations between BIS and EEG suppression ratio [90–91]. These results are consistent with those obtained in the comatose post-cardiac arrest patient population, which is discussed in a prior section.
Numerous studies have sought to create algorithms to quantitatively evaluate burst suppression patterns of adult patients in the ICU setting. For example, one single-center study developed an automated algorithm that detects burst suppression patterns in adult ICU patients [92]. This automated algorithm produced excellent sensitivity and specificity for separately detecting bursts and suppressions (both ranging 93–97%), with higher algorithm-human agreement than human-human agreement. In another multi-center study of adult ICU patients, an automated algorithm was able to detect burst suppression patterns with 90% sensitivity, 84% specificity, and substantial interrater agreement (κ=0.69) [93]. Notably, this algorithm was also able to quantify the durations of these events in real-time, and was not significantly affected by EEG artifacts or periodic patterns. Commercial EEG software packages, such as Persyst, also include panels for detecting EEG continuity, such as the suppression ratio trend. However, such algorithms were developed in non-ICU settings, as previously discussed, and thus, not optimized specifically for ICU patients.
Artifact Reduction
Artifact reduction is a qEEG feature that attempts to remove artifact EEG signals from the raw EEG. The successful removal of artifact signals is particularly useful in the ICU setting, which is laden with electrical interference signals from machines, such as ECMO, ICP monitors, ventilators, and continuous veno-venous hemofiltration devices. Commercially available EEG software packages often include artifact reduction features, such as notch filter. However, accurate artifact reduction is extremely difficult to achieve. First, non-artifactual EEG signals may unintentionally be removed or reduced. This may cause EEG reviewers to overlook subtle yet important findings, including epileptiform discharges, or underestimate qEEG metrics, such as amplitude. Second, even when the fundamental artifact signals are correctly removed, their harmonic frequencies may not be completely accounted for and remain in the EEG, thereby still affecting the final EEG interpretation. Thus, artifact reduction software must be used with caution and should not be relied upon at all times. In certain EEG software packages, including Persyst, both the raw EEG and qEEG panels can be viewed simultaneously. Viewing them side-by-side and toggling the artifact reduction on/off can allow the evaluating neurophysiologists to better understand the extent of artifact reduction, and determine how reliable it may be in that particular epoch.
General Limitations of qEEG in the Adult ICU
While the use of qEEG has become more widespread with encouraging results in the adult ICU setting, it still has critical limitations. First, qEEG analysis is not purely quantitative, and requires subjective, qualitative evaluation of the visual data produced by qEEG. Second, while some studies have sought to create customized algorithms to make the qEEG analysis more numerical and objective, the vast majority of these algorithms have not been validated through multi-center studies. Third, the detailed effects of individual sedating medications on EEG signals in critically ill patients is highly complex and not fully understood, as their pharmacokinetics and pharmacodynamics may differ from the general patient population. Fourth, many studies have not accounted for the time dependence of qEEG features, and the optimal timepoints and frequency to monitor patients with EEG is not well-established.
Conclusion
The use of qEEG in the adult ICU setting has unique challenges that warrant investigation separate from those conducted in other inpatient or outpatient settings. This field of research and its clinical application have expanded rapidly within the past decade. Overall, studies have reported that the integration of qEEG into clinical practice in adult ICUs can reveal important information in faster, more time-efficient, and more easily understandable visual manner than typically possible with traditional methods of reviewing the raw cEEG only. There is also evidence that qEEG review may result in reasonable sensitivity. However, it is crucial to emphasize that qEEG must be reviewed in conjunction with raw cEEG, video monitoring of the patients if available, and in the context of understanding the patients’ clinical status. Moreover, because each qEEG panel only focuses on a single or few aspects of the entire cEEG, different combinations of qEEG panels may be required for optimal analyses of each medical condition and sometimes even each individual patient. Despite its “quantitative” nature, qEEG still has qualitative and subjective aspects in its interpretations, as most qEEG panels still require visual inspection of those trends by trained personnel, preferably neurophysiology experts. Beyond the validated, commercially available software, research tools for qEEG assessment have also been developed but usually lack validation. Currently in practical terms, qEEG can serve as a complementary, valuable tool for portions of the cEEG that require more careful and detailed review. Further multi-center collaborative studies are needed to ultimately develop standardized methods of employing qEEG that are generalizable across institutions. Additionally, as qEEG techniques continue to advance, including those that involve machine learning, qEEG will likely benefit from algorithms specifically suited for the ICU settings that specifically diminishes EEG artifact signals and improves the abilities of quantitative scoring and detecting signals of interest.
Figure 3:
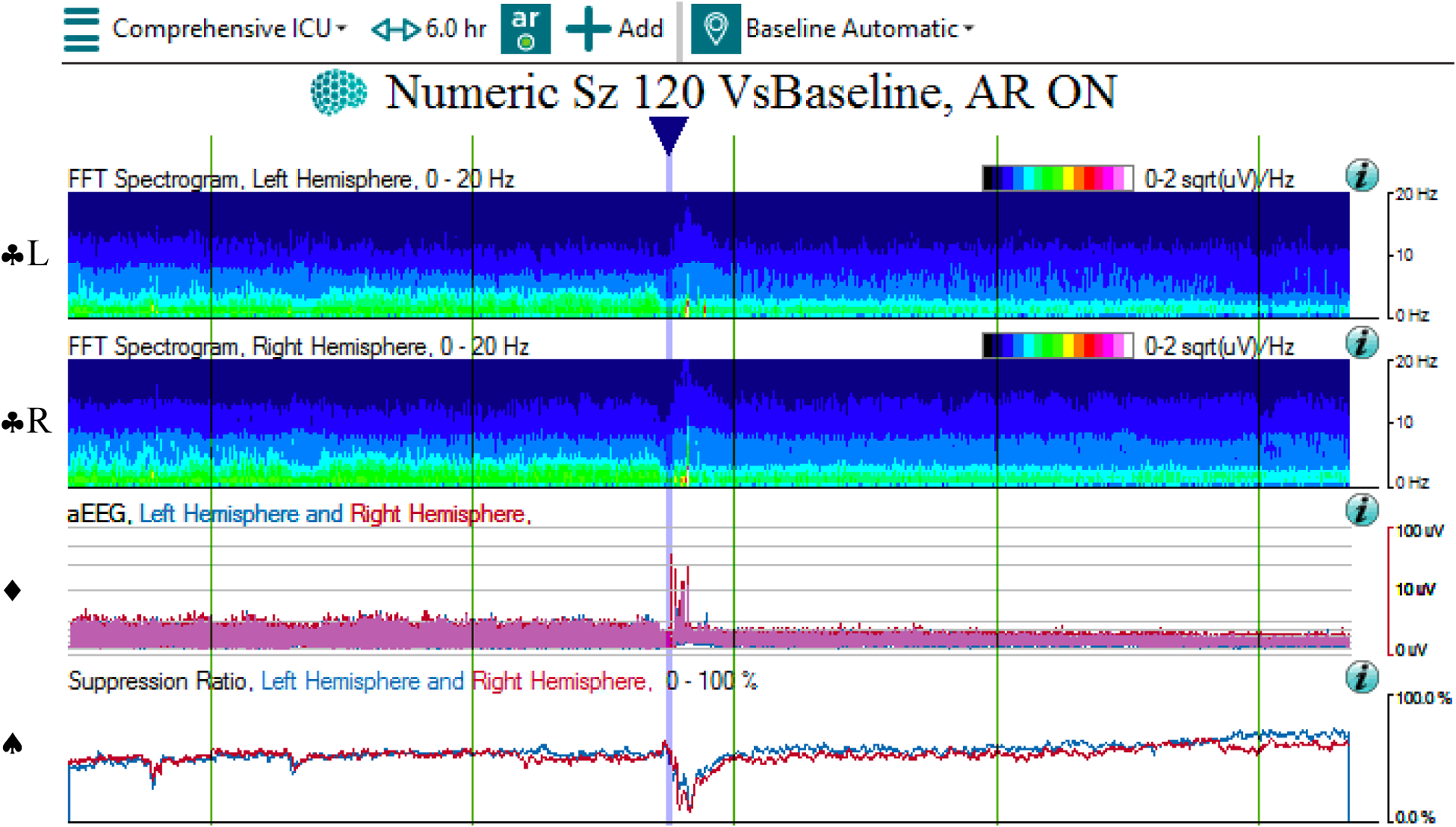
QEEG and raw EEG of suppression
3a: The qEEG shows progressive decline in amplitude and power on the CDSA (♣L, ♣R) and aEEG (♦) panels across 6 hours, as well as rise in suppression ratio (♠), interrupted by artifact (at blue arrow). Note that the time window of 6 hours was chosen to better show the trend of decreased electrocerebral activity over time.
Abbreviations: ♣L: CDSA spectrogram of left hemisphere; ♣R: CDSA spectrogram of right hemisphere; ♦: aEEG spectrogram; ♠: Suppression ratio spectrogram
3b: The corresponding raw EEG shows a suppressed background with impoverished frequency mix across 10 seconds.
3c: The corresponding raw EEG contains superimposed EEG artifact signals that appeared during patient care, which is reflected in the qEEG panel (in 3a at blue arrow).
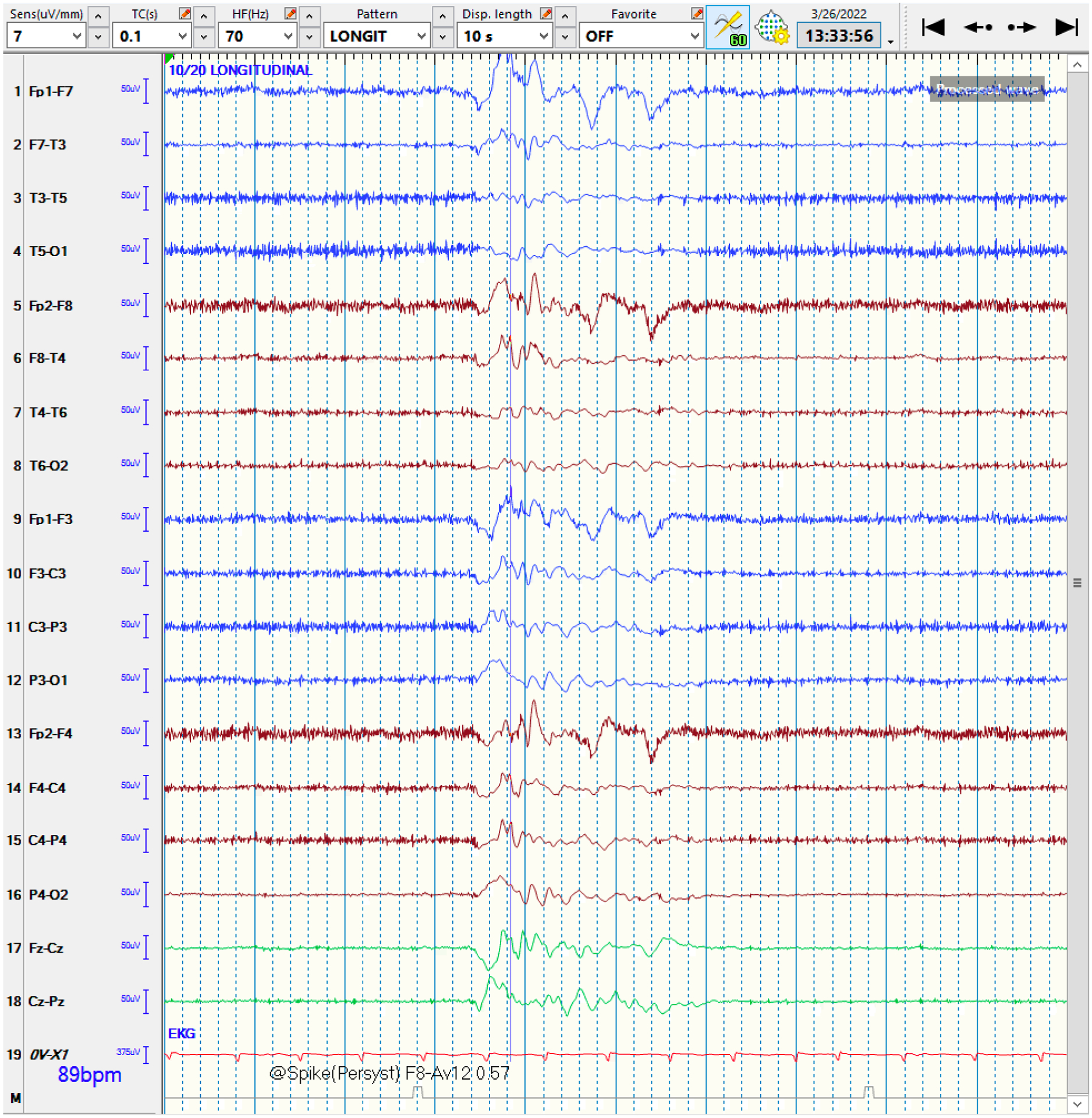
Figure 4:
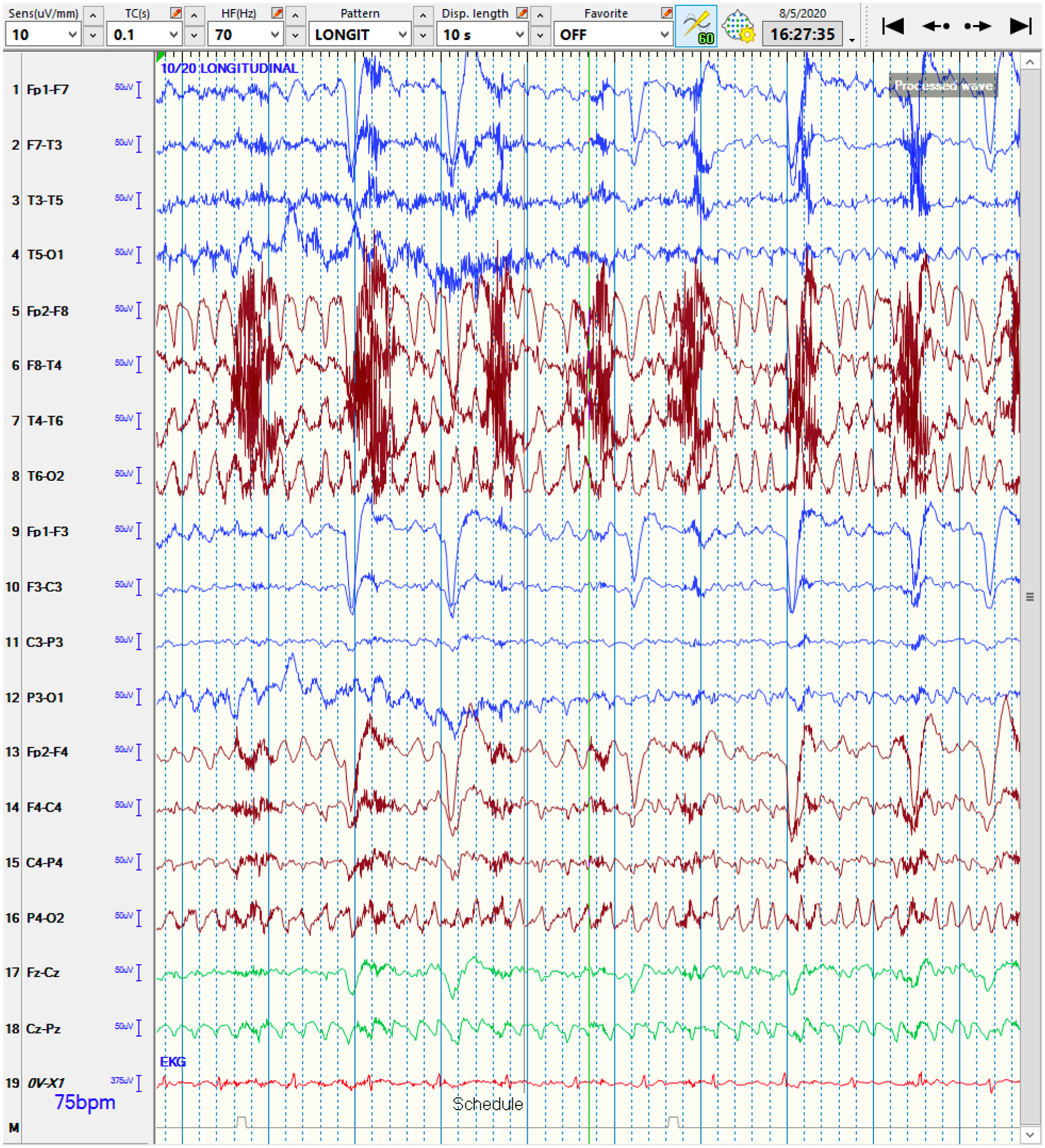
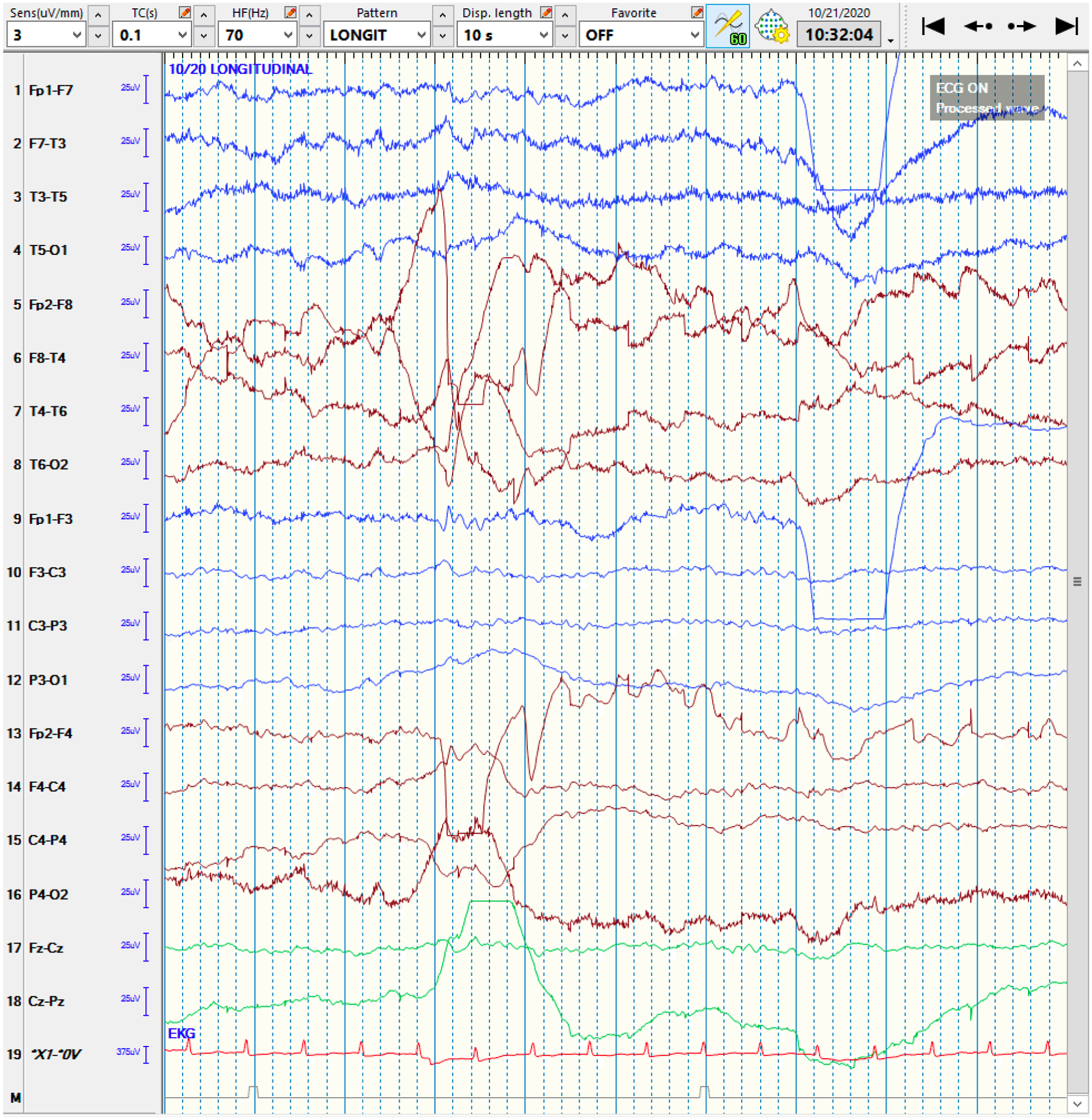
QEEG and raw EEG of a right-hemispheric focal seizure
4a: The qEEG shows evidence of a lateralized seizure in the right hemisphere, lasting roughly 90 seconds. At the blue arrow, the Seizure Probability (◼) panel identifies the seizure. The rhythmicity panel (♠L, ♠R) shows evolution of rhythmicity across frequency bands. The CDSA (♣L, ♣R) and aEEG (♥) panels show greater increase in amplitude and power in the right-hemisphere. The asymmetry panel (♦) shows the right-predominant nature of the seizure. Note that the time window of 30 minutes was chosen to better depict the evolution of rhythmicity across the frequency bands.
Abbreviations: ◼ Seizure probability panel; ♠L: Rhythmicity spectrogram of left hemisphere; ♠R: Rhythmicity spectrogram of right hemisphere; ♣L: CDSA spectrogram of left hemisphere; ♣R: CDSA spectrogram of right hemisphere; ♥: aEEG spectrogram; ♦: Asymmetry spectrogram
4b: The corresponding raw EEG shows the rhythmic activity in the right-hemisphere, corresponding to a focal seizure, with superimposed EEG artifact signals from eye blinking and muscle activation. Note that this epoch is from the middle of the seizure rather than the onset for clarity.
Funding:
No funding, grants, or other support was received.
Footnotes
Competing Interests: Eva K Ritzl is a medical legal expert witness and council member of American Clinical Neurophysiology Society. The remaining authors have no relevant financial or non-financial interests to disclose.
Ethics Approval: This is a literature review. The Johns Hopkins Research Ethics Committee has approved the use of our cEEG database for the extraction of de-identified sample images.
Consent: This is a literature review.
Data and/or Code Availability:
This is a literature review. All publications included in this manuscript are available online, as referenced in the Reference section.
References
- [1].Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, Gerard EE, Hahn CD, Husain AM, Kaplan PW, LaRoche SM, Nuwer MR, Quigg M, Riviello JJ, Schmitt SE, Simmons LA, Tsuchida TN, Hirsch LJ; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society (2015) Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol 32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Swisher CB, Sinha SR (2016) Utilization of Quantitative EEG Trends for Critical Care Continuous EEG Monitoring: A Survey of Neurophysiologists. J Clin Neurophysiol 33: 538–544. doi: 10.1097/WNP.0000000000000287. [DOI] [PubMed] [Google Scholar]
- [3].Haider HA, Esteller R, Hahn CD, Westover MB, Halford JJ, Lee JW, Shafi MM, Gaspard N, Herman ST, Gerard EE, Hirsch LJ, Ehrenberg JA, LaRoche SM; Critical Care EEG Monitoring Research Consortium (2016) Sensitivity of quantitative EEG for seizure identification in the intensive care unit. Neurology 87:935–944. doi: 10.1212/WNL.0000000000003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sun J, Ma D, Lv Y (2018) Detection of seizure patterns with multichannel amplitude-integrated EEG and the color density spectral array in the adult neurology intensive care unit. Medicine (Baltimore) 97:e12514. doi: 10.1097/MD.0000000000012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moura LM, Shafi MM, Ng M, Pati S, Cash SS, Cole AJ, Hoch DB, Rosenthal ES, Westover MB (2014) Spectrogram screening of adult EEGs is sensitive and efficient. Neurology 83:56–64. doi: 10.1212/WNL.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goenka A, Boro A, Yozawitz E (2018) Comparative sensitivity of quantitative EEG (QEEG) spectrograms for detecting seizure subtypes. Seizure 55:70–75. doi: 10.1016/j.seizure.2018.01.008. [DOI] [PubMed] [Google Scholar]
- [7].Alkhachroum A, Ganesan SL, Koren JP, Kromm J, Massad N, Reyes RA, Miller MR, Roh D, Agarwal S, Park S, Claassen J (2022) Quantitative EEG-Based Seizure Estimation in Super-Refractory Status Epilepticus. Neurocrit Care 36:897–904. doi: 10.1007/s12028-021-01395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Swisher CB, White CR, Mace BE, Dombrowski KE, Husain AM, Kolls BJ, Radtke RR, Tran TT, Sinha SR (2015) Diagnostic Accuracy of Electrographic Seizure Detection by Neurophysiologists and Non-Neurophysiologists in the Adult ICU Using a Panel of Quantitative EEG Trends. J Clin Neurophysiol 32:324–330. doi: 10.1097/WNP.0000000000000144. [DOI] [PubMed] [Google Scholar]
- [9].Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, Gerard EE, Hahn CD, Husain AM, Kaplan PW, LaRoche SM, Nuwer MR, Quigg M, Riviello JJ, Schmitt SE, Simmons LA, Tsuchida TN, Hirsch LJ; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society (2015) Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol 32:96–108. doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Akman CI, Micic V, Thompson A, Riviello JJ Jr (2011) Seizure detection using digital trend analysis: Factors affecting utility. Epilepsy Res 93:66–72. doi: 10.1016/j.eplepsyres.2010.10.018. Erratum in: Epilepsy Res. 2011 May;94(3):222. [DOI] [PubMed] [Google Scholar]
- [11].Sierra-Marcos A, Scheuer ML, Rossetti AO (2015) Seizure detection with automated EEG analysis: a validation study focusing on periodic patterns. Clin Neurophysiol 126:456–462. doi: 10.1016/j.clinph.2014.06.025. [DOI] [PubMed] [Google Scholar]
- [12].Sackellares JC, Shiau DS, Halford JJ, LaRoche SM, Kelly KM (2011) Quantitative EEG analysis for automated detection of nonconvulsive seizures in intensive care units. Epilepsy Behav 22 Suppl 1:S69–73. doi: 10.1016/j.yebeh.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kelly KM, Shiau DS, Kern RT, Chien JH, Yang MC, Yandora KA, Valeriano JP, Halford JJ, Sackellares JC (2010) Assessment of a scalp EEG-based automated seizure detection system. Clin Neurophysiol 121:1832–43. doi: 10.1016/j.clinph.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wilson SB, Scheuer ML, Emerson RG, Gabor AJ (2004) Seizure detection: evaluation of the Reveal algorithm. Clin Neurophysiol 115:2280–91. doi: 10.1016/j.clinph.2004.05.018. [DOI] [PubMed] [Google Scholar]
- [15].Bogaarts JG, Hilkman DM, Gommer ED, van Kranen-Mastenbroek VH, Reulen JP (2016) Improved epileptic seizure detection combining dynamic feature normalization with EEG novelty detection. Med Biol Eng Comput 54:1883–1892. doi: 10.1007/s11517-016-1479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, Mani R, Arif H, Jette N, Minazad Y, Kerrigan JF, Vespa P, Hantus S, Claassen J, Young GB, So E, Kaplan PW, Nuwer MR, Fountain NB, Drislane FW (2013) American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- [17].Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, Lee JW, Wusthoff CJ, Hahn CD, Westover MB, Gerard EE, Herman ST, Haider HA, Osman G, Rodriguez-Ruiz A, Maciel CB, Gilmore EJ, Fernandez A, Rosenthal ES, Claassen J, Husain AM, Yoo JY, So EL, Kaplan PW, Nuwer MR, van Putten M, Sutter R, Drislane FW, Trinka E, Gaspard N (2021) American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol 38:1–29. doi: 10.1097/WNP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zafar SF, Amorim E, Williamsom CA, Jing J, Gilmore EJ, Haider HA, Swisher C, Struck A, Rosenthal ES, Ng M, Schmitt S, Lee JW, Brandon Westover M (2020) A standardized nomenclature for spectrogram EEG patterns: Inter-rater agreement and correspondence with common intensive care unit EEG patterns. Clin Neurophysiol 131:2298–2306. doi: 10.1016/j.clinph.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mueller TM, Gollwitzer S, Hopfengärtner R, Rampp S, Lang JD, Stritzelberger J, Madžar D, Reindl C, Sprügel MI, Dogan Onugoren M, Muehlen I, Kuramatsu JB, Schwab S, Huttner HB, Hamer HM (2021) Alpha power decrease in quantitative EEG detects development of cerebral infarction after subarachnoid hemorrhage early. Clin Neurophysiol 132:1283–1289. doi: 10.1016/j.clinph.2021.03.005. [DOI] [PubMed] [Google Scholar]
- [20].Baang HY, Chen HY, Herman AL, Gilmore EJ, Hirsch LJ, Sheth KN, Petersen NH, Zafar SF, Rosenthal ES, Westover MB, Kim JA (2022) The Utility of Quantitative EEG in Detecting Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. J Clin Neurophysiol 39:207–215. doi: 10.1097/WNP.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rots ML, van Putten MJ, Hoedemaekers CW, Horn J (2016) Continuous EEG Monitoring for Early Detection of Delayed Cerebral Ischemia in Subarachnoid Hemorrhage: A Pilot Study. Neurocrit Care 24:207–216. doi: 10.1007/s12028-015-0205-y. [DOI] [PubMed] [Google Scholar]
- [22].Stuart RM, Waziri A, Weintraub D, Schmidt MJ, Fernandez L, Helbok R, Kurtz P, Lee K, Badjatia N, Emerson R, Mayer SA, Connolly ES, Hirsch LJ, Claassen J. Intracortical EEG for the detection of vasospasm in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2010. Dec;13(3):355–8. doi: 10.1007/s12028-010-9414-6. [DOI] [PubMed] [Google Scholar]
- [23].Gollwitzer S, Groemer T, Rampp S, Hagge M, Olmes D, Huttner HB, Schwab S, Madžar D, Hopfengaertner R, Hamer HM (2015) Early prediction of delayed cerebral ischemia in subarachnoid hemorrhage based on quantitative EEG: A prospective study in adults. Clin Neurophysiol 126:1514–1523. doi: 10.1016/j.clinph.2014.10.215. [DOI] [PubMed] [Google Scholar]
- [24].Gollwitzer S, Müller TM, Hopfengärtner R, Rampp S, Merkel J, Hagge M, Jukic J, Lang J, Madžar D, Onugoren MD, Huttner HB, Schwab S, Hamer HM (2019) Quantitative EEG After Subarachnoid Hemorrhage Predicts Long-Term Functional Outcome. J Clin Neurophysiol; 36:25–31. doi: 10.1097/WNP.0000000000000537. [DOI] [PubMed] [Google Scholar]
- [25].Rathakrishnan R, Gotman J, Dubeau F, Angle M (2011) Using continuous electroencephalography in the management of delayed cerebral ischemia following subarachnoid hemorrhage. Neurocrit Care 14:152–161. doi: 10.1007/s12028-010-9495-2. [DOI] [PubMed] [Google Scholar]
- [26].Yu Z, Wen D, Zheng J, Guo R, Li H, You C, Ma L (2019) Predictive Accuracy of Alpha-Delta Ratio on Quantitative Electroencephalography for Delayed Cerebral Ischemia in Patients with Aneurysmal Subarachnoid Hemorrhage: Meta-Analysis. World Neurosurg 126:e510–e516. doi: 10.1016/j.wneu.2019.02.082. [DOI] [PubMed] [Google Scholar]
- [27].Balança B, Dailler F, Boulogne S, Ritzenthaler T, Gobert F, Rheims S, Andre-Obadia N (2018) Diagnostic accuracy of quantitative EEG to detect delayed cerebral ischemia after subarachnoid hemorrhage: A preliminary study. Clin Neurophysiol 129:1926–1936. doi: 10.1016/j.clinph.2018.06.013. [DOI] [PubMed] [Google Scholar]
- [28].Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- [29].Jin T, Jin H, Lee SM (2022) Using Electroencephalogram Biosignal Changes for Delirium Detection in Intensive Care Units. J Neurosci Nurs 54:96–101. doi: 10.1097/JNN.0000000000000639. [DOI] [PubMed] [Google Scholar]
- [30].Hunter A, Crouch B, Webster N, Platt B (2020) Delirium screening in the intensive care unit using emerging QEEG techniques: A pilot study. AIMS Neurosci 7:1–16. doi: 10.3934/Neuroscience.2020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Dellen E, van der Kooi AW, Numan T, Koek HL, Klijn FA, Buijsrogge MP, Stam CJ, Slooter AJ (2014) Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology 121:328–335. doi: 10.1097/ALN.0000000000000329. [DOI] [PubMed] [Google Scholar]
- [32].Zubler F, Koenig C, Steimer A, Jakob SM, Schindler KA, Gast H (2016) Prognostic and diagnostic value of EEG signal coupling measures in coma. Clin Neurophysiol 127: 2942–2952. doi: 10.1016/j.clinph.2015.08.022. [DOI] [PubMed] [Google Scholar]
- [33].Johnson E, Hannawi Y, Martinez NC, Ritzl EK (2016) Cefepime-Associated SIRPIDs in a Patient With Normal Renal Function. Neurohospitalist 6:167–169. doi: 10.1177/1941874415611180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johnson EL, Kaplan PW, Ritzl EK (2017) Termination patterns of stimulus-induced rhythmic, periodic, or ictal patterns and spontaneous electrographic seizures. Clin Neurophysiol 128:2279–2285. doi: 10.1016/j.clinph.2017.09.006. [DOI] [PubMed] [Google Scholar]
- [35].Williams Roberson S, Azeez NA, Taneja R, Pun BT, Pandharipande PP, Jackson JC, Ely EW (2022) Quantitative EEG During Critical Illness Correlates With Patterns of Long-Term Cognitive Impairment. Clin EEG Neurosci 53:435–442. doi: 10.1177/1550059420978009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Amorim E, Rittenberger JC, Zheng JJ, Westover MB, Baldwin ME, Callaway CW, Popescu A; Post Cardiac Arrest Service (2016) Continuous EEG monitoring enhances multimodal outcome prediction in hypoxic-ischemic brain injury. Resuscitation 109:121–126. doi: 10.1016/j.resuscitation.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rossetti AO, Tovar Quiroga DF, Juan E, Novy J, White RD, Ben-Hamouda N, Britton JW, Oddo M, Rabinstein AA (2017) Electroencephalography Predicts Poor and Good Outcomes After Cardiac Arrest: A Two-Center Study. Crit Care Med 45:e674–e682. doi: 10.1097/CCM.0000000000002337. [DOI] [PubMed] [Google Scholar]
- [38].Westhall E, Rossetti AO, van Rootselaar AF, Wesenberg Kjaer T, Horn J, Ullén S, Friberg H, Nielsen N, Rosén I, Åneman A, Erlinge D, Gasche Y, Hassager C, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wetterslev J, Wise MP, Cronberg T; TTM-trial investigators (2016) Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 86:1482–1490. doi: 10.1212/WNL.0000000000002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Backman S, Cronberg T, Friberg H, Ullén S, Horn J, Kjaergaard J, Hassager C, Wanscher M, Nielsen N, Westhall E (2018) Highly malignant routine EEG predicts poor prognosis after cardiac arrest in the Target Temperature Management trial. Resuscitation 131:24–28. doi: 10.1016/j.resuscitation.2018.07.024. Erratum in: Resuscitation. 2019 Dec;145:82. [DOI] [PubMed] [Google Scholar]
- [40].Westhall E, Rosén I, Rossetti AO, van Rootselaar AF, Wesenberg Kjaer T, Friberg H, Horn J, Nielsen N, Ullén S, Cronberg T (2015) Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol 126:2397–2404. doi: 10.1016/j.clinph.2015.03.017. [DOI] [PubMed] [Google Scholar]
- [41].Amorim E, van der Stoel M, Nagaraj SB, Ghassemi MM, Jing J, O’Reilly UM, Scirica BM, Lee JW, Cash SS, Westover MB (2019) Quantitative EEG reactivity and machine learning for prognostication in hypoxic-ischemic brain injury. Clin Neurophysiol 130:1908–1916. doi: 10.1016/j.clinph.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Admiraal MM, Ramos LA, Delgado Olabarriaga S, Marquering HA, Horn J, van Rootselaar AF (2021) Quantitative analysis of EEG reactivity for neurological prognostication after cardiac arrest. Clin Neurophysiol 132:2240–2247. doi: 10.1016/j.clinph.2021.07.004. [DOI] [PubMed] [Google Scholar]
- [43].Johnsen B, Nøhr KB, Duez CHV, Ebbesen MQ (2017) The Nature of EEG Reactivity to Light, Sound, and Pain Stimulation in Neurosurgical Comatose Patients Evaluated by a Quantitative Method. Clin EEG Neurosci 48:428–437. doi: 10.1177/1550059417726475. [DOI] [PubMed] [Google Scholar]
- [44].Liu G, Su Y, Jiang M, Chen W, Zhang Y, Zhang Y, Gao D (2016) Electroencephalography reactivity for prognostication of post-anoxic coma after cardiopulmonary resuscitation: A comparison of quantitative analysis and visual analysis. Neurosci Lett 626:74–78. doi: 10.1016/j.neulet.2016.04.055. [DOI] [PubMed] [Google Scholar]
- [45].Noirhomme Q, Lehembre R, Lugo Zdel R, Lesenfants D, Luxen A, Laureys S, Oddo M, Rossetti AO (2014) Automated analysis of background EEG and reactivity during therapeutic hypothermia in comatose patients after cardiac arrest. Clin EEG Neurosci 45:6–13. doi: 10.1177/1550059413509616. [DOI] [PubMed] [Google Scholar]
- [46].Duez CHV, Ebbesen MQ, Benedek K, Fabricius M, Atkins MD, Beniczky S, Kjaer TW, Kirkegaard H, Johnsen B (2018) Large inter-rater variability on EEG-reactivity is improved by a novel quantitative method. Clin Neurophysiol 129:724–730. doi: 10.1016/j.clinph.2018.01.054. [DOI] [PubMed] [Google Scholar]
- [47].Hermans MC, Westover MB, van Putten MJAM, Hirsch LJ, Gaspard N (2016) Quantification of EEG reactivity in comatose patients. Clin Neurophysiol 127:571–580. doi: 10.1016/j.clinph.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sugiyama K, Miyazaki K, Ishida T, Tanabe T, Hamabe Y (2018) Categorization of post-cardiac arrest patients according to the pattern of amplitude-integrated electroencephalography after return of spontaneous circulation. Crit Care 22:226. doi: 10.1186/s13054-018-2138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ihara S, Sakurai A, Kinoshita K, Yamaguchi J, Sugita A (2019) Amplitude-Integrated Electroencephalography and Brain Oxygenation for Postcardiac Arrest Patients with Targeted Temperature Management. Ther Hypothermia Temp Manag 9:209–215. doi: 10.1089/ther.2018.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Oh SH, Park KN, Shon YM, Kim YM, Kim HJ, Youn CS, Kim SH, Choi SP, Kim SC (2015) Continuous Amplitude-Integrated Electroencephalographic Monitoring Is a Useful Prognostic Tool for Hypothermia-Treated Cardiac Arrest Patients. Circulation 132:1094–103. doi: 10.1161/CIRCULATIONAHA.115.015754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tian G, Qin K, Wu YM, Ji Z, Wang JX, Pan SY (2012) Outcome prediction by amplitude-integrated EEG in adults with hypoxic ischemic encephalopathy. Clin Neurol Neurosurg 114:585–589. doi: 10.1016/j.clineuro.2011.12.011. [DOI] [PubMed] [Google Scholar]
- [52].Sugiyama K, Kashiura M, Akashi A, Tanabe T, Hamabe Y (2016) Prognostic value of the recovery time of continuous normal voltage in amplitude-integrated electroencephalography in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia: a retrospective study. J Intensive Care 4:25. doi: 10.1186/s40560-016-0152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rundgren M, Westhall E, Cronberg T, Rosén I, Friberg H (2010) Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med 38:1838–1844. doi: 10.1097/CCM.0b013e3181eaa1e7. [DOI] [PubMed] [Google Scholar]
- [54].Ruijter BJ, van Putten MJ, Hofmeijer J (2015) Generalized epileptiform discharges in postanoxic encephalopathy: Quantitative characterization in relation to outcome. Epilepsia 56:1845–54. doi: 10.1111/epi.13202. [DOI] [PubMed] [Google Scholar]
- [55].Asgari S, Moshirvaziri H, Scalzo F, Ramezan-Arab N (2018) Quantitative measures of EEG for prediction of outcome in cardiac arrest subjects treated with hypothermia: a literature review. J Clin Monit Comput 32:977–992. doi: 10.1007/s10877-018-0118-3. [DOI] [PubMed] [Google Scholar]
- [56].Ruijter BJ, Hofmeijer J, Tjepkema-Cloostermans MC, van Putten MJAM (2018) The prognostic value of discontinuous EEG patterns in postanoxic coma. Clin Neurophysiol 129:1534–1543. doi: 10.1016/j.clinph.2018.04.745. [DOI] [PubMed] [Google Scholar]
- [57].Hofmeijer J, van Putten MJ (2016) EEG in postanoxic coma: Prognostic and diagnostic value. Clin Neurophysiol 127:2047–2055. doi: 10.1016/j.clinph.2016.02.002. [DOI] [PubMed] [Google Scholar]
- [58].Yang Q, Su Y, Hussain M, Chen W, Ye H, Gao D, Tian F (2014) Poor outcome prediction by burst suppression ratio in adults with post-anoxic coma without hypothermia. Neurol Res 36:453–460. doi: 10.1179/1743132814Y.0000000346. [DOI] [PubMed] [Google Scholar]
- [59].Sekar K, Schiff ND, Labar D, Forgacs PB (2019) Spectral Content of Electroencephalographic Burst-Suppression Patterns May Reflect Neuronal Recovery in Comatose Post-Cardiac Arrest Patients. J Clin Neurophysiol 36:119–126. doi: 10.1097/WNP.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Seder DB, Fraser GL, Robbins T, Libby L, Riker RR (2010) The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med 36:281–288. doi: 10.1007/s00134-009-1691-1. [DOI] [PubMed] [Google Scholar]
- [61].Elmer J, Gianakas JJ, Rittenberger JC, Baldwin ME, Faro J, Plummer C, Shutter LA, Wassel CL, Callaway CW, Fabio A; Pittsburgh Post-Cardiac Arrest Service (2016) Group-Based Trajectory Modeling of Suppression Ratio After Cardiac Arrest. Neurocrit Care 25:415–423. doi: 10.1007/s12028-016-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ghassemi MM, Amorim E, Alhanai T, Lee JW, Herman ST, Sivaraju A, Gaspard N, Hirsch LJ, Scirica BM, Biswal S, Moura Junior V, Cash SS, Brown EN, Mark RG, Westover MB; Critical Care Electroencephalogram Monitoring Research Consortium (2019) Quantitative Electroencephalogram Trends Predict Recovery in Hypoxic-Ischemic Encephalopathy. Crit Care Med 47:1416–1423. doi: 10.1097/CCM.0000000000003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Elmer J, Jones BL, Zadorozhny VI, Puyana JC, Flickinger KL, Callaway CW, Nagin D (2019) A novel methodological framework for multimodality, trajectory model-based prognostication. Resuscitation 137:197–204. doi: 10.1016/j.resuscitation.2019.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wiley SL, Razavi B, Krishnamohan P, Mlynash M, Eyngorn I, Meador KJ, Hirsch KG (2018) Quantitative EEG Metrics Differ Between Outcome Groups and Change Over the First 72 h in Comatose Cardiac Arrest Patients. Neurocrit Care 28:51–59. doi: 10.1007/s12028-017-0419-2. [DOI] [PubMed] [Google Scholar]
- [65].Kustermann T, Nguepnjo Nguissi NA, Pfeiffer C, Haenggi M, Kurmann R, Zubler F, Oddo M, Rossetti AO, De Lucia M (2019) Electroencephalography-based power spectra allow coma outcome prediction within 24 h of cardiac arrest. Resuscitation 142:162–167. doi: 10.1016/j.resuscitation.2019.05.021. [DOI] [PubMed] [Google Scholar]
- [66].Efthymiou E, Renzel R, Baumann CR, Poryazova R, Imbach LL (2017) Predictive value of EEG in postanoxic encephalopathy: A quantitative model-based approach. Resuscitation 119:27–32. doi: 10.1016/j.resuscitation.2017.07.020. [DOI] [PubMed] [Google Scholar]
- [67].Bauerschmidt A, Eliseyev A, Doyle KW, Velasquez A, Egbebike J, Chiu W, Kumar V, Alkhachroum A, Der Nigoghossian C, Al-Mufti F, Rabbani L, Brodie D, Rubinos C, Park S, Roh D, Agarwal S, Claassen J (2021) Predicting early recovery of consciousness after cardiac arrest supported by quantitative electroencephalography. Resuscitation 165:130–137. doi: 10.1016/j.resuscitation.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tjepkema-Cloostermans MC, Hofmeijer J, Beishuizen A, Hom HW, Blans MJ, Bosch FH, van Putten MJAM (2017) Cerebral Recovery Index: Reliable Help for Prediction of Neurologic Outcome After Cardiac Arrest. Crit Care Med 45:e789–e797. doi: 10.1097/CCM.0000000000002412. [DOI] [PubMed] [Google Scholar]
- [69].Tjepkema-Cloostermans MC, van Meulen FB, Meinsma G, van Putten MJ (2013) A Cerebral Recovery Index (CRI) for early prognosis in patients after cardiac arrest. Crit Care 17:R252. doi: 10.1186/cc13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nagaraj SB, Tjepkema-Cloostermans MC, Ruijter BJ, Hofmeijer J, van Putten MJAM (2018) The revised Cerebral Recovery Index improves predictions of neurological outcome after cardiac arrest. Clin Neurophysiol 129:2557–2566. doi: 10.1016/j.clinph.2018.10.004. [DOI] [PubMed] [Google Scholar]
- [71].Selig C, Riegger C, Dirks B, Pawlik M, Seyfried T, Klingler W (2014) Bispectral index (BIS) and suppression ratio (SR) as an early predictor of unfavourable neurological outcome after cardiac arrest. Resuscitation 85:221–226. doi: 10.1016/j.resuscitation.2013.11.008. [DOI] [PubMed] [Google Scholar]
- [72].Riker RR, Stone PC Jr, May T, McCrum B, Fraser GL, Seder D (2013) Initial bispectral index may identify patients who will awaken during therapeutic hypothermia after cardiac arrest: a retrospective pilot study. Resuscitation 84:794–797. doi: 10.1016/j.resuscitation.2012.10.014. [DOI] [PubMed] [Google Scholar]
- [73].Stammet P, Collignon O, Werer C, Sertznig C, Devaux Y (2014) Bispectral index to predict neurological outcome early after cardiac arrest. Resuscitation 85:1674–1680. doi: 10.1016/j.resuscitation.2014.09.009. [DOI] [PubMed] [Google Scholar]
- [74].Haesen J, Eertmans W, Genbrugge C, Meex I, Demeestere J, Vander Laenen M, Boer W, Mesotten D, Dens J, Jans F, Ernon L, De Deyne C (2018) The validation of simplified EEG derived from the bispectral index monitor in post-cardiac arrest patients. Resuscitation 126:179–184. doi: 10.1016/j.resuscitation.2018.01.042. [DOI] [PubMed] [Google Scholar]
- [75].Eertmans W, Genbrugge C, Haesen J, Drieskens C, Demeestere J, Vander Laenen M, Boer W, Mesotten D, Dens J, Ernon L, Jans F, De Deyne C (2019) The Prognostic Value of Simplified EEG in Out-of-Hospital Cardiac Arrest Patients. Neurocrit Care 30:139–148. doi: 10.1007/s12028-018-0587-8. [DOI] [PubMed] [Google Scholar]
- [76].Eveson L, Vizcaychipi M, Patil S (2017) Role of bispectral index monitoring and burst suppression in prognostication following out-of-hospital cardiac arrest: a systematic review protocol. Syst Rev 6:191. doi: 10.1186/s13643-017-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Drohan CM, Cardi AI, Rittenberger JC, Popescu A, Callaway CW, Baldwin ME, Elmer J (2018) Effect of sedation on quantitative electroencephalography after cardiac arrest. Resuscitation 124:132–137. doi: 10.1016/j.resuscitation.2017.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Purdon PL, Sampson A, Pavone KJ, Brown EN (2015) Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures. Anesthesiology 123:937–60. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ruijter BJ, van Putten MJAM, van den Bergh WM, Tromp SC, Hofmeijer J (2019) Propofol does not affect the reliability of early EEG for outcome prediction of comatose patients after cardiac arrest. Clin Neurophysiol 130:1263–1270. doi: 10.1016/j.clinph.2019.04.707. [DOI] [PubMed] [Google Scholar]
- [80].Kobata H, Tucker A, Sarapuddin G, Negoro T, Kawakami M (2020) Continuous amplitude-integrated electroencephalography for prognostication of cardiac arrest patients undergoing extracorporeal cardiopulmonary resuscitation with targeted temperature management. Resuscitation 156:107–113. doi: 10.1016/j.resuscitation.2020.08.123. [DOI] [PubMed] [Google Scholar]
- [81].Jouffroy R, Lamhaut L, Guyard A, Philippe P, An K, Spaulding C, Baud F, Carli P, Vivien B (2017) Early detection of brain death using the Bispectral Index (BIS) in patients treated by extracorporeal cardiopulmonary resuscitation (E-CPR) for refractory cardiac arrest. Resuscitation 120:8–13. doi: 10.1016/j.resuscitation.2017.08.217. [DOI] [PubMed] [Google Scholar]
- [82].Sheikh ZB, Maciel CB, Dhakar MB, Hirsch LJ, Gilmore EJ (2022) Nonepileptic Electroencephalographic Correlates of Episodic Increases in Intracranial Pressure. J Clin Neurophysiol 39:149–158. doi: 10.1097/WNP.0000000000000750. [DOI] [PubMed] [Google Scholar]
- [83].Alsallom F, Casassa C, Akkineni K, Lin L (2022) Early Detection of Cerebral Herniation by Continuous Electroencephalography and Quantitative Analysis. Clin EEG Neurosci 53:133–137. doi: 10.1177/15500594211018535. [DOI] [PubMed] [Google Scholar]
- [84].Tian J, Zhang L, Di P, Liu H, Zhou Y, Liu L (2022) Continuous Quantitative Electroencephalogram (EEG) Monitoring for Early Detection of Brain Herniation in Large Hemispheric Infarction (LHI): A Case Report. J Stroke Cerebrovasc Dis 31:106158. doi: 10.1016/j.jstrokecerebrovasdis.2021.106158. [DOI] [PubMed] [Google Scholar]
- [85].Mullaguri N, Beary JM, Newey CR (2020) Early detection of brainstem herniation using electroencephalography monitoring - case report. BMC Neurol 20:406. doi: 10.1186/s12883-020-01988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Peedicail J, Mehdiratta N, Zhu S, Nedjadrasul P, Ng MC (2021) Quantitative burst suppression on serial intermittent EEG in refractory status epilepticus. Clin Neurophysiol Pract 6:275–280. doi: 10.1016/j.cnp.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Johnson EL, Martinez NC, Ritzl EK (2016) EEG Characteristics of Successful Burst Suppression for Refractory Status Epilepticus. Neurocrit Care 25:407–414. doi: 10.1007/s12028-016-0294-2. [DOI] [PubMed] [Google Scholar]
- [88].Rubin DB, Angelini B, Shoukat M, Chu CJ, Zafar SF, Westover MB, Cash SS, Rosenthal ES (2020) Electrographic predictors of successful weaning from anaesthetics in refractory status epilepticus. Brain 143:1143–1157. doi: 10.1093/brain/awaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].An J, Jonnalagadda D, Moura V Junior , Purdon PL , Brown EN, Westover MB (2018) Variability in pharmacologically-induced coma for treatment of refractory status epilepticus. PLoS One 13:e0205789. doi: 10.1371/journal.pone.0205789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Arbour RB, Dissin J (2015) Predictive value of the bispectral index for burst suppression on diagnostic electroencephalogram during drug-induced coma. J Neurosci Nurs 47:113–122. doi: 10.1097/JNN.0000000000000124. [DOI] [PubMed] [Google Scholar]
- [91].Musialowicz T, Mervaala E, Kälviäinen R, Uusaro A, Ruokonen E, Parviainen I (2010) Can BIS monitoring be used to assess the depth of propofol anesthesia in the treatment of refractory status epilepticus? Epilepsia 51:1580–1586. doi: 10.1111/j.1528-1167.2009.02514.x. [DOI] [PubMed] [Google Scholar]
- [92].Westover MB, Shafi MM, Ching S, Chemali JJ, Purdon PL, Cash SS, Brown EN (2013) Real-time segmentation of burst suppression patterns in critical care EEG monitoring. J Neurosci Methods 219:131–141. doi: 10.1016/j.jneumeth.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fürbass F, Herta J, Koren J, Westover MB, Hartmann MM, Gruber A, Baumgartner C, Kluge T (2016) Monitoring burst suppression in critically ill patients: Multi-centric evaluation of a novel method. Clin Neurophysiol 127:2038–2046. doi: 10.1016/j.clinph.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a literature review. All publications included in this manuscript are available online, as referenced in the Reference section.


