Abstract
One major goal of regenerative medicine is the production of pancreatic endocrine islets to treat insulin-dependent diabetic patients. Among the different methods developed to achieve this goal, a particularly promising approach is direct lineage reprogramming, in which non-β-cells are directly converted to glucose-responsive, insulin-secreting β-like cells. Efforts by different research groups have led to critical insights in the inducing factors necessary and types of somatic tissues suitable for direct conversion to β-like cells. Nevertheless, there is limited understanding of the molecular mechanisms underlying direct cell fate conversion. Significant challenges also remain in translating discoveries into therapeutics that will eventually benefit diabetic patients. This review aims to cover the advances made in the direct reprogramming of somatic cells into β-like cells and discuss the remaining challenges.
Keywords: beta (β) cell, direct reprogramming, transdifferentiation, endocrine pancreas, type 1 diabetes (T1D)
Introduction
Diabetes, a chronic disease in which a person's body loses the ability to properly maintain its blood glucose levels, has reached epidemic scale worldwide. A principal regulator of blood glucose is the pancreatic β-cells which, by secreting insulin, instruct metabolic organs, such as the liver and muscle, to remove blood glucose from circulation. While the majority of diabetic patients suffer from type 2 diabetes (T2D), characterized by malfunction of the β-cells, type 1 diabetes (T1D) patients are afflicted with an autoimmune disease, in which immune cells aberrantly attack and destroy β-cells, rendering patients unable to produce requisite insulin, leaving them hyperglycemic. One therapy proven to be effective at combating T1D is islet transplantation from deceased donors.
However, a shortage of donors means that few patients have benefited from this promising treatment. In addition, a larger population of insulin-dependent T2D patients could also be treated with a transplantation therapy and relieved of the burden of daily insulin injections and the many complications associated with long-term insulin use. Therefore, a major aim of regenerative medicine in recent years has been to generate an abundant supply of functional β-cells, which can be used therapeutically in transplantation procedures.
Different strategies for developing a robust reservoir of new β-cells have been pursued. One approach centers around the self-replication of β-cells, finding ways to spur β-cells to divide. Another avenue has been using embryonic stem cells or induced pluripotent stem cells and leading them down the developmental path of the body toward a β-cell fate. A third approach for β-cell generation is direct reprogramming, converting a differentiated cell lineage directly into another with no pluripotent interim step (Cavelti-Weder et al, 2015; Zhou and Melton, 2018).
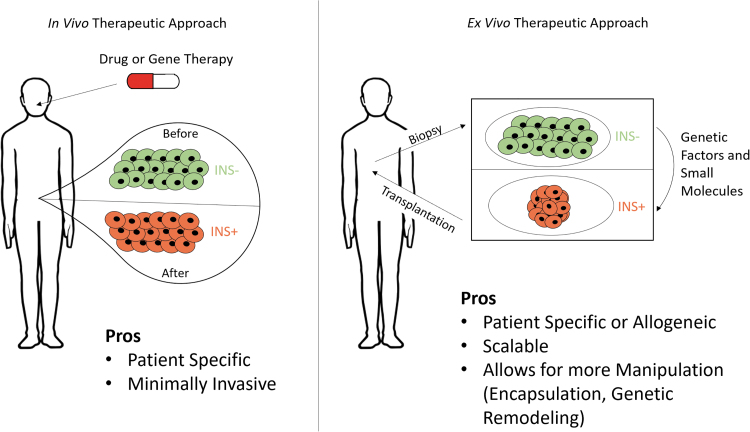
Direct reprogramming, or transdifferentiation, as a technique for β-cell generation is appealing in that it could be tailored to individual patients in autologous therapies. Reprogramming techniques explored thus far can be broadly categorized as in vivo or ex vivo approaches, each with its distinct advantages and challenges (Fig. 1). The advances made and obstacles remaining in the reprogramming for β-cells will be the focus of this review.
FIG. 1.
Two general reprogramming approaches for regenerating and replenishing β-cells to treat diabetes. The in vivo method (left) relies on the administration of a drug or gene therapy to a patient to directly create new β-like cells in the body. Alternatively, the ex vivo method (right) utilizes cells harvested from a patient which can be grown and reprogrammed in culture to β-like cells, before being transplanted back into the patient.
Models of Direct Lineage Conversion to β-Like Cells
Conversion of pancreatic cell lineages
Conversion of acinar cells to β-like cells
The role of the pancreas can be divided into two distinct components. The endocrine pancreas is composed of islet clusters within which reside different specialized cell types, each responsible for secreting a distinct hormone. The exocrine pancreas serves to secrete digestive enzymes, which are produced by acinar cells. These digestive enzymes, once produced, travel to the small intestine via ducts where they interact with consumed food, breaking it down into components for absorption. Among the enzymes secreted are lipases and proteinases for fat and protein breakdown, respectively.
The acinar cells represent an enticing differentiated cell niche to reprogram into β-cells. During embryonic development, the acinar cells derive from the same pancreatic endoderm layer as β-cells. This developmental similarity lends credence to the belief that they would be among the most amenable cells to a reprogramming technique. In addition, acinar cells are abundant in the pancreas, existing in many times greater numbers than β-cells. This results in a situation where most of a donor pancreas is discarded after islets are isolated to be used in a transplantation. If a method to reliably alter acinar cells to β could be established, much more of a donor pancreas would be usable and more people could receive treatment per donor.
A major stride in the reprogramming toward a β-cell fate was made with the identification of three developmental transcription factors (TFs), which are expressed during the inception and maturation of β-cells. Neurogenin3 (NGN3) is a transcriptional regulator necessary for the development of pancreatic progenitors, while pancreatic and duodenal homeobox 1 (PDX1) and MAF BZIP transcription factor A (MafA) are necessary in tandem for β-cell maturation and maintenance (Li et al, 2014a). PDX1 is critical for pancreatic fate determination in embryogenesis as well as maintaining adult β-cell fate. MafA is a transcription factor which activates numerous β-cell genes. There are excellent reviews on the function of these pancreatic factors (Edlund 2002; Jensen, 2004; Murtaugh 2007).
The first experiment which used these TFs to reprogram acinar cells to β-cells did so in vivo using an adenovirus containing all three TF genes. The adenovirus selectively targeted acinar cells and postinfection resulted in cells morphologically and functionally similar to β-cells, but these cells did not reorganize into islet-like clusters. The induced differentiated cells were even capable of providing benefit to hyperglycemic animals (Zhou et al, 2008). This work was then expanded on by a study that more than doubled the reprogramming efficiency of the technique and enabled the development of longer lasting β-cells, which formed into islet-like clusters (Li et al, 2014b). These induced β-cells were capable of ameliorating diabetes to a larger extent and showcase the necessity of creating islet-like clusters to enable better survival and higher functionality of the reprogrammed cells.
Further research into NGN3, PDX1, and MafA's roles in reprogramming led to the discovery that acinar cells can be converted to α, β, or δ-cells using different combinations of the three factors. With only NGN3 present, acinar cells reprogram to somatostatin (SST)-positive δ-like cells. A combination of NGN3 with MafA converts the targets to glucagon (GCG)-positive α-like cells. It is only by a combined expression of all three, NGN3, PDX1, and MafA, that insulin positive β-like cells can be produced (Li et al, 2014a). This study revealed some of the key steps and mechanisms in acinar reprogramming. Acinar cells must first be directed off an exocrine path to an endocrine one before being converted to the desired cell type.
Studies have also cultured and reprogrammed acinar cells in vitro. One study used NGN3, PDX1, and MafA and infected acinar cells using an adenovirus. This resulted in cells capable of secreting insulin but unable to respond to glucose stimulation (Akinci et al, 2012). It was also reported that treatment of acinar culture with epidermal growth factor and leukemia inhibitory factor results in increased insulin, PDX1, and GLUT2 expression (Baeyens et al, 2005). An additional technique showed that human acinar cells could be reprogrammed by delivery of activated MAPK and STAT3 by a lentivirus. These cells displayed NGN3 positivity and were capable of ameliorating diabetes (Lemper et al, 2015).
Conversion of pancreatic endocrine to β-like cells
The functional endocrine component of the pancreas is the islet of Langerhans. The islet is a cluster of several different endocrine cells, each with an associated principal hormone: α-cells release GCG, β-cells release insulin, δ-cells release SST, ɛ-cells release ghrelin, and PP-cells release pancreatic polypeptide for which they are named. The developmental proximity of these endocrine cells makes them an appealing target for reprogramming to a β-like fate as they all form from the endoderm and NGN3+ progenitor cells. In addition, due to their placement within the islet, there is the advantage that any regenerated new β-cells from these subpopulations would be in its native niche environment, suitable for survival and function.
The majority of research into inducing β-cells from non-β endocrine cells has focused on α-cells as the target of interest. This is in part due to the intriguing observation that in a situation of complete β-cell ablation by means of diphtheria toxin, over several months, the β-cell population partially recovered. Using lineage tracing methods, it was elucidated that the α-cell population in the islets had been converted to a β fate (Thorel et al, 2010).
One study suggests that the cell lineage reprogramming is age deterministic with ablation in adults leading to α conversion, while in juveniles, δ-cells are converted (Chera et al, 2014). While the mechanism behind α conversion is not fully understood, one study provides evidence that alterations of the epigenetic profile, specifically histone methylation, is at play (Bramswig et al, 2013). In addition, two genes, paired box 4 (Pax4) and aristaless-related homeobox (Arx), have been implicated as main determinants of this process.
It was shown that Pax4 was necessary for the development and maturation of both β- and δ-cells but not the other pancreatic endocrine cell types (Sosa-Pineda et al, 1997). Furthering this research, one study ectopically expressed Pax4 throughout the pancreas and saw a persistent recruitment of α-cells to a β-phenotype as well as NGN3+ cells converting to replace the lost α-cells, leading to an overall swelling of islet mass (Collombat et al, 2009).
While Pax4 expression is necessary for β-cell development, Arx expression is necessary to maintain an α-like state. One study points to Arx as a potential key regulator in α reprogramming. Using a conditional knockout of Arx, it was shown that α-cells rapidly convert to β-like cells (Courtney et al, 2013). A separate study showed that while Arx inhibition is sufficient to convert α to β, the process works to a greater degree with the coinhibition of DNA methyltransferase I (DNMT1) (Chakravarthy et al, 2017). Interestingly, this study also examined human T1D islets and saw a similar downregulation of Arx in the α-cells present.
Other studies reprogrammed α-cells without the manipulation of Pax4 or Arx. One study directly delivered PDX1 and MafA and saw a similar α to β transformation. Intriguingly, these converted β-like cells appeared to resist autoimmune attacks in the nonobese diabetic mouse model of T1D (Xiao et al, 2018). Recently, it was shown that coexpression of PDX1 and MafA endowed purified primary human α-cells with certain β-cell properties; the hybrid cell types secreted insulin and ameliorated hyperglycemia in mouse diabetic models (Furuyama et al, 2019). It was also shown that the small molecule γ-aminobutyric acid (G-ABA) can elicit a conversion in α-cells, as long-term GABA treatment was able to restore the β-cell population and reverse diabetes after repeated instances of streptozotocin ablation (Ben-Othman et al, 2017).
Conversion of pancreatic ductal cells to β-like cells
Pancreatic ductal cells are epithelial cells which function as a component of the exocrine pancreas, acting as a passageway for the digestive enzymes produced by acinar cells. While not as abundant as acinar cells, ductal cells compose about 30% of the pancreatic tissue. In development, the endocrine cells of the pancreas derive from a primitive ductal epithelial layer, making ductal cells a potentially good target to reprogram.
Initial experiments attempted to transdifferentiate ductal cells using an NGN3 vector. This led to insulin secretion and an uptick in Pax4 but no ability to respond to glucose-stimulated insulin secretion (GSIS) (Heremans et al, 2002). This method was later expanded upon when it was discovered that NGN3, in conjunction with MafA and Pax6, led to reprogramming of ductal cells capable of secreting insulin under GSIS conditions, although only 40%–70% as efficiently as β-cells (Lee et al, 2013). An additional genetic approach found was focused on the role of F-box and WD repeat domain-containing protein 7 (Fbw7) on pancreatic development. When Fbw7 was deleted in ductal cells, it unlocked a latent endocrine potency, as it stabilized NGN3 expression and developed the cells mostly into a β character but generated α and δ-cells as well (Sancho et al, 2014).
There has also been work done to discover optimal culture conditions which augment ductal cell reprogramming toward β-like. One group found that expanding ductal cells in vitro and then growing the cells over Matrigel resulted in budding growth of endocrine cell clusters. These growths were similar in structure and function to islets and capable of GSIS responsiveness (Bonner et al, 2000). More recently, it was found that culturing ductal cells with rho associated kinase to deter the transitioning to a mesenchymal state resulted in robust generation of β-like cells (Lima et al, 2013).
Conversion of extra-pancreatic cell lineages
Conversion of liver to β-like cells
When assessing potential extrapancreatic targets for reprogramming, tissue from the liver stands out as an excellent contender. The liver is primarily composed of hepatocytes, which serve a myriad of functions, including detoxification and synthesis of various proteins, such as serum albumin. Hepatocytes have been sought after as a reprogramming target because of their intrinsic ability to regenerate. In addition, while not as developmentally close as pancreatic tissue to β-cells, the liver likewise derives from the endoderm.
Initial studies looking at the reprogramming capacity of hepatocytes transiently activated PDX1 by adenoviral delivery. This yielded some long-lasting effects, rendering cells with the ability to ameliorate hyperglycemia by insulin secretion and which expressed a number of genes expressed by endocrine cells of the pancreas (Ferber et al, 2000). However, this method left much to be improved upon as the overall reprogramming efficiency was low. When a similar experiment was attempted with a constitutively activated PDX1 construct, key endocrine hormones, including insulin, GCG, and SST, were secreted from reprogrammed hepatocytes (Miyatsuka et al, 2003). However, the constitutive expression caused morphological damage to the tissue, including abnormal lobe structures and the appearance of cystic lesions.
One study found a potentially crucial gene to the reprogramming of hepatocytes by studying the effects of TGFB-induced factor homeobox 2 (TGIF2). TGIF2 is a gene expressed at the branch point in development when pancreatic and hepatic tissue diverge. By overexpressing TGIF2 in hepatocytes, the cells were reprogrammed to a pancreatic progenitor state which could serve as a critical step in the generation of a methodology to work from hepatocyte to β-cell (Cerdá-Esteban et al, 2017).
When a combination of NGN3, PDX1, and MafA was inserted into the liver, a conversion of nonhepatocyte cells in the periportal region of the liver was observed. While the reprogramming was sufficient to ameliorate hyperglycemia in a rodent model, the cells were not truly β-like in character. Several endocrine hormones, including SST and GCG, were secreted from the cells, and the cells took on a physiology entirely distinct from β-cells (Banga et al, 2012). They could only be classified as their own unique cell type. When a similar method was performed to elucidate the nature of the transformed cells, two main candidate cell lineages emerged. Most cells were identified as hepatoblasts, a progenitor cell type able to differentiate further into either a hepatocyte or a biliary cell.
The second lineage was Sox9+ cells, cells that likely compose small bile ducts (Banga et al, 2012; Yang et al, 2013). In addition, the study found that younger livers, at least in mice, are more amenable to reprogramming (Yang et al, 2013). As is the case for methods reprogramming hepatocytes, more work is needed in the targeting of these cell niches as, to date, no cells have been reprogrammed which closely mimic endogenous β-cells nor have there been any studies which look at the reprogramming effects in the liver long term.
Conversion of biliary to β-like cells
Biliary tissue refers to the epithelial network that escorts bile. The bile ducts, gallbladder, and ducts within the liver are the main subsets of biliary tissue. Biliary epithelium represents a potentially enticing niche for transdifferentiation due to the fact that the gallbladder can be excised without severely negative repercussions on a patient. In addition, much like the other cell lineages discussed, a similar developmental history exists; the ventral pancreas and the biliary tissues both derive from the developmental endoderm.
Cells from the biliary network began to be evaluated as a source of β-cells when it was discovered that pancreatic endocrine cells, including β-cells, can reside within the bile duct and that these endocrine cells appear to have developed from the duct itself, not pancreatic tissue (Dutton et al, 2007). This finding was confirmed by one study that further showed that the proportion of endocrine cells within the biliary tissue can be augmented by altering the culture conditions (Eberhard et al, 2008). Hes1 is likely implicated in the mechanism of their production. Hes1 inhibits NGN3 expression in biliary tissue and its repression leads to the development of pancreatic acinar and endocrine cells (Sumazaki et al, 2004).
Several studies have gone forward and tried to directly produce β-cells from biliary tissue in vitro. The TFs PDX1, neurogenic differentiation factor (NeuroD1), and a conjugate of PDX1 with the viral transcription activator, VP16, all led to transdifferentiation into insulin producing cells after being expressed ectopically, although none of these reprogrammed cells demonstrated glucose sensitivity (Nagaya et al, 2009). A separate study induced reprogramming with a combination of NGN3, PDX1, and MafA in a culture which included the Notch inhibitors dibenzazepine and retinoic acid. Both were found to potentiate the reprogramming effect. These cells did not exhibit GSIS, but were able to survive and secrete insulin after engraftment (Hickey et al, 2013).
A very promising discovery came from one study that found a method to vastly expand gallbladder epithelium over 12 passages. The study also proceeded to reprogram the expanded cells using a vector for coexpression of PDX1, NGN3, Pax6, and MafA (Galivo et al, 2017).
Conversion of gut epithelial cells to β-like cells
Tissue from the gastrointestinal (GI) tract, primarily the stomach and proximal small intestine, serve as an additional source of cells, which may be effectively reprogrammed to β-like. In addition to the shared endodermal development pattern of the pancreas and GI system, the unique nature of the GI makes it an attractive target. The GI is home to scattered enteroendocrine cells which express NGN3, a shared TF in pancreatic endocrine and enteroendocrine development. In addition, the GI is richly imbued with stem cells, allowing it to constantly regenerate. This feature serves as a marked advantage for use in therapeutic treatment since cells lost to reprogramming can be readily replaced.
FOXO1 has been identified as a key TF in the determination of cell fate between pancreas and GI tissue. FOXO1 may inhibit the notch pathway, which plays a pivotal role in driving NGN3 expression. When FOXO1 is ablated in NGN3+ enteroendocrine progenitor cells, insulin producing cells develop in vivo in mice as well as in vitro in cultured intestinal organoids (Bouchi et al, 2014; Talchai et al, 2012).
Other studies have induced expression of key TFs instead of repressing them. Ptf1a ectopic expression results in the transdifferentiation to pancreatic endocrine and acinar cell lineages in stomach and intestinal tissue (Jarikji et al, 2007). In antral stomach tissue, it was shown that a combination of NGN3, PDX1, and MafA can lead to reprogramming capable of suppressing hyperglycemia after transplantation (Ariyachet et al, 2016).
A separate study reprogrammed cells from the duodenum and proximal jejunum using the same TF combination. The induced expression resulted in the formation of “neo-islet” clusters within the crypts of the GI capable of responding to insulin and ameliorating hyperglycemia (Chen et al, 2014). These techniques have the advantage of preserving FOXO1's expression, which is thought to be potentially pivotal for β-cell health and stability long term.
Challenges and Future Directions
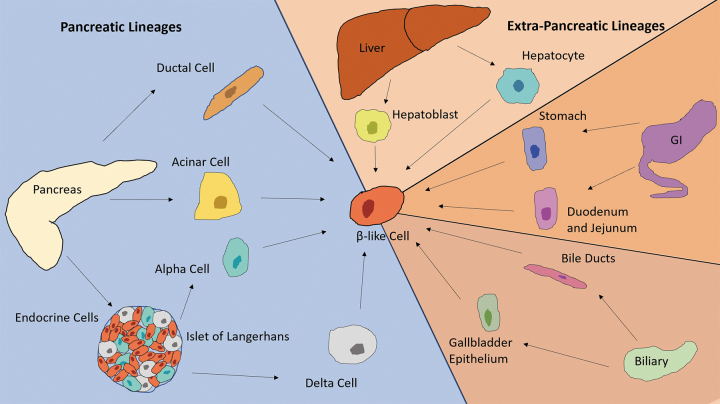
Many impactful strides have been made in the field of direct reprogramming toward a β-like lineage. Studies so far suggest that endodermal tissues are more amenable for conversion to β-like cells, whereas tissues derived from other germ layers such as fibroblasts (mesoderm in origin) are more resistant (Fig. 2). The molecular underpinning of this tissue restriction is not well understood at present, but one can speculate that the ease of lineage conversion may depend on the preexisting epigenetic landscape in the target cells, and that the more similar the epigenome of the target cells is to β-cells, the more likely it is for the conversion to occur. Knowledge of the underlying mechanism may lead to the discovery of protocols which result in better functionality, closer to that of endogenous β-cells as well as increased stability posttransplantation.
FIG. 2.
Summary of the different cell lineages which can be reprogrammed toward a β-like fate. These include pancreatic cells such as ductal, acinar, alpha, and delta cells, and other endodermal cell types such as hepatocytes and hepatoblasts from the liver, stomach, duodenal and jejunal epithelial cells from the GI tract, and bile duct and gallbladder epithelium from the biliary system. GI, gastrointestinal.
At present, molecular and functional analyses of β-like cells were often inadequate and relied on a small number of marker genes. As a result, the identities of the various β-like cells are often unclear. For instance, how similar are the β-like cells compared with islet β-cells at the transcriptomic, proteomic, and epigenomic levels? How much of the original transcriptomic signatures do the β-like cells still retain? Do they resemble β-cells or perhaps represent hybrid cell types? Do they express multiple hormones? Are they functionally mature? To move the field forward, more comprehensive and sophisticated studies will be needed to evaluate the β-like cells, for instance, with single-cell RNA-sequencing, calcium imaging, dynamic GSIS, and long-term transplantation observations.
Nevertheless, we posit that one does not need to make “perfect” β-cells that fully resemble islet β-cells to have a therapeutic impact. The final cell products have to be safe in regard to tumorigenic and hypoglycemic risks, durable, and demonstrate efficacy in terms of sufficient insulin output and GSIS ability. Does it matter if the β-like cells express residual non-β genes or in fact are a hybrid cell type? As long as they demonstrate safety, efficacy, and durability, then, in our opinion, they could be very useful indeed.
Significant hurdles remain to translate laboratory discoveries to therapeutics. As most studies of direction conversion were conducted in mouse models, to assess clinical potential, one must work with human tissues. The research focus should be increasingly shifted to using human tissues to evaluate conversion efficiency and β-like cell phenotypes such as functionality and transplant stability. Small molecule compounds that can induce fate switches are preferred agents of reprogramming, but advances in gene delivery, such as adeno-associated virus, nanoparticles, and modified RNAs, can enable safe expression of genetic factors in the target cells. Genetic engineering tools such as CRISPR-CAS9 may facilitate construction of cell lines for mass production.
Among the two broad approaches for direct reprogramming, direct in vivo induction is currently focused on pancreatic α-cells and gut cells. If successful, either of these targets would readily achieve patient-specificity. However, evaluation of this approach for clinical use will likely require extensive testing in large animal models such as nonhuman primates (NHPs). Given the fast turnover rate of the gut epithelium, an additional challenge of reprogramming gut tissues in vivo is to devise ways to retain the new β-like cells in the lamina propria so that they can aggregate, mature, and persist.
Compared with the in vivo method, the ex vivo approach may not need extensive validation in NHPs. It also has the advantages of being readily scalable and lending itself to opportunities for genetic engineering or encapsulation. However, it is more challenging to develop autologous ex vivo therapies because each batch of cell product requires rigorous quality control.
An additional challenge in developing cell products for T1D treatment is the recurrent autoimmunity that persists in patients, which will destroy any transplanted or regenerated β-cells. To combat this, many studies are ongoing in the development of encapsulation devices to shield transplanted cells from the immune system. Another approach is to render the new β-cells undetectable or impervious to immune system attack via genetic engineering of their immune system, for instance, by partially or completely disabling the MHCI and II complexes with or without concomitant expression of immune suppressive factors such as PDL1 and CD47 (Deuse et al, 2019; Gornalusse et al, 2017; Han et al, 2019).
Given the significant progress in both the reprogramming and immune protection methods, we are hopeful that new treatments for T1D will soon emerge from the confluence of these advances.
Acknowledgment
The authors thank Wei Gu and Xiaofeng Huang for their creative input in the figure design.
Authors' Contributions
J.L.C. and Q.Z. performed the research and wrote and edited the article. J.L.C. designed the figures.
Author Disclosure Statement
The authors declare they have no conflicting financial interests.
Funding Information
This work was supported by awards from NIDDK (R01 DK106253 and UC4DK116280) to Q.Z.
References
- Akinci E, Banga A, Greder LV, et al. Reprogramming of pancreatic exocrine cells towards a beta (β) cell character using Pdx1, Ngn3 and MafA. Biochem J 2012;442(3):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyachet C, Tovaglieri A, Xiang G, et al. Reprogrammed stomach tissue as a renewable source of functional β cells for blood glucose regulation. Cell Stem Cell 2016;18:410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens L, De Breuck S, Lardon J, et al. In vitro generation of insulin-producing β cells from adult exocrine pancreatic cells. Diabetologia 2005;48:49–57. [DOI] [PubMed] [Google Scholar]
- Banga A, Akinci E, Greder LV, et al. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci U S A 2012;109:15336–15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Othman N, Vieira A, Courtney M, et al. Long-term GABA administration induces α-cellmediated β-like cell neogenesis. Cell 2017;168:73–85. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 2000;97(14):7999–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchi R, Foo KS, Hua H, et al. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun 2014;5:4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramswig NC, Everett L, Schug J, et al. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest 2013;123:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelti-Weder C, Li W, Zumsteg A, et al. Direct reprogramming for pancreatic beta-cells using key developmental genes. Curr Pathobiol Rep 2015;3(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Esteban N, Naumann H, Ruzittu S, et al. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat Commun 2017;8:14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy H, Gu X, Enge M, et al. Converting adult pancreatic islet alpha cells into beta cells by targeting both Dnmt1 and Arx. Cell Metab 2017;25:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Finkbeiner S, Weinblatt D, et al. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep 2014;6:1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera S, Barronier D, Ghila L, et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 2014;514:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 2009;138:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M, Gjernes E, Druelle N, et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet 2013;9:e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Hu X, Gravina A, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 2019;37(3):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton JR, Chillingworth NL, Eberhard D, et al. Beta cells occur naturally in extrahepatic bile ducts of mice. J Cell Sci 2007;120(Pt 2):239–245. [DOI] [PubMed] [Google Scholar]
- Eberhard D, Tosh D, Slack JM. Origin of pancreatic endocrine cells from biliary duct epithelium. Cell Mol Life Sci 2008;65(21):3467–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund H, Pancreatic organogenesis—Developmental mechanisms and implications for therapy. Nat Rev Genet 2002;3(7):524–32. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 2000;6(5):568–572. [DOI] [PubMed] [Google Scholar]
- Furuyama K, Chera S, van Gurp L, et al. Diabetes relief in mice by glucosesensing insulin-secreting human α-cells. Nature 2019;567:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F, Benedetti E, Wang Y, et al. Reprogramming human gallbladder cells into insulin-producing β-like cells. PLoS One 2017;12:e0181812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornalusse GG, Hirata RK, Funk SE, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol 2017;35(8):765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wang M, Duan S, et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc Natl Acad Sci U S A 2019;116(21):10441–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans Y, Van de Casteel M, in't Veld P, et al. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol 2002;159:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey RD, Galivo F, Schug J, et al. Generation of islet-like cells from mouse gall bladder by direct ex vivo reprogramming. Stem Cell Res 2013;11(1):503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarikji ZH, Vanamala S, Beck CW, et al. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum and liver to pancreas. Dev Biol 2007;304(2):786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Gene regulatory factors in pancreatic development. Dev Dyn 2004;229(1):176–200. [DOI] [PubMed] [Google Scholar]
- Lee J, Sugiyama T, Liu Y, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife 2013;2:e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemper M, Leuckx G, Heremans Y, et al. Reprogramming of human pancreatic exocrine cells to β-like cells. Cell Death Difer 2015;22:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cavelti-Weder C, Zhang Y, et al. Long-term persistence and development of induced pancreatic β cells generated by lineage conversion of acinar cells. Nat Biotechnol 2014a;32:1223–1230. [DOI] [PubMed] [Google Scholar]
- Li W, Nakanishi M, Zumsteg A, et al. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. eLife 2014b;3:e01846; doi: 10.7554/eLife.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima MJ, Muir KR, Docherty HM, et al. Suppression of epithelial-to-mesenchymal transitioning enhances ex vivo reprogramming of human exocrine pancreatic tissue toward functional insulin-producing beta-like cells. Diabetes 2013;62(8):2821–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Kajimoto Y, et al. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun 2003;310(3):1017–1025. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Pancreas and beta-cell development: From the actual to the possible. Development, 2007;134(3):427–38. [DOI] [PubMed] [Google Scholar]
- Nagaya M, Katsuta H, Kaneto H, et al. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol 2009;201(1):37–47. [DOI] [PubMed] [Google Scholar]
- Sancho R, Gruber R, Gu G, et al. Loss of Fbw7 reprograms adult pancreatic ductal cells into α, δ, and β cells. Cell Stem Cell 2014;15:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, et al. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 1997;386(6623):399–402. [DOI] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, et al. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet 2004;36(1):83–87. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Kitamura T, et al. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet 2012;44:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 2010;464:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Guo P, Shiyota C, et al. Endogenous reprogramming of α cells into β cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell 2018;22:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Akinci E, Dutton JR, et al. Stage specific reprogramming of mouse embryo liver cells to a beta cell-like phenotype. Mech Dev 2013;130:602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008;455:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Melton DA. Pancreas regeneration. Nature 2018;557:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]