ABSTRACT
Approximately 15% of the world's couples suffer from infertility during their reproductive period of which the male factor is responsible for 50% of cases. Male factor infertility is multifactorial in origin, and sperm DNA fragmentation (SDF) has also been linked to male infertility including idiopathic male infertility. Some degree of controlled DNA nicking is essential for adequate DNA compaction, but excessive SDF is usually associated with reduced male fertility potential, reduced fertilisation, poor embryo quality, recurrent pregnancy loss and poor assisted reproductive techniques (ARTs) outcomes. Although semen analysis remains the gold standard for diagnosis of male factor infertility worldwide, its limitations motivated the search and the development of complementary tests of sperm function and integrity. SDF assay is an emerging diagnostic tool in infertile men, and several indications for SDF testing in infertile couples have also been proposed. The use of SDF in routine male infertility assessment is, however, still controversial. Furthermore, both direct and indirect SDF tests are now available. Hence, the present review was conducted to summarise the recent evidence of SDF, underlying mechanisms, clinical indications, diagnostic tests, as well as the role of SDF in male factor infertility, pregnancy and ART outcomes.
KEYWORDS: Assisted reproductive techniques, male infertility, pregnancy, sperm DNA fragmentation
INTRODUCTION
Infertility is defined as the failure of couples to achieve pregnancy within 12 consecutive months of unprotected intercourse, affecting approximately 15% of couples of reproductive ages.[1] Infertility has medical, psychological, social, and financial consequences. Factors underlying male infertility include genetic and anatomical abnormalities, varicocele, endocrine disorders, systemic diseases, infections, immunological and environmental toxins, lifestyle, radiotherapy, chemotherapy and medications.[2] In approximately 30% of infertility cases, the underlying cause of semen abnormalities is unknown and referred to as idiopathic male infertility (IMI). Oxidative stress (OS) and sperm DNA fragmentation (SDF) have been suggested as potential mechanisms for IMI.[3,4] SDF involves sperm DNA single- or double-stranded (ss or ds) breaks and has been linked to reduced male fertility potential, reduced fertilisation, decreased pregnancy rates, suboptimal embryo quality, increased risk of spontaneous abortions and poor assisted reproductive technique (ART) outcomes.[5] Recent studies have also reported an increased incidence of childhood malignancies and genetic and neuropsychological diseases in the offspring of men with high SDF.[6,7] Lower semen parameters have been observed in men with high SDF, and a recent study has also observed a correlation between SDF and sperm morphology among infertile men.[8,9]
Although semen analysis remains the gold standard for the evaluation of male factor infertility worldwide, it has many limitations including data obtained from fertile rather than infertile men, unequal population distribution, inability to assess sperm function and the lack of cut-off values to differentiate fertile from infertile patients.[1,10] Therefore, additional markers of male fertility such as genetic markers, OS, SDF and sperm function tests have also been explored to overcome the limitations of conventional semen analysis.
Although the routine use of SDF testing in assessing infertile men is still controversial, the American Urological Association and European Association of Urology guidelines have acknowledged the value of test.[11] The evidence supporting the utilisation of SDF testing in the clinical setting of infertility is increasing, and the guidelines for SDF testing have been proposed.[12] However, data on the impact of SDF on male fertility potential, pregnancy and ART outcomes, indications of SDF tests, optimal techniques for SDF tests and treatments that effectively reduce SDF are limited.[13,14] Therefore, in this review, we have provided updated evidence regarding SDF, underlying mechanisms, diagnostic tests and the impact of SDF on male fertility potential, pregnancy and ART outcomes.
METHODS
This narrative review included a systematic search of electronic scientific databases PubMed, Medline, Google Scholar, and Cochrane review to include published articles from 2010 to 2022. The search involved keywords and combinations of search terms ’male infertility’, 'sperm DNA fragmentation’, 'sperm DNA damage’, 'sperm DNA fragmentation tests’, 'semen parameters’, ’pregnancy’, ’fertilization’, ’assisted reproductive techniques’, ’IUI’, ’IVF’ and ’ICSI’, and ’genetic diseases’. Articles were perused and their reference lists were checked for relevant publications. We included articles published in English only.
CAUSES OF SPERM DNA FRAGMENTATION
Sperm DNA undergoes compaction during the process of spermatogenesis. For effective condensation, the sperm DNA encircles histone proteins which are gradually substituted by highly basic protamine.[1] Torsional stress is exerted by dsDNA during the process of condensation resulting in nicks and breaks in the DNA, followed by a restoration of appropriate reordering of chromatin.[15]
A range of cellular events is implicated in the impairment of fertility and SDF [Figure 1]. The reduction of protamination from failure to repair the nicks could result in sperm DNA damage.[1] Abnormal chromatin packing and remodelling during spermatogenesis,[16] as well as apoptosis during sperm maturation within the epididymis[17] also contribute to SDF. SDF is also caused by OS, varicocele, infections, inflammation of the male genital tract, drugs, chemotherapy, radiotherapy, cancer, obesity, advanced age, as well as environmental pollutants and toxins.[18,19,20,21,22] Furthermore, SDF is a potential mechanism that may explain the inability to conceive in couples with idiopathic infertility.[23] We and others have reported lower seminal antioxidants markers and higher SDF in infertile men compared to fertile controls and these abnormalities were ameliorated with oral antioxidants.[1,3,5,6,24,25,26]
Figure 1.
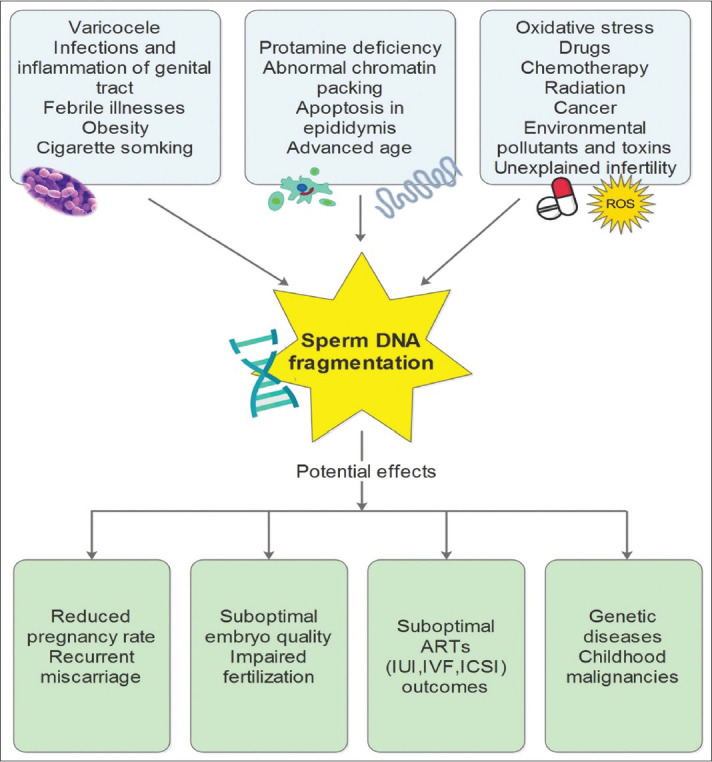
Causes of SDF and consequences of SDF on fertility potential and well-being of the newborn. IUI: Intrauterine insemination; IVF: In vitro fertilisation; ICSI: Intracytoplasmic sperm injection; SDF: Sperm DNA fragmentation
TESTS FOR SPERM DNA FRAGMENTATION
The available tests for the assessment of SDF are generally categorised into two main types: direct and indirect tests. Direct tests are used to measure the degree of sperm DNA damage through the use of probes and dyes. The indirect tests are used for evaluating the DNA vulnerability to denaturation, which is more characteristic of fragmented DNA.[27] The most frequently used tests for the evaluation of SDF include terminal deoxynucleotifyl transferase dUTP nick end labelling (TUNEL), the sperm chromatin structure assay (SCSA), and the sperm chromatin dispersion (SCD) assay.[1] SDF tests are summarised in Table 1.
Table 1.
Sperm deoxyribonucleic acid fragmentation testing techniques, principle, advantages, and disadvantages
| Test | Principle | Method | Interpretation | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Direct assays | |||||
| AO test | AO is nucleic acid selective dye and causes a metachromatic shift in fluorescence upon binding to DNA breaks | Air-dried semen sample smears are first fixed in Carnoy’s fixative for 2 h followed by AO staining for 5 min. Spermatozoa stained with AO are then excited at 488-nm wavelength | Intact DNA gives green fluorescence and damaged DNA red | Rapid, easy, and cost-effective test | Interlaboratory variations |
| AB staining | AB test detects the degree of sperm chromatin maturation and chromatin defects | Optical microscopic visualization of AB stained chromatin | Histone-rich nuclei of immature sperm stain blue, while mature protamine-rich nuclei remain unstained | Rapid, easy, and cost-effective test | Interlaboratory variations and results are dependent on staining efficiency |
| TUNEL | TUNEL assay identifies DNA breaks by the addition of template-independent DNA polymerase to the 3′hydroxyl (OH) breaks-ends of ssDNA and dsDNA | Fluorescein isothiocyanate conjugates with dUTPs and the fluorescent signal thus received is measured by flow cytometer or fluorescence microscopy | Sperm having DNA breaks show fluorescence and the results are presented as a percentage of fluorescent sperms | Direct test Can be used to detect dsDNA and ssDNA breaks Can be performed on fresh/frozen samples |
Inconsistent with high variability in the reference values Time-consuming |
| SCGE/comet assay | SCGE assessment of fragmented DNA | Sperm are embedded in agarose and lysed using detergent and high salt to form nucleoids with supercoiled DNA loops followed by electrophoresis | Fragmented DNA appears as a tail, while intact DNA remains in the sperm head | Direct assay High sensitivity and specificity Can detect different types of DNA damage in single sperm |
Inter-observer variation Time-consuming Requires fresh semen samples Expensive |
| Indirect assays | |||||
| TB staining | TB is an acidophilic metachromatic dye with a high affinity for sperm DNA phosphate residues | Optical microscopic visualization of stained damaged DNA chromatin | Sperm heads with high chromatin DNA integrity are stained blue and damaged ones are stained violet-blue/purple | Rapid, easy, and cost-effective test | Inter-observer variations and results are dependent on staining efficiency |
| CMA3 staining | CMA3 is an anthraquinone antibiotic glycoside that binds reversibly to DNA and competes with protamine for the same site | Air-dried seminal smear fixed with glacial acetic acid-methanol (1:3) solution for 20 min at 4 °C followed by staining with CMA3 | Spermatozoa with low protamination stains light yellow, and those with high DNA damage stains bright yellow | Strong correlation with other SDF assays | Interlaboratory and inter-observer variations |
| SCSA | SCSA is a flow cytometric test that identifies sperm DNA breaks indirectly by acid-induced DNA denaturation | Acid-induced denaturation of sperm DNA, followed by AO staining and measurement by flow cytometry | Intact DNA fluoresces green and denatured DNA as orange-red | Rapid, simple, precise, and repeatable test Can be performed on fresh/frozen semen samples |
Indirect assay Requires expensive set-up and skilled technicians |
| SCD/halo test | Detects fragmented DNA dispersion after acid denaturation | Sperm embedded in agarose microgel are acid denatured to remove nuclear proteins followed by staining with nuclear stain DAPI | Sperm with fragmented DNA do not produce the halo of dispersed DNA loops as produced by sperm with non-fragmented DNA, following acid denaturation | A simple test with easy availability of commercial kits Can be performed on neat and washed seminal samples |
Indirect assay Time-consuming Inter-observer variations |
SCD=Sperm chromatin dispersion, TUNEL=Terminal deoxynucleotidyl transferase dUTP nick end labeling, SCSA=Sperm chromatin structure assay, SCGE/COMET: Single-cell gel electrophoresis, AO=Arcidine orange, AB=Anilinie blue, TB=Toluidine blue, DNA=Deoxyribonucleic acid, SDF=Sperm DNA fragmentation, ssDNA=Single-stranded DNA, dsDNA=Double-stranded DNA, CMA3=Chromomycin A3, DAPI=4′,6-diamidino-2-phenylindole, dUTPs= 2’-Deoxyuridine 5’-Triphosphate, OH=Hydroxyl
Direct sperm DNA fragmentation tests
Acridine orange assay
The principle underlying acridine orange (AO) test is similar to that of SCSA which is based on the evaluation of the degree of DNA denaturation by quantification of the metachromatic shift of AO from green to red.[28] Visual interpretation is utilised in carrying out AO tests by using fluorescent microscopy without the use of flow cytometry. The test does not require extensive training.[28] This makes AO test more affordable and simpler than the SCSA test. However, the test lacks reproducibility and is associated with significant interlaboratory differences.[29]
Terminal deoxynucleotifyl transferase dUTP nick end labelling
TUNEL assay is utilised to identify ’nicks’, or free ends of DNA by using fluorescent nucleotides.[30] The TUNNEL assay was invented by Mitchell et al. by relaxing the whole chromatin structure with dithiothreitol (DIT) before fixation to permit contact with all ’nicks’.[31] A recently modified TUNEL protocol utilising bench top flow has been used recently.[32] The assay measures the integration of dUTP into dsDNA or ssDNA breaks via an enzymatic reaction. It further creates an indication that is multiplied by the number of DNA breaks. Evaluation of the sample is carried out through the use of flow cytometry or a standard fluorescence microscope. The test is limited by the lack of strict standardisation which makes the comparison between laboratories more difficult.[19]
In situ nick translation assay
In situ nick translation test is used to detect DNA strand breaks in the tissue section at the cellular level. Therefore, it can be used for sperm cells as well. NT test can also be applied to detect DNA damage in a single cell, and hence, it is useful to assess DNA damage, stress and apoptosis. The NT and TUNEL assays are similar. Both of them quantify the integration of dUTP into DNA breaks. The difference is that while TUNEL targets the identification of both ssDNA and dsDNA breaks, the NT assay targets the identification of ss breaks in a reaction catalysed by DNA polymerase I. The test is simple, but it is less sensitive than the other tests.[33]
Single-cell gel electrophoresis assay (Comet)
Single-cell gel electrophoresis or Comet assay quantifies the aggregate of DNA damage per spermatozoon, as a single cell can be followed on the gel.[34] Because the test can detect sperm DNA damage at a single cell level, it can be used for the assessment of cases with severe oligozoospermia.[35] The staining power of the comet test depends on the quantity of migrated DNA, which is an indication of different degrees of SDF.[36] Comet assay can not only detect ss and ds breaks but also identify altered bases. The method is inappropriate for quick diagnosis and needs highly experienced staff for the analysis of results. However, the method is informative because of its ability to analyse different kinds of DNA damage in a single cell by utilising electrophoresis.[16]
Indirect sperm DNA fragmentation tests
Toluidine blue staining
Toluidine blue (TB) staining is used for evaluating the integrity of sperm chromatin DNA. TB is a thiazine metachromatic dye with great attraction for sperm DNA phosphate residues. This microscopic assay stains the damaged chromatin nuclear structure of the spermatozoa. Optical microscopy is utilised for viewing the extent of damage after staining. It should be noted that intermediate coloration increases the interobserver variability.[1]
Chromomycin A3 staining
Chromomycin A3 (CMA3) and protamine compete for the same binding sites on the DNA. In CMA3 staining, a highly positive test is an indicator of a low DNA protamination state which is related to poorly packaged sperm chromatin.[37] CMA3 is a guanine-cytosine-specific fluorochrome, and its result has been shown to correlate well with that of aniline blue staining for sperm chromatin assessment.[1]
Sperm chromatin structure assay
Sperm chromatin structure assay (SCSA) evaluates the susceptibility of sperm DNA to denaturation. The test uses metachromatic characteristics of AO for this purpose.[38] The principle underlying SCSA is based on increased susceptibility of abnormal chromatin structure in the sperm DNA to acid or heat denaturation.[33] SCSA is a flow cytometry-based assay that assesses a large number of cells quickly and strongly.[39]
The degree of DNA denaturation is evaluated by the quantification of the metachromatic shift of AO from green to red after treatment with acid. This is done by utilising flow cytometry.[40] SCSA has the advantage that it has a standardised protocol for lowering interlaboratory differences. DNA fragmentation index (DFI) is used in SCSA as the measure of SDF.[1]
Sperm chromatin dispersion test
The principle underlying the sperm chromatin dispersion (SCD) test is that sperm with fragmented DNA fails to produce the characteristic halo of dispersed DNA loops after acid denaturation and removal of nuclear proteins, normally seen in sperms with nonfragmented DNA. The test is also known as Halosperm®test.[33] The test is limited by the interobserver variation resulting from its feature of subjective evaluation under the microscope. One advantage of the SCD test is that there is no need for complex instruments.
Indications for sperm DNA fragmentation testing
Measurement of SDF in infertile men is a promising diagnostic and prognostic tool and many indications for SDF tests have been suggested.[41,42] The main indications for SDF testing are summarised in Table 2.
Table 2.
Recommended indications for sperm DNA fragmentation testing
| Clinical varicocele |
| Unexplained infertility |
| Recurrent pregnancy loss |
| Borderline semen analysis |
| ART (IUI/IVF/ICSI) failure |
| Prognosis of ART treatment and natural pregnancy |
| Lifestyle and environmental factors |
ART=Assisted reproductive techniques, IUI=Intrauterine insemination, IVF=In vitro fertilization, ICSI=Intracytoplasmic sperm injection
Clinical varicocele
Various clinical reports have suggested a significant relationship between SDF and varicocele, in both infertile and fertile men.[10] SDF levels in men with varicocele were significantly high, and it was observed that, after varicocelectomy, the levels were significantly reduced, thereby leading to enhanced fertility potential.[43] Varicocele results in venous stasis and OS that are implicated in the development of SDF and testicular dysfunction.[44]
The existing body of literature shows little evidence of the clinical usage of SDF testing in low-grade varicocele, but there is stronger evidence for men with high-grade varicoceles.[43] Recent studies have revealed that DNA fragmentation testing could help clinicians to select varicocelectomy candidates.[44,45,46] SDF tests are applicable in men with grade II/III varicocele with conventional semen factors and endorsed in men with grade I varicocele with borderline/abnormal semen parameters.[19]
Assisted reproductive techniques (intrauterine insemination, in vitro fertilisation/intracytoplasmic sperm injection) failure
It has been reported that in IVF and ICSI, high SDF is related to a higher incidence of pregnancy loss.[4] Systematic reviews by Zini and Sigman[47] and Osman[48] have demonstrated that there is a significant correlation between high SDF and poor ART outcomes. However, these two systematic reviews were not without limitations due to heterogeneous study design and several potential confounding factors that could affect the results of the reviews which were not controlled. Nonetheless, there is sufficient evidence to suggest an association between high SDF and pregnancy loss.[47]
A significantly higher level of SDF was observed in ejaculated sperm compared to testicular sperm.[49] Moreover, recent studies have shown that the testicular sperm was associated with a higher success rate of IVF and ICSI.[50] Hence, testicular sperm instead of ejaculated sperm in ICSI might have several benefits in men with recurring IVF failure, high SDF and oligozoospermia. SDF can therefore provide useful prognostic information, especially on the outcome ART cycles in patients with recurring ART failure [Table 3]. Different therapeutic approaches, including oral antioxidants and sperm selection methods, have been tried to reduce SDF and its impact on ART results.
Table 3.
Summary of studies on sperm deoxyribonucleic acid fragmentation in male infertility
| Author and year | SDF test | Participants/intervention | Outcome measures | Results |
|---|---|---|---|---|
| Zandieh et al., 2018[51] | SCD | Fertile men (n=30), unexplained infertility group (n=28) | Semen parameters, hydrogen peroxide, superoxide anion levels, and SDF | In the infertile group, sperm motility and normal morphology were significantly lower than in the control group Levels of hydrogen peroxide and superoxide anion were higher in the infertile group SDF was higher (15%) in the infertile group |
| Carlini et al., 2017[52] | TUNEL | Fertile men with proven fertility (n=114), recurrent pregnancy loss (n=112) | Seminal parameters and SDF | Sperm DNA integrity was impaired in the recurrent pregnancy loss group and the values were higher (8%) when compared to the fertile group |
| Atig et al., 2017[53] | TUNEL | Fertile men (n=50), infertile men (n=100; 40 OAT, 31 teratozoospermia, 29 asthenozoospermia) | SDF, nalondialdehyde levels, and semen parameters | SDF and malondialdehyde levels were increased in the infertile group and SDF correlated with semen parameters |
| Wiweko and Utami, 2017[54] | SCD | Fertile group (n=36), the infertile group with abnormal semen analysis except for azoospermia (n=78) | Sperm DFI | Sperm DFI was significantly higher in the infertile group when compared to the control fertile group |
| Martínez-Soto et al., 2016[55] | TUNEL | Treatment group (n=32), docosahexaenoic acid (1500 mg for 10 weeks), placebo group (n=25) | SDF and semen parameters | Significantly lower SDF levels in treatment group (−17.2%±2.8%) versus placebo group (+11.2%±1.9%) Insignificant effect on sperm parameters |
| Malić Vončina et al., 2016[56] | TUNEL | Fertile group (n=51), unexplained couple infertility (n=85) | SDF, MMP levels, semen parameters and natural conception rate | 31% of infertile men conceived naturally Infertile group SDF values <25% and with MMP values >62.5% had significantly increased odds for conception (odds ratio 5.22) |
| Bareh et al., 2016[57] | TUNEL | Fertile (normozoospermic) group (n=31), unexplained recurrent pregnancy loss (n=26) | SDF | SDF was significantly higher (27%) in men with RPL compared to fertile controls |
| Ni et al., 2016[58] | SCSA | Healthy donors (n=25), abnormal semen analysis, and varicocele (n=15) | DFI and malondialdehyde | Sperm DFI was significantly lower in healthy donors with proven fertility A strong correlation between sperm DFI and malondialdehyde levels |
| Muratori et al., 2015[59] | TUNEL | Fertile group with proven fertility (n=86), couples with unexplained infertility (n=348) | SDF with receiver operating characteristic curves | After matching for both age and semen parameters, only brighter and total SDF predicts male fertility At high values of total SDF, brighter SDF predicts natural conception better than total SDF |
| Gual-Frau et al., 2015[60] | SCD | 20 infertile men with Grade 1 varicocele treated with combined antioxidants for 3 months | SDF and sperm concentration | Significantly lower SDF levels (−22.1%). 31.3% fewer highly degraded sperm cells, significantly higher sperm concentration |
| Abad et al., 2013[61] | SCD measurement done following various periods of sperm storage 37°C | 20 infertile patients with asthenoteratozoospermia treated with combined antioxidants for 3 months | SDF and sperm parameters | Significant decrease in SDF with time Significant improvement in sperm concentration, motility, and morphology |
| Mangiarini et al., 2013[62] | TUNEL | Normzoospermia (n=18), teratozoospermia (n=14) | SDF and sperm morphology | In normozoospermic individuals, TUNEL positive normal morphology sperm was 4% Spermatozoa with vacuolated head or a small non-oval head had a significantly higher incidence of DNA fragmentation in both groups (12 and 13%, 19 and 13% respectively) |
| Vani et al., 2012[63] | COMET | 120 healthy human subjects, 120 men exposed to lead received treatment with Vitamin C (1000 mg) 5 consecutive days per week for 3 months | SDF and semen parameters | Significantly lower alkaline-labile sites and mean tail length of the COMET when compared to the control group Significant improvement in all semen parameters |
| Brahem et al., 2011[64] | TUNEL | Fertile group with proven fertility (n=30), men with teratozoospermia (n=70) | SDF and chromosomal aneuploidy | Teratozoospermic individuals show increased levels of SDF and chromosomal aneuploidy |
| Venkatesh et al., 2011[65] | SCSA | Fertile men (n=50), couples with unexplained couple infertility (n=100) | DFI, sperm count, motility, and morphology | DFI in infertile men was significantly higher (9%) in infertile men when compared to controls Semen parameters were negatively correlated with DFI |
| Alahmar et al.[9] | SCD | Fertile controls (n=40), infertile men with idiopathic oligoasthenospermia (n=65) treated with CoQ10 | Semen parameters, seminal SDF, seminal antioxidant markers and seminal CoQ10 level | Increased sperm concentration, motility, seminal antioxidant markers, and CoQ10 level and reduced SDF and ROS |
| Alahmar et al.[66] | SCD | Fertile controls (n=50), infertile men with idiopathic oligoasthenoteratospermia (n=50) treated with CoQ10 | Semen parameters, seminal SDF, seminal antioxidant markers, seminal CoQ10 level and sex hormones | Increased sperm concentration, progressive and total motility, seminal antioxidant and CoQ10 levels, and reduced SDF and ROS |
| Alahmar and Singh[26] | SCD | Fertile controls (n=58), infertile men with idiopathic oligoasthenospermia (n=130) treated with CoQ10 or centrum multivitamin | Semen parameters, seminal SDF, seminal antioxidant markers, seminal CoQ10 level and sex hormones | Both CoQ10 and centrum were effective in improving semen parameters, antioxidant capacity, and SDF, but the improvement was greater with centrum than with CoQ10 |
| Alahmar and Naemi[25] | SCD | Fertile controls (n=84), infertile men with idiopathic oligoasthenospermia (n=178) treated with CoQ10 and followed up for 24 months | Semen parameters, seminal SDF, seminal antioxidant markers, seminal CoQ10 level and pregnancy rate, time to pregnancy, and their predictors | Increased sperm concentration, motility, seminal antioxidant markers, and CoQ10 level and reduced SDF and ROS CoQ10 level, sperm concentration, motility male age, ROS and GPx were independent predictors of pregnancy and time to pregnancy |
SCD=Sperm chromatin dispersion, TUNEL=Terminal deoxynucleotidyl transferase dUTP nick end labeling, DNA=Deoxyribonucleic acid, SDF=Sperm DNA fragmentation, DFI=DNA fragmentation index, OAT=Oligoasthenoteratospermia, SCSA=Sperm chromatin structure assay, COMET=Single-cell gel electrophoresis, ROS=Reactive oxygen species, COQ10=Coenzyme Q10, MMP=Mitochondrial membrane potential, dUTP=2’-Deoxyuridine 5’-Triphosphate, RPL=Recurrent Pregnancy Loss, GPx=Glutathione Peroxidase
Borderline semen parameters
A key contributing factor to male infertility is OS.[67] Antioxidants in the semen counteract excessive reactive oxygen species (ROS). The imbalance between the antioxidant and ROS levels could trigger a state of OS which could damage sperm.[68] According to Majzoub et al.,[69] antioxidant treatment with selenium and zinc resulted in a statistically significant fall in SDF levels by 19%, with a significant decrease in SDF and improvement in sperm concentration.
Age and smoking are other important factors linked with increased sperm DNA defects, reduced overall fertility, reduced fertilisation and reduced semen parameters.[70] It has been shown that DNA fragmentation is considerably lower in infertile non-smokers than in infertile smokers.[71] Abnormalities in semen parameters have been also linked to obesity.[72] Certain organochlorine pollutants such as metabolites of dichlorodiphenyltrichloroethane and polychlorinated biphenyls are also known to cause SDF.[61] Infertility and DNA damage are also associated with environmental and occupational exposure to metals such as cadmium and lead.[73]
Unexplained infertility/recurrent pregnancy loss
The prevalence of unexplained infertility is believed to exist in 20% of fertile couples.[19] SDF is considered an independent predictor of male fertility and helps in assessing unexplained infertility and impairment in sperm DNA integrity in men with normal conventional semen parameters.[74] Studies have shown that SDF could be used as a prognostic factor for natural pregnancy and IUI success rate.[19,75] Some reports have also revealed significantly higher SDF levels in couples with recurring pregnancy loss (RPL) than in controls [Table 3].[76,77,78]
Effect of sperm DNA fragmentation on male infertility
Conventional semen analysis remains the standard initial investigation for male infertility evaluation.[1] Conventional semen analysis has limitations and is unable to predict male fertility, and there is a need to identify new diagnostic and prognostic markers for male infertility.[2] In the last years, the SDF test has been generally recognised as a valuable tool for the evaluation of male infertility.[12]
SDF can be induced by OS, poor sperm compaction and abortive apoptosis.[4] OS impairs spermatogenesis, resulting in the generation of sperm with poorly chromatin condensation; these defective cells initiate an apoptotic pathway associated with an increase in ROS production by the mitochondria.[5] OS is also implicated in the activation of endogenous caspases and endonucleases that increase the damage of sperm DNA.[6] Mature sperms have limited mechanisms to repair DNA damage which makes the sperm vulnerable to oxidative stress-mediated DNA damage.[7]
The impact of SDF on male fertility potential has been supported by numerous studies.[8] Current data advocate different levels of SDF among patients with male infertility.[9] Further, SDF is negatively correlated with male fertility, and infertile men with poor reproductive outcomes exhibit high levels of SDF.[10] Progress in research on SDF has enhanced our understanding of the mechanisms involved in different infertility pathologies. SDF has been proposed to be the underlying cause of poor semen quality in men with IMI[12] and can be considered a promising diagnostic, prognostic and therapeutic tool in the management of male infertility.[13]
Effect of sperm DNA fragmentation on natural pregnancy
Sperm DNA damage negatively correlates with the chances of natural conception. The likelihood of natural pregnancy is decreased when the SDF index assessed by SCSA is 20%–30%[16] [Figure 1]. Some studies also suggest that SDF levels of more than 30% are associated with reduced pregnancy rates.[16,39] Similarly, SDF has been correlated with recurring miscarriages.[76] A study on 30 recurrent spontaneous abortion couples and 30 controls found higher SDF in the patients’ group.[77]
A relatively large number of couples fail to achieve pregnancy despite the absence of a male or female factor of infertility.[13] Gestation and subsequent embryo development depend on the integrity of the gamete's DNA.[15] Both sperm and oocyte contribute equally to the formation of the embryonic DNA; therefore, normal sperm chromatin structure is essential for safe and healthy transmission of parental genetic materials. The defect in sperm DNA structure compromises fertilisation and subsequent development of the embryo.[16]
Evidence from numerous studies supports the association between high SDF and failure to achieve natural pregnancy.[17] Different studies have shown that sperms with high SDF are associated with a longer time to achieve pregnancy and lower rates of natural conception.[20] Bungum et al. showed that a significant proportion of couples with unexplained infertile have a significantly high level of SDF. In addition, men with SDF level of more than 30% have a low probability of achieving natural pregnancy.[13] Miscarriage is a common pregnancy complication, occurring in 15% of all clinically recognised pregnancies, and is also related to SDF level.[16] Couples whose natural pregnancy resulted in miscarriage have lower sperm DNA integrity.[79]
Sperm DNA fragmentation and assisted reproductive techniques outcomes
There are controversies regarding the incorporation of DNA damage tests in the routine assessment of male factor infertility. While some reports showed that sperm DNA damage had a crucial effect on pregnancy and should be integrated as a part of routine clinical examination,[80] others suggested that SDF tests should not be integrated into the routine clinical examination.[39]
In a recent study carried out by Simon et al., out of the 92 studies that were evaluated for the association between SDF and ART, it was observed that only 35 studies indicated a considerably inverse association between SDF and the fertilisation rate.[81] The remaining 57 studies showed no substantial association. The association between sperm DNA damage and low fertilisation rate for every method was higher in IVF (59%) than in ICSI (24%).[33]
Intrauterine insemination
SDF levels higher than 30% may predict reduced pregnancy rates after IUI.[5] A study showed that the pregnancy was lower in men with SDF level of 12% or above.[33] However, Kimura and Nagao[82] reported no association between sperm DNA damage and clinical pregnancy rates after IUI. Measurement of SDF could help in the prediction of IUI results. When SDF is high, other therapeutic approaches such as ICSI could be adopted to treat infertile couples.
In vitro fertilisation
Several studies that have explored the impact of SDF on IVF and IVF/ICSI outcomes have provided variable results. While some studies suggested that SDF does not affect fertilisation or embryo quality,[83] others have implicated paternal factors and SDF in poor embryo development and early pregnancy loss.[80] In a review carried out by Zini et al.,[84] a significant association was observed between abnormal sperm DNA damage tests and lower pregnancy rates. Another study conducted by Zhang et al.[85] showed that higher clinical pregnancy rates were correlated with a DFI lower than 27%. A major challenge associated with these studies is the interpretation of results because of the heterogeneous designs and different protocols used. In a study to evaluate the clinical outcomes of SDF in 550 Chinese couples of which 415 underwent IVF and 135 ICSI, it was concluded that high levels of SDF were not related to alterations in pregnancy or live birth rates in both ICSI and IVF groups.[86]
Intracytoplasmic sperm injection
It has been reported that SDF has a considerable effect on pregnancy rates in different meta-analyses on IVF and ICSI.[33] A meta-analysis conducted by Zini and Sigman[47] revealed that the variation in the pregnancy rate between a group with high SDF and a group with low SDF was 11%. Another study revealed no significant correlation between the percentage of SDF and clinical pregnancy rates.[87] The percentage of SDF was also associated with the clinical pregnancy rate after ICSI.[88]
The probable implication of SDF in ART outcomes has been presented in numerous meta-analysis studies, but the strength of association and the association with ICSI are still debated. A meta-analysis by Sugihara et al. showed a limited capacity of SDF in predicting IUI outcome (risk ratio [RR]: 3.15, 95% confidence interval [CI]: 1.46–6.79),[1] while Chen et al. indicated that high SDF was significantly associated with lower pregnancy rate (RR: 0.34, 95% CI: 0.22–0.52) and birth rate of IUI cycle (RR 0.14, 95% CI: 0.04–0.56).[29]
Another two different studies on of total 25,639 and 8068 IVF/ICSI cycles, respectively, concluded that SDF has negative effect on clinical pregnancy (RR = 0.83; 95% CI: 0.73–0.94(and odds ratio [OR] = 1.31; 95% CI: 1.08–1.59), respectively, following IVF and/or ICSI treatment.[29,30]
Effects of sperm DNA fragmentationon birth defects
There are natural mechanisms that prevent the transmission of the defective genome to the next generation. However, ART treatment for infertility may facilitate the transmission of the defective genome, which could affect the well-being of children born using assisted reproduction. However, increased aneuploidy and high prevalence of genomic abnormalities in ICSI candidates were related to increased SDF levels.[68] Animal studies in mouse models have indicated adverse effects of increased SDF on offspring including abnormal growth and behaviour, premature ageing and increased prevalence of tumours.[37] Recent studies have linked increased SDF to a higher incidence of childhood malignancies, inherited diseases and neuropsychiatric disorders in the offspring.[66,9,90] Nonetheless, the evidence of the association between SDF and genetic diseases is inconsistent.
Treatment and Prevention of sperm DNA fragmentation
OS is a key player in the development of SDF.[31,32] OS triggers a harmful chain reaction that leads to sperm membrane lipid peroxidation and nuclear DNA damage.[33] Treatment and prevention of OS-induced damage could be achieved by, oral antioxidant therapy, varicocele repair, infection control and lifestyle modifications.[36] Although there are no standardised guidelines for the treatment of OS-induced sperm injury, antioxidant therapy is a promising strategy for the treatment of infertile men with high levels of oxidative damage.[37] The rationale behind antioxidant therapy is that SDF is recognised as a significant factor in infertility, pregnancy loss and ART failure, and antioxidants are believed to counteract these negative effects.[12]
There is growing evidence of antioxidant supplementation to minimise SDF in infertile men. However, beneficial effects on pregnancy and live birth outcomes are inconsistent.[41] Different antioxidants showed beneficial effects against SDF.[89] Early in vitro studies showed a protective effect for antioxidants flavonoids and catalase against OS-induced SDF.[42] A combination of zinc, D-aspartate and coenzyme Q10 protected human sperm against SDF using in vitro culture.[39] Ménézo et al. observed that an oral antioxidant treatment consisting of Vitamin C, Vitamin E, β-carotene, zinc and selenium could decrease SDF.[91]
The integrity of the sperm genome is a critical factor in ART success, and pregnancy and implantation rates may improve after antioxidant therapy in couples undergoing ICSI treatment.[19] In another study, the administration of Menevit© combined antioxidants significantly improves pregnancy rates in couples subjected to IVF/ICSI treatment.[46] Therefore, antioxidant treatment may improve fertilisation potential and reproductive outcomes.[41] However, large well-designed randomised placebo-controlled trials are essential to formulate a definitive conclusion.
CONCLUSION
SDF is a major factor involved in IMI and poor reproductive outcomes including reduced semen quality, reduced fertilisation, embryo quality, pregnancy rates, recurrent pregnancy loss and poor ART outcomes. With the advent of various novel, validated, sensitive SDF assays, SDF could provide diagnostic, therapeutic and prognostic information that allows better management of infertile couples, and can predict the outcomes of natural pregnancy and ART. Although the routine use of SDF tests is still not widely recommended, SDF testing in selected patients could still provide complementary data that cannot be provided by the traditional semen analysis. Hence, there is fair evidence indicating that SDF testing is a useful diagnostic and prognostic tool in male fertility evaluation in selected patients. Future studies should be focussed on optimising SDF tests, target patients groups, impact on fertility and ART outcomes as well as therapies that could reduce SDF levels in infertile men.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Panner Selvam MK, Agarwal A. A systematic review on sperm DNA fragmentation in male factor infertility: Laboratory assessment. Arab J Urol. 2018;16:65–76. doi: 10.1016/j.aju.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteves SC, Sharma RK, Gosálvez J, Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–52. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 3.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005.e8. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Alahmar AT, Calogero AE, Singh R, Cannarella R, Sengupta P, Dutta S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin Exp Reprod Med. 2021;48:97–104. doi: 10.5653/cerm.2020.04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vishvkarma R, Alahmar AT, Gupta G, Rajender S. Coenzyme Q10 effect on semen parameters: Profound or meagre? Andrologia. 2020;52:e13570. doi: 10.1111/and.13570. [DOI] [PubMed] [Google Scholar]
- 7.Bracke A, Peeters K, Punjabi U, Hoogewijs D, Dewilde S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod Biomed Online. 2018;36:327–39. doi: 10.1016/j.rbmo.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen HT, Dang HN, Nguyen TT, Nguyen TV, Dang TC, Nguyen QH, et al. Correlations between abnormalities of morphological details and DNA fragmentation in human sperm. Clin Exp Reprod Med. 2022;49:40–8. doi: 10.5653/cerm.2021.04777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alahmar AT, Calogero AE, Sengupta P, Dutta S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm dna fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J Mens Health. 2021;39:346–51. doi: 10.5534/wjmh.190145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evgeni E, Charalabopoulos K, Asimakopoulos B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J Reprod Infertil. 2014;15:2–14. [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: Recent advances in diagnosis and treatment. Reprod Biomed Online. 2013;27:325–37. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: A new guideline for clinicians. World J Mens Health. 2020;38:412–71. doi: 10.5534/wjmh.200128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteves SC, Gosálvez J, López-Fernández C, Núñez-Calonge R, Caballero P, Agarwal A, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol. 2015;47:1471–7. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 14.Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, Kammoun M, Meniaoui I, Sallem A, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril. 2016;105:58–64. doi: 10.1016/j.fertnstert.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935–50. doi: 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosálvez J, Lopez-Fernandez C, Fernandez JL, Esteves SC, Johnston SD. Unpacking the mysteries of sperm DNA fragmentation: Ten frequently asked questions. J Reprod Biotechnol Fertility. 2015;4:1–16. [Google Scholar]
- 18.Rex AS, Aagaard J, Fedder J. DNA fragmentation in spermatozoa: A historical review. Andrology. 2017;5:622–30. doi: 10.1111/andr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho CL, Agarwal A, Majzoub A, Esteves SC. Clinical utility of sperm DNA fragmentation testing: Concise practice recommendations. Transl Androl Urol. 2017;6:S366–73. doi: 10.21037/tau.2017.07.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amor H, Hammadeh ME, Mohd I, Jankowski PM. Impact of heavy alcohol consumption and cigarette smoking on sperm DNA integrity. Andrologia. 2022;54:e14434. doi: 10.1111/and.14434. [DOI] [PubMed] [Google Scholar]
- 21.Ho CL, Vaughan-Constable DR, Ramsay J, Jayasena C, Tharakan T, Yap T, et al. The relationship between genitourinary microorganisms and oxidative stress, sperm DNA fragmentation and semen parameters in infertile men. Andrologia. 2022;54:e14322. doi: 10.1111/and.14322. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez DC, Ory J, Blachman-Braun R, Nackeeran S, Best JC, Ramasamy R. Advanced paternal age and sperm DNA fragmentation: A systematic review. World J Mens Health. 2022;40:104–15. doi: 10.5534/wjmh.200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neill CL, Parrella A, Keating D, Cheung S, Rosenwaks Z, Palermo GD. A treatment algorithm for couples with unexplained infertility based on sperm chromatin assessment. J Assist Reprod Genet. 2018;35:1911–7. doi: 10.1007/s10815-018-1270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alahmar AT. The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med. 2019;46:112–8. doi: 10.5653/cerm.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alahmar AT, Naemi R. Predictors of pregnancy and time to pregnancy in infertile men with idiopathic oligoasthenospermia pre- and post-coenzyme Q10 therapy. Andrologia. 2022;54:e14385. doi: 10.1111/and.14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alahmar AT, Singh R. Comparison of the effects of coenzyme Q10 and centrum multivitamins on semen parameters, oxidative stress markers, and sperm DNA fragmentation in infertile men with idiopathic oligoasthenospermia. Clin Exp Reprod Med. 2022;49:49–56. doi: 10.5653/cerm.2021.04910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majzoub A, Esteves SC, Gosálvez J, Agarwal A. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl. 2016;18:205–12. doi: 10.4103/1008-682X.172642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: A committee opinion. Fertil Steril. 2015;103:e18–25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 29.Shamsi MB, Imam SN, Dada R. Sperm DNA integrity assays: Diagnostic and prognostic challenges and implications in management of infertility. J Assist Reprod Genet. 2011;28:1073–85. doi: 10.1007/s10815-011-9631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell LA, De Iuliis GN, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: Development of an improved methodology. Int J Androl. 2011;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 31.Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril. 2014;101:58–63.e3. doi: 10.1016/j.fertnstert.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of human semen. Eur Urol. 2016;70:635–45. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Kim GY. What should be done for men with sperm DNA fragmentation? Clin Exp Reprod Med. 2018;45:101–9. doi: 10.5653/cerm.2018.45.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29:2402–12. doi: 10.1093/humrep/deu228. [DOI] [PubMed] [Google Scholar]
- 35.Küçük N. Sperm DNA and detection of DNA fragmentations in sperm. Turk J Urol. 2018;44:1–5. doi: 10.5152/tud.2018.49321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enciso M, Sarasa J, Agarwal A, Fernández JL, Gosálvez J. A two-tailed comet assay for assessing DNA damage in spermatozoa. Reprod Biomed Online. 2009;18:609–16. doi: 10.1016/s1472-6483(10)60003-x. [DOI] [PubMed] [Google Scholar]
- 37.Cho CL, Agarwal A. Role of sperm DNA fragmentation in male factor infertility: A systematic review. Arab J Urol. 2018;16:21–34. doi: 10.1016/j.aju.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakkas D, Alvarez JG. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–36. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 39.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 40.Jin J, Pan C, Fei Q, Ni W, Yang X, Zhang L, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril. 2015;103:910–6. doi: 10.1016/j.fertnstert.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Esteves SC, Zini A, Coward RM, Evenson DP, Gosálvez J, Lewis SE, et al. Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia. 2021;53:e13874. doi: 10.1111/and.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farkouh A, Finelli R, Agarwal A. Beyond conventional sperm parameters: The role of sperm DNA fragmentation in male infertility. Minerva Endocrinol (Torino) 2022;47:23–37. doi: 10.23736/S2724-6507.21.03623-X. [DOI] [PubMed] [Google Scholar]
- 43.Malhotra V. Should sperm DNA fragmentation testing be routinely used in assessing male infertility? Transl Androl Urol. 2017;6:S699–701. doi: 10.21037/tau.2017.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril. 2011;96:1283–7. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, Dohle GR. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189:S146–50. doi: 10.1016/j.juro.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Lira Neto FT, Roque M, Esteves SC. Effect of varicocelectomy on sperm deoxyribonucleic acid fragmentation rates in infertile men with clinical varicocele: A systematic review and meta-analysis. Fertil Steril. 2021;116:696–712. doi: 10.1016/j.fertnstert.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Zini A, Sigman M. Are tests of sperm DNA damage clinically useful. Pros and cons? J Androl. 2009;30:219–29. doi: 10.2164/jandrol.108.006908. [DOI] [PubMed] [Google Scholar]
- 48.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod Biomed Online. 2015;30:120–7. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Moskovtsev SI, Jarvi K, Mullen JB, Cadesky KI, Hannam T, Lo KC. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93:1142–6. doi: 10.1016/j.fertnstert.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Pabuccu EG, Caglar GS, Tangal S, Haliloglu AH, Pabuccu R. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia. 2017;49:e12609. doi: 10.1111/and.12609. [DOI] [PubMed] [Google Scholar]
- 51.Zandieh Z, Vatannejad A, Doosti M, Zabihzadeh S, Haddadi M, Bajelan L, et al. Comparing reactive oxygen species and DNA fragmentation in semen samples of unexplained infertile and healthy fertile men. Ir J Med Sci. 2018;187:657–62. doi: 10.1007/s11845-017-1708-7. [DOI] [PubMed] [Google Scholar]
- 52.Carlini T, Paoli D, Pelloni M, Faja F, Dal Lago A, Lombardo F, et al. Sperm DNA fragmentation in Italian couples with recurrent pregnancy loss. Reprod Biomed Online. 2017;34:58–65. doi: 10.1016/j.rbmo.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Atig F, Kerkeni A, Saad A, Ajina M. Effects of reduced seminal enzymatic antioxidants on sperm DNA fragmentation and semen quality of Tunisian infertile men. J Assist Reprod Genet. 2017;34:373–81. doi: 10.1007/s10815-013-9936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiweko B, Utami P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl. 2017;27:1. doi: 10.1186/s12610-016-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Soto JC, Domingo JC, Cordobilla B, Nicolás M, Fernández L, Albero P, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst Biol Reprod Med. 2016;62:387–95. doi: 10.1080/19396368.2016.1246623. [DOI] [PubMed] [Google Scholar]
- 56.Malić Vončina S, Golob B, Ihan A, Kopitar AN, Kolbezen M, Zorn B. Sperm DNA fragmentation and mitochondrial membrane potential combined are better for predicting natural conception than standard sperm parameters. Fertil Steril. 2016;105:637–44.e1. doi: 10.1016/j.fertnstert.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 57.Bareh GM, Jacoby E, Binkley P, Chang TC, Schenken RS, Robinson RD. Sperm deoxyribonucleic acid fragmentation assessment in normozoospermic male partners of couples with unexplained recurrent pregnancy loss: A prospective study. Fertil Steril. 2016;105:329–36.e1. doi: 10.1016/j.fertnstert.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 58.Ni K, Steger K, Yang H, Wang H, Hu K, Zhang T, et al. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology. 2016;4:816–24. doi: 10.1111/andr.12210. [DOI] [PubMed] [Google Scholar]
- 59.Muratori M, Marchiani S, Tamburrino L, Cambi M, Lotti F, Natali I, et al. >DNA fragmentation in brighter sperm predicts male fertility independently from age and semen parameters. Fertil Steril. 2015;104:582–90.e4. doi: 10.1016/j.fertnstert.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Gual-Frau J, Abad C, Amengual MJ, Hannaoui N, Checa MA, Ribas-Maynou J, et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb) 2015;18:225–9. doi: 10.3109/14647273.2015.1050462. [DOI] [PubMed] [Google Scholar]
- 61.Abad C, Amengual MJ, Gosálvez J, Coward K, Hannaoui N, Benet J, et al. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia. 2013;45:211–6. doi: 10.1111/and.12003. [DOI] [PubMed] [Google Scholar]
- 62.Mangiarini A, Paffoni A, Restelli L, Ferrari S, Guarneri C, Ragni G, et al. Specific sperm defects are differentially correlated with DNA fragmentation in both normozoospermic and teratozoospermic subjects. Andrology. 2013;1:838–44. doi: 10.1111/j.2047-2927.2013.00138.x. [DOI] [PubMed] [Google Scholar]
- 63.Vani K, Kurakula M, Syed R, Alharbi K. Clinical relevance of vitamin C among lead-exposed infertile men. Genet Test Mol Biomarkers. 2012;16:1001–6. doi: 10.1089/gtmb.2012.0027. [DOI] [PubMed] [Google Scholar]
- 64.Brahem S, Mehdi M, Elghezal H, Saad A. Detection of DNA fragmentation and meiotic segregation in human with isolated teratozoospermia. J Assist Reprod Genet. 2011;28:41–8. doi: 10.1007/s10815-010-9482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011;18:1005–13. doi: 10.1177/1933719111401662. [DOI] [PubMed] [Google Scholar]
- 66.Alahmar AT, Sengupta P, Dutta S, Calogero AE. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin Exp Reprod Med. 2021;48:150–5. doi: 10.5653/cerm.2020.04084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–24. doi: 10.1016/j.aju.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–40. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 69.Majzoub A, Agarwal A, Esteves SC. Antioxidants for elevated sperm DNA fragmentation: A mini review. Transl Androl Urol. 2017;6:S649–53. doi: 10.21037/tau.2017.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;12:CD007411. doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 71.Zini A, Al-Hathal N. Antioxidant therapy in male infertility: Fact or fiction? Asian J Androl. 2011;13:374–81. doi: 10.1038/aja.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2009;18:761–8. doi: 10.1016/s1472-6483(10)60024-7. [DOI] [PubMed] [Google Scholar]
- 73.Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: Diagnosis and management. Int Braz J Urol. 2012;38:576–94. doi: 10.1590/s1677-55382012000500002. [DOI] [PubMed] [Google Scholar]
- 74.Oleszczuk K, Augustinsson L, Bayat N, Giwercman A, Bungum M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology. 2013;1:357–60. doi: 10.1111/j.2047-2927.2012.00041.x. [DOI] [PubMed] [Google Scholar]
- 75.Rima D, Shiv BK, Bhavna CH, Shilpa B, Saima KH. Oxidative stress induced damage to paternal genome and impact of meditation and yoga – Can it reduce incidence of childhood cancer? Asian Pac J Cancer Prev. 2016;17:4517–25. [PubMed] [Google Scholar]
- 76.Ford HB, Schust DJ. Recurrent pregnancy loss: Etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2:76–83. [PMC free article] [PubMed] [Google Scholar]
- 77.Khadem N, Poorhoseyni A, Jalali M, Akbary A, Heydari ST. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia. 2014;46:126–30. doi: 10.1111/and.12056. [DOI] [PubMed] [Google Scholar]
- 78.Absalan F, Ghannadi A, Kazerooni M, Parifar R, Jamalzadeh F, Amiri S. Value of sperm chromatin dispersion test in couples with unexplained recurrent abortion. J Assist Reprod Genet. 2012;29:11–4. doi: 10.1007/s10815-011-9647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barbăroşie C, Agarwal A, Henkel R. Diagnostic value of advanced semen analysis in evaluation of male infertility. Andrologia. 2021;53:e13625. doi: 10.1111/and.13625. [DOI] [PubMed] [Google Scholar]
- 80.Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90. doi: 10.4103/1008-682X.182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simon L, Emery BR, Carrell DT. Review: Diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract Res Clin Obstet Gynaecol. 2017;44:38–56. doi: 10.1016/j.bpobgyn.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Kimura M, Nagao K. Role of varicocele repair for male infertility in the era of assisted reproductive technologies. Reprod Med Biol. 2014;13:185–92. doi: 10.1007/s12522-014-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95:124–8. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 84.Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: A clinical perspective. J Assist Reprod Genet. 2009;26:427–32. doi: 10.1007/s10815-009-9343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Z, Zhu L, Jiang H, Chen H, Chen Y, Dai Y. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: A meta-analysis. J Assist Reprod Genet. 2015;32:17–26. doi: 10.1007/s10815-014-0374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xue LT, Wang RX, He B, Mo WY, Huang L, Wang SK, et al. Effect of sperm DNA fragmentation on clinical outcomes for Chinese couples undergoing in vitro fertilization or intracytoplasmic sperm injection. J Int Med Res. 2016;44:1283–91. doi: 10.1177/0300060516664240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anifandis G, Bounartzi T, Messini CI, Dafopoulos K, Markandona R, Sotiriou S, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia. 2015;47:295–302. doi: 10.1111/and.12259. [DOI] [PubMed] [Google Scholar]
- 88.Wdowiak A, Bakalczuk S, Bakalczuk G. The effect of sperm DNA fragmentation on the dynamics of the embryonic development in intracytoplasmatic sperm injection. Reprod Biol. 2015;15:94–100. doi: 10.1016/j.repbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Alahmar AT. Role of oxidative stress in male infertility: An updated review. J Hum Reprod Sci. 2019;12:4–18. doi: 10.4103/jhrs.JHRS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Selyam M, Sengupta P, Agarwal A, editors. In: Genetics of Male Infertility. Cham, Switzerland: Springer; 2020. Sperm DNA fragmentation and male infertility; pp. 155–72. [Google Scholar]
- 91.Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: An unexpected adverse effect. Reprod Biomed Online. 2007;14:418–21. doi: 10.1016/s1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]


