Abstract
Antimicrobial resistance is a major public health challenge described by the World Health Organization as one of the top 10 public health challenges worldwide. Drug-resistant microbes contribute significantly to morbidity and mortality in the hospital, especially in the critical care unit. The primary etiology of increasing antibiotic resistance is inappropriate and excessive use of antibiotics. The alarming rise of drug-resistant microbes worldwide threatens to erode our ability to treat infections with our current armamentarium of antibiotics.
Unfortunately, the pace of development of new antibiotics by the pharmaceutical industry has not kept up with rising resistance to expand our options to treat microbial infections. The costs of antibiotic resistance include death and disability, extended hospital stays due to prolonged sickness, need for expensive therapies, rising healthcare expenditure, reduced productivity from time out of the workforce, and rising penury. This review sums up the common mechanisms, trends, and treatment options for hospital-acquired multidrug-resistant microbes.
Keywords: hospital-acquired infections, multidrug resistance, antibiotic treatment, extensive drug resistance, pan drug resistance, antimicrobial resistance, antibiotic resistance
Introduction and background
Hospital-acquired multidrug-resistant microbes are a significant cause of morbidity and mortality, especially in the critical care unit. It is a significant public health threat that prolongs hospital stays and increases healthcare costs. In 2019, an estimated 4.95 million deaths were associated with bacterial antimicrobial resistance alone [1]. Approximately, 1.2 million deaths per year are directly attributable to bacterial antimicrobial resistance alone [1,2].
Antibiotic resistance is classified into three broad groups according to the sensitivity pattern to the different antibiotic classes, namely, pan-drug-resistant (PDR), extensively drug-resistant (XDR), and multidrug-resistant (MDR) microbial infections. MDR microbes are resistant to at least one agent from three or more antibiotic classes. XDR microbes are resistant to at least one agent from each antibiotic class except two or fewer classes. Lastly, PDR microbes are resistant to an agent from all antibiotic classes [3].
The rising incidence of MDR microbes is a safety concern for patients, clinicians, and healthcare administrators. Risk factors for acquiring MDR infections are associated with medical treatment and healthcare facilities, i.e., recent antibiotic use (<90 days), catheter or medical device carriage, and prolonged stay in a healthcare facility [4]. Hospital-acquired or nosocomial infections occur at least 48 hours after admission in a healthcare delivery setting, including hospitals and long-term care facilities. They may also arise after discharge from a healthcare facility [5]. Nosocomial infections put patients and healthcare staff at risk. Long-term care facilities are a proposed connecting link in spreading MDR infections between the hospital and the community [6].
The emergence of antimicrobial resistance is a result of the indiscriminate use of antibiotics in the healthcare, veterinary, and agricultural industries. Wrong antibiotic choice, inadequate dosing, and unnecessarily extended treatment drive antibiotic resistance within hospitals and other healthcare settings, such as nursing homes and the community. Much work has been done to describe antimicrobial resistance genes. However, we need to interrogate the trends in antimicrobial resistance and treatment options for MDR infections to inform and guide public health policy, antimicrobial stewardship programs, and clinical treatment guidelines. This systematic review describes recent antibiotic resistance trends and treatment options for hospital-acquired MDR infections.
Review
Reporting guideline
This systematic review was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [7].
Databases and search strategy
A systematic search was conducted using PubMed, PubMed Central (PMC), ScienceDirect, and Cochrane Library register for all databases on January 16, 2022. The field search was done on PubMed using Medical Subject Headings (MeSH) and keywords. We searched the other databases using the keywords hospital-acquired infection, cross-infection, microbial drug resistance, and treatment. PubMed Search Builders were created using the Boolean scheme, as shown in Table 1.
Table 1. The bibliographic search strategy.
| Concept | Keywords | PubMed Search Builder |
| Hospital-acquired infection | Hospital-acquired infection, cross-infection | (“Cross Infection/drug therapy”[MeSH] OR “Cross Infection/etiology”[MeSH] OR “Cross Infection/microbiology”[MeSH] OR “Cross Infection/prevention and control”[MeSH] OR “Cross Infection/therapy”[MeSH] OR “Cross Infection/transmission”[MeSH]) |
| Microbial drug resistance | (“Drug Resistance, Microbial/analysis”[MeSH] OR “Drug Resistance, Microbial/drug effects”[MeSH] OR “Drug Resistance, Microbial/epidemiology”[MeSH] OR “Drug Resistance, Microbial/etiology”[MeSH] OR “Drug Resistance, Microbial/prevention and control”[MeSH] OR “Drug Resistance, Microbial/statistics and numerical data”[MeSH] OR “Drug Resistance, Microbial/therapy”[MeSH] OR “Drug Resistance, Microbial/trends”[MeSH]) |
We pooled the keywords using the Boolean term “OR” and combined their corresponding search builders that we obtained from PubMed using MeSH terms. In addition, we applied restrictions to MeSH-major topics. All concepts and keywords were combined into a final search strategy using the Boolean term “AND,” as shown in Table 2.
Table 2. The MeSH strategy and corresponding filters.
| Full MeSH strategy | Number of articles |
| (“Cross Infection/drug therapy”[MeSH] OR “Cross Infection/etiology”[MeSH] OR “Cross Infection/microbiology”[MeSH] OR “Cross Infection/prevention and control”[MeSH] OR “Cross Infection/therapy”[MeSH] OR “Cross Infection/transmission”[MeSH]) AND (“Drug Resistance, Microbial/analysis”[MeSH] OR “Drug Resistance, Microbial/drug effects”[MeSH] OR “Drug Resistance, Microbial/epidemiology”[MeSH] OR “Drug Resistance, Microbial/etiology”[MeSH] OR “Drug Resistance, Microbial/prevention and control”[MeSH] OR “Drug Resistance, Microbial/statistics and numerical data”[MeSH] OR “Drug Resistance, Microbial/therapy”[MeSH] OR “Drug Resistance, Microbial/trends”[MeSH]) | 2,895 articles obtained after applying filters (filters: articles published in the last five years, articles published in the English language, patients older than 12) |
Inclusion and exclusion criteria
The population of interest includes patients admitted to the hospital for at least 48 hours with a culture or antigen/polymerase chain reaction (PCR)-confirmed microbial diagnosis. Our intervention is any novel antibiotic treatment. The comparator is the standard of care antibiotic treatment, and the outcome of interest is recovery as defined by clinical and microbiological cure-culture negative after antibiotic treatment.
The literature search was conducted to identify relevant studies that examine antibiotic resistance trends and treatment for hospital-acquired MDR infections. Inclusion criteria were studies conducted on the adult population and published in English as full-text papers in the past five years. Studies in the pediatric population, unpublished literature, papers older than 2016, irrelevant, non-full-text, gray, case reports, editorials, and non-English reports were excluded.
Screening of articles
After obtaining the relevant articles from the databases, we removed duplicates using Microsoft Excel. We subsequently screened the articles based on title, abstract, and reading full-text articles. Articles were screened based on their likelihood of yielding clinically significant practice changes as determined by the writing committee. Finally, we subjected all short-listed articles to a quality appraisal.
Quality appraisal
As displayed in Table 3, we assessed the short-listed articles for quality and risk of bias using tools depending on the study type. Each assessment tool had its criteria and scoring. A score of at least 60% for each assessment tool was accepted.
Table 3. Quality appraisal tools used to assess the various types of studies.
| Type of study | Quality appraisal tool |
| Narrative reviews | Scale for the Assessment of Narrative Review Articles 2 (SANRA 2) |
| Observational studies | Newcastle-Ottawa Scale |
| Randomized controlled trials | Cochrane Collaboration Risk of Bias Tool |
| Systematic reviews and meta-analyses | Assessing the Methodological Quality of Systematic Reviews (AMSTAR 2) |
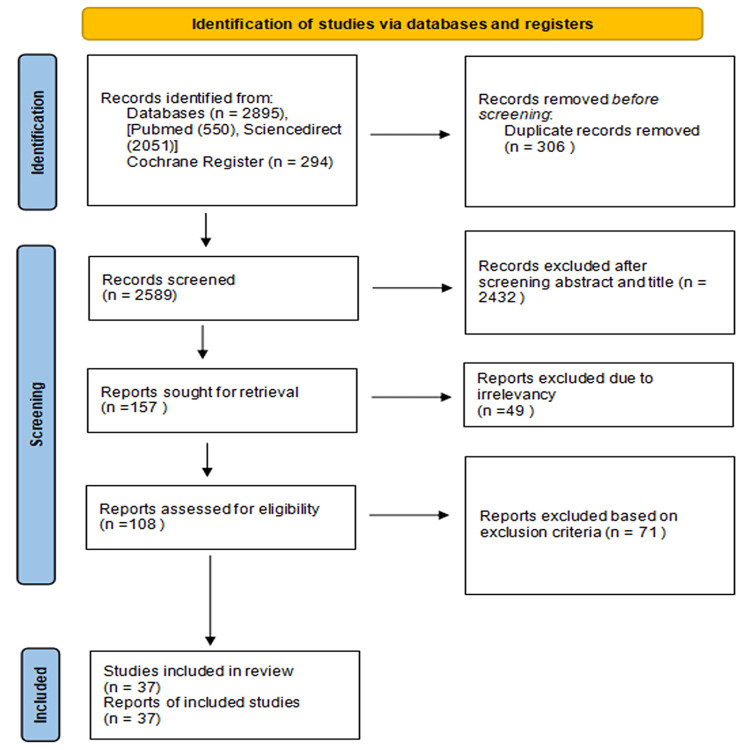
A total of 2,895 articles were found upon employing the appropriate keywords. A total of 306 duplicates were filtered out before screening; 2,589 articles underwent the screening process, of which 2,432 articles were removed based on their titles and abstracts. The authors retrieved 157 articles to assess the full text for relevancy and screened 108 reports for eligibility. In total, 37 articles were finally included in the review upon an in-depth analysis of quality, inclusion/exclusion criteria, and study designs. The first two authors conducted the data extraction and appraised the studies independent of each other. Whenever there arose a difference of opinion, the writing committee settled the outcome. The search strategy and the process of selecting the final studies included in this review are depicted in Figure 1.
Figure 1. The PRISMA flow diagram of the study search of databases and registers.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Because of the variability, such as heterogeneity of participants, interventions, and outcome measures, between studies, this systematic review describes these trials and reviews based on their outcomes, applicability, and limitations on a narrative synthesis rather than conducting a meta-analysis.
Two independent investigators (the first and second author) performed article selection, assessment, and analyses in each step. If there was a contradictory result regarding an article’s eligibility, its full text was assessed by consensus within the group.
We evaluated randomized controlled trials (RCTs) in this study using the Cochrane Collaboration risk of bias tool. Seven RCTs were reviewed. Four were included, and three were rejected due to at least one substantial risk of bias in any domain. The results of the quality assessment are shown in Table 4.
Table 4. Quality Assessment using the Cochrane Collaboration risk of bias tool.
| Article | Random sequence generation | Allocation concealment | Double blinding | Attrition bias | Reporting bias | Blinding outcome assessment | Other bias |
| Khorvash et al. [8] | Low | Low | Low | Low | Unclear | Unclear | Low |
| Harris et al. [9] | Low | Low | High | Low | Low | Unclear | Low |
| Arthur et al. [10] | Low | Low | Low | Low | Unclear | Low | Low |
| Salomão et al. [11] | Low | Low | Low | Low | Unclear | Unclear | Low |
| Davey et al. [12] | Low | Low | Low | Unclear | Low | Low | Low |
| Amin et al. [13] | Low | Low | Low | Low | Unclear | Low | High |
| Maxwell et al. [14] | Low | High | High | Low | Low | Unclear | Low |
We evaluated six systematic reviews using the Assessing the Methodological Quality of Systematic Reviews 2 (AMSTAR 2) criteria., We utilized a passing score of 60% as our cut-off for acceptance. Three articles were included, and three were rejected. The results are displayed in Table 5.
Table 5. Quality assessment using AMSTAR criteria for evaluation of selected systematic review studies.
AMSTAR: Assessing the Methodological Quality of Systematic Reviews
| AMSTAR 2 criteria | Spivak & Hanson [15] | Septimus [16] | Miller et al. [17] | Seo & Song [18] | Effah et al. [19] | Eljaaly et al. [20] |
| Did the research questions and inclusion criteria for the review include the components of PICO? | No | No | No | Yes | No | Yes |
| Was an “a priori” design implemented? | No | No | No | Yes | Yes | Yes |
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? | No | No | No | Unclear | Yes | Yes |
| 4. Did the review authors use a comprehensive literature search strategy? | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Did the review authors perform study selection in duplicate? | Yes | No | Yes | Yes | Yes | Yes |
| 6. Did the review authors perform data extraction in duplicate? | Yes | No | Yes | Yes | Yes | Yes |
| 7. Did the review authors provide a list of excluded studies and justify the exclusions? | No | No | No | No | No | No |
| 8. Did the review authors describe the studies included in adequate detail? | Partial Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Did the review authors use a satisfactory technique for assessing the risk of bias in individual studies that were included in the review? | No | No | No | Unclear | No | Yes |
| 10. Did the review authors report on the sources of funding for the studies included in the review? | Yes | Yes | Yes | Yes | Yes | Yes |
| 11. If a meta-analysis was performed, did the authors use appropriate methods to statistically combine results? | No meta-analysis conducted | No meta-analysis conducted | No meta-analysis conducted | Yes | Yes | Yes |
| 12. If a meta-analysis was performed, did the review authors assess the potential impact of risk of bias in individual studies on the results of the meta-analysis or other evidence synthesis? | No meta-analysis conducted | No meta-analysis conducted | No meta-analysis conducted | Unclear | No | Yes |
| 13. Did the review authors account for risk of bias in individual studies when interpreting/discussing the results of the review? | No | No | No | Unclear | No | No |
| 14. Did the review authors provide a satisfactory explanation for and discussion of any heterogeneity observed in the results of the review? | Yes | Yes | Yes | Unclear | Yes | Yes |
| 15. If they performed quantitative synthesis, did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its impact on the results of the review? | No meta-analysis conducted | No meta-analysis conducted | No meta-analysis conducted | Unclear | No | Yes |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | Yes | Yes | Yes | Yes | Yes | Yes |
| Total score (out of 16) | 6.5 | 5 | 7 | 10 | 10 | 14 |
| Overall methodological quality | Moderate | low | Moderate | Moderate | Moderate | High |
We also reviewed 47 narrative review articles using the Scale for the Assessment of Narrative Review Articles 2 (SANRA 2). A passing score of 70% was utilized as the cut-off. A total of 26 articles were included while 21 narrative review articles were rejected. The results are summarized in Table 6.
Table 6. Quality assessment of narrative reviews using the SANRA criteria.
0 (low standard), 1 (moderate standard), 2 (high standard).
SANRA: Scale for the Assessment of Narrative Review Articles
| Articles | Justification of importance for readership | Statement of aims/formulation of questions | Description of literature search | Referencing | Scientific reasoning | Appropriate presentation of data | Total |
| Luyt et al. [21] | 2 | 1 | 1 | 1 | 1 | 1 | 7 |
| Lee et al. [22] | 2 | 2 | 1 | 1 | 2 | 2 | 10 |
| Fernando al. [23] | 2 | 2 | 1 | 1 | 2 | 1 | 9 |
| Xia et al. [24] | 1 | 2 | 1 | 2 | 2 | 1 | 9 |
| Wong et al. [25] | 2 | 2 | 1 | 2 | 1 | 1 | 9 |
| Martin-Loeches et al. [26] | 1 | 2 | 1 | 1 | 1 | 1 | 7 |
| Tandogdu et al. [27] | 1 | 2 | 2 | 1 | 1 | 1 | 8 |
| Nasr [28] | 1 | 1 | 1 | 2 | 1 | 1 | 7 |
| Forsberg et al. [29] | 2 | 1 | 1 | 2 | 1 | 2 | 9 |
| Navon-Venezia et al. [30] | 2 | 1 | 2 | 1 | 2 | 2 | 10 |
| Gao et al. [31] | 1 | 1 | 1 | 1 | 2 | 1 | 7 |
| Niederman [32] | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Lyons & Kollef [33] | 1 | 1 | 1 | 1 | 1 | 2 | 7 |
| Giuliano et al. [34] | 1 | 2 | 1 | 2 | 1 | 2 | 9 |
| Yim et al. [35] | 1 | 2 | 1 | 1 | 1 | 1 | 7 |
| Lee et al. [36] | 2 | 1 | 1 | 1 | 2 | 2 | 9 |
| Dahiya et al. [37] | 1 | 1 | 1 | 2 | 1 | 1 | 7 |
| Lockhart et al. [38] | 2 | 1 | 1 | 2 | 2 | 2 | 10 |
| Juan et al. [39] | 2 | 2 | 1 | 2 | 2 | 1 | 10 |
| Rao et al. [40] | 1 | 2 | 1 | 1 | 2 | 2 | 9 |
| Lima et al. [41] | 2 | 1 | 2 | 1 | 2 | 1 | 9 |
| D'Accolti et al. [42] | 2 | 1 | 1 | 1 | 2 | 2 | 9 |
| Morley et al. [43] | 2 | 1 | 1 | 1 | 2 | 2 | 9 |
| Valenzuela-Valderrama et al. [44] | 1 | 2 | 1 | 1 | 2 | 1 | 8 |
| Hemeg [45] | 2 | 1 | 1 | 1 | 2 | 2 | 9 |
| Bassetti & Righi [46] | 1 | 1 | 1 | 1 | 2 | 1 | 7 |
| Gomez-Simmonds & Uhlemann [47] | 2 | 1 | 1 | 1 | 2 | 1 | 8 |
| Lynch et al. [48] | 2 | 1 | 1 | 1 | 1 | 1 | 7 |
| Bougnoux et al. [49] | 2 | 2 | 1 | 1 | 1 | 1 | 9 |
| Septimus & Schweizer [50] | 2 | 1 | 1 | 2 | 2 | 1 | 9 |
| Pulzova et al. [51] | 1 | 1 | 1 | 2 | 1 | 1 | 7 |
| Li et al. [52] | 1 | 2 | 1 | 1 | 2 | 1 | 8 |
| Kidd et al. [53] | 2 | 2 | 1 | 1 | 1 | 2 | 9 |
| MacVane [54] | 1 | 1 | 1 | 1 | 2 | 1 | 7 |
| Pettigrew et al. [55] | 2 | 1 | 2 | 2 | 2 | 1 | 9 |
| Kollef et al. [56] | 2 | 2 | 2 | 1 | 2 | 1 | 10 |
| Geisinger & Isberg [57] | 2 | 2 | 1 | 2 | 2 | 1 | 10 |
| Cardozo et al. [58] | 2 | 1 | 1 | 1 | 2 | 1 | 8 |
| Ruiz-Garbajosa & Canton [59] | 1 | 2 | 1 | 1 | 2 | 2 | 9 |
| Chia et al. [60] | 1 | 2 | 1 | 1 | 2 | 1 | 8 |
| Silva-Santana et al. [61] | 2 | 1 | 1 | 2 | 1 | 1 | 8 |
| Mea et al. [62] | 2 | 1 | 1 | 1 | 2 | 2 | 9 |
| Jamal et al. [63] | 1 | 2 | 1 | 2 | 2 | 1 | 9 |
| Zhang et al. [64] | 1 | 1 | 1 | 2 | 2 | 1 | 8 |
| Dey et al. [65] | 2 | 1 | 1 | 1 | 2 | 1 | 8 |
| Pulingam et al. [66] | 2 | 1 | 1 | 1 | 2 | 2 | 9 |
A total of 10 observational/cohort studies were reviewed using the Ottawa Quality Assessment Scale for Cohort Studies. We selected a passing score of 80% as the cut-off. Four studies were included in our final analysis and six studies were not accepted. The results are displayed in Table 7.
Table 7. Newcastle-Ottawa Quality Assessment Scale for Cohort Studies.
0 - 0 star, 1 - 1 star, and 2 - 2 stars according to Newcastle-Ottawa Scale.
| Articles | Representativeness | Selection | Ascertainment | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts | Assessment of outcome | Long follow-up | Adequacy of follow-up | Total score |
| Widerstrom et al [67] | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Alhumaid et al. [68] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Zaha et al. [69] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 |
| Cantón et al. [70] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Puzniak et al. [71] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Wu et al. [72] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Mehl et al. [73] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Bai et al. [74] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Stagliano et al. [75] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Álvarez-Marín et al. [76] | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
Discussion
Antibiotic Resistance Trends
The various mechanisms by which bacteria can become resistant to an antimicrobial agent due to the diverse resistance genes possessed by different microbial species are being elucidated [24,66]. Antibiotic therapy boosts the emergence of MDR strains through selection pressure and the transfer of genetic resistance elements. Drug resistance arises via de novo mutations during antibiotic use and horizontal transfer of genes via the acquisition of plasmids, transposons, and transferable genetic elements. These antibiotic resistance genes (ARGs) involve altered target binding sites, increased efflux pump activity, enzyme induction, and reduced porins. Extensive drug resistance and pan-drug resistance arise from accumulating multiple resistance gene elements [8]. The main antibiotic resistance mechanisms include active efflux pumps, beta-lactamases, carbapenemases, vancomycin resistance gene (Van A) ligases, and porin deficiency. Various gene families, such as the Ambler ampicillin hydrolyzing class C (AmpC), TEM-1, SHV-1, cefotaxime hydrolyzing gene(CTX-M), and oxacillin hydrolyzing gene (OXA), encode beta-lactamases common in bacteria such as Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. Staphylococcal cassette chromosome mecA (SCCmecA) and Mer7 are transferable genetic elements possessed by Staphylococcus aureus strains that encode methicillin resistance.
Enterococci are ubiquitous in the environment and present in the natural gut microbiome. Two clinically significant species are Enterococcus faecalis and Enterococcus faecium. Enterococcus faecium is more responsible for fatal invasive hospital-associated infections. Antimicrobial resistance is prevalent in 80-100% of E. faecium isolates compared to at most 16% of E. faecalis isolates [36]. Enterococci have obtained high-level β-lactam resistance through modification of the penicillin-binding protein gene, resulting in decreased β-lactam affinity and increased β-lactam tolerance due to upregulation of gene expression.
Glycopeptide-resistant enterococci spp. have become clinically significant due to the resistance conferred by gene resistance elements of the Van family. Risk factors for vancomycin-resistant enterococci infection include invasive gastrointestinal, pulmonary, and urologic procedures, indwelling medical devices, and exposure to fourth-generation cephalosporins. The vancomycin (Van) resistance gene family (Van A, B, C, D, G, H, and L) encodes enzymes that lead to decreased affinity at the glycopeptide binding site and substitution of the normal precursors, which end in D-Ala-D-Ala amino acid sequence [24,66]. The Van H gene, also possessed by vancomycin-resistant Staphylococcus aureus (VRSA), encodes a dehydrogenase that converts pyruvate to D-Lac. The Van A gene encodes a ligase which forms an ester bond between D-Ala and D-Lac. Vancomycin can only combine with the D-Ala-D-Ala binding site but not with the D-Ala-D-Lac binding site, thus leading to vancomycin resistance.
K. pneumoniae, belonging to the Enterobacteriaceae family, naturally inhabits the intestinal microbiome. In one study of MDR K. pneumoniae isolates in Eastern and South-Western Europe, 50-60% of isolates were resistant to fluoroquinolones, third-generation cephalosporins, and aminoglycosides [30]. The prevalence rate of resistance to commonly used antibiotics in K. pneumoniae was 40-80% of isolates in one study in Asia [19,22]. Colistin resistance was identified in 2.9% of K. pneumoniae isolates in Asia [19]. The global spread of hypervirulent K. pneumoniae strains with extensive antibiotic resistance is worrying. Half of hypervirulent K. pneumoniae infections affect patients who are non-elderly and do not have comorbidities, with a mortality rate of up to 40% [22].
K. pneumoniae resistance is driven by the accumulation of antibiotic resistance genes leading to XDR strains harboring a super resistome. A super resistome may encompass combinations of carbapenemase genes with aminoglycoside-modifying enzymes or association of CTX-M or New Delhi metalloproteinase(NDM) carbapenemases, 16S ribosomal ribonucleic acid (rRNA) methylases together with porin deficiency and quinolone resistance chromosomal mutations [22]. K. pneumoniae can acquire or transfer mobile genetic elements such as transposons from other gram negatives, including E. coli and Serratia marcescens. Gene elements belonging to the NDM, VIM, IMP-1,and KPC (K. pneumoniae carbapenemases) enzyme families encode carbapenemases that have an increased activity giving rise to extended-spectrum beta-lactamase (ESBL) Enterobacteriaceae. These enzymes can hydrolyze extended-spectrum cephalosporins. They confer resistance to commonly used beta-lactam antibiotics such as ceftazidime, ceftriaxone, and cefotaxime. Plasmid-mediated resistance genes of all classes have also been identified in K. Pneumoniae. The armA gene family encodes for enzymes that prevent aminoglycosides from binding to their 16S rRNA target. Other known plasmids gene-mediated 16S rRNA methylases include the Rmt family and NpmA gene families.
Chromosomal resistance mechanisms that have evolved against aminoglycosides in K. pneumoniae include alterations in AcrAB-TolC and KpnEF efflux pump systems and loss of porins. For fluoroquinolone resistance, the major mechanism is chromosomal mutations in the quinolone binding targets on DNA gyrase involving the gyrA-gyrB subunits and topoisomerase IV involving the parC-parE subunits. These mutations are also seen in other gram negatives such as P. aeruginosa [57]. Overall, fluoroquinolone resistance rates vary geographically but range from 30% to 40% in many countries [43]. Moreover, the K. pneumoniae plasmid encodes the aac and qnr subfamily of genes chromosomally encoded in other gram negatives such as Citrobacter spp., Stenotrophomonas maltophilia, and S. marcescens that confer resistance to aminoglycoside, fluoroquinolones, and beta-lactams [19].
Porin-mediated resistance in P. aeruginosa occurs through mechanisms that downregulate the transcription of the oprD gene leading to the deficiency of porins in the outer membrane resulting in decreased susceptibility. Hydrophilic antibiotics, such as β-lactams, tetracyclines, aminoglycosides, and some fluoroquinolones, have been shown to traverse the outer membrane via porins. On the other hand, a decrease in intracellular antibiotic concentration can occur via extrusion through efflux pumps on the membrane. Efflux pumps are classified into six superfamilies. The superfamilies contain (a) the ATP-binding cassette (ABC) superfamily, (b) the small multidrug resistance (SMDR) superfamily, (c) the major facilitator (MF) superfamily, (d) the resistance-nodulation-division (RND) superfamily, (e) the multidrug and toxic compound extrusion (MTCE) superfamily, and (f) the drug metabolite transporter (DMT) superfamily.
P. aeruginosa is widely prevalent in the hospital environment and usually causes colonization of the alimentary and respiratory tracts. A history of previous rectal colonization is typically present in most patients developing infections. Recent antibiotic therapy is a significant risk factor for rectal colonization by MDR P. aeruginosa in critically ill patients [39,59]. Intestinal colonization and previous use of antibiotics are key risk factors for P. aeruginosa infections. Pathogen-related factors that determine a worse outcome of P. aeruginosa infections include the presence of certain horizontally acquired genomic islands; infection by specific clonal lineages; and expression of virulence factors, such as elastase, type III secretion system (T3SS), and the production of cytotoxins [39]. Host factors such as age, immunosuppression, and underlying disease influence the outcome of Pseudomonas infections. Delayed adequate antimicrobial therapy is also independently associated with increased mortality. In one study, single-agent susceptibility rates for the 11,701 non-duplicate P. aeruginosa isolates ranged from 72.7% for fluoroquinolones and 85.0% for piperacillin-tazobactam [71]. Susceptibility rates were higher for blood isolates than for respiratory isolates [39]. The increasing prevalence of MDR or XDR P. aeruginosa isolates is associated with the spread of high-risk clones, such as ST175 [59].
Acinetobacter spp. is a gram-negative, non-fermenting coccobacillus strictly aerobic, oxidase-negative, catalase-positive, pleomorphic, and non-motile [41]. These bacteria are widespread in the environment in soil, water, and sewage. Acinetobacter baumannii causes opportunistic nosocomial infections involving patients on mechanical ventilation in intensive care units. A. baumannii can colonize new surfaces by the formation of a biofilm. Although polymyxin-resistant A. baumannii represents less than 1% of clinical isolates, its widespread dissemination, multidrug resistance, and multiple virulence factors make it a severe threat to public health worldwide [62]. It has been shown using molecular techniques that A. baumannii outbreaks have been primarily due to specific clones [41]. A. baumannii commonly has extensive resistance to penicillins, cephalosporins, tetracyclines, macrolides, chloramphenicol, fluoroquinolones, aminoglycosides, and carbapenems. Polymyxins are the antibiotic of last resort to treat infections caused by XDR A. baumannii. Polymyxin acts by disrupting membrane integrity through the displacement of divalent cations in the outer membrane by binding to the lipopolysaccharide (LPS) and causing cell lysis. Unfortunately, lineages with low sensitivity to polymyxins have increased in Europe, Asia, and South America [63].
Polymyxin resistance in A. baumannii is attributed to changes in the outer membrane through phosphoethanolamine addition, loss of LPS, changes in osmoprotective amino acids, and overexpression of efflux pumps. Inactivation of the lipid A biosynthetic genes, lpxA, lpxC, or lpxD, results in a complete loss of surface LPS. Thus, the loss of LPS prevents the essential interaction between it and polymyxins [25,63]. Mutations identified in the pmr family of genes are also associated with colistin resistance. This family of genes encodes enzymes involved in the synthesis of lipid A, a component of LPS. Modification of lipid A protects the outer membrane from the binding and action of polymyxins. A. baumannii also possesses genes expressing efflux pumps related to antibiotic resistance, including resistance-nodulation division (RND), major facilitator (MF), multidrug-toxic compound extrusion (MATE), and small multidrug resistance (SMR) families. The mcr-encoding plasmid found initially in E. coli in China has been subsequently reported worldwide in other gram-negative bacteria, including A. baumannii [25].
Fungal Antimicrobial Resistance
The growing incidence of fungal infections in the hospital environment is alarming. Fungi are normal commensals on the human body but can cause invasive infections, particularly in immunocompromised patients. Risk factors for invasive fungal infection include the presence of a central venous catheter, invasive catheterization, diabetes mellitus, immunosuppression, receiving total parenteral nutrition, recent surgery, extended hospital stay, prolonged admission to the ICU, and having received broad-spectrum antibiotics [29,38]. Nosocomial outbreaks due to relatively uncommon fungal species such as Exserohilum rostratum and Sarocladium kiliense have occurred following the contamination of medical products [49]. However, significant MDR fungemia is usually caused by Candida spp., including Candida glabrata, Candida parapsilosis, and Candida auris.
C. auris, an MDR yeast species, is undoubtedly the most problematic species because of its ability to form a biofilm, colonize patients, and persist in the healthcare environment. First reported in 2009 in a Japanese patient, C. auris cases have since been reported, as of February 15, 2021, in 47 countries on all inhabited continents. C. auris isolates have been classified using whole-genome sequencing into four geographically distinct clonal populations [29]. C. auris is clinically significant because it has demonstrated resistance to multiple antifungal drugs, with some isolates resistant to all major antifungal classes (azoles, polyenes, and echinocandins). C. auris candidemia is associated with a 30-60% mortality rate. The transmissibility and extensive antifungal resistance characteristic of C. auris set it apart from other Candida species. In the United States, approximately 90% of isolates have been resistant to fluconazole, 30% to amphotericin B, and 5% to echinocandins compared to 10% of C. glabrata isolates exhibiting fluconazole resistance, and less than 10% exhibit echinocandin resistance [38].
In one study, 41% of patients received systemic antifungal therapy when C. auris was isolated. The median time from admission to infection was 19 days, 61% of patients had bloodstream infections, and 59% died. Interestingly, 41% of isolates were resistant to two antifungal classes, and 4% were resistant to three classes of antifungals [74]. In another study at a large tertiary hospital in China, the average detection rate was 0.29% over a decade. Non-Candida albicans was the main fungus, accounting for 62.5% of isolates. The drug resistance of non-Candida albicans was higher than that of C. albicans, among which C. glabrata had the highest resistance rate [38]. Molecular mechanisms underlying resistance in Candida species include Erg11 mutations, which mediate fluconazole resistance. Efflux pump activity also contributes to azole resistance. It is hypothesized that FKS mutations observed in C. auris isolates, such as the S639F mutation, are responsible for micafungin resistance. A mutation in a gene involved in ergosterol biosynthesis mediates resistance to amphotericin B via a reduction in ergosterol content in the fungal cell wall [29].
Biofilms
Biofilms are aggregates of microorganism communities that adhere irreversibly to abiotic or biotic surfaces through the production of extracellular polymeric material [40]. The self-produced polymeric matrix facilitates the formation of complex structures that promote antibiotic resistance through horizontal gene transfer and persister cells that result in chronic or recurring infections. Persister cells are dormant cells within biofilms that can tolerate high concentrations of antibiotic agents [45]. Biofilms play an essential role in healthcare-associated bacterial and fungal infections. They are more resistant to antimicrobials due to their (a) physiological state, (b) cell density, (c) quorum sensing abilities, (d) protective extracellular matrix, (e) upregulation of drug efflux pumps, (f) increased expression of resistance genes, and (g) presence of persister cells. The significance of the drug efflux pump mechanism in biofilms was observed in a study involving C. albicans that showed that strains lacking Cdr1p, Cdr2p, and Mdr1p pumps were more susceptible to fluconazole at the initial stages of biofilm formation compared to the wildtype. Again, the expression of the efflux pump, AfuMDR4, was notably upregulated in vivo upon exposure to voriconazole [45].
Treatment options
Policy
The use of an antibiotic policy fosters improved prescribing practices and evidence-based antimicrobial use. An antimicrobial stewardship program involves a multifaceted and multidisciplinary approach to achieving the following goals: (a) controlling antimicrobial resistance, (b) improving clinical outcomes, and (c) reducing costs by improving antimicrobial use [56]. The core components of an antibiotic policy must include antimicrobial stewardship, especially the development of prescribing guidelines and standards of care, as well as infection prevention strategies such as hand hygiene, hospital cleaning, and disinfection. Active surveillance is required in outbreaks of MDR, XDR, and PDR infections. Antibiotic resistance surveillance and comparisons of prescribing practices are beneficial feedback activities once effectively communicated to healthcare practitioners [23].
One RCT identified high-certainty evidence that interventions in antimicrobial stewardship programs enabled physicians to improve their antibiotic prescribing practices, reduced the length of stay in hospitals by 1.12 days, and did not increase mortality [12]. Interventions were categorized into restrictive techniques, which incorporate policies to make physicians prescribe properly, and enablement techniques, which provide feedback and advice to help physicians prescribe properly. Enablement interventions were more effective in improving prescribing practices [12]. The best current intervention for optimizing antibiotic use is to have clear guidelines for using an antibiotic regimen. The antibiotic regimen selected should have the highest efficacy for a confirmed infection. The benefits of such intervention include improved clinical cure rates, less antibiotic toxicity, fewer Clostrididoides difficile infections, less disruption of the gut microbiome, and fewer MDR infections. The overarching goal is to provide timely, appropriate antibiotics while avoiding antimicrobial resistance. Timely initial appropriate antibiotics are a critical determining factor of outcomes in severe infections. Several studies have demonstrated that inappropriate initial antimicrobial therapy was independently associated with increased mortality and extended hospital stay [12,56].
Appropriate initial antimicrobial therapy can be achieved using a local antibiogram or rapid molecular identification methods. Keeping a local antibiogram is imperative to guide and periodically review antimicrobial stewardship programs. An antibiogram records the overall profile of antimicrobial susceptibility testing results of specific microbes to a battery of antimicrobial drugs. It helps to guide empiric treatment while microbiology culture and sensitivity results are pending. Additionally, they can be used to detect, monitor, and investigate trends in antimicrobial resistance. Rapid microbiological identification methods, such as PCR, are currently in clinical use to identify resistance genes and quickly guide initial targeted narrow-spectrum antibiotic treatment until final microbiological culture and sensitivity results are known. The ability to determine susceptibility patterns in hours rather than in days is handy to the clinician, especially in severely ill patients or those with bacteremia. The drawback is that these methods do not differentiate colonization from infection.
Non-antibiotic Measures
The healthy microbiota provides protective functions, including preventing colonization and infection via competitive pressure. Antibiotic exposure is associated with disrupting the microbiota that selects for resistance in the gut microbiome. Novel methods to exploit protective mechanisms provided by intact microbiota may provide the key to preventing the spread of MDR organisms in the healthcare setting [55,66]. It is hypothesized that probiotics may effectively decolonize and prevent MDR infections by promoting healthy intestinal microbiota. Some evidence-based analyses from various human studies and animal models have shown the clinical potential of probiotics against infectious diseases, diarrhea, intestinal infections, inflammatory diseases, and antibiotic-associated diarrhea. These studies suggest that it is possible to counteract microbial colonization and antimicrobial resistance spread [42]. However, this is yet to be proven by an RCT. In several RCTs, probiotic drugs were ineffective for decolonizing hospitalized patients harboring MDR gram-negative bacilli and preventing subsequent infections. They did not reduce the in-hospital length of stay, the incidence of adverse events, and in-hospital mortality rates [11].
Antibiotic Measures
Decolonization is a strategy to reduce the incidence of healthcare-associated infections. Decolonization involves the use of topical antimicrobial agents to reduce the bacterial burden on specific sites of the human body, including the nares and the skin. There have been only a few multicenter, randomized trials evaluating decolonization. Of the few that exist, even fewer have compared decolonizing agents head-to-head to determine the superiority of an agent or a decolonizing protocol [50]. The most robust evidence for decolonization is to prevent surgical site infections among surgical patients. The populations that benefit the most from decolonization are cardiac and orthopedic surgery patients. The common agents used for decolonization include chlorhexidine, mupirocin, and povidone-iodine [18,50]. Mupirocin is used for nasal decolonization for methicillin-resistant Staphylococcus aureus (MRSA). Chlorhexidine gluconate (CHG) is the decolonization agent with the most substantial evidence base for oral and skin cleansing. A meta-analysis revealed that 2% chlorhexidine bathing significantly reduced hospital-acquired infection incidence and MDR organisms in ICUs [18].
The reducing sensitivity of MRSA, coagulase-negative Staphylococcus spp., and Enterococcus species to vancomycin is a worrisome threat [36]. The minimum inhibitory concentration (MIC) creep phenomenon is a notable cause of increasing resistance to vancomycin often occurring because of underdosing and excessive use. MIC creep refers to the gradual but steady increase in the levels of MIC standards for MRSA isolates. This results in poor clinical response, high relapse rates, and treatment failures. The two leading alternatives for vancomycin-resistant enterococci (VRE) and VRSA treatment are linezolid and daptomycin, with clinical success rates of 50-80% as a first-line drug and 50-59% as salvage therapy for VRE bacteremia, respectively [75].Enterococci spp. commonly have intrinsic resistance to penicillin monotherapy. However, susceptibility increases when antibiotics with activity against the bacterial cell wall, such as β-lactams, are used synergistically. Double β-lactam therapy is effective in enterococci endocarditis, although no studies have shown efficacy for such therapy in other sites such as deep-seated abscesses and osteomyelitis. Again, ampicillin, an aminopenicillin, is remarkably effective against enterococci infections when used in synergy with gentamicin, an aminoglycoside. Nephrotoxicity commonly limits the use of gentamicin.
For gram-negative bacteria, ceftolozane/tazobactam is especially active against P. aeruginosa (from the intrinsic activity of ceftolozane, a semi-synthetic fifth-generation cephalosporin). In contrast, the addition of tazobactam confers activity against most ESBL)producers. It is approved to treat complicated urinary tract infections, intra-abdominal infections, and nosocomial pneumonia. Avibactam is a novel β-lactamase inhibitor that inactivates class A [including K. pneumoniae carbapenemase (KPC)], class C (AmpC), and some class D (OXA) β-lactamases. The combination of ceftazidime/avibactam inhibited 82% and 76% of MDR and XDR strains, respectively. The susceptibility of P. aeruginosa toward ceftazidime increases from 65% to 94% when used in combination with avibactam [59]. Another novel β-lactamase, relebactam, inhibits Ambler class A and class C cephalosporinases, effectively boosting imipenem activity against resistant K. pneumoniae carbapenemase (KPC) and P. aeruginosa [77]. The combination of imipenem, cilastatin, and relebactam is approved for the treatment of complicated intra-abdominal infections and complicated urinary tract infections.
Newer antibiotics discovered in the last decade include cefiderocol, plazomicin, and eravacycline. Cefiderocol is a novel siderophore cephalosporin that binds to ferric iron which is required for bacterial growth and virulence. Cefiderocol is actively transported across the outer membrane resulting in high concentrations in the periplasmic space, where it exerts a bactericidal effect by binding to penicillin-binding proteins and inhibiting cell wall synthesis. It has been shown to have potent in-vitro activity against MDR gram-negative bacteria including Enterobacterales (>90% of isolates), P. aeruginosa, A. baumannii, and Stenotrophomonas maltophilia [78]. However, cefiderocol has a label warning for higher all-cause mortality versus other antibiotics in critically ill patients with MDR gram-negative bacteria with a mortality rate of 34% for cefiderocol vs. 18% in the best-available therapy group [78]. Plazomicin is a synthetic aminoglycoside approved by the U.S. Food and Drug Administration (FDA) for complicated urinary tract infections active against >95% of Enterobacterales isolates. It is active against ESBL isolates and against 84.6% to 97.6% of carbapenem-resistant isolates. The presence of aminoglycoside-modifying enzymes does not inactivate plazomicin, and it is active against 52.2% of isolates that are resistant to three members of the aminoglycoside drug class [79]. Eravacycline is a fluorocycline of the tetracycline class. The FDA has approved eravacycline for the treatment of complicated intrabadominal infections. Eravacycline is active against ESBL E. coli and K. pneumoniae. It has activity against A. baumannii andcarbapenem-resistant Enterobacterales but has limited activity against P. aeruginosa. Eravacycline has been also investigated for complicated urinary tract infections but showed lower cure rates (84.8% vs. 94.8%) and (60.4% vs. 66.9%) than ertapenem and levofloxacin, respectively [79].
The overall incidence of non-ventilator hospital-acquired pneumonia was 1.6%, representing a rate of 3.63 per 1,000 patient days in the United States [34]. In one study, ceftazidime/avibactam was non-inferior to meropenem to treat healthcare-associated pneumonia/ventilator-associated bacterial pneumonia [53]. It is a valid option against carbapenem-resistant Enterobacteriaceae (CRE). Meropenem/vaborbactam, another novel therapeutic option, displayed a non-significant trend toward lower mortality in patients with CRE infections and penetrated well into the lung [53]. Vaborbactam inhibits class A and C β-lactamases but not class B or D lactamases. In one study, there was no statistical difference in all-cause mortality between monotherapy and combination therapy to treat people with ventilator-associated pneumonia (VAP) [10]. In VAP, a short treatment course of about seven days is validated, even though a longer treatment course may still be recommended for patients with a slower clinical response. Usually, carbapenem monotherapy is used for VAP, including MDR strains. However, there was no statistical difference in all-cause mortality between carbapenem and non-carbapenem therapies. However, carbapenems are associated with a statistically significant increase in clinical cures [10]. On the other hand, a meta-analysis identified significantly higher superinfection with imipenem than non-carbapenems. Superinfection is a new microbial infection occurring after or in addition to an earlier infection usually following treatment with broad-spectrum antibiotics. Superinfection was statistically higher when carbapenems were used compared to other antipseudomonal beta-lactams [20].
Drug concentrations in the airways can be 100-fold higher when antibiotics are administered through the aerosol route in mechanically ventilated patients. Several studies demonstrate a reduction of bacterial load and a good safety profile with aerosolized colistin, or aminoglycosides compared to the intravenous route [8,25]. Accordingly, aerosolized antibiotics are now increasingly used, especially in gram-negative VAP and, more specifically, with MDR strains. However, one rational approach for XDR strains, especially A. Baumannii is to consider combining colistin and carbapenem therapy, particularly when carbapenem MICs are elevated [25]. This approach attempts to supplement the therapeutic effect of the last-line polymyxin therapy with systemic therapy with a carbapenem or another agent.
In VAP caused by MDR A. baumannii, treatment with intravenous meropenem, colistin, and nebulized tobramycin was just as effective as intravenous meropenem, injectable colistin, and nebulized colistin, with no significant difference between clinical pulmonary infection score and creatinine level in both groups, suggesting that nebulized tobramycin is non-inferior to nebulized colistin [8]. Finally, in cases of non-bacteremic XDR Acinetobacter spp. pneumonia, the addition of inhaled colistin minimizes toxicity and maximizes levels delivered to the lung.
Treatment of Fungal Infections
C. auris infections pose a real treatment challenge due to the formation of biofilms and resistance mechanisms. Echinocandins are the recommended first-line treatment in adults. An alternative is liposomal amphotericin B. Antifungal susceptibility testing is required to inform targeted treatment. Outbreaks of Candida require hypervigilance, rapid diagnostic methods, and new molecular typing tools such as whole-genome sequencing (WGS), prompt investigation, and aggressive interventions, including notification of public health agencies [38,49]. For a suspected C. auris infection, the Centers for Disease Control and Prevention (CDC) recommends the identification of species from non-sterile sites when there is an invasive disease, colonization, or infection is detected in a unit or facility, or when a patient has had an overnight stay within the previous year in a healthcare facility in a country with documented C. auris transmission [29].
Alternative Treatments
Antimicrobial lock therapy (ALT) is an alternative therapy for biofilm-related infections associated with medical devices such as central vein catheters. ALT involves instilling antimicrobial agents, which exceed the MIC by 100- to 1,000-fold within an intravascular catheter lumen. The antibiotic will stay locked over a specific time, usually 24 hours for most agents [40]. Its efficacy is disputed, given the lack of source control. However, ALT is used when central catheters cannot be removed for clinical reasons. Several clinical studies indicate that ALT is an effective method for preventing Candida colonization without removing catheters but is yet to be confirmed by a large RCT [40]. In cases of Candida fungemia, however, removal of the catheter remains the standard of care.
Future directions
Antimicrobial coatings are promising options to eradicate biofilm-related infections. Medical devices typically associated with biofilm formation are coated with anti-biofilm layers to prevent the adherence of microbes. These coated surfaces serve as contact-killing surfaces preventing the formation of biofilms, as observed in cases of central line-associated bloodstream infections, catheter-associated urinary tract infections, and VAPs. Nanotechnology is a complementary therapeutic agent that employs quantum-dot, carbon nanotubes, and carbon-based nanoparticles (NPs) to disrupt biofilms and deliver antimicrobials directly to the targeted cells or pathogens without drug degradation. NPs are considered promising alternatives to antibiotics and effective against gram-positive and gram-negative bacteria. Natural NPs, polymer-based nanomaterials, and metallic NPs are cost-effective and may be exploited as antimicrobial coatings on the surface of medical devices for various biomedical applications [45].
Antimicrobial photodynamic therapy (aPDT), or photodynamic activation (PDI), is an alternative treatment modality for localized biofilm infections. Its mechanism of action results from synergism between non-toxic photosensitizer dye, molecular oxygen, and visible light. The principle behind aPDT is that exposure to a light source at a specific wavelength triggers the photosensitizer dyes to generate sufficient reactive oxygen species from molecular oxygen that cause damage and microbial cell death without exerting toxic effects in the host. These modalities are relatively low cost, and widespread adoption will further increase their cost-effectiveness.
Limitations
Although this review is based on a systematic analysis of the medical literature, it reflects the inherent biases of the writing team. We have summarized the most compelling areas of current investigations based on the literature and the experience of the writing committee. As new research becomes available, additions to the priorities of this research agenda should be considered.
Conclusions
Clinicians and scientists have long realized the remarkable ability of microorganisms to survive via evolving resistance to antibiotics. Infections caused by MDR/XDR strains are a cause of concern as they compromise the selection of appropriate empiric and definitive antimicrobial treatments. The knowledge about the variety of molecular mechanisms of antimicrobial resistance has expanded tremendously via advances in genomics and proteomics. Many molecular mechanisms that promote resistance have been elucidated; however, novel antibiotic drug development has not kept pace in tandem. The judicious use of antibiotics through antimicrobial stewardship, good clinical practice, and good public health practices are imperative to stem the tide of increasing drug resistance. There is a need for fundamental studies to answer questions regarding the development and use of new antibiotics and novel strategies for treating and preventing MDR/XDR/PDR bacterial infections.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Cassini A, Högberg LD, Plachouras D, et al. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global prevalence of nosocomial multidrug-resistant Klebsiella pneumoniae: a systematic review and meta-analysis. Mohd Asri NA, Ahmad S, Mohamud R, et al. Antibiotics (Basel) 2021;10:1508. doi: 10.3390/antibiotics10121508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The continuing plague of extended-spectrum β-lactamase-producing Enterobacteriaceae infections. Adler A, Katz DE, Marchaim D. Infect Dis Clin North Am. 2016;30:347–375. doi: 10.1016/j.idc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Patterns of antibiotic use in hospital-acquired infections. Sevin T, Daniau C, Alfandari S, et al. J Hosp Infect. 2021;114:104–110. doi: 10.1016/j.jhin.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Hospital transfer network structure as a risk factor for Clostridium difficile infection. Simmering JE, Polgreen LA, Campbell DR, Cavanaugh JE, Polgreen PM. Infect Control Hosp Epidemiol. 2015;36:1031–1037. doi: 10.1017/ice.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comparison of two therapeutic approaches for the management of ventilator-associated pneumonia due to multidrug-resistant Acinetobacter: a randomized clinical trial study. Khorvash F, Yaghoubi S, Farsaei S, Ataei B, Hakamifard A, Mohajeri F, Gudarzi M. J Immunoassay Immunochem. 2020;41:97–105. doi: 10.1080/15321819.2019.1696818. [DOI] [PubMed] [Google Scholar]
- 9.Deconstructing the relative benefits of a universal glove and gown intervention on MRSA acquisition. Harris AD, Morgan DJ, Pineles L, Perencevich EN, Barnes SL. J Hosp Infect. 2017;96:49–53. doi: 10.1016/j.jhin.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antibiotics for ventilator-associated pneumonia. Arthur LE, Kizor RS, Selim AG, van Driel ML, Seoane L. Cochrane Database Syst Rev. 2016;10:0. doi: 10.1002/14651858.CD004267.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A randomized clinical trial on the effectiveness of a symbiotic product to decolonize patients harboring multidrug-resistant Gram-negative bacilli. Salomão MC, Heluany-Filho MA, Menegueti MG, Kraker ME, Martinez R, Bellissimo-Rodrigues F. Rev Soc Bras Med Trop. 2016;49:559–566. doi: 10.1590/0037-8682-0233-2016. [DOI] [PubMed] [Google Scholar]
- 12.Interventions to improve antibiotic prescribing practices for hospital inpatients. Davey P, Marwick CA, Scott CL, et al. Cochrane Database Syst Rev. 2017;2:0. doi: 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antibiotic treatment for Stenotrophomonas maltophilia in people with cystic fibrosis. Amin R, Jahnke N, Waters V. Cochrane Database Syst Rev. 2020;3:0. doi: 10.1002/14651858.CD009249.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Methicillin-resistant Staphylococcus aureus in a trauma population: does decolonization prevent infection? Maxwell RA, Croft CA, Creech CB, et al. Am Surg. 2017;83:1407–1412. [PubMed] [Google Scholar]
- 15.Candida auris: an emerging fungal pathogen. Spivak ES, Hanson KE. J Clin Microbiol. 2018;56:0–17. doi: 10.1128/JCM.01588-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antimicrobial resistance: an antimicrobial/diagnostic stewardship and infection prevention approach. Septimus EJ. Med Clin North Am. 2018;102:819–829. doi: 10.1016/j.mcna.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Resistance in vancomycin-resistant enterococci. Miller WR, Murray BE, Rice LB, Arias CA. Infect Dis Clin North Am. 2020;34:751–771. doi: 10.1016/j.idc.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.[Effect of 2% chlorhexidine bathing on the incidence of hospital-acquired infection and multidrug-resistant organisms in adult intensive care unit patients: systematic review and meta-analysis] Seo J, Song R. J Korean Acad Nurs. 2021;51:414–429. doi: 10.4040/jkan.21046. [DOI] [PubMed] [Google Scholar]
- 19.Klebsiella pneumoniae: an increasing threat to public health. Effah CY, Sun T, Liu S, Wu Y. Ann Clin Microbiol Antimicrob. 2020;19:1. doi: 10.1186/s12941-019-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Impact of carbapenem versus non-carbapenem treatment on the rates of superinfection: a meta-analysis of randomized controlled trials. Eljaaly K, Enani MA, Al-Tawfiq JA. J Infect Chemother. 2018;24:915–920. doi: 10.1016/j.jiac.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Microbial cause of ICU-acquired pneumonia: hospital-acquired pneumonia versus ventilator-associated pneumonia. Luyt CE, Hékimian G, Koulenti D, Chastre J. Curr Opin Crit Care. 2018;24:332–338. doi: 10.1097/MCC.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 22.Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Lee CR, Lee JH, Park KS, et al. Front Cell Infect Microbiol. 2017;7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healthcare-acquired infections: prevention strategies. Fernando SA, Gray TJ, Gottlieb T. Intern Med J. 2017;47:1341–1351. doi: 10.1111/imj.13642. [DOI] [PubMed] [Google Scholar]
- 24.Nosocomial infection and its molecular mechanisms of antibiotic resistance. Xia J, Gao J, Tang W. Biosci Trends. 2016;10:14–21. doi: 10.5582/bst.2016.01020. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clin Microbiol Rev. 2017;30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Martin-Loeches I, Rodriguez AH, Torres A. Curr Opin Crit Care. 2018;24:347–352. doi: 10.1097/MCC.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 27.Global epidemiology of urinary tract infections. Tandogdu Z, Wagenlehner FM. Curr Opin Infect Dis. 2016;29:73–79. doi: 10.1097/QCO.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 28.Genetics, epidemiology, and clinical manifestations of multidrug-resistant Acinetobacter baumannii. Nasr P. J Hosp Infect. 2020;104:4–11. doi: 10.1016/j.jhin.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Forsberg K, Woodworth K, Walters M, Berkow EL, Jackson B, Chiller T, Vallabhaneni S. Med Mycol. 2019;57:1–12. doi: 10.1093/mmy/myy054. [DOI] [PubMed] [Google Scholar]
- 30.Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. Navon-Venezia S, Kondratyeva K, Carattoli A. FEMS Microbiol Rev. 2017;41:252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 31.Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Gao W, Howden BP, Stinear TP. Curr Opin Microbiol. 2018;41:76–82. doi: 10.1016/j.mib.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Antibiotic treatment of hospital-acquired pneumonia: is it different from ventilator-associated pneumonia? Niederman MS. Curr Opin Crit Care. 2018;24:353–360. doi: 10.1097/MCC.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 33.Prevention of hospital-acquired pneumonia. Lyons PG, Kollef MH. Curr Opin Crit Care. 2018;24:370–378. doi: 10.1097/MCC.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 34.The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Giuliano KK, Baker D, Quinn B. Am J Infect Control. 2018;46:322–327. doi: 10.1016/j.ajic.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Role of combination antimicrobial therapy for vancomycin-resistant Enterococcus faecium infections: review of the current evidence. Yim J, Smith JR, Rybak MJ. Pharmacotherapy. 2017;37:579–592. doi: 10.1002/phar.1922. [DOI] [PubMed] [Google Scholar]
- 36.Antimicrobial-resistant CC17 Enterococcus faecium: the past, the present and the future. Lee T, Pang S, Abraham S, Coombs GW. J Glob Antimicrob Resist. 2019;16:36–47. doi: 10.1016/j.jgar.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Candida auris and nosocomial infection. Dahiya S, Chhillar AK, Sharma N, et al. Curr Drug Targets. 2020;21:365–373. doi: 10.2174/1389450120666190924155631. [DOI] [PubMed] [Google Scholar]
- 38.Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Lockhart SR, Etienne KA, Vallabhaneni S, et al. Clin Infect Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Host and pathogen biomarkers for severe Pseudomonas aeruginosa infections. Juan C, Peña C, Oliver A. J Infect Dis. 2017;215:0–51. doi: 10.1093/infdis/jiw299. [DOI] [PubMed] [Google Scholar]
- 40.Approaches for mitigating microbial biofilm-related drug resistance: a focus on micro- and nanotechnologies. Rao H, Choo S, Rajeswari Mahalingam SR, Adisuri DS, Madhavan P, Md Akim A, Chong PP. Molecules. 2021;26:1870. doi: 10.3390/molecules26071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chromosomally encoded and plasmid-mediated polymyxins resistance in Acinetobacter baumannii: a huge public health threat. Lima WG, Alves MC, Cruz WS, Paiva MC. Eur J Clin Microbiol Infect Dis. 2018;37:1009–1019. doi: 10.1007/s10096-018-3223-9. [DOI] [PubMed] [Google Scholar]
- 42.Fighting AMR in the healthcare environment: microbiome-based sanitation approaches and monitoring tools. D'Accolti M, Soffritti I, Mazzacane S, Caselli E. Int J Mol Sci. 2019;20:1535. doi: 10.3390/ijms20071535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bystander selection for antimicrobial resistance: implications for patient health. Morley VJ, Woods RJ, Read AF. Trends Microbiol. 2019;27:864–877. doi: 10.1016/j.tim.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Photodynamic treatment for multidrug-resistant Gram-negative bacteria: perspectives for the treatment of Klebsiella pneumoniae infections. Valenzuela-Valderrama M, González IA, Palavecino CE. Photodiagnosis Photodyn Ther. 2019;28:256–264. doi: 10.1016/j.pdpdt.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Nanomaterials for alternative antibacterial therapy. Hemeg HA. Int J Nanomedicine. 2017;12:8211–8225. doi: 10.2147/IJN.S132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safety profiles of old and new antimicrobials for the treatment of MRSA infections. Bassetti M, Righi E. Expert Opin Drug Saf. 2016;15:467–481. doi: 10.1517/14740338.2016.1142528. [DOI] [PubMed] [Google Scholar]
- 47.Clinical implications of genomic adaptation and evolution of carbapenem-resistant Klebsiella pneumoniae. Gomez-Simmonds A, Uhlemann AC. J Infect Dis. 2017;215:0–27. doi: 10.1093/infdis/jiw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emergence of antimicrobial resistance among Pseudomonas aeruginosa: implications for therapy. Lynch JP 3rd, Zhanel GG, Clark NM. Semin Respir Crit Care Med. 2017;38:326–345. doi: 10.1055/s-0037-1602583. [DOI] [PubMed] [Google Scholar]
- 49.Healthcare-associated fungal outbreaks: new and uncommon species, new molecular tools for investigation and prevention. Bougnoux ME, Brun S, Zahar JR. Antimicrob Resist Infect Control. 2018;7:45. doi: 10.1186/s13756-018-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decolonization in prevention of health care-associated infections. Septimus EJ, Schweizer ML. Clin Microbiol Rev. 2016;29:201–222. doi: 10.1128/CMR.00049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alterations in outer membrane permeability favor drug-resistant phenotype of Klebsiella pneumoniae. Pulzova L, Navratilova L, Comor L. Microb Drug Resist. 2017;23:413–420. doi: 10.1089/mdr.2016.0017. [DOI] [PubMed] [Google Scholar]
- 52.Considerations in the selection of renal dosage adjustments for patients with serious infections and lessons learned from the development of ceftazidime-avibactam. Li J, Lovern M, Riccobene T, et al. Antimicrob Agents Chemother. 2020;64:0–19. doi: 10.1128/AAC.02105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novel pharmacotherapy for the treatment of hospital-acquired and ventilator-associated pneumonia caused by resistant gram-negative bacteria. Kidd JM, Kuti JL, Nicolau DP. Expert Opin Pharmacother. 2018;19:397–408. doi: 10.1080/14656566.2018.1438408. [DOI] [PubMed] [Google Scholar]
- 54.Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. MacVane SH. J Intensive Care Med. 2017;32:25–37. doi: 10.1177/0885066615619895. [DOI] [PubMed] [Google Scholar]
- 55.The human microbiota: novel targets for hospital-acquired infections and antibiotic resistance. Pettigrew MM, Johnson JK, Harris AD. Ann Epidemiol. 2016;26:342–347. doi: 10.1016/j.annepidem.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Kollef MH, Bassetti M, Francois B, et al. Intensive Care Med. 2017;43:1187–1197. doi: 10.1007/s00134-017-4682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Interplay between antibiotic resistance and virulence during disease promoted by multidrug-resistant bacteria. Geisinger E, Isberg RR. J Infect Dis. 2017;215:0. doi: 10.1093/infdis/jiw402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa. Cardozo C, Rico V, Agüero D, Soriano A. https://seq.es/abstract/suppl2-32-32-34/ Rev Esp Quimioter. 2019;32 Suppl 2:32–34. [PMC free article] [PubMed] [Google Scholar]
- 59.Epidemiology of antibiotic resistance in Pseudomonas aeruginosa. Implications for empiric and definitive therapy. Ruiz-Garbajosa P, Cantón R. http://seq.es/seq/0214-3429/30/suppl1/01ruiz.pdf. Rev Esp Quimioter. 2017;30 Suppl 1:8–12. [PubMed] [Google Scholar]
- 60.The role of hospital environment in transmissions of multidrug-resistant gram-negative organisms. Chia PY, Sengupta S, Kukreja A, S L Ponnampalavanar S, Ng OT, Marimuthu K. Antimicrob Resist Infect Control. 2020;9:29. doi: 10.1186/s13756-020-0685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worldwide survey of Corynebacterium striatum increasingly associated with human invasive infections, nosocomial outbreak, and antimicrobial multidrug-resistance, 1976-2020. Silva-Santana G, Silva CM, Olivella JG, et al. Arch Microbiol. 2021;203:1863–1880. doi: 10.1007/s00203-021-02246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.An overview of Acinetobacter baumannii pathogenesis: motility, adherence and biofilm formation. Mea HJ, Yong PV, Wong EH. Microbiol Res. 2021;247:126722. doi: 10.1016/j.micres.2021.126722. [DOI] [PubMed] [Google Scholar]
- 63.Molecular mechanisms of antimicrobial resistance in Acinetobacter baumannii, with a special focus on its epidemiology in Lebanon. Jamal S, Al Atrouni A, Rafei R, Dabboussi F, Hamze M, Osman M. J Glob Antimicrob Resist. 2018;15:154–163. doi: 10.1016/j.jgar.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Antibiotics and antibiotic resistance genes in landfills: a review. Zhang R, Yang S, An Y, Wang Y, Lei Y, Song L. Sci Total Environ. 2022;806:150647. doi: 10.1016/j.scitotenv.2021.150647. [DOI] [PubMed] [Google Scholar]
- 65.Role of nanomaterials in deactivating multiple drug resistance efflux pumps - a review. Dey N, Kamatchi C, Vickram AS, et al. Environ Res. 2022;204:111968. doi: 10.1016/j.envres.2021.111968. [DOI] [PubMed] [Google Scholar]
- 66.Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome. Pulingam T, Parumasivam T, Gazzali AM, et al. Eur J Pharm Sci. 2022;170:106103. doi: 10.1016/j.ejps.2021.106103. [DOI] [PubMed] [Google Scholar]
- 67.Colonization of patients, healthcare workers, and the environment with healthcare-associated Staphylococcus epidermidis genotypes in an intensive care unit: a prospective observational cohort study. Widerström M, Wiström J, Edebro H, Marklund E, Backman M, Lindqvist P, Monsen T. BMC Infect Dis. 2016;16:743. doi: 10.1186/s12879-016-2094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antimicrobial susceptibility of gram-positive and gram-negative bacteria: a 5-year retrospective analysis at a multi-hospital healthcare system in Saudi Arabia. Alhumaid S, Al Mutair A, Al Alawi Z, et al. Ann Clin Microbiol Antimicrob. 2021;20:43. doi: 10.1186/s12941-021-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Recent advances in investigation, prevention, and management of healthcare-associated infections (HAIs): resistant multidrug strain colonization and its risk factors in an intensive care unit of a university hospital. Zaha DC, Kiss R, Hegedűs C, et al. Biomed Res Int. 2019;2019:2510875. doi: 10.1155/2019/2510875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monitoring the antimicrobial susceptibility of Gram-negative organisms involved in intraabdominal and urinary tract infections recovered during the SMART study (Spain, 2016 and 2017) Cantón R, Loza E, Aznar J, et al. https://seq.es/abstract/february-13-2. Rev Esp Quimioter. 2019;32:145–155. [PMC free article] [PubMed] [Google Scholar]
- 71.A combination antibiogram evaluation for Pseudomonas aeruginosa in respiratory and blood sources from intensive care unit (ICU) and non-ICU settings in U.S. hospitals. Puzniak L, DePestel DD, Srinivasan A, et al. Antimicrob Agents Chemother. 2019;63:0–18. doi: 10.1128/AAC.02564-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tigecycline therapy for nosocomial pneumonia due to carbapenem-resistant gram-negative bacteria in critically ill patients who received inappropriate initial antibiotic treatment: a retrospective case study. Wu X, Zhu Y, Chen Q, Gong L, Lin J, Lv D, Feng J. Biomed Res Int. 2016;2016:8395268. doi: 10.1155/2016/8395268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trends in antimicrobial resistance and empiric antibiotic therapy of bloodstream infections at a general hospital in Mid-Norway: a prospective observational study. Mehl A, Åsvold BO, Kümmel A, et al. BMC Infect Dis. 2017;17:116. doi: 10.1186/s12879-017-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Epidemiological characteristics and drug resistance of fungemia in general hospitals from 2010 to 2019. Bai Y, Zheng Z, Liu T, et al. Biomed Res Int. 2021;2021:2529171. doi: 10.1155/2021/2529171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Epidemiology and outcomes of vancomycin-resistant Enterococcus infections in the U.S. military health system. Stagliano DR, Susi A, Adams DJ, Nylund CM. Mil Med. 2021;186:100–107. doi: 10.1093/milmed/usaa229. [DOI] [PubMed] [Google Scholar]
- 76.Colistin dosage without loading dose is efficacious when treating carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia caused by strains with high susceptibility to colistin. Álvarez-Marín R, López-Rojas R, Márquez JA, et al. PLoS One. 2016;11:0. doi: 10.1371/journal.pone.0168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Activity of imipenem/relebactam against MDR Pseudomonas aeruginosa in Europe: SMART 2015-17. Lob SH, Karlowsky JA, Young K, et al. J Antimicrob Chemother. 2019;74:2284–2288. doi: 10.1093/jac/dkz191. [DOI] [PubMed] [Google Scholar]
- 78.Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Bassetti M, Echols R, Matsunaga Y, et al. Lancet Infect Dis. 2021;21:226–240. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 79.An update on eight "new" antibiotics against multidrug-resistant gram-negative bacteria. Yusuf E, Bax HI, Verkaik NJ, van Westreenen M. J Clin Med. 2021;10:1068. doi: 10.3390/jcm10051068. [DOI] [PMC free article] [PubMed] [Google Scholar]