Abstract
Microphysiological systems (MPSs), also known as organ-on-a-chip models, aim to recapitulate the functional components of human tissues or organs in vitro. Over the last decade, with the advances in biomaterials, 3D bioprinting, and microfluidics, numerous MPS models have emerged with applications to study diseased and healthy tissue models. Various organs have been modeled using MPS technology, such as the heart, liver, lung, and blood-brain barrier. An important aspect of in vitro modeling is the accurate phenotypical and functional characterization of the modeled organ. However, most conventional characterization methods are invasive and destructive and do not allow continuous monitoring of the cells in culture. On the other hand, microfluidic biosensors enable in-line, real-time sensing of target molecules with an excellent limit of detection and in a non-invasive manner, thereby effectively overcoming the limitation of the traditional techniques. Consequently, microfluidic biosensors have been increasingly integrated into MPSs and used for in-line target detection. This review discusses the state-of-the-art microfluidic biosensors by providing specific examples, detailing their main advantages in monitoring MPSs, and highlighting current developments in this field. Finally, we describe the remaining challenges and potential future developments to advance the current state-of-the-art in integrated microfluidic biosensors.
Keywords: Microphysiological systems, Organ-on-a-Chip, Microfluidic biosensor, Disease modeling, Drug discovery
TOC
This review discusses the state-of-the-art microfluidic biosensors by providing specific examples, detailing their main advantages in monitoring MPSs, and highlighting recent advances in the field. We describe the remaining challenges and potential future developments to advance the current state-of-the-art in integrated microfluidic biosensors.
Introduction
Microphysiological systems (MPSs, also known as “organs-on-a-chip”) that can recapitulate human physiology in vitro provide valuable tools for understanding disease mechanisms and accelerating the drug development pipeline.1–3 Various organs and tissues have been fabricated and studied, such as the lung,4 heart,5, 6 liver,7 kidney,8 gut,9, 10 intestine,11 blood vessels,12, 13 and blood-brain barrier.14–17 Recently, MPSs with multi-organ systems were built and used to simulate the complex interactions between different organs in the body (body-on-a-chip).18, 19 Meanwhile, novel MPSs have been reported, including skin-20, bone-21, and muscle-on-a-chip22, which can be integrated with body-on-a-chip systems to create more complex physiological interactions.23–26
Microfluidics and microfabrication play a crucial role in developing and utilizing MPSs.27 Microfluidics is the technology of manipulating a small volume of fluids in micro-size channels. The advantages of microfluidic chips include small scale, dynamic fluid flow, and customized surface modification, which allow the development of diverse MPS platforms. Recent advances in microfluidic components such as microvalves, micropumps, and micromixers allowed the development of automated, integrated, and miniaturized MPSs.28, 29 They also permit researchers to precisely control the physiological microenvironment of cells and make it more in line with the growth microenvironment in the body. Compared with static culture conditions, MPSs have several attractive features as follows: i) dynamic flow conditions that can reconstruct more physiological related settings in vitro; ii) miniaturization to reduce consumption of expensive reagents and cells, ultimately reducing the cost; iii) automated and continuous exchange of nutrients for downstream analysis. These features make MPSs ideal platforms to create physiologically and pharmacologically relevant models for drug testing, disease modeling, and personalized medicine. In addition, these MPSs can serve as pre-clinical models that can complement and potentially replace animal model-based testing.
One of the most significant advantages of MPSs is that researchers can investigate the responses of these tissues under biophysical and/or biochemical stimuli to mimic and trigger physiological phenotypes and functions more efficiently and affordably.30 In the early stage, researchers used modern tissue and cell analysis methods for monitoring MPSs.31, 32 In principle, all modern tissue and cell analysis methods have the potential for monitoring MPSs.32 These methods are robust with accurate detection results. However, most assays were performed off-chip and relied on end-point and single-point tests that were invasive and destructive. MPSs require real-time and non-destructive monitoring of the dynamic process of drug interactions with organoids to obtain detailed information on transient, delayed, and cumulative drug effects. Thus, there is an increasing need for biosensors that can monitor the cellular microenvironment and cell physiology in a continuous, real-time, non-invasive, and non-destructive manner. These biosensors can be integrated into an “on-chip” system for detecting physiological biomarkers, biomolecules, and cell functions. Specifically, several electrical and optical biosensors have been reported and reviewed in recent years.22, 33–35 These biosensors have provided reliable results with high sensitivity, high selectivity, and high-throughput capabilities.
Here, we review recent advances in microfluidic biosensors for monitoring MPSs. First, different types of biosensors, according to the nature of the transducer, are reviewed. For MPSs, electrochemical and optical biosensors are widely used due to their easy integration, fast response, non-invasive, and label-free features. Then, the capabilities of microfluidic biosensors are discussed, such as automation, miniaturization, multiplexing, and integration. Next, the state-of-the-art of different targets in MPSs is examined. We summarize these targets into physiological biomarkers, biomolecule biomarkers, and cell functions. In addition, the applications of microfluidic biosensors in MPSs are also discussed. Finally, we conclude with the description of existing challenges and future advances in microfluidic biosensors for monitoring MPSs.
Biosensors for Monitoring MPSs
A biosensor is a device that utilizes a bioreceptor to “translate” biological events into quantifiable signals.36 The biosensors come in various forms such as optical, mechanical, or chemical-based on the mechanism of detection. Numerous biosensors have been proposed to detect biomolecules from fluids such as sweat, saliva, urine, etc.37 To create a functional MPS and to validate its functionality, one requires real time and continuous monitoring. Biosensors are poised to play a key role in carrying out this activity. Despite the advances in biosensors and MPSs, the applications combining biosensors with MPSs are relatively few. To carry out this task, one needs biosensors that are amenable with microfluidics technology.
Several different biosensing modalities have been proposed for monitoring MPSs. However, the most common modalities found in the literature are optical and electrochemical biosensors, probably due to their simplicity, cost-effectivity, accuracy in measurements, and capacity to be miniaturized and integrated into microfluidic platforms (Table 1). In this section, we review the recent advances in biosensors from a transducer perspective. The goal is to provide the reader with a clearer understanding of the biosensing modalities before discussing the importance of microfluidic technology in enabling the detection performance of these biosensors.
Table 1.
The state-of-the-art biosensors integrated within MPS.
| Sensor Classification | Target | LOD linear range | Probe | Advantages/disadvantages | Represent reference | |
|---|---|---|---|---|---|---|
| Electrochemical Biosensors | Amperometric | Glucose | N/A 0.5-30 mM |
Glucose oxidase | Relatively mature technology Fast response Changes in enzyme activity Calibration required |
42 |
| Lactate | N/A 0.5-20 mM |
Lactase | ||||
| Voltammetric | IL-6 | 8 ng/mL 0- 2 μg/mL |
Antibody | Relatively high sensitivity Multiplexing Changes in antibody activity Relatively long reaction time Signal saturation for long-term monitoring |
22 | |
| TNF-α | 2 ng/mL 0- 2 μg/mL |
Antibody | ||||
| Impedance | CK-MB | 0.0024 ng/mL 0.01- 10 ng/mL |
Antibody | High sensitivity Regeneratable Relatively high linear range Relatively long reaction time Signal saturation for long-term monitoring |
51 | |
| GST-α | 0.01 ng/mL 0.1- 100 ng/mL |
Antibody | ||||
| Albumin | 0.09 ng/mL 0.1- 100 ng/mL |
Antibody | ||||
| Potentiometry | pH | 59 mV/pH (sensitivity) 5- 8 |
Polyaniline | Easy to configure and cheap Fast response Low sensitivity Limitation in targets |
62, 63 | |
| Electrical Biosensors |
TEER | Electrical resistance | N/A | None | High sensitivity Continuous monitoring Relatively high linear range |
54, 55 |
| FET | CEA | 1 fg/ml 10 fg/mL to 1 ng/mL |
Antibody | High sensitivity High detection range High cost Relatively complex manufacturing process |
64 | |
| miRNA | 0.1 fM 1 fM t0 10 pM |
Nucleic acid probe | ||||
| Optical Biosensors | Fluorescence | Oxygen | NM | Silica microparticles | Provide temporal and spatial information Lack portable device |
78 |
| SPR | Insulin | 0.85± 0.13 μg/ mL 0- 100 μg/ mL |
Antibody | Label-free Fast response Relatively low sensitivity Relatively high cost |
93 | |
| FRET | Calcium | NM | Fluorescent probe | Provide intracellular information Relatively high sensitivity Lack portable device |
90 | |
Electrochemical Biosensors
Electrochemical biosensors have attractive features that have led them far ahead of other sensing modalities for monitoring MPSs. In most cases, their working operation relies on the binding of an analyte to its bioreceptor that is immobilized on the working electrode, resulting in a variation of the electrical signal compared to the bare reference electrode.38–41 In some other cases, for example, in enzymatic electrochemical biosensors, such as glucose and lactate sensors, the sensors utilize enzyme-catalyzed reactions. During these processes, the microcurrents of reactions are detected to measure the concentrations of analytes.42 As a function of the type of electrical signal measurement, an electrochemical biosensor can be amperometric, voltammetric, conductometric, and potentiometric.43 Amperometric biosensors measure changes in electric current resulting from a chemical reaction on the electrode surface while maintaining the potential constant. The change in current is proportional to the concentration of target molecules. Conductometric sensors measure the change in conductance of the medium while a constant AC potential is applied between the electrodes. For example, the analyte of interest undergoes an enzymatic reaction that changes the ionic composition that is measured by the conductometric sensors. For voltammetric sensors, the change in electric current between a working and reference electrode as a function of applied potential is measured. The peak current value is used for determining the analyte, and the peak current density reflects the concentration of the analyte. Potentiometric sensors detect the concentration of analytes by monitoring the changes in potential between the reference and working electrodes while keeping a constant current. Ions in samples can change the electric potential of ISEs, which can be detected by measuring the potential difference with respect to the reference electrode. Apart from the electrochemical biosensors, miniaturized electronic systems, such as potentiostats, that can detect these signals are developed.44 Some of them are commercially available.
The first papers regarding amperometric biosensors were published in the 1960s.45 Since then, this sensing modality has been used for rapid biomarker detection from environmental and physiological samples.46–48 Amperometric biosensors were developed to analyze mitochondrial stress in liver-on-a-chip by detecting glucose and lactate.42 The amperometric glucose and lactate sensors were based on the enzymatic reactions on the surface of platinum electrodes. Platinum electrodes detected the catalyzed hydrogen peroxide under polarized conditions (Figure 1a). These glucose and lactate sensors exhibited a linear detection range of 0.5 to 30 mM and 0.5 to 20 mM, respectively (Figure 1b). Another novelty of this work was the development of an automated microfluidic switchboard, which comprises sample and control channels. The microfluidic switchboard was operated automatically in a specific sequence to detect the target analyte reliably (Figure 1c).
Figure 1.
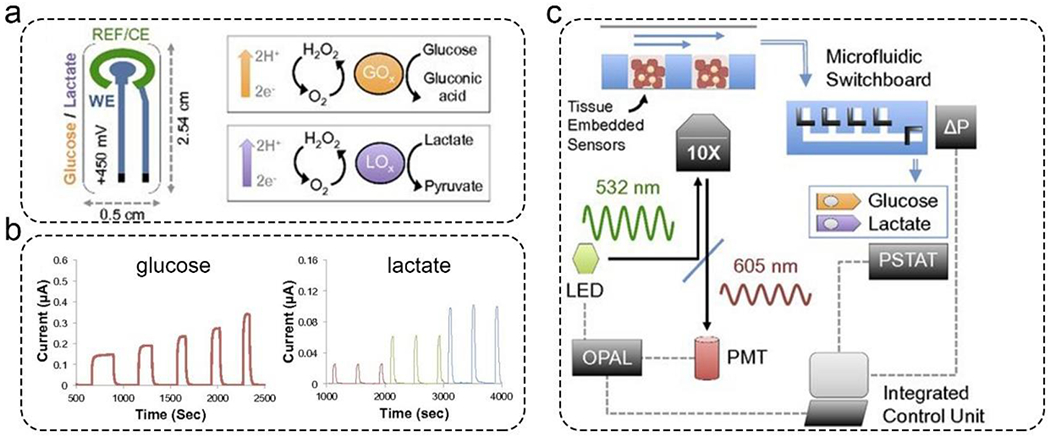
Amperometric glucose and lactate sensors for the real-time analysis of mitochondrial stress in a liver-on-a-chip model. a). The principle of the amperometric glucose and lactate sensors. Platinum electrodes were immobilized with an enzyme (i.e., the bioreceptor), which can catalyze glucose and lactate to produce hydrogen peroxide. b). The standard curve of the amperometric glucose and lactate sensors. c). Automated microfluidic switchboard. This switchboard can operate automatically with an integrated control unit. Reproduced with permission from ref (42). Copyright 2016 National Academy of Sciences.
Voltammetric/amperometric biosensors can monitor the binding activity across a range of applied potentials/currents by detecting well-defined current/potential peaks. Usually, voltammetry is based on a three-electrode system (working, reference, and counter electrodes) in which the working electrode is immobilized with bioreceptors specific to an analyte of interest. These electrodes can be fabricated using microfabrication49, screen-printing20, and 3D-printing50 methods. A voltammetric biosensor was fabricated using screen-printing and was used for in situ and multiplexed monitoring of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) secreted from a muscle-on-a-chip model.22 A highly sensitive and selective sandwich immunoassay realized using gold electrodes functionalized with antibodies was used to detect TNF-α and IL-6. These biosensors can detect IL-6 and TNF-α with a limit of detection (LOD) of 8 ng/mL and 2 ng/mL, respectively. The calibration curves were performed in 0- 2 μg/mL.
Electrochemical impedance biosensors are powerful and sensitive tools for detecting changes in the interfacial properties of electrodes. Zhang et al. published label-free and integrated electrochemical biosensors for the in-line and multiplexed detection of albumin and glutathione S-transferasa α (GST-α) secreted by liver organoids, and creatine kinase (CK-MB) secreted by cardiac organoids.51 In this work, gold electrodes were coated with one layer of 11-mercaptoundecanoic acid, followed by a streptavidin layer. Biotin-conjugated antibodies were immobilized on the gold electrode via biotin/streptavidin interactions. The binding activity can change the interfacial electron transfer kinetics of the labeled probe (Figure 2a). The three impedance biosensors were calibrated to detect GST-α, albumin, and CK-MB with standard titrations from 0 - 100 ng/mL (Figure 2c). The GST-α, albumin, and CK-MB biosensors realized high sensitivities of 1.105, 1.607, and 1.483 log(ng/mL)−1, respectively. Lastly, these three impedance biosensors were integrated with a microfluidic breadboard, which allowed automated sensing cycles comprising biofunctionalization, washing, sensing, and regeneration steps (Figure 2b). For regeneration, electrodes were washed by 10 mM sulfuric acid to break the thiol-gold bonds and etch the thin layers of gold. Then the functionalization reagents, i.e., 11-mercaptoundecanoic acid, streptavidin layer, and biotin-conjugated antibodies, were introduced sequentially.
Figure 2.
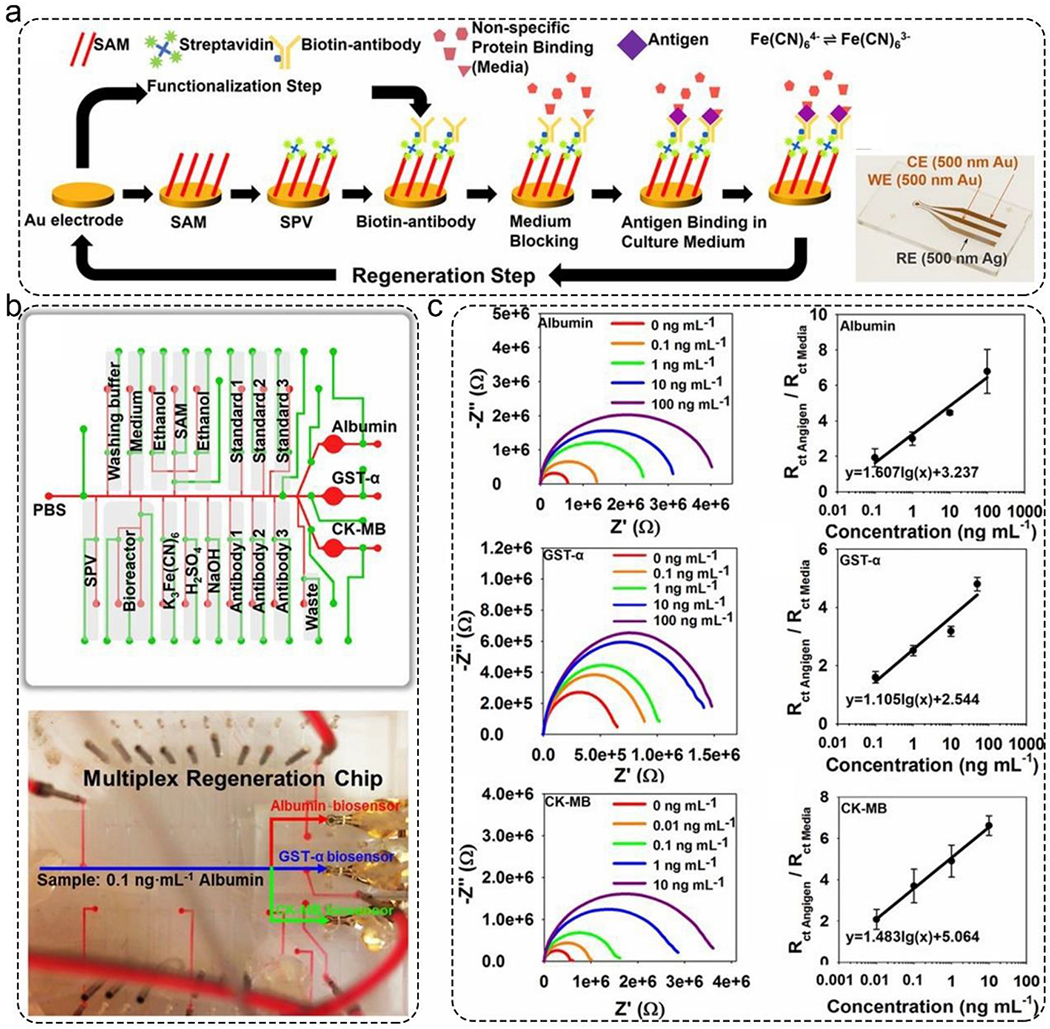
Electrochemical impedance biosensors for automated and in-line detection of albumin, GST-α, and CK-MB secreted by MPSs. a). The functionalization, detection, and regeneration steps of the impedance biosensors. The inset image is the photo of the fabricated gold electrode. b). Schematic illustration of the microfluidic breadboard design. This allowed multiple sensing cycles, including biofunctionalization, washing, sensing, and regeneration. c). The detection performance of these three impedance biosensors. All of them can detect targets from 0 to 100 ng/mL. Reproduced with permission from ref (51). Copyright 2016 National Academy of Sciences.
Another popular application of sensors integrated into organ-on-a-chip devices is measuring trans-endothelial/epithelial electrical resistance (TEER). TEER is often measured for characterization of barrier integrity to generate physiologically relevant models of, for example, the blood-brain-barrier52 and gut53. TEER is influenced by several factors such as the cell type (e.g., human brain microvascular endothelial cells (hBMECs) vs. intestinal epithelial cells), cell source (e.g., immortalized cells vs. stem cell-derived cells), culture type (e.g., monoculture vs. co-culture), extracellular matrix coating, and shear stress.54, 55 Typically, TEER values range widely from 30-150 Ω.cm2 for immortalized hBMECs and 400-1500 Ω.cm2 for induced pluripotent stem cell-derived hBMECs to 1000-4000 Ω.cm2 for human Caco2 intestinal epithelial cells.53, 56, 57 There are multiple approaches available to enable on-chip measurements of TEER. Henry et al. integrated TEER sensors into a dual-channel organ-on-a-chip device by depositing gold electrodes on a polycarbonate substrate.53 The substrate was coated with 3 nm of titanium and 25 nm of gold using an e-beam evaporator and assembled into the device.
Multi-electrode array-based TEER sensors have also been reported.49 This particular type of TEER sensor improved the overall experimental efficiency by simultaneously measuring TEER in 16 different chambers. In addition to the aforementioned sputtered electrodes, platinum-wire-based electrodes have been used as TEER sensors.57 Incorporation of such sensors is done via manual insertion of platinum wires into an assembled chip through dedicated channels. While the sensing abilities of all the TEER sensors described above have previously been demonstrated, the sensor designs, and chip architectures are appreciably different from each other. Given the sensitive nature of the sensors to these variables58, currently, comparing reported TEER values across different studies is nearly impossible. Moreover, the integration of TEER sensors comes with additional challenges, such as the loss of optical transparency due to the presence of electrodes and potential incompatibility with the chip fabrication processes.59 Therefore, addressing these limitations will be critical for the future development of TEER sensors. Future research is warranted to catalyze the efforts to standardize TEER measurement methods and protocols and allow an alternative approach to enable multiple study comparisons to achieve better reproducibility. Additionally, permeability assessment using FITC-dextran is another widely used method to assess barrier integrity. While TEER measurements are highly sensitive to different factors, such as substrate coverage by cells, and tend to vary substantially across different culture platforms, the permeability assay may provide a more platform-agnostic metric to compare barrier properties. As is the case for most previous studies,54 TEER measurements and permeability assays using tracer molecules will present a comprehensive and thus valid way to characterize microfluidic-based barrier models in contrast to either of the methods alone.
Several other electrochemical biosensors have been used to monitor MPSs. Potentiometric biosensors based on ion-selective electrodes (ISEs) are widely used to monitor ions (i.e., sodium ion, potassium ion) and pH.60, 61 These ISEs are modified with ion-exchange resins that can selectively pass-through specific ions. Ions in samples can change the electric potential of ISEs, which can be detected by measuring the potential difference with respect to the reference electrode. These sensors are highly suitable for monitoring cellular ions release, particularly in BBB and adenocarcinoma cancer MPSs.62, 63 Due to its high surface-to-volume ratio, nanowire arrays-based microelectrodes can sensitively detect nitric oxide in a vascular lumen MPS with high temporal and spatial resolution.64 Field-effect transistors (FET) also endow significant impact on real-time monitoring of biomarkers in MPS platforms. This allows the detection of low concentrations of secreted proteins and nucleic acids without enrichment or amplification.33, 65
Optical Biosensors
Optical biosensors are desirable for monitoring of MPSs without the need for electrical wires.66–69 They can quantitatively measure analytes of interest and provide information on molecular interactions. Optical biosensors generally involve monitoring the changes in luminescence intensity, reflective index, and angles of incident/reflected lights. It is worth mentioning that monitoring the color change has been integrated and miniaturized for the quantitative analysis of physiological parameters, such as pH and oxygen.70 Some fiber-coupled on-chip sensors that allow optical detection of pH, oxygen, and carbon dioxide are developed71 and some of them are commercially available. However, the cost is still high, and the lifetime is short, thus limiting their applications for long-term monitoring.
Fluorescence biosensors have been utilized in numerous applications including biomedical diagnostics and environmental monitoring.72–75 The fluorescence signal can be monitored by intra-incubator microscopes. Different dyes, fluorescence probes, and nanoparticles can be used to detect various analytes.76, 77 Meanwhile, researchers can utilize genetically encoded markers and green fluorescent protein to track the cellular distribution of MPSs. For example, silica microparticles encapsulated with tris(4,7-diphenyl-1,10-phenanthroline) ruthenium(II) dichloride (an oxygen-sensitive dye) were introduced into a micro-chamber to form an oxygen-sensitive layer (Figure 3a).78 The oxygen levels in the microchamber were detected using these silica microparticles. In this manner, fluorescent images captured by a camera showed the distribution of oxygen concentration inside the microchamber (Figure 3b). In another work, on-chip AlamarBlue assays were conducted to evaluate the metabolic activity of cardiac and stem cells.79 In this paper, a non-toxic and cell-permeable dye (resazurin) was used to detect oxidation levels during cell respiration. Using microfluidics, these biosensors have the following advantages: low maintenance, higher analysis speed, low cost, enhanced process performance, and reduced reagent consumption.
Figure 3.
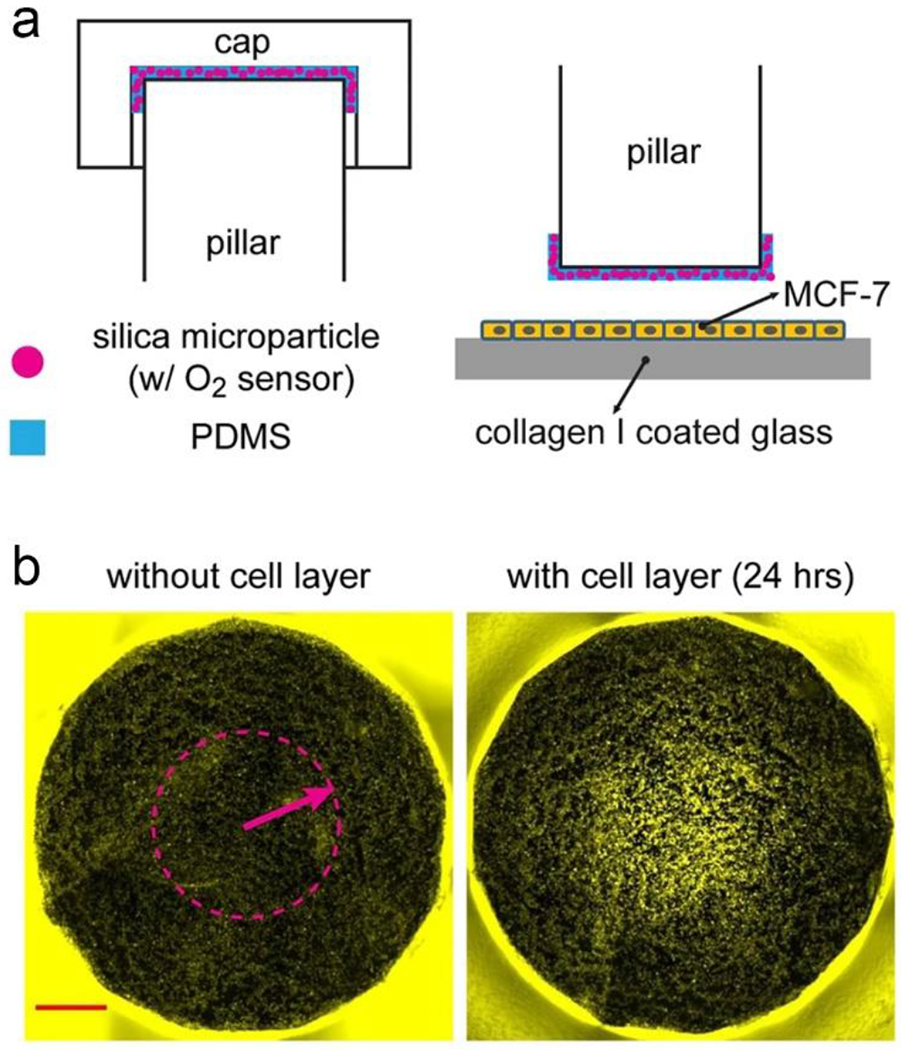
Oxygen levels in the microchamber were measured by silica microparticles encapsulated with an oxygen-sensitive dye. a). Schematic illustration of the silica microparticles inside the microchamber. b). Fluorescent image captured by a camera. These images demonstrated the distribution of oxygen concentration. Reproduced with permission from ref (78). Copyright 2017 Springer.
Fluorescence biosensors have been used to detect various cellular metabolites in MPSs.80–84 For example, fluorescence biosensors that detect hydrogen peroxide or reactive oxygen species (ROS) have been developed to monitor cellular metabolism and processes.85, 86 Total internal reflection fluorescence microscopy has a spatial resolution below 100 nm, providing real-time single-molecule imaging of Fzd8 and Lrp6 in human colon organoid models.87 Another common fluorescence sensor is based on the Förster resonance energy transfer (FRET). The coupling between a fluorophore and a quenching molecule can be observed by measuring the change in the fluorescence signal. FRET sensors have been used for monitoring cellular ions,88, 89 and proteins,90
Among the different optical biosensors, label-free surface plasmon resonance (SPR) biosensors have arisen because they can measure analytes of interest in real-time. SPR has been employed for detecting proteins (i.e., enzymes, antibodies, and antigens) and nucleic acids (i.e., DNA and RNA).91, 92 Some low-cost paper-based SPR sensors are developed for single use.52 For these kinds of biosensors, gold nanomaterials are desirable for the excitation of localized surface plasmons. For example, gold nanorod arrays were fabricated on a glass substrate, and their surfaces were assembled in one layer of 11-mercaptoundecanoic acid and then functionalized with a capture antibody (Figure 4a).93 In the presence of a specific target, the binding between antibody and antigen can change the wavelength of the peak extinction. This sensor showed a LOD of 0.85± 0.13 μg/ mL in an islet-on-a-chip system. In addition, an optofluidic sensor utilizing Fano resonance was developed to monitor live cell secretomes with gold nanoslits (Figure 4b).94 Microchambers contained an arrangement of cell traps that was assembled on the surface of gold nanoslits arrays. The binding events between antibody and cell-secreted matrix metalloproteinase 9 (MMP9) were detected by the transmitted light. In a similar work, Liu et al. developed a real-time monitoring platform using gold nanoparticles-based SPR biosensors to detect the biomarker expression in carcinoma cells.95 In these biochemically stimulated MPSs, the biosensors permitted the detection of cells with an accurate temporal and spatial resolution.
Figure 4.
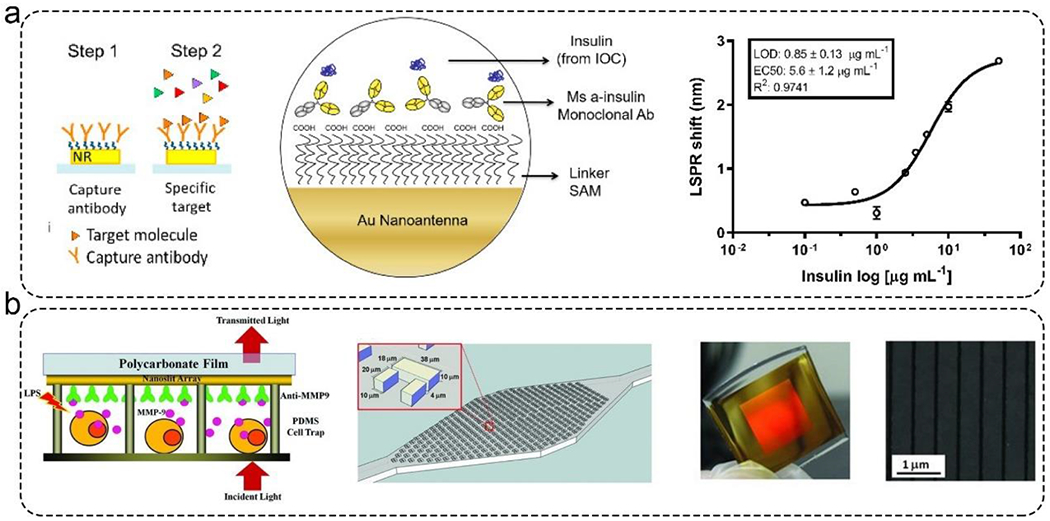
Representative SPR sensor for monitoring MPSs. a). Left: the detection principle of gold nanorods-based SPR sensor. Right: the detection performance of this sensor. This sensor showed a LOD of 0.85± 0.13 μg/ mL when detecting insulin. Reproduced with permission from ref (93). Copyright 2021 MDPI. b). Fano Resonance optofluidic sensor was used to monitor live cell secretomes. Left: the detection principle of gold nanoslits-based SPR sensor. Middle: microchamber contains an array of cell traps for capturing cells. Right: The photo and SEM image of gold nanoslits. Reproduced with permission from ref (94). Copyright 2013 Wiley.
The Merits of Microfluidic Biosensors
The development of microfluidics has revolutionized the field of biosensors owing to its unique characteristics over conventional biosensors. Significantly, the synergistic approach from different areas such as science, engineering, and technology has made microfluidics a popular tool.96–100 As a result, microfluidics-based biosensors have shown potential for commercialization, mainly because they are easy to use, robust, portable, automated, fast, and accurate.101–103
The inherent merit of microfluidics is the manipulation of small volumes of fluids. Most microfluidic biosensors work non-invasively, which is a crucial feature for monitoring MPSs precisely. Microfluidic biosensors can be miniaturized and integrated into complex systems.104, 105 For example, Zhang et al. reported a label-free and multiplexed electrochemical biosensor for in-line detection of albumin and GST-α secreted by liver organoids, and CK-MB secreted by cardiac organoids.51 Simultaneously, physical parameters (i.e., pH, oxygen, and temperature) were monitored in their system. The computer-controlled fluid flow to each sensor or organoid was realized with the support of microfluidic technology (i.e., the use of on-chip microfluidic valves and micropumps). Another example is the development of body-on-a-chip.23, 106 In this paper, eight organ chips (blood-brain barrier, liver, brain, lung, skin, intestine, heart, and kidney) were connected and cultured for 3 weeks.82 The authors used microfluidics for automated manipulation of fluids to achieve multiple processes, which includes reagents perfusion, sample collection, and in situ microscopy imaging.
Another merit of microfluidics is the unique micro/nano-domain effects. Indeed, microfluidics offers a high surface-to-volume ratio, specifically for the reactions within microchannels. Also, the mass transfer, heat transfer, and reaction are efficient due to the available surface area.107 This allows short diffusion distances and rapid mixing within the microfluidic devices. This feature allows researchers to precisely control the cellular microenvironment and accelerates the detection process. As a result, most microfluidic biosensors have shorter detection times compared to conventional methods.108–110 The flow inside the microfluidics follows the laminar flow regime. This feature allows researchers to generate oxygen and chemical gradients and to study their influence on cell behavior.111, 112 The use of microfluidics here is very beneficial since the generation of oxygen and chemical gradients by traditional methods is laborious and consume many reagents.
The Biomarkers detected in MPSs
In MPSs, it is crucial to detect physical parameters related to the microenvironment and biochemical parameters related to cellular metabolism and function.34, 113 In addition, we can apply different physical and biochemical stimuli to MPSs and measure their physical and biochemical response. This is crucial for testing their functionality and their response to drugs. Physical parameters (i.e., oxygen, temperature, and pH) are widely monitored and studied using commercial and customized physical sensors. The primary purpose is to achieve and maintain a controllable and reproducible cell culture microenvironment. For biochemical analytes, such as glucose, lactate, ROS, and cell secretome, most of these are considered as indicators of the metabolic activity of cellular constructs within MPSs. By monitoring these analytes, researchers can study, evaluate, and control cellular maturation, viability, differentiation, and function of MPSs for many biomedical applications.
Parameters of the microenvironment
The changes in oxygen, temperature, and pH can dramatically influence cellular maturation, viability, differentiation, and function. Therefore, sensors to monitor the microenvironment of MPSs have been extensively developed in past decades, and some have been commercialized since. Recently, Tanumihardja et al. developed a ruthenium oxide (RuOx) based electrode to monitor human pluripotent stem cell-derived cardiomyocytes’ metabolism by measuring extracellular acidification rate (ECARs) and oxygen consumption rates (OCRs) (Figure 5a).114 They have used a single electrode to monitor both parameters with a precise spatial resolution by measuring OH−, a by-product of oxygen reduction that increases the pH of the microenvironment. Moreover, their multi-analyte optical sensing module enabled the continuous monitoring of the microenvironments within MPSs. To allow seamless and in situ detection of temperature, a silicon-based temperature sensor was integrated onto a complementary metal oxide semiconductor (CMOS) chip. Cells were directly cultured on the temperature sensor surface (Figure 5b).115 This sensor provided results in approximately 15 seconds with a resolution of ± 0.2°C within a temperature range of 30 to 40 °C.
Figure 5.
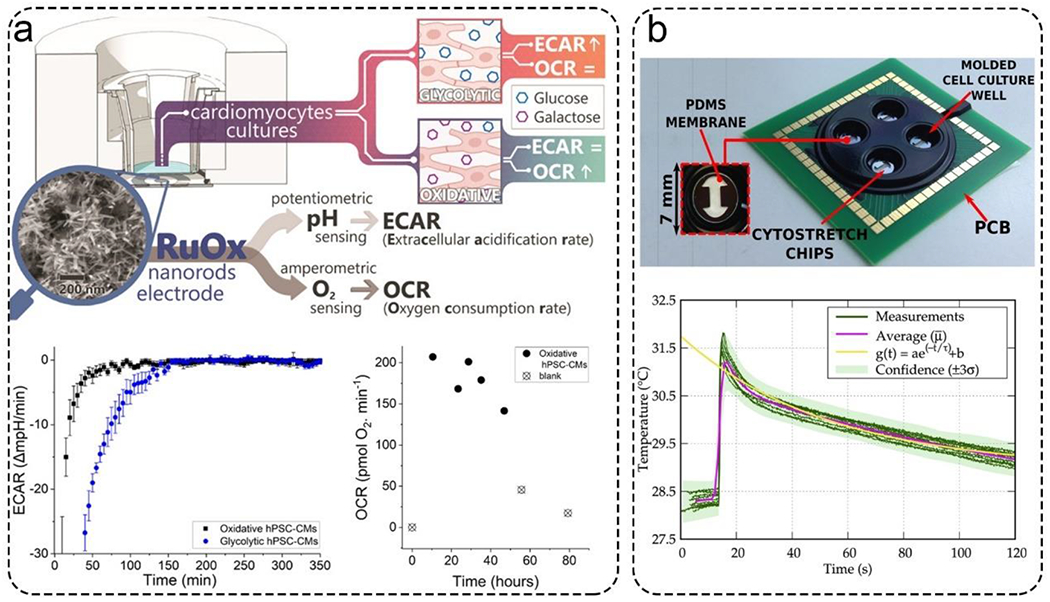
Representative biosensors for monitoring the microenvironment. a). A ruthenium oxide (RuOx) based electrode measured extracellular acidification rate (ECARs) and oxygen consumption rates (OCRs). This nanorods electrode continuously measured ECARs and OCRs in cardiomyocytes cultures over 48 hours. Reproduced with permission from ref (114). Copyright 2021 American Chemical Society. b). A silicon-based sensor integrated into an organ-on-a-chip for temperature monitoring. The sensor could respond in about 15 seconds. Reproduced with permission from ref (115). Copyright 2021 Elsevier.
Monitoring multiple physical parameters is important for maintaining proper microenvironments for MPSs. Optical oxygen and pH sensors were integrated with microfluidics bioreactors for real-time monitoring of the human dermal fibroblasts culture microenvironment.70 In this work, the change in pH caused a color change of the phenol red solution. The oxygen was measured using tris(4,7-diphenyl-1,10-phenanthroline)ruthenium(II) chloride (an oxygen-sensitive fluorophore). A multi-analyte sensing module was used to monitor the color and fluorescence intensities in real-time which was converted into an oxygen level. Another study reported a liver-on-a-chip integrated with multiple electrochemical sensors located along the microfluidic channel to monitor oxygen with high temporal and spatial resolution. Three electrochemical sensors were integrated on the bottom part of a very thin, porous membrane to allow the local measurement of oxygen gradients for primary human hepatocytes.116
Electrical and mechanical cellular activities are important physical parameters to assess the function of specific tissues such as the heart, muscle, and brain.117 Oleaga et al. reported a platform that allowed organ-to-organ communication between four human organs, namely the heart, liver, skeletal muscle, and nervous system. This multi-organ-on-a-chip module monitored electrical and mechanical activities in real-time and non-invasively. Importantly, they demonstrated and monitored long-term cellular viability for up to 28 days.118 They used a custom multi-electrode array integrated into a microfluidic device to measure the electrical activities of neurons and cardiac cells and the mechanical activities of cardiac and skeletal muscle MPSs. Another example is the integration of microcantilevers with different geometries into human heart MPSs. Researchers not only applied specific forces but also measured the mechanical activities and modeled the elastomechanic responses.119, 120
Another critical parameter of the microenvironment that requires monitoring is osmolarity. Fernandes et al. developed an osmotic hydration sensor using a semi-permeable membrane as a core element to monitor osmotic pressure.121 They integrated piezo resistors into the membrane and arranged them in a Wheatstone bridge configuration to detect 20% of dynamic hydration change, which is the limit of hydration that a human body can resist. When water passed through the membrane, osmotic pressure was generated by the ions restricted to one side of the membrane. The output signal from the dual pressure transducer was amplified and detected.
Biochemical Parameters
Glucose and lactate are widely detected in MPSs as cellular stress and dysfunction indicators.35 By detecting glucose, lactate, oxygen, and pH, researchers can evaluate the toxicity of drugs. Most glucose and lactate sensors are based on their respective oxidase enzymes to catalyze hydrogen peroxide production. Since this process is accompanied by electron transfer, electrodes can detect the reaction process.60, 122–124 As a by-product of the reaction, these enzymatic sensors consume oxygen and generate hydrogen peroxide, which may affect cell viability if the sensors are in the same chamber as the device. Enzyme activity changes with reaction conditions. The enzymatic degradation is also an important issue. This results in poor sensor stability that requires calibration for long-term use. Electrode biofouling typically involves the passivation of the electrode surface by forming an impermeable layer on the electrode that inhibits the direct contact of the target analyte. The use of valves is critical in the calibration of these biosensors.125 In this paper, an automated multi-pumps system was used for in-situ calibration of an electrochemical glutamate sensor. The sensor was calibrated every four hours during the 76-hour experiment.
Glucose and lactate from human colon carcinoma cell spheroids within hanging drops were detected by integrating microsensors into the base of hanging drops.126 They used 400 μm diameter platinum electrodes for each drop. In this case, electrodes were functionalized with a hydrogel containing glucose or lactate oxidase that catalyzed the oxidation reaction upon glucose or lactate binding. Sensitivities were calculated as a function of the specific area of the electrodes, 322 nA/ mM*mm2 for glucose and 433 nA/ mM*mm2 for lactate. In another work, electrochemical microsensors monitored lactate production and oxygen consumption in real-time.127 When the microsensors were inserted into the culture medium, lactate oxidase immobilized on the surface of the electrode could convert lactate to hydrogen peroxide. The concentration of lactate was measured by the oxidation current density with a LOD of 5 μM and a detection range of 0-1 mM.
Monitoring the cell secretome can assess the function of organoids and related responses under stimuli.128 For example, Wilmer et al. developed a kidney-on-a-chip to test drug efficacy by studying drug-induced kidney injuries. Biomarkers for nephrotoxicity for these in vitro chip models are kidney injury molecule 1, clusterin, heme oxygenase 1.129 As organs on a chip models advance, there is a strong interest in coupling immune components to these models.130 Some of the immune cells are cultured with cancer cells to study the crosstalk in the era of immunotherapy.131 These cells can be characterized with the following electrochemical sensors. Cytokines secreted from the immune cells represent and regulate many cellular functions of MPSs. Cytokines, such as interleukin and TNF-α, are key immune regulators of inflammation.132 However, the sizes of cytokines are small, and the concentrations are low, which are hard to detect even by traditional methods. In a muscle-on-a-chip model,20 the release levels and release time of IL-6 and TNF-α secreted from muscle cells were on-site measured by amperometric biosensors. Gold electrodes were screen-printed and then functionalized with antibodies. Zhou et al. reported a voltammetry sensor for monitoring transforming growth factor-β (TGF-β) secreted from the liver-on-a-chip model.133 In this sensor, a gold electrode was modified with aptamers that specifically capture target analytes. This sensor can perform in real-time and carry out label-free detection of TGF-β from hepatocyte-stellate cell co-cultures with a LOD of 1 ng/mL and linear range of 0- 250 ng/mL. Another work used aptamer-functionalized electrodes to detect interferon-gamma (IFN-γ) and TNF-α secreted from activated T-cells.134 They measured these two cell-secreted cytokines from the same microelectrode over 2 hours.
For monitoring biochemical parameters, the saturation of the electrode sensor surface is a potential problem. This hinders the long-time stability of these sensors. The development of regenerative processes to clean the electrode surfaces allows the reusability of electrochemical sensors in long-time monitoring of MPSs. Ideally, regenerative processes should be fast and not affect the detection performance. In this paper,51 the author conducted repeated regeneration of the electrode surface for up to four cycles without significant change in electrode performance, which allows five total measurements.
Applications of Microfluidic Biosensors for Monitoring MPSs
Due to advances in microfluidic-based biosensors over the past decade, there have been several publications that demonstrate the use of biosensors for in situ and real-time monitoring of MPSs. Thus, we will highlight the ability of microfluidic biosensors to be used as a versatile tool for various biomedical applications inherent in MPSs. This section outlines some typical applications of microfluidic biosensors for monitoring MPSs, such as drug discovery and screening, personalized medicine, and pre-clinical models.
In tissue models, detecting physical and biochemical cues is crucial to studying disease and evaluating drugs efficacies. For example, CK-MB was detected by an aptamer-based electrochemical biosensor with very high sensitivity, selectivity, and stability compared to antibody-based sensors (Figure 6a).135 In this work, the CK-MB secreted by the cardiac organoids was correlated with the beating rates and cellular viability. Modular biochemical, physical, and optical sensing platforms have been integrated into MPSs using a microfluidics breadboard that connects multiple MPSs and routes fluids in an automated, continuous, and dynamic manner. Using this platform, microenvironmental parameters (temperature, pH, and oxygen), biochemical parameters (CK-MB, GST-α, and albumin), and organoid morphologies were monitored. Also, the group demonstrated acetaminophen-induced toxicity using a normal human heart-liver-on-chips and doxorubicin-induced organoid toxicity using a heart-liver-cancer-on-chip MPSs (Figure 6b).51 All measurements were performed automatically and non-invasively using on-chip pneumatic valves for 5 days. The performance of this platform demonstrated a high potential in drug screening applications. The liver plays a crucial role in metabolizing drugs, and many in vitro liver-on-a-chip devices were developed for continuous monitoring of drug-induced toxicity or drug efficacy. To this end, Shin et al. have developed microfluidic biosensors for continual and non-invasive measurement of the metabolic activity of liver organoids in response to acetaminophen (a toxic drug to liver) by measuring GST-α and albumin secretion from the liver organoids for 7 days. Finally, they validated the accuracy of their biosensors by comparing their results using in vitro cell viability assays and ELISA (Figure 6c).136
Figure 6.
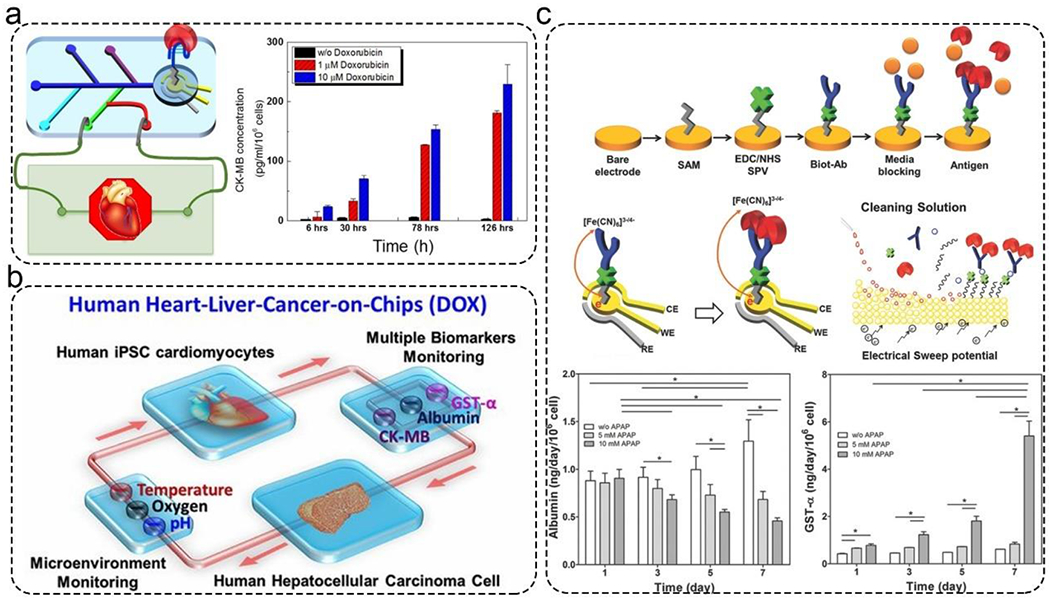
Applications of microfluidic biosensors for monitoring MPSs. a). CK-MB was detected by an aptamer-based electrochemical impedance spectroscopy biosensor. Reproduced with permission from ref (135) Copyright 2016 American Chemical Society. b). Automated multiple biosensors for monitoring acetaminophen-induced toxicity in normal human heart-liver-on-chips and doxorubicin-induced toxicity from heart-liver-cancer-on-chip MPSs. Reproduced with permission from ref (51) Copyright 2016 National Academy of Sciences. c). Sensors for monitoring the metabolic activity of the liver organoids in response to drugs for 7 d. Top: The principle and surface chemistry of electrochemical impedance spectroscopy-based biosensor. Middle: Schematic illustration of the electrode and regeneration process. Bottom: Real-time monitoring of GST-α and albumin for 7 d. Reproduced with permission from ref (136) Copyright 2017 Wiley.
For cardiac and neural tissue, another important parameter is their electrical activity, which indicates myocardial and neural functions.137 In this context, researchers have developed microfluidic heart-on-a-chip models for real-time recording of electrophysiological signals from cardiac tissues. In most cases, the electrode signals were measured with micro-electrode arrays (MEAs), on which cells were cultured. For example, a cardiac MPS used a platinum wire electrode for applying electrical stimulation, gold MEAs for acquiring electrophysiological signals, and a microfluidic chamber for long-term culturing cells (Figure 7a).138 Real-time electrical stimulation and monitoring of cultured cells can significantly increase the maturation of cardiomyocytes and enhance the generation of functional cardiac tissues.
Figure 7.
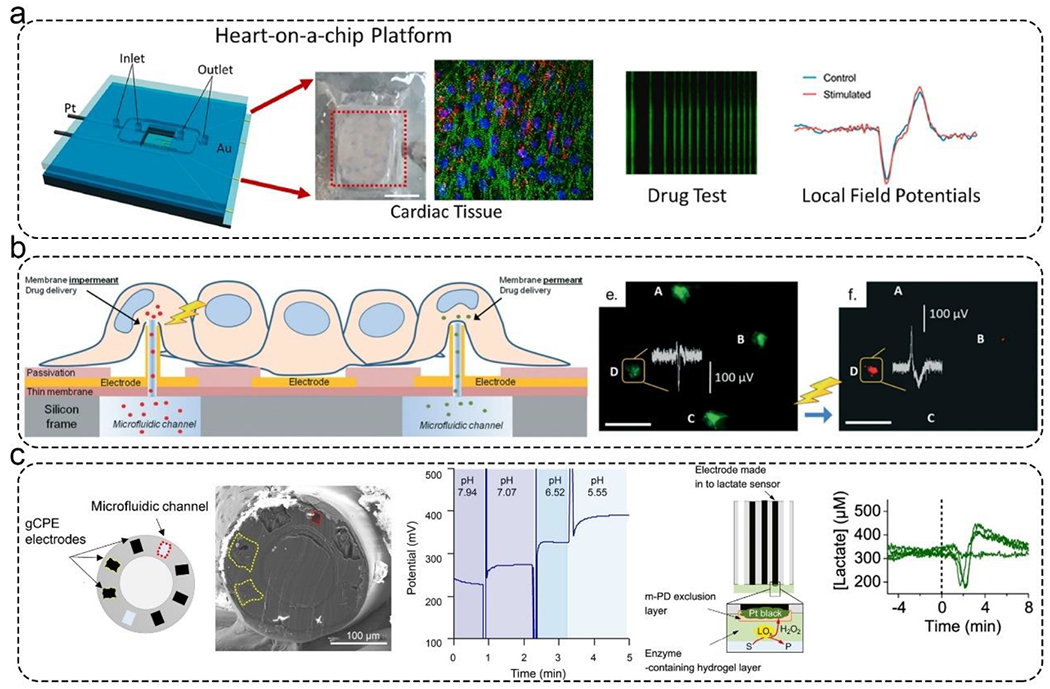
Applications of microfluidic biosensors for monitoring MPSs. a). An integrated cardiac MPS with a platinum wire electrode for applying electrical stimulation, a gold MEAs for acquiring electrophysiological signals, and a microfluidic chamber for long-term culturing cells. This platform can test drug responses with local field potentials. Reproduced with permission from ref (138) Copyright 2021 Elsevier. b). An integrated cardiac MPS. The MEAs were decorated with 3D hollow nanostructures that could deliver calcein-AM and propidium iodide into cardiac cells. The MEAs could monitor this process. Reproduced with permission from ref (139) Copyright 2018 Royal Society of Chemistry. c). Potentiometric fiber electrodes were used to monitor pH and transient neurometabolic lactate in neural tissue. Left: Schematic illustration of the fiber-based biosensor. Middle: Real-time monitoring pH. Right: The detection principle of lactate sensor and the real-time monitoring lactate. Reproduced with permission from ref (142) Copyright 2021 American Chemical Society.
In a similar work, MEAs with 3D hollow nanostructures were integrated on the bottom of microfluidic channels (Figure 7b).139 The 3D hollow nanostructures delivered calcein-AM and propidium iodide into cardiac cells. The MEAs recorded extra- and intracellular activity of electrogenic cells with high-quality spatial-temporal control during the whole process. Using this platform, they were able to study the pathologies at an early stage and examine the toxicity of nanoparticles and drugs on single cells. Developing tools capable of monitoring transient neurochemical dynamics is vital in many areas such as understanding brain physiological function, drug development, and personalized medicine. Mishra and Vazquez have developed a brain-on-a-chip model with the ability to monitor neural cell migration in response to electrical and chemical stimuli either alone or in combination. Using real-time imaging, they demonstrated that in response to a combination of stimuli, neural cells migrated longer distances with higher velocities, thus implicating cooperative behavior.140
Researchers have developed microfluidic chips for monitoring developmental activity and inter- and intra-nodal connectivity of the formed neural networks. Using their chip, they demonstrated the ability of their microfluidic chips to measure nodal dynamic responses to chemical stimulation and examine the immediate activity response of the neural networks in response to nodal functional connectivity disruption.141 Booth et al. developed potentiometric fiber electrodes for monitoring pH and transient neurometabolic lactate (Figure 7c).142 Microfluidic channels and electrodes were integrated into this fiber sensor. The pH and lactate sensors showed responses to pH and lactate levels varying between 5- 8 and 0- 3 mM, respectively. These sensors can be used to directly monitor lactate levels inside a brain following injury.
Conclusions
MPSs are powerful in vitro tools for understanding disease mechanisms and accelerating the drug development pipeline. In these MPS models, researchers can input different stimuli and monitor the response of physiologically relevant tissues for pre-clinical applications. Thus, the integration of biosensors into MPSs is mandatory and urgently needed to advance the potential of MPSs to be used as pre-clinical models and guide clinical trial design. This review summarizes the latest progress of microfluidic biosensors for monitoring MPSs.
For biosensors, several different biosensing methodologies have been proposed for monitoring MPSs. The most popular are optical and electrochemical biosensors for their simplicity, cost-effectivity, accuracy in measurements, and capacity to be miniaturized in microfluidic platforms. To develop and fabricate integrated MPSs, sensors that can detect and maintain microenvironments in MPSs and monitor molecules from a small volume of liquid are highly needed.143 However, most of the current sensors mentioned in this manuscript are academic exercises rather than commercially feasible technologies. As biosensors applied in MPSs, these are the following requirements for future development:
Non-invasive.
To make MPSs recapitulate the physiology of human organs, the detection process should be performed in a non-invasive and non-destructive manner. Microfluidics has excellent potential in this regard. For example, researchers can build in vitro organ models directly on the sensor surface, non-invasively extract culture fluid/cells for subsequent detection or acquisition of high-resolution images. In modular multi-organ-on-a-chip systems, the ability to connect and disconnect the organ modules and sensor systems will enable the long-term operation of the devices by removing the nonfunctional chips.
Real-time and fast response.
In integrated MPSs, researchers need to know the status of the system in real-time. This is easy for physical parameters such as temperature, pH, and oxygen because they exhibit a fast sample-to-answer time. However, monitoring the cell secretome relies on biochemical reactions or interactions on the sensor surface that often takes a relatively long time. As such, biosensors with fast responses are still warranted. For example, some label-free and real-time protein sensors are recommended and could be the future direction in this field.144, 145 In this article, a microwell-based impedance sensor was developed for real-time and in vivo detection of cytokine (interleukin 8, IL-8).146 This kind of sensor could be applied to real-time and continuous monitoring of cell secretome of MPSs.
Long time monitoring.
MPSs often run for extended periods that can be weeks or months. This requires that biosensors can also provide long-term and high-quality signal recording data. However, most biomolecular biosensors are still limited to single and end-point use. Since the detection signal is related to the surface state of the sensor, researchers should focus on developing reusable biosensors with reproducible and regeneratable surfaces. To avoid the saturation of the sensor, a surface with regeneration properties should also be developed. The ability to resist biofouling also needs to be improved for long-term monitoring. For example, the use of Nafion film to prevent protein fouling of electrodes used for measuring oxygen and pH.147 This is particularly important for the Clark electrode used to measure oxygen.148 Meanwhile, there is a strong need for making modular sensing systems with plug-and-play biosensors to move this field forward.
Sensitivity and selectivity.
For biosensors, detection performance depends on the sensitivity, selectivity, detection range, and detection time. It can be noted that each sensor has its benefits and limitations. Therefore, the selection of suitable biosensors is crucial for developing integrated MPSs. In other words, the detection performance of the biosensor needs to be consistent with the requirements of the detection targets. Meanwhile, high-sensitivity biosensors are still lacking when the concentration of cellular secretion reaches sub-picogram/ml concentrations.
Easy to use.
Ideally, MPSs should operate in a fully automated manner to reduce variability and errors introduced by human operations and increase the robustness of the integrated MPS platform. This requires biosensors to be easily integrated and can withstand long-term repeated usage. Microfluidic components such as microvalves, micropumps, and micromixers have been well developed in recent years. For example, traditional pneumatic valves are widely applied in the microfluidic area in the last 30 years. However, this technique requires bulk high-pressure air tank and control systems. Some recently developed on-chip valves are easier to use.125, 149, 150 The on-chip valves are actuated by rolling the roller and pressing the PC bar with through hole. These technologies offer the possibility of developing fully automated, integrated, and miniaturized MPSs, which could provide a better understanding of disease mechanisms and accelerate the development of drugs in the future.
Multiplexing and high throughput.
To utilize MPSs in the early stages of drug discovery and development, attention must be paid to the parallelization and automation of MPSs to increase the throughput. Using microfluidics, several HT-MPS have been recently reported.151–153 They utilize multi-well plate and microscale manufacturing methods to achieve multi-cellular cell culture environments in one chip. During drug development, the companies need to screen a large library of compounds. Accordingly, there is a strong push to build HT-MPS. In parallel, there is a strong need to develop HT sensors for monitoring the MPS. Individual MPS are custom designed for their intended applications. Corresponding biosensors will also need to be designed appropriately for sampling fluids from MPS. Interfacing HT-MPS (e.g., 1000 MPS) with HT-biosensors (e.g., 1000 biosensors) remains an unmet challenge in the field which needs to be addressed. Several papers reported integrating sensors next to the MPS systems within the same chamber, yet the complexity of fabrication prevents facile translation to industry. Current approach of connecting the MPS with biosensor chips using tubing works fine for small number of chips, yet this approach is not amenable to HT systems. Another key area to consider is the readout methods and/or biosensor selection. Electrical biosensors can be fabricated with HT yet the need for wires increase the complexity specifically when the number of chips exceed 100.154, 155 Optical sensors do not require wires yet may not have the needed sensitivity and face the same problem of building HR-readout systems in excess of 100 chips. Another area that needs work is the fluid control between the MPS and the biosensors. Since many biosensors require an incubation period, there is a strong need to be able to start and stop flow to the biosensor chips. These operations can be done with on chip valves yet increase complexity of the fabrication or require additional fluid control chips. Meanwhile, machine learning and big-data technologies capable of handling the results are still needed.
In conclusion, fully automated, non-invasive, real-time, and easy-to-use biosensors are critical before the eventual realization of commercially available in vitro systems for disease modeling and pre-clinical applications. From the papers outlined in the review, it is clear that significant development has been achieved in the field of biosensors and MPSs. These sensors can detect physical parameters related to the microenvironment and biochemical parameters related to the cellular metabolism and function of MPSs. Meanwhile, some fully integrated MPSs with multi-sensors and multi-organs are proposed for pre-clinical applications. This makes MPSs more relevant and valuable in understanding disease mechanisms and etiologies while potentially accelerating the drug discovery, development, and screening processes. The development of integrated MPSs is a very interdisciplinary endeavor that requires the efforts of chemists, materials scientists, physicists, and biologists. We believe that MPSs are an important topic in various disciplines because they have immense application potential, and they will eventually benefit human beings all over the world.
Funding:
The research was sponsored by the Office of the Secretary of Defense through the Advanced Regenerative Manufacturing Institute (ARMI|BioFabUSA) and was accomplished under Agreement Number W911NF-17-3-003. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Office of the Secretary of Defense or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes, notwithstanding any copyright notation here. This paper was partially funded by the National Institutes of Health (R01AR074234 and R01GM126571).
Footnotes
Conflicts of Interest:
The authors declare no conflict of interest.
References
- 1.Wang K, Man K, Liu J, et al. , ACS Biomater. Sci. Eng, 2020, 6, 3231–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth A and Berlin M-W, Science, 2021, 373, 1304–1306. [DOI] [PubMed] [Google Scholar]
- 3.Ashammakhi N, Darabi MA, Çelebi-Saltik B, et al. , Small Methods, 2020, 4, 1900589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh D, Matthews BD, Mammoto A, et al. , Science, 2010, 328, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosberg A, Alford PW, McCain ML, et al. , Lab Chip, 2011, 11, 4165–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Goss JA, Cho A, et al. , Lab Chip, 2013, 13, 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H, Chae S, Kim JY, et al. , Biofabrication, 2019, 11, 025001. [DOI] [PubMed] [Google Scholar]
- 8.Gijzen L, Yengej FAY, Schutgens F, et al. , Nat. Protoc, 2021, 16, 2023–2050. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Huh D, Hamilton G, et al. , Lab Chip, 2012, 12, 2165–2174. [DOI] [PubMed] [Google Scholar]
- 10.Shim K-Y, Lee D, Han J, et al. , Biomed. Microdevices, 2017, 19, 37. [DOI] [PubMed] [Google Scholar]
- 11.Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, et al. , Nat. Biomed. Eng, 2019, 3, 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie J, Gao Q, Wang Y, et al. , Small, 2018, 14, 1802368. [Google Scholar]
- 13.Cheng S, Hang C, Ding L, et al. , Matter, 2020, 3, 1664–1684. [Google Scholar]
- 14.Kim J, Lee K-T, Lee JS, et al. , Nat. Biomed. Eng, 2021, 5, 830–846. [DOI] [PubMed] [Google Scholar]
- 15.Park T-E, Mustafaoglu N, Herland A, et al. , Nat. Commun, 2019, 10, 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthiesen I, Voulgaris D, Nikolakopoulou P, et al. , Small, 2021, 17, 2101785. [DOI] [PubMed] [Google Scholar]
- 17.Vernetti L, Gough A, Baetz N, et al. , Sci. Rep, 2017, 7, 42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung JH, Wang YI, Narasimhan Sriram N, et al. , Anal. Chem, 2018, 91, 330–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picollet-D’hahan N, Zuchowska A, Lemeunier I, et al. , Trends Biotechnol., 2021, 39, 788–810. [DOI] [PubMed] [Google Scholar]
- 20.Sutterby E, Thurgood P, Baratchi S, et al. , Small, 2020, 16, 2002515. [DOI] [PubMed] [Google Scholar]
- 21.Chou DB, Frismantas V, Milton Y, et al. , Nat. Biomed. Eng, 2020, 4, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega MA, Fernández-Garibay X, Castaño AG, et al. , Lab Chip, 2019, 19, 2568–2580. [DOI] [PubMed] [Google Scholar]
- 23.Xiao S, Coppeta JR, Rogers HB, et al. , Nat. Commun, 2017, 8, 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewart L and Roth A, Nat. Rev. Drug Discov, 2021, 20, 327–328. [DOI] [PubMed] [Google Scholar]
- 25.Low LA, Mummery C, Berridge BR, et al. , Nat. Rev. Drug Discov, 2021, 20, 345–361. [DOI] [PubMed] [Google Scholar]
- 26.Trapecar M, Wogram E, Svoboda D, et al. , Sci. Adv, 2021, 7, eabd1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz AUR, Geng C, Fu M, et al. , Bioengineering, 2017, 4, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Yan S, Yuan D, et al. , Lab Chip, 2016, 16, 10–34. [DOI] [PubMed] [Google Scholar]
- 29.Nge PN, Rogers CI and Woolley AT, Chem. Rev, 2013, 113, 2550–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HN, Habbit NL, Su CY, et al. , Adv. Funct. Mater, 2019, 29, 1807553. [Google Scholar]
- 31.Enders JR, Marasco CC, Wikswo JP, et al. , Anal. Chem, 2012, 84, 8467–8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marx U, Andersson TB, Bahinski A, et al. , ALTEX, 2016, 33, 272–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalmykov A, Huang C, Bliley J, et al. , Sci. Adv, 2019, 5, eaax0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y, Mandal K, Hernandez AL, et al. , Curr. Opin. Biomed. Eng, 2021, 19, 100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrari E, Palma C, Vesentini S, et al. , Biosensors, 2020, 10, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thévenot DR, Toth K, Durst RA, et al. , Biosens. Bioelectron, 2001, 16, 121–131. [DOI] [PubMed] [Google Scholar]
- 37.Estevez MC, Alvarez M and Lechuga LM, Laser Photonics Rev., 2012, 6, 463–487. [Google Scholar]
- 38.He L, Huang R, Xiao P, et al. , Chin. Chem. Lett, 2021, 32, 1593–1602. [Google Scholar]
- 39.Zhu C, Yang G, Li H, et al. , Anal. Chem, 2015, 87, 230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maduraiveeran G, Sasidharan M and Ganesan V, Biosens. Bioelectron, 2018, 103, 113–129. [DOI] [PubMed] [Google Scholar]
- 41.Du X, Zhang Z, Zheng X, et al. , Nat. Commun, 2020, 11, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bavli D, Prill S, Ezra E, et al. , Proc. Natl. Acad. Sci. U.S.A, 2016, 113, E2231–E2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui F, Zhou Z and Zhou HS, J. Electrochem. Soc, 2019, 167, 037525. [Google Scholar]
- 44.Dinis H and Mendes PM, Biosens. Bioelectron, 2021, 172, 112781. [DOI] [PubMed] [Google Scholar]
- 45.Borgmann S, Schulte A, Neugebauer S, et al. , Adv. Electrochem. Sci. Eng, 2011, 2. [Google Scholar]
- 46.Kotanen CN, Moussy FG, Carrara S, et al. , Biosens. Bioelectron, 2012, 35, 14–26. [DOI] [PubMed] [Google Scholar]
- 47.Bollella P and Gorton L, Curr. Opin. Electrochem, 2018, 10, 157–173. [Google Scholar]
- 48.Stasyuk N, Gayda G, Zakalskiy A, et al. , Food Chem., 2019, 285, 213–220. [DOI] [PubMed] [Google Scholar]
- 49.Mi S, Xia J, Xu Y, et al. , RSC Adv., 2019, 9, 9006–9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharafeldin M, Jones A and Rusling JF, Micromachines, 2018, 9, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang YS, Aleman J, Shin SR, et al. , Proc. Natl. Acad. Sci. U.S.A, 2017, 114, E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang Y and Yoon J-Y, Sens. Actuators Rep, 2021, 3, 100031. [Google Scholar]
- 53.Henry OY, Villenave R, Cronce MJ, et al. , Lab Chip, 2017, 17, 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivasan B, Kolli AR, Esch MB, et al. , J. Lab. Autom, 2015, 20, 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong S, Kim S, Buonocore J, et al. , IEEE Trans. Biomed. Eng, 2017, 65, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vatine GD, Barrile R, Workman MJ, et al. , Cell Stem Cell, 2019, 24, 995–1005. e1006. [DOI] [PubMed] [Google Scholar]
- 57.Griep LM, Wolbers F, de Wagenaar B, et al. , Biomed. microdevices, 2013, 15, 145–150. [DOI] [PubMed] [Google Scholar]
- 58.Vigh JP, Kincses A, Ozgür B, et al. , Micromachines, 2021, 12, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Odijk M, van der Meer AD, Levner D, et al. , Lab Chip, 2015, 15, 745–752. [DOI] [PubMed] [Google Scholar]
- 60.Mou L, Xia Y and Jiang X, Anal. Chem, 2021, 93, 11525–11531. [DOI] [PubMed] [Google Scholar]
- 61.Mou L, Xia Y and Jiang X, Biosens. Bioelectron, 2022, 197, 113765. [DOI] [PubMed] [Google Scholar]
- 62.Mir M, Palma-Florez S, Lagunas A, et al. , ACS Sens., 2022, DOI: 10.1021/acssensors.2c00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chmayssem A, Verplanck N, Tanase CE, et al. , Talanta, 2021, 229, 122275. [DOI] [PubMed] [Google Scholar]
- 64.Li L-M, Wang X-Y, Hu L-S, et al. , Lab Chip, 2012, 12, 4249–4256. [DOI] [PubMed] [Google Scholar]
- 65.Gao A, Yang X, Tong J, et al. , Biosens. Bioelectron, 2017, 91, 482–488. [DOI] [PubMed] [Google Scholar]
- 66.Borisov SM and Wolfbeis OS, Chem. Rev, 2008, 108, 423–461. [DOI] [PubMed] [Google Scholar]
- 67.Chen C and Wang J, Analyst, 2020, 145, 1605–1628. [DOI] [PubMed] [Google Scholar]
- 68.Liao Z, Zhang Y, Li Y, et al. , Biosens. Bioelectron, 2019, 126, 697–706. [DOI] [PubMed] [Google Scholar]
- 69.Eslami Amirabadi H, Donkers JM, Wierenga E, et al. , Lab Chip, 2022, 22, 326–342. [DOI] [PubMed] [Google Scholar]
- 70.Mousavi Shaegh SA, De Ferrari F, Zhang YS, et al. , Biomicrofluidics, 2016, 10, 044111–044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shevchenko Y, Camci-Unal G, Cuttica DF, et al. , Biosens. Bioelectron, 2014, 56, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho S, Islas-Robles A, Nicolini AM, et al. , Biosens. Bioelectron, 2016, 86, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma A, Khan R, Catanante G, et al. , Toxins, 2018, 10, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Long F, Zhu A and Shi H, Sensors, 2013, 13, 13928–13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee H, Shin W, Kim HJ, et al. , Anal. Chem, 2021, 93, 16123–16132. [DOI] [PubMed] [Google Scholar]
- 76.Wang S, Zheng L, Cai G, et al. , Biosens. Bioelectron, 2019, 140, 111333. [DOI] [PubMed] [Google Scholar]
- 77.Zhong W, Anal. Bioanal. Chem, 2009, 394, 47–59. [DOI] [PubMed] [Google Scholar]
- 78.Ando Y, Ta HP, Yen DP, et al. , Sci. Rep, 2017, 7, 15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobuszewska A, Kolodziejek D, Wojasinski M, et al. , Biosensors, 2021, 11, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kratz SRA, Höll G, Schuller P, et al. , Biosensors, 2019, 9, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Măriuţa D, Colin S, Barrot-Lattes C, et al. , Microfluid. Nanofluidics, 2020, 24, 65. [Google Scholar]
- 82.Senutovitch N, Vernetti L, Boltz R, et al. , Exp. Biol. Med, 2015, 240, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuchs S, Johansson S, Tjell AØ, et al. , ACS Biomater. Sci. Eng, 2021, 7, 2926–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okkelman IA, Neto N, Papkovsky DB, et al. , Redox Biol., 2020, 30, 101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao X, Ding C, Zhu A, et al. , Anal. Chem, 2014, 86, 7071–7078. [DOI] [PubMed] [Google Scholar]
- 86.Azimzadeh M, Khashayar P, Amereh M, et al. , Biosensors, 2022, 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miao Y, Ha A, de Lau W, et al. , Cell Stem Cell, 2020, 27, 840–851.e846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang H, Geng S, Hao M, et al. , Nat. Commun, 2021, 12, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Linden FH, Mahlandt EK, Arts JJG, et al. , Nat. Commun, 2021, 12, 7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ponsioen B, Post JB, Buissant des Amorie JR, et al. , Nat. Cell Biol, 2021, 23, 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y and Zhang X, Micromachines, 2021, 12, 826.34357236 [Google Scholar]
- 92.Lopez GA, Estevez M-C, Soler M, et al. , Nanophotonics, 2017, 6, 123–136. [Google Scholar]
- 93.Ortega MA, Rodríguez-Comas J, Yavas O, et al. , Biosensors, 2021, 11, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu SH, Lee KL, Chiou A, et al. , Small, 2013, 9, 3532–3540. [DOI] [PubMed] [Google Scholar]
- 95.Liu C, Lei T, Ino K, et al. , Chem. Commun, 2012, 48, 10389–10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berlanda SF, Breitfeld M, Dietsche CL, et al. , Anal. Chem, 2021, 93, 311–331. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y, Chen Y, Tang H, et al. , Small Methods, 2020, 4, 1900451. [Google Scholar]
- 98.Song Y, Lin B, Tian T, et al. , Anal. Chem, 2018, 91, 388–404. [DOI] [PubMed] [Google Scholar]
- 99.Mou L, Hong H, Xu X, et al. , ACS Nano, 2021, 15, 13077–13084. [DOI] [PubMed] [Google Scholar]
- 100.Yin J, Mou L, Yang M, et al. , Anal. Chim. Acta, 2019, 1060, 133–141. [DOI] [PubMed] [Google Scholar]
- 101.Elvira KS, i Solvas XC, Wootton RCR, et al. , Nat. Chem, 2013, 5, 905–915. [DOI] [PubMed] [Google Scholar]
- 102.Mou L, Dong R, Hu B, et al. , Lab Chip, 2019, 19, 2750–2757. [DOI] [PubMed] [Google Scholar]
- 103.Mou L, Li Z, Qi J, et al. , CCS Chem, 2021, 3, 1562–1572. [Google Scholar]
- 104.Dincer C, Kling A, Chatelle C, et al. , Analyst, 2016, 141, 6073–6079. [DOI] [PubMed] [Google Scholar]
- 105.Araci IE and Quake SR, Lab Chip, 2012, 12, 2803–2806. [DOI] [PubMed] [Google Scholar]
- 106.Novak R, Ingram M, Marquez S, et al. , Nat. Biomed. Eng, 2020, 4, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kwapiszewski R, Ziolkowska K, Zukowski K, et al. , Anal. Bioanal. Chem, 2012, 403, 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tahirbegi IB, Ehgartner J, Sulzer P, et al. , Biosens. Bioelectron, 2017, 88, 188–195. [DOI] [PubMed] [Google Scholar]
- 109.Matatagui D, Fontecha JL, Fernández MJ, et al. , Sensors, 2014, 14, 12658–12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schuller P, Rothbauer M, Kratz SR, et al. , Sens. Actuators B Chem, 2020, 312, 127946. [Google Scholar]
- 111.Chang C-W, Cheng Y-J, Tu M, et al. , Lab Chip, 2014, 14, 3762–3772. [DOI] [PubMed] [Google Scholar]
- 112.Shourabi AY, Kashaninejad N and Saidi MS, J. Sci.: Adv. Mater. Devices, 2021, 6, 280–290. [Google Scholar]
- 113.Luo Z, Zhou X, Mandal K, et al. , Adv. Drug Deliv. Rev, 2021, 176, 113839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanumihardja E, Slaats RH, van der Meer AD, et al. , ACS Sensors, 2021, 6, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.da Ponte RM, Gaio N, van Zeijl H, et al. , Sens. Actuator A Phys, 2021, 317, 112439. [Google Scholar]
- 116.Moya A, Ortega-Ribera M, Guimerà X, et al. , Lab Chip, 2018, 18, 2023–2035. [DOI] [PubMed] [Google Scholar]
- 117.Zhao H, Kim Y, Wang H, et al. , Proc. Natl. Acad. Sci. U.S.A, 2021, 118, e2100077118.33941674 [Google Scholar]
- 118.Oleaga C, Lavado A, Riu A, et al. , Adv. Funct. Mater, 2019, 29, 1805792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sidorov VY, Samson PC, Sidorova TN, et al. , Acta Biomater, 2017, 48, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schroer AK, Shotwell MS, Sidorov VY, et al. , Acta Biomater, 2017, 48, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernandes LA, Häfliger P, Azadmehr M, et al. , IEEE J Transl Eng Health Med, 2013, 1, 2700309–2700309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin Y, Yu P, Hao J, et al. , Anal. Chem, 2014, 86, 3895–3901. [DOI] [PubMed] [Google Scholar]
- 123.Weltin A, Slotwinski K, Kieninger J, et al. , Lab Chip, 2014, 14, 138–146. [DOI] [PubMed] [Google Scholar]
- 124.Han J, Kim B, Shin J-Y, et al. , ACS Nano, 2015, 9, 2805–2819. [DOI] [PubMed] [Google Scholar]
- 125.Miller DR, Schaffer DK, Neely MD, et al. , Sens. Actuators B Chem, 2021, 341, 129972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Misun PM, Rothe J, Schmid YR, et al. , Microsyst. Nanoeng, 2016, 2, 16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weltin A, Hammer S, Noor F, et al. , Biosens. Bioelectron, 2017, 87, 941–948. [DOI] [PubMed] [Google Scholar]
- 128.Dornhof J, Kieninger J, Muralidharan H, et al. , Lab Chip, 2022, 22, 225–239. [DOI] [PubMed] [Google Scholar]
- 129.Wilmer MJ, Ng CP, Lanz HL, et al. , Trends Biotechnol, 2016, 34, 156–170. [DOI] [PubMed] [Google Scholar]
- 130.Sasserath T, Rumsey JW, McAleer CW, et al. , Adv. Sci, 2020, 7, 2000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parlato S, Grisanti G, Sinibaldi G, et al. , Lab Chip, 2021, 21, 234–253. [DOI] [PubMed] [Google Scholar]
- 132.Zhu J, He J, Verano M, et al. , Lab Chip, 2018, 18, 3550–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou Q, Patel D, Kwa T, et al. , Lab Chip, 2015, 15, 4467–4478. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, Liu Y, Matharu Z, et al. , Biosens. Bioelectron, 2015, 64, 43–50. [DOI] [PubMed] [Google Scholar]
- 135.Shin SR, Zhang YS, Kim D-J, et al. , Anal. Chem, 2016, 88, 10019–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shin SR, Kilic T, Zhang YS, et al. , Adv. Sci, 2017, 4, 1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu Y, Sun L, Wang Y, et al. , Adv. Mater, 2022, 34, 2108972. [DOI] [PubMed] [Google Scholar]
- 138.Zhang F, Qu KY, Zhou B, et al. , Biosens Bioelectron, 2021, 179, 113080. [DOI] [PubMed] [Google Scholar]
- 139.Cerea A, Caprettini V, Bruno G, et al. , Lab Chip, 2018, 18, 3492–3500. [DOI] [PubMed] [Google Scholar]
- 140.Mishra S and Vazquez M, Biosensors, 2017, 7, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van de Wijdeven R, Ramstad OH, Valderhaug VD, et al. , Biosens Bioelectron, 2019, 140, 111329. [DOI] [PubMed] [Google Scholar]
- 142.Booth MA, Gowers SAN, Hersey M, et al. , Anal Chem, 2021, 93, 6646–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wikswo JP, Block IFE, Cliffel DE, et al. , IEEE. Trans. Biomed. Eng, 2013, 60, 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu D, Zhou L, Huang L, et al. , Analyst, 2021, 146, 5380–5388. [DOI] [PubMed] [Google Scholar]
- 145.Li X, Soler M, Szydzik C, et al. , Small, 2018, 14, 1800698. [DOI] [PubMed] [Google Scholar]
- 146.Song N, Xie P, Shen W, et al. , Microsyst. Nanoeng, 2021, 7, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu J and Cao Y, Anal. Methods, 2021, 13, 685–694. [DOI] [PubMed] [Google Scholar]
- 148.Han J-H, Kim S, Choi J, et al. , Sens. Actuators B Chem, 2020, 306, 127465. [Google Scholar]
- 149.Hu B, Liu Y, Deng J, et al. , Anal. Methods, 2018, 10, 2470–2480. [Google Scholar]
- 150.Im SB, Uddin MJ, Jin GJ, et al. , Lab Chip, 2018, 18, 1310–1319. [DOI] [PubMed] [Google Scholar]
- 151.Bircsak KM, DeBiasio R, Miedel M, et al. , Toxicology, 2021, 450, 152667. [DOI] [PubMed] [Google Scholar]
- 152.Yu J, Lee S, Song J, et al. , Nano Converg., 2022, 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Azizgolshani H, Coppeta JR, Vedula EM, et al. , Lab Chip, 2021, 21, 1454–1474. [DOI] [PubMed] [Google Scholar]
- 154.Khalid MAU, Kim KH, Chethikkattuveli Salih AR, et al. , Lab Chip, 2022, 22, 1764–1778. [DOI] [PubMed] [Google Scholar]
- 155.Probst C, Schneider S and Loskill P, Curr. Opin. Biomed. Eng, 2018, 6, 33–41. [Google Scholar]


