Abstract
The current worldwide monkepox outbreak has reaffirmed the continued threat monkeypox virus (MPXV) poses to public health. JYNNEOS, a Modified Vaccinia Ankara (MVA)-based live, non-replicating vaccine, was recently approved for monkeypox prevention for adults at high risk of MPXV infection in the United States. Although the safety and immunogenicity of JYNNEOS have been examined previously, the clinical cohorts studied largely derive from regions where MPXV does not typically circulate. In this study, we assess the quality and longevity of serological responses to two doses of JYNNEOS vaccine in a large cohort of healthcare workers from the Democratic Republic of Congo (DRC). We show that JYNNEOS elicits a strong orthopoxvirus (OPXV)-specific antibody response in participants that peaks around day 42, or 2 weeks after the second vaccine dose. Participants with no prior history of smallpox vaccination or exposure have lower baseline antibody levels, but experience a similar fold-rise in antibody titers by day 42 as those with a prior history of vaccination. Both previously naïve and vaccinated participants generate vaccinia virus and MPXV-neutralizing antibody in response to JYNNEOS vaccination. Finally, even though total OPXV-specific IgG titers and neutralizing antibody titers declined from their peak and returned close to baseline levels by the 2-year mark, most participants remain IgG seropositive at the 2-year timepoint. Taken together, our data demonstrates that JYNNEOS vaccination triggers potent OPXV neutralizing antibody responses in a cohort of healthcare workers in DRC, a monkeypox-endemic region. MPXV vaccination with JYNNEOS may help ameliorate the disease and economic burden associated with monkeypox and combat potential outbreaks in areas with active virus circulation.
Keywords: JYNNEOS, MVA, Monkeypox, Orthopoxvirus, Serology, Antibody, Immunogenicity
1. Introduction
Following the global eradication of smallpox, monkeypox virus (MPXV) has emerged as the most critical orthopoxvirus (OPXV) for public health. MPXV is endemic in several West and Central African countries, and until recently, the sporadic and isolated cases reported outside these regions were largely linked to recent travel to Nigeria [1], [2], [3], [4], [5], or the importation of infected animals [6]. In May 2022, the first clusters of monkeypox infections involving no recent travel to endemic areas were reported in the United Kingdom. As of October 24, 2022, over 75,000 cases have been confirmed in 102 locations that have not historically reported monkeypox, including the United States [7]. Infected individuals develop vesicular-papular lesions and sometimes experience a febrile prodrome and lymphadenopathy [8]. Severe illness may occur in those with immunocompromising conditions, such as HIV [9], and can be marked by secondary bacterial infections leading to bronchopneumonia, sepsis and sometimes death [8], [10].
MPXV and variola virus, the causative virus of smallpox, share a high degree of genetic homology. Consequently, smallpox vaccines have historically been highly effective in preventing or reducing the severity of monkeypox [11]. Past studies have demonstrated that the severe complications and sequelae associated with monkeypox are more common in unvaccinated than vaccinated patients [10]. However, until recently, pregnant women and populations with immunological, cardiac, and dermatological conditions, who are precluded them from receiving live, replicating smallpox vaccine (ACAM2000), were left without immunization options against monkeypox [12], [13]. In 2019 JYNNEOS (also known as IMVAMUNE, IMVANEX), a Modified Vaccinia Ankara (MVA)-based live, non-replicating vaccine developed for smallpox preparedness, was approved by the FDA for smallpox prevention and monkeypox prevention for adults at high risk for MPXV infection in the United States [8], [14]. In severe disease or lethal MPXV challenge studies in non-human primates, vaccination with JYNNEOS demonstrated a clear survival benefit [15], [16], [17]. Additionally, safety studies evaluating JYNNEOS in previously vaccinated and unvaccinated healthy adults, and in adults with atopic dermatitis or HIV, did not find similar serious adverse events and systemic reactions compared to those seen with replicating vaccines [18], [19], [20], [21], [22], [23], [24]. As enrollment in these clinical studies has largely been limited to persons from the United States and Europe, there is an incomplete understanding of the safety and effectiveness of JYNNEOS in monkeypox-endemic regions.
Resuming smallpox vaccination to protect against monkeypox in Africa has been discussed since shortly after the eradication of smallpox, where markedly different attack rates of monkeypox were seen in children born after cessation of vaccination [25]. Continued vaccination was deemed unnecessary at the time, partly because infection clusters were expected to be self-limiting, but also due to the known adverse reactions associated with live virus vaccines [26], [27]. Recent studies have reported an increased number of human MPXV cases in the Congo Basin area, indicating that monkeypox remains a public health threat in the Democratic Republic of the Congo (DRC) [28]. However, widespread immunization using historical smallpox vaccines presents safety concerns that are heightened by the high burden of tropical infectious diseases, HIV and malnutrition-linked immune deficiencies [27]. The development and licensure of JYNNEOS provides an opportunity to minimize the worsening morbidity and mortality of monkeypox in endemic areas.
In 2017, a study was initiated in DRC as a collaborative effort between the U.S. Centers for Disease Control and Prevention (CDC), the DRC Ministry of Health (MOH), and the Kinshasa School of Public Health (KSPH) to evaluate the safety and immunogenicity of the JYNNEOS smallpox vaccine against monkeypox virus infection in healthcare workers, including laboratory personnel [29]. Healthcare workers in Tshuapa Province are at a much higher risk than the general population for exposure to and development of monkeypox [29]. As part of this open-label prospective cohort study, nearly 1000 participants received two doses of the liquid frozen formulation of the JYNNEOS vaccine, and their systemic/local adverse events, immunological responses, and any potential monkeypox exposures or infections were monitored over the next two years. In this manuscript, we describe the kinetics, magnitude, and quality of serological responses against VACV and MPXV in a select cohort of vaccinees with and without any history of prior OPXV exposure. These analyses may shed light on the immunological efficacy of JYNNEOS in a MPXV endemic region. In addition, the data presented herein may further our understanding of the roles that genetic and environmental factors play in the development and maintenance of serological responses against OPXVs.
2. Methods
2.1. Ethics statement
The human serum samples used in this study were collected as part of the clinical trial NCT02977715 described previously [29]. The study was reviewed and approved by the CDC Institutional Review Board (Protocol #6859) and the Kinshasa School of Public Health.2 Each participant agreed to study procedures via written informed consent prior to specimen collection and vaccination.
2.2. Study design and clinical samples
A total of 999 healthcare personnel 18 years of age or older were recruited on a voluntary basis in Kinshasa and Tshuapa Province in the DRC to receive two subcutaneous doses of the liquid frozen formulation of JYNNEOS (then, IMVAMUNE) vaccine. Vaccine doses were administered on days (D) 0 and 28. A pregnancy test was administered to females of childbearing age prior to receipt of vaccine; if positive, vaccine was not administered, with individuals excluded from the study if pregnant at D0. Blood samples were collected on D0, 14, 28, 42, 180, 365, 545, and 730 for all individuals who received at least one dose of vaccine. D14 screenings were only performed in Kinshasa and the provincial capital, Boende, for 324 of participants. Specimens were centrifuged and then stored at 4 °C in double gel SST tubes for up to 3 weeks until shipment to the Institut National pour la Researche Biomedicale (INRB) in Kinshasa was feasible. There, serum was aliquoted and stored at −20 °C before shipment to CDC’s Poxvirus Laboratory in Atlanta, Georgia, USA for immunological analysis.
2.3. Virus strains
Virus strains used for immunogenicity studies include: JYNNEOS (MVA) and Dryvax (VACV) for ELISAs, and VACV WR and MPXV WA for plaque reduction neutralization tests (PRNT)s. Viruses were propagated in BSC-40 cells using RPMI media containing 2 % FBS and 1 % penicillin/streptomycin antibiotics. Working aliquots of all viruses were stored at −20 °C or −80 °C prior to use.
2.4. ELISA
All serum specimens were tested for the presence of OPXV-specific IgG and IgM antibody at 1:100 and 1:50 dilution, respectively. The IgG and IgM ELISAs were performed as previously described [30] using JYNNEOS vaccine as the viral antigen. JYNNEOS antigen ELISA was optimized for comparability with our in-house developed Dryvax-based assay. A secondary cutoff was used to determine positivity in IgG [Optical Density (OD) – cutoff value (COV) > 0.1) and IgM (OD-COV > 0.12)]. These values were selected based on being 1 standard deviation higher from the mean OD-COV result in Naïve Participants at D0 (IgG), and based on the same criteria at D0, D180, D365, and D730 (IgM). In addition, based upon the availability of matched samples, a select subset of matched D0, D42 and D730 sera were tested at a wider range of dilutions to measure the IgG endpoint titer (EPT). OD values were plotted for each dilution tested and the EPT was interpolated using a non-linear regression equation (Sigmoidal, 4PL, X is log[concentration]) on GraphPad Prism software (version 8, GraphPad). The cutoff value to determine the EPT was defined as the mean OD of 5 negative control serum samples plus 3 standard deviations. IgG EPT ELISAs were performed using the VACV strain Dryvax as the viral antigen.
2.5. PRNT
VACV and MPXV-specific neutralizing antibody titers were measured by PRNT. Serum samples were heat inactivated for 1 h at 56 °C, and serially diluted to cover a range of dilutions starting at 1:7.5. Diluted sera were incubated with 50–80 pfu of VACV WR or MPXV WA overnight at 37 °C, and then added to Vero E6 cell monolayers seeded in 96-well plates. The virus, serum and cell mixtures were incubated for 1 h at 37 °C, and a 2 % methylcellulose overlay was added to the cells thereafter. After a 30 h (VACV) or 48 h (MPXV) incubation at 37 °C, cells were stained and inactivated with crystal violet containing 10 % buffered formalin. Plates were imaged using the Cellular Technology Limited (CTL) ImmunoSpot reader (S6 Micro Analyzer, ImmunoSpot), and plaques were enumerated using BioSpot software (7.0.23.2 Professional, ImmunoSpot). The number of plaques observed at each dilution were plotted on GraphPad Prism software (version 8; GraphPad) and PRNT50 values were determined using the non-linear regression equation (Sigmoidal, 4PL, X is log[concentration]).
3. Results
3.1. Clinical cohort
Our clinical cohort consisted of 999 participants who agreed to receive two doses of the liquid frozen formulation of JYNNEOS. Age, sex and other cohort characteristics are provided in Table 1 . Participants were classified into two groups initially: Naïve and Prior Vaccination. The DRC was declared smallpox free in 1977 and discontinued routine vaccination at that time. Thus, individuals that were 40 years of age and older at enrollment may have been vaccinated. As worldwide eradication did not occur until 1980, a more conservative minimum age criteria of 37 years was used as a cutoff for individuals to be included in the Prior Vaccination group. There were no age limits for inclusion in the Naïve group. Group classification was primarily based upon agreement between participant date of birth, age, self-reported vaccination status at the time of recruitment, and the presence or absence of a vaccine take; groups were further refined as individuals were excluded if they had missing age information, there was an incompatibility between Naïve vaccination status and a positive IgG ELISA OD on D0, or there was a notation of a prior MPXV-like disease. A total of 150 participants were excluded from this analysis. Both groups comprised more men than women, but no significant difference in gender distribution was observed between the groups. The Prior Vaccination group was significantly older than the Naïve (p < 0.0001).
Table 1.
Characteristics of the clinical cohort.
| n | Sex |
Age (Median, Range) | ||
|---|---|---|---|---|
| F | M | |||
| Naïve | 273 | 88 (32.2 %) | 185 (67.8 %) | 33.5 (18–59) |
| Prior Vaccination | 576 | 180 (31.2 %) | 396 (68.8 %) | 52 (37–84) |
A majority of participants were not screened at D14 due to logistical difficulties in reaching the secondary study sites. Some individuals missed one or more scheduled visits and/or vaccinations. Participant adherence for all 8 scheduled time points is shown in Supplementary Table 1.
3.2. Robust antibody production and seroconversion post vaccination
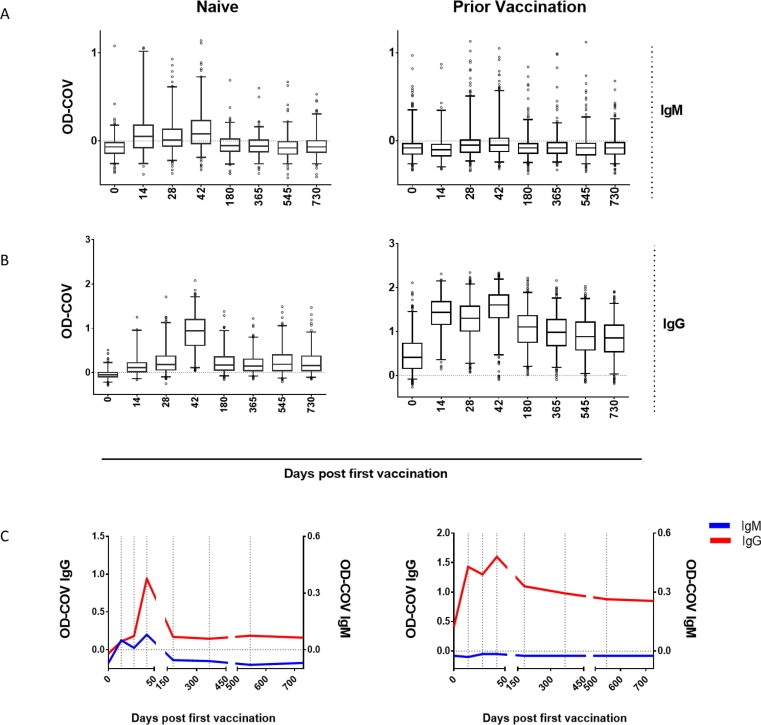
IgM and IgG ELISAs were performed using sera from all time points at a single dilution (1:50 for IgM and 1:100 for IgG) to check for the presence of VACV-specific antibody (Fig. 1 ). Between D0 and D42, an increase in median IgM OD-COV values was observed in both the Naïve and Prior Vaccination groups (Fig. 1A). While the rise in OD-COV values was more pronounced and rapid for the Naïve group, IgM values for the Prior Vaccination group remained relatively stable overall (Fig. 1A, C). These differences in magnitude and kinetics were also reflected in the IgM seropositivity rates shown in Table 2 . IgG trends, in contrast, showed a faster and greater increase in median ODs for Prior Vaccination samples compared to the Naïve group (Fig. 1B, C). In addition, later time points for Prior Vaccination participants remained IgG positive, while seropositivity waned for the Naïve group over time (Table 2). A peak in IgG OD-COV values was observed at D42 for both the Naïve and Prior Vaccination groups. IgM seronegative participants in both groups showed a higher average IgG seropositivity rate at D0 than those who were IgM positive at one or more time points (Supplementary Table 2). After D0, IgG seropositivity rates were largely consistent between IgM seronegative and seropositive participants in both groups.
Fig. 1.
Detection of IgM and IgG antibody after JYNNEOS vaccination. Serum specimens from participants were tested at a single dilution by ELISA to check for the presence of VACV-specific IgM and IgG at different time points after vaccination. IgM (A) and IgG (B) OD-COV values shown for Naïve (left column) and Prior Vaccination (right column) groups. OD-COV values plotted in box-and-whiskers format, wherein the horizontal band shows the median, boxes and whiskers represent 25–75 percentile and 2.5–97.5 percentile, respectively. (C) Magnitude and kinetic trends of VACV-specific IgM and IgG in Naïve vs Prior Vaccination groups. Median IgM (blue) and IgG (red) OD-COV values from (A) and (B) shown with respect to days post primary vaccination. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
VACV-specific IgM and IgG seropositivity rates post vaccination.
| Percent Seropositivity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| D0 | D14 | D28 | D42 | D180 | D365 | D545 | D730 | ||
| IgM | Naïve | 4.9 | 37 | 27.3 | 43.5 | 7.0 | 4.1 | 5.5 | 4.8 |
| Prior Vaccination | 6.3 | 7.3 | 9.9 | 12.5 | 5.8 | 5.1 | 5.3 | 4.8 | |
| IgG | Naïve | 7.8 | 57.6 | 64.4 | 97.7 | 63.8 | 60.5 | 64.3 | 61.4 |
| Prior Vaccination | 82.2 | 100 | 99.5 | 98.7 | 99.3 | 98.8 | 95.3 | 95.6 | |
3.3. Antibody titers strongly boosted by vaccination but decline to baseline levels two years post vaccine
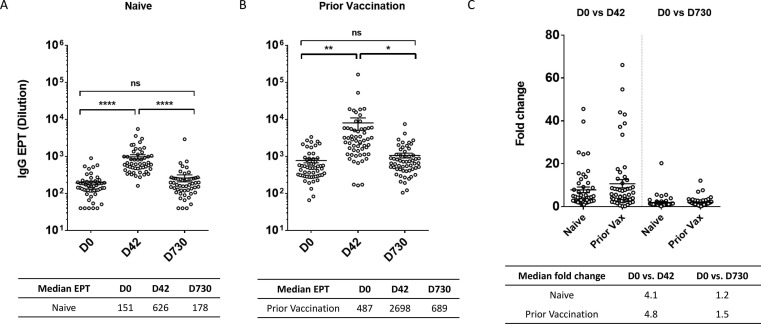
To further examine the magnitude and longevity of antibody responses to JYNNEOS vaccine, we determined VACV-specific endpoint IgG titers at D0 (baseline), D42 (peak response) and D730 (end of study, 2 years post vaccination) for a subset of participants from the Naïve (n = 51) and Prior Vaccination (n = 56) groups (Fig. 2 ). For both participant groups, IgG titers significantly increased between D0 and D42, and then declined significantly between the peak at D42 and D730 (Fig. 2A, 2B). IgG titers for all three time points were higher for Prior Vaccination participants compared to the Naïve. However, the rise in titers between D0 and D42, when expressed as a fold change, was comparable between the two groups (4.1, Naive vs 4.8, Prior Vaccination), indicating that vaccination elicited a robust antibody response for both participant types despite magnitude differences in median IgG titer. In aggregate, D730 IgG titers for both groups returned to baseline levels (Table 2, Fig. 2C).
Fig. 2.
Robust IgG production after JYNNEOS vaccination. D0, D42 and D730 serum specimens from participants were serially diluted and tested by ELISA to determine VACV-specific IgG endpoint titers (EPTs). IgG EPTs shown for Naïve (A) and Prior Vaccination (B) groups. Statistical significance determined using one-way ANOVA with post-hoc Tukey’s multiple comparisons test. P-values represented as asterisks. *p < 0.05, **p < 0.01 and ****p < 0.0001, ns: not significant. C) Fold change in VACV-specific IgG EPTs between D0 and D42 (left) and D0 and D730 (right) for Naïve and Prior Vaccination groups. Graphs show individual data points and mean values +/- SEM. Median values tabulated below respective graphs.
3.4. Marked increase in VACV and MPXV neutralizing titers post vaccination
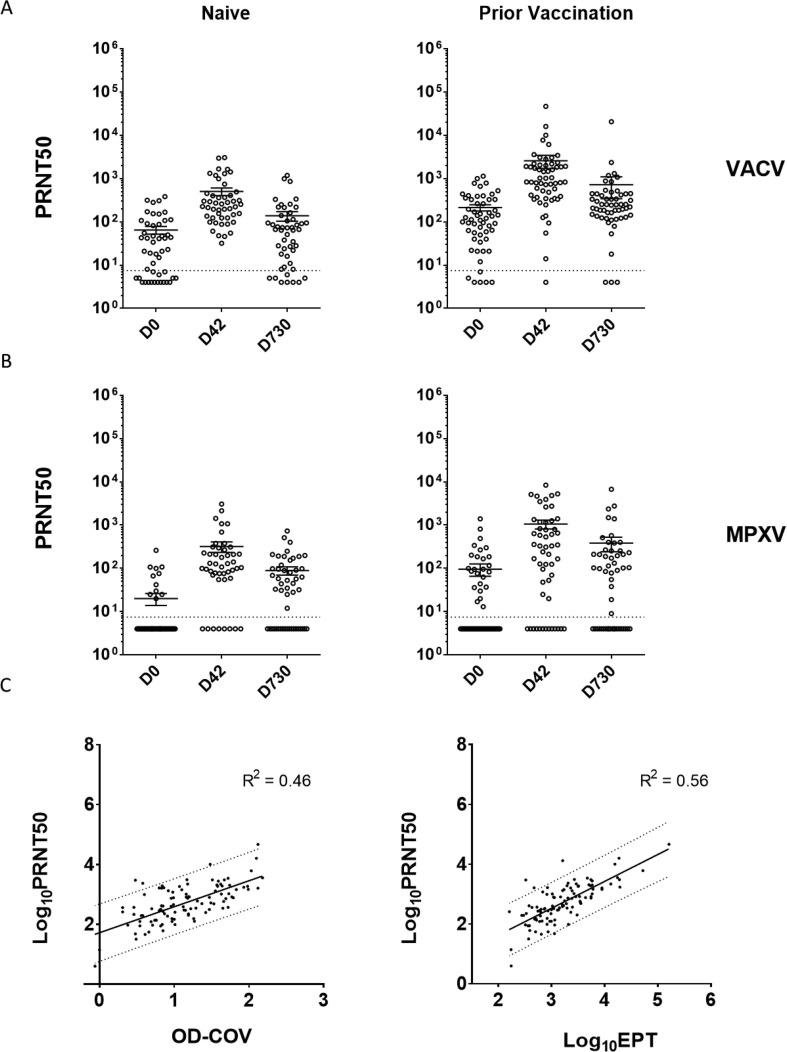
To assess whether the antibodies generated post vaccination were protective, we determined the in vitro neutralization titers of sera against VACV and MPXV by PRNT (Fig. 3 ). Both Naïve and Prior Vaccination participants saw an increase in PRNT50 titers against VACV and MPXV between D0 and D42 (Fig. 3A and 3B). Consistent with ELISA trends, a decline in PRNT50s was observed at D730, suggesting that titers wane considerably by 2 years post vaccination. Statistical significance and fold changes were not calculated due to the sizeable number of participants without a detectable antibody titer in the Naïve group. However, PRNT50 values were higher against VACV than MPXV for both participant groups. VACV PRNT50 values correlated moderately with IgG ELISA results, showing a slightly stronger correlation with EPTs (R2 = 0.56) than with single dilution OD-COV values (R2 = 0.46) (Fig. 3C).
Fig. 3.
VACV and MPXV neutralizing antibodies generated post JYNNEOS vaccination. D0, D42 and D730 serum specimens were serially diluted and tested by PRNT to determine serum neutralization capacity. PRNT50 values against VACV (A) and MPXV (B) shown for Naïve (left column) and Prior Vaccination (right column) participants. Dotted lines signify the highest serum dilution tested (1:7.5). Graphs show individual data points and mean values +/- SEM. Samples with PRNT50 values below the limit of detection were assigned a value of y = 4 so they could be presented graphically. (C) Linear regression plots of log10 transformed PRNT50 vs IgG OD-COV values (left) and log10 transformed PRNT50 vs log10 transformed IgG EPT (right). The dotted lines mark the 95 % prediction band of the best-fit line (bold).
4. Discussion
The safety and efficacy of JYNNEOS has been demonstrated in both non-human primate studies and in clinical trials involving US persons [15], [20], [22], [23], [24]. However, the immunogenicity of this vaccine was previously untested in individuals who live in areas with active OPXV circulation [29]. Study participants, both those with and without prior smallpox vaccination, had a robust production of VACV-specific IgG that peaked on D42, mimicking IgG kinetics reported previously for JYNNEOS vaccinees [22]. Expectedly, IgM production was more pronounced in the Naïve participants, driven by the generation of a de novo B cell response to the vaccine. Naïve participants that remained IgM seronegative throughout the study had a higher average baseline IgG seropositivity rate compared to their IgM seropositive counterparts, suggesting possible unknown prior OPXV exposures. The lack of an IgM response did not impair the generation of IgG antibodies, as both IgM seronegative and seropositive subgroups showed comparable IgG seropositivity rates after D0.
Interestingly, although the median peak IgG titer for the Prior Vaccination group was over 4-fold higher than in Naïve participant groups at any given timepoint, both groups experienced a comparable fold-rise in IgG titers between D0 and D42. These data suggest that JYNNEOS vaccination triggers a potent B cell expansion in all recipients, irrespective of prior vaccination status. Neutralizing antibody titers correlated moderately with ELISA values, with higher median baseline and peak titers for Prior Vaccination participants compared to Naïve. Overall, higher PRNT titers were detected against VACV than MPXV for both participant groups, though the significance of this difference remains unclear given the lack of a defined serological correlate of protection against MPXV. One possible cause for this difference is that VACV WR is more homologous to the vaccine than MPXV WA. Of note, we observed higher IgG titers by both ELISA and PRNT in our clinical cohort, including Naïve baseline (D0) samples, compared to some previously studied cohorts [20], [21], [22], [24]. Whether the higher titers observed in the DRC cohort are indicative of prior asymptomatic exposures, better immunological responses or are linked to serum or assay sensitivity differences, warrants further investigation.
Prior studies evaluating JYNNEOS immunogenicity have monitored responses for up to 30 weeks post vaccination and show a decline in IgG titers by the 6-month time point [20], [21], [22], [24]. In our study, immune responses were tracked up to 2 years after primary vaccination, providing a unique glimpse into the longevity of JYNNEOS-driven antibody responses. Starting at the 6-month visit, ELISA ODs declined steadily until D730, at which median IgG titers for both groups returned close to baseline levels. In a study by Crotty et al. involving Dryvax vaccinees, total VACV-specific and neutralizing IgG titers decreased > 1 year post vaccination [31]. However, titers did not drop to baseline and in some recipients, were maintained for 40–60 years thereafter. Crotty et al. observed that VACV-specific memory B cells post Dryvax vaccination decreased initially (with a half-life of < 1 year), but were then maintained at a 10-fold lower plateau compared to the peak over the next several decades. Long term maintenance of T-cell responses has also been observed [31], [32]. However, the specific mechanisms involved in this prolonged maintenance of OPXV-specific T and B cells decades post Dryvax vaccination are unknown [32]. The cause for the seemingly faster decline of total and neutralizing serum antibody titers in JYNNEOS recipients similarly remains unclear. Although all participants self-reported as healthy at the time of enrollment, the possibility that prior immunodeficiencies or poor nutrition could have impacted immune responses to JYNNEOS, or the possible contribution by the different genetic background of the current study population, cannot be ruled out and is a potential limitation of this study. However, only three individuals reported being HIV +. Additionally, each was vaccinated as a child, had OPXV-specific antibodies on day 0, and a normal boost response like others in the prior vaccinated group.
Our study demonstrated that two doses of JYNNEOS can generate a strong OPXV-neutralizing antibody response in individuals from an MPXV-endemic region. Titers wane by the 2-year mark, and more rapidly in individuals with no prior history of OPXV vaccination compared to previously vaccinated participants. The ongoing 2022 monkeypox outbreak is a stark reminder of the continued threat MPXV poses to public health. MPXV vaccination with JYNNEOS could serve as an effective strategy to mitigate monkeypox disease burden and combat potential outbreaks, especially for individuals in high-risk groups who live in areas with active virus circulation. We anticipate a follow-up study to provide a booster dose of JYNNEOS to individuals vaccinated in this study; it is of interest to examine serum antibody levels at enrollment, which will be more than 5 years post the two-dose vaccination here, and the kinetics of the boost response. Future studies that examine the magnitude and kinetics of cellular mediators of OPXV immunity, including plasmablasts, memory B cells and CD4 T-cells, could provide greater insight into the protective capacity in the absence of a detectible titer and longevity of OPXV-specific responses post JYNNEOS vaccination. Studies that dissect the main targets of the serological responses seen here may shed light on the breadth of the antibody repertoire and its protective capacity against MPXV infection.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to offer their greatest appreciation to the many colleagues that facilitated this multi-year year study in the equatorial forest of The Democratic Republic of the Congo. We thank: the Monkeypox Team in Tshuapa, including Bienvenu Botuli, Théophile Yoka, Papy Bosimbi; the Poxvirus and Rabies Branch staff Yoshinori Nakazawa, Sarah Guagliardo, Whitni Davidson, Mary Reynolds, Jillybeth Burgado, Douglas Green, and Liz LeMasters; INCEF staff Kadi Enanga, Clever Demokolo; and Kinshasa School of Public Health staff Marx Mavoka.
Footnotes
See 45 C.F.R. part 46; 21 C.F.R. part 56.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.10.078.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38) doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erez N., Achdout H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yong S.E.F., Ng O.T., Ho Z.J.M., Mak T.M., Marimuthu K., Vasoo S., et al. Imported Monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobson G., Adamson J., Adler H., Firth R., Gould S., Houlihan C., et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26(32) doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello V., Sowash M., Gaur A., Cardis M., Pasieka H., Wortmann G., et al. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022;28(5):1002–1005. doi: 10.3201/eid2805.220292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease C, Prevention. Update: multistate outbreak of monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:642-6. [PubMed]
- 7.Minhaj F.S., Ogale Y.P., Whitehill F., Schultz J., Foote M., Davidson W., et al. Monkeypox Outbreak - Nine States, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71(23):764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priyamvada L., Satheshkumar P.S. 4 ed: Academic Press; 2021. Variola and Monkeypox Viruses (Poxviridae). Encyclopedia of Virology; pp. 868–874. [Google Scholar]
- 9.Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCollum A., Disu Y., et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jezek Z., Marennikova S.S., Mutumbo M., Nakano J.H., Paluku K.M., Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154(4):551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 12.Petersen B.W., Harms T.J., Reynolds M.G., Harrison L.H. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses - Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257–262. doi: 10.15585/mmwr.mm6510a2. [DOI] [PubMed] [Google Scholar]
- 13.Cono J, Casey CG, Bell DM, Centers for Disease C, Prevention. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm Rep 2003; 52: 1–28. [PubMed]
- 14.Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(22):734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatch G.J., Graham V.A., Bewley K.R., Tree J.A., Dennis M., Taylor I., et al. Assessment of the protective effect of Imvamune and Acam 2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol. 2013;87(14):7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigam P., Earl P.L., Americo J.L., Sharma S., Wyatt L.S., Edghill-Spano Y., et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366(1):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earl P.L., Americo J.L., Wyatt L.S., Anne Eller L., Montefiori D.C., Byrum R., et al. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;366(1):84–97. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Krempelhuber A., Vollmar J., Pokorny R., Rapp P., Wulff N., Petzold B., et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine. 2010;28(5):1209–1216. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vollmar J., Arndtz N., Eckl K.M., Thomsen T., Petzold B., Mateo L., et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24(12):2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg RN, Hurley MY, Dinh DV, Mraz S, Vera JG, von Bredow D, et al. A Multicenter, Open-Label, Controlled Phase II Study to Evaluate Safety and Immunogenicity of MVA Smallpox Vaccine (IMVAMUNE) in 18-40 Year Old Subjects with Diagnosed Atopic Dermatitis. PLoS One. 2015; 10: e0138348. [DOI] [PMC free article] [PubMed]
- 21.Frey S.E., Winokur P.L., Salata R.A., El-Kamary S.S., Turley C.B., Walter E.B., Jr, et al. Safety and immunogenicity of IMVAMUNE(R) smallpox vaccine using different strategies for a post event scenario. Vaccine. 2013;31:3025–3033. doi: 10.1016/j.vaccine.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittman P.R., Hahn M., Lee H.S., Koca C., Samy N., Schmidt D., et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N Engl J Med. 2019;381(20):1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg R.N., Overton E.T., Haas D.W., Frank I., Goldman M., von Krempelhuber A., et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis. 2013;207(5):749–758. doi: 10.1093/infdis/jis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg R.N., Hay C.M., Stapleton J.T., Marbury T.C., Wagner E., Kreitmeir E., et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN(R)) in 56–80-Year-Old Subjects. PLoS ONE. 2016;11:e0157335. doi: 10.1371/journal.pone.0157335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khodakevich L., Jezek Z., Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- 26.Fine P.E.M., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 27.Rimoin A.W., Graham B.S. Whither monkeypox vaccination. Vaccine. 2011;29(Suppl 4):D60–D64. doi: 10.1016/j.vaccine.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitehouse ER, Bonwitt J, Hughes CM, Lushima RS, Likafi T, Nguete B, et al. Clinical and Epidemiological Findings from Enhanced Monkeypox Surveillance in Tshuapa Province, Democratic Republic of the Congo During 2011-2015. J Infect Dis 2021; 223: 1870-8. [DOI] [PubMed]
- 29.Petersen B.W., Kabamba J., McCollum A.M., Lushima R.S., Wemakoy E.O., Muyembe Tamfum J.-J., et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res. 2019;162:171–177. doi: 10.1016/j.antiviral.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karem K.L., Reynolds M., Braden Z., Lou G., Bernard N., Patton J., et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12(7):867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S., Felgner P., Davies H., Glidewell J., Villarreal L., Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 32.Amanna I.J., Slifka M.K., Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211(1):320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.