Abstract
Since the initial clinical approval in the late 1990s and remarkable anticancer effects for certain types of cancer, molecular targeted therapy utilizing small molecule agents or therapeutic monoclonal antibodies acting as signal transduction inhibitors has served as a fundamental backbone in precision medicine for cancer treatment. These approaches are now used clinically as first-line therapy for various types of human cancers. Compared to conventional chemotherapy, targeted therapeutic agents have efficient anticancer effects with fewer side effects. However, the emergence of drug resistance is a major drawback of molecular targeted therapy, and several strategies have been attempted to improve therapeutic efficacy by overcoming such resistance. Herein, we summarize current knowledge regarding several targeted therapeutic agents, including classification, a brief biology of target kinases, mechanisms of action, examples of clinically used targeted therapy, and perspectives for future development.
Subject terms: Targeted therapies, Targeted therapies
Cancer: A focused approach to therapy
Molecular targeted therapies have transformed cancer treatment, enabling personalized treatment of tumors in which growth is being powered by specific mutations. In contrast to traditional chemotherapies, which are toxic to both healthy and tumor cells, these targeted agents are designed to specifically block the effects of particular signaling proteins whose activity is largely restricted to cancerous tissue. Hye-Young Min and Ho-Young Lee at Seoul National University, South Korea, briefly review the history of the development of these drugs, and outline the mechanisms by which different targeted therapies work and how the acquisition of new tumor mutations can confer drug resistance. They also examine recent progress in developing agents that target proteins that have previously proven challenging therapeutic targets, such as the well-known oncogene KRAS, and explore potential opportunities for future therapeutic development.
Introduction
Cancer is one of the main causes of disease-related death worldwide. According to Global Cancer Observatory (GLOBOCAN) estimates of cancer incidence and mortality, there were approximately 19.3 million new cancer cases and almost 10.0 million cancer deaths in 2020 globally1. The cancer-related burden (such as incidence and mortality) is expected to be 28.4 million cases in 2040, which is a 47% increase compared with that in 2020, largely due to increases in risk factors, such as aging, socioeconomic development, overweight status, and smoking1,2. Therefore, it is necessary to develop efficacious treatment strategies for patients with cancer.
Several therapeutic modalities, such as surgery, radiation therapy, and systemic anticancer therapy, have been applied clinically for cancer treatment, either alone, in combination, or sequentially, depending on the stage, resectability, biology, comorbidities, and patient’s overall functional performance3,4. Systemic anticancer therapy, involving a wide range of anticancer drugs for treatment, palliation, symptom alleviation, and quality of life improvement, includes cytotoxic chemotherapy, hormonal agents, targeted therapy, and antitumor immunotherapy5,6. Cytotoxic chemotherapy inhibits the survival of actively proliferating cells by disrupting the synthesis of DNA and RNA, blocking mitosis, and/or forming covalent bonds with DNA, RNA, and proteins7, and it has been extensively used in adjuvant or neoadjuvant therapy as well as in palliative therapy7. Due to the disadvantages of chemotherapy, including side effects and toxicity associated with nonselective action against actively proliferating normal cells2,8, there has been innovative development of ‘targeted’ cancer treatment with increased cancer cell specificity8. Targeted therapy may include the following: conventional molecular targeted agents, such as small molecule inhibitors or antibodies that specifically inhibit signal transduction pathways involved in growth, proliferation, and survival9,10; hormonal agents such as estrogen receptor (ER) antagonists and aromatase inhibitors, which have been used for treatment of hormone receptor (HR)-dependent breast cancer and male and female reproductive cancers11; immune checkpoint inhibitors [e.g., antibodies against programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)], which activate host antitumor immunity in a direct or indirect manner8,12; and even targeted cytotoxic therapy that interferes with a specific cellular target (e.g., methotrexate, a dihydrofolate reductase inhibitor)10. Despite the anticancer effectiveness of these targeted therapies, these drugs are only applicable for patients harboring targetable driver mutations or aberrations13,14. In addition, side effects or toxicity caused by unexpected cross-reactivity with normal cells and emergence of intrinsic or acquired drug resistance hamper their effectiveness13,14. Notwithstanding some limitations, targeted therapy has resulted in remarkable survival benefits in some types of cancer and has led to a revolution in the fundamental concept of cancer treatment, providing the fundamental backbone for evolution toward precision or personalized medicine in cancer13,15. Herein, we summarize current knowledge with respect to molecular targeted therapy, including the history, types, and mechanism of action, and provide examples of clinically available targeted therapy. In this paper, ‘targeted therapy’ is confined to conventional molecular targeted therapy (signal transduction inhibitors).
Brief history of molecular targeted therapy
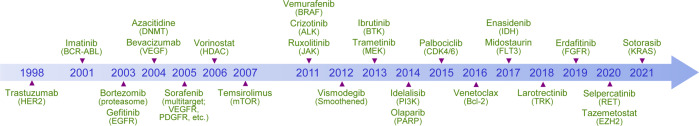
Paul Ehrlich first proposed the concept of targeted therapy in the 1890s as a “magic bullet” that would be completely specific for the target and thus safe without any additional toxicity14,16. This theory was initially applied to infectious diseases but not to anticancer therapy due to insufficient knowledge of the etiology and biology of cancer14,16; however, this concept has since been expanded to cancer treatment14,16. Trastuzumab, an anti-HER2 monoclonal antibody, and imatinib, a small molecule tyrosine kinase inhibitor targeting the BCR-ABL fusion-mediated aberrantly activated ABL kinase, were developed and clinically approved in 1998 and 2001 for treatment of HER2-positive breast cancer and Philadelphia chromosome-positive chronic myelogenous leukemia, respectively14,17–19. The success of imatinib in the clinic has served as the paradigm for extensive use of small molecule kinase inhibitors as anticancer therapy8,17, and a number of anticancer molecular targeted therapies have been approved for clinical use in cancer patients8,17. The timeline for the development of the main molecular targeted therapy is illustrated in Fig. 1.
Fig. 1. Timeline for the approval of selected molecular targeted therapeutic agents.
The first FDA-approved targeted therapeutic agent for each cellular target (denoted in blankets) is indicated in the timeline.
Types, mechanisms of action and resistance, and adverse effects/toxicity of molecular targeted therapy
To date, numerous molecular targeted therapeutic agents have been used clinically for cancer treatment. The classification of molecular targeted therapeutic agents and their targets, mechanism of action, side effects, and toxicity are described below.
Types of molecular targeted therapy
The two major types of molecular targeted therapy are monoclonal antibodies (mAbs) and small molecule kinase inhibitors (SMKIs)8,14. mAbs target extracellular ligands (e.g., bevacizumab targets vascular endothelial growth factor [VEGF]), membrane receptors (e.g., trastuzumab targets HER2 and cetuximab; panitumumab targets EGFR), and membrane-bound proteins (e.g., rituximab targets CD20), acting through ligand-binding blockade, ligand‒receptor interaction neutralization, or target molecule internalization/degradation14,20. Except for inhibitors targeting nonkinase cellular proteins (e.g., mutated KRAS and proteasome) or epigenetic modulators (e.g., histone deacetylases), most SMKIs suppress protein kinases involved in the transformation, growth, proliferation, and survival of cancer cells. As deregulation of protein kinases (e.g., activation by gain-of-function genetic mutation, gene amplification, autonomous activation, and chromosomal rearrangement) has been associated with cancer development and progression21–24, protein kinases have been regarded as important targets for developing molecular targeted therapies. Protein kinases are classified into receptor tyrosine kinases, nonreceptor (cytoplasmic) tyrosine kinases, serine/threonine kinases, and lipid kinases based on their subcellular localization, substrate type, and hallmark roles in cancer21 (Fig. 2). A detailed explanation of the signal transduction by receptor tyrosine kinase is described in previous studies24,25.
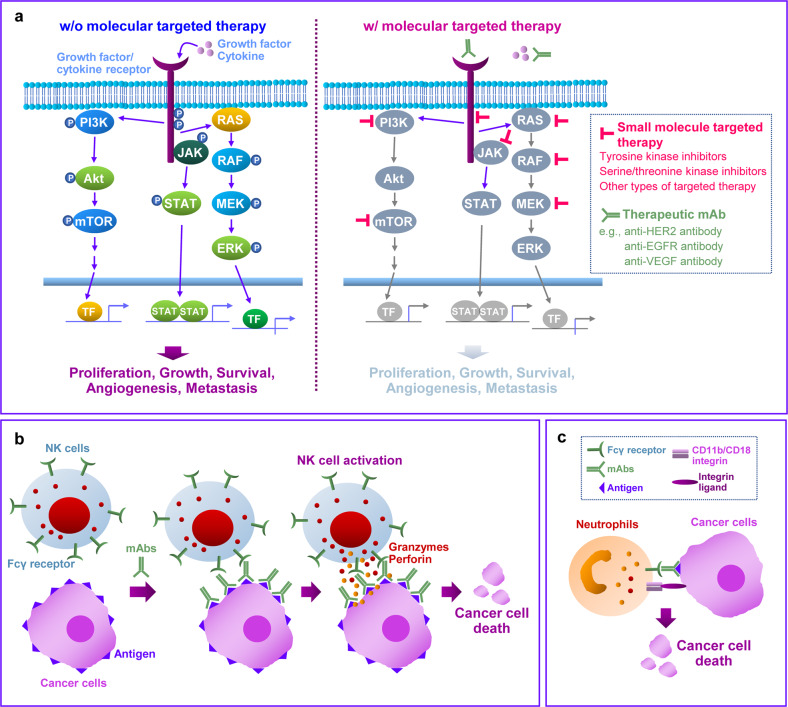
Fig. 2. Mechanism of the anticancer effect of molecular targeted therapy.
a Schematic diagrams of the main protumor signal transduction pathways and their inhibition by molecular targeted therapeutic agents. b, c Schematic diagrams for antibody-dependent cellular cytotoxicity b and trogoptosis c. See the text and relevant references for details.
SMKIs block the enzymatic activity of the aforementioned kinases via several modes of action26. Type I kinase inhibitors bind to the ATP-binding pocket of the active conformation of the enzyme [DFG (Asp-Phe-Gly)-in and αC-helix-in]26, whereas type I1/2 or type II inhibitors bind the enzyme in an inactive conformation (type I1/2: DFG-Asp in; type II: DFG-Asp out)21,26. Type III and type IV inhibitors allosterically suppress kinase activity by binding either to a site next to the ATP-binding pocket or one remote from the ATP-binding pocket located in the kinase substrate-binding site21,26,27. Type V inhibitors act as bivalent inhibitors binding to two different portions of the kinase lobe21,26. Type VI inhibitors covalently bind an enzyme to inhibit kinase activity26,28. A recent paper describes the detailed mode of action of each type of kinase inhibitor26, and some examples are listed in Table 1.
Table 1.
| Class | Mechanism of action | Examples |
|---|---|---|
| Type I | Binding in the ATP-binding pocket of the active conformation of the enzyme (DFG-in and αC-helix-in) | cabozantinib, ceritinib, gefitinib, palbociclib, pazopanib, ponatinib, ruxolitinib, tofacitinib |
|
Type I1/2 Type II |
Binding in the ATP-binding pocket of the inactive conformation of the enzyme (type I1/2: DFG-Asp in; type II: DFG-Asp out) | dasatinib, imatinib, lapatinib, lenvatinib, nilotinib, regorafenib, sorafenib, sunitinib, vemurafenib |
|
Type III Type IV |
Allosteric inhibitors binding to a site in the kinase domain either next to the ATP-binding pocket or remote from the ATP-binding pocket | trametinib, everolimus, sirolimus, temsirolimus |
| Type V | Bivalent inhibitors that bind two different portions of the kinase lobe | lenvatinib28 |
| Type VI | Covalent inhibitors | afatinib, ibrutinib |
In Ref. 26, lenvatinib is classified as a type I1/2 inhibitor.
Mechanisms of the anticancer effects of molecular targeted therapy
Molecular targeted therapies achieve anticancer effects through various mechanisms, such as inhibition of cell proliferation, metastasis, and angiogenesis, induction of apoptosis, and reversal of multidrug resistance2 (Fig. 2a). Several molecular targeted therapeutic agents also facilitate host antitumor immunity by potentiating CD8+ T-cell recruitment and natural killer cell cytotoxicity, downregulating immunosuppressive myeloid cells, and inducing immunogenic cell death, either alone or in combination with chemotherapeutic agents29. Therapeutic mAbs create a bridge between tumor cells and immune cells via Fab region-mediated binding to a target protein of tumor cells and recognition of immune cells through the Fc region of antibodies30, resulting in opsonization and antibody-dependent cellular cytotoxicity (ADCC) toward tumor cells30 (Fig. 2b). A recent study demonstrated that neutrophils mediate trogoptosis (Fig. 2c), the phenomenon of transferring surface molecules of interacting cells onto immune cells31,32, which causes lytic/necrotic death of antibody-opsonized cancer cells33. mAbs and SMIs also exert immune cell-induced cytotoxic effects on cancer cells by activating complement and complement-dependent cytotoxicity30,34, facilitating antigen processing by increasing expression of major histocompatibility complex molecules30,35,36 and regulating cytokine/chemokine expression30,37.
Mechanisms underlying resistance to molecular targeted therapy
The emergence of drug resistance is a major hurdle of efficacious anticancer treatment. Primary (intrinsic) resistance is defined as a refractory status to initial therapy due to intrinsic cellular, genetic, and/or epigenetic alterations. Hyperactivation of compensatory signaling pathways [e.g., truncated HER2 expression (p95HER2) for resistance to anti-HER2 mAbs38; KRAS mutation or MET amplification for resistance to anti-EGFR therapy38,39], mutations in kinase domains (e.g., EGFR exon 20 insertion for resistance to anti-EGFR therapy38), isoform switching (e.g., BRAF/CRAF switching for resistance to anti-BRAF therapy40), and metabolic reprogramming40 during disease development are involved in primary resistance to molecular targeted therapy.
Human cancers often exhibit substantial intratumor heterogeneity, which is a main driver for emerging acquired therapy resistance as a result of expansion of rare preexisting refractory populations during treatment in initial responders39,41,42. Various molecular and cellular alterations [e.g., development of secondary mutations [EGFR T790M and C797S38,43,44, BCR-ABL T315I44, BRAF V600E40,44, Bruton’s tyrosine kinase (BTK) C418S44, anaplastic lymphoma kinase (ALK) G1202R, and ROS1 G2032R and D2033N44], alterations in noncoding RNAs44, activation of bypassing signaling pathways, including MET, HER2, type I insulin-like growth factor receptor (IGF-1R), and AXL43,45, mutations in BRAF, PTEN, PIK3CA, and MAP2K143,45, interaction with stromal cells in the tumor microenvironment43,46, alterations in E3 ubiquitin ligases47, reactivation of developmental processes, such as the epithelial-mesenchymal transition (EMT), acquisition of cancer stem cell (CSC)-associated phenotypes, and transdifferentiation to small-cell lung cancer43,48] have also been shown to induce acquired therapy resistance. The mechanisms of resistance to each molecular targeted therapy are summarized in Tables 2–6.
Table 2.
Receptor tyrosine kinase inhibitors that have been clinically used for cancer treatment.
| Target | Generic name (Code name) | Brand name (Company) | First approved indication (Year) | Additional indication | Drug resistance mechanism (selected) | Side effects/toxicity (selected) | References |
|---|---|---|---|---|---|---|---|
| EGFR1 |
Gefitinib (ZD1839) |
Iressa (AstraZeneca) |
Advanced NSCLC2 after failure of both platinum-based and docetaxel chemotherapies (2003) | Metastatic NSCLC harboring EGFR mutations (first-line therapy, 2015) |
EGFR T790M mutation MET amplification HER2 amplification Small-cell lung cancer transformation |
Skin rash, nausea, diarrhea, transaminitis, ILD3-like disorders, hematuria | 8,52,63,195–197 |
| EGFR |
Erlotinib (OSI-744) |
Tarceva (Roche/Astellas) |
Locally advanced or metastatic NSCLC after failure of prior chemotherapy regimen (2004) | Metastatic NSCLC harboring EGFR mutations (first-line therapy, 2013) |
EGFR T790M mutation HGF overexpression MET amplification HER2 amplification Small-cell lung cancertransformation |
Skin rash, diarrhea, ocular toxicity | 8,50,52,64,195–197 |
| EGFR |
Afatinib (BIBW2992) |
Gilotrif (Boehringer Ingelheim) |
Metastatic NSCLC with kinase activating mutations (2013) | Advanced squamous cell carcinoma of the lung after treatment with platinum-based chemotherapy (2016) |
EGFR T790M mutation MET amplification EGFR V843I mutation |
Skin rash, diarrhea | 196,198,199 |
| EGFR |
Dacomitinib (PF-00299804) |
Vizimpro (Pfizer) |
Metastatic NSCLC with kinase activating mutations (2018) | EGFR T790M/C797S mutation | Skin toxicity, dermatitis acneiform, paronychia, diarrhea | 67,200,201 | |
| EGFR |
Osimertinib (AZD9291) |
Targrisso (AstraZeneca) |
1st- or 2nd-generation EGFR-TKI-refractory NSCLC (2015) | Advanced NSCLC with mutated EGFR, regardless of T90M mutation (2018) |
Loss of EGFR T790M mutation EGFR C797S mutation MET amplification Wild-type EGFR amplification |
Skin rash, diarrhea, mucositis/stomatitis, paronychia, pneumonitis, cardiac failure | 8,79,195,202–204 |
| EGFR | Lazertinib4 (YH25448) |
Leclaza (Yuhan/Janssen) |
Advanced or metastatic NSCLC (2021) |
Loss of EGFR T790M mutation EGFR activating mutation/amplification EGFR C797S mutation |
Skin rash, itchiness, paresthesia, muscle spasm, headache, diarrhea, anorexia | 72 | |
| EGFR | Cetuximab |
Erbitux (ImClone) |
Metastatic CRC5 (2004) | Head and neck squamous cell carcinoma (2006) |
RAS/BRAF mutation EGFR S492R mutation MET amplification PTEN loss |
Infusion reactions, acneform skin rash, nail disorder | 87–89,205–208 |
| EGFR | Panitumumab |
Vectibix (Abgenix/Amgen) |
Metastatic CRC (2006) |
RAS/BRAF mutation MET amplification PTEN loss |
Integument toxicity, skin toxicity, diarrhea | 88,89,206,208 | |
| EGFR |
Amivantamab (JNJ-61186372) |
Rybrevant (Janssen Biotech) |
Advanced NSCLC with EGFR exon 20 insertion mutations progressing after platinum-based chemotherapy (2021) |
Infusion reactions ocular toxicity, peripheral edema, hypoalbuminemia |
79,80 | ||
| EGFR |
Mobocertinib (TAK-788) |
Exkivity (Takeda Pharmaceuticals) |
Advanced NSCLC with EGFR exon 20 insertion mutations progressing after platinum-based chemotherapy (2021) | Diarrhea, skin toxicity | 79,81 | ||
| HER2 | Trastuzumab |
Herceptin (Genentech/Roche) |
Metastatic breast cancer (1998) | Locally advanced unresectable or metastatic HER2+ gastric or gastroesophageal junction (GEJ) adenocarcinoma in combination with pembrolizumab (2021) |
Truncation of HER2 extracellular domain (p95 HER2) PTEN loss IGF-1R expression PIK3CA mutation |
Cardiotoxicity | 8,56,90,209 |
| HER2 | Pertuzumab |
Perjeta (Genentech/Roche) |
HER2+ early breast cancer (EBC) with high risk of recurrence (2017) | Diarrhea, nausea, alopecia, fatigue, peripheral neuropathy, vomiting | 8,90,210 | ||
| HER2 |
Zanidatamab (ZW25) |
(Zymeworks) | Advanced/metastatic HER2-expressing biliary tract cancers | Diarrhea, infusion-related reactions | 91 | ||
| HER2 |
Lapatinib (GW-572016) |
Tykerb (GlaxoSmithKline/ Novartis) |
HER2+ metastatic breast cancer progressing with prior therapy (in combination with capecitabine, 2007) | Triple-positive metastatic breast cancer (in combination with letrozole, 2010) |
Crosstalk with ER HER2 mutation PIK3CA mutation AXL elevation HER2 L755S mutation |
Diarrhea, skin rash, asymptomatic cardiotoxicity | 82,83,209,211 |
| HER2 |
Neratinib (HKI-272) |
Nerlynx (Puma Biotechnology) |
Extended adjuvant therapy for HER2+ breast cancer (2017) | Advanced or metastatic HER2+ breast cancer progressing with prior therapy (in combination with capecitabine, 2020) |
TORC1 hyperactivation RAS upregulation |
Diarrhea | 56,84,209,211,212 |
| HER2 |
Tucatinib (ONT-380) |
Tukysa (Seattle Genetics) |
Advanced or metastatic HER2+ breast cancer (in combination with trastuzumab and capecitabine, 2020) | HER2 L755S mutation |
Diarrhea cardiotoxicity |
85,211 | |
|
ALK ROS1 MET |
Crizotinib (PF-02341066) |
Xalkori (Pfizer) |
Locally advanced or metastatic ALK+ NSCLC (2011) | ROS1-positive NSCLC (2016) | ALK mutation (G1269A, C1156Y, E1210K, I1171T, S1206C/Y, I1151T/N/S, 1174 C/L/V, V1180L, L1196M) | Nausea, vomiting, diarrhea, visual disturbance, sinus bradycardia, liver enzyme abnormalities | 79,96,98,99,213 |
| ALK |
Ceritinib (LDK378) |
Zykadia (Novartis) |
ALK+ metastatic NSCLC after failure of crizotinib therapy (2014) | ALK+ metastatic NSCLC (first-line therapy, 2017) | ALK mutation (G1202R, F1174C/L/V, 1151Tins, L1152P, C1156Y) | Diarrhea, nausea, vomiting, fatigue, elevated level of transaminase | 95,96,213 |
| ALK | Alectinib (CH5424802) |
Alecensa (Chugai Pharmaceutical/ Roche |
ALK-rearranged advanced/recurrent NSCLC with crizotinib resistance (2015) | ALK+metastatic NSCLC (first-line therapy, 2017) |
ALK mutation (G1202R, V1180L and I1171T/N/S) MET amplification |
Photosensitivity, dysgeusia, myalgia, upregulated creatinine phosphokinase | 79,96,213 |
|
ALK EGFR |
Brigatinib (AP26113) |
Alunbrig (ARIAD Pharmaceuticals) |
ALK-rearranged metastatic NSCLC (2017) | ALK double mutation (G1202R, E1210K and S1206C or D1203N) | Pneumonitis, nausea, diarrhea, fatigue | 79,101,102,213 | |
|
ALK ROS1 |
Lorlatinib (PF-6463922) |
Lorbrena (Pfizer) |
ALK-rearranged metastatic NSCLC (2018) (second/third-line treatment, accelerated approval) |
ALK+ metastatic NSCLC (2021) (regular approval) |
Compound ALK mutation including G1202R, I1171N/T/S, and L1198F ALK L1256F mutation MET amplification |
Edema, cholesterolemia, peripheral neuropathy, hypertriglyceridemia, CNS effects | 8,92,203. |
| MET | Capmatinib (INC280) |
Tabrecta (Novartis) |
Metastatic NSCLC harboring MET exon 14 skipping (2020) | MET mutation at D1228 and Y1230 (D1228 A/E/G/H/N/V/Y, Y1230 C/D/H/N/S) | Nausea, diarrhea, peripheral edema, hypoalbuminemia, increased blood creatinine | 79,99,214 | |
| MET |
Tepotinib (EMD 1214063) |
Tepmetko (Merck) |
Metastatic NSCLC harboring MET exon 14 skipping (2021) | MET mutation at D1228 and Y1230 (D1228 A/E/G/H/N/V/Y, Y1230 C/D/H/N/S) | Nausea, vomiting, peripheral edema, hypoalbuminemia, increased blood creatinine | 79,99,214 | |
| TRK | Larotrectinib (LOXO-101) |
Vitrakvi (Loxo Oncology/Bayer) |
Locally advanced or metastatic solid tumors with NTRK gene fusion (2018) | TRKA F589L/G595R/G667C, TRKC G623R/G696A mutation | Upregulation of serum AST/ALT, dizziness, fatigue, nausea, constipation | 106,107 | |
|
TRK ALK ROS1 |
Entrectinib (RXDX-101) |
Rozlytrek (Genentech) |
Solid tumors with NTRK gene fusion and NSCLC harboring ROS1 rearrangement (2019) | TRKA G595R/G667C, TRKC G623R mutation | Fatigue, dysgeusia, nausea, vomiting, paresthesia, myalgia, diarrhea | 106,107 | |
|
FLT3 c-Kit PDGFR Src VEGFR |
Midostaurin (PKC412, CGP 41251) |
Rydapt (Novartis) |
AML6 harboring FLT3 mutations (2017) |
FLT3 N676K, F691L mutation FLT3 ligand overexpression RAS/MAPK mutation JAK, PI3K/Akt activation |
Nausea, febrile neutropenia, mucositis, vomiting, headache, petechiae, fever | 109,215–217 | |
|
FLT3 AXL |
Gilteritinib (ASP2215) |
Xospata (Astellas Pharma) |
FLT3-mutated refractory AML (2018) |
FLT3 F691L mutation RAS/MAPK mutation JAK, PI3K/Akt activation |
upregulation of hepatic transaminase/creatine phosphokinase, edema, cytopenia, febrile neutropenia | 109,215–217 | |
|
VEGFRs PDGFR-β c-Kit FLT3 RET RAFs |
Sorafenib (BAY 43-9006) |
Nexavar (Bayer/Onyx Pharmaceuticals) |
Advanced RCC7 (2005) |
HCC8 (2008) Locally recurrent or metastatic, progressive DTC9 refractory to radioactive iodine treatment (2013) |
FLT3 F691L, Y842C/H, D835F/V/Y mutation FLT3 ligand overexpression JAK, PI3K/Akt activation |
Hand-foot syndrome, asthenia, gastrointestinal irritation, cytopenia, infection, diarrhea, cardiovascular toxicity, fatigue | 109,215–217 |
|
PDGFR-α/β VEGFR1/2/3 CSF-1R c-Kit, RET FLT3 |
Sunitinib (SU11248) |
Sutent (Pfizer) |
Advanced RCC (2006) Imatinib-resistant GIST10 (2006) |
Pancreatic neuroendocrine tumor (2011) |
Angiogenic factor upregulation Autophagy Metabolic adaptation Stromal cell recruitment |
Mucositis, diarrhea, skin abnormality, taste alteration | 8,126,218,219 |
|
VEGFR1/2/3 PDGFR-α/β FGFR1 FGFR3 c-Kit |
Pazopanib (GW786034) |
Votrient (GlaxoSmithKline/Novartis) |
Advanced/metastatic RCC (2009) | Advanced soft-tissue sarcoma previously treated with chemotherapy (2012) |
Angiogenic factor upregulation Stromal cell recruitment |
Hepatic injury, fatigue, hand-food syndrome, myelosuppression | 220 |
|
VEGFR1/2/3 PDGFR-α FGFRs c-Kit RET |
Lenvatinib (E7080) |
Lenvima (Eisai/Merck) |
Progressive radioactive iodine-refractory thyroid cancer (2015) |
Advanced RCC (recurrent or metastatic) (2016) Unresectable HCC (2018) Advanced RCC in combination with pembrolizumab (2021) |
Angiogenic factor upregulation Stromal cell recruitment |
Hypertension, diarrhea, fatigue/asthenia | 220 |
|
MET VEGFR2 c-Kit RET AXL Tie2 FLT3 |
Cabozantinib (XL184) |
Cometriq (capsule) Cabometyx (tablet) (Exelixis) |
Cometriq: medullary thyroid cancer (2012) Cabometyx: RCC (2016) |
Cabometyx: HCC (second-line, 2019) |
Angiogenic factor upregulation Stromal cell recruitment |
Diarrhea, palmar-plantar erythrodysesthesia syndrome | 220 |
| VEGFRs |
Axitinib (AG 013736) |
Inlyta (Pfizer) |
Advanced or metastatic RCC (2012) |
Angiogenic factor upregulation Stromal cell recruitment |
Hypertension, diarrhea, fatigue | 220 | |
|
VEGFR2 EGFR3 GFR ET |
Vandetanib ZD6474) |
Zactima aprelsa AstraZeneca) |
Medullary thyroid cancer (2011) |
RET V804M/L mutation Activation of RAS/ RAF/MEK pathway |
Diarrhea, skin rash, folliculitis, nausea, fatigue, hypertension, QT interval prolongation | 221 | |
|
VEGFR1/2/3 Tie2 PDGFR-α/β FGFR1/2 c-Kit RET RAFs |
Regorafenib BAY 73-4506) |
Stivarga (Bayer) |
Metastatic CRC (2012) |
Advanced GIST (2013) Advanced HCC (2018) |
KIT V654A, D816V mutation | Hypertension, hand-food skin reaction, diarrhea, fatigue | 220,222 |
|
VEGFR1/2/3 PDGFR-β c-Kit |
Tivozanib (AV-951, KRN-951) |
Fotivda (AVEO Pharmaceuticals /Kyowa Kirin) |
Relapsed or refractory RCC (2021) | Infiltration of myeloid cells | Hypertension, hoarseness, fatigue, headache, diarrhea, rash | 223 | |
|
PDGFR-α c-Kit |
Avapritinib (BLU-285) |
Ayvakit (Blueprint Medicines) |
Unresectable or metastatic GIST harboring PDGFRA exon 18 mutations, including D842V (2020) | Memory impairment, cognitive disorder, intracranial bleeding | 222 | ||
|
PDGFR-α c-Kit |
Ripretinib (DCC-2618) |
Qinlock (Deciphera Pharmaceuticals) |
Advanced GIST treated with three or more kinase inhibitors, including imatinib (2020) | Alopecia | 222 | ||
| FGFR |
Erdafitinib (JNJ‑42756493) |
Balversa (Janssen Pharmaceuticals) |
Metastatic urothelial cancer (2018) | Metastatic or locally advanced bladder cancer with an FGFR3 or FGFR2 alteration (2019) |
FGFR1 V561M/F mutation FGFR2 N549H mutation p.E565A and p.L617M single-nucleotide variants |
Hyperphosphatemia, dry mouth, diarrhea, fatigue, stomatitis | 91,224,225 |
| FGFRs |
Pemigatinib (INCB054828) |
Pemazyre (Incyte Corporation) |
Previously treated, unresectable, locally advanced, or metastatic cholangiocarcinoma with FGFR2 fusion or other rearrangements (2020) |
FGFR1 V561M/F mutation FGFR2 N549H mutation |
Hyperphosphatemia, dry mouth, diarrhea, fatigue, stomatitis | 91,224–226 | |
| FGFRs |
Futibatinib (TAS-120) |
(Taiho Pharmaceutical) | Locally advanced/metastatic cholangiocarcinoma with FGFR2 gene rearrangement (2021) |
p.E565A and p.L617M single-nucleotide variants FGFR2 V564F mutation |
Hyperphosphatemia, dry mouth, diarrhea, paronychia, | 91,224,225 | |
| FGFRs |
Infigratinib (BGJ398) |
Truseltriq (QED Therapeutics /Helsinn) |
Locally advanced/metastatic cholangiocarcinoma with FGFR2 gene rearrangement (2021) | FGFR2 N549H, N550H/K, V564F, E565A, K660M, L618V, K641R mutation | Hyperphosphatemia, dry mouth, diarrhea, fatigue, stomatitis | 91,224,225 | |
| FGFRs |
Derazantinib (ARQ 087) |
(Basilea Pharmaceutica /Merck) |
Intrahepatic cholangiocarcinoma (2021) | Hyperphosphatemia, dry mouth, diarrhea, fatigue, stomatitis | 91,224,225 | ||
| RET |
Selpercatinib (LOXO-292) |
Retevmo (Eli Lilly/Loxo Oncology) |
Metastatic RET fusion-positive NSCLC (2020) Advanced or metastatic thyroid cancer with RET alterations (2020) |
RET mutation at G810, Y806 | AST/ALT elevation, hypertension | 227,228 | |
| RET |
Pralsetinib (BLU-667) |
Gavreto (Blueprint Medicines) |
Metastatic RET fusion-positive NSCLC (2020) | RET mutation at G810, L730 | AST/ALT elevation, anemia, hypertension | 227,228 |
1EGFR: epidermal growth factor receptor.
2NSCLC: non-small cell lung cancer.
3ILD: interstitial lung disease.
4Approved in Republic of Korea.
5CRC: colorectal cancer.
6AML: acute myeloid leukemia.
7RCC: renal cell carcinoma.
8HCC: hepatocellular carcinoma.
9DTC: differentiated thyroid carcinoma.
10GIST: gastrointestinal stromal tumor.
Table 6.
Additional targeted therapies that have been clinically used for cancer treatment.
| Target | Generic name (Code name) | Brand name (Company) | First approved indication (Year) | Additional indication | Drug resistance mechanism (selected) | Side effects/toxicity (selected) | References |
|---|---|---|---|---|---|---|---|
| PARP |
Olaparib (AZD2281) |
Lynparza (AstraZeneca) |
Advanced ovarian cancer (2014) |
Maintenance treatment of ovarian cancer (2017, 2018, 2020) BRCA-mutated metastatic breast cancer (2018, 2022) Metastatic pancreatic cancer (2019, 2020) |
Restoration of homologous recombination repair and ADP-ribosylation (PARylation) reversion mutations |
Ileus, myelodysplastic syndrome, interstitial lung disease | 8,178,247 |
| PARP |
Rucaparib (AG014699) |
Rubraca (Clovis Oncology) |
Advanced ovarian cancer (2016) | Maintenance treatment of ovarian cancer (2018) |
Restoration of homologous recombination repair and ADP-ribosylation (PARylation) reversion mutations |
Nausea, vomiting, diarrhea, constipation, red blood cell count decrease, photosensitivity, renal impairment, dysgeusia | 8,178,247 |
| PARP |
Niraparib (MK-4827) |
Zejula (Tesaro) |
Recurrent ovarian cancer (2017) | Maintenance treatment for patients with platinum-responsive ovarian cancer regardless of biomarker status (2020) |
Restoration of homologous recombination repair and ADP-ribosylation (PARylation) reversion mutations |
Nausea, constipation, platelet/red blood cells count decrease, lymphangioleiomyomatosis | 8,178,247 |
| PARP |
Talazoparib (BMN-673) |
Talzenna (Pfizer) |
BRCA1/2-mutated advanced or metastatic HER2- breast cancer (2018) |
Restoration of homologous recombination repair and ADP-ribosylation (PARylation) reversion mutations |
Hematopoietic erythropenia, anemia, thrombocytopenia, pancytopenia, neutropenia | 8,178,247 | |
| DNMT1 |
Azacitidine (5-azacytidine) |
Vidaza (Pharmion Corporation) |
MDS2 (2004) | Adaptive responses of the pyrimidine metabolism network | Fatigue, constipation, mucositis, pneumonia, febrile neutropenia | 8,247,248 | |
| DNMT |
Decitabine (NSC 127716) |
Dacogen (Janssen-Cilag/Otsuka Pharmaceutical) Inqovi (oral tablet) (Otsuka Pharmaceutical) |
Dacogen : MDS (2006) |
Inqovi: MDS in combination with cedazuridine (2020) | Adaptive responses of the pyrimidine metabolism network | Fatigue, constipation, mucositis, pneumonia, febrile neutropenia | 8,247,249 |
| HDAC3 |
Vorinostat (SAHA) |
Zolinza (Merck) |
Relapse/refractory CTCL4 (2006) |
Overexpression of Bcl-2 family proteins JAK/STAT3 pathways HDAC alterations Epigenetic alterations Protection of oxidative stress Alterations in apoptosis/autophagy |
Diarrhea, fatigue, nausea, anorexia, dysgeusia, thrombocytopenia, pulmonary embolism, cardiac abnormalities | 250,251 | |
| HDAC |
Romidepsin (FK228, FR901228) |
Istodax (Celgene Corp./Bristol-Myers Squibb) |
CTCL (2009) | PTCL5 (2011) |
P-glycoprotein-mediated drug efflux HDAC alterations Epigenetic alterations Protection of oxidative stress Alterations in apoptosis/autophagy |
Thrombocytopenia, anemia, neutropenia, fatigue, nausea, vomiting, anorexia, tumor lysis syndrome | 250,251 |
| HDAC |
Belinostat (PXD-101) |
Beleodaq (Spectrum Pharmaceuticals) |
PTCL (2014) |
HDAC alterations Epigenetic alterations Alterations in apoptosis/autophagy |
Nausea, vomiting, tumor lysis syndrome, hepatic failure, cardiac abnormalities | 250,251 | |
| HDAC | Panobinostat (LBH-589) |
Farydak (Novartis/Secura Bio) |
MM7 (2015) |
HDAC alterations Epigenetic alterations Protection of oxidative stress Alterations in apoptosis/autophagy |
Severe diarrhea, nausea, vomiting, cardiac abnormalities | 250,251 | |
| EZH26 | Tazemetostat (E7438/EPZ6438) |
Tazverik (Epizyme) |
Relapsed/refractory follicular lymphoma (2020) | Metastatic or locally advanced epithelioid sarcoma (2020) | EZH2 Y726F, C663Y mutation | Nausea, asthenia, fatigue, alopecia, dry skin, diarrhea, neutropenia, thrombocytopenia | 252 |
| IDH18 |
Ivosidenib (AG-120) |
Tibsovo (Servier Pharmaceuticals) |
Relapse/refractory AML9 with an IDH1 mutation (2018) |
Frontline in AML patients with comorbidities (2019) IDH1-mutated cholangiocarcinoma (2021) |
Elevated 2-hydroxyglutarate Hypermethylation |
QT interval prolongation, IDH differentiation syndrome, anemia, thrombocytopenia | 8,91,216,253 |
| IDH2 |
Enasidenib (AG-221) |
Idhifa (Agios Pharmaceuticals) |
Relapse/refractory AML with an IDH2 mutation (2017) |
Elevated 2-hydroxyglutarate Hypermethylation |
Hyperbilirubinemia, thrombocytopenia, IDH differentiation syndrome |
216,253 | |
| Proteasome |
Bortezomib (PS-341) |
Velcade (Millennium/Takeda/Janssen Pharmaceutical) |
Relapse/refractory MM (2003) |
Proteasome mutation/overexpression Heat shock protein upregulation Autophagy Increased drug efflux Alterations in glutathione metabolism |
Peripheral neuropathy, hematologic toxicities, diarrhea, fatigue, dyspnea, zoster reactivation |
254,255 | |
| Proteasome |
Carfilzomib (PR-171) |
Kyprolis (Onyx Pharmaceuticals) |
Advanced MM (2012) |
Proteasome mutation Autophagy Increased drug efflux |
Hematologic toxicities, pneumonia, hyponatremia, fatigue, hypophosphatemia, infusion reactions, chest pain, heart failure |
254,255 | |
| Proteasome | Ixazomib (MLN2238) |
Ninlaro (Takeda) |
MM (2015) |
Proteasome mutation Autophagy |
Hematologic toxicities, fatigue, rash, decreased appetite, diarrhea, vomiting | 254,255 | |
| Bcl-2 |
Venetoclax (ABT-199) |
Venclexta (AbbVie/Genentech) |
CLL10 (2016) | AML (2018) |
BCL2 mutation Activation of the MAPK/Akt pathway Deregulation of energy metabolism Interaction with stromal cells |
Bone marrow suppression, nausea, vomiting, diarrhea | 8,256 |
| Smoothened | Vismodegib (GDC-0449) |
Erivedge (Genentech/Roche) |
BCC11 (2012) |
SMO mutations (e.g., D473H) SUFU/GLI2 copy number variation/mutation |
Muscle spasm, weight loss, alopecia, dysgeusia | 8,257 | |
| Smoothened |
Sonidegib (NVP-LDE225) |
Odomzo (Novartis) |
Locally advanced BCC (2015) |
SMO mutations SUFU/GLI2 copy number variation/mutation |
Nausea, dysgeusia, anorexia, muscle spasm, fatigue, creatine kinase elevation | 8,257 | |
| Smoothened |
Glasdegib (PF-04449913) |
Daurismo (Pfizer) |
AML (2018) |
SMO mutations SUFU/GLI2 copy number variation/mutation |
Thrombocytopenia, anorexia, peripheral edema, fatigue, neutropenia | 8,257 |
1DNMT: DNA methyltransferase.
2MDS: myelodysplastic syndrome.
3HDAC: histone deacetylase.
4CTCL: cutaneous T-cell lymphoma.
5PTCL: peripheral T-cell lymphoma.
6EZH2: enhancer of zeste homolog 2.
7MM: multiple myeloma.
8IDH: isocitrate dehydrogenase.
9AML: acute myeloid leukemia.
10CLL: chronic lymphocytic leukemia.
11BCC: basal cell carcinoma.
12ALCL: anaplastic large cell lymphoma.
Adverse effects and toxicity of molecular targeted therapy
Despite improved specificity for cancer cells, epidemiological studies have indicated that cancer patients who receive targeted therapy may experience various side effects and toxicity. The side effects of targeted therapy include asthenia, anorexia, dyspnea, diarrhea, nausea, vomiting, mucositis, skin rash, fever, hand-foot syndrome, fatigue, cardiotoxicity, hypertension, and bleeding49,50. Specifically, acneiform rash, a skin rash with an acne-like appearance, is a common side effect of anti-EGFR therapy50,51, and hypertension is a common side effect of bevacizumab and anti-VEGF receptor (VEGFR) therapy52. These common side effects are related to therapy response52. Severe toxicities, such as colitis, digestive perforation, toxic cardiomyopathy, pneumonitis/interstitial lung disease, acute respiratory distress syndrome, posterior reversible encephalopathy syndrome, necrotizing fasciitis, acute renal failure, and hypersensitivity, have been observed in patients receiving molecular targeted therapy, such as antiangiogenic agents, anti-EGFR therapy, and anti-HER2 therapy53. The side effects and toxicity of each molecular targeted therapy are summarized in Tables 2–6.
SMKIs and mAbs in targeted cancer therapy
By focusing on U.S. Food and Drug Administration (FDA)-approved kinase inhibitors, target kinases and examples of clinically used inhibitors are briefly introduced below.
Receptor tyrosine kinase inhibitors
Inhibitors targeting the EGFR family
The human EGFR family comprises four members of the ErbB lineage of proteins (ErbB1/EGFR, ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4)8,54,55. Except for HER2, due to its inability to bind ligand54, EGFR family members form homo- and heterodimers and are activated via binding of ligands, such as EGF, epiregulin, transforming growth factor-α (TGF-α), and neuregulins8,54,55. Approximately 25% of all types of breast cancer patients show HER2 gene amplification or overexpression56. EGFR kinase-activating mutations [e.g., exon 19 microdeletions and L858R point mutations in the cytoplasmic tyrosine kinase domain, truncation of extracellular domain (EGFRvIII)] as well as overexpression without genetic alterations may occur in solid tumors57–59. These genetic changes cause abnormal EGFR activation in a ligand-independent fashion60. Exon 19 microdeletions and L858R point mutations are commonly found in patients with non-small cell lung cancer (NSCLC), particularly in nonsmoking east Asian females59,61, and EGFRvIII is frequently observed in glioblastoma57–59. Additional EGFR mutations, including E884K, D761Y, T854A, and exon 20 insertion, have been detected in NSCLC and found to confer EGFR TKI resistance62.
Several EGFR TKIs have been developed over the past decades and are clinically used for treatment of patients with NSCLC harboring kinase-activating mutations (Table 2). Gefitinib and erlotinib are first-generation EGFR-TKIs8,63,64 that interact with the ATP-binding pocket of EGFR in either the active or inactive conformation26. Second-generation EGFR TKIs, such as afatinib and dacomitinib, are irreversible EGFR inhibitors that covalently bind to the ATP-binding pocket of EGFR8,65,66. Despite the great efficacy of first- and second-generation EGFR-TKIs in patients with kinase-activating mutations in EGFR64,67, the EGFR T790M mutation in exon 2068 is associated with acquired resistance to these first- and second-generation EGFR-TKIs67,69 (e.g., approximately half of NSCLC patients acquire resistance to first-generation EGFR-TKIs69). EGFR T790M provides advantages for the growth and survival of cancer cells69 and limits the therapeutic efficacy of EGFR TKIs through both steric hindrance and potentiated ATP binding62,69. Accordingly, EGFR TKIs targeting the T790M mutation have been developed and clinically utilized. Osimertinib, a third-generation EGFR TKI, inhibits EGFR kinase activity by forming a covalent bond with the cysteine-797 residue in the ATP-binding pocket and shows an approximately 200 times greater inhibitory effect on mutant EGFR [L858R or exon 19 deletion mutations additionally harboring T790M (L858R/T790M or exon19del/T790M)] than on wild-type EGFR70,71. Another third-generation EGFR TKI, lazertinib, is an orally available, CNS-penetrable, and irreversible EGFR TKI that inhibits EGFR T790M and kinase-activating mutations72. Despite the approval of these agents for clinical use, clinical trials evaluating recently developed EGFR TKIs, including canertinib (CI-1033, a pan-ErbB inhibitor), naquotinib (ASP8273, third-generation EGFR TKI), and rociletinib (CO-1686, third-generation EGFR TKI), have been discontinued owing to safety and risk/benefit issues73. Nonetheless, EGFR cysteine-797 mutation was found in 14% of NSCLC patients with acquired osimertinib resistance, leading to the development of fourth-generation EGFR TKIs74,75. Several fourth-generation EGFR TKIs (e.g., BLU-945, EAI045, and OBX02-011) that target EGFR T790M and EGFR C797S have been evaluated in preclinical and clinical settings74,76–78. Additionally, two inhibitors targeting EGFR exon 20 insertions, such as amivantamab and mobocertinib, have been recently approved for the treatment of patients with advanced NSCLC with progression after platinum-based chemotherapy79–81 (Table 2). SMKIs approved to date for clinical use in patients with HER2-positive breast cancer include lapatinib, neratinib, and tucatinib8,56,82. Lapatinib is an orally available TKI that reversibly interacts with the ATP-binding site of EGFR and HER283, and neratinib is an orally available agent that covalently binds to the ATP-binding site of the tyrosine kinase domain of EGFR and HER2, resulting in irreversible EGFR/HER2 inhibition84. Tucatinib is an orally available, selective, and reversible HER2 inhibitor that competitively interacts with the ATP-binding site of HER285. Several clinical trials for recently developed HER2-targeting TKIs are also ongoing86.
In addition to SMKIs, mAbs targeting EGFR and HER2 have been used in the clinic (Table 2). EGFR mAbs, including cetuximab and panitumumab, have been clinically used for treatment of patients with metastatic colorectal cancer87–89. HER2-targeting mAbs, such as trastuzumab and pertuzumab, are approved for clinical use in patients with HER2-positive breast cancer90. Recently, the HER2-bispecific antibody zanidatamab was approved for patients with HER2-expressing biliary tract cancers91, and several clinical trials for recently developed HER2-targeting monoclonal antibodies are ongoing86.
ALK inhibitors
ALK is an receptor tyrosine kinase (RTK) with structural homology to leukocyte tyrosine kinase (LTK), which belongs to the insulin receptor superfamily92. In normal tissues, ALK expression is predominant in the nervous system and is known to play an important role in physiological regulation of nervous system development and function92,93. Chromosomal rearrangement of the ALK gene and consequent generation of a fusion protein with a number of partner proteins, including echinoderm microtubule-associated protein-like 4 (EML4), nucleophosmin (NPM), tropomyosin 3 (TPM3), and tropomyosin 4 (TPM4), ALK gene amplification, or ALK mutations lead to overexpression of a constitutively activated ALK protein92. ALK alterations have been found in several types of cancer, such as anaplastic lymphoma, neuroblastoma, and NSCLC92. Approximately 3–7% of patients with NSCLC, especially for those with the adenocarcinoma subtype, have been reported to harbor ALK rearrangements; ALK mutations are mutually exclusive with KRAS and EGFR mutations94,95.
Several ALK inhibitors are currently available in the clinical setting (Table 2), and these drugs are approved for the treatment of NSCLC patients. Crizotinib, a first-generation ALK inhibitor, is an orally available ATP-competitive inhibitor that was clinically approved in 201195,96. Crizotinib was initially developed as a MET inhibitor; however, based on the inhibitory effect of crizotinib on ALK at pharmacologically relevant concentrations and the structural homology of the ATP-binding site between ALK and ROS1, the clinical efficacy of crizotinib has been evaluated in patients carrying alterations in these genes95,97. Consequently, crizotinib has been used as a first- or second-line therapy in patients with NSCLC harboring ALK, ROS1, or MET alterations96,98,99. However, due to the rapid emergence of resistance to crizotinib and its weak ability to penetrate the central nervous system (CNS)95,96,100, additional ALK inhibitors have been developed. The second-generation ATP-competitive ALK/ROS1 inhibitor ceritinib and the ATP-competitive ALK inhibitor alectinib have been approved for treatment of patients with crizotinib resistance96. In contrast to crizotinib and ceritinib, alectinib can penetrate the CNS, curing NSCLC patients with brain metastasis and preventing progression of CNS metastasis8,96. Additional blood‒brain barrier (BBB)-permeable ATP-competitive ALK TKIs have been developed, including brigatinib, which is effective against FMS-like tyrosine kinase 3 (FLT3), insulin-like growth factor receptor (IGF-1R), EGFR, and several ALK mutations associated with resistance to crizotinib, ceritinib, and alectinib101,102, and lorlatinib, with inhibitory effects against all recognized ALK mutations except the L1198F mutation8,92.
MET inhibitors
MET is an RTK activated by hepatocyte growth factor (HGF) and mediates several physiological processes, such as embryogenesis and tissue repair; aberrant activation of MET by genetic alterations plays an important role in the proliferation, invasion, and metastasis of tumor cells103. Alterations in the MET gene, such as amplification, mutation, and alternative splicing (MET exon 14 skipping), have been detected in NSCLC and other solid tumors8,99. MET overexpression is associated with poor prognosis and resistance to chemotherapeutic agents, including EGFR targeted therapy8,104. In addition, MET gene exon 14 skipping leads to constitutive activation of the MET signaling pathway and confers sensitivity to MET inhibitors105. MET inhibitors, such as orally available ATP-competitive small-molecule TKIs and monoclonal antibodies, have been developed and evaluated in preclinical and clinical trials99. Among them, capmatinib and tepotinib are approved for clinical use in treatment of patients with metastatic NSCLC harboring MET exon 14 skipping99 (Table 2).
TRK and FLT3 inhibitors
Neurotrophic tyrosine receptor kinases (NTRKs) are oncogenes that encode tropomyosin receptor kinase (TRK) proteins, including TRKA, TRKB, and TRKC106. TRKs are activated by binding of intrinsic neurotrophin ligands, such as nerve growth factor (NGF) for TRKA, brain-derived neurotrophic factor (BDNF) and neurotrophin 4 (NT-4) for TRKB, and neurotrophin 3 (NT-3) for TRKC106,107. NTRK gene fusion caused by chromosomal rearrangements of NTRK genes with various fusion partners drives ligand-independent, constitutive activation of TRKs, which has been found in a wide range of cancer types, including mammary analog secretory carcinoma, secretory breast carcinoma, and infantile fibrosarcoma106,107. FLT3 (CD135), a class III RTK, is exclusively expressed in hematopoietic stem and progenitor cell populations108. Constitutive activation of FLT3 kinase through internal tandem duplications (FLT3-ITD) or missense mutations in the FLT3 tyrosine kinase domain109 has been observed in approximately 30% of patients with acute myeloid leukemia (AML) and a normal karyotype109,110. Several TKIs targeting TRKs (e.g., larotrectinib and entrectinib) or FLT3-ITD (e.g., midostaurin, sorafenib, and gilteritinib) have been developed and approved for clinical use. Examples are listed in Table 2.
Inhibitors targeting PDGFR, VEGFR, or FGFR family receptors and Ret
Tumor angiogenesis is a hallmark of cancer. Several growth factors and their receptors, such as platelet-derived growth factor (PDGF)/PDGFR, vascular endothelial growth factor (VEGF)/VEGFR, fibroblast growth factor (FGF)/FGFR, stem cell factor (SCF)/c-Kit, glial cell line-derived neurotrophic factor (GDNF)-family ligands/rearranged during transfection (RET), and angiopoietin/Tie22,111, regulate the growth, differentiation and migration of cancer cells and angiogenic activities of vascular endothelial cells22,111. PDGFs are members of the ‘cysteine knot’ growth factor superfamily, the members of which contain at least three disulfide bridges and forms homo- or heterodimers112. Five types of PDGF dimers (PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD) have been identified, and these PDGFs transduce signals by binding to two isotypes of PDGFRs (PDGFR-α and PDGFR-β)113. PDGF-AA and PDGF-CC, ligands that bind to these PDGFRs with different affinities, have a high affinity for PDGFR-α, whereas PDGF-BB and PDGF-DD transduce signaling through PDGFR-β113. PDGFR-α plays both general and specific roles in the development of mesenchymal and fibroblastic cell compartments; PDGFR-β plays an important role in the formation of vascular mural cells, including vascular smooth muscle cells and pericytes113. Alterations in PDGFR-α and PDGFR-β are associated with vascular diseases and mesenchymal cell/fibroblast-driven pathological conditions, respectively113. Alterations in PDGFR-α, such as point mutations and amplification, exist in approximately 5% of patients with gastrointestinal stromal tumors (GISTs) and 5–10% of patients with glioblastoma multiforme114.
The VEGF family is composed of five glycoproteins, including VEGFA (VEGF), VEGFB, VEGFC, VEGFD (c-Fos-induced growth factor, FIGF), and placental growth factor (PIGF or PGF)115. VEGF is expressed as multiple alternative splicing isoforms, with pro- or antiangiogenic effects; among them, VEGF165 is the predominant proangiogenic isoform that is overexpressed in various solid tumors115,116. VEGF activates signal transduction by binding to VEGFR family receptors, VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4)25,115. VEGFR2 is primarily expressed in vascular endothelial cells, and VEGF/VEGFR2 signaling plays a crucial role in angiogenesis by controlling vascular permeability, proliferation, migration, and survival of vascular endothelial cells115,117. VEGF also stimulates vasculogenesis in tumors by recruiting bone marrow-derived hematopoietic progenitor cells and endothelial progenitor cells115. VEGFC and VEGFD bind VEGFR3 and regulate lymphangiogenesis, contributing to metastatic spread through the lymphatic system118. In addition to these angiogenic effects on vascular endothelial cells, VEGF exerts several tumor-promoting effects, such as increased cancer cell proliferation, migration, invasion, stemness119–121, immune suppression115, and premetastatic niche formation122.
The FGF family growth factors, comprising 18 members that are categorized into six subfamilies, activate signal transduction by binding to FGFRs123. Five FGFRs (FGFR1-FGFR5) are known123,124. FGFR1-FGFR4 possess tyrosine kinase activity; in contrast, FGFR5 lacks the intracellular tyrosine kinase domain but acts as a coreceptor of FGFR1 and modulates ligand-mediated signaling123–125. Heparan sulfate glycosaminoglycan (HSGAG) binds to both FGF and FGFR, protecting FGFs from degradation, stabilizing the interaction between ligand and receptor, and facilitating dimerization of FGF-bound FGFR123. In cancer cells, aberrant activation of FGF/FGFR signaling caused by FGFR amplification, activating FGFR mutations, FGFR single-nucleotide polymorphisms, FGFR fusion protein formation with various binding partners, and deregulation of phospholipase Cγ1 (PLCγ1, FRS1) and FGFR substrate 2 (FRS2) all promote cell survival, cell proliferation, angiogenesis, acquisition of an epithelial-mesenchymal transition (EMT) phenotype, invasion, and metastasis in cancer cells124,126.
Similar to the aforementioned RTKs, ligand binding causes receptor dimerization, autophosphorylation, and activation of colony-stimulating Factor 1 receptor (CSF-1R)/FMS, c-Kit/stem cell factor receptor (SCFR), RET, and Tie127–130. Binding of GDNF family ligands to coreceptor GDNF family receptors (GFRα 1–4) is required to stimulate RET kinase128. In cancer, these signaling pathways promote the proliferation, survival, migration, and invasion of cancer cells and angiogenesis127–130. Alterations in CSF-1R, c-Kit, RET, and Tie caused by overexpression, genetic mutations, gene rearrangement, and fusion protein formation have been found in various types of cancer, including clear cell renal cell carcinoma (RCC, CSF-1R), GIST (c-Kit), acute myeloid leukemia (c-Kit), thyroid cancer (RET), and breast cancer (Tie1)114,127,128,131,132.
The kinase domain of RET is similar to that of VEGFR2, and PDGFR-α/β, c-Kit, CSF-1R, VEGFR1/2/3, Flt3, Tek, and Tie protein kinases are regulated by a similar autoinhibitory brake mechanism133; multikinase inhibitors concurrently targeting these kinases have been developed and clinically utilized. Examples are sorafenib, sunitinib, pazopanib, lenvatinib, regorafenib, vandetanib, cabozantinib, axitinib, tivozanib, avapritinib, ripretinib, erdafitinib, pemigatinib, infigratinib, derazantinib, futibatinib, selpercatinib, and pralsetinib. Moreover, monoclonal antibodies (e.g., bevacizumab and ramucirumab) or recombinant proteins (e.g., aflibercept) have been used clinically134. Several clinically approved inhibitors targeting these RTKs and additional angiogenesis inhibitors are listed in Tables 2 and 3.
Table 3.
Monoclonal antibodies or recombinant proteins that inhibit angiogenesis modulators.
| Class (Target) | Generic name (Code name) | Brand name (Company) | First approved indication (Year) | Additional indication (selected) | Drug resistance mechanism (selected) | Side effects/toxicity (selected) | References |
|---|---|---|---|---|---|---|---|
|
Monoclonal antibody (VEGF) |
Bevacizumab |
Avastin (Genentech/Roche) |
Metastatic CRC1 with standard chemotherapy treatment (2004) |
Metastatic CRC with 5-FU-based therapy (second-line, 2006) Advanced nonsquamous NSCLC2 in combination with chemotherapy (2006) Metastatic RCC (2009) Recurrent GBM3 (2009) Metastatic cervical cancer (2014) Platinum-resistant recurrent ovarian cancer in combination with chemotherapy (2014) |
Activation of the proangiogenic pathway Adaptation of an alternative mode of vessel formation |
Bleeding, pulmonary hemorrhage, proteinuria, hypertension, would healing complications, cardiovascular toxicity, hypersensitivity | 229 |
|
Monoclonal antibody (VEGFR2) |
Ramucirumab (LY3009806, IMC-1121B) |
Cyramza (Eli Lilly) |
Advanced gastric cancer (2014) Aggressive NSCLC (2014) |
Metastatic colorectal cancer in combination with FOLFIRI5 (2015) HCC (2019) EGFR mutated metastatic NSCLC (2020) |
Neutropenia, thrombocytopenia, diarrhea, nausea, vomiting | 230 | |
|
Recombinant protein (VEGFs, VEGF-trap) |
Aflibercept |
Zaltrap, Eylea (Regeneron Pharmaceuticals) |
Eylea: Wet age-related Macular Degeneration (2011) Zaltrap: previously treated metastatic CRC (2012) |
Endophthalmitis, conjunctivitis, muscle volitantes, headache, arrythmia | 231 |
1CRC: colorectal cancer.
2NSCLC: non-small cell lung cancer.
3GBM: glioblastoma multiforme.
4HCC: hepatocellular carcinoma.
5FOLFIRI: drug combination containing 5-fluorouracil, leucovorin calcium (folic acid), and irinotecan hydrochloride.
Nonreceptor tyrosine kinase inhibitors
BCR-ABL and SFK inhibitors
Abelson (ABL) family kinases (ABL1 and ABL2) are nonreceptor tyrosine kinases that commonly contain a specific domain cassette consisting of the Src homology 3 (SH3) domain (a protein module that binds to proline-rich sequences), the SH2 domain (a protein module that binds to tyrosine phosphorylated sites), the tyrosine kinase domain (SH1 domain), the PXXP motif mediating interaction with SH3 domain-containing proteins, and the C-terminal F-actin binding domain135,136. ABL1, but not ABL2, additionally includes a DNA-binding domain, nuclear localization signal motifs, and nuclear export signal motif and mediates DNA damage repair135,136. ABL2 is mainly found at actin-rich sites, including focal adhesion and invadopodia in the cytoplasm, through its F-actin and microtubule-binding domains and mediates cytoskeletal remodeling135,136. Activation of ABL kinases is tightly regulated through autoinhibitory intramolecular interactions, intermolecular interactions with other proteins to disrupt or maintain autoinhibitory conformation, and posttranslational modifications such as trans- or Src-mediated tyrosine phosphorylation (e.g., activation of ABL1 by phosphorylation at Y245 and Y412), serine/threonine phosphorylation, acetylation, myristoylation, and polyubiquitination135,136. Oncogenic alterations in ABLs, including fusion protein formation caused by chromosome translocations in leukemia [e.g., BCR-ABL1 in Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML)] and amplification and somatic mutations in solid tumors, constitutively activate ABL-mediated signaling pathways and promote survival, proliferation, dedifferentiation, migration, and invasion in cancer cells135.
Several kinase inhibitors targeting the BCR-ABL fusion protein have been developed and used clinically (Table 4). Imatinib is an orally active first-generation BCR-ABL inhibitor. Imatinib is an ATP-competitive type II TKI that binds to the inactive conformation of the ABL kinase (DFG-out conformation137)135,137. Mutation in the ATP-interacting gatekeeper residue of the ATP-binding pocket (T315I) leads to maintenance of the active conformation of ABL and resistance to imatinib and related TKIs137. The amide substitution in the central aminophenyl ring of imatinib is crucial for tyrosine kinase inhibition, and the 6-methyl residue in the aminophenyl ring increases selectivity for BCR-ABL137. Due to the structural similarity among ABL, c-Kit, and PDGFRs (class III RTK)25, imatinib also inhibits PDGFR and c-Kit8,135,137,138. Second-generation BCR-ABL inhibitors have been developed and clinically utilized to overcome imatinib resistance caused by ABL kinase point mutations. Nilotinib is an ATP-competitive and orally active type II kinase inhibitor with greatly improved potency compared to imatinib137,138. Similar to imatinib, nilotinib inhibits the inactivated conformation of the ABL kinase, and resistance in the presence of BCR-ABL harboring the T315I mutation has been reported; however, nilotinib suppresses most imatinib-resistant BCR-ABL mutants and is not a substrate of drug influx/efflux transporters137–139. In addition, nilotinib displays inhibitory effects regarding activation of multiple kinases, such as c-Kit, PDGFR, the ABL-related kinase ARG, DDR1 kinase, oxidoreductase NQO2, and ephrin receptor EPHB4138. Bosutinib is an orally active and ATP-competitive dual SFK/ABL inhibitor135,137,138 showing similar inhibitory effects against mutated or amplified BCR-ABL associated with imatinib resistance137,140 and BCR-ABL harboring the T315I mutation137,140. Accordingly, bosutinib has been used for treatment of patients with Ph+ CML who are resistant to or intolerant of imatinib141. Other agents approved in the clinic include radotinib, an orally active second-generation BCR-ABL inhibitor that exhibits inhibitory effects on wild-type and some imatinib-resistant mutant forms of BCR-ABL and PDGFR8,142, and asciminib, an allosteric inhibitor that binds to the myristate pocket of BCR-ABL and is effective against T315I-mutant BCR-ABL143,144. Ponatinib, an orally available third-generation inhibitor against both wild-type and T315I-mutant BCR-ABL135,137,145, also displays inhibitory effects on the activity of multiple kinases, including FLT3, c-Kit, VEGFR, PDGFR, and Src145. Since 2012, ponatinib has been used for treatment of patients with T315I-positive CML (including accelerated phase, chronic phase, or blast phase) or those with T315I-positive Ph+ ALL145. Additional BCR-ABL-targeting inhibitors have been developed and evaluated preclinically and clinically137.
Table 4.
Nonreceptor tyrosine kinase inhibitors that have been clinically used for cancer treatment.
| Target | Generic name (Code name) | Brand name (Company) | First approved indication (Year) | Additional indication | Drug resistance mechanism (selected) | Side effects/toxicity (selected) | References |
|---|---|---|---|---|---|---|---|
|
BCR-ABL PDGFR c-Kit |
Imatinib (STI-571) |
Gleevec (Novartis) |
Ph+1 CML2 (2001) |
GIST3 (2012) Ph+ ALL4 (2013) |
BCR-ABL T315I mutation | Fatigue, rashes, fluid retention, bone pain, diarrhea | 8,135,137,138,145,232 |
|
BCR-ABL c-Kit PDGFR SFKs5 |
Dasatinib (BMS-354825) |
Sprycel (Bristol-Myers Squibb) |
Ph+ ALL (2006) | Ph+ CML with resistance to or intolerance of prior therapy including imatinib (2009) | BCR-ABL T315I mutation | Neutropenia, thrombocytopenia, diarrhea, rash, fluid retention | 8,137,138,141,232,233 |
|
BCR-ABL c-Kit PDGFR ARG DDR1 NQO2 EPHB4 |
Nilotinib (AMN107) |
Tasigna (Novartis) |
Ph+ CML with resistance or intolerance to existing therapies (2007) | BCR-ABL T315I mutation | Thrombocytopenia, myalgia, headache | 137–139,141 | |
|
BCR-ABL SFKs5 c-Kit PDGFR |
Bosutinib (SKI-606) |
Bosulif (Pfizer) |
Ph+ CML with resistance or intolerance to imatinib (2012) | BCR-ABL T315I mutation | Diarrhea, nausea, vomiting | 135,137,138,140 | |
|
BCR-ABL PDGFR |
Radotinib6 (IY-5511) |
Supect (Ilyang Pharmaceutical) |
CML (2012) | BCR-ABL T315I mutation | Thrombocytopenia, anemia, fatigue, asthenia, nausea, myalgia, pruritis | 8,142,234 | |
|
BCR-ABL FLT3 c-Kit VEGFR PDGFR Src |
Ponatinib (AP24534) |
Iclusig (ARIAD Pharmaceuticals) |
Resistant or intolerant CML and Ph+ ALL (2012) | BCR-ABL compound mutation at T315, E255 | Diarrhea, nausea, vomiting, headache | 135,137,145 | |
| BCR-ABL |
Asciminib (ABL001) |
Scemblix (Novartis) |
Ph+ CML (2021) | BCR-ABL mutation at A337, W464, P465, V468, I502 | Diarrhea, nausea | 143,144,232 | |
| BTK |
Ibrutinib (PCI-32765) |
Imbruvica (Pharmacyclics/AbbVie/Janssen) |
MCL7 (2013) |
CML (2014) Waldenström’s Macroglobulinemia (2015) CLL8 (first line) and SLL9 (2016) Relapsed/refractory MZL10 (2017) |
BTK C481S, T474I/M mutation | Atrial fibrillation, bleeding, hypertension, diarrhea, nausea, vomiting | 151,152,235,236 |
| BTK |
Acalabrutinib (ACP-196) |
Calquence (Acerta Pharma/ AstraZeneca) |
Relapsed/refractory MCL (2017) | Relapsed/refractory CLL (2019) | BTK C481S mutation | Atrial fibrillation, bleeding, hypertension, diarrhea, nausea, vomiting | 151,152,235,236 |
| BTK |
Zanubrutinib (BGB-3111) |
Brukinsa (BeiGene) |
MCL (2019) |
Waldenström’s Macroglobulinemia (2021) Relapsed/refractory MZL (2021) |
BTK C481S mutation | Diarrhea, nausea, vomiting | 151,152,235,236 |
| JAK |
Ruxolitinib (INC424) |
Jakafi (Incyte/Novartis) |
Myelofibrosis (2011) | Cytopenia, diarrhea, nausea, vomiting | 154,155,237 | ||
| JAK | Fedratinib (SAR302503, TG101348) |
Inrebic (Celgene/Bristol-Myers Squibb) |
Myelofibrosis (2019) | Diarrhea, nausea, vomiting | 154,155,237 |
1Ph+: Philadelphia chromosome-positive.
2CML: chronic myeloid leukemia.
3GIST: gastrointestinal stromal tumor.
4ALL: acute lymphocytic leukemia.
5SFKs: Src-family kinases.
6Approved in Republic of Korea.
7MCL: mantle cell lymphoma.
8CLL: chronic lymphocytic leukemia.
9SLL: small lymphocytic lymphoma.
10MZL: marginal zone lymphoma.
Src-family kinases (SFK: Blk, Fgr, Frk, Fyn, Hck, Lck, Lyn, Src, Yes, and Yrk) contain a conserved domain organization consisting of a myristoylated N-terminal segment (SH4 domain), followed by SH3, SH2, linker, and tyrosine kinase domains and a short C-terminal tail146,147. Among SFKs, Src, Fyn, and Yes are ubiquitously expressed; Hck, Lck, Lyn, Blk, Yrk, and Fgr are primarily expressed in hematopoietic cells and Frk-related kinases in epithelial-derived tissues. Similar to ABL, SFKs adopt an inactive conformation through autoinhibitory intramolecular interactions involving phosphorylation at Y527/Y530146. Dephosphorylation of Y527/Y530 causes destabilization of intramolecular interactions, leading to SFK activation by interaction with RTKs, G protein-coupled receptors, and focal adhesion kinase via its SH2 or SH3 domains and subsequent autophosphorylation at Y416/Y419146,147. Activated SFKs play a crucial role in cell proliferation, adhesion, migration, invasion, metastasis, angiogenesis, and therapeutic resistance in cancer and act as key nodes of multiple oncogenic signal transduction pathways147,148, indicating the potential of SFK targeting for efficacious anticancer therapeutic regimens. ABL in the active conformation is structurally similar to SFKs138,149, and dasatinib, which preferentially interacts with the active conformation of the ABL kinase domain135,137,138, shows inhibitory effects on SFKs149. Dasatinib targets multiple kinases, including c-Kit, PDGFR, and SFK (Src, Fgr, Fyn, Hck, Lck, Lyn, and Yes)138,146,149, but not BCR-ABL harboring the T315I mutation137. Currently, there are no clinically approved anticancer regimens targeting SFKs, and some clinical trials evaluating the effectiveness of SFK inhibitors are still ongoing150.
BTK and JAK inhibitors
BTK is a nonreceptor tyrosine kinase that plays an essential role in the development and function of B cells151. BTK contains five typical domains, including from the N-terminus to the C-terminus the pleckstrin homology (PH) domain required for binding to phosphatidylinositol lipids, the proline-rich TEC homology (TH) domain, a zinc-finger motif for optimal activity and stability of the protein, SH3 and SH2 domains, and the catalytic domain151,152. Antigen engagement by the B-cell receptor causes activation of BTK through phosphorylation at Y551 in the kinase domain by spleen tyrosine kinase (Syk), Lyn, or Src152, which leads to subsequent autophosphorylation at Y223 in the SH3 domain and activation of downstream signaling pathways, including phospholipase Cγ, mitogen-activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB), and Akt, leading to regulation of B cell survival, proliferation, differentiation, and antibody secretion151,152 Overexpression and hyperactivation of BTK have been observed in a number of non-Hodgkin B-cell malignancies, including chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and mantle cell lymphoma (MCL)151,152. The Janus kinase (JAK) family comprises the nonreceptor tyrosine kinases JAK1, JAK2, JAK3, and TYK2153,154. Cytokine binding to receptors leads to receptor dimerization and recruitment, trans-autophosphorylation, and activation of JAK, resulting in phosphorylation and activation of downstream signaling cascades such as phosphatidylinositol-3-kinase (PI3K)/Akt, MAPK, and signal transducer and activator of transcription (STAT) transcription factors153–155. Deregulation of JAK through hyperactivation and activating mutations (e.g., JAK2 V617F) has been reported in myeloproliferative neoplasms, including myelofibrosis154. Examples of clinically approved inhibitors targeting BTK (e.g., ibrutinib, acalabrutinib, and zanubrutinib) or JAK (e.g., ruxolitinib and fedratinib) are listed in Table 4.
Inhibitors targeting downstream signaling pathways: RAS inhibitor and serine/threonine kinase inhibitors
Activated tyrosine kinases trigger phosphorylation and activation of downstream signaling mediators that are mostly serine/threonine kinases. The main relevant downstream signaling pathways are the PI3K/Akt/mammalian target of rapamycin (mTOR) and RAS/RAF/MEK/ERK pathways. Alterations in several components of these pathways (e.g., RAS, RAF, MEK, and PI3K) have been found in various types of cancer and thus considered druggable targets156–158. Cyclins are also downstream effector molecules of these signaling cascades and play an important role in regulating cell cycle progression and various cellular processes, such as gene transcription, DNA damage repair, and metabolism, by associating with cyclin-dependent kinases (CDKs)159. Alterations in cyclins and CDKs have been observed in various cancer types, and several CDK inhibitors have been developed and approved for clinical use160. Examples of these targeted therapeutic drugs are described below.
RAS/RAF/MEK inhibitors
RAS is a guanine nucleotide-binding protein that plays an important role in cell proliferation and differentiation, and farnesylation of RAS by RAS farnesyltransferase (FTase) is crucial for RAS to associate with membranes and its transforming activity161. Mutations in RAS result in constitutive activation161. Among the three RAS isoforms (KRAS, HRAS, and NRAS), KRAS is the most frequently mutated isoform, and five mutations (G12D, G12V, G12C, G13D, and Q61R) are the most prominent RAS mutations observed in cancer patients156. Based on the important role of RAS FTase in the regulation of RAS transforming activity, several FTase inhibitors have been developed and evaluated, yet none of them have been clinically used because of limited efficacy162. Recently, a small molecule inhibitor targeting mutated KRAS (KRASG12C) was developed and approved for clinical use. Sotorasib is an orally available inhibitor that binds to inactive guanosine diphosphate (GDP)-bound KRAS via a covalent bond between the C12 residue and the acrylamide warhead and noncovalent bonds between the isopropylpyridine substituent and a cryptic pocket comprising H95, Y96, and Q99 residues; this results in inhibition of KRASG12C without affecting wild-type KRAS163,164. Another KRASG12C inhibitor, adagrasib (MRTX849), is under clinical trial evaluation165.
Activated RAS in the GTP-bound state leads to association of RAF proteins, causing formation of RAF homo- or heterodimers, RAF phosphorylation, and consequent activation of the downstream signaling mediators MEKs and ERKs157,166. Among the three isoforms of RAF (ARAF, BRAF, and CRAF), mutations in BRAF, especially at the V600 residue (e.g., V600E) in the activation loop, are frequently observed in several types of cancer, including melanoma, papillary thyroid cancer, and colorectal cancer157,166,167. Indeed, the V600E mutation, which causes RAS-independent activation of BRAF, accounts for more than 90% of BRAF mutation cases in cancer157,166,167. Thus far, three RAF inhibitors and three MEK inhibitors have been used for anticancer treatment. Currently available RAF inhibitors target monomeric V600E-mutant BRAF; thus, for dimeric RAF, inhibition of one protomer by the drug paradoxically leads to transactivation of the other protomer and downstream signaling157. Therefore, a combination of MEK inhibitors (e.g., vemurafenib plus cobimetinib, dabrafenib plus trametinib, and encorafenib plus binimetinib) has been clinically utilized168. Examples of clinically approved BRAF and MEK inhibitors are listed in Table 5.
Table 5.
Serine/threonine kinase inhibitors that have been clinically used for cancer treatment.
| Target | Generic name (Code name) | Brand name (Company) | First approved indication (Year) | Additional indication | Drug resistance mechanism (selected) | Side effects/toxicity (selected) | References |
|---|---|---|---|---|---|---|---|
| KRAS |
Sotorasib (AMG 510) |
Lumakras (Amgen) |
Locally advanced or metastatic NSCLC harboring G12C-mutant KRAS with at least one prior systemic therapy (2021) |
BRAF/RAS mutation KRAS G12V, G13D mutation |
Nausea, vomiting, diarrhea, elevated aminotransferase level, fatigue, arthralgia | 163,164,238 | |
| BRAF |
Vemurafenib (PLX4032) |
Zelboraf (Genentech) |
Melanoma harboring V600E-mutant BRAF (2011) | Advanced melanoma with BRAF mutation in combination with cobimetinib (2015) |
NRAS mutation CRAF overexpression secondary BRAF mutation MEK1/2 mutation |
Rash, diarrhea, fatigue, arthralgia | 157,167,168,239–241 |
| BRAF |
Dabrafenib (GSK2118436) |
Tafinlar or Rafinlar (Novartis/GlaxoSmithKline) |
Melanoma harboring V600E-mutant BRAF (2013) |
Advanced melanoma with BRAF mutation in combination with trametinib (2014) BRAF V600E-mutant metastatic NSCLC1 in combination with trametinib (2017) BRAF V600E-mutant anaplastic thyroid cancer in combination with trametinib (2018) |
NRAS mutation CRAF overexpression secondary BRAF mutation MEK1/2 mutation |
Rash, diarrhea, fatigue, arthralgia | 157,167,168,239–241 |
| BRAF |
Encorafenib (LGX818) |
Braftovi (Novartis/Array BioPharma) |
Unresectable or metastatic melanoma with BRAF mutations in combination with binimetinib (2018) | BRAF V600E-mutant metastatic CRC2 in combination with cetuximab (2020) |
NRAS mutation CRAF overexpression secondary BRAF mutation MEK1/2 mutation |
Rash, diarrhea, fatigue, arthralgia | 157,167,168,239–241 |
| MEK |
Trametinib (GSK1120212, JTP-74057) |
Mekinist (GlaxoSmithKline/Novartis) |
BRAF V600E-mutant advanced melanoma (2013) |
Advanced melanoma with BRAF mutation in combination with dabrafenib (2014) BRAF V600E-mutant metastatic NSCLC1 in combination with dabrafenib (2017) BRAF V600E-mutant anaplastic thyroid cancer in combination with dabrafenib (2018) |
RTK reactivation PI3K, STAT3 activation |
Rash, diarrhea, fatigue, arthralgia | 157,167,168,239–241 |
| MEK |
Cobimetinib (GDC-0973, RG7420) |
Cotellic (Genentech) |
Advanced melanoma with BRAF mutation in combination with vemurafenib (2015) |
RTK reactivation PI3K/STAT3 activation |
Rash, diarrhea, fatigue, arthralgia | 157,167,168,239–242 | |
| MEK |
Binimetinib MEK162, ARRY-162, ARRY-438162) |
Mektovi (Array Biopharma) |
Unresectable or metastatic melanoma with BRAF mutations in combination with encorafenib (2018) |
RTK reactivation PI3K/STAT3 activation |
Rash, diarrhea, fatigue, arthralgia | 157,167,168,239–241 | |
| MEK |
Selumetinib (AZD6244, ARRY-142886) |
Koselugo (Array Biopharma/ AstraZeneca) |
Neurofibromatosis type 1 plexiform neurofibroma (2020) |
RTK reactivation PI3K/STAT3 activation |
Rash, diarrhea, fatigue, arthralgia | 157,167,168,239–241 | |
| PI3Kδ |
Idelalisib (CAL-101, GS-1101) |
Zydelig (Gilead Sciences) |
Relapsed CLL3 (2014) | RTK reactivation | Hyperglycemia, rash, stomatitis, fatigue, nausea, diarrhea | 171,172,243,244 | |
|
PI3Kγ PI3Kδ |
Duvelisib (IPI-145, INK1197) |
Copiktra (Intellikine/ Secura Bio) |
Relapsed or refractory CLL, SLL4, and FL5 (2018) |
Isoform switching Akt/mTOR activation Loss of PTEN |
Hyperglycemia, rash, stomatitis, fatigue, nausea, diarrhea | 171,172,243,244 | |
|
pan-PI3K (p110α and p110δ) |
Copanlisib (BAY 80–6946) |
Aliqopa (Bayer) |
Relapsed or refractory FL (2017) | RTK reactivation | Hyperglycemia, rash, stomatitis, fatigue, nausea, diarrhea | 171,172,243,244 | |
| PI3Kα |
Alpelisib (NVP-BYL719) |
Piqray (Novartis) |
HR+ and HER2- advanced/metastatic breast cancer with a PIK3CA mutation with prior endocrine therapy (in combination with fulvestrant, 2019) |
RTK reactivation | Hyperglycemia, rash, stomatitis, fatigue, nausea, diarrhea | 171,172,243,244 | |
| mTOR |
Sirolimus (AY-22989, rapamycin) |
Rapamune (Pfizer) |
Lymphangioleiomyomatosis (2015) | Locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumor (2021) | RTK reactivation | Hyperglycemia, rash, stomatitis, fatigue, nausea, diarrhea | 171,172,243,244 |
| mTOR |
Temsirolimus (CCI-779) |
Torisel (Pfizer) |
Advanced RCC6 (2007) | RTK reactivation | Hyperglycemia, rash, stomatitis, fatigue, nausea, diarrhea | 171,172,243,244 | |
| mTOR |
Everolimus (RAD001) |
Afinitor (Novartis) |
RCC after failure of sunitinib or sorafenib treatment (2009) |
Advanced pancreatic neuroendocrine tumor (2011) HR+ and HER2- breast cancer for use in combination with exemestane (2012) Subependymal giant-cell astrocytoma (2012) |
RTK reactivation | Hyperglycemia, rash, stomatitis, fatigue, nausea, diarrhea | 171,172,243,244 |
| CDK4/6 |
Palbociclib (PD 0332991) |
Ibrance (Pfizer) |
Advanced or metastatic breast cancer (2015) | HR+ and HER2- metastatic breast cancer (2016) | CDK4/6 overexpression | Fatigue, nausea, diarrhea, vomiting | 8,173,245 |
| CDK4/6 |
Ribociclib (LEE011) |
Kisqali (Novartis) |
HR+ and HER2- metastatic breast cancer (2017) | CDK4/6 overexpression | Fatigue, nausea, diarrhea, vomiting | 8,174,245 | |
| CDK4/6 |
Abemaciclib (LY2835219) |
Verzenio (Eli Lilly) |
HR+ and HER2- advanced or metastatic breast cancer (2017) | HR+, HER2-, and node-positive early breast cancer with a high risk of recurrence and a Ki-67 score ≥20% (2021) | CDK4/6 overexpression | Fatigue, nausea, diarrhea, vomiting | 8,245,246 |
1NSCLC: non-small cell lung cancer.
2CRC: colorectal cancer.
3CLL: chronic lymphocytic leukemia.
4SLL: small lymphocytic lymphoma.
5FL: follicular lymphoma.
6RCC: renal cell carcinoma.
PI3K/mTOR inhibitors
The PI3K/Akt/mTOR pathway plays a central role in regulating cell proliferation, survival, growth, and metabolism158,169. Deregulation of the PI3K/Akt/mTOR pathway through mutation or amplification of PIK3CA (encoding the p110α subunit of PI3K), loss or inactivation of phosphatase and tensin homolog (PTEN), and hyperactivation of mTOR have been commonly found in various cancer types158,169 and related anticancer drug resistance158,170. Hence, inhibitors targeting PI3K, Akt, and mTOR have been evaluated in preclinical studies and clinical trials, and some inhibitors have been used clinically for cancer treatment.
Because of the specific expression of PI3K, p110γ, and p110δ subunits in the hematopoietic system, the association of the PI3K pathway with regulating B-cell receptor (BCR) signaling, and the undesirable toxicity of pan-PI3K or dual PI3K/mTOR inhibitors171,172, PI3K inhibitors that specifically target PI3Kδ or PI3Kγ have been employed for treatment of patients with lymphoma. Some mTOR inhibitors, especially rapamycin analogs (rapalogs) that form a complex with FK506-binding protein 12 (FKBP12) and inhibit mTORC1 (but not mTORC2) activity, have been approved for clinical use8. Additionally, ATP-competitive mTOR inhibitors have been developed and are under preclinical and clinical evaluation8. Examples of clinically utilized PI3K (e.g., idelalisib, duvelisib, copanlisib and alpelisib) and mTOR inhibitors (e.g., sirolimus, temsirolimus, and everolimus) are listed in Table 5.
CDK inhibitors
Among more than 20 members of CDK family proteins159, CDK4 and CDK6 (in complex with cyclin D) play a crucial role in promoting cell cycle progression by sequestering CDK inhibitors and inducing various proteins involved in cell cycle progression from G1 to S phase, DNA replication, chromatin structure, chromosome segregation, and the spindle assembly checkpoint through phosphorylation of various targets, including retinoblastoma protein (RB), and activating E2F-mediated transcription160. Hence, CDK4/6 has been considered attractive] for targeted anticancer therapy. Three CDK4/6 inhibitors have been used clinically for treatment of patients with HR-positive advanced breast cancer (Table 5). Palbociclib, ribociclib, and abemaciclib are orally available, reversible, and selective CDK4/6 inhibitors that have been used clinically in combination with an aromatase inhibitor for treatment of postmenopausal women with ER-positive and HER2-negative advanced or metastatic breast cancer8,173,174.
Other targeted anticancer agents
In addition to PARP inhibitors, other types of clinically used or recently approved targeted therapies, including epigenetic modulators (e.g., DNA methyltransferase inhibitors, histone deacetylase inhibitors, EZH2 inhibitors, and isocitrate dehydrogenase inhibitors), proteasome inhibitors, Bcl-2 inhibitors, and smoothened inhibitors, are summarized in Table 6.
PARP inhibitors
The PARP family plays a crucial role in regulating DNA repair processes upon the DNA damage response (DDR) and chromatin modulation175,176. PARP family proteins, especially PARP1 and PARP2, bind to DNA lesions and mediate poly-ADP ribosylation (PARylation) of chromatin and DNA damage response components, resulting in DNA repair by recruiting DNA repair effectors such as XRCC1175,176. After autoPARylation, PARP dissociates from DNA, and the DNA repair process is completed by recruitment of DNA repair proteins176. BReast CAncer gene 1 (BRCA1) and BRCA2 (BRCA1/2) are tumor-suppressor genes that play a key role in repair of double-strand DNA breaks via the conservative homologous recombination repair (HRR) process175,177. Mutations in BRCA1/2 genes have been found in some cancer types, including breast, ovarian, pancreatic, and prostate cancers177. Defects in BRCA function due to BRCA1/2 gene mutations cause loss of the HRR process and mediate the DNA repair process in a nonconservative manner, such as nonhomologous end joining, leading to DNA alteration175. As BRCA mutant cancer cells are vulnerable to blockade of the DNA repair process, treatment of BRCA-deficient cells with PARP inhibitors leads to unsustainable genomic instability and cancer cell death176. This synthetic lethal interaction between PARP blockade and BRCA1/2 mutation suggests a therapeutic strategy targeting PARP for treatment of cancer types harboring BRCA mutations. Based on these findings, some orally available PARP inhibitors, such as olaparib, rucaparib, niraparib, and talazoparib, have been clinically used for treatment of BRCA-mutated cancers, including ovarian, breast, and prostate cancers (Table 6)8,178. Additional investigations to evaluate the effectiveness of combinatorial treatment with chemotherapeutic agents, PI3K inhibitors, and anticancer immunotherapy have been conducted in preclinical and clinical settings178.
Summary and future perspectives in the development of molecular targeted therapy
Owing to advances in molecular diagnosis, genome-wide analysis, and in-depth understanding of cancer biology, numerous tyrosine kinase inhibitors have recently been developed, tested preclinically and clinically, and utilized for cancer treatment in the clinic. Nevertheless, poor efficacy, toxicity, and tumor relapse due to drug resistance are major obstacles for targeted therapy-based efficacious anticancer treatment. Therefore, further investigation is required to develop efficacious personalized targeted therapies that overcome drug resistance and reduce side effects and toxicity.
To this end, a fundamental template for drug discovery by identifying additional druggable targets through in-depth biochemical, genomic, and molecular studies and structural investigations is needed. Drug discovery with different chemical entities or modes of action is also necessary for the development of molecular targeted therapy. In addition to direct or allosteric modulation of cellular targets, strategies for indirect manipulation of cellular targets [e.g., posttranslational modification179 or targeted protein degradation using proteolysis-targeting chimera (PROTAC)180] based on biological and functional studies for cancer-specific modulation would be applicable. Furthermore, the development of small molecule inhibitors that concurrently block signaling pathways associated with cancer cell proliferation and drug resistance and design of optimized combinatorial therapeutic strategies using molecular targeted therapy, either alone or in combination with other types of anticancer therapy (e.g., chemotherapy and immune checkpoint inhibitors), would be of importance for increased efficacy, limited toxicity, and minimal drug resistance.
Because the side effects and toxicity of targeted therapy are mediated by nonspecific inhibition of the same target in normal cells10, strategies for cancer cell-specific targeting are also important. A relevant example is the recent development of KRASG12C inhibitors. Since the clinical failure of farnesyltransferase inhibitors, KRAS has been considered an undruggable target181. In a recent study utilizing the high reactivity of cysteine, compounds that covalently bind to KRAS via the mutated cysteine residue and allosterically inhibit GTP binding to KRAS were designed182; this approach can inhibit KRAS without occupying the GTP/GDP-binding pocket on the surface and achieve specificity for mutant KRAS beyond wild-type KRAS, thus avoiding the unfavorable effects caused by inhibition of wild-type KRAS182,183. Based on this innovative study and a better understanding of the crystal structure of mutant KRAS, several potent KRASG12C inhibitors have been developed and approved for clinical use183,184; agents targeting other types of mutant KRAS, such as KRASG12D, have also been developed and evaluated in preclinical settings185,186. Studies on molecular diagnosis and discovery of predictive biomarkers are necessary to properly select eligible populations for better efficacy and reduced toxicity183. Several newly developed approaches, such as next-generation sequencing technology187, whole-genome sequencing188, and machine learning189, can be applied to this end. In fact, artificial intelligence (AI)-based strategies190 are expected to be extensively utilized for the design of the structure and chemical synthetic procedures, identification of potential hits, prediction of pharmacokinetic profiles, assessment of side effects and toxicity, and drug repurposing.
Finally, emerging evidence has shown the role of the host microbiome in cancer development and progression, drug responsiveness, and therapy-induced side effects191,192. For example, the gut microbiome promotes the function of mutant p53 toward oncogenicity193 and modulates responsiveness to antitumor therapy such as anti-PD-1 immunotherapy194. A number of investigations into the influence of the gut microbiome on chemotherapy and anticancer immunotherapy are ongoing; however, the effect of the host microbiome on molecular targeted therapy remains elusive. Further studies are necessary to investigate the role of the host microbiome in the efficacy and toxicity of molecular targeted therapy and to identify key factors to develop safer and more efficacious therapeutic strategies based on microbiome-targeted therapy.
In summary, the present paper briefly reviews the current status of molecular targeted therapy and discusses future directions, providing novel therapeutic strategies with better efficacy and safety to improve the prognosis of cancer patients.
Acknowledgements
This research was supported by a grant from the National Research Foundation of Korea (NRF), the Ministry of Science and ICT (MSIT), Republic of Korea (No. NRF-2016R1A3B1908631).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ke X, Shen L. Molecular targeted therapy of cancer: the progress and future prospect. Front. Lab. Med. 2017;1:69–75. [Google Scholar]
- 3.Jemal A, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J. Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saini KS, Twelves C. Determining lines of therapy in patients with solid cancers: a proposed new systematic and comprehensive framework. Br. J. Cancer. 2021;125:155–163. doi: 10.1038/s41416-021-01319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usborne CM, Mullard AP. A review of systemic anticancer therapy in disease palliation. Br. Med. Bull. 2017;125:43–53. doi: 10.1093/bmb/ldx045. [DOI] [PubMed] [Google Scholar]
- 6.Lind MJ. Principles of systemic anticancer therapy. Medicine. 2016;44:20–24. [Google Scholar]
- 7.Jones R. Cytotoxic chemotherapy: clinical aspects. Medicine. 2016;44:25–29. [Google Scholar]
- 8.Zhong L, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021;6:201. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton P, Spicer J. Targeted therapy in cancer. Medicine. 2016;44:34–38. [Google Scholar]
- 10.Peters GJ. From ‘targeted therapy’ to targeted therapy. Anticancer Res. 2019;39:3341–3345. doi: 10.21873/anticanres.13476. [DOI] [PubMed] [Google Scholar]
- 11.Abraham J, Staffurth J. Hormonal therapy for cancer. Medicine. 2016;44:30–33. [Google Scholar]
- 12.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–658. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keefe DMK, Bateman EH. Potential successes and challenges of targeted cancer therapies. J. Natl Cancer Inst. Monogr. 2019;2019:lgz008. doi: 10.1093/jncimonographs/lgz008. [DOI] [PubMed] [Google Scholar]
- 14.Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur. J. Pharmacol. 2018;834:188–196. doi: 10.1016/j.ejphar.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Habeeb NW-A, et al. The use of targeted therapies for precision medicine in oncology. Clin. Chem. 2016;62:1556–1564. doi: 10.1373/clinchem.2015.247882. [DOI] [PubMed] [Google Scholar]
- 16.Valent P, et al. Paul Ehrlich (1854–1915) and his contributions to the foundation and birth of translational medicine. J. Innate Immun. 2016;8:111–120. doi: 10.1159/000443526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colomer R, et al. When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine. 2020;25:100487. doi: 10.1016/j.eclinm.2020.100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillman RO. Perceptions of Herceptin: a monoclonal antibody for the treatment of breast cancer. Cancer Biother. Radiopharm. 1999;14:5–10. doi: 10.1089/cbr.1999.14.5. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MH, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin. Cancer Res. 2002;8:935–942. [PubMed] [Google Scholar]
- 20.Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9:34. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhullar KS, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol. Cancer. 2018;17:48. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Cohen P, Cross D, Jänne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat. Rev. Drug Discov. 2021;20:551–569. doi: 10.1038/s41573-021-00195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer. 2018;17:58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roskoski R., Jr. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol. Res. 2016;103:26–48. doi: 10.1016/j.phrs.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Smaill JB, Ding K. New promise and opportunities for allosteric kinase inhibitors. Angew. Chem. Int. Ed. Engl. 2020;59:13764–13776. doi: 10.1002/anie.201914525. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto K, et al. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med. Chem. Lett. 2015;6:89–94. doi: 10.1021/ml500394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JY, Chen YP, Li YQ, Liu N, Ma J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol. Cancer. 2021;20:27. doi: 10.1186/s12943-021-01317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersh AE, et al. Targeted therapies: immunologic effects and potential applications outside of cancer. J. Clin. Pharmacol. 2018;58:7–24. doi: 10.1002/jcph.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat. Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 32.Miyake K, Karasuyama H. The role of trogocytosis in the modulation of immune cell functions. Cells. 2021;10:1255. doi: 10.3390/cells10051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matlung HL, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23:3946–3959. doi: 10.1016/j.celrep.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 34.Hsu YF, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol. Cancer. 2010;9:139. doi: 10.1186/1476-4598-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lizotte PH, et al. A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol. Res. 2018;6:1511–1523. doi: 10.1158/2326-6066.CIR-18-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donia M, et al. BRAF inhibition improves tumor recognition by the immune system: potential implications for combinatorial therapies against melanoma involving adoptive T-cell transfer. Oncoimmunology. 2012;1:1476–1483. doi: 10.4161/onci.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayeni D, et al. Tumor regression mediated by oncogene withdrawal or erlotinib stimulates infiltration of inflammatory immune cells in EGFR mutant lung tumors. J. Immunother. Cancer. 2019;7:172. doi: 10.1186/s40425-019-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis LM, Hicklin DJ. Resistance to targeted therapies: refining anticancer therapy in the era of molecular oncology. Clin. Cancer Res. 2009;15:7471–7478. doi: 10.1158/1078-0432.CCR-09-1070. [DOI] [PubMed] [Google Scholar]
- 39.Hou J, et al. Evolution of molecular targeted cancer therapy: mechanisms of drug resistance and novel opportunities identified by CRISPR-Cas9 screening. Front. Oncol. 2022;12:755053. doi: 10.3389/fonc.2022.755053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebecca VW, Herlyn M. Nongenetic mechanisms of drug resistance in melanoma. Annu. Rev. Cancer Biol. 2020;4:315–330. [Google Scholar]
- 41.Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the rosetta stone of therapy resistance. Cancer Cell. 2020;37:471–484. doi: 10.1016/j.ccell.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 43.Meador CB, Hata AN. Acquired resistance to targeted therapies in NSCLC: updates and evolving insights. Pharmacol. Ther. 2020;210:107522. doi: 10.1016/j.pharmthera.2020.107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, et al. The multi-molecular mechanisms of tumor-targeted drug resistance in precision medicine. Biomed. Pharmacother. 2022;150:113064. doi: 10.1016/j.biopha.2022.113064. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni Y, et al. The role of tumor-stroma interactions in drug resistance within tumor microenvironment. Front. Cell Dev. Biol. 2021;9:637675. doi: 10.3389/fcell.2021.637675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Duan C, Zhang C. E3 ubiquitin ligase in anticancer drugdsla resistance: recent advances and future potential. Front. Pharmacol. 2021;12:645864. doi: 10.3389/fphar.2021.645864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin S, et al. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020;5:228. doi: 10.1038/s41392-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roger S, et al. Adverse events of targeted therapies reported by patients with cancer treated in primary care. Eur. J. Gen. Pract. 2020;26:202–209. doi: 10.1080/13814788.2020.1846713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widakowich C, de Castro G, Jr., de Azambuja E, Dinh P, Awada A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007;12:1443–1455. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- 51.Fabbrocini G, Panariello L, Caro G, Cacciapuoti S. Acneiform rash induced by EGFR inhibitors: review of the literature and new insights. Ski. Appendage Disord. 2015;1:31–37. doi: 10.1159/000371821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Kurzrock R. Toxicity of targeted therapy: implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 2014;40:883–891. doi: 10.1016/j.ctrv.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Assoun S, Lemiale V, Azoulay E. Molecular targeted therapy-related life-threatening toxicity in patients with malignancies. A systematic review of published cases. Intensive Care Med. 2019;45:988–997. doi: 10.1007/s00134-019-05650-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roskoski R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin. Cancer Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 56.Dean-Colomb W, Esteva FJ. Her2-positive breast cancer: herceptin and beyond. Eur. J. Cancer. 2008;44:2806–2812. doi: 10.1016/j.ejca.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Pirker R, et al. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J. Thorac. Oncol. 2010;5:1706–1713. doi: 10.1097/JTO.0b013e3181f1c8de. [DOI] [PubMed] [Google Scholar]
- 58.Thomas R, Weihua Z. Rethink of EGFR in cancer with its kinase independent function on board. Front. Oncol. 2019;9:800. doi: 10.3389/fonc.2019.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo G, et al. Ligand-independent EGFR signaling. Cancer Res. 2015;75:3436–3441. doi: 10.1158/0008-5472.CAN-15-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ha SY, et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget. 2015;6:5465–5474. doi: 10.18632/oncotarget.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amelia T, Kartasasmita RE, Ohwada T, Tjahjono DH. Structural insight and development of EGFR tyrosine kinase inhibitors. Molecules. 2022;27:819. doi: 10.3390/molecules27030819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giaccone G. The role of gefitinib in lung cancer treatment. Clin. Cancer Res. 2004;10:4233s–4237s. doi: 10.1158/1078-0432.CCR-040005. [DOI] [PubMed] [Google Scholar]
- 64.Johnson JR, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin. Cancer Res. 2005;11:6414–6421. doi: 10.1158/1078-0432.CCR-05-0790. [DOI] [PubMed] [Google Scholar]
- 65.Li D, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau SCM, Batra U, Mok TSK, Loong HH. Dacomitinib in the management of advanced non-small-cell lung cancer. Drugs. 2019;79:823–831. doi: 10.1007/s40265-019-01115-y. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi Y, et al. EGFR T790M and C797S mutations as mechanisms of acquired resistance to dacomitinib. J. Thorac. Oncol. 2018;13:727–731. doi: 10.1016/j.jtho.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 69.Suda K, Onozato R, Yatabe Y, Mitsudomi T. EGFR T790M mutation: a double role in lung cancer cell survival? J. Thorac. Oncol. 2009;4:1–4. doi: 10.1097/JTO.0b013e3181913c9f. [DOI] [PubMed] [Google Scholar]
- 70.Cross DA, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Disco. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santarpia M, et al. Osimertinib in the treatment of non-small-cell lung cancer: design, development and place in therapy. Lung Cancer. 2017;8:109–125. doi: 10.2147/LCTT.S119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhillon S. Lazertinib: first approval. Drugs. 2021;81:1107–1113. doi: 10.1007/s40265-021-01533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah RR, Shah DR. Safety and tolerability of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in oncology. Drug Saf. 2019;42:181–198. doi: 10.1007/s40264-018-0772-x. [DOI] [PubMed] [Google Scholar]
- 74.Duggirala KB, Lee Y, Lee K. Chronicles of EGFR tyrosine kinase inhibitors: targeting EGFR C797S containing triple mutations. Biomol. Ther. 2022;30:19–27. doi: 10.4062/biomolther.2021.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leonetti A, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer. 2019;121:725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S, Song Y, Liu D. EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 2017;385:51–54. doi: 10.1016/j.canlet.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Schalm SS, et al. 1296P BLU-945, a highly potent and selective 4th generation EGFR TKI for the treatment of EGFR T790M/C797S resistant NSCLC. Ann. Oncol. 2020;31:S839. [Google Scholar]
- 78.Kim DS, et al. Abstract 5350: OBX02-011, a reversible fourth-generation EGFR-TKI, overcomes C797S-mediated resistance in non-small cell lung cancer. Cancer Res. 2022;82:5350. [Google Scholar]
- 79.Rivera-Concepcion J, Uprety D, Adjei AA. Challenges in the use of targeted therapies in non-small cell lung cancer. Cancer Res. Treat. 2022;54:315–329. doi: 10.4143/crt.2022.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kohler J, Janne PA. Amivantamab: treating EGFR Exon 20-mutant cancers with bispecific antibody-mediated receptor degradation. J. Clin. Oncol. 2021;39:3403–3406. doi: 10.1200/JCO.21.01494. [DOI] [PubMed] [Google Scholar]
- 81.Pacheco JM. Mobocertinib: a potential treatment for NSCLC with EGFR Exon 20 insertions. Cancer Disco. 2021;11:1617–1619. doi: 10.1158/2159-8290.CD-21-0379. [DOI] [PubMed] [Google Scholar]
- 82.Rusnak D, Gilmer TM. The discovery of lapatinib (GW572016) Mol. Cancer Ther. 2011;10:2019. doi: 10.1158/1535-7163.MCT-11-0697. [DOI] [PubMed] [Google Scholar]
- 83.Tsang RY, Sadeghi S, Finn RS. Lapatinib, a dual-targeted small molecule inhibitor of EGFR and HER2, in HER2-amplified breast cancer: from bench to bedside. Clin. Med. Insights: Ther. 2011;3:CMT.S3783. [Google Scholar]
- 84.Martin, M. & S. López-Tarruella. Emerging therapeutic options for HER2-positive breast cancer. Am. Soc. Clin. Oncol. Educ. Book, e64–e70 (2016). [DOI] [PubMed]
- 85.Kulukian A, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 2020;19:976–987. doi: 10.1158/1535-7163.MCT-19-0873. [DOI] [PubMed] [Google Scholar]
- 86.Chow LWC, Lie EF, Toi M. Advances in EGFR/HER2-directed clinical research on breast cancer. Adv. Cancer Res. 2020;147:375–428. doi: 10.1016/bs.acr.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Galizia G, et al. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26:3654–3660. doi: 10.1038/sj.onc.1210381. [DOI] [PubMed] [Google Scholar]
- 88.Li QH, et al. Anti-EGFR therapy in metastatic colorectal cancer: mechanisms and potential regimens of drug resistance. Gastroenterol. Rep. 2020;8:179–191. doi: 10.1093/gastro/goaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keating GM. Panitumumab: a review of its use in metastatic colorectal cancer. Drugs. 2010;70:1059–1078. doi: 10.2165/11205090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 90.Nami B, Maadi H, Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers. 2018;10:342. doi: 10.3390/cancers10100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho SM, Esmail A, Raza A, Dacha S, Abdelrahim M. Timeline of FDA-approved targeted therapy for cholangiocarcinoma. Cancers. 2022;14:2641. doi: 10.3390/cancers14112641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Della Corte CM, et al. Role and targeting of anaplastic lymphoma kinase in cancer. Mol. Cancer. 2018;17:30. doi: 10.1186/s12943-018-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webb TR, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev. Anticancer Ther. 2009;9:331–356. doi: 10.1586/14737140.9.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takeuchi K, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin. Cancer Res. 2008;14:6618–6624. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 95.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin. Cancer Res. 2015;21:2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC) Mol. Cancer. 2018;17:52. doi: 10.1186/s12943-018-0810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou HY, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 98.Shaw AT, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 2019;30:1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong L, Zhang J, Heymach JV, Le X. Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021;13:1758835921992976. doi: 10.1177/1758835921992976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ernani V, Stinchcombe TE. Management of brain metastases in non–small-cell lung cancer. JCO Oncol. Pract. 2019;15:563–570. doi: 10.1200/JOP.19.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naito T, Shiraishi H, Fujiwara Y. Brigatinib and lorlatinib: their effect on ALK inhibitors in NSCLC focusing on resistant mutations and central nervous system metastases. Jpn. J. Clin. Oncol. 2021;51:37–44. doi: 10.1093/jjco/hyaa192. [DOI] [PubMed] [Google Scholar]
- 102.Zhang S, et al. The potent ALK inhibitor Brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin. Cancer Res. 2016;22:5527–5538. doi: 10.1158/1078-0432.CCR-16-0569. [DOI] [PubMed] [Google Scholar]
- 103.Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin. Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 104.Turke AB, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frampton GM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Disco. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 106.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laetsch TW, Hong DS. Tropomyosin receptor kinase inhibitors for the treatment of TRK fusion cancer. Clin. Cancer Res. 2021;27:4974–4982. doi: 10.1158/1078-0432.CCR-21-0465. [DOI] [PubMed] [Google Scholar]
- 108.Kazi JU, Ronnstrand L. FMS-like tyrosine kinase 3/FLT3: from basic science to clinical implications. Physiol. Rev. 2019;99:1433–1466. doi: 10.1152/physrev.00029.2018. [DOI] [PubMed] [Google Scholar]
- 109.Perl AE. Availability of FLT3 inhibitors: how do we use them? Blood. 2019;134:741–745. doi: 10.1182/blood.2019876821. [DOI] [PubMed] [Google Scholar]
- 110.Kohlmann A, et al. Gene expression profiling in AML with normal karyotype can predict mutations for molecular markers and allows novel insights into perturbed biological pathways. Leukemia. 2010;24:1216–1220. doi: 10.1038/leu.2010.73. [DOI] [PubMed] [Google Scholar]
- 111.Crook T, et al. Angiogenesis inhibitors in personalized combination regimens for the treatment of advanced refractory cancers. Front. Mol. Med. 2021;1:749283. doi: 10.3389/fmmed.2021.749283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen PH, Chen X, He X. Platelet-derived growth factors and their receptors: structural and functional perspectives. Biochim. Biophys. Acta. 2013;1834:2176–2186. doi: 10.1016/j.bbapap.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013;11:97. doi: 10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat. Rev. Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 116.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat. Rev. Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br. J. Pharmacol. 2013;170:712–729. doi: 10.1111/bph.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scavelli C, Vacca A, Di Pietro G, Dammacco F, Ribatti D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia. 2004;18:1054–1058. doi: 10.1038/sj.leu.2403355. [DOI] [PubMed] [Google Scholar]
- 119.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr. Relat. Cancer. 2006;13:905–919. doi: 10.1677/erc.1.01221. [DOI] [PubMed] [Google Scholar]
- 120.Kong D, et al. VEGF-C mediates tumor growth and metastasis through promoting EMT-epithelial breast cancer cell crosstalk. Oncogene. 2021;40:964–979. doi: 10.1038/s41388-020-01539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao D, et al. VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene. 2015;34:3107–3119. doi: 10.1038/onc.2014.257. [DOI] [PubMed] [Google Scholar]
- 122.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 125.Regeenes R, et al. Fibroblast growth factor receptor 5 (FGFR5) is a co-receptor for FGFR1 that is up-regulated in beta-cells by cytokine-induced inflammation. J. Biol. Chem. 2018;293:17218–17228. doi: 10.1074/jbc.RA118.003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xie Y, et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020;5:181. doi: 10.1038/s41392-020-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liang J, et al. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int. J. Biol. Sci. 2013;9:435–443. doi: 10.7150/ijbs.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salvatore D, Santoro M, Schlumberger M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat. Rev. Endocrinol. 2021;17:296–306. doi: 10.1038/s41574-021-00470-9. [DOI] [PubMed] [Google Scholar]
- 129.Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell. Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- 130.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 131.Soares MJ, et al. CSF1R copy number changes, point mutations, and RNA and protein overexpression in renal cell carcinomas. Mod. Pathol. 2009;22:744–752. doi: 10.1038/modpathol.2009.43. [DOI] [PubMed] [Google Scholar]
- 132.Yang XH, Hand RA, Livasy CA, Cance WG, Craven RJ. Overexpression of the receptor tyrosine kinase Tie-1 intracellular domain in breast cancer. Tumor Biol. 2003;24:61–69. doi: 10.1159/000071078. [DOI] [PubMed] [Google Scholar]
- 133.Roskoski R., Jr. The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder. Pharmacol. Res. 2020;151:104567. doi: 10.1016/j.phrs.2019.104567. [DOI] [PubMed] [Google Scholar]
- 134.Yamaoka T, Kusumoto S, Ando K, Ohba M, Ohmori T. Receptor tyrosine kinase-targeted cancer therapy. Int. J. Mol. Sci. 2018;19:3491. doi: 10.3390/ijms19113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat. Rev. Cancer. 2013;13:559–571. doi: 10.1038/nrc3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci. Signal. 2010;3:re6. doi: 10.1126/scisignal.3139re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J. Hematol. Oncol. 2018;11:84. doi: 10.1186/s13045-018-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giles FJ, O’Dwyer M, Swords R. Class effects of tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2009;23:1698–1707. doi: 10.1038/leu.2009.111. [DOI] [PubMed] [Google Scholar]
- 139.Weisberg E, et al. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br. J. Cancer. 2006;94:1765–1769. doi: 10.1038/sj.bjc.6603170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Puttini M, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 141.Hochhaus A, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv41–iv51. doi: 10.1093/annonc/mdx219. [DOI] [PubMed] [Google Scholar]
- 142.Eskazan AE, Keskin D. Radotinib and its clinical potential in chronic-phase chronic myeloid leukemia patients: an update. Ther. Adv. Hematol. 2017;8:237–243. doi: 10.1177/2040620717719851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schoepfer J, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J. Med. Chem. 2018;61:8120–8135. doi: 10.1021/acs.jmedchem.8b01040. [DOI] [PubMed] [Google Scholar]
- 144.Hughes TP, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N. Engl. J. Med. 2019;381:2315–2326. doi: 10.1056/NEJMoa1902328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tan FH, Putoczki TL, Stylli SS, Luwor RB. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. Onco. Targets Ther. 2019;12:635–645. doi: 10.2147/OTT.S189391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 147.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14:667–678. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol. Sci. 2012;33:122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Das, J. et al. 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J. Med. Chem. 49, 6819–6832 (2006). [DOI] [PubMed]
- 150.Martellucci S, et al. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned so Far. Cancers. 2020;12:1448. doi: 10.3390/cancers12061448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances. Leukemia. 2021;35:312–332. doi: 10.1038/s41375-020-01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer. 2018;17:57. doi: 10.1186/s12943-018-0779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seavey MM, Dobrzanski P. The many faces of Janus kinase. Biochem. Pharmacol. 2012;83:1136–1145. doi: 10.1016/j.bcp.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 154.Lee HJ, Daver N, Kantarjian HM, Verstovsek S, Ravandi F. The role of JAK pathway dysregulation in the pathogenesis and treatment of acute myeloid leukemia. Clin. Cancer Res. 2013;19:327–335. doi: 10.1158/1078-0432.CCR-12-2087. [DOI] [PubMed] [Google Scholar]
- 155.Quintas-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin. Cancer Res. 2013;19:1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Prior IA, Hood FE, Hartley JL. The frequency of Ras mutations in cancer. Cancer Res. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yaeger R, Corcoran RB. Targeting alterations in the RAF-MEK pathway. Cancer Disco. 2019;9:329–341. doi: 10.1158/2159-8290.CD-18-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang J, et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol. Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 160.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cho KN, Lee KI. Chemistry and biology of Ras farnesyltransferase. Arch. Pharm. Res. 2002;25:759–769. doi: 10.1007/BF02976989. [DOI] [PubMed] [Google Scholar]
- 162.Wang J, Yao X, Huang J. New tricks for human farnesyltransferase inhibitor: cancer and beyond. Medchemcomm. 2017;8:841–854. doi: 10.1039/c7md00030h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang SS, Nagasaka M. Spotlight on Sotorasib (AMG 510) for KRAS (G12C) positive non-small cell lung cancer. Lung Cancer. 2021;12:115–122. doi: 10.2147/LCTT.S334623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Blair HA. Sotorasib: first approval. Drugs. 2021;81:1573–1579. doi: 10.1007/s40265-021-01574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Janne PA, et al. Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C) mutation. N. Engl. J. Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 166.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell. Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 167.Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020;13:113. doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koelblinger P, Thuerigen O, Dummer R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr. Opin. Oncol. 2018;30:125–133. doi: 10.1097/CCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu R, et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11:797. doi: 10.1038/s41419-020-02998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chauhan AF, Cheson BD. Copanlisib in the treatment of relapsed follicular lymphoma: utility and experience from the clinic. Cancer Manag. Res. 2021;13:677–692. doi: 10.2147/CMAR.S201024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128:331–336. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lu J. Palbociclib: a first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer. J. Hematol. Oncol. 2015;8:98. doi: 10.1186/s13045-015-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hortobagyi GN. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res. 2018;20:123. doi: 10.1186/s13058-018-1050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pilie PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin. Cancer Res. 2019;25:3759–3771. doi: 10.1158/1078-0432.CCR-18-0968. [DOI] [PubMed] [Google Scholar]
- 177.Lord CJ, Ashworth A. BRCAness revisited. Nat. Rev. Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 178.Rose M, Burgess JT, O’Byrne K, Richard DJ, Bolderson E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front. Cell Dev. Biol. 2020;8:564601. doi: 10.3389/fcell.2020.564601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meng F, Liang Z, Zhao K, Luo C. Drug design targeting active posttranslational modification protein isoforms. Med. Res. Rev. 2021;41:1701–1750. doi: 10.1002/med.21774. [DOI] [PubMed] [Google Scholar]
- 180.Bekes M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 2022;21:181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moore AR, Rosenberg SC, McCormick F, Malek S. RAS-targeted therapies: is the undruggable drugged? Nat. Rev. Drug Discov. 2020;19:533–552. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lietman CD, Johnson ML, McCormick F, Lindsay CR. More to the RAS story: KRAS(G12C) inhibition, resistance mechanisms, and moving beyond KRAS(G12C) Am. Soc. Clin. Oncol. Educ. Book. 2022;42:1–13. doi: 10.1200/EDBK_351333. [DOI] [PubMed] [Google Scholar]
- 184.Lim SM, et al. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew. Chem. Int. Ed. Engl. 2014;53:199–204. doi: 10.1002/anie.201307387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang X, et al. Identification of MRTX1133, a noncovalent, potent, and selective KRAS(G12D) inhibitor. J. Med. Chem. 2022;65:3123–3133. doi: 10.1021/acs.jmedchem.1c01688. [DOI] [PubMed] [Google Scholar]
- 186.Mao Z, et al. KRAS(G12D) can be targeted by potent inhibitors via formation of salt bridge. Cell Disco. 2022;8:5. doi: 10.1038/s41421-021-00368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McCutcheon JN, Giaccone G. Next-generation sequencing: targeting targeted therapies. Clin. Cancer Res. 2015;21:3584–3585. doi: 10.1158/1078-0432.CCR-15-0407. [DOI] [PubMed] [Google Scholar]
- 188.Udagawa C, Zembutsu H. Pharmacogenetics for severe adverse drug reactions induced by molecular-targeted therapy. Cancer Sci. 2020;111:3445–3457. doi: 10.1111/cas.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keshava N, et al. Defining subpopulations of differential drug response to reveal novel target populations. NPJ Syst. Biol. Appl. 2019;5:36. doi: 10.1038/s41540-019-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Luo Y, Peng J, Ma J. Next Decade’s AI-based drug development features tight integration of data and computation. Health Data Sci. 2022;2022:9816939. doi: 10.34133/2022/9816939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ting NL, Lau HC, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71:1412–1425. doi: 10.1136/gutjnl-2021-326264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ramirez-Labrada AG, et al. The influence of lung microbiota on lung carcinogenesis, immunity, and immunotherapy. Trends Cancer. 2020;6:86–97. doi: 10.1016/j.trecan.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 193.Kadosh E, et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586:133–138. doi: 10.1038/s41586-020-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gopalakrishnan V, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cells. 2018;7:212. doi: 10.3390/cells7110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Wekken AJ, et al. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit. Rev. Oncol. Hematol. 2016;100:107–116. doi: 10.1016/j.critrevonc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 197.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dungo RT, Keating GM. Afatinib: first global approval. Drugs. 2013;73:1503–1515. doi: 10.1007/s40265-013-0111-6. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Y, et al. The efficacy and toxicity of afatinib in advanced EGFR-positive non-small-cell lung cancer patients after failure of first-generation tyrosine kinase inhibitors: a systematic review and meta-analysis. J. Thorac. Dis. 2017;9:1980–1987. doi: 10.21037/jtd.2017.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shirley M. Dacomitinib: first global approval. Drugs. 2018;78:1947–1953. doi: 10.1007/s40265-018-1028-x. [DOI] [PubMed] [Google Scholar]
- 201.Reungwetwattana T, Rohatgi N, Mok TS, Prabhash K. Dacomitinib as first-line treatment for EGFR mutation-positive non-small cell lung cancer. Expert Rev. Precis. Med. Drug Dev. 2021;6:161–171. [Google Scholar]
- 202.Greig SL. Osimertinib: first global approval. Drugs. 2016;76:263–273. doi: 10.1007/s40265-015-0533-4. [DOI] [PubMed] [Google Scholar]
- 203.Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022;19:499–514. doi: 10.1038/s41571-022-00639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vogel WH, Jennifer P. Management strategies for adverse events associated with EGFR TKIs in non-small cell lung cancer. J. Adv. Pract. Oncol. 2016;7:723–735. [PMC free article] [PubMed] [Google Scholar]
- 205.Cetuximab approved by FDA for treatment of head and neck squamous cell cancer. Cancer Biol. Ther. 5, 340–342 (2006). [PubMed]
- 206.Peeters M, Karthaus M, Rivera F, Terwey JH, Douillard JY. Panitumumab in metastatic colorectal cancer: the importance of tumour RAS status. Drugs. 2015;75:731–748. doi: 10.1007/s40265-015-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Thomas M. Cetuximab: adverse event profile and recommendations for toxicity management. Clin. J. Oncol. Nurs. 2005;9:332–338. doi: 10.1188/05.CJON.332-338. [DOI] [PubMed] [Google Scholar]
- 208.Zhao B, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8:3980–4000. doi: 10.18632/oncotarget.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Boekhout AH, Beijnen JH, Schellens JH. Trastuzumab. Oncologist. 2011;16:800–810. doi: 10.1634/theoncologist.2010-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Howie LJ, et al. FDA approval summary: pertuzumab for adjuvant treatment of HER2-positive early breast cancer. Clin. Cancer Res. 2019;25:2949–2955. doi: 10.1158/1078-0432.CCR-18-3003. [DOI] [PubMed] [Google Scholar]
- 211.Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer. 2021;7:56. doi: 10.1038/s41523-021-00265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sudhan DR, et al. Hyperactivation of TORC1 drives resistance to the Pan-HER tyrosine kinase inhibitor neratinib in HER2-mutant cancers. Cancer Cell. 2020;37:183–199. doi: 10.1016/j.ccell.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat. Rev. Clin. Oncol. 2018;15:694–708. doi: 10.1038/s41571-018-0081-4. [DOI] [PubMed] [Google Scholar]
- 214.Fujino T, et al. Foretinib can overcome common on-target resistance mutations after capmatinib/tepotinib treatment in NSCLCs with MET exon 14 skipping mutation. J. Hematol. Oncol. 2022;15:79. doi: 10.1186/s13045-022-01299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cerchione C, et al. Safety of FLT3 inhibitors in patients with acute myeloid leukemia. Expert Rev. Hematol. 2021;14:851–865. doi: 10.1080/17474086.2021.1969911. [DOI] [PubMed] [Google Scholar]
- 216.Desikan SP, et al. Resistance to targeted therapies: delving into FLT3 and IDH. Blood Cancer J. 2022;12:91. doi: 10.1038/s41408-022-00687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Friedman R. The molecular mechanisms behind activation of FLT3 in acute myeloid leukemia and resistance to therapy by selective inhibitors. Biochim. Biophys. Acta Rev. Cancer. 2022;1877:188666. doi: 10.1016/j.bbcan.2021.188666. [DOI] [PubMed] [Google Scholar]
- 218.Goodman VL, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin. Cancer Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 219.Joosten SC, et al. Resistance to sunitinib in renal cell carcinoma: from molecular mechanisms to predictive markers and future perspectives. Biochim. Biophys. Acta. 2015;1855:1–16. doi: 10.1016/j.bbcan.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 220.Qin S, et al. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol. 2019;12:27. doi: 10.1186/s13045-019-0718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fallahi P, et al. Selective use of vandetanib in the treatment of thyroid cancer. Drug Des. Devel. Ther. 2015;9:3459–3470. doi: 10.2147/DDDT.S72495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bauer S, George S, von Mehren M, Heinrich MC. Early and next-generation KIT/PDGFRA kinase inhibitors and the future of treatment for advanced gastrointestinal stromal tumor. Front. Oncol. 2021;11:672500. doi: 10.3389/fonc.2021.672500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cowey CL. Profile of tivozanib and its potential for the treatment of advanced renal cell carcinoma. Drug Des. Devel. Ther. 2013;7:519–527. doi: 10.2147/DDDT.S31442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kommalapati A, Tella SH, Borad M, Javle M, Mahipal A. FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice. Cancers. 2021;13:2968. doi: 10.3390/cancers13122968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yue S, et al. FGFR-TKI resistance in cancer: current status and perspectives. J. Hematol., Oncol. 2021;14:23. doi: 10.1186/s13045-021-01040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jiang K, et al. GZD824 overcomes FGFR1-V561F/M mutant resistance in vitro and in vivo. Cancer Med. 2021;10:4874–4884. doi: 10.1002/cam4.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thein KZ, Velcheti V, Mooers BHM, Wu J, Subbiah V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer. 2021;7:1074–1088. doi: 10.1016/j.trecan.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lin JJ, Gainor JF. An early look at selective RET inhibitor resistance: new challenges and opportunities. Br. J. Cancer. 2021;124:1757–1758. doi: 10.1038/s41416-021-01344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Garcia J, et al. Bevacizumab (Avastin(R)) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 230.Young K, Smyth E, Chau I. Ramucirumab for advanced gastric cancer or gastro-oesophageal junction adenocarcinoma. Ther. Adv. Gastroenterol. 2015;8:373–383. doi: 10.1177/1756283X15592586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ha D, Choi SR, Kwon Y, Park HH, Shin JY. Pattern of adverse events induced by aflibercept and ranibizumab: a nationwide spontaneous adverse event reporting database, 2007–2016. Medicine. 2019;98:e16785. doi: 10.1097/MD.0000000000016785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Braun TP, Eide CA, Druker BJ. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell. 2020;37:530–542. doi: 10.1016/j.ccell.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Conchon M, Freitas CM, Rego MA, Braga Junior JW. Dasatinib - clinical trials and management of adverse events in imatinib resistant/intolerant chronic myeloid leukemia. Rev. Bras. Hematol. Hemoter. 2011;33:131–139. doi: 10.5581/1516-8484.20110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kim SH, et al. Efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 tyrosine kinase inhibitors. Haematologica. 2014;99:1191–1196. doi: 10.3324/haematol.2013.096776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematol. Am. Soc. Hematol. Educ. Program. 2020;2020:336–345. doi: 10.1182/hematology.2020000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Estupinan HY, et al. BTK gatekeeper residue variation combined with cysteine 481 substitution causes super-resistance to irreversible inhibitors acalabrutinib, ibrutinib and zanubrutinib. Leukemia. 2021;35:1317–1329. doi: 10.1038/s41375-021-01123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Talpaz M, Kiladjian JJ. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia. 2021;35:1–17. doi: 10.1038/s41375-020-0954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhao Y, et al. Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature. 2021;599:679–683. doi: 10.1038/s41586-021-04065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Proietti I, et al. Mechanisms of acquired BRAF inhibitor resistance in melanoma: a systematic review. Cancers. 2020;12:2801. doi: 10.3390/cancers12102801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sanchez JN, Wang T, Cohen MS. BRAF and MEK inhibitors: use and resistance in BRAF-mutated cancers. Drugs. 2018;78:549–566. doi: 10.1007/s40265-018-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther. Adv. Med. Oncol. 2015;7:122–136. doi: 10.1177/1758834014566428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kun E, Tsang YTM, Ng CW, Gershenson DM, Wong KK. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 2021;92:102137. doi: 10.1016/j.ctrv.2020.102137. [DOI] [PubMed] [Google Scholar]
- 243.Armaghani AJ, Han HS. Alpelisib in the treatment of breast cancer: a short review on the emerging clinical data. Breast Cancer. 2020;12:251–258. doi: 10.2147/BCTT.S219436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wright SCE, Vasilevski N, Serra V, Rodon J, Eichhorn PJA. Mechanisms of resistance to PI3K inhibitors in cancer: adaptive responses, drug tolerance and cellular plasticity. Cancers. 2021;13:1538. doi: 10.3390/cancers13071538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Li Z, et al. Mechanisms of CDK4/6 inhibitor resistance in luminal breast cancer. Front. Pharmacol. 2020;11:580251. doi: 10.3389/fphar.2020.580251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Royce M, et al. FDA approval summary: abemaciclib with endocrine therapy for high-risk early breast cancer. J. Clin. Oncol. 2022;40:1155–1162. doi: 10.1200/JCO.21.02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Awad MM, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N. Engl. J. Med. 2021;384:2382–2393. doi: 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005;10:176–182. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- 249.Kim N, et al. FDA approval summary: decitabine and cedazuridine tablets for myelodysplastic syndromes. Clin. Cancer Res. 2022;28:3411–3416. doi: 10.1158/1078-0432.CCR-21-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bondarev AD, et al. Recent developments of HDAC inhibitors: emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021;87:4577–4597. doi: 10.1111/bcp.14889. [DOI] [PubMed] [Google Scholar]
- 251.Fantin VR, Richon VM. Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin. Cancer Res. 2007;13:7237–7242. doi: 10.1158/1078-0432.CCR-07-2114. [DOI] [PubMed] [Google Scholar]
- 252.Julia E, Salles G. EZH2 inhibition by tazemetostat: mechanisms of action, safety and efficacy in relapsed/refractory follicular lymphoma. Future Oncol. 2021;17:2127–2140. doi: 10.2217/fon-2020-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McMurry H, Fletcher L, Traer E. IDH inhibitors in AML-promise and pitfalls. Curr. Hematol. Malig. Rep. 2021;16:207–217. doi: 10.1007/s11899-021-00619-3. [DOI] [PubMed] [Google Scholar]
- 254.Merin NM, Kelly KR. Clinical use of proteasome inhibitors in the treatment of multiple myeloma. Pharmaceuticals. 2014;8:1–20. doi: 10.3390/ph8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bennett, M. K., Pitson, S. M. & Wallington-Beddoe, C. T. In Resistance to Targeted Therapies in Multiple Myeloma (eds S. C. W. Ling & S. Trieu) 39–59 (Springer International Publishing, 2021).
- 256.Yue X, Chen Q, He J. Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int. 2020;20:524. doi: 10.1186/s12935-020-01614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Xie H, Paradise BD, Ma WW, Fernandez-Zapico ME. Recent advances in the clinical targeting of hedgehog/GLI signaling in cancer. Cells. 2019;8:394. doi: 10.3390/cells8050394. [DOI] [PMC free article] [PubMed] [Google Scholar]