Abstract
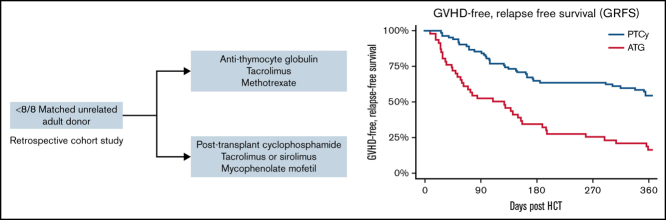
A common method to prevent graft-versus-host disease after allogeneic hematopoietic cell transplantation (HCT) from an HLA-mismatched unrelated donor (MMUD) is tacrolimus, methotrexate, and antithymocyte globulin (ATG). The use of posttransplant cyclophosphamide (PTCy) showed promise in a prospective trial for MMUD HCT. We compared 1-year graft-versus-host disease–free, relapse-free survival (GRFS) in 128 recipients of prophylaxis based on tacrolimus/methotrexate/ATG (ATG group, n = 46) vs PTCy, mycophenolate mofetil, and tacrolimus or sirolimus (PTCy group, n = 82) after MMUD HCT. Patients receiving HCT from a MMUD mismatched at ≥1 locus among HLA-A, HLA-B, HLA-C, and HLA-DRB1 were included. The 2 groups were well matched for HCT indication, high-risk disease, and HCT comorbidity index, whereas more patients on PTCy received bone marrow (50% vs 26%; P = .01) and >1 locus HLA-mismatched (30.5% vs 2.2%; P = .001) grafts. The 1-year GRFS was 16% (95% confidence interval (CI): 8%-31%) vs 54% (95% CI: 44%-66%; P < .001) in the ATG and PTCy groups, respectively. The multivariable adjusted hazard ratio for GRFS was 0.34 (95% CI: 0.21-0.55; P < .001) with the use of PTCy. The 1-year overall survival in the ATG group was 45% (95% CI: 32%-62%) vs 75% (95% CI: 66%-85%) in the PTCy group (P < .001). Relapse incidence was similar. One-year nonrelapse mortality was greater after ATG-based prophylaxis: 38% (95% CI: 23%-52%) vs 16% (95 CI: 9%-25%), P < .001. In summary, PTCy-based prophylaxis resulted in superior GRFS and overall survival in recipients of MMUD.
Key Points
-
•
PTCy improved GRFS compared with recipients of ATG-based prophylaxis after MMUD allogeneic HCT.
-
•
Allograft recipients of ATG-based prophylaxis had higher nonrelapse mortality and impaired humoral immune reconstitution.
Introduction
Outcomes after allogeneic hematopoietic cell transplantation (HCT) for hematologic malignancies improve with the use of HLA-matched sibling or unrelated donors (URDs) compared with the use of a donor mismatched at any of HLA-A, HLA-B, HLA-C, or HLA-DRB1.1,2 Because an HLA-matched sibling is available for only ∼30% of patients, URDs are often sought for transplantation.3 Variegations in HLA haplotype frequency among populations of different racial or ethnic backgrounds result in dramatically different likelihoods that a patient will find a suitable, HLA-matched URD based on their background.4 For example, among persons that self-describe as Euro-Caucasian, the probability of identifying an HLA-matched URD is 75%, compared with 15% to 45% in racial or ethnic minorities.5,6 Therefore, HLA-mismatched HCT is required for many adult patients, particularly those of non-European ancestry.
The most common transplant-specific complication of HLA-mismatched unrelated donor (MMUD) HCT is graft-versus-host disease (GVHD); however, the optimal strategy to prevent GVHD in this setting is unclear. The current standard of care is to administer either tacrolimus or cyclosporine with short-course methotrexate (MTX). In vivo T-cell depletion with peritransplant antithymocyte globulin (ATG) is performed in some patients undergoing MMUD HCT. For example, in Center for International Blood and Marrow Transplant Research (CIBMTR) analyses, among 7/8 HLA-matched URD recipients, ATG was used in 25% to 45% of recipients.7,8 In subjects treated for acute myeloid leukemia, the 1- and 3-year overall survival (OS) estimates were 45% and 34%, respectively, after HLA 7/8 matched URD HCT. In an analysis of patients with mixed disease histology, the 1- and 3-year OS estimates were 48.8% and 37.4%.
More recently, the use of posttransplant cyclophosphamide (PTCy) was first described in the context of HLA-haploidentical (haplo)-related donor recipients,9,10 and later in HLA-MMUD recipients.11,12,13 Encouraging results were described in a prospective study sponsored by the National Marrow Donor Program using PTCy with sirolimus and mycophenolate mofetil after bone marrow (BM)-derived MMUD grafts.12 Notably, many of these study subjects identified as racial or ethnic minorities. This study was not designed to determine whether a PTCy-based strategy improved outcomes compared with HCT with the use of a calcineurin inhibitor with MTX and ATG. Battipaglia et al14 examined this question in a retrospective analysis of patients undergoing MMUD that included HLA-DQB1–mismatched donor recipients; they found beneficial use of PTCy-based regimens in this more heterogeneous population.
The current study sought to compare transplant outcomes in subjects undergoing MMUD HCT using either “ATG-based” prophylaxis, including tacrolimus, short-course MTX, and peritransplant ATG, or a “PTCy-based” program of PTCy with either tacrolimus or sirolimus and mycophenolate mofetil. To minimize heterogeneity in the study population, we restricted this analysis to 2 centers with similar transplant volumes and peritransplant supportive care practices. Given that donor/recipient HLA-DQB1 mismatch variably associates with adverse outcomes in URD HCT, we limited this analysis to subjects undergoing transplant from a donor mismatched at ≥1 of HLA-A, HLA-B, HLA-C, or HLA-DRB1 alleles. The primary end point of this retrospective study was to compare 1-year GVHD-free, relapse-free survival (GRFS) in both cohorts, with similar analyses for the secondary end points of relapse, relapse-free survival (RFS), and OS.15 We also examined potential contributors to adverse outcomes after transplantation in this population.
Methods
Subject inclusion criteria and transplant methodology
Eligible subjects were adult recipients with high-risk hematologic malignancies who underwent HCT from an HLA-MMUD from 2010 to 2020. Donors mismatched at ≥1 locus among HLA-A, HLA-B, HLA-C, and HLA-DRB1 were included. Subjects who received HLA-matched donor transplant, HLA-mismatched-related donor transplant, or whose donor product underwent ex vivo graft manipulation (eg, CD34+ selection) were excluded (n = 6). Subjects were grouped according to GVHD prophylaxis as follows: the PTCy-based group included subjects treated with 50 mg/kg IV cyclophosphamide on days 3 and 4, post-allograft infusion, as previously described.9 All subjects received mycophenolate mofetil 10 to 15 mg/kg by mouth or IV, three times daily, from days 5 to 35, and dose-adjusted tacrolimus (goal trough level, 5-10 ng/mL) or sirolimus (goal trough level, 3-12 ng/mL) starting 5 days post-graft infusion, and were tapered between 3 and 6 months post-HCT. In this cohort, 30 subjects were treated on a prospective, multicenter study (#NCT02793544). The ATG group included patients receiving MTX 5 to 15 mg/m2 IV, on days 1, 3, 6, and 11 post-HCT, peritransplant dose-adjusted tacrolimus (noted here), and 5 to 7.5 mg/kg rabbit ATG, before allograft infusion.
GRFS was defined as a composite end point of death from any cause, relapse of malignancy, grade 3 to 4 acute GVHD, or chronic GVHD requiring systemic immune suppression therapy as previously described.15 GVHD grading was based on IBMTR and the National Institutes of Health chronic GVHD consensus criteria.16,17 Relapse was determined by using standard, disease-specific staging indices. Neutrophil engraftment was defined as the first day of neutrophil count >500/µL for 3 consecutive days, and platelet engraftment was defined as a platelet count ≥20 000/µL, without transfusion within 7 days. Clinically significant cytomegalovirus infection (csCMV) and Epstein-Barr virus infection were defined as blood viremia requiring therapy or visceral organ involvement. Subjects were treated according to institutional standards.18 Letermovir prophylaxis for CMV was instituted at both centers in 2018. Subjects were included from 2 centers: Memorial Sloan Kettering Cancer Center and University of Miami Sylvester Comprehensive Cancer Center. This research project was approved by the institutional review boards and privacy boards of both centers.
Statistical methodology
The study cohort was summarized by using descriptive characteristics, including medians, ranges, and frequencies. Differences in patient characteristics were compared across treatment groups by using χ2, Fisher's exact, or Wilcoxon rank sum tests. The primary end point of this retrospective study was to compare 1-year GRFS between the 2 treatment groups. Secondary end points were 1- and 2-year OS, RFS, nonrelapse mortality (NRM), cumulative incidence of relapse, and cumulative incidence of acute and chronic GVHD. Outcomes were measured from the time of HCT, and surviving patients were censored at their last follow-up. OS and RFS were estimated by using Kaplan-Meier methodology and compared by using the log-rank statistic. NRM and incidence of relapse were calculated by using cumulative incidence functions to account for the competing risk of death and were compared by using Gray's test. Univariable and multivariable cause-specific hazard ratios (HRs) were estimated by using Cox proportional hazards model. Variables deemed conceptually important were included in the univariable model: ATG vs PTCy, recipient's age, recipient and donor CMV serostatus, CIBMTR risk category, HCT comorbidity index, and graft source. Variables deemed significant on univariable analysis (P < .05) were included in the multivariable model.
All tests were two-sided. The type I error rate was fixed at 0.05 for the determination of factors associated with time-to-event outcomes. All regression models were stratified according to center. Statistical analyses were performed by using R version 4.0 (R Foundation for Statistical Computing).
Results
Subjects
Subject demographic characteristics are described in Table 1. The total number of subjects included was 128 (ATG, n = 46; PTCy, n = 82). Donors mismatched at >1 HLA locus were more common in recipients of PTCy-based (25 of 82) compared with ATG-based (1 of 46) prophylaxis. The use of fludarabine and melphalan-based conditioning was the most common platform in both groups, and myeloablative conditioning was similar in both groups. Most subjects underwent HCT in remission in either group. Disease histology was similar between groups (P = .1). The most common indication for transplant was acute leukemia in both groups (54% in ATG, 51% in PTCy) followed by myelodysplastic syndrome (24% in ATG, 26% in PTCy) and non-Hodgkin lymphoma (17% in ATG, 13% in PTCy). Patients who identified as a racial or ethnic minority comprised 46 (56%) of 82 patients in the PTCy group, and 28 (61%) of 46 patients in the ATG group. Female patients comprised 22 (48%) of 46 patients in the ATG group and 37 (45%) of 82 in the PTCy group. Tacrolimus was used in all patients in the ATG group; in the PTCy group, tacrolimus was used in 41 (50%) subjects, and sirolimus was used in 41 (50%) subjects.
Table 1.
Demographic characteristics of the study cohort
| Characteristic | Total (n = 128) | ATG (n = 46) | PTCy (n = 82) | P |
|---|---|---|---|---|
| Transplant center | ||||
| MSKCC | 51 (40%) | 13 (28%) | 38 (46%) | |
| UM SCCC | 77 (60%) | 33 (72%) | 44 (54%) | |
| Age, median (range), y | 60 (21-75) | 55 (21-72) | 60 (21-75) | .05 |
| HLA matching | <.001 | |||
| <7/8 | 26 (20%) | 1 (2.2%) | 25 (30.5%) | |
| 7/8 | 102 (80%) | 45 (98%) | 57 (70%) | |
| Myeloablative intensity | 60 (47%) | 26 (57%) | 34 (41%) | .1 |
| Disease status | .02 | |||
| Complete response | 93 (73%) | 30 (65%) | 63 (77%) | |
| Partial response | 11 (9%) | 7 (15%) | 4 (5%) | |
| Stable disease | 10 (8%) | 1 (2%) | 9 (11%) | |
| No remission | 14 (11%) | 8 (17%) | 6 (7%) | |
| Regimen | .1 | |||
| Busulfan based | 43 (34%) | 18 (39%) | 25 (31%) | |
| Fludarabine/Cy/TBI-200 | 27 (21%) | 5 (11%) | 22 (27%) | |
| Melphalan based | 44 (34%) | 16 (35%) | 28 (34%) | |
| TBI based | 14 (11%) | 7 (15%) | 7 (9%) | |
| HCT comorbidity index | .05 | |||
| 0-2 | 51 (40%) | 13 (28%) | 38 (46%) | |
| ≥3 | 77 (60%) | 33 (72%) | 44 (54%) | |
| Graft source | .008 | |||
| BM | 53 (41%) | 12 (26%) | 41 (50%) | |
| PB | 75 (59%) | 34 (74%) | 41 (50%) | |
| Recipient CMV-seropositive | 92 (72%) | 37 (80%) | 55 (67%) | .1 |
| CD34 dose, median (range) | 2.34 (0.08-10.8) | 2.38 (0.18-9.0) | 2.34 (0.08-10.8) | >.9 |
| CD3 dose, median (range) | 2.30 (0.07-21.8) | 3.81 (0.22-21.8) | 2.12 (0.07-9.5) | .006 |
| CIBMTR risk | .2 | |||
| High | 31 (24%) | 15 (33%) | 16 (20%) | |
| Intermediate | 50 (39%) | 18 (39%) | 32 (39%) | |
| Low | 47 (37%) | 13 (28%) | 34 (41%) | |
| Year of HCT | ||||
| 2010-2013 | 13 (10%) | 13 (28%) | 0 (0%) | |
| 2014-2017 | 38 (30%) | 18 (39%) | 20 (24%) | |
| 2018-2020 | 77 (60%) | 15 (33%) | 62 (76%) | |
| Follow-up, median (range), mo | – | 45.7 (3.7-106) | 27 (6.6-58.7) | |
MSKCC, Memorial Sloan Kettering Cancer Center; TBI, total body irradiation; UM SCCC, University of Miami Sylvester Comprehensive Cancer Center.
GRFS and secondary outcomes
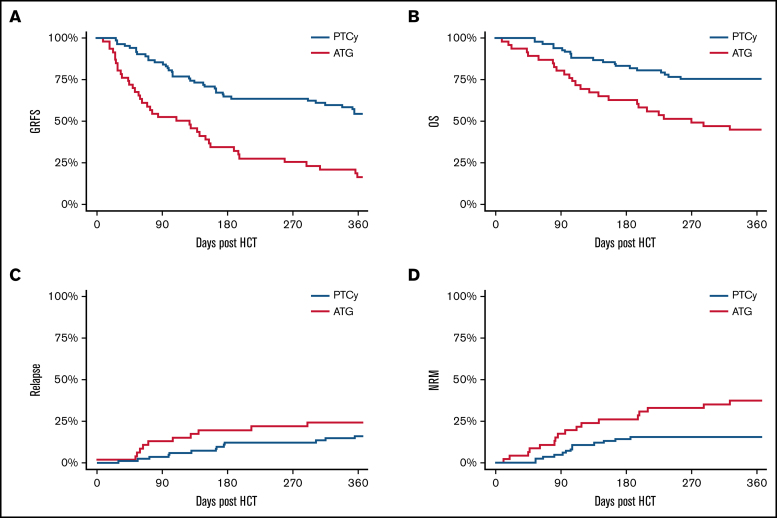
GRFS was superior in subjects receiving PTCy-based vs ATG-based prophylaxis (Figure 1; Table 2). In univariable analysis, CIBMTR disease risk score and graft source were also significantly associated with GRFS (univariable HRs are given in supplemental Table 1). Adjusting for these covariates, the HR for GRFS in PTCy recipients compared with ATG was 0.34 (95% confidence interval (CI): 0.21-0.55; P < .001) (Table 3). Point estimates for the likelihood of OS, RFS, relapse, and NRM are given in Table 2.
Figure 1.
GRFS, OS, relapse, and RFS in both cohorts. GRFS (A), OS (B), cumulative incidence of relapse (C), and cumulative incidence of death without relapse (D).
Table 2.
Point estimates of primary and secondary clinical outcomes
| Variable | ATG group (95% CI) | PTCy group (95% CI) | P |
|---|---|---|---|
| GRFS | |||
| 1 y, total | 0.16 (0.08-0.31) | 0.54 (0.44-0.66) | <.001 |
| 1 y, BM only | 0.08 (0.01-0.54) | 0.61 (0.48-0.78) | |
| 1 y, PB only | 0.19 (0.09-0.38) | 0.47 (0.34-0.66) | |
| OS | <.001 | ||
| 1 y | 0.45 (0.32-0.62) | 0.75 (0.66-0.85) | |
| 2 y | 0.29 (0.18-0.46) | 0.66 (0.56-0.78) | |
| Event-free survival | <.001 | ||
| 1 y | 0.38 (0.26-0.55) | 0.69 (0.59-0.80) | |
| 2 y | 0.27 (0.17-0.44) | 0.63 (0.53-0.75) | |
| Relapse | .3 | ||
| 1 y | 0.24 (0.13-0.38) | 0.16 (0.09-0.25) | |
| 2 y | 0.26 (0.15-0.4) | 0.19 (0.11-0.29) | |
| NRM | <.001 | ||
| 1 y | 0.38 (0.23-0.52) | 0.16 (0.09-0.25) | |
| 2 y | 0.47 (0.31-0.60) | 0.19 (0.11-0.28) | |
| Acute GVHD (grades 3-4) | |||
| 180 d, total | 0.31 (0.18-0.45) | 0.15 (0.08-0.23) | .03 |
| 180 d, BM only | 0.25 (0.05-0.52) | 0.10 (0.03-0.21) | |
| 180 d, PB only | 0.33 (0.17-0.49) | 0.20 (0.09-0.33) | |
| Chronic GVHD | |||
| 1 y, total | 0.22 (0.11-0.35) | 0.09 (0.04-0.17) | .03 |
| 1 y, BM only | 0.08 (0.00-0.33) | 0.10 (0.03-0.21) | |
| 1 y, PB only | 0.26 (0.13-0.42) | 0.08 (0.02-0.19) | |
Table 3.
Multivariable adjusted HRs for clinical outcomes
| Group | GRFS (95% CI) | P | OS (95% CI) | P | RFS (95% CI) | P | Relapse (95% CI) | P |
|---|---|---|---|---|---|---|---|---|
| ATG group | Ref | <.001 | Ref | <.001 | Ref | <.001 | Ref | .2 |
| PTCy group | 0.34 (0.21-0.55) | 0.36 (0.21-0.61) | 0.37 (0.23-0.61) | 0.62 (0.29-1.32) | ||||
Variables deemed significant on univariable analysis (CIBMTR disease risk and graft source) were included in the multivariable model.
Previous studies support an association between the use of peripheral blood (PB) grafts and increased incidence of GVHD.19,20 Similar to prior studies, we found that the median (interquartile range) CD3+ cell dose was greater in PB grafts than in BM grafts (4.07 [2.18-6.53] vs 1.39 [0.36-2.15]; P < .001). Because BM grafts were more frequently used in the PTCy arm, we compared the primary end point of GRFS separately in both subcohorts of patients based on graft source. When analyzed separately, PTCy resulted in improved GRFS in both BM graft recipients and PB graft recipients (Table 2; supplemental Figure 1). No interaction was found between graft source and GVHD prophylaxis group with respect to GRFS (P = .1), indicating that the magnitude of benefit in GRFS associated with the use of PTCy was similar in recipients of BM and PB graft sources. Graft source was not associated with increased risk of relapse (HR, 1.1; 95% CI: 0.5-2.4; P = .2).
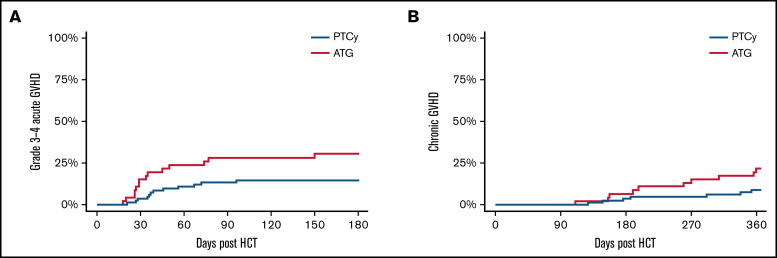
GVHD-specific outcomes are given in Figure 2 and Table 2. The cumulative incidence of grade 3 to 4 acute GVHD at day 180 post-HCT was 31% (95% CI: 18%-45%) vs 15% (95% CI: 8%-23%) in the ATG and PTCy groups, respectively (P = .03). The 1-year cumulative incidence of chronic GVHD requiring systemic immunosuppressive therapy was 22% (95% CI: 11%-35%) vs 9% (95% CI: 4%-17%) in the ATG and PTCy groups (P = .03). The highest risk for chronic GVHD was in the ATG group using a PB graft source, whereas the preventive benefit of PTCy in acute and chronic GVHD was more modest in BM graft recipients.
Figure 2.
Cumulative incidence of acute and chronic GVHD. Cumulative incidence of acute, grade 3 to 4 GVHD (A) and chronic GVHD requiring systemic immune suppressive therapy (B) based on GVHD prophylaxis.
We additionally examined the role of tacrolimus vs sirolimus as an adjunctive immunosuppressant in the PTCy group. One-year GRFS was similar in patients treated with PTCy/tacrolimus (55%; 95% CI: 41%-73%) compared with PTCy/sirolimus (53%; 95% CI: 40%-71%; P = .8). The 2-year OS, EFS, and relapse were also statistically similar between these 2 groups: 75% (95% CI: 60%-89%), 68% (95% CI: 52%-83%), and 23% (95% CI: 7%-37%), respectively, in PTCy/tacrolimus–treated subjects and 58% (43%-73%), 58% (95% CI: 43%-73%), and 15% (95% CI: 4%-26%) in PTCy/sirolimus–treated subjects (supplemental Figure 4). NRM was more frequent in subjects treated with PTCY/sirolimus (1-year NRM, 24% [95% CI: 13%-38%] vs 7% [95% CI: 2%-18%]; P = .05). There was no difference in grade 3 to 4 acute GVHD between these groups (12% [4%-24%] vs 17% [7%-30%]).
Analysis of HCTs performed after 2016
In this cohort, PTCy-based transplants occurred primarily during 2016 and later, whereas ATG-based transplants occurred at all time points. We did not appreciate an effect of HCT timing on GRFS on univariable analysis. To further address any potential bias that may arise from this time discrepancy, we compared the primary end point of GRFS in the subcohort of patients who underwent HCT from 2016 to 2020 (n = 112 [ATG, n = 33; PTCy, n = 79]). Here we found that the use of PTCy similarly resulted in an improved GRFS (56% [95% CI: 46%-69%] vs 16% [95 CI: 7%-36%]; P < .001). Furthermore, in the multivariable adjusted Cox model, the year of transplant did not associate with GRFS (HR, 0.99; 0.86-1.14; P = .9). These results suggest that transplant timing did not have a significant impact on the benefit of PTCy.
Engraftment, immune reconstitution, and causes of death
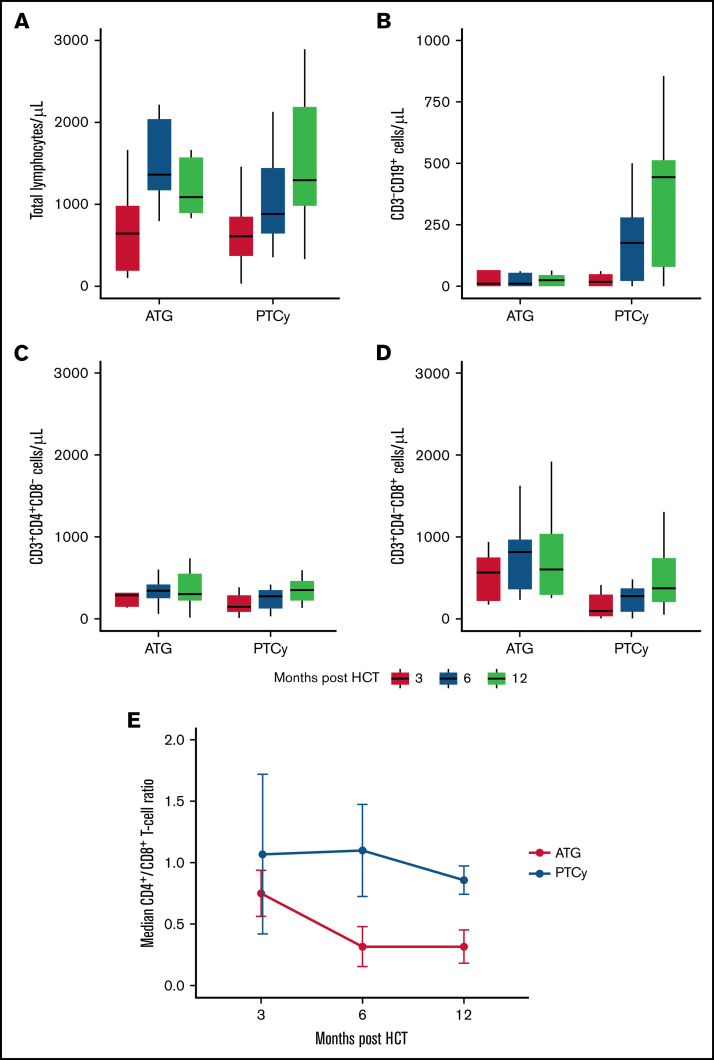
The rate of primary neutrophil engraftment at day 28 post-HCT was similar between the 2 treatment groups: 91% (95% CI: 77%-97%) in the ATG group and 91% (95% CI: 82%-96%) in the PTCy group (supplemental Figure 2). The median day of neutrophil engraftment was day 12 in the ATG group and day 18 in the PTCy group. Platelet engraftment at day 100 was 85% (95% CI: 70%-93%) and 87% (95% CI: 77%-92%) in the ATG and PTCy groups, respectively. We analyzed PB lymphocyte immune reconstitution (IR) in a subset of patients treated at Memorial Sloan Kettering Cancer Center (Figure 3) surviving at months 3, 6, and 12. Total lymphocyte IR and CD3+CD4+ T-cell IR were similar between both groups. However, early CD3–CD8+ T-cell IR was more rapid in ATG-treated patients (median, 566/µL in the ATG group compared with 97/µL in the PTCy group; Wilcoxon rank sum test, P = .02), leading to lower CD4+/CD8+ ratios in patients treated with ATG. Notably, CD3–CD19+ B-cell IR was delayed in subjects treated with ATG, whereas there was a linear B-cell IR in PTCy, resulting in improved B-cell counts at 1-year post HCT (median 25/µL vs 472/µL in the ATG- and PTCy-treated patients; P = .0003).
Figure 3.
Quantitative immune reconstitution in surviving subjects. Blood lymphocyte subsets were determined in a subcohort of patients treated at Memorial Sloan Kettering Cancer Center. Total lymphocyte reconstitution (A), CD3–CD19+ B-cell reconstitution (B), CD3+CD4+ T-cell reconstitution (C), CD3+CD8+ T-cell reconstitution (D), and median of the CD4+/CD8+ T-cell ratio at 3, 6, and 12 months post-HCT (E). Measurements in panels A to D are organized according to approximate time of collection post-HCT.
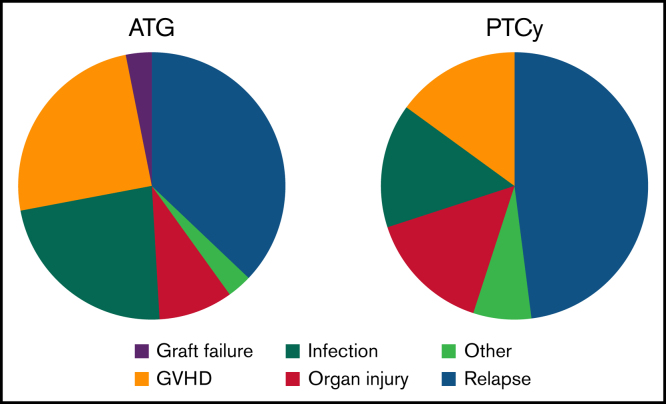
Among subjects who survived 3 months post-HCT and underwent engraftment analyses, full donor chimerism (≥95% donor) was achieved in 23 (92%) of 25 patients in the ATG group and in 61 (89.7%) of 68 patients in the PTCy group (supplemental Figure 3). To evaluate the etiology of increased NRM risk in the ATG arm, cause of death was examined in both groups (Figure 4). Relapse was the most frequent cause of death in both groups; however, GVHD and infection-related deaths were greater in those receiving ATG. The 1-year cumulative incidence of csCMV infection in seropositive recipients was 57% (95% CI: 41%-73%) in the ATG group compared with 26% (95% CI: 14%-37%; P = .0009) in the PTCy group (supplemental Figure 5).
Figure 4.
Causes of death. Relative causes of death in each study group.
To correct for potential confounding of greater letermovir use in the PTCy group, we examined the subcohort of patients who underwent allogeneic HCT during or before 2017 (N = 51). The cumulative incidence of csCMV infection at 1 year after allogeneic HCT was similarly 57% (95% CI: 36%-77%) in the ATG group compared with 30% (95% CI: 2%-58%) in the PTCy group (P = .1). The cumulative incidence of clinically significant Epstein-Barr virus infection was similar between groups (ATG group 5% [95% CI: 2%-20%] vs PTCy group 8% [95% CI: 1%-15%]; P = .4).
Analysis of outcomes in recipients of highly HLA-MMUD donor grafts
Based on the strength of data in HLA-haplo–related donor recipients, we routinely performed highly HLA-MMUD transplants at our centers, here defined as any donor mismatched at >1 HLA locus among HLA-A, HLA-B, HLA-C, or HLA-DRB1. Among the PTCy-treated patients, highly mismatched donors were used in 25 subjects (Table 1). Both class I and HLA-DRB1 mismatched donors were 16 of 25 (64%) compared with multiple class I mismatched donors in 9 of 25 (36%) of these subjects. We found that GRFS was similar between 7/8 and highly HLA-MMUD recipients (52% [95% CI: 36%-76%] vs 55% [95% CI: 43%-70%]; P = .5). Similarly, 1-year OS was comparable between 7/8-matched and highly mismatched HCT recipients (75% [95% CI: 65%-87%] vs 76% [95% CI: 61%-95%], respectively; P > .9).
Discussion
The results of the current study indicate that PTCy resulted in improved clinical outcomes after MMUD HCT despite use in an older population and among patients receiving HCT from donors mismatched at >1 HLA locus. PTCy should be favored as the primary mechanism to prevent GVHD in this setting, in the absence of randomized, prospective clinical trial data.
Strengths of the study cohort are that patients received both BM and PB grafts, as well as significant representation of recipients of highly HLA-mismatched grafts (20.3%). There were uniform immunosuppressive strategies within treatment arms and similar peritransplant supportive care practices between our centers. In addition, the study population included here is diverse, with significant representation of patients of racial and ethnic minority backgrounds (58%). These data are congruous with those from a recent multicenter, prospective study sponsored by the National Marrow Donor Program (15-MMUD). This study was a phase 2 trial showing safety and feasibility of the PTCy approach in BM graft MMUD HCT.12 Growing numbers of retrospective series also suggest superiority of PTCy compared with classic ATG-based in vivo T-cell depletion.14,21,22 Many of these analyses are limited by short follow-up, low representation of PTCy or marrow graft recipients, inclusion of HLA-DQB1 mismatch, incomplete patient race and ethnicity information, and variable adjunct immunosuppressive strategies. Importantly, prior reports were mostly restricted to single-antigen MMUD HCT recipient cohorts.
If related haplo donors are not available, an MMUD may be the sole available graft source for patients without an HLA-matched sibling. GVHD prevention programs in MMUD HCT based on calcineurin inhibitors and ATG were marred by relatively higher rates of NRM and worse OS.7 In addition, the use of donors mismatched at >1 HLA locus with this approach led to excessive GVHD and was not considered feasible.23,24 Although PTCy-based GVHD prophylaxis has overcome barriers to HLA-haplo–related donor HCT, limitations to haplo donor availability such as small family size, medical comorbidities, presence of donor-specific anti-HLA antibodies, or advanced donor age leave many patients without an available familial donor.25,26 Problems of donor availability disproportionately affect patients from racial or ethnic minority backgrounds, and these disparities have an impact on patient outcomes. A previous analysis conducted by the CIBMTR suggested that Euro-Caucasian patients with blood malignancies were more likely to proceed to HCT compared with African-American patients.27 Donor availability was one of several barriers identified in that study. Ciurea et al28 found that patients with uncommon HLA haplotypes, particularly those from minority backgrounds, had a low probability of finding an HLA-matched URD and a reduced likelihood of proceeding to HCT. The authors concluded that an extensive search for a matched URD may harm some patients. A prospective study sponsored by the Blood and Marrow Transplant Clinical Trials Network (#NCT03904134) is ongoing to address these questions in a multicenter setting.
In the context of this study, PTCy resulted in superior GRFS in recipients of both BM-derived and PB-derived allograft sources. In contrast to data reported by Bashey et al19 in haplo HCT with PTCy, we found that the choice of graft was not an independent predictor of GRFS (HR, 1.2; 95% CI: 0.8-1.9; P = .5) in a multivariable Cox regression model. The benefit of PTCy in prevention of chronic GVHD was more pronounced in subjects who received a PB graft source. Prospective examination of PTCy after a PB allograft from an MMUD is the subject of a forthcoming clinical trial sponsored by the National Marrow Donor Program. The finding of higher incidence of NRM in PTCy-treated patients with sirolimus compared with tacrolimus is unexplained in the context of this analysis. The 2-year OS and GRFS were 75% and 48%, respectively, in PTCy/tacrolimus–treated patients and 29% and 11% in ATG-treated patients. Given the size of the study cohort presented here, a larger registry-based analysis may have sufficient power to define whether tacrolimus or sirolimus should be preferred in combination with PTCy.
One-year survival in the PTCy arm was comparable to the survival reported in the 15-MMUD study, evaluating PTCy use in BM MMUD HCT and comparable to haplo PTCy-based HCT outcomes. These high survival rates are very encouraging, especially considering the significant representation by highly mismatched grafts, older age, high HCT comorbidity index, and active disease or partial response status before HCT. Similar to what was described in National Marrow Donor Program's 15-MMUD, a significant proportion of PTCy recipients received a highly HLA-mismatched graft. We did not appreciate a significant impact in GRFS comparing highly MMUD recipients vs those who received a single-antigen MMUD donor HCT. The flexibility to select highly HLA-mismatched donors while maintaining acceptable rates of NRM and GVHD suggests that PTCy can effectively expand HCT access to patients of racial and ethnic minority backgrounds without other suitable donor options.
The primary adverse outcome in both groups in this study remains relapse of the primary malignancy. Increased NRM and overall inferior clinical outcomes noted in our ATG cohort are in line with those published by others.14,22 Compared with the analysis conducted by Soiffer et al,29 including HLA matched and mismatched donors, we noted a higher incidence of NRM and lower incidence of relapse, consistent with the inclusion of only HLA mismatched donors here. We noted a greater incidence of csCMV infection in recipients of ATG-based prophylaxis, although this conclusion may be influenced by the greater use of letermovir in the PTCy group in this study population. Infections were a more frequent, relative cause of death in recipients of ATG-based GVHD prophylaxis, perhaps reflecting dysfunctional immune reconstitution. Increased early CD8+ T-cell IR was noted, suggesting that early infections may have skewed the T-cell subset IR. We additionally found limited B-cell IR in subjects treated with ATG. Quantitative differences in IR as well as differences in NRM could be attributed to higher rates of acute GVHD in the ATG arm. Further analysis of immune subsets in a larger cohort is necessary to clarify the impact of GVHD prophylaxis on quantitative IR.
There are some limitations to this study. First, the historical use of HLA MMUD before the advent of PTCy was known to confer relatively inferior outcomes and was reserved for patients perceived to have a higher disease risk index or higher risk remission status as noted in Table 1. We did not appreciate a significant difference in relapse between groups in this study; however, the PTCy group had significantly more patients undergoing transplant in complete response. The PTCy platform has been used more recently at both of our centers, leading to some differences in the timing of transplantation between cohorts; however, we found that the benefit of PTCy-based GVHD prophylaxis on GRFS was maintained, irrespective of era of treatment. Nevertheless, it is impossible to eliminate all sources of confounding, and thus these results should be interpreted with some caution. Ultimately, a randomized trial would be required to address this issue; however, given the strength of results with PTCy and other emerging therapies for GVHD prophylaxis such as abatacept, such a trial is unlikely to be feasible.
The current study suggests that HLA MMUD HCT is effective and feasible, particularly when combined with PTCy-based GVHD prophylaxis. Other emerging methodologies such as the use of abatacept (a selective inhibitor of T-cell costimulation), in conjunction with tacrolimus and MTX,30 have shown activity in GVHD prevention after minimally mismatched HCT and may be a viable candidate to test against or combined with PTCy in a prospective study. Furthermore, emerging data suggest that pharmacokinetic dosing of ATG may improve immune reconstitution and reduce NRM after this intervention.31 Although a randomized study of ATG-based vs PTCy-based GVHD prophylaxis is not likely to be feasible, combinatorial strategies using targeted ATG dosing with other agents such as abatacept or PTCy may represent novel pathways to prevent both acute and chronic GVHD.
Acknowledgments
The authors gratefully acknowledge the following funding sources: National Institutes of Health, National Heart, Lung, and Blood Institute (K23 HL140134-01A1, B.C.S.) and National Cancer Institute Cancer Center Support Grants (P30 CA008748, Memorial Sloan Kettering Cancer Center authors; P30 CA240139, University of Miami Sylvester Comprehensive Cancer Center authors), and Sylvester Comprehensive Cancer Center Intramural Funding (PG013790, A.J.J.).
Footnotes
The full-text version of this article contains a data supplement.
Authorship
Contribution: B.C.S. and A.J.J. designed the study, collected data, conducted the analysis, and authored the manuscript; M.-A.P. and K.K. designed the study, reviewed the data, and edited the manuscript; B.C.S., S.B., and S.D. reviewed the data and conducted the statistical analysis; and all coauthors contributed to data collection and reviewed the final manuscript.
Conflict-of-interest disclosure: B.C.S. reports consulting for Hansa Biopharma and Gamida Cell; and research funding from Miltenyi Biotec. A.J.J. reports research funding from Takeda and AbbVie. M.-A.P. reports honoraria from AbbVie, Astellas, Bristol Myers Squibb, Celgene, Equillium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, Orca Bio, Takeda, VectivBio AG, and Vor Biopharma; serves on Data and Safety Monitoring Boards for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, as well as the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; and has received research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis. C.S. reports consulting for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, and GSK; and research funding for clinical trials from Juno Therapeutics, Celgene/BMS, Bristol Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals, and Sanofi-Genzyme. D.P. reports consulting for Kadmon Corporation, Ceramedix, and Generon Corporation; and research funding from Takeda Pharmaceuticals. K.K. reports consulting for Kite/Gilead, Novartis, Celgene, Atara, Takeda, Autolus, Kiadis, Gamida Cell, Incyte, Kadmon, and Genentech. The remaining authors declare no competing financial interests.
Supplementary Material
References
- 1.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 2.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26(8):e177–e182. doi: 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68(9):779–788. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker JN, Boughan K, Dahi PB, et al. Racial disparities in access to HLA-matched unrelated donor transplants: a prospective 1312-patient analysis. Blood Adv. 2019;3(7):939–944. doi: 10.1182/bloodadvances.2018028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119(17):3908–3916. doi: 10.1182/blood-2011-09-381699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verneris MR, Lee SJ, Ahn KW, et al. HLA mismatch is associated with worse outcomes after unrelated donor reduced-intensity conditioning hematopoietic cell transplantation: an analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(10):1783–1789. doi: 10.1016/j.bbmt.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciurea SO, Zhang M-J, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasamon YL, Ambinder RF, Fuchs EJ, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1(4):288–292. doi: 10.1182/bloodadvances.2016002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. National Marrow Donor Program-sponsored multicenter, phase II trial of HLA-mismatched unrelated donor bone marrow transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2021;39(18):1971–1982. doi: 10.1200/JCO.20.03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Malki MM, Tsai N-C, Palmer J, et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv. 2021;5(12):2650–2659. doi: 10.1182/bloodadvances.2021004192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battipaglia G, Labopin M, Kröger N, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134(11):892–899. doi: 10.1182/blood.2019000487. [DOI] [PubMed] [Google Scholar]
- 15.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 18.Hakki M, Aitken SL, Danziger-Isakov L, et al. American Society for Transplantation and Cellular Therapy Series: #3 – prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(9):707–719. doi: 10.1016/j.jtct.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bashey A, Zhang M-J, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide [published correction appears in J Clin Oncol. 2019;37(6):528] J Clin Oncol. 2017;35(26):3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anasetti C, Logan BR, Lee SJ, et al. Blood and Marrow Transplant Clinical Trials Network Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta RS, Saliba RM, Chen J, et al. Post-transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br J Haematol. 2016;173(3):444–455. doi: 10.1111/bjh.13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nykolyszyn C, Granata A, Pagliardini T, et al. Posttransplantation cyclophosphamide vs. antithymocyte globulin as GVHD prophylaxis for mismatched unrelated hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55(2):349–355. doi: 10.1038/s41409-019-0682-2. [DOI] [PubMed] [Google Scholar]
- 23.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124(16):2596–2606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134(12):924–934. doi: 10.1182/blood.2019001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciurea SO, Cao K, Fernandez-Vina M, et al. The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the Detection and Treatment of Donor-specific Anti-HLA Antibodies (DSA) in Haploidentical Hematopoietic Cell Transplantation [published correction appears in Bone Marrow Transplant. 2019;54(5):784] Bone Marrow Transplant. 2018;53(5):521–534. doi: 10.1038/s41409-017-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perales M-A, Tomlinson B, Zhang M-J, et al. Alternative donor transplantation for acute myeloid leukemia in patients aged ≥50 years: young HLA-matched unrelated or haploidentical donor? Haematologica. 2020;105(2):407–413. doi: 10.3324/haematol.2018.215202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshua TV, Rizzo JD, Zhang MJ, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116(14):3469–3476. doi: 10.1002/cncr.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciurea SO, Bittencourt MCB, Milton DR, et al. Is a matched unrelated donor search needed for all allogeneic transplant candidates? Blood Adv. 2018;2(17):2254–2261. doi: 10.1182/bloodadvances.2018021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021;39(17):1865–1877. doi: 10.1200/JCO.20.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Admiraal R, Nierkens S, Bredius R, et al. Prospective open-label phase II trial of individualized anti-thymocyte globulin for improved T-cell reconstitution after pediatric allogeneic hematopoietic cell transplantation: the Parachute-Study. Biol Blood Marrow Transplant. 2020;26:S33–S34. suppl 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.