Abstract
In an effort to identify potential cytotoxins expressed by Neisseria gonorrhoeae, we have identified a locus that, when mutated in the gonococcus, results in a significant increase in toxicity of the strain to human fallopian tube organ cultures (HFTOC). This locus, gly1, contains two open reading frames (ORFs) which are likely cotranscribed. ORF1 encodes a polypeptide of 17.8 kDa with a signal sequence that is recognized and processed in Escherichia coli and N. gonorrhoeae. The 15.6-kDa processed polypeptide has been observed in membrane fractions and filtered spent media from cultures of E. coli expressing gly1 and in outer membrane preparations of wild-type N. gonorrhoeae. The gly1 locus is not essential for bacterial survival, and it does not play a detectable role in epithelial cell adhesion, invasion, or intracellular survival. However, a gly1 null mutant causes much more damage to fallopian tube tissues than its isogenic wild-type parent. A strain complemented in trans for the gly1 mutation showed a level of toxicity to HFTOC similar to the level elicited by the wild-type parent. Taken together, these results indicate an involvement of the gly1 locus in the toxicity of N. gonorrhoeae to human fallopian tubes.
Neisseria gonorrhoeae (gonococci [GC]) is a major sexually transmitted pathogen that infects only humans. The gram-negative diplococcus normally infects mucosa of the urogenital tract and causes urethritis or cervicitis that, in most cases, is easily treated with antibiotics. In women, gonococcal infection can lead to pelvic inflammatory disease, and damage to the epithelium of the fallopian tube from gonococcal salpingitis is a major cause of infertility.
There is no animal model for gonococcal infections. Studies with human volunteers are restricted to male subjects, limited by sample size, and examine only the initial events of gonococcal urethritis (8, 57). Most studies on gonococcal virulence have therefore relied on tissue (35, 51) and organ culture techniques. Studies with cells grown on solid support have yielded detailed information on bacteria-host receptor interactions (6, 7, 17, 19, 34, 52, 59), while the human fallopian tube organ culture (HFTOC) model, initially established by McGee et al. (29), has made possible the ex vivo analysis of selected early events of a gonococcal infection at this site (10, 28, 30, 32).
In vitro studies with tissue and organ culture have identified the key early events of gonococcal infection. GC attach to nonciliated epithelial cells via multiple adhesins, including pili (46, 56), the opacity protein (Opa, PII; 26, 60), and LOS (42). Following attachment to nonciliated cells of the epithelium, bacteria enter cells by an undefined mechanism. They transcytose the cell and exocytose through the basal membrane to enter the subepithelial stroma (30, 31, 35). They are usually localized at this site, where they initiate inflammation, although occasionally the bacteria enter the bloodstream to disseminate disease.
Damage to infected HFTOC tissues begins early, before GC enter cells (32). Shortly after bacterial colonization, the ciliary activity of neighboring cells diminishes and ciliated cells slough from the tissues (28, 31). These observations suggested that damage to ciliated cells may be brought about by toxic factors secreted by the bacteria. This toxicity was first reproduced by incubation of uninfected HFTOC with sterilized filtrates from GC-infected HFTOC (32) and later by purified lipopolysaccharide (LPS) (18) and peptidoglycan (33).
In this study, we report the identification of a locus that, when mutated in GC, results in a significant increase in toxicity to HFTOC tissues. We have characterized the locus, gly1, at the molecular level and have partially characterized one of the two proteins it encodes. gly1 null mutants were also examined for their effects on a number of human epithelial cell lines in culture. Possible roles for gly1 in the toxicity of the GC are discussed.
MATERIALS AND METHODS
DNA manipulations.
N. gonorrhoeae DNA isolation (47) and transformation (49) was as described previously. Southern blot analysis was performed essentially as previously described (48), except that DNA was transferred to Zeta Probe blotting membranes (Bio-Rad Laboratories, Richmond, Calif.) and cross-linked with a UV Stratalinker 2400 (Stratagene, La Jolla, Calif.) as per the manufacturer’s instructions. DNA probes were generated by the random priming method with the digoxigenin (DIG) nonradioactive DNA labelling and detection kit (Boehringer Mannheim, Indianapolis, Ind.). Transfer of shuttle plasmids from Escherichia coli to N. gonorrhoeae was done by the filter mating method described by Nassif et al. (40).
E. coli recombinant DNA manipulations were as described previously (27). Cloning vectors used were pHSS6 (50), pMGC18.1 (40), pET24a (Novagen, Madison, Wis.), and the pBluescript series (Stratagene). Restriction enzymes (New England Biolabs, Beverly, Mass.) and T4 DNA ligase (Boehringer Mannheim) were used according to the manufacturer’s recommendations. PCR was done with Taq DNA polymerase (Gibco BRL, Gaithersburg, Md.) in a thermocycler (model 9600; Perkin-Elmer Cetus, Norwalk, Conn.). DNA sequence determination was done by the Core Facility of the Department of Molecular Microbiology and Immunology at Oregon Health Sciences University with an ALF automated DNA sequencer (Pharmacia Biotech, Piscataway, N.J.).
Determination of the transcriptional start site.
Total RNA from CA201(pGly1) was isolated by acid guanidinium thiocyanate-phenol-chloroform extraction with the RNA STAT-60 reagent (Tel-Test “B”, Inc., Friendswood, Tex.). An oligonucleotide corresponding to the antisense strand of bp 817 to 835 (see Fig. 2) was synthesized and labelled at the 5′ end with fluorescein. The primer was annealed to the RNA and extended with Moloney murine leukemia virus reverse transcriptase (Gibco BRL), and the products were analyzed on an ALF automated DNA sequencer (Pharmacia) at the DNA Core Facility of the Molecular Microbiology and Immunology Department at Oregon Health Sciences University.
FIG. 2.
Nucleotide sequence of the 2,245-bp insert from pGly1. Important restriction sites are underlined and labelled. The predicted amino acid sequence is shown below the DNA sequence. Putative promoter (−10 and −35) and ribosome binding sites (rbs) are double underlined. The transcriptional start point is the bold-faced A at position 708 and is indicated as +1. The amino-terminal 20-amino-acid residues determined by protein sequencing are shown in bold type. The boxed region of ORF2 denotes the region of homology with hemD (see text). Neisserial uptake sequences (15) are indicated by broken underline. This sequence has been deposited in GenBank with accession no. AF003941.
Growth and construction of bacterial strains.
E. coli strains used were P90C (37) BL21λDE3 (55), JM109, and CA201. CA201 is a derivative of GC1 (36) that was constructed by P1 transduction of Δlacpro from P90C. E. coli was routinely grown in Luria broth (LB) supplemented as necessary with carbenicillin (CB) at 100 mg/liter, kanamycin (KN) at 100 mg/liter, or erythromycin (EM) at 300 mg/liter. Hemolysis of plasmid-bearing E. coli strains was determined by plating on LB agar containing 5% (vol/vol) washed sheep erythrocytes (RBC) (defibrinated; PML Microbiologicals, Tualitin, Oreg.). Plates were incubated at 37°C for 2 days to observe maximum hemolysis.
N. gonorrhoeae strains used were derivatives of MS11A (P+, Tr [47]) and were maintained in a humidified 5% CO2 atmosphere on GC agar (Difco, Detroit, Mich.) with supplements (20) or 1% (wt/vol) IsoVitaleX (BBL Microbiology Systems, Cockeysville, Md.). Antibiotics (EM [3 mg/liter] or KN [100 mg/liter]) were added as needed. Growth of GC in liquid was done in supplemented GC broth containing 0.042% NaHCO3. Construction of nonrevertible, otherwise-isogenic nonpiliated derivatives has been described elsewhere (35). Nonpathogenic Neisseria species were obtained from the American Type Culture Collection and cultured essentially the same way as N. gonorrhoeae strains.
Protein preparation and analysis.
Bacterial cell fractionations were based on the methods described in Nikaido (41) and performed as follows. Cells from approximately 2 × 1010 CFU were chilled on ice, harvested by centrifugation, and resuspended in 0.7 ml of 0.2 M Tris (pH 8.0)-0.5 M sucrose. A total of 37.5 μl of 10 mM EDTA and 75 μl of lysozyme (3 mg/ml in 50 mM Tris, pH 8.0) were added, and samples were mixed gently and incubated at room temperature for 10 min to allow spheroplasts to form. A total of 0.7 ml of 0.2 M Tris-0.5 mM EDTA, protease inhibitors (1 mM phenylmethylsulfonyl fluoride and 0.5 μg of leupeptin per ml), and 10 μl of 1 M MgSO4 were added, and samples were held on ice for 15 min. Samples were microcentrifuged for 15 min. Supernatants (periplasm) were decanted, and pellets were resuspended in 100 μl of 0.2 M Tris (pH 8.0) and 20 μl of DNase (5 mg/ml) and incubated at 37°C for 10 min. Samples were next microcentrifuged for 30 min, and supernatants (cytoplasm) were decanted. Total membranes were resuspended in 150 μl of 2% Triton X-100 in 50 mM Tris (pH 8.0)-10 mM MgCl2, and samples were incubated for 30 min at room temperature with gentle agitation. Outer membranes were pelleted by microcentrifugation for 10 min. The supernatant (inner membranes) was decanted, and outer membranes were washed with 1 ml of H2O and resuspended in 100 μl of H2O. E. coli culture filtrates (CF) were obtained by removing bacteria from overnight cultures by centrifugation, followed by filtration of culture supernatants through 0.22-μm-pore-size filters and precipitation of proteins with acetone.
N. gonorrhoeae outer membrane (OM) blebs were prepared by scraping eight plates of bacteria grown for 18 to 20 h into 25 ml of LiAc buffer (0.2 M lithium acetate [Sigma Chemical Co., St. Louis, Mo.], 5 mM EDTA [pH 6.0]). Samples were shaken at 37°C overnight. Blebs were sheared off by passage through 22-gauge needles six times. Bacteria were removed by centrifugation twice, and blebs were precipitated by ultracentrifugation at 100,000 × g for 3 h. Aliquots were precipitated with 10% trichloroacetic acid and resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer for electrophoresis.
Protein concentrations were determined by the bicinchoninic acid method (Pierce, Rockford, Ill.) or by the Bradford method (Bio-Rad), depending on the presence of reducing agents and detergents in the samples. Proteins were separated by SDS-PAGE (22) and stained with Coomassie blue or transferred to nitrocellulose for immunoblots.
Immunoblot analysis was done in phosphate-buffered saline (PBS) with nonfat dry milk at 10% (wt/vol) for blocking and 2% for antibody incubations. The secondary antibody, goatαrabbit conjugated to alkaline phosphatase, was used according to the manufacturer’s instructions (Pierce). Detection was carried out with the colorimetric substrates BCIP (5-bromo-4-chloro-3-indoyl-phosphate) and nitroblue tetrazolium.
Protein sequence analysis.
Amino-terminal protein analysis was performed on proteins excised by SDS-PAGE. Excised bands were blotted onto polyvinylidene difluoride membranes (Bio-Rad) and sequenced on an Applied Biosystems model 475A protein sequencer.
Production of antibodies.
BL21λDE3 cells harboring pGly1ORF1-His (see Results) were grown to mid-logarithmic phase in LB Kan100 broth and induced with 400 μM isopropyl-β-d-thiogalactopyranoside (IPTG) overnight with aeration at 37°C. Cells were removed by centrifugation, followed by filtration through a 0.45-μm-pore-size filter. Proteins in the culture filtrate were precipitated with (NH4)2SO4, resuspended in PBS, and dialyzed against buffer I (50 mM NaPO4 [pH 8.0]-300 mM NaCl). Proteins were loaded onto a Ni-NTA agarose column (Qiagen, Chatsworth, Calif.) equilibrated with buffer I. The column was first washed with 5 column volumes of buffer I, followed by 5 column volumes of buffer I adjusted to pH 6.0, and then eluted with buffer I at pH 4.0. The eluate was dialyzed against buffer I (pH 8.0) and reapplied to the Ni-NTA agarose column. The column was then washed with buffer I containing 5 mM imidazole and eluted with buffer I containing 500 mM imidazole. Resulting protein was dialyzed against PBS and concentrated to approximately 1 mg/ml. Samples were analyzed by Coomassie blue staining of SDS-PAGE gels and estimated to be >99% pure. Approximately 900 mg of protein was mixed with Freund’s complete adjuvant and used to immunize three female New Zealand White rabbits. At 14 days postimmunization, a booster of the same preparation was administered, and a second boost of a similar amount of protein in incomplete adjuvant was administered 7 days after the first. At 42 days postimmunization, blood was recovered by exsanguination and allowed to coagulate overnight at 4°C. Erythrocytes were removed by centrifugation, and serum was stored in aliquots at −70°C. An aliquot of serum was adsorbed with an acetone powder preparation of BL21λDE3(pET24a) cells grown and induced with 400 μM IPTG overnight to reduce background, and this serum used at a 1:1,000 dilution for immunoblot analysis.
Cell culture.
Human epithelial cell lines were grown at 37°C in a humidified 5% CO2 atmosphere. A431 (ATCC CRL 1555) and HEC-1-B (ATCC HTB 113) cells were maintained in Dulbecco modified Eagle medium (DMEM) (Gibco) supplemented with 10% fetal calf serum (Gibco). T84 cells (ATCC CCL 248) were maintained in a 1:1 mix of DMEM and Ham’s F12 medium (BioWhittaker, Walkersville, Md.) supplemented with 5% fetal calf serum. Bacterial adhesion and invasion assays were performed as described previously (60). Growth of GC inside A431 cells was done as described by Lin et al. (24).
Human FTOC.
Organ cultures were prepared from human fallopian tubes as described previously (29). Briefly, fallopian tubes were obtained from nonpregnant, premenopausal women during the course of a hysterectomy for surgical indications. The tubes were collected, freed of adventitial tissue, opened longitudinally, cut into 3- to 4-mm2 pieces, and placed in 0.5 M HEPES-buffered Eagle’s minimal essential medium (MEM; Sigma) with l-glutamine. The medium was also supplemented with gentamicin (GM; Gibco, Grand Island, N.Y.) at 0.01 mg/ml. After 24 h of incubation, the medium was removed and placed into HEPES-MEM without antibiotics. After 20 min, the medium was replaced with fresh medium to remove residual antibiotics.
For infection studies, 18- to 20-h N. gonorrhoeae cultures were diluted to 106 CFU per ml. Of this dilution, 0.3 ml was added per well to a 6-well tissue culture plate. Each well contained three pieces of tissue (3 to 4 mm2) in 3 ml of media prepared as described above. Infected (and uninfected control) cultures were incubated for various times as described in the text. At the start of the assay and at various time points thereafter, the wells were sampled and serial dilutions were plated to enumerate the bacteria present.
SEM analysis of infected tissue.
Tissues were processed for scanning electron microscopy (SEM) as described previously (9). Briefly, tissues were washed in HEPES-MEM and then fixed overnight at 4°C in 2% glutaraldehyde (pH 7.3) in 0.2 M sodium cacodylate. Postfixation was in the same buffer containing osmium tetroxide for 1 h at room temperature. The tissues were then rinsed, dehydrated with ethanol, and critical point dried. Samples were coated with gold palladium and examined with a scanning electron microscope (model 500; Hitachi Scientific Instruments, Mountain View, Calif.).
Nucleotide sequence accession number.
The GenBank accession number of the primary sequence reported in Fig. 2 is AF003941.
RESULTS
Isolation of pGly1 from an N. gonorrhoeae gene bank.
N. gonorrhoeae is normally nonhemolytic. However, on rare occasions, narrow zones of hemolysis have been observed to surround gonococcal colonies grown on GCB blood agar plates (2). Additionally, a weak hemolytic activity has been detected in extracts of GC (11). These observations suggest that GC may produce a toxin(s) that acts on erythrocytes and other eukaryotic cells. In an effort to identify potential gonococcal hemolysins, an E. coli library containing GC DNA from strain MS11A, constructed in the vector pHSS6 (50), was plated on blood agar plates and screened for zones of hemolysis surrounding the bacterial colonies. Four hemolytic clones were identified from this screen. Analysis of these inserts indicated that they contained nonoverlapping gonococcal chromosomal fragments (data not shown). One such clone, designated pGly1 (gonolysin 1), was chosen for further analysis.
Analysis of the pGly1 insert.
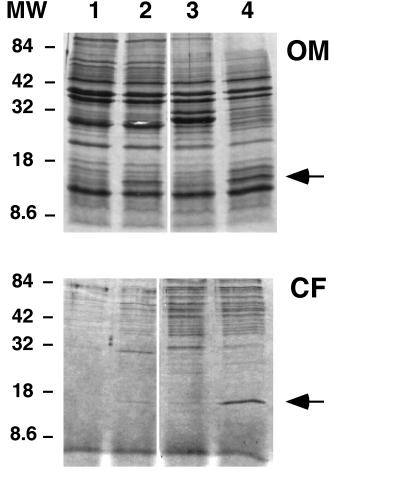
To determine the size and abundance of the protein(s) encoded by pGly1, E. coli cells expressing gly1 were fractionated and subjected to SDS-PAGE analysis. For this analysis, an additional plasmid was constructed in which a 1.4-kb EcoRI fragment of pGly1 was cloned into pBluescript II SK−. This plasmid, pBluGly31, confers a greater degree of hemolysis on the E. coli strain into which it is transformed than does pGly1, which is likely due to the high copy number of the plasmid and also to the presence of a strong promoter (Plac) on the parent vector. A band of approximately 15 kDa was visible in samples from strains containing pGly1 and pBlyGly31 but not in samples from strains containing vectors alone (Fig. 1). This band was observed in both OM and filtered culture supernatants (CF) from these strains.
FIG. 1.
SDS-PAGE analysis of OM preparations and culture CF of E. coli strains expressing gly1. Lanes: 1, CA201(pHSS6) (vector); 2, CA201(pGly1); 3, JM109(pBluescript II SK−); 4, JM109(pBluGly31; pBluescript II SK− plus 1.4 kb EcoRI fragment of pGly1). Eight micrograms of protein was loaded onto each lane of 15% polyacrylamide gels. The gels were stained with Coomassie brilliant blue. MW, molecular weight standards in thousands. Arrows indicate the position of the 15.6-kDa band encoded by ORF1 that was excised and sequenced.
Fragments from the pGly1 insert were subcloned into pBluescript II (Stratagene) and sequenced. The DNA sequence of the 2,245-bp insert, analyzed with the programs ASSEMBLYLIGN and MACVECTOR (Oxford Molecular Group, Oxford, United Kingdom), and its deduced amino acid sequences are presented in Fig. 2. The insert contains two open reading frames (ORF1 and ORF2) oriented in the same direction and separated by 38 nucleotides (nt). A ribosome binding site (RBS) is present 8 nt upstream of the ORF1 ATG start codon. The transcriptional start point was determined by primer extension (data not shown) and maps 151 nt upstream of the ORF1 ATG codon. Immediately upstream of this site are putative −10 (GATACG) and −35 (TTAAAA) sequences typical of ς70 promoters (24).
ORF1 encodes a relatively basic protein of 140 amino acids, with a predicted molecular mass of 17.8 kDa and a pI of 9.57. BLAST searches (1) indicated that this protein has no homology to any proteins registered in the GenBank or EMBL databases. However, the first 21 residues of this protein have strong homology to conserved sequences required for protein export in E. coli (44). If this signal sequence is indeed cleaved, the mature protein should have a molecular mass of 15.6 kDa. To determine if the 15-kDa protein in OM and CF preparations from E. coli strains expressing gly1 (Fig. 1) is the ORF1-encoded processed protein, their N-terminal sequences were determined. Results showed that the first 20 residues of the 15-kDa protein from OM and CF preparations were identical to each other and to the predicted amino acid sequence beginning at residue 22 of ORF1 (shown in bold type in Fig. 2). These results indicate that the ORF1 signal sequence is recognized and processed in E. coli. A hydrophilicity plot of the predicted sequence of ORF1 done with the Kyte and Doolittle scale (21) indicated that, aside from the N-terminal 25 residues, the ORF1 polypeptide is relatively hydrophilic. Therefore the 15-kDa ORF1 protein is unlikely to be a cytoplasmic membrane protein. The predicted ORF1 sequence was analyzed with the program PSORT (39), which predicts OM localization based on analysis of the amino acid composition of a mature protein. The results suggest that Gly1ORF1 is an OM protein. However, analysis of the C-terminal residues of the predicted ORF1 sequence show that it does not have the motif characteristic of some integral OM proteins described by Struyvé et al. (54).
ORF2 begins 38 nucleotides after the ORF1 stop codon. An RBS (GAAG) is present 5 nt upstream of the ORF2 ATG start codon; however, no obvious −10 or −35 sequences were detected. Thus, it is likely that the ORF1 and ORF2 genes are cotranscribed. ORF2 encodes a slightly acidic polypeptide of 247 residues, with a predicted molecular mass of 31.8 kDa and a pI of 6.25. This polypeptide was not visible on Coomassie-stained SDS-PAGE gels of total proteins, OM preparations, or CF from any E. coli strain expressing gly1. BLAST searches of the GenBank and EMBL databases revealed a region of partial homology between the ORF2 polypeptide (residues 86 to 187) and the product of the hemD genes from E. coli (27% identity, 46% similarity) and Pseudomonas aeruginosa (39% identity, 57% similarity). HemD encodes the enzyme uroporphyrinogen III synthase, which catalyzes the cyclization of hydroxymethylbilane to uroporphyrinogen III, the final common precursor for all end product tetrapyrroles, essential components of all heme groups (3).
Presence of the gly1 locus in the Neisseriae.
High-stringency Southern blotting was performed to determine the distribution of the gly1 locus among the Neisseria family of bacteria. ClaI-digested chromosomal DNA from 10 Neisseria species and the closely related Branhamella catarrhalis was probed with the 2.2-kb gly1 insert. Results from this experiment are summarized in Table 1. The gly1 probe hybridized to a 10-kb fragment in N. gonorrhoeae and N. meningitidis, the two pathogenic members of the Neisseria family, suggesting that the gly1 locus is situated within the same chromosomal region in these bacteria. The gly1 probe also hybridized strongly to a 2.3-kb fragment in Neisseria lactamica. N. lactamica, an occasional pathogen (5, 23), is very similar to N. meningitidis and is thought to provide an increased natural immunity to N. meningitidis to carriers (14). The gly1 probe also hybridized weakly to a band of 1.9 kb in Neisseria cinerea, although extended exposure was required for this observation. DNA from the other seven nonpathogenic species showed no hybridization of the gly1 probe, indicating its absence from these organisms.
TABLE 1.
Southern blot analysis of gly1 in Neisseria species
| Organism (strain) | Hybridizing band (kb) |
|---|---|
| B. catarrhalis | None |
| N. cinerea | 1.9a |
| N. flava | None |
| N. gonorrhoeae (MS11A)b | 10.0 |
| N. lactamicab | 2.3 |
| N. meningitidis (8013; [39])b | 10.0 |
| N. mucosa | None |
| N. perflava | None |
| N. polysaccharea | None |
| N. sicca | None |
| N. subflava | None |
Visible only upon extended exposure.
Contains a band of an intensity equal to those in the other two species indicated.
Production of antibodies against the ORF1 polypeptide.
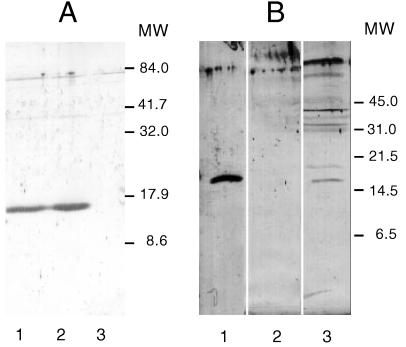
A derivative of pGly1 was constructed to introduce a His6 tag at the C terminus of ORF1. gly1 ORF1 was PCR amplified from pGly1 with the oligonucleotides Wit1 (5′ GGAAATTCATATGAAAAAAATGTTCCTTTCTGC 3′; position 849 to 881 in Fig. 2) which introduces an NdeI site (containing the AUG start codon) at the 5′ end, and Wit2 (5′ CCTTATCCGCGCGGCCGCCCGGGAAAAATCG 3′; position 1266 to 1296), which introduces a NotI site at the 3′ end of the 447-bp amplified fragment. Insertion of this fragment digested with NdeI and NotI into pET24a creates an in-frame fusion, adding a His6 tag at the C terminus of the protein. The resulting plasmid, pGly1ORF1-His, was transformed into E. coli BL21λDE3, which contains the T7 RNA polymerase gene under the control of a lac promoter (55). Transformants produced a protein of approximately 16 kDa in the culture supernatant following IPTG induction (data not shown). Large amounts of ORF1-His were purified from culture supernatants of BL21λDE3(pGly1ORF1-His) as described in Materials and Methods and were used to immunize rabbits. Immune serum from one rabbit, at a 1:1,000 dilution, reacted with a 15.6-kDa protein in CF and OM preparations and total proteins of E. coli strains harboring pBluGly31 (Fig. 3A). This serum did not react with proteins from the vector control, pBluescript II SK−, which was also induced with IPTG. Figure 3 also shows that there is a similar amount of Gly1ORF1 in CF from a strain containing the plasmid pGlyBssErm, which has an insertion in the unique BssHII site of ORF2 (Fig. 2). This finding indicates that ORF2 is not required for expression and processing of the ORF1 polypeptide.
FIG. 3.
Immunoblot analysis with antibodies raised against the Gly1ORF1 polypeptide. Samples were run on 15% acrylamide SDS-PAGE gels and transferred to nitrocellulose electrophoretically. Immunoblotting was carried out as described in Materials and Methods. (A) Culture filtrates from E. coli strains. Lanes: 1, JM109(pGlyBssErm) (2 μg); 2, JM109(pBluGly31) (2 μg); 3, JM109(pBluescript II SK−) (2 μg). (B) OM blebs from N. gonorrhoeae strains. Lanes: (E. coli) JM109(pBluGly31) (2 μg) (CF); 2, 120 (Δgly1) blebs (9 μg); 3, MS11A (wild-type) blebs (9 μg).
Construction of N. gonorrhoeae strains mutated at the gly1 locus.
In order to examine the role of gly1 in N. gonorrhoeae, null mutations were introduced into the gly1 locus in MS11A. A deletion spanning ORF1 and ORF2 was generated by replacing the 822-bp SspI fragment of pGly1 (Fig. 2) with a 1,226-bp erythromycin resistance (Ermr) cassette (58) to generate the plasmid pGly1ΔSsp-Erm. This plasmid was used to transform MS11A (P+, Tr), and the transformants were selected on supplemented GCB Erm plates. Since pGly1ΔSsp-Erm cannot replicate in Neisseria, Ermr transformants occur as a result of a double crossover event between the plasmid and the chromosome to yield a strain that has the gly1 locus replaced by the Ermr cassette. Isogenic P− derivatives of these mutants were also constructed as previously described (35). DNA from the resulting transformants was analyzed by Southern blotting to determine that the Ermr cassette had indeed replaced the wild-type genomic copy of gly1 and that no duplication events had occurred (data not shown). The results shown by these blots indicate that the gly1 locus is present in only one copy on the GC genome and confirmed that all of the constructions were correct. None of the mutants were heterodiploids (one wild-type copy along with a mutated copy), and all grew as well as the parent strains. These results indicate that gly1 is not an essential locus.
Immunoblotting experiments with the Gly1ORF1 antiserum were performed next to determine the location of the protein in GC and confirm its absence in the gly1 null mutant. Initial experiments failed to detect ORF1 in up to 20 μg of total proteins from MS11A. In E. coli, this protein is found in both OM and CF (Fig. 1). We therefore reasoned that Gly1ORF1 might be more abundant in the naturally elaborated OM blebs of GC. Blebs were prepared from bacteria swabbed from a total of eight (100-mm diameter) GCB agar plates of the wild-type parental strain MS11A and also from 120, the isogenic gly1 mutant, as described in Materials and Methods. Proteins from these preparations (approximately 9 μg/lane) were separated by SDS-PAGE and immunoblotted with Gly1ORF1 antiserum. In these experiments, a 15.6-kDa protein was observed in blebs from MS11A and in CF of JM109(pBluGly31) but not in blebs from 120, the gly1 mutant (Fig. 3B). This result confirms the null mutation of the gly1 locus in 120. These results also indicate that the 15.6-kDa protein in GC OM blebs is Gly1ORF1 and that its signal sequence is processed in GC. A formal fractionation of wild-type and gly1 mutant GC cells was also done to localize the Gly1ORF1 protein. Immunoblot analysis of these samples showed that Gly1ORF1 was present only in the Triton X-100 insoluble (OM)-fraction of MS11A, consistent with the result obtained with OM blebs (data not shown). The inability to detect Gly1ORF1 in total GC proteins is likely due to the small quantity of the protein in these bacteria, as blebs from nearly 1010 bacteria were required for immunologic detection of this protein.
Adhesion and invasion indices of GC gly1 null mutants.
No animal model exists for studying Neisseria-host cell interactions, as the pathogenic Neisseria infect only humans. In vitro, these interactions have been studied in detail by using several human cell lines of epithelial origin, including A431, Chang, HEC-1-B, ME-180, and T84. A431, derived from an epidermoid carcinoma (13), has been used recently to study Neisseria intracellular survival (25). HEC-1-B, a human endometrial epithelial line derived from a cervical carcinoma, has been used frequently to study N. gonorrhoeae and N. meningitidis adhesion and invasion (6, 51, 52, 60). T84, a line derived from colonic epithelia (12), was used to establish a polarized cell model for studying Neisseria trafficking across the epithelium (35, 45).
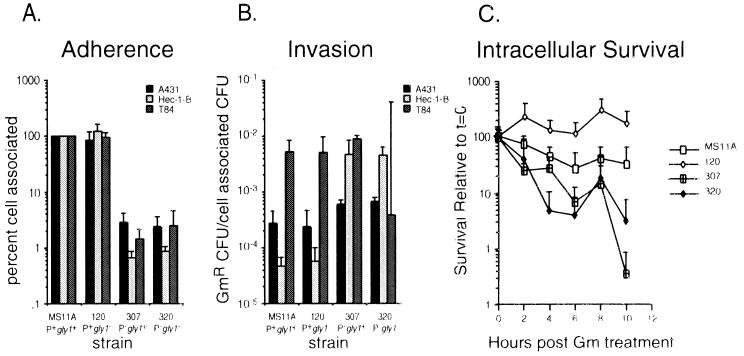
The role played by gly1 in the initial stages of adherence and invasion was assessed by using three of these cell lines, A431, HEC-1-B, and T84. Epithelial cells were infected for 7 h with P+gly1+; P+gly1; P−gly1+ and P−gly1 derivatives of MS11A, and the adherence and invasion indices of these strains were determined. All bacterial strains used in these studies were predominantly Opa−, as judged by Western blots of a portion of each inoculum (data not shown). Adhesion was scored as the number of CFU adhered/total CFU at the end of the experiment, while invasion was scored as the number of bacteria protected from GM killing/number of cell-associated bacteria. The results of these experiments are summarized in Fig. 4. Otherwise-isogenic gly1+ and gly1 strains with the same piliation state showed no statistically significant differences in adhesion and invasion frequencies in all three epithelial cell lines tested. The differences in adherence and invasion that were observed were correlated with the piliation state of the derivatives, not with the gly1 genotype. Our results therefore suggest that gly1 does not play a role in GC adherence to or invasion of epithelial cells.
FIG. 4.
Interaction of N. gonorrhoeae gly1 mutants with epithelial cells in tissue culture. MS11A: P+gly1+; 120: P+gly1; 307: P−gly1+; 320: P−gly1. (A) Adherence to A431, HEC-1-B, and T84 cells. Adhesion data are presented as a percentage of the wild-type control (MS11A). (B) Invasion of A431, Hec-1-B, and T84 cells. Invasion data are presented as the ratio of GM-resistant (Gmr) bacteria per number of cell associated bacteria. (C) Survival within A431 cells. Survival is expressed as the number of GM-resistant (Gmr) CFU at each time point relative to the number of Gmr CFU immediately following GM treatment. Error bars indicate average deviations.
Intracellular survival of gly1 null mutants in A431 cells.
Survival of piliated and nonpiliated gly1+ and gly1 strains in A431 cells was as described by Lin et al. (24). A431 monolayers were infected at a multiplicity of infection of 10 and incubated for 14 h. Nonadherent bacteria were washed off, and extracellular bacteria were killed by incubation with GM for 2 h. Cells were washed again, and fresh medium was added. At 2-h time points, cells were lifted and intracellular bacteria were enumerated by plating for CFU. The medium was also plated to ensure that no extracellular bacteria were present. Figure 4C shows the results of these experiments. Otherwise-isogenic gly1+ and gly1 strains with the same piliation state showed similar levels of survival in A431 cells, indicating that gly1 does not greatly affect the intracellular survival of GC, although the P+gly1 strain does show slightly better survival than the P+gly1+ parent (120 versus MS11A). Interestingly, our data also show that nonpiliated bacteria do not survive inside cells nearly as well as their piliated counterparts, which may indicate a role for pili in intracellular survival.
Infection of HFTOC with gly1 null mutants.
Strain 120, a gly1 null mutant (P+, Tr), was next characterized for its effects on human fallopian tubes in culture. Fallopian tube segments were collected and incubated in media for 24 h prior to infection. Bacteria were diluted in cell culture media and added to tissues at a concentration of approximately 105 CFU/ml. At 24, 48, and 72 h postinfection, medium was sampled and plated for CFU and the tissues were processed for SEM.
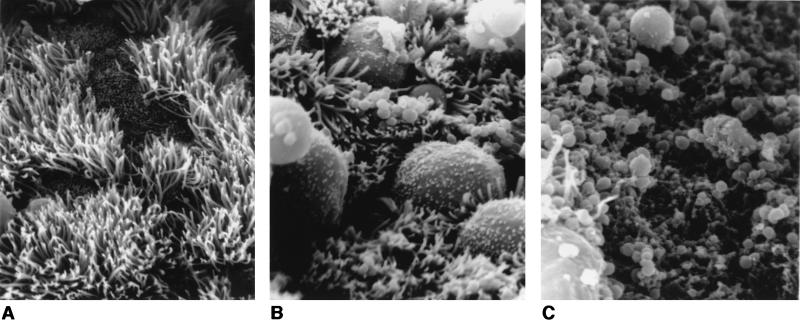
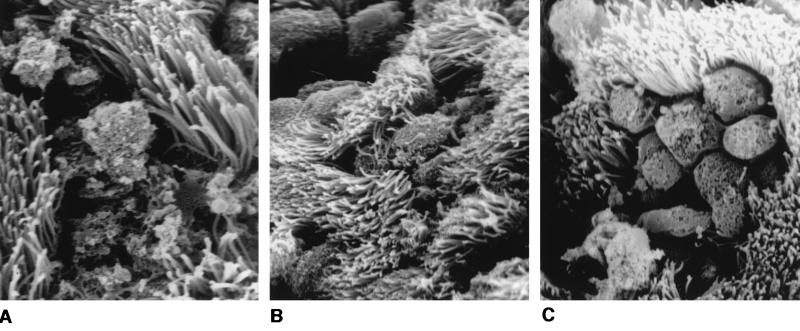
Each fallopian tube segment was examined microscopically in its entirety by SEM, and a representative field was photographed. Electron micrographs from the 24 h time point of one such experiment are presented in Fig. 5. Uninfected tissues appear healthy; ciliated cells are abundant, and the surface of the epithelium, with regular and dense microvillar projections, is fairly uniform in height. In infected tissues, bacteria are seen adhering as clumps or microcolonies to the surfaces of nonciliated cells. At this time point (24 h), tissues infected with MS11A, the wild-type parental strain, are beginning to show signs of damage (Fig. 5B). Colonized cells appear more rounded, and the junctions between cells are more visible. Such responses by HFTOC to GC infection have been described previously (30). Interestingly, tissues infected with strain 120 are badly damaged at 24 h postinfection (Fig. 5C). The cells are deeply pitted, and the tissue surfaces are strewn with debris that may be of bacterial or epithelial cell origin. These tissues have fewer cilia than those infected with MS11A. Those cells that are not effaced still appear to have lost normal morphology; their surfaces are now smooth and devoid of microvilli (Fig. 5C, lower left-hand corner of field). Toxicity was more dramatic at the 48 h time point; tissues infected with strain 120 from the 72 h time point were not successfully processed due to the friability of the samples (data not shown). In these experiments, mutant and wild-type strains yielded similar CFU at each time point (Table 2). Thus, increased toxicity of the gly1 mutant is not due to increased numbers of bacteria. We also examined otherwise-isogenic nonpiliated gly1 mutants that were independently constructed and observed similar effects in HFTOC experiments (data not shown). The electron micrographs shown in Fig. 5, from one experiment, are consistent with the results obtained from two additional experiments performed with tissues from different donors and two independent isolates of the gly1 mutant strain 120.
FIG. 5.
Scanning electron micrographs of human fallopian tubes infected for 24 h with wild-type N. gonorrhoeae strains and deleted for the gly1 locus. Tissues were infected with bacteria and examined 24 h postinfection as described in the text. (A) Uninfected tissue (magnification, ×2,925). (B) Tissue infected with the wild-type parent, MS11A (magnification, ×3,250). (C) Tissue infected with the gly1 mutant strain 120 (magnification, ×5,200).
TABLE 2.
Quantitation of viable bacteria in the supernatants of infected HFTOCa
| Time after infection (h) | CFU per well ± SE of indicated strain (genotype)
|
|
|---|---|---|
| MS11A (P+gly1+) | 120 (P+gly1) | |
| 0 | 3.2 × 105 ± 7.4 × 105 | 2.4 × 105 ± 2.5 × 105 |
| 24 | 1.1 × 109 ± 10.3 × 105 | 1.6 × 109 ± 11.3 × 105 |
| 48 | 1.8 × 109 ± 2.5 × 105 | 2.1 × 109 ± 5.3 × 105 |
Bacteria were sampled from the media immediately prior to processing of tissues for SEM. These data, obtained from the experiment shown in Fig. 5, are similar to data from similar experiments done with tissues from different donors.
Molecular complementation of the gly1 mutation in N. gonorrhoeae.
The gly1 mutation in 120 was complemented in trans to confirm the involvement of the locus in the increased toxicity of this strain to HTOC. The 2.2-kb insert encoding the entire gly1 locus (Fig. 2) was cloned into the E. coli-Neisseria shuttle plasmid pMGC18.1 (40). The recombinant, pGly18.2.2, was then transferred by filter mating into MS11A and its isogenic gly1 mutant derivative 120. These strains were used to infect HFTOC for 24 h as described above. As observed in our previous experiments, there was extensive pitting and effacement of cells in tissues infected with 120 (Fig. 6A). Few healthy cells were visible, and ciliated cells were beginning to slough from these tissues. In contrast, tissues infected with 120(pGly18.2.2) (Fig. 6B) or MS11A(pGly18.2.2) (Fig. 6C) showed little evidence of effacement, and cilia are present in abundance, although nonciliated cells in these tissues have become noticeably rounded and pitted. The fallopian tube tissues used in this set of experiments were not of the highest quality, and the uninfected tissues had a higher baseline toxicity than previous samples. Results from these complementation studies confirm the involvement of the gly1 locus in the toxicity of GC to human fallopian tube tissues in vitro.
FIG. 6.
Scanning electron micrographs of human fallopian tubes infected for 24 h with gly1 mutant N. gonorrhoeae strains expressing gly1 in trans. Tissues were infected and examined as in Fig. 6. (A) Tissue infected with the gly1 mutant strain 120 (magnification, ×6,636). (B) Tissue infected with the complemented mutant, 120(pGly18.2.2) (magnification, ×3,634). (C) Tissue infected with MS11A(pGly18.2.2) (magnification, ×3,634).
DISCUSSION
In an effort to identify potential cytotoxins expressed by N. gonorrhoeae, an E. coli library containing chromosomal sequences from GC strain MS11A was screened for the ability to lyse RBC. This screening technique takes advantage of the fact that agents that are lytic to RBC are often toxic for other mammalian cell types (4) and is therefore often useful for the identification of bacterial toxins. Four different loci were identified in this screen, and one of these, gly1, was chosen for further analysis.
Sequence analysis of pGly1 revealed two open reading frames, ORF1 and ORF2, oriented in the same direction and separated by 38 nt (Fig. 2). An RBS is present 8 nt upstream of the ATG translational start codon of ORF1 and another 6 nt upstream of the ATG of ORF2. Putative −10 and −35 promoter sequences are present upstream of ORF1, 8 nt upstream of the mapped transcriptional start (+1), but none were found upstream of ORF2. The proximity of ORF2 to ORF1 and the apparent lack of promoter sequences in ORF2 suggest that these genes are cotranscribed.
ORF1 encodes a small polypeptide with a 21-residue signal sequence that is processed in E. coli to yield a mature 15.6-kDa protein that is present in both OM and CF from overnight cultures of E. coli strains expressing gly1 (Fig. 1). A protein of this size is also present in OM preparations from GC strain MS11A, indicating that the signal sequence is also processed in N. gonorrhoeae (Fig. 3). The OM localization ORF1 is consistent with the result of an analysis of the predicted protein sequence of Gly1ORF1 obtained with the program PSORT (39). Gly1ORF1 has no homology with any sequences in the GenBank or EMBL databases.
ORF2 encodes a protein with a predicted molecular size of 31.8 kDa. This protein was not visible by Coomassie blue-stained SDS-PAGE of E. coli cells harboring pGly1, suggesting that this protein is made in relatively small amounts compared to Gly1ORF1. The central 100 residues of Gly1ORF2 show partial homology with the E. coli and P. aeruginosa uroporphyrinogen III synthases, products of the hemD gene. This enzyme catalyzes the final step in the synthesis of uroporphyrinogen III, a precursor of the biosynthesis of heme, an essential component of all bacterial cytochromes and catalases (16). The significance of the limited sequence similarity between gly1 ORF2 and hemD is not yet clear but is currently under investigation. It is possible that the ORF2 and HemD polypeptides interact with related molecules. If so, they would most likely be reaction intermediates, as uroporphyrinogen III synthase is reported to have no reversibly bound cofactors or metal ions (3). Interestingly, the P. aeruginosa hemC and hemD homologs are physically linked to AlgR, the response regulator required for the expression of algD (38). AlgD encodes the enzyme that is essential for the mucoid phenotype, an important virulence determinant expressed by this organism in cystic fibrosis patients. Mutations in the hemCD region affect algD expression, which may imply a link between the physiological processes dependent on heme and expression of a virulence determinant. This may have implications for the role for ORF2, although at this time its function is unclear.
GC gly1 null mutants are viable and have normal growth rates in bacterial and cell culture media (GCB and DMEM, respectively). Thus, these proteins are not essential for the survival of GC, at least not under standard laboratory conditions. Piliated and nonpiliated gly1 mutants adhered to and invaded three different human epithelial cell lines as efficiently as their isogenic wild-type parental strains (Fig. 4A and B). Gly1 mutants also survived as well as the parent strains within epithelial cells in tissue culture (Fig. 4C). The lack of an effect on intracellular growth indicates that Gly1 does not function like the hemolysin of Listeria monocytogenes (listeriolysin), whose mutants have a significantly decreased period of intracellular survival (43).
Interestingly, Gly1 appears to have a role in the toxicity of GC for human fallopian tubes. The gly1 null mutant, 120, caused dramatically more damage to human fallopian tube tissues in the HFTOC model than the wild-type parent, MS11A (Fig. 5). Complementation of the gly1 mutation in trans reduced the level of toxicity of strain 120 to that observed for MS11A (Fig. 6), confirming the involvement of the gly1 locus in modulating the toxicity of GC for human fallopian tube tissues.
The exact function of the gly1 proteins is unknown. pGly1 was initially isolated based on its ability to confer a weak hemolytic activity on its E. coli host. GC are not normally hemolytic in vitro; however, the possibility that they have a hemolytic activity in vivo cannot be ruled out. We have occasionally observed zones of weak hemolysis surrounding gonococcal colonies grown on GCB blood agar plates, and Desai and Genco (11) have reported a weak hemolytic activity in GC extracts. On the other hand, numerous infection studies with human epithelial cells in culture have not revealed the presence of a GC toxin other than LPS (18) and peptidoglycan (33). Studies with polarized epithelial cells show that traversal of GC across the monolayer does not destroy the integrity of the barrier, although extended bacterial growth in these chambers did lead to the accumulation of bacterial by-products that can affect the electrical resistance of the barrier (35). Since our results show that gly1 mutants are more toxic (instead of less toxic) to HFTOC, the Gly1 proteins are unlikely to be toxins. The hemolytic activity in the E. coli recombinants may be due to an indirect effect of the gly1 proteins on their host. For instance, the expression of one or both of these proteins may affect the integrity of the E. coli membrane and result in the leakage of host compounds that act negatively on erythrocyte membranes. Indeed, while pGly1 confers a hemolytic phenotype on strains CA201, DH5α, and JM109, it confers a mucoid phenotype on E. coli P90C, supporting this hypothesis. This could also indicate a regulatory function for gly1, which may be manifested as different phenotypes in E. coli and N. gonorrhoeae.
The null mutation in gly1 increased the toxicity of strain 120 for HFTOC. A possible explanation for this gain of phenotype effect is that the gly1 proteins are involved in the production of bacterial components known to be toxic to HFTOC, such as peptidoglycan (33) or the Neisserial LPS (10, 18, 28). Another possibility is that the gly1 proteins play a role in autolysis or blebbing, two processes that can affect the concentration of peptidoglycan and LOS in the culture medium. Thus, a mutation in gly1 causing a change in either of these processes could alter the toxicity of the strain. Alternatively, gly1 may affect the levels of the GC hemolysin(s) detected by Desai and Genco (11). Regardless of its function, the presence of gly1 in the three clinically most important Neisseria species strongly suggests that this locus plays an important role in virulence. Further analysis of the gly1 proteins should shed light on the basis of toxicity of GC for epithelial tissues of fallopian tubes and, it is hoped, further our understanding of how this organism causes sterility in women.
ACKNOWLEDGMENTS
We thank Noelle Fukushima for analysis of primer extension products, Susana Anic for DNA sequencing, and Barbara Robbins, at the Oregon State University Center for Gene Research and Biotechnology, for protein sequencing.
This work was supported by NIH grant RO1 AI34560 to M.S.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Arvidson, C. G., and M. So. Unpublished data.
- 3.Beale S I. Biosynthesis of hemes. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 731–748. [Google Scholar]
- 4.Bernheimer A W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- 5.Brown N M, Ragge N K, Speller D C. Septicaemia due to Neisseria lactamica—initial confusion with Neisseria meningitidis. J Infect Dis. 1987;15:243–245. doi: 10.1016/s0163-4453(87)92703-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen J C-R, Bavoil P, Clark V L. Enhancement of the invasive ability of Neisseria gonorrhoeae by contact with HEC-1-B, and adenocarcinoma endometrial cell line. Mol Microbiol. 1991;5:1531–1538. doi: 10.1111/j.1365-2958.1991.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Gotschlich E C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen M S, Cannon J G, Jerse A E, Charniga L M, Isbey S F, Whicker L G. Human experimentation with Neisseria gonorrhoeae: rationale, methods and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- 9.Cooper M D, Jeffery C, Dever C A. Electron microscope studies of attachment to human fallopian tube mucosa by a gonococcal IgA1 protease deficient mutant and wild-type parent. Scanning Electron Microsc. 1984;1984:1925–1930. [PubMed] [Google Scholar]
- 10.Cooper M D, McGraw P A, Melly M A. Localization of gonococcal lipopolysaccharide and its relationship to toxic damage in human fallopian tube mucosa. Infect Immun. 1986;51:425–430. doi: 10.1128/iai.51.2.425-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai P J, Genco C A. Abstracts of the Tenth International Pathogenic Neisseria Conference, Baltimore, Md. 1996. Specificity of the gonococcal heme transport system; p. 576. [Google Scholar]
- 12.Dharmsathaphorn K, McRoberts J A, Mandel K G, Tisdale L D, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 13.Giard D J, Aaronson S A, Todaro G J, Arnstein P, Kersey J H, Dosik H, Parks W P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 14.Gold R, Goldschneider I, Lepow M L, Draper T F, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978;137:112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- 15.Goodman S D, Scocca J J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granick S, Beale S I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- 17.Grassme H U, Ireland R M, van Putten J P M. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregg C R, Melly M A, Hellerqvist C G, Coniglio J G, McGee Z A. Toxic activity of lipopolysaccharide of Neisseria gonorrhoeae for the human fallopian tube mucosa. J Infect Dis. 1981;143:432–439. doi: 10.1093/infdis/143.3.432. [DOI] [PubMed] [Google Scholar]
- 19.Källström H, Liszewski M K, Atkinson J P, Jonsson A-B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 20.Kellogg D S, Peacock W L, Deacron W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1968;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lauer B A, Fisher C E. Neisseria lactamica meningitis. Am J Dis Child. 1976;130:198–199. doi: 10.1001/archpedi.1976.02120030088017. [DOI] [PubMed] [Google Scholar]
- 24.Lin L, Ayala P, Larson J, Mulks M, Enns C, So M. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24:1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- 25.Lisser S, Margalit H. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 1993;21:1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makino S, van Putten J P M, Meyer T F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 28.Mardh P-A, Baldetorp B, Hakansson C H, Fritz H, Westrom L. Studies of ciliated epithelia of the human genital tract. 3. Mucociliary wave activity in organ cultures of human Fallopian tubes challenged with Neisseria gonorrhoeae and gonococcal endotoxin. Br J Vener Dis. 1979;55:256–264. doi: 10.1136/sti.55.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGee Z A, Johnson A P, Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976;13:608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGee Z A, Johnson A P, Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of type 1 or type 4. J Infect Dis. 1981;143:413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- 31.McGee Z A, Melly M A, Gregg C R, Horn R G, Taylor-Robinson D, Johnson A P, McCutchan J A. Virulence factors of gonococci: studies using human fallopian tube organ cultures. In: Brooks G F, Gotschlich E C, Holmes K K, Sawyer W D, Young F E, editors. Immunobiology of Neisseria gonorrhoeae. Washington, D.C: American Society for Microbiology; 1978. pp. 258–262. [Google Scholar]
- 32.Melly M A, Gregg C R, McGee Z A. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981;143:423–431. doi: 10.1093/infdis/143.3.423. [DOI] [PubMed] [Google Scholar]
- 33.Melly M A, McGee Z A, Rosenthal R S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984;149:378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- 34.Merz A J, So M. Attachment of piliated, Opa− and Opc− gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect Immun. 1997;65:4341–4349. doi: 10.1128/iai.65.10.4341-4349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merz A J, Rifenbery D B, Arvidson C G, So M. Traversal of a polarized epithelium by pathogenic Neisseria: facilitation by type IV pili and maintenance of epithelial barrier function. Mol Med. 1996;2:745–754. [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer T F, Mlawer N, So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982;30:45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- 37.Miller J H, Ganem D, Lu P, Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J Mol Biol. 1977;109:275–301. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- 38.Mohr C D, Sonsteby S K, Deretic V. The Pseudomonas aeruginosa homologs of hemC and hemD are linked to the gene encoding the regulator of mucoidy AlgR. Mol Gen Genet. 1994;242:177–184. doi: 10.1007/BF00391011. [DOI] [PubMed] [Google Scholar]
- 39.Nakai K, Kanehisa M. Expert system for predicting localization sites in gram-negative bacteria. Proteins Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 40.Nassif X, Puaoi D, So M. Transposition of Tn1545-Δ3 in the pathogenic Neisseriae: a genetic tool for mutagenesis. J Bacteriol. 1991;173:2147–2154. doi: 10.1128/jb.173.7.2147-2154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikaido H. Isolation of outer membranes. Methods Enzymol. 1994;235:225–234. doi: 10.1016/0076-6879(94)35143-0. [DOI] [PubMed] [Google Scholar]
- 42.Porat N, Apicella M A, Blake M S. A lipooligosaccharide-binding site on HepG2 cells similar to the gonococcal opacity-associated surface protein Opa. Infect Immun. 1995;63:2164–2172. doi: 10.1128/iai.63.6.2164-2172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pujol C, Eugene E, de Saint Martin L, Nassif X. Interaction of Neisseria meningitidis with a polarized monolayer of epithelial cells. Infect Immun. 1997;11:4836–4842. doi: 10.1128/iai.65.11.4836-4842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudel T, Scheuerpflug I, Meyer T F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 47.Segal E, Billyard E, So M, Storzbach S, Meyer T F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985;40:293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- 48.Segal E, Hagblom P, Seifert H S, So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci USA. 1986;83:2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seifert H S, Ajioka R S, Paruchuri D, Heffron F, So M. Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence. J Bacteriol. 1990;172:40–46. doi: 10.1128/jb.172.1.40-46.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seifert H S, Chen E V, So M, Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1986;83:735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw J H, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon D, Rest R F. Escherichia coli expressing a Neisseria gonorrhoeae opacity-associated outer membrane protein invade human cervical and endometrial cell lines. Proc Natl Acad Sci USA. 1992;89:5512–5516. doi: 10.1073/pnas.89.12.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens D S, McGee Z A, Melly M A, Hoffman L H, Gregg C R. Attachment of pathogenic Neisseria to human mucosal surfaces: role in pathogenesis. Infection. 1982;10:192–195. doi: 10.1007/BF01640777. [DOI] [PubMed] [Google Scholar]
- 54.Struyvé M, Moons M, Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991;218:141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- 55.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 56.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 1990;18:3660. doi: 10.1093/nar/18.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virji M, Watt S M, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesin molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 60.Waldbeser L S, Ajioka R S, Merz A J, Puaoi D, Lin L, Thomas M, So M. The opaH locus of Neisseria gonorrhoeae MS11A is involved in epithelial cell invasion. Mol Microbiol. 1994;13:919–992. doi: 10.1111/j.1365-2958.1994.tb00483.x. [DOI] [PubMed] [Google Scholar]