Abstract
Immunization with a particulate fraction of blood-stage antigens was shown previously to protect mice against Plasmodium yoelii malaria. To identify antigens inducing the protective response, sera from immunized mice were used to screen a P. yoelii cDNA expression library. Sequence analysis of one 2.6-kb cDNA clone indicated that the identified gene, pypag-1, encoded a novel plasmodial antigen. Two nonoverlapping regions of pypag-1 were expressed in Escherichia coli. The first recombinant antigen, pAg-1N, contained the N-terminal 337 residues, which included a putative transmembrane domain and a region relatively rich in tryptophan residues. The second recombinant antigen, pAg-1C, contained the remaining C-terminal 211 residues, which included 31 copies of a 5-amino-acid degenerative repeat. Immunoblot studies using rabbit antiserum raised against recombinant pAg-1N showed that the native pypAg-1 protein migrated at approximately 98 kDa, considerably slower than its predicted molecular mass of 66 kDa. Immunofluorescence studies localized the expression of the native pypAg-1 protein both to the cytoplasm and at the surface of P. yoelii-infected erythrocytes. Immunization with either pAg-1N or pAg-1C induced a four- to sevenfold reduction in P. yoelii blood-stage parasitemia. As such, pypAg-1 appears to contain at least two distinct protective epitopes. To our knowledge, this is the first characterization of a protective antigen of P. yoelii that is associated with the erythrocyte membrane.
Malaria is clearly a major public health problem with significant economic and social consequences for many developing countries, particularly those of sub-Saharan Africa (47). There is a great need to implement effective malaria control programs, with the construction of a multivalent subunit vaccine being a major consideration (18). The vaccine effort has focused primarily on Plasmodium falciparum, the protozoan parasite responsible for the majority of severe disease and death worldwide. Several pre-erythrocytic-stage, asexual blood-stage, and sexual-stage antigens have been identified as vaccine candidates. Although limited, some encouraging results have been reported from P. falciparum vaccine trials (12). Nevertheless, additional efforts in the selection of vaccine antigens, adjuvants, and delivery systems will be necessary to improve on the first generation of subunit malaria vaccines.
Studies utilizing various animal models of malaria have identified protective antigens and protective immune responses. In murine models, the resolution of blood-stage infection results in sterilizing immunity which can be primarily cell mediated, as with Plasmodium chabaudi, or primarily antibody mediated, as with Plasmodium yoelii (31). Protection against P. yoelii malaria can also be induced by immunization with crude preparations of blood-stage antigens in various adjuvants (4, 30, 32, 33, 44). Furthermore, studies with P. yoelii homologues of P. falciparum pre-erythrocytic stage (10, 27, 40) and asexual blood-stage (5, 9, 19, 23, 24) antigens have been particularly useful in augmenting vaccine development efforts.
Previously, we utilized the P. yoelii murine model to investigate immunization-induced protective responses (4). Upon immunization with a particulate fraction of a blood-stage antigen preparation, we induced protection against nonlethal and lethal P. yoelii infection. Both Th1- and Th2-type cytokines were produced in protected mice, with a bias toward a Th2 phenotype evident. In addition, the protection was shown to be B-cell dependent and associated with the production of parasite-specific immunoglobulin G1 (IgG1) and IgG2b antibodies. This particulate antigen fraction, designated pAg, represented 25 to 30% of the total P. yoelii blood-stage antigen preparation. Of particular interest, protective immunization induced antibodies that recognized a limited subset of six to eight P. yoelii antigens. We have employed this model to identify novel vaccine candidate antigens that were selected for their ability to immunize against blood-stage infection. In this paper, we report the identification and characterization of one of the P. yoelii blood-stage antigens that contributed to the pAg-induced protective response.
MATERIALS AND METHODS
Experimental infections.
Male C57BL/6 or CByB6F1/J (BALB/cByJ × C57BL/6J) mice, 5 to 6 weeks of age, were purchased from The Jackson Laboratories (Bar Harbor, Maine) and housed in the American Association for Accreditation of Laboratory Animal Care-approved Animal Care Facility of Meharry Medical College, Nashville, Tenn. The lethal 17XL and nonlethal 17X strains of P. yoelii were originally obtained from William P. Weidanz (University of Wisconsin, Madison) and maintained as cryopreserved stabilates. Blood-stage infections were initiated by intraperitoneal injection of parasitized erythrocytes obtained from donor mice. Resulting parasitemias were monitored by enumerating parasitized erythrocytes in thin tail-blood smears stained with Giemsa stain (17). Routine screenings were conducted throughout these studies to ensure that mice were free of infection with common mouse pathogens. Sentinel animals housed with immunized and/or infected mice remained seronegative for a panel of 19 viral and bacterial pathogens (Assessment Plus Profile; Charles River Laboratories, Wilmington, Mass.).
Sera.
Sera from CByB6F1/J mice (n = 5) were obtained 1 week following secondary immunization with pAg, but prior to infection. Nonimmune control sera were similarly obtained from adjuvant control mice (n = 5) immunized with Quil A alone. The generation and characterization of these sera have been previously reported (4).
A high-titer rabbit antiserum against purified recombinant pAg-1N was commercially prepared (Lampire Biological Laboratories, Pipersville, Pa.). Briefly, rabbits received a total of five immunizations over an 8-week period. Each dose contained 200 μg of purified antigen. The first immunization was with antigen emulsified in complete Freund’s adjuvant. For subsequent boosters, antigen was administered in incomplete Freund’s adjuvant. Preimmune serum was collected prior to the first immunization. Immune serum was collected 2 weeks following the last booster.
cDNA cloning and sequence analysis.
Parasitized blood was collected from 40 C57BL/6 mice infected with P. yoelii 17XL when parasitemias averaged 35 to 40%. This blood contained a mixture of ring, trophozoite, and schizont blood-stage parasites. Mouse leukocytes were removed by passage over columns of microcrystalline cellulose (11). Erythrocytes were collected and lysed with 0.01% saponin. Total RNA was extracted from pelleted parasites, and 30 μg of poly(A)+ RNA representing 1.5% of total RNA was isolated. A cDNA library was constructed in the lambda Uni-ZAP XR expression vector, in a directionally oriented manner according to the manufacturer’s protocols (Stratagene, La Jolla, Calif.). The library contained 3.25 × 106 clones, 94.8% of which were recombinant. The majority of cDNA inserts were between 600 bp and 4 kbp in length.
A pool of sera from mice immunized with pAg was preadsorbed to remove Escherichia coli reactivities (38), and used to screen the P. yoelii cDNA expression library. Bound antibody was detected with 125I-labeled protein A (>30 μCi/μg; ICN Biomedicals, Inc., Irvine, Calif.) followed by autoradiography. Excision of pBSK(−) phagemid sequences from identified clones was carried out according to the manufacturer’s protocol. Nested deletions of clone pypag-1 were generated by exonuclease III digestion (15) and used to obtain the complete sequences of the coding and noncoding strands. Single-stranded template was prepared (6) and the sequence was obtained by Sanger dideoxynucleotide chain termination with [35S]dATP (1,250 Ci/mmol; NEN Life Science Products, Boston, Mass.) (39) or by fluorescence-based sequencing with an ABI Prism 377 automated DNA sequencer (Molecular Biology Core Facility, Meharry Medical College). Sequence analysis utilized a DNASIS for Windows software package (Hitachi Software, South San Francisco, Calif.). Homologies with data bank sequences were determined by BLAST search (1) through the National Center for Biotechnology Information, National Library of Medicine.
Expression and purification of recombinant pypAg-1.
Two pypag-1-encoded recombinant proteins were produced in E. coli by using the pET plasmid vectors and T7 RNA polymerase expression system (42). The N-terminal nonrepeat domain, pAg-1N (amino acids 1 to 337), was PCR amplified from the pypag-1 cDNA clone by using oligonucleotide primers 5′-AAAAATCCATATGAGTGGGCAACTTAC-3′ (primer A, based on nucleotides 593 to 619) and 5′-AGCTCGAGCCTATACGGAAGTTGATTCAGATGCAG-3′ (primer B, based on nucleotides 1591 to 1625) as 5′ and 3′ primers. The C-terminal repeat domain, pAg-1C (amino acids 339 to 549), was PCR amplified from the pypag-1 cDNA clone by using oligonucleotide primers 5′-CTTCCGTACATATGAACGCTGACG-3′ (primer C, based on nucleotides 1606 to 1629) and 5′-GTAATCTCGAGTCACTATATAAGTATCG-3′ (primer D, based on nucleotides 2239 to 2266) as 5′ and 3′ primers. To facilitate subcloning, NdeI and XhoI restriction sites were incorporated into the 5′ and 3′ primers, respectively. The amplified fragments were gel purified, digested with NdeI and XhoI, and ligated into NdeI/XhoI-digested pET-15b (Novagen, Madison, Wis.). E. coli BL21(DE3)(pLysS) was used for expression. Each recombinant protein contained 20 plasmid-encoded amino acids fused to its N terminus, including six histidine residues.
Pellets of induced bacterial cultures (approximately 1 g [wet weight]) were resuspended in 15 ml of TNE (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 10 mM EDTA) and lysed by treatment with lysozyme and sonication. The 43-kDa pAg-1N recombinant protein was purified from an insoluble inclusion body fraction. The inclusion body fraction was recovered by centrifugation for 10 min at 2,000 × g and washed twice in TNE containing 0.1% deoxycholate and twice in TNE containing 2 M urea. The final pellet was solubilized in 10 ml of 100 mM Tris-HCl (pH 8.5)–10 mM EDTA containing 6 M guanidine-HCl. Following dialysis, pAg-1N was purified by nickel-chelate affinity chromatography in the presence of 6 M guanidine-HCl. Eluted material remained soluble following dialysis into 5 mM glycine-HCl (pH 3.0).
Following induction and lysis as described above, the 27-kDa pAg-1C protein was purified from a soluble fraction of lysed bacteria obtained following centrifugation for 30 min at 25,000 × g. A 50 to 80% ammonium sulfate fraction of the initial lysate was separated by preparative isoelectric focusing with a Rotofor IEF cell (Bio-Rad Laboratories, Hercules, Calif.) in the presence of 1% 3/10 ampholytes and 8 M urea. Fractions containing recombinant pAg-1C were pooled. Final antigen purification was by nickel-chelate affinity chromatography in the presence of 6 M urea. The eluted protein was dialyzed into 10 mM Tris-HCl (pH 8.0). Protein concentrations were determined by using the Pierce bicinchoninic acid protein assay (Pierce, Rockford, Ill.), and purity was assessed by Coomassie blue staining following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (26). Endotoxin levels were monitored by using a chromogenic Limulus amebocyte lysate assay (BioWhittaker, Inc., Walkersville, Md.). Endotoxin levels were less than 2.5 endotoxin units (EU) per mg of purified recombinant antigen.
Immunoblot analysis.
Total P. yoelii 17XL blood-stage antigens and the pAg fraction were prepared as previously described (4) and solubilized in SDS-PAGE sample buffer containing 2.5% SDS. Total blood-stage antigens (10 μg/lane), the pAg fraction (10 μg/lane), and purified recombinant pAg-1 antigens (1 μg/lane) were separated by SDS-PAGE on 10% polyacrylamide gels as described by Laemmli (26). Separated proteins were transferred electrophoretically to nitrocellulose membranes (46). Membranes were blocked for 1 h with TBS (25 mM Tris-HCl [pH 8], 150 mM NaCl) containing 5% nonfat dried milk. Blots were incubated for 1 h with pAg immunization or control mouse sera (1:100) or preimmune or immune rabbit serum (1:500) diluted in TBS–0.1% Tween 20 containing 1% bovine serum albumin. Bound antibody was detected with 125I-labeled protein A (ICN Biomedicals) followed by autoradiography.
Indirect immunofluorescence assay.
Parasitized blood was collected from mice infected with P. yoelii 17XL when parasitemias averaged 15 to 20%. Erythrocytes were pelleted by centrifugation for 10 min at 700 × g and washed three times in phosphate-buffered saline (PBS). The final pellet was resuspended in an equal volume of PBS containing 1% gelatin. Thin blood smears were prepared, air dried, and fixed in acetone-methanol (1:1) for 20 min at −20°C. Fixed slides were air dried and stored at 4°C until use. Prior to use, slides were warmed to room temperature and equilibrated with PBS. Fixed cells were incubated for 30 min at 37°C in a humidified chamber with preimmune or immune rabbit serum (1:200) diluted in PBS. Slides were washed three times in PBS and then incubated as above with a fluorescein isothiocyanate-conjugated goat anti-rabbit IgG serum (Sigma Chemical Company, St. Louis, Mo.) diluted 1:160 in PBS. Stained cells were then washed, mounted with Fluoromount-G (Southern Biotechnology Associates, Inc., Birmingham, Ala.), and visualized by fluorescence microscopy.
Immunizations.
Recombinant pAg-1N and pAg-1C were each encapsulated into negatively charged liposomes containing l-α-phosphatidylcholine, dicetyl phosphate, and cholesterol at a ratio of 7:2:1 (Sigma Chemical Company). The encapsulation procedure was essentially as described previously (35), with minor modifications. Briefly, the liposome mixture in chloroform was evaporated to dryness under a stream of nitrogen, leaving a thin coat on the walls of a 15-ml glass Corex tube. Recombinant antigens were added in aqueous solution at a concentration of 1.25 mg/ml and vortexed for 10 min. The mixture was diluted with 4 volumes of PBS, and liposomes were pelleted by centrifugation for 15 min at 3,500 × g. The supernatant was removed, and liposomes were resuspended in PBS. Encapsulation efficiency was monitored by SDS-PAGE and immunoblot analysis using known quantities of purified recombinant pAg-1 antigens as standards.
Groups of CByB6F1/J mice (n = 5) were immunized subcutaneously with 10 to 20 μg of liposome-associated recombinant antigen, utilizing 5 μmol of lipid per mouse per immunization. Quil A (Accurate Chemical and Scientific Corporation, Westbury, N.Y.) was added to the liposome preparation prior to immunization, at a dose of 25 μg per mouse. Control animals were immunized with empty liposomes plus Quil A. Following the primary immunization, animals were boosted twice at 3-week intervals with the same dose of antigen and adjuvant. Seven days later, mice were challenged with 106 P. yoelii 17X-parasitized erythrocytes, and blood parasitemias were monitored. The statistical significance of differences in the mean peak parasitemia between groups was calculated with Student’s t test.
Nucleotide sequence accession number.
The sequence reported in this paper has been deposited in the GenBank database under accession no. AF103869.
RESULTS
Cloning and sequence analysis of the pypag-1 gene of P. yoelii.
A pool of sera from mice protected against P. yoelii malaria by pAg immunization was used to screen a P. yoelii cDNA expression library. Screening of approximately 23,000 recombinants yielded 26 seroreactive clones, with cDNA inserts ranging from 500 bp to 2.75 kb. Through cross-hybridization studies, 23 of the P. yoelii cDNA clones were grouped and shown to represent four distinct antigen genes (data not shown). The working designations assigned to these genes were pypag-1, pypag-2, pypag-3, and pypag-4. Sixteen clones contained pypag-1 gene sequences.
The complete coding and noncoding sequences of pypag-1 were determined (Fig. 1). This cDNA contains 2,642 bp, with a single open reading frame encoding a protein of 549 amino acids. The predicted molecular mass of the encoded protein is 65.7 kDa, with an isoelectric point of 4.60. The C-terminal half of the protein contains 31 copies of a hydrophilic, 5-amino-acid degenerative repeat of (E30/D1)-(V25/E6)-(K31)-(N16/T8/K7)-(D30/Y1). Overall, this represents a highly charged domain of pypAg-1 which includes approximately 25% basic and 43% acidic residues. A single transmembrane domain is predicted near its N terminus between amino acid residues 40 and 59. A stretch of sequence relatively rich in tryptophan is apparent within the nonrepetitive coding sequence, with 27 tryptophan residues found between amino acids 100 and 320. Homology searches of data bank sequences revealed little homology between the nonrepetitive sequence of pypAg-1 and available protein sequences. The repeat domain showed some random similarity with degenerative repeats of otherwise unrelated malarial antigens.
FIG. 1.
Sequence analysis of pypag-1. The DNA and deduced amino acid sequences of the 2,642-bp insert of pypag-1 are shown. Tryptophan residues are in bold. Each 5-amino-acid repeat is underlined. A putative transmembrane segment (residues 40 to 59) is double underlined.
Expression and purification of recombinant pAg-1 proteins.
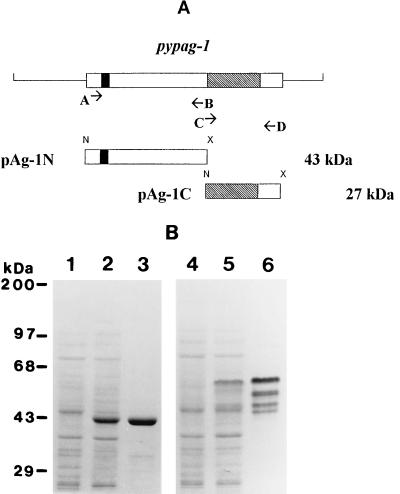
Constructs were made to express two nonoverlapping regions of pypag-1 in E. coli (Fig. 2A). Recombinant antigen pAg-1N contained residues 1 to 337 of the N terminus of pypAg-1. Recombinant antigen pAg-1C contained residues 339 to 549 of the C terminus of pypAg-1. pAg-1C contained the repeat domain followed by 54 nonrepetitive amino acids. Both recombinant proteins contained an N-terminal six-residue histidine tag which was utilized for purification by nickel-chelate affinity chromatography. A Coomassie blue-stained polyacrylamide gel of the purified recombinant proteins is shown in Fig. 2B. pAg-1N migrated close to its predicted molecular mass of 43 kDa (Fig. 2B, lane 3). pAg-1C migrated much slower than its predicted molecular mass of 27 kDa, as a cluster of four bands ranging from 45 to 55 kDa (Fig. 2B, lane 6). The aberrant mobility of pAg-1C was most likely related to the repetitive nature of this protein sequence.
FIG. 2.
Expression and purification of recombinant antigens of pypag-1. (A) Diagram showing the sequences of pypag-1 PCR amplified by using primers A through D and ligated into the pET-15b bacterial expression vector. Recombinant pAg-1N contains amino acids 1 to 337 of pypAg-1, including a potential membrane-spanning sequence (black box). Recombinant pAg-1C contains amino acids 339 to 549 of pypAg-1, including the repeat domain (hatched box). N and X represent NdeI and XhoI restriction sites introduced to facilitate subcloning. (B) Coomassie blue-stained SDS-polyacrylamide gel containing E. coli lysates of uninduced (lane 1) and induced (lane 2) cells expressing pAg-1N, purified pAg-1N (2.5 μg, lane 3), E. coli lysates of uninduced (lane 4) and induced (lane 5) cells expressing pAg-1C, and purified pAg-1C (2.5 μg, lane 6). Molecular mass markers are indicated.
Immunoblot analysis of pypAg-1 antigens.
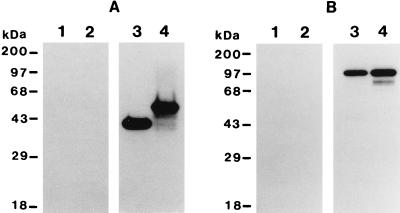
The reactivity of pAg-1N and pAg-1C with sera from mice protected against P. yoelii by pAg-plus-Quil A immunization was assessed by immunoblot. As shown in Fig. 3A, both pAg-1N (lane 3) and pAg-1C (lane 4) were strongly recognized by pAg immunization sera. No reactivity was observed with control sera from mice immunized with Quil A alone (Fig. 3A, lanes 1 and 2). As such, protective immunization with a mixture of particulate blood-stage antigens of P. yoelii induced antibodies that recognized both the repeat and nonrepeat domains of pypAg-1.
FIG. 3.
Immunoblot analysis of recombinant and native pypAg-1. (A) Immunoblot of purified recombinant pAg-1N (lane 1 and 3) and pAg-1C (lanes 2 and 4) probed with sera from mice immunized with the pAg preparation of P. yoelii blood-stage antigens (lanes 3 and 4) or with sera from adjuvant control mice (lanes 1 and 2). (B) Immunoblot of P. yoelii total blood-stage antigens (lanes 1 and 3) or particulate blood-stage antigens (lanes 2 and 4) probed with preimmune rabbit serum (lanes 1 and 2) or rabbit antiserum raised against recombinant pAg-1N (lanes 3 and 4). Molecular mass markers are indicated.
To identify native pypAg-1, a high-titer polyclonal rabbit serum was raised against recombinant antigen pAg-1N. Immunoblot analysis using this antiserum identified a 98-kDa protein present in a total antigen preparation (Fig. 3B, lane 3) as well as the particulate antigen fraction (Fig. 3B, lane 4) of P. yoelii 17XL blood-stage parasites. As with recombinant pAg-1C, native pypAg-1 migrated considerably slower than its predicted molecular mass of 65 to 66 kDa. No reactivity was observed with preimmune rabbit serum (Fig. 3B, lanes 1 and 2).
Localization of pypAg-1 expression.
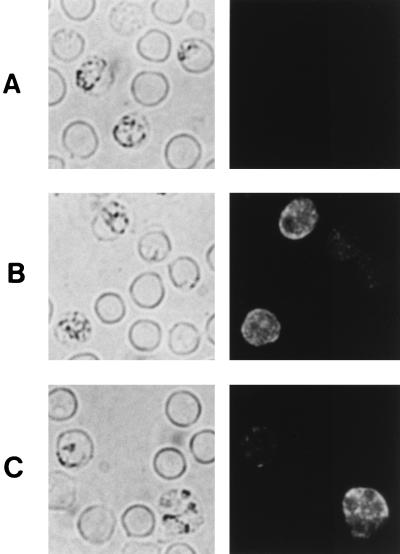
The localization of pypAg-1 expression in parasitized erythrocytes was assessed by indirect immunofluorescence. The reactivity of the polyclonal rabbit serum raised against recombinant pAg-1N was assayed with thin blood films of a mixture of P. yoelii 17XL blood-stage parasites that were air dried and fixed. As shown in Fig. 4B and C, a granular pattern of fluorescence was observed within the cytoplasms of infected erythrocytes. This fluorescence was not associated with the trophozoite itself. Of significance, positive fluorescence was also noted at the erythrocyte membrane. However, pypAg-1-specific fluorescence was not observed on surface of P. yoelii-infected erythrocytes when wet-mount preparations of unfixed cells were assayed (data not shown). No fluorescence of P. yoelii-parasitized erythrocytes incubated with preimmune rabbit serum was observed (Fig. 4A).
FIG. 4.
Immunofluorescence staining of fixed smears of erythrocytes infected with P. yoelii. Thin blood smears were incubated with preimmune rabbit serum (A) or rabbit antiserum raised against recombinant pAg-1N (B and C), followed by a fluorescein isothiocyanate-conjugated secondary antibody. The same fields of cells were examined by phase-contrast (left panels) and fluorescence (right panels) microscopy.
Immunization with recombinant pAg-1.
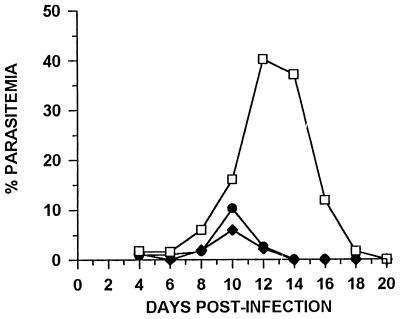
To assess the vaccine potential of pypAg-1, mice were immunized with liposome-encapsulated pAg-1N or pAg-1C, along with Quil A as the adjuvant. Initially, animals that were immunized and given one booster with pAg-1N or pAg-1C exhibited 2.4-fold and 5.8-fold reductions, respectively, in mean peak parasitemia following P. yoelii challenge. In an attempt to improve efficacy in subsequent studies, mice received three immunizations with recombinant antigen prior to challenge with P. yoelii 17X. As shown in Fig. 5, blood parasitemia in control mice peaked on day 12 of infection, with a mean peak parasitemia of 40.2% ± 8.2%. In contrast, mean peak parasitemia was reduced in pAg-1N-immunized mice to 10.3% ± 5.9% and in pAg-1C-immunized mice to 6.0% ± 1.9%. Thus, a four- to sevenfold reduction in P. yoelii parasitemia was induced by immunization with recombinant antigen pAg-1N (P < 0.001) or pAg-1C (P < 0.001) compared to adjuvant controls. Similarly, P. yoelii parasites were cleared from the blood of protected animals approximately 4 days earlier than in control mice. Combined, these data suggest that pypAg-1 contributed to the protection against P. yoelii induced by immunization with the particulate fraction of blood-stage antigens.
FIG. 5.
Protection against P. yoelii malaria induced by immunization with recombinant antigens of pypAg-1. Groups of CByB6F1/J mice (n = 5) were immunized with liposome-encapsulated pAg-1N (•) or pAg-1C (⧫) along with Quil A as the adjuvant. Control animals (n = 5) were immunized with empty liposomes plus Quil A (□). Animals were given boosters twice at 3-week intervals and challenged with 106 P. yoelii 17X-parasitized erythrocytes. Resulting parasitemias were monitored by enumerating parasitized erythrocytes in thin tail-blood smears stained with Giemsa stain.
DISCUSSION
The ability to immunize against P. yoelii malaria with crude preparations of blood-stage antigens was exploited in an attempt to identify novel protective malarial antigens. On the basis of our previous protection studies (4), we focused our efforts on a subset of antigens found within a particulate fraction of whole blood-stage antigens. The ability to induce protective responses against P. yoelii was the primary criteria in the selection of antigens. With this approach, we identified four P. yoelii antigen genes, including pypag-1. The characterization of pypag-1 showed this cDNA to encode a novel plasmodial antigen, expressed in the cytoplasm and associated with the membrane of P. yoelii-infected erythrocytes. Most importantly, immunization with pypAg-1 recombinant antigens induced protective responses against P. yoelii challenge infection.
Data suggesting an association of pypAg-1 with a cell membrane included its initial fractionation into a membrane-enriched pellet and the prediction of a membrane-spanning domain near the N terminus of the deduced amino acid sequence. This was confirmed in immunofluorescence studies with fixed cells, which indicated that pypAg-1 was associated with the membranes of infected erythrocytes. In similar studies with unfixed cells, we were unable to detect pypAg-1 epitopes exposed on the surfaces of P. yoelii-infected erythrocytes. These data suggest that pypAg-1 may be primarily expressed within or on the cytoplasmic side of the erythrocyte membrane. A similar localization has been reported for P. falciparum histidine-rich protein-1 (HRP-1), HRP-2, erythrocyte membrane protein-2 (EMP-2), and the ring-infected erythrocyte surface antigen (8, 13, 20, 21). Expression of pypAg-1 was also observed in the cytoplasm of infected erythrocytes. This is consistent with the transport of pypAg-1 from the intracellular parasite to the erythrocyte membrane. Likewise, P. falciparum HRP-1, HRP-2, and EMP-2 have been localized to membranous structures within the cytoplasms of parasitized erythrocytes (8, 20, 21). An import/export function has been attributed to these membrane-bound vesicles.
The deduced amino acid sequence of the N-terminal, nonrepeat portion of pypAg-1 revealed the presence of 27 tryptophan residues within a 220-amino-acid domain. In a second antigen identified in our initial screen, pypAg-3, we also found a similar tryptophan-rich domain. A 230-amino-acid domain of pypAg-3 has 32% identity with the nonrepeat portion of pypAg-1, including the positional conservation of 24 tryptophan residues (unpublished data). The similarity in this region among different malarial blood-stage antigens raises the possibility that this domain may be functionally important. Tryptophan residues and tryptophan-containing protein motifs have been shown to contribute to the conformation of functional domains in a variety of proteins (3, 29, 43). Among P. falciparum proteins, tryptophan-containing protein motifs have also been found in the circumsporozoite protein (14), in the thrombospondin-related anonymous protein (37), and most recently in EMP-1 (2). Further investigation of the significance of the tryptophan-rich sequence for the function and/or trafficking of pypAg-1 will be of interest.
Protective immunization with our original crude pAg preparation of native P. yoelii blood-stage antigens induced antibodies against the N-terminal and C-terminal domains of pypAg-1. In agreement with this finding, significant protection against P. yoelii malaria was induced by immunization with recombinant pAg-1N or pAg-1C. As only 25% of pAg-1C residues are nonrepetitive, the protective response against pAg-1C may have involved a repetitive epitope. If so, the highly charged repeat domain of pypAg-1 may not serve simply as a component of an immune evasion mechanism (25). Repetitive determinants of other plasmodial antigens have been shown to be potent B-cell epitopes, with some implicated in the induction of protective immune responses (7, 22, 41, 45). However, the sequence polymorphisms associated with the repeats of many plasmodial proteins represent a potential problem for vaccine development. As such, the protection data with recombinant pAg-1N is of considerable importance. A fourfold reduction in P. yoelii blood parasitemia was induced by immunization with the nonrepetitive pAg-1N alone. As the sequences encoding pAg-1N and pAg-1C are nonoverlapping, pypAg-1 appears to contain at least two distinct protective epitopes.
Protective responses against P. yoelii were achieved by immunization with recombinant pypAg-1 encapsulated in liposomes with Quil A as an additional adjuvant. Liposomes have been used effectively as a delivery vehicle in immunization studies with other malarial antigens as well (16, 28, 34, 36). We specifically chose this delivery system in an attempt to mimic our previous crude-antigen immunization studies with pAg plus Quil A (4). In these studies, and in our initial studies with recombinant pAg-1 antigens, the particulate nature of the antigen preparation appeared to be important for the induction of a protective response. Nevertheless, additional work will be necessary to determine if the optimal protective response is induced by immunization with recombinant pAg-1 encapsulated in liposomes. Likewise, improvement in immunization efficacy should be possible using combinations of pAg-1N and pAg-1C, along with other blood-stage antigens that were present in the original pAg fraction.
To our knowledge, pypAg-1 is the first protein of P. yoelii to be characterized that is associated with the erythrocyte membrane and can induce a protective response against challenge infection. This is particularly important as limitations on the direct testing of P. falciparum vaccine antigens in vivo have been an impediment to vaccine development efforts. Fortunately, P. yoelii pre-erythrocytic-stage antigens (CSP and HEP-17) as well as blood-stage antigens (MSP-1, AMA-1, and MAEBL) have provided excellent tools for studying the vaccine potential of antigens with known homologues in P. falciparum (5, 9, 10, 19, 23, 24, 27, 40). Our preliminary cross-hybridization studies suggest that an antigen gene homologous to pypag-1 is also present in P. falciparum. We believe that continued studies of pypAg-1 in vivo will contribute to efforts examining the function and vaccine potential of plasmodial proteins expressed in association with the erythrocyte membrane.
ACKNOWLEDGMENTS
We would like to thank Donna M. Russo, Department of Microbiology, Meharry Medical College, for helpful discussions and critical review of the manuscript.
This work was supported by NIH-NIAID grant AI35661 and NIH Research Centers in Minority Institutions grant G12RR03032. E. Adeeku is supported by National Research Service Award 5F31GM18916.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruch D I, Ma X C, Singh H B, Bi X, Pasloske B L, Howard R J. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 3.Bazan J F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J M, Jr, Dunn P D, Russo D M. Protective immunity against Plasmodium yoelii malaria induced by immunization with particulate blood-stage antigens. Infect Immun. 1997;65:3138–3145. doi: 10.1128/iai.65.8.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns J M, Jr, Daly T M, Vaidya A B, Long C A. The 3′ portion of the gene for a Plasmodium yoelii merozoite surface antigen encodes the epitope recognized by a protective monoclonal antibody. Proc Natl Acad Sci USA. 1988;85:602–606. doi: 10.1073/pnas.85.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns J M, Jr, Shreffler W G, Rosman D E, Sleath P R, Reed S G. Identification and synthesis of a major conserved antigenic epitope of Trypanosoma cruzi. Proc Natl Acad Sci USA. 1992;89:1239–1243. doi: 10.1073/pnas.89.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins W E, Anders R F, Pappaioanou M, Campbell G H, Brown G V, Kemp D J, Coppel R L, Skinner J C, Andrysiak P M, Favaloro J F, Corcoran L M, Broderson J R, Mitchell G F, Campbell C C. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986;323:259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- 8.Coppel R L, Culvenor J G, Bianco A E, Crewther P E, Stahl H, Brown G V, Anders R F, Kemp D J. Variable antigen associated with the surface of erythrocytes infected with the mature stages of Plasmodium falciparum. Mol Biochem Parasitol. 1986;20:265–277. doi: 10.1016/0166-6851(86)90107-6. [DOI] [PubMed] [Google Scholar]
- 9.Daly T M, Long C A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 10.Doolan D L, Hedstrom R C, Rogers W O, Charoenvit Y, Rogers M, de la Vega P, Hoffman S L. Identification and characterization of the protective hepatocyte erythrocyte protein 17 kDa gene of Plasmodium yoelii, homolog of Plasmodium falciparum exported protein 1. J Biol Chem. 1996;271:17861–17868. doi: 10.1074/jbc.271.30.17861. [DOI] [PubMed] [Google Scholar]
- 11.Eling W. Ficoll fractionation for the separation of parasitized erythrocytes from malaria infected blood. Bull W H O. 1977;55:105–114. [PMC free article] [PubMed] [Google Scholar]
- 12.Engers H D, Godal T. Malaria vaccine development: current status. Parasitol Today. 1998;14:56–64. doi: 10.1016/s0169-4758(97)01184-8. [DOI] [PubMed] [Google Scholar]
- 13.Foley M, Tilley L, Sawyer W H, Anders R F. The ring-infected erythrocyte surface antigen of Plasmodium falciparum associates with spectrin in the erythrocytes membrane. Mol Biochem Parasitol. 1991;46:137–147. doi: 10.1016/0166-6851(91)90207-m. [DOI] [PubMed] [Google Scholar]
- 14.Goundis D, Reid K B M. Properdin, the terminal complement components, thrombospondin and the circumsporozoite protein of malaria parasites contain similar sequence motifs. Nature. 1988;335:82–85. doi: 10.1038/335082a0. [DOI] [PubMed] [Google Scholar]
- 15.Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- 16.Heppner D G, Gordon D M, Gross M, Wellde B, Leitner W, Krzych U, Schneider I, Wirtz R A, Richards R L, Trofa A, Hall T, Sadoff J C, Boerger P, Alving C R, Sylvester D R, Porter T G, Ballou W R. Safety, immunogenicity, and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J Infect Dis. 1996;174:361–366. doi: 10.1093/infdis/174.2.361. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman E J, Weidanz W P, Long C A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984;43:981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman S L, Miller L H. Perspectives on malaria vaccine development. In: Hoffman S L, editor. Malaria vaccine development: a multi-immune response approach. Washington, D.C: ASM Press; 1996. pp. 1–13. [Google Scholar]
- 19.Holder A A, Freeman R R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 20.Howard R J. Plasmodium falciparum proteins at the host erythrocyte membrane: their biological and immunological significance and novel parasite organelles which deliver them to the cell surface. In: Englund P T, Sher A, editors. The biology of parasitism. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 111–145. [Google Scholar]
- 21.Howard R J, Uni S, Aikawa M, Aley S B, Leech J H, Lew A M, Wellems T E, Rener J, Taylor D W. Secretion of malaria histidine-rich protein (PFHRPII) from Plasmodium falciparum infected erythrocytes. J Cell Biol. 1986;103:1269–1277. doi: 10.1083/jcb.103.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inselburg J, Bathurst I C, Kansopon J, Barchfeld G L, Barr P J, Rossan R N. Protective immunity induced in Aotus monkeys by a recombinant SERA protein of Plasmodium falciparum: adjuvant effects on induction of protective immunity. Infect Immun. 1993;61:2041–2047. doi: 10.1128/iai.61.5.2041-2047.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kappe S H, Adams J H. Sequence analysis of the apical membrane antigen-1 genes (ama-1) of Plasmodium yoelii yoelii and Plasmodium berghei. Mol Biochem Parasitol. 1996;78:279–283. doi: 10.1016/s0166-6851(96)02619-9. [DOI] [PubMed] [Google Scholar]
- 24.Kappe S H I, Noe A R, Fraser T S, Blair P L, Adams J H. A family of chimeric erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1998;95:1230–1235. doi: 10.1073/pnas.95.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemp D J, Coppel R L, Anders R F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lal A A, de la Cruz V F, Welsh J A, Charoenvit Y, Maloy W L, McCutchan T F. Structure of the gene encoding the circumsporozoite protein of Plasmodium yoelii. J Biol Chem. 1987;262:2937–2940. [PubMed] [Google Scholar]
- 28.Ling I T, Ogun S A, Momin P, Richards R L, Garcon N, Cohen J, Ballou W R, Holder A A. Immunization against the murine malaria parasite Plasmodium yoelii using recombinant protein with adjuvants developed for clinical use. Vaccine. 1997;15:1562–1567. doi: 10.1016/s0264-410x(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 29.Macias M J, Hyvonen M, Baraldi E, Schultz J, Sudol M, Saraste M, Oschkinat H. Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature. 1996;382:646–649. doi: 10.1038/382646a0. [DOI] [PubMed] [Google Scholar]
- 30.McColm A A, Bomford R, Dalton L. A comparison of saponin for the potentiation of protective immunity by killed Plasmodium yoelii vaccine in the mouse. Parasite Immunol. 1982;4:337–347. doi: 10.1111/j.1365-3024.1982.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 31.Melancon-Kaplan J, Burns J M, Jr, Vaidya A B, Webster H K, Weidanz W P. Malaria. In: Warren K, editor. Immunology and molecular biology of parasitic infections. 3rd ed. Cambridge, Mass: Blackwell Scientific Publications, Inc.; 1993. pp. 302–351. [Google Scholar]
- 32.Playfair J H L, De Souza J B. Recombinant gamma interferon is a potent adjuvant for a malaria vaccine in mice. Clin Exp Immunol. 1987;67:5–10. [PMC free article] [PubMed] [Google Scholar]
- 33.Playfair J H L, De Souza J B, Cottrell B J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against Plasmodium yoelii and Plasmodium berghei. Immunology. 1977;33:507–515. [PMC free article] [PubMed] [Google Scholar]
- 34.Pye D, Vandenberg K L, Dyer S L, Irving D O, Goss N H, Woodrow G C, Saul A, Alving C R, Richards R L, Ballou W R, Wu M, Skoff K, Anders R F. Selection of an adjuvant for vaccination with the malaria antigen, MSA-2. Vaccine. 1997;15:1017–1023. doi: 10.1016/s0264-410x(96)00289-7. [DOI] [PubMed] [Google Scholar]
- 35.Reed S G, Barral-Netto M, Inverso J A. Treatment of experimental visceral leishmaniasis with lymphokine encapsulated in liposomes. J Immunol. 1984;132:3116–3119. [PubMed] [Google Scholar]
- 36.Richards R L, Rao M, Wassef N M, Glenn G M, Rothwell S W, Alving C R. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T cell responses against RTS,S malaria antigen. Infect Immun. 1998;66:2859–2865. doi: 10.2307/1366431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robson K J H, Hall J R S, Jennings M W, Harris T J R, Marsh K, Newbold C I, Tate V E, Weatherall D J. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M, Ballou W R, Cohen J D. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 42.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 43.Taylor K M, Trimby A R, Campbell A K. Mutation of recombinant complement component C9 reveals the significance of the N-terminal region for polymerization. Immunology. 1997;91:20–27. doi: 10.1046/j.1365-2567.1997.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ten Hagen T M L, Sulzer A J, Kidd M R, Lal A A, Hunter R L. Role of adjuvants in the modulation of antibody isotype, specificity, and induction of protection by whole blood-stage Plasmodium yoelii vaccines. J Immunol. 1993;151:7077–7085. [PubMed] [Google Scholar]
- 45.Theisen M, Soe S, Oeuvray C, Thomas A W, Vuust J, Danielsen S, Jepsen S, Druilhe P. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun. 1998;66:11–17. doi: 10.1128/iai.66.1.11-17.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Tropical Disease Research Thirteenth Programme Report. Geneva, Switzerland: World Health Organization; 1997. pp. 40–61. [Google Scholar]