Abstract
The goal of this study was to examine social feedback processing among emerging adults with borderline personality features (BPF). Participants (N=118; 66.9% female) completed ratings of BPF and a computerized peer interaction task designed to measure processing of rejection and acceptance cues at the neurophysiological (i.e., electroencephalogram [EEG]), behavioral, and self-report levels. When covarying symptoms of depression and social anxiety, greater BPF were associated with heightened neural processing of social acceptance cues, accounting for reactivity to neutral and rejection cues, as demonstrated by an enhanced reward positivity (RewP) component. Additionally, BPF were associated with less adaptive voting in response to peer acceptance, such that emerging adults with higher BPF made fewer votes to keep peers in the fame who had provided acceptance feedback to participants. These patterns of neural and behavioral patterns associated with BPF highlight the potential role of social reward processing in borderline personality. Specifically, emerging adults high in BPF show a hyper-responsiveness to social acceptance at the neural level but difficulty modulating behavioral responses in an adaptive way to obtain more social rewards. Future research replicating these effects across development may guide efforts to address and prevent the profound social dysfunction associated with BPF.
Keywords: borderline personality, event-related potentials, electroencephalogram, social processing, social reward
Borderline personality disorder is a complex and debilitating disorder associated with instability in affect regulation, poor impulse control, and profound social dysfunction (Lieb et al., 2004). Although identified in 1–3% of the general population, borderline personality disorder is represented disproportionately in mental health systems, often due to the presence of high risk behaviors such as self-harm and suicidal behavior (Lewinsohn et al., 1997; Zanarini et al., 2011). These high risk behaviors represent some of the most severe correlates of the disorder, but they are transient and are not always present in borderline personality disorder (Choi-Kain et al., 2010; Westlund Schreiner et al., 2015). Instead, social processing dysfunction may be a more central marker of borderline personality disorder (Gunderson & Lyons-Ruth, 2008; Herpertz et al., 2014), as social processing deficits persist even after remission from borderline personality disorder, and have been identified among adults with elevated borderline personality features (BPF; Choi-Kain et al., 2010; Lazarus et al., 2014). Sensitivity to social rejection has been a primary clinical focus of borderline personality disorder (Linehan, 2020), and extant research has primarily focused on how adults with the disorder process social rejection cues (Lazarus et al., 2014). However, similar social processing deficits are also identified among adults with depression and social anxiety. As depression and social anxiety co-occur with borderline personality at high rates (Tomko, Trull, Wood, & Sher, 2014; Zanarini et al., 1998), it is not clear whether sensitivity to rejection is unique to borderline personality disorder (Gao, Assink, Cipriani, & Lina, 2017; Domsalla et al., 2014).
Emerging evidence suggests that adults with borderline personality disorder and adults with borderline personality features (BPF) demonstrate deficits in processing social rewards, such as acceptance cues, that persist even when controlling for co-occurring psychopathology (Domsalla et al., 2014; Malejko et al., 2019). However, the specific nature of social acceptance processing alterations underlying BPF is unclear, perhaps due to considerable variability in the methods used to detect these processes. Prominent models of social processing posit that social information is processed in a set of stages with early stages, including encoding and interpreting social cues, influencing later stages of social processing, such as selecting and enacting behavioral responses to social cues (e.g., Crick & Dodge, 1994). To date, self-report and behavioral methods have been used to identify deficits in later stages of social processing. Adults with BPF have been shown to report lower levels of social connection relative to those without BPF after being included (De Panfilis et al., 2015; Gutz et al., 2015) and are less likely compared to those without BPF to trust others and act cooperatively after receiving acceptance feedback (Liebke et al., 2018). Deficits in earlier stages of social processing are also likely, as adults with BPF report lower expectations for social acceptance (De Panfilis et al., 2015). However, self-report methods are limited in being able to discern alterations in the early encoding and interpretation of social cues as social processing is posited to be a mostly automatic process (Crick & Dodge, 1994), and adults with BPF may have limited self-awareness of these processes (Leible & Snell, 2004).
Neural measures may hold promise in further elucidating deficits in social acceptance processing in borderline personality. Among adults with BPF, studies using functional magnetic resonance imaging (fMRI) demonstrate enhanced reactivity to inclusion feedback in the dorsomedial prefrontal cortex (Domsalla et al., 2014; Malejko et al., 2019), a region implicated a number of cognitive functions, including emotional conflict monitoring and self-referential mentalizing about social knowledge. While this enhanced reactivity is speculated to reflect conflict between the desire to be socially accepted and the internal belief of those with borderline personality that others will reject them (Malejko et al., 2019), fMRI methods lack temporal specificity, leaving questions about alterations in more immediate stages of social acceptance processing in BPF. Event-related potentials (ERPs) derived from the electroencephalogram (EEG) provide excellent temporal resolution (Kappenman & Luck, 2016) that is ideal for assessing dysfunction in the most immediate stages of social processing (Kujawa, Kessel, Carroll, Arfer, & Klein, 2017; Kujawa et al., 2014; Pegg et al., 2022). Some BPF research using the Cyberball paradigm has identified an enhanced P3 ERP component in response to being included (Gutz et al., 2015; Weinbrecht et al., 2018). As the P3 amplitude increases when expectations are violated (Luck, 2014; Ruchsow et al., 2008), this may reflect social acceptance feedback is unexpected among those with BPF. However, it is important to note that there is also some disagreement about whether the inclusion condition in the Cyberball task specifically elicits social acceptance (Simard & Dandeneau, 2018), as the task simulates inclusion (i.e., being included in a ball toss) and exclusion (i.e., being excluded from a ball toss), but does not necessarily provide the experiences of being socially evaluated and subsequently rejected or accepted (Cacioppo et al., 2013; Hartgerink, van Beest, Wicherts, & Williams, 2015). Thus, social paradigms that include the pretense of more direct social communication and both positive and negative evaluative feedback may more precisely elucidate early social processing dysfunction.
A virtual, real-time peer interaction task, called Island Getaway (Kujawa, Kessel, Carroll, Arfer, & Klein, 2017; Kujawa et al., 2014), offers an ideal paradigm to examine neurophysiological alterations involved in social acceptance processing in BPF. In the task, participants play a game with simulated peers, in which they exchange personal information, vote to reject and accept simulated peers, and then receive rejection and acceptance feedback from peers while EEG data are simultaneously collected. Principal component analysis (PCA) conducted on ERP data obtained from adolescents and from young adults completing the Island Getaway show a series of ERPs differentially sensitive to rejection versus acceptance feedback (Kujawa et al., 2017; Pegg et al., 2021). Of particular relevance, the reward positivity (RewP) component emerges enhanced (i.e., more positive) for acceptance compared to rejection feedback. RewP is a frontocentral ERP component appearing approximately 300 ms after stimuli onset that is correlated with self-report and behavioral measures of reward responsiveness and may reflect individual differences in approach motivation and reward sensitivity (Bress & Hajcak, 2013). Further, it is correlated with activation of reward-related brain regions, including the ventral striatum, ventromedial prefrontal cortex, midcingulate and anterior cingulate (Becker et al., 2014; Carlson et al., 2011). While originally developed for use with adolescents, the Island Getaway task has also been shown to reliably elicit RewP in young adults (Pegg et al., 2019; Weinberg et al., 2021), and it may also likely be relevant to young adults with BPF.
The RewP may be an important neural indicator of social dysfunction in young adults BPF. Although we are unaware of any extant work that has examined alterations in the RewP in response to social acceptance among young adults with BPF, there is some research showing a blunted RewP in response to non-social feedback in BPF (Endrass, Schuermann, Roepke, Kessler-Scheil, & Kathmann, 2016; Stewart et al., 2019), raising the possibility that altered reward processing more generally may be relevant to the pathophysiology of BPF. Further, previous work has identified a blunted RewP in response to social acceptance feedback among adolescents and young adults with other mental health problems that commonly co-occur with BPF such as depression (Nelson & Jarcho, 2021; Kujawa et al., 2017). However, there is also evidence that RewP is enhanced in response to social acceptance feedback among youth with social anxiety symptoms (Nelson & Jarcho, 2021), and as BPF have been associated with enhanced neural processing of social acceptance cues (Domsalla et al., 2014; Gutz et al., 2015; Malejko et al., 2019; Weinbrecht et al., 2018), it is more likely that BPF may be associated with an enhanced RewP to social acceptance feedback.
The Island Getaway task provides assessments of social acceptance processing at the neurophysiological, behavioral, and self-report levels. Such an approach is well aligned with the Research Domain Criteria (RDoC) initiative of the National Institute of Mental Health, which emphasizes the importance of integrating multiple levels of analysis to more precisely identify important constructs in the development and treatment of psychopathology. At the behavioral level, alterations in social acceptance processing are assessed by examining individual differences in how participants adjust their behavior in response to peer acceptance feedback. As some peers in the task are programmed to more frequently provide rejection feedback while others are programmed to more frequently provide acceptance feedback, young adults who vote to keep more accepting versus rejecting peers demonstrate adaptive responses to social acceptance. At the self-report level, young adults who report greater liking of peers who accept them is related to a larger RewP (Weinberg et al., 2021). Although adults with BPF may display impairments in processing social acceptance feedback across levels of analysis, research that integrates findings across these domains is needed.
The goal of this study is to examine associations between BPF and social feedback processing measured across neurophysiology, behavior, and self-report using a computerized peer interaction task. BPF were examined dimensionally in an unselected sample of emerging adults. It was hypothesized that BPF would be associated with alterations in social acceptance processing across levels of analysis. Based on extant fMRI (Domsalla et al., 2014; Malejko et al., 2019) and some emerging EEG research (Gutz et al., 2015; Weinbrech et al., 2018) examining social acceptance processing in BPF, it was hypothesized that BPF would be associated with an enhanced RewP to peer acceptance relative to neutral and rejection feedback. Less adaptive behavioral and self-reported responses to acceptance were also expected. Specifically, it was hypothesized that BPF would be associated with a lower likelihood of voting to keep and like peers in the task who provided acceptance feedback based on past work showing lower levels of social connection and trust for others among adults with BPF (De Panfilis et al., 2015; Gutz et al., 2015; Liebke et al., 2018). Given that depression and social anxiety frequently co-occur alongside BPF (Tomko et al., 2014; Zanarini et al., 1998) and may be associated with distinct alterations in social feedback processing (Liebke et al., 2018; Malejko et al., 2019; Nelson & Jarcho, 2021), depression and social anxiety were included as covariates in analyses. It was hypothesized that unique associations between BPF and alterations in social acceptance processing variables would emerge when controlling for co-occurring psychopathology.
Methods
Participants
Emerging adult participants were recruited via flyers and the Vanderbilt University psychology research participant pool. Participants were compensated with course credit or money. Written informed consent was obtained from all participants, followed by self-report questionnaires and a series of EEG tasks. These tasks included a monetary reward task and picture viewing task, results of which have been published elsewhere (Dickey et al., 2021a; Dickey et al., 2021b; Pegg et al., 2021). A total of 128 completed the peer interaction task, and nine were excluded for poor EEG data quality and one because they requested their data not be used following debriefing, leaving 118 participants included in the present study. Participants were on average 19.31 years old (SD = 1.15, Min = 18 years); 66.9% female; 52.5% White/Caucasian, 26.3% Asian, 11.0% Black/African American, 0.8% American Indian/Alaska Native, and 9.3% identified as other or mixed race (9.3%); 11.0% identified as Hispanic/Latinx.
Measures
BPF.
The McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD) is a 10-item self-report measure of DSM-IV borderline personality disorder symptoms (Zanarini et al., 2003). Each item corresponds to one of the nine DSM-IV symptoms of the disorder, with two items assessing paranoia/dissociation (Zanarini et al., 2003). Participants are instructed to indicate if a symptom is present (Yes = 1) or absent (No = 0). A sum score is created based on the number of symptoms rated as present, with scores of 7 or higher indicating good sensitivity (.81) and specificity (.85) for a borderline personality diagnosis (Zanarini et al., 2003). In the current sample, Cronbach’s alpha was acceptable (.80). Sum scores ranged from 0 to 9, with 10 (8.5%) participants reporting symptoms in the clinical range.
Internalizing symptoms.
The Inventory of Depression and Anxiety Symptoms (IDAS) was used to assess depression and social anxiety (Watson et al., 2007). The IDAS is a 64-item self-report scale with item responses ranging from 1 (Not at all) to 5 (Extremely). The IDAS includes 12 scales designed to measure dimensions of internalizing problems, and current analyses focused on two subscales expected to be associated with social feedback processing and BPF: Social Anxiety (5 items) and General Depression (20 items). IDAS scale scores have been shown to have good reliability as well as convergent, discriminant, criterion, and incremental validity (Watson et al., 2007, 2008). In the current sample, the General Depression and Social Anxiety scores were considered as covariates, and demonstrated good reliability (Cronbach’s alphas = .90 and .85, respectively).
Island Getaway task.
Participants completed a modified version of the Island Getaway peer interaction task (Kujawa et al., 2014) while EEG data were collected. The task code for this and previous versions of the task is available at: http://arfer.net/projects/survivor (branch: vanderbilt-undergrad). The premise of this 6-round game is that players are traveling with a group to each of the Hawaiian Islands and must vote one of the players out of the game at the end of each round. Participants played with a group of 13 confederate peers (actually computer-controlled) that they were led to believe were other college students interacting with them in real-time from other universities. Prior to the start of the game, participants completed a personal profile that included their photograph, name, age, university, and interests. Participants were told that they would be completing several rounds of the game in which they would vote on each peer (i.e., vote to accept the peer/keep them in the game or vote to reject the peer/kick them out of the game) and would then receive feedback on how each peer “voted” for them. At the end of each round, the player with the most reject votes would be kicked out of the game, and the goal of the game was to make it to the final island following round six without being voted off by their peers. During each round, participants were presented with the profiles of each peer one at a time and decided to vote to accept (i.e., “Keep”) or reject (i.e., “Kick out”) that peer while the peer simultaneously voted to accept or reject the participant. Participants had 5000 ms to vote on each trial. This was followed by a fixation cross for 2000 ms. Participants were then presented with feedback about how that peer voted for them for 2000 ms. Participants were told that a green thumbs up indicated a vote to stay in the game, a red thumbs down indicated a vote to leave the game, and a yellow rectangle indicated no vote was received possibly due to a network error or because the peer did not respond in time (i.e., neutral feedback). After feedback, a fixation cross was presented for 1500 ms before the start of the next trial. The participant continued to vote until they had made a vote on and received feedback from each peer remaining in the game for the round. Prior to the start of the next round, participants completed a poll question to facilitate the gradual exchange of personal information (e.g., “Who is your favorite fictional character?”) and reviewed the responses of their peers to increase believability of the task, as well as the exchange of more personal information.
The task was programmed such that the participant always made it to the final island following six rounds of voting and received equal proportions (i.e., 21 trials each) of acceptance, rejection, and neutral feedback across the task for a total of 63 feedback trials. Each peer profile was randomly assigned to a voting pattern to examine participants’ behavioral responses as a function of peer feedback. Specifically, three peers were considered “allies” and voted to accept the participant on three to five rounds. The ally with three acceptance votes voted to reject the participant once and gave neutral feedback on the remaining two rounds. The allies with four and five acceptance votes provided neutral feedback on the remaining rounds. Three of the peers were considered “enemies” and voted to reject the participant on three to five rounds. The enemy with three rejection votes voted to accept the participant once and gave neutral feedback on the remaining two rounds. The enemies with four and five rejection votes provided neutral feedback on the remaining rounds. One peer was ambiguous in their voting pattern, as they provided neutral feedback on four rounds, voted to accept the participant once, and voted to reject the participant once. The remaining six peers gave each of the three kinds of feedback twice and were voted off one at a time during the game. Participants were fully debriefed following completion of the study and informed that all peer responses were computerized and no one outside of the research study viewed their information.
Following completion of the task, participants responded to three self-report items rated on a 5-point scale: “I really wanted to stay in the game,” “I would’ve liked to play this game again,” and “After a while I lost interest in staying in the game (reverse scored).” Scores were averaged to derive a measure of task engagement ranging from 1 to 5, with higher scores indicating greater engagement. The average self-rating of task engagement for the sample was 3.12 (SD = 1.04). Task engagement was not associated with BPF (r = − .02, p = 0.817).
EEG Data Acquisition
A 64 electrode BrainProducts actiCHamp system (Munich, Germany) was used to collect continuous EEG data. Facial electrodes were attached 1 cm above and below the right eye and 1 cm on each outer corner of the eyes to measure electrooculogram. The sampling rate was 1000 Hz, impedances were lowered below 30 kΩ, and online data acquisition was referenced to Cz. BrainVision Analyzer (Munich, Germany) was used to process collected data. A band-pass filter with cutoffs of 0.1 and 30 Hz was used, and data were re-referenced offline to linked mastoids TP9 and TP10. Per the BrainProducts system design, a single scalp electrode is used for referencing during data recording and then re-referencing to other combinations of electrodes can be conducted offline, which is consistent with recommendations to avoid online data collection using a linked reference (Yao et al., 2019). Although there is no perfect reference for EEG data, the average of both mastoid electrodes is a commonly used reference particularly for ERPs over midline sites because it accounts for both left and right electrical activity and is assumed to be close to a neutral baseline (Yao et al., 2019). Further, the use of this reference allows for direct comparison to ours and others prior research on the RewP (Bress & Hajcak, 2013; Kujawa et al., 2017; Nelson, Perlman, Klein, Kotov, & Hajcak, 2016; Pegg et al., 2021).
Data were segmented −200 ms before to 1000 ms after feedback. Ocular correction was completed using Gratton’s algorithm (Gratton et al., 1983). Semiautomatic artifact rejection was completed with a voltage step greater than 50 μV/ms between sample points, maximum voltage difference of 175 μV within trials, a minimal allowed amplitude of −200 μV and maximal allowed amplitude of 200 μV, and lowest allowed activity of 0.5 μV within 100 ms intervals. Data were visually inspected to remove any remaining artifacts. Single electrodes with faulty recordings were interpolated using the signal from surrounding electrodes. For three participants with poor data from one of the mastoid electrodes (TP9/TP10), data were interpolated at one or both mastoids prior to mastoid re-reference1. (analyses were also conducted with these participants excluded and no substantive changes were observed). Data were averaged by type of feedback (acceptance, rejection, and neutral), and baseline corrected −200 to 0 ms before feedback onset. Participants had on average 20.92 (SD = 0.30) trials for the acceptance feedback condition, 20.87 (SD = 0.44) for rejection feedback condition, and 20.88 (SD = 0.37) for the neutral feedback condition at Cz following artifact rejection. Consistent with prior work with this sample (e.g., Pegg et al., 2021) and visual inspection of the grand averages data (Figure 1), RewP was scored 275–325 ms after feedback onset at Cz. Although we focus analyses on RewP given conceptual links with BPF and associated forms of psychopathology, we also conducted exploratory analyses with other closely related components (P2, P3), with results presented in the Supplement.
Figure 1.
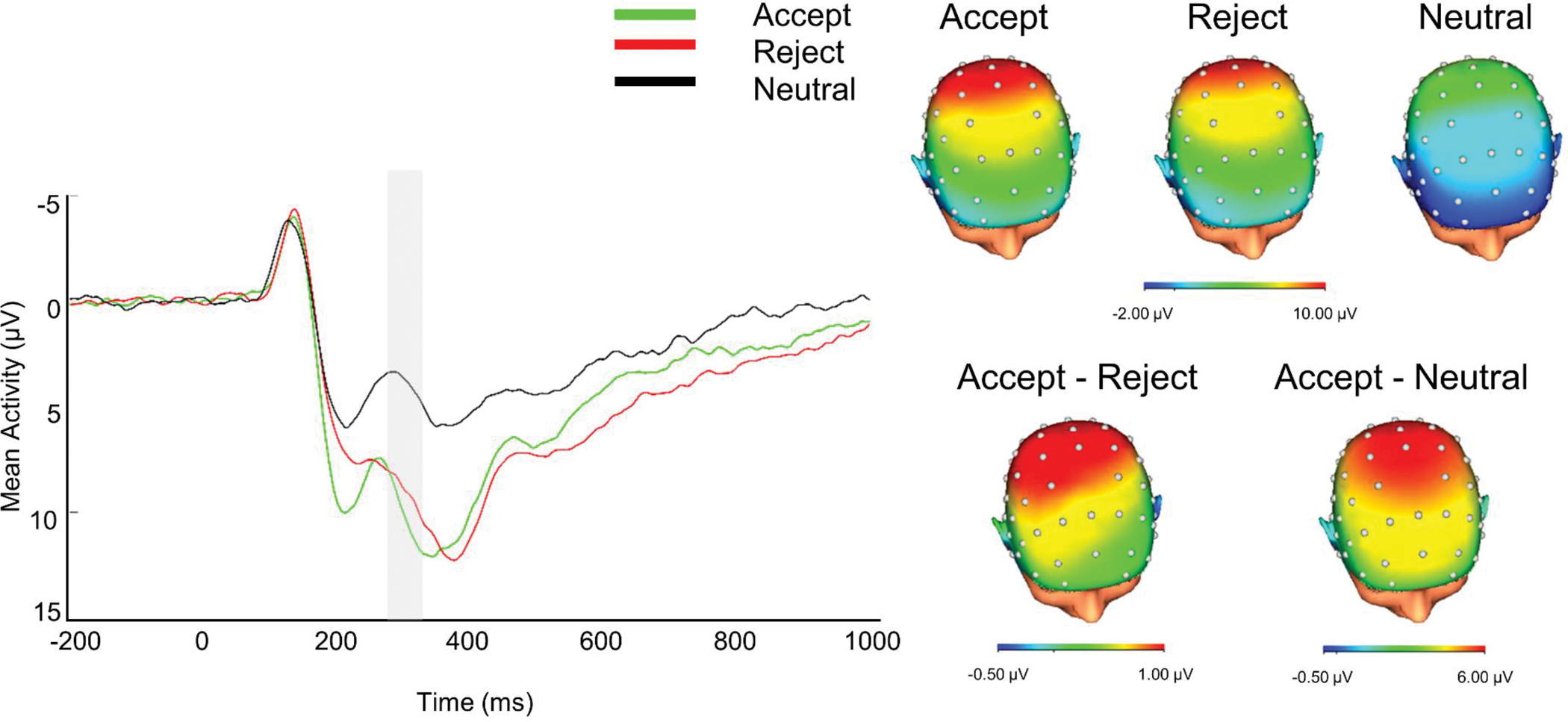
ERP Waveform and Scalp Distributions Depicting Neural Responses to Social Feedback in Emerging Adults
Note. ERP waveform is at Cz. The 275–325ms time window is highlighted in the ERP waveform representing the reward positivity. Negative values are plotted up. Scalp distributions represent the 275–325 ms time window.
Behavioral Measure of Social Processing
As a behavioral measure of social feedback processing, we examined individual differences in the extent to which participants adjusted behavior in response to peer feedback in an adaptive way. Specifically, as three peers were “allies” who tended to accept the participant, and three peer were “enemies” who tended to reject the participant, participant votes to keep allies and kick out enemies would be adaptive for obtaining more positive social feedback, and ultimately, staying in the game. At the same time, variability in peer voting patterns were subtle and there are individual differences in the extent to which participants learn this pattern and update their behavior accordingly. As a measure of adaptive voting patterns, we calculated each participant’s (Pearson) correlation between each co-players’ proportion of votes to accept the participant and the participant’s proportion of votes to accept that same peer. This variable included the seven peers that all participants interacted with throughout the game (i.e., three allies, three enemies, and one ambiguously voting peer). More positive values reflect greater adaptive voting in that participants exhibit more of a tendency to vote to keep participants in the game when they give the participant more acceptance feedback.
Self-Report Measure of Social Processing
At the completion of the task, participants reviewed each peer profile and were asked to rate how much they like the player on a scale from 1 to 5. The (Kendall’s tau-b) correlation between co-player acceptance votes and participants’ liking ratings of the co-player was used as a measure of adaptive liking. Five participants did not show any variability in their liking ratings, and were excluded from liking score analyses. Similar to the adaptive voting variable, this variable included the seven peers that all participants interacted without throughout the six rounds of the game.
Analytic Plan
Outliers were replaced using a 95% Winsorization. First, bivariate correlations were analyzed to examine associations between BPF, depression, social anxiety, gender, and social acceptance processing variables (i.e., RewP to acceptance feedback, adaptive voting and liking patterns). Next, to identify associations between BPF and each of the social acceptance variables accounting for co-occurring problems and covariates, hierarchical linear regression models were calculated. In Step 1, RewP to rejection and neutral were included to isolate variance in the ERP specific to processing of acceptance feedback. In Step 2, gender, self-reported task engagement, and associated symptoms thought to impact social processing (i.e., depression and social anxiety) were included. In Step 3, BPF were entered. For analyses examining adaptive voting and liking patterns, gender, self-reported task engagement, depression, and social anxiety symptoms were included in Step 1, and BPF were included in Step 2. Change in variance explained (i.e., R2) was compared across steps. Standardized regression coefficients are presented as an estimate of effect size to demonstrate the relative strength of associations and their practical meaning (Nieminen, Lehtiniemi, Vähäkangas, Huusko, & Rautio, 2013), with .10, .30, and .50 consistent with small, medium, and large effects, respectively (Cohen, 1988).
Results
Bivariate correlations between study variables are presented in Table 1. As expected, BPF were positively associated with depressive symptoms and social anxiety symptoms. BPF were negatively associated with adaptive voting patterns, but not correlated with RewP in any of the feedback conditions. RewP to rejection feedback was positively correlated with adaptive voting, while RewP to acceptance and RewP to rejection feedback were both positively correlated with adaptive liking. Adaptive voting and liking were also positively associated.
Table 1.
Bivariate Correlations between Study Variables
| M/% | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1. Female gender | 66.9 | -- | -- | |||||||||
| 2. BPF | 2.92 | 2.65 | .12 | -- | ||||||||
| 3. Depressive symptoms | 42.59 | 11.98 | .09 | .61*** | -- | |||||||
| 4. Social anxiety symptoms | 9.69 | 4.31 | .11 | .31*** | .45*** | -- | ||||||
| 5. RewP accept | 9.90 | 7.64 | −.09 | .12 | .02 | −.14 | -- | |||||
| 6. RewP reject | 8.95 | 6.74 | .003 | .14 | .14 | −.03 | .81*** | -- | ||||
| 7. RewP neutral | 3.74 | 6.12 | −.15 | −.01 | −.04 | −.08 | .75*** | .71*** | -- | |||
| 8. Residual RewP | -- | 4.06 | −.07 | .10 | −.08 | −.19* | .53*** | −.02 | 2.30e−16 | -- | ||
| 9. Adaptive voting | 0.32 | 0.36 | .13 | −.21* | −.13 | −.02 | .14 | .19* | .03 | .04 | -- | |
| 10. Adaptive liking | 0.41 | 0.33 | .05 | −.07 | −.05 | −.10 | .27** | .30** | .14 | .10 | .54*** | -- |
Note.
p < .05
p < .01
p < .001 level (2-tailed).
Unstandardized residual RewP was calculated adjusting for RewP to rejection and neutral conditions.
Next, hierarchical regression analyses were conducted to examine the unique associations of BPF with social acceptance processing variables accounting for gender, self-reported task engagement, and symptoms of depression and social anxiety. Results for RewP to acceptance feedback are presented in Table 2. At Step 1, which included RewP to neutral and rejection feedback, the overall model was significant, F(2,115)=145.670, p<.001 and explained 71.7% of the variance. RewP in both conditions explained unique variance in RewP to acceptance. At Step 2, adding gender, self-reported task engagement, along with depression and social anxiety symptoms explained an additional 1.2% of the variance, but this change in R2 was not significant, ΔF(4,111)=1.219, p=.307. These variables were not uniquely associated with RewP to acceptance, although RewP observed in rejection and neutral conditions continued to contribute unique variance to explaining RewP to acceptance. At Step 3, BPF were entered, and an additional 1.1% of variance was explained. This change in R2 was significant, ΔF(1, 110)=4.813, p=.030. RewP to rejection and neutral conditions as well as BPF contributed unique variance to explaining RewP to acceptance. BPF were associated with an enhanced (more positive) RewP to social acceptance feedback (Figure 2). A scatterplot of the association between BPF and the residual RewP to social acceptance feedback adjusting for RewP to neutral and rejection conditions is depicted in Figure 3. We also tested the model with RewP to reject as the dependent variable to examine whether BPF associations with RewP were specific to acceptance feedback or also apparent for rejection feedback. BPF were not associated with RewP to rejection accounting for RewP to other conditions (B=−0.118, SE B=.170, St. β =−.046, p=.489).
Table 2.
Hierarchical Regression Analyses Examining the Association between BPF and RewP to Acceptance Feedback
| Variables | B | SE B | St. β | p | R2 | R2Δ | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Step 1 | RewP to reject and neutral conditions | .717 | -- | ||||
| RewP to reject | 0.627 | 0.080 | 0.553 | <.001 | |||
| RewP to neutral | 0.449 | 0.088 | 0.359 | <.001 | |||
|
| |||||||
| Step 2 | Gender, task engagement, and co-occurring psychopathology | .729 | .012 | ||||
|
| |||||||
| RewP to reject | 0.645 | 0.083 | 0.569 | <.001 | |||
| RewP to neutral | 0.423 | 0.091 | 0.338 | <.001 | |||
| Gender | −0.471 | 0.823 | −0.029 | .568 | |||
| Task engagement | −0.195 | 0.365 | −0.027 | .595 | |||
| Depressive symptoms | −0.001 | 0.036 | −0.001 | .981 | |||
| Social anxiety symptoms | −0.178 | 0.099 | −0.100 | .074 | |||
|
| |||||||
| Step 3 | BPF | .740 | .011 | ||||
|
| |||||||
| RewP to reject | 0.632 | 0.082 | 0.558 | <.001 | |||
| RewP to neutral | 0.428 | 0.090 | 0.343 | <.001 | |||
| Gender | −0.607 | 0.811 | −0.038 | .456 | |||
| Task engagement | −0.190 | 0.359 | −0.026 | .596 | |||
| Depressive symptoms | −0.050 | 0.042 | −0.079 | .237 | |||
| Social anxiety symptoms | −0.189 | 0.097 | −0.106 | .055 | |||
| BPF | 0.391 | 0.178 | 0.136 | .030 | |||
Note. Gender was coded with 0=male and 1=female; RewP = reward positivity.
Figure 2.
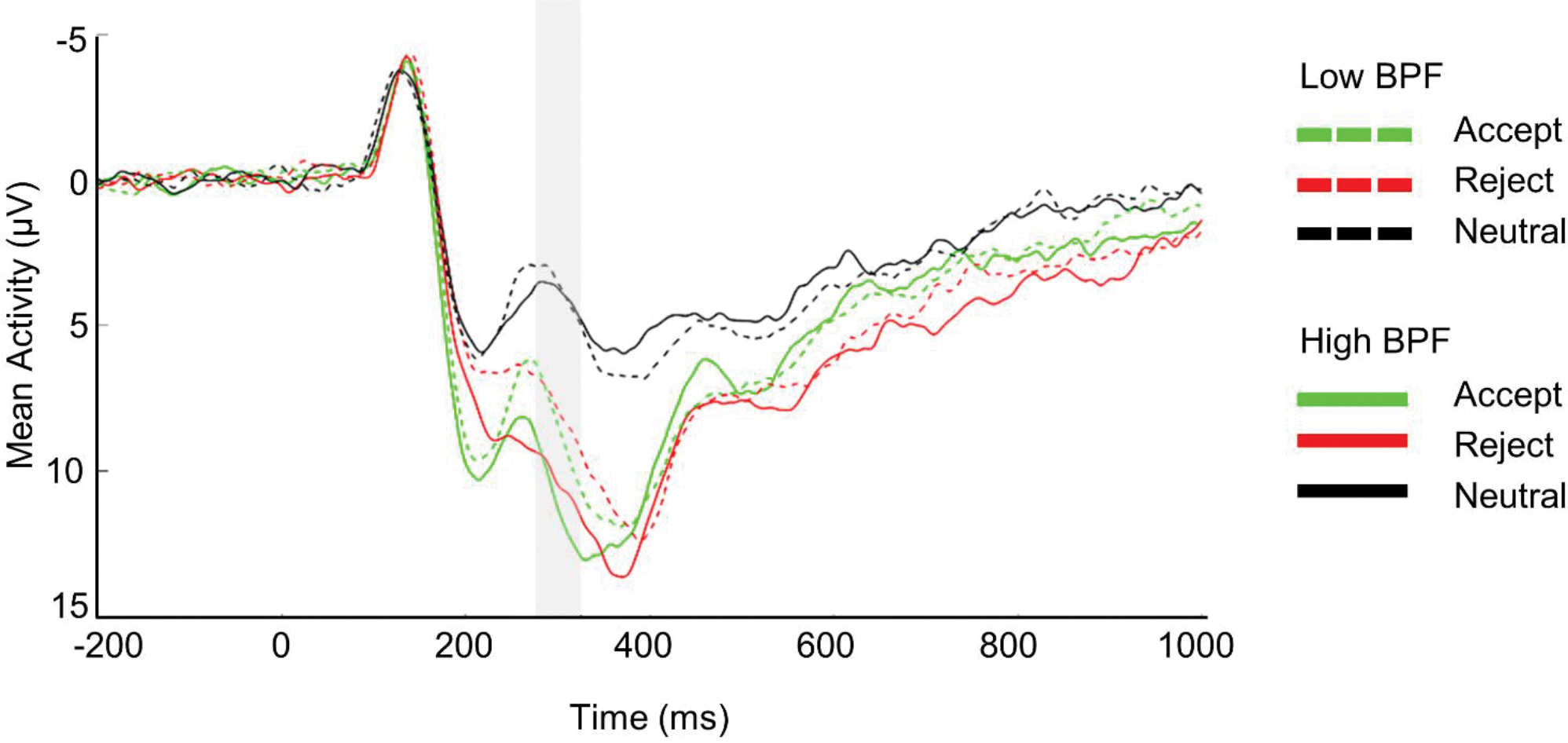
ERP Waveform Depicting Neural Responses to Social Feedback in Emerging Adults Low and High in BPF
Note. ERP waveforms are at Cz. Negative values are plotted up. A median split was computed for illustrative purposes to group participants with low or high BPF.
Figure 3.
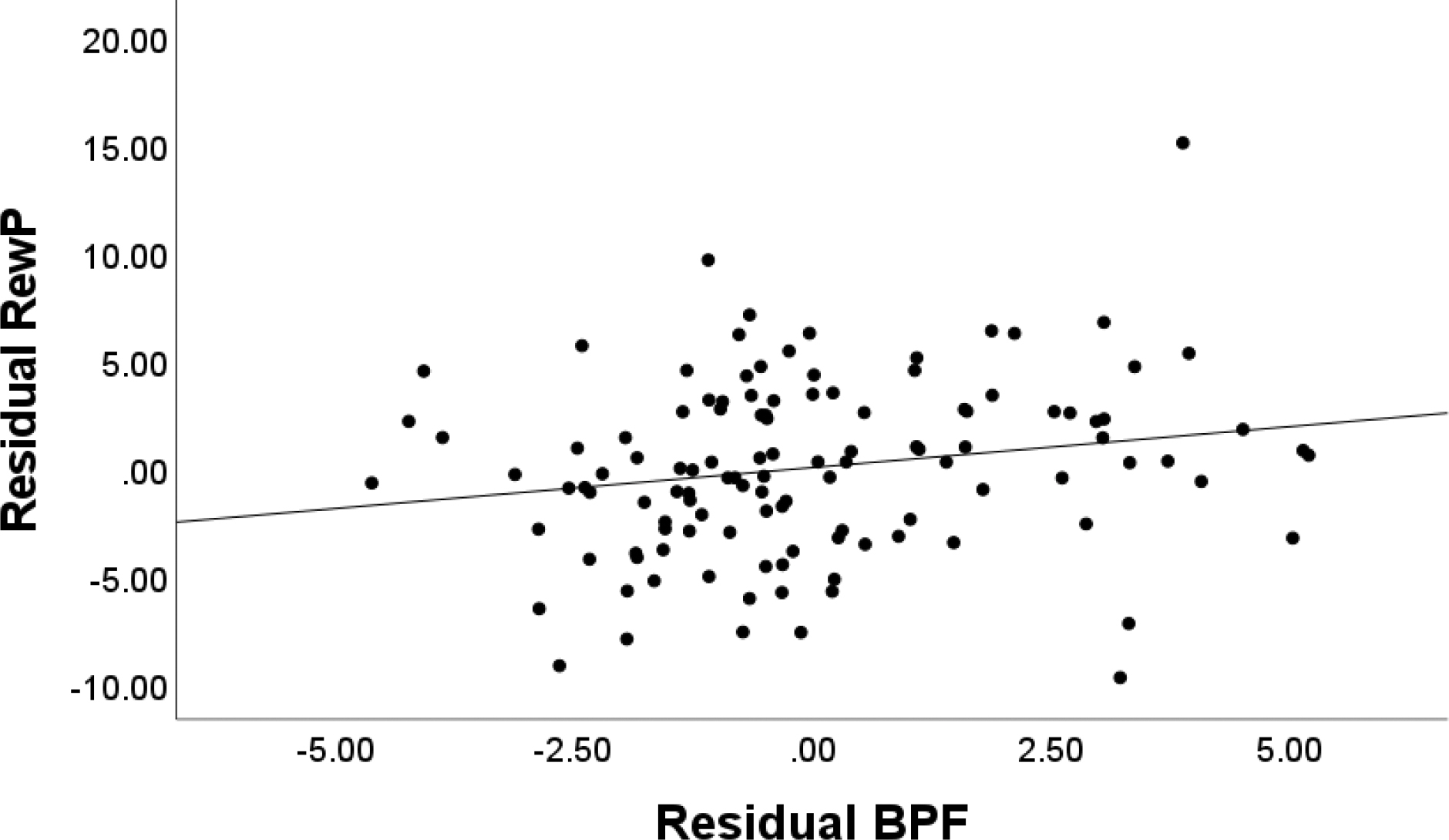
Scatterplot Depicting the Association Between Residual BPF and Residual RewP to Acceptance
Note. Unstandardized residual BPF was adjusted for depressive and social anxiety symptoms. Unstandardized RewP was adjusted for RewP to reject and neutral conditions.
The overall model for adaptive voting at Step 1, when considering gender, self-reported task engagement, depression, and social anxiety was not significant, F(4, 113)=1.545, p=.194. At Step 2, there was a trend for change in R2 when adding in BPF, ΔF(1, 112)=3.916, p=.050 (Table 3). There was a trend for the negative association between BPF and adaptive voting (p = .050), such that higher BPF scores were associated with less adaptive voting. A scatterplot of the association between BPF and the adaptive voting is depicted in Figure 4. Similarly, the overall model for adaptive liking was not significant at Step 1, when considering the effects of gender, self-reported task engagement, depression, and social anxiety, F(4, 108)=1.001, p=.411. At Step 2, when adding BPF, unique variance was not explained, ΔF(1, 107)=0.376, p=.541 (Table 3). None of the coefficients reached significance in the model accounting for other types of symptoms.
Table 3.
Hierarchical Regression Analyses Examining the Associations between BPF and Adaptive Voting and Liking
| Variables | B | SE B | St. β | p | R2 | R2Δ | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Adaptive Voting | |||||||
|
| |||||||
| Step 1 | Gender, task engagement, and co-occurring psychopathology | .052 | -- | ||||
|
| |||||||
| Gender | 0.109 | 0.071 | 0.141 | .128 | |||
| Task engagement | 0.040 | 0.032 | 0.115 | .213 | |||
| Depressive symptoms | −0.005 | 0.003 | −0.154 | .135 | |||
| Social anxiety symptoms | 0.003 | 0.009 | 0.035 | .735 | |||
|
| |||||||
| Step 2 | BPF | .084 | .032 | ||||
|
| |||||||
| Gender | 0.121 | 0.071 | 0.156 | .090 | |||
| Task engagement | 0.040 | 0.032 | 0.116 | .205 | |||
| Depressive symptoms | −0.001 | 0.004 | −0.021 | .861 | |||
| Social anxiety symptoms | 0.004 | 0.009 | 0.044 | .668 | |||
| BPF | −0.031 | 0.016 | −0.227 | .050 | |||
|
| |||||||
| Adaptive Liking | |||||||
|
| |||||||
| Step 1 | Gender, task engagement, and co-occurring psychopathology | .036 | -- | ||||
|
| |||||||
| Gender | 0.043 | 0.066 | 0.062 | .521 | |||
| Task engagement | 0.048 | 0.030 | 0.149 | .119 | |||
| Depressive symptoms | −3.63*10−4 | 0.003 | −0.013 | .904 | |||
| Social anxiety symptoms | −0.007 | 0.008 | −0.091 | .400 | |||
|
| |||||||
| Step 2 | BPF | .039 | .003 | ||||
|
| |||||||
| Gender | 0.046 | 0.067 | 0.066 | .492 | |||
| Task engagement | 0.048 | 0.031 | 0.150 | .117 | |||
| Depressive symptoms | 0.001 | 0.004 | 0.028 | .827 | |||
| Social anxiety symptoms | −0.006 | 0.008 | −0.087 | .423 | |||
| BPF | −0.009 | 0.015 | −0.073 | .541 | |||
Note. Adaptive voting: each participant’s correlation between acceptance votes received from each co-player and acceptance votes cast for that co-player; adaptive liking: the correlation between co-player acceptance votes and participants’ liking ratings of the co-player. Gender was coded with 0=male and 1=female.
Figure 4.
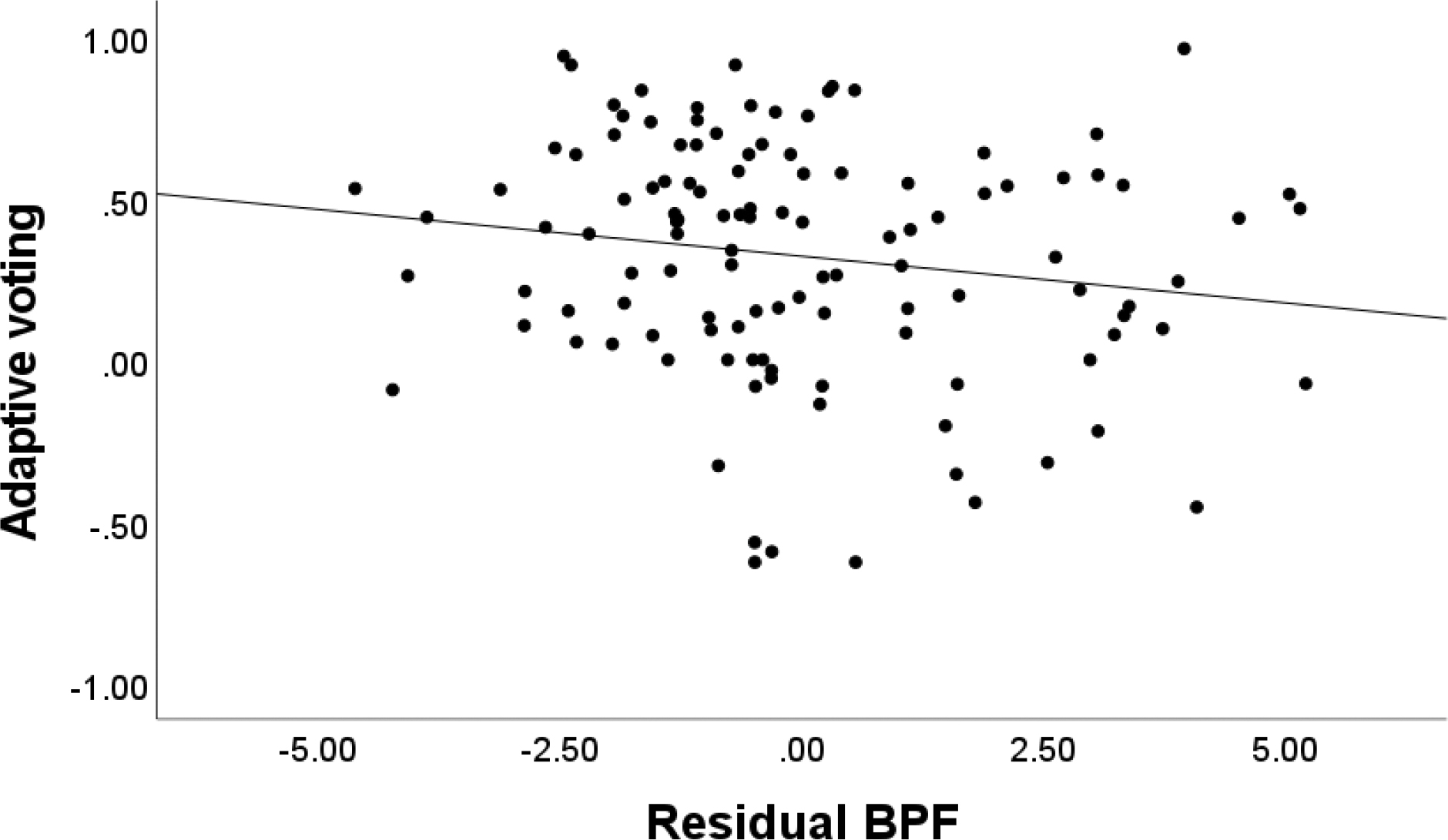
Scatterplot Depicting the Association Between Residual BPF and Adaptive Voting
Note. Unstandardized residual BPF was adjusted for depressive and social anxiety symptoms.
Discussion
Using a computerized peer interaction task with EEG, we identified associations between BPF in emerging adults and neural and behavioral alterations in processing of social acceptance cues. At the behavioral level, BPF were associated with less adaptive voting, as young adults with elevated levels of BPF showed less correspondence between the amount of acceptance feedback obtained from each player and their own votes to keep that player in the game. At the neurophysiological level, BPF were associated with an enhanced RewP to acceptance controlling for RewP to rejection and neutral feedback, indicating hyper-responsiveness to social acceptance feedback. However, the association between BPF and enhanced RewP to acceptance was only significant when controlling for co-occurring social anxiety and depression symptoms in regression analyses.
BPF were modestly but significantly associated with less correspondence between frequency of acceptance feedback from peers and likelihood of offering acceptance feedback in turn. This effect is consistent with extant research showing adults with BPF are less trusting and cooperative with others in response to social acceptance compared to adults without BPF (De Panfilis et al., 2015; Liebke et al., 2018). Notably, this effect emerged only for BPF, whereas social anxiety and depression were not associated with voting patterns. As the measure of adaptive voting used in the current study accounted for the voting patterns of the pre-programmed peers, the behavioral effect identified herein may point to deficits in social reward learning for adults with elevated BPF. That is, BPF may be associated with difficulty learning from social interactions and appropriately updating perceptions of others after receiving new information. For example, Liebke and colleagues (2018) have previously shown that in response to social acceptance feedback, adults with BPF did not adjust their initially low expectations for acceptance even after repeated presentation of positive social feedback, and this effect was not attributed to co-occurring depression. Interestingly, BPF were not associated with difficulty adjusting responses to social rejection, pointing further to potentially unique effects in processing acceptance rather than rejection feedback in BPF.
At the neural level, BPF were associated with an enhanced RewP to social acceptance relative to rejection. This effect is notable, considering that other forms of comorbid psychopathology, such as depression, are typically associated with a blunted RewP response to social acceptance at the neural level (Kujawa et al., 2014; Nelson & Jarcho, 2021), and that previous borderline personality research has shown a blunted RewP in response to non-social feedback (Endrass et al., 2016; Stewart et al., 2019). Yet among emerging research using both EEG (Gutz et al., 2015; Weinbrecht et al., 2018) and fMRI methods (Domsalla et al., 2014; Malejko et al., 2018), enhanced processing of social acceptance feedback has been demonstrated among adults with borderline personality. As the amplitude of the RewP is associated with the degree of positive reward error (i.e., receiving better than expected outcomes; Baker and Holroyd, 2011, Cavanagh, 2015, Holroyd and Umemoto, 2016), the enhanced RewP to acceptance associated with BPF identified herein may be consistent with previous EEG research that has identified an enhanced P3 component in response to inclusion. (Gutz et al., 2015; Weinbrecht et al., 2018). However, combined ERP-fMRI studies have linked RewP to activation in reward-related brain regions including the ventral striatum, ventromedial prefrontal cortex, midcingulate and anterior cingulate (Becker et al., 2014; Carlson et al., 2011, and the enhanced processing of social acceptance identified in the current study may specifically reflect abnormalities in social reward responsiveness and social approach motivation. The enhanced RewP may signal the heightened drive to connect with others, which may underlie some of the most persistent BPF, including dependency and frantic efforts to avoid being alone (Choi-Kahn et al., 2010). More research combining ERP and fMRI methods is needed to advance understanding about the mechanisms underlying the profound social dysfunction associated with borderline personality features.
The heightened neural responsiveness to social acceptance feedback coupled with lower levels of adaptive voting of accepting peers suggests that BPF are associated with difficulties appropriately modulating responses following social acceptance. Prominent models of social information processing describe that social processing occurs in a set of stages, with early stages of social processing, namely encoding and interpreting social cues, impacting later stages of social processing involved in generating and enacting behavioral responses to peers. (e.g., Crick & Dodge, 1994). Our cross-sectional findings raise the possibility that alterations in early encoding and interpretation of social acceptance, indicated by an enhanced RewP, may contribute to generating and enacting maladaptive voting patterns identified among young adults with BPF in this study. Longitudinal multi-method research is needed to examine how these social acceptance processing patterns emerge and how they are associated with real world social functioning for individuals with elevated BPF.
Despite these intriguing findings, several limitations should be discussed. First, effects, while significant, were of modest size, which is common given the large sample size and lack of shared method variance (Patrick et al. 2013). Large effects in clinical neuroscience may be due, at least in part, to overestimates of effect sizes in studies with small samples (Button et al. 2013). Studies integrating multiple measures and levels of analysis of emotional and cognitive constructs of interest are essential in order to identify the clinical significance of findings. This point is particularly notable as an association between BPF did not emerge based on self-reported adaptive liking, and there was notable range in participant data (Figures 3 and 4). Second, we did not assess individual differences in whether participants believed peers were real in the full sample and this could moderate associations between BPF and RewP. Third, results from this non-referred sample of undergraduate students, measuring BPF dimensionally, may not generalize to the larger clinical population of adults diagnosed with borderline personality disorder. However, based on screening criteria, nearly 8% of our sample had elevated BPF traits, in line with previous estimates conducted in college-aged youth (Meaney, Hasking, & Reupert, 2016). Effects may be expected to be even more pronounced in a clinical sample. Fourth, we relied on self-report measures of psychopathology, and information from additional informants may clarify these results. Fifth, analyses controlled for co-occurring depression and social anxiety, some of the most common clinical concerns that co-occur alongside BPF. Interestingly, an association between BPF and RewP to social acceptance did not emerge at the bivariate level, but rather emerged when controlling for co-occurring depression and social anxiety. Given the considerable overlap in the features of depression, social anxiety, and borderline personality (Tomko, Trull, Wood, & Sher, 2014; Zanarini et al., 1998), our findings suggest unique effects of BPF on neural processing of social acceptance. Future work may consider the effects of co-occurring externalizing problems and ADHD, which often co-occur with BPF (Matthies & Philipsen, 2014).
These findings are preliminary and require replication and extension. Research examining how social acceptance processing deficits are associated with social functioning outside of the Island Getaway task is needed. Furthermore, as there is mounting evidence that the developmental origins of BPF date back to childhood (Crowell et al., 2009), future work that seeks to understand whether these patterns emerge in younger populations is needed to mitigate long-term risk for developing borderline personality disorder. Psychosocial treatment for borderline personality has generally focused on coping with negative social experiences (Linehan, 2020). However, but it may be that addressing adaptive responses to positive social feedback are also worthwhile in treating and preventing borderline personality.
Supplementary Material
Highlights.
BPF were associated with alterations in processing peer acceptance cues.
At the neural level, BPF were associated with an enhanced RewP to acceptance.
BPF were also associated with less adaptive voting in response to peer acceptance.
Findings suggest impairments in modulating responses to acceptance unique to BPF.
Targeting adaptive responses to acceptance may be relevant to intervention for BPF.
Acknowledgements
This work was supported by UL1 TR000445 from NCATS/NIH, and T32-MH18921 and F31-MH127817, and MH125052 from NIMH.
Footnotes
Conflicts of Interest
All authors declare that they have no conflicts of interests to declare with respect to the current study.
CRediT author contribution statement
Dara E. Babinski: Conceptualization, Formal Analysis, Writing – Original Draft, Review and Editing
Samantha Pegg: Data collection and curation, Formal Analysis, Writing – Original Draft, Review and Editing
Michael West: Data collection and curation, Writing – Review and Editing
Kodi B. Arfer: Methodology, Writing – Review and Editing
Autumn Kujawa: Conceptualization, Methodology, Writing – Review and Editing, Supervision
Analyses were conducted excluding participants with data interpolated at one or both mastoids prior to mastoid re-reference. No substantive changes were observed with one exception: in Step 3 of the hierarchical regression model predicting RewP to acceptance feedback when BPF are added to the model (Table 2), social anxiety symptoms were significantly associated with RewP to acceptance feedback (B=−0.198, SE B=.096, St. β =−.111, p=.041) when excluding these participants while it previously was not significant (p = .055).
References
- Baker TE, & Holroyd CB (2011). Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biological Psychology, 87(1), 25–34. 10.1016/j.biopsycho.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Becker MP, Nitsch AM, Miltner WH, & Straube T (2014). A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. Journal of Neuroscience, 34(8), 3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein MJ, & Claypool HM (2012). Not all social exclusions are created equal: Emotional distress following social exclusion is moderated by exclusion paradigm. Social Influence, 7(2), 113–130. doi: 10.1080/15534510.2012.664326 [DOI] [Google Scholar]
- Bress JN, & Hajcak G (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50(7), 610–616. 10.1111/psyp.12053 [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek B. a., Flint J, Robinson ESJ, & Munafò MR (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14, 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, & Cacioppo JT (2013). A quantitative meta-analysis of functional imaging studies of social rejection. Scientific Reports, 3(1), 1–3. doi: 10.1038/srep02027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, & Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. NeuroImage, 57(4), 1608–1616. 10.1016/j.neuroimage.2011.05.037 [DOI] [PubMed] [Google Scholar]
- Cavanagh JF (2015). Cortical delta activity reflects reward prediction error and related behavioral adjustments, but at different times. NeuroImage, 110, 205–216. 10.1016/j.neuroimage.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Choi-Kain LW, Zanarini MC, Frankenburg FR, Fitzmaurice GM, & Bradford Reich D (2010). A longitudinal study of the 10-year course of interpersonal features in borderline personality disorder. Journal of Personality Disorders, 24(3), 365–376. 10.1521/pedi.2010.24.3.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: Erlbaum [Google Scholar]
- Crick NR, & Dodge KA (1994). A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychological Bulletin, 115(1), 74–101. 10.1037/0033-2909.115.1.74 [DOI] [Google Scholar]
- Crowell SE, Beauchaine TP, & Linehan MM (2009). A biosocial developmental model of borderline personality: Elaborating and extending Linehan’s theory. Psychological Bulletin, 135(3), 495–510. 10.1037/a0015616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Panfilis C, Riva P, Preti E, Cabrino C, & Marchesi C (2015). When social inclusion is not enough: Implicit expectations of extreme inclusion in borderline personality disorder. Personality Disorders: Theory, Research, and Treatment, 6(4), 301–309. 10.1037/per0000132 [DOI] [PubMed] [Google Scholar]
- Dickey L, Pegg S, & Kujawa A (2021a). Neurophysiological responses to interpersonal emotional images: Associations with symptoms of depression and social anxiety. Cognitive, Affective & Behavioral Neuroscience, 21(6), 1306–1318. 10.3758/s13415-021-00925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey L, West M, Pegg S, Green H, & Kujawa A (2021b). Neurophysiological responses to interpersonal emotional images prospectively predict the impact of COVID-19 pandemic-related stress on internalizing symptoms. Biological psychiatry. Cognitive neuroscience and neuroimaging, 6(9), 887–897. 10.1016/j.bpsc.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsalla M, Koppe G, Niedtfeld I, Vollstädt-Klein S, Schmahl C, Bohus M, & Lis S (2014). Cerebral processing of social rejection in patients with borderline personality disorder. Social Cognitive and Affective Neuroscience, 9(11), 1789–1797. 10.1093/scan/nst176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Roepke S, Kessler-Scheil S, & Kathmann N (2016). Reduced risk avoidance and altered neural correlates of feedback processing in patients with borderline personality disorder. Psychiatry Research, 243, 14–22. 10.1016/j.psychres.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Gao S, Assink M, Cipriani A, & Lin K (2017). Associations between rejection sensitivity and mental health outcomes: A meta-analytic review. Clinical Psychology Review, 57, 59–74. 10.1016/j.cpr.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55, 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Gunderson JG, & Lyons-Ruth K (2008). BPD’s interpersonal hypersensitivity phenotype: A gene-environment- developmental model. Journal of Personality Disorders, 22(1), 22–41. 10.1521/pedi.2008.22.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz L, Renneberg B, Roepke S, & Niedeggen M (2015). Neural processing of social participation in borderline personality disorder and social anxiety disorder. Journal of Abnormal Psychology, 124(2), 421–431. 10.1037/a0038614 [DOI] [PubMed] [Google Scholar]
- Hartgerink CH, Van Beest I, Wicherts JM, & Williams KD (2015). The ordinal effects of ostracism: A meta-analysis of 120 Cyberball studies. PloS one, 10(5), e0127002. doi: 10.1371/journal.pone.0127002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Jeung H, Mancke F, & Bertsch K (2014). Social dysfunctioning and brain in borderline personality disorder. Psychopathology, 47(6), 417–424. 10.1159/000365106 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Umemoto A (2016). The research domain criteria framework: The case for anterior cingulate cortex. Neuroscience & Biobehavioral Reviews, 71, 418–443. 10.1016/j.neubiorev.2016.09.021 [DOI] [PubMed] [Google Scholar]
- Kappenman ES, & Luck SJ (2016). Best practices for event-related potential research in clinical populations. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(2), 110–115. 10.1016/j.bpsc.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Kessel EM, Carroll A, Arfer KB, & Klein DN (2017). Social processing in early adolescence: Associations between neurophysiological, self-report, and behavioral measures. Biological Psychology, 128, 55–62. 10.1016/j.biopsycho.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Arfer KB, Klein DN, & Proudfit GH (2014). Electrocortical reactivity to social feedback in youth: A pilot study of the Island Getaway task. Developmental Cognitive Neuroscience, 10, 140–147. 10.1016/j.dcn.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus SA, Cheavens JS, Festa F, & Zachary Rosenthal M (2014). Interpersonal functioning in borderline personality disorder: A systematic review of behavioral and laboratory-based assessments. Clinical Psychology Review, 34(3), 193–205. 10.1016/j.cpr.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Leible TL, & Snell WE (2004). Borderline personality disorder and multiple aspects of emotional intelligence. Personality and Individual Differences, 37(2), 393–404. 10.1016/j.paid.2003.09.011 [DOI] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR, & Klein DN (1997). Axis II psychopathology as a function of Axis I disorders in childhood and adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 36(12), 1752–1759. 10.1097/00004583-199712000-00024 [DOI] [PubMed] [Google Scholar]
- Lieb Zanarini, Schmahl Linhehan, & Bohus. (2004). Borderline personality disorder. The Lancet, 364, 453–461. 10.1016/S0140-6736(04)16770-6 [DOI] [PubMed] [Google Scholar]
- Liebke L, Koppe G, Bungert M, Thome J, Hauschild S, Defiebre N, Izurieta Hidalgo NA, Schmahl C, Bohus M, & Lis S (2018). Difficulties with being socially accepted: An experimental study in borderline personality disorder. Journal of Abnormal Psychology, 127(7), 670–682. 10.1037/abn0000373 [DOI] [PubMed] [Google Scholar]
- Linehan MM (2020). Dialectical behavior therapy in clinical practice. Guilford Publications. [Google Scholar]
- Malejko K, Neff D, Brown RC, Plener PL, Bonenberger M, Abler B, & Graf H (2019). Neural signatures of social inclusion in borderline personality disorder versus non-suicidal self-injury. Brain Topography, 32(5), 753–761. 10.1007/s10548-019-00712-0 [DOI] [PubMed] [Google Scholar]
- Malejko K, Neff D, Brown R, Plener PL, Bonenberger M, Abler B, & Graf H (2018). Neural correlates of social inclusion in borderline personality disorder. Frontiers in Psychiatry, 9, 1–11. 10.3389/fpsyt.2018.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies SD, & Philipsen A (2014). Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)-review of recent findings. Borderline Personality Disorder and Emotion Dysregulation, 1(1). 10.1186/2051-6673-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney R, Hasking P, & Reupert A (2016). Prevalence of borderline personality disorder in university samples: systematic review, meta-analysis and meta-regression. PloS one, 11(5), e0155439. 10.1371/journal.pone.0155439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA (2018). Use of event-related potentials in the study of typical and atypical development. Journal of the American Academy of Child and Adolescent Psychiatry, 47(11), 1252–1261. 10.1097/CHI.0b013e318185a6d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, & Jarcho JM (2021). Neural response to monetary and social feedback demonstrates differential associations with depression and social anxiety. Social Cognitive and Affective Neuroscience, 16(10), 1048–1056. 10.1093/scan/nsab055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry, 173(12), 1223–1230. doi: 10.1176/appi.ajp.2016.15121524 [DOI] [PubMed] [Google Scholar]
- Nieminen P, Lehtiniemi H, Vähäkangas K, Huusko A, & Rautio A (2013). Standardised regression coefficient as an effect size index in summarizing findings in epidemiological studies. Epidemiology, Biostatistics and Public Health, 10, 1–15. 10.2427/8854 [DOI] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122, 902–916. 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Arfer KB, & Kujawa A (2021). Altered reward responsiveness and depressive symptoms: An examination of social and monetary reward domains and interactions with rejection sensitivity. Journal of Affective Disorders, 282, 717–725. 10.1016/j.jad.2020.12.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Ethridge P, Shields GS, Slavich GM, Weinberg A, & Kujawa A (2019). Blunted social reward responsiveness moderates the effect of lifetime social stress exposure on depressive symptoms. Frontiers in Behavioral Neuroscience, 13, 1–12. 10.3389/fnbeh.2019.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport BI, Hennefield L, Kujawa A, Arfer KB, Kelly D, Kappenman ES, Luby JL, & Barch DM (2019). Peer victimization and dysfunctional reward processing: ERP and behavioral responses to social and monetary rewards. Frontiers in Behavioral Neuroscience, 13, 1–11. 10.3389/fnbeh.2019.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runions KC, Wong J, Pace G, & Salmin I (2020). Borderline personality disorder and peers: A scoping review of friendship, victimization and aggression studies. Adolescent Research Review (Issue 0123456789). Springer International Publishing. 10.1007/s40894-020-00137-y [DOI] [Google Scholar]
- Simard V, & Dandeneau S (2018). Revisiting the Cyberball inclusion condition: Fortifying fundamental needs by making participants the target of specific inclusion. Journal of Experimental Social Psychology. doi: 10.1016/j.jesp.2017.08.002 [DOI] [Google Scholar]
- Stewart JG, Singleton P, Benau EM, Foti D, Allchurch H, Kaplan CS, ... & Auerbach RP (2019). Neurophysiological activity following rewards and losses among female adolescents and young adults with borderline personality disorder. Journal of Abnormal Psychology, 128(6), 610. 10.1037/abn0000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RL, Trull TJ, Wood PK, & Sher KJ (2014). Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. Journal of personality disorders, 28(5), 734–750. 10.1521/pedi_2012_26_093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Chmielewski M, McDade-Montez EA, Koffel E, Naragon K, & Stuart S (2008). Further validation of the IDAS: evidence of convergent, discriminant, criterion, and incremental validity. Psychological Assessment, 20(3), 248. 10.1037/a0012570 [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, ... & Stuart S (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19(3), 253. 10.1037/1040-3590.19.3.253 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Ethridge P, Pegg S, Freeman C, Kujawa A, & Dirks MA (2021). Neural responses to social acceptance predict behavioral adjustments following peer feedback in the context of a real-time social interaction task. Psychophysiology, July, 1–15. 10.1111/psyp.13748 [DOI] [PubMed] [Google Scholar]
- Weinbrecht A, Niedeggen M, Roepke S, & Renneberg B (2018). Feeling excluded no matter what? Bias in the processing of social participation in borderline personality disorder. NeuroImage: Clinical, 19(January), 343–350. 10.1016/j.nicl.2018.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund Schreiner M, Klimes-Dougan B, Begnel ED, & Cullen KR (2015). Conceptualizing the neurobiology of non-suicidal self-injury from the perspective of the Research Domain Criteria Project. Neuroscience and Biobehavioral Reviews, 57, 381–391. 10.1016/j.neubiorev.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Qin Y, Hu S, Dong L, Bringas Vega ML, & Valdés Sosa PA (2019). Which reference should we use for EEG and ERP practice?. Brain Topography, 32(4), 530–549. doi: 10.1007/s10548-019-00707-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, & Reynolds V (1998). Axis I comorbidity of borderline personality disorder. American Journal of psychiatry, 155(12), 1733–1739. 10.1176/ajp.155.12.1733 [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Horwood J, Wolke D, Waylen A, Fitzmaurice G, & Grant BF (2011). Prevalence of DSM-IV borderline personality disorder in two community samples: 6,330 English 11-year-olds and 34,653 American adults. Journal of Personality Disorders, 25(5), 607–619. 10.1521/pedi.2011.25.5.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, & Hennen J (2003). A screening measure for BPD: The Mclean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Journal of Personality Disorders, 17(6), 568–573. 10.1521/pedi.17.6.568.25355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


