Abstract
Background.
Vitamin D insufficiency is a potentially modifiable risk factor for poor outcomes in newly diagnosed large B-cell lymphoma (LBCL). However, the role of circulating vitamin D concentrations in relapsed/refractory LBCL treated with CD19-directed chimeric antigen receptor T-cell therapy (CAR-T) is currently unknown.
Objective and Study Design.
This was a single-center, observational study that evaluated the association of pre-CAR-T 25-hydroxyvitamin D (25-OHD) status with 100-day complete response, progression-free survival, overall survival, and CAR-T related toxicity in 111 adult relapsed/refractory LBCL patients. Vitamin D insufficiency was defined as ≤30 ng/ml in accordance with the Endocrine Society guidelines.
Results.
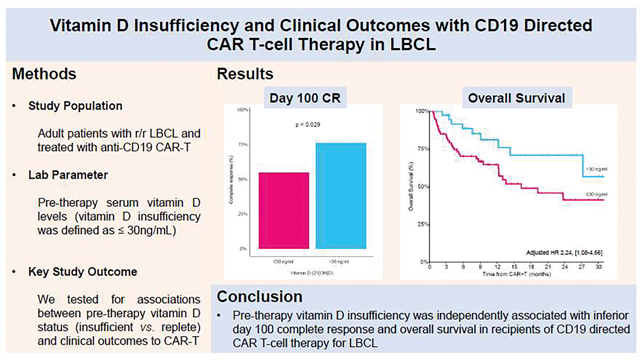
The median pre-CAR-T 25-hydroxyvitamin-D concentration was 24 ng/ml (IQR 18–34). Vitamin D insufficient patients (≤30 ng/ml; n=73 [66%]) were significantly younger than their vitamin D replete (>30 ng/ml; n=38 [34%]) counterparts (p=0.039). The vitamin D insufficient cohort was enriched for de novo LBCL as the histological subtype (p=0.026) and had a higher proportion of tisagenlecleucel as the CAR-T product (p=0.049). There were no other significant differences in the baseline characteristics between the two groups. In vitamin D insufficient compared to replete patients, 100-day complete response was 55% vs. 76% (p=0.029) and 2-year overall survival was 41% vs. 71% (p=0.061), respectively. In multivariate analysis, vitamin D insufficiency remained significantly associated with 100-day complete response (OR 2.58 [1.05–6.83]; p=0.045) and overall survival (HR 2.24 [1.08–4.66], p=0.030). In recipients of tisagenlecleucel, vitamin D insufficiency was associated with significantly lower cell viability of the infused CAR-T product (p=0.015). Finally, pretreatment vitamin D insufficiency did not predict for subsequent CAR-T related toxicity.
Conclusion.
This is the first report to demonstrate that vitamin D insufficiency is associated with inferior clinical outcomes in CAR-T recipients. Further study into the mechanistic insights of this finding, and the potential role of vitamin D supplementation to optimize CAR-T are warranted.
Graphical Abstract
Introduction
Although the primary role of vitamin D relates to calcium and bone mineral homeostasis, multiple non-classical immune functions including T-cell immunomodulation are well described (1, 2). Vitamin D has also been shown to augment rituximab-mediated cellular cytotoxicity (3). Indeed, insufficiency of 25-hydroxyvitamin-D (25-OHD) has been identified as a negative prognostic biomarker in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma treated with chemoimmunotherapy (3–5). Furthermore, achieving normalization of vitamin D levels with supplementation has been associated with improved outcomes in DLBCL (6). Based on these findings, the incorporation of vitamin D supplementation to rituximab in non-Hodgkin lymphoma is being actively investigated in a phase III clinical trial (NCT03078855). If deemed to be efficacious, vitamin D supplementation presents an attractive therapeutic strategy that is inexpensive and well tolerated.
Immunotherapy with chimeric antigen receptor T-cell therapy (CAR-T) has led to a transformational advancement in the management of relapsed/refractory large B-cell lymphoma (LBCL) and there are currently three anti-CD19-CAR-T products approved for this indication (7–9). However, the identification of patients with relapsed/refractory LBCL destined to respond poorly to anti-CD19-CAR-T remains elusive. To this end, clinical characteristics, laboratory biomarkers, T-cell intrinsic factors, and features of the infused CAR-T product have been evaluated to identify such poorly responding patients (10–13). However, the prognostic role of pre-therapy vitamin D insufficiency, which is a potentially modifiable risk factor, has not been studied in recipients of anti-CD19-CAR-T. Vitamin D has been suggested to control T-cell activation and antigen receptor signaling; this is directly relevant to CAR-T efficacy given that CAR T-cells are designed to recognize and eliminate cells bearing target antigens (2).
Given the poor outcomes of patients who do not achieve remission following CAR-T, it is critical to identify new interventions that may optimize outcomes of CAR-T recipients. Towards this end, we assessed the relationship between pretherapy vitamin D status and clinical and safety outcomes of anti-CD19-CAR-T in relapsed/refractory LBCL.
Materials and Methods
Study Participants
This study was approved by the Memorial Sloan Kettering Cancer Center (MSKCC) institutional review board. We retrospectively analyzed consecutive patients with relapsed/refractory LBCL who were treated with anti-CD19-CAR-T (axicabtagene ciloleucel [axi-cel], tisagenlecleucel [tisa-cel], and lisocabtagene maraleucel [liso-cel]) at our center between April 2016 and April 2021. Axi-cel and tisa-cel were given as standard therapy and liso-cel under the TRANSCEND-NHL-001 study (NCT02631044).
Vitamin D Testing
Vitamin D levels were not routinely monitored, and routine supplementation was not implemented. However, patients who had routine clinical 25-OHD testing by means of a quantitative chemiluminescent immunoassay up to one-year prior to CAR-T infusion were included. Vitamin D measurements were performed at a median of 14 days (IQR 3 – 209) prior to the CAR-T infusion and a median of 20 days (IQR: 154 days prior to leukapheresis and 34 days after leukapheresis) post-leukapheresis. Vitamin D insufficiency was defined as ≤30 ng/ml as recommended by the Endocrine Society (14). Furthermore, it corresponds with our institutional threshold for vitamin D inadequacy. Of the 183 patients with relapsed/refractory LBCL treated with CAR-T therapy, 111 patients (primary cohort) had a vitamin D measurement available within one-year preceding their CAR-T infusion. Compared with patients without vitamin D measurements, the primary cohort was more likely to be treated with axi-cel and tisa-cel, and less likely to be treated with liso-cel (p<0.001). Other baseline characteristics were not significantly different when comparing the two groups.
CAR-T infusion product
Cellular features of the CAR-T infusion product were available in patients treated with tisa-cel. This included the CAR transduction efficiency, CAR surface expression, proportion of CAR-T cells with cell viability ≥80%, dose of CAR-T cells, absolute number of viable cells, and in vitro IFN-γ release in response to CD19 expressing target cells.
Study Outcomes
The primary objective was to evaluate whether circulating vitamin D status was associated with CR at 100 days post CAR-T infusion. Response was calculated relative to the most recent disease assessment before infusion of CAR-T and response assessment was performed according to the Lugano criteria (15). Secondary endpoints included progression-free survival (PFS), overall survival (OS), and rates of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). PFS was defined as the time from the date of CAR-T infusion to the date of event defined as the first documented progression or death due to any cause, and OS was the time from CAR-T infusion to death due to any cause. Grading for CRS and ICANS was defined as per the American Society for Transplantation and Cellular Therapies (ASTCT) consensus guidelines (16). Finally, we tested for potential associations between vitamin D levels and the cellular features of the tisa-cel infusion product.
Statistical Analysis
Descriptive statistics, including median and interquartile range [IQR] for continuous variables, and percentages for categorical variables, are provided. Fisher’s exact test or χ2 test was used to evaluate the association between two categorical variables. Wilcoxon rank-sum test or Kruskal-Wallis test was used to assess the difference in a continuous variable between/among patient groups. Kaplan-Meier method was used to estimate overall survival (OS) and progression-free survival (PFS). Relapse or progression incidence was estimated using cumulative incidence; death without relapse or progression was considered as a competing event. Univariable logistic regression was performed to assess the association between disease-related features and response and were studied as binary categorical variable (complete response [CR] vs. non-CR). We also evaluated vitamin D status in a multivariable model adjusted for age, primary refractory disease and CAR-T construct costimulatory domain. Univariable Cox proportional hazard regression was performed to evaluate OS and PFS risk associated with disease-related determinants. Vitamin D status was also studied in multivariable Cox-regression models, adjusting for the same covariate as in the multivariable logistic regression model. Potential associations between vitamin D levels and the cellular features of the tisa-cel infusion product were tested using Spearman Rho correlation. All analyses and graphics were produced using R version 4.0.3.
Results
The 111 patients in the primary cohort had mainly de novo LBCL (57%), were heavily pre-treated (>3 prior lines; 55%), and treated with axi-cel (54%), tisa-cel (40%), and liso-cel (6%). The overall response rate (ORR) at 100 days was 77% (CR 62%). With a median follow-up of 18.6 months (IQR 8.8–30.3), the median PFS and OS were 6.3 months (95% CI 3.4 −19.3) and 27.3 months (95% CI 14.3 – NR), respectively. At 1 year, the cumulative incidence of relapse or progression was 56% (46% to 65%). Grade ≥3 CRS and grade ≥3 ICANS was observed in 8% and 14% of patients, respectively.
The median pre-CAR-T vitamin D concentration was 24ng/mL (IQR 18–34), reflecting vitamin D insufficiency in 66% (n=73) using our threshold (≤ 30 ng/ml). Patients with vitamin D insufficiency were more likely to be ≤ 65-years (p=0.039), have a de novo DLBCL histology (p=0.026) and have received tisa-cel (p=0.049). There were no other significant differences in baseline characteristics, including the Karnofsky performance score (Table 1).
Table 1.
Baseline characteristics by vitamin D status
| Characteristic | Overall N = 1111 | Vitamin D replete (> 30ng/ml) N = 381 | Vitamin D insufficient (≤ 30ng/ml) N = 731 | p-value2 |
|---|---|---|---|---|
| Age | 0.039 | |||
| ≤ 65-years | 53 (48%) | 13 (34%) | 40 (55%) | |
| > 65-years | 58 (52%) | 25 (66%) | 33 (45%) | |
| Sex | 0.69 | |||
| Male | 70 (63%) | 23 (61%) | 47 (64%) | |
| Female | 41 (37%) | 15 (39%) | 26 (36%) | |
| Karnofsky Performance Status | 0.37 | |||
| ≥ 90 | 84 (79%) | 28 (74%) | 56 (81%) | |
| < 90 | 23 (21%) | 10 (26%) | 13 (19%) | |
| Unknown | 4 | 0 | 4 | |
| LBCL subtype | 0.026 | |||
| de novo LBCL | 62 (57%) | 15 (42%) | 47 (65%) | |
| transformed follicular lymphoma | 26 (24%) | 14 (39%) | 12 (17%) | |
| Other transformed low-grade | 20 (19%) | 7 (19%) | 13 (18%) | |
| Unknown | 3 | 2 | 1 | |
| Cell of origin | 0.83 | |||
| GCB | 49 (49%) | 17 (50%) | 32 (48%) | |
| Non-GCB | 52 (51%) | 17 (50%) | 35 (52%) | |
| Unknown | 10 | 4 | 6 | |
| Double/Triple hit | 11 (12%) | 5 (16%) | 6 (10%) | 0.50 |
| Unknown | 18 | 6 | 12 | |
| History of CNS disease | 21 (19%) | 5 (14%) | 16 (22%) | 0.32 |
| Unknown | 2 | 2 | 0 | |
| Primary refractory disease 3 | 32 (29%) | 9 (24%) | 23 (32%) | 0.36 |
| Unknown | 1 | 0 | 1 | |
| Previous autologous HCT | 27 (24%) | 8 (21%) | 19 (26%) | 0.56 |
| Previous allogeneic HCT | 7 (6%) | 3 (8%) | 4 (5%) | 0.69 |
| Number of previous lines | 0.61 | |||
| ≤ 3 lines | 50 (45%) | 16 (42%) | 34 (47%) | |
| 4 – 5 lines | 43 (39%) | 17 (45%) | 26 (36%) | |
| ≥ 6 lines | 18 (16%) | 5 (13%) | 13 (18%) | |
| Bulky disease at apheresis | 10 (9%) | 2 (5%) | 8 (11%) | 0.49 |
| Stage at Apheresis | 0.39 | |||
| ≤ II | 30 (27%) | 12 (32%) | 18 (25%) | |
| III – IV | 80 (73%) | 25 (68%) | 55 (75%) | |
| Unknown | 1 | 1 | 0 | |
| Bridging | 79 (72%) | 30 (79%) | 49 (68%) | 0.23 |
| Unknown | 1 | 0 | 1 | |
| Status at CAR-T infusion | 0.85 | |||
| CR | 9 (8%) | 3 (8%) | 6 (8%) | |
| PR | 25 (23%) | 10 (27%) | 15 (21%) | |
| SD/PD | 74 (69%) | 24 (65%) | 50 (70%) | |
| Unknown | 3 | 1 | 2 | |
| Time from diagnosis to CAR-T infusion (days) | 554 (316 – 924) | 687 (359 – 1,335) | 468 (314 – 803) | 0.12 |
| CAR-T Product | 0.049 | |||
| Axicabtagene ciloleucel | 60 (54%) | 22 (58%) | 38 (52%) | |
| Tisagenlecleucel | 44 (40%) | 11 (29%) | 33 (45%) | |
| Lisocabtagene maraleucel | 7 (6%) | 5 (13%) | 2 (3%) | |
| CAR-T costimulatory domain | 0.56 | |||
| 41BB | 51 (46%) | 16 (42%) | 35 (48%) | |
| CD28 | 60 (54%) | 22 (58%) | 38 (52%) |
Median (IQR); n (%)
Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test
Primary refractory disease pre-apheresis
LBCL, large B-cell lymphoma; GCB, germinal center B-cell; CNS, central nervous system; HCT, hematopoietic stem cell transplant; LDH, lactate dehydrogenase; CAR-T, chimeric antigen receptor T-cell therapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease
Given the important role of vitamin D in modulating T-cell responses (1, 2), we examined whether vitamin D insufficiency pre-CAR-T was associated with response rates. In a univariable analysis, only vitamin D insufficiency (odds ratio [OR] 2.66, [95% CI 1.14–6.68]; p=0.029) and primary refractory disease (OR 2.55, [1.10–6.01]; p=0.030 were associated with a lower likelihood of achieving CR. The day 100 CR rate was 76% (ORR 97%) in vitamin D insufficient compared to 55% (ORR 67%) in the replete group (p=0.029). Vitamin D insufficiency remained an independent predictor of day 100 CR (OR 2.58, [1.05–6.83]; p=0.045) in a multivariable model adjusting for age, primary refractory disease and the CAR-T costimulatory molecule (CD28 or 4–1BB) – Table 2. The only other variable that maintained its association with day 100 CR on multivariable analysis was primary refractory disease (OR 2.58, [1.05–6.83], p=0.029).
Table 2.
Multivariate Analysis of Complete Response and Overall Survival
| Characteristic | Logistic Regression for CR | Cox Proportional Hazard for OS | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | OR | 95% CI | P | No. | HR | 95% CI | P | |
| Vitamin D status | ||||||||
| > 30ng/mL | 38 | — | — | 38 | — | — | ||
| ≤ 30ng/mL | 73 | 2.58 | 1.05 to 6.83 | 0.045 | 73 | 2.24 | 1.08 to 4.66 | 0.030 |
| Age | ||||||||
| ≤ 65-years | 53 | — | — | 53 | — | — | ||
| > 65-years | 58 | 1.36 | 0.56 to 3.37 | 0.49 | 58 | 1.99 | 1.02 to 3.91 | 0.044 |
| Primary refractory disease | ||||||||
| No | 78 | — | — | 78 | — | — | ||
| Yes | 32 | 2.73 | 1.12 to 6.91 | 0.029 | 32 | 2.42 | 1.23 to 4.74 | 0.010 |
| CAR-T costimulatory domain | ||||||||
| 41BB | 51 | — | — | 51 | — | — | ||
| CD28 | 60 | 0.75 | 0.30 to 1.83 | 0.52 | 60 | 0.72 | 0.37 to 1.40 | 0.33 |
CR, complete response; OS overall survival; CAR-T, chimeric antigen receptor T-cell therapy
In a univariable analysis for OS, the risk of death was greater in vitamin D insufficient versus vitamin D replete patients and approached statistical significance (hazard ratio [HR] 1.97 [0.97–4.02]; p=0.061; Figure 1). The 2-year survival rate in vitamin D insufficient versus vitamin D replete patients was 41% (95% CI 28% to 60%) and 71% (55% to 91%), respectively. In a multivariate model, vitamin D insufficiency was a significant independent predictor of OS (HR 2.24, [1.08–4.66]; p=0.030) – Table 2. Vitamin D insufficiency was not associated with lower PFS in univariable (HR 1.35, [0.80–2.66]; p=0.26, Supplementary Figure 1) and multivariable models (HR 1.39, [0.81–2.38]; p=0.23). Both the OS and PFS multivariable models were adjusted for the same factors as the CR model. The most common cause of death in both groups was relapse/progression of the underlying malignancy, which accounted for 90% of deaths in both groups (vitamin D insufficient, n=28 and vitamin D replete, n=9). Among non-relapse mortality (NRM), viral infection was the leading cause in both groups (vitamin D insufficient, 6% [n=2] and vitamin D replete, 10%, [n=1]). Cumulative incidence of relapse, and cumulative incidence of NRM curves are provided in Supplementary Figures 2–3, respectively.
Figure 1.
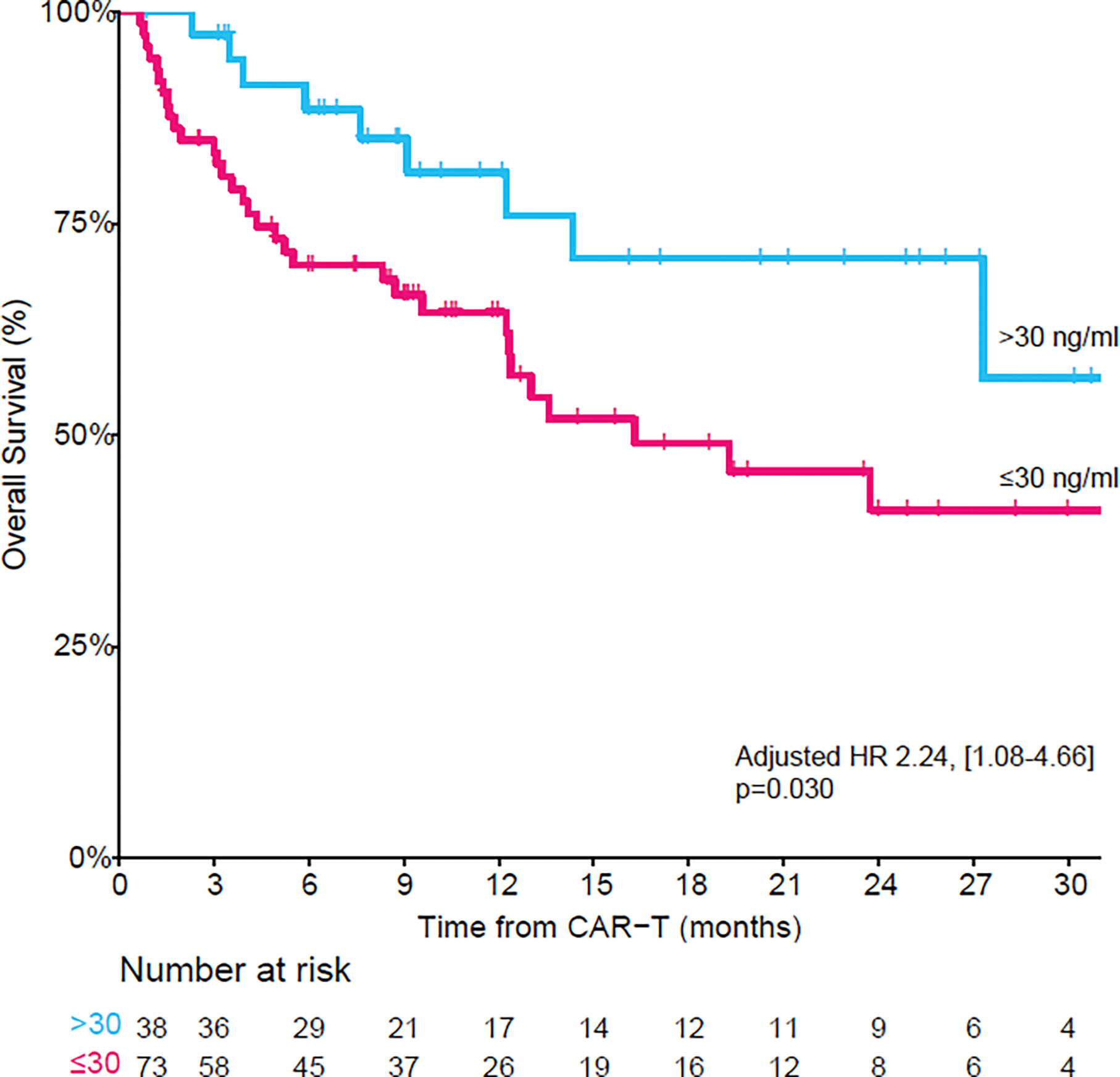
Overall survival by vitamin D status demonstrating significantly inferior overall survival in patients with vitamin D insufficiency. (In a multivariate model adjusted for vitamin D status, age, primary refractory disease and CAR-T costimulatory domain, vitamin D insufficiency remained a significant predictor of overall survival).
Previous studies have suggested that vitamin D promotes a shift from a pro-inflammatory to a tolerogenic immune state (17). However, we found no significant association between vitamin D status and CAR-T toxicity. In vitamin D insufficient compared to replete patients, grade ≥3 CRS was present in 7% vs. 8% (p>0.99), and grade ≥3 ICANS in 13% vs. 16% (p=0.61) of patients, respectively.
In the 44 patients treated with tisa-cel, we assessed whether pre-therapy vitamin D status associated with cellular features of the infusion product. The proportion of CAR-T cells with ≥80% viability was significantly higher in vitamin D replete patients compared to their vitamin D insufficient counterparts (p=0.015). When tested as a continuous variable, there was a moderate correlation between serum 25-OHD and CAR-T viability (Spearman’s correlation 0.34, p=0.023) – Figure 2. There was no significant association between pre-CAR-T therapy vitamin D levels and the other cellular features of the tisa-cel product.
Figure 2.
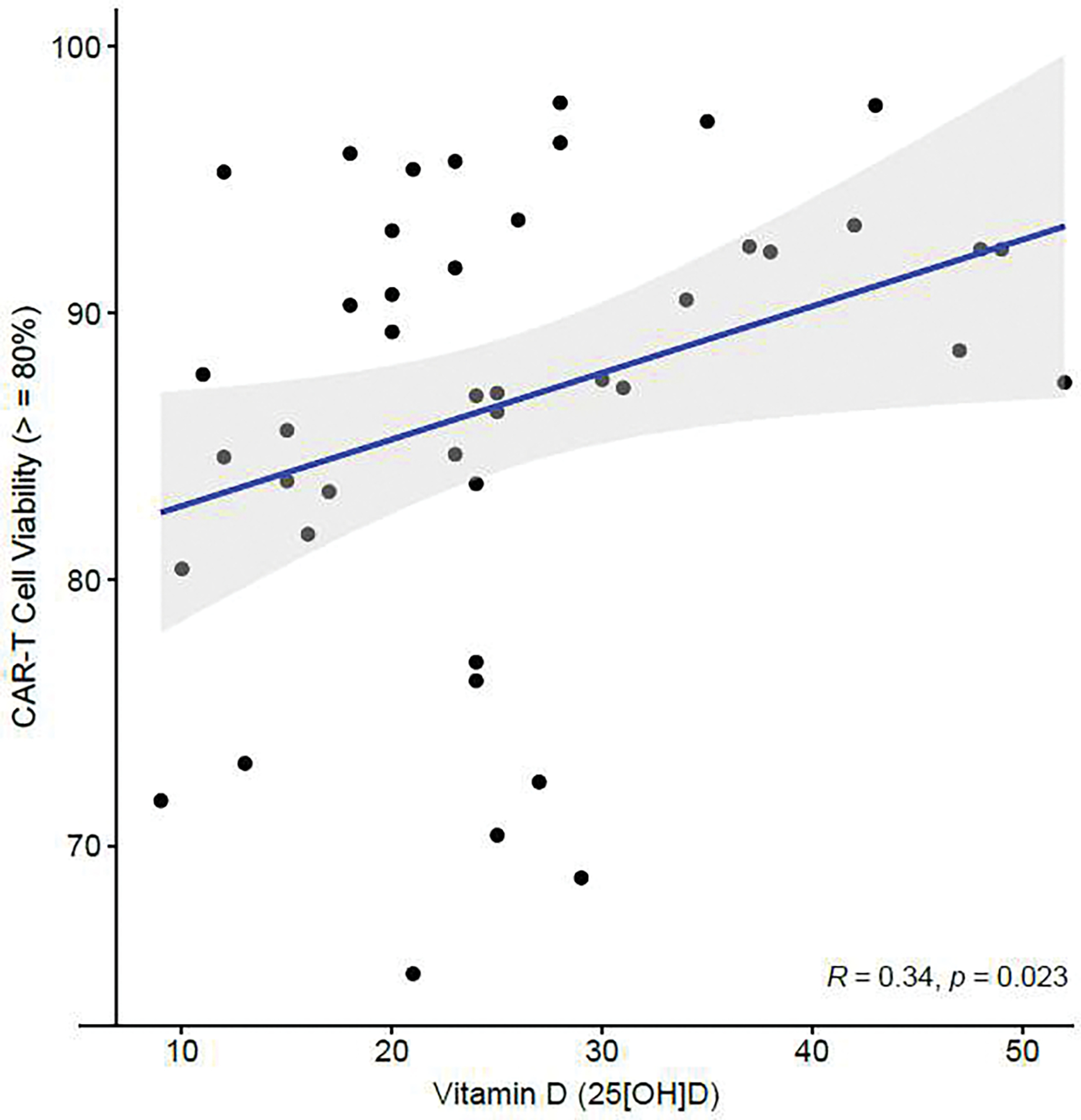
Correlation between pre-CAR-T serum vitamin D levels and proportion of CAR-T cells with ≥80% viability within the tisa-cel infusion product (n=44). The Spearman correlation was applied.
Discussion
The identification of potentially modifiable factors that determine the clinical performance of CAR-T remains a substantial challenge. Vitamin D insufficiency has not been previously evaluated in CAR-T recipients. In this study, we performed analysis of serum vitamin D levels collected prior to anti-CD-19 CAR-T administration in relapsed/refractory LBCL patients and found that pre-CAR-T vitamin D insufficiency was independently associated with inferior day 100 CR and OS rates. We observed no difference in CAR-T toxicity between vitamin D insufficient and replete patients. Pre-therapy serum 25-OHD levels positively correlated with the proportion of viable CAR-T cells within the infusion product.
Prior studies of lymphoma patients treated with chemoimmunotherapy have identified low pretreatment vitamin D levels as a negative prognostic factor for overall survival (3–5, 18). Correlative laboratory studies showed that vitamin D acts synergistically with cytostatic drugs and amplifies their anti-proliferative effects (18). Indeed, the vitamin D receptor is expressed on cells of the immune system, including B-cells, where vitamin D has been shown to induce B-cell apoptosis (19). Vitamin D is also known to play an important role in immune regulation and demonstrated to augment rituximab-mediated cellular cytotoxicity in LBCL (3, 20). The impact of vitamin D to regulate immune signaling pathways may have implications for CAR-T efficacy, which is a form of cellular immunotherapy. The possible association between pre-therapy vitamin D and CAR-T viability within the tisa-cel infusion product is intriguing and suggests that vitamin D may play a role in the subsequent function of the engineered anti-CD19 CAR T-cell product. Though speculative, the potential fortuitous impact of vitamin D to enhance the T-cell phenotypes within the leukapheresis product warrants consideration. Deeper biological mechanistic insights of vitamin D’s impact on microenvironmental signals and T-cell phenotypes in CAR-T recipients is required.
In our study, there was no association between vitamin D status and pre-lymphodepletion bulky disease, which has been previously demonstrated to predict response to axi-cel in LBCL (11). Other studies have suggested that there may be a confounding effect of poor performance status and older age with vitamin D insufficiency (21, 22). Both these clinical factors have been shown to influence outcomes to frontline chemoimmunotherapy and are included within current LBCL prognostic scores (23). However, there was no significant association between vitamin D insufficiency and performance status in our cohort. Additionally, vitamin D insufficient patients were younger than their vitamin D replete counterparts. This suggests that vitamin D insufficiency was not simply a surrogate for older age or poor performance status in this cohort.
This study has several limitations. Given its retrospective nature, vitamin D testing was performed across various timepoints within the 12 months prior to CAR-T infusion and the reasons for vitamin D testing were not able to be collected. We suggest the need for additional studies to prospectively validate our findings. Future study design considerations should include a uniform timepoint to test serum vitamin D levels, ideally just before leukapheresis, and with liquid chromatography-tandem mass spectrometry for 25(OH)D testing, which is considered the gold standard for vitamin D testing. The potential impact of vitamin D on T-cell phenotypes within the apheresis and subsequent CAR-T infusion product should also be explored. Although there was no enrichment of infectious complications as a cause of death in those with vitamin D insufficiency, this was a relatively small cohort with few death events. Further evaluation of infectious complications and post-CAR-T immune reconstitution by vitamin D status warrants consideration. Indeed, in allogeneic hematopoietic stem cell transplant recipients, it has been suggested that relapse prevention in the setting of higher vitamin D levels can potentially be related to enhanced immune reconstitution (24). Pending validation, this study may provide support for an interventional trial of vitamin D supplementation in future CAR-T recipients. Furthermore, adjunctive investigational intervention of potential CAR-T enhancement with vitamin D is appealing given its low-cost and low-risk.
In conclusion, to the best of our knowledge, this is the first study evaluating the association between vitamin D, a potentially modifiable risk factor, and clinical outcomes of CAR-T recipients. We found that pre-therapy vitamin D insufficiency is independently associated with inferior clinical outcomes. Given the retrospective nature of this analysis, additional studies are required to validate our findings. Further investigation of the potential mechanisms by which vitamin D may augment CAR-T responses are also required.
Supplementary Material
Highlights:
Vitamin D insufficiency is associated with inferior CR to CAR-T in LBCL
Vitamin D insufficiency is associated with inferior OS to CAR-T in LBCL
There is no association between vitamin D status and CAR-T related toxicity
Acknowledgments
This research is supported by the Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748) from the National Institutes of Health/National Cancer Institute. RS was supported by the American Society of Transplantation and Cellular Therapy New Investigator Award, the American Society of Hematology Fellow Scholar Award, a grant from the Long Island Sound Chapter, Swim Across America, the Robert Hirschhorn Award, and the Memorial Sloan Kettering Steven Greenberg Lymphoma Research Award. A.A.T. was supported by a grant from the Alfonso Martin Escudero Foundation. J.A.F. was supported by a grant from the American Society of Hematology.
Footnotes
Disclosure of Conflicts of Interest
Roni Shouval. Consulting or Advisory Role: Medexus, MyBiotics; Connie Lee Batlevi Stock and Other Ownership Interests: Moderna Therapeutics, Novavax, Pfizer, Bristol Myers Squibb, Regeneron, Viatris. Honoraria: DAVA Oncology. Consulting or Advisory Role: LifeSci Capital, GLG, Juno Therapeutics, Celgene, Seattle Genetics, Kite, a Gilead company, TG Therapeutics, Karyopharm Therapeutics. Research Funding: Janssen Biotech (Inst), Novartis (Inst), Epizyme (Inst), Xynomic Pharma (Inst), Bayer (Inst), Roche (Inst), Autolus (Inst). Open Payments Link: https://openpaymentsdata.cms.gov/physician/2778694; Parastoo B. Dahi. Consulting or Advisory Role: Kite, a Gilead company; Sergio A. Giralt. Honoraria: Celgene, Takeda, Amgen, Jazz Pharmaceuticals, Sanofi. Consulting or Advisory Role: Celgene, Takeda, Sanofi, Jazz Pharmaceuticals, Amgen, Janssen, Actinuum, Bristol Myers Squibb, Johnson & Johnson, Pfizer. Research Funding: Celgene (Inst), Miltenyi Biotec, Johnson & Johnson, Amgen, Actinuum, Sanofi. Travel, Accommodations, Expenses: Celgene, Sanofi, Amgen, Jazz Pharmaceuticals; Gilles Salles. Honoraria: Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys. Consulting or Advisory Role: Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, BMS, BeiGene, Incyte, Miltenyi Biotec, Ipsen; Craig S. Sauter. Consulting or Advisory Role: Spectrum Pharmaceuticals, Juno Therapeutics, Sanofi, Gilead Sciences, Novartiş Precision BioSciences, Gamida Cell, Karyopharm Therapeutics, GlaxoSmithKline, Genmab. Research Funding: Juno Therapeutics (Inst), Sanofi (Inst), Precision BioSciences (Inst), BMS (Inst), Actinium Pharmaceuticals (Inst). Travel, Accommodations, Expenses: Juno Therapeutics, Sanofi, Gilead Sciences, Novartis; Michael Scordo. Honoraria: i3 CME. Consulting or Advisory Role: McKinsey & Company, Angiocrine Bioscience, Omeros. Research Funding: Angiocrine Bioscience, Omeros (Inst). Travel, Accommodations, Expenses: Kite/Gilead; Gunjan L. Shah. Research Funding: Amgen (Inst), Janssen (Inst); Miguel-Angel Perales. Stock and Other Ownership Interests: NexImmune and Omeros. Honoraria: MorphoSys. Consulting or Advisory Role: Incyte, Merck, Servier/Pfizer, NexImmune, Novartis, MolMed, Medigene, Takeda, Nektar, AbbVie, Cidara Therapeutics, Celgene, Kite/Gilead, Bristol Myers Squibb, Omeros, Vor Biopharma. Research Funding: Incyte (Inst), Miltenyi Biotec (Inst), Novartis (Inst), Kite, a Gilead company (Inst), Nektar (Inst); Maria Lia Palomba. Stock and Other Ownership Interests: Seres Therapeutics (I). Honoraria: Flagship Biosciences (I), Evelo Therapeutics (I), Jazz Pharmaceuticals (I), Therakos (I), Amgen (I), Merck (I), Seres Therapeutics (I). Consulting or Advisory Role: Flagship Biosciences (I), Novartis (I), Evelo Therapeutics (I), Jazz Pharmaceuticals (I), Therakos (I), Amgen (I), Merck (I), Seres Therapeutics (I), Kite, a Gilead company, Novartis, BeiGene, Synthekine. Research Funding: Seres Therapeutics (I) Patents, Royalties, Other Intellectual Property: Intellectual Property Rights (I), Juno intellectual property rights (Inst); Jonathan U. Peled. Research funding, intellectual property fees, and travel reimbursement: Seres Therapeutics. Consulting fees: DaVolterra, CSL Behring, and from MaaT Pharma. Advisory board and holds equity in Postbiotics Plus Research; No other potential conflicts of interest were reported by the other co-authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–7. [DOI] [PubMed] [Google Scholar]
- 2.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Ødum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nature Immunology. 2010;11(4):344–9. [DOI] [PubMed] [Google Scholar]
- 3.Bittenbring JT, Neumann F, Altmann B, Achenbach M, Reichrath J, Ziepert M, et al. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol. 2014;32(29):3242–8. [DOI] [PubMed] [Google Scholar]
- 4.Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(27):4191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly JL, Salles G, Goldman B, Fisher RI, Brice P, Press O, et al. Low Serum Vitamin D Levels Are Associated With Inferior Survival in Follicular Lymphoma: A Prospective Evaluation in SWOG and LYSA Studies. Journal of Clinical Oncology. 2015;33(13):1482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohaus S, Tisi MC, Bellesi S, Maiolo E, Alma E, Tartaglia G, et al. Vitamin D deficiency and supplementation in patients with aggressive B-cell lymphomas treated with immunochemotherapy. Cancer Medicine. 2018;7(1):270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine. 2018;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 9.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet. 2020;396(10254):839–52. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Advances. 2020;4(19):4898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Iyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137(23):3272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nature Medicine. 2020;26(12):1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. [DOI] [PubMed] [Google Scholar]
- 17.Macedo R, Pasin C, Ganetsky A, Harle D, Wang XK, Belay K, et al. Vitamin D deficiency after allogeneic hematopoietic cell transplantation promotes T-cell activation and is inversely associated with an EZH2-ID3 signature. Transplant Cell Ther. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borchmann S, Cirillo M, Goergen H, Meder L, Sasse S, Kreissl S, et al. Pretreatment Vitamin D Deficiency Is Associated With Impaired Progression-Free and Overall Survival in Hodgkin Lymphoma. Journal of Clinical Oncology. 2019;37(36):3528–37. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–47. [DOI] [PubMed] [Google Scholar]
- 20.Wei R, Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients. 2015;7(10):8251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran DS, McClung JP, Kohen T, Lieberman HR. Vitamin d and physical performance. Sports Med. 2013;43(7):601–11. [DOI] [PubMed] [Google Scholar]
- 22.Meehan M, Penckofer S. The Role of Vitamin D in the Aging Adult. J Aging Gerontol. 2014;2(2):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–61. [DOI] [PubMed] [Google Scholar]
- 24.Radujkovic A, Kordelas L, Krzykalla J, Beelen DW, Benner A, Lehners N, et al. Pretransplant Vitamin D Deficiency Is Associated With Higher Relapse Rates in Patients Allografted for Myeloid Malignancies. J Clin Oncol. 2017;35(27):3143–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


