Abstract
Background
Patients with premenopausal breast cancer (PMBC) have been historically excluded from some clinical trials because of the limitations of using endocrine therapy (ET) in this population. We analyzed breast cancer randomized clinical trials (RCTs) to determine the rates of and factors associated with inclusion of PMBC patients to provide a benchmark for PMBC inclusion in RCTs moving forward.
Methods
Using ClinicalTrials.Gov, we identified breast cancer phase III RCTs and extracted inclusion criteria and patient enrollment information. Multiple binary logistic regression modeling was used to assess trial-related factors that were associated with PMBC patient inclusion.
Results
Of 170 breast cancer RCTs identified, 131 (77.1%) included PMBC patients. Sixty-five (38.2%) trials analyzed patients with hormone-receptor-positive (HR+) and HER2-negative (HER2-) breast cancer, of which 31 (47.7%) allowed for enrollment of PMBC patients. Lower rates of PMBC inclusion were seen in trials that studied HR+/HER2-patients (47.7% PMBC inclusion in HR+/HER2-trials vs. 94.3% in non-HR+/HER2-trials, aOR 0.07 [95% CI: 0.02–0.19], p < 0.001) and in trials that randomized or mandated ET (44.4% in ET trials vs. 83.2% in non-ET trials, aOR 0.21 [95% CI: 0.10–0.83], p = 0.02). Trials studying chemotherapy (CT) were associated with inclusion of PMBC patients (100% in CT trials vs. 70.5% in non-CT trials, a OR 14.02 [95% CI: 1.54–127.91], p = 0.01). All surgical and radiation therapy clinical trials allowed for the inclusion of PMBC patients in their eligibility criteria.
Conclusions
Breast cancer clinical trials should carefully select their enrollment criteria and consider inclusion of premenopausal patients when appropriate.
Keywords: Premenopausal, Breast cancer, Eligibility criteria, Randomized controlled trial, Food and drug administration
Highlights
-
•
We investigated the inclusion of premenopausal breast cancer (PMBC) patients in clinical trials.
-
•
There was lower inclusion of PMBC in trials studying HR+/HER2-patients.
-
•
There was lower inclusion of PMBC in trials studying endocrine therapy.
-
•
Trials should carefully select their enrollment criteria and include PMBC patients when appropriate.
1. Introduction
Breast cancer (BC) in premenopausal women accounts for 30.9% of all breast cancer cases diagnosed globally [1]. The National Comprehensive Cancer Network's (NCCN) treatment recommendations are similar for premenopausal breast cancer (PMBC) and postmenopausal breast cancer patients, with the most notable exception being the type of endocrine therapy (ET) recommended [2]. Some studies have shown that non-metastatic PMBC patients have higher rates of hormone-receptor-positive (HR+) status, breast-conserving surgery, radiation therapy, chemotherapy, and ET as compared to their postmenopausal counterparts [[3], [4]]. Regardless of treatment received, younger patients have a higher likelihood of recurrence and worse cancer-specific mortality [[5], [6]].
HR+ breast cancer is the most common breast cancer type in premenopausal women [[7], [8]]. ET is a recommended component of the treatment regimen for HR+ breast cancer; however, some ETs, like aromatase inhibitors, do not work in PMBC patients due to the active estrogen-promoting hormonal axis in premenopausal women [9]. To date, trial investigators have been restricted in their ability to include PMBC patients in randomized controlled trials (RCTs) potentially due to ET requirements in the trial [10]. Since PMBC patients are excluded from some RCTs, additional studies are required to show safety and efficacy of new therapies in PMBC, and this may delay their approval and use in premenopausal patients. We investigated trial factors associated with premenopausal patient inclusion in breast cancer phase III RCTs to provide a benchmark for PMBC inclusion in RCTs for future studies.
2. Methods
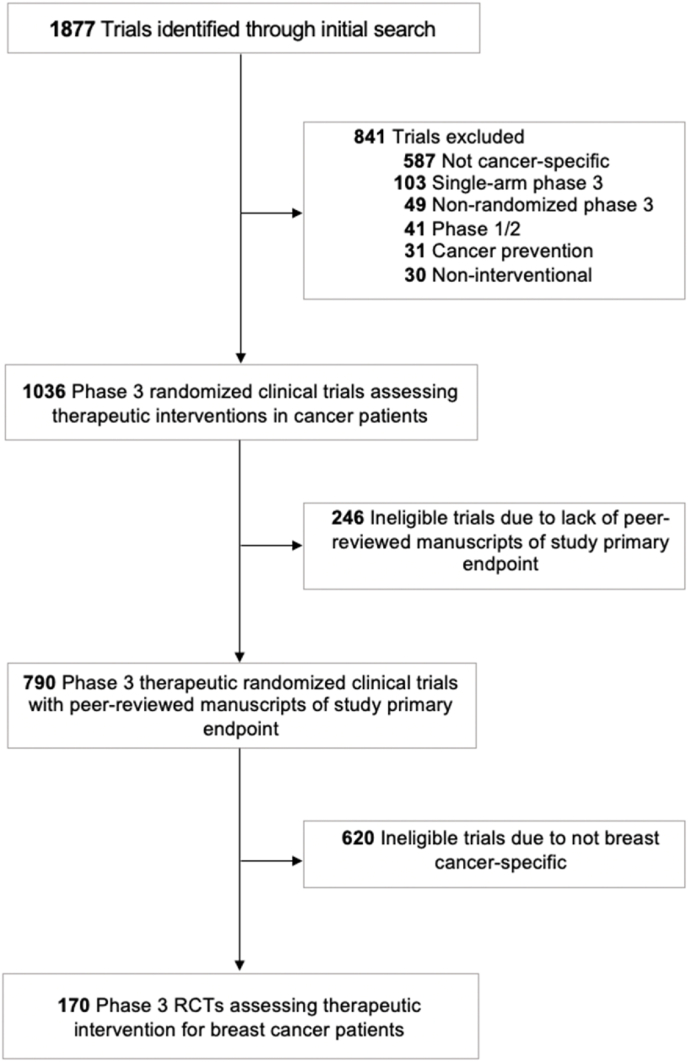
Breast cancer RCTs were identified through a search of ClinicalTrials.gov using the following parameters: Terms: “cancer”; Status: excluded: “Not yet recruiting”; Phase: Phase 3; and Study Results: “With Results.” This search yielded 1877 trials, which were screened for breast cancer-specific RCTs that addressed a therapeutic intervention (Fig. 1). Information regarding enrollment criteria was collected from ClinicalTrials.gov, the study protocol, and the primary publication of endpoint results (as available) for included trials. Two individuals independently performed trial screening and data collection. Menopause was determined according to the trial protocol or the NCCN's previously stated definition: prior bilateral oophorectomy, age ≥60 years, age <60 years and amenorrheic for 12 or more months in the absence of chemotherapy, ET, and ovarian suppression and FSH and estradiol are in the postmenopausal range, and FSH and estradiol in the postmenopausal range in women aged <60 years and who are taking ET.2 Trial-related factors were assessed for association with PMBC patient inclusion in BC trials using Pearson's Chi-squared testing for univariate analyses. Trial-related factors that had p < 0.05 on univariate analysis were included in multiple binary logistic regression modeling for multivariable analysis (SPSS, Version 22.0).
Fig. 1.
Study flowchart of included trials.
3. Results
One-hundred seventy BC-specific RCTs were identified that opened for enrollment between 2002 and 2015 and were included for analysis (Fig. 1). One-hundred thirty-one (77.1%) allowing for enrollment of PMBC patients. Among these 131 trials, 79 reported the number of PMBC patients included. These 79 trials had a total enrollment of 133,834 patients with 36,758 premenopausal women (27.5%); median percent of PMBC enrollment per trial was 45.8% (interquartile range: 30.1%–86.5%). Of the 170 BC-specific RCTs, 65 (38.2%) studied HR+/HER2-patients, 43 (25.3%) studied HER2+ patients, 5 (2.9%) studied triple-negative patients, and 57 (33.5%) had no hormone receptor eligibility requirements. Among trials studying HR+/HER2-patients, 31 (47.7%) allowed for enrollment of PMBC patients. Among all trials, four (2.4%) allowed for inclusion of premenopausal women taking ovarian suppression (OS) that induced hormone levels consistent with the post-menopausal state.
Table 1 shows trial-related factors associated with inclusion of PMBC in phase III clinical trials. On univariate analysis, industry-funding (p = 0.69), cooperative group sponsorship (p = 0.41), and trials meeting their primary endpoint (p = 0.37) were not associated with differences in inclusion of patients with PMBC (Table 1). Trials that studied a targeted therapy (TT) or included patients with metastatic disease had less PMBC patient inclusion, but those results did not show statistical significance (68% PMBC inclusion in TT trials vs. 83% in non-TT trials, p = 0.27; 80% PMBC inclusion in non-metastatic trials vs. 61% in metastatic trials, p = 0.42; Table 1).
Table 1.
Trial-related factors associated with inclusion of pre-menopausal breast cancer patients in phase III clinical trials. Abbreviations: pre-menopausal breast cancer (PMBC), hormone-receptor-positive (HR+), triple-negative breast cancer (TNBC), adjusted odds ratio (aOR), confidence interval (CI), endocrine therapy (ET).
| Premenopausal Inclusion N/Ntotal (%) |
Univariate Analysisa | Multiple Binary Logistic Regression |
||
|---|---|---|---|---|
| aOR (95% CI) | P-Value | |||
| Cancer Stage | ||||
| Stages 0 – lll | 52/65 (80%) | 0.42 | ||
| Stage IV | 64/105 (60.9%) | |||
| Receptor Status | <0.001 | |||
| HER2+, TNBC, or any | 99/105 (94.3%) | – | – | |
| HR + HER2- | 31/65 (47.7%) | 0.07 (0.02–0.19) | <0.001 | |
| Endocrine Therapy | <0.001 | |||
| No ET | 119/143 (83.2%) | – | – | |
| Randomized or mandated ET | 12/27 (44.4%) | 0.21 (0.10–0.83) | 0.02 | |
| Cytotoxic Chemotherapy | <0.001 | |||
| No chemotherapy | 93/132 (70.5%) | – | – | |
| Randomized or mandated chemotherapy | 38/38 (100%) | 15.13 (1.78–128.42) | 0.01 | |
| Targeted Therapy | ||||
| No targeted therapy | 110/132 (83.3%) | 0.27 | ||
| Randomized or mandated targeted therapy | 21/31 (67.7%) | |||
| Immunotherapy | ||||
| No immunotherapy | 88/122 (72.1%) | 0.04 | – | – |
| Randomized or mandated immunotherapy | 43/48 (89.6%) | 2.19 (0.68–7.12) | 0.19 | |
| Industry Sponsorship | ||||
| No | 90/115 (78.3%) | 0.69 | ||
| Yes | 41/55 (75.5%) | |||
| Cooperative Group Sponsorship | ||||
| No | 39/47 (83%) | 0.41 | ||
| Yes | 92/123 (74.8%) | |||
| Trials that met their primary endpoint (PEP) | ||||
| No | 23/36 (63.9%) | 0.37 | ||
| Yes | 99/121 (81.8%) | |||
a Pearson's Chi-squared testing for univariate analyses was used in univariate analyses to assess the association between trial-related factors and PMBC inclusion. Trial-related factors that had p < 0.05 were included in multiple binary logistic regression analysis.
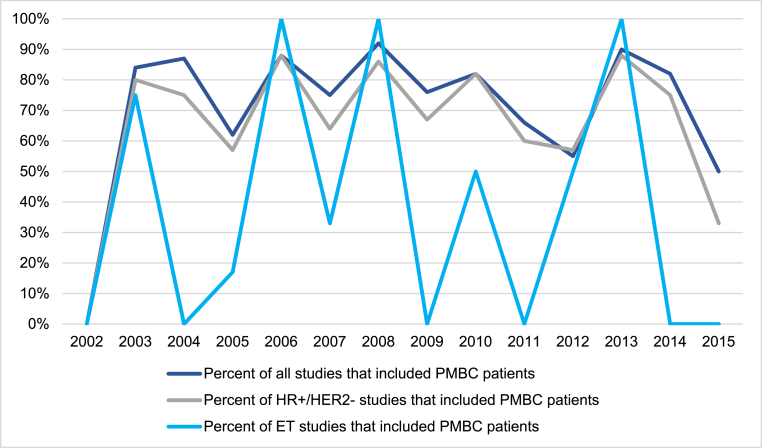
On multivariable regression, trials that studied patients with HR+/HER2-receptor subtype had significantly less PMBC inclusion as compared to trials that studied patients with HER2+, HR-HER2-receptor subtypes, or did not include receptor subtype in their inclusion criteria (47.7% PMBC inclusion in HR+/HER2-trials vs. 94.3% in non-HR+/HER2-trials, aOR 0.07 [95% CI: 0.02–0.19], p < 0.001; Table 1). Trials that randomized or mandated ET as part of the study protocol were also associated with less PMBC patient inclusion compared to trials that did not include ET in their inclusion criteria (44.4% PMBC inclusion in ET trials vs. 83.2% in non-ET trials, aOR 0.21 [95% CI: 0.10–0.83], p = 0.02; Table 1). Conversely, studies that investigated cytotoxic chemotherapy (CT) were associated with including PMBC patients compared to trials that did not study CT (100% PMBC inclusion in CT trials vs. 70.5% in non-CT trials, aOR 15.13 [95% CI: 1.78–128.42], p = 0.01; Table 1). All trials studying radiation therapy and surgery as a mandated or randomized intervention included PMBC patients in their eligibility criteria. Analysis of PMBC inclusion in trials over time revealed no significant trends, including in trials studying the HR+/HER2-subtype and ET (Fig. 2).
Fig. 2.
Trend of inclusion of pre-menopausal breast cancer patients in phase III clinical trials over time. Year represents the year of clinical trial opening. Abbreviations: pre-menopausal breast cancer (PMBC), hormone-receptor-positive and HER2-negative breast cancer subtype (HR+/HER2-), endocrine therapy (ET).
4. Discussion
This study provided an overview of premenopausal women inclusion in BC phase III RCTs. We found that BC trials studying the HR+/HER2-subtype and ET were associated with lower rates of inclusion of PMBC, which may reflect the use of ET that is ineffective in premenopausal women (such as an aromatase inhibitor without OS) in these trials. A minority of trials allowed for inclusion of PMBC women with medically-induced menopause from OS. We also found that trials studying chemotherapy were associated with greater PMBC inclusion whereas trials studying TT or patients with metastatic disease had less PMBC patient inclusion.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) recently reported a meta-analysis investigating the efficacy of aromatase inhibitors among PMBC patients undergoing OS, finding that aromatase inhibitors were efficacious in this population and, notably, lowered the rate of breast cancer recurrence as compared to the standard-of-care ET, tamoxifen [11]. This finding is significant as it breaks ground for new and more effective ET for premenopausal women. Accordingly, the Food and Drug Administration (FDA) published a draft guidance calling for greater inclusion of premenopausal women in BC trials [9]. Specifically, the FDA recommends for trials studying BC drug development to consider removing menopausal status as the basis for participant exclusion, view premenopausal women with adequate OS and postmenopausal women as equally eligible for a trial, and to use menopausal status as a stratification within trial participants if there are efficacy or safety concerns regarding the drug in question.
Our study showed that PMBC patients have high rates of exclusion from clinical trials that study HR+ patients or involve ET. This may have been appropriate in the past before OS was used alongside ET for PMBC or for trials studying low-risk PMBC for which OS may not be appropriate due to toxicity and lack of proven benefit [[12], [13]]. However, now, based on the results of the EBCTCG meta-analysis, allowance of OS with ET for PMBC women should be considered to permit their inclusion in BC clinical trials when appropriate [[10], [11]]. Thus, in light of our findings, and in concordance with the FDA,9 we call for trialists to carefully select their enrollment criteria for BC trials to optimize inclusion and applicability of trial data to PMBC patients.
This study also found that BC clinical trials studying TT or metastatic disease had lower rates of PMBC patient inclusion. This is concerning given the increasing innovation of novel targeted therapies that may greatly impact this population's lifespan and quality of life [14]. Further, excluding PMBC patients from metastatic-disease trials may be unnecessary as it prevents PMBC patients from receiving new therapies and increased medical attention regardless of their hormone-receptor status [15]. Some trials, such as MONALEESA 7 and MONARCH 2, have now been published showing the safety and efficacy of targeted therapy in metastatic PMBC, demonstrating the benefit of designing trials that allow for PMBC inclusion [[16], [17]]. Thus, along with the FDA's call for greater PMBC patient inclusion in trials, our findings corroborate the importance of including these patients in a wider breadth of RCTs when appropriate.
Our analysis has some limitations. First, we analyzed RCTs from a single clinical trials registry (ClinicalTrials.gov), potentially missing RCTs from other registries with trial representation from different geographies. Additionally, some trials allowed for premenopausal patient inclusion, however did not publish enrollment information regarding this population, which limited our ability for additional analyses.
5. Conclusions
Historically, some breast cancer clinical trials have excluded patients with PMBC. Some trials, particularly those studying early-stage HR+/HER2-disease, may still not be appropriate for inclusion of premenopausal women. However, our study highlights opportunities for more inclusive eligibility criteria, and provides a benchmark for PMBC inclusion. Future studies should perform similar analyses to investigate the temporal changes in PMBC inclusion moving forward. We are optimistic that with continued advances, premenopausal patients will be included and meaningfully represented in breast cancer clinical trials.
Funding
EBL is supported by the Sabin Family Fellowship Foundation and the Fund for Innovation in Cancer Informatics. The work is also supported by NIH Cancer Center Support Grant P30 CA016672.
Data sharing
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Declaration of competing interest
SFS has COI unrelated to this work (funding/contracted research agreements from Artios Pharma, Alpha Tau, TAE Life Sciences, Exact Sciences, Emerson Collective Foundation). The authors declare no other conflicts of interests.
Contributor Information
Kelsey L. Corrigan, Email: klcorrigan@mdanderson.org.
Ethan B. Ludmir, Email: EBLudmir@mdanderson.org.
References
- 1.Heer E., Harper A., Escandor N., Sung H., McCormack V., Fidler-Benaoudia M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. Aug. 2020;8(8):e1027–e1037. doi: 10.1016/s2214-109x(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 2.NCCN clinical practice guidelines in oncology: breast cancer. 2021. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Version 2. Accessed. [DOI] [PubMed] [Google Scholar]
- 3.Kocaöz S., Korukluoğlu B., Parlak Ö., Doğan H.T., Erdoğan F. Comparison of clinicopathological features and treatments between pre- and postmenopausal female breast cancer patients - a retrospective study. Prz Menopauzalny. Jun. 2019;18(2):68–73. doi: 10.5114/pm.2019.85786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng F., Wei Y., Zheng K., et al. Comparison of epidemiological features, clinicopathological features, and treatments between premenopausal and postmenopausal female breast cancer patients in western China: a retrospective multicenter study of 15,389 female patients. Cancer Med Jun. 2018;7(6):2753–2763. doi: 10.1002/cam4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge A.H., Hughes M.E., Warner E.T., et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. Sep 20 2016;34(27):3308–3314. doi: 10.1200/jco.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 6.Arvold N.D., Taghian A.G., Niemierko A., et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. Oct 10 2011;29(29):3885–3891. doi: 10.1200/jco.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson W.F., Luo S., Chatterjee N., et al. Human epidermal growth factor receptor-2 and estrogen receptor expression, a demonstration project using the residual tissue repository of the Surveillance, Epidemiology, and End Results (SEER) program. Breast Cancer Res Treat. Jan. 2009;113(1):189–196. doi: 10.1007/s10549-008-9918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SEER cancer statistics review: incidence rates by age at diagnosis. 2018. [Google Scholar]
- 9.Premenopausal women with breast cancer: developing drugs for treatment. U.S. Department of Health and Human Services; 2020. https://www.fda.gov/media/142638/download [Google Scholar]
- 10.Jankowitz R.C., McGuire K.P., Davidson N.E. Optimal systemic therapy for premenopausal women with hormone receptor-positive breast cancer. Breast. Aug 2013;22(Suppl 2):S165–S170. doi: 10.1016/j.breast.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol Mar. 2022;23(3):382–392. doi: 10.1016/s1470-2045(21)00758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sella T., Ruddy K.J., Carey L.A., Partridge A.H. Optimal endocrine therapy in premenopausal women: a pragmatic approach to unanswered questions. JCO Oncology Practice. 2022;18(3):211–216. doi: 10.1200/op.21.00482. [DOI] [PubMed] [Google Scholar]
- 13.Francis P.A. Adjuvant endocrine therapy for premenopausal women: risk stratification, type and duration. Breast Nov. 2019;48(Suppl 1):S85–s88. doi: 10.1016/s0960-9776(19)31131-2. [DOI] [PubMed] [Google Scholar]
- 14.Bardia A., Hurvitz S. Targeted therapy for premenopausal women with HR(+), HER2(-) advanced breast cancer: focus on special considerations and latest advances. Clin Cancer Res Nov 1. 2018;24(21):5206–5218. doi: 10.1158/1078-0432.Ccr-18-0162. [DOI] [PubMed] [Google Scholar]
- 15.Seidman A.D., Maues J., Tomlin T., Bhatnagar V., Beaver J.A. The evolution of clinical trials in metastatic breast cancer: design features and endpoints that matter. Am Soc Clin Oncol Educ Book Mar. 2020;40:1–11. doi: 10.1200/edbk_280451. [DOI] [PubMed] [Google Scholar]
- 16.Im S.A., Lu Y.S., Bardia A., et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. Jul 25 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 17.George W., Sledge J., Toi M., Neven P., et al. Monarch 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi: 10.1200/jco.2017.73.7585. [DOI] [PubMed] [Google Scholar]