Abstract
Background
There is a paucity of the literature on the relationship between frailty and excess mortality due to the COVID-19 pandemic.
Methods
The entire community-dwelling adult population of Ontario, Canada, as of January 1st, 2018, was identified using the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) cohort. Residents of long-term care facilities were excluded. Frailty was categorized through the Johns Hopkins Adjusted Clinical Groups (ACG® System) frailty indicator. Follow-up was until December 31st, 2020, with March 11th, 2020, indicating the beginning of the COVID-19 pandemic. Using multivariable Cox models with patient age as the timescale, we determined the relationship between frailty status and pandemic period on all-cause mortality. We evaluated the modifier effect of frailty using both stratified models as well as incorporating an interaction between frailty and the pandemic period.
Results
We identified 11,481,391 persons in our cohort, of whom 3.2% were frail based on the ACG indicator. Crude mortality increased from 0.75 to 0.87% per 100 person years from the pre- to post-pandemic period, translating to ~ 13,800 excess deaths among the community-dwelling adult population of Ontario (HR 1.11 95% CI 1.09–1.11). Frailty was associated with a statistically significant increase in all-cause mortality (HR 3.02, 95% CI 2.99–3.06). However, all-cause mortality increased similarly during the pandemic in frail (aHR 1.13, 95% CI 1.09–1.16) and non-frail (aHR 1.15, 95% CI 1.13–1.17) persons.
Conclusion
Although frailty was associated with greater mortality, frailty did not modify the excess mortality associated with the pandemic.
Keywords: Frailty, Excess mortality, COVID-19, Population-based study
Introduction
Each successive wave of the global COVID-19 pandemic has been associated with both substantial direct health impacts through infection [1], as well as indirect health impacts through delays or avoidance of non-COVID medical care [2, 3]. Excess mortality is a metric that captures both effects at a population level, by estimating the difference between observed deaths during the pandemic, and the counterfactual expected deaths had the pandemic not occurred [4, 5].
Excess mortality during the COVID-19 pandemic has been estimated in multiple jurisdictions, including the USA [6–8] and Canada [7–9]. These have shown, on average, between 20 and 40% increases in all-cause mortality [6]. Of these excess deaths, there have been wide ranges in the proportion directly attributable to COVID-19 illness, due to differences in testing practices, as well as inconsistencies in how cause of death is ascertained, over time and between jurisdictions [4, 5]. Notwithstanding these issues, there is a paucity of the literature examining the risk factors associated with excess mortality [10, 11], in particular the impact of frailty.
Frailty is defined by declining function across several homeostatic systems leading to increased vulnerability to stressors; it is increasingly recognized as an important driver of morbidity and mortality, across multiple health conditions, care settings and procedures [12, 13]. It has been well documented that residing in congregate living facilities and the presence of comorbidities, such as frailty, are associated with worse outcomes with direct COVID-19 illness [14, 15]. In contrast, less is known about the association of frailty with indirect COVID-19 effects. Understanding if frailty is associated with excess mortality at a population level, and if so, the underlying mechanisms will allow for mitigating strategies during subsequent waves of current and future pandemics.
Accordingly, to address this gap in knowledge, we studied the relationship between frailty and all-cause mortality before and during the COVID-19 pandemic in Ontario, Canada. We anticipated that we would confirm previous reports of excess mortality during the pandemic period and sought to elucidate if frailty was an effect modifier of this observation. Our hypothesis was that frail individuals would experience greater excess deaths during the pandemic compared to non-frail comparators.
Methods
Study design and setting
We conducted an observational retrospective cohort study using population-based administrative data held at ICES, Ontario (previously known as the Institute for Clinical Evaluative Sciences). The use of ICES data in this retrospective cohort study was authorized under Sect. 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board or individual consent. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for reporting of observational studies.
Context
The study was conducted in Ontario, which is Canada’s most populous province with a population of approximately 14.8 million as of 2022. All residents receive universal healthcare insurance provided by a single third-party payer, the Ministry of Health (MOH). The first report of a person with COVID-19 in Ontario was on January 23rd, 2020. On March 11th, 2020, the MOH issued a directive requiring all hospitals reduce non-urgent/emergent care in anticipation of the surge of the first wave of the pandemic. We used this date to signify the beginning of the COVID-19 pandemic in Ontario.
Population
We utilized the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) cohort of the adult population of Ontario, which has been described in detail previously [16]. Briefly, this is a cohort of almost all Ontario residents aged 20 to 105 years eligible for Ontario’s Health Insurance Plan (OHIP) identified from the Ontario Registered Persons Database and then linked deterministically at ICES, using unique encoded identifiers, to over 20 population-based health databases, electronic health records as well as community health survey data [16]. This allows for detailed socio-demographic and health data to be captured on the entire Ontario population of Ontario on a pre-specified inception date and then be followed prospectively forward in time. For the purpose of these analyses, the inception date was January 1, 2018, allowing for 2 years of follow-up prior to the pandemic. Individuals were followed to the earliest of death, loss of OHIP eligibility or December 31st, 2020. We excluded any individual residing in a long-term care (LTC) facility on the cohort inception date.
Frailty
Frailty was based on status at the time of cohort inception and defined using the Johns Hopkins Adjusted Clinical Groups (ACG® System Version 10) frailty indicator. The Johns Hopkins frailty indicator is a dichotomous tag. Any individual who falls into one of the any one of the expanded diagnostic clusters of malnutrition, dementia, impaired vision, decubitus ulcer, incontinence of urine or feces, loss of weight, obesity, poverty, barriers to access of care and difficulty walking is classified as frail [17, 18]. We chose this measure given that our population was community dwelling and therefore ambulatory diagnoses would be most relevant.
SARS-CoV-2 Status
As an indicator of direct COVID-19 illness, we utilized test data from SARS-CoV-2 viral RNA PCR (COVID) testing conducted in the population of Ontario, as previously described [19]. SARS-CoV-2 status for each person in our cohort was determined from all tests captured in the Ontario Laboratories Information System Database (OLIS) at any point during the study period. We categorized SARS-CoV-2 status into the following four groups: never tested, indeterminate, positive or negative.
Outcomes
The primary outcome was all-cause mortality as recorded in the Ontario Registered Persons Database.
Comorbidities
Comorbidities were based on a look-back window of 5 years from the cohort inception date of January 1st, 2018, using previously described CANHEART definitions [16]. The Ontario Marginalization Index [20] was used as a measure of neighborhood ethnic concentration, dependency, material deprivation and residential instability.
Statistics
Baseline characteristics were compared between frailty and non-frail groups using t tests for continuous variables and Chi-square tests for categorical variables. The unadjusted mortality rate per 100 person-years was compared in the pre-pandemic and pandemic eras within each frailty category. A Cox-proportional hazards model was developed using the patient’s age as the timescale, allowing us to compare frail versus non-frail persons of the same age in the pre- versus post-pandemic periods [21, 22]. We used age as the timescale because each individual in the cohort would be, on average, 2 years older in the pandemic period versus the non-pandemic era; by modeling the hazard as a function of age, we account for this by comparing the effect of a given covariate on the hazard function for two subjects who are of the same age [21, 22]. The model was also adjusted for sex, rural residence, income quintile, diabetes, COPD, asthma, cancer, hyperlipidemia, atrial fibrillation and presence of myocardial infarction, stroke, heart failure or history of revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) in the past. We did not adjust for age, given that we used age as the timescale of the model.
To determine whether frailty had a differential impact on mortality in each era, we used two approaches. First, stratified multivariable models based on frailty status were developed, with a separate model for each of the following cohorts: ACG frail, ACG non-frail. The main covariate of interest was time, categorized as being in the pre- versus pandemic era. Second, using a random 30% sample of the full cohort for computational efficiency, we created a multivariable model with the interaction of ACG*era. This allowed us to estimate the effect of pandemic era within each frailty category.
We used SAS version 9.4 (SAS Institute Inc. Cary, North Carolina). Statistical significance was two-sided p-values of ≤ 0.05.
Results
Cohort
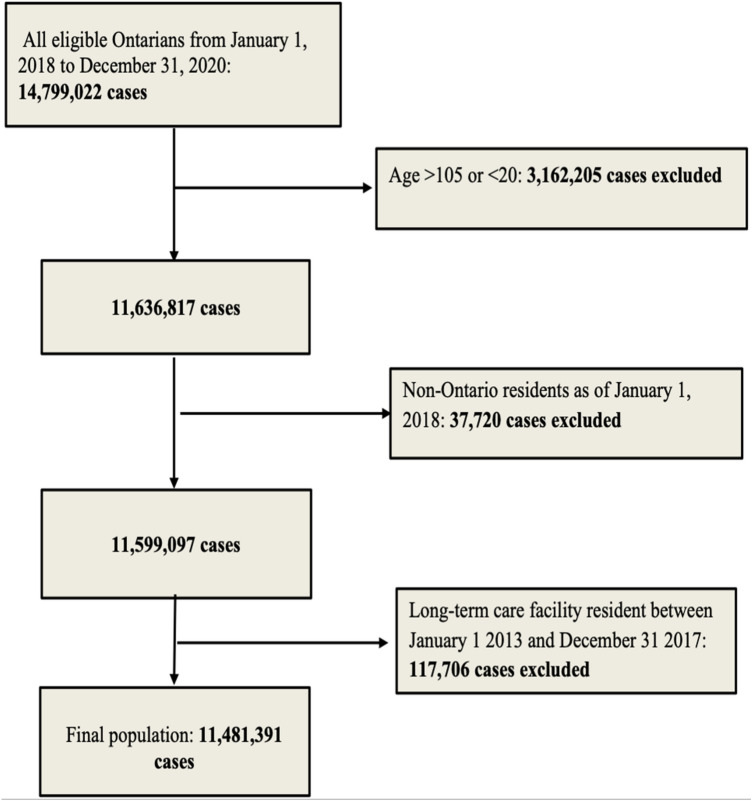
A total of 14,799,022 persons were identified in the CANHEART cohort as of January 1st, 2018. After applying exclusions by age and LTC residence, our study cohort consisted of 11,481,391 persons (Fig. 1). Of these, 376,238 (3.2%) were frail based on the ACG criteria.
Fig. 1.
Cohort selection
Baseline characteristics are shown in Table 1. In general, frail subjects were older, more likely to be female, had greater comorbidities and higher levels of dependency, material deprivation and housing instability as measured by neighborhood-level marginalization quintiles. However, this was not seen with neighborhood ethnic concentration, which was more pronounced in the non-frail categories.
Table 1.
Baseline characteristics
| Variable | Total | Johns Hopkins | ||
|---|---|---|---|---|
| Frailty (No) | Frailty (Yes) | P-Value | ||
| N = 11,481,391 | N = 11,105,153 | N = 376,238 | ||
| Age—Mean ± SD | 48.98 ± 17.66 | 48.29 ± 17.24 |
69.55 ± 17.68 |
< 0.001 |
| Age—Median (IQR) | 48 (34–62) | 48 (34–61) | 73 (59–83) | < 0.001 |
| Sex—female | 5,860,456 (51.0%) | 5,649,930 (50.9%) | 210,526 (56.0%) | < 0.001 |
| Low-income neighborhood* | 4,621,197 (40.2%) | 4,444,729 (40.0%) | 176,468 (46.9%) | < 0.001 |
| Rural residence | 1,159,990 (10.1%) | 1,116,070 (10.1%) | 43,920 (11.7%) | < 0.001 |
| Dependency quintile | ||||
| Missing | 103,354 (0.9%) | 99,145 (0.9%) | 4,209 (1.1%) | < 0.001 |
| 1 (lowest) | 2,977,367 (25.9%) | 2,918,230 (26.3%) | 59,137 (15.7%) | |
| 2 | 2,313,886 (20.2%) | 2,251,327 (20.3%) | 62,559 (16.6%) | |
| 3 | 2,018,602 (17.6%) | 1,955,697 (17.6%) | 62,905 (16.7%) | |
| 4 | 1,952,523 (17.0%) | 1,880,807 (16.9%) | 71,716 (19.1%) | |
| 5 (highest) | 2,115,659 (18.4%) | 1,999,947 (18.0%) | 115,712 (30.8%) | |
| Material deprivation quintile | ||||
| Missing | 103,354 (0.9%) | 99,145 (0.9%) | 4,209 (1.1%) | < 0.001 |
| 1 (lowest) | 2,570,956 (22.4%) | 2,499,595 (22.5%) | 71,361 (19.0%) | |
| 2 | 2,364,542 (20.6%) | 2,293,489 (20.7%) | 71,053 (18.9%) | |
| 3 | 2,174,055 (18.9%) | 2,104,350 (18.9%) | 69,705 (18.5%) | |
| 4 | 2,118,834 (18.5%) | 2,044,215 (18.4%) | 74,619 (19.8%) | |
| 5 (highest) | 2,149,650 (18.7%) | 2,064,359 (18.6%) | 85,291 (22.7%) | |
| Ethnic concentration quintile | ||||
| Missing | 103,354 (0.9%) | 99,145 (0.9%) | 4,209 (1.1%) | < 0.001 |
| 1 (lowest) | 1,807,688 (15.7%) | 1,730,802 (15.6%) | 76,886 (20.4%) | |
| 2 | 1,884,975 (16.4%) | 1,812,303 (16.3%) | 72,672 (19.3%) | |
| 3 | 2,034,455 (17.7%) | 1,964,545 (17.7%) | 69,910 (18.6%) | |
| 4 | 2,408,304 (21.0%) | 2,336,338 (21.0%) | 71,966 (19.1%) | |
| 5 (highest) | 3,242,615 (28.2%) | 3,162,020 (28.5%) | 80,595 (21.4%) | |
| Residential instability quintile | ||||
| Missing | 103,354 (0.9%) | 99,145 (0.9%) | 4,209 (1.1%) | < 0.001 |
| 1 (lowest) | 2,383,434 (20.8%) | 2,331,693 (21.0%) | 51,741 (13.8%) | |
| 2 | 2,112,090 (18.4%) | 2,051,870 (18.5%) | 60,220 (16.0%) | |
| 3 | 2,048,370 (17.8%) | 1,980,924 (17.8%) | 67,446 (17.9%) | |
| 4 | 2,057,339 (17.9%) | 1,980,639 (17.8%) | 76,700 (20.4%) | |
| 5 (highest) | 2,776,804 (24.2%) | 2,660,882 (24.0%) | 115,922 (30.8%) | |
| Comorbidities | ||||
| Diabetes | 1,360,392 (11.8%) | 1,241,463 (11.2%) | 118,929 (31.6%) | < 0.001 |
| Hyperlipidemia | 752,294 (6.6%) | 703,679 (6.3%) | 48,615 (12.9%) | < 0.001 |
| Hypertension | 2,977,438 (25.9%) | 2,730,487 (24.6%) | 246,951 (65.6%) | <0 .001 |
| COPD | 876,684 (7.6%) | 780,382 (7.0%) | 96,302 (25.6%) | < 0.001 |
| Asthma | 1,651,591 (14.4%) | 1,581,377 (14.2%) | 70,214 (18.7%) | < 0.001 |
| Stroke | 44,598 (0.4%) | 26,462 (0.2%) | 18,136 (4.8%) | < 0.001 |
| Heart failure | 78,415 (0.7%) | 52,572 (0.5%) | 25,843 (6.9%) | < 0.001 |
| Myocardial infarction | 91,583 (0.8%) | 74,052 (0.7%) | 17,531 (4.7%) | < 0.001 |
| PCI | 93,048 (0.8%) | 81,612 (0.7%) | 11,436 (3.0%) | < 0.001 |
| CABG | 36,189 (0.3%) | 28,783 (0.3%) | 7,406 (2.0%) | < 0.001 |
| Ischemic heart disease | 253,365 (2.2%) | 208,144 (1.9%) | 45,221 (12.0%) | < .001 |
| Valvular heart surgery | 19,549 (0.2%) | 15,497 (0.1%) | 4,052 (1.1%) | < 0.001 |
| TIA | 13,665 (0.1%) | 9,078 (0.1%) | 4,587 (1.2%) | < 0.001 |
| Liver disease | 38,281 (0.3%) | 30,694 (0.3%) | 7,587 (2.0%) | < 0.001 |
| Peripheral vascular disease | 28,991 (0.3%) | 22,080 (0.2%) | 6,911 (1.8%) | < .001 |
| Atrial fibrillation | 256,839 (2.2%) | 203,140 (1.8%) | 53,699 (14.3%) | < 0.001 |
| Chronic kidney disease | 241,893 (2.1%) | 196,137 (1.8%) | 45,756 (12.2%) | < 0.001 |
| Cancer | 622,358 (5.4%) | 557,390 (5.0%) | 64,968 (17.3%) | < 0.001 |
*Low-income neighborhood is defined by lowest income quintile of 1 and 2; dependency: neighborhood concentrations of individuals have no income including seniors, children, and adults whose work is not compensated, material deprivation: neighborhood levels of income, quality of housing, educational attainment and family structure characteristics, ethnic concentration: neighborhood concentration of recent immigrants, and/or belonging to visible minority group as defined by statistics Canada
Residential instability: neighborhood concentration of types and density of residential accommodations and family structure characteristics
COPD chronic obstructive lung disease, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, TIA transient ischemic attack
COVID-19 status
In the total cohort, 31.9% had a SARS-CoV-2 test in the study period, with 2.7% having a positive test. SARS-CoV-2 test frequency and positivity were higher in frail persons, with 40% and 3.3% of ACG frail individuals having testing and positivity, respectively (both p < 0.001 compared to non-frail persons, Appendix Table 3).
Table 3.
COVID testing and positivity
| TOTAL | Frailty (No) | Frailty (Yes) | P-VALUE | |
|---|---|---|---|---|
| N = 11,481,391 | N = 11,105,153 | N = 376,238 | ||
| Had COVID-19 tested up to May 10, 2021 (yes/no) | 3,666,744 (31.9%) | 3,516,424 (31.7%) | 150,320 (40.0%) | < 0.001 |
| Had COVID-19 test positive up to May 10, 2021 (yes/no) | 314,402 (2.7%) | 302,047 (2.7%) | 12,355 (3.3%) | < 0.001 |
Unadjusted mortality
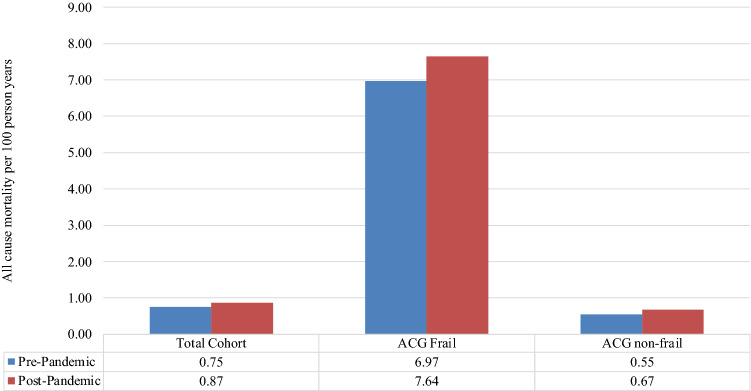
In Fig. 2, the crude mortality per 100 person-years is shown in the pre- versus pandemic periods. In the total cohort, all-cause mortality increased from 0.75 per 100 person-years to 0.87 per 100 person-years. This translates to ~ 13,800 excess deaths across the Ontario adult community-dwelling population of 11.5 million.
Fig. 2.
Mortality pre- and post-pandemic by frailty category
When categorized by ACG frailty status, the same trend was seen for both frail and non-frail residents, albeit with substantially higher mortality in the former (6.97 to 7.64 deaths per 100 person-years in the frail versus 0.55 to 0.67 in the non-frail).
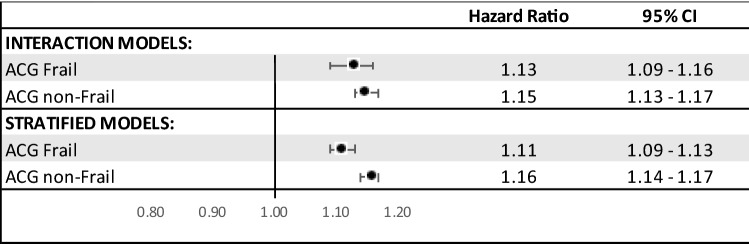
Adjusted mortality and effect modification of frailty on pandemic
In our Cox models, both frailty and the pandemic period were significantly associated with increased mortality (Table 2). In Fig. 3, the effect of the pandemic period was compared by strata of frailty status, using both adjusted stratified and interaction models. The two models showed consistent findings. Mortality was higher in the post-pandemic period for both frail and non-frail persons which was of similar magnitude for both groups (Frail HR 1.13 (95% CI 1.09–1.16); non-Frail HR 1.15 (95% CI 1.13–1.17) for interaction models; Frail HR 1.11 (95% CI 1.09–1.13); non-Frail HR 1.16 (95% CI 1.14–1.17) for stratified models.
Table 2.
Cox model on all-cause mortality
| Parameter | Hazard Ratio |
95% Lower Confidence Limit |
95% Upper Confidence Limit |
p-value |
|---|---|---|---|---|
| ACG | ||||
| ACG frailty (yes/no) | 3.02 | 2.99 | 3.05 | < 0.0001 |
| Pandemic era (after vs before March 11, 2020) | 1.10 | 1.09 | 1.11 | < 0.0001 |
Fig. 3.
Adjusted stratified and interaction Cox model comparing post-pandemic period to pre-pandemic period by frailty status
Discussion
In this population-based study in Ontario, Canada, we found an increase in all-cause mortality in the pandemic period, with approximately 13,800 excess deaths among community-dwelling adults compared to the pre-pandemic period. Frailty was a strong predictor of mortality. Both frail and non-frail individuals exhibited higher mortality in the pandemic compared to the pre-pandemic period.
A sole focus on confirmed deaths due to COVID-19 may underestimate the impact of the pandemic due to several issues [4, 5]. First, confirmed deaths are highly dependent on testing practices, which have evolved as testing capacity has fluctuated [5]. For example, in the first COVID-19 wave in early 2020, many jurisdictions restricted testing to only hospitalized patients. Second, the unintended consequences of the public health measures designed to mitigate the spread of the virus have been profound [5]. These include delays in healthcare provision for time-sensitive conditions, such as cancer or cardiac and vascular care. Third, masking and social mobility restrictions may have reduced the impact of other seasonal respiratory viruses such as influenza [5]. Therefore, excess mortality is a useful metric, as it captures all of these competing impacts, by calculating difference between expected mortality, using historical trends, and observed mortality over a set time period. Indeed, in 77 of 79 countries evaluated by Kapitsinis et al., deaths in 2020 were on average 13% higher than the average annual mortality between 2015 and 2019 [23]; Aburto and colleagues found the same with reductions in life expectancy in 27 out of 29 countries they evaluated [9]. Notwithstanding testing variation, the number of confirmed COVID-19 deaths per population was a strong driver of excess mortality in this analysis [23]. Previous work has focused on the relationship between health system preparedness as well as public health containment and mitigation strategies with excess mortality [23]; others have focused on person-level risk factors including comorbidities [10, 11]as well as location of residency [10].
Early in the pandemic, it was clear that older patients were at increased risk for both acquiring COVID-19 illness and to having a more severe course once infected [14, 15, 24]. Understanding the underlying mechanism of this increased vulnerability of older patients is an important area of research, to both mitigate the increased risk and predict prognosis and thereby inform a fair and efficient framework for the use of scarce healthcare resources such as intensive care beds. Frailty is a strong prognostic factor in patients infected with COVID-19, due to various potential mechanisms including a decreased immune response [14].
In our study, unsurprisingly, we found that mortality increased in the pandemic period and that frailty in general was associated with higher mortality risk. That said, our study builds on previous knowledge by specifically addressing if there is a differential impact in frail versus non-frail person in terms of COVID-19 era excess mortality. Interestingly, while frail persons had higher mortality than non-frail persons, and on average, mortality increased in the pandemic period, counter to our original hypothesis, this increased pandemic mortality was similar in magnitude for both groups. Our work sets the stage for further research to understand potential drivers of the excess mortality. In particular, further research is needed to evaluate patterns of healthcare utilization before and during the pandemic.
Our study must be interpreted in the context of several limitations that merit discussion. First, although our population-level databases were comprehensive, our analyses are nonetheless at risk for residual confounding from factors that we could not measure and therefore adjust for.
Second, we did not account for COVID-19 status in our models, due to the substantial evolution of testing strategies in the first months of the pandemic. Third, we defined frailty using only one measure, the ACG. We did not use other scores such as the Hospital Frailty Risk Score (HFRS) [25], which make use of predominantly hospital-based diagnoses, given that our cohort was community dwelling. Finally, ours is an observational study and should be considered hypothesis generating and not conclusive.
In conclusion, we found that both frail and non-frail individuals experienced similar relative increases in rates of mortality during the pandemic; the mechanism for these findings merit further study.
Appendix
See Table 3 .
Funding
This project was supported by a grant from the Canadian Frailty Network (CFN-006) and a Canadian Institutes of Health Research, COVID-19 Rapid Research Funding Opportunity grant (# VR4 172736). Dr. Wijeysundera is supported by a Canada Research Chair in Structural Heart Disease Policy and Outcomes. Dr. Austin is supported by a Mid-Career Investigator Award from the Heart and Stroke Foundation. Dr. Lee is the Ted Rogers Chair in Heart Function Outcomes, University Health Network, University of Toronto. Dr. Sun was named National New Investigator by the Heart and Stroke Foundation of Canada and is the Clinical Research Chair in Big Data and Cardiovascular Outcomes at the University of Ottawa. Dr. Yu is supported by a National New Investigator Award from the Heart & Stroke Foundation of Canada. Dr. Kapral holds the Lillian Love Chair in Women’s Health at the University Health Network/University of Toronto. Dr. Kwong is supported by a Clinician-Scientist Award from the University of Toronto Department of Family Community Medicine.
Declarations
Compliance with ethical standards
All standards were followed.
Conflict of interest
The authors have no conflicts.
Ethical Approval and Informed Consent
The use of ICES administrative data in this retrospective cohort study was authorized under Sect. 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board or individual consent.
Role of sponsor
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and information compiled and provided by: CIHI, Ontario Health (OH) and the Ontario Community Health Profiles Partnership (for ONMARG data). The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI and OH. However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors, and not necessarily those of CIHI or OH. The corresponding author affirms that he has listed everyone who contributed significantly to the work. The authors had access to all the study data, take responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. The corresponding author confirms that all authors read and approve the manuscript. The Canadian Frailty Network had no involvement in the design or conduct of the study, data management or analysis, or manuscript preparation, review, or authorization for submission. We thank the Toronto Community Health Profiles Partnership for providing access to the Ontario Marginalization Index.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Medicine JHUo. Coronavirus Resource Center. Published 2022. Accessed January 31st, 2022, 2022
- 2.Roifman I, Arora RC, Bewick D, et al. Cardiovascular care delivery during the second wave of COVID-19 in Canada. Can J Cardiol. 2021;37:790–793. doi: 10.1016/j.cjca.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam DY, Qiu F, Manoragavan R, et al. The impact of the COVID-19 pandemic on cardiac procedure wait list mortality in Ontario. Canada Can J Cardiol. 2021;37:1547–1554. doi: 10.1016/j.cjca.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstantinoudis G, Gomez-Rubio V, Cameletti M et al. A framework for estimating and visualising excess mortality during the COVID-19 pandemic. ArXiv. 2022.
- 5.Barnard S, Chiavenna C, Fox S, et al. Methods for modelling excess mortality across England during the COVID-19 pandemic. Stat Methods Med Res. 2021:9622802211046384. [DOI] [PMC free article] [PubMed]
- 6.Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19-related mortality among US adults aged 25–44 years, March-July 2020. JAMA. 2021;325:785–787. doi: 10.1001/jama.2020.24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhtar-Danesh N, Baumann A, Crea-Arsenio M, et al. COVID-19 excess mortality among long-term care residents in Ontario, Canada. PLoS ONE. 2022;17:e0262807. doi: 10.1371/journal.pone.0262807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zalla LC, Mulholland GE, Filiatreau LM, et al. Racial/Ethnic and age differences in the direct and indirect effects of the COVID-19 pandemic on US mortality. Am J Public Health. 2022;112:154–164. doi: 10.2105/AJPH.2021.306541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aburto JM, Scholey J, Kashnitsky I, et al. Quantifying impacts of the COVID-19 pandemic through life-expectancy losses: a population-level study of 29 countries. Int J Epidemiol. 2022;51:63–74. doi: 10.1093/ije/dyab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strongman H, Carreira H, De Stavola BL, et al. Factors associated with excess all-cause mortality in the first wave of the COVID-19 pandemic in the UK: a time series analysis using the clinical practice research Datalink. PLoS Med. 2022;19:e1003870. doi: 10.1371/journal.pmed.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey IM, Cook DG, Harris T, et al. Risk factors for excess all-cause mortality during the first wave of the COVID-19 pandemic in England: a retrospective cohort study of primary care data. PLoS ONE. 2021;16:e0260381. doi: 10.1371/journal.pone.0260381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee DS, Ma S, Chu A, et al. Predictors of mortality among long-term care residents with SARS-CoV-2 infection. J Am Geriatr Soc. 2021;69:3377–3388. doi: 10.1111/jgs.17425. [DOI] [PubMed] [Google Scholar]
- 14.Lee C, Frishman WH. Implications of Frailty in COVID-19. Cardiol Rev. 2021;29:285–288. doi: 10.1097/CRD.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maltese G, Corsonello A, Di Rosa M, et al. Frailty and COVID-19: A Systematic Scoping Review. J Clin Med. 2020;9:2106. doi: 10.3390/jcm9072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu JV, Chu A, Donovan LR, et al. The Cardiovascular Health in ambulatory care research team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. doi: 10.1161/CIRCOUTCOMES.114.001416. [DOI] [PubMed] [Google Scholar]
- 17.https://www.johnshopkinssolutions.com/wp-content/uploads/2014/04/ACG-White-Paper-General-Dec-2012.pdf. Accessed Jun 1, 2022.
- 18.Sternberg SA, Bentur N, Abrams C, et al. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18:e392–397. [PubMed] [Google Scholar]
- 19.McAlister FA, Wang T, Wang X, et al. Statins and SARS-CoV-2 Infection: results of a population-based prospective cohort study of 469 749 adults from 2 Canadian Provinces. J Am Heart Assoc. 2021;10:e022330. doi: 10.1161/JAHA.121.022330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ingen T, Matheson FI. The 2011 and 2016 iterations of the Ontario Marginalization Index: updates, consistency and a cross-sectional study of health outcome associations. Can J Public Health. 2022;113:260–271. doi: 10.17269/s41997-021-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin BA, Anderson GL, Shih RA, et al. Use of alternative time scales in Cox proportional hazard models: implications for time-varying environmental exposures. Stat Med. 2012;31:3320–3327. doi: 10.1002/sim.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canchola AJ, Stewart SL, Bernstein L et al (2003) Cox regression using different time-scales. https://www.lexjansen.com/wuss/2003/DataAnalysis/i-cox_time_scales.pdf. Accessed 31 Jan 2022
- 23.Kapitsinis N. The underlying factors of excess mortality in 2020: a cross-country analysis of pre-pandemic healthcare conditions and strategies to cope with Covid-19. BMC Health Serv Res. 2021;21:1197. doi: 10.1186/s12913-021-07169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DS, Wang CX, McAlister FA, et al. Factors associated with SARS-CoV-2 test positivity in long-term care homes: a population-based cohort analysis using machine learning. Lancet Reg Health Am. 2022;6:100146. doi: 10.1016/j.lana.2021.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]