Abstract
Melanoma is the deadliest of skin cancers and has a high tendency to metastasize to distant organs. Calcium and metabolic signals contribute to melanoma invasiveness; however, the underlying molecular details are elusive. The MCU complex is a major route for calcium into the mitochondrial matrix but whether MCU affects melanoma pathobiology was not understood. Here, we show that MCUA expression correlates with melanoma patient survival and is decreased in BRAF kinase inhibitor‐resistant melanomas. Knockdown (KD) of MCUA suppresses melanoma cell growth and stimulates migration and invasion. In melanoma xenografts, MCUA_KD reduces tumor volumes but promotes lung metastases. Proteomic analyses and protein microarrays identify pathways that link MCUA and melanoma cell phenotype and suggest a major role for redox regulation. Antioxidants enhance melanoma cell migration, while prooxidants diminish the MCUA_KD‐induced invasive phenotype. Furthermore, MCUA_KD increases melanoma cell resistance to immunotherapies and ferroptosis. Collectively, we demonstrate that MCUA controls melanoma aggressive behavior and therapeutic sensitivity. Manipulations of mitochondrial calcium and redox homeostasis, in combination with current therapies, should be considered in treating advanced melanoma.
Keywords: calcium, MCU, melanoma, mitochondria, ROS
Subject Categories: Cancer, Membranes & Trafficking, Metabolism
The MCU channel complex is the main route for Ca2+ into the mitochondrial matrix. MCU controls cellular redox state, disease aggressiveness and therapeutic response and is a favorable prognostic marker in melanoma.

Introduction
Melanoma is the most aggressive of all skin cancers (Schadendorf et al, 2018). Despite recent therapy improvements, advanced melanoma is difficult to cure and many patients still succumb to the disease; this is mostly influenced by low response rates to immunotherapies and drug resistance which commonly occur due to the high heterogeneity and plasticity of melanoma cells (Ribas & Wolchok, 2018; O'Donnell et al, 2019; Jenkins & Fisher, 2020).
Mitochondria were identified as important regulators of melanoma pathobiology (Haq et al, 2013; Roesch et al, 2013; Theodosakis et al, 2014; Zhang et al, 2019). Moreover, mitochondria are essential determinants of melanoma response to targeted therapies and immune checkpoint blockade (Vazquez et al, 2013; Harel et al, 2019). It is also becoming increasingly apparent that the tight interplay between mitochondria, bioenergetics, and reactive oxygen species (ROS), plays an important role in melanoma biology and therapeutic sensitivity (Roesch et al, 2013; Piskounova et al, 2015; Chio & Tuveson, 2017). Our previous findings showed that highly aggressive, tumor‐maintaining, and therapy‐resistant melanoma cells rely on mitochondrial bioenergetic output (Roesch et al, 2010, 2013; Cappello et al, 2021). Other studies also demonstrated that increased mitochondrial capacity and resistance to oxidative stress define aggressive melanoma cell subsets (Haq et al, 2013; Vazquez et al, 2013) and that antioxidants and the environmental redox status control melanoma but also lung cancer cell metastatic spread (Sayin et al, 2014; Le Gal et al, 2015; Piskounova et al, 2015; Ubellacker et al, 2020). In addition, inhibitors of the antioxidant enzyme thioredoxin reductase 1, which is elevated in cancer, efficiently eliminated cancer cells including melanoma without affecting healthy cells (Stafford et al, 2018).
Similar to the redox signals, ionic Ca2+ is an important regulator of cancer cell biology (Prevarskaya et al, 2011; Hoth, 2016; Marchi et al, 2020). In mitochondria, the mitochondrial Ca2+ uniporter (MCU) complex is the main transporter of Ca2+ across the inner mitochondrial membrane (IMM). The MCU complex is composed of several proteins located within the IMM or in the mitochondrial intermembrane space (IMS) (Fig 1A). These include the bona fide channel pore MCUA, its inhibiting isoform MCUB, EMRE, MCUR1, and the three regulators MICU1‐3, which sense the Ca2+ concentration in the IMS and thus control the activity of the whole MCU complex (Fig 1A) (Foskett & Philipson, 2015; Mammucari et al, 2018; Nemani et al, 2018; Pathak & Trebak, 2018; Pallafacchina et al, 2021). Given the functional importance of this complex, the relevance of MCU in cancers such as breast and hepatocellular carcinoma was investigated in the past (summarized in Vultur et al, 2018). These studies identified several important cancer‐relevant mechanisms controlled by the MCU complex. However, they also presented opposing results, thus indicating that the role of MCU in tumor biology is far more complex and demands additional investigation.
Figure 1. MCUA controls melanoma patient survival.
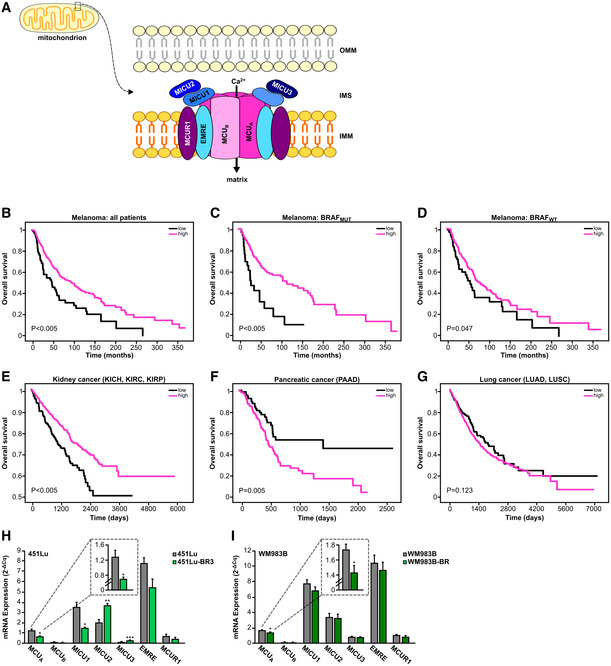
-
ASchematic representation of the MCU complex. EMRE, essential MCU regulator; IMM, inner mitochondrial membrane; IMS, intermembrane space; MCU, mitochondrial calcium uniporter; MCUR, mitochondrial calcium uniporter regulator; MICU, mitochondrial calcium uptake; OMM, outer mitochondrial membrane.
-
B–DKaplan–Meier survival plots comparing the survival probability of all melanoma patients (B), BRAF‐mutated patients (C) and BRAF wild‐type (WT) patients (D) with high‐ (magenta) and low (black) MCUA mRNA expression.
-
E–GKaplan–Meier survival plots comparing the survival probability of patients of indicated cancer types that are divided into either high‐ (magenta) or low (black) MCUA mRNA expression. P‐values were calculated using a log‐rank test. KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PAAD, pancreatic adenocarcinoma.
-
H, ImRNA expression of MCU complex (MCUA, MCUB, MICU1, MICU2, MICU3, EMRE, and MCUR1) in 451Lu (H) and WM983B (I) melanoma cells with and without resistance to BRAF inhibitors (“BR”), normalized to housekeeping gene TBP, quantified by RT–qPCR (n ≥ 3 biological replicates). Data are presented as mean ± SEM. Statistical significance was assessed using unpaired, two‐tailed Student's t‐test, *P < 0.05; **P < 0.01; ***P < 0.005; no asterisk means no statistical significance (P > 0.05).
Calcium signals are also important determinants of melanoma cell pathobiology (Stanisz et al, 2012, 2014, 2016; Hooper et al, 2015; Barceló et al, 2020). However, the role of the MCU complex and thereby mitochondrial Ca2+ (mitoCa2+) homeostasis in melanoma remains elusive.
Using an array of experimental techniques, proteomic and microarray screens, and bioinformatic approaches, we examined the role of the MCUA in a panel of genetically diverse melanoma cells, in melanoma xenografts and in melanoma patient samples and datasets. Our findings demonstrate that MCUA impacts aggressive melanoma cell behavior. Furthermore, we identify redox and metabolic signals as essential links between mitochondrial Ca2+ and melanoma cell aggressiveness. We also show that MCUA controls melanoma cell sensitivity to immunotherapies and to inducers of ferroptotic cell death. Collectively, we propose that MCUA can be utilized as a prognostic biomarker as well as a therapeutic target in treating melanoma.
Results
MCUA expression determines melanoma patient survival
To evaluate the clinical relevance of the MCU complex, and thus mitochondrial Ca2+ homeostasis in melanoma, we performed bioinformatic processing of publicly available melanoma patient datasets (TCGA). To this end, we examined MCUA expression and classified melanoma patients as high and low expressers. Using Kaplan–Meier analyses, we found that melanoma patients with lower MCUA expression have a strongly reduced survival expectancy (Fig 1B). Further analyses suggested that this MCUA association with patient survival is more pronounced in patients with mutations in the BRAF kinase, which is the most commonly altered molecule in cutaneous melanoma patients (Fig 1C). The association of MCUA expression with patient survival was also observed in BRAFWT patients but this was less evident (Fig 1D). As BRAF kinase inhibitors such as vemurafenib or dabrafenib elevate cellular ROS production (Cesi et al, 2017), this finding indicated a potential role of redox signaling in the context of MCUA expression and patient survival. The important role of MCUA expression was also evident in the early stages (I–II) and in the late stages (III–IV) of the disease (Fig EV1A and B). These findings were intriguing since an inverse correlation between MCUA expression and patient survival (high expression = low survival) was suggested in several other cancers such as breast and liver (Li et al, 2020; Zheng et al, 2020). To understand the role of MCUA in a broader cancer‐related context, we evaluated the influence of its expression on patient survival in a panel of additional cancer types. Our results showed that in some cancers such as kidney, bladder, cervical, colorectal, stomach, and thyroid, similarly to melanoma, MCUA expression correlates with patient survival (i.e., low expression = low survival; Figs 1E and EV1C–G). We also found that in other cancer types such as pancreas, breast, and liver, this correlation is inverse (high expression = low survival) (Figs 1F and EV1H and I) and in cancers such as lung, brain, head and neck, ovarian, uterine, prostate, and testicular, MCUA expression did not significantly affect patient survival (Figs 1G and EV1J–O). Given the potential clinical relevance of these findings, we sought to identify factors that regulate MCUA expression and, more importantly, parameters that link low MCUA levels with decreased melanoma patient survival. We thus first tested whether key genetic alterations such as BRAF or NRAS play a role in this context. Grouping melanomas by BRAF or NRAS status did not indicate that driver mutations play a role in the regulation of the MCUA expression (Fig EV1P and Q). Moreover, our analyses also showed that other parameters such as disease stage, patients' age, or gender do not influence MCUA expression (Fig EV1R–T). Considering the possible link between BRAF kinase inhibitors and MCUA, we examined whether resistance to these therapeutics might affect the expression of MCU components. As seen in Fig 1H and I, MCUA expression was decreased in both BRAF kinase inhibitor‐resistant cell lines (451Lu‐BR3 and WM983B‐BR) as compared with their control cell lines (451Lu and WM983B). Notably, also EMRE and MICU1 were decreased in the resistant lines to some extent, suggesting that mitoCa2+ is an important parameter of acquired therapeutic resistance in melanoma.
Figure EV1. MCUA expression affects cancer patient survival (related to Fig 1).
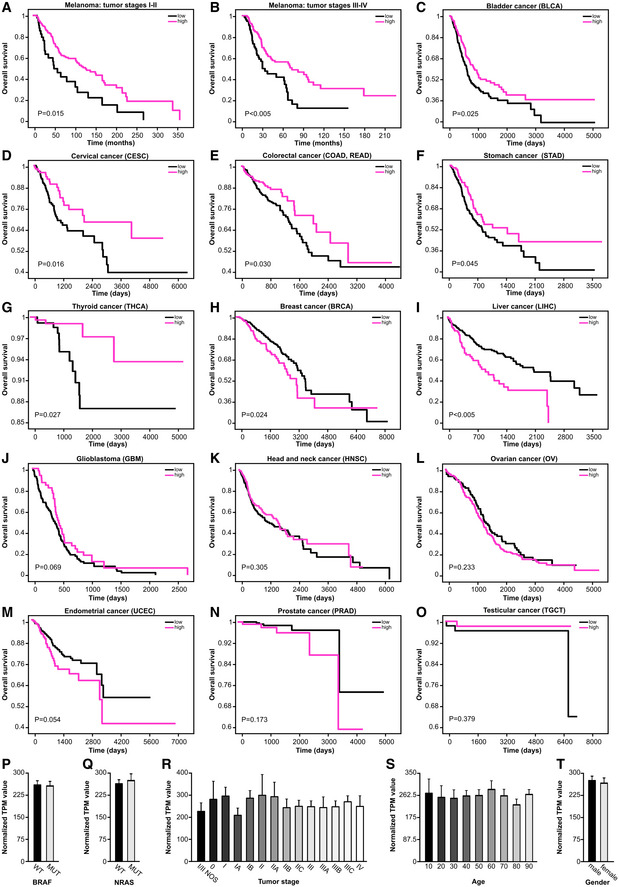
-
A–OKaplan–Meier survival plots depicting the correlation between MCUA mRNA expression levels and survival probability of stage I‐II (A) and stage III‐IV (B) melanoma patients and of patients with indicated cancer types (C–O). BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; LIHC, liver hepatocellular carcinoma; OV, ovarian serous cystadenocarcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma. P‐values are determined by log‐rank test.
-
P–TMCU expression levels in wild‐type versus BRAF‐mutant patients (P) and WT versus NRAS‐mutant melanoma patients (Q), in melanoma patients with different tumor stages (R), in melanoma patients of different age intervals (S) and in male versus female melanoma patients (T). NOS, not otherwise specified; TPM, transcripts per million reads.
Data information: Data in (P–T) are presented as mean ± SEM (n = 463 patients).
Taken all together, we conclude that the role of MCUA in cancer is tumor‐type specific, and that low MCUA expression is associated with decreased melanoma patient survival and therapeutic resistance.
MCUA controls mitochondrial Ca2+ dynamics in melanoma cells
Upregulation of MCUA induces elevated mitochondrial Ca2+ uptake while its downregulation suppresses mitoCa2+ in HEK cells (Petrungaro et al, 2015). To test whether this regulation applies to melanoma cells, we quantified mitoCa2+ using the 4mt‐D3cpV FRET‐based biosensor (Fig 2A; Zhang et al, 2019). To this end, we generated two “stable” MCUA knockdown (KD) cell lines using lentiviral transduction. Indeed, MCUA downregulation caused a decrease of the resting mitoCa2+ as well as the thapsigargin‐induced mitochondrial Ca2+ uptake in both 1205Lu and WM3734 cell lines (Fig 2B–G). The KD efficiency was confirmed by RT–qPCR and Western blot (WB) analyses and showed an inhibition of around 70% at the mRNA level and around 95% at the protein level (Fig EV2A–D). siRNA‐based silencing of MCUA in WM3734 cells also decreased mitoCa2+ (Fig 2H), while transient overexpression caused elevation of the resting mitoCa2+ concentration (Fig 2I). The efficiency of the siRNA transfection was again confirmed by RT–qPCR and WB analyses (Fig EV2E–H). Importantly, downregulation of MCUA did not significantly affect the expression levels of other MCU complex components (Fig EV2I–L).
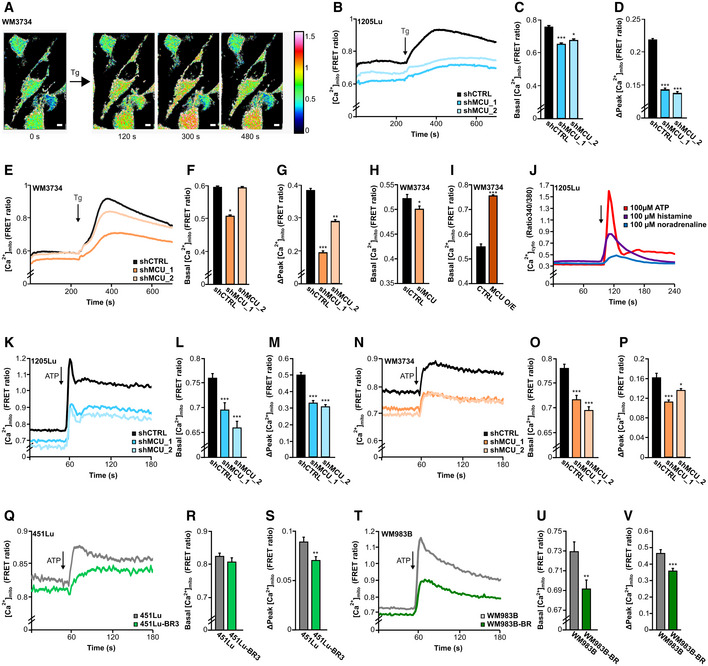
Figure 2. MCUA controls mitochondrial calcium dynamics in melanoma cells.
-
ARepresentative images of mitochondrial Ca2+ measurements in WM3734 cells before and after thapsigargin (Tg; 1 μM) stimulation. Scale bar: 10 μm.
-
B–DMitochondrial calcium uptake (represented as FRET ratio) in stable 1205Lu shCTRL (black; n = 72 cells from nine biological replicates), 1205Lu shMCU_1 (darker blue; n = 67 cells from three biological replicates) and 1205Lu shMCU_2 (lighter blue; n = 64 cells from nine biological replicates) cells upon stimulation with thapsigargin (Tg; 1 μM). (C, D) Quantification of basal levels (C) and mitochondrial Ca2+ uptake (Δpeak) (D) in 1205Lu stable lines with or without MCUA_KD.
-
E–GMitochondrial Ca2+ uptake (represented as FRET ratio) in stable WM3734 shCTRL (black; n = 143 cells from nine biological replicates), WM3734 shMCU_1 (darker orange; n = 156 cells from nine biological replicates) and WM3734 shMCU_2 (lighter orange; n = 135 cells from nine biological replicates) cells upon stimulation with thapsigargin (Tg; 1 μM). (F, G) Quantification of basal levels (F) mitochondrial Ca2+ influx (Δpeak) (G) in WM3734 stable lines with or without MCUA_KD.
-
HBasal mitochondrial Ca2+ levels in WM3734 after transient siMCUA_KD (siCTRL: n = 58 cells from three biological replicates; siMCU: n = 68 cells from three biological replicates).
-
IBasal mitochondrial Ca2+ levels in overexpressing (O/E) MCU WM3734 cells (CTRL: n = 52 cells from nine biological replicates; MCU O/E: n = 66 cells from nine biological replicates).
-
JCytosolic Fura‐2 AM‐based Ca2+ measurements in 1205Lu cells upon physiological stimulation with ATP (100 μM), histamine (100 μM) and noradrenaline (100 μM) (n ≥ 50 cells from three biological replicates).
-
K–MMitochondrial calcium uptake (represented as FRET ratio) in stable 1205Lu shCTRL (black; n = 168 cells from nine biological replicates), 1205Lu shMCU_1 (darker blue; n = 91 from eight biological replicates) and 1205Lu shMCU_2 (lighter blue; n = 104 from eight biological replicates) cells upon physiological stimulation with ATP (100 μM). (L‐M) Quantification of basal levels (L) and Ca2+ uptake (Δpeak) (M).
-
N–PMitochondrial Ca2+ uptake (represented as FRET ratio) in stable WM3734 shCTRL (black; n = 147 cells from 16 biological replicates), WM3734 shMCU_1 (darker orange; n = 175 from 17 biological replicates) and WM3734 shMCU_2 (lighter orange; n = 140 from 17 biological replicates) cells upon physiological stimulation with ATP (100 μM). (O, P) Quantification of basal levels (O) and Ca2+ uptake (Δpeak) (P).
-
Q–SMitochondrial calcium uptake (represented as FRET ratio) in 451Lu (gray; n = 157 cells from 11 biological replicates) and BRAF inhibitor‐resistant 451Lu (451Lu‐BR3; green; n = 164 cells from 12 biological replicates) upon physiological stimulation with ATP (100 μM). (R, S) Quantification of basal levels (R) and Ca2+ uptake (Δpeak) (S).
-
T–VMitochondrial calcium uptake (represented as FRET ratio) in WM983B (gray; n = 151 cells from 14 biological replicates) and BRAF inhibitor‐resistant WM983B (WM983B‐BR; green; n = 133 cells from 11 biological replicates) upon physiological stimulation with ATP (100 μM). (U, V) Quantification of basal levels (U) and Ca2+ uptake (Δpeak) (V).
Data information: Data in (B–I) were obtained by imaging single cells in Ringer's buffer containing 1 mM Ca2+, data in (J–V) were obtained in Ringer's buffer containing 0.5 mM Ca2+ and are presented as mean ± SEM. Statistical significance was determined using unpaired, two‐tailed Student's t‐test (shMCU, siMCU, O/E or BR cells were compared to their respective control), *P < 0.05; **P < 0.01; ***P < 0.005; no asterisk means no statistical significance (P > 0.05).
Figure EV2. Evaluation of siRNA and shRNA‐mediated MCUA depletion efficacy and their influence on store‐operated Ca2+ entry (related to Fig 2).
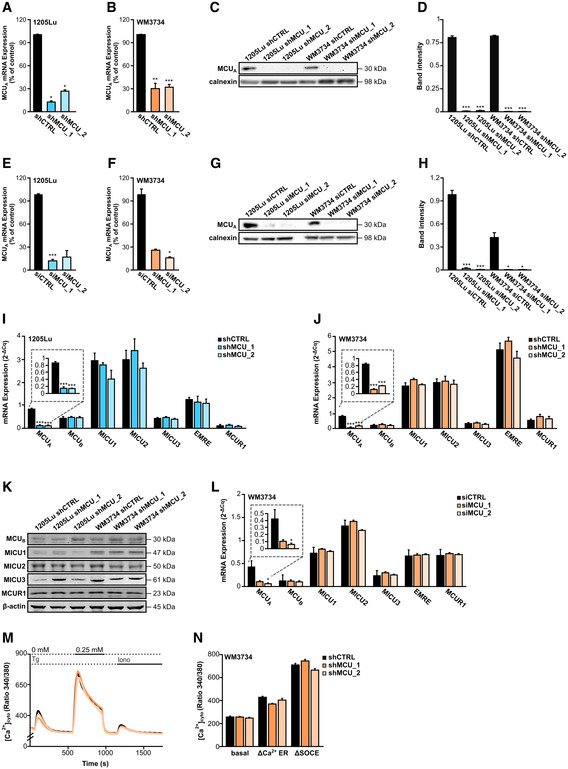
-
A, BmRNA expression of MCUA in 1205Lu (A) and WM3734 (B) shCTRL and MCUA_KD cells (n ≤ 4 biological replicates).
-
CWB for MCUA in 1205Lu and WM3734 MCUA_KD cells.
-
DCorresponding band quantification, shown as the ratio between MCUA and the loading control calnexin (n = 4 biological replicates).
-
E, FmRNA expression of MCUA in 1205Lu (A) and WM3734 (B) MCUA transient (siRNA) KD cells (n ≤ 4 biological replicates).
-
GWB for MCUA in 1205Lu and WM3734 transient MCUA_KD cells.
-
HCorresponding band quantification, shown as the ratio between MCUA and the loading control calnexin (n = 3 biological replicates).
-
I, JmRNA expression of MCU complex components (MCUA, MCUB, MICU1, MICU2, MICU3, EMRE, MCUR1) in 1205Lu (n ≥ 3 biological replicates) (I) and WM3734 (n ≥ 3 biological replicates) (J) cells with and without stable MCUA_KD, normalized to housekeeping gene TBP, quantified by RT–qPCR.
-
KWB for proteins of the MCU complex (MCUB, MICU1, MICU2, MICU3, MCUR1) in 1205Lu and WM3734 cells with and without stable MCUA_KD. β‐actin was used as loading control.
-
LmRNA expression of MCU complex components (MCUA, MCUB, MICU1, MICU2, MICU3, EMRE, and MCUR1) in WM3734 transient MCUA_KD cells, normalized to housekeeping gene TBP, quantified by RT–qPCR (n = 5 biological replicates).
-
MCytosolic Ca2+ measurements in stable WM3734 shCTRL (black), WM3734 shMCU_1 (darker orange) and WM3734 shMCU_2 (lighter orange) cells.
-
NCorresponding quantification of basal cytosolic Ca2+ before thapsigargin (Tg, 1 μM) stimulation, of ΔCa2+ ER and of ΔSOCE (shCTRL: n = 186 cells from seven biological replicates; shMCU_1: n = 133 cells from six biological replicates; shMCU_2: n = 167 cells from seven biological replicates).
Data information: Data are presented as mean ± SEM. Statistical significance was assessed using unpaired, two‐tailed Student's t‐test (KD cells were compared with their respective control), *P < 0.05; **P < 0.01; ***P < 0.005; no asterisk means no statistical significance (P > 0.05).
Source data are available online for this figure.
To examine the contribution of MCUA on mitoCa2+ following physiological stimulation, we first tested the effect of different stimuli on the store‐operated Ca2+ entry (SOCE) in melanoma cells. As depicted in Fig 2J, ATP, histamine, and noradrenaline induced increase in SOCE. Because the effect of ATP was the strongest, we next evaluated its effect on mitoCa2+ in both 1205Lu and WM3734 cells. Our findings demonstrate that physiological stimulation‐induced mitoCa2+ uptake is also strongly suppressed in the MCUA_KD cells (Fig 2K–P).
Given that MCUA expression is decreased in the BRAF kinase inhibitor‐resistant cells, we next measured mitoCa2+ in the resistant (451Lu‐BR3 and WM983B‐BR) and the control (451Lu and WM983B) melanoma cells. Our results showed that ATP‐induced mitoCa2+ uptake is decreased in the resistant lines (Fig 2Q–V). These data thus strengthen the theory that low mitoCa2+ promotes melanoma aggressiveness.
In immune cells, mitochondrial Ca2+ uptake and thus MCU control SOCE by buffering Ca2+ and by preventing premature closure of the Orai Ca2+ channels (Hoth et al, 1997; Samanta et al, 2014). However, in other cell types, this mitochondria‐controlled SOCE regulation was not observed. Because STIM‐gated Orai channels affect melanoma cell biology (Stanisz et al, 2016), we measured SOCE in WM3734 MCUA_KD cells and found no overt differences between the control and the MCUA_KD cells. These results suggest that STIM‐gated Orai channels are not involved in the MCUA‐controlled melanoma cell phenotype (Fig EV2M and N).
In summary, we confirm that MCUA and thereby the MCU complex are central regulators of the mitochondrial Ca2+ homeostasis in melanoma cells. Moreover, we demonstrated that mitoCa2+ is activated by physiological stimuli and is decreased in BRAF kinase inhibitor‐resistant cells.
MCUA controls melanoma cell growth and invasion in vitro
Given that MCUA expression determines mitoCa2+, melanoma patient survival and therapeutic resistance, we explored its effect on cell growth and invasive potential. To this end, we determined cell proliferation of the WM3734 MCUA_KD and 1205Lu MCUA_KD cells. After 24 h, we recorded a mild increase in cell growth in the MCUA_KD cells. However, after 72 h, three out of four MCUA_KD cell lines displayed significantly decreased cell proliferation (Fig 3A and B). To test the invasive potential of the MCUA_KD cells, we performed transwell migration assays and observed a robust increase in transwell migration of 1205Lu MCUA_KD cell lines (Fig 3C and D). As many cellular traits are affected by cell culture conditions and the microenvironment, especially when comparing 2D and 3D cellular cultures (Pampaloni et al, 2007; Kapałczyńska et al, 2018), we next examined the role of MCUA in 3D collagen‐embedded melanoma spheroids. As shown in Fig 3E–H, the spheroid size was reduced in all MCUA_KD cell lines. Importantly, quantification of melanoma cell collagen invasion indicated that following a longer period (10 days), MCUA_KD promotes melanoma cell invasive properties (Fig 3I). Next, we examined migration and invasion potentials of the BRAF kinase inhibitor‐resistant cell lines and found that in both resistant lines, the transwell migration and spheroid invasion were elevated as compared with the control lines (Fig 3J–N).
Figure 3. MCUA knockdown suppresses tumor growth and promotes invasion in vitro and in vivo .
-
A, BProliferation of 1205Lu (A) and WM3734 (B) stable MCUA_KD lines over 72 h, shown as percent of the respective control (shCTRL at 24 h) (n ≥ 7 biological replicates/condition/day).
-
CRepresentative images of the migrated stained 1205Lu shCTRL (black frame), shMCU_1 (darker blue frame) and shMCU_2 (lighter blue frame) cells on the lower side of the insert.
-
DQuantification of the transwell migration in 1205Lu stable MCUA_KD lines, based on the number of stained cells (n = 4 biological replicates/condition, shown also by individual data points).
-
ERepresentative images of 1205Lu shCTRL (black frame), shMCU_1 (darker blue frame) and shMCU_2 (lighter blue frame) melanoma spheroids after 72‐h invasion in collagen. Live cells are shown in green. Scale bar: 100 μm.
-
FQuantification of 1205Lu stable MCUA_KD spheroid core size (n = 6 biological replicates/condition, shown also by individual data points).
-
GRepresentative images of WM3734 shCTRL (black frame), shMCU_1 (darker orange frame) and shMCU_2 (lighter orange frame) melanoma spheroids. Live cells are shown in green. Scale bar: 100 μm.
-
HQuantification of WM3734 stable MCUA_KD spheroid core size (n = 5 biological replicates/condition, shown also by individual data points).
-
IInvasion potential of WM3734 shCTRL (black), shMCU_1 (darker orange) and shMCU_2 (lighter orange) over a period of 10 days (n ≤ 4 biological replicates/condition/day, shown also by individual data points).
-
J, KMigration potential of 451Lu and 451Lu BRAF inhibitor‐resistant (451Lu‐BR3) (J) and WM983B and WM983B BRAF inhibitor‐resistant (WM983B‐BR) cells (K) over 24 h (n = 3 biological replicates/condition, shown also by individual data points).
-
LRepresentative images of 451Lu wild‐type (gray frame), 451Lu‐BR3 (lighter green frame), WM983B wild‐type (gray frame) and WM983B‐BR (darker green frame) melanoma spheroids after 72‐h invasion in collagen. Live cells are shown in green. Scale bar: 100 μm.
-
M, NQuantification of 451Lu versus 451Lu‐BR3 (M) and WM983B versus WM983B‐BR (N) spheroid core size (n ≥ 9 biological replicates/condition, shown also by individual data points).
-
ORepresentative images of mice in the shCTRL group and the shMCU_1 group after 36 days of tumor growth (n = 10 mice/group).
-
PTumor volume growth over 36 days postinoculation.
-
QAverage weight of the tumors at the end point (36 days) upon removal (individual values shown as points).
-
RExemplary image of 1205Lu shCTRL (black frame) and shMCU (blue frame) metastases in mouse lung tissue sections; paraffin sections were made from the removed lungs; H&E staining was carried out and the sections were scanned using an Axio Scan.Z1 microscope. Scale bar: 100 μm.
-
SQuantitative evaluation of the incidence (shown in percent) of lung metastases, total amount of positive animals identified with metastases, total area of all metastases in a group, and the average area per metastasis in shCTRL group (black) and shMCU group (blue).
Data information: Data are presented as mean ± SEM. Statistical significance was determined using unpaired, two‐tailed Student's t‐test (shMCU cells were compared with their respective control, shCTRL), *P < 0.05; **P < 0.01; ***P < 0.005; no asterisk means no statistical significance (P > 0.05).
Collectively, our findings suggested that lowered MCUA levels cause decreased melanoma cell growth but augmented migration and invasion.
MCUA regulates melanoma growth and invasion in vivo
The importance of MCUA in vivo was examined using melanoma xenografts in immunodeficient mice. Two clones of the 1205Lu MCUA_KD cells and a control transduced cell line were inoculated, and the tumor volume was measured over 36 days. As seen in Fig 3O–Q, both MCUA_KD clones resulted in smaller tumors. The difference in tumor volume and weight was detected 2 week after inoculation and reached 40% smaller volumes after 6 week (Fig 3P and Q). These findings confirmed the in vitro observations regarding the role of MCUA in melanoma cell growth. To examine whether this conclusion also applies to the invasive behavior, we evaluated the metastatic burden in a postmortem examination of the mouse lungs. We found that the number of metastatic lesions per mouse, the number of metastasis‐positive mice, the total metastatic area, and the area/metastatic lesion ratio were higher in the mice inoculated with 1205Lu MCUA_KD cells compared with the control cell line (Fig 3R and S). The MCUA_KD efficiency was maintained in vivo over the 6 week after inoculation, as confirmed by WB analyses of tumor lysates (Fig EV3A and B).
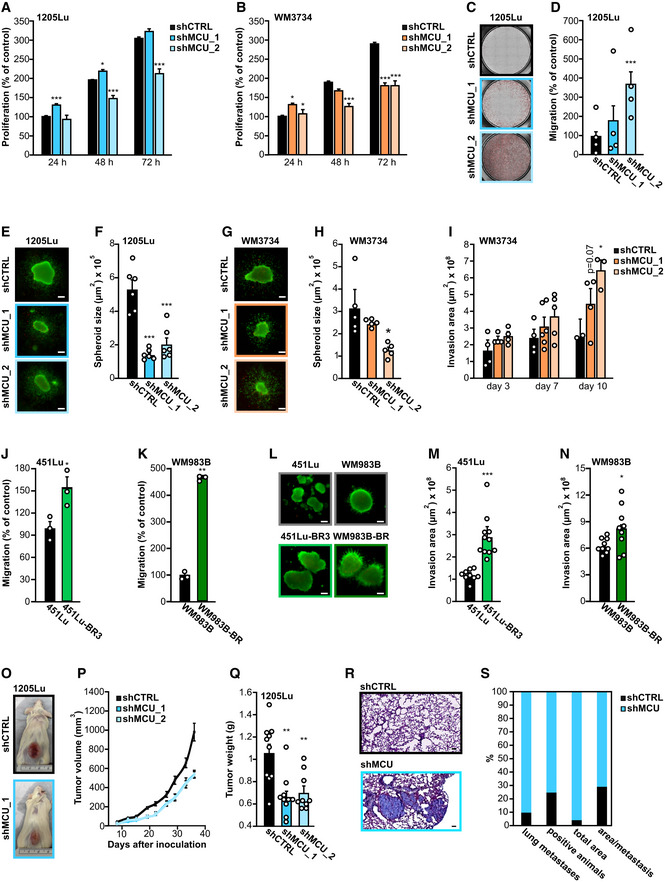
Figure EV3. MCUA controls metabolic signaling in 1205Lu melanoma cells (related to Figs 3 and 4).
-
A, BValidation of MCUA knockdown in melanoma xenografts. WB for MCUA in 1205Lu MCUA_KD cells isolated from xenograft tumors (A). Corresponding band intensity, shown as the ratio between MCUA and the loading control plasma membrane Ca2+ ATPase (PMCA) (B).
-
CVolcano plot of proteomics data for 1205Lu MCUA_KD cells versus MCUA_CTRL. Protein entities with significant P‐values (P < 0.05) are marked with red dots. Protein entities with significance in both P‐value and FDR (false discovery rate) (P < 0.05; FDR < 0.05) are marked with green dots.
-
D, EProteomap analyses with protein entities that display significant difference (absolute Log2 fold change > 0.5 and P < 0.05) between 1205Lu MCUA_KD and 1205Lu shCTRL cells.
-
FKEGG‐based analysis of cellular components and processes based on protein hits that show differential expression between in 1205Lu MCUA_KD and shCTRL cells, revealed via proteomics.
-
GCancer hallmark‐based enrichment analysis of proteins differentially expressed in 1205Lu MCUA_KD versus shCTRL cells, based on proteomics data shown in (A). Percentage of hits in the specific gene set is displayed.
Source data are available online for this figure.
Our in vivo experiments suggest that MCUA and thereby the mitochondrial Ca2+ homeostasis play an important role as regulators of melanoma pathobiology.
Proteomic analyses identify proteins and signaling pathways regulated by MCUA
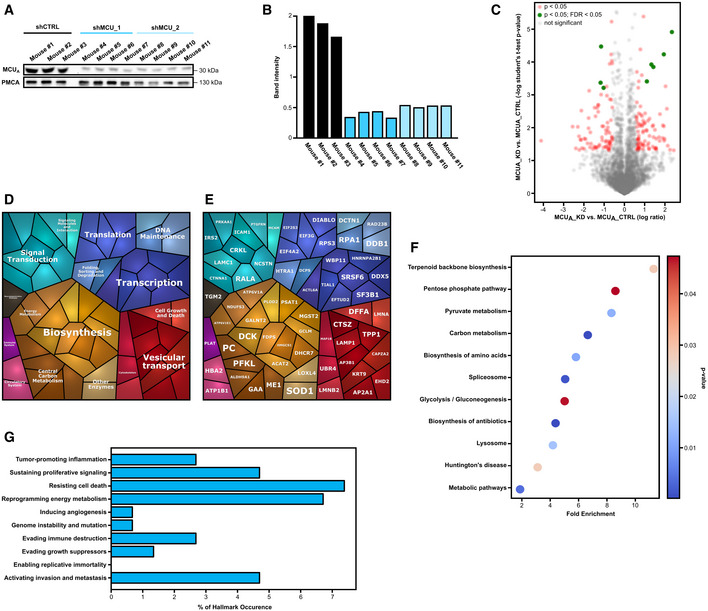
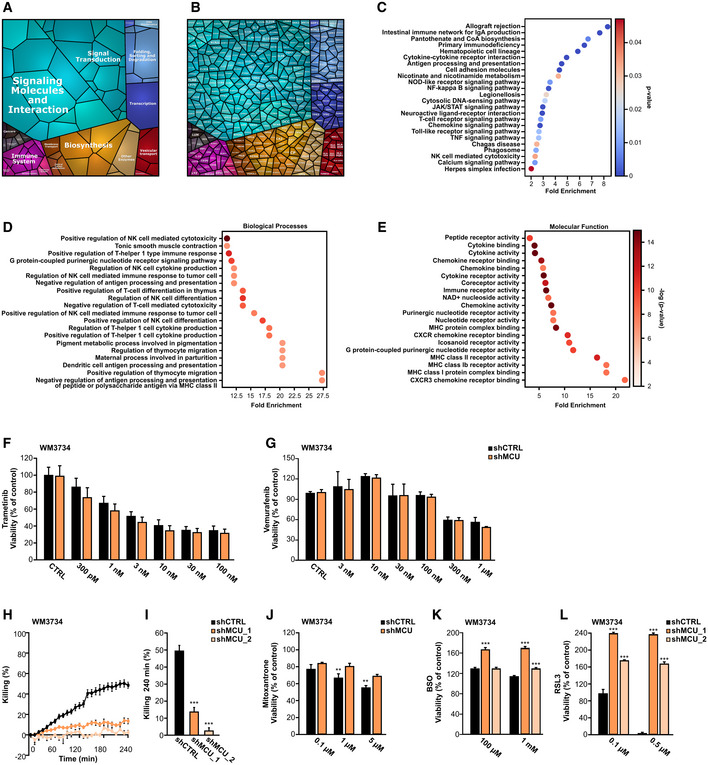
To identify molecular determinants and signaling mechanisms that are influenced by the abundance and activity of MCUA, we performed proteomic and microarray analyses of MCUA_KD cells and the corresponding control cell lines. The volcano plot depicting the data from proteome analyses indicates that the abundance of a number of proteins is significantly altered (Fig 4A, red and green, see also Dataset EV1) following MCUA knockdown in the WM3734 cells. A “proteomap”‐based pathway analysis indicated a high contribution of cellular metabolism and bioenergetics, protein folding, sorting and translation, as well as vesicular transport and cell adhesion, in the MCUA_KD‐regulated proteins (Fig 4B and C). Similar findings were observed when we analyzed the proteomic data obtained from control and MCUA_KD 1205Lu cells (Fig EV3C–E, see also Dataset EV2). The subsequent KEGG‐based pathway analysis depicted a robust melanoma cell metabolic reprograming as a number of metabolic pathways such as the pentose phosphate pathway, the TCA cycle, and the glutathione metabolism were significantly enriched following MCUA downregulation (Figs 4D and EV3F). In addition, the evaluation of the affected biological processes and molecular functions identified response and binding to ascorbic acid, dioxygenase activity as well as NADPH regeneration and NAD binding as processes controlled by MCUA. These data thus suggested that in the context of the MCUA_KD‐induced metabolic reprograming, redox regulation plays a central role in melanoma cell phenotype control (Fig 4E and F). We also assessed the proteomic data according to the “Hallmarks of Cancer” classification and calculated the fraction of MCUA‐controlled proteins into each hallmark as defined by Hanahan & Weinberg (2011) (Figs 4G and EV3G). This analysis identified MCUA‐controlled proteins involved in several cancer hallmarks such as reprograming energy metabolism, activating invasion and metastasis and resisting cell death and further strengthened the hypothesis that MCUA is a critical regulator of melanoma cell pathobiology.
Figure 4. MCUA controls metabolic signaling in melanoma cells.
-
AVolcano plot of proteomics data for WM3734 MCUA_KD versus shCTRL. Protein hits with significant P‐values (P < 0.05) are marked in red. Protein hits with both significant P‐value and FDR (false discovery rate) (P < 0.05; FDR < 0.05) are marked in green.
-
B, CProteomap analyses in WM3734 MCUA_KD cells, based on data shown in (A) (red dots).
-
DKEGG‐based analysis of cellular components and processes based on protein hits that show differential expression between in WM3734 MCUA_KD and shCTRL cells, revealed via proteomics.
-
E, FEnrichment analyses of GO terms and pathways in WM3734 MCUA_KD cells, based on proteomic analysis. GO analyses were performed according to two GO categories: biological processes (E) and molecular function (F).
-
GCancer hallmark‐based enrichment analysis based on proteomics data shown in (A). Percentage of hits in the specific gene set is displayed.
-
HHeatmap encompassing RPPA values of proteins detected in WM3734 and 1205Lu MCUA_KD (shMCU_1 and shMCU_2) cells.
-
I, JProteomap‐based pathway analyses in WM3734 and 1205Lu MCUA_KD cells, based on RPPA data shown in (H).
-
KKEGG‐based analysis of cellular components and processes involved in WM3734 and 1205Lu MCUA_KD cell regulation.
-
L, MEnrichment analyses of GO (gene ontology) terms and pathways in WM3734 and 1205Lu MCUA_KD cells, based on RPPA data. GO analyses were performed according to two GO categories: biological processes (L) and molecular function (M).
-
NHallmark of cancer‐based enrichment analysis in WM3734 and 1205Lu MCUA_KD cells, based on RPPA data. Percentage of hits in the specific gene set is displayed. Gene sets (10 hallmarks): http://bio‐bigdata.hrbmu.edu.cn/CHG/index.html.
To further decipher the role of MCUA in melanoma, we performed reverse phase protein array (RPPA), an antibody‐based screening tool that allows evaluation of abundance and/or activation state of cancer‐relevant proteins (Lu et al, 2016). As shown in the cluster map (Fig 4H, see also Dataset EV3), MCUA_KD significantly affected the abundance and/or activity of several proteins. The proteomaps and KEGG‐based pathway analyses again suggested a significant contribution of MCUA in cell metabolism but also in transcription, signaling, protein quality control and cell growth, death, and invasion (Fig 4I and J). The subsequent pathway and functional analyses (Fig 4K–M) indicated that a number of metabolic and signaling pathways, T cell‐relevant biological processes, kinase and phosphatase activity and cell death mechanisms are under control of or are influenced by the mitochondrial Ca2+ homeostasis. The Hallmarks of Cancer analyses indicated a high occurrence of the identified “hits” and confirmed the proteomic findings that MCUA is involved in hallmarks such as activating invasion and metastasis, sustaining proliferation, and reprograming energy metabolism (Fig 4N).
Redox signals underlie the MCUA ‐driven melanoma cell phenotype
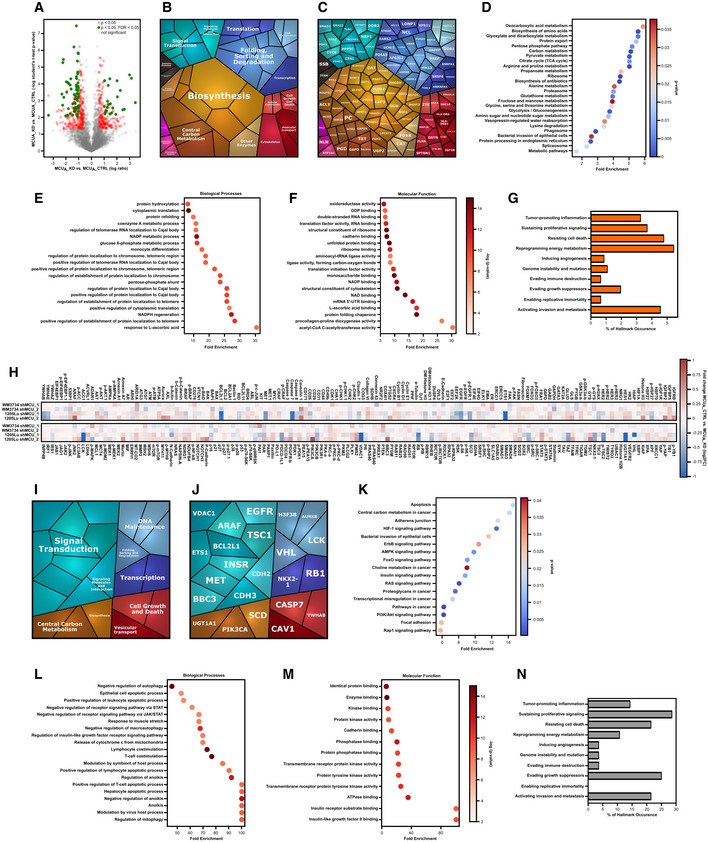
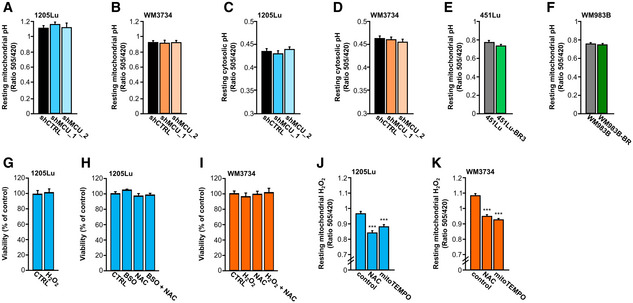
Our proteomic and RPPA screens indicated that MCUA and thereby mitoCa2+ play a distinctive role in several cancer‐relevant signaling pathways and cellular functions. Based on these findings and given that mitoCa2+ is an important regulator of mitochondrial metabolic output and redox signaling (Hoffman et al, 2014; Tosatto et al, 2016; Joseph et al, 2019; Zhang et al, 2019; Booth et al, 2021), we next examined the role of MCUA in mitochondrial redox homeostasis, that is, H2O2 dynamics. Fluorescence live‐cell microscopy using H2O2 biosensors (HyPer and mito‐HyPer) demonstrated that the cytosolic and mitochondrial H2O2 levels in both 1205Lu and WM3734 MCUA_KD cells were significantly decreased (Fig 5A–F). Moreover, transient silencing of MCUA caused decreased cytosolic H2O2 levels (Fig 5G). On the contrary, overexpression of MCUA, which led to elevated mitoCa2+, induced an increase in the cytosolic H2O2 concentration (Fig 5H). Measurements of the mitochondrial glutathione redox potential with another mitochondria‐targeted biosensor (mitoGrx1‐roGFP2) demonstrated a more reducing redox state in the mitochondrial matrix of all four MCUA_KD cell lines (Fig 5I–K). Measurements of mitochondrial H2O2 in the BRAF kinase inhibitor‐resistant cells showed decreased mitochondrial H2O2 levels relative to the control cells (Fig 5L and M). Of note, the control SypHer‐based measurements indicated that mitochondrial and intracellular pH was not altered in the MCUA_KD cells (Fig EV4A–F). Collectively, the redox measurements strengthen the hypothesis that MCUA is an important regulator of the mitochondrial and cellular redox homeostasis.
Figure 5. MCUA controls redox signaling in melanoma cells.
-
A–CMitochondrial hydrogen peroxide (H2O2) measurement in 1205Lu and WM3734 with and without stable MCUA_KD using mito‐HyPer. Exemplary ratiometric images (F505 nm/F420 nm) are shown for all conditions (A). Scale bar: 10 μm. Quantification of mito‐HyPer ratio in 1205Lu (B) and WM3734 (C) under resting state (1205Lu—shCTRL: n = 217 cells from six biological replicates; shMCU_1: n = 197 cells from seven biological replicates; shMCU_2: n = 212 cells from seven biological replicates; WM3734—shCTRL: n = 104 cells from six biological replicates; shMCU_1: n = 107 cells from seven biological replicates; shMCU_2: n = 114 cells from seven biological replicates).
-
D–FCytosolic hydrogen peroxide (H2O2) measurement in 1205Lu and WM3734 with and without stable MCUA_KD using HyPer. Exemplary ratiometric images (F505 nm/F420 nm) are shown for all conditions (D). Scale bar: 10 μm. Quantification of HyPer ratio in 1205Lu (E) and WM3734 (F) under resting state (1205Lu—shCTRL: n = 232 cells from six biological replicates; shMCU_1: n = 290 cells from six biological replicates; shMCU_2: n = 290 cells from six biological replicates; WM3734—shCTRL: n = 443 cells from nine biological replicates; shMCU_1: n = 297 cells from nine biological replicates; shMCU_2: n = 233 cells from nine biological replicates).
-
G, HQuantification of resting cytosolic H2O2 levels (HyPer) in WM3734 upon siRNA‐mediated MCUA_KD (siCTRL: n = 58 cells from nine biological replicates; siMCU: n = 68 cells from nine biological replicates) (G) and MCUA overexpression (O/E) (CTRL: n = 52 cells from nine biological replicates; MCU O/E: n = 66 cells from nine biological replicates) (H).
-
I–KMitochondrial glutathione redox potential, measured with mito‐Grx1‐roGFP2 in 1205Lu and WM3734 with and without stable MCUA_KD. Representative ratiometric images (F385 nm/F475 nm) are shown for all conditions (I). Scale bar: 10 μm. Quantification in 1205Lu (J) and WM3734 (K) under resting state (1205Lu—shCTRL: n = 231 cells from 10 biological replicates; shMCU_1: n = 176 cells from seven biological replicates; shMCU_2: n = 164 cells from seven biological replicates; WM3734—shCTRL: n = 127 cells from seven biological replicates; shMCU_1: n = 142 cells from seven biological replicates; shMCU_2: n = 116 cells from seven biological replicates).
-
L, MResting mitochondrial hydrogen peroxide (H2O2; mito‐HyPer) in 451Lu and BRAF inhibitor‐resistant 451Lu‐BR3 (L) and WM983B and BRAF inhibitor‐resistant WM983B‐BR (M) (451Lu: n = 227 cells from eight biological replicates; 451Lu‐BR3: n = 166 cells from six biological replicates; WM983B: n = 193 cells from eight biological replicates; WM983B‐BR: n = 208 cells from nine biological replicates).
-
NMigration (4 h) of 1205Lu wild‐type cells upon 4‐h pretreatment with 1 mM BSO, 200 μM NAC and 1 mM BSO + 200 μM NAC (n = 6 biological replicates/condition, shown also by individual data points).
-
OMigration (4 h) of WM3734 wild‐type cells upon 4‐h pretreatment with 100 μM H2O2, 200 μM NAC and 100 μM H2O2 + 200 μM NAC (n = 4 biological replicates/condition, shown also by individual data points).
-
PMigration of 1205Lu shCTRL and shMCU untreated and shMCU pretreated for 4 h with 100 μM H2O2 (n = 6 biological replicates/condition, shown also by individual data points).
Data information: All data are presented as mean ± SEM. (A–F) and (I–M) were measured in Ringer's buffer containing 0.5 mM Ca2+ and (G‐H) in Ringer's buffer containing 1 mM Ca2+. For (A–M), statistical significance was determined using unpaired, two‐tailed Student's t‐test, *P < 0.05; **P < 0.01; ***P < 0.005. For (N, O), statistical significance was determined using paired, one‐tailed Student's t‐test, *P < 0.05; **P < 0.01. For (P), statistical significance was assessed using paired, two‐tailed Student's t‐test, **P < 0.01. In all statistical analyses, KD, O/E or treated cells were compared with their respective control.
Figure EV4. Effect of redox agents on melanoma cell viability and mitochondrial ROS (related to Fig 5).
-
A, BQuantification of basal mito‐SypHer ratios (pH control for mito‐HyPer) in 1205Lu (A) and WM3734 (B) with and without stable MCUA_KD (1205Lu—shCTRL: n = 210 cells from six biological replicates; shMCU_1: n = 174 cells from six biological replicates; shMCU_2: n = 117 cells from six biological replicates; WM3734—shCTRL: n = 74 from three biological replicates; shMCU_1: n = 51 cells from three biological replicates; shMCU_2: n = 67 cells from three biological replicates).
-
C, DQuantification of basal SypHer ratios (pH control for HyPer) in 1205Lu (C) and WM3734 (D) with and without stable MCUA_KD (1205Lu—shCTRL: n = 149 cells from four biological replicates; shMCU_1: n = 176 cells from four biological replicates; shMCU_2: n = 158 cells from four biological replicates; WM3734—shCTRL: n = 187 cells from six biological replicates; shMCU_1: n = 189 cells from six biological replicates; shMCU_2: n = 193 cells from six biological replicates).
-
E, FQuantification of basal mito‐SypHer ratios (pH control for mito‐HyPer) in 451Lu (E) and WM983B (F) and their respective BRAF inhibitor‐resistant versions (451Lu: n = 73 cells from four biological replicates; 451Lu‐BR3: n = 136 cells from four biological replicates; WM983B: n = 103 cells from four biological replicates; WM983B‐BR: n = 91 cells from four biological replicates).
-
GViability assay upon 100 μM H2O2 treatment for 24 h in 1205Lu melanoma cells (n = 4 biological replicates).
-
HViability assay upon 4‐h treatment with 1 mM BSO, 200 μM NAC and 1 mM BSO + 200 μM NAC in 1205Lu melanoma cells (n = 12 wells from three biological replicates).
-
IViability assay upon 4‐h treatment with 100 μM H2O2, 200 μM NAC and 100 μM H2O2 + 200 μM NAC in WM3734 melanoma cells (n = 12 wells from three biological replicates).
-
J, KResting mitochondrial hydrogen peroxide (H2O2; mito‐HyPer) in 1205Lu (J) and WM3734 (K) with and without overnight pre‐treatment with NAC (200 μM) or mitoTEMPO (1 μM) (1205Lu—control: n = 191 cells from 3 biological replicates; NAC: n = 148 cells from three biological replicates; mitoTEMPO: n = 133 cells from three biological replicates; WM3734—control: n = 237 cells from four biological replicates; NAC: n = 263 cells from five biological replicates; mitoTEMPO: n = 255 cells from three biological replicates).
Data information: (A–F) and (J–K) were measured in Ringer's buffer containing 0.5 mM Ca2+. Data are presented as mean ± SEM. Statistical significance was determined using unpaired, two‐tailed Student's t‐test, ***P < 0.005; no asterisk means no statistical significance (P > 0.05).
Redox signals have been identified as regulators of melanoma invasive potential and metastatic spread in vitro and in vivo (Le Gal et al, 2015; Piskounova et al, 2015); hence, we hypothesized that MCUA‐controlled H2O2 production, that is, redox regulation, is a molecular mechanism that links decreased mitoCa2+ and increased melanoma cell invasiveness. We tested this theory by measuring transwell migration of melanoma cells treated with antioxidants such as N‐acety‐cystein (NAC) and prooxidants such as buthionine sulphoximine (BSO) and H2O2. Our results confirmed that antioxidants promote melanoma cell migration and also showed that this increase can be reversed by the addition of oxidants (Fig 5N and O). Moreover, we found that the MCUA_KD‐induced increase in melanoma cell migration is diminished when cells are pretreated with H2O2 (Fig 5P). Of note, the BSO, NAC, and H2O2 concentrations used for these experiments did not affect the viability of 1205Lu and WM3734 melanoma cells following 4 h of stimulation (Fig EV4G–I) but NAC and the mitochondria‐targeted antioxidant mitoTEMPO suppressed the mitochondrial H2O2 production (Fig EV4J and K). These findings thus corroborated the hypothesis that MCUA‐controlled redox signals are involved in regulating melanoma aggressive behavior.
MCUA controls mitochondrial ATP production
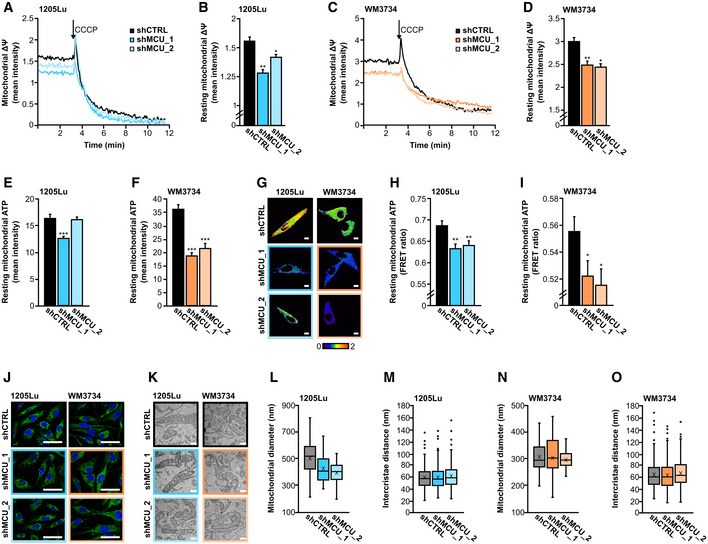
To evaluate the impact of MCUA on mitochondrial function, we determined the inner mitochondria membrane (IMM) potential. Our single‐cell‐based fluorescence measurements showed that in the MCUA_KD melanoma cells, the IMM potential is significantly reduced (Fig 6A–D). These findings suggested that mitochondrial ATP production might also be affected by MCUA_KD. We thus measured ATP levels using a fluorescent dye as well as a genetically encoded mitochondrial ATP sensor, mito‐ATEAM. Both measurements demonstrated that mitochondrial ATP levels are diminished in the 1205Lu‐ and the WM3734 MCUA_KD cells (Fig 6E–I).
Figure 6. MCUA controls mitochondrial membrane potential and ATP production.
-
A–DResting mitochondrial membrane potential (∆Ψ), measured with TMRE in 1205Lu (A, B) and WM3734 (C, D) with and without stable MCUA_KD (1205Lu—shCTRL: n = 81 cells from three biological replicates; shMCU_1: n = 81 cells from three biological replicates; shMCU_2: n = 156 cells from three biological replicates; WM3734—shCTRL: n = 108 cells from three biological replicates; shMCU_1: n = 73 cells from three biological replicates; shMCU_2: n = 110 cells from three biological replicates).
-
E, FResting mitochondrial ATP levels, measured using the ATP‐Red dye in 1205Lu (E) and WM3734 (F) with and without stable MCUA_KD (1205Lu—shCTRL = 67 cells from at least three biological replicates; shMCU_1: n = 80 cells from at least three biological replicates; shMCU_2: n = 69 cells from at least three biological replicates; WM3734—shCTRL: n = 40 cells from at least three biological replicates; shMCU_1: n = 38 cells from at least three biological replicates; shMCU_2: n = 21 cells from at least three biological replicates).
-
G–IMitochondrial ATP, measured using mito‐ATEAM in 1205Lu and WM3734 with and without stable MCUA_KD. Exemplary ratiometric images (FRET/CFP) are shown for all conditions (G). Scale bar: 10 μm. Quantification of basal levels in 1205Lu (H) and WM3734 (I) (1205Lu—shCTRL: n = 141 cells from 14 biological replicates; shMCU_1: n = 143 cells from 12 biological replicates; shMCU_2: n = 143 cells from 13 biological replicates; WM3734—shCTRL: n = 122 cells from 12 biological replicates; shMCU_1: n = 103 cells from 12 biological replicates; shMCU_2: n = 99 cells from 13 biological replicates).
-
JExemplary confocal microscope images of the mitochondrial network (blue: DAPI staining of the nucleus; green: TOMM20 staining of mitochondria); scale bar: 50 μm. (K–O) Electron microscopy of mitochondria of stable MCUA_KD cell lines.
-
KExemplary images of stable MCUA_KD cells' mitochondria. Scale bar: 500 nm.
-
L–OQuantification of mitochondrial diameter (L and N) and intercristae distance (M and O) of 1205Lu (L, M) and WM3734 (N, O) cells with and without stable MCUA_KD, presented as boxplot. The box presents the 25%‐ quartile, median and 75%‐quartile, the X represents the mean and the whiskers the minimum and maximum, outliers are represented as dots (mitochondrial diameter: 1205Lu—shCTRL: n = 47 technical replicates; shMCU_1: n = 44 technical replicates; shMCU_2: n = 40 technical replicates; WM3734—shCTRL: n = 35 technical replicates; shMCU_1: n = 52 technical replicates; shMCU_2: n = 38 technical replicates; intercristace distance: 1205Lu—shCTRL: n = 330 technical replicates; shMCU_1: n = 285 technical replicates; shMCU_2: n = 255 technical replicates; WM3734—shCTRL: n = 352 technical replicates; shMCU_1: n = 433 technical replicates; shMCU_2: n = 357 technical replicates).
Data information: Statistical significance was determined using unpaired, two‐tailed Student's t‐test, *P < 0.05; **P < 0.01; ***P < 0.005; no asterisk means no statistical significance (P > 0.05).
Mitochondrial Ca2+ induces dehydrogenase activity in the matrix and thus fuels ATP synthesis. Accordingly, the decreased ATP levels following MCUA_KD were not an unexpected finding; however, MCUA could have additional effects that may influence mitochondrial bioenergetics. For example, MCUA could modulate mitochondrial dynamics and structure. We explored this possibility by performing confocal‐ and electron microscopy. As seen in Fig 6J–O, neither the overall morphology of the mitochondrial network, nor the parameters such as mitochondrial diameters and the distances between cristae were altered in the MCUA_KD melanoma cells, indicating that the regulation of melanoma biology by MCUA is mostly governed by the mitoCa2+‐ATP‐redox axis.
MCUA affects melanoma cell therapeutic sensitivity
Current treatments of advanced melanoma encompass targeted‐ and immunotherapies (Pasquali et al, 2018; Schadendorf et al, 2018; Jenkins & Fisher, 2020). Given the effects of MCUA expression and its effects on melanoma cell growth, invasion, and patient survival, we assessed the involvement of MCUA in melanoma cell therapeutic sensitivity. To this end, we first identified transcripts that correlate with those of MCUA in the TCGA‐derived melanoma patient dataset (Fig 1). The proteomaps (Fig 7A and B) showed that in addition to metabolism, genetic information processing and signal transduction are processes whose expression correlated with MCUA and are also involved in regulating immune responses (Dataset EV4). The subsequent KEGG‐based pathway analysis further strengthened these findings (Fig 7C). As shown, the correlating genes were involved in a number of immune cell‐relevant and metabolic pathways. This was further corroborated by the GO term‐based evaluation of biological processes and molecular functions which demonstrated a robust enrichment of transcripts involved in antigen presentation, MHC activity, cytokine secretion, and other functions that can determine melanoma cell interaction with T‐ and NK cells (Fig 7D and E). These findings thus suggested that MCUA is an important determinant of melanoma cell therapeutic responses. To test this hypothesis, we first treated MCUA_KD cells with clinically relevant small molecule inhibitors, that is, trametinib (MEK inhibitor) and vemurafenib (BRAF inhibitor). As expected, both drugs reduced melanoma cell viability in a concentration‐dependent manner (Figs 7F and G, and EV5A and B). However, we did not observe differences between the parental and the MCUA_KD cells. To test their sensitivity to immunotherapies, we used primary human natural killer (NK) cells from healthy human donors and determined NK cell‐mediated melanoma cell killing (NKmK; Cappello et al, 2021). Notably, NKmK of MCUA_KD cells was diminished compared with the control cells (Fig 7H and I). Next, we treated the cells with the antineoplastic chemotherapeutic agent mitoxantrone, which is not used to treat melanoma but was recently reported as a potent inhibitor of MCU (Arduino et al, 2017). To this end, we examined its effect on melanoma cell viability and found that MCUA_KD cells are less sensitive to this inhibitor (Fig 7J). Based on our findings regarding the role of MCUA on the redox status of melanoma cells and the fact that ferroptosis is a cell death mechanism controlled by oxidative stress (Jiang et al, 2021), we treated both control and MCUA_KD cells with two ferroptosis inducers (FINs). As demonstrated, MCUA_KD cells were more resistant to BSO, an agent that lowers glutathione levels and thus induces ferroptosis (Fig 7K). This ferroptotic resistance was even more prominent when melanoma cells were treated with RSL3 (RAS‐selective lethal), a drug that induces ferroptosis by inhibiting the glutathione peroxidase 4 (GPX4) (Jiang et al, 2021) (Figs 7L and EV5C).
Figure 7. MCUA controls melanoma cell therapeutic sensitivity.
-
A, BProteomap analyses of genes significantly correlating with MCUA expression in the TCGA‐derived melanoma patient dataset.
-
CKEGG‐based analysis of cellular components and processes based on protein hits shown in A and B.
-
D, EEnrichment analyses of GO terms and pathways based on protein hits shown in A and B. GO analysis were performed according to two GO categories: biological processes (D) and molecular function (E). Significant cellular components and biological processes (P < 0.05) are indicated and ranked by fold enrichment.
-
F, GWM3734 shCTRL and MCUA_KD cell viability following incubation with different concentrations of trametinib (F) and vemurafenib (G) for 72 h (n = 3 biological replicates).
-
H, I(H) Kinetics and (I) quantification of NK cell‐mediated melanoma killing in WM3734 shCTRL and the two MCUA_KD clones (n = 6 biological replicates).
-
JWM3734 shCTRL and shMCU cell viability upon mitoxantrone treatment. Results are depicted as percent of control (DMSO‐treated cells, not shown) (n = 3 biological replicates).
-
K, LWM3734 cell viability upon incubation with different concentrations of BSO (K) and RSL3 (L) for 72 h. Data are shown as percent of control (untreated cells, not shown) (n ≤ 12 wells from three biological replicates/condition). BSO, L‐buthionine‐sulfoximine; RSL, RAS‐selective lethal.
Data information: Data are presented as mean ± SEM. Statistical significance was determined using unpaired, two‐tailed Student's t‐test, **P < 0.01; ***P < 0.005; no asterisk means no statistical significance (P > 0.05).
Figure EV5. MCUA controls melanoma cell therapeutic sensitivity (related to Fig 7).
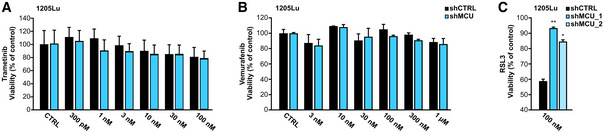
-
A, B1205Lu shCTRL and MCUA_KD cell viability following incubation with different concentrations of trametinib for 144 h (A) and vemurafenib for 96 h (B). Data are shown as percent of control (untreated cells = CTRL) (n = 3 biological replicates).
-
C1205Lu cell viability upon incubation with 100 nM RSL3 for 72 h. Data are shown as percent of control (untreated cells, not shown) (n ≤ 8 biological replicates). RSL, RAS‐selective lethal.
Data information: Data are presented as mean ± SEM. Statistical significance was determined using unpaired, two‐tailed Student's t‐test, *P < 0.05; **P < 0.01; no asterisk means no statistical significance (P > 0.05).
In summary, the presented bioinformatic‐ and experimental data indicate that MCUA regulates the immunogenicity of melanoma cells and thus their sensitivity to immune cell‐based therapies. In addition, the findings that MCUA abundance determines the sensitivity to ferroptosis established a direct link between the altered metabolism, that is, redox status and melanoma aggressive behavior.
Discussion
The importance of Ca2+ and redox regulation in cancer is nowadays widely accepted, and the number of genes and proteins involved in controlling molecular mechanisms that link Ca2+ and redox signals with cancer biology is constantly increasing (Chio & Tuveson, 2017; Monteith et al, 2017; Marchi et al, 2020). In this context, several studies suggest that the MCU complex is an important determinant of cancer biology (summarized in Vultur et al, 2018). However, the current knowledge regarding the role of MCU in cancer is incomplete, and the molecular mechanisms involved and controlled by the Ca2+‐transporting complex are not fully understood.
Our findings revealed that MCUA and thereby mitoCa2+ control melanoma patient survival and melanoma cell aggressive behavior. In this context, we demonstrated that acquired resistance to BRAF kinase inhibitors causes reduced MCUA expression and reduced mitochondrial Ca2+ uptake. We also found that melanoma cell phenotype switching, a concept known to control melanoma progression and therapeutic sensitivity, is of central importance in this regard (Rambow et al, 2019). Indeed, we observed a decreased cell growth and increased invasive properties of MCUA_KD cells in vitro and in vivo. The proteomic and RPPA‐based screens demonstrated that the highest fraction of proteins affected by MCUA_KD are involved in controlling cellular metabolism and redox signaling, in addition to environmental‐ and genetic information processing. Moreover, measurements of parameters, such as ATP production, IMM potential, and mitochondrial structure, suggest that MCUA controls mitochondrial function and bioenergetic output in melanoma cells. Given that bioenergetics, metabolism, and redox signaling play a critical role in cancer pathobiology (Diebold & Chandel, 2016; Panieri & Santoro, 2016; Hempel & Trebak, 2017; Zhang et al, 2019; Tasdogan et al, 2020), we sought to decipher the role of MCUA in melanoma by exploring the contribution of the involved signaling mechanisms and pathways. In addition, we pursued this direction based on studies which suggested that redox signals determine the melanoma cell invasive phenotype, whereby ROS, antioxidants, and the redox state of the cellular environment, that is, the blood and the lymph system control melanoma metastatic spread and sensitivity to ferroptotic cell death (Le Gal et al, 2015; Piskounova et al, 2015; Ubellacker et al, 2020).
Our findings are in line with these studies but also provide novel insights and identify molecular players that are involved in the Ca2+‐controlled metabolic regulation of melanoma. This study also indicates that manipulations of mitoCa2+ as a tool to modulate redox signaling might be applicable in treating melanoma and other cancers. More concretely, our results suggest that mitochondrial Ca2+ uptake alters the cellular redox status and causes a more proliferative but less invasive melanoma cell phenotype. Given that such growth‐oriented phenotypes are in general more responsive to conventional anticancer therapies, we hypothesize that the activation of MCU will increase the therapeutic sensitivity of melanoma cells and have a positive effect on patient survival. To this end, not only activators of MCUA can be applied but also modulators of the other MCU complex components such as MICU1‐2, EMRE, and MCUR1 (Di Marco et al, 2020; De Mario et al, 2021). Inhibitors of other mitochondrial proteins responsible for Ca2+ transport across the IMM such as NCLX, UCPs, and Letm1 might have similar therapeutic effects (Bondarenko et al, 2015; Lin & Stathopulos, 2019; Pathak et al, 2020; Madreiter‐Sokolowski et al, 2021).
Our therapeutic assays demonstrate that MCUA affects melanoma cell sensitivity to immunotherapies and ferroptosis. To this end, the bioinformatic analyses of melanoma patient datasets identified genes whose expression correlates with the one of MCUA. Notably, most of these genes are involved in shaping the immunogenicity of melanoma tumors. These molecular determinants, together with MCUA, have thus the potential to be explored as biomarkers and predictors of patient therapeutic responses. In addition, the identification of the molecular determinants underlying the robust resistance toward ferroptosis inducers in cells lacking MCUA might provide novel therapeutic targets for treating metastatic melanoma.
Our study demonstrates that in melanoma, MCUA inhibits tumor aggressiveness by controlling a signaling cascade which encompasses mitochondrial‐ Ca2+ dynamics, bioenergetics, and ROS production. Based on this model, increased MCUA expression causes elevated mitoCa2+, increased ATP synthesis but also higher ROS production that ultimately causes oxidative stress. Through a regulation of several pathways and cellular functions, this transformation induces a switch that drives melanoma cells toward a more proliferative and less invasive phenotype. Moreover, this MCU‐Ca2+‐ATP‐ROS axis‐induced phenotypic transformation will influence the immunogenicity and the therapeutic sensitivity of melanoma cells. According to this concept, melanomas with high MCUA abundance/activity are associated with higher therapeutic sensitivity and will manifest into a less aggressive disease (Fig 8).
Figure 8. MCU controls melanoma aggressive behavior via regulation of mitochondrial calcium, ATP and redox signals.
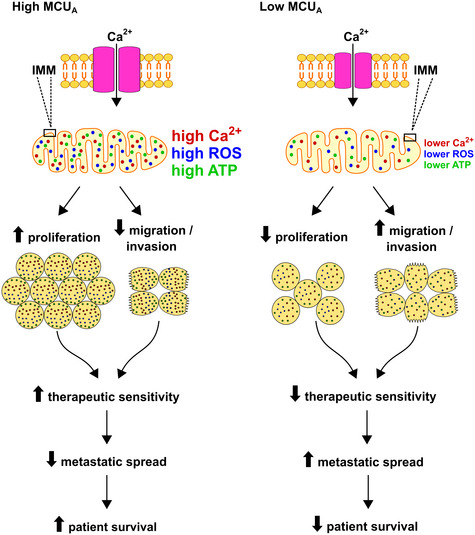
High MCUA abundance results in elevated mitoCa2+, which in turn enhances mitochondrial ROS and ATP production. This Ca2+‐induced alterations induce metabolic and redox reprograming of melanoma cells and promote a more proliferative yet less invasive cellular phenotype. The high mitoCa2+‐induces phenotype sensitizes melanoma cells to therapeutic treatments, decreases metastatic spread and has a positive effect on melanoma patient survival. Conversely, low MCUA abundance causes lower mitoCa2+, lower ROS and ATP production, promotes a less proliferative yet more invasive melanoma cell phenotype, promotes melanoma cell resistance to therapeutic treatments and decreases patient survival rate.
Within this study, we demonstrate that MCUA controls melanoma aggressive behavior and therapeutic sensitivity. We identify novel biomarkers and signaling pathways that control melanoma cell aggressiveness and suggest that manipulations of mitochondrial Ca2+ and redox homeostasis, in combination with current therapies, should be considered in treating advanced melanoma.
Materials and Methods
Cell culture and reagents
Human melanoma cell lines (1205Lu, 451Lu, 451Lu‐BR3, WM983B, WM983B‐BR, and WM3734) and HEK293T cells for lentiviral production were provided by Meenhard Herlyn (The Wistar Institute, Philadelphia, USA). Cells were cultured in TU 2% medium (80% MCDB153 basal medium (#P04‐80062, PAN Biotech), 20% Leibovitz's L‐15 medium (#P04‐27055, PAN Biotech) supplemented with 2 mM L‐Glutamin, 1.68 mM CaCl2 and 2% FCS) and were maintained at 37°C in 5% CO2. All cell lines tested negative for Mycoplasma using regularly the PCR Mycoplasma Test Kit I/C (PromoKine #PK‐CA91‐1048, PromoCell GmbH, Heidelberg, Germany). Chemicals were purchased from Sigma‐Aldrich (Munich, Germany), unless otherwise indicated. Small molecule inhibitors were purchased from Selleckchem (Absource, Munich, Germany).
Protein knockdown
Transient KD was performed using siRNA sequences from Qiagen (Hilden, Germany) and MicroSynth (Balgach, Switzerland), delivered to the cells by nucleofection (Amaxa Nucleofector, Lonza GmbH, Cologne, Germany). Two million cells and 4 μl of 20 pmol siRNA were used for each transfection. Measurements were taken 48 h post‐transfection. All siRNA sequences are listed in Table EV1.
For stable KD, plasmids expressing shRNA (pLKO.1) were obtained from the Broad Institute's RNAi Consortium (Sigma‐Aldrich, St. Louis, MO, USA). Lentiviral vector pLKO.1 was used as an empty vector control (shCTRL) or with the shRNA sequences as follows: shMCU_1 (#TRCN133861) and shMCU_2 (#TRCN420533). Lentiviruses were produced by transfecting HEK293T cells with the packaging plasmids (pPAX2 and pMD2.G) along with 4 μg lentiviral shRNA vector using Lipofectamine 2000 reagent (#116680, Invitrogen, Waltham, MA, USA), according to the manufacturer's instructions. Melanoma cells were transduced with virus in the presence of 8 μg/ml polybrene (Sigma‐Aldrich, St. Louis, MO, USA) for 18 h. Transduced cell populations were selected with 1 μM puromycin (Sigma‐Aldrich, St. Louis, MO, USA). shRNA KD efficiency was confirmed by WB analysis and RT–qPCR.
Fura‐2‐AM‐based Ca2+ imaging
Cytosolic Ca2+ measurements were performed as described in (Saul et al, 2016). Shortly, melanoma cells were loaded with 1 μM Fura‐2 AM (#F1221, Thermo Fisher Scientific GmbH, Schwerte, Germany) in culture medium and incubated at room temperature for 30 min. All measurements were taken in Ringer's buffer (pH = 7.4) containing 145 mM NaCl, 4 mM KCl, 10 mM glucose, 10 mM HEPES (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid), 2 mM MgCl2, and 0–0.5 mM Ca2+ (the 0 mM CaCl2 buffer was supplemented with 1 mM EGTA). One μM thapsigargin (Tg) (#T9033, Sigma‐Aldrich, Munich, Germany) and 4 μM ionomycin (Iono) (#407950, Merck Millipore Calbiochem, Burlington, MA, USA) were used to facilitate Ca2+ store depletion. Time‐lapse ratiometric imaging was carried out using an Olympus IX70 microscope equipped with Xenon‐lamp, Polychrome V Monochromator, and CCD‐Kamera T.I.L.L. Imago (Olympus, Hamburg, Germany).
Fluorescence microscopy
Imaging experiments were performed at 37°C in 0.5 mM or 1 mM Ca2+ Ringer's buffer (as indicated in the figure legends) using either a Zeiss Cell Observer Z1 microscope equipped with a 40× oil “Fluar” (N.A.: 1.3) objective, multi‐filter system, fast acquisition EMCCD camera (Evolve® 512 Delta) and LED system (Colibri, Zeiss) or a Zeiss Observer D1 equipped with a 40X oil Neofluar (N.A.: 1.3) objective, Axiocam 702 mono and LED system (Colibri, Zeiss) or a Zeiss Axio Observer 7 equipped with 40× oil “Neofluar” (N.A.: 1.3), Axiocam 702 mono and LED system (Colibri 7, Zeiss). Subsequent obtained data were processed with the AxioVision, Zen 2.6, or Zen 3.2 softwares (Zeiss, Oberkochen, Germany). All plasmids and constructs used are listed in Table EV2.
FRET measurements
Mitochondrial Ca2+ (4mt‐D3cpV) and mitochondrial ATP (mito‐ATEAM) were measured using fluorescence resonance energy transfer (FRET) sensors (CFP excitation: 420/40 nm; emission: 483/32 nm and YFP excitation: 505/15 nm; emission: 542/27 nm). Ca2+ uptake was initiated using 1 μM thapsigargin (Tg) or 100 μM ATP. FRET was calculated using the background and bleed‐through corrected FRET/donor ratio (equation 1).
| (1) |
*CF: correction factor for donor (d) and acceptor (a) bleed‐through.
H2O2 and glutathione measurements
Hydrogen peroxide (H2O2) measurements were carried out using the ratiometric protein sensor HyPer (excitation: 420/40 and 505/15 nm; emission: 542/28 or 539/25 nm). SypHer sensor was used as a pH sensitivity control. Glutathione redox potential was assessed using the mito‐Grx1‐roGFP2 ratiometric sensor (excitation 385/30 and 469/28 nm; emission 525/50 nm).
TMRE
Mitochondrial membrane potential (∆Ψ) was measured with TMRE (Tetramethylrhodamine, Ethyl Ester, Perchlorate; #T669; ThermoFisher) on a single cell level. Cells were loaded with 100 nM TMRE for 15 min and imaged (excitation: 550/32 nm; emission: 630/92 nm), 1 μM CCCP (carbonylcyanid‐3‐chlorophenylhydrazone) was added as a control.
ATP measurements
Mitochondrial ATP was measured using the BioTracker™ ATP‐Red Live Cell dye (# SCT045; Merck) on a single cell level. Cells were loaded with 5 μM ATP‐Red dye for 15 min at 37°C and imaged (excitation: 550/32 nm; emission: 630/92 nm).
Electron microscopy
Cells grown on glass coverslips were immobilized using a prewarmed solution consisting of 2.5% glutaraldehyde in 0.1 M cacodylic buffer at pH 7.4. Sample immobilization was continued for 1 h at room temperature and was completed at 4°C overnight. Staining and secondary fixation was started using 1% osmium tetroxide in 1.5% K4[Fe(CN)6] in 0.1 M cacodylic buffer at pH 7.4 for 1 h at room temperature. Without any washing in‐between, the solution was replaced with 1% osmium tetroxide in 0.1 M cacodylic buffer at pH 7.4 and incubated for an additional hour at room temperature. During three extensive washing steps using double distilled water for 10 min each, the Walton's lead aspartate solution was prepared. Therefore, 40 mg aspartic acid was dissolved in double distilled water, and the solution brought up to 60°C in an oven. Next, 66 mg lead nitrate was added to the solution. The pH of the mixture was then adjusted using 1N sodium hydroxide solution. The samples were then immersed in the lead aspartate solution and kept at 60°C for 1 h. After three washing steps with double distilled water for 10 min each, the samples were dehydrated with a graded ethanol series starting with 30% over 50, 70 up to 100% with several exchanges in‐between. Final dehydration prior to resin infiltration was facilitated with two exchanges of propylene oxide for 5 min each. Resin infiltration was mediated with a starting solution of 1:1 Epon resin and propylene oxide for 1 h at room temperature followed by placing the samples into fresh 100% Epon resin for an additional hour. For overnight infiltration, the samples were placed again in fresh Epon resin on a rocker table overnight at room temperature. The following day, the samples were embedded in BEEM capsules, and resin polymerization took place over 48 h at 60°C.
Thin sections of 60 nm thickness were prepared from the final resin blocks and collected on formvar‐coated mesh grids. The electron micrographs were recorded on a Philips CM120 transmission electron microscope equipped with a LaB6‐source and a TVIPS 2x2 slow‐scan CCD camera at an original magnification of 8,600×.
Immunolabeling and confocal microscopy
Cells were cultured on glass coverslips and fixed with prewarmed (37°C) 8% formaldehyde in PBS for 10 min at room temperature. After washing with PBS, cells were permeabilized with 0.5% Triton X‐100 in PBS for 5 min and blocked with 5% BSA in PBS for 5 min. Anti‐TOMM20 antibody coupled to Alexa Fluor 488 (1:100, #ab205486, Abcam) was diluted in 5% BSA in PBS and incubation was performed in dark for 1 hour at room temperature. Cells were washed in PBS and 2.5 μg/ml 40,6‐Diamidin‐2‐phenylindol (DAPI) (Sigma‐Aldrich) was added to the PBS during one washing step. Cells were then mounted in Mowiol with 0.1% 1,4‐Diazabicyclo[2.2.2]octan (DABCO).
Confocal microscopy was performed with a TCS SP8 (Leica, Wetzlar, Germany) with a 63X oil immersion objective (HC PL APO 63×/1,40 oil CS2). No image processing was applied except for adjustment of brightness and contrast.
Proliferation, viability, migration, and invasion assays
Melanoma cell proliferation was evaluated by seeding 10,000 cells/well in 24‐well plates and incubating them at 37°C over a period of 72 h. Following cell growth, cells were fixed with methanol for 15 min and stained with a 0.05% crystal violet solution for 30 min at room temperature. Next, cells were destained with a 40% acetic acid solution, and absorbance at 595 nm was measured using a Mithras LB 940 plate reader (Berthold Technologies, Bad Wildbad, Germany).
Cell viability was assessed using the CellTiter‐Blue® Cell Viability Assay kit (Promega GmbH, Walldorf, Germany), according to the manufacturer's instructions. Briefly, 5,000 cells/well were seeded in 96‐well plates and allowed to attach overnight. Cells were afterward treated with small molecule inhibitors or ferroptosis inducers for 24–72 h. The resazurin‐based reagent was added 3 h prior to fluorescence measurement using a CLARIOstar® plate reader (BMG LABTECH, Ortenberg, Germany).
To assess the in vitro migration ability of cells, a transwell migration setup featuring 8 μm pore size inserts (Corning®, Kennebunk ME, USA) was used. Briefly, 200,000 cells in 150 μl of fresh, serum‐free medium were pipetted into the top compartment of an insert and were allowed to migrate for 4–48 h toward preconditioned medium containing 10% FCS. Prior to imaging, nonmigrated cells were washed out from the inserts, and the remaining migrated cells were stained with either 1 μM calcein‐AM (#C1430, Thermo Fisher Scientific, Waltham, Massachusetts, USA) or with 0.5 μg/ml Hoechst 33342 (#H1399, Thermo Fisher Scientific, Waltham, Massachusetts, USA). Fluorescence intensity was measured using an Infinite 200 PRO plate reader (Tecan, Männedorf, Switzerland), or images of migrated cells were acquired using a Zeiss Axiovert S100TV inverted microscope (Oberkochen, Germany) featuring a sCMOS pco.edge camera and later analyzed using the ImageJ software.
Spheroids were generated as previously described (Roesch et al, 2013). Shortly, 5,000 cells/well were seeded in 96‐well plates on top of a nonadhesive layer of agar. Following 96 h and the formation of a 3D structure, spheroids were harvested and embedded in a collagen I mixture and were allowed to invade for a given period of time. Spheroids were then stained with the Live/Dead™ Viability/Cytotoxicity Kit (#L3224, Invitrogen, Carlsbad, California, USA) and were imaged using a Zeiss Primo Vert and a Zeiss Axiovert S100TV microscope equipped with a 10× objective. Images were acquired using the AxioVision and the VisiView® softwares. Spheroid size evaluation was determined using the ImageJ software. Spheroid invasion was measured by subtracting the mask for the core of each spheroid from the total area covered by all the cells of a given spheroid (invasion area [μm2] = total area − spheroid core) using ImageJ.
RT–qPCR
The total RNA (800 ng of template RNA was used per condition) was isolated and reverse transcribed to cDNA using Superscript™ II (#18064022, Invitrogen), according to the manufacturer's instructions. 0.5 μl of cDNA was used for RT–qPCR using the QuantiTect SYBR Green Kit (#204145, Qiagen) and Bio‐Rad CFX96™ Real‐Time System. The CT values of the target mRNAs were normalized to the CT values of TATA box binding protein (TBP) which was used as the housekeeping gene. Data were quantified using the 2−ΔCT method. Gene‐specific primer sets were purchased from Qiagen or Sigma‐Aldrich. Primer sequences are listed in Table EV3.
Western blots
For Western blot (WB), proteins were extracted as previously described in (Stanisz et al, 2014). Shortly, 40–75 μg of protein were resolved on a 10% SDS–polyacrylamide gel and transferred onto a 0.45 μm nitrocellulose membrane (#10600003, Amersham Protran Premium, GE Healthcare). After transfer, membranes were blocked in 5% BSA or 5% skim milk powder solution, followed by an overnight incubation with primary antibodies. Membranes were incubated with secondary antibodies for 1 h in the dark at room temperature. Imaging and quantification of the blots was performed using an Odyssey infrared imaging system (LI‐COR, Lincoln, Nebraska, USA). Primary and secondary antibodies used for immunoblotting are listed in Tables EV4 and EV5.
Real‐time killing assay
Human primary NK cell isolation and the subsequent melanoma killing assay were performed as described in (Cappello et al, 2021). Briefly, NK cells were obtained by negative bead isolation (#11349D, Dynabeads™ Untouched Human NK cell Kit) from human peripheral blood mononuclear cells (PBMCs) of healthy thrombocyte donors of the local blood bank (University Medical Center Göttingen, Ethics approval 2/3/18). Melanoma cells were seeded in a black, clear bottom 96‐well plates and loaded with 0.5 μM calcein‐AM (#C1430, Thermo Fisher Scientific, Waltham, MA, USA). Interleukin‐2‐stimulated (0.05 μg/ml; #15596–026, Thermo Fisher Scientific GmbH, Schwerte, Germany) primary human NK cells were added to melanoma cells in a NK cell to target cell ratio of ~ 5:1. NK cell‐mediated melanoma killing was measured for 4 h at 37°C in 5% CO2 using a CLARIOstar® plate reader (BMG LABTECH, Ortenberg, Germany). Killing efficiency was evaluated by fluorescence signal decrease.
Melanoma xenografts
All animal experiments were performed in accordance with The Wistar IACUC in NOD/LtSscidIL2Rγnull mice (NSG). Mice were kept in the same room, in germ‐free environment, socially caged as five mice/unit cage and fed with sterile food pellets and water. Wistar Animal Facility has a quality control program in place wherein 5% of mice in each holding room were periodically tested serologically for common murine viruses, Mycoplasma pulmonis and Helicobacter. Human melanoma cells were injected with the MCUA_KD cells and their respective control (1205Lu shCTRL, shMCU_1 or shMCU_2); 10 animals were used per group, randomized. Each mouse was inoculated subcutaneously with 400,000 melanoma cells in a 1:1 suspension of Matrigel (#354230, BD Matrigel™ Basement Membrane Matrix, Growth Factor Reduced; Becton Dickinson, Franklin Lakes, NJ, USA) and complete media. Seven days after cell injection, tumor growth was measured every 2–3 days, for 36 days, using a caliper and volumes calculated according to the formula V = (W × D × H)/2 (mm3). On the last experimental day, a final measurement of the tumor was taken before tumor extraction. Other organs such as liver, lung, kidneys, and brain were isolated for further examinations. Tumor samples were snap‐frozen in liquid nitrogen for subsequent protein analyses or fixed in formalin for the generation of paraffin blocks for immunostaining.
Immunohistochemistry
Hematoxylin and eosin (H&E) staining was performed on sections of lung tissue from mice; these were deparaffinized and returned to an aqueous medium by consecutively immersing them in xylene, 100% isopropanol, and 70% isopropanol. For the hematoxylin staining, a hemalum solution diluted 1:10 in water was prepared. Following dipping for 6–10 times into the hemalum solution, tissue sections were rinsed with tap water which resulted in a color change from reddish‐brown to blue‐violet. Tissue sections were consequently counterstained with eosin and were scanned using an Axio Scan.Z1 microscope.
Reverse Phase Protein Array (RPPA)
The RPPA assay was performed by the MD Anderson Center RPPA core facility, as previously described (Paweletz et al, 2001; Grote et al, 2008). Shortly, serial‐diluted lysates were printed on nitrocellulose‐coated slides using a 2470 Microarray printer from Aushon Biosystems (Billerica, Massachusetts, USA). Slides were probed with primary antibodies plus biotin‐conjugated secondary antibodies. The signal obtained was amplified using a Dako signal amplification system (Copenhagen, Denmark) and visualized by DAB colorimetric reaction. The intensity of each spot was calculated using the MicroVigeneTM (VigeneTech, Billerica, Massachusetts, USA). The data obtained contained normalized and linearized values of protein. The values of the KD cells were normalized to the associated control. Primary and secondary antibodies used for the RPPA assay can be found on the website of the MD Anderson Cancer Center (https://www.mdanderson.org/research/research‐resources/core‐facilities/functional‐proteomics‐rppa‐core/antibody‐information‐and‐protocols.html).
Mass spectrometric proteome analysis
A total of 50 μg of protein per sample were loaded onto a 4–12% NuPAGE Novex Bis‐Tris Minigels (Invitrogen) and run into the gel for 1.5 cm. Following Coomassie staining, the protein areas were cut out, diced, and reduced with dithiothreitol, alkylation with iodoacetamide, and digested overnight with trypsin. Tryptic peptides were extracted from the gel, the solution dried in a Speedvac, and kept at −20°C for further analyses (Atanassov & Urlaub, 2013). For the generation of a peptide library, equal amount of aliquots from each sample were pooled to the total amount of 80 μg, and separated into eight fractions using a reversed phase spin column (Pierce High pH Reversed‐Phase Peptide Fractionation Kit, Thermo Fisher Scientific).
Digested proteins were analyzed on a nanoflow chromatography system (Eksigent nanoLC425) hyphenated to a hybrid triple quadrupole‐TOF mass spectrometer (TripleTOF 5600+) equipped with a Nanospray III ion source (Ionspray Voltage 2400 V, Interface Heater Temperature 150°C, Sheath Gas Setting 12) and controlled by Analyst TF 1.7.1 software build 1163 (all AB Sciex). In brief, peptides were dissolved in loading buffer (2% acetonitrile, 0.1% formic acid in water) to a concentration of 0.3 μg/μl. For each analysis, 1.5 μg of digested protein was enriched on a self‐packed precolumn (0.15 mm ID x 20 mm, Reprosil‐Pur120 C18‐AQ 5 μm, Dr. Maisch, Ammerbuch‐Entringen, Germany) and separated on an analytical RP‐C18 column (0.075 mm ID x 250 mm, Reprosil‐Pur 120 C18‐AQ, 3 μm, Dr. Maisch) using a 100‐min linear gradient of 5–35% acetonitrile/0.1% formic acid (v:v) at 300 nl min−1.
Qualitative LC/MS/MS analysis was performed using a Top30 data‐dependent acquisition method with an MS survey scan of m/z 380–1,250 accumulated for 250 ms at a resolution of 35,000 full width at half maximum (FWHM). MS/MS scans of m/z 180–1,500 were accumulated for 100 ms at a resolution of 17,500 FWHM and a precursor isolation width of 0.7 FWHM, resulting in a total cycle time of 3.4 s. Precursors above a threshold MS intensity of 200 cps with charge states 2+, 3+, and 4+ were selected for MS/MS, and the dynamic exclusion time was set to 15 s. MS/MS activation was achieved by CID using nitrogen as a collision gas and the manufacturer's default rolling collision energy settings. Two technical replicates per reversed phase fraction were analyzed to construct a spectral library.
For quantitative SWATH analysis, MS/MS data were acquired using 100 variable size windows across the 400–1,200 m/z range (Zhang et al, 2015). Fragments were produced using rolling collision energy settings for a charge state of 2+, and fragments acquired over an m/z range of 180–1,500 for 40 ms per segment. An overall cycle time of 4.3 s was the result of a 250 ms survey scan. Two replicate injections were acquired for each biological sample.
Protein identification was achieved using ProteinPilot Software version 5.0 build 4769 (AB Sciex) at the appropriate settings. A total of 126,301 MS/MS spectra from the combined qualitative analyses were searched against the UniProtKB Homo sapiens reference proteome (revision 04–2018, 93,610 entries) augmented with a set of 51 known common laboratory contaminants to identify proteins at a false discovery rate (FDR) of 1%.
Spectral library generation and SWATH peak extraction were achieved in PeakView Software version 2.1 build 11041 (AB Sciex) using the SWATH quantitation microApp version 2.0 build 2003. Following retention time correction on endogenous peptides spanning the entire retention time range, peak areas were extracted using information from the MS/MS library at a FDR of 1% (Lambert et al, 2013). The resulting peak areas were then summed to peptide and finally protein area values, which were used for further statistical analyses. A 1.698 proteins could be quantified consistently across all samples at a FDR of 1%
Bioinformatic analyses
Patient survival data
To generate survival plots, melanoma patient datasets (TCGA) were downloaded from cBioPortal on 26.08.2020. Data on other cancers were downloaded from Human Protein Atlas on 18.03.2021. Kaplan–Meier plots were calculated using lifelines (10.5281/zenodo.4816284). Patients were divided into patients with either low or high MCUA expression by scanning 20–80% of MCUA's expression value and determining the optimal separation in terms of survival based on a log‐rank test. For detailed analyses, patients were separated by different melanoma tumor stages (I–II and III–IV), by age, gender, or by BRAF‐ and NRAS‐wild‐type or ‐mutant. Melanoma patient survival data (low and high MCUA) were utilized for further analyses (based on hits evaluation). Hits were determined by calculating P‐values (< 0.001) using Wilcoxon test adjusted by the Benjamini–Hochberg procedure, (FDR < 0.05) and selected according to an absolute logarithmic fold change higher than 1.
Volcano plots
Volcano plots were created to display protein hits. Student's t‐tests were calculated for each shCTRL and MCUA_KD cell line independently. Hits were shown in red if the unadjusted P‐value was significant (P < 0.05) and the absolute logarithmical fold change was higher than 0.5. Hits were shown in green if the adjusted (Benjamini–Hochberg procedure, FDR < 0.05) P‐value was significant and an absolute logarithmical fold change higher than 1 occurred. Only the hits shown in red in the volcano plot were further analyzed.
RPPA heatmap
To assess the RPPA data, a heatmap was generated. Hits were selected by considering a logarithmical fold change higher than 1 for each MCUA_KD (WM3734 shMCU_1, WM3734 shMCU_2, 1205Lu shMCU_1, 1205Lu shMCU_2) cell line independently.
Proteomaps
Proteomaps were generated via (http://bionic‐vis.biologie.uni‐greifswald.de/) by importing patient data‐, proteomic‐, and RPPA hits. All proteomaps were generated considering all human genes as a background. The size of each polygon represents the protein abundance.
KEGG‐based enrichment analyses
KEGG (Kyoto encyclopedia of genes and genomes)‐based enrichment analysis were generated using DAVID bioinformatics resources that analyzed patient data‐, proteomic‐, and RPPA hits (red) (Huang da et al, 2009). All results were filtered by FDR adjusted P‐value threshold of 0.05 and sorted by fold enrichment.
GO Terms
Patient data‐, proteomic‐, and RPPA hits were analyzed using GO (Gene Ontology) terms (Ashburner et al, 2000). All results were filtered by FDR adjusted P‐value threshold of 0.05 and sorted by fold enrichment.
Hallmarks
Cancer Hallmarks Gene sets were taken from (Zhang et al, 2020). Proteomic‐ and RPPA hits were analyzed considering their occurrence in each gene set. The occurrence of each hallmark was calculated and shown in percentage. P‐values were calculated using hygeometric tests.
Statistical analyses
Data were analyzed and processed using Zeiss AxioVision, Zeiss Zen, VisiView®, TILLVISION, Bio‐Rad Quantity One, ImageJ/FIJI and Microsoft Excel. Data are shown as mean ± SEM. Unpaired, two‐tailed Student's t‐tests were carried out to test statistical significance, unless otherwise specified. The significant differences are marked with asterisks: *P < 0.05, **P < 0.01, and ***P < 0.005; a P‐value higher than 0.05 means that no significant difference was observed.
Author contributions
Ioana Stejerean‐Todoran: Formal analysis; investigation; visualization; writing – original draft. Katharina Zimmermann: Formal analysis; investigation; visualization. Christine Gibhardt: Formal analysis; investigation; visualization. Adina Vultur: Formal analysis; supervision; investigation; writing – original draft. Christian Ickes: Formal analysis; investigation; visualization. Batool Shannan: Formal analysis; investigation. Zurine Bonilla del Rio: Formal analysis; investigation. Anna Wölling: Formal analysis; investigation. Sabrina Cappello: Formal analysis; investigation. Hsu‐Min Sung: Formal analysis; investigation. Magdalena Shumanska: Formal analysis; investigation; visualization. Xin Zhang: Formal analysis; investigation. Maithily Nanadikar: Formal analysis; investigation. Muhammad U Latif: Formal analysis; investigation; visualization. Anna Wittek: Formal analysis; investigation; visualization. Felix Lange: Formal analysis; investigation; visualization. Andrea Waters: Investigation. Patricia Brafford: Investigation. Jörg Wilting: Formal analysis; supervision; investigation. Henning Urlaub: Resources; supervision; funding acquisition. Dörthe M Katschinski: Supervision; funding acquisition. Peter Rehling: Supervision; funding acquisition. Christof Lenz: Supervision; investigation; methodology. Stefan Jakobs: Conceptualization; funding acquisition. Volker Ellenrieder: Conceptualization; supervision; funding acquisition. Alexander Roesch: Supervision; funding acquisition. Michael P Schön: Supervision; funding acquisition. Meenhard Herlyn: Resources; supervision; funding acquisition. Hedwig Stanisz: Conceptualization; supervision; funding acquisition; writing – original draft; writing – review and editing. Ivan Bogeski: Conceptualization; supervision; funding acquisition; writing – original draft; writing – review and editing.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Dataset EV1
Dataset EV2
Dataset EV3
Dataset EV4
Source Data for Expanded View
PDF+
Acknowledgments
We thank Prof. Markus Hoth for his continuous support. Many thanks also to Sandra Janku, Alexandra Stark, Andrea Paluschkiwitz, Josh Wang, Annette Bennemann, Sonja Schwoch, and Berti Manshausen for their technical assistance. This work was supported by German Research Foundation (DFG) projects: RO 3577/3‐2 (to A.R.), RO 3577/7‐1 (to A.R.), SFB1430 project A11 (to A.R.), SFB1190 projects 1 (to S.J.), 13 (to P.R.) and 17 (to I.B.), SFB1027 Project C4 (to I.B.), IRTG1816 (to I.B.), TRR 274 Z01 (to S.J.). H.S. acknowledges financial support from the Medical School, University of Saarland via the HOMFOR program. Open Access funding enabled and organized by Projekt DEAL.
EMBO reports (2022) 23: e54746
Data availability
The datasets produced in this study are available in the following databases:
RPPA: These data are available in Dataset EV3.
Mass spectrometry proteomics: These data are available in Datasets EV1 and EV2 and on the ProteomeXchange Consortium via the PRIDE partner repository, dataset identifier: PXD029132 (http://www.ebi.ac.uk/pride/archive/projects/PXD029132).
References
- Arduino DM, Wettmarshausen J, Vais H, Navas‐Navarro P, Cheng Y, Leimpek A, Ma Z, Delrio‐Lorenzo A, Giordano A, Garcia‐Perez C et al (2017) Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol Cell 67: 711–723.e717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT et al (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassov I, Urlaub H (2013) Increased proteome coverage by combining PAGE and peptide isoelectric focusing: comparative study of gel‐based separation approaches. Proteomics 13: 2947–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló C, Sisó P, Maiques O, de la Rosa I, Martí RM, Macià A (2020) T‐type calcium channels: a potential novel target in melanoma. Cancer 12: 391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko AI, Parichatikanond W, Madreiter CT, Rost R, Waldeck‐Weiermair M, Malli R, Graier WF (2015) UCP2 modulates single‐channel properties of a MCU‐dependent Ca(2+) inward current in mitochondria. Pflugers Arch 467: 2509–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DM, Várnai P, Joseph SK, Hajnóczky G (2021) Oxidative bursts of single mitochondria mediate retrograde signaling toward the ER. Mol Cell 81: 3866–3876.e3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S, Sung HM, Ickes C, Gibhardt CS, Vultur A, Bhat H, Hu Z, Brafford P, Denger A, Stejerean‐Todoran I et al (2021) Protein signatures of NK cell‐mediated melanoma killing predict response to immunotherapies. Cancer Res 81: 5540–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesi G, Walbrecq G, Zimmer A, Kreis S, Haan C (2017) ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells. Mol Cancer 16: 102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio IIC, Tuveson DA (2017) ROS in cancer: The Burning Question. Trends Mol Med 23: 411–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mario A, Tosatto A, Hill JM, Kriston‐Vizi J, Ketteler R, Vecellio Reane D, Cortopassi G, Szabadkai G, Rizzuto R, Mammucari C (2021) Identification and functional validation of FDA‐approved positive and negative modulators of the mitochondrial calcium uniporter. Cell Rep 35: 109275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco G, Vallese F, Jourde B, Bergsdorf C, Sturlese M, De Mario A, Techer‐Etienne V, Haasen D, Oberhauser B, Schleeger S et al (2020) A high‐throughput screening identifies MICU1 targeting compounds. Cell Rep 30: 2321–2331.e2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold L, Chandel NS (2016) Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med 100: 86–93 [DOI] [PubMed] [Google Scholar]
- Foskett JK, Philipson B (2015) The mitochondrial Ca(2+) uniporter complex. J Mol Cell Cardiol 78: 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote T, Siwak DR, Fritsche HA, Joy C, Mills GB, Simeone D, Whitcomb DC, Logsdon CD (2008) Validation of reverse phase protein array for practical screening of potential biomarkers in serum and plasma: accurate detection of CA19‐9 levels in pancreatic cancer. Proteomics 8: 3051–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Haq R, Shoag J, Andreu‐Perez P, Yokoyama S, Edelman H, Rowe GC, Frederick DT, Hurley AD, Nellore A, Kung AL et al (2013) Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell 23: 302–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel M, Ortenberg R, Varanasi SK, Mangalhara KC, Mardamshina M, Markovits E, Baruch EN, Tripple V, Arama‐Chayoth M, Greenberg E et al (2019) Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell 179: 236–250.e218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel N, Trebak M (2017) Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium 63: 70–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW et al (2014) SLC25A23 augments mitochondrial Ca2+ uptake, interacts with MCU, and induces oxidative stress‐mediated cell death. Mol Biol Cell 25: 936–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Zhang X, Webster M, Go C, Kedra J, Marchbank K, Gill DL, Weeraratna AT, Trebak M, Soboloff J (2015) Novel protein kinase C‐mediated control of orai1 function in invasive melanoma. Mol Cell Biol 35: 2790–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M (2016) CRAC channels, calcium, and cancer in light of the driver and passenger concept. Biochim Biophys Acta 1863: 1408–1417 [DOI] [PubMed] [Google Scholar]
- Hoth M, Fanger CM, Lewis RS (1997) Mitochondrial regulation of store‐operated calcium signaling in T lymphocytes. J Cell Biol 137: 633–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RW, Fisher DE (2020) Treatment of advanced melanoma in 2020 and beyond. J Invest Dermatol 141: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22: 266–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Booth DM, Young MP, Hajnóczky G (2019) Redox regulation of ER and mitochondrial Ca(2+) signaling in cell survival and death. Cell Calcium 79: 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, Lamperska K (2018) 2D and 3D cell cultures: a comparison of different types of cancer cell cultures. Arch Med Sci 14: 910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JP, Ivosev G, Couzens AL, Larsen B, Taipale M, Lin ZY, Zhong Q, Lindquist S, Vidal M, Aebersold R et al (2013) Mapping differential interactomes by affinity purification coupled with data‐independent mass spectrometry acquisition. Nat Methods 10: 1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyurek LM, Lindahl P, Nilsson J et al (2015) Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7: 308re8 [DOI] [PubMed] [Google Scholar]
- Li CJ, Lin HY, Ko CJ, Lai JC, Chu PY (2020) A novel biomarker driving poor‐prognosis liver cancer: overexpression of the mitochondrial calcium gatekeepers. Biomedicine 8: 451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q‐T, Stathopulos PB (2019) Molecular mechanisms of leucine zipper EF‐hand containing transmembrane protein‐1 function in health and disease. Int J Mol Sci 20: 286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ling S, Hegde AM, Byers LA, Coombes K, Mills GB, Akbani R (2016) Using reverse‐phase protein arrays as pharmacodynamic assays for functional proteomics, biomarker discovery, and drug development in cancer. Semin Oncol 43: 476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madreiter‐Sokolowski CT, Gottschalk B, Sokolowski AA, Malli R, Graier WF (2021) Dynamic control of mitochondrial Ca(2+) levels as a survival strategy of cancer cells. Front Cell Dev Biol 9: 614668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Raffaello A, Vecellio Reane D, Gherardi G, De Mario A, Rizzuto R (2018) Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models. Pflugers Arch 470: 1165–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Giorgi C, Galluzzi L, Pinton P (2020) Ca2+ fluxes and cancer. Mol Cell 78: 1055–1069 [DOI] [PubMed] [Google Scholar]
- Monteith GR, Prevarskaya N, Roberts‐Thomson SJ (2017) The calcium‐cancer signalling nexus. Nat Rev Cancer 17: 367–380 [DOI] [PubMed] [Google Scholar]
- Nemani N, Shanmughapriya S, Madesh M (2018) Molecular regulation of MCU: implications in physiology and disease. Cell Calcium 74: 86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell JS, Teng MWL, Smyth MJ (2019) Cancer immunoediting and resistance to T cell‐based immunotherapy. Nat Rev Clin Oncol 16: 151–167 [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Zanin S, Rizzuto R (2021) From the identification to the dissection of the physiological role of the mitochondrial calcium uniporter: an ongoing story. Biomolecules 11: 786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8: 839–845 [DOI] [PubMed] [Google Scholar]
- Panieri E, Santoro MM (2016) ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7: e2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali S, Hadjinicolaou AV, Sileni VC, Rossi CR, Mocellin S (2018) Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev 2: CD011123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak T, Gueguinou M, Walter V, Delierneux C, Johnson MT, Zhang X, Xin P, Yoast RE, Emrich SM, Yochum GS et al (2020) Dichotomous role of the human mitochondrial Na(+)/Ca2(+)/Li(+) exchanger NCLX in colorectal cancer growth and metastasis. Elife 9: e59686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak T, Trebak M (2018) Mitochondrial Ca(2+) signaling. Pharmacol Ther 192: 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweletz CP, Charboneau L, Bichsel VE, Simone NL, Chen T, Gillespie JW, Emmert‐Buck MR, Roth MJ, Petricoin IE, Liotta LA (2001) Reverse phase protein microarrays which capture disease progression show activation of pro‐survival pathways at the cancer invasion front. Oncogene 20: 1981–1989 [DOI] [PubMed] [Google Scholar]
- Petrungaro C, Zimmermann KM, Küttner V, Fischer M, Dengjel J, Bogeski I, Riemer J (2015) The Ca(2+)‐dependent release of the Mia40‐induced MICU1‐MICU2 dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab 22: 721–733 [DOI] [PubMed] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ (2015) Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527: 186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevarskaya N, Skryma R, Shuba Y (2011) Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer 11: 609–618 [DOI] [PubMed] [Google Scholar]
- Rambow F, Marine JC, Goding CR (2019) Melanoma plasticity and phenotypic diversity: therapeutic barriers and opportunities. Genes Dev 33: 1295–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359: 1350–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga‐Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M (2010) A temporarily distinct subpopulation of slow‐cycling melanoma cells is required for continuous tumor growth. Cell 141: 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA, Philipp SE et al (2013) Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow‐cycling JARID1B(high) cells. Cancer Cell 23: 811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta K, Douglas S, Parekh AB (2014) Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store‐operated Ca2+ entry and Ca2+−dependent gene expression in response to receptor stimulation. PLoS One 9: e101188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul S, Gibhardt CS, Schmidt B, Lis A, Pasieka B, Conrad D, Jung P, Gaupp R, Wonnenberg B, Diler E et al (2016) A calcium‐redox feedback loop controls human monocyte immune responses: the role of ORAI Ca2+ channels. Sci Signal 9: ra26 [DOI] [PubMed] [Google Scholar]
- Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO (2014) Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6: 221ra15 [DOI] [PubMed] [Google Scholar]
- Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A, Ugurel S (2018) Melanoma. Lancet 392: 971–984 [DOI] [PubMed] [Google Scholar]
- Stafford WC, Peng X, Olofsson MH, Zhang X, Luci DK, Lu L, Cheng Q, Trésaugues L, Dexheimer TS, Coussens NP et al (2018) Irreversible inhibition of cytosolic thioredoxin reductase 1 as a mechanistic basis for anticancer therapy. Sci Transl Med 10: eaaf7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisz H, Saul S, Müller CS, Kappl R, Niemeyer BA, Vogt T, Hoth M, Roesch A, Bogeski I (2014) Inverse regulation of melanoma growth and migration by Orai1/STIM2‐dependent calcium entry. Pigment Cell Melanoma Res 27: 442–453 [DOI] [PubMed] [Google Scholar]
- Stanisz H, Stark A, Kilch T, Schwarz EC, Müller CS, Peinelt C, Hoth M, Niemeyer BA, Vogt T, Bogeski I (2012) ORAI1 Ca(2+) channels control endothelin‐1‐induced mitogenesis and melanogenesis in primary human melanocytes. J Invest Dermatol 132: 1443–1451 [DOI] [PubMed] [Google Scholar]
- Stanisz H, Vultur A, Herlyn M, Roesch A, Bogeski I (2016) The role of Orai‐STIM calcium channels in melanocytes and melanoma. J Physiol 594: 2825–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdogan A, Faubert B, Ramesh V, Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin M et al (2020) Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577: 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosakis N, Micevic G, Kelly DP, Bosenberg M (2014) Mitochondrial function in melanoma. Arch Biochem Biophys 563: 56–59 [DOI] [PubMed] [Google Scholar]
- Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G et al (2016) The mitochondrial calcium uniporter regulates breast cancer progression via HIF‐1α. EMBO Mol Med 8: 569–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin‐Sandoval MS, Gu Z, McCormick ML, Durham AB, Spitz DR et al (2020) Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585: 113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K, Clish CB, Granter SR, Widlund HR, Spiegelman BM et al (2013) PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 23: 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vultur A, Gibhardt CS, Stanisz H, Bogeski I (2018) The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflugers Arch 470: 1149–1163 [DOI] [PubMed] [Google Scholar]
- Zhang D, Huo D, Xie H, Wu L, Zhang J, Liu L, Jin Q, Chen X (2020) CHG: a systematically integrated database of cancer hallmark genes. Front Genet 11: 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gibhardt CS, Will T, Stanisz H, Körbel C, Mitkovski M, Stejerean I, Cappello S, Pacheu‐Grau D, Dudek J et al (2019) Redox signals at the ER–mitochondria interface control melanoma progression. EMBO J 38: e100871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bilbao A, Bruderer T, Luban J, Strambio‐De‐Castillia C, Lisacek F, Hopfgartner G, Varesio E (2015) The use of variable Q1 isolation windows improves selectivity in LC‐SWATH‐MS acquisition. J Proteome Res 14: 4359–4371 [DOI] [PubMed] [Google Scholar]
- Zheng X, Lu S, He Z, Huang H, Yao Z, Miao Y, Cai C, Zou F (2020) MCU‐dependent negative sorting of miR‐4488 to extracellular vesicles enhances angiogenesis and promotes breast cancer metastatic colonization. Oncogene 39: 6975–6989 [DOI] [PubMed] [Google Scholar]


