Abstract
Understanding weight-related differences in functional connectivity provides key insight into neurocognitive factors implicated in obesity. Here, we sampled three groups from human connectome project data: 1) 47 pairs of BMI-discordant twins (n=94; average BMI-discordancy 6.7 ± 3.1 kg/m2), 2) 47 pairs of gender and BMI matched BMI-discordant, unrelated individuals, and 3) 47 pairs of BMI-similar twins to test for body mass dependent differences in between network functional connectivity. Across BMI discordant samples, three networks appeared to be highly sensitive to weight status; specifically, a network compromised of gustatory processing regions, a visual processing network, and the default mode network (DMN). Further, individuals with a lower BMI relative to their twin had stronger connectivity between striatal/thalamic and prefrontal networks (pFWE = 0.04) in the BMI-discordant twin sample. We also observed that individuals with a higher BMI than their twin had stronger connectivity between cerebellar and insular networks (pFWE = 0.04). Connectivity patterns observed in the BMI-discordant twin sample were not seen in a BMI-similar sample, providing evidence that the results are specific to BMI discordance. Beyond the involvement of gustatory and visual networks and the DMN, little overlap in results were seen between the two BMI-discordant samples. In concordance with previous research, we hypothesize that stronger cortical-striatal-thalamic connectivity associated with lower body mass in twins may facilitate increased regulation of hedonically motivated behaviors. In twins with higher body mass, increased cerebellar-insula connectivity may be associated with compromised satiation signaling, an interpretation dovetailing prior research. The lack of overlapping results between the two BMI discordant samples may be a function of the higher study design sensitivity in the BMI-discordant twin sample, relative to the more generalizable results in the unrelated sample. These findings demonstrate that distinct connectivity patterns can represent weight variability, adding to mounting evidence that implicates atypical brain functioning with the accumulation and/or maintenance of elevated weight.
Keywords: functional connectivity, twins, BMI, Default mode network, DMN, insula, obesity, dlPFC, striatum, satiation
Obesity is a chronic disease that affects two thirds of American adults (Flegal et al., 2012), and is associated with increased risk of metabolic disorders (Alberti et al., 2005), cardiovascular disease (Hubert et al., 1983), certain cancers (Calle and Kaaks, 2004), and mortality (Masters et al., 2013). Moreover, the prevalence of obesity is rapidly increasing worldwide (Collaborators, 2017). This is highly concerning given that the efficacy of behavioral weight loss efforts are highly variable (Dombrowski et al., 2014; Loveman et al., 2011). A myriad of internal mechanisms and external stimuli influence weight regulation, such as: physiological appetitive feedback systems, reinforcement-driven eating habits, emotional and social cues, and stimuli from the food environment (Berthoud, 2006). Additionally, behavioral constructs such as impulsivity (Nederkoorn et al., 2006), inhibitory control (Lavagnino et al., 2016; Weygandt et al., 2013), and taste sensitivity (Grinker et al., 1972) are theorized to impact weight. The brain is the key integration point for processing these factors, incorporating inputs (e.g., homeostatic satiation signaling, attentional processing) to direct food intake behaviors, thereby influencing weight (Morton et al., 2006). The examination of dynamic temporal correlations in the brain, i.e., functional connectivity, has provided considerable insight into aberrant resting-state brain networks (RSNs) associated with cognitive disease states (Van Den Heuvel and Pol, 2010; Woodward and Cascio, 2015). Therefore, a thorough understanding of how neural patterning varies with weight status is needed to accurately comprehend the neural correlates of weight regulation. Limited, but increasing evidence identify an association between elevated weight and altered neural functioning. For example, relative to healthy-weight, obesity was associated with decreased functional connectivity within networks comprising the medial prefrontal cortex and default mode network in response to viewing food stimuli (García‐García et al., 2013), and with decreased functional connectivity within prefrontal and feeding circuits during taste administration of a milkshake (Geha et al., 2016). Further, obesity also was linked to increased connectivity within the attention network (premotor areas, superior parietal lobule, and visual cortex), as well as, stronger hypothalamic-striatal and amygdala-insular connectivity (Lips et al., 2014). Collectively, these data provide early indication that elevated weight is associated with disruption in the functional integration of brain regions and networks that encode aspects of hedonically motivated behaviors, gustatory, and attentional processing. Though current data are limited to smaller sample sizes, so a more detailed understanding of alterations in functional connectivity associated with elevated weight in a large sample is warranted.
Genetic composition also influences functional connectivity of RSNs (Glahn et al., 2010; Yang et al., 2016), which introduces variability in between-group investigations of weight status and RSN connectivity. Twin study designs provide a powerful approach to control for unmeasured confounds such as heritability and food environment during childhood when examining factors impacting weight status (Barsh et al., 2000; Farooqi and O’rahilly, 2000). In a small sample of female twins, increased weight was associated with decreased lateral functional connectivity within the striatum, providing evidence that brain-level effects studied with twin designs are sensitive to relatively small differences in weight (mean BMI-discordancy 3.96 kg/m2)(Doornweerd et al., 2017). Broadly, existing investigations of weight-related RSN connectivity have compared effects between traditional BMI categories (e.g., healthy weight [BMI=18.5–24.9] vs. overweight [BMI=25.0–29.9] vs. obese [BMI>30.0]) (García‐García et al., 2013; Geha et al., 2016; Lips et al., 2014). However, BMI categories are arbitrary in nature, providing little clinically relevant information when individuals are near a cut-point). Importantly, relatively small fluctuations in body weight can significantly impact health (Jensen et al., 2013). For example, decreasing weight by ~5% can improve physiological predictors of disease (Blackburn, 1995; Douketis et al., 2005). As such, weight loss interventions typically do not use transitions from one BMI category to another as a metric of success, instead focusing on within-subject weight change. A twin study design that draws on the strength of testing relative weight difference, agnostic to BMI categories, can provide a highly sensitive test of the impact of elevated weight on RSNs, while maintaining direct relevance to weight loss recommendations and interventions. Despite these advantages, twin study designs are limited in their generalizability to the population at large (Kukull and Ganguli, 2012). However, a parallel analysis in unrelated, BMI-discordant individuals can be leveraged to test the impact of weight on RSN connectivity simultaneously providing a metric of reliability and increasing generalizability.
Here, we sought to determine weight dependent differences in functional connectivity patterning. To achieve this aim, we performed functional connectivity analyses in three independent samples: 1) a BMI-discordant twin sample; 2) a gender and BMI discordancy-matched, unrelated sample; and 3) a BMI-similar twin sample. BMI dependent connectivity patterns observed in both the BMI-discordant twin and unrelated samples allows for identification of weight dependent altered functional connectivity that is stable across two samples and is generalizable to unrelated individuals. Confirmation that these RSNs patterns are not observed in a weight-similar sample provides support that the findings are not a result of an unseen third-variable confound. Based on studies of obese versus healthy weight groups (García‐García et al., 2013; Geha et al., 2016), we hypothesized that differences in BMI-discordant twins will be observed in connectivity between insular and frontal RSNs, specifically where lighter twins will show stronger connections to the insular and frontal networks.
Methods
Sample selection.
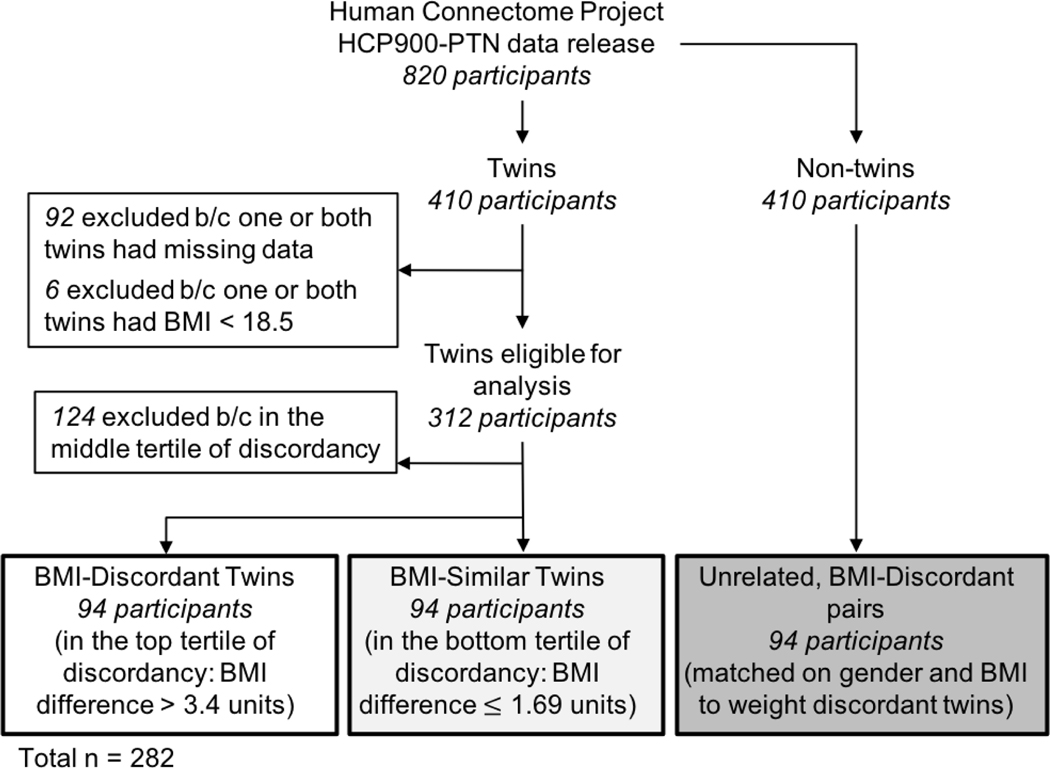
All data were drawn from the HCP900-PTN data release the from the Human Connectome Project (HCP) (Van Essen et al., 2012). A visual representation of the sample selection procedures can be seen in Figure 1. HCP900-PTN data included 820 young adults, of which 410 individuals were twins. From the twin subsample, 98 participants were excluded from the analysis due to: incomplete data/not having a corresponding twin in the sample (n = 92), or a BMI < 18.5 (n = 6). Of the remaining 312 twin participants (npairs = 156), twin pair BMI discordancy, defined as the between twin difference in BMI (heavier twin’s BMI - lighter twin’s BMI), was calculated. BMI-discordant twin sample were defined using the upper tertile of BMI discordancy of the 312 twin participants. The BMI-similar twin sample was selected to have the same proportion of monozygotic and dizygotic twin pairs as the BMI-discordant sample, while maintaining a similar BMI in the twin pairs. Thus, the two twin analyses included a total sample of 188 twins (npairs = 94; 47 pairs per sample). To examine aspects of generalizability, unrelated, BMI-discordant participants (npairs = 47) were also selected from the non-twin participants included in the HCP900-PTN data. These unrelated, BMI-discordant pairs were selected to match the discordant twin sample on both BMI and gender. No significant differences between the unrelated and twin BMI-discordant samples were observed for gender, BMI, or BMI discordancy (p’s > 0.95).
Figure 1.
The selection procedure used to generate the three main samples for the study analyses from the Human Connectome Project HCP900-PTN data release.
Data description and preprocessing.
Participants completed 4 resting state functional MRI (rfMRI) runs over two days (2 runs per day) totaling 58 minutes and 12 seconds of rfMRI data per participant. Scanning details for this sample have been published previously (Van Essen et al., 2012). Briefly each rfMRI scan used an eight-factor multiband, gradient echo EPI sequence with the following parameters: TR: 720ms, TE: 33.1ms, flip angle: 52 degrees, slice thickness: 2.0mm (Van Essen et al., 2012). During the rfMRI scan, participants were instructed to look at a light crosshair on a dark background projected into their field of view.
The human connectome project preprocessed all downloaded data in the HCP900-PTN release, and no additional preprocessing was performed locally. The HCP preprocessing pipeline is extensively published elsewhere and further documented is available on their website (https://www.humanconnectome.org/study/hcp-young-adult/documentation) and therefore will only be summarized here. Data were preprocessed using the recommended minimal preprocessing pipeline (Glasser et al., 2013). Independent component analysis (ICA) and FIX (FMRIB ICA-based X-noisifer) were used to assess and remove noise per participant per run (Salimi-Khorshidi et al., 2014; Smith et al., 2013). Individual participant data was registered using the areal-feature-based alignment and the Multimodal Surface Matching algorithm (‘MSMAII’) (Glasser et al., 2016; Robinson et al., 2014).
Group ICA.
MELODIC’s Incremental Group-PCA (MIGP) was used to generate dense connectomes of all participants’ individual timeseries (Smith et al., 2014). This dense connectome was then parcellated using group-ICA to create spatial-ICA network maps at dimensionalities of 15, 25, 50, 100, 200, and 300 distinct components (Glasser et al., 2016). From the HCP documentation, each component is a continuous range of values that may contain multiple spatially separate anatomical regions. The higher the number of components per map in general means the significant area per component is smaller. Given these traits of the network maps, the 25 and 100 component network maps were selected to examine the larger and more established resting state networks (using the 25 dimensionality), as well as potentially discreet regions that may drive effects (using the 100 dimensionality) (Ray et al., 2013). Given that multiple components in a given network map may include the same anatomical region, components will be referred to as independent components (ICs) with their number and anatomical region(s) comprised within e.g., occipital pole (IC 9) relative to occipital pole (IC 3). Independent components that were considered to be noise were identified by two independent researchers. Components were flagged as noise when BOLD activity was primarily following the gyri and/or solely following the surface of brain/skull (Poldrack et al., 2011). As a result, ICs 18 and 24 were determined to be noise and were removed from consideration in the 25-component parcellation.
Individual component timeseries.
Per dimensionality, individual participant timeseries were concatenated and spatially mapped to the corresponding network map described above. This created a single timeseries per component per participant. Therefore, each participant had a component timeseries made up of the 4800 time points (1200 time points over 4 runs) by the number of components (15, 25, 50, 100, 200, and 300). For this analysis, each participant had a component timeseries made up of either 4800×25 or 4800×100.
Creation of netmats.
The 25 and 100 component group network maps were each regressed against the corresponding individual component timeseries using the “dual-regression stage-1” approach to create individual participant network matrices (netmats) (Filippini et al., 2009).
Heritability factor calculation.
To determine the effect of heritability on connectivity of each network, a heritability score (Hb2) was calculated for each network, based on Falconer’s formula (Falconer and Mackay, 1996).
We calculated the correlation between each twin pair per component in the netmat (both 25 and 100 dimensionalities separately) and averaged the correlations in both the monozygotic and dizgotic twin groups respectively to create two average correlation matrices: monozygotic (rmz) and dizygotic (rdz). The difference between the rmz and rdz matrices was calculated and multiplied by 2. The resulting heritability matrix contained a Hb2 value for each pairwise connection between components. Individual level netmats were then each weighted by the Hb2 matrix to adjust for heritability effects.
Statistical analyses.
FSLNets (Version 0.6, FMRIB, Oxford, UK) was used to assess BMI-dependent differences between RSN connectivity. For each sample (BMI-discordant twins, BMI-similar twins, and unrelated BMI-discordant pairs), full correlations with normalized covariances were run on the netmats described above to create a correlation matrix. A single group paired T test was performed on the correlation matrices with the following contrasts: 1) higher BMI individual compared to lower BMI individual (higher BMI > lower BMI), and 2) lower BMI individual compared to higher BMI individual (lower BMI > higher BMI). To correct for potential false positives, non-parametric permutation testing was used through FSL’s randomise tool with 10,000 permutations per twin pair (or unrelated pair) (Winkler et al., 2014). Results were considered significance at pFWE < 0.05 (Eklund et al., 2016). Negative correlations were not included in analyses, as they are not interpretable in the present context (Murphy et al., 2009).
To account for generalizability of results and heritability confounding, connectivity results within the BMI-discordant twin sample were contrasted to that of the 1) unrelated, BMI-discordant sample as a test of generalizability; and 2) in BMI-similar twin sample as a test of heritability confounding. Significant network connectivity in both contrasts was compared between BMI-discordant twins, unrelated BMI-discordant pairs, and BMI-similar twins. Equivalent results were identified when significant connectivity of two networks was observed in both samples. Connectivity results present in the both BMI-discordant pair samples (twin and unrelated), but not seen in the BMI-similar twin pairs were considered reliable and generalizable body mass contingent alterations in functional connectivity.
Secondary analyses included examination of within pair and between group differences in behavioral, mood, substance abuse, and physiological characteristics theorized to relate to eating behavior and weight regulation or that may represent unique physiological differences between BMI discordant groups not encompassed by BMI alone: response inhibition and executive functioning as measured by the NIH Toolbox’s Flanker Task (age adjusted) (Lavagnino et al., 2016; Weintraub et al., 2013), self-regulation and impulsivity as measured by the Delay Discounting Task (Green et al., 1994; Nederkoorn et al., 2007), taste sensitivity as measured by the NIH Toolbox’s Taste Intensity Test (age adjusted) (Coldwell et al., 2013; Grinker et al., 1972), major depressive episodes, number depressive symptoms, alcohol use, tobacco use, illicit drug use or marijuana use and dependence, hemoglobin A1c and systolic and diastolic blood pressure. Mixed linear models were used to test for within-pair and between-group differences in the behavioral and physiological measures via PROC MIXED in SAS (Version 9.4, SAS Institute Inc., Cary, NC, USA). Significance was thresholded at p < 0.05.
Preregistration and analytic scripts for this study can be found via the Open Science Framework (Center for Open Science, Charlottesville, VA, USA, DOI 10.17605/OSF.IO/VTMPW). All scripts for this analysis can be found on Github at https://github.com/niblunc/twin_HCP_paper.
Results
Sample Characteristics.
Participant data including age, gender, race/ethnicity, BMI and BMI discordancy, and zygosity can be found in Table 1. The BMI-discordant twins (npairs = 47) had a difference > 3.40 kg/m2, and BMI-similar twins (npairs = 47) had a difference ≤ 1.69 kg/m2. The distribution of BMI in the three samples can be seen in Figure 2.
Table 1:
Participant Characteristics (n=282a)
| BMI-discordant twins (n=94) | Unrelated, BMI-discordant pairs (n=94) | Group Difference BMI-discordant twins & unrelated pairs | BMI- similar twins (n=94) | Group Difference BMI-discordant twins & BMI- similar twins | |
|---|---|---|---|---|---|
|
| |||||
| Age (mean ± SD; years) | 29.4 ± 3.5 | 28.5 ± 3.6 | p = 0.08 | 29.3 ± 3.5 | p = 0.71 |
| Body Mass Index (kg/m2) | 28.1 ± 5.6 | 28.1 ± 5.7 | p = 0.95 | 25.6 ± 4.1 | p < 0.001 |
| BMI Discordancy (kg/m2) | 6.7 ± 3.1 | 6.7 ± 3.1 | p = 0.98 | 0.7 ± 0.5 | p < 0.001 |
| Race (percent) | p = 0.002 | p = 0.02 | |||
| Asian/Pacific Islander | 0 | 9 (10%) | 7 (7%) | ||
| African American | 14 (15%) | 21 (22%) | 10 (11%) | ||
| Caucasian | 78 (83%) | 58 (62%) | 77 (82%) | ||
| Multiracial | 0 | 3 (3%) | 0 | ||
| Not Reported | 2 (2%) | 3 (3%) | 0 | ||
| Ethnicity (percent) | p = 0.003 | p = 0.51 | |||
| Hispanic/Latino | 1 (1%) | 12 (13%) | 0 | ||
| Non-Hispanic/Latino | 91 (97%) | 82 (87%) | 93 (99%) | ||
| Not Reported | 2 (2%) | 0 | 1 (1%) | ||
| Zygosity (percent) | N/A | p = 1.0 | |||
| Monozygotic | 44 (47%) | N/A | 44 (47%) | ||
| Dizygotic | 50 (53%) | N/A | 50 (53%) | ||
| Gender (percent) | p =1.0 | p = 0.05 | |||
| Male | 32 (34%) | 32 (34%) | 46 (49%) | ||
| Female | 62 (66%) | 62 (66%) | 48 (51%) | ||
Total unique subjects across the three samples included in analyses
Significant group difference between the BMI-discordant twin sample and the unrelated, BMI-discordant pair sample or BMI-similar twin sample, as determined by t-test (age, BMI, BMI discordancy) or χ2 test (race, ethnicity, zygosity, gender) (p < 0.05)
Figure 2.
BMI distributions of the higher and lower BMI individuals within the BMI discordant twin group, BMI discordant unrelated pair group, and BMI similartwin group.
Connectivity in Body Mass Discordant Twins.
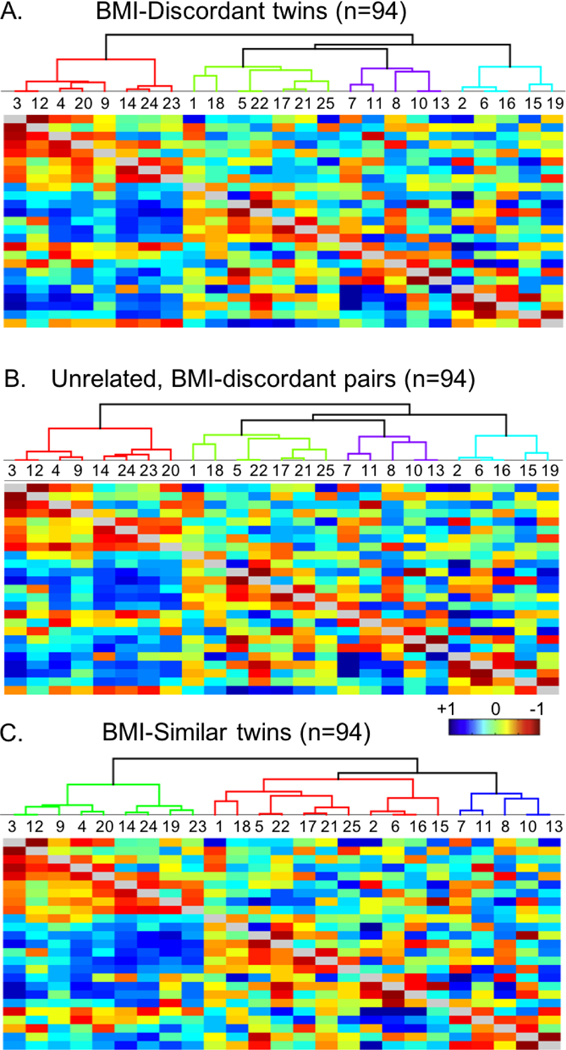
Body mass discordant twins (npairs= 47) had a mean BMI discordancy of 6.7 ± 3.1 kg/m2, representing a difference greater than one BMI category (NIH, 2017). A within-pair comparison confirmed significant difference in BMI (T = 7.4, p < 0.0001) between higher BMI and lower BMI twins. Network hierarchy, represented by the brackets in the upper portion of the image, and partial (below the diagonal) and full correlations (above the diagonal) of the 25-network parcellation for the BMI-discordant sample can be seen in Figure 3A. Between network connectivity results, controlled for heritability, are summarized in Table 2.
Figure 3.
Hierarchy and correlation heatmaps of resting state network connectivity in the three independent samples tested: A) BMI- discordant twins; B) unrelated, BMI-discordantpairs; C) BMI similar twins, for the 25-network parcellation. Each map shows the parcel hierarchy at the top (brackets); independent component (1C) numbers listed in the row below the hierarchy; full correlation values in the upper portion of the heatmap, and partial correlation values in the lower portion of the heatmap.
Table 2:
Significant Between Network Connectivity in BMI-discordant Twins (n=94) and Unrelated, BMI-Discordant Pairs (n=94)
| BMI-Discordant Twins | ||||
|---|---|---|---|---|
| Contrast | IC | Network | T-value | p FWE |
| Higher BMI > Lower BMI | 9 | Occipital pole | 4.30 | 0.004 |
| 2 | Default Mode Network | |||
|
| ||||
| 16 | Right crus I | 3.64 | 0.037 | |
| 7 | Insula, central operculum, precentral gyrus, and anterior cingulate | |||
|
| ||||
| 10 | Dorsolateral prefrontal cortex, ventrolateral prefrontal cortex | 4.57 | 0.001 | |
| 22 | Left crus II | |||
|
| ||||
| Lower BMI > Higher BMI | 9 | Occipital pole | 4.04 | 0.003 |
| 3 | Occipital pole, intracalcarine cortex, and lingual gyrus | |||
|
| ||||
| 9 | Occipital pole | 4.19 | 0.005 | |
| 7 | Insula, central operculum, precentral gyrus, and anterior cingulate | |||
|
| ||||
| 10 | Dorsolateral prefrontal cortex, ventrolateral prefrontal cortex | 3.70 | 0.035 | |
| 25 | Dorsal striatum, ventral striatum, insula | |||
|
| ||||
| Unrelated, BMI-Discordant Pairs | ||||
| Contrast | IC | Network | T-value | p FWE |
|
| ||||
| Higher BMI > Lower BMI | 2 | Default Mode Network | 4.71 | 0.002 |
| 7 | Insula, parietal operculum, and dorsal anterior cingulate cortex | |||
|
| ||||
| 4 | Lateral occipital cortex | 4.62 | 0.003 | |
| 12 | Lingual gyrus, occipital cortex | |||
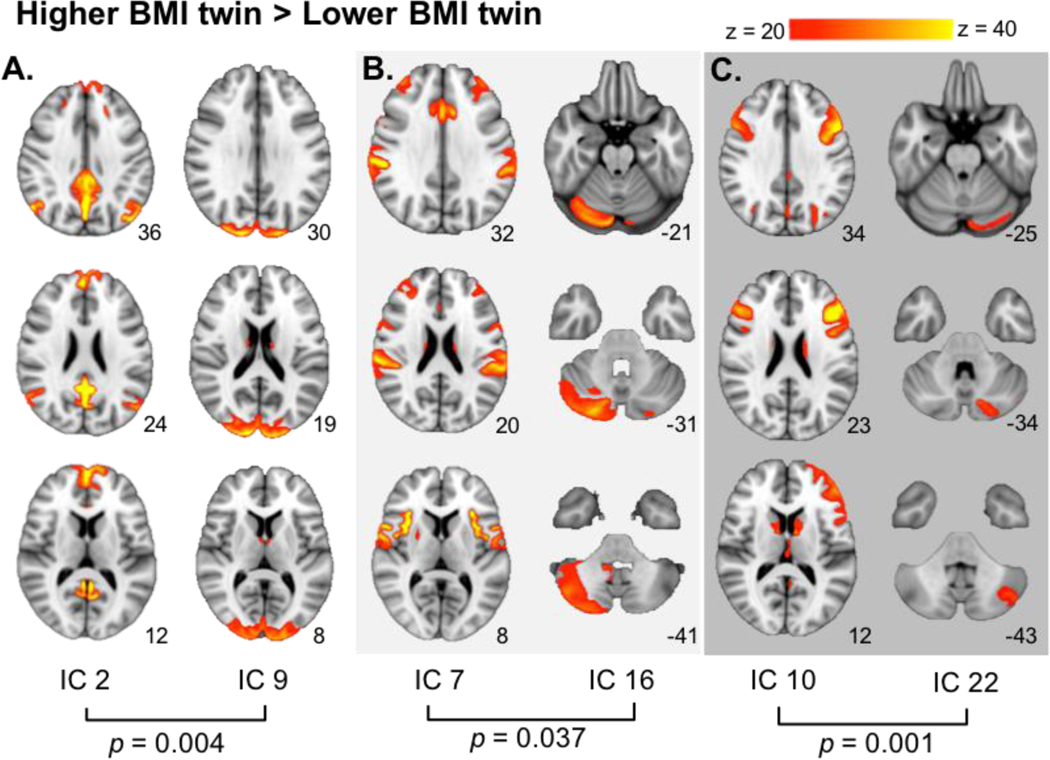
When comparing the higher BMI twin to their lower BMI counterpart (higher BMI > lower BMI), we observed significantly stronger connectivity between the occipital pole (IC 9) and a network (IC 2) including the medial orbitofrontal cortex (mOFC), posterior cingulate cortex/precuneus, temporoparietal junction, and hippocampus, putatively representing the default mode network (DMN; T = 4.30; pFWE = 0.004; Table 2; Figure 4A). Stronger connectivity was also observed between the cerebellar right crus I (IC 16) and a network containing the anterior cingulate, insula, central operculum, and precentral gyrus (IC 7; T = 3.64; pFWE = 0.037; Table 2; Figure 4B), and between a network inclusive of the dorsolateral prefrontal cortex (dlPFC) and ventrolateral prefrontal cortex (vlPFC; IC 10) and left crus II of the cerebellum (IC 22; T = 4.57; pFWE = 0.001 Table 2; Figure 4C).
Figure 4.
Differential network connectivity in the BMI-discordanttwin sample. When contrasting higher BMI > lower BMI, stronger connectivity was seen between: A. the default mode network (1C 2) and the occipital pole (1C 9): B. the insula, central operculum, precentral gyrus and anterior cingulate (1C 7) and right crus I of the cerebellum (1C 16); C. the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex (IC 10) and the left crus II (IC 22). MNI coordinates of each slice on the z-axis are listed below each slice.
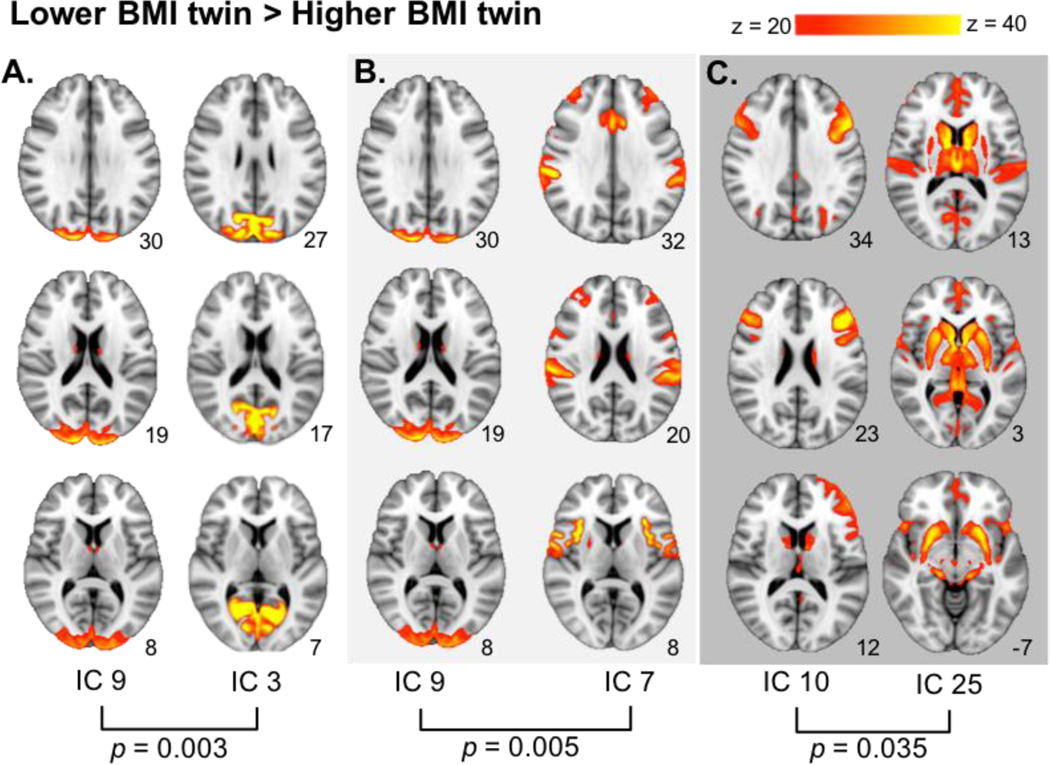
When comparing connectivity of the lower BMI twin relative to the higher BMI twin (lower BMI > higher BMI), significantly stronger connectivity was found between the occipital pole (IC 9) and a network containing the occipital pole (IC 3), intracalcarine cortex, and lingual gyrus (T = 4.04; pFWE = 0.003; Table 2; Figure 5A). Stronger connectivity was also found between the occipital pole (IC 9) network and the network inclusive of the insula, anterior cingulate, central operculum, and precentral gyrus networks (IC 7; T = 4.19; pFWE = 0.005; Table 2; Figure 5B). Additionally, stronger connectivity was seen between the dlPFC/vlPFC network (IC 10) and a network including the dorsal and ventral striatum and insula (IC 25; T = 3.70; pFWE = 0.035; Table 2; Figure 5C).
Figure 5.
Differential network connectivity in the BMI-discordanttwin sample. When contrasting lower BMI > higher BMI, stronger connectivity was found between: A. the occipital pole, intracalcarine cortex, and lingual gyrus (IC 3) and the occipital pole (IC 9); B. the occipital pole(IC 9) and the insula, central operculum, precentral gyrus and anterior cingulate (IC 7); C. the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex (IC 10) and the dorsal striatum, ventral striatum, and insula (IC 25). MNI coordinates of each slice on the z-axis are listed below each slice.
Unique connectivity in Unrelated, Body Mass Discordant Pairs.
Network hierarchy and full and partial correlations of the 25-network parcellation for the unrelated, BMI-discordant pairs can be seen in Figure 3B. The mean BMI difference between the unrelated, BMI-discordant sample was also 6.7 ± 3.1 kg/m2, only 0.12 kg/m2 different relative to BMI-discordant twin sample (T = 0.06, p = 0.95). Among the higher vs. lower BMI individuals, stronger connectivity was identified between a network identified as the default mode network (IC 2) and a network including the insula, central operculum, precentral gyrus, and anterior cingulate (IC 7; T = 4.71, p = 0.002; Table 2). Stronger connectivity was also observed between a network including the lateral occipital cortex (IC 4) and the lingual gyrus and occipital cortex (IC 12; T = 4.62; pFWE = 0.003). Stronger connectivity was not observed in the lower BMI individuals relative to their higher BMI counterparts in the unrelated pairs.
Unique connectivity in Body Mass Similar Twins.
The mean body mass discordance in the BMI-similar twin pairs (npairs = 47) was 0.7 ± 0.5 kg/m2. Network hierarchy and full and partial correlations of the 25-network parcellation can be seen in Figure 3C. Connectivity identified in the BMI-similar twin sample was not equivalent with connectivity observed in the BMI-discordant twins. When controlled for heritability, there was no significant connectivity observed in either contrast.
100-Network ICA Parcellation.
To probe whether connectivity patterns observed in the BMI-discordant twin sample were driven by smaller parcels of networks, we completed the same functional connectivity analyses (including heritability weighting) using the 100-network ICA parcellation in the BMI-discordant twin sample. While significant connectivity was observed in comparing higher BMI > lower BMI twins and lower BMI > higher BMI twins (Supplement Table 1), none of the connected networks using the 100 network parcellation reflected similar connectivity patterns seen in the larger networks stemming from the 25-network ICA parcellation.
Secondary Analyses: Behavioral and Physiological Correlates and Differences as a Function of Zygosity.
Post-hoc examination of behavioral and physiological characteristics between the two body mass discordant samples revealed no significant interaction of BMI and twin-status on the following: Flanker task scores (T = 0.51, p = 0.61), area under the curve for the $2,000 delay discounting task (T = −0.49, p = 0.62), area under the curve for the $40,000 delay discounting task (T = 0.35, p = 0.73), taste sensitivity task (T = 0.59, p = 0.56), systolic blood pressure (T = 0.56, p = 0.58), diastolic blood pressure (T = 0.76, p = 0.45), and hemoglobin A1c (T = 0.06, p = 0.95), collectively indicating no behavioral or physiological differences between the two body mass discordant samples. Additionally, there were no between group or within pair differences in measures of mood and substance use disorders between the BMI-discordant samples (p’s < 0.05). Visual representation of results can be seen in Supplemental Figure 1.
Discussion
Leveraging samples of body mass discordant twins, unrelated body mass discordant individuals, and body mass similar twins we sought to determine whether resting state network connectivity was impacted relative differences in BMI. Across the BMI discordant samples, we show that connectivity of networks inclusive of regions previously implicated in encoding gustatory processing (insula/parietal operculum), a network including the primary visual cortex, and the default mode network (DMN) are particularly sensitive to differences in BMI. In both BMI discordant samples, higher BMI individuals show stronger connectivity between default mode network with other networks, specifically the occipital pole (twin sample), and the network inclusive of gustatory processing regions (unrelated sample). Although the between network connectivity stemming from the DMN is different between the two samples, both are in line with previous investigations that indicate elevated BMI is associated with aberrant gustatory and visual processing (Small et al., 2001; Stice et al., 2008; Stoeckel et al., 2008) and with increased connectivity within the DMN (Tregellas et al., 2011). Further, results from BMI discordant samples were not evident in BMI-similar twins, providing support that connectivity results are a function of BMI. Beyond the aforementioned three networks that appear to be consistently sensitive to differences in weight, below we discuss broader implications of the specific BMI dependent between network results.
We observed that the lower BMI twins showed stronger connectivity between a network centered in the dlPFC and a network comprising the striatum/thalamus relative to their higher BMI twins. The predominate regions in these networks are present in mesocorticolimbic dopaminergic circuitry, specifically the mesolimbic (striatum/thalamus) and the mesocortical (dlPFC) dopaminergic pathways (Le Moal and Simon, 1991). Cortical-striatal connectivity is thought to integrate behavioral motivation with decision-making and executive control (Di Martino et al., 2008). Altered cortical-striatal connectivity and decreased dlPFC connectivity has been observed in obesity (García‐García et al., 2013; Kullmann et al., 2013, 2012; Moreno-Lopez et al., 2016; Nummenmaa et al., 2012). Here, increased connectivity between these regions confers more efficient communication, indicating lower BMI individuals may be more responsive to changes in reward valuation and present with increased cognitive control during decision-making (Rangel and Hare, 2010), possibly specific to decisions about food intake. This interpretation posits that lighter individuals may possess firmer appetite regulation when presented with highly palatable, energy-dense foods. Previous works supports, the current findings and reinforce the hypothesis that cortical-striatal-thalamic network connectivity is involved with weight regulation through mechanisms where stronger connectivity contributes to better regulation of hedonically motivated food-related behaviors.
Higher BMI twins showed increased connectivity between a network including the insula, central operculum, anterior cingulate, and precentral gyrus with the right crus I of the cerebellum. Regions included in the insular network are consistently implicated in encoding gustatory processing (Veldhuizen et al., 2011), where the right crus I is involved in encoding motor control and working memory (Habas et al., 2009; Stoodley et al., 2012). Positron emission tomography in healthy-weight men demonstrated stronger cerebral blood flow in both the insula and cerebellum when fasted for 36 hours versus a postprandial state (when fed) (Tataranni et al., 1999), indicating the strength of his dynamic connectivity may be associated with caloric deprivation. In support, functional connectivity between insular and cerebellar networks in healthy weight individuals was also elevated following an overnight fast (Wright et al., 2016). Moreover, insular-cerebellar connectivity is seen in healthy individuals during hypoglycemia (Bolo et al., 2015), a physiologic characteristic of being fasted for extended time periods. Taken together, these data provide strong evidence that stronger insula and cerebellar connectivity is reflective of caloric deprivation/hunger signaling. Interestingly, in obese individuals, increased insula and cerebellar BOLD response were seen following gastric distension by balloon, mimicking fullness without a caloric load (Tomasi et al., 2009). This supports the notion that higher BMI individuals show an insular-cerebellar response that is thought to encode aspects of being calorically deprived in a time their stomach is ‘full’. This BMI dependent incongruent valence of insula and cerebellar connectivity could act to perpetuate food intake in a postprandial state, commonly referred to as eating in the absence of hunger. In support, behavioral reports demonstrate that obesity is associated with increased appetite and decreased satiation (Cabanac and Duclaux, 1970; Schachter, 1968). In sum, these data signify that, unlike being a healthy weight, higher BMI is associated with a stronger insula-cerebellar connection that is unresponsive to fullness, theoretically, contributing to increased postprandial food intake.
Elevated BMI was also related to stronger connectivity between the primary visual cortex and the DMN, which the latter is reflective of internal mental awareness and is diminished during goal-directed tasks (Buckner et al., 2008). At rest, functional connectivity within the DMN is positively correlated with BMI (Tregellas et al., 2011), and obesity is associated with increased connectivity within the salience network, which comprises the visual cortex and superior parietal lobule (including the precuneus/PCC) (García‐García et al., 2013). Moreover, evoked studies utilizing food stimuli (pictures of foods, cue-elicited anticipation of food receipt) consistently demonstrate that elevated weight is associated increased BOLD response in these regions that encode visual and attentional processing. This pattern of higher propensity towards strong visual response with elevated weight is hypothesized to play a key role forming eating habits (Gilbert and Burger, 2016).
The general approach used here, i.e., comparison the higher BMI versus lower BMI, does not directly align with comparisons of ‘standard’ weight status groupings, i.e., obese versus healthy weight (NIH, 2017). While our study is cross-sectional, the present approach is more relevant to longitudinal and experimental designs. Prospective designs typically use within-subject continuous BMI change as a sensitive test for weight change, for example a shift from a ‘healthy’ BMI of 24.5 to an ‘overweight’ BMI of 25.3 is clinically insignificant. In the present sample, there are relatively broad distributions of BMI in both the ‘higher BMI’ and ‘lower BMI’ groups. Results from these analyses cannot provide valid information regarding the relationship between functional connectivity and absolute BMI. Due to the analysis used, one cannot infer that any given BMI is related to a specific connectivity pattern based on solely this data, one can only interpret the results in terms of relative higher and lower body mass. In support of the present approach, it has been suggested that absolute BMI is not the best standard of health, as it does not always align with health and mortality outcomes (Nuttall, 2015). Moreover, when individuals are at an elevated weight, a 5% weight loss is physiologically beneficial (Douketis et al., 2005). The present design dovetails with this clinically relevant ‘relative weight difference’ approach, making results more applicable to prospective and intervention studies. Though, the approach does require consideration of the magnitude of BMI difference within pairs. Here, BMI-discordant twin pairs had an average BMI difference of 6.7 kg/m2. Although this difference greater than a full BMI category, within some pairs, the heavy and lighter individuals were in the same BMI category, or both obese and overweight. As such, we were not surprised that the patterns of connectivity observed in this study differ somewhat from previous analyses based on BMI categories (García‐García et al., 2013; Kullmann et al., 2012; Park et al., 2016; Wijngaarden et al., 2015).
No equivalent connectivity was observed between BMI-discordant twins and the unrelated, BMI-discordant pairs. Twins inherently have shared genetics, likely have a similar food environment during youth (Barsh et al., 2000; Farooqi and O’rahilly, 2000), demonstrate less between-subject variability in common fMRI confounds, i.e., head motion (Hodgson et al., 2016), and show similar resting state network connectivity (Fu et al., 2015). By largely controlling for these possible confounds (Falconer and Mackay, 1996), results from the BMI-discordant twin sample may provide a robust test for BMI-dependent differences in connectivity. However, this approach cannot completely account for every aspect of heritability and/or shared early life environment. The remaining possible confounders could be represented in the lack of consistent results between two BMI-discordant samples. This possibility demonstrates a decision point in study design, specifically, the comparing relative importance of internal sensitivity and/or high methodological study control, relative to generalizability of results. Here, the twin sample approach is more highly controlled, and thus the results are likely to be closer to true effect of body mass on functional connectivity. However, even if that notion is correct, the ‘true’ results are of limited utility as they only generalize to twins, where a case can be made that the results from the unrelated sample are more meaningful as they generalize to a larger population. Ultimately, replication of findings in independent samples, across multiple studies, utilizing different data acquisition techniques will serve to establish both validity and generalizability of the results.
We did not find significant within pair differences between BMI-discordant twins or unrelated BMI-discordant pairs in behavioral (executive functioning, impulsivity, and taste sensitivity) and physiological measures (hemoglobin A1c and blood pressure) that are thought to be associated with weight. Eligibility criteria excluded individuals with weight-related diseases, e.g. individuals with high blood pressure and/or diabetes, thereby reducing the variability in these behavioral and physiological measures and thus the ability to detect BMI-related differences between higher BMI and lower BMI twins. Additionally, the differences in functional connectivity observed may not manifest behaviorally in the measures included in the present sample, specifically given these generalized tasks, i.e., not food specific. In support, previous weight discordant twin research, heavier twins reported stronger preference for fat and a greater tendency to overeat, but no difference on non-food related psychological measures (Rissanen et al., 2002). This suggests that testing food/eating behaviors specific measures may better identify differences as a function of weight status, but generalized assessments may have lower sensitivity when assessing BMI-related differences.
In the primary analysis, we assessed between network connectivity within the 25 networks identified by ICA parcellation, though it reasonable to hypothesize that additional differences between weight discordant twins may emerge at a higher dimensionality, as it potentially parses apart large networks into smaller sub-regions. The exploratory connectivity analysis using a 100-network parcellation attempted to address this limitation, however this did not elicit identifiable ‘sub’-networks for more specific interpretation. These data indicate that broader networks that incorporate more regions, may provide more meaningful information relating to our hypotheses. However, this approach did lend itself to significant connectivity patterns of networks that are anatomically located next to one another, i.e., (IC 9 with IC 3; IC 4 with IC 12). As the regions in each network are too similar in function for meaningful interpretation, for the brevity of this discussion these patterns were not discussed. The present study is restricted by its cross-sectional design, limiting inferences regarding the temporal precedence of the relations between functional connectivity and BMI. We are unable to determine a causal nature of the observed relationships between weight and differential functional connectivity and can only state that these differences in connectivity may play a role associated with weight regulation.
In sum, this investigation examined functional connectivity between weight discordant twins and unrelated samples. We observed that, across all of the samples tested, a network compromised of gustatory processing regions, a visual processing network, and the default mode network all appear to be highly sensitivity to weight status. Further, reduced weight is associated with greater integration of regions that encode reward learning and executive control which underpin hedonically motived behaviors. This, theoretically, may contribute to better behavioral control when lighter individuals are faced with highly palatable foods. Lastly, higher weight is associated with functional connectivity pattern that has consistently been reflective of caloric deprivation/hunger. Here, we demonstrate that unique, distinct connectivity patterns can represent weight variability adding to the mounting evidence that implicates aberrant brain functioning with the accumulation and/or maintenance of elevated weight.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Steve Smith for assistance with utilization of Human Connectome Project data and selection of the analytic approach.
Footnotes
Competing financial interests statement
The authors declare no competing financial interests.
References
- Alberti KGMM, Zimmet P, Shaw J, 2005. The metabolic syndrome—a new worldwide definition. Lancet 366, 1059–1062. [DOI] [PubMed] [Google Scholar]
- Barsh GS, Farooqi IS, O’rahilly S, 2000. Genetics of body-weight regulation. Nature 404, 644–651. [DOI] [PubMed] [Google Scholar]
- Berthoud H, 2006. Homeostatic and non‐homeostatic pathways involved in the control of food intake and energy balance. Obesity 14. [DOI] [PubMed] [Google Scholar]
- Blackburn G, 1995. Effect of degree of weight loss on health benefits. Obesity 3. [DOI] [PubMed] [Google Scholar]
- Bolo NR, Musen G, Simonson DC, Nickerson LD, Flores VL, Siracusa T, Hager B, Lyoo IK, Renshaw PF, Jacobson AM, 2015. Functional connectivity of insula, basal ganglia, and prefrontal executive control networks during hypoglycemia in type 1 diabetes. J. Neurosci 35, 11012–11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL, 2008. The brain’s default network. Ann. N. Y. Acad. Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Duclaux R, 1970. Obesity: Absence of satiety aversion to sucrose. Science (80-. ) 168, 496–497. [DOI] [PubMed] [Google Scholar]
- Calle EE, Kaaks R, 2004. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579. [DOI] [PubMed] [Google Scholar]
- Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smutzer G, Cowart BJ, Breslin PAS, Bartoshuk LM, Hastings L, 2013. Gustation assessment using the NIH Toolbox. Neurology 80, S20–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators G, 2017. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP, 2008. Functional connectivity of human striatum: a resting state FMRI study. Cereb. cortex 18, 2735–2747. [DOI] [PubMed] [Google Scholar]
- Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF, 2014. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. Bmj 348, g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doornweerd S, van Duinkerken E, de Geus EJ, Arbab‐Zadeh P, Veltman DJ, IJzerman RG, 2017. Overweight is associated with lower resting state functional connectivity in females after eliminating genetic effects: A twin study. Hum. Brain Mapp 38, 5069–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douketis JD, Macie C, Thabane L, Williamson DF, 2005. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int. J. Obes 29, 1153. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci 201602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC, 1996. Introduction to quantitative genetics. Longmans Green, Harlow, Essex, UK. Introd. to Quant. Genet 4th ed. Longmans Green, Harlow, Essex, UK. [Google Scholar]
- Farooqi IS, O’rahilly S, 2000. Recent advances in the genetics of severe childhood obesity. Arch. Dis. Child 83, 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE, 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U. S. A 106, 7209–7214. doi: 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL, 2012. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999–2010. Jama-Journal Am. Med. Assoc 307, 491–497. doi: 10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- Fu Y, Ma Z, Hamilton C, Liang Z, Hou X, Ma X, Hu X, He Q, Deng W, Wang Y, 2015. Genetic influences on resting‐state functional networks: A twin study. Hum. Brain Mapp 36, 3959–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐García I, Jurado MÁ, Garolera M, Segura B, Sala‐Llonch R, Marqués‐Iturria I, Pueyo R, Sender‐Palacios MJ, Vernet‐Vernet M, Narberhaus A, 2013. Alterations of the salience network in obesity: a resting‐state fMRI study. Hum. Brain Mapp 34, 2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha P, Cecchi G, Todd Constable R, Abdallah C, Small DM, 2016. Reorganization of brain connectivity in obesity. Hum. Brain Mapp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JR, Burger KS, 2016. Neuroadaptive processes associated with palatable food intake: present data and future directions. Curr. Opin. Behav. Sci 9, 91–96. [Google Scholar]
- Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, Curran JC, Olvera RL, Laird AR, Smith SM, 2010. Genetic control over the resting brain. Proc. Natl. Acad. Sci 107, 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fristoe N, Myerson J, 1994. Temporal discounting and preference reversals in choice between delayed outcomes. Psychon. Bull. {&} Rev 1, 383–389. [DOI] [PubMed] [Google Scholar]
- Grinker J, Hirsch J, Smith DV, 1972. Taste sensitivity and susceptibility to external influence in obese and normal weight subjects. J. Pers. Soc. Psychol 22, 320. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD, 2009. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci 29, 8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson K, Poldrack RA, Curran JE, Knowles EE, Mathias S, Göring HHH, Yao N, Olvera RL, Fox PT, Almasy L, 2016. Shared genetic factors influence head motion during mri and body mass index. Cereb. Cortex 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert HB, Feinleib M, McNamara PM, Castelli WP, 1983. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67, 968–977. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, 2013. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 01–cir. [Google Scholar]
- Kukull WA, Ganguli M, 2012. Generalizability The trees, the forest, and the low-hanging fruit. Neurology 78, 1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H-UHH-U, Fritsche A, Preissl H, 2012. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp 33, 1052–1061. doi: 10.1002/hbm.21268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Pape A-A, Heni M, Ketterer C, Schick F, Häring H-U, Fritsche A, Preissl H, Veit R, 2013. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb. Cortex 23, 1247–1256. doi: 10.1093/cercor/bhs124 [DOI] [PubMed] [Google Scholar]
- Lavagnino L, Arnone D, Cao B, Soares JC, Selvaraj S, 2016. Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci. Biobehav. Rev 68, 714–726. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Simon Herv, 1991. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol. Rev 71, 155–234. [DOI] [PubMed] [Google Scholar]
- Lips MA, Wijngaarden MA, van der Grond J, van Buchem MA, de Groot GH, Rombouts SA, Pijl H, Veer IM, 2014. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am J Clin Nutr 100, 524–531. doi: 10.3945/ajcn.113.080671 [DOI] [PubMed] [Google Scholar]
- Loveman E, Frampton GK, Shepherd J, Picot J, Cooper K, Bryant J, Welch K, Clegg A, 2011. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG, 2013. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am. J. Public Health 103, 1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Contreras-Rodriguez O, Soriano-Mas C, Stamatakis EA, Verdejo-Garcia A, 2016. Disrupted functional connectivity in adolescent obesity. NeuroImage Clin. 12, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW, 2006. Central nervous system control of food intake and body weight. Nature 443, 289–295. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA, 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A, 2006. Why obese children cannot resist food: the role of impulsivity. Eat. Behav 7, 315–322. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, Jansen A, 2007. Impulsivity predicts treatment outcome in obese children. Behav. Res. Ther 45, 1071–1075. [DOI] [PubMed] [Google Scholar]
- NIH, 2017. Body Mass Index Table [WWW Document]. Natl. Hear. Lung, Blood Inst [Google Scholar]
- Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P, 2012. Dorsal Striatum and Its Limbic Connectivity Mediate Abnormal Anticipatory Reward Processing in Obesity. PLoS One 7. doi: 10.1371/journal.pone.0031089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall FQ, 2015. Body mass index: obesity, BMI, and health: a critical review. Nutr. Today 50, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B-Y, Seo J, Park H, 2016. Functional brain networks associated with eating behaviors in obesity. Sci. Rep 6, 23891. doi: 10.1038/srep23891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE, 2011. Handbook of functional MRI data analysis. Cambridge University Press. [Google Scholar]
- Rangel A, Hare T, 2010. Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol 20, 262–270. [DOI] [PubMed] [Google Scholar]
- Ray KL, McKay DR, Fox PM, Riedel MC, Uecker AM, Beckmann CF, Smith SM, Fox PT, Laird AR, 2013. ICA model order selection of task co-activation networks. Front. Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen A, Hakala P, Lissner L, Mattlar CE, Koskenvuo M, Ronnemaa T, Rönnemaa T, 2002. Acquired preference especially for dietary fat and obesity: a study of weight-discordant monozygotic twin pairs. Int. J. Obes 26, 973. doi: 10.1038/sj.ijo.0802014 [DOI] [PubMed] [Google Scholar]
- Robinson EC, Jbabdi S, Glasser MF, Andersson J, Burgess GC, Harms MP, Smith SM, Van Essen DC, Jenkinson M, 2014. MSM: A new flexible framework for Multimodal Surface Matching. Neuroimage 100, 414–426. doi: 10.1016/j.neuroimage.2014.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM, 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S, 1968. Obesity and eating. Science (80-. ) [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M, 2001. Changes in brain activity related to eating chocolate from pleasure to aversion. Brain 124, 1720–1733. [DOI] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, 2013. Resting-state fMRI in the human connectome project. Neuroimage 80, 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Hyvärinen A, Varoquaux G, Miller KL, Beckmann CF, 2014. Group-PCA for very large fMRI datasets. Neuroimage 101, 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM, 2008. Relation of Reward From Food Intake and Anticipated Food Intake to Obesity: A Functional Magnetic Resonance Imaging Study. J. Abnorm. Psychol 117, 924–935. doi: 10.1037/a0013600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook III EW, Twieg DB, Knowlton RC, Cox JE, 2008. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 41, 636–647. doi: 10.1016/j.neuroimage.2008.02.031 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD, 2012. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59, 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier J-F, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E, 1999. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci 96, 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang G-J, Wang R, Backus W, Geliebter A, Telang F, Jayne MC, Wong C, Fowler JS, Volkow ND, 2009. Association of body mass and brain activation during gastric distention: implications for obesity. PLoS One 4, e6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, Cordes D, Cornier M-A, 2011. Altered default network activity in obesity. Obesity (Silver Spring). 19, 2316–2321. doi: 10.1038/oby.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Pol HEH, 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol 20, 519–534. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage 62, 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundström JN, 2011. Identification of human gustatory cortex by activation likelihood estimation. Hum. Brain Mapp 32, 2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, 2013. Cognition assessment using the NIH Toolbox. Neurology 80, S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygandt M, Mai K, Dommes E, Leupelt V, Hackmack K, Kahnt T, Rothemund Y, Spranger J, Haynes J-D, 2013. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage 83, 669–678. [DOI] [PubMed] [Google Scholar]
- Wijngaarden MA, Veer IM, Rombouts S, van Buchem MA, van Dijk KW, Pijl H, van der Grond J, 2015. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav. Brain Res 287, 127–134. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. Neuroimage 92, 381–397. doi: 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Cascio CJ, 2015. Resting-state functional connectivity in psychiatric disorders. JAMA psychiatry 72, 743–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H, Li X, Fallon NB, Crookall R, Giesbrecht T, Thomas A, Halford JCG, Harrold J, Stancak A, 2016. Differential effects of hunger and satiety on insular cortex and hypothalamic functional connectivity. Eur. J. Neurosci 43, 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zuo X-N, McMahon KL, Craddock RC, Kelly C, de Zubicaray GI, Hickie I, Bandettini PA, Castellanos FX, Milham MP, 2016. Genetic and environmental contributions to functional connectivity architecture of the human brain. Cereb. Cortex 26, 2341–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.