Abstract
The revised Standards for Adult Immunization Practice (“Standards”), published in 2014, recommend routine vaccination assessment, strong provider recommendation, vaccine administration or referral, and documentation of vaccines administered into immunization information systems (IIS). We assessed clinician and pharmacist implementation of the Standards in the United States from 2016 to 2018. Participating clinicians (family and internal medicine physicians, obstetricians-gynecologists, specialty physicians, physician assistants, and nurse practitioners) and pharmacists responded using an internet panel survey. Weighted proportion of clinicians and pharmacists reporting full implementation of each component of the Standards were calculated. Adjusted prevalence ratio (APR) estimates of practice characteristics associated with self-reported implementation of the Standards are also presented. Across all medical specialties, the percentages of clinicians and pharmacists implementing the vaccine assessment and recommendation components of the Standards were >80.0%. However, due to low IIS documentation, full implementation of the Standards was low overall, ranging from 30.4% for specialty medicine to 45.8% in family medicine clinicians. The presence of an immunization champion (APR, 1.40 [95% confidence interval {CI}, 1.26 to 1.54]), use of standing orders (APR, 1.41 [95% CI, 1.27 to 1.57]), and use of a patient reminder-recall system (APR, 1.39 [95% CI, 1.26 to 1.54]) were positively associated with adherence to the Standards by clinicians. Similar results were observed for pharmacists.
Nonetheless, vaccination improvement strategies, i.e., having standing orders in place, empowering an immunization champion, and using patient recall-reminder systems were underutilized in clinical settings; full implementation of the Standards was inconsistent across all health care provider practices.
Keywords: Adult, Vaccination, Standards, Immunization, Providers
1. Introduction
In the United States, adult vaccination coverage generally falls below Healthy People 2020 targets [1], and adult populations have the highest burden of vaccine preventable illness [2–4]. Although evidence-based vaccination improvement strategies (e.g., designating immunization champions; use of standing orders and patient reminder-recall systems; and utilizing electronic health records) and evidence-based guidelines improve vaccine delivery, inadequate implementation of these approaches has negatively influenced adult vaccine uptake [5–7].
The Advisory Committee on Immunization Practices (ACIP) annually updates vaccination recommendations by age, underlying medical conditions, vaccination history, occupation, and other factors [8]. In 2014, in response to persistently low immunization rates for adults, the National Vaccine Advisory Committee (NVAC) updated the Standards for Adult Immunization Practice (the Standards). The Standards outlined evidence-based clinical practice guidelines to improve vaccine delivery strategies for adults in outpatient settings [5,9]. The revised Standards emphasize the responsibility of all providers to: (1) routinely perform vaccination assessments of adult patients at all clinical encounters, (2) provide a strong recommendation for vaccinations for which the patient is eligible, (3) administer or provide referrals for needed vaccines, and (4) document vaccines administered in state or local immunization information systems (IIS), where available.
Studies of vaccine assessment, recommendation, and administration are available for primary care settings [10–17]. However, studies on implementation of the Standards in alternative vaccination settings, such as pharmacies or subspecialist clinics, are limited [18–20]. To address this gap, the current evaluation includes obstetricians-gynecologists (ob-gyns), other specialty care providers, and pharmacists. Because of the significant and increasing role of advanced practitioners in clinical practices, physician assistants (PAs) and nurse practitioners (NPs) in primary and specialty care practices were included in addition to physicians. This evaluation describes self-reported use of vaccination improvement strategies and implementation of the Standards over a three-year period two years after the publication of the Standards.
2. Methods
2.1. Survey development
Survey instruments were jointly developed by the Centers for Disease Control and Prevention (CDC) and Abt Associates Inc. (Abt; Cambridge, MA) to evaluate adherence to NVAC’s revised Standards. To account for different patient flows in their respective practice settings, separate survey instruments were developed for clinicians and pharmacists. Survey questions assessed implementation of each component of the Standards—vaccination assessment, recommendation, administration or referral, and documentation—and administrative processes at the practice level. Data on respondent characteristics, vaccination knowledge, and attitudes toward vaccination were also collected.
Although efforts were made to keep the survey instrument as consistent as possible across survey years, minor revisions were made to improve survey design. Each year, question phrasing, response options, and question order were modified to introduce more user-friendly language for better readability and comprehension of survey questions.
2.2. Sampling strategy
Non-probability samples of clinician and pharmacist respondents in the United States were recruited using the membership roster from Medscape, a medical member list with more than two million U.S. members managed by WebMD Professional Network. For each survey year, the minimum target sample size was 1,500 clinicians and 250 pharmacists. A minimum of 250 respondents each were sampled from family physicians, general internal medicine physicians, and pharmacists; and at least 125 respondents were sampled from ob-gyn physicians, non–ob-gyn specialty care physicians, PAs, and NPs. Hispanic and non-Hispanic black clinicians and pharmacists were oversampled.
2.3. Survey administration
The Internet panel surveys for clinicians and pharmacists were conducted in 2016, 2017, and 2018 in February and March of each year. Medscape members who indicated in their Medscape profile that they worked as a physician, PA, NP, or pharmacist were invited to participate in the survey. Upon acceptance of the survey invitation, respondents were transferred to a Web survey platform operated by Abt. Although it is possible that the same respondent might have submitted completed surveys in more than 1 year, the small sample relative to the overall pool of approximately 2 million Medscape members makes this unlikely.
Participant eligibility was determined via pre-screener questions. Respondents who reported completion of formal education and training, worked at least partly in an outpatient setting, and currently treated patients (clinicians) or dispensed pharmaceuticals directly to patients (pharmacists) aged 19 years or older were eligible for participation. Mobile optimization programming facilitated respondents to take the self-administered survey on a variety of popular mobile platforms including smartphones or tablet computers. This project was determined to be non-research by the Abt Associates Inc. Institutional Review Board (IRB); therefore, CDC determined that additional IRB review was not needed.
As survey questions pertained to implementation of the Standards at the practice level, it was assumed NPs’ and PAs’ adherence was likely reflective of their collaborating supervisory physician. Additionally, a sub-group analysis of NP and PA implementation of the Standards demonstrated analogous results to those observed for physicians. Therefore, all three professions were combined for the final analyses assessing clinician implementation of the Standards.
2.4. Measures
Respondents were asked if they routinely assessed adult patients’ vaccination status, recommended necessary vaccinations, administered the vaccines or referred patients for vaccination, and documented administered vaccines to their state or local immunization information system (IIS). A composite measure for full implementation of the Standards—i.e., adherence to all individual components of the Standards for immunizing providers (assessment, recommendation, administration, documentation in IIS, patient referral) or select components (assessment, recommendation, patient referral, patient follow-up) for non-immunizing providers—was then evaluated.
Clinician and pharmacist responses regarding implementation of the Standards were analysed by medical specialty; practice characteristics, including practice location, type, and size; and use of immunization improvement strategies (e.g., use of immunization champions, standing orders, patient reminder-recall systems, and electronic health records) to estimate associations with adherence to the Standards.
2.5. Statistical analysis and weighting
To produce survey estimates more generalizable to the U.S. clinician and pharmacist population, sampling weights were developed using estimated numbers of persons in each major occupational category by sex, age, race/ethnicity, and U.S. Census region. To reduce the effect of under-coverage and non-response, calibrated weights were obtained using the raking method [21,22]. For the raking procedure, population control estimates were determined using the U.S. Bureau of Labor Statistics National Industry-Specific Occupational Employment and Wage Estimates [23] and the Current Population Survey [24]. The raking method can substantially reduce bias in estimates resulting from disproportionate sampling. All survey estimates were computed using these final weights.
Recruitment of survey respondents through an online opt-in survey sample does not permit calculation of a response rate due to the inability to enumerate the denominator at each individual sampling stage. Therefore, cooperation rates for survey completion were calculated instead. For this study, the cooperation rate was defined as the number of eligible respondents who completed the survey divided by the number of respondents who were eligible and started the survey.
For each survey, weighted proportions were calculated for practice characteristics and provider demographics. Multivariable logistic regression was used to calculate adjusted prevalence ratios (APR) and 95% confidence intervals (CI) for practice characteristics associated with full implementation of the Standards. All statistical analyses were conducted using SAS version 9.3 (Cary, NC) and SUDAAN version 11.0 (Research Triangle Park, NC). Statistical measures were calculated with an assumption of random sampling. However, as non-probability sampling was used, observed associations should be interpreted only as a guide.
2.6. Role of funding source
This work was funded by CDC. This project was also supported in part by an appointment to the Research Participation Program for the CDC, National Center for Immunization and Respiratory Diseases, Immunization Services Division, administered by the Oak Ridge Institute for Science and Education through an agreement between the U.S. Department of Energy and CDC.
3. Results
The cooperation rate for clinician and pharmacist respondents for each survey year was > 86%. From 2016 to 2018, a total of 5,068 clinicians and 794 pharmacists completed the survey. Upon review of participant responses, 157 clinician surveys were excluded as implementation of the Standards was not an expected function of their practices. One pharmacist survey was excluded because the respondent reported being unemployed at the time of the survey. As a result, 4,911 clinicians and 793 pharmacists were included in the analysis. The rate of missing data by survey question varied from 0% to 2.1% for clinician and pharmacist surveys.
The proportion of male and female respondents was approximately equally distributed in both surveys (Table 1). Most clinicians (65.1%) and pharmacists (72.8%) identified themselves as non-Hispanic white, and 32.5% of clinicians and 38.8% of pharmacists reported that they had<10 years of clinical or pharmacy practice experience.
Table 1.
| Provider Characteristics | Clinicians‡ (n = 4,911) |
Pharmacists§ (n = 793) |
|---|---|---|
| Age group, n (%) | ||
| <40 years | 1,497 (30.5) | 310 (45.3) |
| 40–49 years | 1,503 (26.8) | 194 (19.4) |
| 50–59 years | 1,156 (23.4) | 161 (17.5) |
| ≥60 years | 736 (19.3) | 125 (17.7) |
| Sex, n (%) | ||
| Male | 1,858 (48.5) | 378 (49.1) |
| Female | 3,053 (51.5) | 415 (50.9) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic white | 3,668 (65.1) | 608 (72.8) |
| Non-Hispanic black | 178 (9.5) | 24 (7.1) |
| Hispanic | 250 (6.6) | 30 (4.8) |
| Asian | 624 (15.5) | 105 (12.7) |
| Other | 162 (3.3) | 19 (2.6) |
| Years of practice, n (%) | ||
| <10 years | 1,510 (32.5) | 244 (38.8) |
| 10–19 years | 1,687 (29.4) | 203 (19.9) |
| 20–29 years | 1,031 (21.8) | 141 (14.9) |
| ≥30 years | 676 (16.3) | 204 (26.5) |
| Profession, n (%) | ||
| Physician | 2,349 (71.5) | NA |
| Nurse practitioner | 1,293 (15.7) | NA |
| Physician assistant | 1,269 (12.8) | NA |
| Pharmacist | NA | 793 (100.0) |
| Employment status at practice, n (%) | ||
| Owner | 867 (24.6) | 40 (5.2) |
| Direct hire employee | 3,722 (67.6) | 711 (89.7) |
| Contractor/other | 322 (7.8) | 39 (5.1) |
| Percentage of patients served who are non-White, n (%) | ||
| 76% or more | 663 (15.1) | 76 (11.5) |
| 51–75% | 1,742 (35.3) | 199 (25.4) |
| 26–50% | 1,523 (30.9) | 253 (32.0) |
| 25% or less | 949 (18.7) | 259 (31.1) |
NA = not applicable.
Values are unweighted numbers (weighted percentages).
Weighted percentages may not sum to 100 due to rounding.
Data for clinicians were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Healthcare Providers Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Data for pharmacists were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Pharmacists Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Because slightly different surveys were used for clinicians and pharmacists to account for different workflows, their analyses are reported separately below.
3.1. Implementation of the Standards
3.1.1. Clinicians
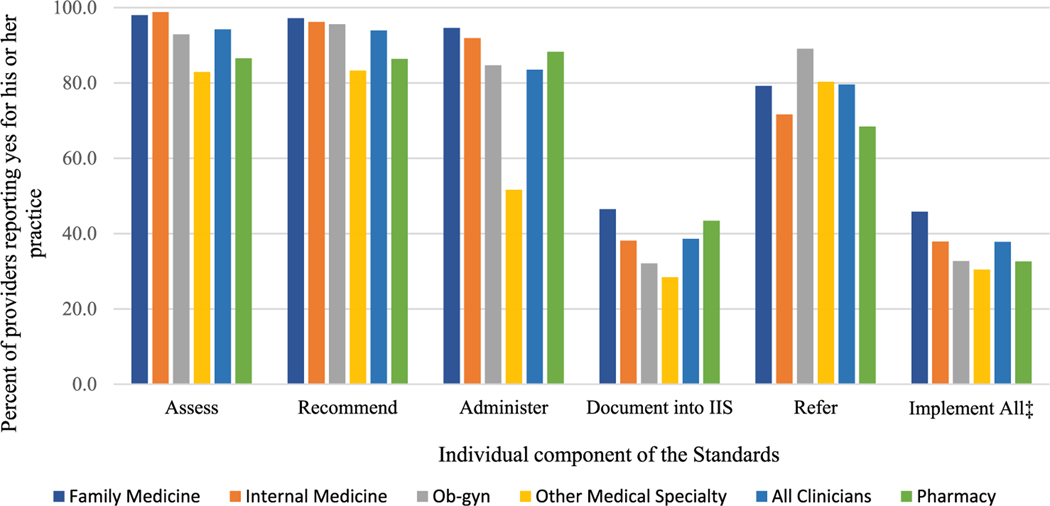
Overall, for the 3 survey years combined, full implementation for the Standards was low, and ranged from 30.4% for non–ob–gyn specialty care clinicians to 45.8% for family medicine clinicians (Fig. 1). However, regardless of clinical specialty, the percentage of clinicians implementing the vaccine assessment, recommendation, and vaccine administration components of the Standards were each > 80.0%. For all specialties, the individual component of the Standards with the lowest reported adherence was documentation of adult vaccinations in the IIS among immunizing clinicians, ranging from 28.4% among non-ob-gyn specialists to 46.5% among family medicine clinicians.
Fig. 1.
Proportion of clinicians* and pharmacists† reporting implementation of each component of the Standards. *Data for clinicians were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Healthcare Providers Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc. †Data for pharmacists were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Pharmacists Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc. ‡Full implementation of the Standards defined as: adherence to all individual components of the Standards for immunizing providers (assessment, recommendation, administration, documentation in IIS, patient referral) or select components of the Standards (assessment, recommendation, patient referral, patient follow-up components) for non-immunizing providers.
More clinicians worked in the private practice office setting (45.4%) and indicated that their practice stocked 4 or more ACIP-recommended adult vaccines (73.9%) (Table 2). However, clinicians who worked in either community health center or public health clinic settings were more likely to implement all components of the Standards (50.1% and 67.9%, respectively) (Table 3). As compared to clinical practices that did not stock any ACIP-recommended adult vaccines (14.9%), those that stocked were more likely to implement the Standards, particularly in practices that reported stocking 4 or more ACIP-recommended adult vaccines (44.2%).
Table 2.
| Provider Characteristics | Clinicians‡ (n = 4,911) |
Pharmacists§ (n = 793) |
|---|---|---|
| Primary work setting - Clinicians, n (%) | ||
| Private practice office | 2,250 (45.4) | NA |
| Office practice owned by a hospital | 1,863 (38.6) | NA |
| Urgent care clinic | 129 (2.7) | NA |
| Community health center | 295 (5.7) | NA |
| Public health clinic | 84 (1.5) | NA |
| Veterans Administration clinic | 101 (2.4) | NA |
| Other practice setting | 189 (3.7) | NA |
| Primary work setting - Pharmacists, n (%) | ||
| Chain drug store pharmacy | NA | 307 (39.9) |
| Retail store pharmacy | NA | 89 (10.8) |
| Supermarket pharmacy | NA | 161 (19.4) |
| Independent community pharmacy | NA | 168 (21.1) |
| Other | NA | 68 (8.7) |
| Number of specialties, n (%) | ||
| Single-specialty practice | 3,307 (65.1) | NA |
| Multi-specialty practice | 1,604 (34.9) | NA |
| Main medical specialty, n (%) | ||
| Family medicine | 1,413 (31.7) | NA |
| Internal medicine | 1,056 (26.8) | NA |
| Obstetrician/gynecologist | 1,251 (22.5) | NA |
| Other specialty care | 1,159 (19.1) | NA |
| Practice size, n (%) | ||
| Small (1–2 physicians) | 1,535 (30.7) | 332 (41.1) |
| Medium (3–5 physicians) | 1,313 (27.2) | 399 (51.3) |
| Large (6 or more physicians) | 1,960 (42.2) | 62 (7.6) |
| U.S. Census region, n (%) | ||
| Northeast | 1,177 (20.8) | 170 (18.3) |
| Midwest | 1,031 (20.2) | 190 (22.5) |
| South | 1,679 (38.1) | 280 (40.0) |
| West | 1,024 (20.9) | 153 (19.2) |
| Presence of immunization champion, n (%) | ||
| Yes | 1,972 (43.9) | 441 (56.4) |
| No | 2,936 (56.1) | 352 (43.6) |
| Presence of standing orders or protocols, n (%) | ||
| Yes | 2,170 (55.1) | 681 (97.0) |
| No | 1,784 (44.9) | 25 (3.0) |
| Reminder-recall system in place, n (%) | ||
| Yes | 2,084 (45.6) | 323 (41.5) |
| No | 2,720 (54.4) | 456 (58.5) |
| Practice uses electronic health records, n (%) | ||
| Yes | 4,489 (92.7) | 730 (93.9) |
| No | 327 (7.3) | 50 (6.1) |
| Number of ACIP-recommended vaccines stocked, n (%) | ||
| 4 or more | 2,737 (73.9) | 667 (94.5) |
| 1–3 | 1,191 (25.3) | 38 (5.3) |
| None | 34 (0.8) | 1 (0.2) |
NA = not applicable; ACIP = Advisory Committee on Immunization Practices.
Values are unweighted numbers (weighted percentages).
Weighted percentages may not sum to 100 due to rounding.
Data for clinicians were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Healthcare Providers Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Data for pharmacists were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Pharmacists Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Table 3.
Practice characteristics associated with implementation of the Standards reported among clinicians and pharmacists.
| Practice characteristics | Clinicians* (n = 4,911) |
Pharmacists† (n = 793) |
||
|---|---|---|---|---|
|
|
|
|||
| Proportion‡ (95% CI) | APR§ (95% CI) |
Proportion (95% CI) |
APRǁ (95% CI) |
|
| Primary work setting - Clinicians | ||||
| Private practice office | 36.0 (33.6, 38.4) | reference | NA | NA |
| Office practice owned by a hospital | 38.3 (35.6, 41.0) | 0.99 (0.88, 1.11) | NA | NA |
| Urgent care clinic | 30.3 (21.5, 41.0) | 0.84 (0.60, 1.18) | NA | NA |
| Community health center | 50.1 (43.0, 57.3) | 1.21 (1.01, 1.45) | NA | NA |
| Public health clinic | 67.9 (55.4, 78.3) | 1.92 (1.61, 2.30) | NA | NA |
| Veterans Administration clinic | 32.8 (22.3, 45.3) | 0.81 (0.55, 1.19) | NA | NA |
| Other | 33.9 (26.1, 42.7) | 0.69 (0.49, 0.98) | NA | NA |
| Primary work setting - Pharmacists | ||||
| Chain drug store pharmacy | NA | NA | 27.8 (22.7, 33.6) | reference |
| Retail store pharmacy | NA | NA | 65.3 (54.3, 74.8) | 2.27 (1.76, 2.92) |
| Supermarket pharmacy | NA | NA | 33.7 (26.1, 42.2) | 1.19 (0.88, 1.61) |
| Independent community pharmacy | NA | NA | 25.4 (19.1, 33.1) | 1.26 (0.91, 1.75) |
| Other | NA | NA | 29.7 (19.6, 42.1) | 1.71 (1.17, 2.50) |
| Number of specialties | ||||
| Single-specialty practice | 35.9 (33.9, 38.0) | reference | NA | NA |
| Multi-specialty practice | 41.5 (38.6, 44.4) | 1.07 (0.96, 1.19) | NA | NA |
| Medical Specialty | ||||
| Family medicine | 45.8 (42.7, 49.0) | reference | NA | NA |
| Internal medicine | 37.9 (34.6, 41.4) | 0.85 (0.75, 0.96) | NA | NA |
| Obstetrician/gynecologist | 32.7 (29.6, 36.1) | 0.91 (0.79, 1.05) | NA | NA |
| Other specialty care | 30.4 (27.2, 33.9) | 0.79 (0.65, 0.95) | NA | NA |
| Practice/Pharmacy size | ||||
| Small (1–2 physicians/pharmacists) | 36.8 (33.8, 39.8) | reference | 30.5 (25.3, 36.3) | reference |
| Medium (3–5 physicians/pharmacists) | 38.4 (35.2, 41.7) | 1.05 (0.92, 1.20) | 34.9 (30.0, 40.1) | 0.96 (0.77, 1.19) |
| Large (6 or more physicians/pharmacists) | 38.3 (35.8, 41.0) | 1.08 (0.95, 1.23) | 29.2 (18.9, 42.3) | 0.94 (0.61, 1.47) |
| U.S. Census region | ||||
| Northeast | 31.0 (27.9, 34.3) | reference | 22.4 (16.5, 29.7) | reference |
| Midwest | 42.4 (38.8, 46.1) | 1.24 (1.07, 1.43) | 25.9 (19.6, 33.3) | 0.98 (0.68, 1.42) |
| South | 38.3 (35.5, 41.3) | 1.16 (1.01, 1.33) | 35.2 (29.4, 41.5) | 1.46 (1.06, 2.00) |
| West | 39.4 (35.9, 43.0) | 1.12 (0.97, 1.30) | 44.9 (36.8, 53.3) | 1.63 (1.18, 2.26) |
| Number of ACIP-recommended vaccines stocked | ||||
| 4 or more | 44.2 (41.9, 46.4) | 2.76 (1.01, 7.54)¶ | 37.7 (33.8, 41.8) | 0.75 (0.28, 2.05)¶ |
| 1–3 | 17.8 (15.3, 20.5) | 1.21 (0.44, 3.30)¶ | 12.5 (5.1, 27.4)¶ | 0.26 (0.07, 0.96)¶ |
| None | 14.9 (5.3, 35.5)¶ | reference | 0.0 (., .)¶ | reference |
APR = adjusted prevalence ratio; NA = not applicable; ACIP = Advisory Committee on Immunization Practices.
Data for clinicians were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Healthcare Providers Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Data for pharmacists were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Pharmacists Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Weighted proportion calculated for implementing the Standards not adjusted for practice characteristics or vaccination improvement strategies.
Adjusted prevalence ratio, adjusted for practice setting, number of specialties at practice, medical specialty, practice size, total number of healthcare providers at practice, region, and number of ACIP-recommended adult vaccines stocked.
Adjusted prevalence ratio, adjusted for pharmacy setting, pharmacy size, total number of staff at practice, region, and number of ACIP-recommended adult vaccines stocked.
Estimate may be unreliable due to small sample size (n < 30) and/or relative standard error (standard error/estimates) > 0.3.
Among all clinical settings, only public health clinics (APR, 1.92 [95% CI, 1.61 to 2.30]) outperformed private practice office settings in adhering to the Standards (Table 3). Internal medicine (APR, 0.85 [95% CI, 0.75 to 0.96]) and specialty care clinicians (APR, 0.79 [95% CI, 0.65 to 0.95]) were significantly less likely to adhere to the Standards compared with family medicine clinicians. Clinicians who stocked four or more vaccines in their practice (APR, 2.76 [95% CI, 1.01 to 7.54]) were significantly more likely to adhere to the Standards compared with their respective reference groups.
3.1.2. Pharmacists
Among pharmacists, full implementation of the Standards was 32.6% (Fig. 1). Although implementation of the vaccination assessment, recommendation, and administration components were high (86.5%, 86.4%, and 88.3%, respectively), documentation in an IIS was low (43.4%). Among pharmacists, 68.4% of respondents reported referring adult patients to another location for vaccination.
More pharmacists worked in a chain drug store setting (39.9%) than other settings and nearly all responded that their pharmacies stocked 4 or more ACIP-recommended adult vaccines (94.5%) (Table 2). Among all pharmacy settings, pharmacists working in a retail store setting were most likely to implement all components of the Standards (65.3%) (Table 3). Implementation of the Standards was also more likely in pharmacies located in either the Western or Southern regions (44.9% and 35.2%, respectively) compared to the Northeast (22.4%) and Midwest (25.9%).
Retail store pharmacies (APR, 2.27 [95% CI, 1.76 to 2.92]) were more than twice as likely as chain drug store pharmacies to adhere to the Standards (Table 3). Pharmacies located in the Western (APR, 1.63 [95% CI, 1.18 to 2.26]) and Southern regions (APR, 1.46 [95% CI, 1.06 to 2.00]) were both significantly more likely to adhere to the Standards when compared to the Northeast region.
3.2. Vaccination improvement strategies
3.2.1. Clinicians
Reported use of vaccination improvement strategies by clinicians were: 43.9% employing an immunization champion, 55.1% having standing orders, and 45.6% using patient reminder-recall systems for adult vaccination (Table 2). Clinicians whose practices employed either an immunization champion (46.1%) or patient reminder-recall system (46.3%) were more likely to implement all components of the Standards than clinicians who did not employ these strategies (29.1% and 28.6%, respectively) (Table 4).
Table 4.
Vaccination improvement strategies associated with implementation of the Standards among clinicians and pharmacists who administer adult vaccines.
| Vaccination improvement strategies | Clinicians* (n = 3,962) |
Pharmacists† (n = 706) |
||
|---|---|---|---|---|
|
|
|
|||
| Proportion‡ (95% CI) |
APR§ (95% CI) |
Proportion (95% CI) |
APRǁ (95% CI) |
|
| Presence of immunization champion | ||||
| Yes | 46.1 (43.4, 48.9) | 1.40 (1.26, 1.54) | 39.3 (34.2, 44.5) | 1.20 (0.96, 1.49) |
| No | 29.1 (26.8, 31.5) | reference | 32.0 (26.5, 38.0) | reference |
| Presence of standing orders/protocols | ||||
| Yes | 45.5 (43.0, 48.0) | 1.41 (1.27, 1.57) | 36.7 (32.8, 40.7) | 1.51 (0.79, 2.91)¶ |
| No | 27.1 (24.6, 29.8) | reference | 23.1 (10.9, 42.5)¶ | reference |
| Reminder-recall system in place | ||||
| Yes | 46.3 (43.6, 49.0) | 1.39 (1.26, 1.54) | 38.8 (33.1, 44.8) | 1.27 (1.02, 1.57) |
| No | 28.6 (26.2, 31.0) | reference | 33.9 (28.9, 39.1) | reference |
| Practice uses electronic health records | ||||
| Yes | 38.4 (36.5, 40.4) | 1.88 (1.39, 2.54) | 36.7 (32.8, 40.8) | 1.27 (0.74, 2.16) |
| No | 18.6 (13.2, 25.5) | reference | 29.4 (17.2, 45.4) | reference |
APR = adjusted prevalence ratio
Data for clinicians were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Healthcare Providers Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Data for pharmacists were obtained from the 2015–16, 2016–17, and 2017–18 National Survey of Pharmacists Regarding Vaccination Practices for Adults, conducted for CDC by Abt Associates Inc.
Weighted proportion calculated for implementing the Standards not adjusted for practice characteristics or vaccination improvement strategies.
Adjusted prevalence ratio, adjusted for practice setting, number of specialties at practice, medical specialty, practice size, total number of healthcare providers at practice, region, and number of ACIP-recommended adult vaccines stocked.
Adjusted prevalence ratio, adjusted for pharmacy setting, pharmacy size, total number of staff at practice, region, presence of an immunization champion, use of standing orders, use of reminder systems, and use of electronic medical records.
Estimate may be unreliable due to small sample size (n < 30) and relative standard error (standard error/estimates) > 0.3.
Among clinicians who indicated their practice administered adult vaccinations, adherence to the Standards was significantly more prevalent in practices that used vaccination improvement strategies (Table 4). Clinicians using patient reminder-recall systems (APR, 1.39 [95% CI, 1.26 to 1.54]), immunization champions (APR, 1.40 [95% CI, 1.26 to 1.54]), standing orders for adult vaccination (APR, 1.41 [95% CI, 1.27 to 1.57]), and electronic health records (APR, 1.88 [95% CI, 1.39 to 2.54]) were significantly more likely to adhere to the Standards compared with their respective reference groups.
3.2.2. Pharmacists
Most pharmacists responded that their pharmacy had an immunization champion (56.4%) (Table 2). Because standing orders are often necessary for vaccine administration within the pharmacy setting, 97.0% of pharmacists reported use of this strategy. However, pharmacist use of a patient reminder-recall system for adult vaccination was only 41.5%. In pharmacies where pharmacists are permitted to administer adult vaccines, full implementation of the Standards was higher in pharmacies where any vaccination improvement strategies were present (Table 4).
Vaccination improvement strategies implemented at the pharmacy level demonstrated positive associations with full adherence to the Standards overall (Table 4). However, for pharmacists, only the use of a patient reminder-recall system was significantly associated with adherence to the Standards (APR, 1.27 [95% CI, 1.02 to 1.57]).
4. Conclusions
This survey evaluated implementation of the Standards for Adult Immunization Practice for ACIP-recommended adult vaccines from 2016 to 2018 among a range of health care provider types and settings. Family medicine, internal medicine, ob-gyn, and specialty care clinicians, and pharmacists reported high adherence to routine vaccination assessment, recommendation, and administration. However, full implementation of all components of the Standards was<46% across all health care professions and settings, largely due to low percentages of clinicians and pharmacists documenting administered adult vaccines in IIS. Additionally, vaccination improvement strategies, i.e., having standing orders in place, empowering an immunization champion, and using patient recall-reminder systems were underutilized in clinical settings. Apart from having standing orders in place, low use of these strategies was also observed in the pharmacy setting.
A previous study [12] reported that although family practice and general internal medicine physicians had higher rates of adult vaccination assessment for annual and initial patient visits, only 32% of family medicine and 29% of general internal physicians reported assessment at every visit. In contrast, this survey found both specialties reported routine assessment of adult vaccination at higher rates (98.0% and 98.8%, respectively). The proportion of family practice and general internal medicine clinicians reporting they refer adult patients for vaccines not in stock, however, is consistent with data previously reported [12], with the majority reporting they referred adult patients for vaccination in our survey. Although this might suggest the impact of the Standards published in 2014 may have become more widespread in 2016–2018, the study methodologies are different, and results are not comparable. Follow-up evaluations will be needed to assess whether the Standards have become routine practice for adult care among family practice and general internal medicine clinicians.
Vaccine recommendations given by health care providers have been shown to be positively associated with increased vaccination uptake in adult patients [25–27]. However, recent examination of clinician recommendation processes suggest recommendation quality may also impact patient vaccine acceptance [28,29]. Interest in the use of presumptive announcement communication techniques as a method to strengthen provider vaccination recommendation has increased in the primary care setting. Although limited to parental acceptance of human papillomavirus vaccine, studies have demonstrated that recommendations using the presumptive announcement method are stronger than those observed using conversation-based techniques [30] or usual care practices [31]. The present evaluation only assessed routine use of provider recommendation; therefore, the high adherence observed for the recommendation component of the Standards is likely not reflective of strong, or presumptive, recommendation method implementation. As such, despite high adherence, low adult vaccination coverage may in part reflect health care providers not using more effective communication strategies.
Findings from a survey of IIS documentation practices in the primary care setting in 2015 indicated low IIS use by family practice and internal medicine physicians. Study investigators found 38% of family practice and 20% of internal medicine physicians reported entering adult vaccinations in IIS [32]. Among family medicine clinicians included in our survey, 46.5% reported IIS use for adult immunizations. However, IIS documentation practices by internal medicine clinicians were substantially higher in the present evaluation (38.1%) compared to that observed by Kempe and colleagues [32]. In a study of community pharmacists, researchers found 53.8% of pharmacists documented vaccinations in an IIS, which is 10 percentage points higher than what was observed in the present evaluation (43.4%) [33]. The low percentage of IIS use among clinicians and pharmacists possibly reflects some current state mandates that characterize IIS documentation of adult immunizations as an optional practice for most health care providers [34,35]. When asked about potential barriers to IIS documentation, health care providers cited lack of awareness or access [32,33], poor interoperability between private office and state IIS systems [36] and end-user difficulty at the individual level [19].
Multivariable modeling suggests that regardless of clinician type, vaccination improvement strategies such as the presence of an immunization champion, use of standing orders, or patient reminder-recall systems are all strongly associated with implementation of the Standards. Expanded use of these strategies could result in more effective implementation of the Standards within the clinical practice setting. Vaccination improvement strategies were not significantly associated with implementation of the Standards in the pharmacy setting. Although pharmacists in retail settings are typically permitted to administer vaccines to adults through standing orders, those who work in independent pharmacies may not have access to licensed physicians who authorize standing orders. Improved bidirectional communication between state and local IIS and pharmacy data management systems could encourage pharmacists to implement patient reminder-recall systems.
This survey has several limitations. The findings presented here are based on self-report of selected providers. Self-report by medical providers can be unreliable [37,38]; overestimation of clinician and pharmacist implementation of the Standards is possible. Non-probability sampling methods were used to recruit study participants and all statistical measures were calculated under the assumption of random sampling. Therefore, presented estimates should be interpreted with caution as estimates of sampling error from on-random samples are not usually considered valid [39]. Additionally, although the raking method was used to calculate weighted adjustments, under-coverage and non-response biases may remain. Because no national sampling frame of health care providers exists, presented results may not be generalizable to all U.S. health care providers in the outpatient care setting. As respondents answered for their practice in general, results may not reflect individual-level provider-patient interactions. Furthermore, as investigators did not explicitly define the term “routine,” respondents may have interpreted survey questions differently and thus influenced survey findings. Lastly, the order and definition of the vaccination assessment and follow-up survey measures were modified after the first survey year. Efforts were made to harmonize responses after question modification; however, estimates for the assessment and follow-up components of the Standards may be inconsistent across survey years.
This analysis highlights incomplete implementation of the Standards for Adult Immunization Practice since they were published in 2014. Although most clinicians and pharmacists report assessment for and recommendation of adult vaccines to their patients, and either administer those vaccines or refer them to a vaccination service provider, implementation of the Standards in their entirety is low because of low documentation in the IIS. Efforts to reduce barriers to routine IIS documentation are needed. Documentation of vaccinations administered in the IIS and vaccination improvement strategies, such as immunization champions, standing orders, and patient reminder-recall systems should become routine in clinical practice [40]. Improved application of the Standards, where they are integrated into the standard of clinical care for adults, comparable to preventive care standards that currently exist for diabetes [41], hypertension [42], and colorectal cancer [43], can increase adult vaccination coverage rates. Health care providers should thus be encouraged to implement the Standards as a whole more consistently across all practice settings.
5. Disclosure
The findings and conclusions of this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Regarding the present manuscript, the following co-authors do not report and conflicts of interest: Charleigh J. Granade, Amy Parker-Fiebelkorn, Carla L. Black, Anup Srivastav, Chelsea S. Lutz, Carolyn B. Bridges, and David K. Kim. Because the data presented in this manuscript was collected through a contractual agreement between CDC and Abt. Associates, the following co-authors report a conflict of interest: Sarah W. Ball, Rebecca G. Devlin, and Ann J. Cloud..
Footnotes
CRediT authorship contribution statement
Charleigh J. Granade: Formal analysis, Writing - original draft. Amy Parker Fiebelkorn: Methodology, Writing - review & editing. Carla L. Black: Methodology, Conceptualization, Writing - review & editing. Chelsea S. Lutz: Methodology, Investigation, Writing - review & editing. Anup Srivastav: Methodology, Writing - review & editing. Carolyn B. Bridges: Project administration, Conceptualization, Methodology, Writing - review & editing. Sarah W. Ball: Software, Investigation, Resources, Data curation, Writing - review & editing. Rebecca G. Devlin: Software, Investigation, Resources, Data curation, Writing - review & editing. Ann J. Cloud: Software, Investigation, Resources, Data curation, Writing - review & editing. David K. Kim: Project administration, Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision.
References
- [1].U.S. Department of Health and Human Services. Healthy People 2020: immunization and infectious diseases objectives. Updated 2019 October 17. Accessed at https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives on 17 October 2019.
- [2].Thompson M, Shay D, Zhou H, et al. Estimates of deaths associated with seasonal influenza-United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010;59:1057–62. [PubMed] [Google Scholar]
- [3].Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822–5. [PMC free article] [PubMed] [Google Scholar]
- [4].National Foundation for Infectious Diseases. Call to action: Adult vaccination saves lives. 2012. Accessed at https://www.nfid.org/wp-content/uploads/2019/08/cta-adult-1.pdf on 01 October 2019.
- [5].National Vaccine. Advisory Committee– Recommendations from the National Vaccine Advisory Committee: Standards for Adult Immunization Practice. Public Health Rep 2014;129:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ding H, Black CL, Ball S, et al. Influenza vaccination coverage among pregnant women—United States, 2016–17 influenza season. MMWR Morb Mortal Wkly Rep 2017;66:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yonas MA, Nowalk MP, Zimmerman RK, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthc Qual 2012;34:34–42. [DOI] [PubMed] [Google Scholar]
- [8].Kim DK, Hunter P. Recommended adult immunization schedule, United States, 2019. Ann Intern Med 2019;170:182–92. [DOI] [PubMed] [Google Scholar]
- [9].National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127 Suppl 1:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Elkin Z, Cohen EJ, Goldberg JD, et al. Studying physician knowledge, attitudes, and practices regarding the herpes zoster vaccine to address perceived barriers to vaccination. Cornea 2013;32:976–81. [DOI] [PubMed] [Google Scholar]
- [11].Freed GL, Clark SJ, Cowan AE, et al. Primary care physician perspectives on providing adult vaccines. Vaccine. 2011;29:1850–4. [DOI] [PubMed] [Google Scholar]
- [12].Hurley LP, Bridges CB, Harpaz R, et al. U.S. physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014;160:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Steiner JF, Hurley LP, Kempe A, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008;197(Suppl 2):S216–23. [DOI] [PubMed] [Google Scholar]
- [14].Hurley LP, Lindley MC, Allison MA, et al. Primary care physicians’ perspective on financial issues and adult immunization in the era of the Affordable Care Act. Vaccine 2017;35:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med. 2010;152:555–60. [DOI] [PubMed] [Google Scholar]
- [16].Kempe A, Hurley L, Stokley S, et al. Pneumococcal vaccination in general internal medicine practice: current practice and future possibilities. J Gen Intern Med 2008;23:2010–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nichol KL, Zimmerman R. Generalist and subspecialist physicians’ knowledge, attitudes, and practices regarding influenza and pneumococcal vaccinations for elderly and other high-risk patients: a nationwide survey. Arch Intern Med 2001;161:2702–8. [DOI] [PubMed] [Google Scholar]
- [18].Lutz CS, Kim DK, Black CL, et al. Clinicians’ and pharmacists’ reported implementation of vaccination practices for adults. Am J Prev Med 2018;55:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Srivastav A, Black CL, Lutz CS, et al. U.S. clinicians’ and pharmacists’ reported barriers to implementation of the Standards for Adult Immunization Practice. Vaccine 2018;36:6772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].O’Leary ST, Riley LE, Lindley MC, et al. Immunization practices of U.S. obstetrician/gynecologists for pregnant patients. Am J Prev Med 2018;54:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Battaglia MP, Hoaglin DC, Frankel MR. Practical considerations in raking survey data. Surv Pract 2009;2:1–10. [Google Scholar]
- [22].Deville JC, Sarndal CE. Calibration estimators in survey sampling. J Am Stat Assoc 1992;87:376–82. [Google Scholar]
- [23].Bureau of Labor Statistics. National industry-specific occupational employment and wage estimates. 2015. Accessed at https://www.bls.gov/oes/special.requests/oesm15in4.zip on 17 October 2019.
- [24].U.S. Census Bureau. Current population survey monthly labor force data. 2017. Accessed at www.census.gov/programs-surveys/cps/data-detail.html on 17 October 2019.
- [25].Lu PJ, Srivastav A, Amaya A, et al. Association of provider recommendation and offer and influenza vaccination among adults aged 18 years—United States. Vaccine 2018;36:890–8. [DOI] [PubMed] [Google Scholar]
- [26].Benedict KM, Santibanez TA, Black CL, et al. Recommendations and offers for adult influenza vaccination, 2011–2012 season United States. Vaccine 2017;35:1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Centers for Disease Control and Prevention. Influenza vaccination coverage among pregnant women: 2011–12 influenza season, United States. MMWR Morb Mortal Wkly Rep. 2012;61:758–63. [PubMed] [Google Scholar]
- [28].Gilkey MB, Calo WA, Moss JL, et al. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine 2016;34:1187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rosenthal SL, Weiss TW, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine 2011;29:890–5. [DOI] [PubMed] [Google Scholar]
- [30].Malo TL, Hall ME, Brewer NT, et al. Why is announcement training more effective than conversation training for introducing HPV vaccination? A theory-based investigation. Implement Sci 2018;13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother 2019;15:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kempe A, Hurley LP, Cardemil CV, et al. Use of immunization information systems in primary care. Am J Prev Med 2017;52:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Westrick SC, Patterson BJ, Kader MS, et al. National survey of pharmacy-based immunization services. Vaccine 2018;36:5657–64. [DOI] [PubMed] [Google Scholar]
- [34].Centers for Disease Control and Prevention. Progress in immunization information systems-United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005–8. [PMC free article] [PubMed] [Google Scholar]
- [35].Martin DW, Lowery NE, Brand B, et al. Immunization information systems: a decade of progress in law and policy. J Public Health Manag Pract 2015;21:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rockwell Pamela G, Hunter Paul. Vaccine science and immunization guideline. In: Rockwell DO Pamela G, editor. Vaccine Science and Immunization Guideline. Cham: Springer International Publishing; 2017. p. 199–234. [Google Scholar]
- [37].Adams A, Soumerai S, Lomas J, et al. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care 1999;11:187–92. [DOI] [PubMed] [Google Scholar]
- [38].Berry JA. Nurse practitioners’ use of clinical preventive services. J Am Acad Nurse Pract 2009;21:454–60. [DOI] [PubMed] [Google Scholar]
- [39].Baker R, Brick JM, Bates NA, et al. Summary report of the AAPOR Task Force on non-probability sampling. J Surv Stat Methodol 2013;1:90–143. [Google Scholar]
- [40].Community Preventive Services Task Force. The Community Guide: vaccination. Accessed at https://www.thecommunityguide.org/topic/vaccination on 17 October 2019.
- [41].American Diabetes Association. Standards of medical care in diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Siu AL. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2015;163:778–86. [DOI] [PubMed] [Google Scholar]
- [43].Wolf AM, Fontham ET, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]