Abstract
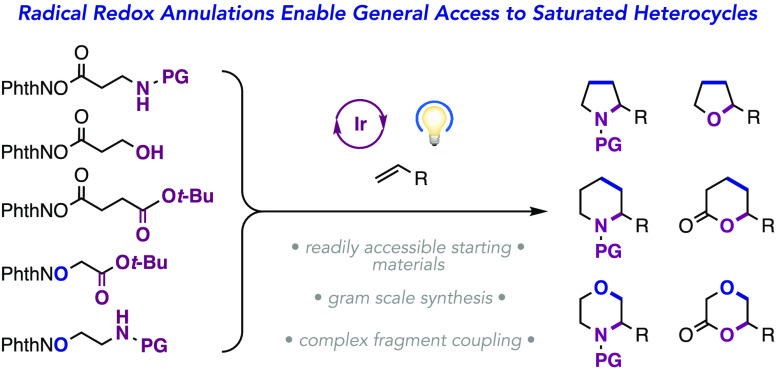
We introduce here a two-component annulation strategy that provides access to a diverse collection of five- and six-membered saturated heterocycles from aryl alkenes and a family of redox-active radical precursors bearing tethered nucleophiles. This transformation is mediated by a combination of an Ir(III) photocatalyst and a Brønsted acid under visible-light irradiation. A reductive proton-coupled electron transfer generates a reactive radical which undergoes addition to an alkene. Then, an oxidative radical-polar crossover step leading to carbocation formation is followed by ring closure through cyclization of the tethered nucleophile. A wide range of heterocycles are easily accessible, including pyrrolidines, piperidines, tetrahydrofurans, morpholines, δ-valerolactones, and dioxanones. We demonstrate the scope of this approach through broad structural variation of both reaction components. This method is amenable to gram-scale preparation and to complex fragment coupling.
Keywords: annulation, photocatalysis, PCET, heterocycles, carbocations
Annulation reactions enabling the synthesis of saturated carbocyclic and heterocyclic ring systems are of central importance in organic synthesis. Indeed, ring formation is a key consideration in the retrosynthetic analysis of complex target molecules,1−5 and general annulation methods are often indispensable synthetic technologies. In this regard, two-component annulation reactions are particularly valuable as they allow for the rapid construction of molecular complexity from simpler, often acyclic, starting materials. Many classical methods for two-component annulation still find frequent use, including pericyclic Diels–Alder [4 + 2] cycloadditions,6,7 Paternò–Büchi [2 + 2] photocycloadditions,8,9 and polar Robinson annulations (Figure 1A).10−12 Moreover, annulation chemistry remains an active area of research, and many novel (photo)catalytic strategies have been developed in recent years to broaden the scope and impact of these approaches (Figure 1B).13
Figure 1.
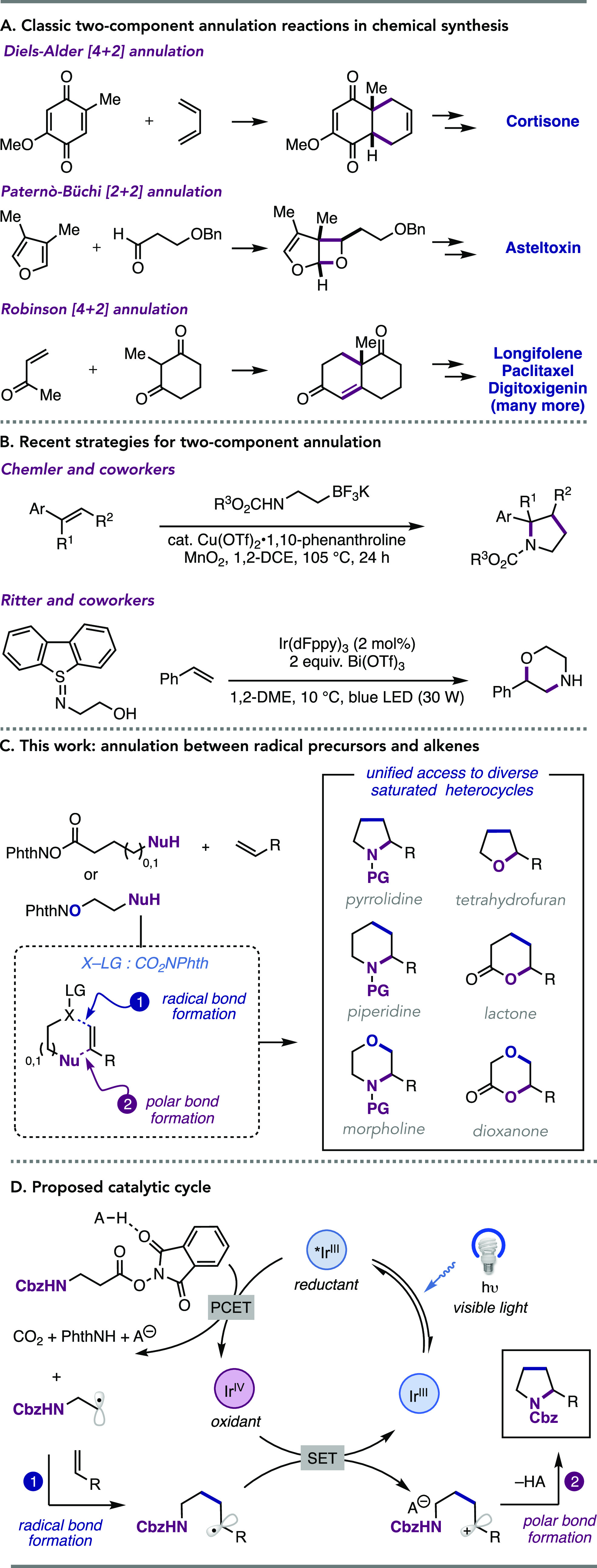
(A) Examples of classic annulation reactions in chemical synthesis. (B) Recent related catalytic methods for two-component annulation. (C) This work: a photocatalytic, two-component annulation of redox-active phthalimides and alkenes. (D) Proposed catalytic cycle.
While being powerful, annulation methods generally place limitations on (i) the nature of the functional groups present in the two reaction partners, (ii) the nature of the atoms involved in the bond formation (carbon or heteroatom), and (iii) the ring size of the annulation product (by necessarily requiring correct spacing between two reactive sites). While being widely applied, most are specific to a narrow subset of (hetero)cyclic products, and each transformation requires different reaction conditions and/or catalysts to proceed. We envisioned a distinct annulation strategy that would enable access to a diverse set of saturated heterocycles in a predictable and modular manner from a common set of reagents under a common set of reaction conditions (Figure 1C). Key to this design was the integration of excited-state electron transfer processes, which allow the two bond-forming events in the annulation to proceed through distinct elementary steps via oxidative radical-polar crossover (ORPC), thus circumventing many of the limitations enumerated above. This report describes the successful realization of these aims and introduces a broadly applicable method for the synthesis of saturated heterocycles—including pyrrolidines, piperidines, tetrahydrofurans, morpholines, δ-valerolactones, and dioxanones—via visible light-driven [n + 2] annulation between redox-active N-hydroxyphthalimide (NHPI) ester or ether reagents and aryl alkene or diene coupling partners.
Our reaction design consists of a bifunctional reagent14 carrying an NHPI ester and a tethered nucleophile, which we hypothesized would pair with an aryl alkene partner through the following sequence of elementary steps (Figure 1D). First, single-electron reduction of the NHPI ester by the excited state of a photocatalyst, followed by fragmentation, would initiate the reaction via radical generation. Then, anti-Markovnikov addition of this nascent radical to the partner aryl alkene would forge a new bond and yield a linear intermediate bearing both a benzylic radical and the tethered nucleophile. Finally, an ORPC event would follow, wherein single-electron oxidation of the benzylic radical by the oxidized state of the photocatalyst furnishes a reactive carbocation.15−30 Cyclization would then occur through addition of the tethered nucleophilic group to the electrophilic cation to yield the desired annulation product. Importantly, this reaction design should accommodate the use of both a variety of radical types and numerous nucleophilic functional groups with varying tether lengths. Thus, we anticipated that this redox-neutral, catalytic method would provide access to a diverse range of saturated heterocyclic scaffolds through a single experimental protocol.
Similar mechanistic scenarios have been successful in promoting a number of three-component alkene 1,2-difunctionalization reactions, including oxyalkylation,31−33 fluoroalkylation,34 bisalkylation,35 hydroesterification,36,37 oxyamination,38 and diamination.39,40 We specifically highlight the work of Chemler and Um, who demonstrated that carbamate-appended trifluoroborate salts serve as reagents for the net-oxidative synthesis of pyrrolidines from styrenes,41 and recent work from Ritter and co-workers demonstrated a similar concept of nucleophile-tethered reductive N-centered radical precursors for the synthesis of morpholines and homomorpholines from styrenes (Figure 1B).42
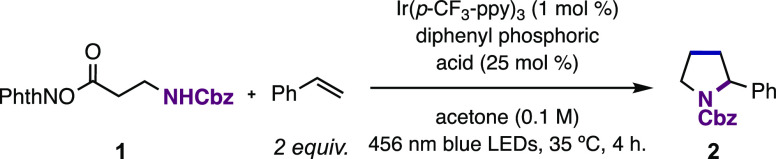
With this framework in mind, our model system for reaction discovery consisted of an N-protected β-alanine NHPI ester and styrene, where the desired product of the reaction would be the corresponding N-protected α-phenyl pyrrolidine (2) via a [3 + 2] annulation (Table 1). In this planning stage, we reasoned that inclusion of a Brønsted acid additive would facilitate a proton-coupled electron transfer (PCET) mechanism for reduction of the NHPI ester substrate.43−54 This would allow for a single photocatalyst to span a greater range of potentials between the excited-state Ir(III) photoreductant and the corresponding Ir(IV) ground-state oxidant, thus increasing the driving force available for the coupled ORPC step. A Cbz-protected β-alanine NHPI ester was readily synthesized via Steglich esterification on multigram scale through a chromatography-free protocol. With this reagent in hand, we set about optimization of the desired process through systematic variation of these highlighted reaction parameters.
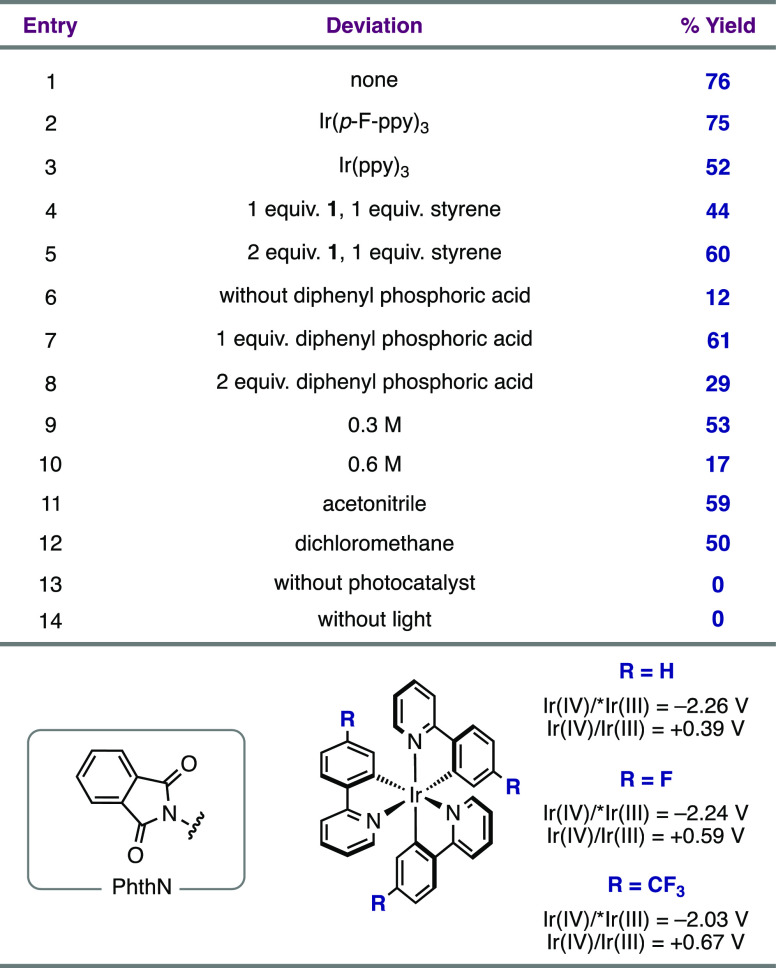
Table 1. Reaction Sensitivity Screena.
We found that with NHPI ester 1 (Ep/2 = −1.52 V vs Fc+/Fc in MeCN) and styrene as model substrates in a 1:2 ratio, pyrrolidine 2 was formed in 76% yield in the presence of 1 mol % Ir(p-CF3-ppy)3 (E1/2 Ir(IV)/*Ir(III) = −2.03 V vs Fc+/Fc in MeCN)55 and 25 mol % diphenyl phosphoric acid in acetone under blue light irradiation (Table 1, entry 1). The annulation could be performed with a range of other photoreductants; for example, Ir(p-F-ppy)3 (E1/2 Ir(IV)/*Ir(III) = −2.24 V vs Fc+/Fc in MeCN)55 and Ir(ppy)3 (E1/2 Ir(IV)/*Ir(III) = −2.26 V vs Fc+/Fc in MeCN)56 were also viable. These photocatalysts facilitated both the reduction of 1 and the oxidation of the resulting secondary benzylic radical (e.g., for the benzylic radical derived from ethylbenzene, E1/2 = −0.01 V vs Fc+/Fc in MeCN)57 to deliver 2 in 75% and 52% yields, respectively (Table 1, entries 2–3). Either decreasing the styrene loading to 1 equiv. or using NHPI ester 1 in excess led to formation of pyrrolidine 2 in moderate to good yields (entries 4–5); this flexibility in stoichiometry can be useful for planning complex fragment couplings (vide infra). The annulation reaction proceeds with poor efficiency in the absence of diphenyl phosphoric acid; the reaction yield is the highest with 25 mol % acid as opposed to 1 or 2 equiv. (entries 6–8). The dependence of the reaction yield on the exogenous acid concentration is consistent with a reductive PCET event initiating radical generation. This hypothesis is further supported by an observed increase in the luminescence quenching of *Ir(p-CF3-ppy)3 by 1 in the presence of diphenyl phosphoric acid (Ksv = 1146 M–1 with acid vs Ksv = 603 M–1 without acid) (see the Supporting Information for details). While reactions run in either acetonitrile or dichloromethane afforded pyrrolidine 2 in moderate yields (entries 11–12), acetone proved to be the optimal solvent for the transformation. Finally, control experiments demonstrated that both light and photocatalyst are required for product formation (entries 13–14). We provide additional details of further optimization experiments for other substrate classes in the Supporting Information.
With optimal conditions established, we first explored pyrrolidine synthesis with respect to the alkene coupling partner (Figure 2). An investigation of electronically varied 4-substituted styrenes showed that electron-neutral and electron-rich olefins undergo annulation in good yields (2–5). Additionally, 1,1-disubstituted and trisubstituted styrenes are effective annulation partners, providing pyrrolidines 6 and 9 bearing fully substituted α-centers, a challenging structural motif to access in a general fashion.58−60
Figure 2.

Substrate scope of [3 + 2] and [4 + 2] annulation reactions. Reactions run on 0.5 mmol scale unless otherwise noted. Yields are for isolated material and are the average of two runs. All products generated from achiral starting materials are racemic. aGram-scale reaction performed using 0.2 mol % photocatalyst with 24 h. reaction time. bReaction performed on 0.1 mmol scale. cGram-scale reaction performed using 0.5 mol % photocatalyst. See the Supporting Information for details on relative stoichiometries of reagents.
Although simple, unactivated alkyl olefins were ineffective substrates under the standard conditions, we found that aryl-substituted α,β-unsaturated carbonyls and dienes proved amenable to annulation. Specifically, pyrrolidines 7 and 8 were generated in 42% and 60% yields from an α-phenyl acrylate ester and an α-phenyl acrylamide, respectively. The success of these alkenes is notable, given the difficulty of accessing α-carbonyl carbocations.61 Additionally, simple dienes such as cyclopentadiene and 1,3-cyclohexadiene afforded fused bicycles 10 and 11 in 60% and 33% yields, respectively, with excellent diastereoselectivity. Alternative routes to related fused bicyclic pyrrolidines generally require multistep sequences via linear cyclization precursors.62−64 Furthermore, an exocyclic diene, which is an intermediate in the synthesis of the insect antifeedant sesquiterpene polygodial,65 underwent annulation to furnish 12 in 62% yield. A 3-vinylindole also undergoes [3 + 2] annulation to provide 13 in good yield.
Next, we found that a variety of commonly used amine protecting groups could be introduced onto the amine-tethered NHPI ester partner, providing N-Ts, N-Bz, and N-Boc pyrrolidines 14, 15, and 16 in good yield. No evidence of competing O-cyclization of the protecting group was observed, in contrast to some examples of carbocation cyclization reactions of carbamates and amides.38,66,67 Due to the synthetic accessibility of β-amino acid derivatives,68 a number of α- and β-functionalized NHPI esters could be readily prepared. These reagents then gave the corresponding pyrrolidines bearing C-4 and C-5 substituents in good yields and modest d.r. (17–23). The synthesis of an α-arylated proline derivative 22 from an l-aspartic acid-derived annulation reagent and a densely functionalized spirocyclic pyrrolidine 23 proceeded in 70% and 54% yields, respectively. Prior access to structures such as 22 required multistep synthetic routes,69 typically deriving from pyroglutamic acid or N-protected prolines via Shono oxidation.70−73
We next sought to investigate the adaptability of the annulation strategy to access other heterocycle classes. Specifically, by changing the pendent nucleophile on the NHPI ester reagent to an alcohol, we anticipated access to tetrahydrofuran products. Using styrene as the annulation partner together with NHPI esters derived from β-hydroxy acids, the desired α-aryl- and α-carboxylate ester-substituted tetrahydrofurans 24 and 26 were formed in good yield under conditions similar to those used in the pyrrolidine-forming reactions. Using this method, a rare 2-trifluoromethyl-substituted tetrahydrofuran 25 was prepared. The introduction of spirocyclic scaffolds into lead structures is also of broad interest in medicinal chemistry; however, it is often accompanied by increased synthetic effort.74,75 We found that the annulation protocol also offers a straightforward route to α-substituted spirocyclic tetrahydrofurans in serviceable yields (27–29). Here, we highlight pyrrolidine 23 and tetrahydrofuran 29 as examples of nitrogen- and oxygen-containing analogues of otherwise-identical spirocyclic scaffolds.
The annulation reaction also enables the synthesis of a variety of six-membered saturated heterocycles through the use of NHPI ester partners with an extended tether to the nucleophile. To achieve efficient reactivity with these precursors, use of 1–3 equiv. of diphenyl phosphoric acid was necessary. With this modification, piperidines 35 and 36 were prepared in good yield, with 36 arising from annulation of a readily available derivative of l-glutamic acid. A tethered t-Bu ester can also serve as a pendent nucleophile in the synthesis of six-membered δ-valerolactone 37. Further highlighting the modularity of this protocol, we found that use of NHPI ether reagents76−80 in place of the esters, without otherwise altering reaction conditions, enabled the preparation of a distinct set of heterocycle classes bearing two heteroatoms. Here, the reaction proceeds via generation of an oxygen-centered radical that undergoes alkene addition, ORPC, and nucleophilic cyclization. For example, morpholine 38 and dioxanone 39 were formed in 70% and 75% yields, respectively. Notably, this protocol provides the alternative regiochemical outcome in the cyclization compared to the recent report of morpholine synthesis from Ritter and co-workers.42
All of the NHPI ester and ether reagents studied above were prepared on >1.0 g scale, and the majority are accessible through chromatography-free protocols. All pyrrolidine, piperidine, morpholine, lactone, and dioxanone reagents above are bench-stable, and comparable yields of pyrrolidines 2 and 16 were realized when using a ca. 12 month old batch of NHPI ester reagent compared to a batch that was freshly prepared. We opted to store the tetrahydrofuran precursors at −20 °C, where they demonstrate stability and reaction viability without deterioration over ca. 6 months. This annulation methodology suffers from some limitations with respect to the electronic character of the alkene. For example, in reactions of more electron-deficient styrenes, products resulting from either carbocation hydration and/or elimination predominate. For cases where ORPC does not occur, linear radical reduction products are observed. Cyclization of piperidine substrates also appears limited to electron-rich styrenes. A list of modestly performing and unsuccessful substrates is included in the Supporting Information.
The modular nature of this annulation protocol enables the generation of a library of diverse heterocycles from a single alkene substrate (Figure 3). To highlight this ability, we selected two pharmaceutically relevant alkenes—deriving from estrone and fenofibrate—bearing styrenyl and 1,1-diarylethylene motifs, respectively, and exposed them to an array of coupling partners. Pyrrolidine (42), δ-valerolactone (43), and morpholine (44) fragments could all be appended to vinyl estrone in good-to-excellent yields. Fenofibrate was also readily derivatized to the corresponding pyrrolidine 45 and tetrahydrofuran 46 analogues.
Figure 3.
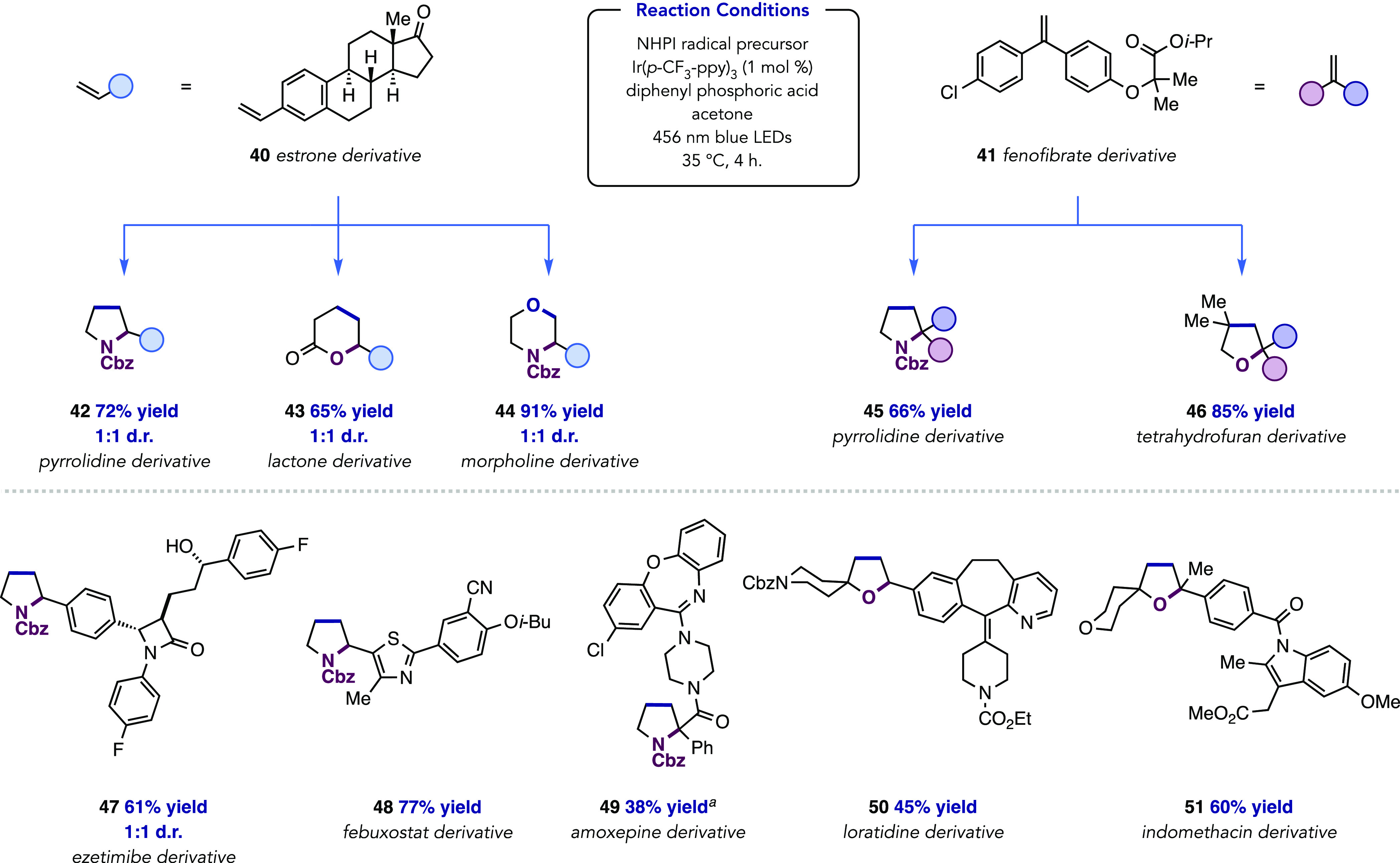
Substrate scope of [3 + 2] and [4 + 2] annulation reactions with complex alkenes. Reactions run on 0.1 mmol scale unless otherwise noted. Yields are for isolated material and are the average of two runs. aReaction performed in acetonitrile. See the Supporting Information for details on relative stoichiometries of reagents.
Numerous complex alkenes were amenable to annulation, highlighting the functional group tolerance of the method toward β-lactams (47), unprotected benzylic alcohols, nitriles, amides, tertiary amidines, and esters. In the case of loratidine as an alkene partner, complete selectivity is observed for radical addition to the less substituted styrenyl fragment as opposed to an internal, tetrasubstituted alkene (50). Finally, this suite of complex alkenes contains an array of heterocyclic functionality that is tolerated under the annulation conditions, including thiazoles (48), piperazines and oxazepines (49), pyridines (50), and indoles (51).
We next examined the reactivity of pharmaceutical-derived NHPI esters for olefin annulation (Figure 4). These reagents are practical and convenient to prepare in two to four steps from the commercial drug substances in 68–90% overall yields (see the Supporting Information for details). A complex sulfonamide-tethered NHPI ester derived from COX-2 inhibitor celecoxib (52) was a competent partner in the annulation, delivering pyrrolidine 55 in 77% yield. Baclofen, an unnatural amino acid used as a muscle relaxant, could be easily derivatized to its corresponding NHPI ester 53 and subjected to annulation conditions with vinyl anisole to deliver piperidine 56 in 77% yield.
Figure 4.
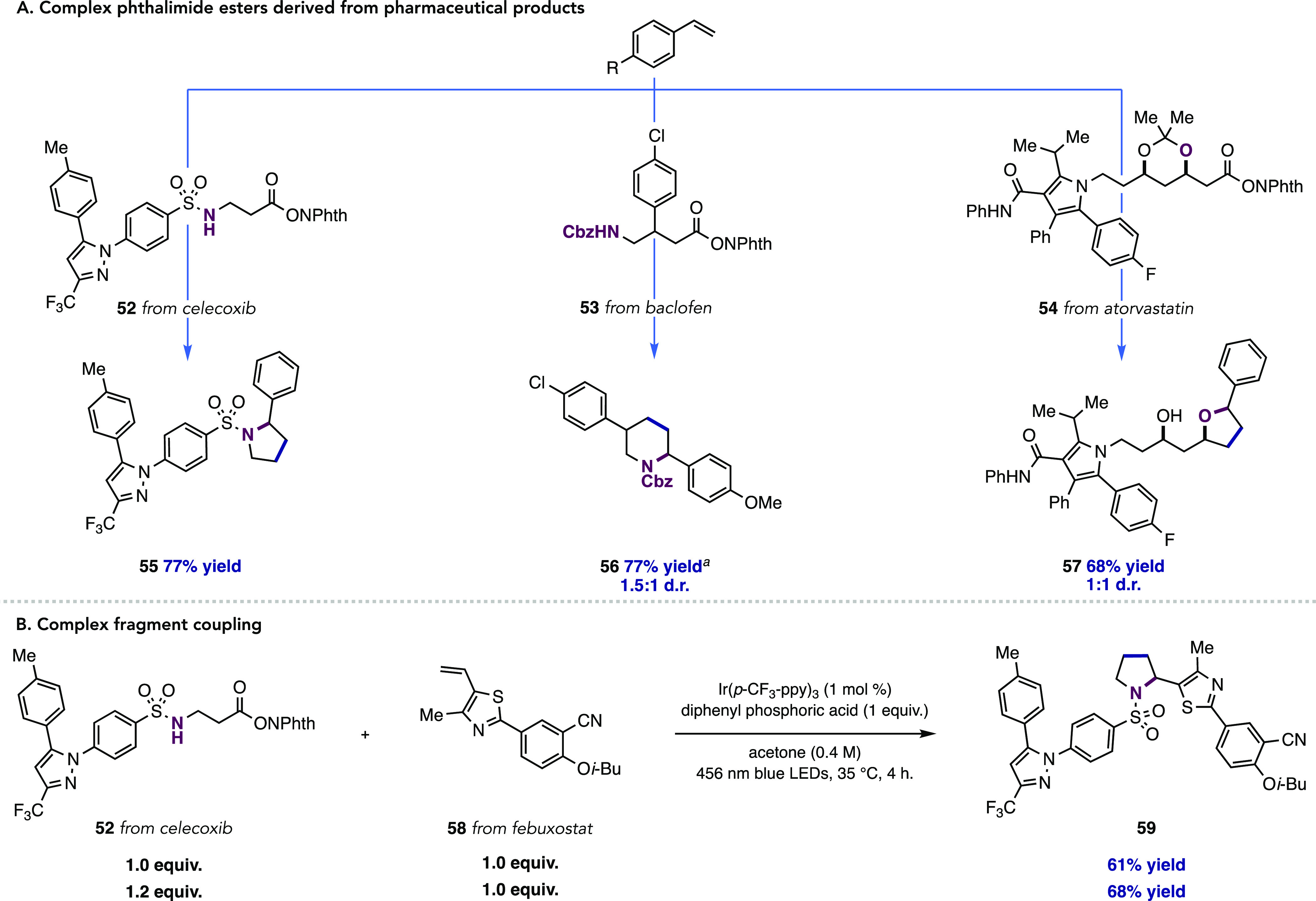
Annulation reactions with complex NHPI ester substrates. Reactions run on 0.1 mmol scale unless otherwise noted. Yields are for isolated material and are the average of two runs. (A) Examples of [3 + 2] and [4 + 2] annulation reactions from pharmaceutical-derived phthalimide esters. (B) Complex fragment coupling. See the Supporting Information for more details on relative stoichiometries of reagents. aReaction performed on 0.5 mmol scale.
Additionally, an acetonide-protected NHPI ester derived from atorvastatin (54)—a drug used for prevention of cardiovascular disease—was synthesized and subjected to the annulation conditions. Here, we observed that the acetonide was a competent nucleophile in pairing with the carbocation intermediate, delivering tetrahydrofuran 57 in 68% yield and 1:1 d.r.. Whereas these examples were conducted using excess styrene relative to the more valuable phthalimide ester, we found that complex fragment couplings can be accomplished efficiently using matched stoichiometries of both redox-active ester and alkene partners; for example, an annulation reaction between a febuxostat-derived alkene 58 and celecoxib-derived NHPI ester 52 proceeded in 61% yield with 1:1 relative stoichiometry. A slight increase in yield was noted with 1.2 equiv of 52 (68% yield). Finally, to highlight the utility of this protocol for preparative-scale synthesis, we carried out annulation reactions on a 6 mmol scale, delivering gram quantities of pyrrolidines 2 and 23 (Figure 2), with reduced photocatalyst loadings of 0.2 mol % and 0.5 mol %, respectively. Gratifyingly, these products were delivered in nearly identical yield compared to those performed on 0.5 mmol scale.
In summary, we introduce here a photocatalytic, two-component annulation strategy for the general synthesis of valuable five- and six-membered saturated heterocycles from alkenes and redox-active radical precursors bearing tethered nucleophiles. A number of distinct heterocycle classes were accessed using this approach, including pyrrolidines, piperidines, tetrahydrofurans, morpholines, δ-valerolactones, and dioxanones. We demonstrate the utility of this methodology for late-stage derivatization, heterocycle library synthesis, and gram-scale preparation. Furthermore, this annulation protocol readily accommodates complexity in both the redox-active radical precursor and alkene components, a feature particularly demonstrated through an example of complex fragment coupling between a celecoxib-derived NHPI ester and a febuxostat-derived alkene. We anticipate that other classes of heterocycles, other ring sizes, and more diverse bicyclic structures should all be accessible using the approach presented here. Efforts toward these ends are ongoing.
Acknowledgments
Funding for this work was provided by the NIH (R35 GM134893 to R.R.K.) and the NSF (CHE-2102266 to A.G.D.). P.R.D.M. wishes to thank the European Commission for a Marie-Skłodowska-Curie Individual Fellowship (grant number: 886224). We thank Nicholas D. Chiappini and Jacob M. Ganley of the Princeton University for helpful discussions. We thank Erik J. Sorensen, Nicholas A. Falcone, and John F. Hoskin of the Princeton University for providing complex diene starting material for annulation example 12 and for helpful discussions. We thank Brandon Kennedy of Lotus separations and Alberto Castanedo of the Princeton University for assistance with the purification of various annulation products. We thank István Pelczer and Kenith Conover for assistance with NMR experiments and John Eng for assistance with mass spectrometry.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.2c04316.
Experimental details, characterization data, and NMR spectra (PDF)
Author Contributions
§ P.R.D.M. and I.N.-M.L. contributed equally to this work.
Author Contributions
# S.M.H. and E.V. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Woodward R. B.; Sondheimer F.; Taub D. The Total Synthesis of Cortisone. J. Am. Chem. Soc. 1951, 73, 4057. 10.1021/ja01152a551. [DOI] [Google Scholar]
- Schreiber S. L.; Satake K. Total Synthesis of (±)-Asteltoxin. J. Am. Chem. Soc. 1984, 106, 4186–4188. 10.1021/ja00327a020. [DOI] [Google Scholar]
- Corey E. J.; Ohno M.; Mitra R. B.; Vatakencherry P. A. Total Synthesis of Longifolene. J. Am. Chem. Soc. 1964, 86, 478–485. 10.1021/ja01057a039. [DOI] [Google Scholar]
- Danishefsky S. J.; Masters J. J.; Young W. B.; Link J. T.; Snyder L. B.; Magee T. V.; Jung D. K.; Isaacs R. C. A.; Bornmann W. G.; Alaimo C. A.; et al. Total Synthesis of Baccatin III and Taxol. J. Am. Chem. Soc. 1996, 118, 2843–2859. 10.1021/ja952692a. [DOI] [Google Scholar]
- Stork G.; West F.; Lee H. Y.; Isaacs R. C. A.; Manabe S. The Total Synthesis of a Natural Cardenolide: (+)-Digitoxigenin. J. Am. Chem. Soc. 1996, 118, 10660–10661. 10.1021/ja962163m. [DOI] [Google Scholar]
- Diels O.; Alder K. Synthesen in Der Hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. 10.1002/jlac.19284600106. [DOI] [Google Scholar]
- Nicolaou K. C.; Snyder S. A.; Montagnon T.; Vassilikogiannakis G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem., Int. Ed. 2002, 41, 1668–1698. . [DOI] [PubMed] [Google Scholar]
- Büchi G.; Inman C. G.; Lipinsky E. S. Light-Catalyzed Organic Reactions. I. The Reaction of Carbonyl Compounds with 2-Methyl-2-Butene in the Presence of Ultraviolet Light. J. Am. Chem. Soc. 1954, 76, 4327–4331. 10.1021/ja01646a024. [DOI] [Google Scholar]
- D’Auria M. The Paternò-Büchi Reaction-a Comprehensive Review. Photochem. Photobiol. Sci. 2019, 18, 2297–2362. [DOI] [PubMed] [Google Scholar]
- Rapson W. S.; Robinson R. 307. Experiments on the synthesis of substances related to the sterols. Part II. A new general method for the synthesis of substituted cyclohexenones. J. Chem. Soc. 1935, 1285–1288. 10.1039/jr9350001285. [DOI] [Google Scholar]
- Bradshaw B.; Bonjoch J. The Wieland-Miescher Ketone: A Journey from Organocatalysis to Natural Product Synthesis. Synlett 2012, 23, 337–356. 10.1055/s-0031-1290107. [DOI] [Google Scholar]
- Gallier F.; Martel A.; Dujardin G. Enantioselective Access to Robinson Annulation Products and Michael Adducts as Precursors. Angew. Chem., Int. Ed. 2017, 56, 12424–12458. 10.1002/anie.201701401. [DOI] [PubMed] [Google Scholar]
- For a collection of recently reported methods for the synthesis of saturated carbocycles and heterocycles with emphasis on photocatalysis, please refer to the Supporting Information.
- Huang H.-M.; Bellotti P.; Ma J.; Dalton T.; Glorius F. Bifunctional Reagents in Organic Synthesis. Nat. Rev. Chem. 2021, 5, 301–321. 10.1038/s41570-021-00266-5. [DOI] [PubMed] [Google Scholar]
- Webb E. W.; Park J. B.; Cole E. L.; Donnelly D. J.; Bonacorsi S. J.; Ewing W. R.; Doyle A. G. Nucleophilic (Radio)Fluorination of Redox-Active Esters via Radical-Polar Crossover Enabled by Photoredox Catalysis. J. Am. Chem. Soc. 2020, 142, 9493–9500. 10.1021/jacs.0c03125. [DOI] [PubMed] [Google Scholar]
- Leibler I. N. M.; Tekle-Smith M. A.; Doyle A. G. A General Strategy for C(Sp3)–H Functionalization with Nucleophiles Using Methyl Radical as a Hydrogen Atom Abstractor. Nat. Commun. 2021, 12, 6950. 10.1038/s41467-021-27165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J.; DeGlopper K. S.; Yoon T. P. Site-Selective Alkoxylation of Benzylic C–H Bonds by Photoredox Catalysis. Angew. Chem., Int. Ed. 2020, 59, 197–202. 10.1002/anie.201910602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed N. L.; Lutovsky G. A.; Yoon T. P. Copper-Mediated Radical-Polar Crossover Enables Photocatalytic Oxidative Functionalization of Sterically Bulky Alkenes. J. Am. Chem. Soc. 2021, 143, 6065–6070. 10.1021/jacs.1c02747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Y.; Gockel S. N.; Lutovsky G. A.; DeGlopper K. S.; Baldwin N. J.; Bundesmann M. W.; Tucker J. W.; Bagley S. W.; Yoon T. P. Decarboxylative Cross-Nucleophile Coupling via Ligand-to-Metal Charge Transfer Photoexcitation of Cu(Ii) Carboxylates. Nat. Chem. 2022, 14, 94–99. 10.1038/s41557-021-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani S.; Kodo T.; Takeda M.; Nagao K.; Tokunaga N.; Sasaki Y.; Ohmiya H. Organophotoredox-Catalyzed Decarboxylative C(Sp3)–O Bond Formation. J. Am. Chem. Soc. 2020, 142, 1211–1216. 10.1021/jacs.9b12335. [DOI] [PubMed] [Google Scholar]
- Kobayashi R.; Shibutani S.; Nagao K.; Ikeda Z.; Wang J.; Ibáñez I.; Reynolds M.; Sasaki Y.; Ohmiya H. Decarboxylative N-Alkylation of Azoles through Visible-Light-Mediated Organophotoredox Catalysis. Org. Lett. 2021, 23, 5415–5419. 10.1021/acs.orglett.1c01745. [DOI] [PubMed] [Google Scholar]
- Nakagawa M.; Nagao K.; Ikeda Z.; Reynolds M.; Ibáñez I.; Wang J.; Tokunaga N.; Sasaki Y.; Ohmiya H. Organophotoredox-Catalyzed Decarboxylative N-Alkylation of Sulfonamides. ChemCatChem 2021, 13, 3930–3933. 10.1002/cctc.202100803. [DOI] [Google Scholar]
- Kodo T.; Nagao K.; Ohmiya H. Organophotoredox-Catalyzed Semipinacol Rearrangement via Radical-Polar Crossover. Nat. Commun. 2022, 13, 2684. 10.1038/s41467-022-30395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Zbieg J. R.; Terrett J. A. A Platform for Decarboxylative Couplings via Photoredox Catalysis: Direct Access to Carbocations from Carboxylic Acids for Carbon-Oxygen Bond Formation. ACS Catal. 2021, 11, 10997–11004. 10.1021/acscatal.1c03251. [DOI] [Google Scholar]
- Li P.; Zbieg J. R.; Terrett J. A. The Direct Decarboxylative N-Alkylation of Azoles, Sulfonamides, Ureas, and Carbamates with Carboxylic Acids via Photoredox Catalysis. Org. Lett. 2021, 23, 9563–9568. 10.1021/acs.orglett.1c03761. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Jiang Y.; Wang Y.; Sun T.; Meng Y.; Huang Y.; Lv X.; Gao J.; Zhang X.; Zhang S.; et al. Photoredox/Copper Dual-Catalyzed Benzylic C-H Esterification via Radical-Polar Crossover. Org. Lett. 2022, 24, 2679–2683. 10.1021/acs.orglett.2c00763. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Fitzpatrick N. A.; Das M.; Bedre I. P.; Yayla H. G.; Lall M. S.; Musacchio P. Z. A Photoredox-Catalyzed Approach for Formal Hydride Abstraction to Enable Csp3–H Functionalization with Nucleophilic Partners (F, C, O, N, and Br/Cl). Chem. Catal. 2022, 2, 292–308. 10.1016/j.checat.2021.12.010. [DOI] [Google Scholar]
- Narobe R.; Murugesan K.; Haag C.; Schirmer T. E.; König B. C(Sp3)–H Ritter Amination by Excitation of in Situ Generated Iodine(Iii)–BF3 Complexes. Chem. Commun. 2022, 58, 8778. 10.1039/d2cc03283j. [DOI] [PubMed] [Google Scholar]
- Reich D.; Noble A.; Aggarwal V. K. Facile Conversion of α-Amino Acids to α-Amino Phosphonates by Decarboxylative Phosphorylation Using Visible-Light Photocatalysis. Angew. Chem., Int. Ed. 2022, 134, e202207063 10.1002/ange.202207063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a related method accessing carbocations via electron trasnfer, see:Zhu Q.; Gentry E. C.; Knowles R. R. Catalytic Carbocation Generation Enabled by the Mesolytic Cleavage of Alkoxyamine Radical Cations. Angew. Chem., Int. Ed. 2016, 55, 9969–9973. 10.1002/anie.201604619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlahuext-Aca A.; Garza-Sanchez R. A.; Glorius F. Multicomponent Oxyalkylation of Styrenes Enabled by Hydrogen-Bond-Assisted Photoinduced Electron Transfer. Angew. Chem., Int. Ed. 2017, 56, 3708–3711. 10.1002/anie.201700049. [DOI] [PubMed] [Google Scholar]
- Sha W.; Ni S.; Han J.; Pan Y. Access to Alkyl-Substituted Lactone via Photoredox-Catalyzed Alkylation/Lactonization of Unsaturated Carboxylic Acids. Org. Lett. 2017, 19, 5900–5903. 10.1021/acs.orglett.7b02899. [DOI] [PubMed] [Google Scholar]
- Shibutani S.; Nagao K.; Ohmiya H. Organophotoredox-Catalyzed Three-Component Coupling of Heteroatom Nucleophiles, Alkenes, and Aliphatic Redox Active Esters. Org. Lett. 2021, 23, 1798–1803. 10.1021/acs.orglett.1c00211. [DOI] [PubMed] [Google Scholar]
- Jang E.; Kim H. I.; Jang H. S.; Sim J. Photoredox-Catalyzed Oxidative Radical-Polar Crossover Enables the Alkylfluorination of Olefins. J. Org. Chem. 2022, 87, 2640–2650. 10.1021/acs.joc.1c02607. [DOI] [PubMed] [Google Scholar]
- Cabrera-Afonso M. J.; Sookezian A.; Badir S. O.; El Khatib M.; Molander G. A. Photoinduced 1,2-Dicarbofunctionalization of Alkenes with Organotrifluoroborate Nucleophilesviaradical/Polar Crossover. Chem. Sci. 2021, 12, 9189–9195. 10.1039/d1sc02547c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L.; Ready J. M. Hydroesterification and Difunctionalization of Olefins with N-Hydroxyphthalimide Esters. ACS Catal. 2021, 11, 13714–13720. 10.1021/acscatal.1c03969.35982833 [DOI] [Google Scholar]
- Quach L.; Dutta S.; Pflüger P. M.; Sandfort F.; Bellotti P.; Glorius F. Visible-Light-Initiated Hydrooxygenation of Unactivated Alkenes–A Strategy for Anti-Markovnikov Hydrofunctionalization. ACS Catal. 2022, 12, 2499–2504. 10.1021/acscatal.1c05555. [DOI] [Google Scholar]
- Reed N. L.; Herman M. I.; Miltchev V. P.; Yoon T. P. Photocatalytic Oxyamination of Alkenes: Copper(II) Salts as Terminal Oxidants in Photoredox Catalysis. Org. Lett. 2018, 20, 7345–7350. 10.1021/acs.orglett.8b03345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Q.; Yu W. L.; Wei Y. L.; Li T. H.; Xu P. F. Photoredox-Induced Functionalization of Alkenes for the Synthesis of Substituted Imidazolines and Oxazolidines. J. Org. Chem. 2017, 82, 243–249. 10.1021/acs.joc.6b02377. [DOI] [PubMed] [Google Scholar]
- Govaerts S.; Angelini L.; Hampton C.; Malet-Sanz L.; Ruffoni A.; Leonori D. Photoinduced Olefin Diamination with Alkylamines. Angew. Chem., Int. Ed. 2020, 59, 15021–15028. 10.1002/anie.202005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um C.; Chemler S. R. Synthesis of 2-Aryl- and 2-Vinylpyrrolidines via Copper-Catalyzed Coupling of Styrenes and Dienes with Potassium β-Aminoethyl Trifluoroborates. Org. Lett. 2016, 18, 2515–2518. 10.1021/acs.orglett.6b01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q.; Bai Z.; Tewari S.; Ritter T. Bifunctional Sulfilimines Enable Synthesis of Multiple N-Heterocycles from Alkenes. Nat. Chem. 2022, 14, 898. 10.1038/s41557-022-00997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayla H. G.; Knowles R. R. Proton-Coupled Electron Transfer in Organic Synthesis: Novel Homolytic Bond Activations and Catalytic Asymmetric Reactions with Free Radicals. Synlett 2014, 25, 2819. 10.1055/s-0034-1379304. [DOI] [Google Scholar]
- Gentry E. C.; Knowles R. R. Synthetic Applications of Proton-Coupled Electron Transfer. Acc. Chem. Res. 2016, 49, 1546–1556. 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R. D.; Cox J. H.; Chiappini N. D.; Roos C. B.; McLoughlin E. A.; Hejna B. G.; Nguyen S. T.; Ripberger H. H.; Ganley J. M.; Tsui E.; et al. Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis. Chem. Rev. 2022, 122, 2017–2291. 10.1021/acs.chemrev.1c00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J.; Espelt L. R.; Guzei I. A.; Yoon T. P. Photocatalytic Reductive Cyclizations of Enones: Divergent Reactivity of Photogenerated Radical and Radical Anion Intermediates. Chem. Sci. 2011, 2, 2115–2119. 10.1039/c1sc00357g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino K. T.; Liu P.; Knowles R. R. Catalytic Ketyl-Olefin Cyclizations Enabled by Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2013, 135, 10022–10025. 10.1021/ja404342j. [DOI] [PubMed] [Google Scholar]
- Rono L. J.; Yayla H. G.; Wang D. Y.; Armstrong M. F.; Knowles R. R. Enantioselective Photoredox Catalysis Enabled by Proton-Coupled Electron Transfer: Development of an Asymmetric Aza-Pinacol Cyclization. J. Am. Chem. Soc. 2013, 135, 17735–17738. 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]
- Sherwood T. C.; Xiao H. Y.; Bhaskar R. G.; Simmons E. M.; Zaretsky S.; Rauch M. P.; Knowles R. R.; Dhar T. G. M. Decarboxylative Intramolecular Arene Alkylation Using N-(Acyloxy)Phthalimides, an Organic Photocatalyst, and Visible Light. J. Org. Chem. 2019, 84, 8360–8379. 10.1021/acs.joc.9b00432. [DOI] [PubMed] [Google Scholar]
- Sayre H.; Ripberger H. H.; Odella E.; Zieleniewska A.; Heredia D. A.; Rumbles G.; Scholes G. D.; Moore T. A.; Moore A. L.; Knowles R. R. PCET-Based Ligand Limits Charge Recombination with an Ir(III) Photoredox Catalyst. J. Am. Chem. Soc. 2021, 143, 13034–13043. 10.1021/jacs.1c01701. [DOI] [PubMed] [Google Scholar]
- Nakajima M.; Fava E.; Loescher S.; Jiang Z.; Rueping M. Photoredox-Catalyzed Reductive Coupling of Aldehydes, Ketones, and Imines with Visible Light. Angew. Chem., Int. Ed. 2015, 54, 8828–8832. 10.1002/anie.201501556. [DOI] [PubMed] [Google Scholar]
- Qi L.; Chen Y. Polarity-Reversed Allylations of Aldehydes, Ketones, and Imines Enabled by Hantzsch Ester in Photoredox Catalysis. Angew. Chem., Int. Ed. 2016, 55, 13312–13315. 10.1002/anie.201607813. [DOI] [PubMed] [Google Scholar]
- de Arriba A. L.; Urbitsch F.; Dixon D. J. Umpolung Synthesis of Branched α-Functionalized Amines from Imines via Photocatalytic Three-Component Reductive Coupling Reactions. Chem. Commun. 2016, 52, 14434–14437. 10.1039/C6CC09172E. [DOI] [PubMed] [Google Scholar]
- Caron A.; Morin É.; Collins S. K. Bifunctional Copper-Based Photocatalyst for Reductive Pinacol-Type Couplings. ACS Catal. 2019, 9, 9458–9464. 10.1021/acscatal.9b01718. [DOI] [Google Scholar]
- Singh A.; Teegardin K.; Kelly M.; Prasad K. S.; Krishnan S.; Weaver J. D. Facile Synthesis and Complete Characterization of Homoleptic and Heteroleptic Cyclometalated Iridium(III) Complexes for Photocatalysis. J. Organomet. Chem. 2015, 776, 51–59. 10.1016/j.jorganchem.2014.10.037. [DOI] [Google Scholar]
- Wu Y.; Kim D.; Teets T. S. Photophysical Properties and Redox Potentials of Photosensitizers for Organic Photoredox Transformations. Synlett 2021, 33, 1154. 10.1055/a-1390-9065. [DOI] [Google Scholar]
- Wayner D. D. M.; McPhee D. J.; Griller D. Oxidation and Reduction Potentials of Transient Free Radicals. J. Am. Chem. Soc. 1988, 110, 132–137. 10.1021/ja00209a021. [DOI] [Google Scholar]
- Vasu D.; Fuentes de Arriba A. L.; Leitch J. A.; de Gombert A.; Dixon D. J. Primary α-Tertiary Amine Synthesis via α-C-H Functionalization. Chem. Sci. 2019, 10, 3401–3407. 10.1039/c8sc05164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. H.; Kumar R.; Gaunt M. J. Visible-Light-Mediated Carbonyl Alkylative Amination to All-Alkyl α-Tertiary Amino Acid Derivatives. J. Am. Chem. Soc. 2021, 143, 1598–1609. 10.1021/jacs.0c12162. [DOI] [PubMed] [Google Scholar]
- Henry Blackwell J.; Harris G. R.; Smith M. A.; Gaunt M. J. Modular Photocatalytic Synthesis of α-Trialkyl-α-Tertiary Amines. J. Am. Chem. Soc. 2021, 143, 15946–15959. 10.1021/jacs.1c07402. [DOI] [PubMed] [Google Scholar]
- Seebach D. Methods of Reactivity Umpolung. Angew. Chem., Int. Ed. Engl. 1979, 18, 239–258. 10.1002/anie.197902393. [DOI] [Google Scholar]
- Larock R. C.; Hightower T. R.; Hasvold L. A.; Peterson K. P. Palladium(II)-Catalyzed Cyclization of Olefinic Tosylamides. J. Org. Chem. 1996, 61, 3584–3585. 10.1021/jo952088i. [DOI] [PubMed] [Google Scholar]
- Liu G.; Stahl S. S. Two-Faced Reactivity of Alkenes: Cis- versus Trans-Aminopalladation in Aerobic Pd-Catalyzed Intramolecular Aza-Wacker Reactions. J. Am. Chem. Soc. 2007, 129, 6328–6335. 10.1021/ja070424u. [DOI] [PubMed] [Google Scholar]
- Hazelden I. R.; Carmona R. C.; Langer T.; Pringle P. G.; Bower J. F. Pyrrolidines and Piperidines by Ligand-Enabled Aza-Heck Cyclizations and Cascades of N-(Pentafluorobenzoyloxy)Carbamates. Angew. Chem., Int. Ed. 2018, 57, 5124–5128. 10.1002/anie.201801109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K.; Watanabe H. Synthesis of Both the Enantiomers of Polygodial, an Insect Antifeedant Sesouiterpene. Tetrahedron 1986, 42, 273–281. 10.1016/s0040-4020(01)87428-7. [DOI] [Google Scholar]
- Morse P. D.; Nicewicz D. A. Divergent Regioselectivity in Photoredox-Catalyzed Hydrofunctionalization Reactions of Unsaturated Amides and Thioamides. Chem. Sci. 2015, 6, 270–274. 10.1039/c4sc02331e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. C.; Campbell J. M.; Moeller K. D. Cyclization Reactions of Anode-Generated Amidyl Radicals. J. Org. Chem. 2014, 79, 379–391. 10.1021/jo402623r. [DOI] [PubMed] [Google Scholar]
- Weiner B.; Szymański W.; Janssen D. B.; Minnaard A. J.; Feringa B. L. Recent Advances in the Catalytic Asymmetric Synthesis of β-Amino Acids. Chem. Soc. Rev. 2010, 39, 1656–1691. 10.1039/b919599h. [DOI] [PubMed] [Google Scholar]
- Haddad M.; Imogaï H.; Larchevêque M. The First Enantioselective Synthesis of the CIS-2-Carboxy-5- Phenylpyrrolidine. J. Org. Chem. 1998, 63, 5680–5683. 10.1021/jo980396l. [DOI] [Google Scholar]
- Asada S.; Kato M.; Asai K.; Ineyama T.; Nishi S.; Izawa K.; Shono T. Enantioselective Synthesis of the Carbapenem Ring System from (S)-Proline. J. Chem. Soc., Chem. Commun. 1989, 486–488. 10.1039/c39890000486. [DOI] [Google Scholar]
- Collado I.; Ezquerra J.; Pedregal C. Stereoselective Addition of Grignard-Derived Organocopper Reagents to N-Acyliminium Ions: Synthesis of Enantiopure 5- and 4,5-Substituted Prolinates. J. Org. Chem. 1995, 60, 5011–5015. 10.1021/jo00121a020. [DOI] [Google Scholar]
- Scharnagel D.; Prause F.; Kaldun J.; Haase R. G.; Breuning M. (2S,5R)-2-Methylaminomethyl-1-Methyl-5-Phenylpyrrolidine, a Chiral Diamine Ligand for Copper(Ii)-Catalysed Henry Reactions with Superb Enantiocontrol. Chem. Commun. 2014, 50, 6623–6625. 10.1039/c4cc02429j. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Donckele E. J.; Thaisrivongs D. A.; Osipov M.; Masters J. T. A New Class of Non-C2-Symmetric Ligands for Oxidative and Redox-Neutral Palladium-Catalyzed Asymmetric Allylic Alkylations of 1,3-Diketones. J. Am. Chem. Soc. 2015, 137, 2776–2784. 10.1021/jacs.5b00786. [DOI] [PubMed] [Google Scholar]
- Carreira E. M.; Fessard T. C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- Hiesinger K.; Dar’in D.; Proschak E.; Krasavin M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. 10.1021/acs.jmedchem.0c01473. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Li Y.; Zhang F.; Hu C.; Chen Y. Generation of Alkoxyl Radicals by Photoredox Catalysis Enables Selective C(Sp3)–H Functionalization under Mild Reaction Conditions. Angew. Chem., Int. Ed. 2016, 55, 1872–1875. 10.1002/anie.201510014. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Li Y.; Xu R.; Chen Y. Donor–Acceptor Complex Enables Alkoxyl Radical Generation for Metal-Free C(Sp3)–C(Sp3) Cleavage and Allylation/Alkenylation. Angew. Chem., Int. Ed. 2017, 56, 12619–12623. 10.1002/anie.201707171. [DOI] [PubMed] [Google Scholar]
- Jia K.; Chen Y. Visible-Light-Induced Alkoxyl Radical Generation for Inert Chemical Bond Cleavage/Functionalization. Chem. Commun. 2018, 54, 6105–6112. 10.1039/c8cc02642d. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Liu D.; Liu S.; Ge Y.; Lan Y.; Chen Y. Visible-Light-Induced Alkoxyl Radicals Enable α-C(Sp3)-H Bond Allylation. iScience 2020, 23, 100755. 10.1016/j.isci.2019.100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin J. F.; Sorensen E. J. A Concise Synthesis of Pleurotin Enabled by a Nontraditional C-H Epimerization. J. Am. Chem. Soc. 2022, 144, 14042. 10.1021/jacs.2c06504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.