Abstract
Cardiovascular disease (CVD) is highly prevalent in an ageing society. The increased incidence and mortality rates of CVD are global issues endangering human health. There is an urgent requirement for understanding the aetiology and pathogenesis of CVD and developing possible interventions for preventing CVD in ageing hearts. It is necessary to select appropriate models and treatment methods. The D‐galactose‐induced cardiac ageing model possesses the advantages of low mortality, short time and low cost and has been increasingly used in the study of cardiovascular diseases in recent years. Therefore, understanding the latest progress in D‐galactose‐induced cardiac ageing is valuable. This review highlights the recent progress and potential therapeutic interventions used in D‐galactose‐induced cardiac ageing in recent years by providing a comprehensive summary of D‐galactose‐induced cardiac ageing in vivo and in vitro. This review may serve as reference literature for future research on age‐related heart diseases.
Keywords: apoptosis, autophagy, cardiac ageing, cardiac function, D‐galactose
1. INTRODUCTION
Ageing is widely defined as a time‐dependent functional decline that affects most organisms. 1 It is characterized by a gradual loss of physiological integrity, resulting in multifaceted structural and functional microcirculation damage, which damages multiple organ functions. 2 , 3 With the increasing elderly population, there is an increase in age‐related diseases, among which cardiovascular disease is the leading cause of health damage and death in the elderly people in the world. 4 The ageing of the heart is closely related to cardiovascular diseases; therefore, it is essential to understand the mechanism of cardiac ageing and to choose a suitable model for ageing research.
Laboratory methods of cardiac ageing, such as natural and induced ageing models, have been used to study cardiovascular diseases. 5 , 6 , 7 The naturally ageing model is most suitable for studying the characteristics of human ageing and the ageing mechanism. However, the attributes of a long feeding cycle, poor health and high mortality limit the broad application of the natural ageing model. Many studies have chosen artificially induced ageing models, such as the D‐galactose‐induced ageing model wherein D‐galactose, a reducing monosaccharide, is continuously injected into animals within a certain period and is bound to cause glucose metabolism disorders in essential organs such as the heart. The concentration of galactose increases in cells, and it is reduced to galactitol by aldose reductase catalysis. The latter accumulates in the cell, resulting in cell swelling, dysfunction, and eventually cell ageing. Alternatively, D‐galactose through another metabolic pathway produces reactive oxygen species (ROS) and advanced glycation end‐products (AGEs), which accelerate the ageing process. 8 Therefore, D‐galactose‐induced ageing models are suitable for studying cardiac ageing.
This review comprehensively summarizes studies on D‐galactose‐induced cardiac ageing submitted to PubMed (https://www.ncbi.nlm.nih.gov/PubMed) between January 2017 and March 2022; they were searched in PubMed using the terms ‘D‐galactose and heart’, or’ D‐galactose and cardiac’. Then, the studies related to heart ageing were screened to review literature on the mechanisms of D‐galactose, potential interventions, and changes in related test indicators in cardiac ageing (Figure 1).
FIGURE 1.
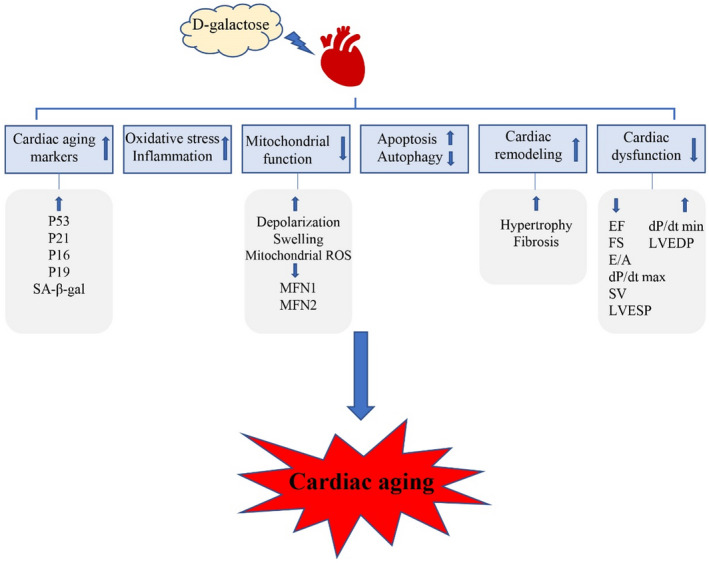
D‐galactose administration induces cardiac ageing. D‐galactose administration increases cardiac ageing markers, oxidative stress, inflammation, apoptosis and cardiac remodelling and reduces mitochondrial function, autophagy and cardiac dysfunction, leading to cardiac ageing
2. EFFECT OF D‐GALACTOSE ADMINISTRATION ON THE MARKERS OF CARDIAC AGEING AND THE CARDIAC INDEX
Numerous studies have shown that D‐galactose administration increases the markers related to cardiac ageing; they are presented in Table 1. The model utilized 2–3‐month‐old mice and rats injected subcutaneously or intraperitoneally with D‐galactose at a dose of 50–200 mg/kg/day, for 6–10 weeks (Table 1). This protocol significantly increases the levels of senescence‐associated β‐galactosidase (SA‐β‐gal) in the cardiac tissues. During cell senescence, there is increased SA‐β‐gal accumulation and activity due to the expansion and increase in lysosomes. D‐galactose administration significantly increases SA‐β‐gal expression in cardiac tissues. 9 , 10 , 11 , 12 , 13 SA‐β‐gal‐positive cells are therefore used as common ageing markers. A series of cell cycle regulatory factors, such as P53, P21, P16 and P19, are also used as ageing markers. As the main participants of the DNA damage response (DDR) path, P53 can trigger a transient cell cycle arrest or permanent cell cycle arrest (cellular senescence). 14 In response to stimuli that induce a DDR, cell growth arrest and senescence occurs through the MDM2‐P53‐P21 and P16‐pRb pathways. 15 D‐galactose administration increases the expression of P53, P21, P19 and P16. 9 , 16 Telomeres are located at the end of eukaryotic cell chromosomes, comprising a tract of tandemly repeated short DNA repeats and associated protective proteins; telomere attrition is closely related to ageing‐related diseases. 17 The telomere stability requires cooperation between multiple factors, including telomerase, TRF1, TRF2 and among others. 18 , 19 , 20 Mice treated with D‐galactose for 10 weeks, at a dose of 100–150 mg/kg/day, showed significantly reduced telomere length and TRF1, TRF2 and TERT expressions in cardiac tissues, with this phenomenon being reversed with the use of protective interventional measures. 16 , 21
TABLE 1.
Effect of D‐galactose administration on the markers of cardiac ageing
| Ref | Study model | Age | Dose (mg/kg/day) | Route | Duration | Intervention | Major findings | Interpretation |
|---|---|---|---|---|---|---|---|---|
| JCR Q2 IF:6.2 9 | C57BL/6 | 3 months | 120 | SC | 8 weeks | Kanglexin(10 and 20 mg/kg/day); Emodin(20 mg/kg/day); 8 weeks; gavage | SA‐β‐gal staining↑——↓; P53, P21↑——↓ | The diastolic dysfunction and cardiac remodelling in mice with D‐gal‐induced ageing were markedly mitigated by KLX and emodin |
| JCR Q2 IF:7.7 10 | Wistar rats | 200–220 g | 150 | SC | 8 weeks | — | SA‐β‐gal staining↑ | D‐galactose‐induced ageing further increased cardiac ageing markers in a time‐dependent manner in the presence of an obese insulin‐resistant condition |
| JCR Q3 IF:3.4 11 | Kunming mice | 6–8 weeks | 500 | SC | 60 days | Polygonatum sibiricum Polysaccharides(PSP); 200, 400 mg/kg/day; 60 days; Ig | SA‐β‐gal staining↑——↓; P53, P21↑——↓ | PSP attenuated D‐gal‐induced cardiac ageing via inhibiting oxidative stress |
| JCR Q3 IF:5.7 12 | Wistar rats | 200–220 g | 150 | SC | 8 weeks (after 12 weeks to induce obese‐insulin resistant condition by eating a high‐fat diet) | Hyperbaric oxygen therapy (HBOT); 100% oxygen (O2) with 250 L/min flow rate; 80 minutes; once daily for 14 days(after 8 weeks d‐gal injection) | SA‐β‐gal staining↑——↓ | HBOT effectively alleviates cardiac dysfunction via attenuating mitochondrial dysfunction in pre‐diabetic rats |
| JCR Q4 IF:2.6 13 | SD rats | 8 weeks | 150 | — | 8 weeks | Alpinate Oxyphyllae Fructus (AOF) 50, 100, 150 mg/kg/day; 10 weeks; orally | SA‐β‐gal↑——↓(50, 100↔); p21↑——↓ | AOF negatively modulated the D‐galactose‐induced cardiac hypertrophy signalling mechanism to attenuate ventricular hypertrophy |
| JCR Q2 IF:5 16 | C57BL/6 | 10‐12 weeks | 100 | SC | 10 weeks | MiR‐21 knockout mice | Telomere length↓——↑; dysregulation of ageing markers (P16INK4a and P19ARF↑——↓; TRF 1, TRF2, and TERT↓——↑) | MiR‐21 knockout had a protective effect against D‐gal‐induced cardiac alterations |
| JCR Q3 IF:5.7 21 | C57BL/6 | 8–10 weeks | 150 | SC | 10 weeks | Natural flavone acacetin; 10, 20, 50 mg/kg/day; 10 weeks; Ig | P53, P21↑——↓; Myocardial telomere length↓——↑ | Acacetin significantly inhibits in vivo cardiac ageing induced by D‐galactose via Sirt1‐mediated activation of Sirt6/AMPK signalling pathway, thereby enhancing mitophagy and preserving mitochondrial function |
| JCR Q3 IF:2.9 22 | ICR mice | 10 weeks | 120 | IP | 6 weeks | Insect tea primary leaf (ITPL); 50 mg/kg, 100 mg/kg; 10 weeks (pretreated 4 weeks and then treated with D‐galactose for 6 weeks); Ig | Cardiac Index↓——↑ | ITPL increased superoxide dismutase, glutathione peroxidase, and glutathione levels and reduced nitric oxide and malondialdehyde levels in the serum in oxidative damaged mice induced by D‐gal |
| JCR Q3 IF:4.4 23 | Kunming mice | 6 weeks | 120 | IP | 6 weeks | Lactobacillus plantarum CQPC11; 1.0 × 109 CFU/kg; four weeks (3rd week to 6th week); Ig | Cardiac Index↓——↑ | LP‐CQPC11 effectively inhibited the reduction in organ indices and alleviated tissue atrophy induced by D‐gal |
| JCR Q1 IF:4 24 | Kunming mice | 10 weeks | 120 | IP | 6 weeks | Lactobacillus plantarum KSFY02; 1.0 × 109 CFU/kg; 10 weeks (pretreat 4 weeks and then 6 weeks during the D‐galactose injections); Ig | Cardiac Index↓——↑ | LP‐KSFY02 effectively inhibited the decrease in organ indices caused by oxidative ageing and alleviated body tissue atrophy caused by D‐galactose |
| JCR Q4 IF:2.4 25 | ICR mice | 10 weeks | 120 | IP | 6 weeks | Apocynum venetum polyphenols(AVP); 50 or 100 mg/kg; 10 weeks (pretreat for 4 weeks and then 6 weeks during the D‐galactose injections); gavage | Cardiac index↓——↑ | AVP inhibited the decline of the Cardiac index caused by oxidative stress‐induced tissue atrophy |
| JCR Q4 IF:2.6 26 | ICR mice | 10 weeks | 120 | IP | 6 weeks | Polyphenol extract of small‐leaved Kuding tea (PSLKDT); 50 or 100 mg/kg; 10 weeks (pretreat for 4 weeks and then 6 weeks during the D‐galactose injection ns); gavage | Cardiac index↓——↑ | PSLKDT inhibited the decline of the cardiac index caused by oxidative stress‐induced tissue atrophy |
| JCR Q4 IF:2.6 27 | SD rats | 3 months | 400 | IP | 14 weeks | Extract of Fructus Cannabis (EFC); 200, 400 mg/kg; 14 weeks; Ig | Cardiac Index↓——↑ | EFC mitigated the features of ageing induced by D‐gal in rats and relieved age‐related memory impairments |
| JCR Q4 IF:2.0 28 | Wistar rats | 180–220 g | 150 | —— | 8 weeks | Mangiferin; 50 mg/kg/day, 100 mg/kg/day; 8 weeks; Ig | Heart index (HI)↑——↓; | Mangiferin suppressed D‐gal‐induced cardiac ageing, and ameliorated cardiac oxidative stress, inflammation, and fibrosis possibly via inhibiting TGF‐β/p38/MK2 signalling pathway |
| JCR Q3 IF:3.6 29 | Kunming mice | 4 weeks | 500 | SC | 4 weeks | Lactobacillus plantarum NJAU‐01; 107, 108, and 109 CFU/ml; 4 weeks; Ig | Heart index↑ | L. plantarum NJAU‐01 alleviated the oxidative damage induced by D‐galactose to the body |
| JCR Q3 IF:3.6 30 | Wistar rats | 170–220 g | 150 | IP | 8 weeks | Resveratrol, 1 mg/kg/day, 8 weeks, Ig; calcitriol, 0.1 μg/kg/day, 8 weeks, IP; resveratrol + calcitriol, 8 weeks | Cardiac klotho↔HW/BW↑——↓ | Co‐administration of resveratrol and vitamin D protected the heart against ageing‐induced damage by the modulation of hemodynamic parameters and antioxidant status of the heart |
| JCR Q2 IF:6.3 32 | C57BL/6 | 6 weeks | 150 | IP | 10 weeks | Camphorquinone (CQ); 5 mg/kg/day; 8 weeks (starting from the 3rd week of D‐Gal injection); IP | P53, P21↑——↓ | CQ possessed antisenescence and cardioprotective properties, and that oxidative‐stress‐induced senescence could be suppressed by AMPK/SIRT1 and autophagy mechanisms |
| JCR Q2 IF:5.3 33 | C57BL/6J | 8 weeks | 200 | SC | 8 weeks | Alginate oligosaccharide(AOS); 50, 100, 150 mg/kg/day; 4 weeks (The last four weeks of the D‐gal injection); Ig | p53, p21↑——↓ | AOS alleviated D‐galactose‐induced cardiac ageing via regulating myocardial mitochondria function and integrity in mice |
| JCR Q3 IF:5.1 38 | C57BL/6J | — | 150 | IP | 8 weeks | CDDO‐imidazolide (CDDO‐Im); 3 μmol/kg/day; 8 weeks; IP | β‐gal, P21↑ | Nrf2 activator CDDO‐Im effectively protected against D‐galactose‐induced cardiac ageing by inhibiting oxidative stress in Nrf2+/+ mice (wild‐type mice) |
| JCR Q2 IF:6.5 40 | C57BL/6 | 8 weeks | 50 | SC | 8 weeks | NaHS; 10, 50, 100 μmol/kg/day; 8 weeks; IP | p16 ↔, p53 ↑——↓(50↔), p21↑——↓(100↔) | NaHS treatment protected against D‐gal‐accelerated ageing by reducing oxidative stress and increasing eNOS expression and NO contents as well as increasing endogenous H2S production |
| JCR Q2 IF:5.9 51 | Wistar rats | 120 ± 20 g | 200 | SC | 42 days | Thymoquinone (20 mg/kg, oral); Curcumin (20 mg/kg, oral); Thymoquinone + Curcumin (20 mg/kg + 20 mg/kg); 42 days | TP53, P21↑——↓ | D‐gal induced histopathological changes in the heart, besides significantly enhancing apoptosis. TQ and Cur defeated the oxidative alterations of the heart activated by D‐gal. The TQ and Cur combination exhibited more protection for brain and heart tissues than TQ or Cur supplemented alone |
| JCR Q2 IF:5.9 53 | C57BL/6 | 6 weeks | 150 | IP | 10 weeks | Licochalcone D (LicoD); 0.5 mg/kg/day; 8 weeks (from the third week of the D‐gal injection); IP | P53, P21↑——↓ | This drug had antioxidant, anti‐ageing, and cardioprotective effects, and the activation of AMPK and autophagy ameliorated oxidative stress‐induced senescenc |
| JCR Q4 IF:2.7 54 | Kunming mice | 8 weeks | 200 | SC | 30 days | 17β‐Estradiol; 0.016 mg/kg/four days; 30 days; SC | pRb/Rb↑——↓ | 17β‐E2 downregulated DNA methylation of the Beclin1, LC3, and Atg5 genes, thereby promoting autophagy and delaying cardiac ageing |
Abbreviations: Ig, intragastric administration; IP, intraperitoneal; SC, subcutaneous; ↑, indicators increased under the action of D‐galactose; ↑——↓, indicators increased under the action of galactose and decreased under the intervention; ↓——↑, indicators decreased under the action of D‐galactose and increased under the intervention; ↔, there was no change in the indicators under D‐galactose or intervention; (↔), under the intervention treatment of this dose, the indicators did not reverse the change caused by D‐galactose; in addition to the special notes in brackets, the intervention works together with D‐galactose.
The cardiac index is an indicator of cardiac ageing. Interestingly, different changes in the cardiac index were observed under different experimental conditions. Intraperitoneal injection of D‐galactose at a dose of 120 mg/kg/day for six weeks induces oxidative stress and ageing, causes tissue atrophy and finally leads to a decline in the cardiac index in mice. 22 , 23 , 24 , 25 , 26 Chen et al. 27 found that intraperitoneal injection of D‐galactose at a dose of 400 mg/kg/day for 14 weeks decreased the cardiac index. However, some studies have reported the opposite result. Intraperitoneal injection of D‐galactose at a dose of 150 mg/kg/day in Wistar rats for eight weeks, or subcutaneous injection of D‐galactose at a dose of 500 mg/kg/day in mice for four weeks increased the cardiac index and led to cardiac fibrosis. 28 , 29 , 30 This difference may be related to the dose and duration of D‐galactose treatment. In conclusion, taking rats as an example, intraperitoneal injection of a higher dose of D‐galactose and for a prolonged period leads to a decrease in the cardiac index. 27 , 30 All discoveries are summarized in Table 1.
3. EFFECT OF D‐GALACTOSE ADMINISTRATION ON CARDIAC OXIDATIVE STRESS AND INFLAMMATION
Previous studies have shown that D‐galactose can aggravate cardiac oxidative stress (Table 2). According to Harman D, the ageing process involves the attack of free radicals (usually produced during cell metabolism) on cellular components. 31 ROS are common free radicals. Excessive D‐galactose administration contributes to increased ROS formation, which subsequently leads to oxidative stress and cardiomyocyte damage. Several studies have assessed the suitability of D‐galactose administration at doses of 50 and 500 mg/kg/day for 6–10 weeks for the establishment of a D‐galactose‐induced cardiac ageing model and reported a close relationship between the initial age of the model animal, dose and duration of treatment. There exists a negative correlation between the age of mice and rats and treatment duration at a dose of 150 or 200 mg/kg/day D‐galactose. 13 , 32 , 33 , 34 , 35 , 36 Moreover, studies have shown that heavier Wistar rats require higher amounts of D‐galactose at the same administration time. 10 , 12 , 28 , 30 , 37
TABLE 2.
Effect of D‐galactose administration on cardiac oxidative stress and inflammation
| Ref | Study model | Age | Dose (mg/kg/day) | Route | Duration | Intervention | Major findings | Interpretation |
|---|---|---|---|---|---|---|---|---|
| JCR Q2 IF:7.7 10 | Wistar rats | 200–220 g | 150 | SC | 4 or 8 weeks | — | MDA↑; TNF‐α↑ | D‐galactose‐induced ageing aggravated cardiac oxidative status in obese insulin‐resistant rats |
| JCR Q3 IF:3.4 11 | Kunming mice | 6–8 weeks | 500 | SC | 60 days | PSP; 200, 400 mg/kg/day; 60 days; Ig | ROS, MDA↑——↓; SOD↓——↑ | PSP attenuated D‐gal‐induced cardiac ageing via inhibiting oxidative stress |
| JCR Q3 IF:5.7 12 | Wistar rats | 200–220 g | 150 | SC | 8 weeks (after 12 weeks to induce obese‐insulin‐resistant condition by eating a high‐fat diet) | HBOT; 100% oxygen (O2) with 250 L/min flow rate; 80 min; once daily for 14 days (after 8 weeks d‐gal injection) | MDA↑——↓; TNF‐α↑——↓ | HBOT effectively alleviated cardiac dysfunction via attenuating mitochondrial dysfunction in pre‐diabetic rats |
| JCR Q4 IF:2.6 13 | SD rats | 8 weeks | 150 | —— | 8 weeks | AOF; 50, 100, 150 mg/kg/day; 10 weeks; orally | HO1 and Cu/ZN SOD↓——↑(50↔) | AOF negatively modulated the D‐galactose‐induced cardiac hypertrophy signalling mechanism to attenuate ventricular hypertrophy |
| JCR Q3 IF:2.9 22 | ICR mice | 10 weeks | 120 | IP | 6 weeks | ITPL; 50 mg/kg, 100 mg/kg; 10 weeks (pretreated 4 weeks and then treated with D‐galactose for 6 weeks); Ig | MDA, NO↑——↓; SOD, GSH, GSH‐Px↓——↑ | ITPL increased superoxide dismutase, glutathione peroxidase, and glutathione levels and reduced nitric oxide and malondialdehyde levels in the serum in oxidative damaged mice induced by D‐gal |
| JCR Q1 IF:4 24 | Kunming mice | 10 weeks | 120 | IP | 6 weeks | Lactobacillus plantarum KSFY02; 1.0 × 109 CFU/kg; 10 weeks(pretreat 4 weeks and then 6 weeks during the D‐galactose injections); Ig | MDA, NO↑——↓; SOD, GSH, G SH‐Px↓——↑ | LP‐KSFY02 effectively inhibited the decrease in organ indices caused by oxidative ageing and alleviated body tissue atrophy caused by D‐galactose |
| JCR Q4 IF:2.0 28 | Wistar rats | 180–220 g | 150 | —— | 8 weeks | Mangiferin; 50 mg/kg/day, 100 mg/kg/day; 8 weeks; Ig | MDA↑——↓; SOD, C AT↓——↑; IL‐1β, IL‐6, T NF‐α↑——↓ | Mangiferin suppressed D‐gal‐induced cardiac ageing, ameliorated cardiac oxidative stress, inflammation and fibrosis possibly via inhibiting TGF‐β/p38/MK2 signalling pathway |
| JCR Q3 IF:3.6 29 | Kunming mice | 4 weeks | 500 | SC | 4 weeks | Lactobacillus plantarum NJAU‐01; 107, 108, and 109 CFU/ml; 4 weeks; Ig | MDA↑——↓; T‐AOC, SOD, GSH‐PX, CAT↓——↑ | L. plantarum NJAU‐01 alleviated the oxidative damage induced by D‐galactose to the body and the strain concentration are related to the antioxidant effect |
| JCR Q3 IF:3.6 30 | Wistar rats | 170–220 g | 150 | IP | 8 weeks | Resveratrol, 1 mg/kg/day, 8 weeks, Ig; Calcitriol, 0.1 μg/kg/day, 8 weeks, IP; Resveratrol + calcitriol; 8 weeks | MDA↑——↓; Cu/ZN SOD, Mn‐SOD, CAT mRNA, A and CAT activity ↓——↑; SOD↔——↑ | Co‐administration of resveratrol and vitamin D protected the heart against ageing‐induced damage by the modulation of hemodynamic parameters and antioxidant status of the heart |
| JCR Q3 IF:2.4 34 | Kunming mice | 7–8 weeks | 200 | SC | 6 weeks | Pine nut protein hydrolysate (PNPH); 150 mg/kg, 300 mg/kg, and 1000 mg/kg; 6 weeks; Ig | MDA↑——↓, SOD↓——↑, GSH‐Px↓——↑(150↔) | PNPH had antioxidant and anti‐ageing activities in vivo. It could reduce the oxidative damage in heart of mice and inhibit lipid peroxidation, thereby delaying the ageing process of mice induced by D‐galactose |
| JCR Q3 IF:6.9 35 | C57BL/6J | 6 weeks | 200 | SC | 10 weeks | 4% H2 inhalation; 4% (v/v) H2 gas for 2 h; 10 weeks | MDA, LPO↑——↓ | H2 prevented oxidative stress in D‐galactose‐induced ageing mice when administered by different routes |
| H2‐rich water drinking; concentration of H2 is above 600 μmol/L and could be drunk freely; 10 weeks | LPO↑——↓ | |||||||
| H2‐rich saline injection; 0.1 ml/10 g bw/day; 10 weeks; IP | — | |||||||
| JCR Q2 IF:6.3 32 | C57BL/6 | 6 weeks | 150 | IP | 10 weeks | CQ; 5 mg/kg/day; 8 weeks (starting from the 3rd week of D‐Gal injection); IP | IL‐1α, IL‐1β, IL‐6↑——↓ | CQ possessed antisenescence and cardioprotective properties, and that oxidative‐stress‐induced senescence was suppressed by AMPK/SIRT1 and autophagy mechanisms |
| JCR Q2 IF:5.3 33 | C57BL/6J | 8 weeks | 200 | SC | 8 weeks | AOS; 50, 100, 150 mg/kg/day; 4 weeks (The last four weeks of the D‐gal injection); Ig | ROS, MDA↑——↓; p47‐phox, p67‐phox and gp91‐phox↑——↓ | AOS alleviated D‐gal‐induced cardiac ageing via regulating myocardial mitochondria function and integrity in mice |
| JCR Q2 IF:4.1 36 | Wistar rats | 18 weeks | 150 | — | 4 weeks | AOF; 100 mg/kg/day; orally administered ADMSCs; administered intravenously with ADMSCs of 107 cells |
Rac‐1, Nox‐2↑——↓; HO‐1 and Cu/ZN SOD↓——↑ IkB↓——↑; p‐NF‐κB, p65, IL‐6↑——↓ |
Synergistic effects of AOF and ADMSCs together possessed therapeutic values against cardiac ageing induced by D‐gal |
| JCR Q2 IF:5.1 37 | Wistarrats | 130–150 g | 200 | IP | 8 weeks | Zeaxanthin heneicosylate(ZH); 250 μg/kg; 4 weeks after 8 weeks d‐gal injection; orally | SOD↓—↑, iNOS↑——↓; IL‐6, NF‐κB↑——↓ | ZH isolated from D. salina ameliorated age‐associated cardiac dysfunction in rats through the activation of retinoid receptors |
| JCR Q3 IF:5.1 38 | C57BL/6J | — | 150 | IP | 8 weeks | CDDO‐Im; 3 μmol/kg/day; 8 weeks; IP | MDA, NO, and PC↑——↓; CAT, SOD, GSH‐Px↓——↑; HO‐1, SOD‐1↓ | Nrf2 activator CDDO‐Im effectively protected against D‐galactose‐induced cardiac ageing by inhibiting oxidative stress in Nrf2+/+ mice (wild‐type mice) |
| JCR Q2 IF: 6.6 39 | ICR mice | 6 weeks | 120 | IP | 6 weeks | Antarctic Ice Microalgae Polysaccharides (AIMP); 50 mg/kg or 100 mg/kg; 6 weeks; Ig | MDA, NO↑——↓; SOD, GSH, GSH‐Px↓——↑ | AIMP effectively inhibited oxidative damage in mice with D‐galactose‐induced oxidative damage |
| JCR Q2 IF:6.5 40 | C57BL/6 | 8 weeks | 50 | SC | 8 weeks | NaHS; 10, 50, 100 μmol/kg/day; 8 weeks; IP |
ROS↑——↓; SOD, GPx, NO↓——↑; CSE↓——↑(10↔); CBS↔ ——↑(10↔)and 3‐MST↔ H2S↓——↑(10, 50↔) |
NaHS treatment protected against D‐gal‐accelerated ageing by reducing oxidative stress and increasing eNOS expression and NO contents as well as increasing endogenous H2S production |
| JCR Q3 IF:1.9 71 | Kunming mice | 18–22 g | 125 | SC | 10 weeks | Dendrobium officinale (DO); DO‐1 (DO juice with a dose of 1 g/kg), DO‐2 (DO Polysaccharide with a dose of 0.32 g/kg); 9 weeks(from ten days after injection of D‐gal); orally | NO↔, SOD↓——↑ | DO had a marked anti‐ageing effect on the D‐galactose‐induced model of ageing |
Abbreviation: Ig, intragastric administration; IP, intraperitoneal; SC, subcutaneous; ↑, indicators increased under the action of D‐galactose; ↓, indicators decreased under the action of D‐galactose; ↑——↓, indicators increased under the action of galactose and decreased under the intervention; ↓——↑, indicators decreased under the action of D‐galactose and increased under the intervention; ↔, there was no change in the indicators under D‐galactose or intervention; (↔), under the intervention treatment of this dose, the indicators did not reverse the change caused by D‐galactose; ↔——↑, the indicators did not change after D‐galactose administration, but increased after intervention; in addition to the special notes in brackets, the intervention works together with D‐galactose.
Evidence suggests that D‐galactose treatment increases oxidants levels and decreases antioxidants levels (Table 2). ROS can attack proteins, lipids and DNA, altering their structures and functions. Many studies have reported that under D‐galactose treatment, the levels of ROS, malondialdehyde (MDA), lactoperoxidase (LPO), and NADPH oxidase 2 (NOX2) are significantly increased, as described in Table 2. Rac‐1 and protein carbonyl (PC) level also increased after D‐galactose injection. 36 , 38 Nrf2, a major stress‐response transcription factor, regulates the expression of heme oxygenase‐1 (HO‐1), Cu/ZN superoxide dismutase (SOD), Nox‐2, Rac‐1 and significantly reduces ageing‐induced oxidative stress in D‐galactose‐induced ageing rat hearts. 36 The activation of nitric oxide synthases (NOS) and nitric oxide (NO) production triggers the production of O2 and OH‐free radicals, leading to cell damage. 39 Previous studies have shown that D‐galactose treatment significantly increases the levels of NO and iNOS. 22 , 37 , 38 , 39 , 40
The antioxidant enzyme system is an antioxidant system and includes SOD, catalase (CAT), glutathione peroxidase (GSH‐Px) and among others. The total antioxidant capacity (T‐AOC) level of mice significantly decreased after subcutaneous administration of 500 mg/kg/day D‐galactose for 4 weeks. 29 As summarized in Table 2, SOD, Cu/ZN SOD, and Mn‐SOD levels, the mRNA expression and activity of CAT, and GSH‐PX levels, which represent antioxidant capacity, significantly decreased following D‐galactose administration. In addition, D‐galactose‐induced cardiac ageing models also show reduced HO‐1 and cystathionine gamma lyase (CSE). 36 , 38 , 40 The other antioxidant system is the no‐enzyme antioxidant system, which includes glutathione (GSH), vitamin C, vitamin E and some tracer elements such as copper and zinc. D‐galactose administration at a dose of 120 mg/kg/day for six weeks has been shown to induce GSH reduction in ICR mice. 22 , 39
Oxidative stress plays a vital role in the activation of transcription factors‐mediated signalling pathways, such as the nuclear factor kappa B (NF‐κB) pathway, which can lead to ageing by regulating the expression of inflammatory genes. 41 D‐galactose through the activation of NF‐κB inflammatory signalling pathway promotes the release of inflammatory factors, accelerating the formation of an inflammatory state and ageing. 36 NF‐κB, a transcription factor, is inhibited and inactivated by inhibitory κB (IκBs) proteins. 42 IκBα inhibits NF‐κB function by binding. Under ROS excess, IκBα is phosphorylated and degraded to form p‐IκBα, and unbound NF‐κB enters the nucleus and activates inflammatory responses. 43 , 44 Studies have shown that p‐NF‐κB, NF‐κB, P65 (a member of the NF‐κB family of proteins), IL‐1α, IL‐1β, and IL‐6 increase, and IkB decreases with D‐galactose administration. 12 , 28 , 32 , 36 , 37
4. EFFECT OF D‐GALACTOSE ADMINISTRATION ON CARDIAC APOPTOSIS AND AUTOPHAGY
As summarized in Table 3, D‐galactose administration aggravates apoptosis and decreases cardiac autophagy. D‐galactose intraperitoneal or subcutaneous injection, at a dose of 150–200 mg/kg/day for 4 and 8 weeks alters apoptosis‐related indices. D‐galactose increased the number of cardiac apoptosis cells. 5 , 10 , 12 , 38 D‐galactose also activates apoptosis pathways, including the mitochondria‐initiated intrinsic pathway and death receptor‐stimulated extrinsic pathway, involved in cardiomyocytes apoptosis. 45 The extrinsic apoptotic pathway is usually triggered by the Fas ligand (FAS‐L), which induces receptor activation and leads to the formation of a death‐inducing signalling complex (DISC). DISC recruits and activates pro‐caspase 8, and then activates the downstream effector caspase‐3, which can directly degrade structural and functional proteins and cause apoptosis. 46 , 47 A study reported that FAS‐L and caspase‐8 levels increased after D‐galactose injection, corroborating the role of D‐galactose in apopttosis. 5 The intrinsic pathway (also known as the mitochondrial pathway) involves many signalling proteins. D‐galactose also induces cardiac cell apoptosis, which is strictly regulated by pro‐apoptotic and anti‐apoptotic factors of Bcl‐2 family. The pro‐apoptotic factor Bax forms holes in the outer mitochondrial membrane, destroying the mitochondrial membrane integrity. This leads to increased mitochondrial cytochrome c release and activation of caspase 3. 48 , 49 , 50 Many studies have shown that D‐galactose‐induced cardiac cell apoptosis is characterized by an increase in Bax and cleaved caspase‐3, and a decrease in the gene and protein expression of anti‐apoptotic factor Blc2. 5 , 10 , 12 , 50 , 51
TABLE 3.
Effect of D‐galactose administration on cardiac autophagy and apoptosis
| Ref | Study model | Age | Dose (mg/kg/day) | Route | Duration | Intervention | Major findings | Interpretation |
|---|---|---|---|---|---|---|---|---|
| JCR Q2 IF:10.5 5 | SD‐rats | 4 weeks | 150 | IP | 8 weeks | Exercise training; In the first two weeks, swimming for 20 min/day, 5 times/week. The duration of swimming was extended to 30 min/−day starting from the 3rd week and to 60/min during the fourth to eighth weeks; 8 weeks | Apoptosis:number of apoptotic cells in the LV↑——↓; Extrinsic pathway: Fas‐L(↑——↔), FADD↔, and caspase‐8↑——↓; Intrinsic pathway: Bax and cleaved caspase‐3(↑——↔), PARP↑——↓ | Exercise training attenuated ageing‐associated cardiac apoptosis and cardiac fibrosis induced by D‐galactose |
| JCR Q2 IF:7.7 10 | Wistar rats | 200–220 g | 150 | SC | 4 weeks or 8 weeks | —— | Apoptosis: Cleaved caspase‐3/caspase‐3↑, TUNEL‐positive cells↑ Autophagy impairment: Beclin‐1↓(4 weeks↔), P62 | D‐galactose‐induced ageing led to a worsening of cardiac cells apoptosis in obese insulin‐resistant rats and exacerbated the impairment of autophagic processes in obese insulin‐resistant rats |
| JCR Q3 IF:5.7 12 | Wistar rats | 200–220 g | 150 | SC | 8 weeks (after 12 weeks to induce obese‐insulin resistant condition by eating a high‐fat diet) | HBOT; 100% oxygen (O2) with 250 L/min flow rate; 80 min; once daily for 14 days (after 8 weeks d‐gal injection) | Apoptosis: TUNEL assay, Bax/Bcl‐2 ratio, and cleaved‐caspase 3/caspase 3↑——↓ Autophagy: Beclin‐1↓——↑, p62 ↑——↓, LC3II↔ | HBOT effectively alleviated cardiac dysfunction via attenuating mitochondrial dysfunction in pre‐diabetic rats |
| JCR Q2 IF:6.3 32 | C57BL/6 | 6 weeks | 150 | IP | 10 weeks | CQ; 5 mg/kg/day; 8 weeks (starting from the 3rd week of D‐Gal injection); IP | Autophagy: LC3II/LC3Iand BECN1↓——↑; SQSTM1↑——↓ | CQ possessed antisenescence and cardioprotective properties, and that oxidative‐stress‐induced senescence was suppressed by AMPK/SIRT1 and autophagy mechanisms |
| JCR Q2 IF:5.3 33 | C57BL/6J | 8 weeks | 200 | SC | 8 weeks | AOS; 50, 100, 150 mg/kg/day; 4 weeks (The last four weeks of the D‐gal injection); Ig | Autophagy: beclin‐1↓——↑; mTOR phosphorylation↑——↓ | AOS alleviated D‐gal‐induced cardiac ageing via regulating myocardial mitochondria function and integrity in mice |
| JCR Q3 IF:5.1 38 | C57BL/6J | — | 150 | IP | 8 weeks | CDDO‐Im; 3 μmol/kg/day; 8 weeks; IP | TUNEL positive↑ | Nrf2 activator CDDO‐Im effectively protected against D‐galactose‐induced cardiac ageing by inhibiting oxidative stress in Nrf2+/+ mice (wild‐type mice) |
| JCR Q2 IF:5.9 51 | Wistar rats | 120 ± 20 g | 200 | SC | 42 days | Thymoquinone (20 mg/kg, oral); Curcumin (20 mg/kg, oral); Thymoquinone + Curcumin (20 mg/kg + 20 mg/kg); 42 days | Apoptosis: cardiac necrosis↑——↓; Necrosis and apoptosis: Casp‐3 and Bax genes, caspase 3 protein↑——↓; Blc2 gene and protein↓——↑ | D‐gal induced histopathological changes in the heart, besides significantly enhancing apoptosis. TQ and Cur defeated the oxidative alterations of the heart activated by D‐gal. The TQ and Cur combination exhibited more protection for brain and heart tissues than TQ or Cur supplemented alone |
| JCR Q2 IF:5.9 53 | C57BL/6 | 6 weeks | 150 | IP | 10 weeks | Lico D; 0.5 mg/kg/day; 8 weeks (From the third week of the D‐gal injection); IP | Autophagy: LC3II, BECN1↓——↑; SQSTM1↑——↓ | This drug had antioxidant, anti‐ageing, and cardioprotective effects, and the activation of AMPK and autophagy ameliorated oxidative stress‐induced senescence |
| JCR Q4 IF:2.7 54 | Kunming mice | 8 weeks | 200 | SC | 30 days | 17β‐Estradiol; 0.016 mg/kg/four days; 30 days; SC | Autophagy: the expression of Beclin1, LC3, and Atg5 gene and protein↓——↑; P62↑——↓; Beclin1, LC3, and Atg5 methylation levels↑——↓ | 17β‐E2 downregulated DNA methylation of the Beclin1, LC3, and Atg5 genes, thereby promoting autophagy and delaying cardiac ageing |
Abbreviations: Bax, Bcl‐2‐associated X protein; Bcl‐2, B‐cell lymphoma 2; Cyt‐c, cytochrome c; FADD, Fas‐associated death domain; Fas, tumour necrosis factor receptor; Ig, intragastric administration; IP, intraperitoneal; mito, mitochondria; SC, subcutaneous; ↑, indicators increased under the action of D‐galactose; ↓, indicators decreased under the action of D‐galactose; ↑——↓, indicators increased under the action of galactose and decreased under the intervention; ↓——↑, indicators decreased under the action of D‐galactose and increased under the intervention; ↔, there was no change in the indicators under D‐galactose or intervention;(↔), under the intervention treatment of this dose, the indicators did not reverse the change caused by D‐galactose; ↑——↔, the indicators increased after D‐galactose administration, but did not change after intervention; in addition to the special notes in brackets, the intervention works together with D‐galactose.
Autophagy, an intracellular catabolic recycling system associated with life and health span extension, is a fundamental process that maintains cardiac and vascular health during ageing. 52 The subcutaneous or intraperitoneal administration of D‐galactose at a dose of 150–200 mg/kg/day for 4–10 weeks, reduced autophagy and accelerated cardiac ageing (Table 3). Several factors regulate the occurrence and development of autophagy. Lower levels of lipidated microtubule‐associated protein light chain 3 (LC3‐II), a marker of autophagosome formation, have been observed in D‐galactose‐induced ageing hearts. 32 , 53 , 54 Beclin1, an essential autophagy adjustment factor, is also reduced in the ageing heart. 10 , 12 , 33 , 54 Beclin1 abnormality in the promoter region is related to the methylation status, and abnormal methylation of autophagy genes can regulate autophagy, inducing the onset and development of cardiac ageing. 54 , 55 Moreover, BECN1 and Atg5 levels significantly decreased, while SQSTM1, P62 and mTOR phosphorylation increased (Table 3). Interestingly, D‐galactose‐induced ageing aggravates autophagy impairment in the cardiac tissues in a time‐dependent manner. 10 Thus, D‐galactose impairs cardiac autophagy, which is essential for maintaining cardiac health during ageing.
5. EFFECT OF D‐GALACTOSE ADMINISTRATION ON CARDIAC MITOCHONDRIA
D‐galactose administration reduces cardiac mitochondrial function. Previous studies have shown that mitochondrial integrity decreases with ageing, demonstrating the importance of mitochondrial dysfunction in cell senescence. 56 In addition to the loss of mitochondrial integrity, age‐related mitochondrial dysfunction is characterized by increased mitochondrial reactive oxygen species (MitoROS) formation, decreased mitochondrial membrane potential (MMP), and mitochondrial biogenesis efficiency, alterations in mitochondrial dynamics, and defective quality control by autophagy. 57 As described in Table 4, subcutaneously injection of D‐galactose at a dose of 125–200 mg/kg/day for 6–10 weeks significantly negatively altered cardiac mitochondrial functions. Some studies have shown that D‐galactose administration significantly increases MitoROS in the heart. 10 , 12 Additionally, D‐galactose also leads to enlarged cardiomyocyte mitochondria with swelling and partial loss of cristae, decreased MMP and increased depolarization of mitochondrial membranes. 10 , 12 , 33 The study demonstrates that mitochondrial biogenesis decreases in the aged hearts, as indicated by the decreases in mtDNA copy number and peroxisome proliferator‐activated receptor‐γ coactivator‐1α(PGC‐1α). 33 SIRT3 also plays a vital role in maintaining mitochondrial bioenergetics. SIRT3 protein expression significantly decreased in D‐galactose‐induced ageing mice. 33 D‐galactose administration at a dose of 150 mg/kg/day, for eight weeks significantly reduced the cardiac mitochondrial fusion marker mitofusin 1 and 2 (MFN1 and MFN2) and increased the levels of dynamin‐related protein 1(Drp1) and pDrp1ser616, indicating mitochondrial dynamics imbalance. 10 , 12 Additionally, impaired mitophagy is associated with an accelerated decline in mitochondrial integrity and heart function. 52 Studies have shown that D‐galactose administration decreased PINK1, Parkin, and Sirt6. 21
TABLE 4.
Effect of D‐galactose administration on the cardiac mitochondria
| Ref | Study model | Age | Dose (mg/kg/day) | Route | Duration | Intervention | Major findings | Interpretation |
|---|---|---|---|---|---|---|---|---|
| JCR Q2 IF:7.7 10 | Wistar rats | 200–220 g | 150 | SC | 4 weeks or 8 weeks | — | Mitochondrial impairment↑; mitochondrial ROS↑; depolarization of mitochondrial membranes and Cardiac mitochondrial swelling↑; MFN1, MFN2↓; pDrp1ser616//Total Drp1↑, Drp1/VDAC↑ | D‐galactose‐induced ageing exacerbated cardiac mitochondrial dysfunction in obese insulin‐resistant rats, and both D‐galactose‐induced ageing and obese insulin resistance led to the impairment of the cardiac mitochondrial fusion process |
| JCR Q3 IF:5.7 12 | Wistar rats | 200–220 g | 150 | SC | 8 weeks (after 12 weeks to induce obese‐insulin resistant condition by eating a high‐fat diet) | HBOT; 100% oxygen (O2) with 250 L/min flow rate; 80 minutes; once daily for 14 days (after 8 weeks d‐gal injection) | Mitochondrial function: mitochondrial ROS level↑——↓; depolarization, and swelling↑——↓Mitochondrial dynamics processes: MFN1 and MFN 2↓——↔ | HBOT effectively alleviated cardiac dysfunction via attenuating mitochondrial dysfunction in pre‐diabetic rats |
| JCR Q3 IF:5.7 21 | C57BL/6 | 8–10 weeks | 150 | SC | 10 weeks | Natural flavone acacetin; 10, 20, 50 mg/kg/day; 10 weeks; Ig | Mitophagy: PINK1, Parkin, and Sirt6 (10 mg/kg/day↔)↓——↑ | Acacetin significantly inhibited in vivo cardiac ageing induced by D‐galactose via Sirt1‐mediated activation of the Sirt6/AMPK signalling pathway, thereby enhancing mitophagy and preserving mitochondrial function |
| JCR Q2 IF:5.3 33 | C57BL/6J | 8 weeks | 200 | SC | 8 weeks | AOS; 50, 100, 150 mg/kg/day; 4 weeks (The last four weeks of the D‐gal injection); Ig | Mitochondria: cardiomyocyte mitochondria were enlarged, swelling and partial loss of cristae↑——↓; MMP↓——↑; PGC‐1α↓——↑; SIRT3↓——↑; mtDNA copy number↓——↑ | AOS alleviated D‐gal‐induced cardiac ageing via regulating myocardial mitochondria function and integrity in mice |
| JCR Q4 IF:3 50 | SD rats | 3 months | 125 | SC | 6 weeks | Melatonin; 10 mg/kg/day; 6 weeks; IP | Mitochondria: Bcl‐2↓——↑; Cyt‐c protein in the cytoplasm↑——↓ | Melatonin exhibited a protective effect on mitochondrial function in a rat model of accelerated ageing |
Abbreviations: Ig, intragastric administration; IP, intraperitoneal; SC, subcutaneous; ↑, indicators increased under the action of D‐galactose; ↓, indicators decreased under the action of D‐galactose; ↑——↓, indicators increased under the action of galactose and decreased under the intervention; ↓——↑, indicators decreased under the action of D‐galactose and increased under the intervention; (↔), under the intervention treatment of this dose, the indicators did not reverse the change caused by D‐galactose; ↓——↔, The indicators decreased after D‐galactose administration, but did not change after intervention; in addition to the special notes in brackets, the intervention works together with D‐galactose.
6. EFFECT OF D‐GALACTOSE ADMINISTRATION ON CARDIAC HISTOPATHOLOGY AND FUNCTION
Cardiac remodelling and left ventricular (LV) dysfunction are the primary manifestations of cardiac ageing. 58 , 59 Several studies have demonstrated an increase in heart weight, cardiac hypertrophy, cardiac fibrosis, and cardiac remodelling in D‐galactose‐induced cardiac ageing models (Table 5). D‐galactose administration of 120–500 mg/kg/day for 4–8 weeks has been reported to cause an increase in the whole heart weight (WHW), left ventricular weight (LVW), and LV wall thickening, an increase in hypertrophic makers such as ANP, BNP, β‐MHC, and MYH7 and a decrease in MYH6. 13 , 28 , 33 , 36 Chang et al. 13 found that the concentric hypertrophy‐related MAPKs such as p‐ERK1/2, p‐c‐JUN, p‐JNK, and p‐p38, pathological hypertrophy‐associated transcription factors such as NFATc3 and p‐GATA4, and the eccentric pathological related protein p‐MEK5, p‐ERK5, and transcription factors STAT3 were significantly increased with D‐galactose administration.
TABLE 5.
Effect of D‐galactose administration on the cardiac morphology and function
| Ref | Study model | Age | Dose (mg/kg/day) | Route | Duration | Intervention | Major findings | Interpretation |
|---|---|---|---|---|---|---|---|---|
| JCR Q2 IF:10.5 5 | SD‐rats | 4 weeks | 150 | IP | 8 weeks | Exercise training; In the first two weeks, swimming for 20 min/day, 5 times/week. The duration of swimming was extended to 30 min/−day starting from the 3rd week and to 60/min during the fourth to eighth weeks; 8 weeks | Cardiac fibrosis and collagen accumulation↑——↓ | Exercise training attenuated ageing‐associated cardiac apoptosis and cardiac fibrosis induced by D‐galactose |
| JCR Q2 IF:6.2 9 | C57BL/6 | 3 months | 120 | SC | 8 weeks | Kanglexin(10 and 20 mg/kg/day); Emodin (20 mg/kg); 8 weeks; Ig | Collagen deposition and fibrosis↑——↓; Cardiac diastolic function↑——↓, E/V↓——↑, LV mass↑——↓, EF, FS ↔ | Diastolic dysfunction and cardiac remodelling in mice with D‐gal‐induced ageing were markedly mitigated by KLX and emodin |
| JCR Q2 IF:7.7 10 | Wistar rats | 200–220 g | 150 | SC | 4 weeks or 8 weeks | —— | LV dysfunction: FS↓; LVESP, dP/dt max and SV↓; LVEDP and dp/dt min↑ Sympathovagal imbalance: The LF/HF ratio of HRV↑ | D‐galactose‐induced ageing aggravated LV dysfunction and sympathovagal imbalance in obese insulin‐resistant rats |
| JCR Q3 IF:3.4 11 | Kunming mice | 6–8 weeks | 500 | SC | 60 days | PSP; 200, 400 mg/kg/day; 60 days; Ig | Disorder of cardiac fibre arrangement↑——↓; CK and cTnT↑——↓ | PSP attenuated D‐gal‐induced cardiac ageing via inhibiting oxidative stress |
| JCR Q3 IF:5.7 12 | Wistar rats | 200–220 g | 150 | SC | 8 weeks (after 12 weeks to induce obese‐insulin resistant condition by eating a high‐fat diet) | HBOT; 100% oxygen (O2) with 250 L/min flow rate; 80 min; once daily for 14 days (after 8 weeks d‐gal injection) | LVEF, FS, LVESP, dP/dt max, SV↓——↑; LVEDP, dP/dt min↑——↓, HR↔; LF/HF↑——↓ | HBOT effectively alleviated cardiac dysfunction via attenuating mitochondrial dysfunction in pre‐diabetic rats |
| JCR Q4 IF:2.6 13 | SD rats | 8 weeks | 150 | —— | 8 weeks | AOF; 50, 100, 150 mg/kg/day; 10 weeks; orally | WHW and LVW↑——↓(50↔); LV wall thickening↑——↓ Cardiac Hypertrophy: p‐ERK1/2, p‐JNK, p‐p38↑——↓(50↔); p‐c‐JUN↑——↓; NFATc3 and p‐GATA4↑——↓(50, 100↔); p‐ERK5↑——↓, p‐MEK5, STAT3↑——↓(50, 100↔); MYH6↓——↑, MYH7↑——↓BNP↑——↓(50↔); LVPWd↑——↓(50↔) | AOF negatively modulated the D‐galactose‐induced cardiac hypertrophy signalling mechanism to attenuate ventricular hypertrophy |
| JCR Q3 IF:5.7 21 | C57BL/6 | 8–10 weeks | 150 | SC | 10 weeks | Natural flavone acacetin; 10, 20, 50 mg/kg/day; 10 weeks; Ig | EF, FS↓——↑; LVAWd, LVPWd↓——↑(10 mg/kg/day↔); LVESD↔ | Acacetin significantly inhibited in vivo cardiac ageing induced by D‐galactose via Sirt1‐mediated activation of Sirt6/AMPK signalling pathway, thereby enhancing mitophagy and preserving mitochondrial function |
| JCR Q4 IF:2.0 28 | Wistar rats | 180–220 g | 150 | —— | 8 weeks | Mangiferin; 50 mg/kg/day, 100 mg/kg/day; 8 weeks; Ig | Cardiac morphology: heart weight ↑——↓; hypertrophic makers ANP, BNP↑——↓, β‐MHC↑——↓(50↔); Cardiac collagen deposition: Masson‐positive and Sirius red‐positive area↑——↓; pro‐fibrogenic proteins TGF‐β, p‐p38/p38, p‐MK2/MK2↑——↓; Col‐I, Col‐III, and α‐SMA↑——↓; Cardiac function: CK and LDH in serum↑——↓ | Mangiferin suppressed D‐gal‐induced cardiac ageing, ameliorated cardiac oxidative stress, inflammation and fibrosis possibly via inhibiting TGF‐β/p38/MK2 signalling pathway |
| JCR Q3 IF:3.6 30 | Wistar rats | 170–220 g | 150 | IP | 8 weeks | Resveratrol, 1 mg/kg/day, gavage, 8 weeks; Calcitriol, 0.1 μg/kg/day, IP, 8 weeks; resveratrol + calcitriol; 8 weeks | Cardiomyocytes size and cardiac fibrosis↑——↓ | Co‐administration of resveratrol and vitamin D protected the heart against ageing‐induced damage by the modulation of hemodynamic parameters and antioxidant status of the heart |
| JCR Q2 IF:5.3 33 | C57BL/6J | 8 weeks | 200 | SC | 8 weeks | AOS; 50, 100, 150 mg/kg/day; 4 weeks (The last four weeks of the D‐gal injection); Ig | Cardia morphology: disordered arrangement of cardiomyocytes and increased intercellular space between cells left ventricular cardiomyocyte area↑——↓; cardiac fibrosis↑——↓ Cardiac function: EF, FS↓——↑; heart rate ↔; LVEDD↔; LVESD↑——↓(low‐dose AOS↔); LVPWd↔; ANP, BNP↑——↓ | AOS alleviated D‐gal‐induced cardiac ageing via regulating myocardial mitochondria function and integrity in mice |
| JCR Q2 IF:4.1 36 | Wistar rats | 18 weeks | 150 | — | 4 weeks | AOF; 100 mg/kg/day; orally administered ADMSCs; administered intravenously with ADMSCs of 107 cells | Size and weight of heart left ventricular weight↑——↓; Cardiac hypertrophy: ANP, BNP↑——↓; Cardiac fibrosis: deposition of collagen↑——↓; CTGF, MMP‐2, and MMP‐9↑——↓; TIMP‐1, −4↓——↑ | Synergistic effects of AOF and ADMSCs together possessed therapeutic values against cardiac ageing induced by D‐gal |
| JCR Q2 IF:5.1 37 | Wistar rats | 130–150 g | 200 | IP | 8 weeks | ZH; 250 μg/kg; 4 weeks after 8 weeks d‐gal injection; orally | An abnormal myocardial architecture decreased cellular volume with an exhibition of spaces between the cells; heart rate, PR, QRS↑——↓; ECG pattern in ST height and T wave has been improved; homocysteine (HS), creatinine kinase isoenzyme (CK‐MB), lactate dehydrogenase (LDH)in serum↑——↓; Cardiac GLUT‐4 in serum↓——↑ | ZH isolated from D. salina ameliorated age‐associated cardiac dysfunction in rats through the activation of retinoid receptors |
| JCR Q4 IF:2.7 54 | Kunming mice | 8 weeks | 200 | SC | 30 days | 17β‐Estradiol; 0.016 mg/kg/four days; 30 days; SC | EF and FS↓——↑; LVVd, LV mass, HR↔; LVVs↑——↓ | 17β‐E2 downregulated DNA methylation of the Beclin1, LC3, and Atg5 genes, thereby promoting autophagy and delaying cardiac ageing |
| JCR Q2 IF:4.1 60 | SD rats | 8 weeks | 150 | — | 8 weeks | AOF; 50, 100, 150 mg/kg/day; 8 weeks; orally | Cardiac fibrosis: MMP 2 and MMP 9↑——↓; cardiac accumulation of collagen fibres↑——↓ (TGFβ1, p‐MEK1/2, p‐ERK1/2, SP1 and CTGF↑——↓); Extracellular collagen degradation: MMP‐9↑——↓ | D‐galactose‐induced ageing activated the process of myocardial fibrosis and caused cardiac remodelling. AOF treatment decreased the risk for myocardial fibrosis via down‐regulation of collagen‐related accumulation/degradative pathways and further maintaining collagen homeostasis |
| JCR Q2 IF:6.5 63 | C57BL/6J | 8 weeks | 125 | SC | 3 months | NaHS; 100 μmol/kg/day; 3 months; IP | EF and FS↓——↑ | H2S Restored the Diurnal Variations of EF and FS in Subacute Ageing Mice |
| JCR Q2 IF:5 16 | C57BL/6 | 10–12 weeks | 100 | SC | 10 weeks | MiR‐21 knockout mice | EF, FS↓——↑ | MiR‐21 knockout had a protective effect against D‐gal‐induced cardiac alterations |
Abbreviations: Ig, intragastric administration; IP, intraperitoneal; SC, subcutaneous; ↑, indicators increased under the action of D‐galactose; ↓, indicators decreased under the action of D‐galactose; ↑——↓, indicators increased under the action of galactose and decreased under the intervention; ↓——↑, indicators decreased under the action of D‐galactose and increased under the intervention; ↔, there was no change in the indicators under D‐galactose or intervention;(↔), under the intervention treatment of this dose, the indicators did not reverse the change caused by D‐galactose; in addition to the special notes in brackets, the intervention works together with D‐galactose.
As summarized in Table 5, D‐galactose administration increases cardiac fibrosis and collagen deposition. 5 , 9 , 28 , 30 , 33 , 36 , 60 After activation, excessive collagen secreted by cardiac fibroblasts is deposited in the extracellular matrix, resulting in myocardial fibrosis. 61 The production of various cytokines and growth factors plays a critical role in the cardiac fibrosis pathway. In the ageing heart, we observed an increase in extracellular matrix (ECM) proteins such as Col‐I, Col‐III, and α‐SMA, as well as pro‐fibrogenic proteins TGF‐β1, p‐p38 and p‐MK2. 28 Moreover, phosphoMEK1/2 (MAP Kinase Kinase), extracellular signal‐regulated kinases 1/2 (ERK1/2), specific protein 1 (SP1), and connective tissue growth factor (CTGF), all elements of a fibrotic response, increased with D‐galactose administration, resulting in excessive ECM protein production and collagen expression. 36 , 60 The homeostatic imbalance of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) lead to cardiac fibrosis. 62 Previous studies have shown that MMP‐2 and MMP‐9 increased and TIMP‐1/4 decreased with D‐galactose administration. 36 , 60
D‐galactose‐induced ageing hearts have been shown to show impaired cardiac fibre arrangement, decreased cellular volume with spaces between cells, increased size of cardiomyocytes and area of left ventricular cardiomyocytes. 11 , 30 , 33 , 37 In summary, tissue regeneration potential is critically affected by the deterioration of the function of stem cells during ageing. D‐galactose‐induced cardiac remodelling can lead to a decline in cardiac function. 36
As summarized in Table 5, cardiac function was significantly reduced in mice and rats injected with D‐galactose at 100–500 mg/kg/day for 4–12 weeks. Markers of cardiac function include homocysteine (HS), creatinine kinase isoenzyme (CK‐MB), lactate dehydrogenase (LDH), glucose transporter 4 (GLUT‐4), and cardiac troponin T (cTnT) and are measured to assess cardiac function. HS, CK, LDH and cTnT levels in serum increased, and GLUT‐4 levels in serum decreased after D‐galactose injection. 11 , 28 , 37 Echocardiography is the standard method used for evaluating cardiac function. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) are common test indicators measured to assess cardiac function. D‐galactose has been shown to induce a decrease in LVEF and FS. 12 , 16 , 21 , 33 , 54 , 63 D‐galactose also worsened LV function as indicated by significantly increased LV end‐diastolic pressure (LVEDP), dP/dt min, and LV volume in end systole (LVVs), and significantly reduced LV end‐systolic pressure (LVESP), dP/dt max, and SV. 10 , 12 , 54 Additionally, D‐galactose injection did not significantly alter heart rate, 12 , 33 , 54 and significantly increased LV end‐systolic diameter (LVESD) and LV mass. 9 , 33 D‐galactose administration at a dose of 150 mg/kg/day for 10 weeks decreased LV anterior wall thickness (LVAWd) and LV posterior wall thickness (LVPWd) in C57 mice. 21 However, another study reported that LVPWd increased after D‐galactose administration. 13 The mitral E/A ratio decreases significantly with the increase in age, indicating impaired ventricular filling. 9 , 62 The low‐frequency /high‐frequency (LF/HF) ratio is measured as an indicator of cardiac sympathovagal balance. At a dose of 150 mg/kg/day, D‐galactose administration for eight weeks significantly increased LF/HF in ageing Wistar rats. 10 , 12 D‐galactose administration resulted in dramatic changes in the electrocardiographic (ECG) measurements in the form of an irregular rhythm of heartbeats, reduced ST height, increased PR and QRS intervals, and negative T wave. 37
7. SUMMARY OF IN VITRO STUDIES ON THE D‐GALACTOSE‐INDUCED CARDIOMYOCYTES AGEING
Hayflick found the limit of cell division through in vitro cell culture and proposed an advanced hypothesis that the finite lifetime of diploid cells in vitro may be an expression of ageing or senescence at the cellular level. 64 Cellular senescence is critical in vivo and is associated with age‐related diseases. 15 Some researchers have used primary cardiomyocytes and H9c2 cell line to study D‐galactose‐induced cardiac ageing. The typical dose of D‐galactose is 10–50 g/L, and the treatment duration is 24–72 h. However, there is one exception, the amount of galactose is tiny, considering that it may be the author's clerical error. 9 D‐galactose increases ageing markers, oxidative stress and inflammation, apoptosis of cardiomyocytes, and decreases cardiomyocyte autophagy. These effects induced by D‐galactose are concentration and time‐dependent. 65 , 66 , 67 , 68 , 69 These results are summarized in Table 6.
TABLE 6.
Summary of in vitro studies on the D‐galactose‐induced cardiac ageing
| Ref | Study model | Dose | Duration | Intervention | Cardiac ageing | Cardiac oxidative stress and inflammation | Apoptosis/autophagy/cardiac mitochondria/cytotoxicity | Interpretation |
|---|---|---|---|---|---|---|---|---|
| JCR Q2 IF:6.2 9 | Mice primary cardiomyocytes | 40 μM (7.2 mg/L) | 24 h | Kanglexin (10 μM and 20 μM), emodin (20 μM) | SA‐β‐gal staining↑——↓; p53, p21↑——↓ | ROS↑——↓ |
Mitochondrial: mitochondrial swelling, myofilament rupture, nuclear constriction, and mitophagy inhibition↑——↓ Mitophagy: P62↑——↓, LC3 II/I, Parkin↓——↑; autolysosome formation↓——↑ |
KLX and emodin treatment reversed the senescence of neonatal mouse cardiomyocytes and mitophagy induced by D‐gal |
| JCR Q3 IF:5.7 21 | H9c2 | 20 g/L | 72 h | Acacetin; (0.3, 1 or 3 μM); 72 h | SA‐β‐gal staining and activity of β‐galactosidase↑——↓; p53, p21↑——↓ | Mitophagy: LC3II/LC3I↓——↑; PINK1 and Parkin↓——↑(0.3 μM↔); depolarization↑—— ↓(0.3 μM↔); sirt6↓——↑ | Acacetin significantly inhibited in vitro cardiac senescence induced by D‐galactose via Sirt1‐mediated activation of the Sirt6/AMPK signalling pathway, thereby enhancing mitophagy and preserving mitochondrial function | |
| JCR Q3 IF:4.4 65 | H9c2 | 10 g/L | 24 h | Klotho; 0.1 μg/ml; 24 h | SA‐β‐gal staining↑——↓; p53, p21, and p16↑——↓ | ROS↑——↓ |
Autophagy: LC3II/LC3I, Beclin1↓——↑; Apoptosis: Bax↑——↓, Bcl2↓——↑, caspase‐3↑——↓; rapid nuclear changes with heterogeneous intensity and chromatin condensation↑——↓ Cytotoxicity: cardiomyocytes size, extensive cytoplasmic vacuolation, and granular cells↑——↓; LDH↑——↓ |
Klotho reduced cardiomyocytes senescence induced by D‐gal |
| JCR Q2 IF:3.5 66 | Rat primary cardiomyocytes | 10 g/L | 48 h | —— | SA‐β‐gal staining↑; p53↑ | Cell viability↓ | D‐gal increased ageing‐related markers and decreased cell viability | |
| JCR Q3 IF:5.7 67 | H9c2 | 10 g/L | 24 h | —— | SA‐β‐gal staining↑; p53, P21↑ | ROS↑ | ROS/NLRP3 pathways contributed to the pathogenesis of cardiocytes ageing | |
| JCR Q2 IF:6.5 68 | H9c2 | 40 g/L | 48 h | Resveratrol;25, 50, 100 μM | SA‐β‐gal staining↑——↓; cardiomyocyte proliferation↓——↑; calcium concentrations↑—— ↓(25 μM↔) | ROS↑—— ↓(25 μM↔) |
Mitochondrial dynamics: Mitochondrial Elongation↑—— ↓(25 μM↔) Mfn1, Mfn2, OPA1↔, Drp1↓——↑(25, 50 μM↔) Proapoptotic: Bcl‐2, BAX↔ |
Resveratrol alleviated cardiomyocytes ageing phenotype and ameliorated mitochondrial elongation via Drp1/Parkin/PINK1 Signalling in Senescent‐Like Cardiomyocytes induced by Carbonyl cyanide 3‐chlorophenylhydrazone |
| JCR Q4 IF:3 69 | H9c2 | 10 g/L | 24 h | Adiponectin (An adiponectin‐overexpression plasmid was transfected into D‐gal‐treated H9c2 cells) | P16, P21↑——↓ | ROS↑——↓; MDA↑——↓ | Adiponectin protected against cardiomyocyte senescence induced by D‐gal via AdipoR1/APPL1 signalling, and it attenuated oxidative stress in senescent H9c2 cells by inhibiting the HO‐1/HMGB1 signalling pathway | |
| JCR Q2 IF:4.6 70 | H9c2 | 10 g/L | 24 h | CD38 knockdown; Nicotinamide dinucleotide (NAD+); 1 mM, 24 h |
CD38 knockdown: SA‐β‐gal‐positive cells↑——↓; p16, p21↑——↓ NAD+:SA‐β‐gal‐positive cells↑——↓ |
CD38 knockdown: ROS↑——↓, NOX4↑——↓; the level of total protein acetylation↑——↓ NAD+: ROS↑——↓; MDA↑——↓; SOD2↓——↑; NOX4↑——↓ |
D‐gal increased cellular senescence and oxidative stress, that CD38 knockdown and NAD+ decreased cellular senescence and oxidative stress | |
| JCR Q2 IF:5.9 72 | H9c2 | 50 g/L | 48 h | Mitochondrial‐targeting antioxidant(MitoTEMPO); 1 μM; 24 h | ROS↑——↓ | Cellular intracellular free Zn2+ ([Zn2+]i), [Zn2+]i in mitochondria ([Zn2+]Mit)↑——↓, [Zn2+]i in S(E)R([Zn2+]SER)↓——↑; MMP↑——↓ |
Mitochondria‐Targeting Antioxidant provided Cardioprotection through regulation of cytosolic and mitochondrial Zn2+ Levels with re‐distribution of Zn2+‐transporters in aged rat cardiomyocytes |
Abbreviations: ↑, indicators increased under the action of D‐galactose; ↓, indicators decreased under the action of D‐galactose; ↑——↓, indicators increased under the action of galactose and decreased under the intervention; ↓——↑, indicators decreased under the action of D‐galactose and increased under the intervention; ↔, there was no change in the indicators under D‐galactose or intervention; (↔), under the intervention treatment of this dose, the indicators did not reverse the change caused by D‐galactose.
8. EFFECTS OF PROTECTIVE INTERVENTIONS ON THE D‐GALACTOSE‐INDUCED CARDIAC AGEING
Numerous studies have reported that many interventions have protective effects against D‐galactose‐induced cardiac ageing in vivo and in vitro. These interventions regulate different signs of ageing. Here, we classify and summarize recent intervention measures from the last five years (Tables 1, 2, 3, 4, 5, 6).
First, cardiac ageing markers are common indicators in the study of cardiac ageing and are the main prognostic markers for measuring the efficacy of interventions. As shown in Table 1, many drugs restore the increase in D‐galactose‐induced markers of cardiac ageing, such as Kanglexin, CQ, Lico D, Thymoquinone, Curcumin, NaHS, AOS, 17β‐Estradiol, PSP, natural flavone acacetin, CDDO‐Im, resveratrol, calcitriol, AOF, Lactobacillus plantarum CQPC11, KSFY02, and NJAU‐01, AVP, PSLKDT, EFC, and Mangiferin. In addition to drug treatment, Bo‐Htay et al. 12 treated rat with HBOT to reduce the number of senescent cells determined by SA‐β‐gal staining. Furthermore, Bei et al. 16 found that miR‐21 knockout has a protective effect against D‐galactose‐induced cardiac alterations.
Second, cardiac oxidative stress is closely related to inflammation and is a critical indicator of the efficacy of interventions on ageing. Some drugs have been used to reduce cardiac oxidative stress and inflammation, including Mangiferin, PNPH, ITPL, AIMP, Lactobacillus plantarum NJAU‐01, ZH, CQ, NaHS, AOS, PSP, CDDO‐Im, AOF, resveratrol, calcitriol, and DO (Table 2). Interestingly, study has shown that 4% H2 inhalation or H2‐rich water drinking can reduce cardiac oxidative stress. 35 As reported in the articles, HBOT has the same effect. 12
Third, apoptosis has been increasingly studied in association with cardiac ageing. Some drugs, such as Thymoquinone, Curcumin, and CDDO‐Im, work through the mitochondria‐initiated intrinsic pathway and the death receptor‐stimulated extrinsic pathway (Table 3). HBOT also has protective effects on D‐galactose‐induced cardiomyocyte apoptosis. 12 Exercise training has also been shown to restore insulin‐like growth factor‐I receptor‐mediated survival signalling in D‐galactose induced‐ageing rats to suppress cardiac apoptosis. 5
Fourth, the relationship between autophagy and cardiac ageing is becoming increasingly clear with growing research. CQ, Lico D, AOS, and 17β‐Estradiol alleviate cardiac ageing by promoting autophagy (Table 3), and HBOT acts similarily. 12
Fifth, medical treatments such as AOS, natural flavone acacetin, melatonin, and HBOT alleviated D‐galactose‐induced cardiac ageing by maintaining myocardial mitochondrial function and integrity (Table 4). As summarized in Table 5, cardiac morphology is essential for maintaining normal cardiac function. Medical treatments, such as Mangiferin, Kanglexin, ZH, AOF, AOS, PSP, resveratrol, and calcitriol, and exercise training, can resist D‐galactose‐induced cardiac remodelling (Table 5). Moreover, Mangiferin, Kanglexin, NaHS, ZH, AOS, 17β‐Estradiol, PSP, natural flavone acacetin, and AOF have been reported to have a positive effect on cardiac function (Table 5). Besides drug therapy, HBOT and miR‐21 knockouts also improve cardiac function. 12 , 16
As shown in Table 6, the interventional therapy of D‐galactose‐induced ageing is very similar in vitro and in vivo. Medical treatments, such as recombinant protein klotho, Nicotinamide dinucleotide (NAD+), mitochondrial‐targeting antioxidant(MitoTEMPO), Kanglexin, emodin, acacetin, and Resveratrol, reduce cardiac ageing markers, oxidative stress, and inflammation (MitoTEMPO has no effect on ageing markers, and acacetin has no effect on oxidative stress and inflammation) (Table 6). Additionally, CD38 knockdown and adiponectin‐overexpression plasmid was used in D‐gal‐treated H9c2 cells to play the same role. 69 , 70 Many studies have shown that klotho, Kanglexin, emodin, acacetin, Resveratrol, and MitoTEMPO have protective effects on D‐galactose‐induced cardiac ageing in vitro by altering apoptosis and Autophagy as well as cardiac mitochondrial function (Table 6).
9. CONCLUSIONS
This review mainly summarizes the literature on D‐galactose‐induced cardiac ageing in the last five years. D‐galactose induces cardiac ageing by increasing ageing‐related markers, upregulating oxidative stress and inflammation, altering autophagy and apoptosis of cardiomyocytes, remodelling cardiac morphology, and impairing cardiac function. We postulate that D‐galactose is a reliable for establishing ageing models for studying ageing‐related diseases and anti‐ageing therapeutic interventions.
10. LATEST ADVANCES
Although this review is not the first to summarize D‐galactose‐induced cardiac ageing (see article for details), herein, we discuss the recent advances in D‐galactose‐induced cardiac ageing research, which has rapidly increased in recent years. We believe that it is necessary to provide an updated review in this field, particularly recent advancements, including the indicators of cardiac function that are rarely discussed in previous reviews. First, the role of autophagy in D‐galactose‐induced cardiac ageing has been widely reported in recent studies. Table 3 summarizes the changes in autophagy after D‐galactose‐induced cardiac ageing. Table 5 describes the changes in cardiac morphology and cardiac function‐related indicators, which is a detailed and comprehensive improvement to previous reviews. Finally, recently, intervention options for D‐galactose‐induced cardiac ageing have become increasingly diverse. Therefore, we marked the protocol of intervention in each table, and summarized the efficacy of various intervention measures on different indicators at the end of the article for convenience.
AUTHOR CONTRIBUTIONS
Sui‐sui Wang: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); resources (lead); software (lead); writing – original draft (lead); writing – review and editing (lead). Xu Zhang: Data curation (equal); supervision (supporting); writing – review and editing (equal). Ze‐zhi Ke: Investigation (equal); writing – review and editing (supporting). Xiu‐yun Wen: Data curation (supporting); investigation (supporting). Wei‐dong Li: Data curation (supporting); writing – review and editing (supporting). WenBin Liu: Investigation (supporting); writing – review and editing (supporting). Xiao‐dong Zhuang: Resources (equal); writing – review and editing (equal). Li‐zhen Liao: Resources (equal); writing – review and editing (equal).
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Funding source: This work was supported by the National Natural Science Foundation of China, No. 82104991, and the Teaching Quality and Teaching Reform Project of Guangdong Province, No.223.
Wang S‐s, Zhang X, Ke Z‐z, et al. D‐galactose‐induced cardiac ageing: A review of model establishment and potential interventions. J Cell Mol Med. 2022;26:5335‐5359. doi: 10.1111/jcmm.17580
Sui‐sui Wang and Xu Zhang contributed equally to this study.
Contributor Information
Xiao‐dong Zhuang, Email: zhuangxd3@mail.sysu.edu.cn.
Li‐zhen Liao, Email: liaolizhen@gdpu.edu.cn.
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.
REFERENCES
- 1. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of ageing. Cell. 2013;153(6):1194‐1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular Ageing. Circ Res. 2018;123(7):849‐867. doi: 10.1161/circresaha.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular Ageing, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):931‐941. doi: 10.1016/j.jacc.2019.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and National Burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1‐25. doi: 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lay IS, Kuo WW, Shibu MA, et al. Exercise training restores IGFIR survival signaling in D‐galactose induced‐ageing rats to suppress cardiac apoptosis. J Adv Res. 2021;28:35‐41. doi: 10.1016/j.jare.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen K, Wang S, Sun QW, Zhang B, Ullah M, Sun Z. Klotho deficiency causes heart ageing via impairing the Nrf2‐GR pathway. Circ Res. 2021;128(4):492‐507. doi: 10.1161/circresaha.120.317348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeh CH, Shen ZQ, Hsiung SY, et al. Cisd2 is essential to delaying cardiac ageing and to maintaining heart functions. PLoS Biol. 2019;17(10):e3000508. doi: 10.1371/journal.pbio.3000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu J, Wu DM, Zheng YL, et al. Ursolic acid attenuates D‐galactose‐induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF‐κB pathway activation. Cereb Cortex. 2010;20(11):2540‐2548. doi: 10.1093/cercor/bhq002 [DOI] [PubMed] [Google Scholar]
- 9. Li HM, Liu X, Meng ZY, et al. Kanglexin delays heart ageing by promoting mitophagy. Acta Pharmacol Sin. 2022;43(3):613‐623. doi: 10.1038/s41401-021-00686-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bo‐Htay C, Shwe T, Higgins L, et al. Ageing induced by D‐galactose aggravates cardiac dysfunction via exacerbating mitochondrial dysfunction in obese insulin‐resistant rats. GeroScience. 2020;42(1):233‐249. doi: 10.1007/s11357-019-00132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma W, Wei S, Peng W, et al. Antioxidant effect of Polygonatum sibiricum polysaccharides in D‐galactose‐induced heart ageing mice. Biomed Res Int. 2021;2021:6688855. doi: 10.1155/2021/6688855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bo‐Htay C, Shwe T, Jaiwongkam T, et al. Hyperbaric oxygen therapy effectively alleviates D‐galactose‐induced‐age‐related cardiac dysfunction via attenuating mitochondrial dysfunction in pre‐diabetic rats. Aging. 2021;13(8):10955‐10972. doi: 10.18632/ageing.202970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang YM, Chang HH, Lin HJ, et al. Inhibition of cardiac hypertrophy effects in D‐galactose‐induced senescent hearts by Alpinate Oxyphyllae fructus treatment. Evid Based Complement Alternat Med. 2017;2017:2624384. doi: 10.1155/2017/2624384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodier F, Campisi J, Bhaumik D. Two faces of p53: ageing and tumor suppression. Nucleic Acids Res. 2007;35(22):7475‐7484. doi: 10.1093/nar/gkm744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729‐740. doi: 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 16. Bei Y, Wu X, Cretoiu D, et al. miR‐21 suppression prevents cardiac alterations induced by D‐galactose and doxorubicin. J Mol Cell Cardiol. 2018;115:130‐141. doi: 10.1016/j.yjmcc.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 17. Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in ageing, disease risks, and protection. Science. 2015;350(6265):1193‐1198. doi: 10.1126/science.aab3389 [DOI] [PubMed] [Google Scholar]
- 18. Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66(3):407‐425, table of contents. doi: 10.1128/mmbr.66.3.407-425.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385(6618):740‐743. doi: 10.1038/385740a0 [DOI] [PubMed] [Google Scholar]
- 20. van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end‐to‐end fusions. Cell. 1998;92(3):401‐413. doi: 10.1016/s0092-8674(00)80932-0 [DOI] [PubMed] [Google Scholar]
- 21. Hong YX, Wu WY, Song F, Wu C, Li GR, Wang Y. Cardiac senescence is alleviated by the natural flavone acacetin via enhancing mitophagy. Ageing. 2021;13(12):16381‐16403. doi: 10.18632/ageing.203163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi R, Chen X, Li W, Mu J, Tan F, Zhao X. Preventive effect of insect tea primary leaf (Malus sieboldii [regal] Rehd.) extract on D‐galactose‐induced oxidative damage in mice. Food Sci Nutr. 2020;8(9):5160‐5171. doi: 10.1002/fsn3.1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qian Y, Zhang J, Zhou X, et al. Lactobacillus plantarum CQPC11 isolated from Sichuan pickled cabbages antagonizes D‐galactose‐induced oxidation and aAgeing in mice. Molecules. 2018;23(11):3026. doi: 10.3390/molecules23113026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao X, Yi R, Zhou X, et al. Preventive effect of Lactobacillus plantarum KSFY02 isolated from naturally fermented yogurt from Xinjiang, China, on D‐galactose‐induced oxidative ageing in mice. J Dairy Sci. 2019;102(7):5899‐5912. doi: 10.3168/jds.2018-16033 [DOI] [PubMed] [Google Scholar]
- 25. Guo H, Kuang Z, Zhang J, Zhao X, Pu P, Yan J. The preventive effect of Apocynum venetum polyphenols on D‐galactose‐induced oxidative stress in mice. Exp Ther Med. 2020;19(1):557‐568. doi: 10.3892/etm.2019.8261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu B, Ma R, Zhang J, Sun P, Yi R, Zhao X. Preventive effect of small‐leaved Kuding tea (Ligustrum robustum [Roxb.] bl.) polyphenols on D‐galactose‐induced oxidative stress and ageing in mice. Evid Based Complement Alternat Med. 2019;2019:3152324. doi: 10.1155/2019/3152324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen NY, Liu CW, Lin W, et al. Extract of fructus cannabis ameliorates learning and memory impairment induced by D‐galactose in an aAgeing rats model. Evid Based Complement Alternat Med. 2017;2017:4757520. doi: 10.1155/2017/4757520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng J, Ren C, Cheng R, et al. Mangiferin ameliorates cardiac fibrosis in D‐galactose‐induced ageing rats by inhibiting TGF‐β/p38/MK2 signaling pathway. Korean J Physiol Pharmacol. 2021;25(2):131‐137. doi: 10.4196/kjpp.2021.25.2.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ge Q, Yang B, Liu R, et al. Antioxidant activity of Lactobacillus plantarum NJAU‐01 in an animal model of ageing. BMC Microbiol. 2021;21(1):182. doi: 10.1186/s12866-021-02248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dehghani A, Hafizibarjin Z, Najjari R, Kaseb F, Safari F. Resveratrol and 1,25‐dihydroxyvitamin D co‐administration protects the heart against D‐galactose‐induced ageing in rats: evaluation of serum and cardiac levels of klotho. Aging Clin Exp Res. 2019;31(9):1195‐1205. doi: 10.1007/s40520-018-1075-x [DOI] [PubMed] [Google Scholar]
- 31. Harman D. Ageing: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298‐300. doi: 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 32. Maharajan N, Cho GW. Camphorquinone promotes the antisenescence effect via activating AMPK/SIRT1 in stem cells and D‐galactose‐induced aAgeing mice. Antioxidants. 2021;10(12):1916. doi: 10.3390/antiox10121916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng W, Liu J, Wang S, et al. Alginate oligosaccharide alleviates D‐galactose‐induced cardiac ageing via regulating myocardial mitochondria function and integrity in mice. J Cell Mol Med. 2021;25(15):7157‐7168. doi: 10.1111/jcmm.16746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Z, Shi Y, Liu H, Jia Q, Liu Q, Tu J. Purification and identification of pine nut (Pinus yunnanensis Franch.) protein hydrolysate and its antioxidant activity in vitro and in vivo. Chem Biodivers. 2021;18(1):e2000710. doi: 10.1002/cbdv.202000710 [DOI] [PubMed] [Google Scholar]
- 35. Liu B, Xie Y, Chen J, et al. Protective effect of molecular hydrogen following different routes of administration on D‐galactose‐induced ageing mice. J Inflamm Res. 2021;14:5541‐5550. doi: 10.2147/jir.S332286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin HJ, Ramesh S, Chang YM, et al. D‐galactose‐induced toxicity associated senescence mitigated by alpinate oxyphyllae fructus fortified adipose‐derived mesenchymal stem cells. Environ Toxicol. 2020;36(1):86‐94 doi: 10.1002/tox.23014 [DOI] [PubMed] [Google Scholar]
- 37. El‐Baz FK, Hussein RA, Saleh DO, Abdel Jaleel GAR. Zeaxanthin isolated from Dunaliella salina microalgae ameliorates age associated cardiac dysfunction in rats through stimulation of retinoid receptors. Mar Drugs. 2019;17(5):6839. doi: 10.3390/md17050290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang X, Jia J, Ding L, Yu Z, Qu C. The role of Nrf2 in D‐galactose‐induced cardiac ageing in mice: involvement of oxidative stress. Gerontology. 2021;67(1):91‐100. doi: 10.1159/000510470 [DOI] [PubMed] [Google Scholar]
- 39. Yi R, Deng L, Mu J, Li C, Tan F, Zhao X. The impact of Antarctic ice microalgae polysaccharides on D‐galactose‐induced oxidative damage in mice. Front Nutr. 2021;8:651088. doi: 10.3389/fnut.2021.651088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu W, Hou CL, Mu XP, et al. H(2)S donor NaHS changes the production of endogenous H(2)S and NO in D‐galactose‐induced accelerated ageing. Oxid Med Cell Longev. 2017;2017:5707830. doi: 10.1155/2017/5707830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Azman KF, Safdar A, Zakaria R. D‐galactose‐induced liver ageing model: its underlying mechanisms and potential therapeutic interventions. Exp Gerontol. 2021;150:111372. doi: 10.1016/j.exger.2021.111372 [DOI] [PubMed] [Google Scholar]
- 42. Vlantis K, Pasparakis M. Role of TNF in pathologies induced by nuclear factor kappaB deficiency. Curr Dir Autoimmun. 2010;11:80‐93. doi: 10.1159/000289198 [DOI] [PubMed] [Google Scholar]
- 43. Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF‐κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res. 2011;89(1):129‐138. doi: 10.1093/cvr/cvq274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maier HJ, Schips TG, Wietelmann A, et al. Cardiomyocyte‐specific IκB kinase (IKK)/NF‐κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2012;109(29):11794‐11799. doi: 10.1073/pnas.1116584109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bo‐Htay C, Palee S, Apaijai N, Chattipakorn SC, Chattipakorn N. Effects of D‐galactose‐induced ageing on the heart and its potential interventions. J Cell Mol Med. 2018;22(3):1392‐1410. doi: 10.1111/jcmm.13472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liou CM, Tsai SC, Kuo CH, Ting H, Lee SD. Cardiac Fas‐dependent and mitochondria‐dependent apoptosis after chronic cocaine abuse. Int J Mol Sci. 2014;15(4):5988‐6001. doi: 10.3390/ijms15045988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bang S, Jeong EJ, Kim IK, Jung YK, Kim KS. Fas‐ and tumor necrosis factor‐mediated apoptosis uses the same binding surface of FADD to trigger signal transduction. A typical model for convergent signal transduction. J Biol Chem. 2000;275(46):36217‐36222. doi: 10.1074/jbc.M006620200 [DOI] [PubMed] [Google Scholar]
- 48. Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol. 2001;2(1):67‐71. doi: 10.1038/35048073 [DOI] [PubMed] [Google Scholar]
- 49. Bank A, Wang P, Du C, Yu J, Zhang L. SMAC mimetics sensitize nonsteroidal anti‐inflammatory drug‐induced apoptosis by promoting caspase‐3‐mediated cytochrome c release. Cancer Res. 2008;68(1):276‐284. doi: 10.1158/0008-5472.Can-07-5242 [DOI] [PubMed] [Google Scholar]
- 50. Guo XH, Li YH, Zhao YS, Zhai YZ, Zhang LC. Anti‐ageing effects of melatonin on the myocardial mitochondria of rats and associated mechanisms. Mol Med Rep. 2017;15(1):403‐410. doi: 10.3892/mmr.2016.6002 [DOI] [PubMed] [Google Scholar]
- 51. El‐Far AH, Elewa YHA, Abdelfattah EA, et al. Thymoquinone and curcumin defeat ageing‐associated oxidative alterations induced by D‐galactose in Rats' brain and heart. Int J Mol Sci. 2021;22(13). doi: 10.3390/ijms22136839 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. Abdellatif M, Sedej S, Carmona‐Gutierrez D, Madeo F, Kroemer G. Autophagy in cardiovascular ageing. Circ Res. 2018;123(7):803‐824. doi: 10.1161/circresaha.118.312208 [DOI] [PubMed] [Google Scholar]
- 53. Maharajan N, Ganesan CD, Moon C, Jang CH, Oh WK, Cho GW. Licochalcone D ameliorates oxidative stress‐induced senescence via AMPK activation. Int J Mol Sci. 2021;22(14):7324. doi: 10.3390/ijms22147324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ye L, Huang J, Xiang X, et al. 17β‐estradiol alleviates cardiac ageing induced by D‐galactose by downregulating the methylation of autophagy‐related genes. Steroids. 2021;170:108829. doi: 10.1016/j.steroids.2021.108829 [DOI] [PubMed] [Google Scholar]
- 55. Peng J, Yang Q, Li AF, et al. Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE−/− mice. Oncotarget. 2016;7(47):76423‐76436. doi: 10.18632/oncotarget.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483‐495. doi: 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 57. Manolis AS, Manolis AA, Manolis TA, et al. Mitochondrial dysfunction in cardiovascular disease: current status of translational research/clinical and therapeutic implications. Med Res Rev. 2021;41(1):275‐313. doi: 10.1002/med.21732 [DOI] [PubMed] [Google Scholar]
- 58. Cheng S, Xanthakis V, Sullivan LM, et al. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham heart study. Circulation. 2010;122(6):570‐578. doi: 10.1161/circulationaha.110.937821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shah AM, Claggett B, Kitzman D, et al. Contemporary assessment of left ventricular diastolic function in older adults: the atherosclerosis risk in communities study. Circulation. 2017;135(5):426‐439. doi: 10.1161/circulationaha.116.024825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang YM, Tamilselvi S, Lin HJ, et al. Alpinia oxyphylla Miq extract ameliorates cardiac fibrosis associated with D‐galactose induced ageing in rats. Environ Toxicol. 2019;34(2):172‐178. doi: 10.1002/tox.22671 [DOI] [PubMed] [Google Scholar]
- 61. Li L, Zhao Q, Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018;68–69:490‐506. doi: 10.1016/j.matbio.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 62. Bonnema DD, Webb CS, Pennington WR, et al. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail. 2007;13(7):530‐540. doi: 10.1016/j.cardfail.2007.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang H, Dai J, Tian D, et al. Hydrogen sulfide restored the diurnal variation in cardiac function of aAgeing mice. Oxid Med Cell Longev. 2021;2021:8841575. doi: 10.1155/2021/8841575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hayflick L. The limited IN vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614‐636. doi: 10.1016/0014-4827(65)90211-9 [DOI] [PubMed] [Google Scholar]
- 65. Li‐Zhen L, Chen ZC, Wang SS, Liu WB, Zhuang XD. Klotho deficiency causes cardiac ageing by impairing autophagic and activating apoptotic activity. Eur J Pharmacol. 2021;911:174559. doi: 10.1016/j.ejphar.2021.174559 [DOI] [PubMed] [Google Scholar]
- 66. Zhang X, Cheng L, Xu L, et al. The lncRNA, H19 mediates the protective effect of hypoxia postconditioning against hypoxia‐reoxygenation injury to senescent cardiomyocytes by targeting microRNA‐29b‐3p. Shock (Augusta, Ga). 2019;52(2):249‐256. doi: 10.1097/shk.0000000000001213 [DOI] [PubMed] [Google Scholar]
- 67. Liao LZ, Chen ZC, Wang SS, Liu WB, Zhao CL, Zhuang XD. NLRP3 inflammasome activation contributes to the pathogenesis of cardiocytes ageing. Ageing. 2021;13(16):20534‐20551. doi: 10.18632/ageing.203435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ren X, Chen L, Xie J, et al. Resveratrol ameliorates mitochondrial elongation via Drp1/parkin/PINK1 signaling in senescent‐like cardiomyocytes. Oxid Med Cell Longev. 2017;2017:4175353‐4175320. doi: 10.1155/2017/4175353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu R, Meng J, Lou D. Adiponectin inhibits D‐gal‐induced cardiomyocyte senescence via AdipoR1/APPL1. Mol Med Rep. 2021;24(4):719. doi: 10.3892/mmr.2021.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang LF, Cao Q, Wen K, et al. CD38 deficiency alleviates D‐galactose‐induced myocardial cell senescence through NAD(+)/Sirt1 signaling pathway. Front Physiol. 2019;10:1125. doi: 10.3389/fphys.2019.01125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liang CY, Liang YM, Liu HZ, et al. Effect of Dendrobium officinale on D‐galactose‐induced ageing mice. Chin J Integr Med. 2017. doi: 10.1007/s11655-016-2631-x [DOI] [PubMed] [Google Scholar]
- 72. Olgar Y, Tuncay E, Turan B. Mitochondria‐targeting antioxidant provides Cardioprotection through regulation of cytosolic and mitochondrial Zn(2+) levels with Re‐distribution of Zn(2+)‐transporters in aged rat cardiomyocytes. Int J Mol Sci. 2019;20(15):3783. doi: 10.3390/ijms20153783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.


