Abstract
The hallmark of Legionnaires’ disease is intracellular replication of Legionella pneumophila within cells in the alveolar spaces. Cytopathogenicity of this bacterium to the host cell has been well demonstrated, but the mechanisms of host cell death due to infection by L. pneumophila are not well understood. In this study, induction of apoptosis in macrophages and alveolar epithelial cells by L. pneumophila during early stages of infection was confirmed by using multiple criteria, including DNA fragmentation by agarose gel electrophoresis, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling, surface exposure of phosphatidylserine, and cellular morphology by transmission electron microscopy. Induction of nuclear apoptosis in L. pneumophila-infected macrophages is mediated by activation of the caspase cascade death machinery. We provide genetic and biochemical evidence that L. pneumophila-induced apoptosis in macrophages and alveolar epithelial cells does not require intracellular bacterial replication or new protein synthesis. In addition, extracellular L. pneumophila is capable of inducing apoptosis. Furthermore, induction of apoptosis by L. pneumophila correlates with cytopathogenicity. We conclude that L. pneumophila-induced apoptosis in macrophages and alveolar epithelial cells plays an important role in cytopathogenicity to the host cell during early stages of infection.
The Legionnaires’ disease bacterium, Legionella pneumophila, is one of the most common etiologic agents of bacterial pneumonia (27). In the aquatic environment, L. pneumophila is a parasite of at least 13 species of amoebae and ciliated protozoa (6). Upon transmission to humans through environmentally generated aerosols, the bacteria invade and replicate within alveolar macrophages and monocytes that have been recruited into the alveolar spaces (see references 1 and 6 for recent reviews). In addition, it has been recently shown that intracellular replication within type I and II alveolar epithelial cells may contribute to the pathogenesis of Legionnaires’ disease (18).
Initial bacterial attachment to mammalian macrophages, alveolar epithelial cells, and protozoa is mediated, at least in part, by type IV pili designated CAP (40). Following entry into mammalian and protozoan cells, L. pneumophila replicates within a ribosome-studded phagosome (1, 6). Within this unique niche, the bacterium undergoes dramatic alterations in gene expression, which may be required for interaction with the host cell, for adaptation to the intracellular microenvironment, and possibly for exiting the host cell upon termination of intracellular replication (2–5, 7, 10, 16, 21).
The hallmark of Legionnaires’ disease is intracellular replication of L. pneumophila within phagosomes that are blocked from fusion to the lysosomes (23). This alteration in endocytic trafficking has been recently shown to be mediated by L. pneumophila proteins encoded by the pmi, mil, dot, and icm loci (19, 20, 39, 41). Mutations in many of these loci render the bacteria defective in both cytopathogenicity and intracellular replication within macrophages (19, 20, 39, 41). Interestingly, some of the pmi and mil loci that are indispensable for infectivity of macrophages are not required for infectivity of alveolar epithelial cells (18). Although cytopathogenicity of L. pneumophila to macrophages and alveolar epithelial cells has been well documented, the mechanisms of cell death as a result of bacterial infection are not well understood (1, 6, 24, 31, 35).
Apoptosis and necrosis are the two commonly observed types of cell death. While necrosis is characterized as accidental cell death due to physical damage, apoptosis is strictly regulated suicide program within the dying cell manifesting morphological and biochemical features distinct from those of necrosis (8, 14). A cascade of activation of a family of cysteine proteases (caspases) that specifically cleave proteins after aspartate (Asp) residues is required for induction of apoptosis (38). A number of intracellular pathogens are capable of manipulating host cell apoptotic pathways. The obligate intracellular bacteria Chlamydia trachomatis and Rickettsia rickettsii inhibit apoptosis in the host cells, allowing their intracellular growth and persistence (13, 15). The facultative intracellular pathogenic bacterium Shigella flexneri induces apoptosis in macrophages, which is accompanied by activation of caspase-1/ICE (43). In contrast, the bacterium Mycoplasma penetrans fragments DNA of the host cell by secretion of a bacterial nuclease that specifically cleaves host cell DNA into internucleosomal fragments of 180 to 200 bp (9). Whether other intracellular bacterial pathogens induce apoptosis by bacterial nucleases or through the activation of caspases is not known.
Induction of apoptosis in macrophages by L. pneumophila has been observed in HL-60 human-derived macrophages after 24 to 48 h of infection when the cells were infected at a multiplicity of infection (MOI) of 10 to 100 (34). On the other hand, when a high MOI was used for infection, necrosis was evident at 20 to 60 min after infection (24, 26). Apoptosis was not detected during this period when infected cells were examined by electron microscopy (26). Therefore, whether L. pneumophila induces apoptosis in the host cell within 24 h after infection at a lower MOI is not known. Since apoptosis may not be recognized by a single strategy or at a time too early to manifest it, multiple criteria and a broad time frame need to be considered in order to detect it.
In this report, we provide genetic and biochemical evidence that L. pneumophila-induced apoptosis in macrophages and alveolar epithelial cells occurs within a few hours of infection prior to intracellular bacterial replication and correlates with cytopathogenicity. Furthermore, extracellular bacteria are capable of inducing apoptosis and are also cytopathogenic to the host cell. We demonstrate that L. pneumophila-induced apoptosis is mediated by activation of the caspase cascade.
MATERIALS AND METHODS
Bacterial strains and growth media.
Virulent strain L. pneumophila AA100 and the characterization of the pmi mutants of L. pneumophila have been described previously (19). L. pneumophila strains were grown on buffered charcoal yeast extract (BCYE) agar plates or in buffered yeast extract broth supplemented with 50-μg/ml kanamycin for the mutant strains.
U937 macrophages and WI-26 alveolar epithelial cells.
The human macrophage-like cell line U937 was maintained and differentiated by using phorbol 12-myristate 13-acetate (Sigma Chemical Co., St. Louis, Mo.) as we described previously (19). Human type I alveolar epithelial cells (WI-26 VA4; American Type Culture Collection) were maintained in supplemented minimal essential medium.
Cytopathogenicity of L. pneumophila to U937 macrophages and WI-26 alveolar epithelial cells.
L. pneumophila strains were grown on BCYE plates for 3 days prior to infection of U937 macrophages or WI-26 alveolar epithelial cells. Infection was performed, in triplicate, in 96-well plates containing 105 cells/well at an MOI of 0.5, 5, or 50 for 1 h, followed by three washes of extracellular unattached bacteria and further incubation at 37°C in 5% CO2. At several time intervals, the monolayers were treated for 4 h with 10% Alamar Blue dye (Alamar Bioscience Inc., Sacramento, Calif.) as we described previously (7). Viability of the monolayers was determined by measurement of the optical density (OD) of Alamar Blue-treated monolayers by using a VMAX Kinetic Microplate Reader (Molecular Devices, Menlo Park, Calif.) and expressed as percent cytopathogenicity compared to uninfected cells by using the formula [1 − (mean OD of treated cells/mean OD of nontreated cells)] × 100%. To examine the effect of inhibition of bacterial protein synthesis and intracellular replication by chloramphenicol or erythromycin on the cytopathogenicity of L. pneumophila to U937 macrophages, the bacteria and cells were separately pretreated with chloramphenicol (20 or 100 μg/ml) or erythromycin (8 or 80 μg/ml) (Sigma) for 1 h and the treated bacteria were used to infect the treated cells at an MOI of 50 in the presence of the respective antibiotic. To confirm that protein synthesis of L. pneumophila within the host cells was inhibited by the antibiotics, infected monolayers were pretreated with cycloheximide (200 μg/ml) to inhibit host cell protein synthesis (3) and then incubated with [35S]methionine (150 μCi/ml) (ICN Pharmaceuticals, Irvine, Calif.) to label bacterial proteins in the presence or absence of the antibiotics as we described previously (2). To examine the effect of inhibition of bacterial uptake by cytochalasin D (CytD) on the cytopathogenicity of L. pneumophila to U937 macrophages, the cells were treated with 1-μg/ml CytD and infected at an MOI of 50 as described previously (20). Inhibition of bacterial uptake was confirmed by complete sterilization of the infected monolayers with 50-μg/ml gentamicin after the 1-h infection period as described previously (19, 20).
Growth kinetics of L. pneumophila strains in U937 macrophages and WI-26 alveolar epithelial cells.
Infections of U937 macrophages by L. pneumophila strains were performed, in triplicate, in 96-well plates containing 105 cells/well at an MOI of 0.5, 5, or 50 for 1 h. At the end of the infection period, the monolayers were either treated (the initial number represents intracellular bacteria) or not treated (the initial number represents cell-associated bacteria) with gentamicin. The number of bacteria in the monolayers at several time intervals after infection was determined as we described previously (19, 20).
Transmission electron microscopy.
U937 macrophages and WI-26 alveolar epithelial cells were infected with L. pneumophila at an MOI of 50 for 1 h, followed by washing of extracellular bacteria. Preparation of ultrathin sections was performed as described previously (20). Briefly, infected macrophages were fixed with 3.5% glutaraldehyde, followed by 1% OsO4, dehydrated with ethanol, and embedded in Eponate 12 resin (Ted Pella, Redding, Calif.). Ultrathin sections were stained with uranyl acetate, followed by lead citrate, and examined by a Hitachi H-7000/STEM electron microscope (Hitachi Inc., Tokyo, Japan) at 75 kV.
DNA fragmentation analysis.
Differentiated U937 macrophages were plated in six-well plates (1.5 × 106 cells/well) and infected with L. pneumophila strains at an MOI of 0.5, 5, or 50 for 1 h. At the end of the infection period, the monolayers were washed three times to remove unattached extracellular bacteria and maintained at 37°C in culture medium. At 3 h postinfection, the cells in each well were lysed in 500 μl of lysis buffer (10 mM Tris [pH 7.5], 20 mM EDTA [pH 8.0], 0.5% Triton X-100) for 30 min on ice. The lysates were treated with 0.5% sodium dodecyl sulfate and 300-μg/ml proteinase K for 2 h and extracted with phenol and chloroform before precipitation with ethanol. The precipitates were solubilized in 10 mM Tris (pH 8.0)–1 mM EDTA containing 0.5-μg/ml RNase, electrophoresed in 1.8% agarose gel, and stained with ethidium bromide. As a positive control for induction of apoptosis, the cells were incubated in culture medium containing 10-μg/ml actinomycin D (Act D; Sigma) for 4 h and DNA fragmentation was examined as described above (28, 34). As a negative control, the cells were incubated in culture medium without bacterial infection. To examine inhibition of DNA fragmentation by the broad-specificity caspase inhibitor Z-VAD-FMK (30) (Oncogene Research Products, Cambridge, Mass.), U937 macrophages were pretreated with the inhibitor (100 μM) for 90 min. The monolayers were infected with strain AA100 at an MOI of 50 for 1 h in the presence of the inhibitor, followed by washing of unattached extracellular bacteria and 3 h of incubation in the presence of the inhibitor.
Development of antiserum.
L. pneumophila AA100 grown on a BCYE plate for 3 days was washed three times with saline, and 0.2 ml containing 107 bacteria in complete Freund’s adjuvant was injected subcutaneously into a 12-week-old female New Zealand rabbit. Two booster injections were administered at 2-week intervals. The final titer of the antiserum was approximately 1:105, as examined by enzyme-linked immunosorbent assay.
Analysis of apoptosis by fluorescence microscopy.
Cells attached to glass coverslips in 24-well plates were infected by L. pneumophila or treated with ActD as described above for the DNA fragmentation studies. For labeling of the bacteria, cells were fixed with 4% paraformaldehyde (Sigma) for 20 min, permeabilized with 0.2% Triton X-100 (Sigma) on ice for 5 min, blocked with 1% bovine serum albumin (Sigma) for 1 h, incubated with rabbit polyclonal antiserum (raised against L. pneumophila AA100) for 1 h, and then incubated for 1 h with a goat anti-rabbit immunoglobulin G secondary antibody conjugated to Alexa red (Molecular Probes, Inc., Eugene, Oreg.). For labeling of apoptotic nuclei, the cells were then subjected to fluorescein isothiocyanate (FITC)-conjugated terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) by using an apoptosis detection kit in accordance with the manufacturer’s instructions (Boehringer Mannheim Corporation, Indianapolis, Ind.). Cells were examined with a Zeiss Axiophot Photomicroscope (Carl Zeiss Inc., Oberkochen, Germany) or with a Leica TCS NT confocal laser scanning microscope. A minimum of 100 cells per sample were counted, and apoptosis was quantitated as the percentage of apoptotic cells (TUNEL-positive nuclei) among all of the cells counted by phase-contrast microscopy. Multiple independent samples were examined.
Apoptosis was also detected and quantified by an assay based on the detection of surface exposure of phosphatidylserine (PS) (29). Unfixed cells were stained with FITC-conjugated annexin-V by using the Annexin-V-FLUOS Staining Kit in accordance with the manufacturer’s instructions (Boehringer GmbH, Mannheim, Germany). Apoptotic cells with surface-exposed, labeled PS were visualized immediately by fluorescence microscopy. The monolayers were simultaneously stained with 0.5-μg/ml propidium iodide (PI) for examination of changes in the permeability of the plasma membrane. To quantitate percentages of apoptotic (annexin-V-positive) or PI-positive cells, a minimum of 100 cells per sample were counted and multiple independent samples were examined.
RESULTS
Cytopathogenicity of L. pneumophila to macrophages and alveolar epithelial cells during early stages of infection is dose dependent.
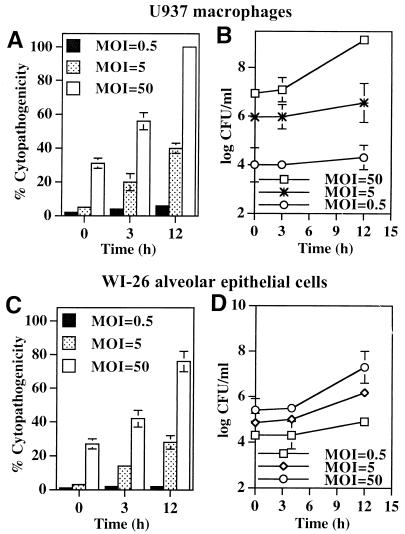
Infection of U937 macrophages by wild-type L. pneumophila AA100 at an MOI of 50 resulted in cytopathogenicities of 31 and 56% to the monolayers immediately after the 1-h infection period and at 3 h postinfection, respectively, which increased to 100% by 12 h postinfection (Fig. 1A). However, a longer period of time was required for L. pneumophila to induce similar levels of cytopathogenicity to the monolayers when the infection was performed at a lower MOI of 5 or 0.5 (Fig. 1A), suggesting that an increase in intracellular bacterial numbers or accumulation of bacterial products may be required to induce cytopathogenicity. No detectable intracellular replication was observed during the 3-h period after infection at the different MOIs examined (the lag phase is approximately 4 h) (Fig. 1B).
FIG. 1.
L. pneumophila is cytopathogenic to U937 macrophages (A) and WI-26 alveolar epithelial cells (C) in a dose-dependent manner. The cells were infected by the bacteria at an MOI of 0.5, 5, or 50 for 1 h, and the extracellular bacteria were washed away. The time points indicate the times at which Alamar Blue was added. The values are means of triplicate samples, and the error bars represent standard deviations. Intracellular growth kinetics of strain AA100 within U937 macrophages and WI-26 alveolar epithelial cells are shown in panels B and D, respectively. Infection of the monolayers was performed exactly as for cytopathogenicity assays, except that at the end of the 1-h infection period the monolayers were treated with gentamicin for 1 h to kill extracellular bacteria. At several time intervals, infected cells were hypotonically lysed and the number of intracellular bacteria was determined following growth on agar plates.
Type I and II alveolar epithelial cells constitute approximately 95% of the surface area of alveoli, which are the sites of infection, and both cell types support intracellular replication of L. pneumophila (1, 11, 12, 18, 32). Intracellular replication of L. pneumophila within type I and II alveolar epithelial cells has been recently shown to contribute to the pathogenesis of Legionnaires’ disease (18). Therefore, we examined the cytopathogenicity of L. pneumophila to type I alveolar epithelial cells. Results similar to those obtained by infection of U937 macrophages were obtained by infection of WI-26 type I human alveolar epithelial cells under the same experimental conditions (Fig. 1C and D).
Taken together, our data showed that L. pneumophila induced cytopathogenicity to macrophages and alveolar epithelial cells during early stages of infection prior to intracellular bacterial replication in a dose-dependent manner.
Induction of apoptosis by L. pneumophila in macrophages and alveolar epithelial cells is dose dependent.
Apoptosis has been observed in L. pneumophila-infected HL-60 macrophages at 24 to 48 h postinfection at an MOI of 10 to 100 (34), but not in L. pneumophila-infected mouse bone marrow-derived macrophages at a high MOI of 500 during the period of 20 to 60 min postinfection (26). Whether L. pneumophila induces apoptosis in host cells within 24 h postinfection at lower MOIs has not been examined. Therefore, we used multiple criteria to examine apoptosis in macrophages and alveolar epithelial cells during early stages of infection by L. pneumophila at different MOIs.
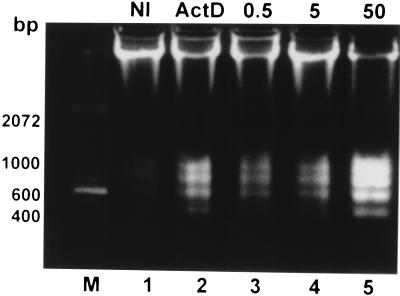
Apoptosis in L. pneumophila-infected U937 macrophages was first examined by agarose gel electrophoresis for detection of DNA fragmentation. Monolayers of U937 macrophages were infected by strain AA100 at an MOI of 0.5, 5, or 50 for 1 h, washed to remove unattached extracellular bacteria, and incubated at 37°C for 3 h before isolation of chromosomal DNA. DNA fragmentation was prominent in cells infected at an MOI of either 5 or 50 (Fig. 2, lanes 4 and 5, respectively) and detectable in cells infected at an MOI of 0.5 (Fig. 2, lane 3). At an MOI of 50, DNA fragmentation was detected as early as 90 min postinfection and most of the chromosomal DNA was fragmented at 3 h postinfection (Fig. 2, lane 5, and data not shown). The pattern of DNA fragmentation of U937 macrophages induced by L. pneumophila was similar to that induced by ActD, a positive inducer of apoptosis (Fig. 2, lane 2).
FIG. 2.
Kinetics of DNA fragmentation in L. pneumophila-infected (lanes 3 to 5) or ActD-treated (lane 2) U937 macrophages examined by agarose gel electrophoresis. The monolayers were infected by strain AA100 at an MOI of 0.5, 5, or 50 exactly as described in the legend to Fig. 1A. DNA isolated from uninfected or infected cells at 3 h post-1-h infection or 4 h after ActD treatment was subjected to electrophoresis and stained with ethidium bromide. Lane M contained a 100-bp molecular size marker (Gibco BRL, Gaithersburg, Md.). Lane 2 contained DNA from cells incubated with 1-μg/ml ActD. NI, noninfected.
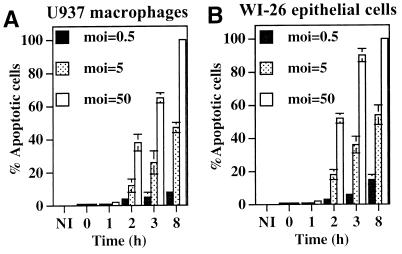
We further examined and quantitated DNA fragmentation in infected U937 macrophages (Fig. 3 and 4) by TUNEL, which labels fragmented DNA at the 3′ free ends with FITC-conjugated dUTP (22). L. pneumophila was specifically labeled with L. pneumophila-specific polyclonal antiserum followed by a secondary antibody conjugated to Alexa red, and the apoptotic cells were scored by TUNEL-positive nuclei, which fluoresced green (Fig. 4). Apoptotic cells were not detected at 1 h postinfection or earlier in U937 macrophages infected at any MOI examined (Fig. 3A). At 2 h postinfection at MOIs of 5 and 50, 12 and 38% of the cells were apoptotic, respectively, and the levels increased to 26 and 65% at 3 h postinfection (Fig. 3A and 4), prior to any detectable intracellular bacterial replication (Fig. 1B). Induction of similar levels of apoptosis was delayed with lower MOIs, suggesting that an increase in the number of bacteria following intracellular replication or the accumulation of certain bacterial products may be required to induce apoptosis. In contrast, less than 1% of the uninfected cells were apoptotic (Fig. 3A and 4H). TUNEL of L. pneumophila-infected macrophages was similar to that of ActD-treated cells (32 and 51% positive at 2 and 3 h posttreatment, respectively) (Fig. 4F). These data showed that L. pneumophila induced apoptosis during early stages of infection, prior to intracellular bacterial replication and in a dose-dependent manner, consistent with that of DNA fragmentation examined by agarose gel electrophoresis (Fig. 2). Examination of apoptosis in L. pneumophila-infected WI-26 alveolar epithelial cells demonstrated that approximately 60 and 90% of the cells infected at an MOI of 50 were apoptotic at 2 and 3 h postinfection, respectively (Fig. 3B). Dose-dependent induction of apoptosis was also evident in L. pneumophila-infected alveolar epithelial cells (Fig. 3B).
FIG. 3.
Apoptosis in L. pneumophila-infected U937 macrophages (A) and WI-26 alveolar epithelial cells (B) quantitated by TUNEL (see Fig. 4). The monolayers were infected by L. pneumophila as described in the legend to Fig. 2. One hundred cells were counted randomly, and the percentages of TUNEL-positive cells were calculated and expressed as mean percentages of apoptotic cells. NI, noninfected.
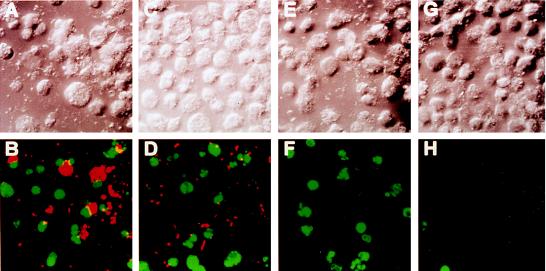
FIG. 4.
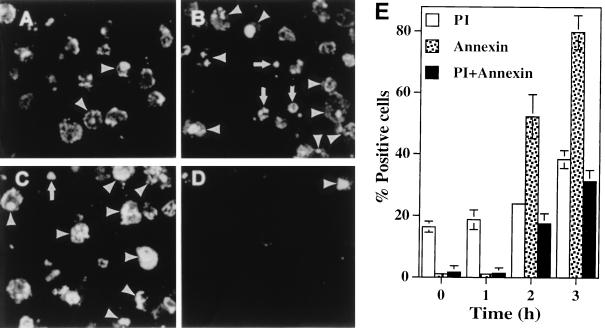
Representative confocal laser scanning images of TUNEL of L. pneumophila-infected U937 macrophages undergoing apoptosis. L. pneumophila infection and ActD treatment of the monolayers were performed exactly as described in the legend to Fig. 2. Monolayers were labeled simultaneously (bottom panels) with FITC-conjugated dUTP (green) and a polyclonal antiserum specific for L. pneumophila detected by a secondary antibody conjugated to Alexa red (red). Panels: B, L. pneumophila infected; D, L. pneumophila infected in the presence of CytD; F, ActD treated; H, uninfected. Phase-contrast images A, C, E, and G correspond to B, D, F, and H, respectively.
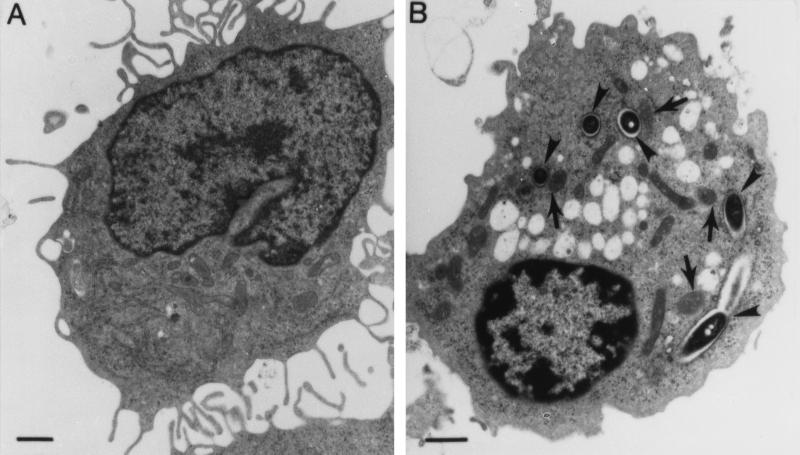
Morphological changes in L. pneumophila-infected U937 macrophages and WI-26 alveolar epithelial cells were also examined by transmission electron microscopy. Compared to uninfected cells (Fig. 5A), U937 macrophages examined at 4 h postinfection at an MOI of 50 manifested morphological features characteristic of apoptosis, including cytoplasmic vacuolation, chromatin condensation, and margination, but intact organelles such as mitochondria (indicated by arrows in Fig. 5B) (33, 42). Similar results were obtained with WI-26 alveolar epithelial cells infected by L. pneumophila (data not shown).
FIG. 5.
Detection of L. pneumophila-induced apoptosis in U937 macrophages by transmission electron microscopy. Infected cells at 4 h postinfection (B) showed apoptotic nuclear morphology distinct from that of uninfected cells (A). The arrowheads indicate ribosome-surrounded phagosomes containing the bacteria. The arrows indicate intact mitochondria within the vicinity of the phagosomes.
Surface exposure of PS, another indicator of apoptosis, was examined in L. pneumophila-infected U937 macrophages. PS is a phospholipid that is mostly distributed in the inner leaflet of the membrane bilayer in a healthy cell but is exposed to the outer leaflet during early stages of induction of apoptosis (29). U937 macrophages were infected with strain AA100 at an MOI of 0.5, 5, or 50 for 1 h, followed by washing of unattached bacteria (t0). At several time points postinfection, surface exposure of PS was examined by labeling with FITC-conjugated annexin-V and quantitated by random counting of at least 100 cells. The monolayers were simultaneously stained with PI to monitor the integrity of the plasma membrane. Increased permeability of the plasma membrane to PI was detected immediately after infection (∼20% of the cells were positive) and increased slightly (∼35%) at 3 h postinfection, indicating an immediate cytotoxic effect of the inoculated bacteria (24). In contrast, surface exposure of PS was initially detected at 2 h postinfection (56%) (Fig. 6A and E), and the level increased to 80% at 3 h postinfection (Fig. 6B and E). Importantly, since approximately 50% of the cells at 3 h postinfection were annexin-V (molecular mass is 33 kDa) positive but not permeable to PI (molecular mass is 668 Da), the data clearly indicated that binding of annexin-V to the plasma membrane was not due to increased permeability. Less than 1% of uninfected cells were annexin-V positive (Fig. 6D and E). The pattern of annexin-V labeling of L. pneumophila-infected cells was similar to that of ActD-treated cells (Fig. 6C) (43 and 68% annexin-V positive at 2 and 3 h posttreatment, respectively) and was consistent with that examined by TUNEL assays (Fig. 3 and 4). However, more ActD-treated cells became permeable to PI (data not shown).
FIG. 6.
Representative confocal laser scanning images of annexin-V labeling of L. pneumophila-infected U937 macrophages undergoing apoptosis and comparison to ActD-treated cells. The Monolayers were labeled simultaneously with PI and FITC-conjugated annexin-V. Panels: A, 2 h postinfection; B, 3 h postinfection; C, 4 h post-ActD treatment; D, uninfected cells. The arrowheads indicate double-labeled cells, the arrows in panels B and C indicate cells labeled with PI only, and the rest of the fluorescent cells were with annexin-V only. Quantitation of annexin-V-positive and PI-positive U937 macrophages is shown in panel E. One hundred cells were counted randomly, and multiple samples were examined.
Induction of apoptosis by L. pneumophila does not require intracellular bacterial replication or new protein synthesis and correlates with cytopathogenicity.
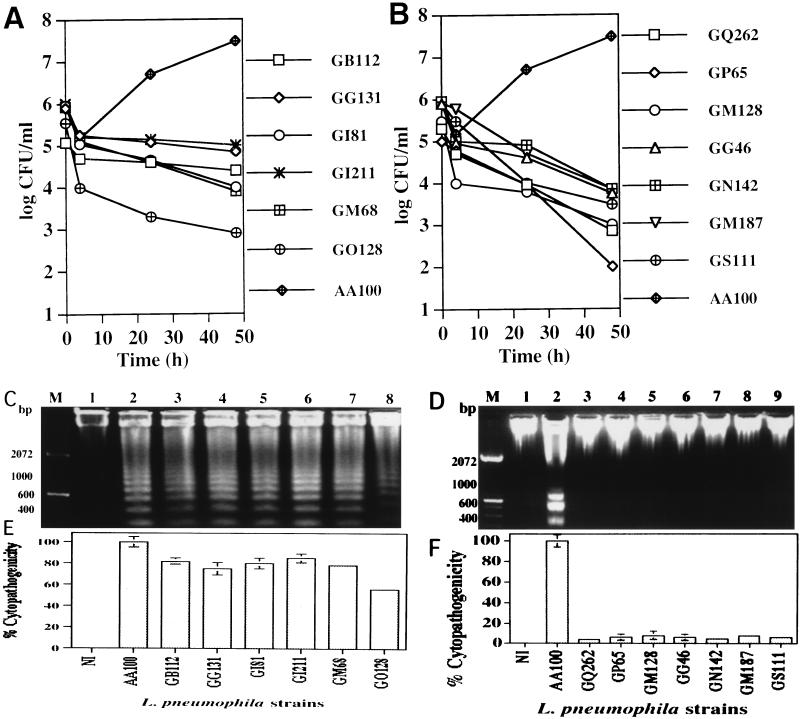
Our data suggested that intracellular replication was not required for L. pneumophila to induce apoptosis (Fig. 1). Two strategies were used to confirm that induction of apoptosis did not require intracellular bacterial replication or new protein synthesis and that apoptosis correlated with cytopathogenicity. First, we examined genetically defined mutants of L. pneumophila that are defective in intracellular replication for the ability to induce apoptosis and cytopathogenicity. We have recently isolated and characterized a collection L. pneumophila mutants that are defective, to various degrees, in intracellular replication within both human macrophages and protozoan cells, and the defective genetic loci have been designated protozoan and macrophage infectivity (pmi) loci (19). As shown in Table 1 and Fig. 7A and B, we examined all of the mutants belonging to pmi mutant groups 1, 2, and 3, all of which are severely defective in intracellular replication within U937 macrophages (19), for the ability to induce apoptosis. Agarose gel electrophoresis of chromosomal DNA of U937 macrophages isolated at 3 h postinfection by the mutants at an MOI of 50 showed that 36 of the 46 mutants induced various degrees of DNA fragmentation (Table 1). Seventeen of these 36 mutants (GB111 to GQ61, Table 1) induced apoptosis at levels similar to that induced by the parental strain and were also highly cytopathogenic to U937 macrophages at 12 h postinfection, despite their intracellular replication defect (Fig. 7). Nineteen of the mutants (Table 1, GN266 to GH37) induced various degrees of apoptosis in U937 macrophages at lower levels than parental strain AA100 and were also less cytopathogenic to these cells (Table 1), and an example of these (GO128) is shown in Fig. 7. In contrast to the 36 mutants that induced apoptosis, the other 10 mutants were completely defective in the induction of DNA fragmentation in U937 macrophages (Table 1 and Fig. 7D). Correlated with their defect in apoptosis induction, these 10 mutants were also severely defective in cytopathogenicity to these cells (Fig. 7F). All three dotA and icmWXYZ mutants (GG105, GL10, and GS95) (19) were completely defective in both apoptosis induction (Fig. 7D, lanes 3, 5, and 7) and cytopathogenicity (Fig. 7F). Importantly, the level of apoptosis consistently correlated with the level of cytopathogenicity in multiple experiments.
TABLE 1.
Correlation of cytopathogenicity of L. pneumophila mutantsa to U937 macrophages with induction of apoptosis
| L. pneumophila strain | DNA fragmentation | % Cytopathogenicity |
|---|---|---|
| AA100 | + | 100 |
| GB111 | + | 100 |
| GD113 | + | 97 |
| GE42 | + | 80 |
| GB112 | + | 82 |
| GI211 | + | 85 |
| GE193 | + | 91 |
| GT282 | + | 86 |
| GO108 | + | 88 |
| GC44 | + | 92 |
| GG104 | + | 79 |
| GI81 | + | 80 |
| GM68 | + | 78 |
| GI178 | + | 82 |
| GG131 | + | 75 |
| GO128 | + | 56 |
| GT128 | + | 48 |
| GQ61 | + | 73 |
| GN266 | + | 52 |
| GN251 | + | 60 |
| GM90 | + | 55 |
| GF35 | + | 68 |
| GF249 | + | 72 |
| GD150 | + | 61 |
| GQ89 | + | 68 |
| GF248 | + | 70 |
| GP130 | + | 57 |
| GM112 | + | 65 |
| GO38 | + | 72 |
| GF162 | + | 42 |
| GG60 | + | 35 |
| GI40 | + | 25 |
| GI25 | + | 32 |
| GS50 | + | 12 |
| GR67 | + | 17 |
| GJ302 | + | 16 |
| GH37 | + | 10 |
| GM128 | − | 4 |
| GG46 | − | 2 |
| GP65 | − | 2 |
| GN142 | − | 1 |
| GS111 | − | 2 |
| GM187 | − | 0 |
| GQ262 | − | 0 |
| GL10 | − | 0 |
| GS95 | − | 3 |
| GG105 | − | 2 |
Representatives of these mutants are shown in Fig. 7.
FIG. 7.
Ability of pmi mutants of L. pneumophila to induce apoptosis in U937 macrophages correlates with cytopathogenicity to the cells. Intracellular growth kinetics of the mutants (A and B) was examined as described in the legend to Fig. 1B but without gentamicin treatment. The ability of these mutants to induce DNA fragmentation in U937 macrophages was examined at 3 h postinfection (C and D). Infection of the monolayers was performed at an MOI of 50 exactly as described in the legend to Fig. 1A. Percent cytopathogenicity of the wild-type and mutant strains of L. pneumophila to U937 macrophages infected at an MOI of 50 at 12 h postinfection is shown in panels E and F and was determined as described in the legend to Fig. 1A. Lane M contained a 100-bp molecular size marker. NI, noninfected.
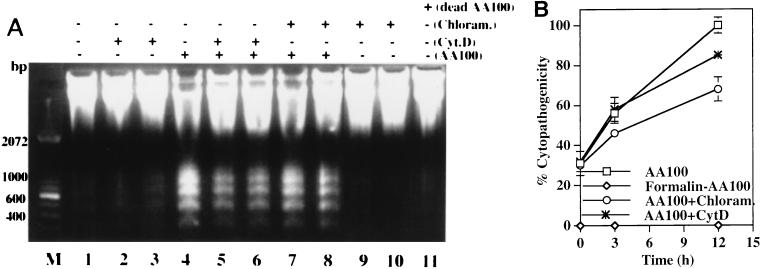
In the second strategy, we examined the effect of inhibition of bacterial protein synthesis on L. pneumophila-induced apoptosis and cytopathogenicity. The bacteriostatic antibiotic chloramphenicol (100 μg/ml) or erythromycin (80 μg/ml) completely inhibited protein synthesis and intracellular replication of L. pneumophila within U937 macrophages (see Materials and Methods), but the intracellular bacteria were viable for more than 5 h after antibiotic treatment (data not shown). Chloramphenicol by itself did not have a detectable effect on DNA fragmentation (Fig. 8A). Inhibition of bacterial protein synthesis and intracellular replication by chloramphenicol did not affect the ability of L. pneumophila to induce apoptosis (Fig. 8A). The antibiotic reduced but did not block the cytopathogenicity of L. pneumophila to U937 macrophages during the 5-h period when intracellular bacteria were still viable in the presence of this antibiotic (Fig. 8B). Similar results were obtained with erythromycin treatment (data not shown).
FIG. 8.
Inhibition of intracellular bacterial replication and inhibition of bacterial uptake do not block L. pneumophila-induced apoptosis and cytopathogenicity to U937 macrophages. (A) Some monolayers were treated with chloramphenicol at 20 (lane 7) or 100 (lane 8) μg/ml for 1 h prior to infection at an MOI of 50 and during the 1-h infection period and the subsequent 3-h incubation time. Some monolayers were pretreated with 1-μg/ml CytD for 30 min and infected at an MOI of 50 for 30 min in the presence of CytD before gentamicin treatment. CytD was either removed (lane 5) or maintained (lane 6) after the 1-h gentamicin treatment. Uninfected monolayers were incubated with chloramphenicol at 0 (lane 1), 20 (lane 9), or 100 (lane 10) μg/ml for 5 h or with CytD at 1 μg/ml for 1 h (lane 2) or 5 h (lane 3). Formalin-killed bacteria were also used to infect U937 macrophages to examine their ability to induce DNA fragmentation (lane 11). Lane M contained a 100-bp molecular size marker. (B) Cytopathogenicity of L. pneumophila to U937 macrophages under different treatment conditions. Infection or treatments were performed as described in the legend to Fig. 7 for detection of DNA fragmentation. Percent cytopathogenicity was determined as described in the legend to Fig. 1A.
Taken together, these data provided genetic and biochemical evidence that intracellular bacterial replication or new protein synthesis is not required for L. pneumophila to induce apoptosis and that induction of apoptosis correlates with cytopathogenicity.
Extracellular L. pneumophila induces apoptosis in U937 macrophages.
The actin microfilament depolymerizer CytD completely inhibits uptake of L. pneumophila by U937 macrophages (25). We observed by transmission electron microscopy and confocal laser scanning microscopy that numerous cells in the infected monolayers that did not contain bacteria were apoptotic (data not shown). Therefore, we examined whether extracellular L. pneumophila induced apoptosis in U937 macrophages during inhibition of bacterial uptake by CytD. We confirmed complete blockage of bacterial uptake by CytD-treated cells by sterilization of the infected monolayers following gentamicin treatment (19, 20). Our data showed that CytD by itself did not have detectable effects on DNA fragmentation and association of the bacteria with the U937 macrophages (Fig. 8A) (19, 20). Inhibition of bacterial uptake by CytD reduced but did not block the ability of L. pneumophila to induce apoptosis in U937 macrophages, as examined by agarose gel DNA fragmentation (Fig. 8A, lanes 5 and 6). This observation was further confirmed by TUNEL assays (Fig. 4D). Viability of L. pneumophila was essential for induction of apoptosis in U937 macrophages since formalin-killed bacteria completely lost the ability to induce apoptosis (Fig. 8A, lane 11). A viable bacterium-cell contact may be required for induction of apoptosis, since bacterial culture supernatants or supernatants of infected monolayers were not able to induce apoptosis (data not shown). In addition, correlated with their ability to induce apoptosis in U937 macrophages, extracellular bacteria were also cytopathogenic to these cells (Fig. 8B), further supporting our observations of a correlation between induction of apoptosis and cytopathogenicity.
L. pneumophila-induced apoptosis is mediated by activation of the caspase cascade.
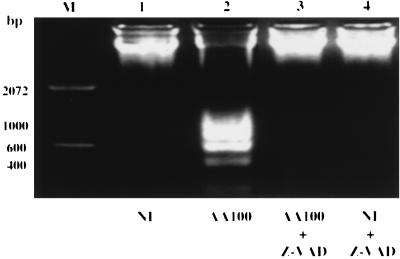
Activation of the cascade of caspases is required for DNA fragmentation in various types of cells induced by a variety of apoptotic stimuli (38). While S. flexneri has been shown to induce DNA fragmentation in the host cell by activation of caspase-1, Mycoplasma penetrans induces DNA fragmentation in the host cell by secretion of a bacterial nuclease that specifically cleaves host cell DNA (9). To examine whether DNA fragmentation in L. pneumophila-infected U937 macrophages was mediated by a bacterial nuclease or by activation of the host cell caspases, a broad-specificity, cell-permeable caspase inhibitor, Z-VAD-FMK, was used to examine whether it could block L. pneumophila-induced apoptosis. Monolayers were pretreated with Z-VAD-FMK for 90 min, infected with strain AA100 at an MOI of 50 for 1 h in the presence of the inhibitor, washed to remove unattached extracellular bacteria, and incubated for an additional 3 h in the presence of the inhibitor. DNA was isolated at the end of the 3-h incubation period and examined by agarose gel electrophoresis for detection of DNA fragmentation. As shown in Fig. 9, DNA fragmentation in L. pneumophila-infected U937 macrophages was completely blocked by Z-VAD-FMK. Similar results were obtained for macrophages infected by L. pneumophila at a lower MOI of 5 (data not shown). Incubation of L. pneumophila with Z-VAD-FMK did not affect bacterial viability or the ability to induce apoptosis in U937 macrophages (data not shown), indicating that inhibition of DNA fragmentation was specifically due to inhibition of the activity of caspases.
FIG. 9.
Inhibition of DNA fragmentation in L. pneumophila-infected U937 macrophages by the caspase inhibitor Z-VAD-FMK. Monolayers were pretreated with a 100 μM concentration of the inhibitor for 90 min and infected with strain AA100 at an MOI of 50 in the presence of the inhibitor, extracellular bacteria were washed off, and monolayers were incubated for an additional 3 h in the presence of the inhibitor before DNA isolation. Lane M contained a 100-bp molecular size marker. NI, noninfected.
DISCUSSION
It has been well documented that L. pneumophila replicates within and is cytopathogenic to macrophages, monocytes, and epithelial cells (1, 6, 24, 31, 35). The mechanisms of cell death due to L. pneumophila infection are not well understood. Both apoptosis and necrosis have been recently observed in L. pneumophila-infected macrophages (24, 26, 34). Müller et al. reported apoptosis in L. pneumophila-infected HL-60 macrophages at 24 and 48 h postinfection when the cells were infected at an MOI of 10 to 100 (34). On the other hand, when a high MOI of 500 was used for infection of mouse bone marrow-derived macrophages, L. pneumophila induced rapid necrosis within 20 to 60 min after infection, without signs of apoptosis during this period, as examined by electron microscopy (26). In this study, we have used multiple criteria to examine apoptosis and shown that, at an MOI of 0.5, 5, or 50, L. pneumophila induces apoptosis in macrophages and alveolar epithelial cells within a few hours of infection in a dose-dependent manner. We also provide genetic and biochemical evidence that induction of apoptosis by L. pneumophila does not require intracellular bacterial replication or new protein synthesis and that apoptosis correlates with cytopathogenicity. Therefore, apoptosis plays an important role in L. pneumophila-induced cell death of macrophages and alveolar epithelial cells during early stages of infection.
Our data show that apoptosis is exhibited in L. pneumophila-infected cells during the first few hours of infection. The ability of L. pneumophila to induce apoptosis is independent of the bacterial growth phase (17). In addition to induction of apoptosis, L. pneumophila becomes cytotoxic and induces rapid necrosis in macrophages and alveolar epithelial cells only upon exiting the exponential phase and entering the postexponential phase of growth (1, 10, 21). Therefore, we propose a model by which L. pneumophila promotes biphasic death of the host cell. In the first phase, host cells are exposed to a low dose of the bacteria, which is what occurs naturally (6). During this phase, the cells undergo apoptosis when the bacterial number reaches a certain threshold, regardless of the growth phase. The second phase is manifested during late stages of the infection, when there is a large number of postexponential-phase bacteria. During this phase, the bacteria become cytotoxic, infected cells become necrotic, intracellular bacteria are released, and the neighboring cells also undergo necrosis upon exposure to a large number of cytotoxic bacteria released from the lysed cells. This second phase of necrotic death is most probably mediated by temporal expression of the pore-forming toxin upon entry of L. pneumophila into the postexponential phase of growth (10, 21, 24, 26). The two strategies (necrosis and apoptosis) utilized by L. pneumophila to kill the host cell may be required to ensure exploitation of host cell resources for intracellular replication and subsequent efficient killing to exit the spent host cell upon termination of intracellular replication.
Different strategies can be used by different species of facultative intracellular bacterial pathogens to induce apoptosis in specific host cells. S. flexneri induces apoptosis in macrophages after escape from the phagosome into the cytoplasm (42) but does not induce apoptosis in epithelial cells (43). Yersinia enterocolitica, which induces apoptosis in macrophages from an extracellular location, also does not induce apoptosis in epithelial cells (37). In contrast, we have demonstrated that extracellular L. pneumophila is capable of inducing apoptosis in both macrophages and alveolar epithelial cells. This induction of apoptosis prior to entry of L. pneumophila may be required for formation of the intracellular niche and alteration of the host cell endocytic pathway.
While S. flexneri has been shown to induce DNA fragmentation in the host cell by activation of caspase-1, M. penetrans induces DNA fragmentation in the host cell by secretion of a bacterial nuclease that specifically cleaves host cell DNA (9). Inhibition of caspase activity by the broad-specificity caspase inhibitor completely blocks nuclear apoptosis in L. pneumophila-infected U937 macrophages. This is further confirmed by our recent observations that activation of caspase-3, but not caspase-1, is essential for nuclear apoptosis induced upon infection by L. pneumophila (17). Therefore, activation of the caspase cascade, but not a bacterial nuclease activity, is responsible for apoptosis induced by L. pneumophila.
Based on our observations that live bacteria are required to induce apoptosis in macrophages from an extracellular location, we speculate that L. pneumophila exerts its apoptotic effect on the host cell through contact-mediated export of a bacterial factor(s) that results in activation of the cascade of caspases. This speculation is supported by our finding that pmi mutants of L. pneumophila with mutations in various dot or icm loci, which encode proteins thought to be involved in the assembly of a type IV-like secretion apparatus (39, 41), are completely defective in the induction of apoptosis in U937 macrophages. It is likely that some of these mutants are defective in components of the secretion apparatus (such as the cytoplasmic membrane protein DotA) (36) or in expression of the apoptosis-inducing factor(s). Further characterization of the mechanisms of induction of apoptosis by L. pneumophila will elucidate these possibilities.
ACKNOWLEDGMENT
This work is supported by Public Health Service award R29AI-38410, awarded to Y.A.K.
REFERENCES
- 1.Abu Kwaik Y. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–696. doi: 10.1046/j.1365-2958.1998.01092.x. [DOI] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect Immun. 1998;66:203–212. doi: 10.1128/iai.66.1.203-212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. doi: 10.1111/j.1365-2958.1994.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Abu Kwaik Y, Gao L-Y, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 6.Abu Kwaik Y, Gao L-Y, Stone B J, Venkataraman C, Harb O S. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl Environ Microbiol. 1998;64:3127–3133. doi: 10.1128/aem.64.9.3127-3133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu Kwaik Y, Pederson L L. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol Microbiol. 1996;21:543–556. doi: 10.1111/j.1365-2958.1996.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson P. Kinase cascades regulating entry into apoptosis. Microbiol Mol Biol Rev. 1997;61:33–46. doi: 10.1128/mmbr.61.1.33-46.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendjennat M, Blanchard A, Loutfi M, Montagnier L, Bahraoui E. Purification and characterization of Mycoplasma penetrans Ca2+/Mg2+-dependent endonuclease. J Bacteriol. 1997;179:2210–2220. doi: 10.1128/jb.179.7.2210-2220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpo J D, Barry B E, Gehr P, Bachofen M, Weibel E R. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;125:740–745. doi: 10.1164/arrd.1982.125.6.740. [DOI] [PubMed] [Google Scholar]
- 12.Cianciotto N P, Stamos J K, Kamp D W. Infectivity of Legionella pneumophila mip mutant for alveolar epithelial cells. Curr Microbiol. 1995;30:247–250. doi: 10.1007/BF00293641. [DOI] [PubMed] [Google Scholar]
- 13.Clifton D R, Goss R A, Sahni S K, Antwerp D V, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J J. Overview: mechanisms of apoptosis. Immunol Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 15.Fan T, Lu H, Shi L, MaClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez R C, Logan S, Lee S H S, Hoffman P S. Elevated levels of Legionella pneumophila stress protein Hsp60 early in infection of human monocytes and L929 cells correlated with virulence. Infect Immun. 1996;64:1968–1976. doi: 10.1128/iai.64.6.1968-1976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, L.-Y., and Y. Abu Kwaik. 1998. Unpublished data.
- 18.Gao, L.-Y., B. J. Stone, J. K. Brieland, and Y. Abu Kwaik. Different fates of Legionella pneumophila pmi and mil mutants within macrophages and alveolar epithelial cells. Microb. Pathog., in press. [DOI] [PubMed]
- 19.Gao L-Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L-Y, Harb O S, Abu Kwaik Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, L.-Y., B. J. Stone, O. S. Harb, and Y. Abu Kwaik. 1998. Unpublished data.
- 22.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King C H, Fields B S, Shotts E B, Jr, White E H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun. 1991;59:758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 27.Marston B J. Epidemiology of community-acquired pneumonia. Infect Dis Clin Pract. 1995;4:S232–S239. [Google Scholar]
- 28.Martin S J, Lennon S V, Bonhan A M, Cotter T G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990;145:1859–1867. [PubMed] [Google Scholar]
- 29.Martin S J, Reutelingsperger C P M, McGahon A J, Rader J A, van Schie R C A A, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy N J, Whyte M K B, Gilbert C S, Evan G I. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCusker K T, Braaten B A, Cho M W, Low D A. Legionella pneumophila inhibits protein synthesis in Chinese hamster ovary cells. Infect Immun. 1991;59:240–246. doi: 10.1128/iai.59.1.240-246.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mody C H, Paine III R, Shahrabadi M S, Simon R H, Pearlman E, Eisenstein B I, Toews G B. Legionella pneumophila replicates within rat alveolar epithelial cells. J Infect Dis. 1993;167:1138–1145. doi: 10.1093/infdis/167.5.1138. [DOI] [PubMed] [Google Scholar]
- 33.Monack D M, Raupach B, Hromocky A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearlman E, Jiwa A H, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 36.Roy C R, Isberg R R. Topology of Legionella pneumophila DotA: an inner membrane protein required for replication in macrophages. Infect Immun. 1997;65:571–578. doi: 10.1128/iai.65.2.571-578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1998;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 39.Segal G, Purcell M, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila chromosome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone B J, Abu Kwaik Y. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 42.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 43.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what we can learn from bacteria-induced cell death. Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]