Abstract
The current study was performed to explore the effects of dietary supplementation of Saccharomyces cerevisiae hydrolysate (SCH) on growth performance, immune function, and intestinal health in broiler chicken. A total of 300 Ross 308 male broilers (1-day-old) were randomly assigned to 2 dietary treatments including a basal diet (control group), and a basal diet supplemented with SCH feed additive (500 mg/kg in starter and grower phase, and 250 mg/kg in finisher phase). Each treatment had 6 replicates with 25 birds each. The results showed that the addition of SCH promoted growth during d 15 to 28 (P < 0.05). Although the addition of SCH had no significant effect on the intestinal relative indexes, it significantly increased the jejunum villus height (VH) and the ratio of villus height to crypt depth (VCR) of jejunum, and decreased the crypt depth (CD) of ileum (P < 0.05). Furthermore, SCH addition significantly downregulated the mRNA expression of immunomodulatory genes (TNF-α, IL-1β, and IL-6), and upregulated the tight junction genes (ZO-1 and Claudin-1) (P < 0.05). High throughput sequencing analysis of bacterial 16S rRNA revealed that dietary SCH supplementation altered cecum microbiota. Alpha diversity analysis showed that a higher bacterial richness in cecum of broilers fed with SCH. The composition of cecum microbiota regulated by SCH addition was characterized by an increased abundance of Firmicutes and a reduced abundance of Bacteroidetes. At the genus level, dietary SCH resulted in a decrease of Bacteroides and an increase of short-chain fatty acids (SCFA) -producing bacteria including Lactobacillus and Faecalibacterium. Taken together, dietary SCH supplementation can stimulate the growth of broilers by regulating the intestinal immunity and barrier function, and improving the intestinal morphology, which may be related to the enhancement of bacterial diversity and the changes of intestinal microbial composition.
Key words: Saccharomyces cerevisiae hydrolysate, growth performance, microbiota, broilers
INTRODUCTION
In the context of antibiotic free livestock production, studies on feed additives as antibiotic substitutes have gained more interest. The main concern for the evaluation of antibiotic alternative is to assess their efficacy on animal health and immune response, as well as animal productivity (Caly et al., 2015; Salaheen et al., 2017). Among the alternatives, yeast hydrolysate is a promising product, which are usually obtained by liquid fermentation with Saccharomyces cerevisiae, and then concentrated or dried after autolysis or hydrolysis catalyzed by exogenous enzymes. Saccharomyces cerevisiae hydrolysate (SCH) generally contains abundant nucleotides, B-vitamins, amino acids, and yeast cell wall polysaccharides (such as β-glucan and mannan). As an important component of SCH, nucleotides have a lot of benefits in improving growth performance, regulating immune function, and repairing the gastrointestinal tract of animals (Sauer, 2010; Superchi et al., 2012), and β-glucan and mannan oligosaccharide were generally used as prebiotics to regulate the immune response (Fadl et al., 2020). Meanwhile, SCH have been considered as one of the effective alternatives to antibiotic growth promoters (AGP) in animals, due to their ability to improve intestinal nutrients digestibility (Samarasinghe et al., 2004), promote the intestinal health (Fu et al., 2019), enhance disease resistance (Bu et al., 2019), and relieve inflammatory responses (Bu et al., 2020). The specific benefits of SCH on growth performance of poultry have already been described in considerable studies (Awaad et al., 2011; Santovito et al., 2018; Perricone et al., 2022). However, it is not clear how SCH leads to the improvement of growth performance and modulates the body health in broilers. One hypothesis for the mechanism of action of SCH could be related to an improved intestinal resistance to external injuries or a possible role in the modulation of intestinal microbiota (Khalid et al., 2021).
As the first line of defense against the external environment, the intestinal mucosa is firstly damaged when the inflammatory reaction occurs, which would negatively impact the digestion and absorption of nutrients (Bai et al., 2018). Intestinal microbiota plays an important role in digestion, barrier, and immune function of broilers, contributing to subsequent improvement in the growth performance (Pan and Yu, 2014; Pandit et al., 2018). It is widely accepted that AGP boost animal growth mainly through reducing pathogenic bacteria, increasing beneficial bacteria, and modulating gastrointestinal microbiota (Diarra and Malouin, 2014; Gadde et al., 2017). However, information regarding the effect of SCH on the intestinal microbiota in broilers is still limited. Modulation of immune status and intestinal microbiota may provide a new avenue to explain the potential effects of dietary SCH on growth performance and intestinal health.
Therefore, the purpose of this study was to investigate the action by which dietary SCH improve gut immunity and intestinal health, which would lead to the observed improved performance as result.
MATERIALS AND METHODS
Saccharomyces Cerevisiae Hydrolysate
The Saccharomyces cerevisiae hydrolysate (I-Care, batch number: 210210; production date: February 2021) used in this experiment was obtained from Prosol S.p.A (Madone, Italy), and added to broiler feed at a prescribed level (the optimal additive dosage has been determined in previous studies). The active ingredients presented in SCH supplement were 38% of crude protein, 4.9% of glutamic acid, 3.5% of nucleotides, 23% of β-glucans, and 15% mannan oligosaccharide. The MSDS and COA of I-Care can be found in Supplementary Materials.
Birds and Management
A temperature-controlled house (33°C ± 0.5°C for one-day-old chicks and 21°C ± 1°C for chickens after 4 wk old) was provided for this experiment during the whole rearing stage. Birds for the trial were allocated to 4-tier cages with 25 hens per cage (cage size: 180 cm × 120 cm × 45 cm), and the bottom of each cage was covered with mesh plastic litter. A total of 12 replicates of the 2 treatments were randomly distributed into 12 cages in the chicken house. Each cage is equipped with a feeding trough and 4 nipple drinkers. Chicks were provided with water and feed ad libitum. All birds remained in good health during the feeding period.
Experimental Design and Diets
A total of 300 one-day-old male Ross 308 broiler chicks purchased from a local hatchery were randomly allocated into 2 treatment groups that were fed corn-soybean meal basal diets (control group) and the basal diet supplied with SCH (I-Care, Prosol S.p.A, Italy). Each treatment contained 6 replicates of 25 chicks/cage. The addition amount of SCH was 500 mg/kg in starter & grower diet (d 1–28) and 250 mg/kg in finisher diet (d 29–42). The basal diets (Table 1) were formulated to meet National Research Council (1994).
Table 1.
The ingredient composition of basal diet and nutrient levels.
| Items | Starter diet (1–14 d) | Grower diet (15–28 d) | Finisher diet (29–42 d) |
|---|---|---|---|
| Corn | 54.87 | 59.12 | 57.90 |
| Soybean meal (46 CP) | 25.85 | 20.05 | 18.65 |
| Corn gluten meal (60 CP) | 4.00 | 4.50 | 5.00 |
| Cottonseed meal | 3.00 | 3.00 | 3.65 |
| Rapeseed meal | 2.50 | 2.80 | 3.00 |
| Wheat middlings | 2.00 | 2.00 | 2.50 |
| Soybean oil | 3.35 | 4.65 | 5.58 |
| Dicalcium phosphate | 1.55 | 1.25 | 0.98 |
| Limestone | 1.50 | 1.35 | 1.45 |
| Salt | 0.25 | 0.20 | 0.20 |
| DL-Methionine | 0.25 | 0.21 | 0.20 |
| L-lysine.HCl | 0.35 | 0.32 | 0.30 |
| L-threonine | 0.05 | 0.02 | 0.01 |
| Vitamin Premix1 | 0.02 | 0.02 | 0.02 |
| Mineral Premix2 | 0.20 | 0.20 | 0.20 |
| Choline chloride (50%) | 0.10 | 0.10 | 0.10 |
| Sodium bicarbonate | 0.15 | 0.20 | 0.25 |
| Phytase | 0.01 | 0.01 | 0.01 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels3 | |||
| AME (MJ/kg) | 12.35 | 12.97 | 13.18 |
| Crude protein, % | 22.00 | 21.00 | 20.00 |
| Calcium, % | 1.00 | 0.90 | 0.85 |
| Available phosphorus, % | 0.40 | 0.35 | 0.30 |
| Lysine, % | 1.25 | 1.10 | 1.05 |
| Methionine, % | 0.57 | 0.52 | 0.50 |
| Methionine+cystine, % | 0.90 | 0.81 | 0.80 |
| Threonine, % | 0.81 | 0.72 | 0.68 |
| Tryptophan, % | 0.25 | 0.23 | 0.19 |
The vitamin premix supplied the following per kg of complete feed: vitamin A, 12,500 IU; vitamin D3, 2,500 IU; vitamin K3, 2.65 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B12, 0.025 mg; vitamin E, 30 IU; biotin,0.0325 mg; folic acid, 1.25 mg.
The mineral premix supplied the following per kg of complete feed: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; I, 0.35 mg, Se, 0.15 mg.
Calculated composition.
This study was approved by the Animal Care and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences, Beijing (approval No. FRI-CAAS-20210903). All the management for broilers was performed in accordance with the guidelines of raising Ross 308 broilers (Delezie et al., 2012). The experiment lasted for 42 d, divided into starter (day 1–14), grower (day 15–28), and finisher (d 29–42) stages.
Growth Performance
The birds and feed were weighed by pen at 0, 14, 28, and 42 d post-hatch for determination of growth performance, including body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). Mortalities and postmortem weight were recorded daily for the calculation of mortality, body weight gain, and mortality-corrected FCR.
Intestinal Relative Index and Morphology
On d 14, 28, and 42, 6 birds per treatment (1 bird per cage) were scarified and intestinal tissues were collected to analyze relative index of intestinal weight and morphology. Before that, birds had been fasted for 12 h. The relative index of intestinal weight was calculated according to the weight of duodenum, jejunum, ileum, cecum, and the BW of birds. Intestinal relative index = intestinal segment weight of empty (g) / body weight (g) × 100%. Meanwhile, the middle segments of duodenum, jejunum and ileum (about 2 cm) were collected and fixed in 10% neutral buffered formalin. After wash, dehydration, and clarification, the samples were embedded in paraffin. The serial sections with a thickness of 5 μm were placed on a glass slide for dewaxing, hydration, and staining. The villus height (VH) and crypt depth (CD) were measured by NIKON DS-U3 image processing and analyzing system (NIKON ECLIPE CI, Tokyo, Japan). The ratio of villus height/crypt depth (VCR) was calculated.
Real-Time Quantitative PCR
On d 28 and 42, jejunum and ileum mucosa of 6 birds from each treatment group were collected to analyze the gene expression levels. Total RNA of jejunum and ileum mucosa samples was extracted by Trizol reagent (Thermo Fisher Scientific, Wilmington, DE), and the purity and concentration of total RNA was determined by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Then, 1 μg RNA of each sample was used to reverse-transcribe into cDNA using TransScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) following the manufacturer's guidelines. The PowrUp SYBR Master Mix (Thermo Scientific) was used to carry out real-time quantitative polymerase chain reaction (qRT-PCR) on a QuantStudio 5 real-time PCR Design & Analysis system (Applied Biosystems, Foster City, CA). Each sample was measured in duplicate. Primers sequences used in this study were shown in Table 2. The relative mRNA expression levels were normalized to avian β-actin by the 2–ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Primer sequences used for the real-time PCR analysis.
| Name | Sequence 5’-3’ | GenBank number |
|---|---|---|
| β-actin | F: 5’ -TTGGTTTGTCAAGCAAGCGG-3’ | NM_205518.1 |
| R: 5’ -CCCCCACATACTGGCACTTT-3’ | ||
| TNF-α | F: 5’-TGTGTATGTGCAGCAACCCGTAGT-3’ | NM 204267 |
| R: 5’-GGCATTGCAATTTGGACAGAAGT-3’ | ||
| IL-1β | F: 5’-GCTCTACATGTCGTGTGTGATGAG-3’ | NM_204524 |
| R: 5’-TGTCGATGTCCCGCATGA-3’ | ||
| IL-6 | F: 5’-TCTGTTCGCCTTTCAGACCTA-3’ | AJ309540 |
| R: 5’-GACCACCTCATCGGGATTTAT-3’ | ||
| IFN-γ | F: 5’-CTCCCGATGAACGACTTGAG-3’ | NM_205149.2 |
| R: 5’-CTGAGACTGGCTCCTTTTCC-3’ | ||
| ZO-1 | F: 5’-CTTCAGGTGTTTCTCTTCCTCCTC-3’ | XM_413773.4 |
| R: 5’-CTGTGGTTTCATGGCTGGATC-3’ | ||
| Claudin-1 | F: 5’-ACAACATCGTGACGGCCCA-3’ | NM_001013511.2 |
| R: 5’-CCCGTCACAGCAACAAACAC-3’ | ||
| Occludin | F: 5’-GCAGATGTCCAGCGGTTACTAC-3’ | NM_205128.1 |
| R: 5’-CGAAGAAGCAGATGAGGCAGAG-3’ |
DNA Extraction and PCR Amplification of 16S rRNA Gene Sequences
On d 42, microbial DNA was extracted from 300 mg terminal ileum content samples taken from 6 birds from each treatment group using the E.Z.N.A Soil DNA Kit (Omega Bio-tek, Norcross, GA) according to manufacturer's instructions. The hypervariable region V3–V4 of the bacterial 16S rRNA gene were amplified with primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R(5′-GGACTACHVGGGTWTCTAAT-3′) by an ABI GeneAmp 9700 PCR thermocycler (ABI, CA). The PCR reaction conditions were: initial denaturation at 95 °C for 2 min, followed by 25 cycles consisting of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final extension of 5 min at 72 °C. According to the manufacturer's instructions, amplicons were extracted and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA). The excess primer dimers and dNTPs were removed. Purified amplicons were pooled in equal amounts and paired-end sequenced (2 × 250 bp) throughput analysis was performed at Shanghai Majorbio Bio-Pharm Technology Co., Ltd., using the Illumina MiSeq platform. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database.
Statistical Analysis
Data analysis of growth performance, intestinal relative index, intestinal morphology, gene expression level and differential species identified were performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC). The replicate (each cage) was considered the experimental unit for growth performance and the bird represented the experimental unit for intestinal samples. Data were analyzed using one-way ANOVA and means were separated using Duncan's multiple range test. Differences were considered statistically significant at P < 0.05. Data were expressed as Means ± SD.
For microbiota profiling, raw pair-end sequences were demultiplexed and quality-filtered using The Quantitative Insights Into Microbial Ecology (QIIME, version 1.17; Edgar, 2010). Sequences with length shorter than 150 bp, average Phred scores lower than 20 were filtered through (Dai et al., 2020). The effective reads were clustered into operational taxonomic units (OTUs) based on the 97% similarity. Classification of OTUs at various taxonomic levels were implemented using the Greengenes database. The rarefaction curves and α-diversity analysis were calculated using QIIME (Caporaso et al., 2010). Beta-diversity was estimated using principal coordinate analysis (PCoA) and partial least squares discriminant analysis (PLS-DA). Correlations were analyzed using spearman correlation with the pheatmap package (P < 0.05).
RESULTS
Growth Performance
The effects of SCH on growth performance of broilers at different growing phases are shown in Table 3. There's a clear trend that SCH increased BW, ADG and ADFI during the starter phase (1–14 d). Similarly, during finisher phase (29–42 d), dietary SCH supplementation tended to increase BW, ADG, and improve FCR. In particular, dietary SCH significantly increased broiler BW at 28 d (P < 0.05), and partially increased ADG for grower phase (15–28 d) (P < 0.10). The FCR did not differ, although SCH group had numerically lower FCR (P > 0.05). In general, feeding diet supplemented with SCH tended to improve the performance of broilers.
Table 3.
Effect of dietary SCH supplementation on growth performance of 1 to 42-day-old broilers.
| Item2 | Control | SCH1 | P value |
|---|---|---|---|
| Starter (1–14 d) | |||
| BW at d 14 (g) | 440.6 ± 17.2 | 461.7 ± 23.8 | 0.110 |
| ADG (g) | 26.8 ± 1.2 | 28.3 ± 1.6 | 0.095 |
| ADFI (g) | 33.6 ± 2.1 | 35.1 ± 0.5 | 0.133 |
| FCR | 1.256 ± 0.051 | 1.243 ± 0.067 | 0.715 |
| Grower (15–28 d) | |||
| BW at 28 d (g) | 1,366.3 ± 59.8b | 1,434.6 ± 28.4a | 0.042 |
| ADG (g) | 65.5 ± 4.2 | 69.22 ± 2.01 | 0.074 |
| ADFI (g) | 101.4 ± 7.7 | 106.4 ± 3.3 | 0.176 |
| FCR | 1.551 ± 0.068 | 1.538 ± 0.047 | 0.718 |
| Finisher (29–42 d) | |||
| BW at 42 d (g) | 2,579.8 ± 105.9 | 2,688.7 ± 128.1 | 0.140 |
| ADG (g) | 89.3 ± 6.5 | 92.0 ± 7.5 | 0.513 |
| ADFI (g) | 158.4 ± 12.4 | 159.6 ± 11.4 | 0.863 |
| FCR | 1.775 ± 0.105 | 1.736 ± 0.060 | 0.446 |
| Whole phase (1–42 d) | |||
| ADG (g) | 57.7 ± 2.4 | 60.5 ± 3.2 | 0.112 |
| ADFI (g) | 92.8 ± 6.2 | 95.7 ± 5.2 | 0.407 |
| FCR | 1.608 ± 0.068 | 1.581 ± 0.037 | 0.402 |
| Mortality (%) | 4.74 ± 3.70 | 3.57 ± 2.26 | 0.523 |
The basal diets supplied with 500 mg/kg (d 0–28) and 250 mg/kg (d 29–42) SCH.
Abbreviations: ADG, average daily gain; ADFI, average daily feed intake; BW, body weight; FCR, feed conversion ratio.
Means within a row with no common superscript differ significantly (P < 0.05). Data is presented in mean ± SD (n = 6).
Intestinal Relative Index
Table 4 shows the effect of dietary SCH supplemental on intestinal relative index of broilers. On the whole, there was no significant difference in the relative index of duodenum, jejunum, and ileum between the 2 groups (P > 0.05), but the relative index of duodenum and jejunum in SCH group was numerically higher than the control group at 14, 28, and 42 d of age. In addition, there is a trend that the relative index of ileum increased at 14 and 28 d of age by the SCH diet (P < 0.05).
Table 4.
Effect of dietary SCH supplementation on intestinal relative index of 14, 28, and 42-day-old broilers.
| Item | Control | SCH1 | P value |
|---|---|---|---|
| 14 d | |||
| Duodenum (%) | 1.51 ± 0.11 | 1.54 ± 0.13 | 0.650 |
| Jejunum (%) | 2.48 ± 0.20 | 2.54 ± 0.36 | 0.748 |
| Ileum (%) | 1.74 ± 0.14 | 1.86 ± 0.33 | 0.428 |
| 28 d | |||
| Duodenum (%) | 1.09 ± 0.19 | 1.16 ± 0.40 | 0.696 |
| Jejunum (%) | 2.28 ± 0.21 | 2.79 ± 0.77 | 0.146 |
| Ileum (%) | 2.03 ± 0.39 | 2.33 ± 0.58 | 0.318 |
| 42 d | |||
| Duodenum (%) | 0.70 ± 0.18 | 0.72 ± 0.19 | 0.831 |
| Jejunum (%) | 1.21 ± 0.22 | 1.35 ± 0.29 | 0.384 |
| Ileum (%) | 0.88 ± 0.15 | 0.81 ± 0.22 | 0.528 |
The basal diets supplied with 500 mg/kg (d 0–28) and 250 mg/kg (d 29–42) SCH. Data is presented in mean ± SD (n = 6).
Intestinal Morphology
The effects of basal diet supplemented with SCH on the VH, CD, and VCR of duodenum, jejunum, and ileum are shown in Table 5. At 14 d of age, compared with the control group, dietary SCH supplementation tended to increase the VH and VCR of the 3 intestinal segments, and reduce the CD of three intestinal segments (P > 0.05). At 28 and 42 d of age, dietary SCH significantly increased the VH and VCR of jejunum (P < 0.05). Furthermore, at 42 d of age, dietary SCH increased the VCR and reduced the CD of ileum significantly (P < 0.05). On the whole, feeding diet supplemented with SCH reduced the CD of the duodenum, jejunum and ileum, increase the VH and VCR to a certain extent, and effectively improved intestinal morphology.
Table 5.
Effect of dietary SCH supplementation on intestinal morphology of 14, 28, and 42-day-old broilers.
| Item2 | Control | SCH1 | P value | |
|---|---|---|---|---|
| 14 d | ||||
| Duodenum | VH (μm) | 1,216.05 ± 219.16 | 1,265.14 ± 269.96 | 0.737 |
| CD (μm) | 168.51 ± 21.90 | 160.24 ± 25.91 | 0.564 | |
| VCR | 7.18 ± 0.52 | 7.85 ± 0.59 | 0.067 | |
| Jejunum | VH (μm) | 1,004.34 ± 126.19 | 1,065.50 ± 144.05 | 0.452 |
| CD (μm) | 155.67 ± 23.95 | 151.77 ± 24.08 | 0.785 | |
| VCR | 6.49 ± 0.69 | 7.05 ± 0.51 | 0.144 | |
| Ileum | VH (μm) | 586.96 ± 69.53 | 647.69 ± 59.55 | 0.135 |
| CD (μm) | 140.62 ± 24.20 | 136.89 ± 16.44 | 0.762 | |
| VCR | 4.29 ± 0.88 | 4.76 ± 0.52 | 0.284 | |
| 28 d | ||||
| Duodenum | VH (μm) | 1,492.25 ± 142.91 | 1,614.37 ± 129.39 | 0.152 |
| CD (μm) | 192.07 ± 33.64 | 182.92 ± 33.48 | 0.604 | |
| VCR | 8.01 ± 0.96 | 8.89 ± 0.65 | 0.096 | |
| Jejunum | VH (μm) | 1,132.98 ± 114.54b | 1,291.81 ± 124.78a | 0.045 |
| CD (μm) | 161.53 ± 15.59 | 158.04 ± 20.00 | 0.743 | |
| VCR | 7.02 ± 0.41b | 8.24 ± 0.91a | 0.013 | |
| Ileum | VH (μm) | 663.32 ± 78.23 | 705.53 ± 69.28 | 0.346 |
| CD (μm) | 153.90 ± 16.67 | 141.97 ± 9.51 | 0.159 | |
| VCR | 4.40 ± 1.05 | 5.00 ± 0.71 | 0.276 | |
| 42 d | ||||
| Duodenum | VH (μm) | 1,600.01 ± 213.03 | 1,693.67 ± 315.11 | 0.640 |
| CD (μm) | 193.04 ± 24.12 | 185.76 ± 32.33 | 0.731 | |
| VCR | 8.29 ± 0.31 | 9.11 ± 0.63 | 0.057 | |
| Jejunum | VH (μm) | 1,317.16 ± 123.97b | 1,498.97 ± 80.24a | 0.020 |
| CD (μm) | 185.47 ± 25.98 | 167.07 ± 12.57 | 0.184 | |
| VCR | 7.15 ± 0.46b | 8.99 ± 0.41a | <0.001 | |
| Ileum | VH (μm) | 952.92 ± 85.76 | 1078.36 ± 167.15 | 0.141 |
| CD (μm) | 161.57 ± 9.58a | 144.65 ± 11.81b | 0.022 | |
| VCR | 5.89 ± 0.33 | 7.45 ± 0.97 | 0.005 | |
The basal diets supplied with 500 mg/kg (d 0–28) and 250 mg/kg (d 29–42) SCH.
Abbreviations: CD, crypt depth; VH, villus height; VCR, the ratio of villus height to crypt depth.
Means within a row with no common superscript differ significantly (P < 0.05). Data is presented in mean ± SD (n = 6).
The mRNA Expression Levels of Immunomodulatory Genes and Tight Junction Protein
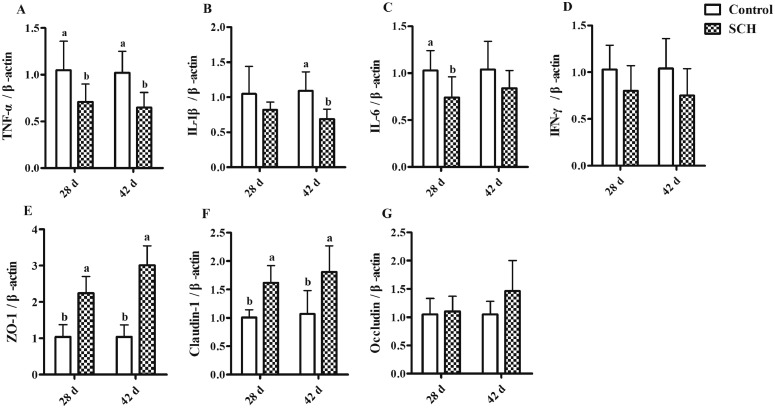
The mRNA expression levels of immunomodulatory genes and tight junction protein in chicken jejunum mucosa are shown in Figure 1. At 28 d of age, compared to the control group, the TNF-α and IL-6 mRNA expressions in jejunum significantly decreased in the SCH groups (P < 0.05, Figures 1A and 1C). Diet supplemented with SCH significantly downregulated the mRNA expression of TNF-α and IL-1β at 42 d of age (P < 0.05, Figures 1A and 1B). Furthermore, the ZO-1 and Claudin-1 mRNA expressions in jejunum of SCH group had a significant increase compared to the control group (P < 0.05, Figures 1E and1 F). There was no significant difference in the Occludin mRNA expression between the 2 groups (P > 0.05). In general, dietary addition of SCH reduced the expression level of intestinal inflammatory factors, increase the expression level of tight junction protein, alleviate the intestinal inflammatory response of broilers, and play an important role in immune regulation.
Figure 1.
The mRNA expression level of immunomodulatory genes and tight junction protein in chicken jejunum mucosa at 28 and 42 d. (A–D) were relative immunomodulatory genes mRNA expression, (E–G) were relative tight junction protein mRNA expression. Control, control group with the basal diets; SCH, the basal diets supplied with 500 mg/kg (d 0–28) and 250 mg/kg (d 29–42) SCH. a, b Means within a row with no common superscript differ significantly (P < 0.05).
Cecum Microbiota Analysis by 16S rRNA
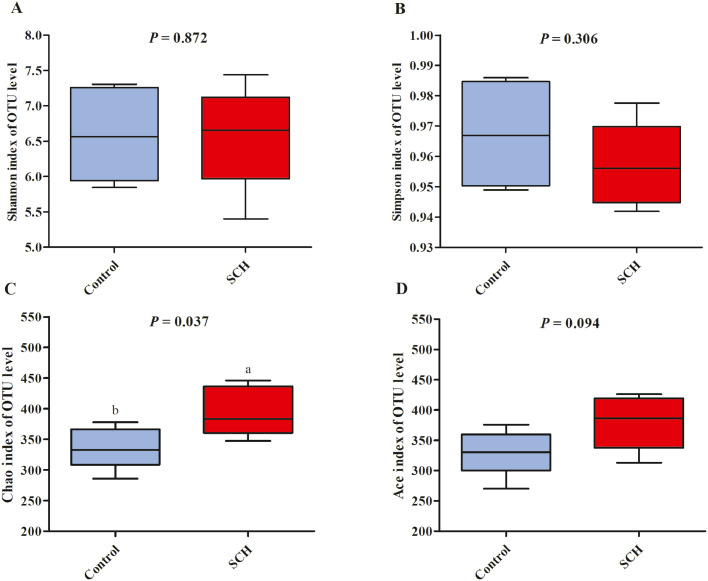
After filtering, an average of 53,524 reads per sample was obtained. First, sequencing depths were examined by plotting the rarefaction curve for richness and the numbers of shared OTUs. Most of the samples reached plateaus, indicating that sampling depth was adequate. Bacterial α-diversity in cecum microbiota was estimated using Shannon, Simpson, and Chao indices of diversity and richness. As shown in Figure 2, there was no significant difference in Shannon, Simpson, or Ace indices between the 2 groups (P > 0.05), but Chao index in SCH group was significantly higher than control group (P < 0.05, Figure 2C).
Figure 2.
Effects of dietary supplementation with SCH on the cecum microbial α-diversity of broilers on day 42. A–D were Shannon, Simpson, Chao, and Ace index of OUT level results respectively. Control, control group with the basal diets; SCH, the basal diets supplied with 500 mg/kg (d 0–28) and 250 mg/kg (d 29–42) SCH. a, b Means within a row with no common superscript differ significantly (P < 0.05).
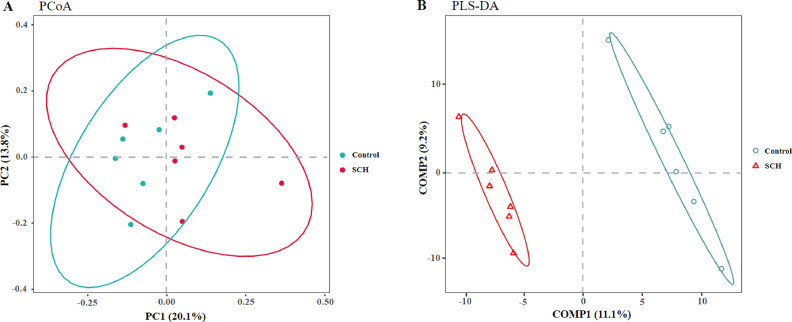
Beta-diversity analysis was performed to compare the overall microbial profiles of 2 groups as displayed in Figure 3. PCoA analysis was performed to present a holistic perception of the microbiota via weighted UniFrac distance metric. Results for PCoA showed that the visual separation effect of microbial samples was not significant between the 2 groups (Figure 3A), but PLS-DA plot defined groups where samples from different groups occupied distinct positions (Figure 3B), which indicated that the microbiota compositions were dissimilar within the two groups.
Figure 3.
Effects of dietary supplementation with SCH on cecum microbial β-diversity of broilers on d 42. (A) principal coordinate analysis (PCoA) based on based on weighted UniFrac distance calculated from OTU abundance matrix; (B) partial least squares discriminant analysis (PLS-DA); Analysis of similarities (ANOSIM) of weighted UniFrac distances. Control, control group with the basal diets; SCH, the basal diets supplied with 500 mg/kg (d 0–28) and 250 mg/kg (d 29–42) SCH.
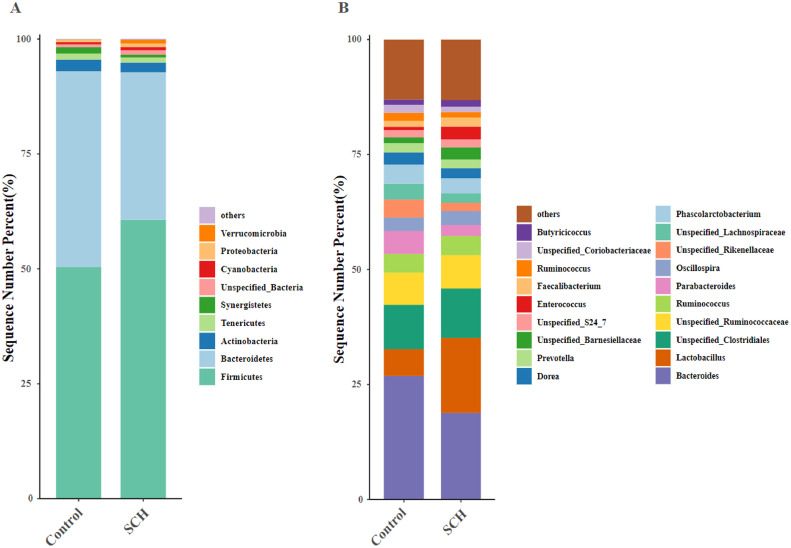
To assess the differences induced by SCH in the cecum microbiota, taxonomic compositions were analyzed at phyla and genus levels in Figure 4. At the phylum level, Bacteroidetes and Firmicutes are 2 dominant bacteria phyla of cecum. Broilers fed with SCH diet were characterized by higher relative abundance of Firmicutes (60.41%: 50.26%) and lower abundance of Bacteroidetes (32.42%: 42.80%) compared with the control group, thus leading to a higher ratio of Firmicutes to Bacteroidetes ratio (Figure 4A). Compared with control group at the genus level, Lactobacillus and Faecalibacterium in SCH group were increased by 2.80- and 3.86-fold (16.31%:5.83% and 2.73%:0.71%, respectively), meanwhile Bacteroides was decreased by 1.42-fold (19.10%:27.13%, Figure 4B).
Figure 4.
Compositions of cecum microbiota and differential species identified in broilers at phylum and genus level on d 42. (A, B) were the bacterial community compositions at phylum and genus level, respectively. Control, control group with the basal diets; SCH, the basal diets supplied with 500 mg/kg (d 0–28) and 250 mg/kg (d 29–42) SCH.
DISCUSSION
Considerable studies have documented that the dietary SCH supplementation improved growth performance of animals (Gao et al., 2008; Afsharmanesh et al., 2010; Superchi et al., 2012). In the current study, our data showed that dietary SCH supplementation increased BW, ADG, and ADFI of broilers during the starter and grower phase. It is worth noting that during the starter, grower and overall period, the SCH group enhanced ADG by 5.6, 5.8, and 5.0%, and enhanced BW by 4.8, 5.0, and 4.3%, respectively. Similarly, positive results were observed on growth performance of broilers fed diets with yeast hydrolysate (Li et al., 2016; Wang et al., 2017). One explanation for the growth promotion effects of SCH may be that the yeast-derived additive or its components could improve the anti-inflammatory effect in animals as previously reported (Salinas-Chavira et al., 2018). In addition, it was reported that SCH could improve the growth performance by increasing the VH, reducing intestinal pH, regulating intestinal microbes, increasing the secretion of auxiliary digestive enzymes, and improves nutrient absorption (Zhang et al., 2014). The main reason for the lack of improvement in growth performance in the current study may be related to the reduction of SCH addition in finisher stage.
Our results showed that feeding SCH only slightly improved the relative index of the jejunum, the finding was in accordance with previous studies (Singh et al., 2017). SCH supplementation may have a trophic effect on jejunum and ileum compared to duodenum as proved by the increased VH and VCR observed in the current study, which was consistent with previous observation (Zhang et al., 2005). The improvement of intestinal morphology suggested an ameliorated intestinal nutrient digestibility and absorption capacity and might further contribute to subsequent enhancement in the growth performance (Montagne et al., 2003). Whereas some others documented that no positive effects on intestinal morphology of broilers fed diet supplemented with hydrolyzed yeast products was observed (Baurhoo et al., 2009; Reisinger et al., 2012), which may be related to the composition of yeast hydrolysate and the farming conditions. Different farming conditions, such as farm environment, breeding mode, litter and so on, may have a certain impact on the microbial diversity and community in the intestinal of broilers, which will further affect the intestinal development and growth performance (Gupta, et al., 2021; Xiao et al., 2021). Therefore, the actual application effect of the SCH needs to be evaluated according to its composition and the conditions of the applied farm.
The size of the gap between intestinal epithelial cells is mainly controlled by tight junction proteins, including occludin, claudin, and zonula occludens (ZO) families (Tang et al., 2015). The intestinal barrier regulated by tight junction proteins performs the crucial role of defense against the passage of pathogens and antigens into the intestinal epithelium (Broom, 2018). Claudins and occludin are transmembrane proteins that are responsible for regulating the size of the intercellular space (Suzuki, 2013). ZO-1 is present in intestinal epithelial cells and can be attached to claudins and occludin to increase the stability of tight junction (Bauer et al., 2010). Our results showed that the mRNA expression of ZO-1 and Claudin-1 in SCH group was higher than control group, which were consistent with previous studies (Chuang et al., 2021a), indicating that broiler chickens fed with SCH had more stable intestinal environment. Therefore, it can be inferred that SCH may improve the integrity of the intestinal epithelium, thereby creating a more friendly gut environment, which could help to resist pathogen infection.
The most important regulator of inflammation is the IL family (Awaad et al., 2011). As one of the most important members of the IL family, IL-1β mediates many pathways involved in apoptosis or inflammation (Gabay et al., 2010). Previous study reported that IL-1β is inhibited by β-glucan (Municio et al., 2013), which is abundant in SCH. Important pro-inflammatory cytokines TNF-α and IL-1β can activate macrophages that regulate cell death and inflammation. IFN-γ is associated with infection and high concentration of IFN-γ may contribute to autoimmune disease (Ivashkiv and Donlin, 2014). The reduction of the pro-inflammatory cytokine related to inflammatory response can decrease energy loss and improve the cell survival rate (Lee et al., 2017). The above results showed that SCH could exert an anti-inflammatory effect by inhibiting the excessive expressions of IL-1β, IL-6, and TNF-α, which was consistent with previous study (Superchi et al., 2012).
The alterations in intestinal microbiota may substantially affect the intestinal barrier function and inflammation reaction (Liu et al., 2020; Desai et al., 2016). In order to better understand the connection between intestinal barrier and gut microflora, the cecum content which contains the most detailed information regarding chicken gut microbiota was analyzed with 16s rRNA methodology (Pourabedin and Zhao, 2015). Similar to the previous reports (Chuang et al., 2021b), data from analyses of α-diversity and β-diversity corroborate the initial hypothesis that SCH improved microbial richness and altered microbiota structure to a certain extent. The diversity of the intestinal tract microbiota community is believed to have a positive effect on the productivity of the bird (Janczyk et al., 2009).
In the current study, higher Firmicutes-to-Bacteroidetes ratio at the phylum level demonstrated that SCH changed cecum microbiome composition of birds. The abundance of Firmicutes has been proved to be positively correlated with energy and nutrient absorption, while the increase in fecal Bacteroidetes is associated with poor nutrient digestibility (Turnbaugh et al., 2006; Jumpertz et al., 2011), which indicated that the higher ratio of Firmicutes to Bacteroidetes may improve nutrient digestibility and lead to greater body weight (Paola et al., 2010; Singh et al., 2013). Therefore, the increase in abundance of Firmicutes accompanied by the decrease in abundance of Bacteroidetes may contribute to the nutrient utilization of broilers.
Further analyses revealed more differential species at various taxonomic levels between the 2 groups. At the genus level, there was an increased abundance of Lactobacillus in cecum of birds fed SCH. As one of the main genera in the chicken gut, Lactobacillus can protect the intestinal barrier by antagonizing pathogens (Servin, 2004). At the same time, the lactic acid produced by the fermentation of Lactobacillus could be used by butyric acid producers, thereby increasing the digestibility of nutrients and improving intestinal morphology. The enrichment of Faecalibacterium in SCH addition group also proved this possible pattern. Faecalibacterium was one of the most abundant symbiotic bacteria in the colon of healthy people and an important butyrate-producing bacteria in the intestine. Furthermore, Faecalibacterium also plays an important role in the intestine of broilers. It can quickly adhere to the intestinal mucosa, inhibit pathogenic bacteria from adhering to the intestinal tract through the exclusion effect, and form the intestinal barrier, so as to protect the intestinal health (Eeckhaut et al., 2011; Liu et al., 2019). Previous studies had confirmed that the abundance of Bacteroides is positively correlated with the expression of IL-1β and TNF-α in birds (Wang et al., 2019), which may disrupt the epithelial barrier function (Matthias et al., 2003). In this study, dietary SCH addition triggered a decreased in the abundances of Bacteroides and the improvement of intestinal barrier function.
In summary, alteration of the bacterial phylotypes indicated that SCH can improve the immune response and intestinal barrier function by regulating the cecum microbiota, such as supporting commensal lactic acid bacteria and diminishing the detrimental bacteria (Wilson et al., 2005; Yang et al., 2008), which can help to maintain the intestinal morphology and improve the production performance of broilers.
CONCLUSIONS
The overall results revealed that dietary SCH supplementation in broilers could improve growth performance, intestinal morphology and barrier function, while regulating intestinal inflammation, which might be attributed to the enhancement of bacterial richness and alteration of microbial composition, particularly the enrichment of SCFAs-producing bacteria. The understanding of the regulatory role of SCH on intestinal health and growth performance in poultry production warrants further study.
ACKNOWLEDGMENTS
This study was supported by Shandong Key Science and Technology Innovation Program (2019JZZY010704), Agricultural Science and Technology Innovation Program (ASTIP) of the Chinese Academy of Agricultural Sciences, and Prosol S.p.A.
DISCLOSURES
All authors approve the submission of this manuscript and declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102237.
Appendix. Supplementary materials
REFERENCES
- Afsharmanesh M., Barani M., Silversides F.G. Evaluation of wet-feeding wheat-based diets containing Saccharomyces cerevisiae to broiler chickens. Br. Poult. Sci. 2010;51:776–783. doi: 10.1080/00071668.2010.531006. [DOI] [PubMed] [Google Scholar]
- Awaad M., Atta A.M., El-Ghany W.A., Elmenawey M., Ahmed A., Hassan A.A., Nada A., Abdelaleem G.A., Kawkab A.A. Effect of a specific combination of mannan-oligosaccharides and β-glucans extracted from yeast cell wall on the health status and growth performance of ochratoxicated broiler chickens. J. Am. Sci. 2011;7:82–96. [Google Scholar]
- Bai K.W., Feng C.C., Jiang L.Y., Zhang L.G., Zhang J.F., Zhang L.L., Wang T. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 2018;97:2312–2321. doi: 10.3382/ps/pey116. [DOI] [PubMed] [Google Scholar]
- Bauer H., Zweimueller-Mayer J., Steinbacher P., Lametschwandtner A., Bauer H.C. The dual role of zonula occludens (ZO) proteins. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurhoo B., Ferket P.R., Zhao X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 2009;88:2262–2272. doi: 10.3382/ps.2008-00562. [DOI] [PubMed] [Google Scholar]
- Broom L.J. Gut barrier function: effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 2018;97:1572–1578. doi: 10.3382/ps/pey021. [DOI] [PubMed] [Google Scholar]
- Bu X.Y., Huang J.S., Tao S.Q., Yang J.M., Liao Y.L., Liu H.J., Yang Y.H. Yeast cultures alleviate gossypol induced inflammatory response in liver tissue of Ussuri catfish (Pseudobagrus ussuriensis) Aquaculture. 2020;518 [Google Scholar]
- Bu X.Y., Lian X.Q., Wang Y., Luo C.Z., Tao S.Q., Liao Y.L., Yang J.M., Chen A.J., Yang Y.H. Dietary yeast culture modulates immune response related to TLR2-MyD88-NF-kB signaling pathway, antioxidant capability and disease resistance against Aeromonas hydrophila for Ussuri catfish (Pseudobagrus ussuriensis) Fish Shellfish Immunol. 2019;84:711–718. doi: 10.1016/j.fsi.2018.10.049. [DOI] [PubMed] [Google Scholar]
- Caly D.L., D'Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang W.Y., Lin L.J., Hsieh Y.C., Chang S.C., Lee T.T. Effects of Saccharomyces cerevisiae and phytase co-fermentation of wheat bran on growth, antioxidation, immunity and intestinal morphology in broilers. Anim. Biosci. 2021;34:1157–1168. doi: 10.5713/ajas.20.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang W.Y., Lin L.J., Shih H., Shy Y.M., Chang S.C., Lee T.T. Intestinal microbiota, anti-inflammatory, and anti-oxidative status of broiler chickens fed diets containing mushroom waste compost by-products. Animals (Basel). 2021;11:2550. doi: 10.3390/ani11092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D., Wu S.G., Zhang H.J., Qi G.H., Wang J. Dynamic alterations in early intestinal development, microbiota and metabolome induced by in ovofeeding of L-arginine in a layer chick model. J. Anim. Sci. Biotechnol. 2020;11:19. doi: 10.1186/s40104-020-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delezie E., Maertens L., Huyghebaert G. Consequences of phosphorus interactions with calcium, phytase, and cholecalciferol on zootechnical performance and mineral retention in broiler chickens. Poult. Sci. 2012;91:2523–2531. doi: 10.3382/ps.2011-01937. [DOI] [PubMed] [Google Scholar]
- Desai M.S., Seekatz A.M., Koropatkin N.M., Stappenbeck T.S., Martens E.C. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra M.S., Malouin F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014;5:282. doi: 10.3389/fmicb.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V., Van Immerseel F., Croubels S., De Baere S., Haesebrouck F., Ducatelle R., Louis P., Vandamme P. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb. Biotechnol. 2011;4:503e12. doi: 10.1111/j.1751-7915.2010.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadl S.E., El-Gammal G.A., Sakr O.A., Salah A.A.B.S., Atia A.A., Prince A.M., Hegazy A.M. Impact of dietary mannan-oligosaccharide and b-glucan supplementation on growth, histopathology, E-coli colonization and hepatic transcripts of TNF-a and NF- kB of broiler challenged with E. coli O78. BMC. Vet. Res. 2020;16:204. doi: 10.1186/s12917-020-02423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R., Chen D., Tian G., Zheng P., Mao X., Yu J., He J., Huang Z., Luo Y., Yu B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2019;5:366–372. doi: 10.1016/j.aninu.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C., Lamacchia C., Palmer G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health. Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H.J., Yu S.H., Wu S.G., Yoon I., Quigley J., Gao Y.P., Qi G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Gupta C.L., Avidov R., Kattusamy K., Saadi I., Varma V.S., Blum S.E., Zhu Y.G., Zhou X.Y., Su J.Q., Laor Y., Cytryn E. Spatial and temporal dynamics of microbiomes and resistomes in broiler litter stockpiles. Comput. Struct. Biotechnol. J. 2021;19:6201–6211. doi: 10.1016/j.csbj.2021.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczyk P., Halle B., Souffrant W.B. Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris. Poult. Sci. 2009;88:2324e32. doi: 10.3382/ps.2009-00250. [DOI] [PubMed] [Google Scholar]
- Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I., Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011;94:58e65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid A.H., Ullah K.S., Naveed S., Latif F., Pasha T.N., Hussain I., Qaisrani S.N. Effects of spray dried yeast (Saccharomyces cerevisiae) on growth performance and carcass characteristics, gut health, cecal microbiota profile and apparent ileal digestibility of protein, amino acids and energy in broilers. Trop. Anim. Health. Prod. 2021;53:252. doi: 10.1007/s11250-021-02684-5. [DOI] [PubMed] [Google Scholar]
- Lee Y., Lee S.H, Gadde U.D., Oh S.T., Lee S.J., Lillehoj H.S. Dietary Allium hookeri reduces inflammatory response and increases expression of intestinal tight junction proteins in LPS-induced young broiler chicken. Res. Vet. Sci. 2017;112:149–155. doi: 10.1016/j.rvsc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Li X.H., Chen Y.P., Cheng Y.F., Yang W.L., Wen C., Zhou Y.M. Effect of yeast cell wall powder with different particle sizes on the growth performance, serum metabolites, immunity and oxidative status of broilers. Anim. Feed. Sci. Technol. 2016;212:81–89. [Google Scholar]
- Liu S.M., Li E.Y., Sun Z.Y., Fu D.J., Duan G.Q., Jiang M.M. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019;9:287e95. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Yu Z., Tian F., Zhao J., Zhang H., Zhai Q. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell. Fact. 2020;19:23. doi: 10.1186/s12934-020-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matthias B., Andreas L., Torsten K., Parkos C.A., Madara J.L., Hopkins A.M. Proin-flammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003;171:6164e72. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Montagne L., Crévieu-Gabriel I., Toullec R., Lallès J.P. Influence of dietary protein level and source on the course of protein digestion along the small intestine of the veal calf. J. Dairy. Sci. 2003;86:934–943. doi: 10.3168/jds.S0022-0302(03)73676-5. [DOI] [PubMed] [Google Scholar]
- Municio C., Alvarez Y., Montero O., Hugo E., Rodríguez M., Domingo E., Alonso S., Fernández N., Crespo M.S. The response of human macrophages to β-glucans depends on the inflammatory milieu. PLoS One. 2013;8:e62016. doi: 10.1371/journal.pone.0062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. 9th rev. ed. Press, Washington, DC; 1994. [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut. Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R., Shah T.M., Limon G., Psifidi A., Guitian J., Hume D.A., Tomley F.M., Rank D.N., Raman M., Tirumurugaan K.G., Blake D.P., Joshi C.G. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paola M.D., Filippo C.D., Cavalieri D., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14691e6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone V., Sandrini S., Irshad N., Savoini G., Comi M., Agazzi A. Yeast-derived products: the role of hydrolyzed yeast and yeast culture in poultry nutrition-a review. Animals (Basel). 2022;12:1426. doi: 10.3390/ani12111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS. Microbiol. Lett. 2015;362:122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Reisinger N., Ganner A., Masching S., Schatzmayr G., Applegate T.J. Efficacy of a yeast derivative on broiler performance, intestinal morphology and blood profile. Livest. Sci. 2012;143:195–200. [Google Scholar]
- Salaheen S., Kim S.W., Haley B.J., Van Kessel J.A.S., Biswas D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 2017;8:2088. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Chavira J., Montano M.F., Torrentera N., Zinn R.A. Influence of feeding enzymatically hydrolysed yeast cell wall+yeast culture on growth performance of calf-fed Holstein steers. J. Appl. Anim. Res. 2018;46:327–330. [Google Scholar]
- Samarasinghe K., Shanmuganathan T., Silva K., Wenk C. Influence of supplemental enzymes, yeast culture and effective micro-organism culture on gut micro-flora and nutrient digestion at different parts of the rabbit digestive tract. Asian-Australas. J. Anim. Sci. 2004;17:830–835. [Google Scholar]
- Santovito E., Greco D., Logrieco A.F., Avantaggiato G. Eubiotics for food security at farm level: Yeast cell wall products and their antimicrobial potential against pathogenic bacteria. Foodborne Pathog. Dis. 2018;15:531–537. doi: 10.1089/fpd.2018.2430. [DOI] [PubMed] [Google Scholar]
- Sauer N. Nucleotides modify growth of selected intestinal bacteria in vitro. Livest Sci. 2010;133:161e3. [Google Scholar]
- Servin A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS. Microbiol. Rev. 2004;28:405e40. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Singh P., Karimi A., Devendra K., Waldroup P.W., Cho K.K., Kwon Y.M. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult. Sci. 2013;92:272–276. doi: 10.3382/ps.2012-02603. [DOI] [PubMed] [Google Scholar]
- Singh S., Singh R., Mandal A. Associated efficiency of Saccharomyces cerevisiae and vitamin E in ameliorating adverse effects of ochratoxin on carcass traits and organ weights in broiler chickens. Indian J. Poult. Sci. 2017;52:22–27. [Google Scholar]
- Superchi P., Saleri R., Borghetti P., Angelis E.D., Ferrari L., Cavalli V., Amicucci P., Ossiprandi M.C., Sabbioni A. Effects of dietary nucleotide supplementation on growth performance and hormonal and immune responses of piglets. Animal. 2012;6:902e8. doi: 10.1017/S1751731111002473. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithet lialperme ability by tight junctions. Cell Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Lee J., Chen W.N. Engineering the fatty acid metabolic pathway in Saccharomyces cerevisiae for advanced biofuel production. Metab. Eng. Commun. 2015;2:58–66. doi: 10.1016/j.meteno.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Vincent M., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027e31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Wang W.W., Jia H.J., Zhang H.J., Wang J., Lv H.Y., Wu S.G., Qi G.H. Supplemental plant extracts from flos ionicerae in combination with baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by salmonella pullorum. Front. Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tsai T.C., Walk C.L., Wilcock P., Maxwell C.V. Effect of yeast cell wall (YCW) inclusion rate on growth performance in nursery pigs. J. Anim. Sci. 2017;952:105. [Google Scholar]
- Wilson J., Tice G., Brash M.L., Hilaire S.S. Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens. Worlds Poult. Sci. J. 2005;61:435–449. [Google Scholar]
- Xiao S.S., Mi J.D., Mei L., Liang J., Feng K.X., Wu Y.B., Liao X.D., Wang Y. Microbial diversity and community variation in the intestines of layer chickens. Animals (Basel). 2021;11:840. doi: 10.3390/ani11030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Iji P.A., Kocher A., Mikkelsen L.L., Choct M. Effects of dietary mannanoligosaccharide on growth performance, nutrient digestibility and gut development of broilers given different cereal-based diets. J. Anim. Physiol. Anim. Nutr (Berl). 2008;92:650–659. doi: 10.1111/j.1439-0396.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- Zhang A.W., Lee B.D., Lee S.K., Lee K.W., An G.H., Song K.B., Lee C.H. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci. 2005;84:1015e21. doi: 10.1093/ps/84.7.1015. [DOI] [PubMed] [Google Scholar]
- Zhang J., Lü F., Shao L., He P. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014;168:252–258. doi: 10.1016/j.biortech.2014.02.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.