Abstract
Background
The U.S. Food and Drug Administration (FDA) Biologics Effectiveness and Safety (BEST) Initiative conducts active surveillance of adverse events of special interest (AESI) after COVID-19 vaccination. Historical incidence rates (IRs) of AESI are comparators to evaluate safety.
Methods
We estimated IRs of 17 AESI in six administrative claims databases from January 1, 2019, to December 11, 2020: Medicare claims for adults ≥ 65 years and commercial claims (Blue Health Intelligence®, CVS Health, HealthCore Integrated Research Database, IBM® MarketScan® Commercial Database, Optum pre-adjudicated claims) for adults < 65 years. IRs were estimated by sex, age, race/ethnicity (Medicare), and nursing home residency (Medicare) in 2019 and for specific periods in 2020.
Results
The study included >100 million enrollees annually. In 2019, rates of most AESI increased with age. However, compared with commercially insured adults, Medicare enrollees had lower IRs of anaphylaxis (11 vs 12–19 per 100,000 person-years), appendicitis (80 vs 117–155), and narcolepsy (38 vs 41–53). Rates were higher in males than females for most AESI across databases and varied by race/ethnicity and nursing home status (Medicare). Acute myocardial infarction (Medicare) and anaphylaxis (all databases) IRs varied by season. IRs of most AESI were lower during March–May 2020 compared with March–May 2019 but returned to pre-pandemic levels after May 2020. However, rates of Bell’s palsy, Guillain-Barré syndrome, narcolepsy, and hemorrhagic/non-hemorrhagic stroke remained lower in multiple databases after May 2020, whereas some AESI (e.g., disseminated intravascular coagulation) exhibited higher rates after May 2020 compared with 2019.
Conclusion
AESI background rates varied by database and demographics and fluctuated in March–December 2020, but most returned to pre-pandemic levels after May 2020. It is critical to standardize demographics and consider seasonal and other trends when comparing historical rates with post-vaccination AESI rates in the same database to evaluate COVID-19 vaccine safety.
Keywords: Background rates, Vaccine safety surveillance, COVID-19, Adverse events
Abbreviations: AESI, Adverse Event of Special Interest; AMI, Acute Myocardial Infarction; BEST, CBER Biologics Effectiveness and Safety; BHI, Blue Health Intelligence; CBER, Center for Biologics Evaluation and Research; CI, Confidence Interval; CMS, Centers for Medicare & Medicaid Services; DIC, Disseminated Intravascular Coagulation; DVT, Deep Vein Thrombosis; EHR, Electronic Health Record; EUA, Emergency Use Authorization; FDA, Food and Drug Administration; GBS, Guillain-Barré Syndrome; ICD-10-CM/PCS, International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System; IR, Incidence Rate; IRR, Incidence Rate Ratio; ITP, Immune Thrombocytopenia; PE, Pulmonary Embolism; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; TTS, Thrombosis with Thrombocytopenia Syndrome
1. Introduction
Coronavirus disease 2019 (COVID-19) is a contagious respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On January 30, 2020, the World Health Organization declared that the COVID-19 outbreak constituted a global public health emergency [1]. As of June 2022, there have been more than 530 million confirmed cases and more than 6 million deaths worldwide—the largest contributor is the United States [2]. Since December 2020, Pfizer-BioNTech, Moderna, Janssen, and Novavax COVID-19 vaccines have been available under emergency use authorization (EUA) or full licensure (Pfizer-BioNTech for ages ≥ 16 years; Moderna for ages ≥ 18 years) by the U.S. Food and Drug Administration (FDA). Additional COVID-19 vaccine candidates are under study in pre-licensure clinical trials [3].
As with all authorized or licensed medical products, clinical trials evaluating COVID-19 vaccine safety have limitations. Even large phase III trials may have limited statistical power to detect rare adverse events [4]. Post-market surveillance of potential adverse events of special interest (AESI) is needed to continue monitoring the safety of authorized or approved COVID-19 vaccines. An AESI is an untoward occurrence of medical concern that follows immunization but does not necessarily have a causal relationship with vaccination [5]. The FDA Center for Biologics Evaluation and Research (CBER) monitors the safety of authorized or approved COVID-19 vaccines using passive and active surveillance systems, in collaboration with other agencies [6]. The FDA Biologics Effectiveness and Safety (BEST) Initiative uses a broad network of large-scale data sources to rapidly monitor vaccine safety where rates of AESI in historical controls serve as comparator (expected) rates.
The background rate is the AESI’s incidence rate (IR) estimated from historical cohorts. Background rates of AESI are important in vaccine safety monitoring because they may serve as one comparator to contextualize the observed IRs of the same AESI following vaccination in a similar population. In addition, stratified AESI background IRs may provide more appropriate comparators than overall IRs for the respective stratum of vaccinated individuals. At the time of this study, published background rates of AESI in the U.S. population using multiple data sources and including both pre-COVID-19 (before 2020) and peri-COVID-19 (after March 2020) periods were limited [7]. Furthermore, the COVID-19 pandemic presented unprecedented challenges to the healthcare system and may have altered patients’ care-seeking patterns and rates of reported AESI. Studies report that healthcare service utilization decreased in 2020 [8], [9], [10] but returned to near pre-pandemic rates in late 2020 [11].
Using administrative claims data sources in the BEST Initiative, this study estimated background rates of 17 AESI, overall and stratified by population characteristics, in six data sources. We evaluated monthly trends in IRs during 2019 and 2020 to better understand how the pandemic may have affected utilization patterns and AESI rates. We also estimated rates of certain negative control events during 2019 and 2020; these are considered unrelated to vaccination but may reflect changes in healthcare utilization over time. A study report with more comprehensive results from these analyses, along with the results of additional analyses not included in this article, is on the BEST Initiative website [12]. The report presents results from an extended study period (2017–2020), background rates of additional AESI and negative control events, and rates of AESI and negative control events in other subpopulations of interest (e.g., population with recent influenza vaccine). This article excludes those results due to limited space.
2. Methods
2.1. Data sources
We used six administrative claims databases from the United States. Individuals aged ≥ 65 years with Medicare coverage were identified from Centers for Medicare & Medicaid Services (CMS) Medicare fee-for-service claims for beneficiaries enrolled in Medicare Parts A/B. We identified data on commercially insured adults aged < 65 years and children aged < 18 years from Blue Health Intelligence® (BHI) commercial claims, CVS Health (Aetna) commercial claims, the HealthCore Integrated Research Database®, the IBM® MarketScan® Commercial Database, and Optum pre-adjudicated commercial claims. BHI data were limited to claims for enrollees who received a biologic product, were pregnant, or were born after October 1, 2015. Appendix A includes descriptions of the data sources.
The study involved no personally identifiable information, and the data used in this study were deidentified and anonymized before use. It was conducted as a public health mandate and not as a research activity. Our study practices were performed in accordance with the Declaration of Helsinki guidelines.
2.2. Study period
The study period was from January 1, 2019, through December 11, 2020, when FDA issued the first EUA for a vaccine to prevent COVID-19. The observation period started January 1, 2018, to evaluate the clean period requirement (described in Section 2.4). For analysis of the MarketScan data, the study period ended October 31, 2020, to ensure data included in the study were at least 80 % complete.
We subset the study period to the pre-COVID-19 period (calendar year 2019) and peri-COVID-19 period (March–October 2020 [MarketScan] and March–December 2020 [all other databases]). We further classified the peri-COVID-19 period into an initial period (March–May 2020) and a later period (June–October 2020 [MarketScan] and June–December 2020 [all other databases]). We chose these subperiods after observing that the rates of negative control events decreased during March–May 2020 but returned to pre-pandemic levels by June 2020.
2.3. AESI and negative control events
In selecting AESI, we considered serious events that have been studied with other vaccines, events that are suspected as possibly related to novel vaccine platforms or adjuvants, and events related to COVID-19 severity that may potentially relate to vaccine failure/immunogenicity (enhanced disease). Other considerations included recommendations from other surveillance research networks, such as the Brighton Collaboration [13], and events specific to certain populations of interest, such as pregnant or immunocompromised individuals [4], [14], [15]. AESI were not selected based on adverse events observed in pre-authorization or pre-licensure studies of COVID-19 vaccines.
AESI were identified using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes. Development of administrative claims-based AESI algorithms was based on literature reviews and consultations with clinical experts. Claims data came from inpatient facilities, emergency departments, and/or other outpatient facilities and individual healthcare providers or professionals. The healthcare settings in which AESI and negative control events were captured differed by the event (see Supplemental Table 1). We evaluated 17 AESI (acute myocardial infarction [AMI], anaphylaxis, appendicitis, Bell’s palsy, deep vein thrombosis [DVT], disseminated intravascular coagulation [DIC], encephalitis/encephalomyelitis, Guillain-Barré syndrome [GBS], hemorrhagic stroke, immune thrombocytopenia [ITP], myocarditis/pericarditis, narcolepsy, non-hemorrhagic stroke, pulmonary embolism [PE], transverse myelitis, unusual site thrombosis with thrombocytopenia [TTS], common site TTS) and three negative control events (colonic diverticulitis, hypertension, well-care visits [i.e., annual preventive care]) (Supplemental Table 1). The publicly posted study protocol includes details on AESI algorithms [16]. Appendix B herein presents ICD-10-CM diagnosis codes used to identify AESI.
2.4. Study cohort construction
Within each data source, we constructed cohorts for each AESI and negative control event. The general study population included any individual who was enrolled in a medical plan for at least 1 day during the study period, met age requirements at cohort entry (≥65 years for Medicare, 18–64 years for commercially insured adults, <18 years for commercially insured children [Supplemental Table 1]), and met a clean period requirement before cohort entry. The clean period requirement was defined as having continuous enrollment for the entire pre-specified clean period and no observed AESI or negative control events (colonic diverticulitis and hypertension only) during the clean period. Clean periods were specific, and some differed for each AESI and negative control event.
2.5. Statistical analysis
Person-time at risk was calculated as the number of days between cohort entry and the end of follow-up. Individuals in the study population entered the cohort beginning January 1, 2019, or the date the clean period requirement was met (specific to each AESI or negative control event), whichever occurred later. Infants who were born before January 1, 2019, and were continuously enrolled from birth but had not reached the full length of the clean period on January 1 were assigned a cohort entry date of January 1, 2019, provided no AESI occurred during the shortened clean period before entry. For infants who were born during the study period and started enrollment within 31 days of birth, the cohort entry date was the date of birth. Individuals were followed until the earliest date of AESI/negative control event occurrence or censoring due to death, disenrollment, exceeding specified age range (e.g., AESI-specific age criteria, commercially insured population reaching 65 years), or the study period’s end. After censoring, individuals could re-enter the same or a different AESI/negative control event cohort if they met another clean period requirement during the study period. Negative control event rates were not estimated in Optum data. Supplemental Fig. 1 illustrates the accumulation of person-time at risk with several examples.
We calculated annual 2019, peri-COVID-19 (overall, initial, and later periods), and monthly 2019–2020 IRs for each AESI and negative control event within each data source by dividing the count of incident events during the time at risk in a specified period by the total person-time at risk during the same period. For example, we calculated the annual 2019 rate for a given AESI by dividing the number of incident events occurring in 2019 by the total person-time at risk in 2019 within the AESI cohort and the monthly rate of an AESI by dividing the number of incident events occurring in a given month by the total person-time at risk in that month. Additionally, 2019 annual IRs were stratified by age and sex (all data sources), as well as race/ethnicity and nursing home residency status (Medicare only). The commercial databases lacked sufficient valid data on race/ethnicity.
IRs are presented as event counts per 100,000 person-years. In descriptive analyses, we calculated incident rate ratios (IRRs) to compare unadjusted IRs between subpopulation strata as well as between 2019 and the peri-COVID-19 periods (initial and later). Exact Poisson 95 % confidence intervals (CIs) were calculated for each IR and IRR. Differences were considered when the 95 % CI of the IRR did not overlap 1 or when the 95 % CIs of IRs for two populations did not overlap. The main results focus on the Medicare population aged ≥ 65 years and adults aged 18–64 years. Findings among children (0–17 years) are presented briefly in Section 3.1.4 and in the supplements.
3. Results
3.1. Incidence rates of AESI and negative control events, pre- and peri-COVID-19 periods
The total number of eligible adults included in the 2019 analysis from all data sources ranged from 55.5 million (for hypertension) to 110.2 million (for anaphylaxis); for children, the number ranged from 17.0 million (well-care visits) to 27.0 million (anaphylaxis) (Supplemental Table 2).
3.1.1. Incidence rates of AESI, pre-COVID-19 (2019)
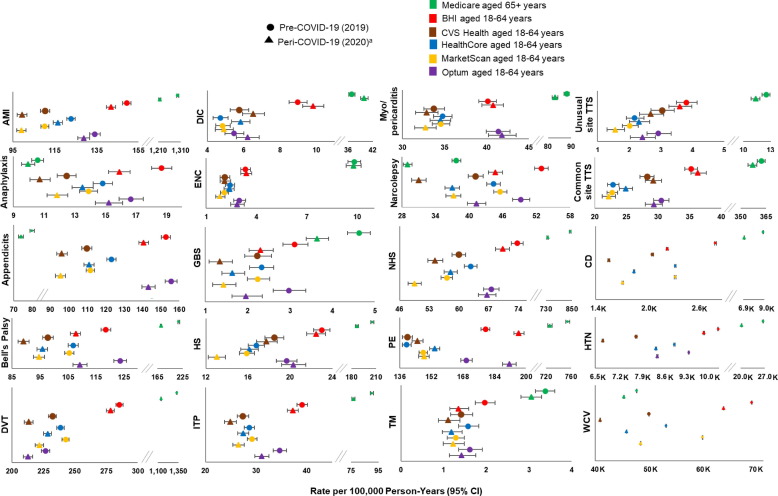
For adults aged ≥ 65 years in Medicare, the top two AESI with the highest IRs per 100,000 person-years in the pre-COVID-19 period were DVT (1,331.0; 95 % CI: 1,326.4–1,355.6) and AMI (1,297.5; 95 % CI: 1,293.0–1,302.0), followed by non-hemorrhagic stroke (842.8; 95 % CI: 839.2–846.4), PE (755.1; 95 % CI: 751.7–758.5), common site TTS (362.3; 95 % CI: 360.0–364.7), Bell’s palsy (215.4; 95 % CI: 213.6–217.2), and hemorrhagic stroke (205.3; 95 % CI: 203.5–207.0) (Fig. 1 , Supplemental Table 3). All other AESI had pre-pandemic IRs under 100 per 100,000 person-years. The rarest events were transverse myelitis (3.4 per 100,000 person-years; 95 % CI: 3.2–3.6), GBS (4.6; 95 % CI: 4.4–4.9), and encephalitis/encephalomyelitis (9.8; 95 % CI: 9.4–10.2).
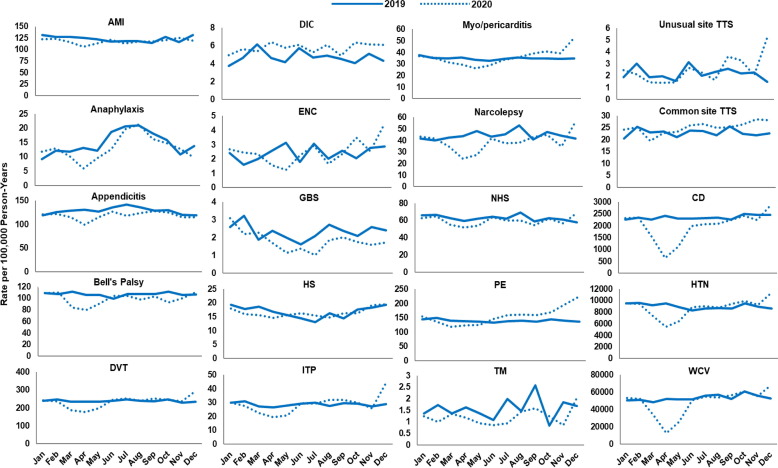
Fig. 1.
Incidence rates of AESI and negative control events in the pre- and peri-COVID-19 periods from six data sources. Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; BHI, Blue Health Intelligence; CD, colonic diverticulitis; CI, confidence interval; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; ITP, immune thrombocytopenia; K, thousands; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit. a For MarketScan, the peri-COVID-19 period includes data from March through October 2020; for all other data sources, the peri-COVID-19 period includes data through December 11, 2020. Statistics on negative control events were not available from Optum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In commercially insured adults aged 18–64 years, AMI, appendicitis, Bell’s palsy, DVT, and PE had IRs of approximately 100 per 100,000 person-years or greater across data sources. IRs were highest for DVT (ranging from 226.5 in Optum to 285.5 in BHI). DIC, encephalitis/encephalomyelitis, GBS, transverse myelitis, and unusual site TTS had a rate below 10 per 100,000 person-years across the commercial insurance data sources. IRs were lowest for transverse myelitis (ranging from 1.3 in MarketScan to 2.0 in BHI).
3.1.2. Heterogeneity in AESI IRs across data sources, pre-COVID-19 (2019)
In 2019, adults aged ≥ 65 years in Medicare had lower IRs of anaphylaxis, appendicitis, narcolepsy, and well-care visits and higher IRs of all other AESI and negative control events compared with commercially insured adults aged 18–64 years (Fig. 1, Supplemental Table 3).
There was also heterogeneity in the IRs of most AESI across the commercial insurance data sources. All 17 AESI showed at least a 20 % difference in rates among some commercial data sources (data not shown). Rates were higher in BHI and Optum (and CIs did not overlap) than in the other commercial databases for 9 of the 17 AESI: AMI, anaphylaxis, appendicitis, Bell’s palsy, ITP, myocarditis/pericarditis, narcolepsy, non-hemorrhagic stroke, and PE (Fig. 1, Supplemental Table 3). The rates of DVT, DIC, hemorrhagic stroke, and TTS (unusual and common site) were higher in BHI than in CVS Health, HealthCore, MarketScan, and Optum. Rates of colonic diverticulitis, hypertension, and well-care visits also were higher in BHI than in CVS Health, HealthCore, and MarketScan.
3.1.3. Variability of incidence rates during the pre- and peri-COVID-19 periods
Six AESI (AMI, appendicitis, Bell’s palsy, DVT, GBS, narcolepsy) and all three negative control events had lower IRs across all data sources during the peri-COVID-19 period than during 2019 (IRR < 1 and 95 % CI does not include 1) (Fig. 1, Supplemental Table 3). IRs decreased during the peri-COVID-19 period in some data sources for anaphylaxis (BHI, CVS Health, HealthCore, MarketScan, Optum), hemorrhagic stroke (Medicare, MarketScan), ITP (Medicare, BHI, CVS Health, MarketScan, Optum), myocarditis/pericarditis (Medicare, MarketScan), non-hemorrhagic stroke (Medicare, BHI, CVS Health, HealthCore, MarketScan), transverse myelitis (BHI, HealthCore), and unusual site TTS (Medicare, MarketScan). Several AESI exhibited elevated IRs during the peri-COVID-19 period among some data sources, including DIC (Medicare, BHI, HealthCore), PE (BHI, CVS Health, HealthCore, Optum), and common site TTS (HealthCore). Rates of encephalitis/encephalomyelitis were similar in the peri-COVID-19 period and 2019 across all six data sources.
3.1.4. Incidence rates among children during the pre- and peri-COVID-19 periods
In 2019, most AESI among children showed low IRs at < 10 per 100,000 person-years across all commercial data sources, except for anaphylaxis (24.5–31.3 per 100,000 person-years), appendicitis (106.6–127.5), Bell’s palsy (21.1–24.9), and ITP (10.7–12.8) (Supplemental Fig. 2 and Supplemental Table 4a). The 2019 rates for appendicitis, Bell’s palsy, DVT, myocarditis/pericarditis, narcolepsy, and PE were higher among children 11 years or older compared with the younger group (Supplemental Table 4b).
3.2. Monthly incidence rates of AESI, 2019 and 2020
3.2.1. Decrease of incidence rates in 2020 and return to pre-pandemic levels
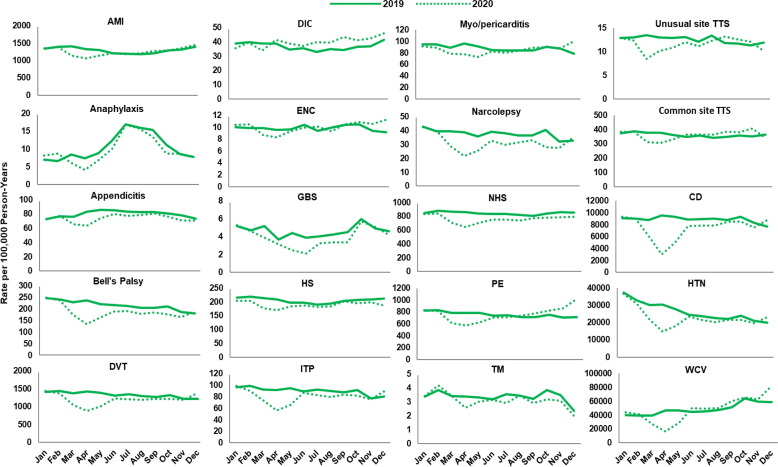
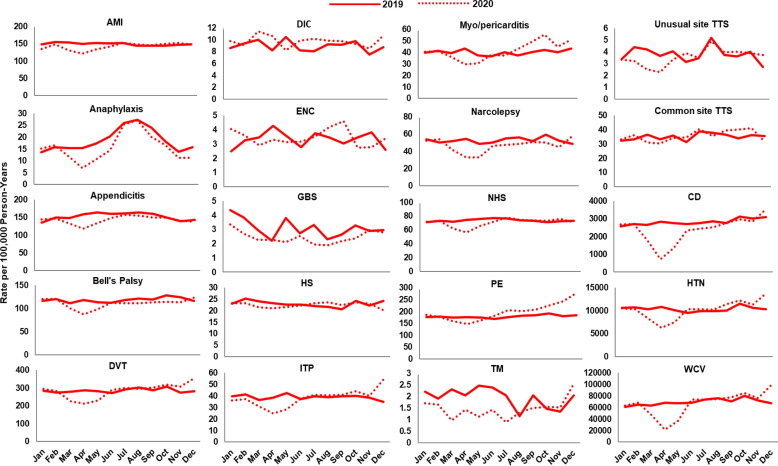
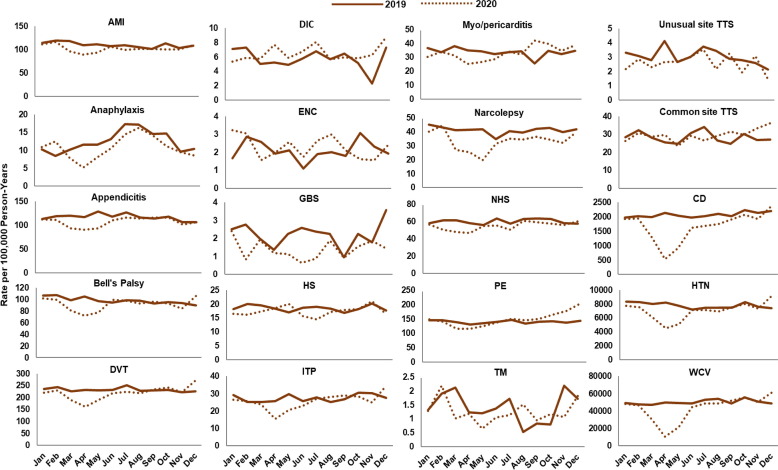
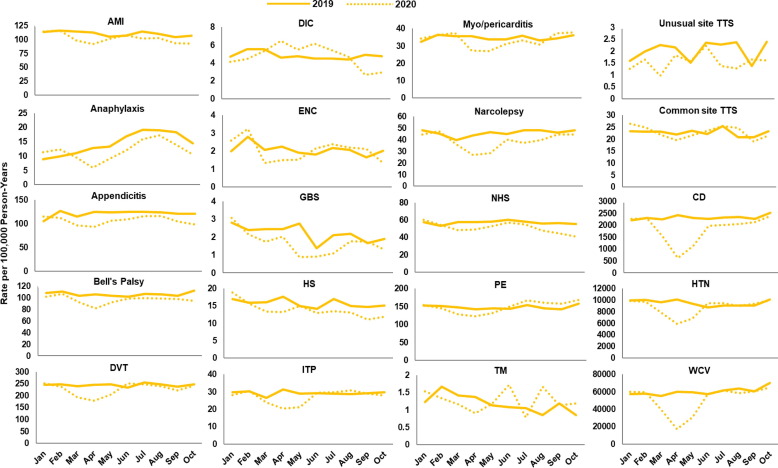
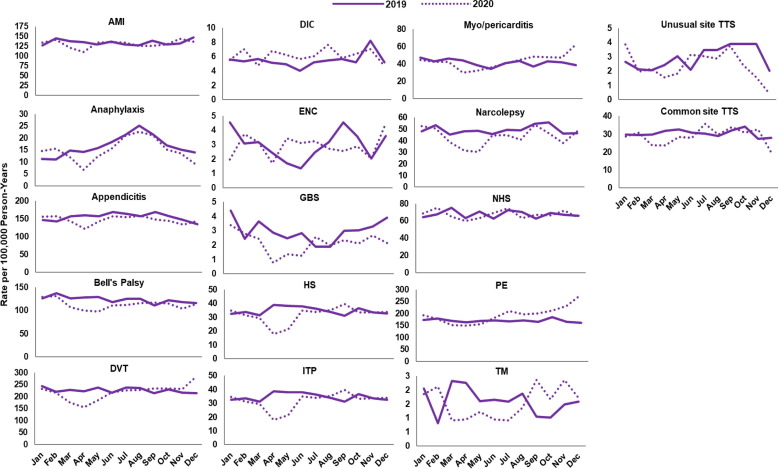
Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7 display monthly rates of the AESI and negative control events. Across all data sources, monthly rates of negative control events showed a marked reduction during the initial peri-COVID-19 period, reaching the lowest value in April 2020. This reduction was followed by a return to similar 2019 levels after May 2020 (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7). For adults aged ≥ 65 years in Medicare (Fig. 2), IRs of most AESI reached their lowest value in April 2020, but exceptions included DIC, GBS, myocarditis/pericarditis, transverse myelitis, and unusual site TTS. For commercially insured adults aged 18–64 years (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7), IRs of five AESI also reached their lowest level in April 2020 (AMI, anaphylaxis, appendicitis, DVT, ITP) consistently across data sources.
Fig. 2.
Monthly incidence rates of AESI and negative control events, CMS Medicare beneficiaries aged ≥ 65 years, 2019 and 2020. Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; CD, colonic diverticulitis; CMS, Centers for Medicare & Medicaid Services; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; ITP, immune thrombocytopenia; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit. Note: December includes data through December 11, 2020.
Fig. 3.
Monthly incidence rates of AESI and negative control events, BHI enrollees aged 18–64 years, 2019 and 2020. Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; BHI, Blue Health Intelligence; CD, colonic diverticulitis; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; ITP, immune thrombocytopenia; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit. Note: BHI data available for this study were limited to claims for enrollees who received a biologic product, were pregnant, or were born after October 1, 2015. December includes data through December 11, 2020. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Monthly incidence rates of AESI and negative control events, CVS Health enrollees aged 18–64 years, 2019 and 2020. Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; CD, colonic diverticulitis; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; ITP, immune thrombocytopenia; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit. Note: December includes data through December 11, 2020.
Fig. 5.
Monthly incidence rates of AESI and negative control events, HealthCore enrollees aged 18–64 years, 2019 and 2020. Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; CD, colonic diverticulitis; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; ITP, immune thrombocytopenia; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit. Note: December includes data through December 11, 2020.
Fig. 6.
Monthly incidence rates of AESI and negative control events, MarketScan enrollees aged 18–64 years, 2019 and 2020. Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; CD, colonic diverticulitis; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; ITP, immune thrombocytopenia; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit. Note: Data go through October 31, 2020.
Fig. 7.
Monthly rates of AESI, Optum enrollees aged 18–64 years, 2019 and 2020. Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; ITP, immune thrombocytopenia; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome. Note: Analyses and results for negative control events are not available for Optum.
Compared with 2019 annual IRs, rates during the initial peri-COVID-19 period were lower by more than 10 % for most AESI across all data sources (IRR ranging from 0.5 to below 0.9 and 95 % CI not including 1) (Supplemental Table 5). There were several exceptions with a higher rate in the initial peri-COVID-19 period or inconsistent trends across data sources. Specifically, DIC rates were elevated during the initial peri-COVID-19 period in BHI, HealthCore, and MarketScan. The IRs for nine AESI decreased 10 % or more in the initial peri-COVID-19 period compared with 2019 in some data sources: AMI (Medicare, CVS Health, BHI, MarketScan), encephalitis/encephalomyelitis (Medicare, HealthCore, MarketScan), GBS (Medicare, BHI, CVS Health, MarketScan, Optum), hemorrhagic stroke (Medicare, MarketScan), non-hemorrhagic stroke (Medicare, BHI, CVS Health, HealthCore, MarketScan), PE (Medicare, BHI, CVS Health, HealthCore, MarketScan), transverse myelitis (BHI), common site TTS (Medicare, Optum), and unusual site TTS (Medicare, BHI, HealthCore, MarketScan, Optum).
During the later period of 2020 (June–October 2020 for MarketScan; June–December 2020 for all other data sources), rates of most AESI returned to levels similar to 2019 annual rates (IRR between 0.9 and 1.1 or the 95 % CI for IRR including 1), although IRs of GBS were still low after May 2020 across all data sources (IRR ranging from 0.6 to 0.8). Five AESI remained lower than 90 % of 2019 annual rates, with an IRR < 0.9 in certain data sources: Bell’s palsy (Medicare), narcolepsy (Medicare, CVS Health), hemorrhagic stroke and non-hemorrhagic stroke (MarketScan), and transverse myelitis (BHI). Six AESI exhibited an IR more than 10 % higher than the corresponding 2019 annual rate in certain data sources: anaphylaxis (Medicare), DIC (Medicare, HealthCore), myocarditis/pericarditis (BHI), PE (BHI, CVS Health, HealthCore, Optum), and common and unusual site TTS (HealthCore).
3.2.2. Seasonality
For adults aged ≥ 65 years in 2019 Medicare data, IRs of anaphylaxis were lowest during the winter months (February: 6.6 per 100,000 person-years) and highest during the summer months (July: 16.9) (Fig. 2). AMI had a peak IR of 1,408.8 per 100,000 person–years in March and a minimum IR of 1,178.8 per 100,000 person-years in August (data not shown). We observed similar seasonal trends in anaphylaxis in the commercial insurance data sources (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7), with the lowest rates in April 2020 at 5.3–6.9 per 100,000 person-years and highest rates in August 2020 at 16.3–27.2 per 100,000 person-years. During 2017 and 2018, similar seasonal fluctuations were observed for AMI and anaphylaxis among adults aged ≥ 65 years in the Medicare data and for anaphylaxis among those aged 18–64 years across commercial data sources (data not shown; results presented in the BEST Initiative study report [12]).
3.3. Incidence rates by population characteristics, 2019
3.3.1. Sex
Table 1 compares 2019 IRs of the AESI between male and female adults via IRRs. IRs of six AESI (AMI, DVT, hemorrhagic stroke, myocarditis/pericarditis, non-hemorrhagic stroke, common site TTS) were higher for males in all data sources, ranging from 3 % higher risk among males compared with females for DVT (IRR = 1.03; 95 % CI: 1.02–1.03) and non-hemorrhagic stroke (IRR = 1.03; 95 % CI: 1.02–1.04) in Medicare to more than threefold higher risk among males for AMI in Optum (IRR = 3.08; 95 % CI: 2.96–3.22). Additionally, six other AESI (appendicitis, DIC, encephalitis/encephalomyelitis, GBS, PE, unusual site TTS) had higher incidence rates for males than females across multiple data sources. Other AESI rates were lower for males than females across multiple data sources, including anaphylaxis (BHI, CVS Health, HealthCore, MarketScan), Bell’s palsy (Medicare, BHI, CVS Health, HealthCore, MarketScan), and transverse myelitis (BHI, HealthCore, MarketScan). Rates of ITP and narcolepsy were lower in males than females across all the commercial insurance data sources but higher among males aged ≥ 65 years in Medicare.
Table 1.
Incidence rates for each AESI and negative control eventa by sex and data source in 2019.
| AESI or negative control and sex | Medicare, 65+ years |
BHI, 18–64 years |
CVS Health, 18–64 years |
HealthCore, 18–64 years |
MarketScan, 18–64 years |
Optum,a 18–64 years |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
|
| AMI | 1297.48 (1293.01–1301.97) |
149.45 (147.45–151.47) |
110.48 (108.31–112.69) |
122.85 (120.81–124.92) |
110.28 (108.44–112.15) |
134.14 (131.63–136.68) |
||||||
| Female | 1105.96 (1100.45–1111.49) |
[ref] | 83.97 (81.97–86.01) |
[ref] | 67.17 (64.83–69.57) |
[ref] | 71.68 (69.49–73.92) |
[ref] | 62.64 (60.72–64.60) |
[ref] | 68.67 (66.24–71.16) |
[ref] |
| Male | 1543.22 (1535.85–1550.61) |
1.40 (1.39–1.41) |
232.04 (228.30–235.84) |
2.76 (2.68–2.85) |
158.24 (154.47–162.09) |
2.36 (2.26–2.46) |
174.61 (171.17–178.11) |
2.44 (2.35–2.53) |
161.97 (158.75–165.24) |
2.59 (2.49–2.68) |
211.67 (207.02–216.40) |
3.08 (2.96–3.22) |
| Anaphylaxis | 10.57 (10.18–10.96) |
18.67 (18.02–19.33) |
12.46 (11.86–13.09) |
14.81 (14.19–15.45) |
13.90 (13.32–14.49) |
16.66 (15.88–17.47) |
||||||
| Female | 10.37 (9.86–10.90) |
[ref] | 20.54 (19.64–21.48) |
[ref] | 14.69 (13.78–15.65) |
[ref] | 17.01 (16.06–17.99) |
[ref] | 16.09 (15.23–16.98) |
[ref] | 16.94 (15.88–18.06) |
[ref] |
| Male | 10.81 (10.23–11.42) |
1.04 (0.97–1.12) |
16.25 (15.34–17.20) |
0.79 (0.74–0.85) |
9.91 (9.13–10.73) |
0.67 (0.61–0.75) |
12.61 (11.80–13.46) |
0.74 (0.68–0.81) |
11.52 (10.77–12.31) |
0.72 (0.66–0.78) |
16.32 (15.19–17.52) |
0.96 (0.87–1.06) |
| Appendicitis | 80.05 (78.95–81.16) |
152.71 (150.69–154.75) |
117.15 (114.91–119.43) |
128.22 (126.13–130.33) |
118.73 (116.81–120.66) |
155.05 (152.35–157.78) |
||||||
| Female | 76.55 (75.11–78.01) |
[ref] | 148.12 (145.45–150.82) |
[ref] | 113.00 (109.95–116.10) |
[ref] | 126.90 (123.98–129.87) |
[ref] | 117.47 (114.83–120.14) |
[ref] | 141.61 (138.12–145.18) |
[ref] |
| Male | 84.51 (82.81–86.25) |
1.10 (1.07–1.14) |
158.50 (155.40–161.64) |
1.07 (1.04–1.10) |
121.67 (118.36–125.05) |
1.08 (1.04–1.12) |
129.55 (126.58–132.57) |
1.02 (0.99–1.05) |
120.09 (117.32–122.91) |
1.02 (0.99–1.06) |
170.95 (166.77–175.20) |
1.21 (1.17–1.25) |
| Bell’s palsy | 215.42 (213.64–217.21) |
118.58 (116.88–120.31) |
97.84 (95.98–99.72) |
106.89 (105.11–108.70) |
105.47 (103.78–107.19) |
123.67 (121.42–125.96) |
||||||
| Female | 220.31 (217.91–222.73) |
[ref] | 122.39 (120.08–124.74) |
[ref] | 104.49 (101.83–107.19) |
[ref] | 115.21 (112.60–117.87) |
[ref] | 110.71 (108.31–113.15) |
[ref] | 121.38 (118.35–124.46) |
[ref] |
| Male | 209.21 (206.58–211.87) |
0.95 (0.93–0.97) |
113.73 (111.21–116.28) |
0.93 (0.90–0.96) |
90.46 (87.88–93.10) |
0.87 (0.83–0.90) |
98.52 (96.10–100.99) |
0.86 (0.83–0.88) |
99.80 (97.44–102.22) |
0.90 (0.87–0.93) |
126.40 (123.03–129.84) |
1.04 (1.00–1.08) |
| DVT | 1330.99 (1326.43–1335.55) |
285.50 (282.73–288.30) |
232.20 (229.03–235.39) |
238.51 (235.67–241.39) |
242.62 (239.89–245.39) |
226.52 (223.25–229.82) |
||||||
| Female | 1315.90 (1309.86–1321.97) |
[ref] | 253.97 (250.48–257.51) |
[ref] | 222.19 (217.91–226.53) |
[ref] | 225.89 (221.98–229.84) |
[ref] | 229.66 (225.97–233.39) |
[ref] | 202.59 (198.40–206.84) |
[ref] |
| Male | 1350.26 (1343.35–1357.21) |
1.03 (1.02–1.03) |
325.26 (320.82–329.75) |
1.28 (1.26–1.31) |
242.97 (238.27–247.72) |
1.09 (1.06–1.12) |
251.28 (247.14–255.47) |
1.11 (1.09–1.14) |
256.69 (252.62–260.80) |
1.12 (1.09–1.14) |
254.85 (249.74–260.05) |
1.26 (1.22–1.30) |
| DIC | 36.91 (36.17–37.67) |
8.96 (8.48–9.47) |
5.75 (5.26–6.27) |
4.72 (4.33–5.14) |
4.84 (4.46–5.25) |
5.47 (4.97–6.00) |
||||||
| Female | 32.80 (31.86–33.76) |
[ref] | 7.91 (7.31–8.56) |
[ref] | 6.27 (5.57–7.03) |
[ref] | 5.21 (4.63–5.84) |
[ref] | 5.31 (4.76–5.90) |
[ref] | 5.23 (4.57–5.95) |
[ref] |
| Male | 42.16 (40.96–43.39) |
1.29 (1.23–1.34) |
10.29 (9.51–11.11) |
1.30 (1.16–1.45) |
5.24 (4.57–5.98) |
0.84 (0.70–1.00) |
4.23 (3.71–4.8) |
0.81 (0.68–0.97) |
4.34 (3.83–4.90) |
0.82 (0.69–0.96) |
5.75 (5.01–6.58) |
1.10 (0.91–1.33) |
| ENC | 9.82 (9.44–10.21) |
3.34 (3.06–3.64) |
2.11 (1.84–2.40) |
2.42 (2.15–2.70) |
2.08 (1.85–2.33) |
2.98 (2.64–3.36) |
||||||
| Female | 8.63 (8.16–9.12) |
[ref] | 2.94 (2.59–3.33) |
[ref] | 2.06 (1.71–2.48) |
[ref] | 2.17 (1.82–2.56) |
[ref] | 1.95 (1.65–2.3) |
[ref] | 2.77 (2.33–3.27) |
[ref] |
| Male | 11.33 (10.73–11.96) |
1.31 (1.21–1.42) |
3.84 (3.39–4.33) |
1.30 (1.09–1.56) |
2.14 (1.76–2.58) |
1.04 (0.79–1.36) |
2.67 (2.28–3.10) |
1.23 (0.98–1.55) |
2.22 (1.88–2.61) |
1.14 (0.90–1.44) |
3.24 (2.72–3.83) |
1.17 (0.92–1.49) |
| GBS | 4.63 (4.36–4.90) |
3.11 (2.83–3.42) |
2.23 (1.93–2.56) |
2.33 (2.05–2.62) |
2.24 (1.99–2.52) |
2.97 (2.61–3.37) |
||||||
| Female | 3.56 (3.25–3.88) |
[ref] | 2.53 (2.19–2.90) |
[ref] | 1.95 (1.56–2.39) |
[ref] | 2.01 (1.66–2.42) |
[ref] | 1.71 (1.40–2.06) |
[ref] | 2.34 (1.91–2.84) |
[ref] |
| Male | 5.99 (5.54–6.46) |
1.68 (1.50–1.90) |
3.85 (3.38–4.37) |
1.52 (1.26–1.85) |
2.51 (2.06–3.04) |
1.29 (0.97–1.73) |
2.64 (2.23–3.10) |
1.31 (1.02–1.69) |
2.83 (2.41–3.29) |
1.66 (1.29–2.13) |
3.71 (3.12–4.38) |
1.58 (1.22–2.07) |
| HS | 205.26 (203.49–207.04) |
22.98 (22.20–23.78) |
18.49 (17.60–19.40) |
16.77 (16.02–17.54) |
15.88 (15.18–16.59) |
19.64 (18.69–20.63) |
||||||
| Female | 186.20 (183.95–188.47) |
[ref] | 18.89 (17.94–19.87) |
[ref] | 15.91 (14.78–17.11) |
[ref] | 13.9 (12.95–14.91) |
[ref] | 13.22 (12.35–14.14) |
[ref] | 14.98 (13.86–16.17) |
[ref] |
| Male | 229.61 (226.79–232.45) |
1.23 (1.21–1.25) |
28.14 (26.84–29.48) |
1.49 (1.39–1.60) |
21.40 (20.03–22.84) |
1.34 (1.22–1.48) |
19.67 (18.52–20.86) |
1.41 (1.29–1.55) |
18.75 (17.67–19.89) |
1.42 (1.30–1.55) |
25.14 (23.56–26.81) |
1.68 (1.52–1.86) |
| ITP | 90.21 (89.03–91.39) |
39.09 (38.07–40.13) |
27.40 (26.32–28.51) |
28.67 (27.69–29.68) |
29.20 (28.25–30.16) |
34.70 (33.43–36.00) |
||||||
| Female | 78.17 (76.72–79.65) |
[ref] | 44.39 (42.93–45.88) |
[ref] | 32.84 (31.21–34.53) |
[ref] | 34.46 (32.95–36.02) |
[ref] | 34.98 (33.55–36.45) |
[ref] | 39.88 (38.03–41.79) |
[ref] |
| Male | 105.58 (103.67–107.51) |
1.35 (1.32–1.39) |
32.41 (31.02–33.85) |
0.73 (0.69–0.77) |
21.48 (20.10–22.92) |
0.65 (0.60–0.71) |
22.83 (21.6–24.12) |
0.66 (0.62–0.71) |
22.93 (21.73–24.18) |
0.66 (0.61–0.70) |
28.57 (26.88–30.34) |
0.72 (0.66–0.77) |
| Myocarditis/pericarditis | 88.09 (86.94–89.26) |
40.11 (39.08–41.16) |
33.75 (32.55–34.98) |
34.79 (33.71–35.90) |
34.58 (33.55–35.63) |
41.36 (39.97–42.78) |
||||||
| Female | 80.60 (79.12–82.09) |
[ref] | 30.22 (29.02–31.45) |
[ref] | 26.73 (25.26–28.26) |
[ref] | 26.87 (25.54–28.25) |
[ref] | 27.22 (25.96–28.53) |
[ref] | 29.86 (28.26–31.52) |
[ref] |
| Male | 97.67 (95.84–99.53) |
1.21 (1.18–1.24) |
52.57 (50.79–54.39) |
1.74 (1.65–1.83) |
41.44 (39.52–43.43) |
1.55 (1.44–1.67) |
42.79 (41.10–44.54) |
1.59 (1.49–1.70) |
42.55 (40.91–44.24) |
1.56 (1.47–1.66) |
54.96 (52.61–57.40) |
1.84 (1.72–1.97) |
| Narcolepsy | 37.71 (36.96–38.48) |
52.89 (51.70–54.09) |
41.17 (39.85–42.53) |
44.32 (43.10–45.57) |
45.53 (44.35–46.74) |
49.23 (47.72–50.78) |
||||||
| Female | 36.05 (35.06–37.05) |
[ref] | 61.77 (60.06–63.53) |
[ref] | 48.24 (46.25–50.28) |
[ref] | 56.15 (54.21–58.14) |
[ref] | 54.75 (52.96–56.59) |
[ref] | 54.96 (52.79–57.19) |
[ref] |
| Male | 39.84 (38.67–41.04) |
1.11 (1.06–1.15) |
41.70 (40.12–43.32) |
0.67 (0.64–0.71) |
33.49 (31.77–35.29) |
0.69 (0.65–0.74) |
32.37 (30.90–33.90) |
0.58 (0.54–0.61) |
35.54 (34.04–37.09) |
0.65 (0.61–0.69) |
42.46 (40.39–44.61) |
0.77 (0.72–0.82) |
| NHS | 842.83 (839.23–846.44) |
73.75 (72.34–75.17) |
59.93 (58.33–61.56) |
62.74 (61.29–64.22) |
57.15 (55.82–58.49) |
67.65 (65.87–69.46) |
||||||
| Female | 830.52 (825.75–835.30) |
[ref] | 55.12 (53.50–56.78) |
[ref] | 50.29 (48.26–52.37) |
[ref] | 49.77 (47.95–51.64) |
[ref] | 46.92 (45.26–48.62) |
[ref] | 51.74 (49.63–53.91) |
[ref] |
| Male | 858.57 (853.09–864.07) |
1.03 (1.02–1.04) |
97.21 (94.80–99.68) |
1.76 (1.70–1.83) |
70.50 (67.99–73.08) |
1.40 (1.33–1.48) |
75.85 (73.59–78.17) |
1.52 (1.45–1.60) |
68.23 (66.15–70.37) |
1.45 (1.39–1.52) |
86.48 (83.51–89.52) |
1.67 (1.58–1.76) |
| PE | 755.10 (751.69–758.53) |
179.46 (177.26–181.67) |
139.95 (137.50–142.44) |
139.64 (137.46–141.84) |
148.04 (145.90–150.20) |
169.82 (166.99–172.68) |
||||||
| Female | 742.23 (737.71–746.77) |
[ref] | 169.35 (166.50–172.24) |
[ref] | 142.45 (139.03–145.94) |
[ref] | 138.56 (135.51–141.66) |
[ref] | 149.60 (146.62–152.62) |
[ref] | 159.80 (156.08–163.58) |
[ref] |
| Male | 771.54 (766.34–776.77) |
1.04 (1.03–1.05) |
192.19 (188.78–195.65) |
1.13 (1.11–1.16) |
137.06 (133.54–140.64) |
0.96 (0.93–1.00) |
140.72 (137.63–143.87) |
1.02 (0.98–1.05) |
146.35 (143.29–149.46) |
0.98 (0.95–1.01) |
181.68 (177.36–186.07) |
1.14 (1.10–1.18) |
| TM | 3.38 (3.15–3.61) |
1.96 (1.74–2.21) |
1.41 (1.17–1.68) |
1.57 (1.35–1.82) |
1.28 (1.08–1.49) |
1.61 (1.35–1.91) |
||||||
| Female | 3.18 (2.89–3.49) |
[ref] | 2.29 (1.97–2.65) |
[ref] | 1.62 (1.28–2.03) |
[ref] | 1.82 (1.48–2.21) |
[ref] | 1.49 (1.21–1.82) |
[ref] | 1.57 (1.22–1.99) |
[ref] |
| Male | 3.63 (3.28–4.00) |
1.14 (0.99–1.31) |
1.55 (1.26–1.89) |
0.68 (0.53–0.87) |
1.19 (0.88–1.56) |
0.73 (0.50–1.06) |
1.32 (1.04–1.66) |
0.73 (0.53–0.99) |
1.04 (0.8–1.34) |
0.70 (0.50–0.97) |
1.67 (1.28–2.14) |
1.06 (0.74–1.52) |
| Unusual site TTS | 12.49 (12.06–12.93) |
3.80 (3.49–4.14) |
3.04 (2.69–3.42) |
2.17 (1.91–2.46) |
2.02 (1.78–2.28) |
2.92 (2.56–3.32) |
||||||
| Female | 10.01 (9.49–10.54) |
[ref] | 2.81 (2.45–3.20) |
[ref] | 2.46 (2.03–2.96) |
[ref] | 1.94 (1.60–2.34) |
[ref] | 1.86 (1.54–2.23) |
[ref] | 2.16 (1.75–2.64) |
[ref] |
| Male | 15.66 (14.93–16.42) |
1.57 (1.46–1.68) |
5.06 (4.52–5.64) |
1.80 (1.51–2.15) |
3.68 (3.12–4.30) |
1.49 (1.16–1.92) |
2.41 (2.02–2.85) |
1.24 (0.96–1.61) |
2.19 (1.83–2.60) |
1.17 (0.91–1.52) |
3.82 (3.22–4.50) |
1.77 (1.35–2.32) |
| Common site TTS | 362.30 (359.95–364.67) |
35.22 (34.25–36.21) |
28.25 (27.15–29.38) |
22.89 (22.01–23.79) |
22.74 (21.91–23.59) |
30.47 (29.28–31.69) |
||||||
| Female | 275.60 (272.86–278.35) |
[ref] | 26.79 (25.67–27.96) |
[ref] | 24.09 (22.69–25.55) |
[ref] | 18.4 (17.3–19.56) |
[ref] | 18.39 (17.36–19.47) |
[ref] | 24.33 (22.89–25.83) |
[ref] |
| Male | 473.24 (469.19–477.33) |
1.72 (1.69–1.74) |
45.83 (44.18–47.54) |
1.71 (1.62–1.81) |
32.91 (31.20–34.69) |
1.37 (1.26–1.48) |
27.42 (26.06–28.82) |
1.49 (1.38–1.61) |
27.45 (26.14–28.82) |
1.49 (1.38–1.61) |
37.73 (35.79–39.76) |
1.55 (1.43–1.68) |
| CD, in thousandsb | 8.91 (8.89–8.92) |
2.82 (2.81–2.83) |
2.08 (2.07–2.09) |
2.34 (2.34–2.35) |
2.34 (2.33–2.35) |
N/A | ||||||
| Female | 8.76 (8.74–8.78) |
[ref] | 2.50 (2.49–2.51) |
[ref] | 2.00 (1.99–2.01) |
[ref] | 2.26 (2.25–2.27) |
[ref] | 2.26 (2.24–2.27) |
[ref] | N/A | N/A |
| Male | 9.10 (9.08–9.11) |
1.04 (1.04–1.04) |
3.23 (3.22–3.24) |
1.29 (1.28–1.30) |
2.16 (2.15–2.17) |
1.08 (1.07–1.09) |
2.43 (2.42–2.44) |
1.07 (1.07–1.08) |
2.44 (2.43–2.45) |
1.08 (1.07–1.09) |
N/A | N/A |
| HTN, in thousandsb | 26.55 (26.52–26.59) |
10.37 (10.35–10.39) |
7.80 (7.78–7.82) |
8.99 (8.97–9.01) |
9.44 (9.42–9.46) |
N/A | ||||||
| Female | 25.62 (25.57–25.67) |
[ref] | 8.63 (8.61–8.65) |
[ref] | 7.06 (7.04–7.09) |
[ref] | 8.00 (7.98–8.03) |
[ref] | 8.46 (8.43–8.48) |
[ref] | N/A | N/A |
| Male | 27.71 (27.65–27.77) |
1.08 (1.08–1.08) |
12.79 (12.76–12.82) |
1.48 (1.48–1.49) |
8.63 (8.60–8.66) |
1.22 (1.22–1.23) |
10.03 (10–10.06) |
1.25 (1.25–1.26) |
10.56 (10.53–10.58) |
1.25 (1.24–1.25) |
N/A | N/A |
| WCV, in thousandsb | 47.88 (47.85–47.91) |
69.73 (69.69–69.77) |
50.23 (50.19–50.28) |
53.47 (53.43–53.51) |
60.41 (60.37–60.45) |
N/A | ||||||
| Female | 50.51 (50.47–50.56) |
[ref] | 83.36 (83.29–83.42) |
[ref] | 66.43 (66.36–66.51) |
[ref] | 71.37 (71.3–71.44) |
[ref] | 78.76 (78.69–78.83) |
[ref] | N/A | N/A |
| Male | 44.59 (44.55–44.64) |
0.88 (0.88–0.88) |
52.56 (52.51–52.62) |
0.63 (0.63–0.63) |
32.36 (32.31–32.42) |
0.49 (0.49–0.49) |
35.37 (35.32–35.42) |
0.50 (0.49–0.50) |
40.51 (40.46–40.57) |
0.51 (0.51–0.52) |
N/A | N/A |
Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; BHI, Blue Health Intelligence; CD, colonic diverticulitis; CI, confidence interval; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; IR, incidence rate; IRR, incidence rate ratio; ITP, immune thrombocytopenia; N/A, not available; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; PY, person-years; ref, reference; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit.
Analyses and results for negative control events are not available for Optum.
Incidence rates of negative control events are reported in thousands (e.g., a value of 1 corresponds to a rate of 1,000 per 100,000 person-years).
3.3.2. Age group
Among commercially insured adults aged < 65 years, 12 of the AESI had higher IRs in older age groups than among individuals aged 18–25 years (reference group) across all five data sources in 2019 (Table 2 ). Rates of transverse myelitis were higher in some older age groups than in the reference group in all data sources except Optum. Rates of anaphylaxis, appendicitis, and narcolepsy in older age groups were lower than or similar to the corresponding rates in those aged 18–25 years. Compared with individuals aged 18–25 years, rates of encephalitis/encephalomyelitis were generally similar or lower for those aged 26–55 years but higher for those aged 56–64 years.
Table 2.
Incidence rates for each AESI and negative control eventa by age group (18–64 years) and data source in 2019.
| AESI or negative control and age, years | BHI |
CVS Health |
HealthCore |
MarketScan |
Optuma |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
IR per 100,000 PY (95 % CI) |
IRR (95 % CI) |
|
| AMI | 149.45 (147.45–151.47) |
110.48 (108.31–112.69) |
122.85 (120.81–124.92) |
110.28 (108.44–112.15) |
134.14 (131.63–136.68) |
|||||
| 18–25 | 5.26 (4.39–6.26) |
[ref] | 3.88 (2.95–5.01) |
[ref] | 4.17 (3.31–5.18) |
[ref] | 4.14 (3.32–5.10) |
[ref] | 6.91 (5.47–8.61) |
[ref] |
| 26–35 | 15.70 (14.13–17.39) |
2.98 (2.43–3.68) |
10.99 (9.44–12.71) |
2.83 (2.10–3.87) |
13.62 (12.03–15.37) |
3.27 (2.54–4.25) |
11.90 (10.45–13.50) |
2.88 (2.25–3.71) |
16.11 (14.18–18.23) |
2.33 (1.80–3.04) |
| 36–45 | 65.13 (62.12–68.25) |
12.37 (10.34–14.92) |
52.05 (48.81–55.44) |
13.41 (10.31–17.76) |
60.35 (57.24–63.58) |
14.48 (11.57–18.34) |
55.58 (52.73–58.54) |
13.44 (10.83–16.86) |
74.06 (70.15–78.14) |
10.72 (8.54–13.63) |
| 46–55 | 195.08 (190.29–199.96) |
37.06 (31.11–44.5) |
152.85 (147.49–158.36) |
39.39 (30.45–51.87) |
171.28 (166.34–176.34) |
41.09 (32.99–51.84) |
148.52 (144.15–152.98) |
35.90 (29.07–44.86) |
192.14 (186.06–198.37) |
27.82 (22.26–35.22) |
| 56–64 | 358.23 (352.04–364.51) |
68.05 (57.18–81.64) |
298.14 (290.33–306.10) |
76.83 (59.47–101.06) |
315.91 (308.81–323.12) |
75.78 (60.89–95.53) |
278.20 (271.94–284.56) |
67.25 (54.51–83.96) |
331.99 (323.23–340.94) |
48.06 (38.50–60.81) |
| Anaphylaxis | 18.67 (18.02–19.33) |
12.46 (11.86–13.09) |
14.81 (14.19–15.45) |
13.90 (13.32–14.49) |
16.66 (15.88–17.47) |
|||||
| 18–25 | 25.96 (24.12–27.90) |
[ref] | 16.07 (14.48–17.79) |
[ref] | 18.33 (16.7–20.07) |
[ref] | 17.72 (16.19–19.37) |
[ref] | 24.11 (21.68–26.73) |
[ref] |
| 26–35 | 18.37 (16.87–19.96) |
0.71 (0.63–0.79) |
11.31 (10.05–12.68) |
0.70 (0.60–0.82) |
12.77 (11.48–14.17) |
0.70 (0.61–0.80) |
13.09 (11.82–14.45) |
0.74 (0.64–0.85) |
15.99 (14.35–17.77) |
0.66 (0.57–0.77) |
| 36–45 | 18.59 (17.12–20.15) |
0.72 (0.64–0.80) |
12.56 (11.23–14.00) |
0.78 (0.67–0.91) |
14.50 (13.16–15.94) |
0.79 (0.69–0.90) |
14.69 (13.40–16.06) |
0.83 (0.73–0.94) |
17.06 (15.40–18.84) |
0.71 (0.61–0.82) |
| 46–55 | 18.11 (16.76–19.53) |
0.70 (0.63–0.78) |
12.87 (11.55–14.28) |
0.80 (0.69–0.93) |
14.81 (13.51–16.21) |
0.81 (0.71–0.92) |
13.42 (12.25–14.68) |
0.76 (0.67–0.86) |
14.88 (13.38–16.51) |
0.62 (0.53–0.72) |
| 56–64 | 14.36 (13.21–15.57) |
0.55 (0.50–0.62) |
9.76 (8.58–11.07) |
0.61 (0.51–0.72) |
13.96 (12.61–15.42) |
0.76 (0.66–0.87) |
11.04 (9.92–12.26) |
0.62 (0.54–0.72) |
13.38 (11.80–15.11) |
0.55 (0.47–0.65) |
| Appendicitis | 152.71 (150.69–154.75) |
117.15 (114.91–119.43) |
128.22 (126.13–130.33) |
118.73 (116.81–120.66) |
155.05 (152.35–157.78) |
|||||
| 18–25 | 201.61 (196.00–207.34) |
[ref] | 148.26 (142.20–154.51) |
[ref] | 167.17 (161.47–173.03) |
[ref] | 154.16 (148.92–159.53) |
[ref] | 223.45 (214.86–232.29) |
[ref] |
| 26–35 | 179.06 (173.67–184.59) |
0.89 (0.85–0.93) |
128.19 (122.77–133.80) |
0.86 (0.81–0.92) |
146.07 (140.73–151.56) |
0.87 (0.83–0.92) |
142.30 (137.17–147.58) |
0.92 (0.88–0.97) |
188.13 (181.37–195.07) |
0.84 (0.80–0.89) |
| 36–45 | 158.59 (153.87–163.42) |
0.79 (0.75–0.82) |
118.75 (113.84–123.82) |
0.80 (0.75–0.85) |
130.01 (125.43–134.71) |
0.78 (0.74–0.82) |
117.36 (113.2–121.64) |
0.76 (0.72–0.80) |
155.10 (149.41–160.96) |
0.69 (0.66–0.73) |
| 46–55 | 136.85 (132.85–140.95) |
0.68 (0.65–0.71) |
108.13 (103.63–112.78) |
0.73 (0.69–0.77) |
110.48 (106.51–114.55) |
0.66 (0.63–0.69) |
104.88 (101.21–108.64) |
0.68 (0.65–0.71) |
129.56 (124.58–134.69) |
0.58 (0.55–0.61) |
| 56–64 | 112.12 (108.67–115.65) |
0.56 (0.53–0.58) |
90.19 (85.93–94.62) |
0.61 (0.57–0.65) |
100.05 (96.08–104.14) |
0.60 (0.57–0.63) |
89.54 (86.02–93.18) |
0.58 (0.55–0.61) |
106.56 (101.62–111.67) |
0.48 (0.45–0.51) |
| Bell’s palsy | 118.58 (116.88–120.31) |
97.84 (95.98–99.72) |
106.89 (105.11–108.7) |
105.47 (103.78–107.19) |
123.67 (121.42–125.96) |
|||||
| 18–25 | 50.21 (47.54–52.98) |
[ref] | 36.65 (33.99–39.47) |
[ref] | 43.52 (40.83–46.35) |
[ref] | 44.29 (41.68–47.01) |
[ref] | 59.49 (55.41–63.79) |
[ref] |
| 26–35 | 88.25 (84.74–91.88) |
1.76 (1.64–1.88) |
68.77 (65.27–72.40) |
1.88 (1.71–2.06) |
78.43 (74.9–82.09) |
1.80 (1.67–1.95) |
79.87 (76.41–83.45) |
1.80 (1.67–1.94) |
97.73 (93.31–102.31) |
1.64 (1.51–1.79) |
| 36–45 | 117.33 (113.45–121.31) |
2.34 (2.19–2.49) |
97.86 (93.79–102.06) |
2.67 (2.45–2.91) |
105.98 (102.10–109.97) |
2.43 (2.26–2.62) |
103.41 (99.75–107.17) |
2.34 (2.18–2.51) |
125.38 (120.58–130.32) |
2.11 (1.95–2.29) |
| 46–55 | 152.05 (147.98–156.21) |
3.03 (2.85–3.22) |
129.76 (125.21–134.42) |
3.54 (3.26–3.85) |
137.83 (133.61–142.14) |
3.17 (2.95–3.40) |
132.42 (128.50–136.42) |
2.99 (2.8–3.20) |
148.96 (143.90–154.16) |
2.5 (2.32–2.71) |
| 56–64 | 157.75 (153.79–161.79) |
3.14 (2.96–3.34) |
144.45 (139.46–149.57) |
3.94 (3.63–4.28) |
153.30 (148.57–158.13) |
3.52 (3.28–3.78) |
149.98 (145.58–154.47) |
3.39 (3.17–3.62) |
165.31 (159.41–171.36) |
2.78 (2.57–3.01) |
| DVT | 285.50 (282.73–288.30) |
232.20 (229.03–235.39) |
238.51 (235.67–241.39) |
242.62 (239.89–245.39) |
226.52 (223.25–229.82) |
|||||
| 18–25 | 57.95 (54.96–61.05) |
[ref] | 39.74 (36.64–43.04) |
[ref] | 44.59 (41.67–47.66) |
[ref] | 46.70 (43.84–49.70) |
[ref] | 54.08 (49.90–58.51) |
[ref] |
| 26–35 | 126.53 (122–131.18) |
2.18 (2.05–2.33) |
89.57 (85.04–94.27) |
2.25 (2.05–2.48) |
97.69 (93.34–102.19) |
2.19 (2.02–2.38) |
103.80 (99.43–108.32) |
2.22 (2.06–2.40) |
105.09 (100.06–110.31) |
1.94 (1.77–2.14) |
| 36–45 | 219.60 (214.04–225.28) |
3.79 (3.58–4.02) |
176.55 (170.54–182.71) |
4.44 (4.07–4.85) |
180.95 (175.53–186.49) |
4.06 (3.77–4.37) |
188.79 (183.50–194.19) |
4.04 (3.77–4.33) |
187.73 (181.45–194.17) |
3.47 (3.19–3.79) |
| 46–55 | 368.41 (361.81–375.11) |
6.36 (6.02–6.72) |
317.13 (309.37–325.02) |
7.98 (7.34–8.69) |
309.04 (302.37–315.82) |
6.93 (6.46–7.44) |
312.43 (306.07–318.88) |
6.69 (6.27–7.15) |
293.96 (286.42–301.66) |
5.44 (5.00–5.92) |
| 56–64 | 520.75 (513.27–528.32) |
8.99 (8.51–9.49) |
480.29 (470.35–490.38) |
12.08 (11.13–13.14) |
489.95 (481.09–498.93) |
10.99 (10.25–11.79) |
477.26 (469.05–485.59) |
10.22 (9.58–10.91) |
425.15 (415.20–435.28) |
7.86 (7.24–8.55) |
| DIC | 8.96 (8.48–9.47) |
5.75 (5.26–6.27) |
4.72 (4.33–5.14) |
4.84 (4.46–5.25) |
5.47 (4.97–6.00) |
|||||
| 18–25 | 3.78 (3.05–4.64) |
[ref] | 1.97 (1.33–2.82) |
[ref] | 2.01 (1.43–2.74) |
[ref] | 1.83 (1.30–2.51) |
[ref] | 3.15 (2.20–4.36) |
[ref] |
| 26–35 | 5.96 (5.01–7.04) |
1.58 (1.20–2.07) |
3.05 (2.26–4.02) |
1.55 (0.96–2.52) |
3.04 (2.32–3.93) |
1.52 (1.00–2.33) |
3.13 (2.41–4.00) |
1.71 (1.13–2.62) |
3.66 (2.77–4.74) |
1.16 (0.75–1.82) |
| 36–45 | 5.93 (5.05–6.93) |
1.57 (1.21–2.05) |
4.86 (3.91–5.97) |
2.46 (1.61–3.86) |
3.20 (2.52–4.02) |
1.60 (1.07–2.41) |
3.36 (2.69–4.15) |
1.83 (1.24–2.75) |
4.15 (3.27–5.20) |
1.32 (0.88–2.02) |
| 46–55 | 10.24 (9.17–11.41) |
2.71 (2.14–3.45) |
6.66 (5.58–7.89) |
3.38 (2.26–5.20) |
5.13 (4.30–6.06) |
2.55 (1.78–3.75) |
4.84 (4.08–5.71) |
2.64 (1.84–3.87) |
6.25 (5.19–7.46) |
1.99 (1.36–2.96) |
| 56–64 | 15.59 (14.32–16.94) |
4.12 (3.30–5.19) |
11.12 (9.66–12.75) |
5.64 (3.83–8.57) |
9.33 (8.14–10.63) |
4.65 (3.30–6.71) |
9.89 (8.74–11.15) |
5.39 (3.84–7.76) |
9.32 (7.90–10.92) |
2.96 (2.05–4.38) |
| ENC | 3.34 (3.06–3.64) |
2.11 (1.84–2.40) |
2.42 (2.15–2.70) |
2.08 (1.85–2.33) |
2.98 (2.64–3.36) |
|||||
| 18–25 | 2.88 (2.27–3.60) |
[ref] | 1.21 (0.76–1.81) |
[ref] | 1.92 (1.39–2.59) |
[ref] | 1.85 (1.35–2.48) |
[ref] | 3.40 (2.48–4.54) |
[ref] |
| 26–35 | 2.71 (2.13–3.41) |
0.94 (0.67–1.32) |
1.90 (1.36–2.58) |
1.57 (0.92–2.75) |
1.82 (1.32–2.45) |
0.95 (0.61–1.48) |
1.32 (0.91–1.85) |
0.71 (0.44–1.14) |
1.81 (1.25–2.53) |
0.53 (0.33–0.85) |
| 36–45 | 2.78 (2.21–3.45) |
0.97 (0.70–1.34) |
1.71 (1.21–2.35) |
1.42 (0.82–2.49) |
1.77 (1.30–2.35) |
0.92 (0.60–1.43) |
1.71 (1.27–2.26) |
0.93 (0.61–1.42) |
2.04 (1.47–2.76) |
0.60 (0.39–0.94) |
| 46–55 | 3.16 (2.60–3.81) |
1.10 (0.81–1.49) |
2.19 (1.63–2.87) |
1.81 (1.09–3.11) | 2.49 (1.95–3.13) |
1.29 (0.88–1.93) |
1.90 (1.45–2.43) |
1.02 (0.69–1.54) |
3.23 (2.52–4.08) |
0.95 (0.65–1.41) |
| 56–64 | 4.69 (4.03–5.43) |
1.63 (1.24–2.16) |
3.42 (2.69–4.29) |
2.84 (1.76–4.74) |
3.96 (3.23–4.80) |
2.06 (1.43–3.01) |
3.50 (2.85–4.24) |
1.89 (1.32–2.75) |
4.67 (3.73–5.79) |
1.38 (0.95–2.02) |
| GBS | 3.11 (2.83–3.42) |
2.23 (1.93–2.56) |
2.33 (2.05–2.62) |
2.24 (1.99–2.52) |
2.97 (2.61–3.37) |
|||||
| 18–25 | 1.56 (1.11–2.15) |
[ref] | 1.25 (0.75–1.95) |
[ref] | 1.65 (1.13–2.32) |
[ref] | 1.13 (0.72–1.68) |
[ref] | 2.01 (1.27–3.02) |
[ref] |
| 26–35 | 2.66 (2.04–3.41) |
1.70 (1.12–2.62) |
1.46 (0.94–2.18) |
1.17 (0.62–2.26) |
2.06 (1.47–2.81) |
1.25 (0.77–2.06) |
1.81 (1.28–2.50) |
1.61 (0.94–2.81) |
2.7 (1.94–3.64) |
1.34 (0.79–2.33) |
| 36–45 | 2.86 (2.25–3.57) |
1.83 (1.22–2.77) |
2.10 (1.50–2.88) |
1.68 (0.95–3.09) |
2.01 (1.48–2.67) |
1.22 (0.76–1.97) |
2.34 (1.79–3.02) |
2.08 (1.27–3.49) |
3.21 (2.44–4.15) |
1.60 (0.97–2.71) |
| 46–55 | 3.16 (2.57–3.83) |
2.02 (1.38–3.01) |
2.98 (2.28–3.84) |
2.39 (1.41–4.24) |
2.86 (2.26–3.59) |
1.74 (1.14–2.72) |
2.57 (2.03–3.22) |
2.28 (1.43–3.78) |
2.85 (2.15–3.70) |
1.42 (0.86–2.41) |
| 56–64 | 4.63 (3.95–5.39) |
2.96 (2.07–4.33) |
3.01 (2.27–3.91) |
2.41 (1.41–4.29) |
2.80 (2.17–3.56) |
1.70 (1.10–2.68) |
2.99 (2.37–3.71) |
2.65 (1.66–4.37) |
3.78 (2.90–4.84) |
1.88 (1.15–3.18) |
| HS | 22.98 (22.2–23.78) |
18.49 (17.60–19.40) |
16.77 (16.02–17.54) |
15.88 (15.18–16.59) |
19.64 (18.69–20.63) |
|||||
| 18–25 | 3.91 (3.16–4.78) |
[ref] | 2.83 (2.05–3.81) |
[ref] | 3.04 (2.31–3.92) |
[ref] | 3.15 (2.44–4.00) |
[ref] | 3.85 (2.80–5.16) |
[ref] |
| 26–35 | 6.95 (5.92–8.11) |
1.78 (1.37–2.32) |
5.25 (4.20–6.48) |
1.86 (1.27–2.74) |
4.64 (3.73–5.71) |
1.53 (1.09–2.16) |
4.80 (3.90–5.85) |
1.52 (1.11–2.11) |
7.12 (5.86–8.58) |
1.85 (1.29–2.69) |
| 36–45 | 14.54 (13.14–16.05) |
3.72 (2.97–4.71) |
10.58 (9.15–12.17) |
3.74 (2.68–5.33) |
8.76 (7.6–10.04) |
2.88 (2.15–3.92) |
9.49 (8.34–10.77) |
3.01 (2.29–4.01) |
12.73 (11.14–14.49) |
3.31 (2.39–4.68) |
| 46–55 | 29.1 (27.27–31.02) |
7.45 (6.03–9.30) |
23.48 (21.41–25.70) |
8.30 (6.07–11.63) |
21.56 (19.83–23.40) |
7.10 (5.43–9.45) |
20.26 (18.67–21.95) |
6.43 (4.99–8.41) |
25.87 (23.67–28.22) |
6.72 (4.94–9.37) |
| 56–64 | 47.3 (45.07–49.62) |
12.11 (9.84–15.05) |
45.42 (42.41–48.59) |
16.06 (11.82–22.36) |
40.29 (37.78–42.91) |
13.27 (10.20–17.56) |
35.47 (33.26–37.78) |
11.26 (8.79–14.65) |
42.65 (39.55–45.93) |
11.09 (8.17–15.4) |
| ITP | 39.09 (38.07–40.13) |
27.40 (26.32–28.51) |
28.67 (27.69–29.68) |
29.2 (28.25–30.16) |
34.70 (33.43–36.00) |
|||||
| 18–25 | 20.37 (18.61–22.24) |
[ref] | 13.03 (11.27–14.97) |
[ref] | 17.71 (15.89–19.68) |
[ref] | 14.48 (12.91–16.19) |
[ref] | 22.13 (19.48–25.03) |
[ref] |
| 26–35 | 38.24 (35.77–40.84) |
1.88 (1.68–2.10) |
24.42 (22.09–26.94) |
1.87 (1.58–2.23) |
24.21 (22.07–26.5) |
1.37 (1.19–1.58) |
26.65 (24.46–28.99) |
1.84 (1.60–2.12) |
30.43 (27.75–33.30) |
1.38 (1.18–1.61) |
| 36–45 | 37.45 (35.17–39.83) |
1.84 (1.65–2.05) |
26.02 (23.75–28.45) |
2.00 (1.69–2.37) |
24.79 (22.81–26.89) |
1.40 (1.22–1.60) |
27.63 (25.63–29.75) |
1.91 (1.67–2.19) |
33.29 (30.68–36.06) |
1.50 (1.30–1.75) |
| 46–55 | 42.74 (40.52–45.06) |
2.10 (1.89–2.33) |
29.06 (26.75–31.51) |
2.23 (1.90–2.63) |
31.00 (28.92–33.19) |
1.75 (1.54–1.99) |
32.44 (30.42–34.56) |
2.24 (1.97–2.56) |
36.05 (33.44–38.80) |
1.63 (1.41–1.89) |
| 56–64 | 50.33 (48.03–52.72) |
2.47 (2.24–2.74) |
41.36 (38.49–44.39) |
3.18 (2.71–3.73) |
42.44 (39.87–45.14) |
2.40 (2.12–2.72) |
40.62 (38.25–43.09) |
2.80 (2.47–3.19) |
47.43 (44.15–50.88) |
2.14 (1.86–2.48) |
| Myocarditis/pericarditis | 40.11 (39.08–41.16) |
33.75 (32.55–34.98) |
34.79 (33.71–35.90) |
34.58 (33.55–35.63) |
41.36 (39.97–42.78) |
|||||
| 18–25 | 29.87 (27.73–32.12) |
[ref] | 25.85 (23.36–28.54) |
[ref] | 28.26 (25.95–30.73) |
[ref] | 28.63 (26.41–31.00) |
[ref] | 36.21 (32.81–39.87) |
[ref] |
| 26–35 | 29.65 (27.48–31.94) |
0.99 (0.89–1.10) |
23.93 (21.62–26.42) |
0.93 (0.80–1.07) |
27.10 (24.83–29.52) |
0.96 (0.85–1.08) |
27.04 (24.83–29.39) |
0.94 (0.84–1.06) |
35.12 (32.23–38.19) |
0.97 (0.85–1.10) |
| 36–45 | 35.4 (33.19–37.72) |
1.19 (1.07–1.31) |
30.83 (28.35–33.46) |
1.19 (1.05–1.36) |
30.51 (28.31–32.83) |
1.08 (0.96–1.21) |
31.65 (29.51–33.91) |
1.11 (0.99–1.23) |
39.10 (36.27–42.09) |
1.08 (0.96–1.22) |
| 46–55 | 45.24 (42.95–47.62) |
1.51 (1.38–1.66) |
38.71 (36.04–41.53) |
1.50 (1.32–1.69) |
37.22 (34.93–39.61) |
1.32 (1.19–1.46) |
36.77 (34.62–39.03) |
1.28 (1.16–1.42) |
41.99 (39.18–44.96) |
1.16 (1.03–1.31) |
| 56–64 | 52.84 (50.48–55.28) |
1.77 (1.62–1.93) |
46.40 (43.36–49.60) |
1.79 (1.59–2.03) |
47.83 (45.10–50.69) |
1.69 (1.53–1.88) |
45.30 (42.80–47.90) |
1.58 (1.43–1.75) |
52.59 (49.14–56.22) |
1.45 (1.29–1.64) |
| Narcolepsy | 52.89 (51.70–54.09) |
41.17 (39.85–42.53) |
44.32 (43.10–45.57) |
45.53 (44.35–46.74) |
49.23 (47.72–50.78) |
|||||
| 18–25 | 60.02 (56.98–63.18) |
[ref] | 41.46 (38.29–44.83) |
[ref] | 46.25 (43.27–49.38) |
[ref] | 48.92 (46.00–51.99) |
[ref] | 66.43 (61.78–71.32) |
[ref] |
| 26–35 | 64.55 (61.33–67.90) |
1.08 (1.00–1.16) |
42.14 (39.06–45.41) |
1.02 (0.91–1.13) |
49.37 (46.29–52.61) |
1.07 (0.97–1.17) |
52.50 (49.4–55.74) |
1.07 (0.98–1.17) |
52.81 (49.26–56.54) |
0.79 (0.72–0.88) |
| 36–45 | 62.05 (59.11–65.10) |
1.03 (0.96–1.11) |
43.86 (40.89–46.98) |
1.06 (0.95–1.18) |
49.98 (47.16–52.93) |
1.08 (0.99–1.18) |
51.74 (48.99–54.60) |
1.06 (0.97–1.15) |
52.98 (49.68–56.45) |
0.80 (0.72–0.88) |
| 46–55 | 49.01 (46.62–51.48) |
0.82 (0.76–0.88) |
41.36 (38.60–44.27) |
1.00 (0.90–1.11) |
43.04 (40.58–45.62) |
0.93 (0.85–1.02) |
43.47 (41.12–45.91) |
0.89 (0.82–0.97) |
44.45 (41.55–47.50) |
0.67 (0.61–0.74) |
| 56–64 | 37.00 (35.03–39.05) |
0.62 (0.57–0.66) |
37.21 (34.49–40.09) |
0.90 (0.80–1.00) |
34.53 (32.21–36.97) |
0.75 (0.68–0.82) |
34.01 (31.84–36.27) |
0.70 (0.64–0.76) |
35.47 (32.65–38.47) |
0.53 (0.48–0.60) |
| NHS | 73.75 (72.34–75.17) |
59.93 (58.33–61.56) |
62.74 (61.29–64.22) |
57.15 (55.82–58.49) |
67.65 (65.87–69.46) |
|||||
| 18–25 | 5.39 (4.50–6.39) |
[ref] | 3.42 (2.55–4.49) |
[ref] | 4.48 (3.59–5.52) |
[ref] | 3.85 (3.07–4.78) |
[ref] | 7.26 (5.78–9.00) |
[ref] |
| 26–35 | 14.54 (13.04–16.18) |
2.70 (2.2–3.33) |
11.17 (9.61–12.91) |
3.27 (2.39–4.53) |
9.60 (8.27–11.08) |
2.14 (1.65–2.80) |
10.63 (9.26–12.14) |
2.76 (2.13–3.60) |
15.15 (13.28–17.21) |
2.09 (1.62–2.71) |
| 36–45 | 36.43 (34.19–38.79) |
6.76 (5.63–8.18) |
32.01 (29.49–34.69) |
9.36 (7.04–12.68) |
30.04 (27.86–32.34) |
6.71 (5.36–8.48) |
30.79 (28.68–33.02) |
7.99 (6.36–10.16) |
37.77 (34.99–40.71) |
5.20 (4.14–6.62) |
| 46–55 | 92.67 (89.38–96.05) |
17.20 (14.44–20.65) |
73.45 (69.75–77.30) |
21.48 (16.29–28.89) |
79.77 (76.41–83.25) |
17.82 (14.37–22.34) |
70.54 (67.54–73.64) |
18.30 (14.67–23.11) |
87.26 (83.18–91.49) |
12.02 (9.64–15.17) |
| 56–64 | 170.22 (165.96–174.56) |
31.59 (26.57–37.86) |
162.35 (156.60–168.24) |
47.47 (36.1–63.71) |
166.44 (161.3–171.69) |
37.17 (30.06–46.51) |
144.44 (139.95–149.04) |
37.47 (30.11–47.22) |
169.07 (162.83–175.48) |
23.3 (18.73–29.34) |
| PE | 179.46 (177.26–181.67) |
139.95 (137.50–142.44) |
139.64 (137.46–141.84) |
148.04 (145.90–150.20) |
169.82 (166.99–172.68) |
|||||
| 18–25 | 43.62 (41.03–46.32) |
[ref] | 29.41 (26.75–32.27) |
[ref] | 31.51 (29.06–34.11) |
[ref] | 35.17 (32.7–37.79) |
[ref] | 47.68 (43.76–51.85) |
[ref] |
| 26–35 | 88.50 (84.72–92.41) |
2.03 (1.88–2.19) |
58.20 (54.57–62.02) |
1.98 (1.77–2.22) |
61.35 (57.91–64.94) |
1.95 (1.76–2.15) |
69.36 (65.79–73.07) |
1.97 (1.80–2.16) |
85.9 (81.35–90.63) |
1.8 (1.63–1.99) |
| 36–45 | 141.96 (137.5–146.54) |
3.25 (3.04–3.49) |
111.83 (107.06–116.75) |
3.8 (3.43–4.22) |
110.07 (105.86–114.41) |
3.49 (3.20–3.82) |
117.66 (113.49–121.94) |
3.35 (3.09–3.63) |
137.11 (131.76–142.62) |
2.88 (2.62–3.16) |
| 46–55 | 229.26 (224.06–234.55) |
5.26 (4.93–5.61) |
186.41 (180.48–192.48) |
6.34 (5.74–7.01) |
178.87 (173.81–184.04) |
5.68 (5.22–6.19) |
189.69 (184.75–194.73) |
5.39 (5–5.83) |
215.54 (209.09–222.14) |
4.52 (4.13–4.95) |
| 56–64 | 315.28 (309.47–321.17) |
7.23 (6.79–7.71) |
280.65 (273.08–288.38) |
9.54 (8.66–10.53) |
276.91 (270.27–283.68) |
8.79 (8.09–9.56) |
279.75 (273.48–286.13) |
7.95 (7.38–8.59) |
316.35 (307.78–325.09) |
6.64 (6.07–7.26) |
| TM | 1.96 (1.74–2.21) |
1.41 (1.17–1.68) |
1.57 (1.35–1.82) |
1.28 (1.08–1.49) |
1.61 (1.35–1.91) |
|||||
| 18–25 | 1.15 (0.77–1.66) |
[ref] | 0.59 (0.27–1.12) |
[ref] | 0.98 (0.59–1.53) |
[ref] | 0.80 (0.47–1.28) |
[ref] | 1.49 (0.87–2.38) |
[ref] |
| 26–35 | 1.59 (1.12–2.19) |
1.38 (0.82–2.34) |
1.46 (0.94–2.18) |
2.47 (1.11–6.05) |
1.39 (0.92–2.03) |
1.42 (0.76–2.71) |
1.13 (0.71–1.69) |
1.41 (0.72–2.81) |
1.09 (0.64–1.75) |
0.73 (0.35–1.53) |
| 36–45 | 2.26 (1.73–2.91) |
1.96 (1.24–3.19) |
1.13 (0.70–1.73) |
1.91 (0.84–4.75) |
1.45 (1.01–2.03) |
1.49 (0.82–2.76) |
1.21 (0.82–1.72) |
1.52 (0.81–2.92) |
1.77 (1.21–2.50) |
1.19 (0.64–2.29) |
| 46–55 | 2.23 (1.74–2.81) |
1.94 (1.23–3.11) |
1.94 (1.38–2.65) |
3.28 (1.56–7.69) |
1.85 (1.37–2.44) |
1.89 (1.09–3.4) |
1.56 (1.14–2.08) |
1.95 (1.10–3.63) |
1.78 (1.24–2.47) |
1.2 (0.65–2.28) |
| 56–64 | 2.30 (1.83–2.85) |
2.00 (1.29–3.18) |
1.72 (1.18–2.43) |
2.91 (1.36–6.92) |
2.01 (1.48–2.66) |
2.05 (1.18–3.7) |
1.51 (1.09–2.05) |
1.89 (1.05–3.55) |
1.83 (1.23–2.61) |
1.23 (0.66–2.38) |
| Unusual site TTS | 3.80 (3.49–4.14) |
3.04 (2.69–3.42) |
2.17 (1.91–2.46) |
2.02 (1.78–2.28) |
2.92 (2.56–3.32) |
|||||
| 18–25 | 0.90 (0.57–1.37) |
[ref] | 0.79 (0.41–1.38) |
[ref] | 0.21 (0.06–0.53) |
[ref] | 0.33 (0.13–0.68) |
[ref] | 0.79 (0.36–1.49) |
[ref] |
| 26–35 | 1.80 (1.30–2.44) |
1.99 (1.16–3.50) |
0.43 (0.17–0.88) |
0.54 (0.18–1.49) |
0.93 (0.55–1.47) |
4.51 (1.49–18.33) |
0.54 (0.27–0.96) |
1.64 (0.58–4.98) |
0.77 (0.40–1.35) |
0.98 (0.38–2.63) |
| 36–45 | 2.45 (1.89–3.11) |
2.71 (1.65–4.60) |
1.35 (0.87–1.99) |
1.71 (0.83–3.73) |
1.58 (1.11–2.18) |
7.68 (2.76–29.65) |
1.52 (1.08–2.08) |
4.63 (2.05–12.27) |
2.49 (1.82–3.33) |
3.17 (1.53–7.37) |
| 46–55 | 4.55 (3.84–5.35) |
5.03 (3.20–8.27) |
3.98 (3.15–4.95) |
5.04 (2.73–10.16) |
2.71 (2.12–3.42) |
13.18 (4.93–49.69) |
2.30 (1.79–2.92) |
7.00 (3.22–18.07) |
3.46 (2.68–4.38) |
4.39 (2.18–10.01) |
| 56–64 | 7.43 (6.56–8.38) |
8.21 (5.31–13.33) |
7.85 (6.63–9.23) |
9.94 (5.52–19.68) |
4.77 (3.93–5.73) |
23.16 (8.80–86.46) |
4.61 (3.84–5.5) |
14.02 (6.61–35.58) |
6.27 (5.12–7.61) |
7.97 (4.04–17.93) |
| Common site TTS | 35.22 (34.25–36.21) |
28.25 (27.15–29.38) |
22.89 (22.01–23.79) |
22.74 (21.91–23.59) |
30.47 (29.28–31.69) |
|||||
| 18–25 | 6.17 (5.22–7.24) |
[ref] | 3.95 (3.01–5.08) |
[ref] | 4.63 (3.72–5.69) |
[ref] | 4.42 (3.57–5.41) |
[ref] | 8.13 (6.56–9.96) |
[ref] |
| 26–35 | 10.42 (9.15–11.82) |
1.69 (1.37–2.09) |
7.75 (6.46–9.22) |
1.96 (1.43–2.72) |
6.66 (5.56–7.91) |
1.44 (1.09–1.90) |
7.88 (6.71–9.20) |
1.78 (1.38–2.33) |
8.28 (6.91–9.84) |
1.02 (0.77–1.34) |
| 36–45 | 18.88 (17.28–20.60) |
3.06 (2.55–3.70) |
14.14 (12.48–15.96) |
3.58 (2.70–4.83) |
12.39 (11.00–13.90) |
2.67 (2.10–3.43) |
12.31 (10.98–13.74) |
2.79 (2.21–3.55) |
17.94 (16.04–20.00) |
2.21 (1.75–2.81) |
| 46–55 | 43.13 (40.89–45.45) |
6.99 (5.90–8.33) |
34.62 (32.10–37.30) |
8.77 (6.73–11.62) |
26.88 (24.94–28.93) |
5.80 (4.66–7.31) |
26.63 (24.8–28.56) |
6.03 (4.86–7.55) |
36.49 (33.87–39.26) | 4.49 (3.61–5.63) |
| 56–64 | 76.43 (73.59–79.36) |
12.39 (10.51–14.70) |
73.34 (69.50–77.34) |
18.58 (14.35–24.49) |
56.74 (53.76–59.84) |
12.25 (9.89–15.34) |
53.93 (51.2–56.77) |
12.21 (9.90–15.20) |
73.68 (69.58–77.95) |
9.06 (7.33–11.31) |
| CD, in thousandsb | 2.82 (2.81–2.83) |
2.08 (2.07–2.09) |
2.34 (2.34–2.35) |
2.34 (2.33–2.35) |
N/A | |||||
| 18–25 | 0.07 (0.06–0.07) |
[ref] | 0.06 (0.05–0.06) |
[ref] | 0.07 (0.07–0.07) |
[ref] | 0.07 (0.06–0.07) |
[ref] | N/A | N/A |
| 26–35 | 0.28 (0.28–0.29) |
4.23 (4.00–4.46) |
0.22 (0.21–0.23) |
3.77 (3.50–4.06) |
0.26 (0.25–0.26) |
3.73 (3.51–3.97) |
0.26 (0.25–0.26) |
3.90 (3.68–4.14) |
N/A | N/A |
| 36–45 | 0.95 (0.94–0.96) |
14.13 (13.44–14.86) |
0.74 (0.72–0.75) |
12.65 (11.81–13.55) |
0.86 (0.84–0.87) |
12.46 (11.79–13.18) |
0.83 (0.82–0.84) |
12.56 (11.90–13.26) |
N/A | N/A |
| 46–55 | 4.13 (4.11–4.15) |
61.48 (58.55–64.60) |
3.26 (3.24–3.29) |
56.05 (52.45–59.96) |
3.61 (3.59–3.63) |
52.53 (49.77–55.49) |
3.54 (3.52–3.56) |
53.62 (50.86–56.56) |
N/A | N/A |
| 56–64 | 6.90 (6.87–6.92) |
102.62 (97.76–107.78) |
5.65 (5.61–5.68) |
97.11 (90.89–103.88) |
6.22 (6.18–6.25) |
90.49 (85.74–95.58) |
6.09 (6.06–6.12) |
92.16 (87.44–97.20) |
N/A | N/A |
| HTN, in thousandsb | 10.37 (10.35–10.39) |
7.80 (7.78–7.82) |
8.99 (8.97–9.01) |
9.44 (9.42–9.46) |
N/A | |||||
| 18–25 | 1.22 (1.21–1.24) |
[ref] | 0.94 (0.92–0.96) |
[ref] | 1.11 (1.10–1.13) |
[ref] | 1.12 (1.11–1.14) |
[ref] | N/A | N/A |
| 26–35 | 3.93 (3.90–3.96) |
3.21 (3.17–3.25) |
2.77 (2.74–2.79) |
2.94 (2.89–3.00) |
3.39 (3.37–3.42) |
3.05 (3.00–3.1) |
3.57 (3.54–3.60) |
3.18 (3.13–3.23) |
N/A | N/A |
| 36–45 | 8.06 (8.02–8.1) |
6.58 (6.50–6.66) |
6.33 (6.29–6.37) |
6.73 (6.62–6.85) |
7.33 (7.30–7.37) |
6.59 (6.5–6.69) |
7.51 (7.48–7.55) |
6.69 (6.60–6.79) |
N/A | N/A |
| 46–55 | 16.15 (16.09–16.2) |
13.19 (13.04–13.34) |
12.57 (12.51–12.62) |
13.37 (13.14–13.60) |
14.23 (14.18–14.28) |
12.79 (12.62–12.96) |
14.74 (14.69–14.79) |
13.13 (12.96–13.3) |
N/A | N/A |
| 56–64 | 24.54 (24.47–24.61) |
20.04 (19.82–20.27) |
19.25 (19.17–19.33) |
20.47 (20.14–20.82) |
22.15 (22.08–22.23) |
19.91 (19.65–20.18) |
23.03 (22.96–23.11) |
20.52 (20.26–20.78) |
N/A | N/A |
| WCV, in thousandsb | 69.73 (69.69–69.77) |
50.23 (50.19–50.28) |
53.47 (53.43–53.51) |
60.41 (60.37–60.45) |
N/A | |||||
| 18–25 | 51.23 (51.14–51.32) |
[ref] | 35.26 (35.16–35.35) |
[ref] | 35.56 (35.48–35.65) |
[ref] | 41.76 (41.67–41.84) |
[ref] | N/A | N/A |
| 26–35 | 60.91 (60.81–61.01) |
1.19 (1.19–1.19) |
44.00 (43.90–44.10) |
1.25 (1.24–1.25) |
45.07 (44.97–45.16) |
1.27 (1.26–1.27) |
52.11 (52.01–52.2) |
1.25 (1.25–1.25) |
N/A | N/A |
| 36–45 | 69.56 (69.46–69.66) |
1.36 (1.36–1.36) |
51.93 (51.83–52.04) |
1.47 (1.47–1.48) |
53.77 (53.68–53.87) |
1.51 (1.51–1.52) |
61.06 (60.97–61.16) |
1.46 (1.46–1.47) |
N/A | N/A |
| 46–55 | 77.44 (77.34–77.54) |
1.51 (1.51–1.51) |
57.33 (57.22–57.43) |
1.63 (1.62–1.63) |
61.17 (61.08–61.27) |
1.72 (1.72–1.72) |
68.74 (68.65–68.83) |
1.65 (1.64–1.65) |
N/A | N/A |
| 56–64 | 81.25 (81.16–81.34) |
1.59 (1.58–1.59) |
58.59 (58.48–58.70) |
1.66 (1.66–1.67) |
65.97 (65.87–66.07) |
1.85 (1.85–1.86) |
71.62 (71.52–71.72) |
1.72 (1.71–1.72) |
N/A | N/A |
Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; BHI, Blue Health Intelligence; CD, colonic diverticulitis; CI, confidence interval; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; IR, incidence rate; IRR, incidence rate ratio; ITP, immune thrombocytopenia; N/A, not available; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; PY, person-years; ref, reference; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit.
Analyses and results for negative control events are not available for Optum.
Incidence rates of negative control events are reported in thousands (e.g., a value of 1 corresponds to a rate of 1,000 per 100,000 person-years).
Among Medicare beneficiaries aged ≥ 65 years, rates of AESI also varied by age (Supplemental Table 6). Compared with individuals aged 65–74 years, 10 AESI (AMI, Bell’s palsy, DVT, DIC, hemorrhagic and non-hemorrhagic stroke, ITP, myocarditis/pericarditis, PE, common site TTS) had higher rates among those aged ≥ 75 years, while rates of anaphylaxis, appendicitis, and unusual site TTS were lower among individuals aged ≥ 75 years.
3.3.3. Nursing home residency status (Medicare only)
Among adults aged ≥ 65 years in Medicare in 2019, nursing home residents had higher rates for 13 AESI, ranging from 35 % higher than rates among non-nursing home residents for Bell’s palsy (IRR = 1.35; 95 % CI: 1.29–1.42) to almost threefold higher for DIC (IRR = 2.93; 95 % CI: 2.69–3.18) (Table 3 ). Additional AESI with higher rates among nursing home residents included AMI, DVT, encephalitis/encephalomyelitis, hemorrhagic stroke, ITP, myopericarditis/pericarditis, narcolepsy, non-hemorrhagic stroke, PE, transverse myelitis, and common site TTS. Rates of anaphylaxis, appendicitis, and GBS were lower for Medicare nursing home residents than non-residents; rates of unusual site TTS did not differ by nursing home residency status.
Table 3.
Incidence rates for each AESI and negative control event by nursing home residence status among CMS Medicare beneficiaries aged 65+ years in 2019.
| AESI or negative control | All Medicare beneficiaries IR per 100,000 PY (95 % CI) |
Nursing home IR per 100,000 PY (95 % CI) |
Non-nursing home IR per 100,000 PY (95 % CI) |
IRR comparing nursing home vs non-nursing home (95 % CI) |
|---|---|---|---|---|
| AMI | 1,297.48 (1,293.01–1,301.97) |
3,037.00 (2,991.55–3,082.96) |
1,257.34 (1,252.88–1,261.80) |
2.42 (2.38–2.45) |
| Anaphylaxis | 10.57 (10.18–10.96) |
7.09 (5.15–9.52) |
10.65 (10.26–11.05) |
0.67 (0.48–0.90) |
| Appendicitis | 80.05 (78.95–81.16) |
50.74 (45.09–56.89) |
80.74 (79.62–81.87) |
0.63 (0.56–0.71) |
| Bell’s palsy | 215.42 (213.64–217.21) |
288.54 (275.06–302.50) |
213.71 (211.92–215.51) |
1.35 (1.29–1.42) |
| DVT | 1,330.99 (1,326.43–1,335.55) |
3,732.56 (3,681.22–3,784.43) |
1,277.11 (1,272.6–1,281.63) |
2.92 (2.88–2.96) |
| DIC | 36.91 (36.17–37.67) |
103.54 (95.41–112.18) |
35.35 (34.61–36.10) |
2.93 (2.69–3.18) |
| ENC | 9.82 (9.44–10.21) |
18.55 (15.26–22.34) |
9.62 (9.24–10.00) |
1.93 (1.58–2.33) |
| GBS | 4.63 (4.36–4.90) |
2.77 (1.58–4.50) |
4.67 (4.40–4.95) |
0.59 (0.34–0.97) |
| HS | 205.26 (203.49–207.04) |
416.60 (400.07–433.63) |
200.32 (198.55–202.09) |
2.08 (2.00–2.17) |
| ITP | 90.21 89.03–91.39) |
122.14 (113.28–131.50) |
89.45 (88.28–90.65) |
1.37 (1.26–1.47) |
| Myocarditis/pericarditis | 88.09 (86.94–89.26) |
155.04 (145.05–165.55) |
86.52 (85.36–87.69) |
1.79 (1.67–1.92) |
| Narcolepsy | 37.71 (36.96–38.48) |
73.33 (66.51–80.66) |
36.88 (36.12–37.65) |
1.99 (1.80–2.19) |
| NHS | 842.83 (839.23–846.44) |
1,757.09 (1,722.59–1,792.11) |
821.80 (818.2–825.40) |
2.14 (2.10–2.18) |
| PE | 755.10 (751.69–758.53) |
1,447.10 (1,415.82–1,478.88) |
739.06 (735.65–742.49) |
1.96 (1.91–2.00) |
| TM | 3.38 (3.15–3.61) |
7.62 (5.53–10.22) |
3.28 (3.06–3.51) |
2.32 (1.67–3.15) |
| Unusual site TTS | 12.49 (12.06–12.93) |
13.54 (10.53–17.13) |
12.47 (12.03–12.92) |
1.09 (0.84–1.38) |
| Common site TTS | 362.30 (359.95–364.67) |
760.53 (736.67–784.96) |
354.12 (351.77–356.48) |
2.15 (2.08–2.22) |
| CD, in thousandsa | 8.91 (8.89–8.92) |
5.65 (5.58–5.71) |
8.99 (8.97–9.00) |
2.32 (1.67–3.15) |
| HTN, in thousandsa | 26.55 (26.52–26.59) |
59.02 (58.42–59.63) |
26.28 (26.24–26.31) |
1.09 (0.84–1.38) |
| WCV, in thousandsa | 47.88 (47.85–47.91) |
16.79 (16.68–16.90) |
48.74 (48.71–48.77) |
0.34 (0.34–0.35) |
Abbreviations: AESI, adverse event of special interest; AMI, acute myocardial infarction; CD, colonic diverticulitis; CI, confidence interval; CMS, Centers for Medicare & Medicaid Services; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ENC, encephalitis/encephalomyelitis; GBS, Guillain-Barré syndrome; HS, hemorrhagic stroke; HTN, hypertension; IR, incidence rate; IRR, incidence rate ratio; ITP, immune thrombocytopenia; NHS, non-hemorrhagic stroke; PE, pulmonary embolism; PY, person-years; TM, transverse myelitis; TTS, thrombosis with thrombocytopenia syndrome; WCV, well-care visit.
Incidence rates of negative control events are reported in thousands (e.g., a value of 1 corresponds to a rate of 1,000 per 100,000 person-years).
3.3.4. Race/ethnicity (Medicare only)
For adults aged ≥ 65 years in Medicare, two AESI had higher IRs among people of color than their White counterparts (Supplemental Table 7). Compared with rates for White beneficiaries, rates of DIC were 92 % higher for Asian (IRR = 1.92; 95 % CI: 1.71–2.15), more than twofold higher for Black (IRR = 2.34; 95 % CI: 2.21–2.48), 61 % higher for Hispanic (IRR = 1.61; 95 % CI: 1.39–1.85), and 69 % higher for North American Native (IRR = 1.69; 95 % CI: 1.31–2.15) beneficiaries. Rates of hemorrhagic stroke were 48 % higher for Asian (IRR = 1.48; 95 % CI: 1.41–1.56), 56 % higher for Black (IRR = 1.56; 95 % CI: 1.51–1.60), 25 % higher for Hispanic (IRR = 1.25; 95 % CI: 1.17–1.33), and 26 % higher for North American Native (IRR = 1.26; 95 % CI: 1.12–1.42) beneficiaries. Rates of other AESI also displayed disparities that vary by race/ethnicity.
4. Discussion
The FDA BEST Initiative comprises large administrative claims data, electronic health records (EHRs), and linked claims-EHR databases. This study used six administrative claims databases in the BEST Initiative and included tens of millions of individuals to conduct one of the first large-scale assessments of AESI background rates in the United States using a common protocol, definitions, and analyses across databases. We report the background IRs of 17 AESI and three negative control events. The background rates are used to produce expected rates of AESI for comparison to the observed rates in populations post-COVID-19 vaccination in active surveillance studies to identify increased risks or safety concerns. Considerations in active surveillance monitoring include confounding, generalizability, and the COVID-19 pandemic’s effect on AESI incidence, which this study may inform.
We found heterogeneity in the IRs of AESI among different data sources. For commercially insured adults younger than 65 years, we observed variability in AESI rates across the five commercial insurance data sources. Insurance companies have similar functions and serve the same sector of the U.S. population (employees and their families insured through employers) but may cover different geographic locations. For most AESI examined, IRs tended to be higher in BHI and Optum than in CVS Health, HealthCore, and MarketScan. The population represented in BHI may differ from other commercially insured populations in this study in that the BHI data were limited to individuals who received a biologic product, were pregnant, or were born after October 1, 2015; the other commercially insured populations included all enrollees. For instance, individuals represented in BHI may be more likely to seek care, resulting in higher IRs. Other factors contributing to the heterogeneity across commercial insurance data may be differences in the populations that insurers serve, formulary and care management rules, and data processing systems. This highlights the need for cross-data source active surveillance to account for the differences in background rates observed among the data sources.
Within each data source evaluated, IRs varied by age group and sex and, among the Medicare population, by nursing home residency status and race/ethnicity. Background rates by population characteristics may inform the selection of appropriate comparators in active safety surveillance. Most AESI had higher rates for males than females, increased with age, were higher among Medicare beneficiaries residing in nursing homes, and displayed racial/ethnic differences, with the highest IRs generally among Black beneficiaries (Medicare); however, there were some exceptions to these general observations. Many patterns we observed are generally consistent with recent research conducted in the United States [7], [17] and other countries [7], [18], [19], [20], [21], with the exception of narcolepsy and transverse myelitis, for which much lower rates were observed in non-U.S. EHR and claims data [7], [18].
Visual inspection of monthly rates demonstrated seasonality patterns for anaphylaxis and AMI. In both Medicare and the commercial insurance data sources, rates of anaphylaxis were highest during the summer and lowest during winter. Additionally, in Medicare, rates of AMI were highest in March and lowest in August. For these AESI, active surveillance may need to account for seasonality when comparing observed rates with expected rates or use different approaches (e.g., concurrent comparator) for appropriate comparisons.
COVID-19 affected healthcare utilization in 2020. This finding is consistent with existing reports [18], [22] and is evidenced by lower rates in 2020 for the negative control events that are acute (colonic diverticulitis) and chronic (hypertension), as well as for the indicator of time-insensitive preventive care (well-care visits) included in this study. Although many AESI rates returned to pre-COVID-19 levels by the end of 2020, some remained low during the peri-COVID-19 period compared with their 2019 annual rates. The patterns may inform the choice of the appropriate comparator background rates, which may vary by AESI and data source. For instance, using 2019 annual rates as the background rates for AESI that decreased in the peri-COVID-19 period may lead to overestimated expected rates and bias toward the null and may miss a potential safety signal.
We also observed elevated rates of some AESI during the peri-COVID-19 period that may be associated with COVID-19 disease (e.g., DIC in Medicare and HealthCore) [23]. Previous research has shown that coagulopathy is common in severe COVID-19 cases [23], [24], [25], [26], [27], [28], [29], [30], [31] and that the risk of myocarditis is elevated among patients with COVID-19 [32]. Related to coagulopathy and myocarditis, we measured the IRs of DIC, DVT, ITP, myocarditis/pericarditis, PE, stroke, and common and unusual site TTS. During June 2020–December 2020, rates of DIC, myocarditis/pericarditis, PE, and common and unusual site TTS were elevated by more than 10 % compared with their pre-pandemic rates in at least one data source, while rates of DVT and ITP also showed an increase to a lesser extent (2–7 %) in at least two data sources. Information on the decrease and elevation of rates during the pandemic period is important when conducting active surveillance.
This study has several strengths. It is a large population-based study consisting of more than 100 million individuals across six administrative claims data sources. The large cohort enables the robust estimation of IRs, particularly for rare AESI. Additionally, the use of pre-adjudicated claims, where available, enabled accumulation of more current data with less delay and allowed for calculation of IR estimates through December 11, 2020, for a broad range of AESI and negative control events. To our knowledge, there has not yet been any prior large-scale study comparing pre- and peri-COVID-19 IRs of AESI, including pandemic-related fluctuations, in the United States. One European study, the vACCine COVID-19 monitoring readinESS (ACCESS) project, funded by the European Medicines Agency, evaluated potential impacts of the pandemic on AESI background rates [18]. Most prior studies on AESI background rates stratified the rates by demographic characteristics such as age and sex only [7], [18], [19], [20], [21]. In addition to age and sex, our study stratified the rates by race/ethnicity and nursing home residence status, where available, and revealed additional heterogeneity in rates by these population characteristics.
Our study also has several limitations. The data reflect only Medicare fee-for-service and certain commercially insured populations. Given the observed variability in rates across data sources, our findings may not be generalizable to uninsured or other publicly insured (i.e., Medicaid, Medicare Advantage) populations. Although claims data are valuable for efficiently examining health events, all claims databases have certain inherent limitations because claims are collected for payment, not clinical management or research purposes. To provide a timely pandemic response, we generated the AESI claims-based algorithms and risk and clean periods used in this study by reviewing published literature with prioritization of validation studies, communicating with other agencies, and consulting clinical subject matter experts. However, not all AESI definitions had been validated, nor was literature available to determine the clean period for all AESI. The presence of a diagnosis code on a medical claim may represent a rule-out or miscoding; therefore, it may not necessarily reflect the presence of a disease. Although unlikely, potential selection of mis-specified clean periods may have led to the capture of a combination of prevalent and incident events. Finally, the presented comparisons were unadjusted for other variables, and we did not account for multiple comparisons.
5. Conclusion
This study presents IRs of 17 AESI using a large U.S. population. The estimated rates can serve as historical control rates in any observational study evaluating the safety of medical products when it is not feasible to use concurrent comparator groups. Given that AESI background rates varied by database and demographics, and some fluctuated during the calendar year, it is critical when evaluating COVID-19 vaccine safety to compare post-vaccination AESI rates with the background rates originating from the same database, standardize demographics, and account for seasonal trends.
A current example of a study in which concurrent comparators may not be feasible is initial safety surveillance of COVID-19 vaccines. The authors have used these rates as historical control rates to evaluate the safety of COVID-19 vaccines, applying rapid-cycle analysis soon after each COVID-19 vaccine received EUA from FDA. Although AESI rates varied across data sources and demographic strata in different study periods, the rates were generated in large populations, and the variation likely reflects heterogeneity in the populations and some of the unexpected events the population and the healthcare system were experiencing during the study period. Because the AESI rates cover all age groups in the U.S. population and are stratified by certain demographics, they provide more granular comparator estimates and can be used in more diverse studies.
Large population-based data, like the BEST Initiative, may be useful in generating historical comparators used to identify potential increases in observed rates of AESI that may represent a safety concern requiring further evaluation and mitigation.
Funding
This work was supported by the U.S. Food and Drug Administration through the Department of Health and Human Services (HHS) Contract number HHSF223201810022I Task Order 75F40120F19003.
CRediT authorship contribution statement
Keran Moll: Conceptualization, Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Funding acquisition, Supervision, Resources, Project administration, Writing – original draft, Writing – review & editing. Bradley Lufkin: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Project administration, Writing – original draft, Writing – review & editing. Kathryn R. Fingar: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Cindy Ke Zhou: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Ellen Tworkoski: Conceptualization, Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Chianti Shi: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Shayan Hobbi: Conceptualization, Funding acquisition, Supervision, Resources, Project administration, Writing – original draft, Writing – review & editing. Mao Hu: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – review & editing. Minya Sheng: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – review & editing. Jillian McCarty: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Shanlai Shangguan: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Timothy Burrell: Conceptualization, Writing – original draft, Writing – review & editing. Yoganand Chillarige: Conceptualization, Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Jeff Beers: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Patrick Saunders-Hastings: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Stella Muthuri: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Kathryn Edwards: Conceptualization, Writing – original draft, Writing – review & editing. Steven Black: Conceptualization, Writing – original draft, Writing – review & editing. Jeff Kelman: Data curation, Writing – original draft, Writing – review & editing. Christian Reich: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Funding acquisition, Supervision, Resources, Writing – original draft, Writing – review & editing. Kandace L. Amend: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Djeneba Audrey Djibo: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – review & editing. Daniel Beachler: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Funding acquisition, Supervision, Resources, Writing – original draft, Writing – review & editing. Rachel P. Ogilvie: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Alex Secora: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Cheryl N. McMahill-Walraven: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – review & editing. John D. Seeger: Data curation, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Funding acquisition, Supervision, Resources, Writing – original draft, Writing – review & editing. Patricia Lloyd: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Project administration, Writing – original draft, Writing – review & editing. Deborah Thompson: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Rositsa Dimova: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Thomas MaCurdy: Data curation, Funding acquisition, Supervision, Resources, Writing – original draft, Writing – review & editing. Joyce Obidi: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Project administration, Writing – original draft, Writing – review & editing. Steve Anderson: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Richard Forshee: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Hui-Lee Wong: Conceptualization, Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing. Azadeh Shoaibi: Formal analysis, Methodology, Investigation, Validation, Visualization, Software, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Shayan Hobbi reports financial support was provided by US Food and Drug Administration, Timothy A Burrell reports financial support was provided by US Food and Drug Administration. Timothy A Burrell reports a relationship with IBM that includes: employment, Dr. Edwards has disclosed the following financial relationships. Grant Recipient from CDC (Vaccine Safety with COVID vaccines) Grant Recipient from NIH (Mentoring young investigators in vaccine sciences) Consultant from BioNet (pertussis vaccines) Consultant from IBM (vaccine safety networks) Consultant from Data safety and Monitoring Boards: Sanofi, X-4 Pharma, Seqirus, Moderna, Pfizer, Merck, GSK, Roche, Dr. Black has disclosed the following financial relationships: I am a consultant for CEPI through the SPEAC project and also a consultant for GSK on meeting planning, Djeneba Audrey Djibo reports financial support was provided by US Food and Drug Administration. Djeneba Audrey Djibo reports a relationship with CVS Health that includes: employment and equity or stocks, Daniel C. Beachler reports a relationship with Elevance Health Inc. that includes: employment and equity or stocks, Patricia Lloyd, Deborah Thompson, Rositsa Dimova, Joyce Obidi, Steve Anderson, Richard Forshee, Hui-Lee Wong, Azadeh Shoaibi, Cheryl N McMahill-Walraven reports financial support was provided by US Food and Drug Administration. Cheryl N McMahill-Walraven reports a relationship with CVS Health that includes: employment, John D. Seeger reports financial support was provided by Optum Inc. John D. Seeger reports a relationship with Optum that includes: employment and equity or stocks, Kandace Amend reports financial support was provided by Optum Inc. Kandace Amend reports a relationship with Optum that includes: employment and equity or stocks, Thomas MaCurdy reports financial support was provided by Acumen LLC. Thomas MaCurdy reports a relationship with Acumen LLC that includes: employment and equity or stocks]. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank the following individuals for their support: Lisa S. Kowarski, Veronica Hernandez-Medina, Yu Sun, Molly Bailey, Mary Beth Schaefer, Nasim Lari, Cameron Joyce, Sandia Akhtar, Yixin Jiao, Kathryn Matuska, Jennifer Song, Ron Parambi, Yiyu Fang, Robin Clifford, Katie Reed, Vivian Wilt, Lisa Weatherby, Mike Kirksey, Charlalynn Harris, Jennifer Pigoga, Anne Marie Kline, Nancy Sheffield, Kristin A Sepúlveda, and Tainya C Clarke.
Disclaimer
The opinions expressed are those of the authors and do not necessarily represent the opinions of their respective organizations.
Footnotes
Appendices A and B and supplemental figures/ tables can be found online at https://doi.org/10.1016/j.vaccine.2022.11.003.
Supplementary material
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.World Health Organization. WHO Director-General's statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV). https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov); 30 January 2020 [accessed 28 March 2022].
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/; last updated 23 June 2022 [accessed 23 June 2022].
- 3.COVID-19 Vaccine Tracker. United States of America, https://covid19.trackvaccines.org/country/united-states-of-america/; last updated 28 March 2022 [accessed 28 March 2022].
- 4.Black S.B., Law B., Chen R.T., Dekker C.L., Sturkenboom M., Huang W.T., et al. The critical role of background rates of possible adverse events in the assessment of COVID-19 vaccine safety. Vaccine. 2021;39(19):2712–3278. doi: 10.1016/j.vaccine.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Council for International Organizations of Medical Sciences. CIOMS Cumulative Pharmacovigilance Glossary Version 1.0, https://cioms.ch/wp-content/uploads/2021/03/CIOMS-Cumulative-PV-Glossary-v1.0.pdf; 25 March 2021 [accessed 11 April 2022].
- 6.U.S. Food and Drug Administration. COVID-19 vaccine safety surveillance. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/covid-19-vaccine-safety-surveillance; 7 December 2021 [accessed 28 March 2022].
- 7.Li X., Ostropolets A., Makadia R., Shoaibi A., Rao G., Sena A.G., et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ. 2021;373 doi: 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantor JH, Sood N, Bravata D, Pera M, Whaley CM. The impact of the COVID-19 pandemic and policy response on health care utilization: evidence from county-level medical claims and cellphone data. https://www.nber.org/papers/w28131; November 2020 [accessed 28 March 2022]. [DOI] [PMC free article] [PubMed]
- 9.Lange S.J., Ritchey M.D., Goodman A.B., Dias T., Twentyman E., Fuld J., et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States, January–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:795–800. doi: 10.15585/mmwr.mm6925e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czeisler M.É., Marynak K., Clarke K.E., Salah Z., Shakya I., Thierry J.M., et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterji P., Li Y. Effects of the COVID-19 pandemic on outpatient providers in the United States. Med Care. 2021;59(1):58–61. doi: 10.1097/MLR.0000000000001448. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. Biologics Effectiveness and Safety (BEST) Initiative. https://www.bestinitiative.org/wp-content/uploads/2022/01/C19-Vaccine-Safety-AESI-Background-Rate-Report-2021.pdf; December 2021 [accessed 28 March 2022].
- 13.Safety Platform for Emergency vACcines (SPEAC). SO2-D2.1.2 Priority list of COVID-19 adverse events of special interest: quarterly update. https://brightoncollaboration.us/wp-content/uploads/2021/01/SO2_D2.1.2_V1.2_COVID-19_AESI-update-23Dec2020-review_final.pdf; December 2020 [accessed 28 March 2022].
- 14.Safety Platform for Emergency vACcines (SPEAC). D2.3 Priority list of adverse events of special interest: COVID-19. https://brightoncollaboration.us/wp-content/uploads/2021/11/SPEAC_D2.3_V2.0_COVID-19_20200525_public.pdf; 25 May 2020 [accessed 28 March 2022].
- 15.European Medicines Agency, European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Background rates of adverse events of special interest for monitoring COVID-19 vaccines. https://www.encepp.eu/encepp/viewResource.htm?id=37274; last updated 26 August 2021 [accessed 28 March 2022].
- 16.U.S. Food and Drug Administration, Center for Biologics Evaluation and Research (CBER) Surveillance Program. Background rates of adverse events of special interest for COVID-19 vaccine safety monitoring: protocol. https://www.bestinitiative.org/wp-content/uploads/2022/01/C19-Vax-Safety-AESI-Bkgd-Rate-Protocol-FINAL-2020.pdf; 12 January 2021 [accessed 28 March 2022]. Addendum: https://bestinitiative.org/wp-content/uploads/2022/01/C19-Vax-Safety-AESI-Bkgd-Rate-Protocol-Addendum-2021.pdf.
- 17.Gubernot D., Jazwa A., Niu M., Baumblatt J., Gee J., Moro P., et al. U.S. population-based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine. 2021;39(28):3666–3677. doi: 10.1016/j.vaccine.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willame C, Dodd C, Gini R, Durán CE, Thomsen RM, Wang L, et al. Background rates of adverse events of special interest for monitoring COVID-19 vaccines. Zenodo. https://zenodo.org/record/5255870#.Ycy2hmjMI2x; 25 August 2021 [accessed 28 March 2022].
- 19.Nyberg F, Lindh M, Vanfleteren LEGW, Hammar N, Wettermark B, Sundström J, et al. Adverse events of special interest for COVID-19 vaccines - background incidences vary by sex, age and time period and are affected by the pandemic. medRxiv. doi: 10.1101/2021.10.04.21263507; 5 October 2020 [accessed 28 March 2022].
- 20.Burn E, Li X, Kostka K, Stewart HM, Reich C, Seager S, et al. Background rates of five thrombosis with thrombocytopenia syndromes of special interest for COVID-19 vaccine safety surveillance: incidence between 2017 and 2019 and patient profiles from 25.4 million people in six European countries. medRxiv. doi: 10.1101/2021.05.12.21257083; 17 September 2021 [accessed 28 March 2022]. [DOI] [PMC free article] [PubMed]
- 21.Nasreen S., Calzavara A.J., Sundaram M.E., MacDonald S.E., Righolt C.H., Pai M., et al. Background incidence rates of hospitalisations and emergency department visits for thromboembolic and coagulation disorders in Ontario, Canada for COVID-19 vaccine safety assessment: a population-based retrospective observational study. BMJ Open. 2021;11:e052019. doi: 10.1136/bmjopen-2021-052019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whaley C.M., Pera M.F., Cantor J., Chang J., Velasco J., Hagg H.K., et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open. 2020;3(11):e2024984. doi: 10.1001/jamanetworkopen.2020.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gómez-Mesa J.E., Galindo-Coral S., Montes M.C., Muñoz Martin A.J. Thrombosis and coagulopathy in COVID-19. Curr Probl Cardiol. 2021;46(3) doi: 10.1016/j.cpcardiol.2020.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asakura H., Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113(1):45–57. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miró Ò., Jiménez S., Mebazaa A., Freund Y., Burillo-Putze G., Martín A., et al. Spanish Investigators on Emergency Situations TeAm (SIESTA) network. Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur Heart J. 2021;42(33):3127–3142. doi: 10.1093/eurheartj/ehab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsoularis I., Fonseca-Rodríguez O., Farrington P., Lindmark K., Connolly A.-M.-F. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riyahi S., Dev H., Behzadi A., Kim J., Attari H., Raza S.I., et al. Pulmonary embolism in hospitalized patients with COVID-19: a multicenter study. Radiology. 2021;301(3):E426–E433. doi: 10.1148/radiol.2021210777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patone M., Handunnetthi L., Saatci D., Pan J., Katikireddi S.V., Razvi S., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q., Tong X., George M.G., Chang A., Merritt R.K. COVID-19 and risk of acute ischemic stroke among Medicare beneficiaries aged 65 years or older: self-controlled case series study. Neurology. 2022;98(8):e778–e789. doi: 10.1212/WNL.0000000000013184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., et al. Association between COVID-19 and myocarditis using hospital-based administrative data — United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.