Abstract
As of this writing, it is estimated that there have been nearly 600 million cases of coronavirus disease 2019 (COVID-19) around the world with over six million deaths. While shocking, these figures do not fully illustrate the morbidity associated with this disease. It is also estimated that between 10% and 30% of those who survive COVID-19 develop persistent symptoms after the acute infection has passed. These individuals, who most often experienced initial infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) considered mild to moderate in severity, often display a broad array of symptoms. Collectively, this disorder or syndrome is now referred to as Long COVID (among other designations), and it represents a national/international health crisis. The most frequently reported symptoms associated with Long COVID include chronic fatigue with post exertional features, neurocognitive dysfunction, breathlessness, and somatic pain. Long COVID can range in severity from mild to severely debilitating, with resultant loss of quality of life and productivity. For now, there are many unanswered questions surrounding Long COVID: how can it be best defined, what is needed for accurate diagnosis, what is causing it, and how should it be best managed. How rheumatologists will engage in the Long COVID pandemic is another question; at the minimum, we will be called upon to evaluate and manage our own patients with immune-mediated inflammatory diseases who have developed it. This review focuses on addressing the disease essentials, providing both declarative and procedural knowledge to prepare rheumatologists for how to address Long COVID: understanding its origins, its current case definitions, epidemiology, pathobiology and clinical manifestations. Finally, it will provide an outline on how to clinically approach patients with possible Long COVID and initiate treatment and/or guide them on how to best manage it.
Keywords: COVID-19, Long COVID, Infection
Introduction
The pandemic of SARS-CoV-2 infection has taken a dramatic toll on the world’s population in terms of acute morbidity and mortality. At the same time, despite emerging viral variants, advances in in-patient and out-patient care and the introduction of highly effective vaccines have been attended by a falling mortality. Given that more than half of the US population is estimated to have been infected with SARS-CoV-2, we have now increasingly begun to focus on the post-acute sequelae of COVID-19 (PASC). An estimated 10%–30% of patients experience debilitating symptoms months after resolution of the acute infection [1]. This disorder has been given various names including Long COVID, PASC, and others, and the patients afflicted are often referred to as “Long Haulers” [2]; given its epidemiologic scope, Long COVID has been referred to as “our next national public health disaster” [3,4].
For now, Long COVID is not a condition with diagnostic biomarkers, nor does it have a universally accepted case definition. Despite these limitations it is clear that Long COVID represents an emerging and growing heath problem for millions of Americans (and far more throughout the world), which has led to a significant burden of loss of productivity, decreased quality of life, and fear. The role of rheumatologists will play in this follow-on epidemic is still evolving; at a minimum, they will be engaged by patients with rheumatic diseases, many of whom they already care for, who develop it. Rheumatologists may also be well-suited to engage in research in disease immunopathogenesis and therapeutics, as emerging data suggest that immune dysregulation and immune activation may contribute to the pathogenesis of Long COVID [5]. Although there are presently no specific therapies for Long COVID, we believe there are emerging diagnostic and therapeutic principles that may be considered Best Practices, which we will attempt to summarize here.
Is long COVID-19 unique?
The introduction of a new disease or syndrome in disease nosology deserves critical historical appraisal. Accordingly, we must be reminded that there is a long history of patients describing chronic non-specific symptoms after infection [6,7]. Ironically, in contrast to the current allocation of resources to Long COVID, medically unexplained symptoms following infections have historically received little attention. Indeed, these syndromes have been to some degree the target of derision by some, regarded as an area of fringe science or in some way not real, based largely on being poorly understood and clinically frustrating for both the patient and the practitioner [8]. These manifestations, conceptually thought of as ‘tails’ of acute infections and referred to as Post-Acute Infection Syndromes (PAIS), were recently elegantly reviewed by Choutka and colleagues [7].
Taken in this context, it is not surprising that we are now observing the phenomenon of Long COVID, which is simply the latest in a long list of unexplained syndromes following bacterial, viral, and parasitic infections. Among the more well-established infections associated with PAIS are viral pathogens such as Epstein-Barr virus, Ebola, Alphaviruses (e.g., Chikungunya and Ross River), dengue, and West Nile, as well as non-viral pathogens such as Coxiella burnetii (Q fever), borrelia (Lyme), and Giardia lamblia [7]. It should be noted that the evidence supporting such associations varies significantly based upon the type of study and degree of clinical curation of cases; collectively, however, they provide unequivocal evidence that such phenomenon are not clinically rare, are potentially debilitating, and are in need of serious investigation and the development of effective treatments and care pathways.
In general, while some infections have pathogen-specific PAIS such as corneal disease in Ebola, arthritis in Chikungunya, and anosmia and ageusia in COVID-19, most PAIS are not disease specific and tend to share many features of a common endotype. The most common manifestations of this non-specific form of PAIS generally include fatigue and lassitude with post-exertional features, non-restorative sleep, neurocognitive complaints, signs and symptoms of dysautonomia, and viscerosomatic pain [7,9]. Collectively these features reflect a strong overlap with many other medically unexplained disorders, especially with myalgic encephalomyelitis and chronic fatigue syndrome (ME/CFS) [10,11], as will be discussed below. The fact that Long COVID is closely clinically related to these medically unexplained disorders adds challenges on many fronts. First and perhaps not surprisingly, the mere concept of Long COVID is attended by some controversy [12] in both medical and media circles, not dissimilar to other post infection disorders that have been labeled as “psychosomatic” or in some way not real [3]. Such controversy merely adds to the clinical and interpersonal challenges facing both clinicians and patients who collectively so often feel compelled to find reasons and solutions for their suffering [6].
How is long COVID defined?
Challenges exist regarding how to classify patients with persistent symptoms following acute COVID-19. On one extreme, there is a small group with severe disease, often needing critical care and experiencing end-organ damage (e.g., infarction, fibrosis and scarring), who then endure prolonged recoveries [13](Jiang JACC 2022) and psychologic stress typical of post-ICU syndromes, which are well recognized outside of COVID-19 [14]. This group clearly deserves extensive diagnostic and rehabilitative care but is, in most ways, distinct from the far larger group, members of which suffer after-effects from mild to moderate acute illness. Accordingly, there are likely patients with admixtures of both; as of now there are no clear distinctions or diagnostic biomarkers to readily differentiate these groups.
For now, Long COVID and PASC serve as umbrella terms used to describe the chronic sequelae of SARS-CoV-2 infections; in general, Long COVID is a heterogeneous, multisystem, relapsing, and remitting illness that can affect patients regardless of the severity of their acute SARS-CoV-2 infection [2] and is unattended by end-organ pathology. Numerous groups and official bodies such as the Center for Disease Control and Prevention (CDC) and the UK National Health Service (NHS) have rendered informal definitions, which overlap in terms of stipulating that symptoms must last for a minimum of 4–12 weeks following presumed or proven COVID-19, (even if asymptomatic or paucisymptomatic) [2,7]. A recent Delphi conference designed to achieve a global consensus among experts and patients convened by the World Health Organization [15] concluded that Long COVID or PASC may be defined as a new onset or persistent symptoms usually 3 months from the onset of probable or confirmed SARS-CoV-2 infusion, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. They further assert that common symptoms include, but are not limited to, fatigue, shortness of breath, and cognitive dysfunction, and generally have an impact on everyday functioning; further, they recognize that the definition will likely change as knowledge increases. We believe that this is both an accurate and practical definition of Long COVID.
Epidemiologic risk factors
The incidence, prevalence, and natural history of Long COVID are unknown; these are issues of great importance and complexity, given the lack of consensus on Long COVID diagnosis and lack of current diagnostic biomarkers. All studies to date are limited by gaping holes in our underlying knowledge of the disorder itself, and thus there is a wide range of estimates in the literature. Variances in estimates stem from numerous factors including how patients were recruited (self-referral versus prospective enrolment), methods of assessment (electronic medical record screening, self-reporting, mail delivered or email delivered questionnaires, in person interview and or examination, and wearable device use) (Ledford), sample size, and geographic, social, and cultural influences. The frequency of persistent symptoms (4–12 weeks or longer) following COVID-19 vary widely, ranging from less than 10% to over 80% with most estimates in the 15–30% range [1,13]. More recent epidemiologic analyses using prospective methodologies found that 1 in 8 patients developed Long COVID at 28 days or longer 18. A large global meta-analysis estimated the prevalence of symptoms 30, 60, 90, and 120 days after infection as 0.37 (95% CI: 0.26, 0.49), 0.25 (95% CI: 0.15,0.38), 0.32 (95% CI: 0.14,0.57) and 0.49 (95% CI: 0.40,0.59), respectively, with fatigue as the most common symptom [19].
Risk factors for the development of Long COVID symptomatology have been exhaustively studied. Several features have been repeatedly associated with a greater likelihood of persistent symptomatology: the severity and symptom burden of COVID-19 disease at onset, increasing age, female sex, white ethnicity, poor pre-pandemic general and mental health, and other comorbidities (e.g., obesity and diabetes) [13,20]. It is clear that Long COVID also occurs after mild infection and, while likely less frequent, imposes a significant burden on afflicted patients [21,22]. Despite these seeming areas of agreement, important questions remain unanswered regarding potential mitigating factors. Some preliminary results suggest a partially protective effect from vaccines [23,24] (possibly through lowered disease frequency) and a lower incidence in breakthrough [25] versus primary infection. Recent data also suggest that Long COVID may be less frequent following infection with Omicron variants [26]; each of these findings has broad public health implications. Lingering questions including whether Long COVID occurs after asymptomatic infection, the effects of variants on the risk of Long COVID, the incidence of Long COVID in infants and toddlers as they age, and many more must wait for long-term prospective studies, such as the 1 billion dollar 40,000 person National Institutes of Health (NIH) sponsored trial, which are just getting started and are unlikely to generate answers for some time [27].
An important question in rheumatology is whether patients with immune-mediated inflammatory diseases are equally or more susceptible to Long COVID, and whether it is more or less severe. Currently, we are just beginning to see studies of varying designs that document that post-COVID sequelae are not uncommon; however, the natural history and comparative severity remain to be determined [28,29].
Clinical endotypes
COVID-19 sequelae can be classified in various ways; we favor separating patients with traditional end organ pathologic damage whether through scaring (such as in those with fibrotic pulmonary disease who have experienced severe pneumonitis), microthrombotic complications (stroke and post recovery sequelae of encephalitis and encephalomyelitis), cardiac complications, (pericarditis and myocarditis) and numerous other such complications which have been thoroughly reviewed [13,30,31]. These are serious and severe sequelae that are generally understandable from a pathophysiologic perspective, and they are reminiscent of complications that have been long observed in patients who have required advanced and often prolonged critical care in hospital settings.
For the purposes of this review, we will focus on the by far larger group of patients with post COVID sequelae who a) have a prior history of mild to moderate COVID-19, generally managed in the out-patient setting and b) lack clear underlying tissue pathology to account for symptomatology (e.g., lung scarring and dyspnea, stroke, and neurocognitive dysfunction). For these patients, the majority, but not all, will report a prior history of a viral syndrome consistent with COVID-19. For this subset of long haulers who have persistent unexplained symptoms despite aggressive diagnostic evaluations, the basis for the majority of the most common symptoms (fatigue, post-exertional malaise, neurocognitive dysfunction, dyspnea, cough, and viscerosomatic pain) is far from clear, leaving both clinicians and patients with significant uncertainty. Unspoken but important to emphasize is that not all patients who have had COVID and then have new-onset unexplained symptoms have Long COVID. As will be subsequently discussed, all such patients are deserving of vigorous and thoughtful clinical evaluations.
The spectrum of symptomatology following COVID-19 is daunting. A widely cited meta-analysis described over 50 long-term effects [32], while a more recent online survey of suspected and confirmed COVID-19 patients screened for 203 symptoms [33], reflecting both the broad array of complaints and the urgent need for careful clinical curation before assigning causality of symptoms in individual cases.
Efforts to sort and stratify this myriad of symptoms have attempted to organize the field through two strategies. The first is to focus on those symptoms that have reproducibly been identified in the vast majority of studies: fatigue (often with post-exertional features), cognitive dysfunction, somatic pains, headache, and recurrent flu like symptoms. Of note, anosmia and ageusia, which were previously well-documented and infection-specific sequelae of COVID-19, have largely regressed with Omicron strain infections [2,34]. Another approach has been to utilize machine learning from large data sets to create clinical endotypes [33,35] by grouping symptoms that currently lack an etiopathogenic distinction. We will briefly summarize three symptoms of particular relevance to rheumatologists.
Fatigue
Fatigue is a challenging symptom to both assess and treat; it is widely discussed [36,37] in rheumatology and is the most dominant symptom in most studies of patients with Long COVID. Fatigue in rheumatic disease patients, as well as the general population, is often attended by numerous cofactors including pain, depression, sleep disturbances, and reduced physical activity. Fatigue in Long COVID patients is also affected by these domains but, in its most severe and chronic forms, has the dominant characteristic of postexertional exacerbation [10,38,39]. This is a cardinal feature of ME/CFS and important to explore with patients with medically unexplained fatigue. This postexertional flare of fatigue intensity may be brought on not only by physical exertion but also mental, psychologic, and postural stressors in a variable number of patients. The type of physical exertion that may induce a flare of fatigue is often modest (e.g., brief walking and shopping) and not characteristic of exhaustive exercise training. In our experience with patients with Long COVID, chronic fatigue is generally reported by patients to be the most dominant and bothersome symptom; such patients relate to us that their fatigue is profound, and poses challenges to maintain a productive lifestyle including their roles in the workplace and at home. Fatigue must be at the top level of priorities in the research agenda for Long COVID; it is possible that any resultant advances may benefit patients with other disease characterized by chronic fatigability including ME/CFS and fibromyalgia.
Neuropsychiatric dysfunction
Ranking high in the complaints among Long COVID sufferers are neurocognitive issues, which are often referred to as ‘brain fog’ - an inadequate colloquial descriptor of a major morbidity afflicting such patients [40]. There is a range of neuropsychiatric complaints that have been documented in Long COVID, which have recently been reviewed and confirmed that neuropsychiatric sequelae can occur in up to 91% of patients for 6 months or longer [41]. Most studies, again in those without underlying gross neuropathology, have reassuringly not revealed clinical signs of dementia but have documented persistent or new onset mood disturbances (3–46%), sleep disturbances (3–27%), post-traumatic stress disorder (6–43%), and headache (5–12%) [42]. In terms of neurocognitive function, Long COVID patients frequently display loss of short-term memory, episodic memory loss, and greater decline in vigilance with time on task [43]. An in-depth study examining neurocognitive profiles in (predominantly white) patients referred for formal neuropsychiatric evaluations found mild cognitive deficits on tests involving attention, speed of processing, or executive function. At the time of testing, 35–40% endorsed moderate to severe mood disturbances, and 85% were chronically fatigued. It is believed that such patients would benefit from resources to manage mood and compensatory strategies to address cognitive inefficiencies [44].
In addition to abnormalities in cognition, new onset or exacerbated disturbances of sleep including un-restful and non-restorative sleep, delayed onset sleep, and frequent awakenings are not uncommon [45]. Exacerbations of pre-existing mood disorders or new onset mood disorders as well as post-traumatic stress are also common and may contribute to loss of quality of life [46].
Pain
Pain is prevalent but poorly characterized in Long COVID; it most frequently involves muscle, soft tissues, joints, and the lower back as well as non musculoskeletal pain such as headache [32]. Epidemiologic studies vary widely in the prevalence of musculoskeletal pain (0.3% −61.5%) [47], suggesting that ascertainment and documentation of painful states are not standardized. There is scant data utilizing standardized clinical tools including structural musculoskeletal exams, tender points/colorimetry, muscle strength testing, or musculoskeletal imaging including ultrasound. Clauw and colleagues suggest that SARS-CoV-2 may be capable of inducing or exacerbating painful conditions via diverse mechanisms, though this is an area that remains poorly understood and in need of detailed investigation [48].
Presently, there is no compelling evidence of a widespread inflammatory basis for such pains despite rare cases of inflammatory arthritis and myositis reported as possible post-COVID sequelae [28]. These should be viewed separately from the musculoskeletal pains that accompany the vast majority of patients with Long COVID. The reCOVer Clinic at the Cleveland Clinic has, as of July 2022, cared for over 2000 patients. Our anecdotal experience based upon patients with musculoskeletal pain referred from the reCOVer Clinic has revealed that virtually all have been non-inflammatory, with many bearing features of fibromyalgia. With this anecdotal background, two important questions loom for rheumatologists that need to be addressed. First: does COVID-19 induce a state of central pain sensitization with features of fibromyalgia? Second: what is the fate of patients with preexisting fibromyalgia when they develop COVID-19?
In terms of the question of whether infections with SARS-CoV-2 can result in a fibromyalgia-like syndrome, it is informative to note that a similar syndrome was reported in a small study of patients with Severe Acute Respiratory Syndrome caused by SARS-CoV-1 over decade ago [49]; chronic pain syndromes have been documented up to 12 months after other infections as well, including Q fever, Ross River virus, and Epstein-Barr virus [48]. A questionnaire-based study designed to explore the evidence of central sensitization as a potential mechanism for pain in a cohort of patients identified as having Long COVID revealed that 70.2% displayed evidence of such [50]. A single study in Italy using a questionnaire-based approach to screen 616 patients with COVID-19 based on American College of Rheumatology Survey criteria for fibromyalgia found that up to 30% of patients may satisfy such criteria and, interestingly, were correlated with male gender and obesity [51]. Further characterization of such patients using physical examinations, biomarkers and imaging is needed. With regards to the impact of COVID-19 on patients with pre-existing fibromyalgia, the data are limited and somewhat mixed. One study demonstrated adverse outcomes in women with pre-existing fibromyalgia [52], while another study found that patients with pre-existing fibromyalgia did not experience an increase in incidence or severity of Long COVID [53]. Clearly, further work is needed.
Other clinical manifestations of long COVID-19 important in rheumatology
Beyond the major categories discussed above, and as noted previously, there are a wide range of other symptoms that have been ascribed to Long COVID, which have previously been reviewed [31,32,54,55]. Breathlessness, cough, and dyspnea are common and, when present, require special attention with careful consideration for underlining cardiac or pulmonary pathology. The algorithm for evaluating such patients is complex and includes imaging and pulmonary function tests, followed by more advanced studies including cardiopulmonary testing (CPT), which requires specialty referral {Vehar, 2021 #2551}. Also of note in the context of the dominant Long COVID phenotype (fatigue with post-exertional features, neurocognitive and neuropsychiatric complaints, and bodily pain) is the variable presence of signs and symptoms consistent with postural orthostatic tachycardia syndrome (POTS) [[56], [57], [58]]. Practitioners should actively screen for possible POTS in patients with post-acute COVID syndrome symptoms by inquiring about lightheadedness, fainting, and rapid heartbeat, all of which may be relieved by lying down [59]. Depending on the severity, such patients should be referred for more detailed cardiovascular assessment.
Pathophysiology
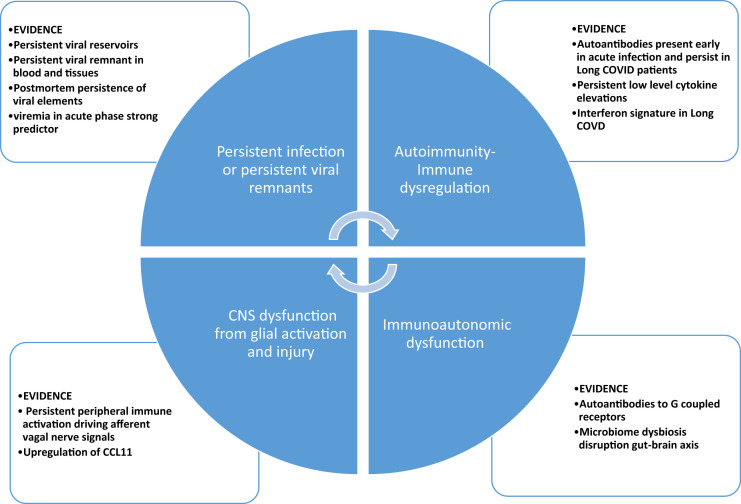
The underlying pathophysiology of Long COVID is likely heterogeneous and has been extensively reviewed [5,7,60]. The most prominent theories with therapeutic implications are summarized and displayed in Fig. 1 . First, it is clear that immune dysregulation is observed in a subset of patients with Long COVID, characterized by persistent, albeit low grade, inflammation [61], dysregulation and persistence of an interferon response [62], and a diverse autoantibody signature [63], all of which could represent therapeutic targets. Unfortunately, at present, it is far from clear that these are drivers of the disorder versus merely markers of disease presence or severity. Growing evidence of viral presence has immediate implications for studying the effects of our increasing armamentarium of antiviral therapies [64]. Mounting evidence for a neuropathologic basis for cognitive dysfunction with evidence of microglial activation [65] and evidence of viral-derived proteins in neural-derived exosomes [66] is providing impetus for trials of anti-inflammatory or immunomodulatory medications, possibly in combination with antiviral therapies. Finally, there is mounting evidence of autonomic dysfunction in a subset of patients [58,67,68], though its origin is unclear. Current theories supported by variable degrees of evidence include autoimmunity, oxidative stress, and/or possible perturbation of the gut-brain axis via dysbiosis. A full discussion of this topic is beyond the scope of this review but as leading mechanisms include immunodysregulation leading to persistent inflammation and/or autoimmunity, they form a basis for potential immune-based therapeutics and are logically of interest to rheumatologists.
Fig. 1.
Proposed major pathogenic mechanisms in long COVID.
Prospects and challenges for therapy
General clinical approach
The role of rheumatologists in diagnosis and management of Long COVID generally occurs in one of two settings. The first is when patients with chronic rheumatic diseases develop Long COVID, and the second is when patients with rheumatic, immunologic, and or inflammatory Long COVID symptoms are referred to rheumatology for further diagnosis and/or treatment.
With regards to the incidence and severity of Long COVID in patients with inflammatory rheumatic diseases we are merely at the beginning of studying these populations. From early in the pandemic {Leon, 2022 #1565} as well as more recently [29], persistent symptoms appear common in patients with inflammatory rheumatic diseases, though the lack of comparator groups makes this difficult to quantify. At the minimum, rheumatologists should expect to see patients from their own practice with lingering symptoms that need to be addressed. Of note the study by DiIorio et al. [29], previously noted, also documented that rheumatic disease patients with COVID-19 frequently disrupted their disease modifying therapies, and that disease flares were common. Though important information, we do not consider this part of the Long COVID spectrum as it is explainable and generally manageable and should be easily recognized.
Clinical evaluation
From the clinical perspective, a guiding principle is that not all patients with post-COVID symptoms have Long COVID and as always, especially given the lack of diagnostic biomarkers or even current classification or diagnostic criteria; a vigorous diagnostic work-up is indicated. Table 1 displays the most common symptoms typically encountered in Long COVID patients along with the most common differential diagnostic considerations. It is important to note that when asking patients about new or exacerbated fatigue, it is critical to explicitly explore the specific features associated with this complaint . Rheumatologists are familiar with fatigue as it is common and often severe in the setting of inflammatory diseases [36]. Fatigue, however, is also clinically challenging; it lacks a formal consensus definition and tools to measure it are both problematic and rarely used in clinical practice. Further, complicating the evaluation of fatigue is its interrelationship with other variables such as pain, sleep, mood, physical inactivity, and autonomic dysfunction - all of which are variably observed in both inflammatory disease states as well as Long COVID [36,69]. The fatigue described in Long COVID, when clinically severe, is generally similar to that of other PAIS and ME/CFS in that it is associated with post-exertional exacerbation. As discussed above, fatigue that is greatly exacerbated by relatively mild to moderate stress (be it physical, intellectual, psychologic, or even orthostatic in nature) should be explicitly searched for in post-COVID patients with lingering symptoms. Pain is of course important, but, as of now, while myalgia and arthralgia have been reported [47], frank articular complaints are relatively uncommon; most pain by description appears to be non-inflammatory and consistent with some form of central sensitization. Signs and symptoms of systemic inflammatory disease including fever, significant weight loss, or features consistent with end-organ dysfunction (e.g., renal, hepatic, and cardiac diseases) are not common in patients who have experienced only mild to moderate infections.
Table 1.
Long COVID – most common signs and symptoms.
| System | Symptom | Differential/must rule out |
|---|---|---|
| Fatigue | Post-exertional fatigue and/or malaise | Anemia Deconditioning |
| Musculoskeletal | Joint pain Myalgia Diffuse pain |
Central sensitization Small fiber neuropathy Inflammatory arthritis Myositis |
| Neuropsychiatric | Anxiety Depression Posttraumatic stress |
Pre-morbid mood disorder |
| Neurocognitive | “Brain fog” Decreased concentration Decreased reaction time Impaired memory Light sensitivity |
Hypothyroidism Autonomic dysfunction Post-ICU syndrome |
| Sleep | Insomnia Hypersomnia Unrefreshing sleep |
Sleep-related disorders – sleep apnea, narcolepsy Mood disorder Medication effect |
| Autonomic Nervous System |
|
Dehydration Orthostatic hypotension Postural Orthostatic Tachycardia Syndrome Small fiber neuropathy |
| Respiratory | Shortness of breath Dyspnea on exertion/exercise intolerance |
Asthma Postviral reactive airways Organizing pneumonia Pulmonary embolus Myocarditis Pulmonary scarring from acute pneumonitis |
Neurocognitive complaints are common in patients with Long COVID. These complaints are often referred to as ‘brain fog’, which is an inadequate and potentially pejorative term considering it is a source of both concern and stress. In general, while the neuropathological basis for this is still unclear, there is no evidence that it is progressive with some reports supporting gradual resolution. The relationship of mood with other complaints such as fatigue, pain, and neurocognitive dysfunction is complex; since there exists an increase in intercurrent mood disturbances in the general population during the COVID pandemic, this is only amplified in patients with Long COVID [70]. There are evidence that Long COVID is also accompanied by varying degrees of social stigmatization that only amplify subjective symptoms [18]When neurocognitive dysfunction is a source of significant distress or concern, a referral for formal neuropsychiatric testing and management is indicated. As noted above, the role of mood disorders in Long COVID is complex, and our understanding as to how it contributes to the multisystemic illness as both risk factor and possible enhancer of other symptomatic domains is evolving [71,72]. Screening for mood disorders with standardized instruments is of value, and referral for treatment is indicated when clinically significant.
Finally, the role and value of extensive laboratory testing including the use of autoantibodies, serum cytokines, flow cytometry, and beyond has no demonstrable value other than to rule out other diseases that may be suggested by such tests. A recent in-depth study of clinical and laboratory features of patients with unexplained post-COVID symptomatology performed at the NIH revealed a high burden of symptoms, but extensive diagnostic evaluation revealed no specific cause in most cases [73].
Outside of the spectrum of Long COVID, there are rare reports of post-COVID occurrences of serious flares of pre-existing immune-mediated diseases or, even rarer, new onset of symptoms that can resemble some autoimmune or inflammatory diseases. The severity of some of these complications or manifestations is also varied, ranging from benign and self-limiting features to life-threatening systemic syndromes; the etiology is far from clear in the majority of cases. Such disorders are not within the working framework of Long COVID, remain largely medically unexplained, and have been extensively reviewed [74].
Therapy of long COVID
Presently, there is no specific therapy for the most vexing and common manifestations of Long COVID including fatigue with post-exertional features, neurocognitive dysfunction, and somatic pain, though there are principles of management largely drawn from the management of other medically unexplained disorders. Above all, the goals of management must be patient-centered, focusing on managing symptoms, self-efficacy, and improving quality of life. Progress is being made in building consensus for management, but much work needs to be done [2]. The CDC emphasizes the treatment of comorbidities (i.e., medical and psychologic), goal setting, and establishing a rehabilitation plan [75]. The US Department of Veterans affairs has recently issued guidelines on the management of multisystem illness, with a particular focus on unexplained fatigue, pain and neurocognitive complaints. These guidelines emphasize the incorporation of holistic and integrative medicine practices such as mindfulness, mind-body medicine, and some form of exercise, as well as the avoidance of therapies attended by negative evidence from clinical trials or with potential for harm [76].
Many major health care institutions have opened multidisciplinary clinics for Long COVID patients; at the Cleveland Clinic, the reCOVer clinic is seeing approximately 125 new patients per month [77]. Most clinics focus on accurate diagnosis combined with non-specific management of comorbidities and referral to specialty services for clinically significant complications such as neurocognitive, neuropsychiatric, immunoautonomic, and cardiopulmonary complications [77]. For those patients for whom fatigability is a prominent issue, referral to some type of cardiopulmonary rehabilitation program and continued follow-up is indicated. Some degree of uncertainty, however, surrounds the type of rehabilitative program that is optimal. There exists a long-standing debate in ME/CFS circles regarding the risks and benefits of graded exercise, which is typical for physically deconditioned individuals, versus programs specifically tailored for patients with postexertional fatigue, in which exercises/activity programs are paced based on how patients feel [10]. A multidisciplinary consensus guideline on assessment and treatment of fatigue in the setting of Long COVID by the American Academy of Physical Medicine and Rehabilitation also recommends an individualized, structured, and titrated return to activity program, which seems to support an approach in line with programs widely utilized for ME/CFS [78]. The nature of fatigue with postexertional features is still poorly understood, and it may be attended by signs of exertional oxygen desaturation and orthostatic intolerance, underscoring the importance of consulting specialists for CPT when clinically significant. Merely telling patients to push through their pain and discomfort rather than titrate their activities is often ill-advised and may lead to postexertional exacerbation and loss of trust in their rehabilitation program.
Fortunately, studies on the natural history of Long COVID do appear to demonstrate gradual improvement over time in unrefreshing sleep and fatigue in many but not all patients, with great variability at the individual level [7]. There is also some evidence that neurocognitive symptoms may linger longer and at times even intensify [79]. In others, there appears to be a waxing and waning of symptoms which patients should be made aware of [2,80]. While there appears to be a small percentage of patients with Long COVID who have been observed to be profoundly and chronically debilitated even several years into their course, this is not the norm in terms of our understanding of the clinical course in the vast majority [80]. Even with this note of optimism, the significant burden of Long COVD should not be minimized for those who are most seriously afflicted; considering the prevalence, the disease must remain a national priority [2]. Finally, multidisciplinary Long COVID clinics are not available to all, and thus medically underserved patients will lack equal access to such a limited service.
We currently believe that in light of so many unanswered questions surrounding Long COVID, the very nature of the clinical encounter and the biopsychosocial factors surrounding the patient–clinician interaction are vitally important and can have meaningful effects on clinical outcomes including quality of life and symptom improvements. Unfortunately, it is not uncommon in the care setting for medically unexplained symptoms, particularly for those occurring in the absence of abnormalities on physical examination and screening diagnostic studies, to be dismissed or minimized by families and clinicians. This lack of support on the most basic level may lead to a clinical encounter attended by toxicity, frustration, and despair [6]. By incorporating empathic communication, which provides listening combined with validation and expungement of guilt, the mere nature of the encounter may provide a positive therapeutic effect. The scientific bases for this is derived from the growing literature in placebo science as recently discussed [81].
Experimental pharmacologic therapies
As there are no approved therapies for Long COVID, we are just at the beginning of a long process of drug discovery. As of August 2022, there were over 280 registered studies focused on some aspect of Long COVID, but most are epidemiologic or clinical discovery (www.clinincal trials.gov access date Aug 13th, 2022). The vast majority of therapeutic studies focus on integrative therapies (e.g., dietary supplements, exercise rehabilitation including mind-body approaches, hyperbaric oxygen, cognitive behavioral therapies, and many others); of these, most are unpublished or of limited design quality compromising their interpretation. A recent well-designed randomized trial of hyperbaric oxygen therapy in a cohort of 37 patients with Long COVID utilizing a sham control arm with documented effective blinding describes statistically and clinically significant improvements in cognitive function, fatigue, and pain. Importantly, MRIs revealed superior perfusion, which correlated with clinical improvements [82]. These data, while intriguing, need to be replicated.
In terms of trials of pharmacologic agents, there are fewer than 30 randomized studies at some stage of development [83] These are partially summarized in Table 2 . It is telling that this list is so short, given the prevalence and public health needs surrounding Long COVID. This lack of effort likely reflects the reluctance of the pharmaceutical industry to invest in a disease with so many unanswered questions.
Table 2.
Summary of pharmacologic therapeutics for long COVID currently registered (August 2022 www.ClinicalTrials.gov).
| Therapeutic Class | Agent/Status | Comment |
|---|---|---|
| Antivirals | Nirmatrelvir– anecdotal reports only, no formal trials registered | Observational descriptions clinical improvement in some [86] |
| Immunomodulatory agents | Leronlimab -pilot study completed [87] | Anti CCR5 receptor monoclonal antibody with the improvement in some associated with modulation of cell surface receptor expression- further trials planned |
| Imatinib or infliximab | Active drugs versus standard of care assessing effects on quality of life; currently recruiting | |
| IV methylprednisolone or IVIg | Placebo-controlled trial for neuro Long COVID; recruiting | |
| Metabolic Agents | AXA1125 | An amino acid mixture with activity against NASH∗∗ in a small RCT with primary endpoint of fatigue |
| Miscellaneous agents [83] | TNX102 (centrally active analgesic), colchicine, statins, cell-based therapies, glucocorticoids, antihistamines, antithrombotics, and dietary supplements | Some of these agents are novel, and some are repurposed |
There are now emerging clinical reports, largely anecdotal and observational, of a variety of therapies that should be interpreted with caution. Such observations include improvement in Long COVID symptoms after subsequent COVID-19 booster vaccination [84]. Several classes of candidate pharmacologic therapeutics are listed in Table 2. Of particular interest are the role of the virus as a driver of Long COVID (largely based on the emerging data for viral persistence in Long COVID-19) [85], as well as a correlation of subsequent Long COVID with initial viremia of SARS-CoV-2 and Epstein-Barr virus in the acute phase of infection [85]. There are now emerging anecdotal reports of improvement in Long COVID in small numbers of patients following a course of oral nirmatrelvir, further supporting the urgent need for randomized and placebo-controlled trials [86]. Based on the growing data surrounding ongoing inflammation, as evidenced by modest but significant elevations of inflammatory cytokines and persistence of dysregulated interferon signaling, there is growing interest in immunomodulatory approaches to treatment; to date, there are no robust studies completed of any such agents. Finally, it is important to note that, not surprisingly in a disease surrounded by so many unanswered questions at a time of intense societal stress and controversy surrounding the infection itself, desperate patients are seeking and receiving a myriad of unproven remedies.
Summary
Long COVID is a disease state of profound public health importance, which is yet early in its evolution. Despite extraordinary progress being made in elucidating its pathobiology, numerous critical issues remain unsettled. Most prominent among these issues are the lack of a uniform and widely accepted set of classification criteria, diagnostic criteria, and diagnostic biomarkers. Patients are in need of care now and rheumatologists will have to assess how they will approach and engage rheumatic disease patients who are afflicted. Furthermore, many of the most common disease manifestations are domains well familiar to the rheumatologist, further emphasizing the important roles we will need to play in order to further progress in the arena of Long COVID. Resources for comprehensive evaluation and treatment are currently and will likely to remain inadequate to manage the vast majority of patients, resulting in practitioners in all specialties needing to “step up” and participate in emerging care pathways. Finally, growing data support some role of viral immune interactions in pathogenesis, thus yielding prospects of immune interventions which may be well suited to the expertise of the rheumatologist.
Practice points.
-
•
Long COVID is an emerging and growing health problem
-
•
Long COVID remains without a universally accepted definition, diagnostic biomarkers, or specific therapies
-
•
The role of rheumatologists in Long COVID is still evolving
Research agenda.
-
•
Long-term prospective studies are needed to better define diagnosis, clinical endotypes and risk factors for Long COVID
-
•
Studies evaluating incidence, severity and clinical characteristics of Long COVID in patients with immune-mediated inflammatory diseases are needed
-
•
Placebo-controlled trials will be essential in discovering effective treatments for Long COVID
Declaration of competing interest
LHC: AstraZeneca.
CC: AstraZeneca.
References
- 1.Ledford H. How common is long COVID? Why studies give different answers. Nature. 2022;606(7916):852–853. doi: 10.1038/d41586-022-01702-2. [DOI] [PubMed] [Google Scholar]
- 2.Hope A.A., Evering T.H. Postacute sequelae of severe acute respiratory syndrome Coronavirus 2 infection. Infect Dis Clin North Am. 2022;36(2):379–395. doi: 10.1016/j.idc.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips S., Williams M.A. Confronting our next national health disaster — long-haul covid. N Engl J Med. 2021;385(7):577–579. doi: 10.1056/NEJMp2109285. [DOI] [PubMed] [Google Scholar]
- 4.Levine R.L. Addressing the long-term effects of COVID-19. JAMA. Aug 3 2022 doi: 10.1001/jama.2022.14089. [DOI] [PubMed] [Google Scholar]
- 5.Peluso M.J., Deeks S.G. Early clues regarding the pathogenesis of long-COVID. Trends Immunol. 2022;43(4):268–270. doi: 10.1016/j.it.2022.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigal L.H. What is causing the 'long-hauler' phenomenon after COVID-19? Cleve Clin J Med. May 3 2021;88(5):273–278. doi: 10.3949/ccjm.88a.21009. [DOI] [PubMed] [Google Scholar]
- 7.Choutka J., Jansari V., Hornig M., Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. May 2022;28(5):911–923. doi: 10.1038/s41591-022-01810-6. [DOI] [PubMed] [Google Scholar]
- 8.Komaroff A.L., Lipkin W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. Sep 2021;27(9):895–906. doi: 10.1016/j.molmed.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler C.X., Wyller V.B.B., Moss-Morris R., et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect Dis. Oct 2021;8(10):ofab440. doi: 10.1093/ofid/ofab440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman L., Bested A.C., Bonilla H.F., et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96(11):2861–2878. doi: 10.1016/j.mayocp.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Komaroff A.L., Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front Med. 2020;7 doi: 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman M. Chronic fatigue syndrome and long covid: moving beyond the controversy. BMJ. Jun 23 2021;373:n1559. doi: 10.1136/bmj.n1559. [DOI] [PubMed] [Google Scholar]
- 13.Jiang D.H., Roy D.J., Gu B.J., Hassett L.C., McCoy R.G. Postacute sequelae of severe acute respiratory syndrome Coronavirus 2 infection: a state-of-the-art review. JACC Basic Transl Sci. Sep-Oct 2021;6(9):796–811. doi: 10.1016/j.jacbts.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi N., Liu K., Kawakami D., et al. Post-intensive care syndrome and its new challenges in Coronavirus disease 2019 (COVID-19) pandemic: a review of recent advances and perspectives. J Clin Med. Aug 28 2021;10(17) doi: 10.3390/jcm10173870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. Apr 2022;22(4):e102–e107. doi: 10.1016/s1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballering A.V., van Zon S.K.R., olde Hartman T.C., Rosmalen J.G.M. Persistence of somatic symptoms after COVID-19 in The Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L., Wu Y., Xiong H., Mei B., You T. Persistence of symptoms after discharge of patients hospitalized due to COVID-19. Front Med. 2021;8 doi: 10.3389/fmed.2021.761314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson E.J., Williams D.M., Walker A.J., et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. Jun 28 2022;13(1):3528. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Townsend L., Dowds J., O'Brien K., et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. Jun 2021;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend L., Dyer A.H., Jones K., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azzolini E., Levi R., Sarti R., et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. Jul 1 2022 doi: 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zisis S.N., Durieux J.C., Mouchati C., Perez J.A., McComsey G.A. The protective effect of Coronavirus disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: a multicenter study from a large national health research network. Open Forum Infect Dis. 2022;9(7):ofac228. doi: 10.1093/ofid/ofac228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. May 25 2022 doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonelli M., Pujol J.C., Spector T.D., Ourselin S., Steves C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–2264. doi: 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subbaraman N. US health agency will invest $1 billion to investigate ‘long COVID’. Nature. 2021;591(7850):356. doi: 10.1038/d41586-021-00586-y. [DOI] [PubMed] [Google Scholar]
- 28.Sapkota H.R., Nune A. Long COVID from rheumatology perspective - a narrative review. Clin Rheumatol. 2022;41(2):337–348. doi: 10.1007/s10067-021-06001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Iorio M., Cook C.E., Vanni K.M.M., et al. DMARD disruption, rheumatic disease flare, and prolonged COVID-19 symptom duration after acute COVID-19 among patients with rheumatic disease: a prospective study. Semin Arthritis Rheum. Aug 2022;55 doi: 10.1016/j.semarthrit.2022.152025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nature Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. Apr 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Res Square. Mar 1 2021 doi: 10.21203/rs.3.rs-266574/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butowt R., Bilińska K., von Bartheld C. Why does the omicron variant largely spare olfactory function? Implications for the pathogenesis of anosmia in COVID-19. J Infect Dis. Apr 25 2022 doi: 10.1093/infdis/jiac113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yelin D., Margalit I., Nehme M., et al. Patterns of long COVID symptoms: a multi-center cross sectional study. J Clin Med. 2022;(4):11. doi: 10.3390/jcm11040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies K., Dures E., Ng W.F. Fatigue in inflammatory rheumatic diseases: current knowledge and areas for future research. Nat Rev Rheumatol. 2021;17(11):651–664. doi: 10.1038/s41584-021-00692-1. [DOI] [PubMed] [Google Scholar]
- 37.Katz P. Fatigue in rheumatoid arthritis. Curr Rheumatol Rep. 2017;19(5):25. doi: 10.1007/s11926-017-0649-5. [DOI] [PubMed] [Google Scholar]
- 38.Twomey R., DeMars J., Franklin K., Culos-Reed S.N., Weatherald J., Wrightson J.G. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther. Apr 1 2022;(4):102. doi: 10.1093/ptj/pzac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong T.L., Weitzer D.J. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-A systemic review and comparison of clinical presentation and symptomatology. Medicina. Apr 26 2021;57(5) doi: 10.3390/medicina57050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabacof L., Tosto-Mancuso J., Wood J., et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am J Phys Med Rehabil. 1 2022;101(1):48–52. doi: 10.1097/phm.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frontera J.A., Yang D., Lewis A., et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. Jul 15 2021;426 doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frontera J.A., Simon N.M. Bridging knowledge gaps in the diagnosis and management of neuropsychiatric sequelae of COVID-19. JAMA Psychiatr. Jun 29 2022 doi: 10.1001/jamapsychiatry.2022.1616. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S., Shibata K., Hellyer P.J., et al. Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun. 2022;4(1):fcab295. doi: 10.1093/braincomms/fcab295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnan K., Miller A.K., Reiter K., Bonner-Jackson A. Neurocognitive profiles in patients with persisting cognitive symptoms associated with COVID-19. Arch Clin Neuropsychol. May 16 2022;37(4):729–737. doi: 10.1093/arclin/acac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neculicioiu V.S., Colosi I.A., Costache C., Sevastre-Berghian A., Clichici S. Time to sleep?-A review of the impact of the COVID-19 pandemic on sleep and mental health. Int J Environ Res Public Health. Mar 16 2022;19(6) doi: 10.3390/ijerph19063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahmoudi H., Saffari M., Movahedi M., et al. A mediating role for mental health in associations between COVID-19-related self-stigma, PTSD, quality of life, and insomnia among patients recovered from COVID-19. Brain Behav. May 2021;11(5) doi: 10.1002/brb3.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoja O., Silva Passadouro B., Mulvey M., et al. Clinical characteristics and mechanisms of musculoskeletal pain in long COVID. J Pain Res. 2022;15:1729–1748. doi: 10.2147/jpr.S365026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clauw D.J., Häuser W., Cohen S.P., Fitzcharles M.A. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. Aug 2020;161(8):1694–1697. doi: 10.1097/j.pain.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. Mar 24 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goudman L., De Smedt A., Noppen M., Moens M. Is central sensitisation the missing link of persisting symptoms after COVID-19 infection? J Clin Med. 2021;28(23):10. doi: 10.3390/jcm10235594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ursini F., Ciaffi J., Mancarella L., et al. Fibromyalgia: a new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open. Aug 2021;7(3) doi: 10.1136/rmdopen-2021-001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazaridou A., Paschali M., Vilsmark E.S., Wilkins T., Napadow V., Edwards R. The impact of COVID-19 pandemic on mental and physical wellbeing in women with fibromyalgia: a longitudinal mixed-methods study. BMC Women Health. Jun 30 2022;22(1):267. doi: 10.1186/s12905-022-01840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivera J., Rodríguez T., Pallarés M., et al. Prevalence of post-COVID-19 in patients with fibromyalgia: a comparative study with other inflammatory and autoimmune rheumatic diseases. BMC Muscoskel Disord. May 19 2022;23(1):471. doi: 10.1186/s12891-022-05436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Healey Q., Sheikh A., Daines L., Vasileiou E. Symptoms and signs of long COVID: a rapid review and meta-analysis. J Glob Health. May 21 2022;12 doi: 10.7189/jogh.12.05014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chippa V., Aleem A., Anjum F. StatPearls Publishing LLC.; 2022. Post Acute Coronavirus (COVID-19) Syndrome. StatPearls. StatPearls Publishing Copyright © 2022. [PubMed] [Google Scholar]
- 56.Ormiston C.K., Świątkiewicz I., Taub P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Heart Rhythm. Jul 16 2022 doi: 10.1016/j.hrthm.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monaghan A., Jennings G., Xue F., Byrne L., Duggan E., Romero-Ortuno R. Orthostatic intolerance in adults reporting long COVID symptoms was not associated with postural orthostatic tachycardia syndrome. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.833650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novak P., Mukerji S.S., Alabsi H.S., et al. Multisystem involvement in post-acute sequelae of Coronavirus disease 19. Ann Neurol. 2022;91(3):367–379. doi: 10.1002/ana.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamal S.M., Landers D.B., Hollenberg S.M., et al. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. J Am Coll Cardiol. Jun 14 2022;79(23):2325–2330. doi: 10.1016/j.jacc.2022.03.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science (New York, NY) 11 2022;375(6585):1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 61.Peluso M.J., Lu S., Tang A.F., et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome Coronavirus 2 infection. J Infect Dis. Dec 1 2021;224(11):1839–1848. doi: 10.1093/infdis/jiab490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phetsouphanh C., Darley D.R., Wilson D.B., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 63.Knight J.S., Caricchio R., Casanova J.L., et al. The intersection of COVID-19 and autoimmunity. J Clin Invest. Dec 15 2021;(24):131. doi: 10.1172/jci154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peluso M.J., Deveau T.-M., Munter S.E., et al. Impact of pre-existing chronic viral infection and reactivation on the development of long COVID. medRxiv. 2022;2022 doi: 10.1101/2022.06.21.22276660. 06.21.22276660. [DOI] [Google Scholar]
- 65.Kao J., Frankland P.W. COVID fog demystified. Cell. Jul 7 2022;185(14):2391–2393. doi: 10.1016/j.cell.2022.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peluso M.J., Deeks S.G., Mustapic M., et al. SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann Neurol. 2022;91(6):772–781. doi: 10.1002/ana.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colombo J., Weintraub M.I., Munoz R., et al. Long COVID and the autonomic nervous system: the journey from dysautonomia to therapeutic neuro-modulation through the retrospective analysis of 152 patients. NeuroSci. 2022;3(2):300–310. [Google Scholar]
- 68.Bisaccia G., Ricci F., Recce V., et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;15(11):8. doi: 10.3390/jcdd8110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Margalit I., Yelin D., Sagi M., et al. Risk factors and multidimensional assessment of long COVID fatigue: a nested case-control study. Clin Infect Dis. Apr 11 2022 doi: 10.1093/cid/ciac283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernández-de-Las-Peñas C., Martín-Guerrero J.D., Cancela-Cilleruelo I., Moro-López-Menchero P., Rodríguez-Jiménez J., Pellicer-Valero O.J. Trajectory curves of post-COVID anxiety/depressive symptoms and sleep quality in previously hospitalized COVID-19 survivors: the LONG-COVID-EXP-CM multicenter study. Psychol Med. 2022;10:1–2. doi: 10.1017/s003329172200006x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Graham E.L., Koralnik I.J., Liotta E.M. Therapeutic approaches to the neurologic manifestations of COVID-19. Neurotherapeutics. Jul 21 2022:1–32. doi: 10.1007/s13311-022-01267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graham E.L., Clark J.R., Orban Z.S., et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. May 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sneller M.C., Liang C.J., Marques A.R., et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med. 2022;175(7):969–979. doi: 10.7326/m21-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramos-Casals M., Brito-Zerón P., Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. 2021;17(6):315–332. doi: 10.1038/s41584-021-00608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.CDC https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-index.html
- 76.Robbins R., Helmer D., Monahan P., et al. Management of chronic multisymptom illness: synopsis of the 2021 US department of Veterans affairs and US department of defense clinical practice guideline. Mayo Clin Proc. May 2022;97(5):991–1002. doi: 10.1016/j.mayocp.2022.01.031. [DOI] [PubMed] [Google Scholar]
- 77.Vehar S., Boushra M., Ntiamoah P., Biehl M. Post-acute sequelae of SARS-CoV-2 infection: caring for the ‘long-haulers’. Cleve Clin J Med. May 3 2021;88(5):267–272. doi: 10.3949/ccjm.88a.21010. [DOI] [PubMed] [Google Scholar]
- 78.Herrera J.E., Niehaus W.N., Whiteson J., et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients. PMR. 2021;13(9):1027–1043. doi: 10.1002/pmrj.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jason L.A., Islam M., Conroy K., et al. COVID-19 symptoms over time: comparing long-haulers to ME/CFS. Fatigue. 2021;9(2):59–68. doi: 10.1080/21641846.2021.1922140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peluso M.J., Kelly J.D., Lu S., et al. Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of SARS-CoV-2 infection: cohort description and approaches for measurement. Open Forum Infect Dis. 2022;9(2):ofab640. doi: 10.1093/ofid/ofab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calabrese L., Colloca L. Long COVID-19 and the role of the patient-clinician interaction in symptom management. J Patient Exp. 2022;9 doi: 10.1177/23743735221077514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zilberman-Itskovich S., Catalogna M., Sasson E., et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci Rep. Jul 12 2022;12(1) doi: 10.1038/s41598-022-15565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ledford H. Long-COVID treatments: why the world is still waiting. Nature. Aug 2022;608(7922):258–260. doi: 10.1038/d41586-022-02140-w. [DOI] [PubMed] [Google Scholar]
- 84.Peluso M.J., Williams M.C., Campbell D.M., et al. SARS-CoV-2 booster vaccination for participants in “HIV cure”-related clinical trials. J Acquir Immune Defic Syndr. 1 2022;89(3):e30. doi: 10.1097/qai.0000000000002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su Y., Yuan D., Chen D.G., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895. doi: 10.1016/j.cell.2022.01.014. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peluso M.J., Anglin K., Durstenfeld M.S., et al. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun. 2022;7(1):95–103. doi: 10.20411/pai.v7i1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaylis N.B., Ritter A., Kelly S.A., et al. Reduced cell surface levels of C-C chemokine receptor 5 and immunosuppression in long Coronavirus disease 2019 syndrome. Clin Infect Dis. Apr 22 2022 doi: 10.1093/cid/ciac226. [DOI] [PMC free article] [PubMed] [Google Scholar]