Abstract
Psychedelics are 5-HT2A receptor agonists that can lead to profound changes in perception, cognition, and mood. In this review, we focus on the basic neurobiology underlying the action of psychedelic drugs. We first discuss chemistry, highlighting the diversity of psychoactive molecules and principles that govern their potency and pharmacokinetics. We describe the roles of serotonin receptors and their downstream molecular signaling pathways, emphasizing key elements for drug discovery. We consider the impact of psychedelics on neuronal spiking dynamics in several cortical and subcortical regions, along with transcriptional changes and sustained effects on structural plasticity. Finally, we summarize neuroimaging results that pinpoint effects on association cortices and thalamocortical functional connectivity, which inform current theories of psychedelic action. By synthesizing knowledge across the chemical, molecular, neuronal, and network levels, we hope to provide an integrative perspective on the neural mechanisms responsible for the acute and enduring effects of psychedelics on behavior.
Editor summary:
Psychedelics are serotonergic drugs that have therapeutic potential. This Review article provides an integrative perspective on the basic neurobiology underlying the actions of psychedelics and highlights open questions in the field.
Introduction
Psychedelics have captured the imagination of neuroscientists since the early 20th century1, as they are molecules that can profoundly bend sensory processing, alter cognition, and produce intense subjective experiences. Psychedelic drugs’ unique abilities to modulate perceptual states provide powerful tools for probing the human mind. Psychedelics are also molecules that afford potential benefits to patients diagnosed with a wide range of neuropsychiatric disorders, including depression, anxiety, and substance use disorders2–4. Unlike current treatment options, only one or a few sessions of psychedelic-assisted psychotherapy has been reported to yield durable reductions of symptoms in Phase II clinical trials. For these reasons, psychedelics hold promise to transform neuroscience and psychiatry. Psychedelic research flourished in the 1950s and 1960s when LSD and psilocybin were synthesized for pharmacological and behavioral research and readily available. Controlled substance laws enacted in the 1970s led to a hiatus lasting several decades, but now there is renewed scientific interest in understanding psychedelics and their effects on the brain and body.
This review article is focused on the neuroscience of psychedelics. We will begin with chemistry, move to receptors and molecular signaling, and finish with recent insights into how psychedelics modulate neurons and neural circuits. Because the emphasis is on basic neurobiology, we will only set the stage with brief descriptions of the behavioral effects and clinical relevance of psychedelics (Text Box 1) and preclinical assays for evaluating psychedelics in animal models (Text Box 2), which have been covered by other excellent reviews5–8. Our aim is to connect multiple levels of investigation to provide both an integrative and in-depth perspective on this topic. We will address questions such as how knowledge at the chemical and molecular levels may accelerate psychedelic-related drug discovery, and whether neuronal and network mechanisms can lead to unified theories explaining psychedelic action. We will complement discussion of recent results with historical findings that are often ignored in the current explosion of research activity. The goal of this review is to synthesize the field’s current knowledge and to highlight open questions that could spark further investigations into the neural basis of psychedelic action.
Text Box 1: Brief overview of behavioral effects and therapeutic potentials.
The term ‘Psychedelic’, from the Greek for mind-manifesting, was coined in 1956 by Humphrey Osmond, who chose the term because “it is clear, euphonious, and uncontaminated by other associations”146. Acutely, psychedelic drugs generate perceptual distortions, psychological experiences, and labile moods147, 148. The effects are often accompanied by imaginary percepts akin to hallucinations; hence ‘hallucinogens’ is another term used in the scientific literature to refer to molecules that include psychedelics. Some psychedelic users experience a reduced sense of self-referential awareness, a subjective feeling termed ‘ego dissolution’. In 2006, an influential study by Roland Griffiths and colleagues reported that psilocybin can evoke mystical-type experiences that impart personal meaning and spiritual significance149. The peak intensity of such an experience is associated with a level of 10 – 20 μg/L of psilocin in plasma, which corresponds to ~60% of 5-HT2A receptor occupancy in the neocortex of humans32. There are noted variations in how individuals may respond to the same dose of a psychedelic. Set and setting, which refers to a person’s internal state and external environment, may impact the psychedelic-induced subjective experience. Intriguingly, the intensity of mystical-type experience has been reported to correlate with therapeutic efficacy150.
It was recognized early that psychedelics may have therapeutic potential for treating mental illnesses. There is a rich history of experimentation with compounds like lysergic acid diethylamide (LSD) for alcoholism and psychiatric distress151, although these early studies lacked the rigor of current clinical trial designs. Recent trials have focused on psilocybin-assisted psychotherapy, starting with a few pilot studies demonstrating improvements in depression and anxiety in terminal cancer patients150, 152, 153. Subsequently, randomized Phase II trials demonstrated a reduction of symptoms following psilocybin-assisted psychotherapy for major depressive disorder and treatment-resistant depression2, 3. Results from these trials are notable for the relatively large effect sizes and enduring benefits lasting up to several weeks or months, although these remain to be confirmed in multi-site, large-scale clinical trials. Psilocybin and other psychedelics have also shown value for overcoming substance-use disorders4. In parallel, although ketamine and MDMA are not considered to be psychedelic, their progressions through clinical trials — ketamine for depression154 and MDMA for post-traumatic stress disorder155 — are part of a paradigm shift in psychiatry to leverage substances with acute psychoactive effects to induce long-term benefits for psychiatric patients.
Text Box 2: Behavioral assays for evaluating psychedelics in animal models.
Subjective experience in humans is assessed through self-reports and questionnaires, which cannot be applied to animals. In preclinical research, the main assays for evaluating psychedelics are drug discrimination and head-twitch response5, 8. For drug discrimination, animals are trained for many weeks to distinguish between a psychoactive substance (e.g., LSD) and vehicle by indicating their response via lever presses. On test day, the experimenter can determine the extent to which a test drug can substitute for the reference psychedelic. For head-twitch response, several species, including mice, rats, and rabbits, exhibit high-frequency, side-to-side head movements following the administration of a psychedelic drug. These assays have predictive validity, because potencies in drug discrimination in rats33 and head-twitch response in mice34 correlate exceptionally well with hallucinogenic potencies in humans across a panel of several dozen distinct molecules. A difference between the assays is that the head-twitch response is an innate behavior, whereas drug discrimination is learned and requires animal training. Moreover, drug discrimination allows for a finer classification of molecules because a drug may act on multiple receptors that collectively contribute to the interoceptive stimulus. A partial substitution in the drug discrimination is possible and would indicate that the test and reference compounds share some but not all features. However, it has been noted that these assays have certain pitfalls156, 157, and ultimately it is important to recognize that these behavioral readouts in animal models are surrogate measures that cannot capture the full spectrum and nuances of the human experience.
Chemistry of psychedelics
All classical psychedelics are derived from a primary pharmacophore consisting of an aromatic group separated from a basic amine by a two-carbon linker (Figure 1a). When protonated, the basic nitrogen engages a key aspartate residue (D1553.32) in the binding pocket of the 5-HT2A receptor, while the aromatic group makes important hydrophobic contacts with other residues in the protein9, 10. A two-carbon linker length appears to provide optimal spacing between these functional groups for activation of 5-HT2A receptors. Shortening the linker length by one carbon converts the 5-HT2A receptor agonist N,N-dimethyltryptamine (DMT) into the 5-HT2A receptor antagonist gramine11, 12, while increasing the distance between the aromatic group and basic amine can reduce affinity13. The specific identity of the aromatic group in the primary pharmacophore divides psychedelics into two broad structural families. Tryptamines possess a C3-substituted indole, while phenethylamines are characterized by a phenyl group. Ergolines, often considered as a distinct group, can be viewed chemically as a specialized case of tryptamines because the N,N-dimethyltryptamine pharmacophore is embedded within the ergoline framework.
Figure 1. Chemical phylogeny of psychedelics.
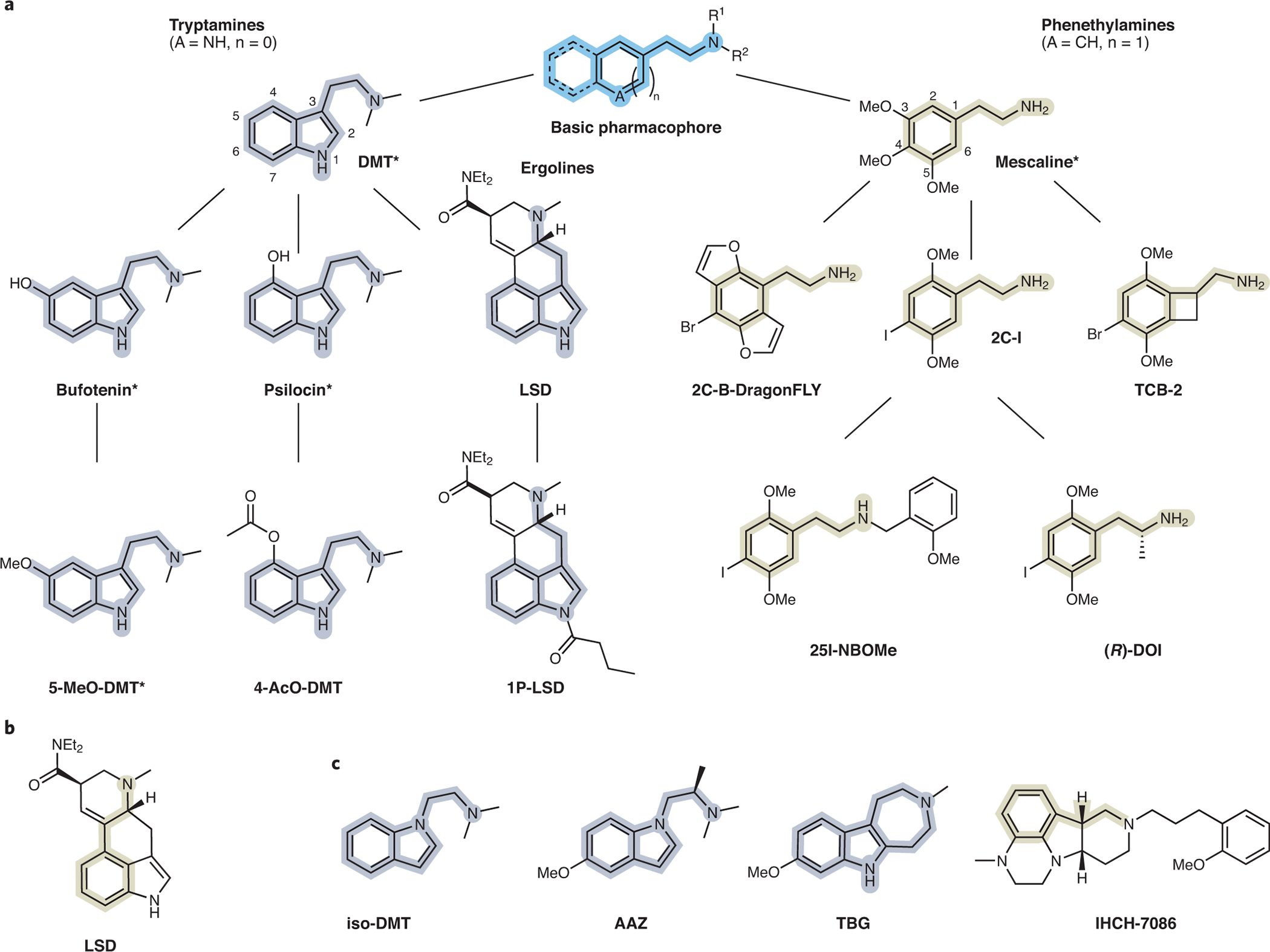
(a) The basic psychedelic pharmacophore is highlighted in blue. Tryptamine and phenethylamine pharmacophores are highlighted in gray and yellow, respectively. Ergolines (LSD, 1P-LSD) can be viewed chemically as a specialized case of tryptamines. Branches indicate structurally related compounds. Natural products are indicated with asterisks. (b) LSD has the phenethylamine substructure (yellow) embedded and can thus contains the key elements of both psychedelic structural families. (c) Structures of non-hallucinogenic psychedelic analogs with therapeutic potential, which may contain the tryptamine-like (gray) or phenethylamine-like (yellow) pharmacophore.
Comparing the conformationally flexible substituted tryptamines, like psilocin and bufotenin, and rigidified ergolines, like LSD, shows that rigidification of a primary pharmacophore can often increase affinity/potency (i.e., the amount of drug necessary to bind to or activate a receptor) by easing entropic penalties to binding, and this may be one of the reasons that LSD is a particularly potent psychedelic with respect to its ability to activate the 5-HT2A receptor and elicit associated behavioral responses. Potencies of the more flexible tryptamines are highly dependent on secondary contacts in the binding pocket, and thus, tryptamine substitution can have a dramatic impact on potency. Typically, 4- and 5-substituted tryptamines are significantly more potent than their 6- and 7-substituted congeners14. Like tryptamines, the potencies of phenethylamines can be modulated by both substitution and scaffold rigidification. The 2,4,5-trisubstitution pattern appears to be more favorable than the 3,4,5-trisubstitution pattern as compounds like 2C-I and 2C-B are significantly more potent than mescaline13. Potency can be further improved by conformationally restricting the ethylamine group or the methoxy substituents as in the cases of R-TCB-215 and 2C-B-dragonFLY16, respectively. In addition to rigidification strategies involving the aromatic ring, the potency of phenethylamines can be improved by structural modifications that promote additional secondary interactions with the 5-HT2A receptor or prevent metabolism. In the case of N-benzylated compounds like 25I-NBOMe, potency is likely enhanced because the benzyl group appended to the basic nitrogen can engage a deep secondary binding pocket in the 5-HT2A receptor9. In the case of R-DOI, simple addition of a methyl group alpha to the basic amine yields amphetamine-like structures that are more resistant to oxidative deamination by monoamine oxidase (MAO)17. Similar effects have been observed for α-methyltryptamines18. The stereochemistry of the α-methyl group is important, with the R- and S-enantiomers of α-methylphenethylamines (i.e., amphetamines) and α-methyltryptamines being the more potent optical isomers, respectively18, 19. While LSD is classified here chemically as a tryptamine derivative, it is interesting to note that the R-amphetamine substructure is embedded within the ergoline scaffold, making LSD a hybrid structure that contains the key elements of both psychedelic structural families (Figure 1b).
A hallmark of psychedelics is their pharmacokinetic properties and high brain penetrance. Many psychedelics adhere to the ‘rule of three’ (i.e., molecular weight is <300 Da, ClogP is ≤3, number of H-bond acceptors is ≤3, and number of H-bond donors is ≤3)20 and exhibit excellent central nervous system multiparameter optimization scores21, as they are small, relatively hydrophobic, and possess few hydrogen bond donors and acceptors. These physical properties enable psychedelics to cross the blood–brain barrier easily and rapidly, leading to high brain to plasma ratios22. Though tryptamine psychedelics bear substantial structural similarities to serotonin and have high affinity for many of the same receptors, the pharmacological properties of serotonin differ substantially from those of psychedelics. While some of these differences can be attributed to how serotonin engages various residues in the binding pocket of the 5-HT2A receptor23, another major factor is pharmacokinetics. Serotonin is a very polar molecule that cannot easily cross nonpolar membranes. In fact, the majority of serotonin in the body is produced in the gut, and its high polarity ensures that peripherally produced serotonin cannot readily access the brain24. In contrast, methylation of serotonin produces bufotenin and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) — compounds that are substantially more hydrophobic, and thus, capable of crossing the blood–brain barrier22. It is interesting to note that psychedelics with greater lipophilicity and/or pKa’s closer to physiological pH tend to be more potent25. The pKa of mescaline is 9.56, while the pKa of LSD is 7.826. Thus, compared to LSD, a substantially lower proportion of mescaline molecules exist in the deprotonated state that is capable of passively diffusing across the nonpolar blood–brain barrier. This property, in addition to mescaline’s very weak partial agonism of 5-HT2A receptors, contribute to its low potency in vivo. The importance of pharmacokinetics in the actions of psychedelics is perhaps best illustrated by comparing psilocin and bufotenin. These compounds are constitutional isomers, and differ only in the position of their phenolic hydroxyl groups (4- and 5-positions for psilocin and bufotenin, respectively). While these compounds exhibit comparable 5-HT2A receptor potencies and efficacies in vitro23, their in vivo effects are drastically different. In contrast to bufotenin, psilocin is orally bioavailable and readily crosses the blood–brain barrier because it can form an intramolecular hydrogen bond that improves lipophilicity by lowering the pKa of its amino group27.
Several psychedelics are natural products produced by plants and fungi, but others are non-natural structures conceived by humans (Figure 1a). While some psychedelics can be obtained directly from natural sources, most are produced via de novo chemical synthesis from simple starting materials. Though LSD is a non-natural compound, it is derived from the natural product lysergic acid through semi-synthesis. Recent advances in synthetic biology have enabled the reconstitution of biosynthetic pathways in organisms such as yeast and E. coli, and these techniques have the potential to enable large-scale production of several psychedelics and psychedelic precursors such as psilocybin and lysergic acid28, 29.
Receptors and molecular signaling
In humans, pre-treatment with the 5-HT2 receptor antagonist ketanserin diminishes, in a dose-dependent manner, the ability of psilocybin30 and LSD31 to alter subjective experience. The occupancy of 5-HT2A receptors in the brain relates closely to the intensity of the psychedelic effect32. These human data are corroborated by animal studies, which showed that human hallucinogenic potencies and potencies in rodent drug discrimination assays scale with 5-HT2A binding affinity33. Moreover, a strong correlation has been observed between human hallucinogenic potencies and potencies in the mouse head-twitch response34, which is abolished in 5-HT2A receptor knockout mice35, 36. Together, the evidence is overwhelming that the 5-HT2A receptor is crucial for the psychedelic effect. However, there are species differences that can affect the properties of 5-HT2A receptors. For example, primate and pig 5-HT2A receptors possess a serine at residue 242 in the binding pocket, whereas the rat and mouse receptors have an alanine at this position. Mutagenesis studies have demonstrated that a S242A mutation can drastically increase the dissociation rate of LSD9.
Notwithstanding the importance of 5-HT2A receptors, psychedelics have a complex pharmacology with actions on many other biogenic amine G protein-coupled receptors8, 37. LSD, for instance, is a high-affinity agonist for most of the 14 distinct human 5-HT receptors and has potent agonist activity at D1, D2, D3 and D4-dopamine and α1 and α2-adrenergic receptors37. In rodents, the actions of LSD at D2 receptors have been postulated to mediate the relatively prolonged drug effects38, while its actions at D4 and 5-HT5A receptors may underlie select behavioral actions39, 40. Likewise, psilocin is a potent agonist at many 5-HT receptors41 and actions at the 5-HT1A receptor have been observed in humans42. It is currently unknown which of these receptors mediate the potential therapeutic actions of psychedelics although recent studies in rodents and humans suggest 5-HT2A receptors may not be the sole determinants42–44. Many psychedelic drugs — including LSD and psilocin — also have high affinities for 5-HT2B and 5-HT2C receptors9, 45–47. The actions at 5-HT2B receptors are especially problematic as it is now well established that 5-HT2B agonists, when chronically administered, cause potentially life-threatening cardiac valvulopathy8, 48. Although valvulopathy has not yet been sufficiently evaluated, there have been reports among individuals who have used MDMA chronically49 due to 5-HT2B receptor activation50.
Downstream of 5-HT2A receptors, 5-HT2A agonists in general51, 52 and psychedelics53, 54 in particular activate Gq-like G proteins to enhance the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) (Figure 2a). This leads to the mobilization of intracellular calcium by released inositol trisphosphate (IP3) and the activation of protein kinase C via diacylglycerol (DAG)51, 52. Other signaling pathways at 5-HT2A receptors include activation of arrestin translocation9, 46, 47, 55 and arachidonic acid release56. Actions at both Gq- and arrestin-signaling pathways have been implicated in the behavioral effects of various psychedelics, although in vivo evidence is sparse57–59. Some investigators have noted activity of mouse 5-HT2A receptors at an apparent Gi-mediated response for regulating arachidonic acid release60.
Figure 2. The 5-HT2A receptors and molecular signaling pathways.
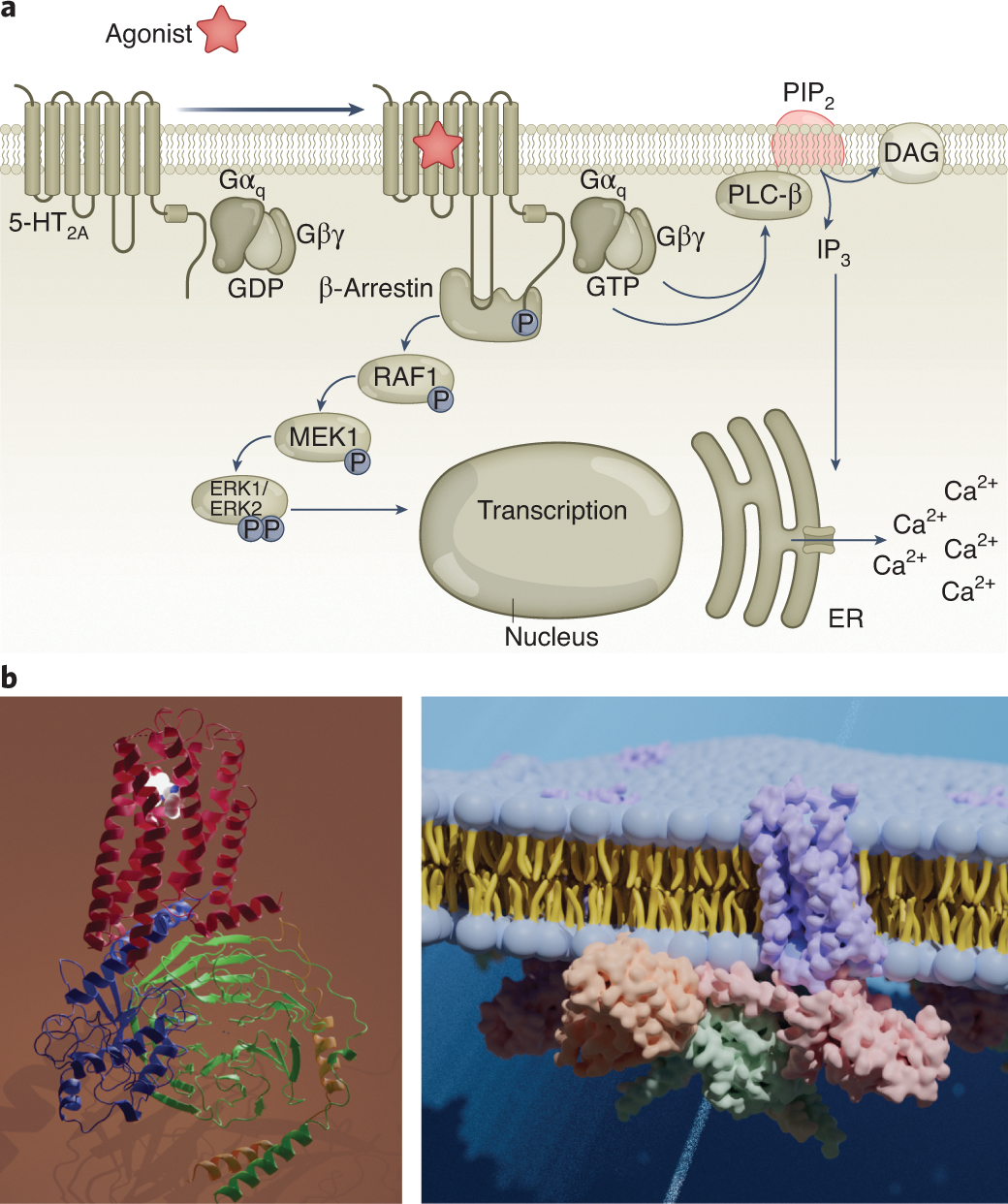
(a) Intracellular signal transduction pathways. Downstream of the 5-HT2A receptor, activation of heterotrimeric G proteins and subsequent intracellular signaling (Ca2+ release and diacylglycerol (DAG) production) synergistically activate additional downstream effects, which ultimately lead to altered neuronal firing. (b) left: Structure of the 5-HT2A receptor; right: A model of the 5-HT2A receptor signaling complex in the membrane.
Although the molecular details responsible for psychedelic drug actions at 5-HT2A receptors have been elusive for many decades, recent breakthrough studies via x-ray diffraction and cryo-electron microscopy have provided crystal structures of psychedelic-bound serotonin receptors (Figure 2b, c). The first study by Wacker and colleagues47 of LSD complexed with the 5-HT2B receptor was largely validated by a second report9 demonstrating that LSD interacts with 5-HT2A receptors via a variety of hydrophobic, ionic and other factors9. The results were further supported by additional structural biology results, including an inverse-agonist stabilized crystal structure of a complex of 5-HT2A receptors with the psychedelic drug 25-CN-NBOH along with the active nucleotide-free Gq hetereotrimer9. The availability of these relatively high-resolution structures of 5-HT2A and other relevant receptors61 promises to produce novel insights into the molecular details of psychedelic drug action.
Integrating chemical and molecular mechanisms to discover new compounds
Molecular advances have led to a surge of medicinal chemistry efforts, which seek to use computational modeling and structure-activity relationships to engineer psychedelic-based therapeutics. The structure of psilocybin often serves as a starting point for these efforts, and though psilocybin is one of the most commonly studied psychedelics in clinical trials, it is not responsible for producing hallucinogenic effects. In fact, psilocybin is rapidly dephosphorylated in vivo and serves as a prodrug for psilocin62. Compared to psilocin, psilocybin exhibits higher chemical stability in the solid state and has a longer shelf life; although not published, this fact is well known among researchers in the field. While clandestine labs have attempted to use prodrugs of psychedelics, such as 4-AcO-DMT and 1P-LSD41, 63 (Figure 1a), to circumvent controlled substance laws, pharmaceutical companies have become interested in psychedelic prodrugs as a means to improve oral bioavailability or modify pharmacokinetic properties. The duration of a psychedelic-induced subjective experience could be a key parameter in the optimization of psychedelic-assisted psychotherapy. Psychedelics producing hallucinogenic effects of shorter durations are likely to be more cost-effective and more scalable to implement at a clinic given that contact time with medical professionals can be minimized64. However, this idea depends crucially on the assumption that the duration of the acute psychedelic effect may be manipulated without affecting efficacy, which has not yet been convincingly demonstrated in humans.
Parallel efforts to improve the scalability of psychedelic-like therapeutics have focused on engineering compounds that lack hallucinogenic/perceptual effects but maintain sustained therapeutic efficacy after a single dose64. Presumably, these compounds could be administered at home, obviating the need for costly in-clinic supervision as is currently required for psychedelics and other intoxicating compounds such as ketamine. Initial work in this area has focused on developing non-hallucinogenic entities (also referred to as non-hallucinogenic psychoplastogens65), such as isoDMT66, TBG67, and AAZ12 by slightly modifying the structures of known hallucinogenic compounds (Figure 1c). On this front, the availability of high-resolution structures of 5-HT2A receptors in complex with psychedelics promises to accelerate the search for novel psychedelic and non-hallucinogenic 5-HT2A agonists. For example, in silico design is beginning to yield potentially non-hallucinogenic 5-HT2A receptor ligands like IHCH-708610 (Figure 1c). The search is boosted by recent ultra-large-scale computational studies of hundreds of millions68 to billions69 of compounds, which have demonstrated the relative ease of discovering new chemotypes for many GPCRs.
What properties are desirable for novel psychedelic-based compounds? Such novel chemical matter may have enhanced selectivity towards 5-HT2A receptors and therefore fewer off-target actions. Selective agonists would also represent useful tools for clarifying the role of 5-HT2A and other receptors in the hallucinatory and therapeutic actions of psychedelics. It should be possible to identify and optimize chemotypes with biased signaling profiles preferring Gq versus arrestin versus other potential signaling pathways to determine which of these pathways are physiologically and therapeutically relevant. Finally, developing psychedelic-like drugs devoid of 5-HT2B agonism is essential for therapeutic interventions which envision chronic dosing. Overall, efforts are aimed at leveraging functional selectivity to maximize efficacy, safety, and tolerability.
Neurons and circuits modulated by psychedelics
Psychedelics modify neural activity dynamics by activating various receptors. Specifically, 5-HT2A receptor agonism increases neuronal excitability through multiple mechanisms including membrane depolarization, diminished afterhyperpolarization, and reduced spike frequency adaptation70. However, some psychedelics also bind to other receptors with opposing functional consequences; for example, tryptamines such as psilocin and 5-MeO-DMT have affinities for 5-HT1A receptors, which act to decrease neuronal excitability70, 71. The relative abundance and subcellular distribution of the receptor subtypes will therefore dictate the overall effects of psychedelics on the electrical activities of a neuron72.
Acute effects
The impact of psychedelics on neurophysiology has been well studied in a few brain regions. In the prefrontal cortex, 5-HT2A receptors are primarily postsynaptic73 and highly enriched in the apical dendrites of deep-layer pyramidal neurons74, 75 (Figure 3a). The localization pattern suggests that psychedelics should increase dendritic excitability and induce excitatory postsynaptic potentials, as has been shown for serotonin76. However, the impact of psychedelics in vivo is likely more complex because cortical microcircuits contain multiple subpopulations of pyramidal neurons and subtypes of GABAergic neurons. These cell types express differential amounts of 5-HT2A and other serotonin receptors72, 77, 78, which are reflected in their heterogeneous responses to serotonin neuromodulation79. In agreement with this, following systemic administration of DOI, frontal cortical neurons in vivo showed varied changes in firing rates across the neuronal population in the rat medial frontal cortex80.
Figure 3. Regional differences in psychedelic action on neurophysiology.
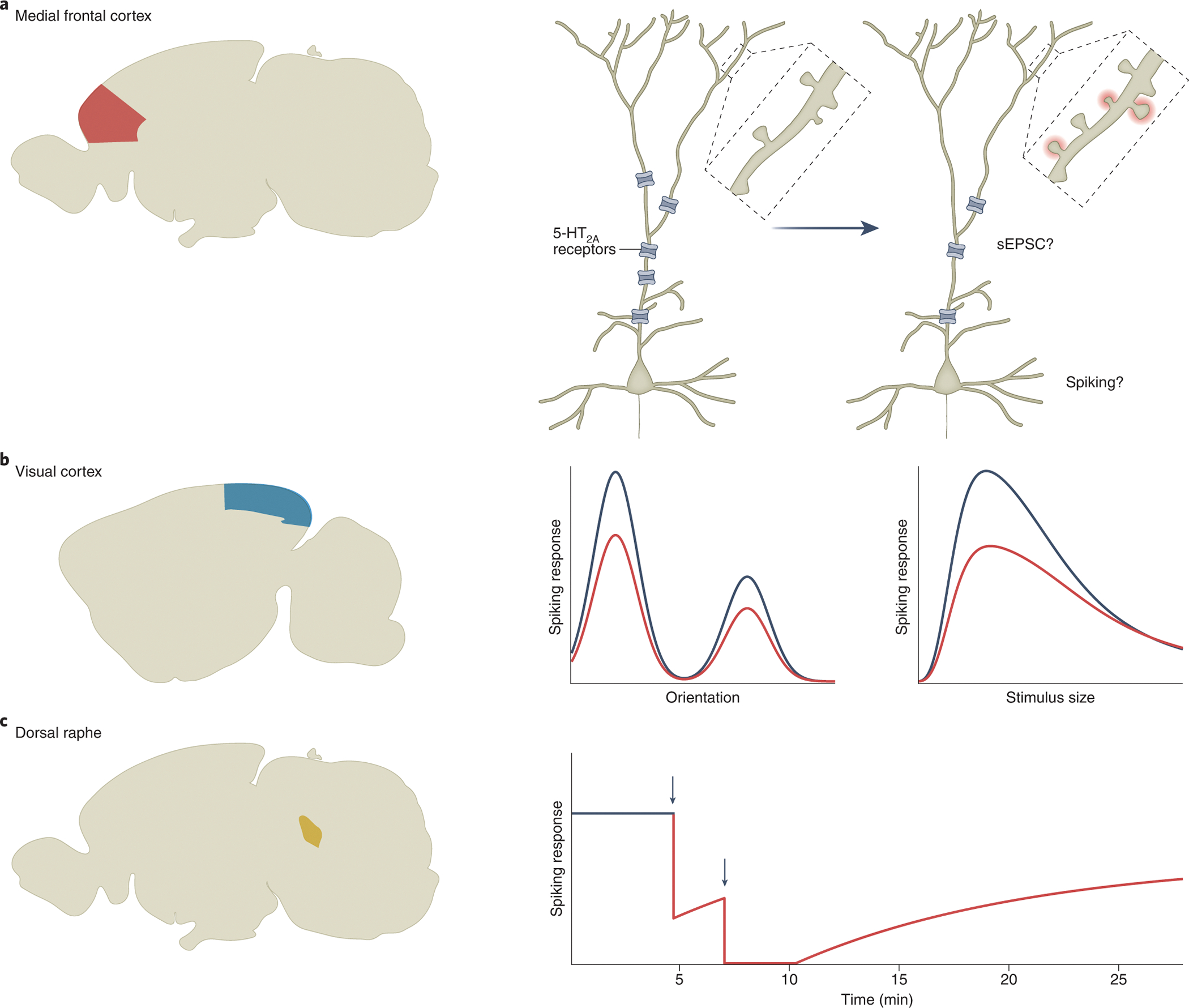
(a) In the medial frontal cortex (red), psychedelics are thought to acutely increase dendritic excitability due to the dendritic localization of 5-HT2A receptors, but the overall effect on postsynaptic currents and spiking activity in vivo remains unclear. On the days following administration, psychedelics cause receptor internalization (resulting in fewer receptors being expressed) and promote the formation of new dendritic spines. (b) In the primary visual cortex (blue), psychedelics reduce visually evoked spiking activity (right panel). Orientation tuning remains intact, but surround suppression is reduced (middle panel). Black, baseline; red, after drug administration (c) In the dorsal raphe (yellow), systemic or local administration of psychedelics causes a cessation of spiking activity. Black, baseline. Red, after drug administration. Black arrows, times of drug infusion. The schematics are based on data from Shao et al.44 for (a), Michaiel et al.83 for (b), and Aghajanian et al.85 for (c).
The visual pathway has been a focus of neurophysiologists who are motivated by the hallucinogenic property of psychedelics. In one of the earliest studies, single-unit activities were recorded from the optic tract, lateral geniculate nucleus, and visual cortex of anesthetized cats81. LSD was found to decrease neuronal firing in lateral geniculate nucleus in response to optic tract stimulation. In visual cortex, although the overall effect is also suppressive, individual neurons’ responses to psychedelics are heterogeneous: some cells increase spiking, whereas others decrease or exhibit no change82. A more recent, systematic investigation corroborates these spiking activity differences and further indicates that feature tuning is intact, but surround suppression is reduced in mice after the administration of DOI83 (Figure 3b), hinting at erroneous processing of contextual information. These results suggest that psychedelics suppress sensory inputs at multiple points along the central visual pathway.
Psychedelics also exert pronounced effects of firing activity in select subcortical nuclei, with the dorsal raphe as a prime example. Dorsal raphe is the largest serotonergic nucleus in the brain. In a series of elegant studies spanning more than 20 years, George Aghajanian and colleagues found that intravenous administration of LSD can lead to a near-complete cessation of firing in dorsal raphe within 1 – 2 minutes, which returns to baseline after 20 – 30 minutes in anesthetized rats84 (Figure 3c). This effect is specific for psychedelics, with comparable effects being evoked by psilocin, DMT, mescaline, and DOM85. By contrast, compounds such as atropine, scopolamine, and phencyclidine do not have appreciable effects on raphe unit firing85. Psychedelic-induced cessation of spiking activity arises from local mechanisms within the dorsal raphe likely through somatodendritic 5-HT1A receptors86. Initially, it was thought that the strong effects on raphe activity may be responsible for the acute behavioral effects of psychedelics. However, subsequent studies in freely moving animals argued against this possibility, because LSD’s effects on raphe firing do not display tolerance87 and are not aligned with behavioral changes88. It remains unclear which brain regions are responsible for the acute subjective effects in humans and which specific neural circuits mediate the head-twitch response in mice. In addition to the prefrontal cortex, visual cortex, and dorsal raphe, psychedelics have been reported to alter synaptic neurotransmission and spiking activities in the hippocampus43, 89, locus coeruleus90, and numerous other cortical and subcortical locations91.
Longer-term effects
The acute effects of psychedelics on molecular signaling and neuronal firing are precursors to long-term modifications in the brain. Indeed, analysis of mRNA transcripts shows that 90 min after a single dose of LSD in rodents, there are several-fold increases in the expression of immediate early genes associated with plasticity such as c-fos, arc, and egr-2 in the neocortex36, 92. Further neural adaptation may rely on the upregulation of neurotrophic factors such as BDNF, which has been reported in some brain regions following the administration of psychedelics93. More comprehensive profiling of the transcriptional impact of psychedelics is underway, adding cell-type and epigenetic information78, 94.
One enduring consequence of psychedelic administration is structural neural plasticity. In primary neuronal cultures, bath application of psychedelics can impact spine size95, increase spine density96, and promote the proliferation of dendrites97. Structural remodeling has likewise been observed in tissues ex vivo94, 97 and in the intact brain in vivo for psilocybin and other psychedelic analogs44, 67, 98. In one recent study, longitudinal two-photon microscopy was used to track dendritic spines in the mouse medial frontal cortex44. The results show that a single dose of psilocybin led to a rapid increase in spine density and size within 24 h (Figure 3a). Strikingly, spine density remained elevated for up to 1 month after the initial administration, which could potentially underlie the long-lasting beneficial effects that follow psilocybin administration. It is worth noting that structural remodeling has also been observed in primary culture99 and in the medial frontal cortex after a single dose of the fast-acting antidepressant ketamine100, potentially via acute actions on dendritic excitability101. However, ketamine is primarily an N-methyl-D-aspartate (NMDA) receptor antagonist. It is still unknown how ketamine and psychedelics, which engage distinct receptors, converge onto seemingly related structural plasticity processes at the neuronal level72, 102. Studies with direct comparisons of multiple compounds99, 103 will be helpful to address this important question.
Networks involved in psychedelic actions
Psychedelic-induced changes in neuronal activity manifest in spontaneous and task-evoked activations of brain regions at the network level. The impact of psychedelics can be observed in the living human brain, using neuroimaging methods such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), functional magnetic resonance imaging (fMRI), electroencephalography (EEG), and magnetoencephalography (MEG). In the first neuroimaging studies of psychedelics, effects of mescaline and psilocybin were assessed using [18F]FDG PET to measure glucose metabolism and SPECT to assess cerebral blood flow104, 105. Across the entire neocortex, psilocybin administration is associated with increased metabolism105, but reduced blood flow106. However, after adjusting for global changes, there is relative hypermetabolism in prefrontal cortical areas and hypometabolism in subcortical and occipital brain regions105. Similar regional differences in cerebral blood flow were confirmed by a subsequent arterial spin labeling study107. Collectively, these results demonstrate differential responses in associative and sensory cortical regions under the influence of psychedelics.
Another approach to assess the effects of psychedelics on neural architecture is to investigate the functional connectivity between or within brain networks, which refers to covarying activities across regions. Unfortunately, it has been challenging to compare results across studies, because there are various measures of functional connectivity, with some of them sensitive to preprocessing methods. Nevertheless, there are a couple of consistent findings. First, psilocybin acutely reduces the activity and functional connectivity within association networks including the default-mode network (DMN)106, 108–110, which consists of medial prefrontal cortex, posterior cingulate cortex and parietal regions and is thought to be activated when people are at rest and focused on internal mental processes. Further analyses using data-driven approaches such as global brain connectivity reveal that, while disintegrating the connectivity in associative brain regions, psilocybin and LSD concurrently induce hyperconnectivity between sensory brain regions108, 111. Second, it has repeatedly been shown that LSD increases thalamo-cortical functional connectivity, in particular between the thalamus and sensory regions as part of the somato-motor network108, 112. These changes are corroborated by effective connectivity, deduced from dynamic causal modeling, which highlights thalamic connectivity increases to the posterior cingulate cortex and decreases to the temporal cortex113. Concurrent neuroimaging and pharmacological blockade confirm that the LSD-induced changes in functional network configuration relies on binding to 5-HT2 receptors108.
An exciting development in neuroscience is the availability of high-resolution atlases of gene expression and receptor binding across the entire brain. For instance, the Allen Institute for Brain Science applied in situ hybridization and single-cell sequencing approaches to map transcript levels of all genes in the brain114. These data have enabled exploratory analyses to correlate gene expression with drug-evoked fMRI signals in humans108, 111, which highlight the importance of 5-HT2A receptors and potentially dopamine and glutamate receptors in shaping region-specific responses to LSD and psilocybin. Another significant advance is a protein density map of serotonin receptors and the serotonin transporter in humans115. The large-scale genomic and proteomic datasets open avenues for incorporating molecular and receptor information into biophysically based computational models116–118, with aims to capture and predict how psychedelics interact within constraints of the neural architecture to modulate activity dynamics.
While detailed network-level measurements of acute psychedelic effects are starting to emerge, longitudinal studies that can relate to enduring behavioral changes remain scarce. Comparisons of before versus after psilocybin administration revealed changes in resting-state network configurations that are detectable for at least 1 week after a single dose exposure119–122. Therefore, there are hints of enduring changes in brain networks, but the prolonged effects and their clinical relevance remain understudied. Part of the challenge is the need to address inter- and intra-individual variations in psychedelic action. So far, it has been shown that an individual’s baseline functional connectivity influences the magnitude of psilocybin-induced changes on network activation111. Additionally, baseline availability of 5-HT2A receptors predicts the intensity and duration of the acute subjective experience123. A better understanding of the time course and heterogeneity of network-level effects will facilitate precision medicine approaches for psychedelic-assisted psychotherapy.
Integrating neuronal and network mechanisms to inform theories of psychedelic action
Building on current knowledge of the neuronal and network mechanisms, different theories have been proposed to explain psychedelic action in the brain. We will highlight the cortico-striato-thalamo-cortical (CSTC) model124, the relaxed beliefs under psychedelics and the anarchic brain (REBUS) model125, the strong prior (SP) model126, and the cortico-claustro-cortical (CCC) model127.
The CSTC model suggests that psychedelics alter information processing in the brain by stimulating 5-HT2A receptors located within cortico-striato-thalamic loops, resulting in a disruption of thalamic gating124. Consequently, an increase in feedforward information may underlie the acute subjective effects experienced under the influence of psychedelics (Figure 4a). This model is supported by behavioral measures of impaired sensorimotor gating in humans after administration of psilocybin and LSD128, 129. Additionally, the model agrees with neuroimaging measures of increased thalamo-cortical functional connectivity108, 112 and synchronization of cortical sensory regions108, 111, 130.
Figure 4. Network-level models of psychedelic action.
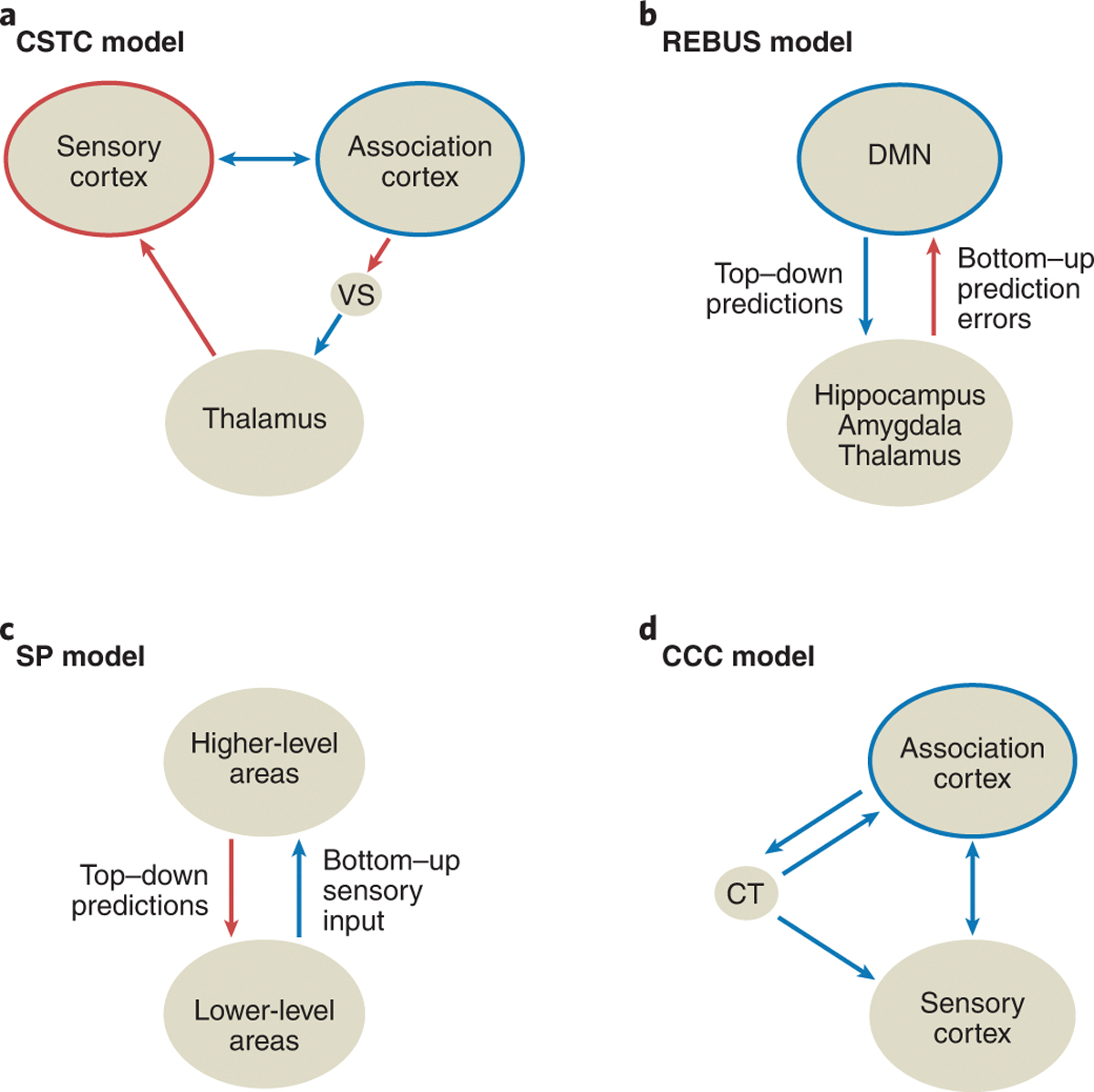
(a) The cortico-striatothalamo-cortical (CSTC) model focuses on altered thalamic gating and subsequent changes in sensory vs. association processing induced by psychedelics108, 124. (b) The ‘relaxed beliefs under psychedelics and the anarchic brain’ (REBUS) model posits increased bottom-up signaling, concurrent with a disintegration of association cortices and reduced top-down predictions, which are postulated to underlie psychedelic-induced effects125. (c) The strong prior (SP) model suggests that psychedelic experiences arise from a reduction of bottom-up sensory inputs and an aberrant reliance on top-down expectations. This model does not make predictions about specific anatomical substrates126. (d) The cortico-claustro-cortical (CCC) model centers on disrupted claustrum activity and functional connectivity, leading to a desynchronization of cortical networks127. Red arrows: increased functional connectivity/control. Blue arrows: decreased functional connectivity/control. Red circles: increased integration. Blue circles: decreased integration. VS: ventral striatum. CT: claustrum.
The REBUS model postulates that psychedelics enhance bottom-up flow of sensory inputs, while reducing the precision of prior beliefs – expectations and past experiences that normally constrain neural processing125 (Figure 4b). The collective effect is predicted to increase entropy in neural dynamics125. Empirical evidence for this model hinges on the findings from neuroimaging of a disintegration of association networks including the DMN106, 109. More complex neural signals can be observed with MEG and EEG after the administration of LSD, psilocybin, and DMT131, 132. Furthermore, frequency-domain analyses can be used to estimate the directionality of signal propagation from multi-electrode EEG recordings, which suggest that DMT weakens alpha-band oscillatory neural signals flowing in the top-down direction while potentiating the bottom-up signals133.
However, the REBUS model is at odds with various neurophysiological and behavioral results, which instead favor the SP model. Specifically, recordings in animals showed mostly reduced stimulus-evoked spiking activity in the visual cortex81, 83. Moreover, it is well known that perceptions are often not altered by vivid stimuli, but rather hallucinations can occur for healthy humans under sensory deprivation in complete darkness134. These results suggest that the psychedelic experience may arise from reduced bottom-up sensory inputs, coupled with aberrant reliance on top-down expectations, which constitute key features of the SP model126 (Figure 4c). Indeed, recent studies of conditioning-induced hallucinations data have provided evidence for such heightened dependence on inappropriate beliefs135.
Beyond the cortico-striato-thalamic loops, other recurrent networks in the brain may be involved, such as the cortico-claustro-cortical (CCC) pathway. In one model127, psychedelics were suggested to disrupt the communication between the prefrontal cortex and claustrum, by aberrantly driving prefrontal inputs and/or activating the claustrum (Figure 4d), which impairs the coordinated responses of association networks to changing task demands. The involvement of claustrum has been suggested based on the high density of 5-HT2A receptors5, and supported by a recent study showing reduced functional connectivity between the claustrum and cortical networks observed after a low dose of psilocybin136.
It should be appreciated that the various models arise from different explanatory focuses. The CSTC and CCC models emphasize implementation, highlighting altered circuits without relying heavily on psychological implications. By contrast, SP is a theory of perceptual and cognitive processes that give rise to hallucinations, which is computational in nature and agnostic to implementation in the brain. SP may be reconciled with REBUS if the hypothesized weakened low-level beliefs eventually culminate in stronger extra-perceptual beliefs, although more precise definitions of priors and beliefs will be helpful for comparing and testing these models. Ultimately, a unified model of psychedelic action should build on the growing body of knowledge at the neuronal and network levels and be tethered firmly to both implementation- and computation-based explanations.
Open questions
Accumulating evidence in humans and rodents strongly suggests that activation of the 5-HT2A receptor is primarily responsible for the hallucinogenic effects of psychedelics. These receptors and their downstream signaling pathways may also mediate some of the therapeutic effects observed following psychedelic administration, considering that the 5-HT2A receptor is a common target shared by all psychedelic compounds. However, no clinical study to date has explicitly tested the molecular basis of psychedelics’ potential therapeutic effects in humans, whether through antagonist pretreatment or comparison with a non-hallucinogenic 5-HT2A agonist. In rodents, it remains debatable whether psychedelic-evoked neural plasticity requires functional 5-HT2A receptors. On the one hand, pretreatment with ketanserin did not affect psilocybin-evoked structural remodeling43, 44, though this manipulation does not block all 5-HT2A receptors in the rodent brain137. On the other hand, other data indicated that 5-HT2A receptor is essential for structural plasticity94, 97, but these studies relied on in vitro preparations or constitutive knockout mice that could affect neurodevelopment.
Another important caveat to consider is that many, if not all, psychedelics also potently activate the closely related 5-HT2C receptor. This receptor is highly expressed in the brain and is known to regulate the function of mesolimbic dopaminergic neurons, making it an ideal therapeutic target for several neuropsychiatric disorders138. Complicating matters further, multiple isoforms of the 5-HT2C receptor exist due to post-transcriptional RNA editing139. Unfortunately, we currently lack pharmacological tools that can adequately differentiate between 5-HT2A and 5-HT2C receptors. To the best of our knowledge, there are no potent 5-HT2A receptor agonists that either lack affinity for 5-HT2C receptors or exhibit 5-HT2C receptor antagonism. Moreover, a truly selective 5-HT2A receptor antagonist has not yet been identified140. This dearth of selective pharmacological tools impedes our ability to achieve a full mechanistic understanding of psychedelic drug action.
In addition to psychedelics, other drugs such as psychostimulants (including amphetamine and cocaine) can induce changes in dendritic architecture. A key difference is that psychostimulant-induced alterations occur after repeated exposure over several weeks141. Another difference is that increases in spine density and dendritic branching after psychostimulant administration are more pronounced in striatal regions such as nucleus accumbens, although they can also be detected in medial frontal cortex and hippocampus. Other chronic exposures such as long-term diazepam treatment can lead to a loss of cortical dendritic spines142. Therefore, although various drugs can modify the density of dendritic spines, they differ in specific parameters such as duration of treatment and affected brain regions. Finding out those specific characteristics unique to psychedelic-induced neural plasticity will be important, particularly if we want to leverage structural remodeling as a biomarker for drug discovery.
The role of cell types in driving the neural responses to psychedelics remain to be clarified. Many of the neurophysiological recordings were performed during a time when the diversity of cell types was less appreciated than it currently is. For example, in the frontal cortex, major classes of GABAergic neurons — including subtypes expressing parvalbumin, somatostatin and other markers — express varying amounts of different serotonin receptor subtypes72, 78, and therefore should have distinct responses to psychedelics. Similarly, although the dorsal raphe nucleus is known for containing serotonergic neurons, those cells only constitute 30–50% of the population in this region, and the remainder consists of glutamatergic, GABAergic, dopaminergic, and other peptidergic neurons that also express subtypes of serotonin receptors143. The local circuit interactions that shape the neural activity dynamics induced by psychedelic compounds are largely unknown.
Taking a step back, an even greater gap in our current knowledge of psychedelics is linking causally these neurobiological actions to the compounds’ behavioral effects. Among the potential multitude of receptor targets, plasticity processes, and cell types involved, it is not understood which of these mechanisms drive the beneficial effects seen in clinical trials. Without this fundamental understanding, we lack a foundation to guide optimal dosing and identify individuals who are likely to respond positively to psychedelics.
Looking ahead
What can we look forward to in the next decade for psychedelic research? A major question in the field is the extent to which the subjective experience may be separable from the potential therapeutic effects144, 145. The question can be reframed at the molecular level by asking whether the acute and long-lasting effects arise from activating the same receptors and/or the same intracellular signaling pathways. We may expect answers soon as more receptor structures become available, and the function of select signaling pathways are tested via genetic manipulations in preclinical species. Furthermore, the distribution of receptors and degree of biased agonism will vary across cell types and neural circuits, which must also play a role in shaping the effects of psychedelics. Current studies have focused on a few brain regions, but we can anticipate systematic investigations of psychedelic effects on dendritic excitability and spiking dynamics across the entire brain using optical imaging and large-scale electrophysiological recordings. It is not obvious whether the same or different neural circuits are responsible for psychedelics’ various perceptual, cognitive, and therapeutic effects. In particular, the contribution of subcortical brain regions remains underappreciated. Such neurophysiological insights will complement the gene expression and receptor binding atlases to lay a foundation for more realistic computational models of psychedelic action. For neuroimaging, high-quality, well-powered studies through individualized, repeated sessions could provide further insights into the fine-grained changes in different brain regions and relate them to psychiatric conditions. After all, most modern psychedelic research shares a common goal, which is to find mechanistic explanations for how psychedelics impact human behavior. By integrating across levels and delving deep to understand the neural basis of psychedelic action, we hope to one day leverage the ability of these molecules to shape and heal minds.
Acknowledgements
A.C.K. thanks Neil Savalia, Pasha Davoudian, and Phil Corlett, and D.E.O. thanks Lee Dunlap, Hunter Warren, and David Nichols for helpful conversations. A.C.K. was supported by the Yale Program in Psychedelic Science, and NIH/NIMH grants R01MH121848 and R01MH128217. D.E.O. was supported by NIH/NIDA grant R01DA056365, NIH/NIGMS grant R01GM128997, and the Camille and Henry Dreyfus Foundation. B.L.R. was supported by grants from NIH/NIMH, NIH/NIDA, DARPA, and the Michael Hooker Distinguished Professorship.
Footnotes
Declaration of Interests
A.C.K. is a member of the Scientific Advisory Board of Empyrean Neuroscience and Freedom Biosciences. A.C.K. has consulted for Biohaven Pharmaceuticals. No-cost compounds were provided to A.C.K. for research by Usona Institute. D.E.O. is a co-founder of Delix Therapeutics, Inc. and serves as the Chief Innovation Officer and Head of the Scientific Advisory Board. K.H.P. is currently an employee of Boehringer Ingelheim GmbH & Co. KG. B.L.R. is a member of the Scientific Advisory Board of Septerna Pharmaceuticals and Escient Pharmaceuticals. These duties had no influence on the content of this article.
Code availability
Not applicable.
Peer review information:
Nature Neuroscience thanks Javier Gonzalez-Maeso, Gitte Knudsen, and Charles Nichols for their contribution to the peer review of this work.
Data availability
Not applicable.
References
- 1.Nichols DE & Walter H The History of Psychedelics in Psychiatry. Pharmacopsychiatry 54, 151–166 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Davis AK, et al. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 78, 481–489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carhart-Harris R, et al. Trial of Psilocybin versus Escitalopram for Depression. N Engl J Med 384, 1402–1411 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Johnson MW, Garcia-Romeu A & Griffiths RR Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 43, 55–60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols DE Psychedelics. Pharmacol Rev 68, 264–355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollenweider FX & Preller KH Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci 21, 611–624 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Kelmendi B, Kaye AP, Pittenger C & Kwan AC Psychedelics. Curr Biol 32, R63–R67 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClure-Begley TD & Roth BL The promises and perils of psychedelic pharmacology for psychiatry. Nat Rev Drug Discov (2022). [DOI] [PubMed] [Google Scholar]
- 9.Kim K, et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 182, 1574–1588 e1519 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao D, et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375, 403–411 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Froldi G, Silvestrin B, Dorigo P & Caparrotta L Gramine: a vasorelaxing alkaloid acting on 5-HT(2A) receptors. Planta Med 70, 373–375 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Dong C, et al. Psychedelic-inspired drug discovery using an engineered biosensor. Cell 184, 2779–2792 e2718 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glennon RA, Liebowitz SM & Mack EC Serotonin Receptor-Binding Affinities of Several Hallucinogenic Phenylalkylamine and N,N-Dimethyltryptamine Analogs. Journal of Medicinal Chemistry 21, 822–825 (1978). [DOI] [PubMed] [Google Scholar]
- 14.Lyon RA, Titeler M, Seggel MR & Glennon RA Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens. Eur J Pharmacol 145, 291–297 (1988). [DOI] [PubMed] [Google Scholar]
- 15.McLean TH, et al. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem 49, 5794–5803 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Halberstadt AL, Chatha M, Stratford A, Grill M & Brandt SD Comparison of the behavioral responses induced by phenylalkylamine hallucinogens and their tetrahydrobenzodifuran (“FLY”) and benzodifuran (“DragonFLY”) analogs. Neuropharmacology 144, 368–376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantle TJ, Tipton KF & Garrett NJ Inhibition of monoamine oxidase by amphetamine and related compounds. Biochem Pharmacol 25, 2073–2077 (1976). [DOI] [PubMed] [Google Scholar]
- 18.Glennon RA, Young R & Jacyno JM Indolealkylamine and Phenalkylamine Hallucinogens - Effect of Alpha-Methyl and N-Methyl Substituents on Behavioral Activity. Biochemical Pharmacology 32, 1267–1273 (1983). [DOI] [PubMed] [Google Scholar]
- 19.Dyer DC, Nichols DE, Rusterholz DB & Barfknecht CF Comparative effects of stereoisomers of psychotomimetic phenylisopropylamines. Life Sci 13, 885–896 (1973). [DOI] [PubMed] [Google Scholar]
- 20.Congreve M, Carr R, Murray C & Jhoti HA ‘Rule of Three’ for fragment-based lead discovery? Drug Discovery Today 8, 876–877 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Wager TT, Hou X, Verhoest PR & Villalobos A Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem Neurosci 1, 435–449 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T, et al. C-11 Labeling of Indolealkylamine Alkaloids and the Comparative-Study of Their Tissue Distributions. International Journal of Applied Radiation and Isotopes 36, 965–969 (1985). [DOI] [PubMed] [Google Scholar]
- 23.Ebersole BJ, Visiers I, Weinstein H & Sealfon SC Molecular basis of partial agonism: orientation of indoleamine ligands in the binding pocket of the human serotonin 5-HT2A receptor determines relative efficacy. Mol Pharmacol 63, 36–43 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Banskota S, Ghia JE & Khan WI Serotonin in the gut: Blessing or a curse. Biochimie 161, 56–64 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Barfknecht CF & Nichols DE Correlation of psychotomimetic activity of phenethylamines and amphetamines with 1-octanol-water partition coefficients. J Med Chem 18, 208–210 (1975). [DOI] [PubMed] [Google Scholar]
- 26.Perrin DD Dissociation constants of organic bases in aqueous solution (Butterworths, London, 1965). [Google Scholar]
- 27.Migliaccio GP, Shieh TLN, Byrn SR, Hathaway BA & Nichols DE Comparison of Solution Conformational Preferences for the Hallucinogens Bufotenin and Psilocin Using 360-Mhz Proton Nmr-Spectroscopy. Journal of Medicinal Chemistry 24, 206–209 (1981). [DOI] [PubMed] [Google Scholar]
- 28.Adams AM, et al. In vivo production of psilocybin in E. coli. Metab Eng 56, 111–119 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Milne N, et al. Metabolic engineering of Saccharomyces cerevisiae for the de novo production of psilocybin and related tryptamine derivatives. Metab Eng 60, 25–36 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H & Hell D Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Preller KH, et al. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27, 451–457 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Madsen MK, et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44, 1328–1334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glennon RA, Titeler M & McKenney JD Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35, 2505–2511 (1984). [DOI] [PubMed] [Google Scholar]
- 34.Halberstadt AL, Chatha M, Klein AK, Wallach J & Brandt SD Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keiser MJ, et al. Predicting new molecular targets for known drugs. Nature 462, 175–181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Maeso J, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Kroeze WK, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol 22, 362–369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marona-Lewicka D, Thisted RA & Nichols DE Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl) 180, 427–435 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Grailhe R, et al. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron 22, 581–591 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Marona-Lewicka D, Chemel BR & Nichols DE Dopamine D4 receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacology (Berl) 203, 265–277 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Klein AK, et al. Investigation of the Structure-Activity Relationships of Psilocybin Analogues. ACS Pharmacol Transl Sci 4, 533–542 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pokorny T, Preller KH, Kraehenmann R & Vollenweider FX Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur Neuropsychopharmacol 26, 756–766 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Hesselgrave N, Troppoli TA, Wulff AB, Cole AB & Thompson SM Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao LX, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544 e2534 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sard H, et al. SAR of psilocybin analogs: discovery of a selective 5-HT 2C agonist. Bioorg Med Chem Lett 15, 4555–4559 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Wacker D, et al. Structural Features for Functional Selectivity at Serotonin Receptors. Science 340, 615–619 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wacker D, et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 168, 377–389 e312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth BL Drugs and valvular heart disease. N Engl J Med 356, 6–9 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Droogmans S, et al. Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am J Cardiol 100, 1442–1445 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Setola V, et al. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 63, 1223–1229 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Roth BL, Nakaki T, Chuang DM & Costa E Aortic recognition sites for serotonin (5HT) are coupled to phospholipase C and modulate phosphatidylinositol turnover. Neuropharmacology 23, 1223–1225 (1984). [DOI] [PubMed] [Google Scholar]
- 52.Roth BL, Nakaki T, Chuang DM & Costa E 5-Hydroxytryptamine2 receptors coupled to phospholipase C in rat aorta: modulation of phosphoinositide turnover by phorbol ester. J Pharmacol Exp Ther 238, 480–485 (1986). [PubMed] [Google Scholar]
- 53.Kristiansen K, et al. A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT(2A) serotonin receptor but does not participate in activation via a “salt-bridge disruption” mechanism. J Pharmacol Exp Ther 293, 735–746 (2000). [PubMed] [Google Scholar]
- 54.Egan C, et al. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse 35, 144–150 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Gray JA, Bhatnagar A, Gurevich VV & Roth BL The interaction of a constitutively active arrestin with the arrestin-insensitive 5-HT(2A) receptor induces agonist-independent internalization. Mol Pharmacol 63, 961–972 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Felder CC, Kanterman RY, Ma AL & Axelrod J Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A 87, 2187–2191 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia EE, Smith RL & Sanders-Bush E Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology 52, 1671–1677 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmid CL, Raehal KM & Bohn LM Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A 105, 1079–1084 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguiz RM, et al. LSD-stimulated behaviors in mice require beta-arrestin 2 but not beta-arrestin 1. Sci Rep 11, 17690 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurrasch-Orbaugh DM, Parrish JC, Watts VJ & Nichols DE A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem 86, 980–991 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Zhuang Y, et al. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell 184, 931–942 e918 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasler F, Bourquin D, Brenneisen R, Bar T & Vollenweider FX Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv 72, 175–184 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Brandt SD, et al. Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test Anal 8, 891–902 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vargas MV, Meyer R, Avanes AA, Rus M & Olson DE Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front Psychiatry 12, 727117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olson DE Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J Exp Neurosci 12, 1179069518800508 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunlap LE, et al. Identification of Psychoplastogenic N,N-Dimethylaminoisotryptamine (isoDMT) Analogues through Structure-Activity Relationship Studies. J Med Chem 63, 1142–1155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cameron LP, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyu J, et al. Ultra-large library docking for discovering new chemotypes. Nature 566, 224–229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadybekov AA, et al. Synthon-based ligand discovery in virtual libraries of over 11 billion compounds. Nature 601, 452–459 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Araneda R & Andrade R 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40, 199–412 (1991). [DOI] [PubMed] [Google Scholar]
- 71.Davies MF, Deisz RA, Prince DA & Peroutka SJ Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res 423, 347–352 (1987). [DOI] [PubMed] [Google Scholar]
- 72.Savalia NK, Shao LX & Kwan AC A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci 44, 260–275 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miner LAH, Backstrom JR, Sanders-Bush E & Sesack SR Ultrastructural localization of serotonin2a receptors in the middle layers of the rat prelimibic prefrontal cortex. Neuroscience 116, 107–117 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Jakab RL & Goldman-Rakic PS 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A 95, 735–740 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willins DL, Deutch AY & Roth BL Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 27, 79–82 (1997). [DOI] [PubMed] [Google Scholar]
- 76.Aghajanian GK & Marek GJ Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36, 589–599 (1997). [DOI] [PubMed] [Google Scholar]
- 77.Santana N, Bortolozzi A, Serrats J, Mengod G & Artigas F Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex 14, 1100–1109 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Martin DA & Nichols CD Psychedelics Recruit Multiple Cellular Types and Produce Complex Transcriptional Responses Within the Brain. EBioMedicine 11, 262–277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avesar D & Gulledge AT Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits 6, 12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wood J, Kim Y & Moghaddam B Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci 32, 3022–3031 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evarts EV, Landau W, Freygang W Jr. & Marshall WH Some effects of lysergic acid diethylamide and bufotenine. Am J Physiol 182, 594–598 (1955). [DOI] [PubMed] [Google Scholar]
- 82.Rose D & Horn G Effects of LSD on the responses of single units in cat visual cortex. Exp Brain Res 27, 71–80 (1977). [DOI] [PubMed] [Google Scholar]
- 83.Michaiel AM, Parker PRL & Niell CM A Hallucinogenic Serotonin-2A Receptor Agonist Reduces Visual Response Gain and Alters Temporal Dynamics in Mouse V1. Cell Rep 26, 3475–3483 e3474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aghajanian GK, Foote WE & Sheard MH Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science 161, 706–708 (1968). [DOI] [PubMed] [Google Scholar]
- 85.Aghajanian GK, Foote WE & Sheard MH Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther 171, 178–187 (1970). [PubMed] [Google Scholar]
- 86.Sprouse JS & Aghajanian GK Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1, 3–9 (1987). [DOI] [PubMed] [Google Scholar]
- 87.Trulson ME, Ross CA & Jacobs BL Lack of tolerance to the depression of raphe unit activity by lysergic acid diethylamide. Neuropharmacology 16, 771–774 (1977). [DOI] [PubMed] [Google Scholar]
- 88.Trulson ME & Jacobs BL Dissociations between the Effects of Lsd on Behavior and Raphe Unit-Activity in Freely Moving Cats. Science 205, 515–518 (1979). [DOI] [PubMed] [Google Scholar]
- 89.Domenico C, Haggerty D, Mou X & Ji D LSD degrades hippocampal spatial representations and suppresses hippocampal-visual cortical interactions. Cell Rep 36, 109714 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rasmussen K & Aghajanian GK Effect of hallucinogens on spontaneous and sensory-evoked locus coeruleus unit activity in the rat: reversal by selective 5-HT2 antagonists. Brain Res 385, 395–400 (1986). [DOI] [PubMed] [Google Scholar]
- 91.Aghajanian GK LSD and CNS transmission. Annu Rev Pharmacol 12, 157–168 (1972). [DOI] [PubMed] [Google Scholar]
- 92.Nichols CD & Sanders-Bush E A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26, 634–642 (2002). [DOI] [PubMed] [Google Scholar]
- 93.Vaidya VA, Marek GJ, Aghajanian GK & Duman RS 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17, 2785–2795 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de la Fuente Revenga M, et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep 37, 109836 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones KA, et al. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A 106, 19575–19580 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida H, et al. Subtype specific roles of serotonin receptors in the spine formation of cortical neurons in vitro. Neurosci Res 71, 311–314 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Ly C, et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 23, 3170–3182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raval NR, et al. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int J Mol Sci 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ly C, et al. Transient Stimulation with Psychoplastogens Is Sufficient to Initiate Neuronal Growth. ACS Pharmacology & Translational Science (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Phoumthipphavong V, Barthas F, Hassett S & Kwan AC Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro 3, ENEURO.0133–0115.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ali F, et al. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun 11, 72 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aleksandrova LR & Phillips AG Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci 42, 929–942 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Hibicke M, Landry AN, Kramer HM, Talman ZK & Nichols CD Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem Neurosci 11, 864–871 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Hermle L, et al. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry 32, 976–991 (1992). [DOI] [PubMed] [Google Scholar]
- 105.Vollenweider FX, et al. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16, 357–372 (1997). [DOI] [PubMed] [Google Scholar]
- 106.Carhart-Harris RL, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109, 2138–2143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lewis CR, et al. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage 159, 70–78 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Preller KH, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muller F, Dolder PC, Schmidt A, Liechti ME & Borgwardt S Altered network hub connectivity after acute LSD administration. Neuroimage Clin 18, 694–701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Madsen MK, et al. Psilocybin-induced changes in brain network integrity and segregation correlate with plasma psilocin level and psychedelic experience. Eur Neuropsychopharmacol 50, 121–132 (2021). [DOI] [PubMed] [Google Scholar]
- 111.Preller KH, et al. Psilocybin Induces Time-Dependent Changes in Global Functional Connectivity. Biol Psychiatry 88, 197–207 (2020). [DOI] [PubMed] [Google Scholar]
- 112.Muller F, et al. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand 136, 648–657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Preller KH, et al. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci U S A 116, 2743–2748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hawrylycz MJ, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beliveau V, et al. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J Neurosci 37, 120–128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Deco G, et al. Whole-Brain Multimodal Neuroimaging Model Using Serotonin Receptor Maps Explains Non-linear Functional Effects of LSD. Curr Biol 28, 3065–3074 e3066 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Burt JB, et al. Transcriptomics-informed large-scale cortical model captures topography of pharmacological neuroimaging effects of LSD. Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kringelbach ML, et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc Natl Acad Sci U S A 117, 9566–9576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McCulloch DE, et al. Lasting effects of a single psilocybin dose on resting-state functional connectivity in healthy individuals. J Psychopharmacol 36, 74–84 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barrett FS, Doss MK, Sepeda ND, Pekar JJ & Griffiths RR Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci Rep 10, 2214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Doss MK, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry 11, 574 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Daws RE, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med 28, 844–851 (2022). [DOI] [PubMed] [Google Scholar]
- 123.Stenbaek DS, et al. Brain serotonin 2A receptor binding predicts subjective temporal and mystical effects of psilocybin in healthy humans. J Psychopharmacol 35, 459–468 (2021). [DOI] [PubMed] [Google Scholar]
- 124.Vollenweider FX & Geyer MA A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull 56, 495–507 (2001). [DOI] [PubMed] [Google Scholar]
- 125.Carhart-Harris RL & Friston KJ REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol Rev 71, 316–344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Corlett PR, et al. Hallucinations and Strong Priors. Trends Cogn Sci 23, 114–127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Doss MK, et al. Models of psychedelic drug action: modulation of cortical-subcortical circuits. Brain (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vollenweider FX, Csomor PA, Knappe B, Geyer MA & Quednow BB The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 32, 1876–1887 (2007). [DOI] [PubMed] [Google Scholar]
- 129.Schmid Y, et al. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol Psychiatry 78, 544–553 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Roseman L, et al. LSD alters eyes-closed functional connectivity within the early visual cortex in a retinotopic fashion. Hum Brain Mapp 37, 3031–3040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK & Muthukumaraswamy SD Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 7, 46421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Timmermann C, et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep 9, 16324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alamia A, Timmermann C, Nutt DJ, VanRullen R & Carhart-Harris RL DMT alters cortical travelling waves. Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vernon J, Marton T & Peterson E Sensory deprivation and hallucinations. Science 133, 1808–1812 (1961). [DOI] [PubMed] [Google Scholar]
- 135.Powers AR, Mathys C & Corlett PR Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 357, 596–600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barrett FS, Krimmel SR, Griffiths RR, Seminowicz DA & Mathur BN Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. Neuroimage 218, 116980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith RL, Barrett RJ & Sanders-Bush E Neurochemical and behavioral evidence that quipazine-ketanserin discrimination is mediated by serotonin2A receptor. J Pharmacol Exp Ther 275, 1050–1057 (1995). [PubMed] [Google Scholar]
- 138.Palacios JM, Pazos A & Hoyer D A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology (Berl) 234, 1395–1418 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Fitzgerald LW, et al. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology 21, 82S–90S (1999). [DOI] [PubMed] [Google Scholar]
- 140.Casey AB, Cui M, Booth RG & Canal CE “Selective” serotonin 5-HT2A receptor antagonists. Biochem Pharmacol 200, 115028 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Robinson TE & Kolb B Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 Suppl 1, 33–46 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Bittner T, et al. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci 29, 10405–10409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang KW, et al. Molecular and anatomical organization of the dorsal raphe nucleus. Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olson DE The Subjective Effects of Psychedelics May Not Be Necessary for Their Enduring Therapeutic Effects. ACS Pharmacology & Translational Science (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yaden DB & Griffiths RR The Subjective Effects of Psychedelics Are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacology & Translational Science (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Osmond H A review of the clinical effects of psychotomimetic agents. Ann N Y Acad Sci 66, 418–434 (1957). [DOI] [PubMed] [Google Scholar]
- 147.Shulgin A & Shulgin A PIHKAL: A Chemical Love Story (Transform Press, Berkeley, CA, 1990). [Google Scholar]
- 148.Shulgin A & Shulgin A TiHKAL: The Continuation (Transform Press, Berkeley, CA, 2002). [Google Scholar]
- 149.Griffiths RR, Richards WA, McCann U & Jesse R Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187, 268–283; discussion 284–292 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Griffiths RR, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30, 1181–1197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abramson HA The use of LSD in psychotherapy and alcoholism (Bobbs-Merrill, Indianapolis, 1967). [Google Scholar]
- 152.Grob CS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68, 71–78 (2011). [DOI] [PubMed] [Google Scholar]
- 153.Ross S, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30, 1165–1180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krystal JH, Abdallah CG, Sanacora G, Charney DS & Duman RS Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron 101, 774–778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mitchell JM, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med 27, 1025–1033 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Canal CE & Morgan D Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4, 556–576 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Appel JB, West WB & Buggy J LSD, 5-HT (serotonin), and the evolution of a behavioral assay. Neurosci Biobehav Rev 27, 693–701 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


