Background: Rates of graft versus host disease (GVHD) and non-relapse mortality (NRM) following myeloablative allogeneic hematopoietic stem cell transplant (MA-alloHSCT) remain unacceptably high. Strategies to reduce GVHD and NRM have been compromised by limited efficacy or increased risk of infection and relapse, emphasizing the need for new approaches that holistically improve outcomes.
Orca-T is a high-precision, allogeneic investigational cell therapy product comprised of stem and immune cells that leverages highly purified, polyclonal donor regulatory T cells to control alloreactive immune responses, reducing the need for pharmacologic GVHD prophylaxis. Orca-T is produced in a central GMP facility and has been successfully scaled to clinical centers throughout the U.S.
Aims: The aim of these studies was to evaluate the safety and efficacy of Orca-T in patients with hematologic malignancies.
Methods: As of 28 February 2022, 138 patients with high-risk hematologic malignancies have received Orca-T in a single-center Phase 1-2 study (NCT01660607, n=41) and a multicenter Phase 1b study (NCT04013685, n=97) and have ≥ 100 days of follow-up. Informed consent was obtained from all transplant recipients and donors, and the studies received IRB approval from participating institutions. Orca-T was produced from G-CSF-mobilized peripheral blood (PB) from matched related donors (n=72), matched unrelated donors (n=62), or mismatched unrelated donors (MMUD, n=4). Median follow-up for recipients was 300 days (range: 27-1941). Median age was 49 years, and diagnoses included AML (43%), ALL (27%), MDS (10%), myelofibrosis (7%), and CML (6%). Patients received myeloablative conditioning (busulfan-based, n=109; TBI-based, n=27; BCNU, n=2) followed by GVHD prophylaxis with either single-agent tacrolimus (tac, n=127), sirolimus (n=7), or tac plus mycophenolate (n=4, MMUD). A contemporaneous CIBMTR-based control arm was obtained that consisted of patients with similar diagnoses who received myeloablative alloHSCT from a PB source followed by tac/methotrexate PPX.
Results: Orca-T was successfully manufactured, distributed, and infused for all patients enrolled. Overall time from donor centers to recipient centers was under 60 hours in all cases. Median time to neutrophil engraftment was 13 days. The rates of grade ≥ 3 acute GVHD in the first 180 days and moderate to severe chronic GVHD through 1 year were low with Orca-T at 4% and 5%, respectively. NRM was infrequent at 4% through 1 year. Orca-T exhibited GRFS of 71% & OS of 90% at 1 year. No formal comparison to the CIBMTR cohort was performed, and a Phase 3 study has been initiated to confirm these findings. Longitudinal immune reconstitution data was collected and will be presented. Clinical data is summarized in Table 1.
*MAGIC Criteria **NIH Consensus Grading
Image:
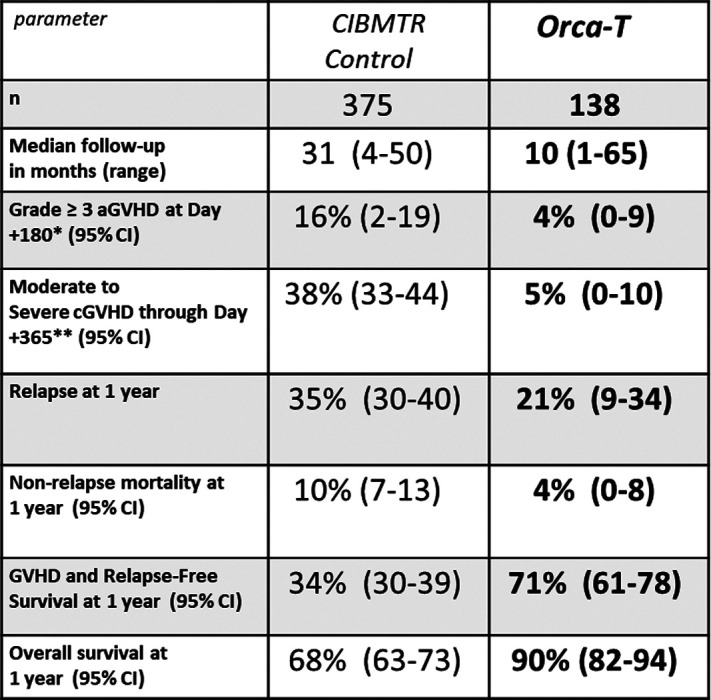
Summary/Conclusion: Results from patients treated with Orca-T, a high-precision Treg-engineered donor product, suggest a reduction in cGVHD, improved GRFS, and low toxicity relative to historic data. Orca-T manufacturing was accomplished with consistent and reliable cell manufacturing and distribution across a wide geographic area. A multicenter randomized-control trial phase 3 trial comparing Orca-T to SOC has been initiated.


