Abstract
Protective immunity against tuberculosis is considered to be essentially cell mediated, and an important role for CD8+ T lymphocytes has been suggested by several studies of murine and human infections. The present work, using an experimental model of infection with Mycobacterium bovis in cattle, showed that live M. bovis elicits the activation of CD8+ T cells in vitro. However, a sonic extract prepared from M. bovis (MBSE) and protein purified derivative (PPDb) also induced a considerable degree of activation of the CD8+ T cells. Analysis of proliferative responses of peripheral blood mononuclear cells, purified CD8+ T cells, and CD8+ T-cell clones to M. bovis and to soluble antigenic preparations (MBSE, PPDb) showed that the responses of all three types of cells were always superior for live mycobacteria but that strong responses were also obtained with complex soluble preparations. Furthermore, while cytotoxic capabilities were not investigated, the CD8+ T cells were found to produce and release gamma interferon in response to antigen (live and soluble), which indicated one possible protective mechanism for these cells in bovine tuberculosis. Finally, it was demonstrated by metabolic inhibition with brefeldin A and cytochalasin D at the clonal level that an endogenous pathway of antigen processing is required for presentation to bovine CD8+ cells and that presentation is also dependent on phagocytosis of the antigen.
Infection with Mycobacterium bovis, the causative organism of bovine tuberculosis, is an important health problem in cattle and other animal species. The disease presents a major barrier to animal-related trade and production, causing significant losses to farming economies worldwide. Furthermore, bovine tuberculosis has serious zoonotic implications, especially in developing regions.
Many countries operate bovine tuberculosis surveillance programs targeted at either maintaining disease-free status or achieving national eradication. Attempts to control infection have often depended on slaughter of skin test-positive cattle, but complications due to wildlife reservoirs of M. bovis have become apparent in recent years. Vaccination of cattle is being considered in some regions as an additional means of controlling and eradicating the disease, and bacillus Calmette-Guérin (BCG) has been used in several trials (2, 32, 47). However, the efficacy of this vaccine in cattle has been variable, as has been the case for human tuberculosis (40). Also, vaccination with BCG may compromise the skin test status, thus interfering with the course of current eradication programs.
The development of novel, improved vaccines is urgently needed (35), and, an increased understanding of the immunology of both human and animal tuberculosis with a more complete definition of T-cell responses, is crucial to logical progression. Particularly for bovine tuberculosis, detailed aspects of cellular immunity in relation to M. bovis infection remain largely undefined.
Protective immunity against mycobacterial infections is considered to be essentially cell mediated, dependent on the interaction of macrophages with T lymphocytes (18). However, the relative contribution of CD8+- and CD4+-T-cell subpopulations has not yet been clearly determined.
An important role for CD8+ T cells in mycobacterial infections has been suggested by a series of experiments in the murine and human models: adoptive transfer of cytotoxic CD8+-T-cell precursors induces protective immunity against infection (34); selective depletion of CD8+ T cells renders mice more susceptible to infection with M. tuberculosis (30); β2-microglobulin knockout mice have enhanced susceptibility to M. tuberculosis infection (12); CD8+-T-cell clones from M. tuberculosis-immunized mice show mycobacterial antigen-specific cytolytic activity (9); immunization of mice with plasmids expressing mycobacterial hsp65, antigen 85A, or the 38-kDa antigen results in the generation of antigen-specific CD8+ cytotoxicity associated with protection from subsequent challenge with M. tuberculosis (17, 45, 50); human CD8+ T cells restricted by CD1b molecule are able to inhibit the growth of M. tuberculosis in vitro (43); and, finally, a recent study demonstrated the presence of classical major histocompatibility complex (MHC) class I-restricted CD8+ cytolysis specific for ESAT-6 in infected humans (22).
Both cytotoxicity and gamma interferon (IFN-γ) production are likely functions for CD8+ T cells in antimycobacterial immunity (4), suggesting the existence of several subsets of such cells. It has been recently recognized that CD8+ T cells can be subdivided based on specific patterns of cytokine secretion, with type I cells secreting primarily interleukin-2 (IL-2) and IFN-γ and type II cells secreting primarily IL-4, IL-5, and IL-10 (14). A variety of signals from both antigen-presenting cells and CD4+ T cells produced during an immune response may result in generation of either cytotoxic or immunoregulatory CD8+ T cells (33).
It has been classically proposed that CD8+-T-cell responses are restricted by MHC class I molecules and that such molecules present endogenous antigens that are synthesized within the presenting cell and processed within the cytosol; on the other hand, the so-called “exogenous” antigens that are internalized from the extracellular space by endocytosis or phagocytosis are processed within vacuolar compartments for presentation by class II MHC molecules. Despite this general rule, a number of recent studies have demonstrated that this segregation of cytoplasmic and soluble antigens into class I and class II presentation pathways is not as absolute as was initially thought (10, 36).
Because the increasing evidence that CD8+ T cells play a crucial role in the protective response against M. tuberculosis in the murine model and in humans, this study has investigated the role of CD8+ T cells in bovine tuberculosis and is, to our knowledge, the first such investigation. The objectives of this work were, first, to determine whether CD8+ T cells respond in bovine tuberculosis by using a model of infection in the natural host; second, to compare the responses of CD8+ T cells to live M. bovis and soluble mycobacterial antigens; and finally, to investigate, at the clonal level, the responses of bovine CD8+ T cells to mycobacterial antigens and the antigen-processing pathways which are involved. These in vitro results indicate clearly that CD8+ T cells play an important role in antimycobacterial immune responses and that these responses may be stimulated by soluble antigen.
MATERIALS AND METHODS
Mycobacterial antigens and cultures.
A Northern Irish field isolate of M. bovis was cultured in air at 37°C in Middlebrook 7H9 supplemented with oleic acid albumin dextrose complex (Difco, Paisley, United Kingdom). Mid-log-phase cultures were washed in 0.01 M phosphate-buffered saline (PBS; pH 7.2), harvested, and sonicated for 20 s to break up the bacterial clumps. Aliquots of the cultures were frozen at −80°C until use. Bacterial counts and viability were checked by thawing several aliquots, plating 10-fold dilutions in Middlebrook 7H10 (Difco), and counting the CFU after 5 weeks.
For production of M. bovis sonic extract (MBSE), mid-log-phase cultures grown under the same conditions were harvested, washed three times in PBS, and subjected to ultrasonication for five periods of 5 min each on ice (Soniprep 150; MSE Sanyo Gallenkamp, Leicester, United Kingdom). MBSE was clarified by centrifugation at 9,000 × g for 1 h and filter sterilized through 0.22-μm-pore-size filters. The protein concentration was estimated by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.) as specified by the manufacturer.
Purified protein derivative prepared from M. bovis (PPDb) was obtained from Central Veterinary Laboratory (Weybridge, United Kingdom).
Experimental animals.
Castrated Friesian-cross males approximately 6 months of age were obtained from herds with no history of tuberculosis for at least 5 years. During the study, all the animals were fed normal diets. Four animals selected for infection were housed in a high-security isolation house under negative pressure with expelled air filtered through absolute filters. Animals 01, 02, 84, and 97 were infected by intranasal instillation of 106 CFU of M. bovis as previously described (31). Noninfected animals 6063 and 2701 were housed in normal farm boxes and were used as controls for the experiments. Blood samples used for proliferation and IFN-γ production experiments were obtained at 50 to 73 weeks postinfection.
Lymphocyte proliferation assay for PBMC.
Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood over Ficoll-Histopaque gradients (Sigma) and resuspended in culture medium (RPMI 1640 [BioWhittaker UK Ltd.] containing 10 mM HEPES buffer [Gibco], 2 mM glutamine [Gibco], and 10% [vol/vol] fetal calf serum [Difco]) as described previously (38). Antigens were added at optimal concentrations (M. bovis, 106 CFU/ml; MBSE and PPDb, 4 μg/ml) to triplicate wells containing 2 × 105 PBMC each in 200 μl of medium. Concanavalin A (4 μg/ml) (Sigma Chemical Co., Poole, United Kingdom) was added to triplicate wells as a positive control for cell viability; for each experiment, three wells remained unstimulated as negative controls. The cultures were incubated at 37°C in 6% CO2 for different periods (1.5, 3.5, 4.5, 6.5, and 7.5 days), and for the final 16 h each well was pulsed with 0.25 μCi of [methyl-3H]thymidine (Amersham International, Little Chalfont, United Kingdom). Incorporated radioactivity was determined as counts per minute (cpm) by liquid scintillation counting with a Wallac 1205 Betaplate counter. The results are expressed as cpm or as stimulation indices, where the stimulation index is defined as (mean cpm of stimulated wells)/(mean cpm of control wells).
Analysis of CD8+-T-cell activation in short-term cultures by flow cytometry.
Aliquots of 2 × 106 PBMC in 1 ml of medium were incubated with or without antigen in 24-well tissue culture plates at 37°C in the presence of 6% CO2. Antigens were added to multiple wells as follows: M. bovis was added at 106 CFU/ml, and MBSE, PPDb, and concanavalin A were added at 4 μg/ml. Control cultures (no antigen) were also established for each time point. On days 1.5, 3.5, 4.5, 6.5, and 7.5, PBMC cultures were harvested and washed once with PBS. The cells were incubated for 30 min at 4°C with optimal concentrations (in RPMI 1640 plus 10% normal rabbit serum) of both monoclonal antibodies CC63 (mouse immunoglobulin G2a [IgG2a]) specific for BoCD8 (16) and CACT 116A (mouse IgG1) specific for cells expressing the low-affinity receptor for IL-2 (IL-2R; BoCD25) (27). After the cells were washed twice with PBS–0.1% NaN3, positive cells were identified by using a mixture of fluorescein isothiocyanate-conjugated goat anti-mouse IgG2a, and phycoerythrin-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates Inc., Birmingham, Ala.). After a further 30-min incubation at 4°C, the cells were washed and fixed in 1% paraformaldehyde in PBS before analysis.
Flow cytometry analysis was performed with a Vantage fluorescence-activated cell sorter (FACS) (Becton Dickinson, La Jolla, Calif.) equipped with an Innova Enterprise ion laser (Coherent Laser Group, San Jose, Calif.). The lymphocyte population was identified on the basis of forward and orthogonal light scatter and gated appropriately. The log integral fluorescence was measured for the gated population, and 5,000 cells were counted for each sample. The percentage of positively stained cells was determined with reference to control aliquots of cells for which the primary antibodies had been omitted.
Magnetic cell sorting of T-cell subpopulations.
CD8+ and CD4+ T cells were positively selected from freshly isolated PBMC by using the magnetically activated cell sorting system (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). Pelleted aliquots of 2 × 107 PBMC were resuspended in 200 μl of the primary monoclonal antibody (CC63 or CC8 [specific for BoCD4] [16]) at optimal dilutions (in PBS–10% normal rabbit serum) for 25 min at 4°C. The cells were then washed with PBS, resuspended in 80 μl of MACS flow (2 parts of PBS, 1 part of FACS flow (Becton Dickinson), 1% bovine serum albumin [Sigma]) and incubated with 4 μl of goat anti-mouse IgG magnetic microbeads (Miltenyi Biotec) for 15 min at 4°C. Positive cells bound by MACS microbeads were separated on LS+ separation columns attached to Midi Magnets as specified by the manufacturer. Two aliquots of 2 × 107 cells were loaded in each column, and MACS flow was used as washing and elution buffer. The final cell population was resuspended in complete medium, and cell viability was checked by trypan blue exclusion. Cell purity was assessed by flow cytometry after staining with the appropriate monoclonal antibodies and was always found to be greater than 95%.
Production and phenotyping of CD8+-T-cell clones.
Antigen (MBSE)-specific T-cell lines were established from animal 02 at 30 weeks postinfection by methods described previously (25). Briefly, 20-ml cultures of PBMC (106 cells/ml) were stimulated with MBSE (4 μg/ml) and incubated for 14 days in 75-cm2 tissue culture flasks. Subsequently, viable cells were resuspended at 2 × 105 cells/ml with MBSE and 10 IU of recombinant human IL-2 (rhIL-2; Sigma) per ml. Autologous antigen-presenting cells (APC) were prepared from freshly isolated PBMC by treatment of 107 cell aliquots with mitomycin C (Sigma) for 35 min at 37°C at a final concentration of 25 μg/ml. After this treatment, the cells were washed three times in 20 ml of PBS, resuspended in complete medium (as described above for proliferation assay), counted by trypan blue exclusion, and used on the same day. APC were added to the T-cell lines at 5 × 105/ml. These T-cell lines were maintained by the addition of fresh APC, MBSE, and rhIL-2 at 7-day intervals.
T-cell clones were produced by limiting dilution after the lines became established. T cells were seeded into 96-well microtiter plates to allow, on average, one cell in alternate wells, along with MBSE, rhIL-2, and 105 APC/well. Proliferating clones were expanded into 24-well plates (Costar) for functional assays and phenotyping.
The phenotype of each T-cell clone was determined by flow cytometry, using indirect staining with monoclonal antibodies CC8, CC63, or CC15 (specific for WC1/γδ T cells) (16) in conjunction with monoclonal antibody CACT 116A. The combined detection of the phenotype and activation markers enabled the detection of active growing clonal cells, thus minimizing the interference of the APC present in the culture. Prior to any proliferation or IFN-γ release experiments, the clones were harvested, washed in PBS, and held for 24 h in the absence of antigen or APC.
Proliferation assays for sorted T cells and T-cell clones.
Microtiter cultures were established with 2.5 × 105 CD8+ or CD4+ T cells and 2 × 105 autologous mitomycin C-prepared APC in 200 μl of medium. Triplicate wells were set up for the antigens as well as for negative controls. Cultures were incubated for 5 days under the same conditions described above for the PBMC. Controls of proliferation for APC were included in each experiment, and the readings were always equivalent to the negative controls for the assay.
Determination of IFN-γ release.
Magnetically sorted cells or T-cell clones and APC were used at the same concentrations described for the proliferation assays. Antigens were added to duplicate microtiter wells at the concentrations given above. Negative (no-antigen) duplicate controls were also established for each experiment. The cultures were incubated for 4 days in 6% CO2 at 37°C, and supernatants were harvested and assayed in duplicate for IFN-γ by enzyme-linked immunosorbent assay (ELISA; CSL). Color development was measured with an ELISA reader (Spectra, STL-Lab Instruments), and the results are expressed as optical densities at 450 nm (OD450). Under the conditions described here and until the saturation level for the ELISA (OD450 = 3.5), the relationship between the IFN-γ concentration and OD450 was linear (data not shown). To exclude a potential interference of IFN-γ produced by APC in response to antigen, these APC (2 × 105 cells/well) were incubated with M. bovis, MBSE, or PPDb and the supernatants of the cultures were tested for IFN-γ release; the levels of IFN-γ detected (OD450) were always <0.8 for cells from animal 01, <0.3 for cells from animal 02, and <0.06 for cells from animals 6063 and 2701.
Inhibition of antigen presentation by chemical treatment.
Brefeldin A (BFA-A) and cytochalasin D (CYT-D) were purchased from Sigma. Freshly mitomycin C-treated APC (2 × 105 cells) were plated into microculture plates and incubated with final concentrations of 1 μg/ml (BFA-A) or 10 μM (CYT-D) for 1 h at 37°C. Antigens were then added at the above concentrations, and the plates were further incubated for 1 h at 37°C. Finally, T-cell clones (2.5 × 105 cells) were added to the corresponding culture wells. The chemicals were present in the media during the total incubation time (5 days) for the proliferation experiments. Results are presented as percent control response as follows: percent control response = (response in the presence of inhibitor [cpm]/response in the absence of inhibitor [cpm]) × 100.
RESULTS
Proliferative responses of PBMC.
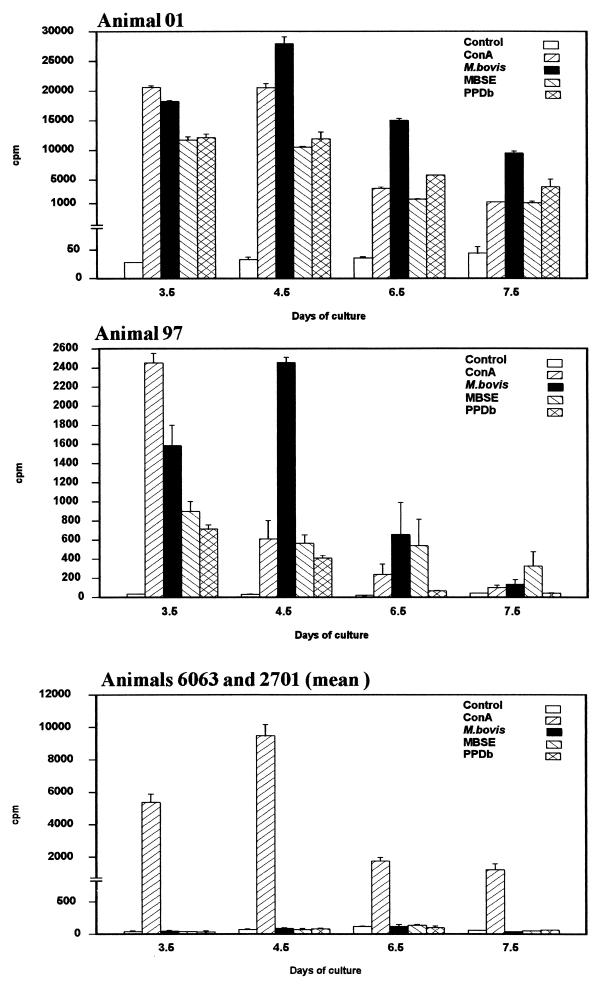
Figure 1 represents the proliferation of PBMC from infected (01 and 97) and non-infected (6063 and 2701) animals after in vitro stimulation with different preparations of mycobacterial antigens (live M. bovis, MBSE, PPDb). At each time point analyzed, proliferation was evaluated by [3H]thymidine incorporation. In general, for animals 01 and 97, the proliferative response to live M. bovis was notably higher than that observed for the soluble antigens at all time points tested and reached its maximum at day 4.5. The responses to MBSE and PPDb were comparable and followed kinetics similar to the response to viable microorganisms. For all the antigens tested, the response diminished after day 6.5. In every case, both infected animals showed a strong response and the stimulation indices were above 2 (the minimum considered to indicate a positive response). No responses to any of the antigens were observed in the noninfected animals at any of the time points.
FIG. 1.
PBMC populations from two experimentally infected animals (01 and 97) and two noninfected controls (6063 and 2701) were stimulated in vitro with M. bovis (106 CFU/ml), MBSE (4 μg/ml), or PPDb (4 μg/ml). Control (no antigen) and concanavalin A (ConA)-stimulated cultures were also included. Results represent lymphocyte proliferation assessed by [methyl-3H]thymidine incorporation at various time points and are expressed as mean counts per minute (cpm) of triplicate values ± standard errors.
Analysis of CD8+-T-cell activation in short-term cultures.
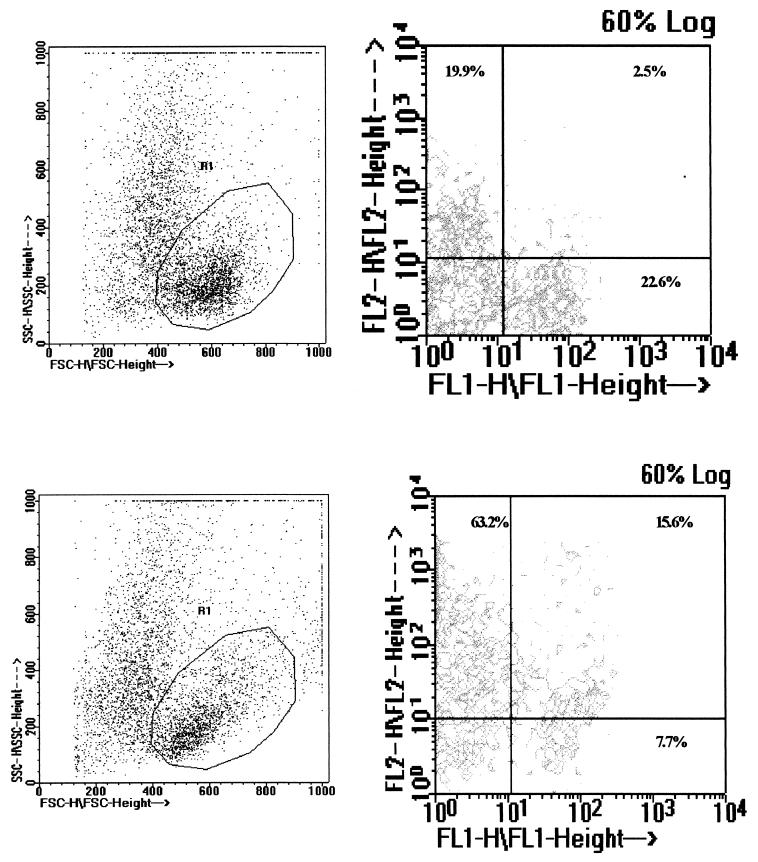
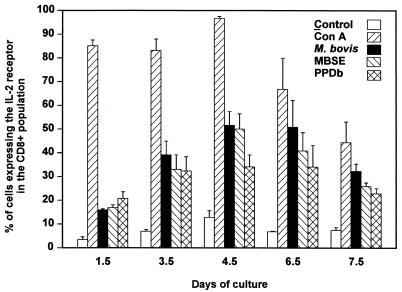
To determine whether live M. bovis was more efficient in generating a response in CD8+-T-cell populations, short-term cultures of PBMC were established, stimulated with M. bovis, MBSE, or PPDb and subsequently analyzed for the degree of CD8+ activation by flow cytometry. Figure 2 shows an example of the gating and FACS analysis of PBMC cultured for 4.5 days in the presence or absence of M. bovis. Figure 3 demonstrates that over a 7.5-day time course, the degree of activation with live M. bovis was only slightly greater than that encountered with the use of soluble antigens. The peak level of activation was found on day 4.5 with all the antigens tested. At this time point, approximately 50% of the CD8+ cells expressed the IL-2R; nonstimulated PBMC had a small population of CD8+ cells (<12%) expressing the IL-2 receptor at all time points. To verify that the IL-2R expression was due to antigen-specific stimulation, the responses of fresh CD8+ T cells sorted from PBMC and of CD8+ T-cell clones were investigated.
FIG. 2.
Flow cytometric analysis of CD8+-T-cell activation. PBMC from animal 01 were cultured with PBS (top) or M. bovis (bottom) and stained with monoclonal antibodies to the surface molecules CD8 (FL1 signal) and IL-2R (FL2 signal) on day 4.5, and viable cells were selected on forward scatter (FSC) versus side scatter (SSC) dot plots (left). Dual-fluorescence contour diagrams for the gated population (R1) (right) were used to assess the percentage of cells positive for both markers. The percentage in the upper right quadrant represents activated (IL-2R+) CD8+ T cells.
FIG. 3.
Kinetics of activation of CD8+ T cells. PBMC from M. bovis-infected animals (01, 84, and 97) were stimulated in vitro with live M. bovis (106 CFU/ml), MBSE (4 μg/ml), or PPDb (4 μg/ml) for different periods (1.5, 3.5, 4.5, 6.5, and 7.5 days). Control (no-antigen) and concanavalin A (Con A)-stimulated cultures were also included. Activation of CD8+ T cells within the short-term cultures was determined by staining for CD8 and IL-2R at each of the time points and analyzed by flow cytometry. Results are representative of two experiments and are expressed as the mean value from the three animals ± standard error.
Antigen-induced proliferation and IFN-γ production from circulating CD8+ and CD4+ T cells.
Purified T-cell (CD8+ or CD4+) populations were selected from PBMC from animals 01, 02, 6063, and 2701 by MACS. Assessment of cell purity by flow cytometry after staining with the appropriate monoclonal antibody always indicated that the purity was above 95%.
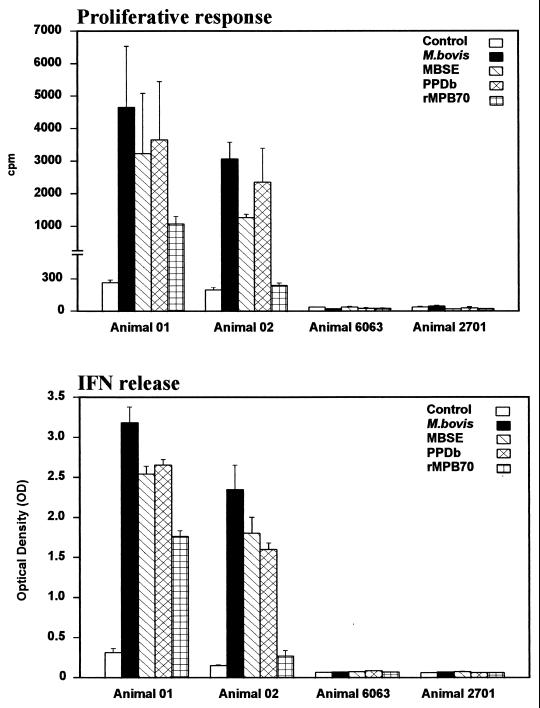
In proliferation and IFN-γ release experiments with CD8+ T cells from animals 01 and 02, stimulation with live M. bovis elicited the highest response in both types of assays (Fig. 4). Both MBSE and PPDb also induced considerable levels of proliferation, with stimulation indices greater than 6. IFN-γ production was also elicited by stimulation with both preparations of soluble antigens to levels well above the values for the controls. These results showed that CD8+ T cells respond powerfully to both live M. bovis and complex soluble antigens in the model used for these experiments. Responses to recombinant protein MPB70 (26) were investigated for comparison and were always lower than those achieved with the previous antigenic preparations. No proliferative or IFN-γ responses were detected with sorted cells from control animals 6063 and 2701.
FIG. 4.
Proliferation (top) and IFN-γ production (bottom) of MACS-separated CD8+ T cells in response to M. bovis (106 CFU/ml), MBSE, PPDb, and rMPB70 (4 μg/ml). The top panel shows the responses as cpm following [methyl-3H]thymidine incorporation. The bottom panel indicates the IFN-γ production assessed by ELISA and presented as OD450. Results are shown for experimentally infected animals 01 (two experiments) and 02 (three experiments) and for two noninfected control animals (animals 6063 and 2701) and are expressed as mean and standard error. The proliferation (cpm) obtained for APC alone in all the animals were similar to the control values. The OD450 obtained for APC alone were <0.8 for animal 01, <0.3 for animal 02, and <0.06 for animals 6063 and 2701.
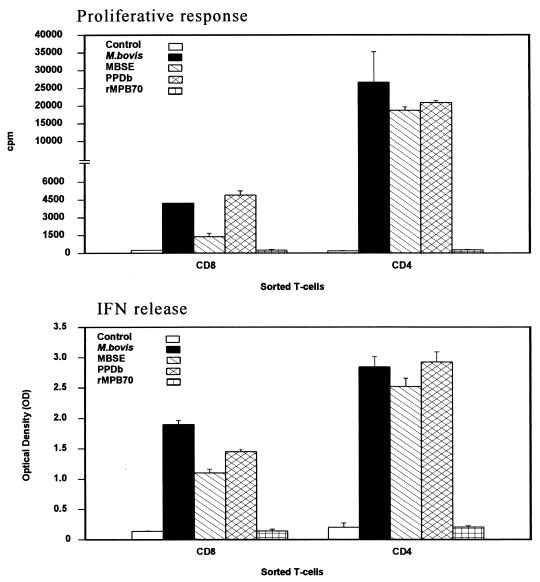
To assess the importance of the IFN-γ production in CD8+ T cells, an experiment was established with freshly sorted CD8+ and CD4+ cells from animal 02. Figure 5 illustrates the responses of both T-cell subsets to the different antigenic preparations. The results show that under these experimental conditions, the CD4+ T cells proliferated at a higher rate than did CD8+ T cells and that this result was consistent for all the antigens tested. The IFN-γ levels detected in the supernatants of CD4+ cultures were also superior to those detected for the CD8+ populations. However, the CD8+ subset also produced a notable amount of the cytokine. Considering the level of IFN-γ detected for both types of cells, this result indicates that CD8+ T cells have an important capacity to produce IFN-γ in response to mycobacterial antigens.
FIG. 5.
Comparison of the proliferative responses (top) and IFN-γ production (bottom) of CD8+ and CD4+ MACS-sorted T cells from animal 02 in response to M. bovis (106 CFU/ml), MBSE, PPDb, and rMPB70 (4 μg/ml). The top panel shows the responses as cpm following [methyl-3H]thymidine incorporation. The bottom panel indicates the IFN-γ production as assessed by ELISA and presented as OD450. The proliferative responses obtained for APC alone were similar to the control value. The OD450 values obtained for APC alone were <0.2.
T-cell cloning and responses to antigen by CD8+ clones.
Table 1 summarizes the panel of clones established at 30 weeks postinfection for animal 02 with MBSE as the antigen. Maintenance of the clones was always dependent on the presence of MBSE and APC. A total of 18 clones were obtained and phenotyped by FACS analysis. The CD8+ phenotype was the most prevalent; a total of 14 CD8+-T-cell clones and 4 CD4+-T-cell clones were isolated from this cloning experiment. The clones were not 100% pure due to interference by a small number of nonviable APC within the analysis gates.
TABLE 1.
Phenotypes of T-cell clones isolated at 30 weeks postinfection from animal 02a
| CLONE | % of cells staining with monoclonal antibody:
|
|
|---|---|---|
| CC8 (anti-CD4) | CC63 (anti-CD8) | |
| 2A3 | 4.1 | 95 |
| 2A5 | 0.5 | 98.4 |
| 2A6 | 3 | 99.4 |
| 2C4 | 1.6 | 96.8 |
| 2D3 | 0.8 | 95 |
| 2F4 | 2.1 | 95.4 |
| 2F7 | 1.2 | 99.3 |
| 2F11 | 5 | 96.1 |
| 2F12 | 0.6 | 98 |
| 2G3 | 13.2 | 92.9 |
| 2G12 | 1.1 | 91 |
| 2H3 | 0.4 | 95.7 |
| 2H6 | 2 | 94.9 |
| 2H7 | 1.1 | 96 |
| 2C9 | 89.9 | 5 |
| 2E1 | 99.2 | 1.1 |
| 2E6 | 99 | 0.9 |
| 2E12 | 70 | 5.7 |
Results are expressed as the percentage of positive cells after analysis by flow cytometry.
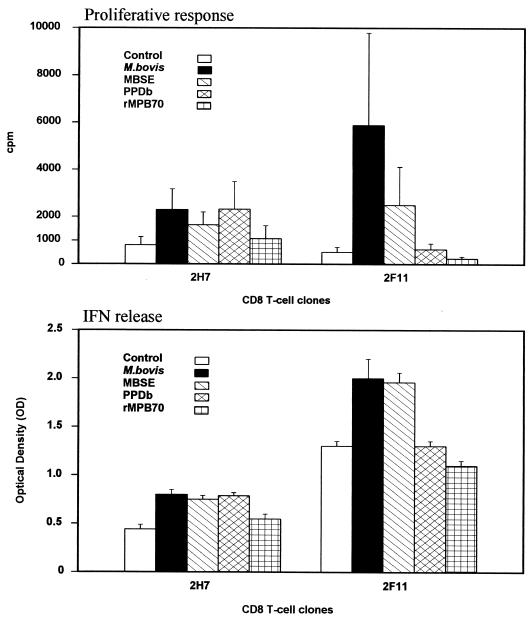
To investigate the nature of the responses within CD8+ clones, the proliferative responses and the IFN-γ secretion of two clones (2H7 and 2F11) in response to stimulation with different antigenic preparations (M. bovis, MBSE, PPDb, and rMPB70) were assessed. The results from a series of experiments are shown in Fig. 6. These data demonstrate that CD8+-T-cell clones were able to proliferate after stimulation with either complex soluble antigens or M. bovis; the stimulation indices were generally greater than 2. Comparable effects were seen for secretion of IFN-γ, indicating the ability of restimulated CD8+-T-cell clones to produce this cytokine in response to antigen. For the rMPB70, a very low response was observed in terms of both proliferation and IFN-γ secretion.
FIG. 6.
Proliferation (top) and IFN-γ production (bottom) of CD8+ T-cell clones in response to M. bovis (106 CFU/ml), MBSE, PPDb, and rMPB70 (4 μg/ml). The top panel shows the responses as cpm following [methyl-3H]thymidine incorporation. The bottom panel indicates the IFN-γ production assessed by ELISA and presented as OD450. Results are mean values of three experiments and standard error. For proliferation, the values obtained for APC alone were similar to the controls. The IFN-γ OD450 values obtained for APC alone were <0.3.
Metabolic inhibition of antigen presentation.
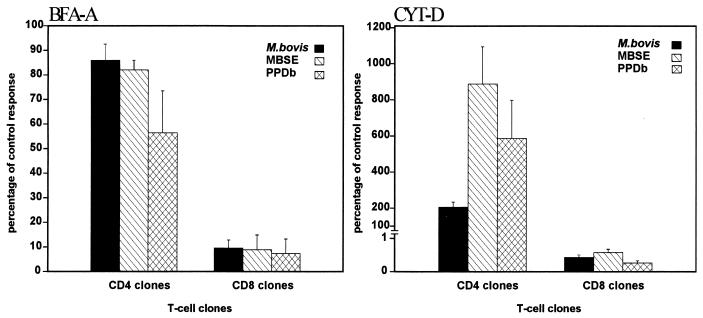
To define the mode of antigen processing required to present the different antigens, two metabolic inhibitors were used in blocking experiments with two CD8+ and two CD4+ T-cell clones: BFA-A, which blocks the egress of newly synthesized proteins from pre-Golgi compartments, causing them to recycle back to the endoplasmic reticulum, and CYT-D, which is a potent inhibitor of microtubule assembly and therefore of some types of phagocytosis. As shown in Fig. 7, CYT-D completely prevented the presentation of all antigens tested to CD8+-T-cell clones (percentage with respect to the control response, <1%). These data indicated that the processing and presentation of both types of preparations (soluble and live) required phagocytosis. In contrast, the same concentration of CYT-D did not inhibit the proliferation of CD4+-T-cell clones. Moreover, an enormous increase of the proliferative response to the antigens was found to occur within these clones as a result of treatment with this chemical.
FIG. 7.
Effect of BFA-A (left) and CYT-D (right) on the presentation of M. bovis, MBSE, and PPDb. Autologous mitomycin C-treated PBMC were used as APC. The APC were treated with BFA-A (1 μg/ml) or CYT-D (10 μM) for 1 h at 37°C. The different antigens were then added to the cultures, which were further incubated for 1 h. Finally, T-cell clones (CD8+ or CD4+) were added to the appropriate wells. Results are presented as percentages of the control response, i.e., (response in the presence of chemical [cpm]/response in absence of chemical [cpm]) × 100, and are representative of two repeated experiments with CD8+ (2H7 and 2F11) and CD4+ (2E1 and 2E6) T-cell clones.
The data presented in Fig. 7 suggest that although BFA-A acted as a potent inhibitor of the proliferative response in the CD8+-T-cell clones, the same treatment did not significantly inhibit the presentation by the same APC of the same antigens to CD4+-T-cell clones, showing that the MHC class II pathway was not significantly affected. Altogether, the results of blocking with BFA-A indicated that an endogenous pathway of antigen processing is required for the presentation to the CD8+ clones. Although further experiments are needed, including specific blocking with appropriate antibodies, it seems most probable that soluble antigens were presented by using a classical MHC class I pathway (the newly synthesized MHC class I molecules are required in the presentation to these CD8+-T-cell clones of these exogenously added antigens).
DISCUSSION
In the present study, we assessed the proliferation of PBMC induced by different kinds of mycobacterial antigen preparations in naive and M. bovis-infected animals. In the infected animals, the higher proliferative response to live bacteria than to soluble antigens may have been because viable microorganisms represent a continuous and good source of early secreted antigens produced by the bacilli multiplying inside mononuclear phagocytes. Also, this production and release of different antigens by M. bovis may involve a wider range of T-cell subsets and T-cell clones in the response. For example, it has been generally accepted that live infections are more likely to involve MHC class I responses, leading to the activation of CD8+ T cells. The response achieved with MBSE or PPDb was also important, suggesting that somatic and cytoplasmic antigens in MBSE, as well as proteinaceous antigens in PPDb, are relevant in the response to mycobacteria. The difference in the level of response in the two infected animals discussed is due to individual variation in the immune response, but in both cases the stimulation indices were sufficient to consider them responders. Also, both infected animals showed the same pattern of response in the time course.
To ascertain the requirement for live mycobacteria to elicit CD8+-T-cell responses, we proceeded to study the activation of CD8+ subsets in a bulk culture of PBMC by the use of different antigenic preparations (M. bovis, MBSE, and PPDb). In general, it is believed that proteinateous antigens added exogenously elicit primarily CD4+-T-cell responses, which are MHC class II restricted. However, recent reports have demonstrated that APC can acquire exogenous antigens by phagocytosis and present them to CD8+ T cells in the context of MHC class I molecules (13, 41, 42). Some of these reports have proposed the existence of a subset of APC located throughout the lymphoid tissues with this specialized function. Our study shows that the level of activation (IL-2R expression) in CD8+ cells is very similar with the three antigenic preparations tested, suggesting that although M. bovis activated a slightly greater percentage of cells, both MBSE and PPDb also led to a considerable degree of activation of CD8+ T cells. These results indicate that soluble mycobacterial antigens can be presented to CD8+ T cells, possibly in association with class I MHC molecules. To prove the antigen specificity of the activation of this subpopulation, we demonstrated that magnetically sorted CD8+ cells from animals 01 and 02 proliferate after stimulation with M. bovis, MBSE, or PPDb. As with PBMC, the proliferation was superior with live M. bovis but a considerable response was achieved with the use of soluble preparations. Around 20% of the total PBMC population expressed IL-2R in the control (no-antigen) cultures; the reason for this result was probably that a considerable percentage of the bovine gamma-delta WC1+ T cells constitutively express this marker even in naive animals (6).
Some studies have reported that cell debris or associated soluble protein elicits cytolytic activity, indicating that the cell membrane rather than the intact cells may be important in the priming of the MHC class I responses (3, 8). Furthermore, inclusion of lipid components into the immunogen formulations could be critical in the cytosolic delivery of antigen. It has been demonstrated that proteins conjugated to lipid molecules can enter the cytosolic compartment of cells (19). Other studies have shown that association of antigen with liposomes targets the antigen to the appropriate APC and/or cellular compartments to induce a MHC class I response (49). Both MBSE and PPD are complex antigen preparations (11) that include not only a large number of different proteins but also nonprotein constituents such as lipoarabinomannan, other phospholipids, and complex carbohydrates, which may influence antigen processing in endosomal compartments.
MHC class I-restricted T cells may be an important population for protection against tuberculosis, although the nature of the protective antigens and the mechanisms by which vacuolar antigens can gain access to the cytoplasm are poorly understood (29). It has been suggested that in some cases, exogenous antigens can escape from phagosomes into the cytosol while intact antigenic particles or intravacuolar bacteria are not directly released into the cytosol and must undergo vacuolar processing to some degree. It is theoretically possible that proteins or peptides generated by vacuolar processing gain access to the cytosol and (with or without additional proteolysis) are subsequently transported into the endoplasmic reticulum to bind nascent MHC class I molecules (15).
The results of the present study with both purified CD8+ cells and CD8+ clones showed that this subset releases IFN-γ specifically in response to restimulation with antigen. Also, it was apparent that the response was greater with live M. bovis, possibly due to the preferential involvement of the MHC class I pathway. However, the responses to soluble antigens were also notable. These data indicate that the CD8+ subset may contribute substantially to the immune response via IFN-γ production. Other authors have found similar results in different models. Su et al. (44) performed intracellular cytokine expression analysis and showed that CD8+ T cells are important IFN-γ producers in the response to viral infections. Breen et al. (5) also proved that in human immunodeficiency virus infection there is an important expression or secretion of IFN-γ in stimulated or cloned peripheral CD8+ cells. The recent literature has reported evidence that in response to tuberculosis infection, CD8+ T cells may play a protective role via several mechanisms, (i) by producing cytokines (IFN-γ and tumor necrosis factor alpha) which are potent macrophage activators, and (ii) by means of the release of granular constituents that promote the destruction of infected cells (4). We did not perform cytotoxicity experiments and cannot conclude if IFN-γ release is the predominant effector mechanism for these cells or if the classical role of cytolysis is the main instrument by which CD8+ cells exert their activity in cattle. However, it seems accepted that CD8+ T cells may contribute their antimycobacterial activity through noncytotoxic pathways. Tascon et al. (46) have recently shown the role of CD8+ cells in IFN-γ production and suggested that such cells may act through classical cytokine-mediated macrophage activation rather than through a cytotoxic mechanism. Also, recent data obtained with perforin and granzyme gene knockout mice has suggested that the role of CD8+ T cells in controlling M. tuberculosis infection may rely on mechanisms such as cytokine secretion, not only on their lytic activity (7, 23).
In an attempt to determine the importance of the CD8+ T cells in the infection, we established a panel of T-cell clones from PBMC collected 30 weeks postinfection. MBSE was chosen as the antigen since CD8+-T-cell clones had resulted from this strategy in previous experiments (39). The present panel included a majority of CD8+-T-cell clones, which was consistent with the previous study, in which the progression of experimental bovine tuberculosis was associated with an increased importance of the CD8+ response (39). Although variation in the level of responses was found between the CD8+ T-cell clones, experiments demonstrated their ability to proliferate and release IFN-γ in response to restimulation with M. bovis, MBSE, or PPDb.
To clarify the pathway used for the antigen presentation to CD8+ clones, we performed several chemical inhibition experiments. Since CYT-D (an inhibitor of microtubule assembly) completely prevented presentation of all three types of antigen to CD8+-T-cell clones, it was concluded that mere attachment of antigen to the APC surface was not sufficient for presentation of antigen via the MHC class I processing pathway and that phagocytosis is necessary for eliciting a CD8+ response. Interestingly, treatment of the APC with the same concentration of CYT-D caused an elevated proliferative response within the CD4+ clones. It has been described previously that when added to T cells, low concentrations of cytochalasins enhance the proliferative response to both antigen or mitogen (28). Cytochalasins act by augmenting early events in T-cell activation, including increasing cellular cyclic AMP levels, intracellular Ca2+ influx, and turnover of phosphatidylinositol after ligand-receptor interactions. The results of this study indicate that CYT-D alone was not capable of activating the signal transduction pathways in the absence of antigen but that when receptor-ligand interactions did occur, CYT-D may have acted in synergism, boosting the proliferative response. Importantly, the increase in CD4+ proliferation was considerably higher with soluble antigens than with live M. bovis. This could be explained by activation of the CD4+ clones by direct binding of peptide antigens (largely present in MBSE and PPDb) to the MHC class II molecules. This finding could also be related to enhanced exocytosis and therefore to further availability of antigen. In a recent study (20), it was reported that pretreatment of monocytes with CYT-D inhibited the proliferation of CD4+ T cells against M. tuberculosis but not against soluble antigens. However, those authors did not report a synergism between the chemical and the antigen, possibly because CYT-D was removed by washing after treatment of the APC in their model. In the method described in our study (continuous presence of the drug), only CD8+-T-cell clones were inhibited by CYT-D treatment of APC.
When BFA-A was used in inhibition experiments, the results suggested that for the CD8+-T-cell clones tested, an endogenous pathway of antigen processing was required for the presentation of both live and exogenously added soluble antigens. Although we do not have definitive evidence, the results suggest that presentation is achieved via a classical MHC class I pathway. In agreement with our results, Kovacsovics-Bankowski et al. (21) reported that presentation of exogenous particulate antigen through class I processing pathways requires Golgi-to-endoplasmic reticulum (ER) transport. Also, Yee et al. (48) demonstrated that presentation of an exogenous polypeptide antigen from the murine AIDS virus to CD8+ clones was possible and that the proliferative response was inhibited by BFA-A and therefore was MHC class I restricted. However, in contrast to our results, Pfeifer et al. (37) found that BFA-A had little effect on the processing of bacterial peptide antigens for MHC class I and that presentation of such antigens to CD8+ cells occurred through a nonclassical pathway. Also, following experiments with BFA-A and M. tuberculosis-reactive CD8+-T-cell clones, Lewinsohn et al. (24) concluded that proteasomally derived peptides did not require Golgi-to-ER transport and therefore were not conventional MHC class I-restricted antigens. In their study, the antigen presentation to the CD8+ lines was possibly achieved by binding of processed peptides to nonpolymorphic MHC class Ib molecules on the cell surface.
Adorini et al. (1) found that BFA-A greatly inhibits the presentation of exogenous protein antigen by MHC class II molecules to T cells. In our experiments, we did not make the same observation. When the CD4+ clones were used as controls for toxicity of the chemical, their responses were not significantly depressed. It could well be that for CD4+ clones, direct binding of antigen to surface class II molecules without further processing represents a possible mechanism for antigen presentation. Another likely mechanism to be involved in this presentation is recycling of class II molecules from the cell surface, with peptide exchange occurring in an endosomal compartment and therefore with no need for Golgi-ER transport.
This study is the first to highlight the importance of the CD8+ responses in bovine tuberculosis. Our data suggest that CD8+ T cells respond strongly to M. bovis but also to complex soluble mycobacterial antigens which are processed within the APC and presented via an endogenous pathway. Furthermore, since the CD8+ T-cells may play an important role in the protective response against M. bovis, the design of improved vaccines requires a better understanding of these processes and the antigens which drive them.
ACKNOWLEDGMENTS
This work was performed entirely in the Department of Veterinary Sciences (Queen’s University of Belfast) and was supported the Fundación Ramón Areces, European Contract ERBFMBICT972853, and funds from DANI.
REFERENCES
- 1.Adorini L, Ullrich S J, Appella S, Fuchs S. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II restricted cells. Nature. 1990;346:63–66. doi: 10.1038/346063a0. [DOI] [PubMed] [Google Scholar]
- 2.Berggren S A. Field experiment with BCG vaccine in Malawi. Br Vet J. 1981;137:88–96. doi: 10.1016/s0007-1935(17)31792-x. [DOI] [PubMed] [Google Scholar]
- 3.Bevan M J. Antigen recognition. Class discrimination in the world of immunology. Nature. 1987;325:192–194. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- 4.Boom W H. The role of T cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis. 1996;5:73–81. [PubMed] [Google Scholar]
- 5.Breen E C, Salazar-Gonzalez J F, Shen L P, Kolberg J A, Urdea M S, Martinez-Maza O, Fahey J L. Circulating CD8+ T cells show increased interferon-γ m-RNA expression in HIV infection. Cell Immunol. 1997;178:91–98. doi: 10.1006/cimm.1997.1115. [DOI] [PubMed] [Google Scholar]
- 6.Collins R A, Sopp P, Gelder K I, Morrison W I, Howard C J. Bovine γ/δ TcR+ T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. Scand J Immunol. 1996;44:444–452. doi: 10.1046/j.1365-3083.1996.d01-332.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russel D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debrick J E, Campbell P A, Staerz U D. Macrophages as accessory cells for class I MHC restricted immune responses. J Immunol. 1991;147:2846–2851. [PubMed] [Google Scholar]
- 9.De Libero G, Flesh I, Kaufmann S H E. Mycobacteria reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18:59–64. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 10.Denis O, Lozes E, Huygen K. Induction of cytotoxic responses against culture filtrate antigens in Mycobacterium bovis BCG-infected mice. Infect Immun. 1997;65:676–684. doi: 10.1128/iai.65.2.676-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desbordes J, Coniver L, Prevot A. Recherches sur les lipides microbiens. Essays d’identification des lipides de quelques produits d’origine biologique. Ann Pharm Fr. 1972;7–8:507–518. [PubMed] [Google Scholar]
- 12.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant E P, Rock K L. MHC class I restricted presentation of exogenous antigen by thymic antigen presenting cells in vitro and in vivo. J Immunol. 1992;148:13–18. [PubMed] [Google Scholar]
- 14.Halverson D C, Schwartz G N, Carter C, Gress R E, Fowler D H. In vitro generation of allospecific human CD8+ T cells of Tc1 and Tc2 phenotype. Blood. 1997;90:2089–2096. [PubMed] [Google Scholar]
- 15.Harding C V, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4934. [PubMed] [Google Scholar]
- 16.Howard C J, Naessens J. Summary of workshop findings for cattle. Vet Immunol Immunopathol. 1993;39:25–48. doi: 10.1016/0165-2427(93)90161-v. [DOI] [PubMed] [Google Scholar]
- 17.Huygen K, Content J, Denis O, Montgomery D L, Yawmann A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann S H E. The roles of conventional and unconventional T cells in antibacterial immunity. ASM News. 1997;63:251–255. [Google Scholar]
- 19.Kavanov A V, Levashov A V, Alakhov V Y. Lipid modification of proteins and their membrane transport. Protein Eng. 1989;3:39–42. doi: 10.1093/protein/3.1.39. [DOI] [PubMed] [Google Scholar]
- 20.Kithiganahalli N B, Boom W H. Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+ αβ and γδ T cells: role of particulate antigen. Infect Immun. 1998;66:98–106. doi: 10.1128/iai.66.1.98-106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock K L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalvani A, Brookes R, Wilkinson R J, Malin A S, Pathan A A, Andersen P, Dockrell H, Pasvol G, Hill A V S. Human cytolytic and interferon gamma secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laochumroonvorapong P, Wang J, Liu C C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewinsohn D M, Alderson M R, Briden A L, Riddell S R, Reed S G, Grabstein K H. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis infected antigen presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lightbody K A, Girvin R M, Pollock D A, Mackie D P, Neill S D, Pollock J M. Recognition of a common mycobacterial T cell epitope in MPB59 of Mycobacterium bovis. Immunology. 1998;93:314–322. doi: 10.1046/j.1365-2567.1998.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lightbody K A, Skuce R A, Neill S D, Pollock J M. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet Rec. 1998;142:295–300. doi: 10.1136/vr.142.12.295. [DOI] [PubMed] [Google Scholar]
- 27.MacHugh N D, Taracha E L, Toye P G. Reactivity of workshop antibodies with COS cell transfectants expressing bovine CD antigens. Vet Immunol Immunopathol. 1993;39:61–67. doi: 10.1016/0165-2427(93)90164-y. [DOI] [PubMed] [Google Scholar]
- 28.Matsuyama T, Yamada A, Deusch K, Sleasman J, Daley J F, Torimoto Y, Abe T. Cytochalasins enhance the proliferation of CD4 cells through the CD3-Ti antigen receptor complex or the CD2 molecule through an effect on early events of activation. J Immunol. 1991;146:3736–3741. [PubMed] [Google Scholar]
- 29.Mazzaccaro R J, Gedde M, Jensen E R, van Santen H M, Ploegh H L, Rock K L, Bloom B R. Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1996;93:11786–11791. doi: 10.1073/pnas.93.21.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller I, Cobbold S P, Waldmann H, Kaufmann S H E. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neill S D, Hanna J, O’Brien J J, McCracken R. Excretion of Mycobacterium bovis by experimantally infected cattle. Vet Rec. 1988;123:340. doi: 10.1136/vr.123.13.340. [DOI] [PubMed] [Google Scholar]
- 32.Newell D G, Hewinson R G. Control of bovine tuberculosis by vaccination. Vet Rec. 1995;136:459–463. doi: 10.1136/vr.136.18.459. [DOI] [PubMed] [Google Scholar]
- 33.Noble A, Macary P A, Kemeny D M. IFN-γ and IL-4 regulate the growth and differentiation of CD8+ T cells into subpopulations with distinct cytokine profiles. J Immunol. 1995;155:2928–2937. [PubMed] [Google Scholar]
- 34.Orme I M, Collins F M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell defficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orme I M. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 36.Pancholi P, Mirza A, Bhardwaj N, Steinman R M. Sequestration from immune CD4+ T cells of Mycobacterium growing in human macrophages. Science. 1993;260:984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer J D, Wick M J, Roberts R L, Findlay K F, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 38.Pollock J M, Douglas A J, Mackie D P, Neill S D. Identification of bovine T cell epitopes for three Mycobacterium bovis antigens: MPB70, 19000 MW and MPB57. Immunology. 1994;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 39.Pollock J M, Pollock D A, Campbell D G, Girvin R M, Crockard A D, Neill S D, Mackie D P. Dynamic changes in circulating and antigen-responsive T cell subpopulations post-Mycobacterium bovis infection in cattle. Immunology. 1996;87:236–241. doi: 10.1046/j.1365-2567.1996.457538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roche W P, Triccas J A, Winter N. BCG vaccination against tuberculosis: past disappointments and future hopes. Trends Microbiol. 1995;3:397–401. doi: 10.1016/s0966-842x(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 41.Rock K L, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990;249:918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 42.Rock K L, Rothstein L, Gamble S, Fleischacker C. Characterization of antigen presenting cells that present exogenous antigens in association with class I MHC molecules. J Immunol. 1993;150:438–446. [PubMed] [Google Scholar]
- 43.Stenger S, Mazzaccaro R J, Uyemura K, Cho S, Barnes P F, Rosat J P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;279:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 44.Su H C, Cousens L P, Fast L D, Slifka M K, Bungiro R D, Ahmed R, Biron C A. CD4+ and CD8+ T cell interactions in IFN-γ and IL-4 responses to viral infections: requirements for IL-2. J Immunol. 1998;160:5007–5017. [PubMed] [Google Scholar]
- 45.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 46.Tascon R E, Stavropoulos E, Lukacs K V, Colston M J. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waddington F G, Ellwood D C. An experiment to challenge the resistance to tuberculosis in BCG vaccinated cattle in Malawi. Br Vet J. 1972;128:541–552. doi: 10.1016/s0007-1935(17)36683-6. [DOI] [PubMed] [Google Scholar]
- 48.Yee S T, Okada Y, Ogasawara K, Omura S, Takatsuki A, Kakiuchi T, Muno D, Kominami E, Mizuochi T. MHC class I presentation of an exogenous polypeptide antigen encoded by the murine AIDS defective virus. Microbiol Immunol. 1997;41:563–570. doi: 10.1111/j.1348-0421.1997.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhou F, Rouse B T, Huang L. Induction of cytotoxic T lymphocytes in vivo with protein antigen entrapped in membranous vehicles. J Immunol. 1992;149:1599–1604. [PubMed] [Google Scholar]
- 50.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordemeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]