Abstract
Background
Acid–base status in full-term pregnant women is characterised by hypocapnic alkalosis. Whether this respiratory alkalosis is primary or consequent to changes in CSF electrolytes is not clear.
Methods
We enrolled third-trimester pregnant women (pregnant group) and healthy, non-pregnant women of childbearing age (controls) undergoing spinal anaesthesia for Caesarean delivery and elective surgery, respectively. Electrolytes, strong ion difference (SID), partial pressure of carbon dioxide (), and pH were measured in simultaneously collected CSF and arterial blood samples.
Results
All pregnant women (20) were hypocapnic, whilst only four (30%) of the controls (13) had an arterial <4.7 kPa (P<0.001). The incidence of hypocapnic alkalosis was higher in the pregnant group (65% vs 8%; P=0.001). The CSF-to-plasma Pco2 difference was significantly higher in pregnant women (1.5 [0.3] vs 1.0 [0.4] kPa; P<0.001), mainly because of a decrease in arterial Pco2 (3.9 [0.3] vs 4.9 [0.5] kPa; P<0.001). Similarly, the CSF-to-plasma difference in SID was less negative in pregnant women (–7.8 [1.4] vs –11.4 [2.3] mM; P<0.001), mainly because of a decreased arterial SID (31.5 [1.2] vs 36.1 [1.9] mM; P<0.001). The major determinant of the reduced plasma SID of pregnant women was a relative increase in plasma chloride compared with sodium.
Conclusions
Primary hypocapnic alkalosis characterises third-trimester pregnant women leading to chronic acid–base adaptations of CSF and plasma. The compensatory SID reduction, mainly sustained by an increase in chloride concentration, is more pronounced in plasma than in CSF, as the decrease in Pco2 is more marked in this compartment.
Clinical trial registration
Keywords: acid–base imbalance, cerebrospinal fluid, hyperventilation, hypocapnic alkalosis, metabolic acidosis, pregnancy, respiratory alkalosis, respiratory physiology, water–electrolyte imbalance
Editor's key points.
-
•
It is unclear whether hypocapnic alkalosis in full-term pregnant women is primary or secondary to changes in CSF electrolytes.
-
•
Simultaneously collected CSF and arterial blood samples from third-trimester pregnant women and healthy non-pregnant women of childbearing age undergoing spinal anaesthesia for Caesarean delivery or elective surgery were analysed.
-
•
Hypocapnic alkalosis was more common in the pregnant (65%) than the control (8%) group.
-
•
The primary hypocapnic alkalosis that characterises pregnancy, likely because of progesterone, leads to a compensatory reduction in strong ion difference (metabolic acidosis) more pronounced in plasma than in CSF.
Cerebrospinal fluid (CSF) is a clear fluid produced by the choroid plexus,1 the acid–base regulation of which is of paramount importance in the control of spontaneous breathing.2 According to Stewart's physico-chemical approach, only two variables regulate the acid–base equilibrium in this extracellular compartment: the partial pressure of carbon dioxide (Pco2) and the difference between strong cation and anion concentrations (strong ion difference [SID]).3 A third independent variable that must also be considered in evaluating the acid–base status of blood is the weak non-volatile acids (ATOT).
By the first 7 weeks of gestation, resting spontaneously breathing pregnant women increase their minute ventilation, progressively developing hypocapnia and respiratory alkalosis.4, 5, 6 Several studies have addressed the possible roles of progesterone and arginine–vasopressin (AVP) in ventilatory regulation in pregnancy.6, 7, 8 For instance, progesterone acts on central chemoreceptors, increasing minute ventilation independently from CSF pH,9 and acts on peripheral chemoreceptors, inducing a hypoxic respiratory response at higher partial pressure of oxygen (Po2).10 Additionally, AVP induces water reabsorption, decreasing osmolality and SID of extracellular fluids.6,11 The reduction in SID and plasma osmolality is associated with increased minute ventilation in humans,12 particularly during pregnancy.13 Although multiple mechanisms have been hypothesised and tested, the relative contributions of different mechanisms underlying this physiological respiratory response have not been addressed. Moreover, previous studies rarely relied on human blood and CSF sampled simultaneously.
The aims of the present study were to (i) describe, applying the physico-chemical approach to acid–base status, the characteristics of CSF and arterial plasma in resting spontaneously breathing third-trimester pregnant women; (ii) compare these results with healthy non-pregnant women of childbearing age; and (iii) discuss the physiological adaptations to hypocapnic alkalosis (HA) in pregnancy.
Methods
The study protocol and the informed consent process were approved by the ethics committee (Milano Area 2; protocol number: 797_2017bis), and written informed consent was obtained from all enrolled patients (Clinical trial registration: NCT03496311).
Study design
Adult healthy pregnant women (pregnant group) with gestational age ≥35 weeks and scheduled for elective Caesarean delivery and healthy fertile non-pregnant women (control group) scheduled for minor elective surgery under spinal anaesthesia at the IRCCS Ca’ Granda Policlinico (Milan, Italy) were enrolled. For both groups, exclusion criteria were ASA physical status >2; age <18 yr; or active brain, lung, or kidney disease. Twin pregnancy was an additional exclusion criterion for the pregnant group.
A CSF sample of 2 ml was drawn anaerobically (avoiding contact with air) directly from the needle used for spinal anaesthesia before local anaesthetic injection. Immediately after that, an arterial blood sample was collected from a radial artery, and a venous blood sample was drawn. Anaesthesiologists were asked to limit i.v. fluids before spinal anaesthesia and choose balanced solutions to avoid i.v. fluid-related acid–base derangement.14 The following clinical data were recorded: anthropometric parameters, medical history, vital signs, and fluids administered on the study day.
Analysis of CSF and blood
Acid–base and electrolyte compositions of CSF and arterial blood samples were immediately measured (ABL800 FLEX; Radiometer, Copenhagen, Denmark). Magnesium, phosphate, and albumin (cobas c 702; Roche Diagnostics GmbH, Mannheim, Germany); haemoglobin concentration; and red and white blood cell counts (XN-9000 V; Sysmex Corporation, Kobe, Japan) were analysed for CSF and blood samples. For both plasma and CSF, osmolality (Osmometer MIR 300-P; E. Mires, Milan, Italy), progesterone (cobas e 801, Roche Diagnostics GmbH), and copeptin concentrations (B·R·A·H·M·S™ Copeptin proAVP KRYPTOR™ assay; Hennigsdorf/Berlin, Germany) were measured. We chose to measure copeptin as a proxy for AVP, as it is more stable in blood at room temperature.
For each CSF and blood sample, the SID was calculated as described in Equation (1):
| (1) |
where Lac– is lactate, all expressed as mM. CSF bicarbonate concentration was computed using a solubility constant for carbon dioxide of 0.234 mM kPa−1 and a pK1 for carbonic acid of 6.130.15 Albumin and phosphate concentrations were used to calculate ATOT.16 The differences between CSF and arterial SID (ΔSID) and CSF and arterial Pco2 (ΔPco2) were computed.17 The CSF:plasma ratio of the concentrations of sodium and chloride was calculated, and the expected chloride concentration () resulting from blood dilution was estimated as
| (2) |
where and were the average values of plasma chloride and sodium, respectively, measured in controls, and was the actual plasma sodium concentration measured for each pregnant woman. Average values of plasma sodium and chloride of controls were chosen as a reference, as they were measured by the same method and are representative of a population of the same sex and similar age. The same calculation was applied to CSF values to estimate expected CSF chloride concentration.
The percentage of SID variation related to renal chloride reabsorption and choroid plexus chloride secretion was calculated (both for arterial plasma and CSF) as
| (3) |
where was the average arterial or CSF SID of the controls.
Clinical definition of hypocapnia and HA
Arterial Pco2 <4.7 kPa (<35 mm Hg) defined hypocapnia, whilst the simultaneous presence of hypocapnia and arterial pH >7.45 defined HA. The pregnant group was divided into patients with HA and patients without HA (non-HA).
Sample size and statistical analysis
We calculated that a sample of 40 subjects (20 subjects per group) would provide a statistical power of 0.95 with an alpha error of 0.05 to detect a difference in arterial SID of 3 mM, assuming a standard deviation (sd) of 2.5 mM using a two-tailed Student's t-test.
Comparison between continuous variables was performed via Student's t-test or Wilcoxon rank-sum test, as appropriate. Differences between categorical variables were assessed using the χ2 test. The relationship between quantitative variables was investigated using linear regression. Differences between expected and measured chloride concentrations were tested using paired t-test. If multiple regression analysis was performed, we reported the adjusted r2. Unless otherwise stated, data are expressed as mean (sd) or median (inter-quartile range). Statistical significance was defined as P<0.05. Analysis was performed with Stata statistical software (Stata Statistical Software, Release 16; StataCorp, College Station, TX, USA) and SigmaPlot version 12.0 (SYSTAT Software; San Jose, CA, USA). The Strengthening the Reporting of Observational Studies in Epidemiology checklist was used to guide reporting of this observational study.
Results
The study population was enrolled between April 2018 and April 2019. Twenty pregnant women (age 37 [6] yr; pre-pregnancy BMI: 22 [3] kg m−2; week of gestation 38 [1]) were studied. Because of the slow recruitment of controls, the study was interrupted after enrolment of 13 non-pregnant controls (age 36 [7] yr; BMI: 23 [3] kg m−2; nine ASA physical status 1; four ASA physical status 2). Five subjects underwent general surgery, five orthopaedic surgery, two vascular surgery, and one urologic surgery (Supplementary Table S1).
The measured ventilatory frequency at rest was 19 (5) bpm for pregnant women and 17 (5) bpm for controls (P=0.49). Before CSF and blood sampling, both pregnant women and controls received a limited volume of balanced crystalloid solution (422 [151] vs 350 [252] ml; P=0.33).
Pregnant women had higher progesterone concentrations both in plasma (179 [137; 221] vs 0 [0; 5] μg L−1; P<0.001) and in CSF (2.9 [2.4; 3.9] vs 0.1 [0.1; 0.1] μg L−1; P<0.001). Copeptin concentration was similar in pregnant and control groups (6.7 [5.0; 11.1] vs 4.0 [2.7; 6.4] pM; P=0.09).
CSF and arterial blood acid–base characteristics
The CSF and arterial blood characteristics are summarised in Table 1, Table 2. The CSF of pregnant women had lower SID and Pco2 than controls. The arterial plasma of pregnant women was characterised by lower SID (31.5 [1.2] vs 36.1 [1.9] mM; P<0.001), Pco2, and ATOT compared with controls. As a result of these changes, both CSF and arterial pH were higher in pregnant women. Pregnant women were characterised by a less negative ΔSID (–7.8 [1.4] vs –11.4 [2.3] mM; P<0.001) and a higher ΔPco2 (1.5 [0.3] vs 1.0 [0.4] kPa; P<0.001).
Table 1.
Acid–base characteristics of CSF in the study population. Data expressed as mean (standard deviation). Student's t-test or Wilcoxon rank-sum tests used as appropriate. ATOT, total amount of weak, non-carbonic acids; Pco2, partial pressure of carbon dioxide; RBC, red blood cell count; SID, strong ion difference; WBC, white blood cell count.
| Variables | Control group, n=13 | Pregnant group, n=20 | P-value |
|---|---|---|---|
| pH | 7.34 (0.02) | 7.36 (0.02) | 0.003 |
|
Pco2 (kPa) (mm Hg) |
5.9 (0.3) 45 (2) |
5.4 (0.3) 41 (2) |
<0.001 |
| ATOT (mM) | 1.2 (0.3) | 1.5 (0.6) | 0.046 |
| SID (mM) | 24.7 (1.2) | 23.7 (1.3) | 0.02 |
| Na+ (mM) | 139 (1) | 137 (1) | 0.001 |
| K+ (mM) | 2.7 (0.1) | 2.7 (0.1) | 0.22 |
| Ca2+ (mM) | 0.99 (0.02) | 0.96 (0.02) | <0.001 |
| Mg2+ (mM) | 1.09 (0.04) | 1.08 (0.02) | 0.45 |
| Cl– (mM) | 120 (1) | 119 (1) | 0.04 |
| Lactate (mM) | 1.4 (0.2) | 1.5 (0.1) | 0.03 |
| HCO3– (mM) | 22.5 (0.9) | 21.5 (1.1) | 0.01 |
| Ionised phosphate (mM) | 0.8 (0.3) | 0.6 (0.1) | 0.01 |
| Ionised albumin (mM) | 0.1 (0.1) | 0.6 (0.4) | 0.001 |
| Urea (mg dl−1) | 20 (4) | 18 (7) | 0.30 |
| Osmolality (mOsm kg−1) | 284 (3) | 276 (3) | <0.001 |
| Glucose (mg dl−1) | 56 (7) | 51 (5) | 0.012 |
| Haemoglobin (g dl−1) | 0.0 (0.0) | 0.0 (0.0) | 0.35 |
| RBC (106 μl−1) | 0.0 (0.0) | 0.0 (0.0) | 0.85 |
| WBC (μl−1) | 0.0 (0.0) | 0.0 (0.0) | 0.85 |
Table 2.
Acid–base characteristics of arterial blood of the study population. Data expressed as mean (standard deviation). Student's t-test or Wilcoxon rank-sum tests used as appropriate. ATOT, total amount of weak, non-carbonic acids; Pco2, partial pressure of carbon dioxide; Po2, partial pressure of oxygen; RBC, red blood cell count; SBE, standard base excess; SID, strong ion difference; WBC, white blood cell count; ΔPco2, CSF-to-plasma partial pressure of carbon dioxide difference; ΔSID, CSF-to-plasma difference in SID.
| Variables | Controls, n=13 | Pregnant group, n=20 | P-value |
|---|---|---|---|
| pH | 7.41 (0.02) | 7.45 (0.02) | <0.001 |
|
Pco2 (kPa) (mm Hg) |
4.9 (0.5) 37 (3) |
3.9 (0.3) 29 (2) |
<0.001 |
| ATOT (mM) | 16.9 (1.4) | 14.8 (1.2) | <0.001 |
| SID (mM) | 36.1 (1.9) | 31.5 (1.2) | <0.001 |
| Po2 (kPa) | 13.1 (0.6) | 15.5 (4.3) | 0.08 |
| Na+ (mM) | 138 (2) | 134 (1) | <0.001 |
| K+ (mM) | 3.8 (0.3) | 3.9 (0.3) | 0.15 |
| Ca2+ (mM) | 1.22 (0.05) | 1.19 (0.04) | 0.02 |
| Mg2+ (mM) | 0.82 (0.05) | 0.76 (0.06) | 0.003 |
| Cl– (mM) | 109 (2) | 110 (2) | 0.16 |
| Lactate (mM) | 1.1 (0.4) | 1.2 (0.3) | 0.23 |
| HCO3– (mM) | 23.4 (1.7) | 20.5 (1.4) | <0.001 |
| Ionised phosphate (mM) | 1.9 (0.3) | 2.1 (0.4) | 0.18 |
| Ionised albumin (mM) | 11.7 (1.0) | 10.1 (0.9) | <0.001 |
| SBE (mM) | –1.0 (1.6) | –3.1 (1.4) | <0.001 |
| Urea (mg dl−1) | 23 (7) | 19 (7) | 0.11 |
| Osmolality (mOsm kg−1) | 281 (4) | 275 (3) | <0.001 |
| Glucose (mg dl−1) | 95 (11) | 83 (6) | <0.001 |
| Albumin (g dl−1) | 4.4 (0.4) | 3.7 (0.3) | <0.001 |
| Haemoglobin (g dl−1) | 12.7 (1.5) | 11.9 (1.5) | 0.13 |
| RBC (106 μl−1) | 4.12 (0.42) | 4.10 (0.27) | 0.03 |
| WBC (103 cells μl−1) | 6.94 (2.10) | 9.34 (3.37) | 0.03 |
| ΔSID (mM) | –11.4 (2.3) | –7.8 (1.4) | <0.001 |
| ΔPco2 (kPa) (mm Hg) |
1.0 (0.4) 7.8 (2.8) |
1.5 (0.3) 11.3 (2.1) |
<0.001 |
| [Na+] CSF/[Na+] plasma | 1.01 (0.02) | 1.02 (0.01) | 0.008 |
| [Cl–] CSF/[Cl–] plasma | 1.10 (0.02) | 1.09 (0.01) | 0.001 |
The chloride concentration in CSF was slightly and significantly lower in the pregnant group. In this population, the actual CSF chloride was higher than the expected CSF chloride (119 [1] vs 118 [1] mM; P=0.02) (i.e. the expected CSF chloride concentration solely attributable to the dilution of CSF with pure water). Regarding arterial plasma, similar chloride concentrations were observed in the two groups. However, the measured chloride concentration in pregnant women was significantly higher than expected (110 [2] vs 106 [1] mM; P<0.001). On average, the relative increase in chloride concentration was responsible for 79 (11)% of observed SID variation in arterial blood and 79 (44)% in CSF.
Pregnant women had significantly lower sodium concentrations both in CSF and in plasma. Overall, the CSF osmolality was higher compared with plasma (279 [5] vs 277 [5] mOsm L−1; P=0.002), and the osmolality of these two compartments was strongly related (r=0.89; P<0.001; Supplementary Fig. S1).
In the pregnant group, the osmolality of both CSF (P=0.001) and plasma (P<0.001) were significantly lower. The CSF-to-plasma osmolality difference was smaller in this population (0 [–1; 3] vs 3 [1; 5] mOsm L−1; P=0.045).
Hypocapnia and hypocapnic alkalosis
All pregnant women were hypocapnic, whilst only four (30%) of the control subjects had Pco2 <4.7 kPa (P<0.001). The incidence of HA was higher in the pregnant group (65% vs 8%; P=0.001). When comparing pregnant women with HA to those without, no differences in weeks of gestation, progesterone concentration, and clinical and acid–base variables were observed (Supplementary Tables S2, S3, and S4, respectively).
Interplay between Stewart's independent variables and pH
Cerebrospinal fluid
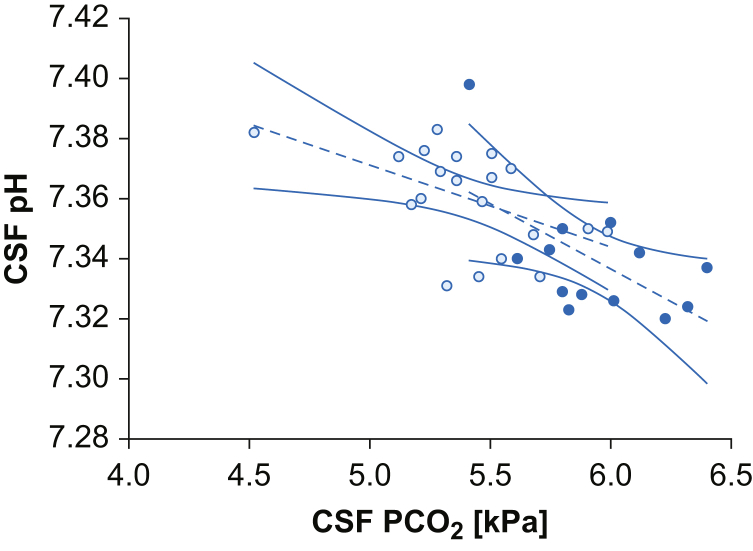
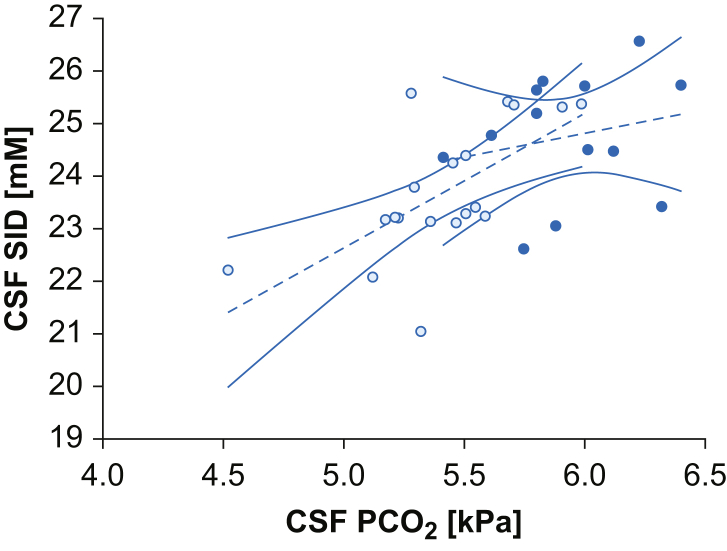
Linear and multilinear regression analyses were used to investigate the association between Stewart's independent variables. In both groups, CSF Pco2 showed a negative association with CSF pH (pregnant group: r=–0.52, P=0.02; controls: r=–0.60, P=0.02) (Fig. 1), whilst no relationship was found between CSF SID and CSF pH (P=0.54; P=0.78). In the pregnant group, CSF SID and CSF Pco2 were strongly correlated (r=0.64; P=0.002), whilst no association was found in controls (P=0.47) (Fig. 2). In the pregnant group, CSF Pco2 remained independently negatively associated with CSF pH when corrected for CSF SID in the multivariate analysis (Supplementary Table S5). No association between CSF osmolality and CSF SID, Pco2, or pH was observed in either group.
Fig 1.
Relationships of CSF Pco2 and pH. Data are represented by scatter plot, linear regression (dashed line), and 95% confidence interval (continuous line) of CSF Pco2 and CSF pH in the two populations. White dots represent the pregnant group (r=–0.52; P=0.02) and black dots the control group (r=–0.60; P=0.03). Pco2, partial pressure of carbon dioxide.
Fig 2.
Relationships of CSF Pco2 and SID. Data are represented as scatter plot, linear regression (dashed line), and 95% confidence interval (continuous line) of CSF Pco2 and CSF SID in the two populations. White dots represent the pregnant group (r=0.64; P=0.002) and black dots the control group (P=0.48). Pco2, partial pressure of carbon dioxide; SID, strong ion difference.
Interplay between CSF and plasma in pregnant women
In the pregnant group, CSF Pco2 was the only parameter that was associated to both arterial Pco2 (r=0.59; P=0.006) and arterial SID (r=0.53; P=0.02). A negative association was found between arterial Pco2 and ΔPco2 in both the pregnant group (r=–0.46; P=0.04) and controls (r=–0.78; P<0.001) (Supplementary Fig. S2). No relationship was found between CSF Pco2 and ΔPco2 either in pregnant women (P=0.051) or in controls (P=0.77). A positive correlation was found between ΔPco2 and ΔSID (Supplementary Fig. S3) in the pregnant group (r=0.54; P=0.01) but not in controls (P=0.22).
Blood
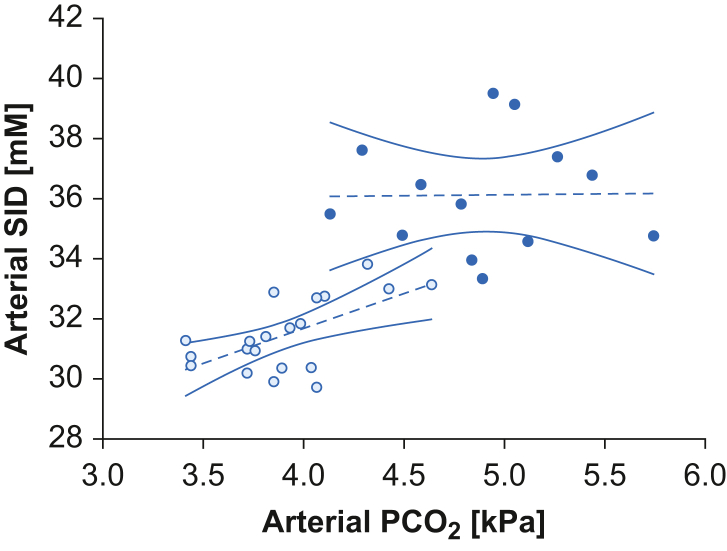
An association was observed between arterial Pco2 and SID in pregnant women (r=0.61; P=0.004), whilst it was lacking in control subjects (P=0.96) (Fig. 3). In both the pregnant and control groups, arterial Pco2 was negatively associated with arterial pH (r=–0.55, P=0.01 and r=–0.58, P=0.04). No relationship between arterial SID or ATOT with arterial pH was found in either group. In pregnant women, when corrected for SID and ATOT, Pco2 remained independently negatively associated with pH at the multilinear analyses, whilst this was not the case in the control group (Supplementary Table S6).
Fig 3.
Relationships of arterial Pco2 and arterial SID. Data represented as scatter plot, linear regression (dashed line), and 95% confidence interval (continuous line) of arterial Pco2 and arterial SID in the two populations. White dots represent the pregnant group (r=0.61; P=0.004) and black dots the control group (P=0.96). Pco2, partial pressure of carbon dioxide; SID, strong ion difference.
Hormones and Stewart's variables
Overall, both CSF (r=–0.78; P<0.001) and blood progesterone (r=–0.68; P<0.001) (Supplementary Fig. S4) concentrations were negatively associated with arterial Pco2. However, this association was lost when the groups were tested separately. Finally, no relationship between copeptin concentration and CSF/plasma SID, Pco2, or osmolality was found in either group.
Discussion
We used a physico-chemical approach to describe the acid–base characteristics of arterial blood and CSF of spontaneously breathing third-trimester pregnant women undergoing spinal anaesthesia for Caesarean delivery, and we compared these results with a group of non-pregnant women of childbearing age undergoing elective surgery under spinal anaesthesia.
Our results confirmed that third-trimester pregnant women are characterised by hypocapnia, frequently leading to HA.4, 5, 6 In line with available literature, we did not observe a difference in ventilatory frequency compared with controls.18 Simultaneous CSF and arterial blood sampling allowed investigation of the mechanisms underlying the ventilatory response to pregnancy.
According to Stewart's physico-chemical approach, SID, ATOT, and Pco2 are three independent determinants regulating acid–base equilibrium of biological solutions. During pregnancy, all three parameters are markedly modified.
Isolated reduction of CSF SID, representative of local CSF metabolic acidosis, is associated with development of sustained hyperventilation both in experimental and clinical settings.17,19,20 In this context, central chemoreceptors sense CSF metabolic acidosis and trigger hyperventilation to lower CSF Pco2 and restore physiological CSF pH.21 During pregnancy, physiological hormonal changes adapt the mother's physiology to fetal development and needs,5,6,18 progressively modifying the electrolytes of plasma and CSF. For example, higher circulating AVP concentrations induce water reabsorption, dilute extracellular fluid, and reduce SID.6,11 For this reason, having observed a positive association between plasma SID and Pco2 during pregnancy, a primary role of SID reduction in the development of hyperventilation has been proposed.11,12,18,22 Whilst we measured a significantly lower CSF SID in pregnant women, our data do not support its role as the primary determinant of the observed hyperventilation for several reasons.
Firstly, CSF pH in the pregnant group was more alkalotic than in the controls. Physiologically, a compensatory response is sustained by the primary insult and usually does not fully compensate. Consequently, if the decreased CSF SID had been the primary acid–base disorder, we would expect a certain degree of residual CSF acidosis. On the contrary, CSF pH was more alkalotic, pointing towards a primary HA with a secondary CSF SID reduction.
Secondly, because of increased AVP-induced free water absorption, pregnant women are characterised by lower sodium concentrations. However, comparing actual and expected chloride concentrations, the actual was significantly higher, underlining the presence of active renal retention and choroid plexus secretion of chloride into extracellular fluids.23, 24, 25, 26 This response is the typical renal compensation of chronic respiratory alkalosis (i.e. a reduction of plasma SID). Notably, ∼80% of the observed SID reduction (both in plasma and in CSF) was attributable to the increased chloride concentration.
Thirdly, despite a lower SID in pregnant women, a relationship between SID and pH was observed neither in CSF nor in plasma.
On the contrary, a strong association was found between Pco2 and pH in both compartments. Of note, SID was positively associated with Pco2 in both CSF and plasma, pointing to compensatory mechanism. The association between Pco2 and pH again supports hypocapnia as the primary derangement. In conclusion, in the context of pregnancy, SID variations are a secondary response to primary chronic hypocapnia and, to a lesser extent, to AVP-induced dilution.
The cause of the primary hyperventilation is likely increased progesterone concentration. Machida27 showed that progesterone crosses the blood–brain barrier, directly stimulating neural sites involved in the control of breathing, independently from the central ventilatory chemoreflex or peripheral ventilatory feedback. Consequently, in line with previous observations, our results support that pregnant women physiologically develop primary central hyperventilation sustained by progesterone.7,28
Other authors have hypothesised a possible role of osmolality as a cause of hyperventilation during pregnancy.6,11,29 In line with the available literature, the pregnant group had lower CSF and plasma osmolality. However, we did not find an association between osmolality and hypocapnia. The main contribution of this AVP-induced osmolality modification was a decrease in sodium and, to a lesser extent, glucose concentration. We measured copeptin, which is the C-terminal fragment of the AVP prohormone, as a proxy for AVP.30 We did not find any difference in copeptin concentration between groups. However, AVP secretion changes during pregnancy. Compared with non-pregnant women, early pregnancy is characterised by higher circulating AVP concentrations,31 whilst late in pregnancy AVP metabolism rises four-fold from non-pregnant values.32 Consequently, in the third trimester, circulating AVP concentration could be similar or lower compared with non-pregnant women.
Another interesting finding is the increased CSF-to-plasma difference of Pco2 observed in pregnant women. We recently described a reduced ΔPco2 in patients with aneurismal subarachnoid haemorrhage and hypothesised reduced cerebral metabolism as the cause of this finding.17 However, we do not think that increased cerebral metabolism could explain the finding in the present context, as several studies described a normal cerebral CO2 production during physiological pregnancy.9
In pregnant women, the reduction in arterial Pco2 was marked, whilst CSF Pco2 varied only slightly. Consequently, arterial Pco2 was the main determinant of ΔPco2 (Supplementary Fig. S2). Together with the primary central hyperventilation, the increase in cardiac output typical of pregnancy could lower systemic Pco2 and increase ΔPco2.5 However, this systemic effect does not apply to brain perfusion because of the particular autoregulation of blood flow and preserved cerebrovascular reactivity to Pco2.33 In the brain, Pco2 is one of the major determinants of cerebral blood flow.34 CSF Pco2 tension is an average between the Pco2 of venous blood flowing into the choroid plexus and brain tissue Pco2.35
Despite some controversy, several studies suggested that during full-term pregnancy the blood flow velocity of large brain vessels decreases36, 37, 38 despite preserved MAP and without variation of large vessel calibre.36 These phenomena could be explained by the simultaneous increase in arterial distensibility and decrease in peripheral arteriolar resistance.37,38 The slower cerebral blood flow could therefore limit the tissue CO2 removal, thus increasing tissue and CSF Pco2 tension.
Similar to ΔPco2, ΔSID differed significantly between pregnant and non-pregnant women, with the latter characterised by less negative values. Physiologically, the CSF compartment and the plasma compartment have different SIDs. The CSF compartment is characterised by a negligible amount of protein, and the negative charges deriving from proteins (approximately 16 mM in plasma) are lacking in this extracellular compartment. However, electrical neutrality is guaranteed by higher concentrations of chloride, leading to a lower SID in CSF compared with plasma. Physiologically, the CSF and plasma compartments have different SIDs with a normal value for the CSF-to-plasma SID difference (ΔSID) of ∼11 mM.17 Results from the present study are in line with these findings. Given these premises, ΔSID has been used to identify the presence of a metabolic derangement confined to one of the two compartments. For example, in patients with subarachnoid haemorrhage, the increase in CSF lactate lowers CSF SID. As plasma SID is not markedly affected, the resulting ΔSID is more negative, pointing towards local acidification of CSF.17 In contrast, in the context of pregnancy, both CSF and plasma SIDs are reduced. These SID variations are the consequences of two different phenomena: dilution attributable to AVP action and compensatory metabolic response to chronic hypocapnia. Because of cerebral CO2 autoregulation, CSF HA is less marked, whilst arterial Pco2 variations are more pronounced. Consequently, to limit the pH variations of each compartment, blood and CSF have modified SID proportionally to local Pco2 variations (Supplementary Fig. S4): a larger decrease in plasma SID and a smaller reduction in CSF SID, leading to a less negative ΔSID. Thus, in this context, ΔSID also describes the entity of the relative compensation of the two compartments to primary hyperventilation.
During pregnancy, AVP-induced water reabsorption generates a blood volume expansion of about 50%, only partially compensated by an increased count of red blood cells. As a result, a relative state of anaemia with lower plasma protein concentration develops.39,40 In line with previous descriptions, our study observed lower albumin concentrations in pregnant women, leading to a small but significant reduction in ATOT, with a small alkalinising effect on plasma pH.
Limitations
We recognise some limitations of our study. First, the enrollment of the control group was interrupted because of the slow recruitment rate. However, the data from the control subjects are consistent with the available literature. Second, no direct measurements of ventilatory (e.g. tidal volume and minute ventilation) or cerebral (e.g. blood flow velocity) parameters were available. Third, analyses exploring the CSF characteristics of hypocapnic alkalosis and subjects without hypocapnic alkalosis could be underpowered. Consequently, the results of these secondary analyses need to be interpreted cautiously.
Conclusions
Third-trimester pregnant women are characterised by marked hypocapnia and a high incidence of hypocapnic alkalosis, likely sustained by progesterone. The decrease in Pco2 differs between CSF and arterial blood, with lower values observed in blood. Consequently, the compensatory metabolic acidification, represented by chloride-induced reduction in strong ion difference, is more pronounced in plasma.
Authors' contributions
Concept and study design: TL, FZ
Patient recruitment: FZ, GG, CFF, MTA
Sample collection: FZ, GG, CFF, MTA
Sample size analysis: FZ, GG, CFF, AM
Data analysis/interpretation: FZ, TL
Drafting of article: FZ, TL
Designing of figures: FZ, TL
Review/editing/approval of article: all authors
All authors confirm that this article, including related data and figures, has not been previously reported or published elsewhere, and it is not under consideration by other journals.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Italian Ministry of Health – Current research IRCCS.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.07.048.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sakka L., Coll G., Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–316. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Fencl V., Miller T., Pappenheimer J.R. Studies on the respiratory response to disturbances of acid–base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966;210:459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- 3.Langer T., Zanella A., Caironi P. Understanding the role of the cerebrospinal fluid in acid–base disorders. Intensive Care Med. 2016;42:436–439. doi: 10.1007/s00134-015-4059-8. [DOI] [PubMed] [Google Scholar]
- 4.Weissgerber T.L., Wolfe L.A., Hopkins W.G., Davies G.A.L. Serial respiratory adaptations and an alternate hypothesis of respiratory control in human pregnancy. Respir Physiol Neurobiol. 2006;153:39–53. doi: 10.1016/j.resp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Weissgerber T.L., Wolfe L.A. Physiological adaptation in early human pregnancy: adaptation to balance maternal–fetal demands. Appl Physiol Nutr Metab. 2006;31:1–11. doi: 10.1139/h05-003. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe L.A., Kemp J.G., Heenan A.P., Preston R.J., Ohtake P.J. Acid–base regulation and control of ventilation in human pregnancy. Can J Physiol Pharmacol. 1998;76:815–827. doi: 10.1139/cjpp-76-9-815. [DOI] [PubMed] [Google Scholar]
- 7.Bayliss D.A., Millhorn D.E. Central neural mechanisms of progesterone action: application to the respiratory system. J Appl Physiol. 1992;73:393–404. doi: 10.1152/jappl.1992.73.2.393. [DOI] [PubMed] [Google Scholar]
- 8.Jensen D., Webb K.A., O’Donnell D.E. Chemical and mechanical adaptations of the respiratory system at rest and during exercise in human pregnancy. Appl Physiol Nutr Metab. 2007;32:1239–1250. doi: 10.1139/H07-120. [DOI] [PubMed] [Google Scholar]
- 9.Jensen D., Duffin J., Lam Y.M., et al. Physiological mechanisms of hyperventilation during human pregnancy. Respir Physiol Neurobiol. 2008;161:76–86. doi: 10.1016/j.resp.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Tatsumi K., Pickett C.K., Jacoby C.R., Weil J.V., Moore L.G. Role of endogenous female hormones in hypoxic chemosensitivity. J Appl Physiol. 1997;83:1706–1710. doi: 10.1152/jappl.1997.83.5.1706. [DOI] [PubMed] [Google Scholar]
- 11.Anderson J.W., Jennings D.B. Osmolality, NaCl dietary intake, and regulation of ventilation by CO2. Am J Physiol. 1988;255:R105–R112. doi: 10.1152/ajpregu.1988.255.1.R105. [DOI] [PubMed] [Google Scholar]
- 12.Jennings D.B. The physicochemistry of [H+] and respiratory control: roles of PCO2, strong ions, and their hormonal regulators. Can J Physiol Pharmacol. 1994;72:1499–1512. doi: 10.1139/y94-216. [DOI] [PubMed] [Google Scholar]
- 13.Heenan A.P., Wolfe L.A. Plasma osmolality and the strong ion difference predict respiratory adaptations in pregnant and nonpregnant women. Can J Physiol Pharmacol. 2003;81:839–847. doi: 10.1139/y03-072. [DOI] [PubMed] [Google Scholar]
- 14.Langer T., Carlesso E., Protti A., et al. In vivo conditioning of acid–base equilibrium by crystalloid solutions: an experimental study on pigs. Intensive Care Med. 2012;38:686–693. doi: 10.1007/s00134-011-2455-2. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell R.A., Herbert D.A., Carman C.T. Acid-base constants and temperature coefficients for cerebrospinal fluid. J Appl Physiol. 1965;20:27–30. doi: 10.1152/jappl.1965.20.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Fencl V., Jabor A., Kazda A., Figge J. Diagnosis of metabolic acid–base disturbances in critically ill patients. Am J Respir Crit Care Med. 2000;162:2246–2251. doi: 10.1164/ajrccm.162.6.9904099. [DOI] [PubMed] [Google Scholar]
- 17.Langer T., Zadek F., Carbonara M., et al. Cerebrospinal fluid and arterial acid–base equilibrium of spontaneously breathing patients with aneurismal subarachnoid hemorrhage. Neurocrit Care. 2022;37:102–110. doi: 10.1007/s12028-022-01450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heenan A.P., Wolfe L.A. Plasma acid-base regulation above and below ventilatory threshold in late gestation. J Appl Physiol. 2000;88:149–157. doi: 10.1152/jappl.2000.88.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Mascheroni D., Kolobow T., Fumagalli R., Moretti M.P., Chen V., Buckhold D. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15:8–14. doi: 10.1007/BF00255628. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook M.A., Hutchinson E.C., Aber G.M. Metabolic studies in subarachnoid haemorrhage and strokes: I. Serial changes in acid-base values in blood and cerebrospinal fluid. Brain. 1973;96:171–190. doi: 10.1093/brain/96.1.171. [DOI] [PubMed] [Google Scholar]
- 21.Jennings D.B. Breathing for protein function and [H+] homeostasis. Respir Physiol. 1993;93:1–12. doi: 10.1016/0034-5687(93)90063-g. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J.W., Sarda I.R., Jennings D.B. Acute changes in osmolality and renin and respiratory control of arterial PCO2 and [H+] Respir Physiol. 1990;80:1–16. doi: 10.1016/0034-5687(90)90002-g. [DOI] [PubMed] [Google Scholar]
- 23.Krapf R., Beeler I., Hertner D., Hulter H.N. Chronic respiratory alkalosis. N Engl J Med. 1991;324:1394–1401. doi: 10.1056/NEJM199105163242003. [DOI] [PubMed] [Google Scholar]
- 24.Gennari F.J., Goldstein M.B., Schwartz W.B. The nature of the renal adaptation to chronic hypocapnia. J Clin Invest. 1972;51:1722–1730. doi: 10.1172/JCI106973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nattie E.E., Romer L. In: The regulation of respiration during sleep and anesthesia. Fitzgerald R.S., Gautier H., Lahiri S., editors. Springer; Boston, MA: 1978. The role of chloride and other anions in cerebrospinal fluid bicarbonate regulation; pp. 211–218. [DOI] [PubMed] [Google Scholar]
- 26.Siesjö B.K. Symposium on acid-base homeostasis. The regulation of cerebrospinal fluid pH. Kidney Int. 1972;1:360–374. doi: 10.1038/ki.1972.47. [DOI] [PubMed] [Google Scholar]
- 27.Machida H. Influence of progesterone on arterial blood and CSF acid–base balance in women. J Appl Physiol Respir Environ Exerc Physiol. 1981;51:1433–1436. doi: 10.1152/jappl.1981.51.6.1433. [DOI] [PubMed] [Google Scholar]
- 28.Bayliss D.A., Cidlowski J.A., Millhorn D.E. The stimulation of respiration by progesterone in ovariectomised cat is mediated by an estrogen-dependent hypothalamic mechanism requiring gene expression. Endocrinology. 1990;126:519–527. doi: 10.1210/endo-126-1-519. [DOI] [PubMed] [Google Scholar]
- 29.Jennings D.B. Respiratory control during exercise: hormones, osmolality, strong ions, and PaCO2. Can J Appl Physiol. 1994;19:334–349. doi: 10.1139/h94-027. [DOI] [PubMed] [Google Scholar]
- 30.Christ-Crain M. Vasopressin and copeptin in health and disease. Rev Endocr Metab Disord. 2019;20:283–294. doi: 10.1007/s11154-019-09509-9. [DOI] [PubMed] [Google Scholar]
- 31.Lindheimer M.D., Davison J.M. Osmoregulation, the secretion of arginine vasopressin and its metabolism during pregnancy. Eur J Endocrinol. 1995;132:133–143. doi: 10.1530/eje.0.1320133. [DOI] [PubMed] [Google Scholar]
- 32.Lindheimer M.D., Barron W.M., Davison J.M. Osmotic and volume control of vasopressin release in pregnancy. Am J Kidney Dis. 1991;17:105–111. doi: 10.1016/s0272-6386(12)81112-7. [DOI] [PubMed] [Google Scholar]
- 33.Bergersen T.K., Hartgill T.W., Pirhonen J. Cerebrovascular response to normal pregnancy: a longitudinal study. Am J Physiol Heart Circ Physiol. 2006;290:1856–1861. doi: 10.1152/ajpheart.00919.2005. [DOI] [PubMed] [Google Scholar]
- 34.Yoon S., Zuccarello M., Rapoport R.M. pCO2 and pH regulation of cerebral blood flow. Front Physiol. 2012;3:365. doi: 10.3389/fphys.2012.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pontén U., Siesjö B.K. Gradients of CO2 tension in the brain. Acta Physiol Scand. 1966;67:129–140. doi: 10.1111/j.1748-1716.1966.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 36.Zeeman G.G., Hatab M., Twickler D.M. Maternal cerebral blood flow changes in pregnancy. Am J Obstet Gynecol. 2003;189:968–972. doi: 10.1067/s0002-9378(03)00820-2. [DOI] [PubMed] [Google Scholar]
- 37.Belfort M.A., Tooke-Miller C., Allen J.C., et al. Changes in flow velocity, resistance indices, and cerebral perfusion pressure in the maternal middle cerebral artery distribution during normal pregnancy. Acta Obstet Gynecol Scand. 2001;80:104–112. [PubMed] [Google Scholar]
- 38.Johnson A.C., Cipolla M.J. The cerebral circulation during pregnancy: adapting to preserve normalcy. Physiology. 2015;30:139–147. doi: 10.1152/physiol.00048.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horowitz K.M., Ingardia C.J., Borgida A.F. Anemia in pregnancy. Clin Lab Med. 2013;33:281–291. doi: 10.1016/j.cll.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Pivarnik J.M., Lee W., Miller J.F., Werch J. Alterations in plasma volume and protein during cycle exercise throughout pregnancy. Med Sci Sports Exerc. 1990;22:751–755. doi: 10.1249/00005768-199012000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.