Abstract
A 55-kDa outer membrane protein of Porphyromonas gingivalis W50 is a significant target of the serum immunoglobulin G antibody response of periodontal disease patients and hence may play an important role in host-bacterium interactions in periodontal disease. The gene encoding the 55-kDa antigen (ragB, for receptor antigen B) was isolated on a 9.5-kb partial Sau3AI fragment of P. gingivalis W50 chromosomal DNA in pUC18 by immunoscreening with a monoclonal antibody to this antigen. The 1.6-kb open reading frame (ORF) encoding RagB was located via subcloning and nested-deletion analysis. Sequence analysis demonstrated the presence of an upstream 3.1-kb ORF (ragA) which is cotranscribed with ragB. A number of genetic characteristics suggest that the ragAB locus was acquired by a horizontal gene transfer event. These include a significantly reduced G+C content relative to that of the P. gingivalis chromosome (42 versus 48%) and the presence of mobility elements flanking this locus in P. gingivalis W50. Furthermore, Southern blotting and PCR analyses showed a restricted distribution of this locus in laboratory and clinical isolates of this bacterium. The association of ragAB+ P. gingivalis with clinical status was examined by PCR analysis of subgingival samples. ragAB+ was not detected in P. gingivalis-positive shallow pockets from periodontal disease patients but was present in 36% of the P. gingivalis-positive samples from deep pockets. These data suggest that the ragAB locus was acquired by certain P. gingivalis strains via horizontal gene transfer and that the acquisition of this locus may facilitate the survival of these strains at sites of periodontal destruction.
Porphyromonas gingivalis is a gram-negative anaerobic bacterium considered to be a major etiological agent in destructive periodontal disease (38). The periodontal diseases are chronic inflammatory conditions of the tooth-supporting tissues which in severe cases result in the destruction of the periodontium, including alveolar bone, leading to eventual tooth loss. In patients with periodontitis, P. gingivalis is frequently isolated in high numbers from heavily inflamed subgingival lesions, where it may constitute a significant proportion of the oral microflora. P. gingivalis may also be isolated from periodontally healthy populations but at a much lower percentage of the total microflora (5, 28). It is not known whether strains colonizing periodontally healthy individuals are genetically distinct from those associated with periodontitis, but DNA fingerprinting studies have identified a large number of distinct P. gingivalis genotypes (23, 25, 30). The pathogenic potentials of different P. gingivalis strains have been investigated in animal models of soft tissue destruction and do vary according to the strain used, suggesting that strain variation may influence the virulence potential of this organism (20, 32).
The interplay between the periodontal microflora and the host in destructive periodontal disease is composed of a complex set of interactions, involving extracellular and surface determinants of the bacteria and the host’s inflammatory and specific immune responses. In order to identify the determinants of P. gingivalis which participate in this dynamic interaction, we have analyzed the specific immunoglobulin G serum antibody responses of periodontal disease patients and matched healthy controls to preparations of P. gingivalis W50 outer membranes and cell sonicates (11). These studies led to the identification of three immunodominant surface antigens (115, 55, and 47 kDa) of this organism which, by extrapolation, are expressed in vivo and interact with the immune systems of periodontal disease patients.
Subsequent molecular genetic and immunochemical investigations have demonstrated that the 47-kDa antigen is a hemagglutinin/adhesin component of a major arginine-specific protease, RgpA, of this organism and that the coding region for this antigen is present in multiple genes of P. gingivalis (1, 12). Members of this family of gene products all have putative roles in proteolytic, adherence, or surface transport processes and are thought to represent key virulence determinants (2, 14, 18). The distribution of these genes has been examined, and the evidence obtained to date suggests that they are present in all laboratory and clinical isolates, although some between-strain genetic polymorphism at some of these loci is detectable. These data are analogous to the findings for another important colonization determinant of P. gingivalis, the surface fimbriae, the gene for which is similarly ubiquitous in different strains (24). To our knowledge, there are no examples to date of virulence determinant genes of this bacterium which show a restricted distribution which could account for between-strain variance in virulence potential.
The properties of the remaining two antigens (115 and 55 kDa), identified on the basis of their reactivity with periodontal disease case sera, have not been described. However, production of a monoclonal antibody to the 55-kDa antigen by Millar et al. (31) facilitated the analysis of the expression of this antigen. These studies confirmed that the 55-kDa antigen constitutes a major fraction of the outer membrane of P. gingivalis W50 and, furthermore, that it is expressed in only approximately 60% of laboratory strains and clinical isolates (31). The purpose of the present study was to identify and characterize at the molecular level the genetic component encoding the 55-kDa antigen.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. gingivalis W50, W50Be1, W50Br1, W83, LB13D-3, 381, 11834, WPH-34, and WPH-35 and Porphyromonas asaccharolytica ATCC 25260 have been described previously (1, 3). Chromosomal DNAs from 38 clinical isolates (3) were used in PCR analysis. Porphyromonas species were grown on a blood agar base containing 5% defibrinated horse blood or in brain heart infusion broth supplemented with hemin (5 μg ml−1) in an anaerobic chamber (Don Whitley, Shipley, United Kingdom) with an atmosphere of 85% N2, 10% H2, and 5% CO2 at 37°C. Escherichia coli XL-1 Blue (Stratagene Ltd., Cambridge, United Kingdom) was used for all cloning experiments and was grown aerobically on Luria-Bertani medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl) with 20 μg of tetracycline ml−1 added for F′ episome selection. The plasmid pUC18 was used for cloning in E. coli XL-1 Blue and was selected on Luria-Bertani medium supplemented with ampicillin (50 μg ml−1). Blue or white colony color selection was used to distinguish between nonrecombinant and recombinant E. coli clones, respectively, by means of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and IPTG (isopropyl-β-d-thiogalactopyranoside). General cloning procedures were as described by Sambrook et al. (35).
Protein analysis.
E. coli XL-1 Blue transformants were immobilized at a high density on nitrocellulose membranes and permeabilized with chloroform vapor for 30 minutes followed by lysis with lysozyme in the presence of pancreatic DNase I for 16 h. Membranes were blocked in 5% bovine serum albumin in phosphate-buffered saline and incubated with the primary antibody (DRU55.5, which recognizes the 55-kDa outer membrane antigen, at a 1:500 dilution) in 1% bovine serum albumin in phosphate-buffered saline. The antibody-antigen reaction was detected with horseradish peroxidase-conjugated antimouse antiserum at a 1:500 dilution, and the reaction was developed by using H2O2 and diaminobenzidine (0.05%).
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed as described by Laemmli (22) on 10% separating gels. Transfer to nitrocellulose membranes for immunoblot analysis was carried out in a bicarbonate transfer buffer (3 mM Na2CO3, 10 mM NaHCO3 [pH 9.9], 20% methanol) at a constant current of 400 mA for 2 h. Immunostaining of the membranes was performed with horseradish peroxidase as described above.
Outer membranes of P. gingivalis W50 were prepared by Sarkosyl solubilization, and P. gingivalis whole cells were also prepared as previously described (11).
DNA manipulations and sequencing.
Two sets of P. gingivalis libraries were constructed by cloning partially Sau3AI-digested P. gingivalis W50 chromosomal DNA in the ranges of 1.8 to 5 kb and 5 to 12 kb into the plasmid pUC18 and transforming it into competent E. coli XL-1 Blue cells. The E. coli transformants were immobilized on nitrocellulose membranes and screened by using the monoclonal antibody DRU55.5, which was raised against the immunodominant 55-kDa antigen. Approximately 10,000 colonies from each library were screened. The library consisting of the smaller insert sizes of 1.8 to 5 kb was nonreactive with the antibody; however, one immunoreactive clone was identified from the library containing the larger inserts of 5 to 12 kb. The recombinant plasmid pPM1, which expressed the 55-kDa protein, was isolated from the E. coli clone for retransformation and characterization. Restriction analysis indicated that pPM1 contained a 9.5-kb P. gingivalis chromosomal insert. Removal of a 2.0-kb SmaI fragment from one end of pPM1 followed by pPM1 religation gave rise to a 7.5-kb insert, pSM, which also expressed the 55-kDa protein. The plasmid pSM was used as a convenient template for the construction of a set of nested-deletion derivatives. The pSM plasmid was digested with SphI and XbaI, both of which specifically restrict the DNA within the multiple cloning site of pUC18, and then was subjected to a progressive unidirectional digestion with exonuclease III. A total of 24 derivatives were prepared by using the Nested Deletion kit (Amersham Pharmacia Biotech, St. Albans, United Kingdom). Plasmid DNA was isolated from E. coli by the method described by Birnboim and Doly (6).
For DNA sequence analysis, sequencing-grade plasmid DNA was prepared by ion-exchange chromatography on columns from Qiagen Ltd. (West Sussex, United Kingdom). The nested-deletion plasmids were subjected to Taq cycle sequencing by using the dideoxy dye termination chemistry from Applied Biosystems and the universal M13 forward and reverse primers. The products were analyzed on an Applied Biosystems model 377 automated DNA sequencer. Complete coverage of both strands of the pSM subclone and linkage of contigs were achieved by primer walking. All oligonucleotide primers were purchased from Amersham Pharmacia.
DNA sequence data were analyzed by using Gene Jockey II (Biosoft, Cambridge, United Kingdom), the Wisconsin Genetics Computer Group software (13), and the Staden DNA and protein analysis software (National Institute for Medical Research, London, United Kingdom).
Southern hybridization analysis.
Genomic DNA was electrophoresed in agarose gels, depurinated in 0.25 M HCl, and then transferred onto Hybond N+ membranes (Amersham Pharmacia) by vacuum blotting (Vacu-Aid; Life Sciences International, Hampshire, United Kingdom) under alkaline conditions (0.4 M NaOH). After blotting, the membranes were prehybridized for 1 h at 65°C in Rapid-Hyb buffer (Amersham Pharmacia) and hybridized for 2 h in the same solution with heat-denatured 32P-labelled probes. The probe DNA for ragA and ragB was prepared by PCR (see below), and that for orf3 was prepared by EcoRV restriction digestion of pPM1. Probes were labelled by the random-primer method with the Ready To Go labelling system (Amersham Pharmacia). Membranes were washed twice in final stringent washes of 0.2× standard saline citrate (SSC) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% SDS at 65°C. Autoradiography was performed at −70°C with intensifying screens for variable exposure times on Kodak-X Omat LS Films (Genetic Research Instrumentation, Essex, United Kingdom).
Northern hybridization analysis.
RNA was prepared from P. gingivalis W50 cells after 6 h of growth in brain heart infusion by using a Total RNA Isolation Reagent Kit (Advanced Biotechnologies, Epsom, United Kingdom). RNA species were resolved in denaturing formaldehyde agarose gel (35) and then transferred to Hybond N+ membranes by vacuum blotting (Vacu-Aid; Life Sciences International) essentially as described above for Southern blotting except that the transfer was performed in 20× SSC. RNA was fixed to the membrane by using UV light (λ = 312 nm) for 5 min. The blot was stained with methylene blue (0.04% in 0.5 M Na-acetate, pH 6) (21) to locate 16S RNA, 23S RNA, and RNA markers (0.24 to 9.5 kb; Life Sciences International) and then briefly destained with water and prehybridized in Rapid-Hyb buffer (Amersham Pharmacia) at 65°C. Northern hybridization was as described above for Southern hybridization.
PCR analysis of ragA and ragB gene distributions.
Probes for Southern analysis of ragA and ragB distributions were prepared by amplification of the complete 1.5-kb ragB gene and an internal 1.6-kb fragment of the ragA gene. The PCR products were purified by using the Wizard cleanup kit (Promega, Southampton, United Kingdom). Amplification was performed in a thermal cycler (Omnigene; Life Sciences International) with Red Hot DNA polymerase (Advanced Biotechnologies). The reaction mixture consisted of approximately 20 ng of pSM plasmid template, 10 μl of 10× PCR amplification buffer (100 mM Tris, 500 mM KCl, 1% [vol/vol] Triton X-100), 0.5 μg of forward and reverse ragA.1 or ragB.1 primers (Table 1), 250 nM deoxynucleoside triphosphates (Amersham Pharmacia), and 2 U of DNA polymerase in a 100-μl volume adjusted by the addition of distilled water. The program conditions for both primer sets were as follows: 2 min at 95°C followed by 25 cycles of 95°C for 1 min, 50°C for 1 min, and 72°C for 3 min. After the final cycle, the extension reaction was allowed to proceed at 72°C for 3 min.
TABLE 1.
PCR amplification primers
| Amplification (reference) | Primer pairs (5′-3′) | Direction | Base position (amplicon length [bp]) |
|---|---|---|---|
| ragA.1 | TGT CTG GTG CGA TAG CGA ATA G | Forward | 3198–4904 (1,600) |
| TAC ATA GGT GAG TTT GAG ATT C | Reverse | ||
| ragB.1 | AAT ACT GAA AAT CCA CGA | Forward | 5275–6686 (1,500) |
| TAG GGG CTG CGA CAA AAA | Reverse | ||
| ragA.2 | CAA CCA CCA AGC ACT CGT | Forward | 4033–4542 (509) |
| CGA CTT GAC CAG GAA CAT AC | Reverse | ||
| ragB.2 | GAC TCT TGG CTC GTT TAC TG | Forward | 6001–6436 (436) |
| ACG CAA ACG ACT ACC CTC A | Reverse | ||
| P. gingivalis 16S RNA (4) | AGG CAG CTT GCC ATA CTG CG | Forward | 729–1132 (404) |
| ACT GTT AGC AAC TAC CGA TGT | Reverse | ||
| P. gingivalis rpgA catalytic domain (3) | AGT GAG CGA AAC TTC GGA GC | Forward | 1199–2902 (1,709) |
| CCT GTC CAA TAT GGG TCA CTA TGG | Reverse |
For analysis of ragA and ragB distributions in clinical isolates, PCR was used to amplify smaller amplicons of 435 and 509 bp of ragB and ragA, respectively. Chromosomal DNAs purified from 38 P. gingivalis clinical isolates (3) were used as templates in the PCR analysis. The reactions were carried out in a 100-μl volume consisting of 50 μl of Taq Master Mix (Qiagen Ltd.), approximately 200 ng of template chromosomal DNA, 0.5 μg of forward or reverse ragA.2 and ragB.2 primers (Table 1), and 47 μl of H2O. The program conditions for both primer sets were as follows: a hot start of 95°C for 2 min followed by 25 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min. After the final cycle, the extension reaction was allowed to proceed at 72°C for 3 min.
PCR detection of ragB in clinical samples.
Subgingival plaque samples from adult periodontitis patients were obtained from patients attending the periodontal clinic (mean age, 44 years; range, 28 to 60 years; n = 28) at the School of Medicine and Dentistry. Control samples were obtained from periodontally healthy students and members of the staff (mean age, 32 years; range, 22 to 50 years; n = 33). Clinical samples were collected by the procedures outlined by Ashimoto et al. (4). Briefly, sample sites were isolated with cotton rolls and air dried. Paper points were inserted to the depth of the pockets. Samples from periodontal disease patients were taken from at least three of the deepest pockets, and a sample was also taken from a site without evidence of severe periodontal destruction (<4-mm pocket depth). Two samples were taken from each member of the control group, from the lower right and lower left first molar. All pockets sampled in the control group were <4 mm. The paper points were removed 5 to 10 s after insertion and placed in a vial containing 1 ml of fastidious anaerobic broth (Oxoid, Basingstoke, United Kingdom). The samples in fastidious anaerobic broth were mixed well on a Vortex mixer, 0.5 ml of the microbial suspension was removed and centrifuged, and the pellet was washed three times with distilled water. The bacterial pellets were resuspended in 0.1 ml of distilled water, boiled for 10 min, and placed on ice. After centrifugation to remove cell debris, the supernatant was used for PCR analysis.
The reactions were carried out in a 100-μl volume consisting of 50 μl of Taq Master Mix (Qiagen Ltd.), 10 μl of sample, and 0.5 μg of forward and reverse P. gingivalis 16S RNA or ragB.2 primers (Table 1) made up to 100 μl with distilled H2O. The program conditions for both primer sets were as follows: 2 min at 95°C followed by 36 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 1 min. After the final cycle, the extension reaction was allowed to proceed at 72°C for 3 min. Primers designed for the catalytic domain of the rgpA protease gene (Table 1) were also used for P. gingivalis detection in samples which were negative for P. gingivalis with the 16S RNA primers but positive with the ragB.2 primers. The PCRs were performed with Taq Master Mix (Qiagen Ltd.) as outlined above, and the conditions used were as described by Allaker et al. (3). The results were statistically analyzed by Spearman’s rho correlation analysis with Stata software.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this study have been assigned GenBank accession no. AJ130872.
RESULTS
Identification of an E. coli clone, PM1, expressing the 55-kDa antigen.
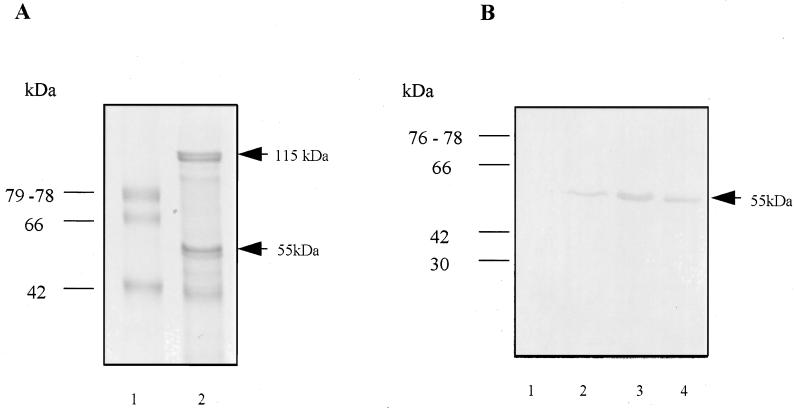
Immunoblot analysis of colony lifts by using a monoclonal antibody (DRU55.5) to the 55-kDa antigen (31) resulted in the identification of one E. coli clone from the library containing plasmids with the larger inserts of 5 to 12 kb only. The E. coli clone identified, PM1, contains a 9.5-kb fragment of P. gingivalis DNA in pUC18 and expresses the 55-kDa protein (Fig. 1B).
FIG. 1.
(A) Protein composition of the outer membrane of P. gingivalis W50 in SDS–10% polyacrylamide gel electrophoresis. Lane 1, low-molecular-weight standards; lane 2, outer membrane preparation of P. gingivalis W50. Arrows indicate the positions of the immunodominant 115- and 55-kDa outer membrane proteins. (B) Western blot analysis of whole-cell preparations of E. coli XL-Blue containing pUC18 (lane 1), E. coli clone PM1 containing a 9.5-kb fragment of P. gingivalis W50 DNA cloned in pUC18 (lane 2), E. coli subclone SM containing a 7.5-kb fragment of P. gingivalis W50 DNA (lane 3), and an outer membrane preparation of P. gingivalis W50 (lane 4). Western analysis was performed with the monoclonal antibody DRU55.5.
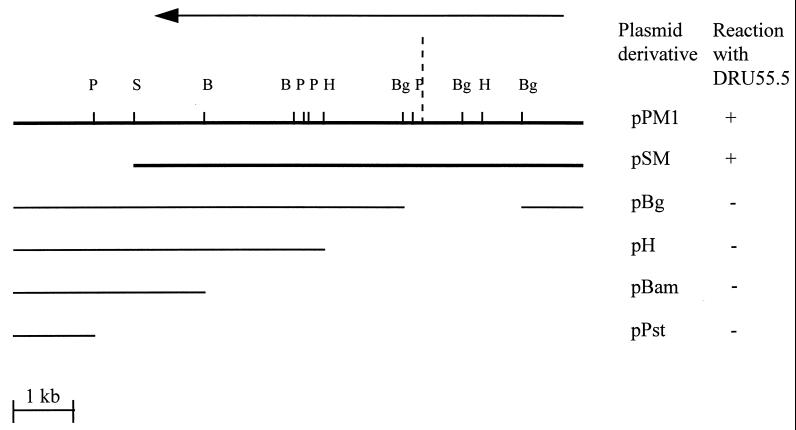
To localize the gene expressing the 55-kDa protein, subclone analysis was performed on the 9.5-kb pPM1 insert by restriction digestion. SmaI digestion of pPM1 and recircularization generated a 7.5-kb subclone, pSM (Fig. 2), which maintained expression of the 55-kDa protein (Fig. 1B). Restriction analysis with the enzymes BglII, HindIII, BamHI, and PstI, whose recognition sites lie within pPM1, did not generate subclones expressing the 55-kDa protein (Fig. 2).
FIG. 2.
Schematic diagram of restriction digest-generated derivatives of pPM1 and their reaction with the monoclonal antibody DRU55.5. The fragment shown is cloned in the BamHI site of pUC18, with the residual pUC18 multiple cloning sites of EcoRI and HindIII located to the left and right of the insert, respectively. Restriction enzyme sites in pPM1: S, SmaI; Bg, BglII; H, HindIII; B, BamHI; and P, PstI. +, positive reaction with DRU55.5 in Western blot analysis; −, nonreactivity with DRU55.5 antibody. The arrow depicts the direction of exonuclease III digestion in the construction of the nested-deletion derivatives. The dashed line represents the point at which loss of reactivity with DRU55.5 occurs, between the first 9 nested deletion derivatives and the remaining 15 derivatives.
Localization of the gene encoding the 55-kDa antigen within pSM.
The 7.5-kb P. gingivalis insert in pSM, which expressed the 55-kDa antigen, was chosen as a template to generate a set of nested-deletion derivatives. pSM was subjected to progressive unidirectional digestion by the enzyme exonuclease III followed by blunting with S1 nuclease. This resulted in the production of 24 pSM derivatives. The insert sizes of these derivatives ranged from 0.2 to 7.5 kb. Western blot analysis of the E. coli clones with the monoclonal antibody DRU55.5 revealed a positive reaction with the nine largest derivatives (5.4 to 7.5 kb), while the smaller derivatives were nonreactive with the antibody. These results, coupled with the results of the Western analysis of the subclones of pPM1 generated by restriction digestion, localized the coding region for the DRU55.5 epitope to within the initial 3 to 4 kb of the pSM subclone (Fig. 2).
Sequence analysis of the pSM insert and identification of the ragAB locus.
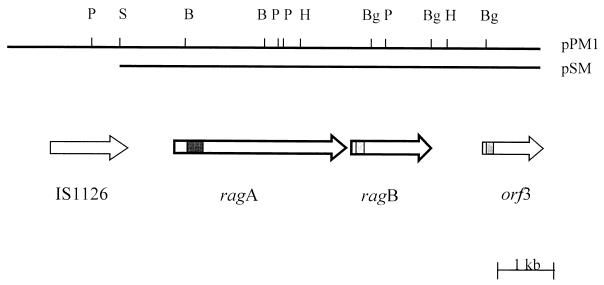
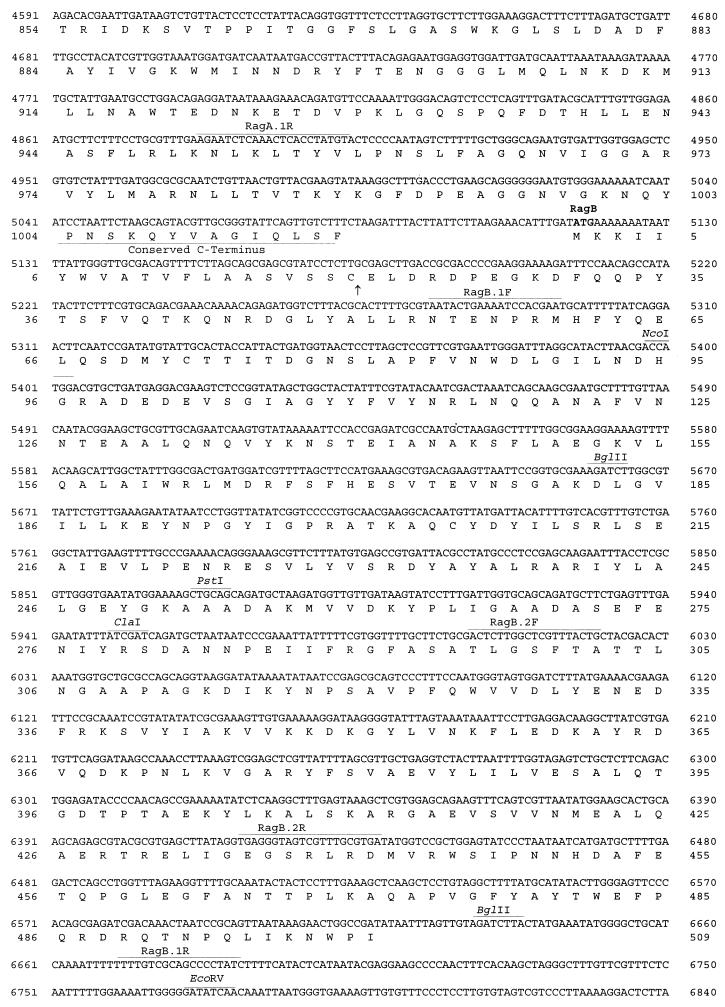
Nucleotide sequence analysis of pSM and pPM1 indicated that there are two major open reading frames (ORFs), which are flanked by two potentially mobile elements. A 1.6-kb ORF encoding a protein of approximately 55 kDa is found in the first 3 to 4 kb of pSM. Western blot analysis of the nested-deletion clones had previously shown the epitope recognized by DRU55.5 to lie within this region, indicating that the 1.6-kb ORF identified encodes the antigenic 55-kDa outer membrane protein. A 3.1-kb ORF encoding a protein with a calculated molecular size of 115 kDa is situated 30 bp upstream of the 1.6-kb gene (Fig. 3). Due to the direction of transcription of these two ORFs and their close proximity to each other, the gene for the 115-kDa protein was named ragA (for receptor antigen A), and the gene for the 55-kDa outer membrane protein was named ragB.
FIG. 3.
Schematic diagram of the genetic organization of P. gingivalis W50 DNA in pPM1 and pSM. The horizontal bar represents the 9.5- and 7.5-kb P. gingivalis DNA fragments cloned in pPM1 and pSM, respectively; the vertical bars indicate positions of restriction enzyme sites. Restriction enzyme sites: S, SmaI; Bg, BglII; H, HindIII; B, BamHI; P, PstI. The arrows indicate the locations and directions of ORFs, with known motifs shaded: ■, TonB box sites; ░⃞, signal sequence motif of lipoproteins. The heavier outlined arrows of ragA and ragB represent the difference in the G+C contents of these genes in comparison to the G+C content of P. gingivalis genes.
An incomplete ORF, orf3, is present at the 3′ end of the pSM insert approximately 1 kb downstream of ragB (Fig. 3). Database homology searches of the region between ragB and orf3 revealed sequence similarity at the amino acid level with localized regions of a number of potentially mobile elements, including a putative transposase from Vibrio cholerae (accession no. AF004384) and the P. gingivalis insertion sequence element PGIS2 (41). In addition, regional similarity was also found with the H repeat-associated protein (42) of E. coli (accession no. P28917).
In the 5′ region of pSM and upstream of ragA is 300 bp corresponding to the C-terminal region of the product of the 1.3-kb P. gingivalis insertion sequence element IS1126 (27). Subsequent sequence analysis of the 5′ end of pPM1 revealed the additional 1 kb of this insertion sequence ORF, thus identifying a complete copy of IS1126 (Fig. 3). Sequence comparison between IS1126W83, sequenced by Maley and Roberts (27) from P. gingivalis W83, and the copy of IS1126W50 present in pPM1 demonstrated 97% homology at the nucleic acid level. Examination of the restriction enzyme sites of IS1126W50 revealed an additional PstI site at position 806 (Fig. 4), indicating heterogeneity within the transposase between the two P. gingivalis strains.
FIG. 4.
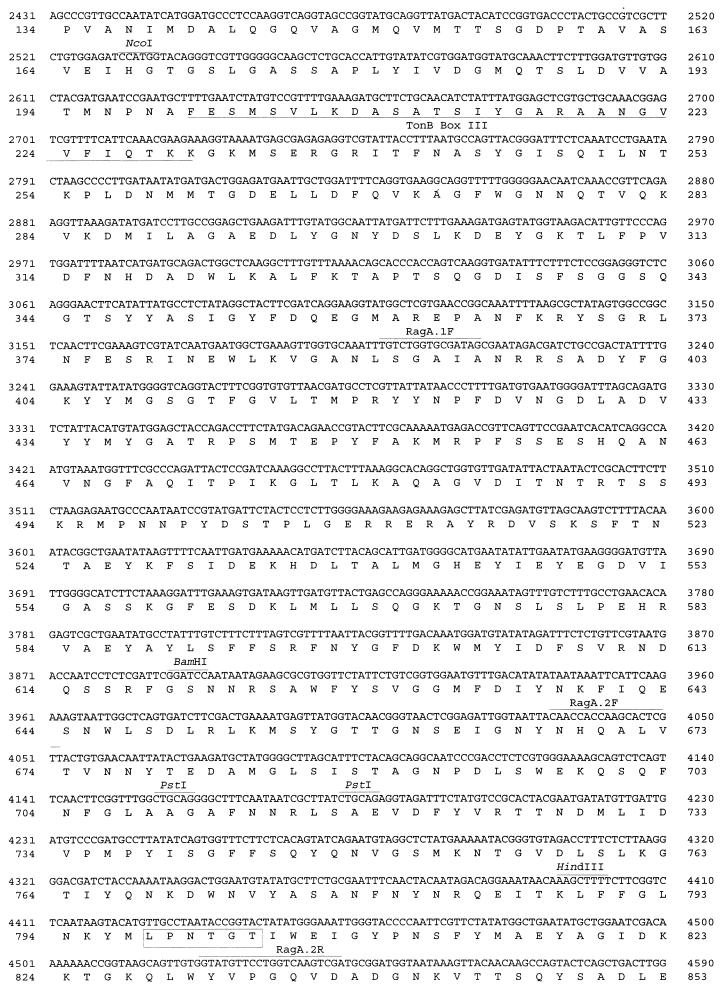
Nucleotide and amino acid sequences of the coding regions of the P. gingivalis W50 insert in pPM1. Sequences shown are those of IS1126, ragA, ragB, and the incomplete orf3. The putative RBS, the E. coli −35 and −10 promoter sites, and the methionine ATG start codons are shown in boldface. Restriction enzyme and primer sites are shown above the sequence and underlined. The 12-bp inverted repeats of IS1126 are depicted in boldface. In the RagA sequence the TonB boxes and conserved TonB C terminus are labelled and underlined, and the conserved hexapeptide motif involved in membrane anchoring is boxed. The signal peptidase II cleavage sites are indicated by arrows at the cysteine residues in the RagB and Orf3 deduced amino acid sequences.
Examination of the G+C content revealed a ratio of 47.5% for IS1126, which is comparable to the expected 46 to 48% ratio for P. gingivalis genes. However, analysis of ragA and ragB revealed a significantly lower G+C ratio of approximately 42%. Furthermore, the noncoding region downstream of ragB with deduced sequence similarity to transposases also displayed a G+C ratio (39%) different from that of the remainder of the chromosome. The difference in the G+C ratios suggests that this entire locus may have originated from a source other than P. gingivalis via a horizontal gene transfer event.
Analysis of the nucleic acid sequence upstream of the ragA start codon revealed putative promoter sequences at positions 1791 to 1796 and 1835 to 1840 which share 100 and 85% homology, respectively, with the −35 and −10 E. coli consensus promoter sequences. An E. coli consensus ribosome binding site (RBS) (37) could not be located. Examination of the sequence upstream of the ragB start codon could not identify a promoter sequence, suggesting either that ragB does not contain a typical E. coli promoter sequence or that transcription of ragB may be linked to that of ragA. Upstream of orf3 are second putative promoter sequences at positions 7126 to 7131 and 7167 to 7173 which share 100 and 85% identity with the E. coli −35 and −10 promoter sequences, respectively. A putative RBS with 85% identity to the E. coli RBS is located at positions 7490 to 7495 (Fig. 4) preceding orf3.
Database analysis of the ragA, ragB, and orf3 genes.
Nucleic acid and amino acid homology searches of the National Center for Biotechnology Information database with the sequence encoding the 55-kDa protein (RagB) did not yield significant similarities with any existing sequences. However, a protein motif search with the deduced amino acid sequence revealed the presence of a signal sequence typical of lipoproteins (19). A cysteine residue at position 20 in the amino acid sequence (Fig. 4) denotes the specific cleavage site for signal peptidase II, the enzyme responsible for signal sequence cleavage of lipoproteins. Other features typical of a signal sequence include a net positive charge at the N terminus (the start methionine followed by two lysine residues), a hydrophobic core (residues 4 to 16), and, following signal peptidase II cleavage, a net negative charge at the N terminus of the mature protein starting at residue 20. Hydropathy plot analysis of the amino acid sequence of RagB also demonstrated the hydrophobic region at the N terminus typical of signal sequences (not shown).
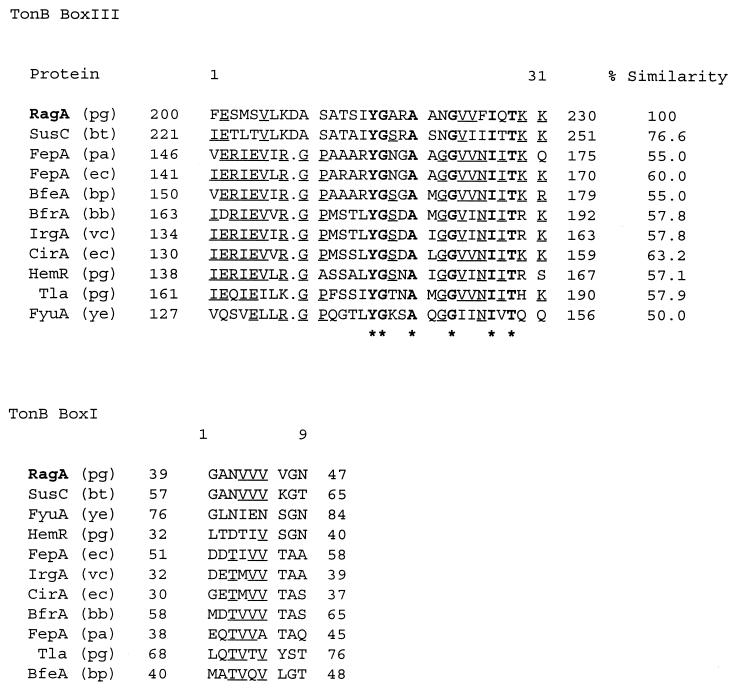
Significant homology was found in database searches with the 3.1-kb ragA gene. RagA was found to have significant sequence similarity to several protein sequences, including the TonB-dependent outer membrane receptor protein SusC (for starch utilization system gene C) of Bacteroides thetaiotamicron (34), the TonB-dependent colicin I receptor (CirA) of E. coli (16), and the TonB-linked adhesin (Tla) of P. gingivalis (2). The homology between RagA and SusC is significantly greater (probability value of −85) than those between RagA and CirA and between RagA and Tla (probability values of −4) and spans the entire length of the SusC protein sequence. SusC is a TonB-linked receptor which forms part of a complex involved in the uptake and utilization of malto-oligosaccharides and starch (34).
The regions of RagA with greatest homology to SusC are located primarily in the first 300 residues of the N terminus and the last 30 residues at the extreme C terminus of the protein and suggest a relationship between RagA and TonB-linked receptors in other gram-negative bacteria. A multiple alignment of the putative TonB boxI and TonB boxIII regions of RagA with the TonB boxes of SusC and other TonB-linked receptors and their percent similarity values are shown in Fig. 5.
FIG. 5.
Multiple alignments of TonB boxes with the highest similarity scores compared to P. gingivalis RagA. The receptors are SusC (starch utilization), FepA (ferric enterochelin), BfeA (ferrichrome-iron), BfrA (exogenous ferric siderophore), IrgA (iron-regulated outer membrane), CirA (colicin 1), HemR (hemin-regulated protein), Tla (TonB linked adhesion), and FyuA (yersiniabactin siderophore). Abbreviations for bacteria: pg, P. gingivalis; bt, B. thetaiotamicron; pa, Pseudomonas aeruginosa; ec, E. coli; bp, Bordetella pertussis; bb, Bordetella bronchiseptica; vc, V. cholerae; and ye, Y. enterocolitica. Sequences are aligned in descending order of relatedness. Boldface amino acid residues correspond to residues conserved throughout the alignment, with asterisks indicating the consensus sequence, while the underlined residues are conserved in more than 50% of the sequences. Amino acid sequence positions are shown at the beginning and end of each sequence. For boxIII the percentage of similarity is calculated with reference to RagA.
The C-terminal amino acid residues of RagA also exhibit similarity to TonB-dependent receptor proteins. A C-terminal phenylalanine residue is highly conserved in TonB-dependent receptors, as are hydrophobic amino acids at positions −3, −5, −7, and −9 from the C terminus (taking the C-terminal phenylalanine residue as −1). These features are thought to be important in outer membrane protein assembly and sorting (40). An additional feature identified by protein motif searches of RagA is a conserved hydrophobic hexapeptide, LPxTGT (amino acid residues 798 to 803), which is involved in the anchoring of the protein to the cell wall (36). Hydropathy plot analysis also indicated a hydrophobic region at the N terminus, which is typical of a signal sequence, and a hydrophobic region at the C terminus due to the presence of the conserved TonB-linked C-terminal motif (not shown). From database searches, sequence analysis, and hydropathy plot analysis of the gene, RagA bears all the features typical of a TonB-linked outer membrane receptor.
Nucleic acid and amino acid sequence searches of the incomplete ORF, orf3, did not yield significant homology with any known sequence. However, a protein motif search did reveal the presence of a lipoprotein signal sequence, with the signal peptidase II cleavage site located before the cysteine residue at amino acid position 26 (Fig. 4).
Northern analysis of ragA and ragB: presence of a rag operon.
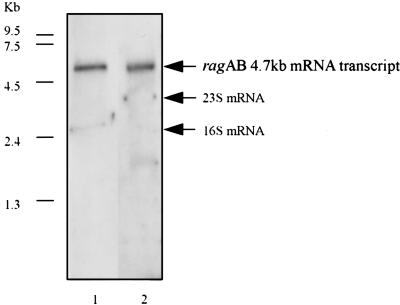
Transcription of ragA and ragB in P. gingivalis W50 was examined by Northern blot analysis of total RNA from exponentially grown cells (Fig. 6). A single mRNA transcript of approximately 4.7 kb hybridized to both the ragA and ragB probes, indicating that ragA and ragB are cotranscribed as a polycistronic message. This result agrees with the earlier finding that an E. coli consensus promoter sequence could not be found for ragB and also with the finding that a 55-kDa-antigen-expressing clone could be isolated only from a P. gingivalis library with insert sizes of between 5 and 12 kb. The finding that ragA and ragB form a single transcriptional unit strongly suggests that the products of these genes are functionally linked.
FIG. 6.
Northern analysis of RagA and RagB expression in exponentially grown P. gingivalis W50. Lane 1, P. gingivalis W50 RNA probed with a PCR-amplified 1.6-kb ragA probe; lane 2, P. gingivalis W50 RNA probed with a PCR-amplified 1.5-kb ragB probe. The arrows indicate the position of the ragAB mRNA transcript of approximately 4.7 kb and the positions of the 23S and 16S mRNAs.
Distribution analysis of ragA, ragB, and orf3 among P. gingivalis laboratory strains.
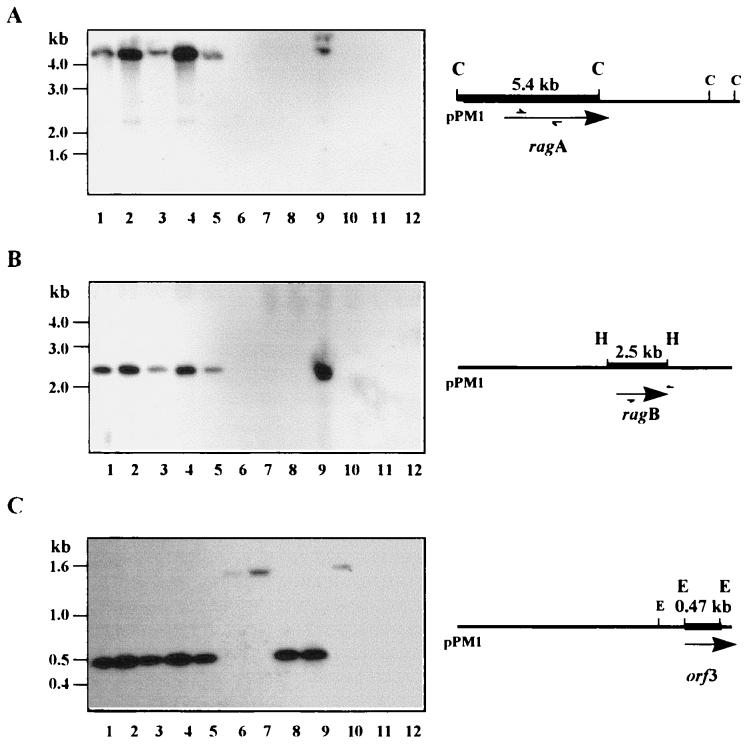
Previous immunochemical investigations had indicated that RagB (the 55-kDa antigen) is expressed in only a proportion of P. gingivalis strains (31). To determine whether this was reflected in a restricted distribution of the ragAB genomic locus, Southern hybridization experiments were performed under stringent conditions. Genomic DNAs from laboratory strains of P. gingivalis, P. asaccharolytica ATCC 25260, and E. coli XL-1 Blue were restricted and probed with a 32P-labelled DNA fragment. Analysis of the ragA, ragB, and orf3 distributions was performed as follows: chromosomal DNA was restricted with ClaI and probed with an internal 1.6-kb PCR-amplified fragment of ragA (Fig. 7A), chromosomal DNA was restricted with HindIII and probed with a 1.5-kb PCR-amplified product of ragB (Fig. 7B), and chromosomal DNA was restricted with EcoRV and probed with a 470-bp EcoRV-restricted fragment of orf3 (Fig. 7C).
FIG. 7.
Southern blot analysis of the distributions of ragA, ragB, and orf3 in laboratory P. gingivalis strains. (A) P. gingivalis chromosomal DNA was digested with ClaI (C) and probed with a 32P-labelled 1.6-kb PCR-amplified product of ragA. (B) P. gingivalis chromosomal DNA was digested with HindIII (H) and probed with a 32P-labelled 1.6-kb amplified product of ragB. (C) P. gingivalis chromosomal DNA was digested with EcoRV (E) and probed with a 0.47-kb 32P-labelled EcoRV-restricted fragment of orf3. Lanes 1 to 10, P. gingivalis W50, W50Be1, W50Br1, W83, LB13D-3, 381, 11834, 23A4, WPH35, and WPH34, respectively; lane 11, E. coli XL-Blue; lane 12, P. asaccharolytica ATCC 25260.
The results indicate that ragA and ragB are codistributed as single copies and show a restricted distribution pattern in the strains examined: ragA and ragB are found in strain W50 and the phenotypic variants W50Be1 and W50Br1 as well as W83, LB13D-3, and WPH35, but both are absent from 381, 11834, 23A4, and WPH34. The codistribution of ragA and ragB is consistent with their transcription as a single unit. This pattern of distribution also complements the immunochemical findings of Millar et al. (31), where 9 of 15 P. gingivalis strains examined were positive in Western blot analysis with the monoclonal antibody DRU55.5. The results from the Western and Southern analyses are exactly correlated except in the case of strain LB13D-3, which contains the ragAB locus but does not express the epitope recognized by DRU55.5.
In contrast, orf3 is present as a single copy in all P. gingivalis strains examined. Strains W50, W50Be1, W50Br1, W83, LB13D-3, 23A4, and WPH35 give the expected 470-bp band size in EcoRV digests of chromosomal DNA, whereas the remaining strains, 381, 11834, and WPH34, which are ragAB negative, give a higher-molecular-size band of greater than 1.5 kb. Thus, the coding sequences of ragA and ragB are found in a restricted number of laboratory strains, while orf3 is found in all P. gingivalis strains examined, although some polymorphism in the restriction profile at the orf3 locus is evident and this partially correlates with the presence or absence of the ragAB operon.
Distribution analysis of the rag locus among clinical isolates.
In order to determine if the restricted distribution pattern of ragA and ragB observed among laboratory P. gingivalis strains is reflected in vivo, PCR analysis with primer ragA.2 or ragB.2 (Table 1) was performed on 38 P. gingivalis clinical isolates. Chromosomal DNAs from these isolates were used as templates in the PCRs. The 38 isolates were obtained from 17 patients with chronic adult periodontitis. One to three sites per patient were sampled, and one to three isolates were obtained from each site (3).
The expected 509- and 436-bp amplicons of ragA and ragB, respectively, were amplified from 17 of the 38 isolates, corresponding to 5 of the 17 patients. ragA and ragB were codistributed in all positive isolates. These results confirm the Southern blot analysis of laboratory strains and also demonstrate that the restricted distribution pattern is not an artifact of repeated subculture of laboratory strains but is also reflected in fresh isolates.
Analysis of the ragAB locus in clinical samples.
The finding that the ragAB locus is present in only a restricted proportion of P. gingivalis clinical isolates while the immune response to RagB is significantly elevated in periodontal disease patients may indicate that strains which harbor this locus are more associated with destructive periodontal disease than ragAB-negative strains or, alternatively, that RagB may be an important antigen expressed by another bacterial species in the periodontal microflora. In order to address these possibilities, we undertook a preliminary investigation to determine by PCR the presence of this locus in subgingival plaque samples from periodontally healthy individuals (n = 33) and periodontal disease patients (n = 28).
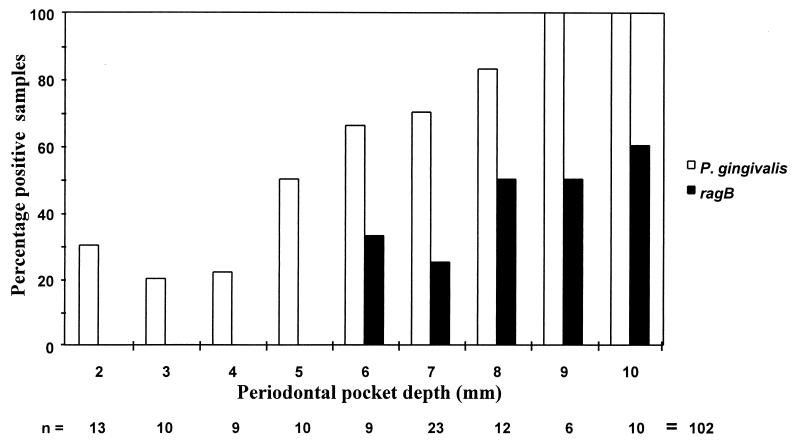
PCR of the P. gingivalis 16S RNA gene was performed on the samples to establish the presence or absence of this organism (Fig. 8). On the basis of the 16S RNA PCR, P. gingivalis was present in 5 of 67 (7.5%) subgingival samples from the 33 periodontally healthy individuals and in 7 of 30 (23%) samples from shallow pockets (periodontal pocket depths of <4 mm) and 55 of 72 (76%) deep pockets (pocket depths of 5 to 10 mm) from periodontal disease patients (n = 28). To establish the presence of the ragAB locus in the samples, PCR was performed with primers to amplify ragB only (Fig. 8), since ragB and ragA were shown to be codistributed in both P. gingivalis laboratory strains and clinical isolates. ragB was not detected in samples which were positive for P. gingivalis 16S RNA from periodontally healthy individuals or in P. gingivalis-positive samples from shallow pockets in periodontal disease patients. However, ragB was detected in 20 of 55 (36%) of P. gingivalis-positive samples from deep pockets in the periodontal disease patients.
FIG. 8.
PCR analysis of subgingival paper point samples from periodontal disease patients to detect the presence of P. gingivalis (A) and ragB (B). The primers used were P. gingivalis 16S RNA primers to amplify a 404-bp product and ragB.2 primers to amplify a 435-bp product. Lanes 1, 1-kb ladder; lanes 2 to 17, subgingival paper point PCRs; lanes 18, negative control (distilled H2O as template); lanes 19, positive control (200 ng of P. gingivalis W50 chromosomal DNA as template). Lanes 12, 15, and 17 represent three of the eight samples which are PCR positive for ragB but PCR negative for P. gingivalis. Ten microliters from a total volume of 100 μl of the PCR mixtures was run on a 1% agarose gel.
The results of the PCR analysis of the clinical samples are shown in Fig. 9. Statistical analysis of these results revealed a positive correlation between detection of P. gingivalis and increasing pocket depth (P = 0.01) and a stronger association between P. gingivalis ragB-positive samples and increasing pocket depth (P = 0.001). Overall, these data suggest that P. gingivalis strains harboring the ragAB locus are associated more with sites of periodontal destruction than with sites from periodontally healthy individuals or shallow pockets in periodontal disease patients.
FIG. 9.
Percentages of P. gingivalis and ragB PCR-positive subgingival samples from periodontal disease patients. The y axis represents the percentages of P. gingivalis- and ragB-positive samples. The x axis represents both the periodontal pocket depth and the total sample number (n) from each pocket depth.
PCR of ragB was also performed on the P. gingivalis-negative samples from periodontal disease patients, and 8 of 40 samples were found to be ragB positive. These samples were subsequently reanalyzed for the presence of P. gingivalis by PCR with primers to amplify the 1.7-kb catalytic domain of the P. gingivalis-specific rgpA protease gene (3) and were confirmed to be negative. These results suggest that the ragAB locus may be present in the genome of another member(s) of the periodontal microbial community.
Together these findings suggest that the elevated immune response to RagB in periodontal disease patients may be due to the association of P. gingivalis RagAB-positive strains with sites of destructive periodontal disease and also to the presence of a RagB homolog in another bacterial species in the periodontal microflora.
DISCUSSION
The exact role that P. gingivalis plays in the initiation and progression of periodontitis remains unclear. It is not yet established whether P. gingivalis is a minor component of the indigenous oral flora and behaves as an opportunistic pathogen, with all strains having equal virulence potential, or whether virulent and avirulent strains exist within this species. DNA fingerprinting and multilocus enzyme electrophoresis studies have shown a diverse range of genotypes among P. gingivalis strains, but no distinct correlation between a specific genotype and disease has been made (23, 25, 30).
The present investigation was prompted by the analysis of the specific immune response of periodontal disease case sera to surface antigens of P. gingivalis W50. The results demonstrate that the 55-kDa outer membrane antigen identified in the earlier investigations is encoded by a gene (ragB) which is cotranscribed with an upstream gene (ragA). Since the predicted size of RagA is approximately 115 kDa, it is possible that this antigen corresponds to the abundant antigen with this molecular mass in P. gingivalis W50 outer membranes which was also identified in the initial case-control investigation (11). On the basis of the signal peptidase II cleavage site motif at the N terminus, the 55-kDa outer membrane antigen, RagB, is a lipoprotein, while RagA is homologous to the family of TonB-linked outer membrane receptors which are involved in the recognition and active transport of specific external ligands by a wide range of gram-negative species (7). These receptors interact with TonB in the periplasm at distinct regions known as the TonB boxes (boxI, boxII, and boxIII), which are located near the N-terminal amino acid sequences of the receptor proteins. The codistribution and cotranscription of ragA and ragB strongly suggest that they are functionally linked, and the products may form a complex on the outer surface of P. gingivalis that is involved in a TonB-linked process.
The genetic arrangement at the ragAB locus is very analogous to those for the lactoferrin and transferrin binding systems in Neisseria and Haemophilus species, respectively. In these organisms a TonB-linked outer membrane receptor is also cotranscribed with an outer membrane lipoprotein, and the resulting surface complex is involved in the active transport of lactoferrin and transferrin iron sources (10, 15, 26). However, multiple alignments between RagB and the analogous transferrin and lactoferrin binding lipoproteins, Tbp2 and Lbp2, did not identify any significant homology between these proteins. Furthermore, database searches with RagA revealed only a low homology with TonB receptors involved in iron uptake but an extremely high similarity to SusC, a TonB-linked receptor involved in maltose uptake in B. thetaiotamicron. It is possible, therefore, that the putative RagAB surface complex of P. gingivalis is involved in either the uptake or recognition of a specific carbohydrate or glycoprotein. Given the asaccharolytic nature of P. gingivalis, it is likely that carbohydrate uptake would be linked to an anabolic process.
The significantly lower G+C content of the ragAB locus than of the rest of the P. gingivalis chromosome and its restricted distribution in laboratory strains and clinical isolates is consistent with its acquisition from a foreign source via horizontal gene transfer. The ragB PCR analysis of subgingival samples demonstrated that this locus can be detected in the absence of P. gingivalis. The other bacterial species which harbor the ragAB locus in these subgingival samples may be the source of this locus in P. gingivalis or, equally likely, may have acquired this locus by a similar horizontal gene transfer event. Several other characteristics indicate that this locus may correspond to one of a rapidly growing family of virulence-associated chromosomal loci termed pathogenicity islands (PAIs).
PAIs are chromosomal elements encoding virulence determinants which are present in pathogenic strains but are absent or rarely found in nonpathogenic strains of the same species. These genetic elements have been reported to range in size from a 1.6-kb single gene to large 35- to 190-kb multigene elements. Frequently observed characteristics of PAIs include a G+C content different from that of the DNA of the host bacterium, mobility or the presence of mobile genetic elements, and the capability of horizontal gene transfer (17). Acquisition of PAIs enhances the genetic flexibility of bacterial species and contributes to the spread and evolution of virulence. PAIs have been found in a range of gram-negative organisms and include the hemolysin gene cluster (hly) of uropathogenic E. coli 536, the cag (cytotoxin-associated antigen genes A to T) gene cluster of Helicobacter pylori, and the hms, fyuA, and irp genes involved in iron uptake and storage in Yersinia species (8, 9, 29, 32).
PAIs are found associated with mobile elements such as integrases, transposons, and insertion sequence elements. The ragAB locus is flanked by two potentially mobile elements: IS1126W50 upstream of ragA and a region with sequence similarity to transposons downstream of ragB. Southern blot analysis of IS1126 has demonstrated that this element is found in all P. gingivalis strains examined to date and is present in multiple copies, ranging from 6 to 12, depending on the strain (27). The 1.3-kb copy of IS1126W50 upstream of ragA encodes a transposase of approximately 41 kDa, indicating that this copy of IS1126W50 is capable of transposition. Unlike IS1126W50, the region downstream of ragB with transposon sequence similarity appears to be noncoding, which may be indicative of a previous integration event between P. gingivalis and another organism.
Database analysis of the region downstream of ragB revealed localized similarity to PGIS2 (41). This putative transposase bears significant homology to several mobile elements, including the predicted product of H repeats in E. coli, the product of the orfH region of Salmonella enterica, and the putative transposase IS1358 of V. cholerae. The H-repeat elements in E. coli are associated with recombination hot spots (Rhs elements). These elements have no known function but have recently been incorporated into the E. coli genome and are found in many but not all wild-type E. coli isolates (42). The IS1358 element in V. cholerae is found in the lipopolysaccharide rfb gene cluster and is associated with novel lipopolysaccharide genes which, on the basis of G+C analysis, are considered to have arisen from a foreign source. IS1358 is thought to be responsible for the generation of a new serotype, 0139, by mediating the incorporation of these novel O-antigen genes into the V. cholerae rfb gene cluster (39). It is conceivable that PGIS2, which has been shown to be mobile in P. gingivalis (41), and related mobile elements are capable of horizontal gene transfer and that this transfer facilitates a greater genetic flexibility in the recipient bacterial species.
PAIs carry virulence-associated genes and have a restricted distribution pattern which correlates with the presence in and absence from virulent and avirulent strains of the same species, respectively. As outlined above, the sequence homologies of RagA and RagB suggest that they may have a role in recognition and transport via a TonB-mediated mechanism. In this respect, the ragAB locus may be analogous to fyuA, a TonB-dependent receptor in the high-pathogenicity island of Yersinia enterocolitica which is absent from low-pathogenicity Y. enterocolitica strains (8). Loss of the high-pathogenicity island in Yersinia pestis does not abolish the capacity to cause disease but prevents the most fulminant expression of bacterial pathogenicity (33). There are few reliable measures of the virulence potentials of different P. gingivalis strains in relation to periodontal disease. However, small-animal models of soft tissue destruction have been employed to examine the effects of gene inactivation or growth conditions on the virulence of this organism (20, 32). Although few strain-to-strain comparative data are available, the ragAB locus is present in the common strains W50 and W83, which are highly invasive in small-animal models, and in WPH35 (unpublished data) and is absent from 381 and 33277, which produce nonspreading localized lesions at the site of inoculation (32).
In the absence of definitive methods for predicting the virulence potentials of P. gingivalis strains which harbor the ragAB locus, in the present study we used PCR of subgingival plaque samples to determine whether ragAB-positive P. gingivalis strains are associated more frequently with sites of periodontal destruction than with periodontally healthy sites. Surprisingly, given the relatively small sample size, ragAB-positive P. gingivalis strains were significantly more associated with diseased sites than with healthy sites. Larger studies of the ragAB-positive status of P. gingivalis from sites of well-defined clinical conditions are required to confirm this association, since there may be significant diagnostic implications. Furthermore, on the basis of the data presented in the present report, analysis of the function of RagA and RagB is warranted, and these studies are under way through the construction and phenotypic analysis of isogenic mutants.
In summary, the 55-kDa immunodominant surface antigen of P. gingivalis W50 is the product of a polycistronic message from a locus we have termed ragAB. The antigen gene is cotranscribed with a second gene, the product of which resembles TonB-linked receptors from other gram-negative bacteria, in particular SusC from B. thetaiotamicron. RagA and RagB may therefore cooperate at the surface of P. gingivalis W50 in the transport or recognition of an external ligand. On the basis of a G+C content different from that of the remainder of the host chromosome, the presence of mobility elements at this locus, a restricted distribution in laboratory strains and clinical isolates, and the presence of this locus in clinical samples which did not contain P. gingivalis, we propose that the ragAB operon has arisen by horizontal gene transfer from a foreign source which may also be a component of the periodontal microflora. The greater prevalence of ragAB-positive P. gingivalis strains at sites of periodontal destruction than at periodontally healthy sites suggests that RagAB may confer a selective advantage to strains which harbor these genes and hence that the ragAB locus may correspond to a novel PAI of this species.
ACKNOWLEDGMENTS
We are grateful to Catherine Williams and Francis Hughes for help with the clinical sampling.
This work was supported in part by the Research Advisory Committee of St. Bartholomew’s and The Royal London School of Medicine and Dentistry and by the Medical Research Council (grants G9711648PB and PG9318173).
REFERENCES
- 1.Aduse-Opoku J, Muir J, Slaney J M, Rangarajan M, Curtis M A. Characterization, genetic analysis, and expression of a protease antigen (PrpRI) of Porphyromonas gingivalisW50. Infect Immun. 1995;63:4744–4754. doi: 10.1128/iai.63.12.4744-4754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aduse-Opoku J, Slaney J M, Rangarajan M, Muir J, Young K A, Curtis M A. The Tla protein of Porphyromonas gingivalisW50: a homolog of the RI protease precursor (PrpRI) is an outer membrane receptor required for growth on low levels of hemin. J Bacteriol. 1997;179:4778–4788. doi: 10.1128/jb.179.15.4778-4788.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaker R P, Aduse-Opoku J, Batten J E, Curtis M A. Natural variation within the principal arginine-specific protease gene, prpRI, of Porphyromonas gingivalis. Oral Microbiol Immunol. 1997;12:298–302. doi: 10.1111/j.1399-302x.1997.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 4.Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 5.Ashley F P, Gallagher J, Wilson R F. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis, Bacteroides intermidiusand spirochaetes in the subgingival microflora of adolescents and their relationship with the amount of supragingival plaque and gingivitis. Oral Microbiol Immunol. 1988;3:77–82. doi: 10.1111/j.1399-302x.1988.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 6.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun V. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 8.Carniel E, Guilvont I, Prentice M. Characterization of a large chromosomal “high-pathogenicity island” in biotype 1B Yersinia enterocolitica. J Bacteriol. 1996;178:6743–6751. doi: 10.1128/jb.178.23.6743-6751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelissen C N, Sparling P F. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin binding proteins. J Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis M A, Slaney J M, Carman R J, Johnson N W. Identification of the major surface protein antigens of Porphyromonas gingivalisusing IgG antibody reactivity of periodontal case-control serum. Oral Microbiol Immunol. 1991;6:321–326. doi: 10.1111/j.1399-302x.1991.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 12.Curtis M A, Aduse-Opoku J, Slaney J M, Rangarajan M, Booth V, Cridland J, Shepherd P. Characterization of an adherence and antigenic determinant of the ArgI protease of Porphyromonas gingivaliswhich is present on multiple gene products. Infect Immun. 1996;64:2532–2539. doi: 10.1128/iai.64.7.2532-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher H M, Schenkein H A, Macrina F L. Cloning and characterization of a new protease gene (prtH) from Porphyromonas gingivalis. Infect Immun. 1994;62:4279–4286. doi: 10.1128/iai.62.10.4279-4286.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray-Owen S D, Schryvers A S. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 16.Griggs D W, Tharp B B, Konisky J. Cloning and promoter identification of the iron-regulated cir gene of Escherichia coli. J Bacteriol. 1987;169:5343–5352. doi: 10.1128/jb.169.12.5343-5352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 18.Han N, Whitlock J, Progulske-Fox A. The hemagglutinin gene A (hagA) of Porphyromonas gingivalis381 contains four large contiguous direct repeats. Infect Immun. 1996;64:4000–4007. doi: 10.1128/iai.64.10.4000-4007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinje V G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989;2:531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]
- 20.Klausen B, Evans R T, Ramamurthy N S, Golub L M, Sfintescu C, Lee C, Bedi G, Zambon J J, Genco R J. Periodontal bone level and gingival proteinase activity in gnotobiotic rats immunized with Bacteroides gingivalis. Oral Microbiol Immunol. 1991;6:193–201. doi: 10.1111/j.1399-302x.1991.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan H B, Lewin A, Fellay R, Broughton W J, Pueppke S G. Differential expression of nods accounts for the varied abilities of Rhizobium fredii USDA257 and Rhizobium sp strain NGR234 to nodulate Leaucaenaspp. Mol Microbiol. 1992;6:3321–3330. doi: 10.1111/j.1365-2958.1992.tb02200.x. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Loos B G, Mayrand D, Genco R J, Dickinson D P. Genetic heterogeneity of Porphyromonas (Bacteroides) gingivalisby genomic DNA fingerprinting. J Dent Res. 1990;69:1488–1493. doi: 10.1177/00220345900690080801. [DOI] [PubMed] [Google Scholar]
- 24.Loos B G, Dyer D W. Restriction fragment length polymorphism analysis of the fimbrillin locus fimA of Porphyromonas gingivalis. J Dent Res. 1992;5:1173–1181. doi: 10.1177/00220345920710050901. [DOI] [PubMed] [Google Scholar]
- 25.Loos B G, Dyer D W, Whittam T S, Selander R K. Genetic structure of populations of Porphyromonas gingivalisassociated with periodontitis and other oral infections. Infect Immun. 1993;61:204–212. doi: 10.1128/iai.61.1.204-212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loosmore S M, Yang Y, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S C, Klein M H. Cloning and expression of the Haemophilus influenzaetransferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 27.Maley J, Roberts I S. Characterisation of IS1126 from Porphyromonas gingivalisW83: a new member of the IS4 family of insertion sequence elements. FEMS Microbiol Lett. 1994;123:219–224. doi: 10.1111/j.1574-6968.1994.tb07225.x. [DOI] [PubMed] [Google Scholar]
- 28.Matto J, Saarella M, Alaluusua S, Oja V, Jousimies-Somer H, Asikainen S. Detection of Porphyromonas gingivalisfrom saliva by PCR by using a simple sample processing method. J Clin Microbiol. 1998;36:157–160. doi: 10.1128/jcm.36.1.157-160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coliK-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 30.Menard C, Mouton C. Clonal diversity of the taxon Porphyromonas gingivalisassessed by random amplified polymorphic DNA fingerprinting. Infect Immun. 1995;63:2522–2531. doi: 10.1128/iai.63.7.2522-2531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millar D J, Scott E E, Slaney J M, Benjamin S U P, Curtis M A. Production and characterisation of monoclonal antibodies to the principle sonicate antigens of Porphyromonas gingivalisW50. FEMS Immunol Med Microbiol. 1993;7:211–222. doi: 10.1111/j.1574-695X.1993.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 32.Neiders M E, Chen P B, Suido H, Reynolds H S, Zambon J J, Shlossman M, Genco R J. Heterogeneity of virulence among strains of Bacteroides gingivalis. J Periodontal Res. 1989;24:192–198. doi: 10.1111/j.1600-0765.1989.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 33.Rakin A, Urbitsch P, Heeseman J. Evidence for two evolutionary lineages of highly pathogenic Yersiniaspecies. J Bacteriol. 1995;177:2292–2298. doi: 10.1128/jb.177.9.2292-2298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves A R, D’Elia J N, Frias J, Salyers A A. A Bacteroides thetaiotamicronouter membrane protein that is essential for utilization of maltooligosaccharides and starch. J Bacteriol. 1996;178:823–830. doi: 10.1128/jb.178.3.823-830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Schneewind O, Jones K F, Fischetti V A. Sequence and structural characteristics of the trypsin-resistant T6 surface protein of group A streptococci. J Bacteriol. 1990;172:3310–3317. doi: 10.1128/jb.172.6.3310-3317.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shine J, Dalgarno L. The 3 prime terminal sequence of Escherichia coli16S ribosomal RNA: complementarity to nonsense triplets and ribosomal binding sites. Proc Natl Acad Sci USA. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermediusin destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 39.Stroeher U H, Jedani K E, Dredge B K, Morona R, Brown M H, Karageorgos L E, Albert M J, Manning P A. Genetic rearrangements in the rfb regions of Vibrio cholerae01 and 0139. Proc Natl Acad Sci USA. 1995;92:10374–10378. doi: 10.1073/pnas.92.22.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Struyve M, Meas M, Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991;218:141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- 41.Wang C Y, Bond V C, Genco C A. Identification of a second endogenous Porphyromonas gingivalisinsertion element. J Bacteriol. 1997;179:3808–3812. doi: 10.1128/jb.179.11.3808-3812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao S, Sandt C H, Feulner G, Vlazny D A, Antal Gray J, Hill C W. Rhs elements of Escherichia coliK-12: complex composites of shared and unique components that have different evolutionary histories. J Bacteriol. 1993;175:2799–2808. doi: 10.1128/jb.175.10.2799-2808.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]