Abstract
Objective
Examine whether the relationship between the pooled cohort equations (PCE) predicted 10-year risk for atherosclerotic cardiovascular disease (ASCVD) and absolute risk for ASCVD is modified by socioeconomic status (SES).
Design
Population-based longitudinal cohort study—Atherosclerosis Risk in Communities (ARIC)—investigating the development of cardiovascular disease across demographic subgroups.
Setting
Four communities in the USA—Forsyth County, North Carolina, Jackson, Mississippi, suburbs of Minneapolis, Minnesota and Washington County, Maryland.
Participants
We identified 9782 ARIC men and women aged 54–73 without ASCVD at study visit 4 (1996–1998).
Primary outcome measures
Risk ratio (RR) differences in 10-year incident hospitalisations or death for ASCVD by SES and PCE predicted 10-year ASCVD risk categories to assess for risk modification. SES measures included educational attainment and census-tract neighbourhood deprivation using the Area Deprivation Index. PCE risk categories were 0%–5%, >5%–10%, >10%-15% and >15%. SES as a prognostic factor to estimate ASCVD absolute risk categories was further investigated as an interaction term with the PCE.
Results
ASCVD RRs for participants without a high school education (referent college educated) increased at higher PCE estimated risk categories and was consistently >1. Results indicate education is both a risk modifier and delineates populations at higher ASCVD risk independent of PCE. Neighbourhood deprivation did modify association but was less consistent in direction of effect. However, for participants residing in the most deprived neighbourhoods (referent least deprived neighbourhoods) with a PCE estimated risk >10%–15%, risk was significantly elevated (RR 1.65, 95% CI 1.05 to 2.59). Education and neighbourhood deprivation inclusion as an interaction term on the PCE risk score was statistically significant (likelihood ratio p≤0.0001).
Conclusions
SES modifies the association between PCE estimated risk and absolute risk of ASCVD. SES added into ASCVD risk prediction models as an interaction term may improve our ability to predict absolute ASCVD risk among socially disadvantaged populations.
Keywords: preventive medicine, cardiac epidemiology, social medicine, coronary heart disease, epidemiology, public health
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Population-based prospective cohort with over three decades of follow-up data to investigate the development of cardiovascular disease across demographic subgroups are major strengths of this study.
Hospitalisations for coronary heart disease and stroke hospitalisations—an outcome measured—was based on the Atherosclerosis Risk in Communities abstraction of hospital data, and some hospitalisations may be missing.
A potential misclassification bias of area-level deprivation exposure possibly exists due to not accounting for Atherosclerosis Risk in Communities participants moving to different neighbourhoods with a different degree of area-level deprivation exposure.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and morbidity in the USA and globally.1–4 A substantially higher burden of ASCVD is experienced among those with lower socioeconomic status (SES).5–14 The pooled cohort equations (PCE) are currently recommended in the USA to estimate the 10-year risk of ASCVD and guide primary prevention treatment decisions.15–18 The PCE does not currently account for SES factors such as educational attainment or neighbourhood deprivation. However, SES measures may have prognostic value in predicting ASCVD outcomes and identifying populations in greatest need of primary ASCVD prevention.
Existing evidence regarding the prognostic value of controlling for SES in ASCVD prediction models is mixed. A recent analysis showed that PCE overestimated ASCVD risk among low SES populations, but including SES measures such as household income or educational attainment in the PCE model did not improve model calibration.19 Conversely, prior research evaluating the use of SES measures, such as household income or neighbourhood deprivation, with the Framingham Risk Score that estimates coronary heart disease (CHD) risk only, showed that such measures improved model fit statistics.20–22 The latter findings eventually led to ASCVD risk models, such as QRISK2, primarily used in the UK that incorporate the Townsend deprivation score, a neighbourhood measure of deprivation.23–25 Such discrepancies have important implications globally and for the USA, creating uncertainty regarding the importance of incorporating SES into ASCVD risk prediction models and the value of SES as a marker to identify individuals in need of additional ASCVD primary prevention interventions and services.
How prior ASCVD prediction models incorporated SES into the model is a potential reason for the discrepancies in understanding the prognostic value and use of SES in ASCVD prediction models. SES traditionally is modelled as an independent risk factor or confounder.19–22 24 However, SES’s prognostic value in predicting ASCVD risk is likely identifying populations most impacted by proximate causes of ASCVD. If true, SES incorporated into risk prediction models as a risk modifier is more appropriate in determining ASCVD risk than an independent risk factor. For example, the health impact of hypertension over 10 years is different for an individual living in abject poverty vs an individual residing in an affluent neighbourhood. SES likely modifies the association between risk estimated from algorithms that use proximate causes of ASCVD (ie, hypertension and smoking) and actual ASCVD incidence.
This study explored whether SES modifies the association of PCE 10-year estimated risk with actual ASCVD 10-year incidence using data from the Atherosclerosis Risk in Communities (ARIC) study. That is, actual observed ASCVD 10-year incidence will vary depending on the PCE estimated risk and the individual’s SES. We defined SES along two dimensions typically used in social epidemiology research: educational attainment and neighbourhood deprivation.26 Educational attainment as a measure of individual SES was selected over other measures—for example, income level—due to being a stable measure of SES that remain relatively stable over an adult life course when compared with other measures. We hypothesise that the long-term effects of proximate causes of ASCVD measured in the PCE (eg, hypertension and smoking) impact on actual ASCVD incidence are dependent on SES (ie, risk modification).
Methods
Data source
Data obtained for our analyses came from the ARIC study. In brief, the ARIC study is an ongoing prospective observational cohort study of 15 792 men and women age 45–64 years, recruited from population-based sampling from four communities in the USA–Forsyth County, North Carolina, Jackson, Mississippi, suburbs of Minneapolis, Minnesota and Washington County, Maryland.27 The study was designed to investigate the development of cardiovascular disease across demographic subgroups. Follow-up has included seven in-person study visits to-date from the baseline visit in 1987–1989; surveillance of the cohort continues with annual telephone interviews and active surveillance of discharges from local hospitals.
Study population
We restricted our analysis to 11 374 ARIC participants who attended Visit 4 (1996–1998) to maintain an observational cohort that reflected similar temporal trends in ASCVD outcomes as the cohorts used to derive the PCE. We excluded visit 4 participants with prevalent CHD (N=1210), prior stroke (N=231), participants missing clinical variables for ASCVD risk assessment (N=155), and participants missing educational attainment information collected at study visit 1 (N=12). Prevalent CHD was defined as self-reported or physician diagnoses of myocardial infarction at baseline and incident CHD occurring between baseline and visit 4. We defined prevalent stroke as self-reported or physician diagnoses of stroke, transient ischaemic attack and stroke-like symptoms at baseline or hospitalisation for a definite or probable stroke between baseline and visit 4. Due to small numbers, we excluded blacks in Minneapolis and Washington County (N=35). Three participants were excluded due to unclear incident ASCVD dates for a final sample of 9728.
Individual-level covariate measures
Trained staff administered in-home interviews that collected information on demographics, socioeconomic factors, lifestyle and medical comorbidities. Race, gender and educational attainment were self-reported. We used the information on race, gender and educational attainment collected at ARIC visit 1; we used data on age and medical comorbidities collected during visit 4 for our analyses.
We categorised smoking status as current or not current smokers. Hypertension was defined as having a systolic blood pressure of 140 mm Hg or greater (mean of two measurements recorded at study visit), diastolic blood pressure 90 mm Hg or greater (mean of two measurements recorded at study visit) or were taking antihypertensive medications. We classified diabetes as having a fasting blood glucose level ≥126 mg/dL, non-fasting blood glucose≥200 mg/dL, use of antidiabetic medications or self-reported history of physician-diagnosed diabetes. We used total cholesterol and high-density lipoprotein (HDL) levels collected at visit 4 to assess ASCVD risk. Pill bottle review, when available, was performed at every ARIC visit to confirm medication use. Statin medication use at visit 4 was self-reported or based on medications brought to the visit.
SES measures
We examined one individual and one neighbourhood exposure of SES. We classified educational level attainment into three categories: no high school degree, high school/some college or college graduate and above. The Area Deprivation Index (ADI) was used to analyse neighbourhood deprivation.28–30 The ADI is a validated measure of neighbourhood deprivation that uses 17 different markers to measure area-level deprivation from 2000 census block group-level data. We used the participants’ census tract according to the nine-digit zip code to assign ADI. The ADI measures neighbourhood deprivation along a continuum; higher values represent higher levels of neighbourhood deprivation. We stratified ADI into three categories according to IQR. Levels chosen to represent lowest (residing in the least deprived neighbourhoods), top (residing in the most deprived neighbourhoods) and middle two ADI quartiles.
Estimation of ASCVD risk
We estimated individual ASCVD risk using the published PCE covariate parameters.15 The following factors were used to estimate ASCVD risk according to the PCE: age, gender, race (black or other), levels of total cholesterol, levels of HDL cholesterol, systolic blood pressure, evidence of treatment for high blood pressure, diabetes status and current smoker status. We used laboratory measures collected at visit 4 to estimate risk using the PCE. We partitioned the ARIC study population into four categories of 10-year PCE predicted ASCVD risk: 0%–5%, >5%–10%, >10%-15% and >15%.
Ascertainment of myocardial infarction and stroke outcomes
Hospital records were abstracted to identify hospitalisations for myocardial infarction and stroke. CHD and stroke events were classified algorithmically and following physician review and adjudication, as previously published.27 31 Criteria for the incidence of definite or probable myocardial infarction for the ARIC cohort were based on combinations of chest pain, electrocardiographic changes and cardiac enzyme levels during hospitalisation. Classification of events as fatal myocardial infarction was based on the following factors: cause of death on the death certificate for both hospitalised or out of hospital deaths; and diagnoses at the time of hospitalisation from medical records before death. The minimum criterion for definite or probable stroke was evidence of sudden or rapid onset of neurological symptoms lasting >24 hours or leading to death, in the absence of a non-stroke aetiology.27 32 We included adjudicated events that occurred within 10 years of participants’ visit 4 date (from 1 January 1996 to 31 December 2008) in our analysis.
Statistical analysis
Univariate descriptive statistics examined baseline participant-level characteristics. We calculated the mean and SD for continuous variables, percentages for dichotomous variables, and median with IQR for ordinal or nominal variables. We performed bivariate analysis using Pearson’s χ2 test or Kruskal-Wallis test for categorical data and a two-sample t-test for continuous variables.
The 10-year incidence rate for hospitalisations or death for CHD or stroke was estimated in subgroups defined by education attainment, ADI categories (IQR) and PCE risk categories (0%–5%, >5%–10%, >10%–15% and >15%). Incidence rates are presented as per 1000 person-years. Individual time at risk was measured from visit 4 until an ASCVD event occurred or one of the censoring events (whichever came first): death, lost to follow-up or end of the observation period.
The absolute risk (AR) was calculated as crude cumulative incidence using the pseudo-values methodology, which accounted for competing risk of death for reasons other than death due to ASCVD.33 We estimated AR according to participant educational attainment and ADI, stratified by the PCE 10-year estimated risk category. We calculated risk ratios (RRs) within each PCE predicted risk category comparing AR across educational attainment levels and ADI categories. AR differences between SES measures were estimated for each PCE 10-year estimated risk category (0%–5%, >5%–10%, >10%–15% and >15%). The referent group for educational attainment level is a college degree or above, and the referent group for ADI is residing in the least deprived neighbourhoods (lowest ADI quartile). Point estimates are reported with 95% CIs.
Generalised linear estimation models with a log-link function were used to predict the probability of ASCVD events. The naïve model included only the PCE predicted risk score category as the predictor. To evaluate the effect of SES on model fit statistics, additional models included: (1) education category added as a predictor and interacted with the PCE score, (2) ADI category added as a predictor and interacted with the PCE category and (3) both education and ADI categories as predictors and interacted with the PCE category. Generalised linear models compared took the following form:
Prob(ASCVD) = β0 +β1(i.Score).
Prob(ASCVD) = β0 + β1(i.Score) + β3(i.Education) + β4(i.Score x i.Education).
Prob(ASCVD) = β0 + β1(i.Score) + β2(i.ADI) + β3(i.Score x i.ADI).
Prob(ASCVD) = β0 + β1(i.Score) + β2(i.Education) + β3(i.ADI) + β4(i.Score x i.Education) + β5(i.Score x i.ADI).
The likelihood ratio test, Akaike information criterion and Bayesian information criterion evaluations were performed to compare model fit statistics of the different models. All analyses were performed using STATA V.13.
Patient and public involvement
Patients or the public were not involved in this specific research project.
Results
Of 9728 ARIC study participants, 1764 (18%) did not have a high school education (table 1). Participants with a 10-year predicted risk of ASCVD >15% were older, less likely to be male and had more comorbid conditions such as diabetes or hypertension, and more likely to smoke. Increases in PCE estimated risk categories corresponded to a higher proportion of participants without a high school degree or residing in the most deprived neighbourhoods.
Table 1.
Participant characteristics by 10-year ASCVD predicted risk category*
| Variable | All (n=9728) |
0%–5% (n=2383) |
>5%–10% (n=2652) |
>10%–15% (n=1880) |
>15% (n=2813) |
P value |
| Demographics | ||||||
| Age, mean (SD) | 62.61 (5.65) | 58.09 (3.29) | 61.44 (4.76) | 64.01 (5.19) | 66.61 (5.10) | <0.001 |
| Male, no (%) | 5728 (59) | 2203 (92) | 1656 (62) | 870 (46) | 999 (36) | <0.001 |
| Race, no (%) | ||||||
| White | 7528 (77) | 2097 (76) | 2027 (76) | 1400 (75) | 2004 (71) | <0.001 |
| Black | 2200 (23) | 286 (12) | 625 (24) | 480 (26) | 809 (29) | |
| Clinical comorbidities | ||||||
| Hypertension, no (%) | 3875 (40) | 460 (19) | 865 (33) | 780 (42) | 1770 (63) | <0.001 |
| Diabetes, no (%) | 1495 (15) | 47 (2) | 143 (5) | 228 (12) | 1077 (38) | <0.001 |
| Total cholesterol, mean (SD), mg/dL | 201.81 (36.48) | 201.22 (35.14) | 200.63 (36.17) | 201.82 (36.91) | 203.4 (37.56) | 0.034 |
| HDL cholesterol, mean (SD), mg/dL | 50.84 (16.69) | 60.11 (16.59) | 50.88 (15.56) | 48.54 (15.73) | 44.48 (14.83) | <0.001 |
| Current smoker, no (%) | 1431 (15) | 147 (6) | 332 (13) | 330 (18) | 622 (22) | <0.001 |
| Medication use | ||||||
| Statin use, no (%) | 845 (9) | 138 (6) | 232 (9) | 177 (9) | 298 (11) | <0.001 |
| ARIC field centre | ||||||
| Forsyth, NC, no (%) | 2343 (24) | 603 (25) | 642 (24) | 461 (25) | 637 (23) | <0.001 |
| Jackson, MS, no (%) | 1955 (20) | 256 (11) | 570 (22) | 424 (23) | 705 (25) | |
| Minneapolis, MN, no (%) | 2902 (30) | 892 (37) | 777 (29) | 511 (27) | 722 (26) | |
| Washington county, MD, no (%) | 2529 (26) | 632 (27) | 663 (25) | 484 (26) | 749 (27) | |
| Social-risk factors | ||||||
| Educational attainmen | ||||||
| College or above, no (%) | 3843 (40) | 1063 (45) | 1097 (41) | 707 (38) | 976 (35) | <0.001 |
| High school/some college, no (%) | 4110 (42) | 1120 (47) | 1132 (43) | 778 (41) | 1080 (39) | |
| No high school, no (%) | 1764 (18) | 199 (8) | 419 (16) | 395 (21) | 751 (27) | |
| ADI, median (IQR)† | 102 (96.3–108.8) | 100 (93.8–104.9) | 101.9 (96.1–108.9) | 102.5 (96.9–109.6) | 103.2 (97.6–111.5) | <0.001 |
*Risk categories estimated using the pooled cohort equations.
†ADI measures area-level social deprivation and estimated using the census-tract of participants’ five-digit zip code; higher values represent higher area-level social deprivation.
ADI, Area Deprivation Index; ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL, high-density lipoprotein; MD, Maryland; MN, Minnesota; MS, Mississippi; NC, North Carolina.
Incidence rates stratified by education level, ADI category and 10-year PCE estimated risk category are shown in table 2. A total of 751 incident ASCVD events occurred over 10 years of follow-up. Mean follow-up was 9.28 years. As expected, 10-year ASCVD incidence rates increased with increases in 10-year PCE estimated risk categories. Conditional on PCE estimated risk category, incidence rates were higher for participants without a high school education than participants with a high school education. Conditional on PCE estimated risk category, incidence rates were higher for participants residing in the most deprived neighbourhoods than less deprived neighbourhoods, except for participants with PCE estimated risk of >5%–10%. Among participants without a high school degree, incidence rates for ASCVD correlated with the 10-year PCE estimated risk categories. The relationship between 10-year estimated ASCVD risk and observed incidence rates of ASCVD varied for all ADI categories with <15% PCE estimated risk, with less variation for the degree of neighbourhood deprivation for participants at the highest PCE estimated risk category of >15%.
Table 2.
Event counts and incidence rates stratified by predicted ASCVD, education and ADI
| ASCVD predicted risk* | Events | 1000 person-years | Rate† per 1000 person-years | Events | 1000 person-years | Rate† per 1000 person-years | Events | 1000 person-years | Rate† per 1000 person-years |
| College or above | High school/some college | No high school degree | |||||||
| 0%–5% | 28 | 10.39 | 2.70 | 25 | 10.87 | 2.30 | 6 | 1.94 | 3.09 |
| >5%–10% | 45 | 10.41 | 4.32 | 62 | 10.66 | 5.72 | 32 | 3.91 | 8.19 |
| >10%–15% | 35 | 6.58 | 5.32 | 50 | 7.23 | 6.91 | 41 | 3.48 | 11.79 |
| >15% | 145 | 8.33 | 17.40 | 147 | 9.30 | 15.81 | 135 | 6.31 | 21.38 |
| Lowest ADI quartile | Middle two ADI quartile | Top ADI quartile | |||||||
| 0%–5% | 19 | 9.68 | 1.96 | 24 | 8.29 | 2.89 | 16 | 5.23 | 3.06 |
| >5%–10% | 56 | 8.52 | 6.57 | 33 | 8.27 | 3.99 | 49 | 8.23 | 5.96 |
| >10%–15% | 30 | 5.45 | 5.51 | 37 | 5.45 | 6.78 | 59 | 6.39 | 9.24 |
| >15% | 119 | 6.62 | 17.96 | 127 | 7.80 | 16.29 | 181 | 9.57 | 18.92 |
*Risk categories were estimated using the pooled cohort equations.
†Incidence rate of combined stroke and coronary heart disease was estimated over 10 years.
‡ADI measures area-level social deprivation and estimated using the census-tract of participants’ 9-digit zip code; higher values represent higher area-level social deprivation and categories were defined using quartiles of distribution.
ADI, Area Deprivation Index; ASCVD, atherosclerotic cardiovascular disease.
Risk modification analysis
Within each PCE predicted risk category, we evaluated if SES modified the relationship between PCE estimated risk and actual ASCVD 10-year observed incidence for each educational attainment level and neighbourhood deprivation (college-educated and least deprived neighbourhood as the referent) (table 3). Large RR differences (ie, more than 10%) within stratum-specific PCE estimated risk categories by SES indicates risk modification. We found that the RR was greater than 1 among those not having a high school degree for all PCE estimated risk categories. This result indicated a heavier burden of ASCVD than in college-educated participants independent of PCE estimated risk. This relative increase in ASCVD risk was statistically significant for groups with >5%–10% and >10%–15% PCE estimated risk; RR 1.78 (95% CI 1.16 to 2.76) and 2.15 (95% CI 1.39 to 3.34), respectively. The risk of ASCVD in the most deprived neighbourhoods (referent least deprived neighbourhoods) was significantly higher only for the 10-year PCE estimated risk category >10%–15%, RR 1.65 (95% CI 1.05 to 2.59).
Table 3.
RRs comparing observed ASCVD incidence rates across education and ADI categories within each predicted risk category
| 10-year ASCVD predicted risk* | Education | ADI | ||||
| No high school RR (95% CI) | High school/some college RR (95% CI) | College† or above RR (95% CI) |
Top ADI quartile RR (95% CI) |
Middle two ADI quartile RR (95% CI) | Lowest‡ ADI quartile RR (95% CI) |
|
| 0%–5% | 1.16 (0.48 to 1.53) | 0.84 (0.46 to 1.53) | 1.00 | 1.61 (0.76 to 3.38) | 1.51 (0.75 to 3.04) | 1.00 |
| >5%–10% | 1.78 (1.16 to 2.76) | 1.29 (0.86 to 1.93) | 1.00 | 0.92 (0.65 to 1.32) | 0.61 (0.38 to 0.97) | 1.00 |
| >10%–15% | 2.15 (1.39 to 3.34) | 1.30 (0.82 to 2.05) | 1.00 | 1.65 (1.05 to 2.59) | 1.22 (0.73 to 2.03) | 1.00 |
| >15% | 1.22 (0.99 to 1.49) | 0.92 (0.99 to 1.49) | 1.00 | 1.07 (0.87 to 1.32) | 0.93 (0.74 to 1.17) | 1.00 |
*Risk categories were estimated using the pooled cohort equations.
†College or above as referent.
‡Lowest ADI as the referent.
ADI, Area Deprivation Index; ASCVD, atherosclerotic cardiovascular disease; RR, risk ratio.
In analyses stratified by educational attainment and neighbourhood deprivation, participants without a high school degree who resided in the most deprived neighbourhoods had a higher risk of ASCVD for all 10-year PCE estimated risk categories than other SES groups (online supplemental table 1 and figure 1). At 10-year PCE estimated risk categories of 0%–5% and >10%–15%, having both individual and neighbourhood measures of low SES (without high school education and residing in the most deprived neighbourhood) meant a substantially higher risk of ASCVD than either measure alone; RR 3.64 (95% CI 1.46 to 9.07) and 4.78 (95% CI 1.62 to 14.09) respectively.
bmjopen-2021-058777supp001.pdf (801.2KB, pdf)
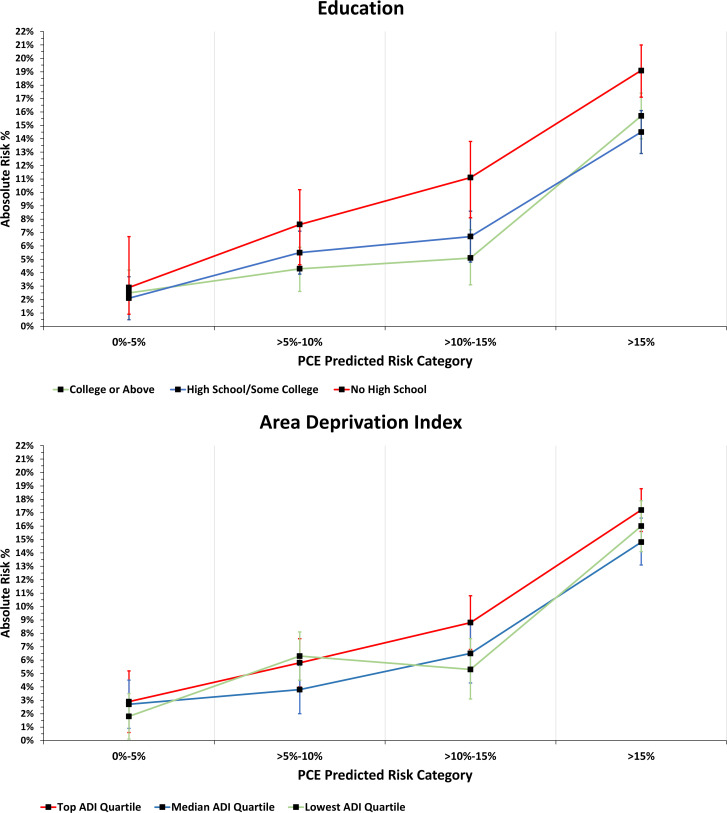
Observed 10-year AR is presented for each education category, and ADI category across PCE estimated risk categories (figure 1). We found heterogeneous differences in AR (ie, risk modification) by SES within stratum-specific PCE estimated risk categories. For example, the difference in AR for participants without a high school degree (referent college-educated) rose by 6 percentage points for PCE estimated risk of >10%–15%; AR difference decreased to 3.4 percentage points for PCE estimated risk >15% (online supplemental figure 2). Heterogeneous differences in AR for ADI categories were also noted, although smaller differences than educational attainment categories. Differences in AR for participants living in the most deprived neighbourhoods (referent least deprived neighbourhoods) were 1.2 percentage points higher for PCE estimated risk of >5%–15%, and 1.6 percentage points higher for PCE estimated risk 10%–15%.
Figure 1.
Observed 10-year incidence rate of ASCVD events by socioeconomic status. A 10-year incidence rate of ASCVD events by education attainment (A) and ADI (B).Area Deprivation Index measures area-level social deprivation and estimated using the census-tract of participants’ 9-digit zip code. ADI, Area Deprivation Index; ASCVD, atherosclerotic cardiovascular disease; PCE, Pooled Cohort Equations.
SES interaction with PCE model analysis
The coefficient for each SES risk factor’s interactions with estimated risk categories was statistically significant, and model fit measures to estimate ASCVD risk improved (table 4). For example, the likelihood ratio test comparing models 1 and 4, which included education and ADI categories, and their interaction with the PCE 10-year predicted ASCVD risk categories (model 4: Prob(ASCVD) = β0 + β1(i.Score) + β2(i.Education) + β3(i.ADI) + β4(i.Score x i.Education) + β5(i.Score x i.ADI)) demonstrated a statistically significant model improvement when measures of SES was added as an interaction term with PCE estimated risk category (p<0.0001). In addition, the Akaike information criterion was smaller, suggesting that educational attainment measures and area deprivation improved model fit for predicting 10-year ASCVD outcomes compared with the PCE predicted risk category alone.
Table 4.
Comparison of models predicting ASCVD 10-year Incident events with and without measures of socioeconomic status
| Model | No | Akaike information criterion* | Bayesian information criterion† | Likelihood ratio tests P value |
| PCE‡ | 9728 | 2371 | 2386 | |
| i.PCE + i.Education§ | 9717 | 2366 | 2395 | 0.004 |
| (i.PCE)x(i.Education) | 9717 | 2331 | 2374 | <0.0001 |
| i.PCE + i.ADI¶ | 9728 | 2371 | 2400 | 0.14 |
| (i.PCE) x (i.ADI) | 9728 | 2346 | 2389 | <0.0001 |
| i.PCE + i.Education + i.ADI | 9717 | 2366 | 2409 | 0.002 |
| (i.PCE) x (i.Education)x(i.ADI) | 9717 | 2328 | 2458 | <0.0001 |
All models that added in the social deprivation factor as a risk factor was compared with the PCE without a social deprivation factor.
All models that added in social deprivation as an interaction term was compared with the PCE model with social deprivation added as a risk factor.
*Akaike information criterion measures goodness-of-fit between observed values and expected values; lower scores compared with referent indicate an improvement in prediction.
†Bayesian information criterion measures goodness-of-fit between observed values and expected values; lower scores compared with a referent model indicate an improvement in prediction.
‡PCE predicted risk was stratified into four categories of risk: 0%–5%; >5%–10%; >10%–15%; >15%.
§Education was stratified into three categories: no high school; high school/some college; college or above (referent).
¶Higher ADI indicates higher neighbourhood deprivation and was stratified into three categories according to the IQR: top ADI quartile; middle two ADI; lowest ADI quartile (referent).
ADI, Area Deprivation Index; ASCVD, atherosclerotic cardiovascular disease; PCE, pooled cohort equations.
Discussion
In this study, we investigated whether SES’s individual and neighbourhood measures modify the association between the PCE risk score and actual 10-year ASCVD observed outcomes. We also described the excess burden of ASCVD events among low-SES populations relative to high-SES populations conditional on PCE estimated risk. The PCE estimated risk underestimated incidence of ASCVD events experienced among low-SES groups, and absolute differences in risk among SES measures became most pronounced at higher PCE predicted risk categories, indicating risk modification by measures of SES. Our results also suggest that SES factors’ value in predicting incident ASCVD events may vary by PCE predicted risk levels.
A potential reason for the inconsistent evidence for SES’s prognostic value to predict 10-year ASCVD outcomes could be the different outcome modelling strategies used in prior studies. Prior studies have historically modelled SES as an independent risk factor or confounder.19–22 24 Classical social epidemiological frameworks such as the ‘fundamentals causes of health inequalities theory’ suggest that despite any 10-year estimated risk of ASCVD for an individual at a given time, the clinical trajectory and outcomes are both influenced and dependent on the individual’s SES.26 34–37 According to the fundamental cause theory, high-SES individuals, possess a variety of flexible resources (ie, knowledge, money, prestige and power) to protect their health in a way that low-SES individuals cannot. As such, the effects of the non-SES traditional ASCVD risk factors used in the PCE (ie, hypertension and total cholesterol) on ASCVD incidence will likely be modified by whether the individual is of lower or higher SES. Our results show that having at least a college-education was protective against ASCVD relative to not having a high school degree across all risk levels, with greater protective effects at higher PCE estimated risk levels. Living in the least deprived neighbourhood was also protective, but likely less consistently than an individual SES exposure measure due to the potential for the ecological fallacy that can occur when making inferences about individuals based on group-level factors.
The substantial model fit improvement by interacting SES factors with the PCE risk score suggests that this modelling strategy will significantly improve ASCVD outcome prediction accuracy, but further analysis is required. Any 10-year ASCVD model that does not account for SES as a risk modifier may lead to measurement error. Prior modelling studies and current ASCVD risk models that incorporate SES into predicting risk do not incorporate SES as an interaction term into the model.
The current PCE model estimates a graded ASCVD risk irrespective of SES status. Our results show that the PCE placed disadvantaged individuals with an inherently higher risk of ASCVD into the corresponding 10-year estimated ASCVD risk categories at the expense of over-estimating risk for higher SES individuals. At the very least, the PCE will direct ASCVD preventive care to our most disadvantaged populations. The same population which research shows are less likely to receive appropriate preventive measures are just as likely to receive needed ASCVD risk management as their higher SES counterparts when the PCE is used to guide ASCVD prevention.38–41
Additional research is needed to improve ASCVD risk prediction among different SES groups and prevent ASCVD among disadvantaged populations. Our data only allow us to describe these epidemiological phenomena of excess ASCVD events experienced among lower SES individuals and possible ways to model future risk, but our analysis does not permit us to identify underlying mechanisms. Many unknown factors exist along the socioecological paradigm that works in concert with individual behavioural and physiological factors to lead to a higher burden of ASCVD among low-SES populations.
These findings have clinical and policy implications, with current guideline recommendations for using the PCE model to guide primary prevention ASCVD strategies in cholesterol management, hypertension management and aspirin use.16 18 42 43 For example, at an estimated 10-year PCE risk of 7.5%, statin therapy is recommended for primary prevention of ASCVD.18 We show that a higher SES is a risk-protecting factor, and the AR of ASCVD does not cross the 7.5% threshold until a PCE 10-year risk of >15% (figure 1). The use of SES in estimating an individual’s risk can potentially improve the efficiency of resource use and more precisely target interventions to achieve population-level objectives to decrease the ASCVD burden globally and in the United States. However, drug therapy decisions for primary prevention of ASCVD should incorporate other qualifying factors such as patient preference and not base decisions solely on ASCVD risk estimates.
We do not advocate for the use of SES in the clinical decision of ASCVD preventive therapies for US patients without a validated ASCVD prediction model that incorporates SES. Our findings do suggest validation of an ASCVD prediction model that appropriately incorporates SES as an ASCVD risk modifier is warranted. Model validation comparison measures such as net risk reclassification—similar to Mosley et al evaluation of PCE risk prediction improvement with adding a polygenic risk score—can help guide decisions on the utility of incorporating SES to guide clinical decision making.44 45 In addition, what and how SES measures are incorporated into an ASCVD prediction model—for example, summation of SES factors versus single SES factors—requires further exploration.46 47
Limitations
The study has several limitations. The ARIC study is restricted to four communities in the USA and is not nationally or internationally representative. Furthermore, some communities have limited diversity with respect to race or SES measures. The measurement of outcomes based on ARIC abstraction of hospitalisation data is a strength since it avoids reliance on self-report of events. However, some hospitalisations may be missing since comparing Medicare claims to ARIC records showed that between 10% and 20% of hospitalisations are missed if only one source is used.48 Internal exploration of this issue suggested that the additional hospitalisations were not correlated with our SES measures and did not substantively affect the results.
Results from our area-level deprivation analyses must be considered in the context of analytical limitations. For example, the use of the ADI as an aggregate measure of SES can potentially introduce ecological fallacy bias. Furthermore, we did not account for possible movement to other neighbourhoods for our sample over 10 years of follow-up. A potential misclassification bias of area-level deprivation exposure may exist over time. We expect that this misclassification bias is likely small. Our results are conservative estimates because bias from random measurement error is towards the null. Also, we did not adjust for ASCVD preventive medication use—for example, statin therapy—as a time-varying covariate in our models. While medication use could influence ASCVD outcome differences by SES, our focus was on the overall differences in prediction and outcome by SES rather than on causal pathways of the differences. Last, we did not control for the ARIC study site in our area-level deprivation analyses. Without controlling for the ARIC study site, homogeneity in participant characteristics (ie, a predominantly African-American/black population vs a predominantly white population) by ARIC study site may have resulted in the loss of statistical power to detect a meaningful difference in ASCVD outcomes according to ADI.
Conclusions
This study extends our understanding of the relationship between socioeconomic factors and the risk of heart disease and stroke outcomes. We find that the associations of PCE risk score and incident ASCVD are dependent on education level and area deprivation. Our findings may partially explain the discrepancy in results from earlier studies evaluating the utility of adding SES as a prognostic measure into ASCVD prediction models. Given the potentially important clinical and policy implications of our results, we suggest further refinement of the PCE model is needed to improve the estimation of risk for all populations, both historically vulnerable and less vulnerable populations. We believe the development of a new ASCVD risk prediction model should apply appropriate validation methods and use a more racially and ethnically diverse observational cohort for validation.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Twitter: @brystana
Contributors: KH, PPC and SS initiated the project. JSR and BK performed all statistical analyses. KH had main responsibility for writing the manuscript and was responsible for the overall content as the guarantor. KH, PPC, SS, JSR, BK, RF, CAAS and PMH all contributed to the statistical analyses, interpretation of outcomes and provided comments on the manuscript. KH, PPC, SS, JSR, BK, RF, CAAS and PMH all read and approved the final manuscript. PPC is the senior author.
Funding: This work was supported in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by institutional review boards at all ARIC centres approved study procedures, and participants gave written informed consent at each visit. The Institutional Review Board at the University of North Carolina at Chapel Hill approved this study (IRB# 18-1187). Participants gave informed consent to participate in the study before taking part.
References
- 1.GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2015. Natl Vital Stat Rep 2017;66:1–76. [PubMed] [Google Scholar]
- 3.Heron M. Deaths: leading causes for 2016. Natl Vital Stat Rep 2018;67:1–77. [PubMed] [Google Scholar]
- 4.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke Statistics-2019 update: a report from the American heart association. Circulation 2019;139:e56–28. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 5.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 6.Brown AF, Liang L-J, Vassar SD, et al. Neighborhood disadvantage and ischemic stroke: the cardiovascular health study (CHS). Stroke 2011;42:3363–8. 10.1161/STROKEAHA.111.622134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addo J, Ayerbe L, Mohan KM, et al. Socioeconomic status and stroke: an updated review. Stroke 2012;43:1186–91. 10.1161/STROKEAHA.111.639732 [DOI] [PubMed] [Google Scholar]
- 8.Grimaud O, Béjot Y, Heritage Z, et al. Incidence of stroke and socioeconomic neighborhood characteristics: an ecological analysis of Dijon stroke Registry. Stroke 2011;42:1201–6. 10.1161/STROKEAHA.110.596429 [DOI] [PubMed] [Google Scholar]
- 9.Rao SV, Kaul P, Newby LK, et al. Poverty, process of care, and outcome in acute coronary syndromes. J Am Coll Cardiol 2003;41:1948–54. 10.1016/S0735-1097(03)00402-9 [DOI] [PubMed] [Google Scholar]
- 10.Spatz ES, Beckman AL, Wang Y, et al. Geographic variation in trends and disparities in acute myocardial infarction hospitalization and mortality by income levels, 1999-2013. JAMA Cardiol 2016;1:255–65. 10.1001/jamacardio.2016.0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucharska-Newton AM, Harald K, Rosamond WD, et al. Socioeconomic indicators and the risk of acute coronary heart disease events: comparison of population-based data from the United States and Finland. Ann Epidemiol 2011;21:572–9. 10.1016/j.annepidem.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–27. 10.1002/ana.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper S, Lynch J, Smith GD. Social determinants and the decline of cardiovascular diseases: understanding the links. Annu Rev Public Health 2011;32:39–69. 10.1146/annurev-publhealth-031210-101234 [DOI] [PubMed] [Google Scholar]
- 14.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American heart association. Circulation 2015;132:873–98. 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 15.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American heart association Task force on practice guidelines. J Am Coll Cardiol 2014;63:2935–59. 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett DK, Khera A, Blumenthal RS. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Part 1, lifestyle and behavioral factors. JAMA Cardiol 2019;4:1043. 10.1001/jamacardio.2019.2604 [DOI] [PubMed] [Google Scholar]
- 17.Whelton PK, Carey RM, Aronow WS. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Hypertension 2018;71. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Circulation 2019;139:e1082–143. 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colantonio LD, Richman JS, Carson AP, et al. Performance of the atherosclerotic cardiovascular disease pooled cohort risk equations by social deprivation status. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.005676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiscella K, Tancredi D, Franks P. Adding socioeconomic status to Framingham scoring to reduce disparities in coronary risk assessment. Am Heart J 2009;157:988–94. 10.1016/j.ahj.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 21.Tunstall-Pedoe H, Woodward M, SIGN group on risk estimation . By neglecting deprivation, cardiovascular risk scoring will exacerbate social gradients in disease. Heart 2006;92:307–10. 10.1136/hrt.2005.077289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodward M, Brindle P, Tunstall-Pedoe H, et al. Adding social deprivation and family history to cardiovascular risk assessment: the assign score from the Scottish heart health extended cohort (SHHEC). Heart 2007;93:172–6. 10.1136/hrt.2006.108167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins GS, Altman DG. Predicting the 10 year risk of cardiovascular disease in the United Kingdom: independent and external validation of an updated version of QRISK2. BMJ 2012;344:e4181. 10.1136/bmj.e4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335:136. 10.1136/bmj.39261.471806.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475–82. 10.1136/bmj.39609.449676.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkman LF, Kawachi I, Glymour MM. Social epidemiology. Second edition. edn. Oxford: Oxford University Press, 2014. [Google Scholar]
- 27.The Atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC Investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 28.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25-64 years by area socioeconomic status, 1969-1998. Int J Epidemiol 2002;31:600–13. 10.1093/ije/31.3.600 [DOI] [PubMed] [Google Scholar]
- 29.Knighton AJ, Savitz L, Belnap T, et al. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS 2016;4:1238. 10.13063/2327-9214.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh GK, Siahpush M, Azuine RE, et al. Increasing area deprivation and socioeconomic inequalities in heart disease, stroke, and cardiovascular disease mortality among working age populations, United States, 1969-2011. Int J MCH AIDS 2015;3:119–33. [PMC free article] [PubMed] [Google Scholar]
- 31.National Heart L, and Blood Institute . Atherosclerosis risk in communities (ARIC) study. operations manual No. 3. surveillance components procedures, version 1.0 1987.
- 32.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis risk in communities (ARIC) cohort. Stroke 1999;30:736–43. 10.1161/01.str.30.4.736 [DOI] [PubMed] [Google Scholar]
- 33.Andersen PK, Perme MP. Pseudo-observations in survival analysis. Stat Methods Med Res 2010;19:71–99. 10.1177/0962280209105020 [DOI] [PubMed] [Google Scholar]
- 34.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav 2010;51 Suppl:S28–40. 10.1177/0022146510383498 [DOI] [PubMed] [Google Scholar]
- 35.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav 1995;Spec No:80–94. 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- 36.Link BG, Phelan JC. McKeown and the idea that social conditions are fundamental causes of disease. Am J Public Health 2002;92:730–2. 10.2105/AJPH.92.5.730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diez Roux AV. Conceptual approaches to the study of health disparities. Annu Rev Public Health 2012;33:41–58. 10.1146/annurev-publhealth-031811-124534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 2018;137:2166–78. 10.1161/CIRCULATIONAHA.117.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosengren A, Smyth A, Rangarajan S, et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the prospective urban rural epidemiologic (PURE) study. Lancet Glob Health 2019;7:e748–60. 10.1016/S2214-109X(19)30045-2 [DOI] [PubMed] [Google Scholar]
- 40.Sherman BW, Gibson TB, Lynch WD, et al. Health care use and spending patterns vary by wage level in employer-sponsored plans. Health Aff 2017;36:250–7. 10.1377/hlthaff.2016.1147 [DOI] [PubMed] [Google Scholar]
- 41.Vargas Bustamante A, Chen J, Rodriguez HP, et al. Use of preventive care services among Latino subgroups. Am J Prev Med 2010;38:610–9. 10.1016/j.amepre.2010.01.029 [DOI] [PubMed] [Google Scholar]
- 42.Whelton PK, Carey RM, Aronow WS. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Hypertension 2017;71. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 43.Bibbins-Domingo K. Force USPST: aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U. S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836–45. [DOI] [PubMed] [Google Scholar]
- 44.Kerr KF, Wang Z, Janes H, et al. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology 2014;25:114–21. 10.1097/EDE.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosley JD, Gupta DK, Tan J, et al. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA 2020;323:627–35. 10.1001/jama.2019.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safford MM, Reshetnyak E, Sterling MR, et al. Number of social determinants of health and fatal and nonfatal incident coronary heart disease in the REGARDS study. Circulation 2021;143:244–53. 10.1161/CIRCULATIONAHA.120.048026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Bacquer D, van de Luitgaarden IAT, De Smedt D, et al. Socioeconomic characteristics of patients with coronary heart disease in relation to their cardiovascular risk profile. Heart 2021;107:799–806. 10.1136/heartjnl-2020-317549 [DOI] [PubMed] [Google Scholar]
- 48.Savitz ST, Stearns SC, Groves JS, et al. Mind the gap: hospitalizations from multiple sources in a longitudinal study. Value Health 2017;20:777–84. 10.1016/j.jval.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058777supp001.pdf (801.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.