Abstract
Myeloid checkpoints are receptors on the myeloid cell surface which can mediate inhibitory signals to modulate anti-tumor immune activities. They can either inhibit cellular phagocytosis or suppress T cells and are thus involved in the pathogenesis of various diseases. In the tumor microenvironment, besides killing tumor cells by phagocytosis or activating anti-tumor immunity by tumor antigen presentation, myeloid cells could execute pro-tumor efficacies through myeloid checkpoints by interacting with counter-receptors on other immune cells or cancer cells. In summary, myeloid checkpoints may be promising therapeutic targets for cancer immunotherapy.
Keywords: Cancer, immune checkpoint, myeloid checkpoint, MDSC, TAM, immunotherapy
Introduction
Anti-tumor immunotherapies, conventionally referred to as immune checkpoint inhibitors (ICIs), target T-cell immune checkpoints and have elicited impressive therapeutic responses in the treatment of human cancers (1). Despite achieving the long-term survival of 10%−30% of treated individuals, immunotherapies are not effective for most patients suffering from cancer (2). A primary challenge of this strategy for extensive anticancer application remains the cell complexity of the tumor immune microenvironment. In addition to many endeavors to seek intrinsic factors to enhance the efficacy of immune therapy in both tumor and T cells (3-5), many studies have revealed that the therapeutic efficacy could be improved by targeting immune regulatory cells, including myeloid cells in the tumor microenvironment (TME) (6,7).
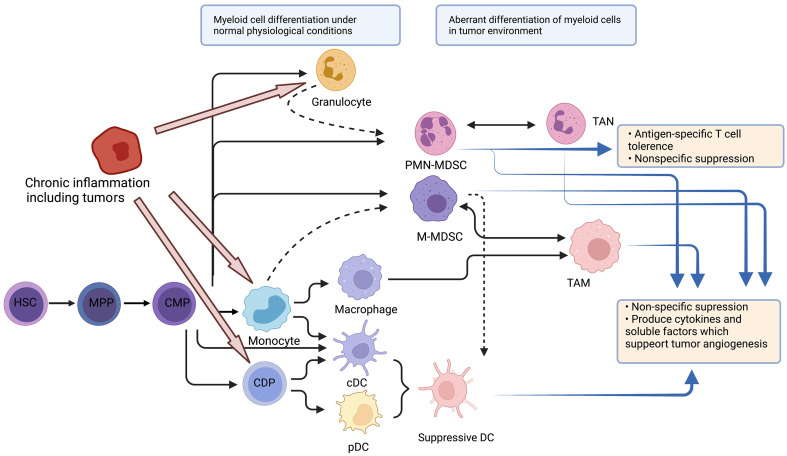
Myeloid cells are the most abundant hematopoietic cells in the human body and have diverse functions. All myeloid cells arise from multipotent hematopoietic stem cells (HSCs) that develop into mature myeloid cells through sequential steps of differentiation (Figure 1) (6). Various tumors show abundant infiltration of myeloid cells. More specifically, tumor-infiltrating myeloid cells (TIMs) are constitutive of several myeloid lineages, including mast cells , plasmacytoid dendritic cells (pDCs) (8), conventional dendritic cells (cDCs) (9), monocytes (10), macrophages (11), and granulocytes (12). A pan-cancer single-cell transcriptional atlas of TIMs across 15 human cancer types showed that monocytes and macrophages accounted for the largest proportion of TIMs, with an average of above 50% in most tumors, and the proportion of cDCs was relatively stable (approximately 10%−20%) across tumor types, while the proportion of mast cells showed great variation across different types of tumors (13).
Figure 1.
Stages of myelopoiesis differentiation. Myeloid cells originate from HSCs and MPPs. Myelopoiesis is amplified during chronic inflammation to assist tumor progression and dissemination. HSCs differentiate into CMP, which can further differentiate through hematopoietic system. In physiological conditions, CMP can differentiate into neutrophils or monocytes, and subsequently into DCs or macrophages. However, with chronic inflammation, pro-inflammatory cytokines can skew monocytopoiesis of CMP into M-MDSC and TAM, and granulopoiesis into PMN-MDSC and TAN. HSC, hematopoietic stem cell; MPP, multipotent progenitor cell; CMP, common myeloid progenitor; DC, dendritic cell; M-MDSC, monocytic myeloid-derived suppressor cell; TAM, tumor-associated macrophage; PMN-MDSC, polymorphonuclear myeloid-derived suppressor cell; TAN, tumor-associated neutrophil; CDP, common DC progenitor; cDC, conventional DC; pDC, plasmacytoid DC.
Single-cell RNA-seq also indicated that in the TME, myeloid cells experience continuous reprogramming (14). Following TME-specific cues, monocytes can differentiate into inflammatory macrophages (M1-like type) and monocyte-derived DCs, whose functions are anti-tumor, or alternatively activated macrophages (M2-like type) with immunosuppressive capacity. The differentiation from monocytes to M2 macrophages may be the predominant path in the process of myeloid reprogramming (15).
These cells have a profound impact on anti-tumor effect and could influence neoangiogenesis, and sustain cancer cell proliferation, metastasis and immunotherapy resistance (16). On the one hand, myeloid cells can directly kill tumor cells by phagocytosis (17) or activate anti-tumor immunity by tumor antigen presentation (6). On the other hand, a group of myeloid cells, including tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs) and inhibitory DCs, support tumor development and metastasis by suppressing infiltrating cytotoxic lymphocytes (18). The activities and functions of these myeloid cells are regulated by stimulatory or inhibitory signals mediated by receptors on the cell surface, and inhibitory signals are considered myeloid checkpoints, which may contain an immune-receptor tyrosine-based inhibitory motif (ITIM)/immune-receptor tyrosine-based switch motif (ITSM) to transduce immune modulatory signals in myeloid cells or interact with counter-receptors on other immune cells to modulate their activities or on cancer cells to execute pro-tumor efficacies. A new type of immunotherapeutic strategy targeting myeloid cells has also emerged (19).
In this review, we will discuss the biological functions and molecular mechanisms of myeloid checkpoints below.
Checkpoints expressed by myeloid cells
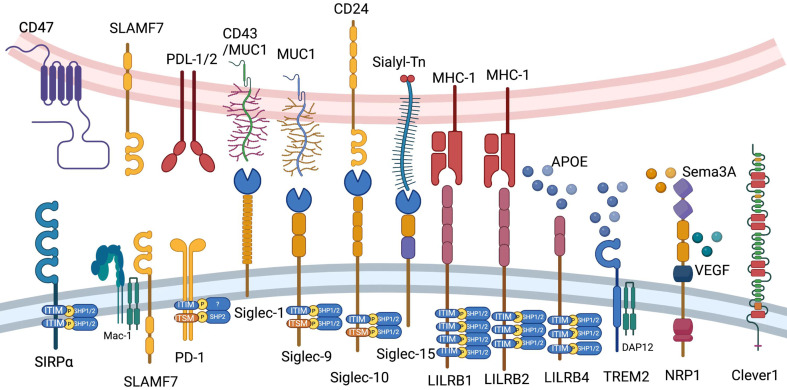
We briefly introduce CD47, signaling lymphocytic activation molecule (SLAM) family, programmed cell death 1 (PD-1), sialic acid-binding immunoglobulin-type lectin (Siglec) family, leukocyte immunoglobulin-like receptor B (LILRB) family, triggering receptor expressed on myeloid cells 2 (TREM2), neuropilin-1 (NRP1) and common lymphatic endothelial and vascular endothelial receptor-1 (Clever-1) in this chapter (Figure 2).
Figure 2.
Current myeloid checkpoints in cancer immunotherapy. Ligands and elementary structures of CD47, SLAMF7, PD-1, LILRB1, LILRB2, LILRB4, Siglec-1, Siglec-9, Siglec-10, Siglec-15, TREM2, NRP1 and Clever1 are shown in this figure. PD-1, programmed cell death 1; LILRB, leukocyte immunoglobulin-like receptor B; Siglec, sialic acid-binding immunoglobulin-type lectin; TREM2, triggering receptor expressed on myeloid cells 2; NRP1, neuropilin-1; Clever1, commonlymphatic endothelial and vascular endothelial receptor-1.
CD47/SIRPα
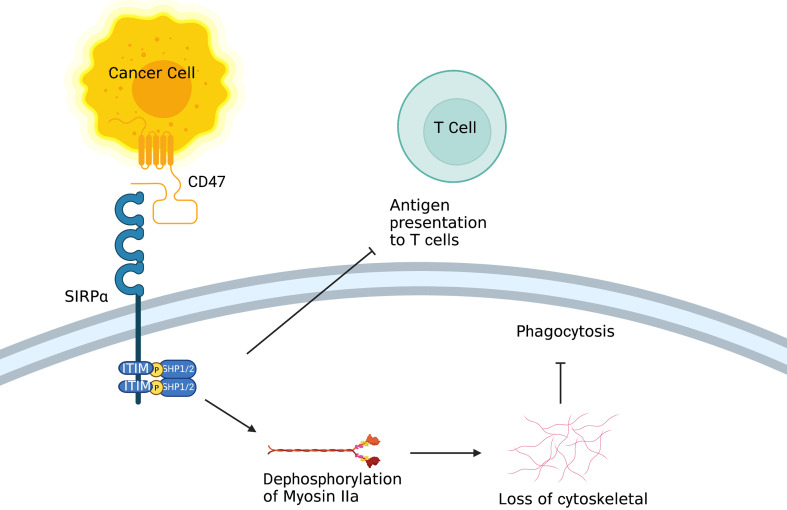
CD47 (also known as inhibitor of apoptosis protein, IAP) is a red blood cell signal that serves to discriminate self and non-self (20). Upon engagement of CD47 by signal regulatory protein alpha [SIRPα; also known as SH2-domain bearing protein tyrosine phosphatase (SHP) substrate-1 (SHPS-1) or CD172a] on macrophages and DCs, the ITIM in the cytoplasmic tail of SIRPα activates the inhibitory tyrosine phosphatases SHP-1 and SHP-2, resulting in inhibition of the actin cytoskeleton rearrangement required for phagocytosis (Figure 3). CD47 is upregulated on the surface of several cancer cell types, enabling these cells to evade phagocytic removal by immune cells (21). In accord with this, high CD47 expression is negatively correlated with disease prognosis and survival in acute myeloid leukemia (AML), non-small cell lung cancer (NSCLC) and high-grade serous ovarian carcinoma (21-24). Furthermore, therapeutic reagents that antagonize the CD47-SIRPα axis in macrophages inhibit the growth of several types of tumors, including AML and non-Hodgkin lymphoma, by enhancing cellular phagocytosis (25-28). Furthermore, CD47 blockade has been shown to robustly increase immune responses against tumor antigens by activating DCs but not macrophage cross-presentation of tumor antigens (29). In recent years, combined therapies of a CD47-SIRPα axis inhibitor and other anticancer therapeutics [e.g., PD-1/programmed cell death ligand-1 (PD-L1) blockade] have been investigated in the context of potentiating anti-tumor immunity in vitro (25,30).
Figure 3.
Role of CD47/SIRPα. On the one hand, after binding to CD47, ITIM in the cytoplasmic tail of SIRPα on macrophage activates inhibitory tyrosine phosphatases SHP-1 and SHP-2, resulting in inhibition of the actin cytoskeleton rearrangement required for phagocytosis. On the other hand, SIRPα on DC inhibits the antigen presentation of tumor cells. ITIM, tyrosine-based inhibitory motif; DC, dendritic cell.
SLAM family
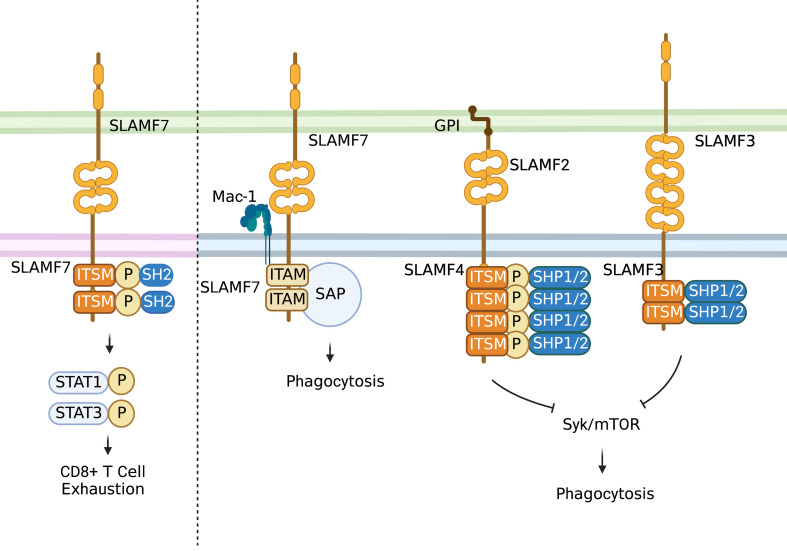
The SLAM family of receptors is expressed on the majority of immune cells. These receptors often serve as self-ligands and play important roles in cellular communication and adhesion, thus modulating immune responses (Figure 4) (31,32). SLAM family receptors (SFRs) play a key role in inhibiting macrophage phagocytosis. SFR deficiency triggers macrophage phagocytosis of hematopoietic cells, resulting in severe rejection of donor hematopoietic grafts in recipient mice (33).
Figure 4.
Role of SLAM family. In T cells, the activation of self-ligand SLAMF7 immune receptor could induce phosphorylation of STAT1 and STAT3 and expression of various inhibitory receptors and transcription factors associated with T cell exhaustion. In macrophages, SLAMF7 could mediate phagocytosis interacting with integrin Mac-1 and do with signals involving immune-receptor tyrosine-based activation motifs. While most SLAM-mediated phagocytosis functions depend on SAP adaptors. SLAMF3 and SLAMF4 are identified as “don’t eat me” receptors on macrophages which inhibit “eat-me” signals such as LRP1-mediated activation of mTOR and Syk through SH2 domain-containing phosphatases. STAT, signal transduction and activator of transcription; SAP, signaling lymphocyte activation molecule-associated protein; GPI, glycosyl phosphatidyl inositol.
SLAMF7
The SLAMF7 [also acknowledged as CD2-like receptor-activating cytotoxic cell (CRACC), CS1 and CD319] receptor belongs to the SLAM family of receptors (34), presenting at diverse periodicities on different types of immune cells, such as macrophages, and restrictively expressed on hematopoietic cells (32,34,35). Except for SLAMF4 (2B4), SLAM family members function as homotypic receptors, which recruit different SH2 domain-containing proteins to their cytoplasmic immune-receptor tyrosine-based switch motifs when activated (34) so that SLAMF7 can regulate immune cell-specific functions across diverse types of immune cells (34-36). SLAM family receptors are correlated with T-cell exhaustion. SLAMF6 is a marker of the progenitor of exhausted CD8+ T cells (37), and both SLAMF6 and SLAMF4 (2B4) are reported as inhibitory receptors of CD8+ T cells (34). As reported, SLAMF7 is also expressed on a CD8+ T-cell subset enriched in melanoma patients who are not responsive to checkpoint blockade immune therapy (38). Moreover, SLAMF7 is expressed on certain kinds of memory-precursor and effector CD8+ T cells, which respond to checkpoint blockade in an indirect way (39). A recent study showed that activation of the self-ligand SLAMF7 immune receptor on T cells could induce phosphorylation of signal transduction and activator of transcription 1 (STAT1) and STAT3, and expression of various inhibitory receptors and transcription factors associated with T-cell exhaustion (31,40). High SLAMF7 expression is reported to indicate poor survival in clear cell renal cell carcinoma (ccRCC) (31). Moreover, SLAMF7highCD38high TAMs show strong relations with exhausted T cells and are an independent prognostic factor of ccRCC (31). Apart from the role of SLAMF7 in modulating T-cell function in the TME, SLAMF7 could mediate phagocytosis by interacting with integrin Mac-1 (41) and with signals involving immune-receptor tyrosine-based activation motifs (42). Most SLAM-mediated phagocytosis functions depend on signaling lymphocyte activation molecule-associated protein (SAP) adaptors (34,43). What’s more, it has been shown that patients whose tumors express SLAMF7 are more likely to respond to SIRPα-CD47 blockade therapy (33).
SLAMF3 & SLAMF4
Specifically, SLAMF3 (Ly-9, CD229) and SLAMF4 (2B4, CD244) were identified as “do not eat me” receptors on macrophages (44). These receptors inhibit “eat-me” signals, such as LRP1-mediated activation of mTOR and Syk, through SH2 domain-containing phosphatases. SFRs bind to but are independent of CD47 to alleviate macrophage phagocytosis, and combined deletion of SFRs and CD47 results in reduced hematopoietic cells in mice. This SFR-mediated tolerance is compromised in patients with hemophagocytic lymphohistiocytosis, a syndrome characterized by inappropriate phagocytosis of hematopoietic cells. Thus, SFR-mediated inhibition of macrophage phagocytosis is critical for hematopoietic homeostasis, and SFR may represent a previously unknown tumor immunotherapy target (44).
PD-1/PD-L1
PD-1 (also known as CD279) is one of the best-characterized immune checkpoint targets for cancer immunotherapy, and PD-1 blockade has proven to be the most successful immunotherapy strategy to date (45). It has been reported that increased galectin-9+ M-MDSCs in the peripheral blood of NSCLC patients are involved in resistance to anti-PD-1 therapy (46). Therefore, the presence of MDSCs in the TME is detrimental for the anti-PD-1/PD-L1 response. As expected, several studies revealed the relationship between MDSC infiltration and PD-1 blockade resistance, and selective depletion of MDSCs could restore anti-PD-1 efficacy (47,48).
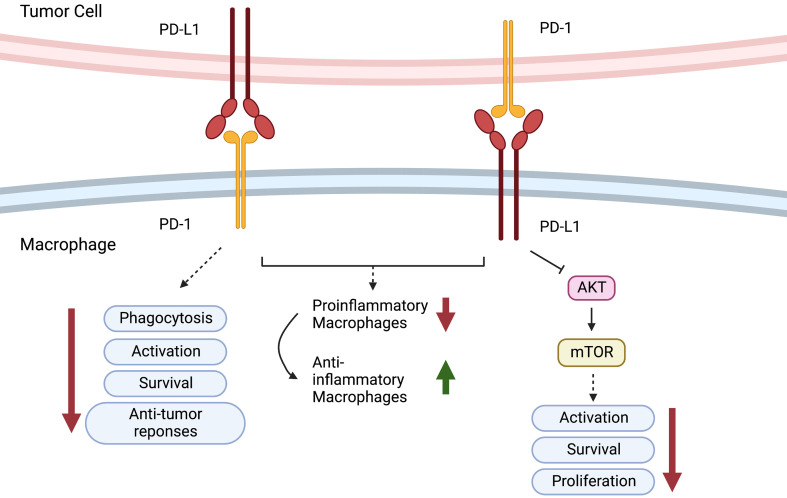
In addition, it has been found that PD-1 is expressed in TAMs but not in circulating monocytes or splenic macrophages and that it can negatively regulate cancer cell phagocytosis (30). In addition, as reported, blockade of PD-1 or PD-L1 enhanced the phagocytic activity of TAMs towards tumor cells in vivo and inhibited tumor growth in a macrophage-dependent fashion, suggesting that the PD-1/PD-L1 axis is an immune checkpoint for regulating both innate and adaptive immunity. They further found that PD-1 blockade also changed the nature of TAMs from immunosuppressive M2-like cells to pro-inflammatory M1-like cells. The presence of TAMs in pancreatic cancer exaggerates immunosuppression within the microenvironment and leads to PD-1/PD-L1 blockade resistance. Inhibition of colony-stimulating factor 1 receptor (CSF1R) on TAMs can upregulate the expression of PD-L1 and increase CD8+ T-cell infiltration, which ablates anti-PD-1/PD-L1 resistance (49). PD-1-expressing macrophages exhibit an anti-inflammatory-like surface profile in tumor settings. PD-1 expression is negatively correlated with the phagocytic ability of macrophages, and blockade of PD-1/PD-L1 enhances antitumor responses and prolongs survival by inhibiting pro-inflammatory to anti-inflammatory macrophage polarization (50,51). PD-L1 antibody treatment promoted the proliferation of cultured bone marrow-derived macrophages, which was related to the activation of the AKT-mTOR pathway (Figure 5) (52). The transcriptomic profiles of macrophages were switched to inflammatory phenotypes following PD-L1 antibody treatment (53). The interaction between the immune checkpoint molecules PD-1 and PD-L1 may also be responsible for TAN-mediated immune suppression. In human hepatocellular carcinoma (HCC) tissues and HCC-bearing mice, PD-L1 and PD-1 were expressed in peritumoral neutrophils and T lymphocytes, respectively, and PD-L1+ neutrophils from patients with HCC effectively suppressed the proliferation and activation of T cells by utilizing PD-1/PD-L1 interactions (54).
Figure 5.
Role of PD-1/PD-L1. PD-1-expressing macrophages exhibit an anti- inflammatory-like surface profile in tumor settings. PD-1 expression is negatively correlated to phagocytic ability of macrophages. PD-L1 antibody treatment promotes cell proliferation of cultured bone marrow-derived macrophages, which is related to the activation of the AKT-mTOR pathway. PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
In addition to PD-L1, B7 superfamily member 1 (B7S1) (also known as B7-H4, B7x, or VTCN1) is expressed by tumor-infiltrating antigen-presenting cells (APCs) and triggers dysfunction and exhaustion of activated CD8+ TILs by Eomes upregulation (55). Murine experiments indicated that B7S1 inhibition suppresses the development of liver cancer and enhances antitumor CTLs and that the combination of B7S1 and PD-1 blockade could inhibit tumor growth in a synergistic way (55).
In summary, together with PD-1, B7S1 could be an intriguing target to reverse T-cell exhaustion and maximize immunotherapeutic efficacy in cancer.
Siglec family
Sialic acid-binding immunoglobulin-type lectins (Siglecs) contain a family of cell surface proteins playing an essential role in regulating immune homeostasis (Figure 6). The dysregulation of Siglecs is reported to be correlated with various diseases, including autoimmunity diseases, infections and cancer (56). Siglecs are type I transmembrane proteins with one V-set immunoglobulin (Ig) domain, including a sialic acid-binding site, and one or more C2-set Ig domains in the extracellular region. Most Siglecs, such as CD22 (Siglec-2) and most CD33 (Siglec-3)-related Siglecs, have ITIMs and/or ITIM-like motifs in the intracellular domain and convey signaling mediated by inhibitory receptors (57). Each Siglec prefers a different kind of sialic acid expressed on all mammalian cells to distinguish self and non-self (58). Mammalian cells have a relatively high density of sialoglycans on their surface in comparison to most pathogens. This high density of sialoglycans, which can be considered self-associated molecular patterns (SAMPs), can lead to the inhibition of immune responses against the self (59). Siglec is mainly expressed on hematopoietic cells, almost on myeloid cells and B cells or other types of cells, such as neurons (56,58).
Figure 6.
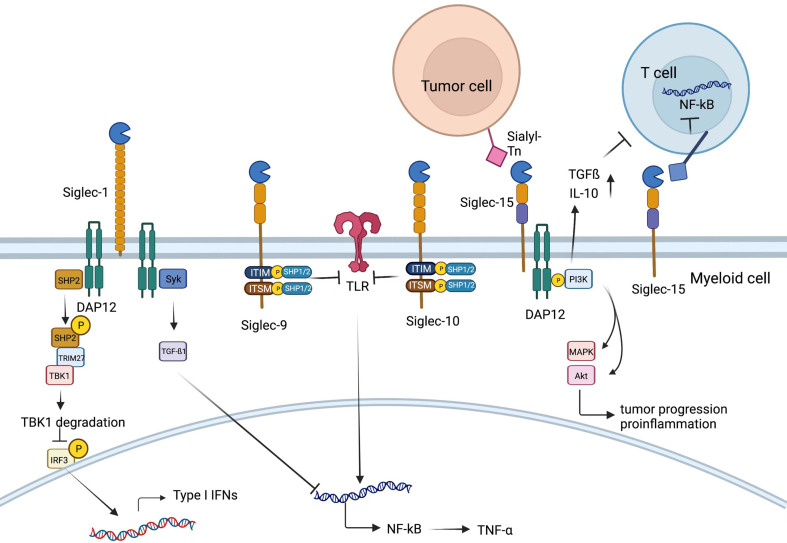
Role of Siglec family. On the one hand, Siglec-1 could associate with DAP12 to recruit and activate SHP2, which then could recruit E3 ubiquitin ligase TRIM27, inducing TBK1 degradation. Therefore, type I IFN production is inhibited and innate immune response could be suppressed. On the other hand, Syk activation occurs when Siglec-1 binds to DAP12, leading to increased production of TGF-β1, which could inhibit production of NF-kB, Siglec-9 and Siglec-10. Membrane-proximal ITIM of Siglec-9 and Siglec-10 could offer docking sites for SHP-1/2 once tyrosine is phosphorylated, which could inhibit the role of Toll-like-receptor, resulting in increased production of NF-kB. SHP, SH2-domain bearing protein tyrosine phosphatase; IFN, interferon; TGF, transforming growth factor; Siglec, sialic acid-binding immunoglobulin-type lectin.
CD169
CD169, which is also called sialoadhesin or Siglec-1, is expressed mostly on macrophages conserved among mammals (60,61). The CD169 molecule consists of 17 Ig-like domains, including an N-terminal V-set domain and 16 C2-set domains (62). CD169 can serve as a functional ligand, different from most Siglecs, which work as receptors (57). The functions of CD169 are involved in cell-to-cell adhesion and cell-pathogen interactions (63) including sialylated pathogen uptake, antigen presentation, and modulating self-antigen tolerance (64). CD169 could potentially contribute to both the damping and the facilitation of antitumor immunity (65). On the one hand, Siglec1 can recognize and bind surface polysaccharides, such as CD43, on T cells to implement cell-to-cell communication (66). CD169+ macrophages in the marginal zone of the spleen recognize phosphatidylserine on the surface of apoptotic cells, acting as APCs to present apoptotic cell antigens so that Tregs are recruited to exhibit their immune tolerance role (67). On the other hand, CD169 in lymph nodes plays an immunoregulatory role by interacting with MUC-1 binding on the surface of breast cancer tumor cells (68). A high density of CD169+ macrophages in the regional lymph nodes of colorectal, endometrial carcinoma and malignant melanoma patients indicates a better clinical prognosis, perhaps owing to an increase in the number of tumor-infiltrating cytotoxic immune cells (69,70). CD169+ macrophages can also directly contact CD43-expressing CD8+ T cells (71). In endometrial carcinoma patients, a high density of CD169+ cells in the regional lymph nodes of the tumor is associated with a higher density of CD8+ T cells and NK cells in the tumor tissue (69,70). The number of intratumoral cells expressing CD169 also positively correlates with the number of infiltrating CD8+ T cells in the tumor and with patient survival (71). In contrast, the subcapsular sinus macrophages in regional lymph nodes of patients with advanced-stage endometrial carcinoma or metastatic carcinoma bear lower levels of CD169 or are completely dismissed from this molecule (70). Furthermore, some results suggested that upon vesicular stomatitis virus (VSV) infection, Siglec1 can associate with DAP12 and SHP2, triggering suppression of type I IFN production (72). There is also evidence that Siglec-1 can interact with and activate the DAP12-Syk pathway to increase the production of TGF-β1, which plays an essential role in sepsis and endotoxin tolerance (73).
Siglec-9
Siglec-9 is expressed on various kinds of human blood leukocytes, including lymphocytes and myeloid cells such as B cells, small groups of T cells, monocytes, neutrophils, and NK cells (74). Siglec-9 is mainly expressed on neutrophils in peripheral blood (75) and on NK cells, B cells and monocytes afterwards (76). As plentiful evidence indicates, Siglec-9 plays inhibitory roles in modulating immune homeostasis (77) by interacting with the membrane-proximal ITIM. ITIMs can offer docking sites for SHP-1/2 once tyrosine is phosphorylated (78). Siglec-9 could also link with transmembrane epithelial MUC, a kind of strongly glycosylated protein basically produced by epithelial tissues, which could trigger immune evasion (79). MUC1 overexpressed on adenocarcinomas and hematological cancers could recruit β-catenin binding to its C-terminal domain to give rise to the growth of tumor cells (80). MUC1-sialylated O-linked glycan binding to Siglec-9 could induce calcium flux, leading to the activation of MEK-ERK kinases instead of recruiting SHP-1/2 (81). Siglec-9 could also interact with MUC16 expressed on epithelial ovarian cancer cells, which protects tumor cells from immune clearance (82).
Siglec-E
Siglec-E is the mouse orthologue of human Siglec-9. Similar to Siglec-9, Siglec-E was first found on neutrophils, macrophages and monocytes (83). Siglec-E could stop inflammatory responses mediated by immune cells when binding to sialoglycan ligands in its extracellular region (83). A Siglec-E-deficient mouse model (84) was utilized to explore the role of Siglecs in myeloid cells (85). As reported, neutrophil-associated Siglec-E could promote extravasation and colony formation of tumor cells in the lungs. The tumoricidal effect and reactive oxygen species (ROS) production mediated by neutrophils are shown to be enhanced in vitro with the deficiency of Siglec-E (85,86). TAMs in mice with Siglec-E deficiency have a higher preference for polarization into M2 macrophages and could enhance subcutaneous tumor growth (85).
Siglec-10
CD24 (also known as heat stable antigen or small-cell lung carcinoma cluster 4 antigen) is a heavily glycosylated glycosylphosphatidylinositol-anchored surface protein (87). It is reported to interact with the inhibitory receptor sialic-acid-binding Ig-like lectin 10 (Siglec-10) on innate immune cells to dampen damaging inflammatory responses to infection (88), sepsis (89), liver damage and chronic graft vs. host diseases (87,90). The binding of CD24 to Siglec-10 triggers an inhibitory signaling cascade, which is mediated by Src homology region 2 domain-containing phosphatases, SHP-1 and/or SHP-2. The phosphatases are associated with the two immune-receptor tyrosine-based inhibition motifs in the cytoplasmic tail of Siglec-10, thereby blocking Toll-like receptor-mediated inflammation and the cytoskeletal rearrangement required for cellular engulfment by macrophages (58,91,92). Moreover, the role of CD24 in modulating tumor immune responses has been reported. CD24 is a potent antiphagocytic “don’t eat me” signal that is capable of directly protecting cancer cells from attack by Siglec-10-expressing macrophages. Many tumors overexpress CD24, and TAMs express high levels of Siglec-10. Genetic ablation of either CD24 or Siglec-10, as well as blockade of the CD24-Siglec-10 interaction using monoclonal antibodies, robustly augmented the phagocytosis of all CD24-expressing human tumors and led to macrophage-dependent reduction of tumor growth in vivo and an increase in survival time (93). Studies have shown that CD24 is expressed by several solid tumors, such as ovarian cancer and breast cancer (94,95), which demonstrate weaker responses to anti-PD-L1/PD-1 immunotherapies than other cancers (52,96), suggesting that an alternative strategy may be required to achieve responses across a wide range of cancers.
Siglec-15
Among the Siglec family members, Siglec-15 has been identified as a very unique member, selectively expressed on myeloid cells and osteoclasts (a bone-specific myeloid lineage) and generally absent in other immune cells and tissues (97,98). Siglec-15 was identified as a novel T-cell-inhibitory molecule, which was originally characterized as an osteoclast modulator (99). The expression of Siglec-15 is normally limited to cells in the myeloid lineage but can be upregulated in many human cancers (97). As a recent study indicated, Siglec-15 strongly suppresses antigen-specific T-cell responses in vitro and in vivo and can mediate immune evasion in the TME (100). As an immune suppressive molecule, the Siglec-15 pathway is nonredundant to the B7-H1/PD-1 pathway (100,101). Siglec-15 can be upregulated by macrophage colony-stimulating factor (M-CSF) released by diverse cell types in response to inflammatory cytokines or by tumor cells, while under physiological conditions, it is expressed on macrophages at a low level (102).
Apart from Siglec-15’s unique induction mechanism by M-CSF, IFNγ, the major inducer of PD-L1 (101), is significantly demonstrated to suppress the expression of Siglec-15 on macrophages (100). Unlike the majority of Siglec members, there are no typical ITIMs or ITIM-like motifs in the intracellular domain of Siglec15 (58). Instead, it was reported to be associated with the adaptors DAP12 and DAP10, which contain an immunoreceptor tyrosine-based activation motif (ITAM) (103). DAP12 can recruit PI3K (104) and then promote an inflammatory response by activating the mitogen-activated protein kinases (MAPK) pathway (105). Furthermore, Siglec-15 could inhibit T cell NF-κB/NFAT signaling in a direct way once binding and could suppress the proliferation of T cells and the production of cytokines, and the inhibitory function could be mediated by IL-10 (99). In addition, Siglec-15 could behave as a macrophage receptor and produce TGFβ when binding with its sialic acid ligand Sialyl-Tn on tumor cells (101). When the levels of IL10 and TGFβ rise in the TME, the immune-suppressive effect of Siglec-15 is magnified (100).
LILRB family
The LILR family comprises a set of paired immunomodulatory receptors expressed among human myeloid and lymphocyte cell populations. The leukocyte immunoglobulin-like receptor (LILR, LIR, ILT, CD85) family can be divided into two classes: the inhibitory LILR subfamily B (LILRB1−5) and the activating LILR subfamily A (LILRA1−6). Inhibitory LILRB receptors were first identified in 1997 (106). Expression is enriched in myeloid cell populations and is primate specific, reflecting rapid gene duplication and evolution within the leukocyte receptor complex of chromosomes (106). LILRs are closely linked to the human killer cell inhibitory receptor (KIR) family, and both LILRs and KIRs share similar Ig-like structures and cytoplasmic signaling domains. Whereas KIR expression is restricted to natural killer (NK) cells, LILRs are expressed on various immune cells, including NK, T, and B lymphocytes and myelomonocytic cells (monocytes, macrophages, DCs and granulocytes). LILRB expression has also been reported in other cell types, including osteoclasts (107), leukemia , stromal and endothelial cells , and various cancers (108). LILRB expression in cancer has been associated with enhanced tumor growth and correlates with poor survival outcomes. LILRB1 is broadly expressed on myeloid cells, B cells and subsets of NK cells and T cells. LILRB2−5 are more restricted to cells of myeloid origin and DCs. LILRB receptors contain cytoplasmic (S/I/V/LxYxxI/V/L) ITIM domains to recruit the Src homology 2 domain-containing phosphatase SHP1/SHP2/SHIP, leading to inhibited immune signaling cascades. SHP/SHIP phosphatase activity is critical in maintaining immune homeostasis (Figure 7) (109). LILRBs act as both immune checkpoint molecules and tumor sustaining factors but may not affect normal development. Thus, they have potential as attractive targets for cancer treatment.
Figure 7.
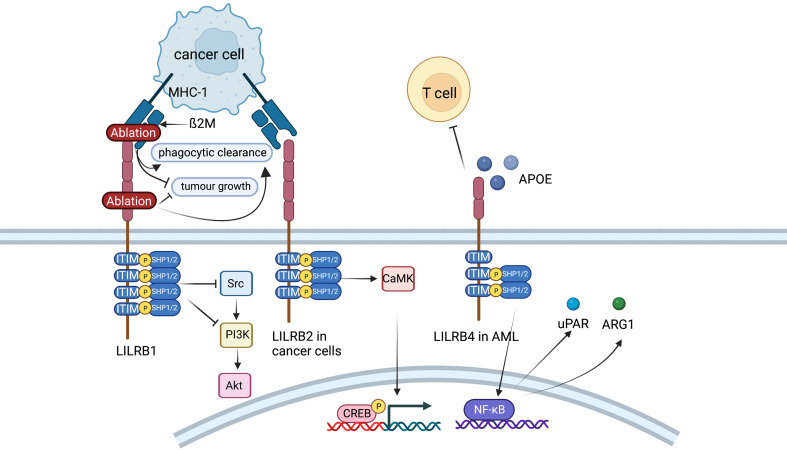
Role of LILRB family. LILRB1 inhibits Fc receptor-mediated signaling in NK cells and monocytes and possibly plays an anti-tumor effect in hepatocarcinoma cells by activating SHP-1, which could then inhibit PI3K/Akt pathway. In endometrial cancer cells, LILRB2 could enhance the SHP2/CaMK1/CREB signaling pathways. LILRB4 supports tumor cell infiltration into tissues and suppresses T cell activity possibly via APOE, LILRB4, SHP-2, uPAR and ARG1 in AML cells. LILRB, leukocyte immunoglobulin-like receptor B; SHP, SH2-domain bearing protein tyrosine phosphatase; NK, natural killer; AML, acute myeloid leukemia.
LILRB1
LILRB1, an immunoreceptor tyrosine-based inhibitory motif-containing receptor, is widely expressed on human immune cells, including B cells, monocytes and macrophages, dendritic cells and subsets of NK cells and T cells (110). The ligands of LILRB1, such as major histocompatibility complex (MHC) class I molecules, activate LILRB1 and transduce a suppressive signal, which inhibits immune responses (111). The MHC class I component β2-microglobulin (β2M) has been found to act as a potential antiphagocytic signal in cancer cells (110). MHC-I on the cancer cell surface is sensed by LILRB1 on TAMs, resulting in negative regulation of cancer cell phagocytosis. LILRB1 is an immunoglobulin-like receptor for the gene product of human cytomegalovirus UL18, a homolog of cellular MHC class I antigens (112). LILRB1 in NK cells inhibits Fc receptor-mediated signaling in monocytes by activating the inhibitory phosphatase SHP-1 (113). It has been reported that LILRB1 possibly plays an antitumor role in hepatocarcinoma cells by interacting with SHP1 (114). Ablation of MHC-I on cancer cells or LILRB1 on macrophages has been reported to promote the phagocytic clearance of tumor cells and inhibit tumor growth in an in vivo mouse model (110).
LILRB2
LILRB2 is known as an immune inhibitory receptor that suppresses the immune system. CD8+CD28− alloantigen-specific T suppressor cells could trigger the expression of LILRB2 on monocytes and DCs, leading to the tolerance effect on these APCs (115). LILRB2high DCs could give rise to the anergy of CD4+CD45RO+CD25+ T cells and suppress their further differentiation into regulatory T cells (116). As recent studies have reported, LILRB2 is highly expressed on hematopoietic stem cells or leukemia stem cells and is critical for the maintenance of stemness supporting hematopoiesis or leukemogenesis (117). LILRB2 is also reported to be expressed in many types of solid cancers. For instance, LILRB2 is linked with fewer infiltrating lymphocytes and more lymphatic metastasis in breast cancer (117). LILRB2 can be detected on the cell membrane and in the intracellular region of NSCLC and enhances progression (118). LILRB2 plays a vital role in sustaining epithelial-mesenchymal transition (EMT) and the early metastatic behavior of pancreatic adenocarcinoma (119). Furthermore, LILRB2 is shown to be highly expressed in endometrial cancer, and the expression levels are conversely correlated with overall survival of patients (119). In vitro experiments indicate that knockdown of LILRB2 leads to a significant decrease in proliferation, colony formation and migration in several endometrial cancer cell lines. LILRB2 could enhance the SHP2/CaMK1/CREB signaling pathways, which supports the expansion and migration of endometrial cancer cells (119).
LILRB4
LILRB4 (also known as CD85K, ILT3, LIR5, and HM18) has two extracellular Ig-like domains (D1 and D2) and three ITIMs. On the one hand, LILRB4 is a marker for monocytic AML (120). Further demonstration is that LILRB4 is more highly expressed on monocytic AML cells than on their normal counterparts and that LILRB4 expression inversely correlates with overall survival of patients with AML (120). Experiments in mouse models and human cells show that LILRB4 supports tumor cell infiltration into tissues and suppresses T-cell activity via a signaling pathway that involves APOE, LILRB4, SHP-2, uPAR and ARG1 in AML cells (120). Furthermore, LILRB4 represents a compelling target for the treatment of monocytic AML. It has been reported that a LILRB4-targeted humanized mAb, which blocks the LILRB4/APOE interaction in a competitive manner, inhibits monocytic AML cell tissue infiltration and reverses T cell suppression (121). On the other hand, LILRB4 is expressed on MDSCs and TAMs (122). Expression of LILRB4 on PMN-MDSCs and M-MDSCs in NSCLC is associated with poor patient outcomes (123). In vitro, the capacity of M-MDSCs from normal human monocytes to induce IL-10-producing Treg cells could be potentiated by prostaglandin E2 through LILRB4 on M-MDSCs (124). Anti-LILRB4 antibodies impaired the T cell suppression induced by M-MDSCs (122). Furthermore, fibronectin expressed by stromal cells in the TME can bind and activate LILRB4 on MDSCs, which recruits SHP-1 to inhibit Syk-mediated FcγR signaling and the immunosuppressive activities of MDSCs (125). Apart from MDSCs, studies have indicated that LILRB4 is expressed on TAMs in various human cancers and mouse models, including melanoma, lung cancer, colon carcinoma and pancreatic carcinoma (108). Blocking LILRB4 or gp49b deficiency could increase the infiltration of antineoplastic immune cells into the TME and lessen the inhibitory effect of Treg cells by regulating IL-1b and iNOS production from TAMs (126). In summary, LILRB4 expressed on MDSCs and TAMs may be an interesting target for cancer immunotherapy.
TREM2
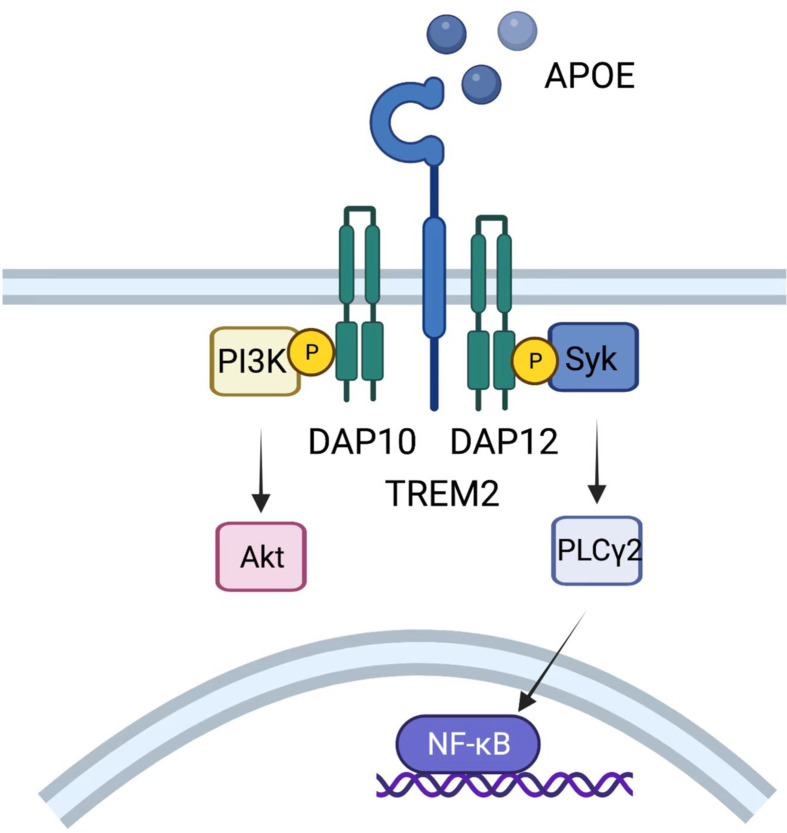
TREM2 was originally recognized in Alzheimer’s disease as a myeloid receptor that transmits intracellular signals to maintain microglial responses. TREM2 is also expressed on TAMs, acting as an activating receptor of the Ig superfamily and transmitting intracellular signals when binding to the adaptor DNAX-activating protein of 12 kDa (DAP12) (127). DAP12, which recruits the protein tyrosine kinase Syk, could conduct a tyrosine phosphorylation cascade activating downstream mediators such as PLCγ2, PI-3K, mTOR and MAPK, finally triggering cell activation (Figure 8). Mouse models show that TREM2−/− mice are more resistant to the growth of diverse kinds of cancers than wild-type mice and are more responsive to anti-PD-1 immunotherapy (128). Moreover, treatment with an anti-TREM2 monoclonal antibody suppresses tumor growth and raises regression when combined with anti-PD-1 (128). Moreover, in colorectal carcinoma (CRC) and triple-negative breast cancer, TREM2 expression is reported to have a converse correlation with higher overall survival and relapse-free survival (128,129).
Figure 8.
Role of TREM2. TREM2 expressed on TAMs could transmit intracellular signals when binding to DAP12, which recruits the protein tyrosine kinase Syk, conducting a tyrosine phosphorylation cascade activating downstream mediators such as PLCγ2, PI-3K, mTOR and MAPK, and finally triggers cell activation. TREM2, triggering receptor expressed on myeloidcells 2; TAM, tumor-associated macrophage; MAPK, mitogen-activated protein kinases.
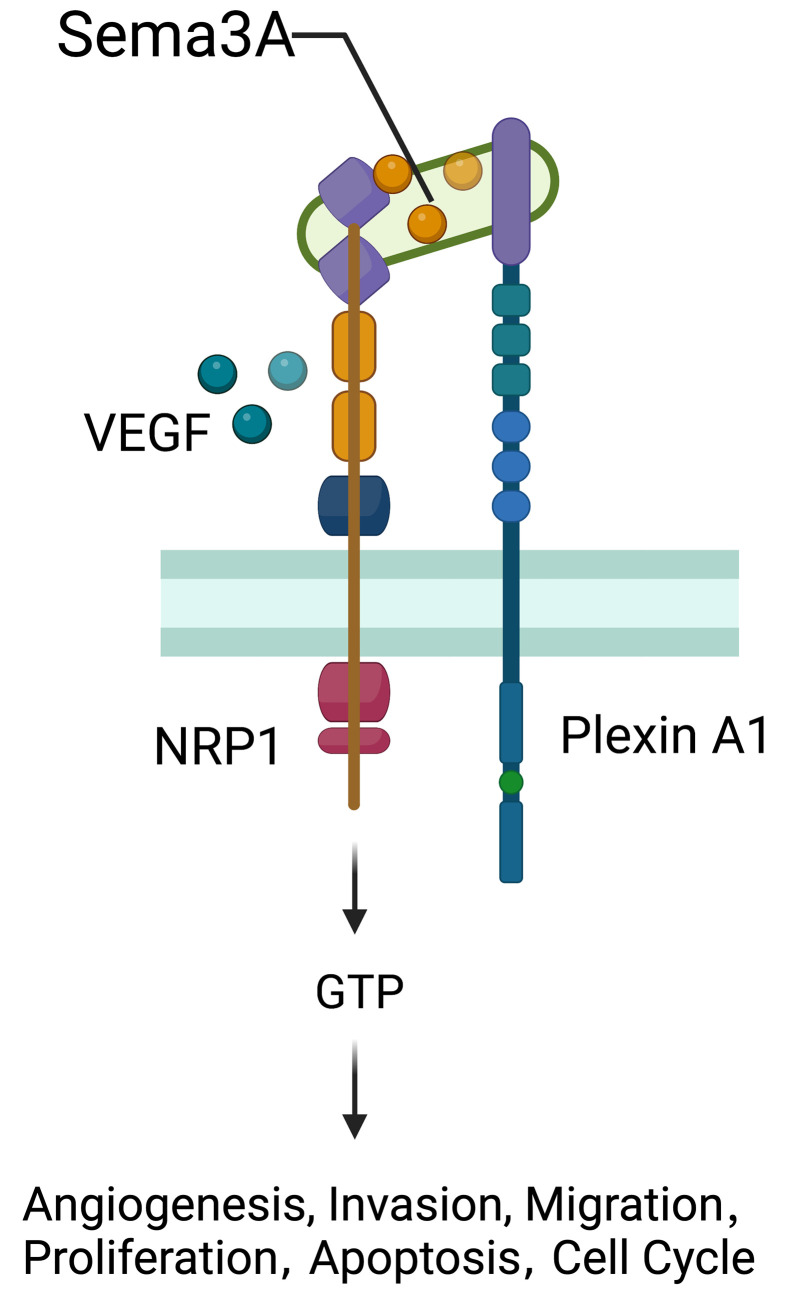
NRP1
NRP1 was originally distinguished as an adhesion molecule in the frog nervous system and found as a transmembrane glycoprotein located on axons of nerve fibers (130). NRP1 located on the cell surface acts as a nontyrosine kinase transmembrane glycoprotein and plays a role as a coreceptor of secreted Semaphorin-3A (Sema-3A) (Figure 9). The NRP1 gene is widely expressed in various kinds of cells, tissues and organs, such as endothelial cells, and the heart, liver, lung, kidney, pancreas, and skeletal muscle (131), playing an essential role in promoting angiogenesis, neural development, cytoskeleton remodelling, inflammation, the initial immune response, and tumor development (132,133). The expression of NRP1 in the immune system is more restricted and regulated. NRP1, which is also known as blood DC antigen 4 (BDCA4, or CD304), was identified as a human DC marker expressed in all pDCs (134). Other types of APCs, including monocytes and macrophages, also express NPR1 (135). NRP1 expressed in monocytes and macrophages generally plays proangiogenic and anti-inflammatory roles, contributing to tissue remodelling and wound healing (136). As recently reported, NPR1 is also expressed in CD8+ T cells in mice and humans and is mainly detected in intratumoral CD8+ T cells marked by high expression of PD-1 (137).
Figure 9.
Role of NRP1. NRP1 located on the cell surface acts as a non-tyrosine kinase transmembrane glycoprotein and plays a role as a co-receptor of secreted Semaphorin-3A (Sema-3A). NRP1 gene widely expresses over various kinds of cells, tissues and organs such as endothelial cells, the heart, liver, lung, kidney, pancreas, and skeletal muscle, playing an essential part in promoting angiogenesis, neural development, cytoskeleton remodeling, inflammation, initial immune response, and tumors development. VEGF, vascular endothelial growth factor.
A recent study indicated the role of NRP1 in the emergence and development of human malignant tumors (138). Higher expression or mutations of NRP1 are linked with the initiation, progression, and prognosis of human malignant tumors such as hepatocellular carcinoma (139), gastric cancer (140,141), breast cancer (142), prostatic cancer, and pancreatic cancer (143). Overexpression of NRP1 significantly reduces the survival rate in NSCLC patients. The reduction of NRP1 expression by utilizing NRP1 monoclonal antibody, RNA interference or NRP1 inhibitor could inhibit tumor cell growth and tumor angiogenesis (144). Overexpression of NRP1 in human oral squamous cell carcinoma shows more spindle filaments, reduces the expression of EMT epithelial markers and increases the expression of mesenchymal markers (145). As studies report, NRP1 promotes the presence of EMT in breast cancer cells by augmenting signaling molecules such as TGF-β (146).
Clever-1
Clever-1, also known as stabilin-1 or FEEL-1, is a multifunctional glycoprotein expressed on a subset of anti-inflammatory macrophages involved in scavenging, angiogenesis and cell adhesion, which are involved in receptor-mediated endocytosis and recycling, intracellular sorting and transcytosis (147,148). Additionally, it is the first adhesion molecule implicated in transmigration through the lymphatic arm of the immune system (147). Attenuated progression of melanoma tumor growth and metastasis has been observed in Clever-1-knockout mice and those treated with anti-Clever-1 therapy (149). Clever-1+ macrophages are found in human cancers and are associated with poorer disease-free survival in colorectal cancers of advanced stage and overall survival in bladder cancer (149). Circulating monocytes and tissue macrophages show higher expression of Clever-1 in an immunosuppressive environment, such as pregnancy and cancer, which could suppress Th1 lymphocyte activation (148). Meanwhile, according to a phase 1/2 clinical trial, bexmarilimab, a novel anti-Clever-1 antibody, has shown significant efficacy in 10 patients with hard-to-treat solid tumors (150).
Clinical application
While myeloid checkpoints have been increasingly recognized as a potentially hopeful target to develop novel immunotherapeutic strategies, several monoclonal antibodies (mAbs) targeting myeloid checkpoints have recently been under development, and clinical trials are ongoing. Here, we labelled the recent advanced antibodies targeting those myeloid checkpoints below (Table 1).
Table 1. Current clinical trials targeting myeloid checkpoints.
| Target | Agent | Interventions | Company | Study phase/status | Clinical trial ID | Conditions or diseases |
| LILRB, leukocyte immunoglobulin-like receptor B; Siglec, sialic acid-binding immunoglobulin-type lectin; NRP1, neuropilin-1; Cleve1, commonlymphatic endothelial and vascular endothelial receptor-1; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CMML, chronic myelomonocytic leukemia. | ||||||
| CD47/ SIRPα |
HX009 | Anti-CD47/PD-1 bifunctional antibody | Waterstone Hanxbio Pty Ltd. | Phase 2/Recruiting | NCT04886271 | Advanced solid tumor |
| IBI188, Rituximab | Anti-CD47 monoclonal antibody | Innovent Biologics (Suzhou) Co. Ltd. | Phase 1/Active | NCT03717103 | Advanced malignancies | |
| IBI188 | Anti-CD47 monoclonal antibody | Innovent Biologics (Suzhou) Co. Ltd. | Phase 1/Completed | NCT03763149 | Advanced malignancies | |
| Magrolimab | Anti-CD47 monoclonal antibody | University of California, San Francisco & Gilead Sciences | Phase 1/Not yet recruiting | NCT05169944 | Children and adults with recurrent or progressive malignant brain tumors | |
| STI-6643 | − | Sorrento Therapeutics, Inc. | Phase 1/Recruiting | NCT04900519 | Advanced solid tumors | |
| CC-90002 Rituximab |
− | Celgene | Phase 1/Completed | NCT02367196 | Advanced solid and hematologic cancers | |
| TTI-621 | Soluble recombinant fusion protein | Trillium Therapeutics Inc. | Phase 1/Recruiting | NCT02663518 | Hematologic malignancies and selected solid tumors | |
| Hu5F9-G4 | Anti-CD47 monoclonal antibody | Gilead Sciences | Phase 1/Phase 1 | NCT02678338 | Hematological malignancies | |
| Hu5F9-G4 | − | Gilead Sciences | Phase 1/Phase 1 | NCT02216409 | Solid tumors | |
| PF-07257876 |
CD47-PD-L1 bispecific antibody | Pfizer | Phase 1/Recruiting | NCT04881045 | Selected advanced tumors | |
| Magrolimab | Monotherapy or in combination with Azacitidine | Gilead Sciences | Phase 1/Recruiting | NCT03248479 | Hematological malignancies | |
| SRF231 | − | Surface Oncology | Phase 1/Completed | NCT03512340 | Advanced solid and hematologic cancers | |
| AK117 | Anti-CD47 | Akeso | Phase 1/Recruiting | NCT04728334 | Malignant neoplasms | |
| TQB2928 | New molecular entity | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Phase 1/Not yet recruiting | NCT04854681 | Advanced solid tumors and hematological malignancies | |
| IMC-002 | Blocking the interaction between CD47 and SIRPα | ImmuneOncia Therapeutics Inc. | Phase 1/Recruiting | NCT04306224 | Metastatic or locally advanced solid tumors and relapsed or refractory lymphomas | |
| IBI322 | Recombinant anti-human CD47/PD-L1 bispecific antibody | Innovent Biologics (Suzhou) Co. Ltd. | Phase 1/Recruiting | NCT04328831 | Advanced malignancies | |
| IMM2902 | HER2/SIRPα bispecific mAb-trap antibody-receptor fusion protein | ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd. | Phase 1/Not yet recruiting | NCT05076591 | HER2-expressing advanced solid tumors | |
| AO-176 | Humanized monoclonal antibody (mAb) targeting CD47 | Arch Oncology | Phase 1/2/Recruiting | NCT03834948 | Solid tumor malignancies | |
| IBC0966 | − | SUNHO (China) BioPharmaceutical CO., Ltd. | Phase 1/2/Not yet recruiting | NCT04980690 | Advanced malignant tumors | |
| IBI322 | Recombinant anti-human CD47/PD-L1 bispecific antibody | Innovent Biologics (Suzhou) Co. Ltd. | Phase 1/Not yet recruiting | NCT04338659 | Advanced malignancies | |
| IBI322 | IBI322 monotherapy or combination therapy | Innovent Biologics (Suzhou) Co. Ltd. | Phase 1/Not yet recruiting | NCT04912466 | Advanced malignant tumors | |
| IBI322+HMA | IBI322 monotherapy or combination therapy with HMA | Innovent Biologics (Suzhou) Co. Ltd. | Phase Ia/Ib/Not yet recruiting | NCT05148442 | Myeloid tumors | |
| TTI-621 | Soluble recombinant fusion protein created by directly linking the sequences encoding the N-terminal CD47 binding domain of human SIRPα with the Fc domain of human immunoglobulin (IgG1) | Trillium Therapeutics Inc. | Phase 1/Terminated | NCT02890368 | Relapsed and refractory solid tumors and mycosis fungoides | |
| TTI-621+TTI-622 | TTI-621 and TTI-622 in combination with daratumumab hyaluronidase-fihj | Memorial Sloan Kettering Cancer Center | Phase 1/Recruiting | NCT05139225 | Multiple myeloma | |
| AK117 | Anti-CD47 antibody | Akeso | Phase 1/2/Recruiting | NCT04980885 | Acute myeloid leukemia | |
| AK117 | Anti-CD47 antibody | Akeso | Phase 1/2/Recruiting | NCT04900350 | Myelodysplastic syndrome | |
| ALX148 (Evorpacept) | − | ALX Oncology Inc. | Phase 1/Active, not recruiting | NCT03013218 | Advanced solid tumors and lymphoma | |
| ALX148 | Combination with trastuzumab, ramucirumab, and paclitaxel | ALX Oncology Inc. | Phase 2/3/Not yet recruiting | NCT05002127 | HER2+ gastric cancer gastric cancer | |
| ALX148 | Evaluating the combination of ALX148, cetuximab, and pembrolizumab | Criterium, Inc. | Phase 2/Not yet recruiting | NCT05167409 | Microsatellite stable metastatic colorectal cancer | |
| AO-176 | AO-176 as monotherapy and in combination with bortezomib/dexamethasone | Arch Oncology | Phase 1/2/Recruiting | NCT04445701 | Relapsed/refractory multiple myeloma | |
| CC-90002 | Monoclonal Ab to CD47 | Celgene | Terminated | NCT02641002 | AML and high-risk MDS | |
| CPO107/ JMT601 | CD20−CD47 bispecific fusion protein | Conjupro Biotherapeutics, Inc. | Phase 1/2/Not yet recruiting | NCT04853329 | CD20 positive non-Hodgkin lymphoma | |
| TG-1801 | Bispecific CD47 and CD19 antibody | TG Therapeutics, Inc. | Phase 1/Recruiting | NCT04806035 | B-cell lymphoma or chronic lymphocytic leukemia | |
| Lemzoparlimab (TJC4) | CD47-SIRPα antibody | AbbVie | Phase 1 | NCT04912063 | AML; MDS | |
| SL-172154 | Fusion protein consisting of human SIRPα and CD40L (SIRPα-Fc-CD40L) linked via a human Fc | Shattuck Labs, Inc. | Phase 1/Recruiting | NCT04502888 | Squamous cell carcinoma of the head and neck or skin | |
| TJ1133 | Anti-CD47 antibody | The First Affiliated Hospital of Bengbu Medical College | Phase 1/2/Recruiting | NCT05148533 | Advanced solid tumors | |
| SLAMF7 | SLAMF7 BATs | Combining anti-CD3 x anti-SLAMF7 bispecific antibody | University of Virginia | Phase 1/Not yet recruiting | NCT04864522 | Relapsed/Refractory multiple myeloma |
| Anti-SLAMF7 | Anti-SLAMF7 monoclonal antibody | University of Arkansas | Phase 2/Withdrawn (No study population. No subjects enrolled.) | NCT03168100 | Multiple myeloma | |
| Anti-SLAMF7 | Anti-SLAMF7 monoclonal antibody | University of Arkansas | Phase 2/Withdrawn (No study population. No subjects enrolled.) | NCT03000634 | Multiple myeloma during maintenance therapy | |
| Elotuzumab | mAb directed against the extracellular domain of SLAMF7 | Tulane University School of Medicine | Phase 2/Not yet recruiting | NCT05170789 | Relapsed refractory multiple myeloma | |
| Elotuzumab | Elotuzumab in combination with carfilzomib, lenalidomide and dexamethasone (E-KRd) | Wuerzburg University Hospital | Phase 3/Active, not recruiting | NCT03948035 | Newly diagnosed multiple myeloma | |
| Elotuzumab | − | University of Arkansas | Phase 2/Withdrawal of study support | NCT03003728 | High-risk myeloma post- autologous stem cell transplant | |
| Elotuzumab | Lenalidomide and dexamethasone with or without elotuzumab | Bristol-Myers Squibb | Phase 3/Terminated (Insufficient enrollment) | NCT01891643 | Newly diagnosed, previously untreated multiple myeloma | |
| Biological: Elotuzumab | Expanded access to elotuzumab (empliciti) for multiple myeloma | Bristol-Myers Squibb | No longer available | NCT03126617 | Multiple myeloma | |
| Biological: BCMA-CS1 cCAR T cells | − | iCell Gene Therapeutics | Early phase 1/Unknown | NCT04156269 | Multiple myeloma | |
| Biological: Elotuzumab | Anti-SLAMF7 monoclonal antibody | M.D. Anderson Cancer Center | Phase 2/Recruiting | NCT04517851 | JAK2-mutated myelofibrosis | |
| LILRB1 | BND-22 | − | Biond Biologics | Phase 1/2/Recruiting | NCT04717375 | Advanced solid tumors |
| NGM707 | LILRB1/LILRB2 bispecific antibody | NGM Biopharmaceuticals, Inc. | Phase 1/2/Recruiting | NCT04913337 | Advanced or metastatic solid tumor malignancies | |
| LILRB2 | JTX 8064 | As monotherapy or in combination with PD-1 inhibitor | Jounce Therapeutics, Inc. | Phase 1/Recruiting | NCT04669899 | Advanced refractory solid tumors |
| IO-108 | As monotherapy or in combination with pembrolizumab | Immune-Onc Therapeutics Inc. | Phase 1/Recruiting | NCT05054348 | Solid tumors | |
| MK-4830 | − | Merck Sharp & Dohme Corp | Phase 1/Recruiting | NCT03564691 | Advanced solid tumors | |
| LILRB4 | IO-202 | Monotherapy or plus azacitidine | Immune-Onc Therapeutics Inc. | Phase 1/Recruiting | NCT04372433 | AML and CMML |
| IO-202 | Monotherapy or in combination with pembrolizumab | Immune-Onc Therapeutics Inc | Phase 1/Not yet recruiting | NCT05309187 | Solid cancer | |
| MK-0482 | In combination with pembrolizumab | Merck Sharp & Dohme LLC | Phase 2 | NCT04165096 | Non-small-cell lung carcinoma, acute leukemia, myeloid, neoplasms | |
| TREM2 | PY314 | PY314 or in combination with pembrolizumab | Pionyr Immunotherapeutics Inc. | Phase 1/Recruiting | NCT04691375 | Advanced solid tumors |
| Siglec15 | NC318 | NC318 or in combination with pembrolizumab | Yale University New Haven, Connecticut, United States | Phase 2/Recruiting | NCT04699123 | Advanced non-small cell lung cancer |
| NRP1 | ASP1948 | ASP1948 or in combination with nivolumab or pembrolizumab | Astellas Pharma Global Development, Inc. | Phase 1b | NCT03565445 | Advanced solid tumors |
| Clever1 | Bexmarilimab | Dose-escalation, six dose levels | The University of Texas Health Science Center at San Antonio San Antonio, Texas, United States | Phase I/II | NCT03733990 | Advanced solid tumors |
Summary and prospects
In this review, we briefly summarized the biological functions of different kinds of myeloid checkpoints expressed on various myeloid cells, which has greatly advanced cancer treatment. To overcome the immune escape mechanisms of tumors and to improve the versatility and raise the efficiency of current immunotherapies, it is necessary to understand myeloid checkpoints in more details and explore novel approaches based on these kinds of checkpoints.
Acknowledgements
This study was supported by the Imported Scholar Project and Startup from Peking University Health Science Center (BMU2021YJ063 to MD); the Biotechnology Innovation Plan from Beijing Sungen Biomedical Technology Co., Ltd (2022066 to MD); and the Excellent Young Scientists Fund Program (overseas) from National Natural Science Fund (HY2021-7 to MD).
References
- 1.O’Donnell JS, Teng MWL, Smyth MJ Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer JR, Tykodi SS, Chow LQ, et al Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manguso RT, Pope HW, Zimmer MD, et al In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–8. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SJ, Sanjana NE, Kishton Rj, et al Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–42. doi: 10.1038/nature23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan D, Kobayashi A, Jiang P, et al A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science. 2018;359:770–5. doi: 10.1126/science.aao1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura K, Smyth MJ Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17:1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen TO, Schmidt H, Møller HJ, et al Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012:118;2476–85. doi: 10.1002/cncr.26511. [DOI] [PubMed] [Google Scholar]
- 9.Goc J, Germain C, Vo-Bourgais TK, et al Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–15. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 10.Huang SH, Waldron JN, Milosevic M, et al Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015:121;545–55. doi: 10.1002/cncr.29100. [DOI] [PubMed] [Google Scholar]
- 11.Lavin Y, Winter D, Blecher-Gonen R, et al Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–26. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engblom C, Pfirschke C, Pittet MJ The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16:447–62. doi: 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S, Li Z, Gao R, et al A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184:792–809.e23. doi: 10.1016/j.cell.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Geissmann F, Manz MG, Jung S, et al Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331><20133564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Q, Hawkins GA, Wudel L, et al Dissecting intratumoral myeloid cell plasticity by single cell RNA-seq. Cancer Med. 2019:8;3072–85. doi: 10.1002/cam4.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiss M, Van Gassen S, Movahedi K, et al Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol. 2018:330;188–201. doi: 10.1016/j.cellimm.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Kim IS Harnessing immune checkpoints in myeloid lineage cells for cancer immunotherapy. Cancer Lett. 2019:452;51–8. doi: 10.1016/j.canlet.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Logtenberg MEW, Scheeren FA, Schumacher TN The CD47-SIRPα immune checkpoint. Immunity. 2020:52;742–52. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg AD, Romano E, Rufo N, et al Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. Cell Death Differ. 2016:23;938–51. doi: 10.1038/cdd.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oldenborg PA, Zheleznyak A, Fang YF, et al Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 21.Majeti R, Chao MP, Alizadeh AA, et al CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009:138;286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrera L, Montes-Servín E, Hernandez-Martinez JM CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. 2017;117:385–97. doi: 10.1038/bjc.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Lu S, Xu Y, et al Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res. 2017;9:2901–10. [PMC free article] [PubMed] [Google Scholar]
- 24.Guo H, Li W, Qian L, et al Clinical challenges in neoadjuvant immunotherapy for non-small cell lung cancer. Chin J Cancer Res. 2021;33:203–15. doi: 10.21147/j.issn.1000-9604.2021.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ring NG, Herndler-Brandstetter D, Weiskopf K, et al Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114:E10578–85. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advani R, Flinn I, Popplewell L, et al CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018;379:1711–21. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sockolosky JT, Dougan M, Ingram JR, et al Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113:E2646–54. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin N, Song Y, Zhu J Immune checkpoint inhibitors in malignant lymphoma: Advances and perspectives. Chin J Cancer Res. 2020;32:303–18. doi: 10.21147/j.issn.1000-9604.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu MM, Pu Y, Han D, et al Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein α signaling. Immunity. 2017;47:363–73.e5. doi: 10.1016/j.immuni.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon SR, Maute RL, Dulken BW, et al PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connell P, Hyslop S, Blake MK, et al SLAMF7 signaling reprograms T cells toward exhaustion in the tumor microenvironment. J Immunol. 2021;206:193–205. doi: 10.4049/jimmunol.2000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell P, Amalfitano A, Aldhamen YA SLAM family receptor signaling in viral infections: HIV and beyond. Vaccines (Basel) 2019;7:184. doi: 10.3390/vaccines7040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Zhong MC, Guo H, et al SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544:493–7. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannons JL, Tangye SG, Schwartzberg PL SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell P, Pepelyayeva Y, Blake MK, et al SLAMF7 is a critical negative regulator of IFN-α-mediated CXCL10 production in chronic HIV infection. J Immunol. 2019;202:228–38. doi: 10.4049/jimmunol.1800847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo H, Cruz-Munoz ME, Wu N, et al Immune cell inhibition by SLAMF7 is mediated by a mechanism requiring src kinases, CD45, and SHIP-1 that is defective in multiple myeloma cells. Mol Cell Biol. 2015;35:41–51. doi: 10.1128/MCB.01107-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller BC, Sen DR, Al Abosy R, et al Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–36. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sade-Feldman M, Yizhak K, Bjorgaard SL, et al Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175:998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurtulus S, Madi A, Escobar G, et al Checkpoint blockade immunotherapy induces dynamic changes in PD-1−CD8+ tumor-infiltrating T cells. Immunity. 2019;50:181–94.e6. doi: 10.1016/j.immuni.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson DE, O’Keefe RA, Grandis JR Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–48. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ffrench-Constant C, Colognato H Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–86. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Hamerman JA, Ni M, Killebrew JR, et al The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu N, Veillette A SLAM family receptors in normal immunity and immune pathologies. Curr Opin Immunol. 2016;38:45–51. doi: 10.1016/j.coi.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Li D, Xiong W, Wang Y, et al SLAMF3 and SLAMF4 are immune checkpoints that constrain macrophage phagocytosis of hematopoietic tumors. Science Immunol. 2022;7:eabj5501. doi: 10.1126/sciimmunol.abj5501. [DOI] [PubMed] [Google Scholar]
- 45.Ferris RL, Blumenschein G Jr, Fayette J, et al Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Limagne E, Richard C, Thibaudin M, et al Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. Oncoimmunology. 2019;8:e1564505. doi: 10.1080/2162402X.2018.1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Highfill SL, Cui Y, Giles AJ, et al Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra267. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Henau O, Rausch M, Winkler D, et al Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539:443–7. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Knolhoff BL, Meyer MA, et al CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014:74;5057–69. doi: 10.1158/0008-5472.Can-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipson EJ, Forde PM, Hammers HJ, et al Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42:587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topalian SL, Hodi FS, Brahmer JR, et al Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alsaab HO, Sau S, Alzhrani R, et al PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mi Y, Han J, Zhu J, et al Role of the PD-1/PD-L1 signaling in multiple sclerosis and experimental autoimmune encephalomyelitis: recent insights and future directions. Mol Neurobiol. 2021;58:6249–71. doi: 10.1007/s12035-021-02495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He G, Zhang H, Zhou J, et al Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:141. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Lee Y, Li Y, et al Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8+ T cells. Immunity. 2018;48:773–86.e5. doi: 10.1016/j.immuni.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Macauley MS, Crocker PR, Paulson JC Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–66. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C, Rauch U, Korpos E, et al Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J Immunol. 2009;182:6508–16. doi: 10.4049/jimmunol.0804247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crocker PR, Paulson JC, Varki A Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 59.Varki NM, Strobert E, Dick EJ Jr, et al Biomedical differences between human and nonhuman hominids: potential roles for uniquely human aspects of sialic acid biology. Annu Rev Pathol. 2011;6:365–93. doi: 10.1146/annurev-pathol-011110-130315. [DOI] [PubMed] [Google Scholar]
- 60.O’Neill AS, van den Berg TK, Mullen GE Sialoadhesin - a macrophage-restricted marker of immunoregulation and inflammation. Immunology. 2013:138;198–207. doi: 10.1111/imm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan S, Paulson JC Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365–95. doi: 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- 62.Nycholat CM, Rademacher C, Kawasaki N, et al In silico-aided design of a glycan ligand of sialoadhesin for in vivo targeting of macrophages. J Am Chem Soc. 2012;134:15696–9. doi: 10.1021/ja307501e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oetke C, Vinson MC, Jones C, et al Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin M levels. Mol Cell Biol. 2006;26:1549–57. doi: 10.1128/MCB.26.4.1549-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez-Gil A, Schnaar RL Siglec ligands. Cells. 2021;10:1260. doi: 10.3390/cells10051260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fraschilla I, Pillai S Viewing Siglecs through the lens of tumor immunology. Immunol Rev. 2017;276:178–91. doi: 10.1111/imr.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edgar LJ, Kawasaki N, Nycholat CM Targeted delivery of antigen to activated CD169+ macrophages induces bias for expansion of CD8+ T cells. Cell Chem Biol. 2019;26:131–6.e4. doi: 10.1016/j.chembiol.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravishankar B, Shinde R, Liu H, et al Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci U S A. 2014;111:4215–20. doi: 10.1073/pnas.1320924111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiota T, Miyasato Y, Ohnishi K, et al The clinical significance of CD169-positive lymph node macrophage in patients with breast cancer. PLoS One. 2016;11:e0166680. doi: 10.1371/journal.pone.0166680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohnishi K, Komohara Y, Saito Y, et al CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013;104:1237–44. doi: 10.1111/cas.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohnishi K, Yamaguchi M, Erdenebaatar C, et al Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016;107:846–52. doi: 10.1111/cas.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Dinther D, Lopez Venegas M, Veninga H, et al Activation of CD8+ T cell responses after melanoma antigen targeting to CD169+ antigen presenting cells in mice and humans. Cancers (Basel) 2019;11:183. doi: 10.3390/cancers11020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Q, Hou J, Zhou Y, et al Siglec1 suppresses antiviral innate immune response by inducing TBK1 degradation via the ubiquitin ligase TRIM27. Cell Res. 2015;25:1121–36. doi: 10.1038/cr.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prenzler S, Rudrawar S, Waespy M, et al. The role of sialic acid-binding immunoglobulin-like-lectin-1 (siglec-1) in immunology and infectious disease. Int Rev Immunol 2021;1-26. [Epub ahead of print]
- 74.Zhang JQ, Nicoll G, Jones C, et al Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275:22121–6. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 75.Yu H, Gonzalez-Gil A, Wei Y, et al Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology. 2017;27:657–68. doi: 10.1093/glycob/cwx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyazaki K, Sakuma K, Kawamura YI, et al Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and -9. J Immunol. 2012;188:4690–700. doi: 10.4049/jimmunol.1100605. [DOI] [PubMed] [Google Scholar]
- 77.Hudak JE, Canham SM, Bertozzi CR, et al Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol. 2014;10:69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avril T, Floyd H, Lopez F, et al The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–9. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 79.Ghasemi Z, Dinarvand R, Mottaghitalab F, et al Aptamer decorated hyaluronan/chitosan nanoparticles for targeted delivery of 5-fluorouracil to MUC1 overexpressing adenocarcinomas. Carbohydr Polym. 2015;121:190–8. doi: 10.1016/j.carbpol.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 80.Tanida S, Akita K, Ishida A, et al Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of beta-catenin and subsequent cell growth. J Biol Chem. 2013;288:31842–52. doi: 10.1074/jbc.M113.471318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beatson R, Tajadura-Ortega V, Achkova D, et al The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. 2016;17:1273–81. doi: 10.1038/ni.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belisle JA, Horibata S, Jennifer GA, et al Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams OJ, Stanczak MA, von Gunten S, et al Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology. 2018;28:640–7. doi: 10.1093/glycob/cwx108. [DOI] [PubMed] [Google Scholar]
- 84.McMillan SJ, Sharma RS, McKenzie EJ, et al Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11bβ2-integrin-dependent signaling. Blood. 2013;121:2084–94. doi: 10.1182/blood-2012-08-449983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Läubli H, Pearce OM, Schwarz F, et al Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci U S A. 2014;111:14211–6. doi: 10.1073/pnas.1409580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwarz F, Pearce OM, Wang X, et al Siglec receptors impact mammalian lifespan by modulating oxidative stress. Elife. 2015;4:e06184. doi: 10.7554/eLife.06184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen GY, Brown NK, Zheng P, et al Siglec-G/10 in self-nonself discrimination of innate and adaptive immunity. Glycobiology. 2014;24:800–6. doi: 10.1093/glycob/cwu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen W, Han C, Xie B, et al Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152:467–78. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 89.Chen GY, Chen X, King S, et al Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. 2011;29:428–35. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu H, Pan Y, Zhang N A breakthrough in liver regeneration for treatment of liver cancer. Cancer Biol Med. 2021;18:631–4. doi: 10.20892/j.issn.2095-3941.2021.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abram CL, Lowell CA Shp1 function in myeloid cells. J Leukoc Biol. 2017;102:657–75. doi: 10.1189/jlb.2MR0317-105R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen RL, Raine T, Haude A, et al Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol. 2001:167;5543–7. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 93.Barkal AA, Brewer RE, Markovic M, et al CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–6. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kleinmanns K, Bischof K, Anandan S, et al CD24-targeted fluorescence imaging in patient-derived xenograft models of high-grade serous ovarian carcinoma. EBioMedicine. 2020;56:102782. doi: 10.1016/j.ebiom.2020.102782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taurin S, Alkhalifa H Breast cancers, mammary stem cells, and cancer stem cells, characteristics, and hypotheses. Neoplasia. 2020;22:663–78. doi: 10.1016/j.neo.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ayers M, Nebozhyn M, Cristescu R, et al Molecular profiling of cohorts of tumor samples to guide clinical development of pembrolizumab as monotherapy. Clin Cancer Res. 2019;25:1564–73. doi: 10.1158/1078-0432.CCR-18-1316. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Sun J, Liu LN, et al Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25:656–66. doi: 10.1038/s41591-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stuible M, Moraitis A, Fortin A, et al Mechanism and function of monoclonal antibodies targeting siglec-15 for therapeutic inhibition of osteoclastic bone resorption. J Biol Chem. 2014;289:6498–512. doi: 10.1074/jbc.M113.494542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hiruma Y, Hirai T, Tsuda E Siglec-15, a member of the sialic acid-binding lectin, is a novel regulator for osteoclast differentiation. Biochem Biophys Res Commun. 2011;409:424–9. doi: 10.1016/j.bbrc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 100.Sun J, Lu Q, Sanmamed MF, et al Siglec-15 as an emerging target for next-generation cancer immunotherapy. Clin Cancer Res. 2021;27:680–8. doi: 10.1158/1078-0432.CCR-19-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong H, Strome SE, Salomao DR, et al Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 102.Zhang YH, He M, Wang Y, et al Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. 2017;8:120. doi: 10.3389/fimmu.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ishida-Kitagawa N, Tanaka K, Bao X, et al Siglec-15 protein regulates formation of functional osteoclasts in concert with DNAX-activating protein of 12 kDa (DAP12) J Biol Chem. 2012;287:17493–502. doi: 10.1074/jbc.M111.324194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao H, Lakner U, de Bono B, et al SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008;38:2303–15. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 105.Ali SR, Fong JJ, Carlin AF, et al Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 2014;211:1231–42. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Samaridis J, Colonna M Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol. 1997;27:660–5. doi: 10.1002/eji.1830270313. [DOI] [PubMed] [Google Scholar]
- 107.Mori Y, Tsuji S, Inui M, et al Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J Immunol. 2008;181:4742–51. doi: 10.4049/jimmunol.181.7.4742. [DOI] [PubMed] [Google Scholar]
- 108.Yang T, Qian Y, Liang X, et al LILRB4, an immune checkpoint on myeloid cells. Blood Sci. 2022;4:49–56. doi: 10.1097/BS9.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ravetch JV, Lanier LL Immune inhibitory receptors. Science. 2000;290:84–9. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 110.Barkal AA, Weiskopf K, Kao KS, et al Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19:76–84. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen H, Chen Y, Deng M, et al Antagonistic anti-LILRB1 monoclonal antibody regulates antitumor functions of natural killer cells. J Immunother Cancer. 2020;8:e000515. doi: 10.1136/jitc-2019-000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cosman D, Fanger N, Borges L, et al A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–82. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 113.Fanger NA, Cosman D, Peterson L, et al The MHC class I binding proteins LIR-1 and LIR-2 inhibit Fc receptor-mediated signaling in monocytes. Eur J Immunol. 1998;28:3423–34. doi: 10.1002/(SICI)1521-4141(199811)28:11<3423::AID-IMMU3423>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 114.Cheng J, Luan J, Chen P, et al Immunosuppressive receptor LILRB1 acts as a potential regulator in hepatocellular carcinoma by integrating with SHP1. Cancer Biomark. 2020;28:309–19. doi: 10.3233/CBM-190940. [DOI] [PubMed] [Google Scholar]
- 115.Chang CC, Ciubotariu R, Manavalan JS, et al Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 116.Stallone G, Pontrelli P, Infante B, et al Rapamycin induces ILT3(high)ILT4(high) dendritic cells promoting a new immunoregulatory pathway. Kidney Int. 2014;85:888–97. doi: 10.1038/ki.2013.337. [DOI] [PubMed] [Google Scholar]
- 117.Zheng J, Umikawa M, Cui C, et al Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature. 2012;485:656–60. doi: 10.1038/nature11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grosskopf S, Eckert C, Arkona C, et al Selective inhibitors of the protein tyrosine phosphatase SHP2 block cellular motility and growth of cancer cells in vitro and in vivo. ChemMedChem. 2015;10:815–26. doi: 10.1002/cmdc.201500015. [DOI] [PubMed] [Google Scholar]
- 119.Shao H, Ma L, Jin F, et al Immune inhibitory receptor LILRB2 is critical for the endometrial cancer progression. Biochem Biophys Res Commun. 2018;506:243–50. doi: 10.1016/j.bbrc.2018.09.114. [DOI] [PubMed] [Google Scholar]
- 120.Deng M, Gui X, Kim J, et al LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018;562:605–9. doi: 10.1038/s41586-018-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gui X, Deng M, Song H, et al Disrupting LILRB4/APOE interaction by an efficacious humanized antibody reverses T-cell suppression and blocks AML development. Cancer Immunol Res. 2019;7:1244–57. doi: 10.1158/2326-6066.CIR-19-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh L, Muise ES, Bhattacharya A, et al ILT3 (LILRB4) promotes the immunosuppressive function of tumor-educated human monocytic myeloid-derived suppressor cells. Mol Cancer Res. 2021;19:702–16. doi: 10.1158/1541-7786.MCR-20-0622. [DOI] [PubMed] [Google Scholar]
- 123.de Goeje PL, Bezemer K, Heuvers ME, et al Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. Oncoimmunology. 2015;4:e1014242. doi: 10.1080/2162402X.2015.1014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tomić S, Joksimović B, Bekić M, et al Prostaglanin-E2 potentiates the suppressive functions of human mononuclear myeloid-derived suppressor cells and increases their capacity to expand IL-10-producing regulatory T cell subsets. Front Immunol. 2019;10:475. doi: 10.3389/fimmu.2019.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paavola KJ, Roda JM, Lin VY, et al The fibronectin-ILT3 interaction functions as a stromal checkpoint that suppresses myeloid cells. Cancer Immunol Res. 2021;9:1283–97. doi: 10.1158/2326-6066.CIR-21-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma N, Atolagbe OT, Ge Z, et al LILRB4 suppresses immunity in solid tumors and is a potential target for immunotherapy. J Exp Med. 2021;218:e20201811. doi: 10.1084/jem.20201811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peng Q, Malhotra S, Torchia JA, et al TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Molgora M, Esaulova E, Vermi W, et al TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell. 2020;182:886–900.e17. doi: 10.1016/j.cell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mantovani A, Marchesi F, Malesci A, et al Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mercurio AM VEGF/Neuropilin signaling in cancer stem cells. Int J Mol Sci. 2019;20:490. doi: 10.3390/ijms20030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wild JR, Staton CA, Chapple K, et al Neuropilins: expression and roles in the epithelium. Int J Exp Pathol. 2012;93:81–103. doi: 10.1111/j.1365-2613.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roy S, Bag AK, Singh RK, et al Multifaceted role of neuropilins in the immune system: Potential targets for immunotherapy. Front Immunol. 2017;8:1228. doi: 10.3389/fimmu.2017.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schellenburg S, Schulz A, Poitz DM, et al Role of neuropilin-2 in the immune system. Mol Immunol. 2017:90;239–44. doi: 10.1016/j.molimm.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 134.Dzionek A, Fuchs A, Schmidt P, et al BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 135.Wallerius M, Wallmann T, Bartish M, et al Guidance molecule SEMA3A restricts tumor growth by differentially regulating the proliferation of tumor-associated macrophages. Cancer Res. 2016;76:3166–78. doi: 10.1158/0008-5472.CAN-15-2596. [DOI] [PubMed] [Google Scholar]
- 136.DeFalco T, Bhattacharya I, Williams AV, et al Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111:E2384–93. doi: 10.1073/pnas.1400057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leclerc M, Voilin E, Gros G, et al Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat Commun. 2019:10;3345. doi: 10.1038/s41467-019-11280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Torres-Salido MT, Sanchis M, Solé C, et al Urinary neuropilin-1: A predictive biomarker for renal outcome in lupus nephritis. Int J Mol Sci. 2019;20:4610. doi: 10.3390/ijms20184601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bergé M, Allanic D, Bonnin P, et al Neuropilin-1 is upregulated in hepatocellular carcinoma and contributes to tumour growth and vascular remodelling. J Hepatol. 2011;55:866–75. doi: 10.1016/j.jhep.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 140.Peng Y, Liu YM, Li LC, et al MicroRNA-338 inhibits growth, invasion and metastasis of gastric cancer by targeting NRP1 expression. PLoS One. 2014;9:e94422. doi: 10.1371/journal.pone.0094422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Z, Xie T, Zhang X, et al Immune checkpoint inhibitors for treatment of advanced gastric or gastroesophageal junction cancer: Current evidence and future perspectives. Chin J Cancer Res. 2020;32:287–302. doi: 10.21147/j.issn.1000-9604.2020.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Naik A, Al-Zeheimi N, Bakheit CS, et al Neuropilin-1 associated molecules in the blood distinguish poor prognosis breast cancer: A cross-sectional study. Sci Rep. 2017;7:3301. doi: 10.1038/s41598-017-03280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tse BWC, Volpert M, Ratther E, et al Neuropilin-1 is upregulated in the adaptive response of prostate tumors to androgen-targeted therapies and is prognostic of metastatic progression and patient mortality. Oncogene. 2017;36:3417–27. doi: 10.1038/onc.2016.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dong JC, Gao H, Zuo SY, et al Neuropilin 1 expression correlates with the radio-resistance of human non-small-cell lung cancer cells. J Cell Mol Med. 2015;19:2286–95. doi: 10.1111/jcmm.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chu W, Song X, Yang X, et al Neuropilin-1 promotes epithelial-to-mesenchymal transition by stimulating nuclear factor-kappa B and is associated with poor prognosis in human oral squamous cell carcinoma. PLoS One. 2014;9:e101931. doi: 10.1371/journal.pone.0101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pires BR, Mencalha AL, Ferreira GM, et al NF-kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS One. 2017;12:e0169622. doi: 10.1371/journal.pone.0169622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salmi M, Koskinen K, Henttinen T, et al. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood;104:3849-57.
- 148.Tadayon S, Dunkel J, Takeda A, et al Lymphatic endothelial cell activation and dendritic cell transmigration is modified by genetic deletion of Clever-1. Front Immunol. 2021;12:602122. doi: 10.3389/fimmu.2021.602122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Karikoski M, Marttila-Ichihara F, Elima K, et al Clever-1/stabilin-1 controls cancer growth and metastasis. Clin Cancer Res. 2014;20:6452–64. doi: 10.1158/1078-0432.CCR-14-1236. [DOI] [PubMed] [Google Scholar]
- 150.Virtakoivu R, Rannikko JH, Viitala M, et al Systemic blockade of Clever-1 elicits lymphocyte activation alongside checkpoint molecule downregulation in patients with solid tumors: Results from a Phase I/II clinical trial. Clin Cancer Res. 2021;27:4205–20. doi: 10.1158/1078-0432.CCR-20-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]