OBJECTIVE:
Acute respiratory distress syndrome (ARDS) is a life-threatening respiratory injury with multiple physiological sequelae. Shunting of deoxygenated blood through intra- and extrapulmonary shunts may complicate ARDS management. Therefore, we conducted a systematic review to determine the prevalence of sonographically detected shunts, and their association with oxygenation and mortality in patients with ARDS.
DATA SOURCES:
Medical literature analysis and retrieval system online, Excerpta Medica dataBASE, Cochrane Library, and database of abstracts of reviews of effects databases on March 26, 2021.
STUDY SELECTION:
Articles relating to respiratory failure and sonographic shunt detection.
DATA EXTRACTION:
Articles were independently screened and extracted in duplicate. Data pertaining to study demographics and shunt detection were compiled for mortality and oxygenation outcomes. Risk of bias was appraised using the Joanna-Briggs Institute and the Newcastle-Ottawa Scale tools with evidence rating certainty using Grading of Recommendations Assessment, Development and Evaluation methodology.
DATA SYNTHESIS:
From 4,617 citations, 10 observational studies met eligibility criteria. Sonographic detection of right-to-left shunt was present in 21.8% of patients (range, 14.4–30.0%) among included studies using transthoracic, transesophageal, and transcranial bubble Doppler ultrasonographies. Shunt prevalence may be associated with increased mortality (risk ratio, 1.22; 95% CI, 1.01–1.49; p = 0.04, very low certainty evidence) with no difference in oxygenation as measured by Pao2:Fio2 ratio (mean difference, –0.7; 95% CI, –18.6 to 17.2; p = 0.94, very low certainty).
CONCLUSIONS:
Intra- and extrapulmonary shunts are detected frequently in ARDS with ultrasound techniques. Shunts may increase mortality among patients with ARDS, but its association with oxygenation is uncertain.
Keywords: acute respiratory distress syndrome, bubble study, echocardiography, respiratory failure, shunt
Key Points
Question: In adult critically ill ARDS patients, what is the prevalence of right-to-left shunts, and what are their effects on mortality and/or oxygenation?
Findings: In this systematic review and meta-analysis, shunts be may prevalent in approximately one in five ARDS patients. They may be associated with a statistically significant increase in mortality, with no difference in oxygenation parameters.
Meaning: Intra- and extrapulmonary shunts are detected frequently in ARDS with ultrasound techniques and may increase mortality among patients with ARDS (although its association with oxygenation is uncertain).
Acute respiratory distress syndrome (ARDS) is a life-threatening lung injury that can occur following a variety of pulmonary insults (1). Researchers describe several mechanisms that contribute to hypoxemia in ARDS (aside from parenchymal disease) (2, 3), including right-to-left shunts (4). Intra or extrapulmonary shunting can occur from dysregulated pulmonary capillary deformation and/or intracardiac shunting via an atrial septal defect or patent foramen ovale (5), exacerbated by acute cor pulmonale from elevated right-sided pressures during positive-pressure ventilation (6–8). This has become of increasing interest, as shunts were hypothesized as a contributor to COVID mortality (9, 10).
Detection of right-to-left shunt has increased with broader application of various sonographic modalities, with increased ease by point-of-care ultrasound providers (11, 12), with support from various societies (13, 14). These include transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and transcranial Doppler sonography (TCD), which can be performed with agitated saline bubble contrast administration to detect shunts (9, 10). Presence of bubbles in left-sided cardiac structures (TTE and TEE) or cerebral vasculature (TCD) indicates shunting of venous blood to the systemic circulation, bypassing pulmonary capillary vasculature (9, 10).
Therefore, we conducted a systematic review to determine if the prevalence of right-to-left shunts (using sonographic methods and contrast bubble studies) and whether presence of shunt detection are associated with negative outcomes on oxygenation and mortality in ARDS in comparison with patients without shunt.
MATERIALS AND METHODS
This systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (15–17) and registered in advance with the international prospective registry of systematic reviews (international prospective register of systematic reviews [PROSPERO] CRD42021245194, registered March 26, 2021, search March 26, 2021). PRISMA checklist is shown in Supplemental Table 1 (http://links.lww.com/CCX/B82).
Search Strategy
Searches were performed by a clinical librarian with experience in conducting electronic literature searches, with adjudication using Peer Review Electronic Search Strategy criteria (11) by a second librarian. We searched Medical literature analysis and retrieval system online (via Ovid), Excerpta Medica dataBASE, Cochrane Library, and database of abstracts of reviews of effects using combinations of Keywords: acute respiratory distress syndrome, intracardiac or transpulmonary shunt, bubble contrast ultrasonography, and related terms. Database search was executed on March 24, 2021, and followed guidelines described in the PRISMA statement (15–17). Detailed search strategy can be found in Supplemental Appendix 1 (http://links.lww.com/CCX/B82).
Study Selection and Eligibility Criteria
Articles were screened by title and abstract by two independent reviewers using the Covidence (18) and selected for full-text review if identified as relevant by at least one reviewer. Full-text review was performed by two independent reviewers, and conflicts were resolved in discussion with a third reviewer. All eligible articles met the following criteria: 1) inclusion of adult patients with ARDS (including COVID-19) and 2) have undergone an agitated bubble saline sonographic study. Animal and pediatric articles were excluded. No date or language restrictions were applied.
Data Abstraction and Analysis
Our pre-piloted data abstraction tables were created in Microsoft Excel Version 14.0.6 (Microsoft Corporation, Redmond, WA) and used by paired, independent reviewers of included articles to extract: study characteristics, patient demographic data, sonographic modality, shunt prevalence, oxygenation, and mortality data (where available). We contacted corresponding authors for retrieval of incomplete data where not directly published. Included data were verified for internal consistency between the paired reviewers by consensus.
Continuous data were presented as means and sd, or medians and interquartile ranges, which were compared (where appropriate) using a t test or Wilcoxon rank-sum test. Categorical variables and proportions will be compared using the Pearson χ² or Fisher exact tests, as appropriate.
Outcome data were compiled for meta-analysis using the RevMan Cochrane software, London, United Kingdom (https://revman.cochrane.org) (Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration 2014) Version 5.4 software (19, 20). Significance was set at 0.05. CIs were reported for 95% CIs, where applicable.
We used the method of DerSimonian and Laird (21) to pool effect sizes for each outcome under a random-effects model for outcomes of interest, with study weights measured using the inverse variance method. We presented the results as relative risk (RR) for dichotomous outcomes. We presented the results as risk differences (RDs) for continuous outcomes.
Heterogeneity was assessed using the I² statistic, the χ2 test for homogeneity (p < 0.1 for significance of substantial heterogeneity), and visual inspection of the forest plots. We considered an I² value greater than 50% indicative of substantial heterogeneity (19, 20). If significant unexplained heterogeneity existed, or if there were an insufficient number of studies for meta-analysis, we described data qualitatively. We assessed evidence of publication bias using funnel plots if there were greater than 10 trials per outcome.
Subgroup Analyses
Potential and expected clinical sources of heterogeneity include different patient demographics and patient illness and diagnosis. To explore significant heterogeneity, we planned the following prespecified subgroup analyses, if enough trials were available (hypothesized direction of effect in parentheses): COVID ARDS versus non-COVID ARDS studies (COVID studies would demonstrate worse shunt rates, hypoxemia, and mortality compared with non-COVID ARDS).
Risk of Bias Assessment and Evidence GRADE Recommendations
We assessed risk of bias (RoB) using the Newcastle-Ottawa Scale (NOS) and Joanna-Briggs Institute (JBI) tools for observational cohort studies as described in our systematic review protocol (22, 23), with domain scoring in the footnotes (Supplemental Tables 2 and 3, http://links.lww.com/CCX/B82).
We reported recommendations using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for mortality, and oxygenation outcome data (24–26).
RESULTS
Study Characteristics
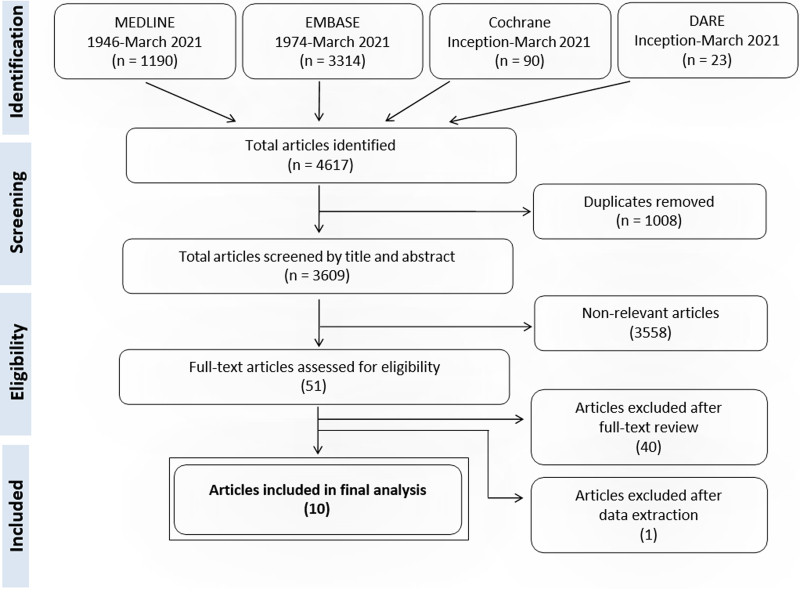
Our search yielded 4,617 citations, with 51 relevant articles retrieved for full-text evaluation. Forty-one articles were excluded for: incorrect study design (e.g., cohort/case-series without control group or case reports) (25), incorrect patient population (e.g., non-ARDS patients or non-ICU) (1), missing outcome data (10), missing ultrasonography bubble study investigation (3), and duplicate study (1). This yielded 10 eligible articles. The PRISMA flowchart is shown in Figure 1.
Figure 1.
Acute respiratory distress syndrome shunt preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart. DARE = database of abstracts of reviews of effects, EMBASE = Excerpta Medica dataBASE, MEDLINE = medical literature analysis and retrieval system online.
Risk of Bias, Critical Appraisal, and Publication Bias
RoB was assessed using the JBI critical appraisal tool for cohort and case control studies (Supplemental Table 2, http://links.lww.com/CCX/B82) and the NOS tools (Supplemental Table 3, http://links.lww.com/CCX/B82). Overall assessment of “good” RoB was present in four of 10 studies (40%) as assessed by the NOS. Similarly, binary deficiencies led to an overall appraisal of “include” in six of 10 studies (60%) using the JBI tool.
The deficiencies leading to RoB assessment of “poor” (NOS) or “exclude” (JBI) were due to: unclear or absent description of comparator (shunt vs nonshunt, two studies) and absence of relevant oxygenation or mortality outcome data (four studies). Given there were less than 10 studies per outcome, no funnel plots were constructed for assessment of publication bias.
Clinical Outcomes and GRADE Assessment
A total of 1,114 patients were pooled between 10 studies with an overall shunt prevalence of 21.8% (range, 14.4–30.0%) as detected by various agitated saline bubble sonography modalities (4, 9, 10, 27–32). The majority of studies included ARDS secondary to infectious pneumonia, with three COVID-19-specific studies (9, 10, 27). Where reported, included studies demonstrated a male predominance (72.3%) and a mean age of 58.5 years. Study demographics are shown in Table 1 (2, 5–7, 21–26).
TABLE 1.
Acute Respiratory Distress Syndrome Shunt Articles Summary Statistics
| Reference | n | Age (yr) | % Male | Simplified Acute Physiology II Score | Primary Diagnosis | Shunt Assessment Modality | Overall Shunt Prevalence |
|---|---|---|---|---|---|---|---|
| Observational cohort (9) | |||||||
| Boissier et al (28)a | 216 | 63 | 69.4 | 53 ± 25 | ARDS | TEE | 57/216 (26.4%) |
| Legras 1999b | 195 | 56 | NR | 46 ± 17 | PNA/ARDS | TEE | 28/195 (14.4%) |
| Lhéritier et al (4)b | 200 | 57 | 68.7 | 46 ± 17 | PNA/ARDS | TTE + TEE | 31/200 (15.5%) |
| Masi et al (10)a | 60 | 62 | 83.3 | NR | COVID-19 ARDS | TTE | 18/60 (30.0%) |
| Mekontso Dessap et al (8)a | 203 | 60 | 72.9 | 55 ± 18 | PNA/ARDS | TEE | 39/203 (19.2%) |
| Mekontso Dessap et al (30)b | 34 | 62 | 79.4 | 57 (40–72) | PNA/ARDS | TEE | 7/34 (20.6%) |
| Salazar-Orellana et al (27)b | 31 | 44 | 80.6 | NR | COVID-19 PNA | TCD | 7/31 (22.6%) |
| Vavlitou 2016a | 108 | 57 | 75.0 | NR | Respiratory failure | TEE | 30/108 (27.8%) |
| Védrinne et al (31)b | 49 | 53 | NR | NR | Respiratory failure | TEE | 11/49 (22.4%) |
| Cross-sectional pilot study (1) | |||||||
| Reynolds et al (9)b | 18 | 59 | 61.1 | NR | COVID-19 ARDS | TCD | 15/18 (83.3%) |
| WEIGHTED AVERAGES | 58.5 ± 16.1 | 629/870 (72.3%) | 50.4 ± 19.7 | 243/1114 (21.8%) | |||
ARDS = acute respiratory distress syndrome, NR = not reported, PNA = pneumonia, TCD = transcranial Doppler, TEE = transesophageal echocardiography, TTE = transthoracic echocardiography
aAgitated saline contrast technique description: injecting 9.5 mL of sterile saline solution aerated with 0.5 mL of room air via two syringes connected with a three-way stopcock into a peripheral vein or central venous line (performed with and without Valsalva).
bAgitated saline contrast technique not well described (aside from agitated saline injection performed).
Data are presented as mean (± sd) or median (interquartile range) (% male, Simplified Acute Physiology Score II score).
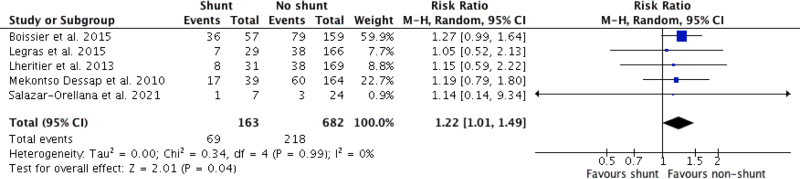
Where ARDS mortality data were reported (n = 5 studies, 845 patients), the meta-analysis of pooled studies yielded 42.3% mortality (69/163 patients; 95% CI, 34.6–50.3%) for shunt presence compared with 32.0% mortality (218/682 patients; 95% CI, 28.5–35.6%) for shunt absence (RD, 10.3% [95% CI, 0.2–18.7%]; RR, 1.22; [95% CI, 1.01–1.49]; p = 0.04, very low certainty). Forest plot for pooled mortality is shown in Figure 2 (4, 27–30). Observational cohort studies were subsequently downgraded to “very low” due to serious RoB in the majority of studies (Supplemental Table 4, http://links.lww.com/CCX/B82).
Figure 2.
Forest plot mortality for acute respiratory distress syndrome shunt versus nonshunt patients M-H = Mantel-Haenszel.
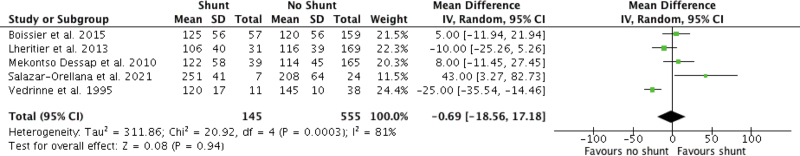
In contrast, oxygenation (as reported by Pao2:Fio2 [PF] ratio) was variable between reported studies (n = 5 studies, 700 patients). In ARDS patients, shunt presence had a mean PF ratio of 123.8 ± 51.0 compared with 124.5 ± 46.3 for shunt absence, with a mean PF ratio difference of –0.7 (95% CI, –18.6 to 17.2; p = 0.94, very low certainty). Forest plot for pooled PF ratio is shown in Figure 3 (4, 27, 28, 30, 31). Oxygenation was downgraded to “very low” certainty of evidence based on high RoB scores detailed above, “very serious” allocation of inconsistency (studies with CI confined on either side of null effect), and imprecision (multiple studies with wide CIs) (Supplemental Table 4, http://links.lww.com/CCX/B82).
Figure 3.
Forest plot oxygenation for acute respiratory distress syndrome shunt versus nonshunt patients.
Subgroup Analyses
Prespecified subgroup forest plots for mortality and PF ratio are shown in Supplemental Figures 1 and 2 (http://links.lww.com/CCX/B82).
For mortality (Supplemental Fig. 1 http://links.lww.com/CCX/B82), there was a similar increased mortality for non-COVID (RR, 1.22; 95% CI, 1.01–1.49; p = 0.04; four studies) compared to COVID-19 patients (RR, 1.14; 95% CI, 0.14–9.34; p = 0.90; one study), although not statistically significant.
For PF ratio (Supplemental Fig. 2, http://links.lww.com/CCX/B82), there was equivocal effects for non-COVID (mean difference, –6.7 (95% CI, –23.0 to 9.6; p = 0.42; four studies) compared with COVID-19 patients 43.0 (95% CI, 3.3–82.7; p = 0.03; one study), which was statistically significant in favor of better PF ratios with shunt presence, which is contradictory to our prior hypothesis that shunts would worsen PF ratios.
DISCUSSION
In this systematic review, current literature demonstrates that right-to-left shunts are common, evident in approximately one in five ARDS patients (2, 5–7, 21–26). Our meta-analysis found increased mortality among critically ill patients with ARDS with sonographically detectable right-to-left shunt compared with no detectable shunt, although there was no difference in oxygenation.
This study attempts to quantify prevalence of shunt in hypoxemic ARDS patients and its association on mortality, which may not be necessarily secondary to oxygenation alone. The incidence of a diagnosis of a right-to-left shunt varies across populations (33), varying with: age of the patient (varying incidence of a PFO 15–35%, with decrease incidence and increasing size with age) (34); underlying disease process (intracardiac vs intrapulmonary); mechanical ventilation strategies (mean airway pressure and positive end-expiratory pressure [PEEP]) (35); and the diagnostic modality (TTE vs TEE vs TCD) (5). Our meta-analysis aligns with prior general population shunt prevalence, including demonstrating association with increased ARDS mortality by worsening shunt physiology with potential interventions (e.g., invasive mechanical ventilation, high mean airway pressures, and pulmonary vasodilators). Diagnosis of an atrial septal defect/patent foramen ovale is important and is associated with potential embolism, resulting in ischemic events and strokes (35). Unfortunately, there was inconsistent reporting of stroke incidence in this meta-analysis, and whether mortality was affected.
Right-to-left shunt is on the differential diagnoses of refractory hypoxemia, but is not necessarily investigated with bubble studies to confirm shunt presence routinely in ARDS patients. With approximately ~22% shunt prevalence, this study demonstrates how many potential right-to-left shunts may be missed if not investigated. Given that bubble studies are routine ultrasonographic practice with standard protocols (5, 36–38) and are safe (<0–0.15% cerebral ischemic events post-agitated saline contrast injection) (39), intracardiac shunt (PFO and ASD) or intrapulmonary shunt investigations should be entertained in patients with ARDS. However, cautious administration of bubble study investigations is still important to avoid air embolism through venous lines (40, 41). Valsalva maneuvers, which are routine during bubble study investigations, can increase right-sided pressures, which may increase embolic events (42, 43). Intravenous filters (44, 45) and possible PFO closures (46) may be warranted for some patients with cryptogenic strokes.
The finding of increased mortality in the shunt groups, which is independent of oxygenation, is difficult to conceptualize. However, limitations in oxygenation measurement using the PF ratio exist. The most accurate standardization uses a consistent Fio2 of 1.0 for PF ratio measurement (47), which is not routinely performed, leading to nonstandardized measures of PF ratio (48). Oxygenation is incompletely evaluated by PF ratio, not accounting for PEEP (48). There are many confounders, which affect the outcomes of shunt presence, including invasive mechanical ventilation parameters; heart-lung mechanics; right ventricle dysfunction; baseline cardiac (e.g., congenital defects), pulmonary (e.g., arteriovenous malformations), and hepatic (e.g., hepatopulmonary syndrome in cirrhosis) comorbidities; temporal changes in physiology; nonstandardized measurements of shunt; and, interrater reliability of ultrasound diagnostics (6, 7). Future research should seek to investigate potential confounders, minimize their impact through adjustment or matching, and standardize data collection.
The strengths of this systematic review (SR) include a comprehensive search strategy; a rigorous process for study selection and data abstraction based on an a priori protocol; and due consideration to study quality, RoB, and overall certainty of the evidence using GRADE methodology.
This SR also has several limitations. Certainty of evidence was very low for all outcomes, driven by: observational nature of studies included; lack of adjustment for baseline characteristics and illness severity; and small sample sizes. Shunt definitions were variable between studies. This ranged from the sonographic detection of right-to-left bubbles quantitatively from any number of detected bubbles by TCD at any time (9, 27) to at least 10 bubbles at any time by TTE/TEE (30) to any number of bubbles within three cardiac cycles (4). Sonography is also limited as an operator-dependent modality (5, 37, 38, 49). Although our study demonstrates increased mortality associated with the sonographic detection of shunt, a causal relationship is difficult to rationalize. The level of data provided in these studies was poor, with many not measuring: type of shunt (intracardiac vs intrapulmonary); shunt fractions; use of cointerventions (e.g., inodilators, pulmonary vasodilators, and diuretics); and ventilator management strategies. Inconsistent reporting of whether there was PFO/ASD closure limits our understanding if any of the intracardiac R-L shunts were clinically relevant to warrant intervention, alongside lack of stroke data. It is also unclear what a clinically significant intrapulmonary shunt would be defined by, as PF ratios showed no difference. Differences in duration of mechanical ventilation or total duration of supplemental oxygen use were not routinely measured. Finally, there were no prespecified ARDS subgroups of note, specifically based on ARDS severity and etiology. Substantial heterogeneity among ARDS patients exists with respect to shunts contributes to their illness, especially as COVID deaths, demonstrating varying complications through different waves of the pandemic (e.g., parenchymal interstitial lung disease vs venous thromboembolism) (50).
CONCLUSIONS
The detection of intra- and extrapulmonary shunts in ARDS using ultrasonography is relatively common in critically ill patients with ARDS. There may be increased mortality among patients with ARDS and evidence of shunt (very low certainty). However, shunt prevalence may have uncertain direct physiologic impacts on oxygenation (very low certainty). Further research is required to solidify the certainty in this finding.
ACKNOWLEDGMENTS
We thank Diane Keto-Lambert (Faculty of Medicine and Dentistry, University of Alberta) for her assistance with the systematic review search, as well as during the peer review electronic search strategies process with Doug Salzwedel.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Odenbach, Dhanoa, Milovanovic, Robinson, Rewa, Bagshaw, Buchanan, and Lau have made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data; drafted the submitted article and revised it critically for important intellectual content; and provided final approval of the version to be published. Drs. Odenbach, Buchanan, and Lau helped in conception. Drs. Odenbach, Mah, Buchanan, and Lau helped in background. Drs. Odenbach, Mah, Rewa, Bagshaw, Buchanan, and Lau helped in design. Drs. Odenbach, Dhanoa, Milovanovic, Robinson, Mah, and Lau helped in acquisition of data. Drs. Odenbach, Dhanoa, Milovanovic, Robinson, Rewa, Bagshaw, Buchanan, and Lau helped in analysis of data. Drs. Odenbach, Dhanoa, Milovanovic, Robinson, Rewa, Bagshaw, Buchanan, and Lau helped draft the article. Drs. Odenbach, Dhanoa, Milovanovic, Robinson, Rewa, Bagshaw, Bagshaw, Buchanan, and Lau helped in revising the article.
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Dr. Bagshaw is supported by a Canada Research Chair in Critical Care Outcomes and Systems Evaluation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
PROSPERO Registration Number: CRD42021245194 (March 26, 2021).
REFERENCES
- 1.Wu C, Chen X, Cai Y, et al. : Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med 2020; 180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer NJ, Gattinoni L, Calfee CS: Acute respiratory distress syndrome. Lancet 2021; 398:622–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334–1349 [DOI] [PubMed] [Google Scholar]
- 4.Lhéritier G, Legras A, Caille A, et al. : Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: A multicenter study. Intensive Care Med 2013; 39:1734–1742 [DOI] [PubMed] [Google Scholar]
- 5.Silvestry FE, Cohen MS, Armsby LB, et al. : Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr 2015; 28:910–958 [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Coppola S, Cressoni M, et al. : COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201:1299–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cressoni M, Caironi P, Polli F, et al. : Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med 2008; 36:669–675 [DOI] [PubMed] [Google Scholar]
- 8.Mekontso Dessap A, Boissier F, Leon R, et al. : Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med 2010; 38:1786–1792 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds AS, Lee AG, Renz J, et al. : Pulmonary vascular dilatation detected by automated transcranial Doppler in COVID-19 pneumonia. Am J Respir Crit Care Med 2020; 202:1037–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masi P, Bagate F, d’Humières T, et al. : Is hypoxemia explained by intracardiac or intrapulmonary shunt in COVID-19-related acute respiratory distress syndrome? Ann Intensive Care 2020; 10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau V, Priestap F, Landry Y, et al. : Diagnostic accuracy of critical care transesophageal echocardiography vs cardiology-led echocardiography in ICU patients. Chest 2019; 155:491–501 [DOI] [PubMed] [Google Scholar]
- 12.Arntfield R, Lau V, Landry Y, et al. : Impact of critical care transesophageal echocardiography in medical-surgical ICU patients: Characteristics and results from 274 consecutive examinations. J Intensive Care Med 2018 [DOI] [PubMed] [Google Scholar]
- 13.Expert Round Table on Echocardiography in ICU: International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med 2014; 40:654–666 [DOI] [PubMed] [Google Scholar]
- 14.Panebianco NL, Mayo PH, Arntfield RT, et al. : Assessing competence in critical care echocardiography: Development and initial results of an examination and certification processes*. Crit Care Med 2021; 49:1285–1292 [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamseer L, Moher D, Clarke M, et al. : Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015; 350:g7647. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 2009; 62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 18.Veritas Health Innovation: Covidence Systematic Review Software [Internet]. 2019. Available at: www.covidence.org. Accessed January 3, 2020
- 19.Higgins JP, Green S: Cochrane handbook for systematic reviews of interventions: Cochrane book series. In: The Cochrane Collaboration. Wiley Global Permissions (Ed). Chichester, UK, John Wiley & Sons, Ltd., 2011, pp. 449–80 [Google Scholar]
- 20.Higgins JPT, Altman DG, Gøtzsche PC, et al. : The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 22.Gomersall J, Jadotte Y, Xue Y, et al. : Joanna Briggs Institute critical appraisal checklist for economic evaluations: Conducting systematic reviews of economic evaluations. Int J Evidence Based Healthcare 2015; 13:170–178 [DOI] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’Connell D, et al. : Ottawa Hospital Research Institute [Internet]. 2019. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 27, 2019
- 24.Schünemann H, Brożek J, Guyatt G, et al. : GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations [Internet]. 2013. Available at: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed March 7, 2019
- 25.Guyatt GH, Oxman AD, Vist GE, et al. : GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santesso N, Glenton C, Dahm P, et al. : GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020; 119:126–135 [DOI] [PubMed] [Google Scholar]
- 27.Salazar-Orellana JLI, García-Grimshaw M, Valdés-Ferrer SI, et al. : Detection of pulmonary shunts by transcranial Doppler in hospitalized non-mechanically ventilated coronavirus disease-19 patients. Rev Invest Clin 2021; 73:61–64 [DOI] [PubMed] [Google Scholar]
- 28.Boissier F, Razazi K, Thille AW, et al. : Echocardiographic detection of transpulmonary bubble transit during acute respiratory distress syndrome. Ann Intensive Care 2015; 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legras A, Dequin PF, Hazouard E, et al. : Right-to-left interatrial shunt in ARDS: Dramatic improvement in prone position. Intensive Care Med 1999; 25:412–414 [DOI] [PubMed] [Google Scholar]
- 30.Mekontso Dessap A, Proost O, Boissier F, et al. : Transesophageal echocardiography in prone position during severe acute respiratory distress syndrome. Intensive Care Med 2011; 37:430–434 [DOI] [PubMed] [Google Scholar]
- 31.Védrinne JM, Duperret S, Gratadour P, et al. : [Effects of mechanical ventilation with PEEP on right to left intra-cardiac shunt caused by patent foramen ovale]. Ann Fr Anesth Reanim 1995; 14:387–392 [DOI] [PubMed] [Google Scholar]
- 32.Vavlitou A, Minas G, Zannetos S, et al. : Hemodynamic and respiratory factors that influence the opening of patent foramen ovale in mechanically ventilated patients. Hippokratia 2016; 20:209–213 [PMC free article] [PubMed] [Google Scholar]
- 33.Teshome MK, Najib K, Nwagbara CC, et al. : Patent foramen ovale: A comprehensive review. Curr Probl Cardiol 2020; 45:100392. [DOI] [PubMed] [Google Scholar]
- 34.McMahon CJ, Feltes TF, Fraley JK, et al. : Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart 2002; 87:256–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guchlerner M, Kardos P, Liss-Koch E, et al. : PFO and right-to-left shunting in patients with obstructive sleep apnea. J Clin Sleep Med 2012; 8:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jauss M, Zanette E: Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis 2000; 10:490–496 [DOI] [PubMed] [Google Scholar]
- 37.Porter TR, Abdelmoneim S, Belcik JT, et al. : Guidelines for the cardiac sonographer in the performance of contrast echocardiography: A focused update from the American Society of Echocardiography. J Am Soc Echocardiogr 2014; 27:797–810 [DOI] [PubMed] [Google Scholar]
- 38.Mulvagh SL, Rakowski H, Vannan MA, et al. : American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr 2008; 21:1179–1201 [DOI] [PubMed] [Google Scholar]
- 39.Bernard S, Churchill TW, Namasivayam M, et al. : Agitated saline contrast echocardiography in the identification of intra- and extracardiac shunts: Connecting the dots. J Am Soc Echocardiogr 2021; 34:1–12 [DOI] [PubMed] [Google Scholar]
- 40.Wilkins RG, Unverdorben M: Accidental intravenous infusion of air: A concise review. J Infus Nurs 2012; 35:404–408 [DOI] [PubMed] [Google Scholar]
- 41.Ruiz Avila HA, García-Araque HF, Acosta-Gutiérrez E: Paradoxical venous air embolism detected with point-of-care ultrasound: A case report. Ultrasound J 2022; 14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schawkat K, Litmanovich D, Appel E, et al. : Embolic events after computed tomography contrast injection in patients with interatrial shunts: A cohort study. J Thorac Imaging 2022; 37:331–335 [DOI] [PubMed] [Google Scholar]
- 43.Myers GJ: Air in intravenous lines: A need to review old opinions. Perfusion 2017; 32:432–435 [DOI] [PubMed] [Google Scholar]
- 44.Bulsara KR, Lee S, Calafiore R: Commentary: Air bubbles in infusion: An easily avoidable potential complication. Oper Neurosurg 2020; 18:E59. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Bulsara KR: Assessing the efficacy of commercially available filters in removing air micro-emboli in intravenous infusion systems. J Extra Corpor Technol 2020; 52:118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kavinsky CJ, Szerlip M, Goldsweig AM, et al. : SCAI guidelines for the management of patent foramen ovale [Internet]. J Soc Cardiovasc Angiogr Interventions 2022; 1:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrick IA, Champion LK, Froese AB: A clinical comparison of indices of pulmonary gas exchange with changes in the inspired oxygen concentration. Can J Anaesth 1990; 37:69–76 [DOI] [PubMed] [Google Scholar]
- 48.Palanidurai S, Phua J, Chan YH, et al. : P/FP ratio: Incorporation of PEEP into the PaO2/FiO2 ratio for prognostication and classification of acute respiratory distress syndrome. Ann Intensive Care 2021; 11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saric M, Armour AC, Arnaout MS, et al. : Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42 [DOI] [PubMed] [Google Scholar]
- 50.Radermacher P, Maggiore SM, Mercat A: Gas exchange in acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 196:964–984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.