Abstract
Chronic pain represents one of the most serious worldwide medical problems, in terms of both social and economic costs, often causing severe and intractable physical and psychological suffering. The lack of biological markers for pain, which could assist in forming clearer diagnoses and prognoses, makes chronic pain therapy particularly arduous and sometimes harmful. Opioids are used worldwide to treat chronic pain conditions, but there is still an ambiguous and inadequate understanding about their therapeutic use, mostly because of their dual effect in acutely reducing pain and inducing, at the same time, tolerance, dependence, and a risk for opioid use disorder. In addition, clinical studies suggest that opioid treatment can be associated with a high risk of immune suppression and the development of inflammatory events, worsening the chronic pain status itself. While opioid peptides and receptors are expressed in both central and peripheral nervous cells, immune cells, and tissues, the role of opioids and their receptors, when and why they are activated endogenously and what their exact role is in chronic pain pathways is still poorly understood. Thus, in this review we aim to highlight the interplay between pain and immune system, focusing on opioids and their receptors.
Keywords: pain, chronic pain, chronic pain biomarkers, chronic pain diagnosis, opioids, opioid receptors, immune system, immune cells, opioid receptor signalling pathway, neuroimmune synapse
Introduction
Chronic pain (CP) places an enormous burden on sufferers and health care systems, leading the European Commission to include pain as a topic in its mission-oriented research (https://ec.europa.eu/health/sites/health/files/policies/docs/ev_20181112_co06_en.pdf; Thematic Network on the Societal Impact of Pain—Recommendations for Policy Actions—05 November 2018). CP is not only a complex health problem but also a real “bio-psycho-social experience,” which requires a more comprehensive approach than many other pathologies (Darnall and others 2017). Even if pain is considered more frequently as a specific symptom of another health issue, CP represents the main and exclusive condition for many patients (Raffaeli and Arnaudo 2017), as underlined in the new “International Classification of Diseases–11th Revision (ICD-11) (Treede and others 2019). Despite the large number of preclinical studies (Burma and others 2017), the intrinsic subjectivity of this pathology makes clinical diagnosis still quite arduous. In particular, pain intensity is the most difficult CP characteristic to quantify and is usually described through a patient self-certification based on numerical, visual, verbal, or facial pain ranking scales. In this context, the gap between preclinical and clinical CP data are larger than for other diseases. Moreover, the lack of specific CP biomarkers complicates diagnostic and prognostic approaches, often resulting in a delay of the appropriate pharmacological treatments (Borsook and others 2011). In CP therapy, opioids are commonly used, but their beneficial effects are often overshadowed by their side effects (Cumenal and others 2021). This is also due to the absence of proper, universally shared and agreed-upon therapeutic guidelines about the right dosages of opioid medication. For the reasons mentioned above, more and more scientists are highlighting the need to strengthen research aimed at discovering novel pain biomarkers. Quantifiable biomarkers of pain would facilitate the identification of new mechanisms and signs to help with pain diagnoses, predict subgroups inside heterogeneous painful conditions, and provide pharmacodynamic modifications associated with a specific dosage and drug formulation (Zhao and others 2015). In this context, recent studies have paid attention to promising peripheral pain biomarkers, which represent an efficient and highly feasible avenue for investigation, given that all is needed is a blood sample (Backryd 2015). For example, Niculescu and colleagues, through a blood microarray analysis (Niculescu and others 2019) have shown that several genes and, recently, specific microRNAs expressions (Tavares-Ferreira and others 2019), are tightly correlated with the intensity of pain.
The interest in peripheral detection of pain biomarkers finds its origin in the hypothesis that hyperalgesia is a result of the interplay between the immune and nervous systems, where the bridge between the two is represented by opioid receptors, expressed in both the central and the peripheral nervous system (Campana and others 2010; Kipnis 2016). In this context, a recent study proposed that Mu opioid receptors (MOR) expressed on B cells may be a potential biological marker (Mu-Lympho-Marker, MLM) to assist health care professionals in crafting a more objective chronic pain diagnosis, both in patients suffering from fibromyalgia (FM) and osteoarthritis (OA) (Raffaeli and others 2020). The major goal of this review is to explore and discuss the latest findings in chronic pain biomarkers in the immune and nervous systems, in order to disentangle the relationship between opioid receptors, uncontrolled pain conditions, and the immune system.
Chronic Pain
For the past four decades, pain has been defined as “An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (Treede 2018). However, the term “describe” has been improperly used, considering that some patients are unable to verbally express their painful experience. Thus, in 2020, the International Association for the Study of Pain (IASP) approved the new definition of pain as “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (Raja and others 2020), where the subjectivity of a single person is highlighted by both sensory and affective components.
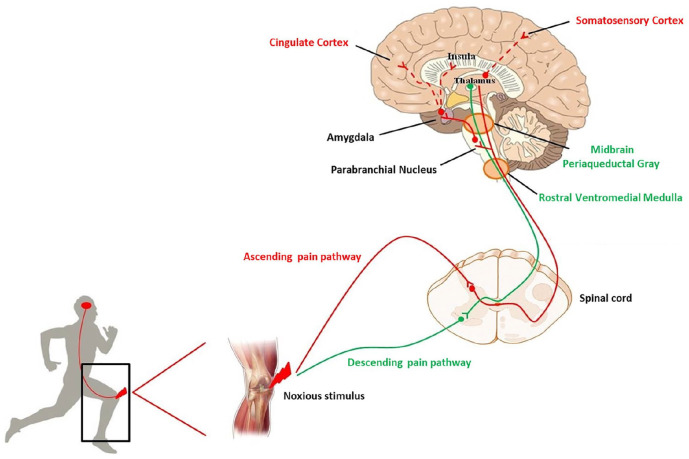
Under physiological conditions, a noxious stimulus activates first order neurons in order to reach specific laminae of the spinal cord. There, second order neurons send the information to different zones of the brain where it is processed. The intensity and the body localization of the stimulus are analyzed at the level of the somatosensory cortex. The cingulate and insular cortices, contribute to the affective component of pain, by receiving projection neurons, via connections in the parabrachial nucleus and amygdala. Then, the rostral ventral medulla and midbrain periaqueductal gray drive the descending feedback system, regulating the output from the spinal cord (Basbaum and others 2009) (Fig. 1).
Figure 1.
Schematic representation of ascending (red line) and descending (green line) pain pathways. After an injury, a noxious stimulus reaches the spinal cord and, from here, the stimulus sensation is conveyed to the brain. The localization and intensity of pain is elaborated in the somatosensory cortex. Cingulate and insula cortices, receiving projection neurons via the parabrachial nucleus and amygdala, contribute to the affective component of pain. At the level of the rostral ventromedial medulla and midbrain periaqueductal gray the descending feedback system departs, regulating the output from the spinal cord.
When this physiological pathway undergoes a pathological alteration and pain persists beyond the resolution of the underlying disorder, the healing of an injury, or sometimes without any cause, this is usually called “chronic pain.” Although CP is considered a persistent or recurrent pain lasting longer than 3 months (Treede and others 2015), it is not appropriate to call it a mere temporal extension of acute pain. In CP, the symptom represents the disease itself and this “bio-psycho-social experience” requires a multidisciplinary approach (Darnall and others 2017; Raffaeli and Arnaudo 2017).
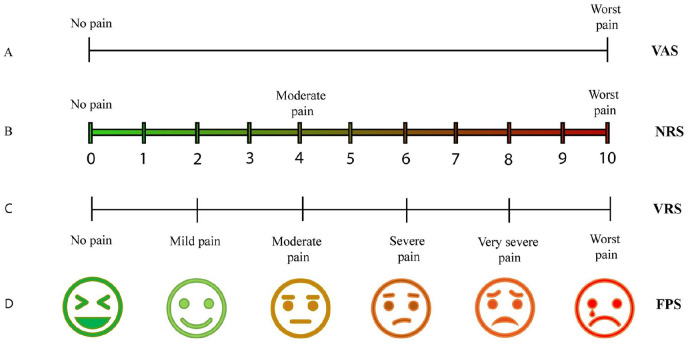
The main problem in CP remains its accurate diagnosis, particularly due to the lack of specific tools that detect subjective pain intensity. Today, evaluation of CP still consists of a patient self-certification using on various ranking scales (Thong and others 2018) (Fig. 2).
Figure 2.
Schematic illustration of different pain ranking scales, based on patient self-certification. (A) Visual analogue scale (VAS) pain ranking scale: Patients mark a point on a line that describes the amount of pain, starting from “no pain” (left) up to the “worst possible pain” (right). (B) Numerical Rating Scale (NRS): Patients describe their pain using numbers, where “0” corresponds to “no pain” and “10” to the “worst pain” possible. (C) Verbal Rating Scale (VRS): Patients verbally describe pain, using adjectives for pain, starting from “no pain” up to “worst pain.” (D) Faces Pain Scale (FPS): Patients describe the level of their pain choosing a face, ranking from a happy face (no pain) up to a crying face (worst pain).
Chronic Pain Biomarkers
In the past decades, the need for CP biomarkers for a specific diagnosis, prognosis, and therapy, has grown exponentially. The concept that pain is a subjective conscious perception, which requires brain activity, has placed brain processes at the center of pain biomarker research (Reckziegel and others 2019). Functional magnetic resonance imaging (fMRI) (Kumbhare and others 2017), positron emission tomography (PET) (Albrecht and others 2019), and proton magnetic resonance spectroscopy (H-MRS) (Levins and others 2019) are increasingly used to detect structural, functional, and neurochemical information as potential biomarkers for CP. However, brain imaging is a complex field, and new findings and approaches are needed to bypass overlapping information due to chronic pain side effects and comorbid conditions (e.g., anxiety, depression, and fatigue) (Reckziegel and others 2019).
Recently, Gunn and others (2020) presented an alternative retrospective biomarker assay, revealing an atypical biochemistry in CP patients. They proposed a panel of functional biomarkers that underlines the role of oxidative stress, cytokine-mediated inflammation, neurotransmitters, and micronutrients in CP conditions (Gunn and others 2020). In particular, the study showed elevated levels of quinolinic acid, pyroglutamate, xanthurenic acid, acrolein metabolite 3-hydroxypropyl mercapturic acid, and methylmalonic acid. They also detected abnormally low levels of 5-hydroxyindoleacetate (metabolite of serotonin) and vanilmandelate (metabolite of norepinephrine). However, more data in this context are needed, considering that medications and other conditions not associated with chronic pain were not analyzed in this study, and may be potential additional causes in the occurrence of atypical biomarkers.
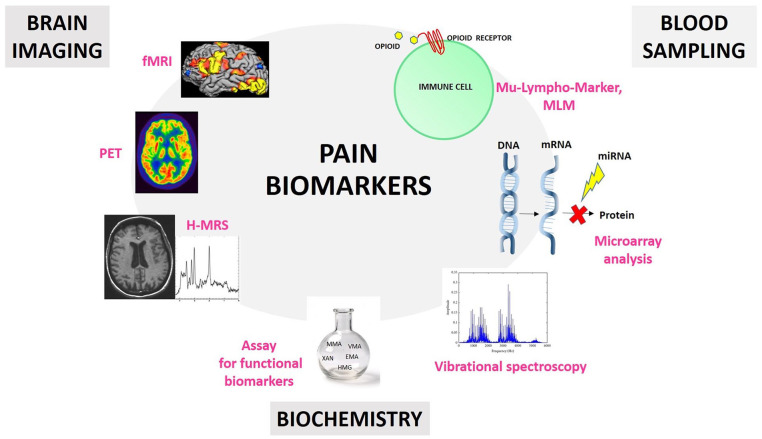
A key feature of a good biomarker is that it has to be easy and fast to detect (Califf 2018). This is why new studies are focusing on peripheral diagnostic approaches involving blood samples (Backryd 2015). For example, Niculescu and others (2019) have shown a blood microarray analysis can identify severe pain risk genes, which are more strongly expressed than putative protective/resilience genes, with the goal of developing a pain markers gene expression database. Other studies have demonstrated the correlation between specific microRNAs expressions (Tavares-Ferreira and others 2019) and CP, such as in low back pain (Hasvik and others 2019), osteoarthritis (Swingler and others 2019), and neuropathic pain (Tavares-Ferreira and others 2019). However, the lack of a miRNAs database for the healthy population and another one specific for CP patients makes miRNA validation as CP biomarkers quite difficult. Moreover, every disease ideally should have a specific miRNA of reference in order to bypass overlapping data (Lopez-Gonzalez and others 2017; Ramanathan and Ajit 2016). Recently, a new study has proposed a pain “molecular fingerprint” construction, using a vibrational spectroscopy technique, in order to identify metabolites associated with different CP conditions as serologic biomarkers (Hackshaw and others 2019). Together, these studies highlight the critical need and utility of blood samples in this field (Fig. 3).
Figure 3.
Schematic illustration of different approaches for pain biomarker research. Blood sampling can be used for the detection of biological markers on the surface of immune cells (e.g., opioid receptors), for miRNA detection through microarray analysis, and for vibrational spectroscopy approaches in order to isolate metabolites associated with different chronic pain (CP) conditions. Brain imaging can involve functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and proton magnetic resonance spectroscopy (H-MRS) techniques in order to detect specific brain activity patterns as pain biomarkers. Biochemical approaches can lead to the detection of atypical biochemistry as biomarkers for patients with CP.
Opioid Receptors
As mentioned above, peripheral detection of pain biomarkers is based on the idea that hyperalgesia is a consequence of the interplay between immune and nervous systems, where the interface is represented by opioids receptors (Campana and others 2010; Kipnis 2016).
Endogenous opioids derive from the precursors proopiomelanocortin, proenkephalin, and prodynorphin, which respectively encode for β-endorphin, enkephalins (Met-enkephalin and Leu-enkephalin), and dynorphins (Bodnar 2017). Opioid peptides share a common opioid-motif containing the Tyr-Gly-Gly-Phe-Met/leu amino acid sequence and explicate their action by binding to specific receptors. β-Endorphin and enkephalins are antinociceptive peptides, linking µ (mu [MOR]/Oprm1) and δ (delta [DOR]/Oprd1) opioid receptors (Hurley and Hammond 2001; Smith and others 1992). Dynorphins can mediate not only antinociceptive effects, via κ (kappa [KOR]/Oprk1) opioid receptors but also pro-nociceptive effects via N-methyl-d-aspartate (NMDA) receptors (Podvin and others 2016; Stein 2016). Opioid receptors are G-protein-coupled receptors, expressed in the central and peripheral nervous systems, and in neuroendocrine and immune tissues and cells (Stein 2016). Endogenous opioids and their receptors mediate analgesia, but also tolerance and dependence after long-term exposure to exogenous full opioid agonists, and withdrawal syndrome after an abrupt interruption of exogenous opioid exposure or after antagonist administration (Stein 2018). MOR mediates side effects such as nausea, sedation, respiratory depression, reward/euphoria, biliary spasms and constipation, urinary retention, and reduced gastrointestinal motility. KOR can lead to aversive, sedative, and diuretic effects, as well as dysphoria. DOR is involved in respiratory depression, constipation, and reward and euphoria. In the 1990s, a new endogenous opioid system was identified. The ligand was defined as nociceptin/orphanin (N/OFQ) and the receptor as nociceptin orphanin peptide receptor (NOP receptor), also called opioid receptor like1 (ORL1) receptor. The sequence of N/OFQ is closely related to that of dynorphin A. Furthermore, N/OFQ is not active at the classical opioid receptors, such as mu, kappa, and delta receptors. Unlike other members of the opioid family, N/OFQ plays a crucial role in pain modulation in a bidirectional manner, exhibiting either pro- or anti-nociceptive effects, depending on a series of complex factors, including type of pain, administration strategies and dosage of opioid agonists (Toll and others 2019). NOP is a G-coupled receptor (Agostini and Petrella 2014; Kiguchi and others 2016). This receptor is involved in spinal analgesia and mediates side effects such as tolerance to morphine after chronic treatment, hyperalgesia at low doses of N/OFQ, exacerbation of CP condition, depression, and Parkinson’s disease (Table 1).
Table 1.
Opioid Receptors: Agonists, Antagonists, Side Effects.
| Receptor | Endogenous Peptides | Opioid Agonists | Effects | Side Effects |
|---|---|---|---|---|
| µ mu [MOR]/Oprm1 | β-Endorphins Endomorphin-1 Endomorphin-2 |
Morphine Fentanyl Meperdine Methadone Codeine Oxymorphone Hydromorphone Levorphanol |
Analgesia | Tolerance and dependence after chronic exposure to opioid
agonists Respiratory depression Nausea Urinary retention Biliary spasms and constipation Sedation Reward/euphoria |
| K kappa [KOR]/Oprk1 | Dynorphin-A Dynorphin-B |
Morphine Levorphanol |
Analgesia | Tolerance and dependence after chronic exposure to opioid
agonists Diuretic effect Sedative effect Dysphoria |
| δ delta [DOR]/Oprd1 | Leu-enkephalin Met-enkephalin |
Meperidine Codeine Hydromorphone Levorphanol |
Analgesia Spinal analgesia |
Tolerance and dependence after chronic exposure to opioid
agonists Antidepressant effect Reward/Euphoria Respiratory depression Constipation |
| NOP | N/OFQ | — | Analgesia (spinally) | Exacerbation of chronic pain condition Tolerance to morphine Depression Hyperalgesia (low dose of N/OFQ) Parkinson’s disease |
Opioid Receptor Signaling Pathways
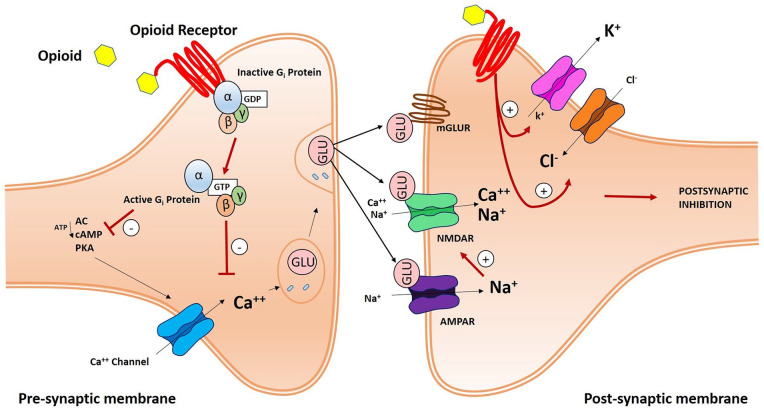
Opioid receptors play a role in synaptic pain transmission, which takes place between C-fiber primary afferents and secondary projection neurons in the dorsal horn of the spinal cord. After binding their specific receptors, opioids lead to the dissociation of the trimeric G-protein complex, which switches from a GDP-bound inactive to a GTP-bound active state, into Gα and Gβγ subunits. Gβγ subunits can directly bind calcium (Ca++) channels and inhibit intracellular Ca++ influx. Activated Gα subunits can inhibit adenylyl cyclase (AC) activity and the consequential production of cyclic AMP (cAMP) and, in a downstream mechanism, protein kinase A (PKA) and Ca++ ion influx. In fact, Ca++ ions have a pivotal role in the synaptic process, enhancing glutamate (Glu) release from presynaptic vesicles. Glu mediates fast excitatory transmission between primary and secondary sensory neurons, by binding ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA receptors, and slow transmission by acting on metabotropic glutamate receptors (mGluRs). Slow synaptic effects are also mediated by other neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP), through metabotropic G protein-coupled receptors and receptor tyrosine kinases. Moreover, opioid peptides act both pre- and postsynaptically to inhibit the pain pathway. Presynaptic inhibition reduces voltage-gated calcium (Ca++) channel activity, while postsynaptic inhibition enhances chloride (Cl−) influx and potassium (K+) efflux (Golan 2008). These events prevent excitation and propagation of action potentials in second order projection neurons and suppresses pain development (Fig. 4).
Figure 4.
Schematic illustration of an opioid receptor-mediated synaptic pain pathway. In the presynaptic membrane, opioid peptides bind opioid receptors and activate G-proteins, thereby inhibiting Calcium (Ca++) influx. G-βγ subunits then directly bind and inhibit Ca++ influx. Activated G proteins can have the same effect by inhibiting adenylyl cyclase (AC) and, as a consequence, cyclic AMP (cAMP) and protein kinase A (PKA) activity. Ca++ channel inhibition blocks glutamate (Glu) release from presynaptic vesicles and fast and slow excitatory transmission between primary and secondary neurons. Fast transmission is mediated by ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors; slow transmission by metabotropic glutamate receptors (mGluR). Moreover, opioid receptors mediate postsynaptic inhibition of pain, by enhancing chloride (Cl−) influx and potassium (K+) efflux.
Peripherally, at the site of injury, the opioid receptor-mediated synaptic pain pathway involves peripheral nociceptors, leukocytes, and anti-inflammatory cytokines (Pinho-Ribeiro and others 2017). Peripheral opioid receptor-containing immune cells reach the inflamed tissue, and their activation leads to the secretion of opioid peptides, which bind their specific peripheral neuronal opioid receptors. At the early stage of the inflammation process, granulocytes are the major opioid-containing leukocytes involved, later monocytes, macrophages, and lymphocytes predominate (Brack and others 2004). Not all the totality of immune cells produce opioids, but it is well known that, during inflammation and leukocytes’ homing, the expression of opioid peptides increases (Hua 2016; Mousa and others 2007). Opioid release is triggered by several endogenous factors, including temperature, low pH, proteolytic activity, or local inflammatory factors (Julius and Basbaum 2001). When released, opioid peptides penetrate the perineural sheath and activate specific receptors on peripheral terminals of sensory neurons, producing analgesia and also anti-inflammatory effects such as the inhibition of substance P, NA (noradrenaline) and TNF (tumor necrosis factor)-α neuronal release (Mambretti and others 2016; Stein and Machelska 2011). This mechanism produces analgesia through the inhibition of activity of nociceptors (Rittner and others 2008; Stein and Kuchler 2012).
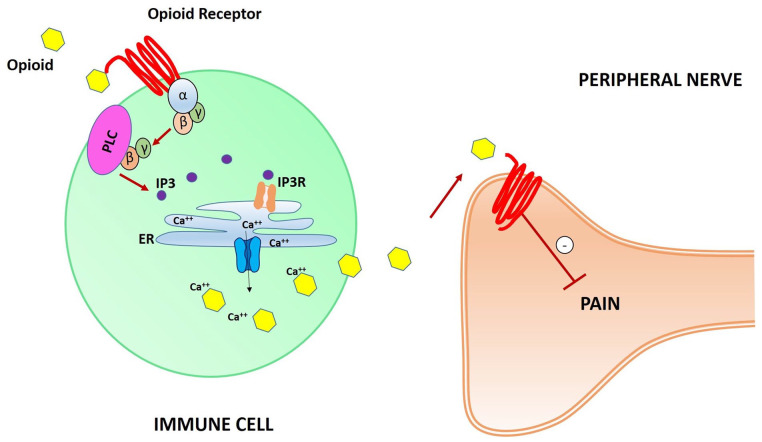
Recently, in a mouse model, an expansion of the classical model of analgesia mediated by opioid receptors was presented, focusing on immune cells containing opioid receptors (Celik and others 2016; Machelska and Celik 2020). In contrast to the previously described model (Fig. 3), in this new model leukocytes opioid receptor mediate analgesia through the release of pain-inhibiting opioid peptides. Gi proteins are still involved in the process, in association with the phospholipase C (PLC), which leads to production of the second messenger inositol 1,4,5-trisphosphate (IP3). IP3 binds and activates the IP3 receptor (IP3R) in the endoplasmic reticulum (ER), leading to the release of intracellular Ca++ ions. This pathway permits extracellular delivery of intracellular Ca++-dependent opioid peptides, which bind specific opioid receptors on peripheral nerves membranes, thereby inhibiting pain (Fig. 5).
Figure 5.
Schematic illustration of opioid receptors on leukocytes’ surface-mediated synaptic pain pathway. Opioid peptides bind opioid receptors, activating Gi proteins. G-βγ subunits activate phospholipase C (PLC), leading to the second inositol 1,4,5-trisphosphate (IP3) messenger production and subsequent IP3 receptor (IP3R) activation in the endoplasmic reticulum (ER). Ca++ ions are released from the ER, leading to the extracellular delivery of intracellular Ca++-dependent opioid peptides. Thus, opioid receptors on peripheral nerve cell membranes are activated and pain pathways are inhibited.
Neuroimmune Synapse
In a rat study, researchers showed that the half-life of endogenous peptides at the site of inflammation is strictly conditioned by peripheral blood proteases: 5 minutes for enkephalins and 40 minutes for β-endorphins. Thus, for the best analgesic effects, exogenous opioids peptides should be ideally released in close proximity to sensory neurons (Hua and others 2006).
Along these lines, several studies provide evidence for the close association between peripheral nerve and opioid-containing immune cells (Hua 2016). Leukocytes have been found physically close to the innervation of many organs, such as the skin (Darsow and Ring 2001), eye (Schafer and others 1994), liver (Kaiser and others 2003), respiratory tract (Kingham and others 2002), and gastrointestinal tract (Tournier and Hellmann 2003). In fact, a bidirectional interaction between immune cells and primary afferent nerves has been described and is supported by three major observations. First, several studies indicate a direct membrane-membrane contact (Crivellato and others 2002; Tian and others 2000) when nerve fibers end on the surface of lymphoid organs. This anatomical connection chemically mediates the bidirectional release of transmitters and postsynaptic receptors activation (Stein 2013). Second, both immune cells and neurons share common ligands and receptors (Shaw and Allen 2001). Third, this ligand-receptor communication influences and activates cellular pathways in both immune and nervous systems (Rittner and others 2008). Traditionally, the term “synapse” indicates a stable adhesive junction between two cells, in which information is relayed by direct secretion. Here, for the neuro-immune synapse we are describing a hybrid structure: a specific zone between immune cells and neurons (Shepherd and others 2005) in which both of the systems share common mediators (Shaw and Allen 2001).
The Impact of Exogenous Opioids on the Immune System in CP Conditions
When the physiological pain pathway is altered, pain becomes the “enemy” to defeat. In this fight, the role of opioid receptors is still ambiguous, particularly their localization on surfaces of immune cells. A better understanding about peripheral opioid receptors function could be critical for informing more effective CP pharmacological treatment approaches (Machelska and Celik 2020). Clinical studies suggest that opioid administration is associated with high risk of immune suppression and the development of inflammatory mechanisms, enhancing CP status itself (Kosciuczuk and others 2020). Moreover, it is well known that opioid consumption has a dual effect in CP patients, by inducing at the same time tolerance, dependence, and a risk for opioid use disorders (Cameron-Burr and others 2021; Thong and others 2018). Thus, understanding the role of peripheral opioid receptors in CP conditions could also be helpful in minimizing central nervous system opioid side effects, such as addiction, sedation, respiratory depression, and nausea. In this context, we have to approach the problem considering that every single immune cell type could have a role in this process.
Immune Cells and Opioid Treatment
Since 1979, several immunomodulatory effects of opioids on T lymphocytes have been described (Sacerdote and others 2003). T lymphocytes, cells of the adaptive immunity, infiltrate the injured central nervous system only after neutrophils and macrophages have already arrived: indeed, macrophages infiltration is essential for lymphocytes recruitment as this event is prevented by macrophages ablation in mice (Ghasemlou and others 2015; Kobayashi and others 2015). T cells interact with neurons in a bidirectional manner. For example, vasoactive intestinal peptide (VIP) induces pro-inflammatory cytokines secretion from CD4+ T-lymphocytes in allergic inflammation. Among these cytokines, secreted interleukin (IL)-5 from lymphocytes in turn enhances neuronal secretion of VIP (Talbot and others 2015). T lymphocytes can stimulate the production of brain-derived neurotropic factor (BDNF) through the action of IL-4 on neurons (Ziv and others 2006). T cells can exert different effects depending on their polarization: Th1 cells, that secrete proinflammatory chemokines (Il-1b, TNF-α, IL.17) enhance pain hypersensitivity while Th2 lymphocytes reduced mechanical allodynia and thermal hyperalgesia in neuropathic models (Moalem and others 2004; Palmer and Weaver 2010). The role of T cells in the induction of pain in inflammatory pain models is poorly understood. For example, T cell–deficient mice do not show a reduction of pain hypersensitivity (Ghasemlou and others 2015; Petrovic and others 2019; Sorge and others 2015) and present a prolonged duration of mechanical allodynia (Laumet and others 2018). In addition, regulatory T cells (T-Reg cells), characterized by the expression of the transcription factor FoxP3 and specialized in dampening inflammation and resolving immune response, can reduce neuropathic pain (Liu and others 2014). Of our interest, T lymphocytes express all the three kinds of opioid receptors on their surface (Liang and others 2016). Human studies have demonstrated that short-term morphine administration induces T-lymphocyte cytokine expression, such as IL-2 and IL-6, by enhancing the differentiation of B-lymphocytes (Campana and others 2010). Work by Campana and others showed that 1-year intrathecal morphine treatment, in chronic non-malignant pain conditions patients, leads to an increase of MOR mRNA level in circulating T-lymphocytes, and this effect is stronger after administration of morphine plus bupivacaine than a pure morphine solution. Assuming a correlation exists between transcription and translation, considerably higher amounts of MOR receptors should be expected in the lymphocytes of patients receiving chronic intrathecal morphine (Campana and others 2010). Moreover, morphine abuse inhibits T-helper 17 (Th17) function and, at the same time, enhances the activity of T-Reg cells. This mechanism could be linked to immune suppression, as reported by Abo-Elnazar and others (2014).
Morphine can also increase KOR mRNA expression on T cells, indicating that opioid drugs can exert their effects through multiple opioid receptor subtypes (Suzuki and others 2001). Moreover, activation of DOR receptors expressed on surfaces of T-lymphocytes by the endogenous ligand met-enkephalin enhances the expansion of CD4+ cells and CD4 molecule expression (Shan and others 2011).
Today, it is well known that all the elements of the immune system play a specific role in pain. For example, B-lymphocytes, which are antibody-producing cells that represent the source of the humoral immune response, also express μ, δ, and κ opioid receptors (Malafoglia and others 2017). MOR agonists increase IgM and IgG production; DOR agonists produce the opposite effect (Liang and others 2016). Interestingly, B cells are influenced by opioids but the ability to produce antibodies also requires the cooperation of other immune cells, for example, monocytes and macrophages. Monocytes are peripheral blood circulating leukocytes, that rapidly infiltrate the site of infection, an injury, or a damaged tissue. These cells can differentiate toward a pro- or an anti-inflammatory phenotype, depending on the extracellular milieu (Yang and others 2014). Monocytes differentiate into macrophages, typically recognized in Immunohistochemistry sections by the staining with CD68. Chemokines and cytokines contribute to the recruitment of monocytes/macrophages into the peripheral nervous system (Ren and Dubner 2010). In particular, the monocyte chemoattractant protein-1 (MCP-1), also known as CCL2, induces peripheral sensitization acting on CCR2 expressing nociceptors (Zhang and others 2013). Lack of CCR2 in murine models results in reduced macrophages infiltration in nerve injury sites (Siebert and others 2000). Fractalkine, also known as CX3CL1, have been shown to increase in dorsal root ganglia after injury, and blocking CX3CL1 reduces allodynia in paclitaxel-induced neuropathy (Huang and others 2014). TNF-α), a prototypic proinflammatory cytokines, seems to be also involved in macrophages recruitment as it is impaired in TNF-α-deficient mice (Shubayev and Myers 2000). Accordingly, IL-1β accumulates in nerve injury sites and support macrophages recruitment (Perrin and others 2005). The role of macrophages in the induction of pain is demonstrated also in murine models, where macrophages are depleted by the injection of clodronate liposomes: in these mice, thermal and mechanical hyperalgesia are reduced (Liu and others 2000). However, macrophages can sustain tissue repair and regenerative process in several tissue, including nervous tissue (Liu and others 2019), thanks to their capacity to differentiate into M2-anti-inflammatory macrophages. These are immunosuppressive cells, that secrete anti-inflammatory cytokines and growth factors to promote tissue repair and resolution of pain (Mokarram and others 2012). Polarization of macrophages into IL-10 producing M2 cells enhances the resolution of inflammation, thus dampening hyperalgesia (Willemen and others 2014), and in vitro generated M2 macrophage have been shown to secrete opioid peptides, including metenkephalin, dynorphin A, and β-endorphin (Pannell and others 2016).
Macrophage cells express opioid receptors themselves. MOR activation by morphine has been shown to regulate macrophages functions, including nitric oxide production and phagocytosis (Brack and others 2004). Macrophage MOR is up-regulated by cytokines, namely IL-1β, IL-4, IL-6, TNF-α, and interferon-γ (IFN-γ). Moreover, an in vitro murine study showed that IFN-γ also stimulates the expression of macrophage’s KOR (Gabrilovac and others 2012).
Neutrophils and mast cells are two other major types of immune cells involved in pain modulation. Neutrophils are innate immune cells that rapidly gather around the damaged or injured tissue (Kanashiro and others 2020). Recruitment of neutrophils, induced by T leukotriene B4 (LTB4) and complement component 5a (C5a) is associated with pain sensitization (Ting and others 2008). Neutrophils can act on neurons by inducing the secretion of chemotactic factors that in turn recruit more neutrophils, thus amplifying the nociceptive response (Grace and others 2014). In humans, neutrophils accumulation in the joints of arthritis patients is associated with the induction of hyperalgesia. On the other hand, neutrophils can also secrete analgesic mediators such as opioid peptides (β-endorphin, met-enkephalin, and dynorphin-A), that in turn can inhibit nociceptive transmission by activating opioid receptors on peripheral sensory neurons (Rittner and others 2009). Neutrophils express opioid receptors on their surface and a murine study demonstrated that morphine can completely attenuate neutrophil migration to the site of inflammation and opioids consumption can impede the bactericidal action of these cells (Kanashiro and others 2020; Roy and others 2011).
Mast cells are leukocytes with a cytoplasm rich in granules that are resident in tissue, in particular in connective ones. They participate to the immune response to injury by secreting their granules’ content, releasing cytokines, chemokines and other mediators such as histamine (Forsythe and Bienenstock 2012). Mast cell have been shown to increase in human inflammatory diseases (Nigrovic and Lee 2005), and in murine models, which lack mast cells, a reduction of pain can be observed (Milenkovic and others 2007). Blocking histamine signaling by using histamine receptor antagonists reduces mechanical and thermal hyperalgesia (Gupta and Harvima 2018; Liu and others 2021). Mast cells express opioid receptors in their surface but the mechanism that describe the communication between these cells and peripheral nerves in pain pathways is still unknown. A common opioid side effect is the activation of mast cells (Nguyen and others 2014). Interestingly, a human study demonstrated that morphine and other opioids with lower MOR affinity induce mast cells activation; differently MOR potent agonists (i.e., naloxone, buprenorphine) did not activate mast cells. These results indicate that MOR is not directly involved in mast cells activation (Blunk and others 2004). In 2017 a silico design study (Lansu and others 2017) proposed a unique atypical opioid-like receptor impor-tant for modulating mast cell degranulation, named MRGPRX2. Later, a preclinical primate study demonstrated that MRGPRX2 is necessary for innate immune cells recruitment at the injury site, mediating neurogenic inflammation and pain (Green and others 2019).
In the past few decades, our group has been focused on MOR expression on natural killer (NK) lymphocytes and lymphokine-activated killer (LAK) cells. These are specific cytotoxic cells of the innate immune system. We demonstrated that, in cancer patients, after chronic in vivo analgesic therapy with morphine, the endogenous cytotoxic activity of NK cells was reduced, while LAK cell cytotoxicity increased. Then, we showed that LAK cell activity mainly increased after an oral morphine administration, rather than an intrathecal one (Provinciali and others 1991). Later, we showed that the effect of morphine on LAK cells activation, but not on NK cell reduction, is related to the modulation of prolactin levels determined by the opioid drug (Provinciali and others 1996). Moreover, we observed an increase of MOR mRNA levels in lymphocytes and a reduction of the percentage of NK cells also in non-cancer-pain patients (CNCP), treated for long time with intrathecal morphine (Campana and others 2010). Interestingly, a recent systematic review (Diasso and others 2020) highlighted the effects of long-term opioids treatment in CNCP, demonstrating that the majority of the articles analyzed had considerable limitations (e.g., cross-sectional design, lack of randomization and/or clinical description, small sample size). According to the authors, very few studies increased our understanding in this field (Campana and others 2010; Tabellini and others 2014), despite that their findings could be not comparable because of diverse opioid formulations and administrations. In addition, although the level of evidence is weak, long-term opioid treatment alters the immune system in CNCP referring not only to NK cells alteration but also to IL-1ß production as a consequence of toll-like receptors (TLRs) stimulation (Dutta and others 2012; Meng and others 2013). In this contest, it has been demonstrated that opioids are TLR4 agonists (Zhang and others 2020b) and an increased IL-1β production has been observed after PBMC stimulation with TLR2 and TLR4 agonists (Kwok and others 2012). These data could be explained and enhanced considering a recent finding (Chang and others 2021), which describes the cross-talk of TLRs and MOR in a preclinical rodent model of chronic constriction injury (CCI). Here the authors suggested that mechanical hyperalgesia might be the result of the cross-talk between TLRs and MOR in a PKCα-dependent manner, opening the way for novel neuropathic pain therapeutic strategies (Table 2).
Table 2.
Pharmacological Evidences of Opioid Therapy in Patients with Chronic Pain (CP).
| Immune Cell Type | Opioid Receptors | Pharmacological Evidences in CP Condition | References |
|---|---|---|---|
| T-lymphocytes | µ | Short-term morphine: cytokines expression
induction Long-term morphine: µ mRNA expression enhancing |
Raffaeli and others (2010) |
| κ | Morphine: κ mRNA expression enhancing | Suzuky and others (2001) | |
| δ | Met-enkephalin: CD4+ cells expression enhancing | Shan and others (2011) | |
| Th17 | µ | Morphine abuse: Th17 function inhibition | Abo-Elnazar and others (2014) |
| Treg cells | µ | Morphine abuse: Treg cells activity enhancing | |
| B-lymphocytes | µ | Agonists: IgM and IgG production enhancing | Liang and others (2016) |
| δ | Agonists: IgM and IgG production inhibition | ||
| NK | µ | Long-term morphine: NK percentage and cytotoxic activity reduction | Provinciali and others (1991) |
| LAK | µ | Long term morphine: cytotoxic LAK activity increasing related to the modulation of prolactin levels | Provinciali and others (1991) Provinciali and others (1996) |
| Macrophages | µ | Morphine: NO production and phagocytosis
regulation IL-1β, IL-4, IL-6, TNF-α, IFN-γ: MOR upregulation |
Brack and others (2004) Gabrilovac and others (2012) |
| κ | IFN-γ: κ expression stimulation | Gabrilovac and others (2012) | |
| Neutrophils | µ | Morphine: attenuation of migration to the site of
inflammation Agonists: impediment of bactericidal action |
Kanashiro and others (2020) |
| Mast cells | µ | Morphine and low agonists: mast cells activation
induction Potent agonists: NO mast cells activation induction |
Blunk and others (2004) |
| MRGPRX2 (atypical opioid-like receptor) | ZINC-3573: neurogenic inflammation and pain mediation | Green and others (2019) |
Interestingly, a recent clinical study (Lassen and others 2021) reported findings about the role of NK cells in pain disorders associated with central pain sensitization, (i.e., herpes zoster neuralgia, polyneuropathy). The authors showed that a low NK-cell frequency in the cerebrospinal fluid (CSF) was associated with central sensitization, unlike a high NK-cell frequency, which seemed to prevent it. Thus, a future project could be focused on analyzing opioid receptors expression and pharmacological modulation on NK cells in the CSF. The comparison between central and peripheral analysis could be pivotal to set up the right opioid and/or immunologic treatment.
Opioid-Related Biomarkers in CP
Recently, inspired by the increasing attention on the role of peripheral opioid receptors in pain pathways, our group decided to analyze the percentage of immune cells expressing opioid receptors in CP patients suffering from fibromyalgia or osteoarthritis. Interestingly, we found that the percentage of B cells expressing MOR was lower in CP patients than in a pain-free control group. This difference was greater in CP patients with severe pain. Thus, for the first time, MOR could be considered as a potential peripheral CP biomarker (Mu-Lympho-Marker, MLM) (Fig. 5), considering that B cells express it in patients with FM and OA (Raffaeli and others 2020). The role of MOR as a marker of pain has been also postulated in an ongoing clinical trial (Malafoglia and others 2017), but the meaning of its immunological characteristic is still a topic of discussion. The low percentage of B cells expressing MOR in CP patients could be due to a reduction of an opioid receptor “reserve,” necessary for the pain inhibition pathway mediated by immune cells. In this study, we did not enroll patients taking opioids. Thus, we can exclude a reduction of the opioid receptor reserve as a consequence of desensitization and/or internalization of MOR (Zhang and others 2020a). Still, several questions are open. There could be additional mechanisms of action, and a new study to first assess CP patients could be greatly helpful to set up the right therapeutic strategy. In particular, once we understand the mechanistic role of the MOR reserve reduction, it could be possible to develop peripheral pharmacological treatments based on the ideal opioid dosage administration for each single CP patient, bypassing central side effects.
Moreover, future analysis should address how gender and sex differences affect the opioids and immune system interaction in patients suffering from CP. In fact, preclinical and clinical studied reported that women, in general, present a higher immune response than men (Schwarz and Bilbo 2012). Importantly, morphine has a stronger analgesic effect in males than in females (Doyle and Murphy 2017). Sex differences in CP are probably underestimated, although it is well known that women are more likely to use anti-inflammatory drugs, which enhance opiate action (Li and others 2021), than men. Thus, even if more data is needed to confirm the MOR reserve hypothesis, preliminary findings could be helpful to also discriminate gender-dependent CP biomarkers, underlining the importance of peripheral opioid receptors in analgesia and paving the way for new peripheral, tailored pharmacological approaches and rehabilitation strategies for CP patients. In conclusion, the review of the current literature seems to suggest that the identification of specific pain biomarkers remains perhaps the most important challenge in the field of CP medicine (Fig. 3).
Acknowledgments
We thank Chloe J. Jordan for proofreading and constructive feedback on the manuscript.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is funded and supported by ISAL Foundation and Nando and Elsa Peretti Foundation, PON03PE_00078_1, PON03PE_00078_2. The study is also supported by Reale Foundation and SGR group and by the Italian Ministry of Health [ricerca corrente] to IRCCS San Raffaele Roma.
ORCID iD: Valentina Malafoglia  https://orcid.org/0000-0002-1533-704X
https://orcid.org/0000-0002-1533-704X
References
- Abo-Elnazar S, Moaaz M, Ghoneim H, Molokhia T, El-Korany W. 2014. Th17/Treg imbalance in opioids and cannabinoids addiction: relationship to NF-κB activation in CD4+ T cells. Egypt J Immunol 21(2):33–47. [PubMed] [Google Scholar]
- Agostini S, Petrella C. 2014. The endogenous nociceptin/orphanin FQ-NOP receptor system as a potential therapeutic target for intestinal disorders. Neurogastroenterol Motil 26(11):1519–26. [DOI] [PubMed] [Google Scholar]
- Albrecht DS, Forsberg A, Sandstrom A, Bergan C, Kadetoff D, Protsenko E, and others. 2019. Brain glial activation in fibromyalgia—a multi-site positron emission tomography investigation. Brain Behav Immun 75:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backryd E. 2015. Pain in the blood? Envisioning mechanism-based diagnoses and biomarkers in clinical pain medicine. Diagnostics (Basel) 5(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. 2009. Cellular and molecular mechanisms of pain. Cell 139(2):267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. 2004. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth Analg 98(2):364–70. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. 2017. Endogenous opiates and behavior: 2015. Peptides 88:126–88. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. 2011. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discov Med 11(58):197–207. [PubMed] [Google Scholar]
- Brack A, Labuz D, Schiltz A, Rittner HL, Machelska H, Schafer M, and others. 2004. Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology 101(1):204–11. [DOI] [PubMed] [Google Scholar]
- Burma NE, Leduc-Pessah H, Fan CY, Trang T. 2017. Animal models of chronic pain: advances and challenges for clinical translation. J Neurosci Res 95(6):1242–56. [DOI] [PubMed] [Google Scholar]
- Califf RM. 2018. Biomarker definitions and their applications. Exp Biol Med (Maywood) 243(3):213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron-Burr KT, Conicella A, Neavyn MJ. 2021. Opioid use and driving performance. J Med Toxicol 17(3):289–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana G, Sarti D, Spampinato S, Raffaeli W. 2010. Long-term intrathecal morphine and bupivacaine upregulate MOR gene expression in lymphocytes. Int Immunopharmacol 10(9):1149–52. [DOI] [PubMed] [Google Scholar]
- Celik MO, Labuz D, Henning K, Busch-Dienstfertig M, Gaveriaux-Ruff C, Kieffer BL, and others. 2016. Leukocyte opioid receptors mediate analgesia via Ca2+-regulated release of opioid peptides. Brain Behav Immun. 57:227–42. [DOI] [PubMed] [Google Scholar]
- Chang C, Liu HK, Yeh CB, Yang ML, Liao WC, Liu CH, and others. 2021. Cross-talk of toll-like receptor 5 and mu-opioid receptor attenuates chronic constriction injury-induced mechanical hyperalgesia through a protein kinase C alpha-dependent signaling. Int J Mol Sci. 22(4):1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E, Soldano F, Travan L. 2002. A light and electron microscopic quantitative analysis of nerve-immune cell contacts in the gut-associated lymphoid tissue of the mouse colon. J Submicrosc Cytol Pathol 34(1):55–66. [PubMed] [Google Scholar]
- Cumenal M, Selvy M, Kerckhove N, Bertin C, Morez M, Courteix C, and others. 2021. The safety of medications used to treat peripheral neuropathic pain, part 2 (opioids, cannabinoids and other drugs): review of double-blind, placebo-controlled, randomized clinical trials. Expert Opin Drug Saf 20(1):51–68. [DOI] [PubMed] [Google Scholar]
- Darnall BD, Carr DB, Schatman ME. 2017. Pain psychology and the biopsychosocial model of pain treatment: ethical imperatives and social responsibility. Pain Med 18(8):1413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow U, Ring J. 2001. Neuroimmune interactions in the skin. Curr Opin Allergy Clin Immunol 1(5):435–9. [DOI] [PubMed] [Google Scholar]
- Diasso PDK, Birke H, Nielsen SD, Main KM, Hojsted J, Sjogren P, and others. 2020. The effects of long-term opioid treatment on the immune system in chronic non-cancer pain patients: a systematic review. Eur J Pain 24(3):481–96. [DOI] [PubMed] [Google Scholar]
- Doyle HH, Murphy AZ. 2017. Sex differences in innate immunity and its impact on opioid pharmacology. J Neurosci Res. 95(1–2):487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Krishnan A, Meng J, Das S, Ma J, Banerjee S, and others. 2012. Morphine modulation of toll-like receptors in microglial cells potentiates neuropathogenesis in a HIV-1 model of coinfection with pneumococcal pneumoniae. J Neurosci 32(29):9917–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe P, Bienenstock J. 2012. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem Immunol Allergy 98:196–221. [DOI] [PubMed] [Google Scholar]
- Gabrilovac J, Cupic B, Zapletal E, Brozovic A. 2012. IFN-γ up-regulates κ opioid receptors (KOR) on murine macrophage cell line J774. J Neuroimmunol 245(1–2):56–65. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. 2015. CD11b+Ly6G− myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 112(49):E6808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan DE. 2008. Principles of pharmacology: the pathophysiologic basis of drug therapy. Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. 2014. Pathological pain and the neuroimmune interface. Nat Rev Immunol 14(4):217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. 2019. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101(3):412–20.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn J, Hill MM, Cotten BM, Deer TR. 2020. An analysis of biomarkers in patients with chronic pain. Pain Physician 23(1):E41–9. [PubMed] [Google Scholar]
- Gupta K, Harvima IT. 2018. Mast cell-neural interactions contribute to pain and itch. Immunol Rev 282(1):168–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw KV, Aykas DP, Sigurdson GT, Plans M, Madiai F, Yu L, and others. 2019. Metabolic fingerprinting for diagnosis of fibromyalgia and other rheumatologic disorders. J Biol Chem 294(7):2555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasvik E, Schjolberg T, Jacobsen DP, Haugen AJ, Grovle L, Schistad EI, and others. 2019. Up-regulation of circulating microRNA-17 is associated with lumbar radicular pain following disc herniation. Arthritis Res Ther 21(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. 2016. Neuroimmune interaction in the regulation of peripheral opioid-mediated analgesia in inflammation. Front Immunol 7:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S, Hermanussen S, Tang L, Monteith GR, Cabot PJ. 2006. The neural cell adhesion molecule antibody blocks cold water swim stress-induced analgesia and cell adhesion between lymphocytes and cultured dorsal root ganglion neurons. Anesth Analg 103(6):1558–64. [DOI] [PubMed] [Google Scholar]
- Huang ZZ, Li D, Liu CC, Cui Y, Zhu HQ, Zhang WW, and others. 2014. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun 40:155–65. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. 2001. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J Neurosci 21(7):2536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. 2001. Molecular mechanisms of nociception. Nature 413(6852):203–10. [DOI] [PubMed] [Google Scholar]
- Kaiser MJ, Tiegs G, Neuhuber WL. 2003. Close apposition of dynorphin-positive nerve fibres to lymphocytes in the liver suggests opioidergic neuroimmunomodulation. Histochem Cell Biol 120(3):213–21. [DOI] [PubMed] [Google Scholar]
- Kanashiro A, Hiroki CH, da Fonseca DM, Birbrair A, Ferreira RG, Bassi GS, and others. 2020. The role of neutrophils in neuro-immune modulation. Pharmacol Res 151:104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Ko MC. 2016. Central N/OFQ-NOP receptor system in pain modulation. Adv Pharmacol 75:217–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham PJ, McLean WG, Sawatzky DA, Walsh MT, Costello RW. 2002. Adhesion-dependent interactions between eosinophils and cholinergic nerves. Am J Physiol Lung Cell Mol Physiol 282(6):L1229–38. [DOI] [PubMed] [Google Scholar]
- Kipnis J. 2016. Multifaceted interactions between adaptive immunity and the central nervous system. Science 353(6301):766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kiguchi N, Fukazawa Y, Saika F, Maeda T, Kishioka S. 2015. Macrophage-T cell interactions mediate neuropathic pain through the glucocorticoid-induced tumor necrosis factor ligand system. J Biol Chem 290(20):12603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosciuczuk U, Knapp P, Lotowska-Cwiklewska AM. 2020. Opioid-induced immunosuppression and carcinogenesis promotion theories create the newest trend in acute and chronic pain pharmacotherapy. Clinics (Sao Paulo) 75:e1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbhare DA, Elzibak AH, Noseworthy MD. 2017. Evaluation of chronic pain using magnetic resonance (MR) neuroimaging approaches: what the clinician needs to know. Clin J Pain 33(4):281–90. [DOI] [PubMed] [Google Scholar]
- Kwok YH, Hutchinson MR, Gentgall MG, Rolan PE. 2012. Increased responsiveness of peripheral blood mononuclear cells to in vitro TLR 2, 4 and 7 ligand stimulation in chronic pain patients. PLoS One 7(8):e44232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, and others. 2017. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 13(5):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen J, Sturner KH, Gierthmuhlen J, Dargvainiene J, Kixmuller D, Leypoldt F, and others. 2021. Protective role of natural killer cells in neuropathic pain conditions. Pain. Epub Mar 24. doi: 10.1097/j.pain.0000000000002274 [DOI] [PubMed] [Google Scholar]
- Laumet G, Edralin JD, Chiang AC, Dantzer R, Heijnen CJ, Kavelaars A. 2018. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology 43(13):2597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins KJ, Drago T, Roman E, Martin A, King R, Murphy P, and others. 2019. Magnetic resonance spectroscopy across chronic pain disorders: a systematic review protocol synthesising anatomical and metabolite findings in chronic pain patients. Syst Rev 8(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang H, Qi X, Huang X, Li Y. 2021. Intrathecal dexmedetomidine improves epidural labor analgesia effects: a randomized controlled trial. J Int Med Res 49(4):300060521999534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Liu R, Chen C, Ji F, Li T. 2016. Opioid system modulates the immune function: a review. Transl Perioper Pain Med 1(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ye M, Guo H. 2019. An updated review of randomized clinical trials testing the improvement of cognitive function of Ginkgo biloba extract in healthy people and Alzheimer’s patients. Front Pharmacol 10:1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JA, Yu J, Cheung CW. 2021. Immune actions on the peripheral nervous system in pain. Int J Mol Sci 22(3):1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, van Rooijen N, Tracey DJ. 2000. Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain 86(1–2):25–32. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, and others. 2014. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res 24(11):1374–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gonzalez MJ, Landry M, Favereaux A. 2017. MicroRNA and chronic pain: from mechanisms to therapeutic potential. Pharmacol Ther. 180:1-15. [DOI] [PubMed] [Google Scholar]
- Machelska H, Celik MO. 2020. Opioid receptors in immune and glial cells–implications for pain control. Front Immunol 11:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafoglia V, Celi M, Muscoli C, Ilari S, Lauro F, Giancotti LA, and others. 2017. Lymphocyte opioid receptors as innovative biomarkers of osteoarthritic pain, for the assessment and risk management of opioid tailored therapy, before hip surgery, to prevent chronic pain and opioid tolerance/addiction development: OpMarkArt (Opioids-Markers-Arthroprosthesis) study protocol for a randomized controlled trial. Trials 18(1):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambretti EM, Kistner K, Mayer S, Massotte D, Kieffer BL, Hoffmann C, and others. 2016. Functional and structural characterization of axonal opioid receptors as targets for analgesia. Mol Pain 12:1744806916628734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, and others. 2013. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8(1):e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic N, Frahm C, Gassmann M, Griffel C, Erdmann B, Birchmeier C, and others. 2007. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron 56(5):893–906. [DOI] [PubMed] [Google Scholar]
- Moalem G, Xu K, Yu L. 2004. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 129(3):767–77. [DOI] [PubMed] [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. 2012. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33(34):8793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, Straub RH, Schafer M, Stein C. 2007. Beta-endorphin, Met-enkephalin and corresponding opioid receptors within synovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann Rheum Dis 66(7):871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J, Luk K, Vang D, Soto W, Vincent L, Robiner S, and others. 2014. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth 113(Suppl 1):i4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu AB, Le-Niculescu H, Levey DF, Roseberry K, Soe KC, Rogers J, and others. 2019. Towards precision medicine for pain: diagnostic biomarkers and repurposed drugs. Mol Psychiatry 24(4):501–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Lee DM. 2005. Mast cells in inflammatory arthritis. Arthritis Res Ther 7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MT, Weaver CT. 2010. Autoimmunity: increasing suspects in the CD4+ T cell lineup. Nat Immunol 11(1):36–40. [DOI] [PubMed] [Google Scholar]
- Pannell M, Labuz D, Celik MO, Keye J, Batra A, Siegmund B, and others. 2016. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation 13(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin FE, Lacroix S, Aviles-Trigueros M, David S. 2005. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and interleukin-1β in Wallerian degeneration. Brain 128(Pt 4):854–66. [DOI] [PubMed] [Google Scholar]
- Petrovic J, Silva JR, Bannerman CA, Segal JP, Marshall AS, Haird CM, and others. 2019. γδ T cells modulate myeloid cell recruitment but not pain during peripheral inflammation. Front Immunol 10:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho-Ribeiro FA, Verri WA, Jr, Chiu IM. 2017. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 38(1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podvin S, Yaksh T, Hook V. 2016. The emerging role of spinal dynorphin in chronic pain: a therapeutic perspective. Annu Rev Pharmacol Toxicol 56:511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provinciali M, Di Stefano G, Raffaeli W, Pari G, Desiderio F, Fabris N. 1991. Evaluation of NK and LAK cell activities in neoplastic patients during treatment with morphine. Int J Neurosci 59(1–3):127–33. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Di Stefano G, Stronati S, Raffaeli W, Pari G, Fabris N. 1996. Role of prolactin in the modulation of NK and LAK cell activity after short- or long-term morphine administration in neoplastic patients. Int J Immunopharmacol 18(10):577–86. [DOI] [PubMed] [Google Scholar]
- Raffaeli W, Arnaudo E. 2017. Pain as a disease: an overview. J Pain Res 10:2003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaeli W, Malafoglia V, Bonci A, Tenti M, Ilari S, Gremigni P, and others. 2020. Identification of MOR-positive B cell as possible innovative biomarker (Mu Lympho-Marker) for chronic pain diagnosis in patients with fibromyalgia and osteoarthritis diseases. Int J Mol Sci 21(4):1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, and others. 2020. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161(9):1976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Ajit SK. 2016. MicroRNA-based biomarkers in pain. Adv Pharmacol 75:35–62. [DOI] [PubMed] [Google Scholar]
- Reckziegel D, Vachon-Presseau E, Petre B, Schnitzer TJ, Baliki MN, Apkarian AV. 2019. Deconstructing biomarkers for chronic pain: context- and hypothesis-dependent biomarker types in relation to chronic pain. Pain 160(Suppl 1):S37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. 2010. Interactions between the immune and nervous systems in pain. Nat Med 16(11):1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner HL, Brack A, Stein C. 2008. Pain and the immune system. Br J Anaesth 101(1):40–4. [DOI] [PubMed] [Google Scholar]
- Rittner HL, Hackel D, Voigt P, Mousa S, Stolz A, Labuz D, and others. 2009. Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog. 5(4):e1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, and others. 2011. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 6(4):442–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P, Limiroli E, Gaspani L. 2003. Experimental evidence for immunomodulatory effects of opioids. Adv Exp Med Biol 521:106–16. [PubMed] [Google Scholar]
- Schafer M, Carter L, Stein C. 1994. Interleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci U S A 91(10):4219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. 2012. Sex, glia, and development: interactions in health and disease. Horm Behav 62(3):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan F, Xia Y, Wang N, Meng J, Lu C, Meng Y, and others. 2011. Functional modulation of the pathway between dendritic cells (DCs) and CD4+ T cells by the neuropeptide: methionine enkephalin (MENK). Peptides 32(5):929–37. [DOI] [PubMed] [Google Scholar]
- Shaw AS, Allen PM. 2001. Kissing cousins: immunological and neurological synapses. Nat Immunol 2(7):575–6. [DOI] [PubMed] [Google Scholar]
- Shepherd AJ, Downing JE, Miyan JA. 2005. Without nerves, immunology remains incomplete—in vivo veritas. Immunology 116(2):145–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. 2000. Upregulation and interaction of TNFα and gelatinases A and B in painful peripheral nerve injury. Brain Res 855(1):83–9. [DOI] [PubMed] [Google Scholar]
- Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W. 2000. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol 110(1–2):177–85. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Robertson B, Monroe PJ, Taylor DA, Leedham JA, Cabral JD. 1992. Opioid receptors mediating antinociception from β-endorphin and morphine in the periaqueductal gray. Neuropharmacology 31(11):1137–50. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, and others. 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18(8):1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. 2013. Opioids, sensory systems and chronic pain. Eur J Pharmacol 716(1–3):179–87. [DOI] [PubMed] [Google Scholar]
- Stein C. 2016. Opioid receptors. Annu Rev Med 67:433–51. [DOI] [PubMed] [Google Scholar]
- Stein C. 2018. New concepts in opioid analgesia. Expert Opin Investig Drugs 27(10):765–75. [DOI] [PubMed] [Google Scholar]
- Stein C, Kuchler S. 2012. Non-analgesic effects of opioids: peripheral opioid effects on inflammation and wound healing. Curr Pharm Des 18(37):6053–69. [DOI] [PubMed] [Google Scholar]
- Stein C, Machelska H. 2011. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev 63(4):860–81. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Doi RH, Bidlack JM, Chuang RY. 2001. Kappa-opioid receptors on lymphocytes of a human lymphocytic cell line: morphine-induced up-regulation as evidenced by competitive RT-PCR and indirect immunofluorescence. Int Immunopharmacol 1(9–10):1733–42. [DOI] [PubMed] [Google Scholar]
- Swingler TE, Niu L, Smith P, Paddy P, Le L, Barter MJ, and others. 2019. The function of microRNAs in cartilage and osteoarthritis. Clin Exp Rheumatol 37 Suppl 120(5): 40–7. [PubMed] [Google Scholar]
- Tabellini G, Borsani E, Benassi M, Patrizi O, Ricotta D, Caimi L, and others. 2014. Effects of opioid therapy on human natural killer cells. Int Immunopharmacol 18(1):169–74. [DOI] [PubMed] [Google Scholar]
- Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, and others. 2015. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87(2):341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares-Ferreira D, Lawless N, Bird EV, Atkins S, Collier D, Sher E, and others. 2019. Correlation of miRNA expression with intensity of neuropathic pain in man. Mol Pain 15:1744806919860323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong ISK, Jensen MP, Miro J, Tan G. 2018. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain 18(1):99–107. [DOI] [PubMed] [Google Scholar]
- Tian L, Kilgannon P, Yoshihara Y, Mori K, Gallatin WM, Carpen O, and others. 2000. Binding of T lymphocytes to hippocampal neurons through ICAM-5 (telencephalin) and characterization of its interaction with the leukocyte integrin CD11a/CD18. Eur J Immunol 30(3):810–8. [DOI] [PubMed] [Google Scholar]
- Ting E, Guerrero AT, Cunha TM, Verri WA, Jr, Taylor SM, Woodruff TM, and others. 2008. Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain. Br J Pharmacol 153(5):1043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Ozawa A, Cippitelli A. 2019. NOP-related mechanisms in pain and analgesia. Handb Exp Pharmacol 254:165–86. [DOI] [PubMed] [Google Scholar]
- Tournier JN, Hellmann AQ. 2003. Neuro-immune connections: evidence for a neuro-immunological synapse. Trends Immunol 24(3):114–5. [DOI] [PubMed] [Google Scholar]
- Treede RD. 2018. The International Association for the Study of Pain definition of pain: as valid in 2018 as in 1979, but in need of regularly updated footnotes. Pain Rep 3(2):e643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, and others. 2015. A classification of chronic pain for ICD-11. Pain 156(6):1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, and others. 2019. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160(1):19–27. [DOI] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, and others. 2014. Monocytes/macrophages control resolution of transient inflammatory pain. J Pain 15(5):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang L, Yu C, Yang XF, Wang H. 2014. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Boyette-Davis JA, Kosturakis AK, Li Y, Yoon SY, Walters ET, and others. 2013. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J Pain 14(10):1031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Bu J, Wu X, Deng L, Chi M, Ma C, and others. 2020. a. Upregulation of µ-opioid receptor in the rat spinal cord contributes to the α2-adrenoceptor agonist dexmedetomidine-induced attenuation of chronic morphine tolerance in cancer pain. J Pain Res. 13:2617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. 2020. b. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol. 11:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Modur V, Carayannopoulos LN, Laterza OF. 2015. Biomarkers in pharmaceutical research. Clin Chem 61(11):1343–53. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, and others. 2006. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9(2):268–75. [DOI] [PubMed] [Google Scholar]