Abstract
Synthetic biology is a relatively new field of science that combines aspects of biology and engineering to create novel tools for the construction of biological systems. Using tools within synthetic biology, stem cells can then be reprogrammed and differentiated into a specified cell type. Stem cells have already proven to be largely beneficial in many different therapies and have paved the way for tissue engineering and regenerative medicine. Although scientists have made great strides in tissue engineering, there still remain many questions to be answered in regard to regeneration. Presented here is an overview of synthetic biology, common tools built within synthetic biology, and the way these tools are being used in stem cells. Specifically, this review focuses on how synthetic biologists engineer genetic circuits to dynamically control gene expression while also introducing emerging topics such as genome engineering and synthetic transcription factors. The findings mentioned in this review show the diverse use of stem cells within synthetic biology and provide a foundation for future research in tissue engineering with the use of synthetic biology tools. Overall, the work done using synthetic biology in stem cells is in its early stages, however, this early work is leading to new approaches for repairing diseased and damaged tissues and organs, and further expanding the field of tissue engineering.
Summary
Stem cells serve as a repair system for the body and hold tremendous promise for replacing and/or regenerating tissues damaged by disease and injury.
Stem cells tightly control their gene expression to either self-renew, or differentiate into more specialized cell types.
Genetic circuits built by synthetic biologists offer a platform technology to regulate gene expression patterns in stem cells to drive their cell fate.
Coupling synthetic biology with stem cells will help to facilitate more robust tissue engineering outcomes.
Introduction
Synthetic biology offers a platform of technologies to unlock the potential to engineer cells as custom-made therapies. A cell’s ability to proliferate, migrate, or differentiate is regulated by various mechanisms including environmental factors, in addition to the level of activation and silencing of various genes including transcription factors. In recent years, synthetic biology has significantly advanced the design of genetic circuits to offer unique expression patterns in mammalian cells [1–16]. This is particularly exciting for engineering stem cells since dynamic control of gene expression patterns is thought to improve differentiation outcomes, which play an important role in the fields of cell therapy, tissue engineering, and regenerative medicine. This review provides a brief introduction to stem cells and synthetic biology. We also describe studies that have implemented synthetic biology in stem cells and discuss their impact on tissue engineering and regenerative medicine.
Stem cells
Stem cells serve as a repair system for the body and therefore hold tremendous promise for replacing and/or regenerating tissues damaged by disease and injury. Stem cells are defined as cells that have the capacity to differentiate into more mature cells, in addition to having the ability to self-renew (Figure 1). They also have many levels of cell potency, which is defined as the cell’s ability to differentiate into other cell types. A greater cell potency indicates a larger number of cell types that a stem cell can become. When cells terminally differentiate, they exit the cell cycle and mature into specialized functional cells of tissues (Figure 1) [17].
Figure 1. Stem cells.
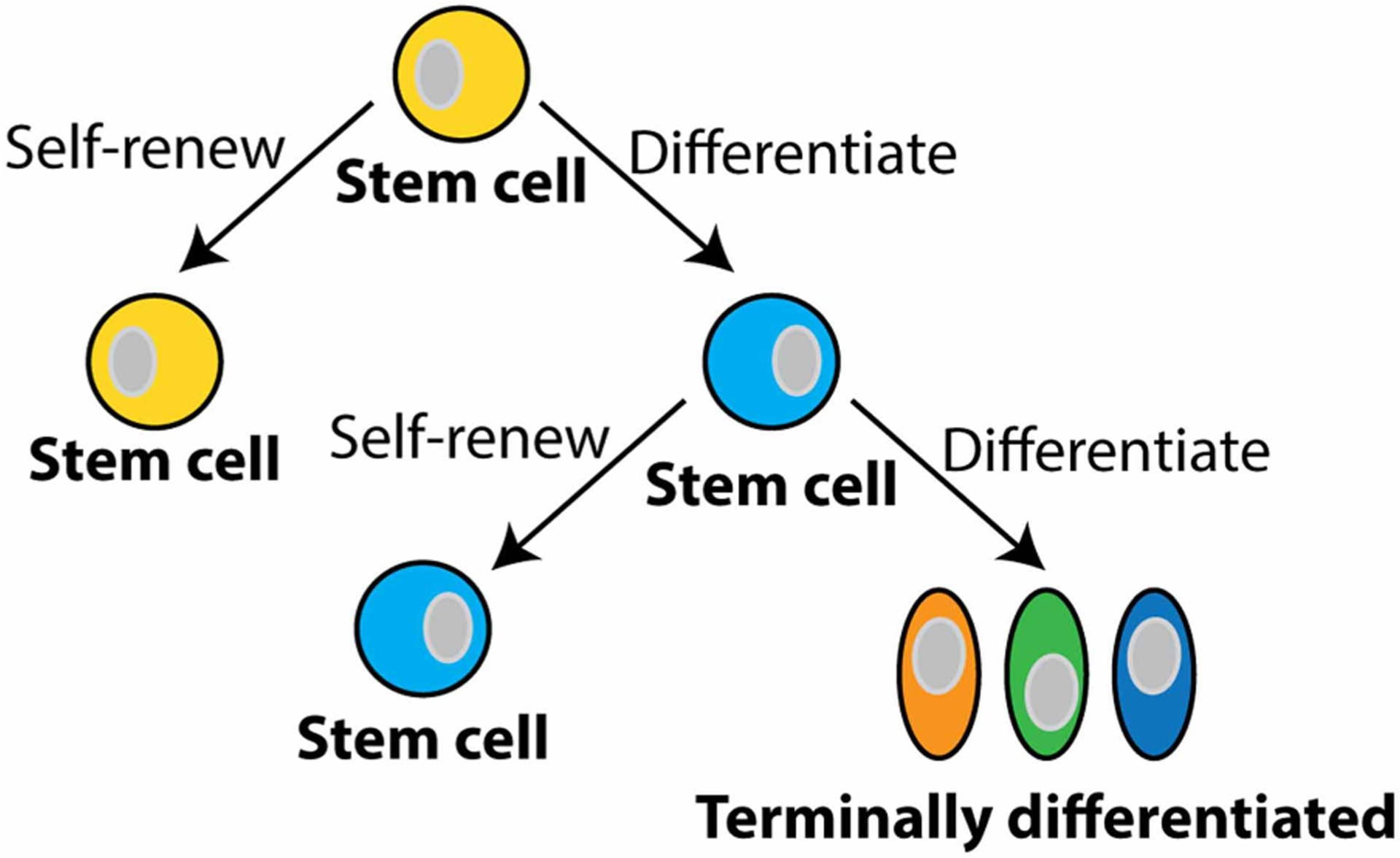
Stem cells are defined as cells that can differentiate into more specialized cells and also self-renew themselves (yellow and light blue cells). Stem cells have many levels of cell potency, which is the cell’s ability to differentiate into other cell types. A greater cell potency indicates a larger number of cell types that a stem cell can become (e.g. the yellow stem cell has more potency than the light blue stem cell). As cells differentiate, they lose their potency. When cells terminally differentiate, they exit the cell cycle and mature into specialized functional cells of tissues.
Pluripotent stem cells
With the exception of the bacteria that make up the gut microbiome, all cells in the human body originate from embryonic stem (ES) cells [18,19]. One of the first major events during embryonic development of vertebrate animals, is the specification of the three embryonic germ layers: ectoderm, mesoderm, and endoderm [20–22]. These germ layers give rise to multipotent stem cells that have the potential to differentiate into specific tissue lineages (Figure 2). The discovery that pluripotent ES cells can be harvested from the inner cell mass of an early stage pre-implanted embryo, be grown in culture in the laboratory and have the capacity to generate any cell type in the body, offered a controversial promise that these cells could be used as replacement cells and/or for generating organs and tissues for treating many different diseases and injuries. However, the more recent discovery that just 4 transcription factors can override previously made cell fate decisions to become pluripotent (Figure 2) has circumvented much of the controversy surrounding pluripotent stem cell research [23–31]. Moreover, iPS cells provide patient-specific models of disease that can be used for drug screening [32–38]. These induced pluripotent stem (iPS) cells are powerful tools that have opened the door to an unlimited source of any type of human cell needed for many therapeutic purposes.
Figure 2. Pluripotent stem cells.
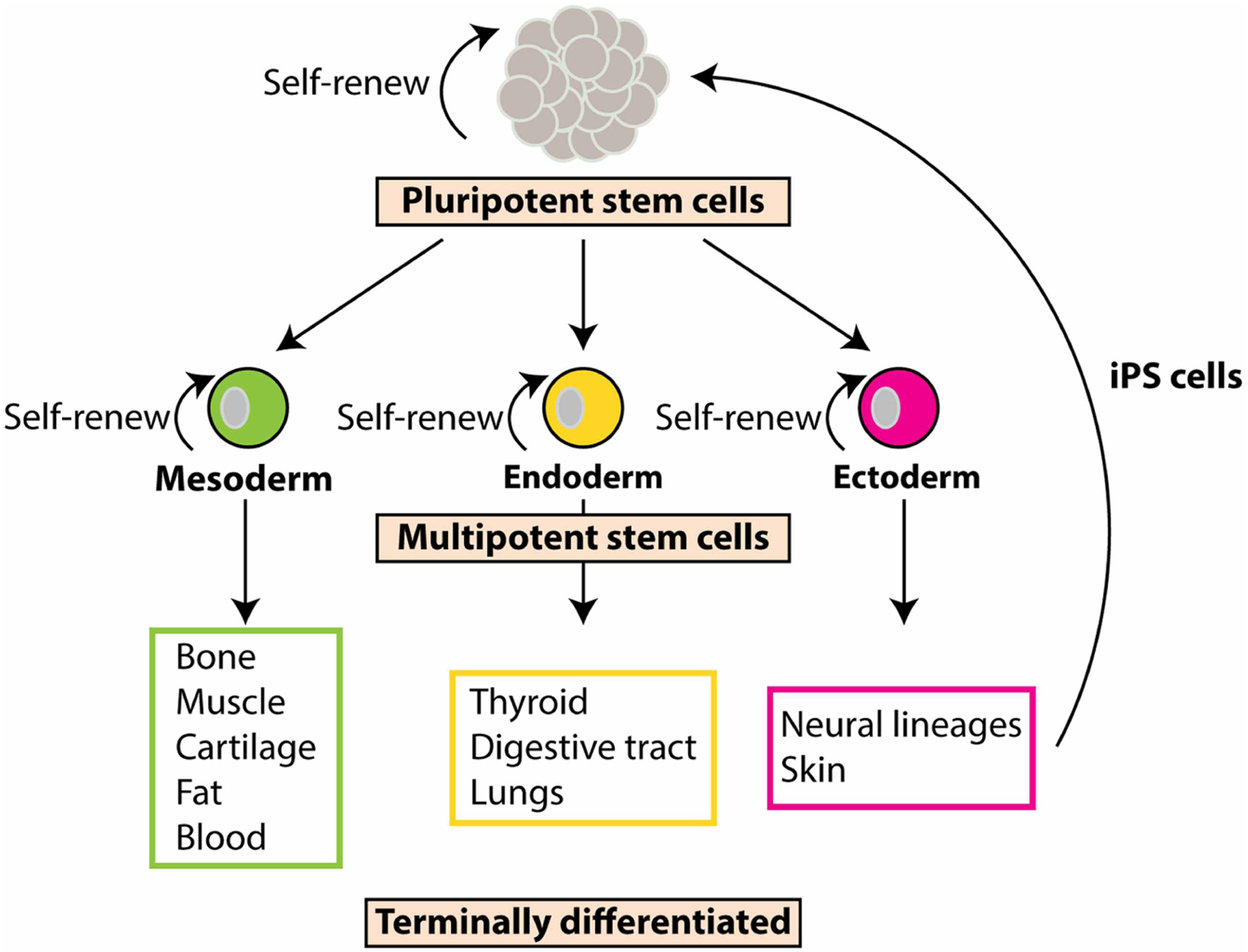
Pluripotent stem cells are undifferentiated cells with the potential to become any cell in the body. These special stem cells are able to form all three germ layer cells: the mesoderm (green cell), endoderm (yellow cell), and ectoderm (pink cell). These cell types are multipotent stem cells. They are somewhat differentiated, but still have the capabilities to differentiate into a more specific cell type. Once multipotent stem cells further differentiate, they are known as terminally differentiated and can no longer self-renew. Induced pluripotent stem cells (iPS cells), start out as terminally differentiated cells, most often skin cells from the ectoderm. The skin cells are then reprogrammed using transcription factors to revert them back into pluripotent stem cells, hence the name ‘induced’ pluripotent stem cells.
Adult stem cells
Adult stem cells are multipotent stem cells that are found among differentiated cells in a tissue or organ. As stem cells, they can self-renew themselves and differentiate into other specialized cell types of tissues or organs (Figure 2), however they have relatively restricted potency; that is, their ability to differentiate into specific cell types is generally limited to the type of tissue in which the adult stem cell resides. Therefore, it is believed that the primary role of adult stem cells is to maintain and repair the tissue in which they are found. The bone marrow has been a site of extensive research because of the two adult stem cells located within it: hematopoietic stem cells and mesenchymal stem cells. Hematopoietic stem cells (HSCs) are responsible for making all of the cells of the blood system, that is, all of the immune cells in addition to the red blood cells and platelets [39–45]. Mesenchymal stem cells (MSCs) were originally identified as cells capable of differentiating into mature cells of several mesenchymal tissues such as bone, muscle, tendon, cartilage, and fat [46–54]; however more recently, studies have shown that MSCs also play a supporting role when growing HSCs in culture, in addition to having unique immunomodulatory properties that make them an important cell type for the repair of damaged and diseased tissues [51,55–67]. Indeed, progress with enlisting adult stem cells for the purpose of regenerating tissues has been a primary focus for many scientists in the stem cell community because these cells are more likely to select the necessary gene expression patterns that are consistent with the more specialized cells.
Altogether, both pluripotent and adult stem cells continue to enhance our understanding of development, disease and drug screening, and provide the cells for tissue replacement, and regeneration [68–76]. Despite these advances, challenges that exist with using stem cells for therapeutic utilization include obtaining large enough numbers of cells for particular applications, producing homogeneous cell populations when needed, along with proper cell and tissue patterning, morphogenesis, and the directed differentiation of these cells to desired tissue outcomes.
Synthetic biology
Synthetic biologists use engineering approaches to build genetic circuits that enable the predictable programming of new functions into cells. This is done by rationally designing individual gene expression parts to engineer and assemble functional genetic devices to perform defined functions inside or outside of a cell [77–91]. These functions may include variations in sensing of environmental conditions [92,93], light [94–102], as well as producing custom proteins and/or changing the expression level of proteins [6,14,103,104].
By piecing together individual functional gene regulatory units, synthetic biologists create genetic circuits that are capable of reprogramming cells. Traditionally, the design of genetic circuits included a trigger element that was usually a small molecule, or different wavelengths of light that act on regulator proteins within the circuit (Figure 3A). For example, the bacterial repressor proteins, LacI and TetR have been extensively used and shown to offer inducible transcriptional control by placing the proteins’ binding domains within promoter regions (Figure 3B). By fusing these regulatory proteins to either a transcriptional repressor domain (e.g. KRAB), or an activator domain (e.g. VP16 or VP64), they can function to repress gene expression or activate gene expression, respectively. The effects of the LacI and the TetR regulatory proteins can be reversed by adding the small molecule inducers, isopropyl β-d-1-thiogalactopyranoside (IPTG) and tetracycline, respectively (Figure 3C). For example, in the case of the LacI repressor proteins, the LacI proteins bind to lacO binding sites that have been placed in the promoter that regulates a downstream gene of interest (GOI). When LacI repressor proteins bind, the proteins block the binding sites for RNA polymerase, the enzyme responsible for starting transcription, resulting in the repression of the downstream gene. Upon adding IPTG, the LacI repressor proteins undergo a conformational change and can no longer bind to the lacO binding sites, enabling RNA polymerase to bind to the DNA and transcribe the GOI. Genetic parts like the LacI and TetR systems have been regularly used for the construction of genetic circuits.
Figure 3. Building genetic circuits.

(A) Genetic circuits are built by assembling genetic parts that can be induced by either (i) small molecules or (ii) light. (B) Traditional approaches to building genetic circuits consist of implementing repressor proteins (blue circles) that are transcribed and translated and bind to their respective binding sites (blue squares) that are placed in or close to the promoter. When the repressor proteins bind to their respective binding sites, preventing RNA polymerase from sitting down and transcribing the downstream gene of interest (GOI, green box). (C) Adding the inducer small molecule, IPTG, binds to the LacI repressor proteins, causing a conformational change in their structure, and they can no longer bind to their binding sites. This opens up the DNA strand for RNA polymerase to bind, and transcription of the downstream GOI ensues. (D) The genetic toggle switch was built by assembling genetic parts to create a genetic circuit. Here, each promoter is inhibited by the repressor that is transcribed by the opposing promoter. The bistability of the toggle switch arises from the mutually inhibitory arrangement of the repressor genes, and the state of the switch can be flipped by adding the appropriate inducer.
The inception of synthetic biology was put forth with the introduction of the genetic toggle switch [103] and the repressilator [104] in bacteria where individual regulatory genetic parts were assembled into genetic circuits to reprogram cells with novel functions. The genetic toggle switch behaves as an on/off switch, and the repressilator implements oscillations of gene expression in cells. Specifically, the genetic toggle switch is a bistable switch that has two stable equilibrium states where each promoter is inhibited by the repressor that is transcribed by the opposing promoter. The bistability of the toggle switch arises from the mutually inhibitory arrangement of the repressor genes, and the state of the switch can be flipped by adding the appropriate inducer (Figure 3D). Synthetic biologists also demonstrated that it is possible to use genetic parts to build gene circuits for programming cells to perform Boolean logic operations upon induction with small molecules that endow cells to perform programmed decision-making capabilities [86,105–111]. Small molecule gene expression systems are the most common genetic parts that synthetic biologists use to engineer gene circuits. Their assembly and function have been extensively reviewed elsewhere [11,16,112–119].
Synthetic biologists have also demonstrated that prokaryotic genetic parts (e.g. repressors and their respective binding sites) can be used to build genetic circuits that function in mammalian cells. However, mammalian cells are more difficult than bacteria to engineer due to the complexity of eukaryotic cell signaling. Despite this challenge, synthetic biologists have successfully engineered genetic circuits to function in mammalian cells by combining prokaryotic and eukaryotic genetic parts [6,120], and constructing daisy-chain interconnections of individual transcription control circuits [121,122] that program cells to display tight levels of gene control that can be used to reprogram the cells to perform specific functions.
Genome engineering and controlling cell fate
Many of the genetic circuits built utilize transcription factors and their respective binding sites. Transcription factors are proteins that regulate gene transcription by binding to specific areas of DNA within the cellular genome and play an important role in stem cell identity and differentiation. They translate intracellular and extracellular signals into dynamic patterns of gene expression that drive stem cell differentiation and define cellular phenotype [90,123,124]. Modeling the natural gene expression patterns during development, it has been demonstrated that the overexpression of specific transcription factors can reprogram cells to obtain desired cellular outcomes [125]. A classic example of this is the reprogramming of adult cells into iPS cells through the overexpression of Oct4, Sox2, Klf4, and cMyc transcription factors [27,28]. While the overexpression of these transcription factors has paved the way for producing tissue-specific cell types, the differentiation of iPS cells into desired adult cell types and tissues remains difficult due to epigenetic memory, and the requirement of spatial and temporal control of gene expression patterns of lineage-specific transcription factors [31].
DNA recombinases
Site-specific DNA recombinase technology was the first widely used technique for engineering the mammalian genome. This technology is based on recombinase enzymes that selectively recognize two target sections of DNA, that have been previously inserted, cutting out the DNA between these target sections, and gluing the strands back together [126]. This approach has been used by molecular biologists for years, specifically for the generation of transgenic and knockout mice. On the other hand, synthetic biologists have used site-specific DNA recombinase technology to build genetic circuits capable of programming cells as memory devices for recording cellular events, performing mathematical computations, in addition to higher-order disease identification [127–134]. Using DNA recombinase technology as a platform, Boolean Logic and Arithmetic through DNA Excision (BLADE) technology was created. BLADE was shown to function in mammalian cells and has six programmed inputs to allow for 16 logic operations [135]. This type of programmed circuitry in cells can be used to custom build cells for targeted therapeutic applications and directing stem cell differentiation.
CRISPR
More recently, it was discovered that DNA nucleases could be programmed to bind to and cleave DNA sequences without the recognition sequences already present in the genome that is required for site-specific DNA recombinase technology. Zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR) are powerful genetic tools that enable precise genetic modifications by inducing targeted DNA breaks that stimulate cellular DNA repair mechanisms that can result in deletions or insertions of the original DNA sequence [136–141]. An alternative to double-stranded breaks in the DNA, targeted genome editing can be done using a D10A nickase Cas endonuclease, cytidine deaminase, and a DNA uracil glycosylase inhibitor [142]. Altogether, these components are capable of introducing a mutation into the DNA by converting cytosine to thymine. To report base editing activity within a cell population, Transient Reporter for Editing Enrichment (TREE) was developed [143], an assay that allows for the real-time, florescent-based identification and isolation of base-edited cell populations. This was done by engineering a blue florescent protein (BFP) variant to switch and express green fluorescent protein (GFP) after the targeted modification of converting a cytosine to thymine occurs. TREE was demonstrated to work in human iPS cells, which enabled the enrichment of edited cells. Since the discovery of CRISPR, methods for improving the targeting of Cas9 to specific locations in the genome continue to advance this technology [144–147].
Synthetic transcription factors
Engineering synthetic transcription factors (Syn-TFs) is a novel approach for targeting endogenous cellular networks within cells to modulate gene expression at specific locations within the genome by binding to DNA sequences. These Syn-TFs are made using DNA domains of one of several well-established genetic parts known for their ability to target specific sequences, such as zinc finger (ZF) proteins, transcription activator-like effectors (TALEs), and the clustered regulatory interspaced short palindromic repeats (CRISPR)/Cas9 system where the Cas9 protein has been engineered to remove the nuclease activity (dCas9) [119,136,148–150]. These proteins can be engineered to target genomic regulatory elements that either suppress or activate expression at a specific DNA sequence by fusing gene regulator domains to the synthetic transcription factors. Syn-TFs have been instrumental in studying endogenous DNA sequences to better understand the epigenetic landscapes of cells, for reprogramming cells to obtain desired cell types [151], and to study the fundamental genomic molecular mechanisms that dictate health and behavior of cells.
Synthetic biology, stem cells, and tissue engineering
Efforts in synthetic biology are currently underway to engineer stem cells with genetic circuits that dynamically control gene expression for the purpose of driving stem cell fate decisions. Recently, a new genetic circuit was engineered by assembling genetic parts from the bacterial LacI repressor system and the bread mold, Neurospora crassa, that functions as an orthogonal genetic switch in mammalian cells [120]. Specifically, the metabolic cluster of regulatory genes, called the Q system, was optimized and moved to mammalian cells. In this genetic circuit, upon the QF transcription factor binding to its QUAS binding sites, gene expression of the downstream gene is turned on. This genetic switch was demonstrated to dynamically control gene expression in pluripotent stem cells. Additional studies have been reported of genetic circuits improving cell fate decisions by engineering a band-pass filter to dynamically control three key transcription factors in pancreatic progenitor stem cells to enable the timely coordination of their expression to produce a homogeneous population of cells that produce insulin [152]. Another study emphasized the need for temporal regulation of gene expression during cell fate decisions with the pulsing of a key transcription factor [153]. In this study, the early pulsing of the transcription factor, Gata6, in iPS cells initiated the formation of a complex three-dimensional multicellular tissue construct that displayed liver tissue phenotypes. All in all, to improve cell fate outcomes, studies demonstrate the need to dynamically control transcription factors throughout differentiation. Having the tools and understanding for directing cell fate decisions will enable the translation of stem cell therapies to clinical practices.
The primary goal of tissue engineering is to repair and regenerate diseased and damaged tissues. While remarkable progress has been made in understanding tissue repair and regeneration from endogenous adult stem cells, it still remains unexplained why mammals have a tendency for imperfect healing and scarring rather than regeneration [154]. One significant difference between embryonic tissue development and repairing injured and diseased tissue is that inflammation is usually present with a disease and after an injury. Studies have shown that the immune system plays a central role in tissue repair and regeneration [155,156]. While an immune system response to tissue injury is essential in determining the recovery time and the outcome of the healing process, a prolonged inflammatory environment for stem cells can drastically change the outcome of the tissue healing process. For example, a prolonged exposure to an inflammatory environment can lead to incomplete healing and scar formation, whereas a tightly controlled immune response will result in complete regeneration where the tissue has total repair and restoration of function. To address this, a synthetic promoter was designed and built to contain five NF-κB recognition sequences and was placed upstream of the gene that encodes for interleukin-1 receptor antagonist (IL-1Ra), an anti-inflammatory protein [157]. In cells, the NF-κB pathway naturally controls the transcription of potent inflammatory cytokines that are responsible for activating inflammatory responses (Figure 4A). An iPS cell line with the synthetic promoter was created and was shown to possess self-regulating and attenuating inflammation properties in vitro with the controlled release of anti-inflammatory molecules. Specifically, iPS cells were virally transduced with the engineered synthetic inducible promoter driving the expression of IL-1Ra, and differentiated into chondrocytes, the cells that make cartilage tissue (Figure 4B). Repairing cartilage is difficult since it is not vascularized, leading to a lack of natural regeneration capabilities, along with a harsh inflammatory response that usually accompanies damaged and diseased tissues. The resulting engineered cartilage tissue is therefore endowed with the ability to sense and respond to inflammatory stimuli by producing anti-inflammatory proteins in an autoregulatory fashion. This study suggests that when the engineered cells are implanted in an animal, it will have the capacity to sense and respond to inflammatory stimuli and lead to improved tissue regeneration (Figure 4C).
Figure 4. Engineered cartilage.

(A) The natural cellular response to inflammatory cytokines, like IL-1. IL-1 binds to the IL-1 receptor, which activates the NF-κB pathway that turns on the transcription of potent inflammatory cytokines that are responsible for activating inflammatory responses (red background). (B) Engineered cells with a synthetic promoter containing five NF-κB recognition sequences upstream of interleukin-1 receptor antagonist (IL-1Ra) was integrated into the genome of iPS cells (dotted line in DNA). In these engineered cells, when IL-1 proteins bind to their receptors, rather than inflammatory cytokines being produced, these cells produce IL-1Ra, a protein that blocks the IL-1 receptors from binding to the IL-1 protein and therefore prevents inflammatory cytokines from being produced (green background). (C) These engineered cells can be implanted into the knee of an animal experiencing inflammation (red background) to aide in the immune response by sensing and responding to inflammatory stimuli and producing anti-inflammatory proteins in an autoregulatory fashion (green background).
Conclusions
Stem cells serve as a repair system for the body and therefore hold tremendous promise for replacing and/or regenerating tissues damaged by disease and injury. However, the molecular mechanisms of tissue repair have yet to be realized, precluding the development of go-to clinical therapies. Furthermore, the complex relationship between tissue regeneration and the immune system during wound healing has further delayed clinical advances. A primary goal in synthetic biology is to engineer genetic circuits that exert fine control over cell behavior by endowing cells with complex functions like sensing and responding. Recent studies have capitalized on the utility of using synthetic biology approaches to drive stem cell differentiation into desired lineages, and to engineer synthetic tissues that mediate the inflammatory environment for improved tissue repair.
Acknowledgements
I would like to thank my amazing team of undergraduate students who worked very hard for this publication.
Funding
We gratefully acknowledge the funding from the University of Utah startup funds, the National Science Foundation CAREER Program (CBET-1554017), the Office of Naval Research Young Investigator Program (N00014-16-1-3012), and the National Institute of Health Trailblazer Award (1R21EB025413-01).
Abbreviations
- BFP
blue florescent protein
- BLADE
Boolean Logic and Arithmetic through DNA Excision
- CRISPR
clustered regulatory interspaced short palindromic repeats
- ES
embryonic stem
- GFP
green fluorescent protein
- GOI
gene of interest
- HSC
hematopoietic stem cell
- iPS
induced pluripotent stem
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- MSC
mesenchymal stem cell
- Syn-TFs
synthetic transcription factors
- TALEs
transcription activator-like effectors
- ZF
zinc finger proteins
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Aubel D and Fussenegger M (2010) Mammalian synthetic biology–from tools to therapies. Bioessays 32, 332–345 10.1002/bies.200900149 [DOI] [PubMed] [Google Scholar]
- 2.Auslander S, Auslander D, Muller M, Wieland M and Fussenegger M (2012) Programmable single-cell mammalian biocomputers. Nature 487, 123–127 10.1038/nature11149 [DOI] [PubMed] [Google Scholar]
- 3.Auslander S and Fussenegger M (2016) Engineering gene circuits for mammalian cell-based applications. Cold Spring Harb. Perspect. Biol 8, 1–17 10.1101/cshperspect.a023895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacchus W, Lang M, El-Baba MD, Weber W, Stelling J and Fussenegger M (2012) Synthetic two-way communication between mammalian cells. Nat. Biotechnol 30, 991–996 10.1038/nbt.2351 [DOI] [PubMed] [Google Scholar]
- 5.Black JB, Perez-Pinera P and Gersbach CA (2017) Mammalian synthetic biology: engineering biological systems. Annu. Rev. Biomed. Eng 19, 249–277 10.1146/annurev-bioeng-071516-044649 [DOI] [PubMed] [Google Scholar]
- 6.Deans TL, Cantor CR and Collins JJ (2007) A tunable genetic switch based on RNAi and repressor proteins for regulating gene expression in mammalian cells. Cell 130, 363–372 10.1016/j.cell.2007.05.045 [DOI] [PubMed] [Google Scholar]
- 7.Gaber R, Lebar T, Majerle A, Ster B, Dobnikar A, Bencina M et al. (2014) Designable DNA-binding domains enable construction of logic circuits in mammalian cells. Nat. Chem. Biol 10, 203–208 10.1038/nchembio.1433 [DOI] [PubMed] [Google Scholar]
- 8.Greber D and Fussenegger M (2007) Mammalian synthetic biology: engineering of sophisticated gene networks. J. Biotechnol 130, 329–345 10.1016/j.jbiotec.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 9.Hemphill J and Deiters A (2013) DNA computation in mammalian cells: microRNA logic operations. J. Am. Chem. Soc 135, 10512–10518 10.1021/ja404350s [DOI] [PubMed] [Google Scholar]
- 10.Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L et al. (2013) Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 10.1038/nature12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lienert F, Lohmueller JJ, Garg A and Silver PA (2014) Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat. Rev. Mol. Cell Biol 15, 95–107 10.1038/nrm3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohmueller JJ, Armel TZ and Silver PA (2012) A tunable zinc finger-based framework for Boolean logic computation in mammalian cells. Nucleic Acids Res. 40, 5180–5187 10.1093/nar/gks142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slomovic S and Collins JJ (2015) DNA sense-and-respond protein modules for mammalian cells. Nat. Methods 12, 1085–1090 10.1038/nmeth.3585 [DOI] [PubMed] [Google Scholar]
- 14.Tigges M, Marquez-Lago TT, Stelling J and Fussenegger M (2009) A tunable synthetic mammalian oscillator. Nature 457, 309–312 10.1038/nature07616 [DOI] [PubMed] [Google Scholar]
- 15.Weber W and Fussenegger M (2009) Engineering of synthetic mammalian gene networks. Chem. Biol 16, 287–297 10.1016/j.chembiol.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Ye H and Fussenegger M (2014) Synthetic therapeutic gene circuits in mammalian cells. FEBS Lett. 588, 2537–2544 10.1016/j.febslet.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 17.Vizeacoumar FJ, Chong Y, Boone C and Andrews BJ (2009) A picture is worth a thousand words: genomics to phenomics in the yeast Saccharomyces cerevisiae. FEBS Lett. 583, 1656–1661 10.1016/j.febslet.2009.03.068 [DOI] [PubMed] [Google Scholar]
- 18.Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP et al. (2004) Derivation of embryonic stem-cell lines from human blastocysts. N. Engl. J. Med 350, 1353–1356 10.1056/NEJMsr040330 [DOI] [PubMed] [Google Scholar]
- 19.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP et al. (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 10.1016/j.cell.2005.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagers AJ and Weissman IL (2004) Plasticity of adult stem cells. Cell 116, 639–648 10.1016/S0092-8674(04)00208-9 [DOI] [PubMed] [Google Scholar]
- 21.Wells JM and Melton DA (1999) Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol 15, 393–410 10.1146/annurev.cellbio.15.1.393 [DOI] [PubMed] [Google Scholar]
- 22.Deans TL and Elisseeff JH (2009) Stem cells in musculoskeletal engineered tissue. Curr. Opin. Biotechnol 20, 537–544 10.1016/j.copbio.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K et al. (2008) Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321, 699–702 10.1126/science.1154884 [DOI] [PubMed] [Google Scholar]
- 24.Ariyachet C, Tovaglieri A, Xiang G, Lu J, Shah MS, Richmond CA et al. (2016) Reprogrammed stomach tissue as a renewable source of functional beta cells for blood glucose regulation. Cell Stem Cell 18, 410–421 10.1016/j.stem.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JS, Choi HW, Choi S and Do JT (2011) Reprogrammed pluripotent stem cells from somatic cells. Int. J. Stem Cells 4, 1–8 10.15283/ijsc.2011.4.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raab S, Klingenstein M, Liebau S and Linta L (2014) A comparative view on human somatic cell sources for iPSC generation. Stem Cells Int. 2014, 768391 10.1155/2014/768391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K and Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka S (2007) Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1, 39–49 10.1016/j.stem.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 30.Yeo NC, Chavez A, Lance-Byrne A, Chan Y, Menn D, Milanova D et al. (2018) An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods 15, 611–616 10.1038/s41592-018-0048-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Healy CP and Deans TL (2019) Genetic circuits to engineer tissues with alternative functions. J. Biol. Eng 13, 39 10.1186/s13036-019-0170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giacomelli E, Mummery CL and Bellin M (2017) Human heart disease: lessons from human pluripotent stem cell-derived cardiomyocytes. Cell. Mol. Life Sci 74, 3711–3739 10.1007/s00018-017-2546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsa E, Ahrens JH and Wu JC (2016) Human induced pluripotent stem cells as a platform for personalized and precision cardiovascular medicine. Physiol. Rev 96, 1093–1126 10.1152/physrev.00036.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blau HM and Daley GQ (2019) Stem cells in the treatment of disease. N. Engl. J. Med 380, 1748–1760 10.1056/NEJMra1716145 [DOI] [PubMed] [Google Scholar]
- 35.Choi SM, Kim Y, Shim JS, Park JT, Wang RH, Leach SD et al. (2013) Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology 57, 2458–2468 10.1002/hep.26237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T et al. (2012) Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med 4, 145ra104 10.1126/scitranslmed.3004052 [DOI] [PubMed] [Google Scholar]
- 37.Jin ZB, Okamoto S, Osakada F, Homma K, Assawachananont J, Hirami Y et al. (2011) Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS ONE 6, e17084 10.1371/journal.pone.0017084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoo N, Baba S, Kaichi S, Niwa A, Mima T, Doi H et al. (2009) The effects of cardioactive drugs on cardiomyocytes derived from human induced pluripotent stem cells. Biochem. Biophys. Res. Commun 387, 482–488 10.1016/j.bbrc.2009.07.052 [DOI] [PubMed] [Google Scholar]
- 39.Lemischka IR, Raulet DH and Mulligan RC (1986) Developmental potential and dynamic behavior of hematopoietic stem cells. Cell 45, 917–927 10.1016/0092-8674(86)90566-0 [DOI] [PubMed] [Google Scholar]
- 40.Mendelson A and Frenette PS (2014) Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med 20, 833–846 10.1038/nm.3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosaad YM (2014) Hematopoietic stem cells: an overview. Transfus. Apher. Sci 51, 68–82 10.1016/j.transci.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 42.Orkin SH and Zon LI (2008) Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 10.1016/j.cell.2008.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park D, Sykes DB and Scadden DT (2012) The hematopoietic stem cell niche. Front Biosci. 17, 30–39 10.2741/3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Q and Frenette PS (2018) Niches for hematopoietic stem cells and their progeny. Immunity 48, 632–648 10.1016/j.immuni.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu VW and Scadden DT (2016) Hematopoietic stem cell and its bone marrow niche. Curr. Top. Dev. Biol 118, 21–44 10.1016/bs.ctdb.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamberlain G, Fox J, Ashton B and Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25, 2739–2749 10.1634/stemcells.2007-0197 [DOI] [PubMed] [Google Scholar]
- 47.Chanda D, Kumar S and Ponnazhagan S (2010) Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J. Cell Biochem 111, 249–257 10.1002/jcb.22701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehninger A and Trumpp A (2011) The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med 208, 421–428 10.1084/jem.20110132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gigante A, Manzotti S, Bevilacqua C, Orciani M, Di Primio R and Mattioli-Belmonte M (2008) Adult mesenchymal stem cells for bone and cartilage engineering: effect of scaffold materials. Eur. J. Histochem 52, 169–174 10.4081/1208 [DOI] [PubMed] [Google Scholar]
- 50.Jackson L, Jones DR, Scotting P and Sottile V (2007) Adult mesenchymal stem cells: differentiation potential and therapeutic applications. J. Postgrad. Med 53, 121–127 10.4103/0022-3859.32215 [DOI] [PubMed] [Google Scholar]
- 51.Murphy MB, Moncivais K and Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med 45, e54 10.1038/emm.2013.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pittenger MF (2008) Mesenchymal stem cells from adult bone marrow. Methods Mol. Biol 449, 27–44 10.1007/978-1-60327-169-1_2 [DOI] [PubMed] [Google Scholar]
- 53.Schipani E and Kronenberg HM (2008) Adult mesenchymal stem cells. Harvard Stem Cell Institute, Cambridge: [PubMed] [Google Scholar]
- 54.Spitkovsky D and Hescheler J (2008) Adult mesenchymal stromal stem cells for therapeutic applications. Minim. Invasive Ther. Allied Technol 17, 79–90 10.1080/13645700801969758 [DOI] [PubMed] [Google Scholar]
- 55.Caplan AI and Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9, 11–15 10.1016/j.stem.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caplan AI and Sorrell JM (2015) The MSC curtain that stops the immune system. Immunol. Lett 168, 136–139 10.1016/j.imlet.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 57.Aggarwal S and Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 10.1182/blood-2004-04-1559 [DOI] [PubMed] [Google Scholar]
- 58.Dai W, Hale SL and Kloner RA (2007) Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats. Regen. Med 2, 63–68 10.2217/17460751.2.1.63 [DOI] [PubMed] [Google Scholar]
- 59.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E et al. (2007) Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol 61, 219–227 10.1002/ana.21076 [DOI] [PubMed] [Google Scholar]
- 60.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H et al. (2005) Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med 11, 367–368 10.1038/nm0405-367 [DOI] [PubMed] [Google Scholar]
- 61.Karussis D, Kassis I, Kurkalli BG and Slavin S (2008) Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J. Neurol. Sci 265, 131–135 10.1016/j.jns.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 62.Li L, Zhang S, Zhang Y, Yu B, Xu Y and Guan Z (2009) Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol. Biol. Rep 36, 725–731 10.1007/s11033-008-9235-2 [DOI] [PubMed] [Google Scholar]
- 63.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA et al. (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uccelli A, Benvenuto F, Laroni A and Giunti D (2011) Neuroprotective features of mesenchymal stem cells. Best Pract. Res. Clin. Haematol 24, 59–64 10.1016/j.beha.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Yan H, Wang Z, Zhu L, Liu J and Guo Z (2012) Cotransplantation of allogeneic mesenchymal and hematopoietic stem cells in children with aplastic anemia. Pediatrics 129, e1612–e1615 10.1542/peds.2011-2091 [DOI] [PubMed] [Google Scholar]
- 66.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O et al. (2012) Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical sjogren syndrome. Blood 120, 3142–3151 10.1182/blood-2011-11-391144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao Y, Huang J, Geng Y, Qian H, Wang F, Liu X et al. (2015) Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS ONE 10, e0129164 10.1371/journal.pone.0129164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen IY, Matsa E and Wu JC (2016) Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat. Rev. Cardiol 13, 333–349 10.1038/nrcardio.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chong JJ and Murry CE (2014) Cardiac regeneration using pluripotent stem cells–progression to large animal models. Stem Cell Res. 13, 654–665 10.1016/j.scr.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A et al. (2018) Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol 36, 328–337 10.1038/nbt.4114 [DOI] [PubMed] [Google Scholar]
- 71.Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D et al. (2017) Global position paper on cardiovascular regenerative medicine. Eur. Heart J 38, 2532–2546 10.1093/eurheartj/ehx248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L et al. (2017) Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332 10.1038/nature24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A et al. (2018) Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol 71, 429–438 10.1016/j.jacc.2017.11.047 [DOI] [PubMed] [Google Scholar]
- 74.Nguyen PK, Rhee JW and Wu JC (2016) Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. 1, 831–841 10.1001/jamacardio.2016.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stern JH, Tian Y, Funderburgh J, Pellegrini G, Zhang K, Goldberg JL et al. (2018) Regenerating eye tissues to preserve and restore vision. Cell Stem Cell 23, 453 10.1016/j.stem.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 76.Stevens KR and Murry CE (2018) Human pluripotent stem cell-derived engineered tissues: clinical considerations. Cell Stem Cell 22, 294–297 10.1016/j.stem.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrianantoandro E, Basu S, Karig DK and Weiss R (2006) Synthetic biology: new engineering rules for an emerging discipline. Mol. Syst. Biol 2, 1–14 10.1038/msb4100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bashor CJ, Horwitz AA, Peisajovich SG and Lim WA (2010) Rewiring cells: synthetic biology as a tool to interrogate the organizational principles of living systems. Annu. Rev. Biophys 39, 515–537 10.1146/annurev.biophys.050708.133652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Callura JM, Cantor CR and Collins JJ (2012) Genetic switchboard for synthetic biology applications. Proc. Natl Acad. Sci. U.S.A 109, 5850–5855 10.1073/pnas.1203808109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cameron DE, Bashor CJ and Collins JJ (2014) A brief history of synthetic biology. Nat. Rev. Microbiol 12, 381–390 10.1038/nrmicro3239 [DOI] [PubMed] [Google Scholar]
- 81.Cheng AA and Lu TK (2012) Synthetic biology: an emerging engineering discipline. Annu. Rev. Biomed. Eng 14, 155–178 10.1146/annurev-bioeng-071811-150118 [DOI] [PubMed] [Google Scholar]
- 82.Collins J (2012) Synthetic biology: bits and pieces come to life. Nature 483, S8–10 10.1038/483S8a [DOI] [PubMed] [Google Scholar]
- 83.Collins JJ, Maxon M, Ellington A, Fussenegger M, Weiss R and Sauro H (2014) Synthetic biology: how best to build a cell. Nature 509, 155–157 10.1038/509155a [DOI] [PubMed] [Google Scholar]
- 84.Deans TL (2014) Parallel networks: synthetic biology and artificial intelligence. ACM J. Emerg. Technol. Comput. Syst 11, 21:21–21:11 10.1145/2667229 [DOI] [Google Scholar]
- 85.Deans TL, Grainger DW and Fussenegger M (2016) Synthetic biology: innovative approaches for pharmaceutics and drug delivery. Adv. Drug Deliv. Rev 105, 1–2 10.1016/j.addr.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 86.Dobrin A, Saxena P and Fussenegger M (2015) Synthetic biology: applying biological circuits beyond novel therapies. Integr. Biol 8, 409–430 10.1039/c5ib00263j [DOI] [PubMed] [Google Scholar]
- 87.Kitada T, DiAndreth B, Teague B and Weiss R (2018) Programming gene and engineered-cell therapies with synthetic biology. Science 359, eaad1067 10.1126/science.aad1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levskaya A, Chevalier AA, Tabor JJ, Simpson ZB, Lavery LA, Levy M et al. (2005) Synthetic biology: engineering Escherichia coli to see light. Nature 438, 441–442 10.1038/nature04405 [DOI] [PubMed] [Google Scholar]
- 89.Purnick PE and Weiss R (2009) The second wave of synthetic biology: from modules to systems. Nat. Rev. Mol. Cell Biol 10, 410–422 10.1038/nrm2698 [DOI] [PubMed] [Google Scholar]
- 90.Weisenberger MS and Deans TL (2018) Bottom-up approaches in synthetic biology and biomaterials for tissue engineering applications. J. Ind. Microbiol. Biotechnol 45, 599–614 10.1007/s10295-018-2027-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie M, Haellman V and Fussenegger M (2016) Synthetic biology-application-oriented cell engineering. Curr. Opin. Biotechnol 40, 139–148 10.1016/j.copbio.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 92.Antunes MS, Morey KJ, Smith JJ, Albrecht KD, Bowen TA, Zdunek JK et al. (2011) Programmable ligand detection system in plants through a synthetic signal transduction pathway. PLoS ONE 6, e16292 10.1371/journal.pone.0016292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng J, Jester BW, Tinberg CE, Mandell DJ, Antunes MS, Chari R et al. (2015) A general strategy to construct small molecule biosensors in eukaryotes. eLife 4, e10606 10.7554/eLife.10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fenno L, Yizhar O and Deisseroth K (2011) The development and application of optogenetics. Annu. Rev. Neurosci 34, 389–412 10.1146/annurev-neuro-061010-113817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim T, Folcher M, Doaud-El Baba M and Fussenegger M (2015) A synthetic erectile optogenetic stimulator enabling blue-light-inducible penile erection. Angew. Chem. Int. Ed. Engl 54, 5933–5938 10.1002/anie.201412204 [DOI] [PubMed] [Google Scholar]
- 96.Muller K and Weber W (2013) Optogenetic tools for mammalian systems. Mol. Biosyst 9, 596–608 10.1039/c3mb25590e [DOI] [PubMed] [Google Scholar]
- 97.Muller K, Zurbriggen MD and Weber W (2015) An optogenetic upgrade for the Tet-OFF system. Biotechnol. Bioeng 112, 1483–1487 10.1002/bit.25562 [DOI] [PubMed] [Google Scholar]
- 98.Olson EJ and Tabor JJ (2014) Optogenetic characterization methods overcome key challenges in synthetic and systems biology. Nat. Chem. Biol 10, 502–511 10.1038/nchembio.1559 [DOI] [PubMed] [Google Scholar]
- 99.Polstein LR, Juhas M, Hanna G, Bursac N and Gersbach CA (2017) An engineered optogenetic switch for spatiotemporal control of gene expression, cell differentiation, and tissue morphogenesis. ACS Synth. Biol 6, 2003–2013 10.1021/acssynbio.7b00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tischer D and Weiner OD (2014) Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol 15, 551–558 10.1038/nrm3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toettcher JE, Voigt CA, Weiner OD and Lim WA (2011) The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat. Methods 8, 35–38 10.1038/nmeth.f.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye H, Daoud-El Baba M, Peng RW and Fussenegger M (2011) A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332, 1565–1568 10.1126/science.1203535 [DOI] [PubMed] [Google Scholar]
- 103.Gardner TS, Cantor CR and Collins JJ (2000) Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 10.1038/35002131 [DOI] [PubMed] [Google Scholar]
- 104.Elowitz MB and Leibler S (2000) A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 10.1038/35002125 [DOI] [PubMed] [Google Scholar]
- 105.Bonnet J, Yin P, Ortiz ME, Subsoontorn P and Endy D (2013) Amplifying genetic logic gates. Science 340, 599–603 10.1126/science.1232758 [DOI] [PubMed] [Google Scholar]
- 106.Chin JW (2006) Programming and engineering biological networks. Curr. Opin. Struct. Biol 16, 551–556 10.1016/j.sbi.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 107.Miyamoto T, Razavi S, DeRose R and Inoue T (2013) Synthesizing biomolecule-based Boolean logic gates. ACS Synth. Biol 2, 72–82 10.1021/sb3001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moon TS, Lou C, Tamsir A, Stanton BC and Voigt CA (2012) Genetic programs constructed from layered logic gates in single cells. Nature 491, 249–253 10.1038/nature11516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mukherji S and van Oudenaarden A (2009) Synthetic biology: understanding biological design from synthetic circuits. Nat. Rev. Genet 10, 859–871 10.1038/nrg2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siuti P, Yazbek J and Lu TK (2013) Synthetic circuits integrating logic and memory in living cells. Nat. Biotechnol 31, 448–452 10.1038/nbt.2510 [DOI] [PubMed] [Google Scholar]
- 111.Way JC, Collins JJ, Keasling JD and Silver PA (2014) Integrating biological redesign: where synthetic biology came from and where it needs to go. Cell 157, 151–161 10.1016/j.cell.2014.02.039 [DOI] [PubMed] [Google Scholar]
- 112.Weber W and Fussenegger M (2011) Molecular diversity–the toolbox for synthetic gene switches and networks. Curr. Opin. Chem. Biol 15, 414–420 10.1016/j.cbpa.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 113.Horner M, Reischmann N and Weber W (2012) Synthetic biology: programming cells for biomedical applications. Perspect. Biol. Med 55, 490–502 10.1353/pbm.2012.0042 [DOI] [PubMed] [Google Scholar]
- 114.Horner M and Weber W (2012) Molecular switches in animal cells. FEBS Lett. 586, 2084–2096 10.1016/j.febslet.2012.02.032 [DOI] [PubMed] [Google Scholar]
- 115.Karlsson M and Weber W (2012) Therapeutic synthetic gene networks. Curr. Opin. Biotechnol 23, 703–711 10.1016/j.copbio.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 116.Weber W and Fussenegger M (2012) Emerging biomedical applications of synthetic biology. Nat. Rev. Genet 13, 21–35 10.1038/nrg3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Litcofsky KD, Afeyan RB, Krom RJ, Khalil AS and Collins JJ (2012) Iterative plug-and-play methodology for constructing and modifying synthetic gene networks. Nat. Methods 9, 1077–1080 10.1038/nmeth.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schwille P (2011) Bottom-up synthetic biology: engineering in a tinkerer’s world. Science 333, 1252–1254 10.1126/science.1211701 [DOI] [PubMed] [Google Scholar]
- 119.MacDonald IC and Deans TL (2016) Tools and applications in synthetic biology. Adv. Drug Deliv. Rev 105, 20–34 10.1016/j.addr.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 120.Fitzgerald M, Gibbs C, Shimpi AA and Deans TL (2017) Adoption of the Q transcriptional system for regulating gene expression in stem cells. ACS Synth. Biol 6, 2014–2020 10.1021/acssynbio.7b00149 [DOI] [PubMed] [Google Scholar]
- 121.Kramer BP, Weber W and Fussenegger M (2003) Artificial regulatory networks and cascades for discrete multilevel transgene control in mammalian cells. Biotechnol. Bioeng 83, 810–820 10.1002/bit.10731 [DOI] [PubMed] [Google Scholar]
- 122.Kiani S, Beal J, Ebrahimkhani MR, Huh J, Hall RN, Xie Z et al. (2014) CRISPR transcriptional repression devices and layered circuits in mammalian cells. Nat. Methods 11, 723–726 10.1038/nmeth.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Black JB and Gersbach CA (2018) Synthetic transcription factors for cell fate reprogramming. Curr. Opin. Genet. Dev 52, 13–21 10.1016/j.gde.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 124.Deans TL and Elisseeff JH (2010) The life of a cell: probing the complex relationships with the world. Cell Stem Cell 6, 499–501 10.1016/j.stem.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 125.Cherry AB and Daley GQ (2012) Reprogramming cellular identity for regenerative medicine. Cell 148, 1110–1122 10.1016/j.cell.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hartley JL, Temple GF and Brasch MA (2000) DNA cloning using in vitro site-specific recombination. Genome Res. 10, 1788–1795 10.1101/gr.143000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sheth RU and Wang HH (2018) DNA-based memory devices for recording cellular events. Nat. Rev. Genet 19, 718–732 10.1038/s41576-018-0052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ajo-Franklin CM, Drubin DA, Eskin JA, Gee EP, Landgraf D, Phillips I et al. (2007) Rational design of memory in eukaryotic cells. Genes Dev. 21, 2271–2276 10.1101/gad.1586107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burrill DR, Inniss MC, Boyle PM and Silver PA (2012) Synthetic memory circuits for tracking human cell fate. Genes Dev. 26, 1486–1497 10.1101/gad.189035.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burrill DR and Silver PA (2010) Making cellular memories. Cell 140, 13–18 10.1016/j.cell.2009.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Purcell O and Lu TK (2014) Synthetic analog and digital circuits for cellular computation and memory. Curr. Opin. Biotechnol 29C, 146–155 10.1016/j.copbio.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang C, Tibbitt MW, Basta L and Anseth KS (2014) Mechanical memory and dosing influence stem cell fate. Nat. Mater 13, 645–652 10.1038/nmat3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang L, Nielsen AA, Fernandez-Rodriguez J, McClune CJ, Laub MT, Lu TK et al. (2014) Permanent genetic memory with >1-byte capacity. Nat. Methods 11, 1261–1266 10.1038/nmeth.3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Friedland AE, Lu TK, Wang X, Shi D, Church G and Collins JJ (2009) Synthetic gene networks that count. Science 324, 1199–1202 10.1126/science.1172005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weinberg BH, Pham NTH, Caraballo LD, Lozanoski T, Engel A, Bhatia S et al. (2017) Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat. Biotechnol 35, 453–462 10.1038/nbt.3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gaj T, Gersbach CA and Barbas CF III (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 10.1016/j.tibtech.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE et al. (2013) CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 10.1016/j.cell.2013.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hsu PD, Lander ES and Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sander JD and Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol 32, 347–355 10.1038/nbt.2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Savic N and Schwank G (2016) Advances in therapeutic CRISPR/Cas9 genome editing. Transl. Res 168, 15–21 10.1016/j.trsl.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 141.He X, Tan C, Wang F, Wang Y, Zhou R, Cui D et al. (2016) Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 44, e85 10.1093/nar/gkw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Komor AC, Kim YB, Packer MS, Zuris JA and Liu DR (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 10.1038/nature17946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Standage-Beier K, Tekel SJ, Brookhouser N, Schwarz G, Nguyen T, Wang X et al. (2019) A transient reporter for editing enrichment (TREE) in human cells. Nucleic Acids Res., gkz713 10.1093/nar/gkz713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE et al. (2013) RNA-guided human genome engineering via Cas9. Science 339, 823–826 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schmid-Burgk JL, Honing K, Ebert TS and Hornung V (2016) CRISPaint allows modular base-specific gene tagging using a ligase-4-dependent mechanism. Nat. Commun 7, 12338 10.1038/ncomms12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ et al. (2016) In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 540, 144–149 10.1038/nature20565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Standage-Beier K, Brookhouser N, Balachandran P, Zhang Q, Brafman DA and Wang X (2019) RNA-guided recombinase-Cas9 fusion targets genomic DNA deletion and integration. CRISPR J. 2, 209–222 10.1089/crispr.2019.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Joung JK and Sander JD (2013) TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol 14, 49–55 10.1038/nrm3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li HL, Fujimoto N, Sasakawa N, Shirai S, Ohkame T, Sakuma T et al. (2015) Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Rep. 4, 143–154 10.1016/j.stemcr.2014.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF et al. (2011) A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol 29, 143–148 10.1038/nbt.1755 [DOI] [PubMed] [Google Scholar]
- 151.Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW et al. (2016) Rapid, Low-cost detection of Zika virus using programmable biomolecular components. Cell 165, 1255–1266 10.1016/j.cell.2016.04.059 [DOI] [PubMed] [Google Scholar]
- 152.Saxena P, Heng BC, Bai P, Folcher M, Zulewski H and Fussenegger M (2016) A programmable synthetic lineage-control network that differentiates human IPSCs into glucose-sensitive insulin-secreting beta-like cells. Nat. Commun 7, 11247 10.1038/ncomms11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guye P, Ebrahimkhani MR, Kipniss N, Velazquez JJ, Schoenfeld E, Kiani S et al. (2016) Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat. Commun 7, 10243 10.1038/ncomms10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Julier Z, Park AJ, Briquez PS and Martino MM (2017) Promoting tissue regeneration by modulating the immune system. Acta Biomater. 53, 13–28 10.1016/j.actbio.2017.01.056 [DOI] [PubMed] [Google Scholar]
- 155.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ et al. (2016) Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366–370 10.1126/science.aad9272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brunger JM, Zutshi A, Willard VP, Gersbach CA and Guilak F (2017) Genome engineering of stem cells for autonomously regulated, closed-loop delivery of biologic drugs. Stem Cell Rep. 8, 1202–1213 10.1016/j.stemcr.2017.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pferdehirt L, Ross AK, Brunger JM and Guilak F (2019) A synthetic gene circuit for self-regulating delivery of biologic drugs in engineered tissues. Tissue Eng. Part A 25, 809–820 10.1089/ten.tea.2019.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]


