Abstract
Logging-induced disturbance can be an important agent of change in tropical forests. Understanding the relative impacts of specific logging regimes on tree community structure is essential for forest management and biodiversity conservation. In this study, we assessed the response of tree community structure to selective and clear-cut logging in a moist semi-deciduous forest in Ghana. We quantified the diversity, composition, density and basal area of trees (diameter at breast height ≥5 cm) in 30 20 × 20 m plots in each of three forest management systems (selectively logged, clear-cut logged, old-growth). Our results showed that the two logged forests harboured significantly lower tree species diversity than the old-growth forest. Nevertheless, the selectively logged forest supported significantly higher tree species diversity than the clear-cut logged forest. Similarly, both logging regimes caused shifts in tree species composition, but the shift was higher in the clear-cut forest than the selectively logged forest, indicating a better recovery in the selective logging stands. Selective and clear-cut logged forests supported similar stem density of trees, but they were lower than that of the old-growth forest. Finally, the old-growth forest exhibited significantly higher basal area than the selectively logged forest, which in turn, had significantly higher basal area than the clear-cut logged forest. Overall, selective logging imprints on tree community structure were lower than clear-cut logging due to faster recovery by the former. Our findings suggest that logged tropical forests may require a long period to fully recover.
Keywords: Clear-cutting, Disturbance, Species composition, Species diversity, Tree stem density
Clear-cutting; Disturbance; Species composition; Species diversity; Tree stem density.
1. Introduction
Tropical forests are both ecologically and biodiversity rich, being exclusive reservoir for much of the world’s biodiversity. They harbour about 50 % of the world’s terrestrial species (Gallery, 2014). The high biodiversity content of tropical forests enables them to offer diverse ecosystem services for the sustenance of human wellbeing. Nevertheless, tropical forests suffer from human disturbance which tends to degrade and destroy them (Morris, 2010). In tropical forests, particularly in developing economies, human interactions with forest ecosystems result in forest destruction and loss, and remain as an important agent of change in forest structure (FAO and UNEP, 2020) and functioning (Simard et al., 2021). Although there are many human activities responsible for this phenomenon, logging and agricultural land use are the major drivers of degradation and deforestation, respectively (Acheampong et al., 2019; Morris, 2010; Nias, 2013; Rajpar, 2018; Shvidenko, 2008). In some forests, logging disturbance facilitates the colonisation and spread of invasive plants that can displace native plant species (Addo-Fordjour et al., 2022; Brown and Gurevitch, 2004). This phenomenon, together with logging-induced changes, may cause much loss of forest productivity, biodiversity, and ecosystem functions and services, although logged forests can recover over time and restore these ecosystem characteristics (Lamb and Gilmour, 2003; Xu et al., 2015).
Post-logging assessment of plant community structure provides a useful information on the extent of vegetation recovery over time, which can increase our understanding of the imprints of logging on plant communities. Logging is a common forest management regime which causes disturbance in tropical forests (Huth and Ditzer, 2001). Traditionally, selective and clear-cut logging have been the most commonly used methods for timber extraction in the tropics (Addo-Fordjour and Afram, 2021). Generally, these two logging regimes exert different effects on forests, with selective logging causing degradation, whereas clear-cut logging results in deforestation (Addo-Fordjour and Afram, 2021; Chaudhary et al., 2016). Selective logging is often targeted at removing selected trees which creates canopy gaps (Seidler and Bawa, 2001). Conversely, clear-cut logging removes either all or almost all trees in the forest (Simard et al., 2021), indicating higher disturbance intensity of this logging regime. Logging related activities such as creation of logging roads and skid trails also contribute to cause further forest destruction (Magrach et al., 2016). Given the differences between the two logging methods, knowledge of the response of plant community structure to selective and clear-cut logging will improve our understanding of their role in shaping plant community structure.
Generally, only a few authors have compared the impacts of selective and clear-cut logging on tree community structure in single studies (Fan et al., 2021). This trend limits our knowledge on the relative response of tree communities to selective and clear-cut logging. Some studies demonstrated that the recovery of tree species diversity, composition, and community structure in selectively logged forests was faster than clear-cut logged forests (Torras and Saura, 2008; Xu et al., 2015). In other words, selective logging left better imprints than clear-cut logging. Unlike the above studies, Ding et al. (2017) reported no difference in tree species diversity between selectively and clear-cut logged forests. Similarly, there was no difference in long-term impacts of selective and clear-cut logging on tree species diversity in a montane rainforest in Madagascar (Brown and Gurevitch, 2004). Furthermore, similar rates of recruitment, mortality and turnover of tree abundance occurred in selectively and clear-cut logged forests in a tropical rainforest in China (Fan et al., 2021).
Despite the contribution of the above-mentioned studies to enhancing our understanding of logging effects on plant communities, the clear-cut logged forests of some of them had longer time span since logging than their corresponding selectively logged forests, and thus the clear-cut logged forests had more time to recover. This means that time span of logging acted as a confounding factor which potentially influenced the findings of the studies. In other studies, logging time span of selectively and clear-cut logged stands was not clearly stated (Torras and Saura, 2008; Xu et al., 2015). Generally, selective logging better preserves seed banks (Dupuy and Chazdon, 1998) and leave residual trees to serve as seed sources (Damptey et al., 2021) for natural regeneration of tree species, while clear-cutting removes seed sources and damages seed banks (Simard et al., 2021) with negative consequences for shade-tolerant species (Negrón-Juárez et al., 2019; Torras and Saura, 2008; Xu et al., 2015). Thus, all things being equal, selectively logged forests have a better chance to recover faster and show lower imprints than clear-cut logged forests. However, the differences in the time span of logging between selectively and clear-cut logged forests in the above studies make it difficult to conclude whether the clear-cut logged forests recovered as much as selectively logged forests. This uncertainty, together with the limited number of studies about the impacts of the two logging methods on tree community structure, necessitates further studies on the subject matter, where logged stands of the same time of recovery are compared. Thus, the findings of our study will deepen our understanding of the impacts of selective and clear-cutting on tree community structure.
Our study was therefore aimed at determining the relative impacts of two logging regimes, selective and clear-cut logging on tree community structure after 26 years of logging in a moist semi-deciduous forest in Ghana. We tested the following hypotheses: (1) selective and clear-cut logging would cause a reduction in tree species diversity, density, and basal area, (2) selective and clear-cut logging would cause shifts in tree species composition, and (3) selectively logged forest would show a higher recovery of tree species diversity, composition, density, and basal area than clear-cut logged forest. The findings generated from this study would be important in forest management, and could also inform the development of future forest management practices.
2. Materials and methods
2.1. Study area
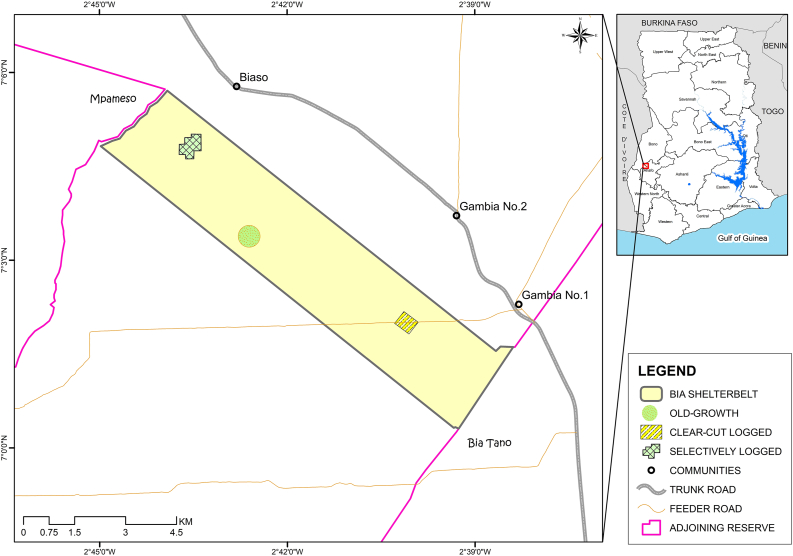
We conducted our study in the Bia Shelterbelt Forest Reserve (Figure 1) which is located in Goaso in the Ahafo Region, Ghana (7°0'18.197" N; 2°43'55.593" W) during the period of October to December 2019. The forest reserve covers a total land area of 2,953 ha in the moist semi-deciduous forest zone of Ghana (Addo-Fordjour and Afram, 2021). The forest receives a double maxima rainfall consisting of major (April to July) and minor (September and October) rainfall seasons. The area experiences a mean annual rainfall in the range of 1250–1750 mm, with humidity ranging from 75 to 80 % in the rainy season and 20–35 % in the dry season (Ghana Statistical Service, 2014). Dry season in the forest occurs from November to March and the mean monthly temperature range in the area is 26–29 °C (Ghana Statistical Service, 2014). The vegetation of the forest is dominated by semi-deciduous tree species including Celtis mildbraedii, Nesogordonia papaverifera, Triplochiton scleroxylon and Terminalis superba (Addo-Fordjour and Afram, 2021).
Figure 1.
Map of Ghana with an insert of the study area showing the three forest management systems (old-growth, selectively logged, clear-cut logged) of a moist semi-deciduous forest in Ghana.
2.2. Sampling design and data collection
We collected our data from three forest management systems namely, selectively logged, clear-cut logged and old-growth forest. Sections of the forest reserve were selectively and clear-cut logged in 1993 (Addo-Fordjour and Afram, 2021), with no other logging activity or any other form of human disturbance occurring afterwards. The inclusion of the old-growth forest enabled us to quantify the logging impacts, since there was no pre-logging data. The three forest management systems selected for the study share similar soil type, elevation (clear-cut: 230–300 m. a.s.l., selective: 250–300 m. a.s.l., old-growth: 240–290 m. a.s.l.), slope angle (0–3 %), and climate (Addo-Fordjour and Afram, 2021). During logging of the clear-cut stand, all trees with diameter at breast height (dbh) ≥ 20 cm together with lianas were removed, and the forest allowed to regenerate. In the case of the selectively logged stand, only a few mature commercial trees (20 individuals/ha) with the minimum felling dbh of 50 cm were harvested. All trees that fell within the above-mentioned criterion, were tagged and randomly selected for logging, starting with bigger stems.
We demarcated a total of 30 20 × 20 m plots in each of the three forest management system, giving a total of 90 plots in the study. In each plot, we identified and counted trees with diameter at breast height ≥5 cm. A minimum distance of 2 km separated the forest management systems, whereas an inter-plot distance of 300 m was maintained. Tree species were identified by plant taxonomists, being supported by identification manuals (Arbonnier, 2004; Hawthorne, 1990; Hawthorne and Jongkind, 2006).
2.3. Data analysis
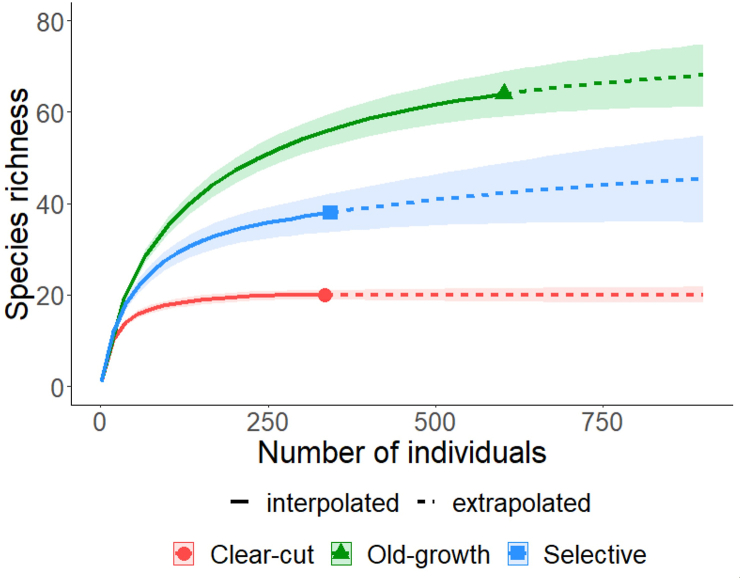
We compared tree species diversity among the three forest management systems using two approaches. Firstly, we compared species richness, Shannon diversity index, and species evenness among the forest management systems using permutation tests in PAST statistical software. The rest of our statistical analysis were all performed in R version 4.0.3. Secondly, since stem density differences among different sites can affect species richness, we employed individual-based rarefaction-extrapolation analysis to standardise stem density of trees in order to compare species richness among the forest management systems (Chao et al., 2014). Rarefaction and extrapolation curves were estimated with iNEXT package using 100 bootstrap replications (Hsieh et al., 2016).
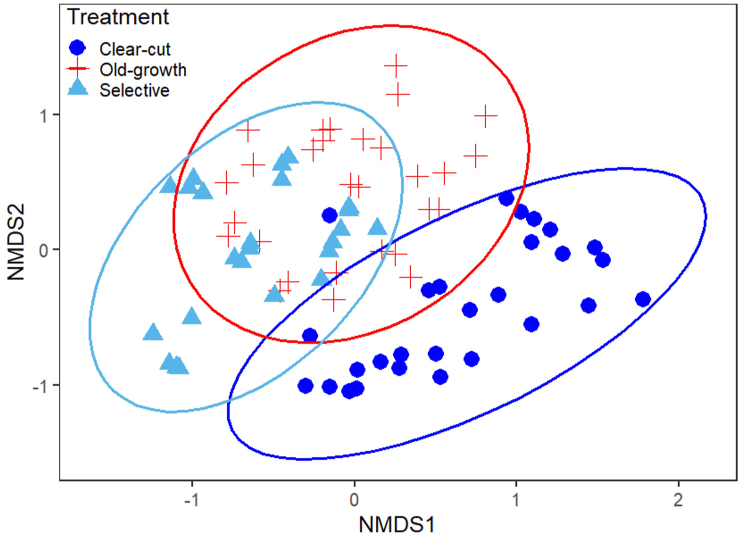
Tree species composition patterns among the forest management regimes were visualised with non-metric multidimensional scaling (NMDS), and the statistical significance of the differences among the forest management regimes tested with permutational multivariate analysis of variance (PERMANOVA). We also tested for differences in multivariate dispersion among the forest management regimes using permutational analysis of multivariate dispersion (PERMDISP). The following functions in the vegan package were used for the above-mentioned analyses: metaMDS (NMDS); adonis (PERMANOVA); betadisper (PERMDISP). We used Bray-Curtis dissimilarity.
One-way ANOVA was run to compare tree stem density among the three forest management regimes using aov function in the stats package. We used Tukey HSD function in the stats package to run Tukey post-hoc test in order to separate group means.
3. Results
3.1. Tree species diversity and composition
A total of 74 tree species were identified across the three forest management systems, which belonged to 63 genera and 23 families (old-growth: 54 genera, 23 families; selectively logged: 30 genera, 15 families; clear-cut logged: 17 genera, 8 families). Tree species richness was significantly higher in the old-growth forest than the selectively logged forest (P = 0.001) and the clear-cut logged forest (P = 0.001) (Tables 1 and 2). The selectively logged forest also supported significantly higher tree species richness than the clear-cut logged forest (P = 0.001). These trends were confirmed by the rarefaction and extrapolation curves in the three forest management systems (Figure 2). The non-overlapping confidence intervals of the curves confirm that indeed, the observed species richness differed significantly among the three forest management systems, and also indicate that the extrapolated species richness of trees differed significantly in accordance with the above mentioned trends. The rarefaction curve in the clear-cut logged forest reached complete asymptote, while those in the selectively logged and old-growth forests approached asymptote. Shannon diversity index differed significantly among the three forest management systems in accordance with the following order: old-growth > selectively logged > clear-cut logged (Table 2; old-growth vs selectively logged: P = 0.002; old-growth vs clear-cut logged: P = 0.001; selectively logged vs clear-cut: P = 0.001). Similarly, tree species evenness differed significantly among the three forest management systems, but the trend was a sharp contrast to that of Shannon diversity index: clear-cut logged > selectively logged > old-growth (old-growth vs selectively logged: P = 0.023; old-growth vs clear-cut logged: P = 0.001; selectively logged vs clear-cut: P = 0.001).
Table 1.
Tree species density (number of stems/plot) in the three forest management systems (OG: old-growth, SL: selectively logged, CC: clear-cut logged) in a moist semi-deciduous forest in Ghana.
| Species | Family | OG | SL | CC |
|---|---|---|---|---|
| Albizia adianthifolia (Schum.) W. Wight | Fabaceae | 8 | 4 | - |
| Alstonia boonei De Wild. | Apocynaceae | 6 | - | - |
| Amphimas pterocarpoides Harms | Fabaceae | 2 | - | - |
| Aningeria robusta (A. Chev.) Aubrév. & Pellegr. | Sapotaceae | 2 | - | - |
| Antiaris toxicaria (Rumph. ex Pers) Lesch. | Moraceae | 6 | - | - |
| Baphia nitida Lodd. | Fabaceae | 27 | 4 | 2 |
| Baphia pubescens Hook. f. | Fabaceae | 1 | 2 | - |
| Blighia sapida Konig | Sapindaceae | 5 | - | - |
| Blighia unijugata Baker | Sapindaceae | 3 | 5 | - |
| Bombax buonopozense P. Beauv. | Malvaceae | 3 | - | 12 |
| Broussonetia papyrifera Vent. | Moraceae | - | 26 | 15 |
| Carapa procera DC. | Meliaceae | 4 | - | - |
| Canarium schweinfurthii Engl. | Burseraceae | 1 | - | - |
| Cedrela odorata L. | Meliaceae | - | 2 | - |
| Ceiba pentandra (L.) Gaertn. | Malvaceae | 10 | 12 | 7 |
| Celtis adolfi-friderici Engl. | Ulmaceae | 19 | 50 | 2 |
| Celtis mildbraedii Engl. | Ulmaceae | 124 | 10 | 25 |
| Celtis zenkeri Engl. | Ulmaceae | 19 | 4 | 5 |
| Chrysophyllum giganteum A. Chev. | Sapotaceae | 3 | - | - |
| Cleidion gabonicum Baill. | Euphorbiaceae | 4 | - | - |
| Cola gigantea A. Chev. | Malvaceae | 6 | - | - |
| Corynanthe pachyceras K. Schum. | Rubiaceae | 5 | - | - |
| Cyathea manniana Hook. | Cyatheraceae | 4 | - | - |
| Cylicodiscus gabunensis Harms | Fabaceae | 2 | 3 | 9 |
| Daniellia ogea (Harms) Holland | Fabaceae | 9 | - | - |
| Discoglypremna caloneura (Pax) Prain | Euphorbiaceae | 1 | - | - |
| Drypetes sp. | Euphorbiaceae | 1 | - | - |
| Elaeis guineensis Jacq. | Palmae | 2 | - | - |
| Entandrophragma angolense (Welw.) C. DC. | Meliaceae | 1 | 18 | 11 |
| Entandrophragma cylindricum (Sprague) Sprague | Meliaceae | 4 | 3 | - |
| Entandrophragma utile (Dawe & Sprague) Sprague | Meliaceae | 4 | 4 | - |
| Ficus lutea Vahl | Moraceae | - | 17 | 3 |
| Funtumia elastica (Preuss) Stapf | Apocynaceae | 5 | - | - |
| Guarea cedrata (A. Chev.) Pellegr. | Meliaceae | - | 1 | - |
| Guibourtia ehie (A. Chev.) J. Leonard | Fabaceae | 2 | - | - |
| Hexalobus crispiflorus A. Rich. | Annonaceae | 4 | 1 | - |
| Holoptelea grandis (Hutch.) Mildbr. | Ulmaceae | 1 | - | - |
| Hymenostegia afzelii (Oliv.) Harms | Fabaceae | 3 | - | - |
| Irvingia gabonensis (Aubry-Lecomte) Baill. | Irvingiaceae | 2 | - | - |
| Isolona campanulata Engl. & Diels | Annonaceae | 2 | - | - |
| Khaya grandifoliola C. DC. | Meliaceae | 2 | - | - |
| Klainedoxa gabonensis Pierre ex Engl. | Irvingiaceae | 1 | - | - |
| Lannea welwitschii (Hiern) Engl. | Anacardiaceae | - | 1 | - |
| Macaranga barteri Müll. Arg. | Euphorbiaceae | - | 1 | - |
| Mansonia altissima (A. Chev.) A. Chev. | Sterculiaceae | 28 | 6 | - |
| Microdesmis puberula Hook. f. ex Planch. | Pandaceae | 1 | - | - |
| Milicia excelsa (Welw.) C.C. Berg | Moraceae | 11 | 3 | 18 |
| Morinda lucida Benth. | Rubiaceae | - | 16 | - |
| Morus mesozygia Stapf | Moraceae | 5 | 4 | 3 |
| Musanga cecropioides R. Br. | Cecropiaceae | - | 4 | - |
| Myrianthus arboreus P. Beauv. | Cecropiaceae | 3 | 8 | - |
| Nesogordonia papaverifera (A. Chev.) R. Capuron | Malvaceae | 56 | 1 | 20 |
| Piptadeniastrum africanum (Hook. f.) Brenan | Fabaceae | 5 | - | - |
| Placodiscus boya Aubrév. & Pellegr. | Sapindaceae | 4 | - | - |
| Pseudospondias microcarpa (A. Rich.) Engl. | Anacardiaceae | 1 | - | - |
| Pterygota macrocarpa K. Schum. | Sterculiaceae | 1 | - | - |
| Pycnanthus angolensis (Welw.) Warb. | Myristicaceae | 24 | 6 | 19 |
| Rauvolfia vomitoria Afzel. | Apocynaceae | 6 | 7 | 15 |
| Rhodognaphalon brevicuspe (Sprague) Roberty | Malvaceae | 1 | - | - |
| Ricinodendron heudelotii (Baill.) pierre ex Pax | Euphorbiaceae | 2 | 3 | - |
| Rinorea oblongifolia (C.H. Wright) C. Marquand ex Chipp | Violaceae | 13 | - | - |
| Senna siamea (Lam.) H.S. Irwin & Barneby | Fabaceae | - | 12 | - |
| Sterculia oblonga Mast. | Malvaceae | 14 | 16 | 18 |
| Sterculia rhinopetala K. Schum. | Malvaceae | 19 | - | - |
| Sterculia tragacantha Lindl. | Malvaceae | 8 | 4 | 19 |
| Terminalia ivorensis A. Chev. | Combretaceae | 9 | 1 | 26 |
| Terminalia superba Engl. & Diels | Combretaceae | 25 | 5 | - |
| Tieghemella heckelii Pierre ex A. Chev. | Sapotaceae | 4 | - | - |
| Trichilia monadelpha (Thonn.) J.J. de Wilde | Meliaceae | - | 57 | - |
| Trichilia prieuriana A. Juss. | Meliaceae | 20 | - | - |
| Trichilia tessmannii Harms | Meliaceae | 2 | 5 | 79 |
| Trilepisium madagascariense DC. | Moraceae | 26 | 16 | - |
| Triplochiton scleroxylon K. Schum. | Malvaceae | 5 | 1 | 6 |
| Vitex ferruginea Schumach. & Thonn. | Verbanaceae | 1 | - | - |
Table 2.
Comparison of tree community structure among the three forest management systems (OG: old-growth, SL: selectively logged, CC: clear-cut logged) in a moist semi-deciduous forest in Ghana. Across the same row, values with different superscripts are significantly different (P < 0.05) as determined by permutation tests (diversity indices) and Tukey’s post-hoc test (density and basal area).
| Tree characteristics | OG | SL | CC |
|---|---|---|---|
| Species richness | 64a | 38b | 20c |
| Shannon diversity index | 3.36a | 3.05b | 2.61c |
| Evenness | 0.45a | 0.55b | 0.68c |
| Density per plot | 21a | 11b | 10b |
| Basal area per plot (m2) | 3.10a | 1.70b | 0.90c |
Figure 2.
Individual-based rarefaction-extrapolation curves of tree species richness patterns in the three forest management systems in a moist semi-deciduous forest in Ghana. The solid lines of the curves are the rarefaction (interpolation) curves from the reference sample, whereas the dashed lines are the extrapolation curves. The symbols at the end of each rarefaction curve (see also legend) represents the observed number of individuals for the particular forest management system.
The NMDS ordination revealed that the old-growth forest plots were distinct from the plots in the two logging regimes in terms of tree species composition (Figure 3). The composition of the old-growth forest was more similar to that in the selectively logged forest than that of the clear-cut logged forest. The selectively and clear-cut logged forests had dissimilar tree species composition. The PERMANOVA results indicated that the differences in tree species composition among all the forest management systems were significantly different (F. model = 11.95, P = 0.001). The difference in multivariate dispersion between the selectively logged and old-growth forests was significant (PERMDISP; P = 0.001). Likewise, the multivariate dispersion of the selectively logged forest was significantly different from that in the clear-cut logged forest (P = 0.010). Nevertheless, there was homogeneity of multivariate dispersion between the clear-cut logged and old-growth forests (P = 0.820).
Figure 3.
Non-metric multidimensional scaling (NMDS) of tree species composition in the sampling sites of the three forest management systems in a moist semi-deciduous forest in Ghana. Ellipses are shown in the diagram, representing 95 % confidence interval based on differences in species composition of trees.
3.2. Tree density and basal area
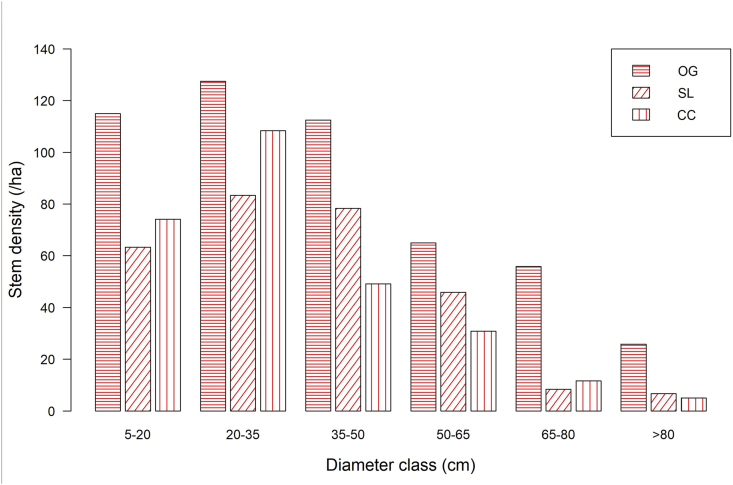
A total of 1259 tree individuals were enumerated across the three forest management systems (Table 1). We counted most trees in the old-growth forest (635 individuals), followed by the selectively logged forest (343 individuals) and then the clear-cut logged forest (314 individuals). There was no significant difference in tree density between the two logged forests (Table 2) (P = 0.158). Nevertheless, tree density in the old-growth forest was significantly higher than that in the selectively (P = 0.001) and clear-cut (P = 0.001) logged forests. The five most abundant species of trees in the old-growth forest contributed about 43.4 % of tree density (Table 1). The species were C. mildbraedii, N. papaverifera, Mansonia altissima, Baphia nitida, and T. scleroxylon. In the selectively logged forest, Cedrela odorata, B. papyrifera, T. superba, Entandrophragma utile, and Hexalobus crispiflorus contributed 49 % of tree density. On the part of clear-cut logged forest, the five most abundant tree species contributed 50.7 % of the total tree stem density in this logging regime. The five most abundant species were T. scleroxylon, Terminalia ivorensis, C. mildbraedii, N. papaverifera, and Pycnanthus angolensis. The three forest management systems showed a similar trend in tree diameter distribution (Figure 4). In general, tree density increased from the lowest diameter class (5–20 cm) up to the 20–35 cm diameter class, and then from this point decreased consistently with diameter class increase. Thus, the diameter distribution of trees in the three forest management systems was nearly a reverse J-shaped curve.
Figure 4.
Diameter class distribution of trees in the three forest management systems in a moist semi-deciduous forest in Ghana (OG: old-growth, SL: selectively logged, CC: clear-cut logged) across the various diameter classes.
Tree basal area differed significantly among all the three forest management systems, being highest in the old-growth forest and lowest in the clear-cut logged forest (P = 0.001). Tree basal area differences between the old-growth and selectively logged forests increased with increasing diameter class (Table 3). For all diameter classes, the old-growth forest had higher basal area than the selectively logged forest, showing that the basal area variations between the two forests were contributed by the old-growth forest. With respect to the old-growth and clear-cut logged forests, basal area differences fluctuated with diameter class. All the basal area differences between the two forests were contributed by the old-growth forest. Tree basal area differences between the selectively and clear-cut logged forests also fluctuated with diameter class. All the variations in basal area between the two forests were contributed by the selectively logged forest, with the exception of the difference with respect to the 5–20 cm diameter class, which came from the clear-cut logged forest.
Table 3.
Distribution of tree basal area differences between pairs of the three forest management systems in a moist semi-deciduous forest in Ghana (OG: old-growth, SL: selectively logged, CC: clear-cut logged) across the various diameter classes. If a value bears a superscript of a particular forest management systems, it means that value was contributed by that forest management system (i.e., that forest management system had a higher basal area value than the other forest management systems in the pair).
| Diameter class | Basal area differences between the forest pairs |
||
|---|---|---|---|
| OG–SL | OG–CC | SL–CC | |
| 5-20 | 0.33OG | 0.10OG | 0.07SL |
| 20-35 | 1.20 OG | 2.40OG | 1.21SL |
| 35-50 | 6.71 OG | 15.22OG | 8.51SL |
| 50-65 | 4.00 OG | 11.00OG | 7.03SL |
| 65-80 | 16.53 OG | 18.23OG | 0.01SL |
| >80 | 47.21 OG | 56.44OG | 1.71SL |
4. Discussion
Unlike previous studies, we sampled trees from selectively and clear-cut logged forest stands with the same recovery time. Thus, our study employed a more objective approach to compare tree community structure between the two logged forests. The findings of the current study therefore add valuable information to existing literature to increase our understanding of the relative impacts of selective and clear-cut logging on tree community structure. Our results showed that selective and clear-cut logging had varied imprints on tree species diversity and composition in the forest, with the latter showing severer impacts. This trend is consistent with previous studies in which clear-cut logging had more pronounced negative impacts on tree species diversity (Torras and Saura, 2008; Xu et al., 2015) and composition (de Avila et al., 2015; Hayward et al., 2021; Osazuwa-Peters et al., 2015) compared to selective logging. Given that logging disturbance influences natural regeneration (Bahati, 2005), the extent or intensity of the logging disturbance could serve as an important determinant of tree species diversity and composition recovery in the logged forests. Clear-cutting destroyed significant number of trees and consequently removed seed sources for regeneration from the clear-cut logged forest. This phenomenon which reduces proximity to seed sources, might have impaired the recovery of tree species diversity and composition in the clear-cut forest stand (Simard et al., 2021). Moreover, in clear-cut forests where light availability is high, tree species exhibit a spectrum of light tolerance physiology, resulting in a negative impact on shade tolerant species (Negrón-Juárez et al., 2019; Torras and Saura, 2008). On the contrary, selective logging often preserves seed sources which contributes to faster recovery of tree species diversity and composition (Damptey et al., 2021; Dupuy and Chazdon, 1998).
In view of the fact that clear-cutting causes higher disturbance intensity than selective logging, clear-cut forests have the tendency to recover at a slower rate than selectively logged forests (Chazdon et al., 2007; Ding et al., 2017; Fan et al., 2021). This assertion is supported by our findings, as tree species diversity and composition of the clear-cut forest shifted more from the old-growth forest compared to the shift of the selectively logged forest from the old-growth forest. In fact, species composition of the selectively logged forest showed a strong convergence towards that of the old-growth forest, suggesting that the selectively logged forest possibly recovered faster than the clear-cut logged forest. Contrarily, Fan et al. (2021) demonstrated that tree species composition of a clear-cut logged forest recovered faster than that of a selectively logged forest. Tree species composition recovery in the selectively logged forest studied by Fan et al. (2021) was perhaps limited by higher intensity of past logging which removed 60 % of tree volume compared to 20 % of tree volume removed in our selectively logged forest. Secondary, the clear-cut logged forest of Fan et al. (2021) had a longer time of recovery, which could have been an undue advantage over the selectively logged forest.
Our findings revealed that stem density of trees in the clear-cut logged forest recovered to the level of the selectively logged forest. Increased light availability associated with canopy openings of clear-cut logged forest might have favoured germination, establishment and recruitment of tree species, especially light demanders, contributing to increased tree stem density in this logged forest (Torras and Saura, 2008). In relation to the old-growth forest, the two logged forests harboured a lower stem density of trees. Thus, natural regeneration and recruitment of trees in the logged forests were not enough to compensate for the number of trees lost due to selective and clear-cut logging. The density of tree stems in the logged forests may be limited by post-logging tree mortality and decreased recruitment due to reduced light availability as canopy closes. Our findings imply that damage inflicted on tree communities by logging can persist for several years, and that a considerable longer time is required for logged sites to fully recover their tree stem density (Xu et al., 2015). A reverse J-shaped distribution curve is a common and representative feature of undisturbed or old-growth forests, which indicates good regeneration of plant species (Borah et al., 2014; Lábusová et al., 2019). Consistent with this trend, we observed a near-perfect reverse J-shaped curve of tree diameter distribution in the old-growth forest. This pattern may be due to active regeneration of trees, particularly shade-tolerant species (Busing, 1994). A similar pattern was recorded in both the selectively and clear-cut logged forests, showing that natural regeneration of trees was active and continuous in the logged forests (Borah et al., 2014).
The results of our study also showed logging-induced changes in tree basal area in both the selectively and clear-cut logged forests, although the adverse changes were more pronounced in the case of clear-cutting. The above trend is supported by previous studies which reported negative impacts of logging on woody plant basal area (Hayward et al., 2021; Tálamo et al., 2020; Xu et al., 2015). Lutz et al. (2013) observed that variation in large-diameter stem density of trees is an important determinant of tree basal area patterns. Similarly, our results revealed that variations in the density of large-diameter stems drove basal area differences among the forest management systems. For example, large diameter trees (dbh ≥50 cm) which were more abundant in the old-growth forest than the two logging regimes, accounted for 89 % of the differences in basal area between the old-growth forest and selectively logged forest, and 86 % of the difference between the old-growth forest and clear-cut logged forest. In fact, whereas tree basal area was driven by large diameter-trees in the old-growth forest, it was limited by large-diameter trees in the two logged forests. These trends reflect the negative impacts of logging and demonstrate that the removal of large-diameter trees by logging inflicts long lasting negative impacts on tree basal area, irrespective of recovery through natural regeneration, recruitment, and stem growth (Tálamo et al., 2020).
Overall, the findings of the current study reveal that the imprints of selective logging and clear-cutting on tree stem density and basal area still remain 26 years after harvesting. Nonetheless, logging impacts in the clear-cut logged forest was more prominent on tree basal area than tree stem density. Xu et al. (2015) reported partial recovery of tree community structure in selectively and clear-cut logged forests, and suggested that the full recovery of tropical forests may require a long period of time. Consistent with the above study, our findings showed partial recovery of tree diversity, composition, density, and basal area in the two logged forests, indicating that indeed post-logging recovery of tropical forests is a long term process. In general, selective logging is considered as a milder form of disturbance that can help maintain biodiversity and forest ecosystem functions, due to the recovery ability of selectively logged forests (Lima et al., 2020). Nevertheless, the persistent of selective logging impacts on tree community structure in our forests after 26 years, shows that selective logging can induce a long term disruption of tree community structure, with ramifications for forest functioning. The long term impacts of selective logging on tree community structure was also reported by previous studies (Asase et al., 2014; Brown and Gurevitch, 2004; Osazuwa-Peters et al., 2015; Xu et al., 2015).
There are other important factors that also possibly accounted for the shifts in tree community structure in the logged forests. Firstly, logging-induced disturbance can facilitate colonisation and establishment of invasive species that can impair native vegetation recovery (Addo-Fordjour et al., 2022; Brown and Gurevitch, 2004). We found an invasive species, B. papyrifera among the native tree species in the two logged forests. Given that B. papyrifera displaces native species (Addo-Fordjour et al., 2022; Yalley et al., 2020), its presence in the logged forests could lower tree species diversity, density, and basal area, and cause shifts in species composition. In fact, previous studies found that the establishment of invasive plant species in logged forests prevent the recolonisation of native tree species (Brown and Gurevitch, 2004) due to a strong competitive edge of invasive plant species over native trees (Bueno et al., 2019; Dyderski and Jagodziński, 2020; Gioria and Osborne, 2014; Kawaletz et al., 2013). Furthermore, although canopy openings in the logged forest might have favoured light demanding tree species, sudden exposure of shade tolerant species to increased light intensity in the logged forests might have caused physiological stress, thus limiting tree regeneration and recruitment (Hall et al., 2003). Finally, it is widely established that logging causes damage to non-targeted trees (Clatterbuck, 2006; Hall et al., 2003). This logging damage could later cause mortality of residual trees and reduce plant community structure (Saiful and Latiff, 2014). Our study has clearly shown that given equal recovery time, selectively logged forests show better recovery than clear-cut forests, although the recovery in both types of logged forests could be partial. This information may be useful in forest management as it highlights the need to use or develop forest management regimes that enhance full recovery of logged forests and maintain their biodiversity. Furthermore, the findings of the current study imply that selective logging can preserve forests better than clear-cut logging. Thus, selective logging should be given priority over clear-cutting in areas where maintenance of forest structure and conservation of biodiversity is desired.
5. Conclusion
We used a more objective approach than previous studies to assess the relative impacts of selective and clear-cut logging on tree community structure by sampling selectively and clear-cut logged forest stands that had the same recovery time. Both selective and clear-cut logging inflicted adverse impacts on tree species diversity and composition, but the imprints were more visible with respect to clear-cutting, indicating that recovery of tree species diversity and composition was faster in the selectively logged forest than the clear-cut logged forest. There were negative and similar imprints of logging on tree stem density in the two logging regimes, but with respect to basal area, a lesser imprint was observed in the selectively logged forest. Overall, our findings suggest that logged tropical forest recovery is a long term process, and so such forests may not recover fully after short to medium term. The information generated in this study calls for the use or development of forest management regimes that favour full recovery of tree community structure in tropical forests. In view of the lower imprints of selective logging on tree community structure, it must be given priority over clear-cutting where forest structure maintenance and biodiversity conservation are desired.
Declarations
Author contribution statement
Patrick Addo-Fordjour: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Isaac Sarfo Afram: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jennifer Oppong: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to Forestry Commission of Ghana for granting access to the forest reserve.
References
- Acheampong E.O., Macgregor C.J., Sloan S., Sayer J. Deforestation is driven by agricultural expansion in Ghana’s forest reserves. Scientific African. 2019;5 [Google Scholar]
- Addo-Fordjour P., Afram I.S. Clearcutting and selective logging have inconsistent effects on liana diversity and abundance but not on liana–tree interaction networks. Biotropica. 2021;53:442–452. [Google Scholar]
- Addo-Fordjour P., Ofosu-Bamfo B., Mbroh E., Arnold C.K., Opoku Boadi A., Mulberry M., Doe D.E.K., Oduro Takyi E. Plant invasion drives liana and tree community assemblages and liana‑tree network structure in two moist semi‑deciduous forests in Ghana. Biol. Invasions. 2022:1–22. [Google Scholar]
- Arbonnier M. CIRAD, MARGRAF Publishers GMBH; MNHN, Weikersheim: 2004. Trees, Shrubs and Lianas of West African Dry Zones. [Google Scholar]
- Asase A., Asiatokor B.K., Ofori-Frimpong K. Effects of selective logging on tree diversity and some soil characteristics in a tropical forest in southwest Ghana. J. For. Res. 2014;25 [Google Scholar]
- Bahati J.B. PhD Thesis. Makerere University; Kampala, Uganda: 2005. Effects of logging on environmental factors, natural regeneration, and distribution of selected mahogany species in budongo forest reserve, Uganda. [Google Scholar]
- Borah N., Athokpam F.D., Garkoti S.C., Das A.K., Hore D.K. Structural and compositional variations in undisturbed and disturbed tropical forests of Bhuban hills in south Assam, India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2014;10 [Google Scholar]
- Brown K.A., Gurevitch J. Long-term impacts of logging on forest diversity in Madagascar. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6045–6049. doi: 10.1073/pnas.0401456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno A., Pritsch K., Simon J. Species-specific outcome in the competition for nitrogen between invasive and native tree seedlings. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busing R.T. Canopy cover and tree regeneration in old-growth cove forests of the Appalachian Mountains. Vegetatio. 1994;115:19–27. [Google Scholar]
- Chao A., Gotelli N.J., Hsieh T.C., Sander E.L., Ma K.H., Colwell R.K., Ellison A.M. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014;84:45–67. [Google Scholar]
- Chaudhary A., Burivalova Z., Koh L.P., Hellweg S. Impact of forest management on species richness: global meta-analysis and economic trade-offs. Sci. Rep. 2016;6 doi: 10.1038/srep23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazdon R.L., Letcher S.G., Van Breugel M., Martínez-Ramos M., Bongers F., Finegan B. Rates of change in tree communities of secondary Neotropical forests following major disturbances. Phil. Trans. Biol. Sci. 2007;362:273–289. doi: 10.1098/rstb.2006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatterbuck W.K. Logging damage to residual trees following commercial harvesting to different overstory retention levels in a mature hardwood stand in Tennessee. Proc. 13th Biennial Southern Silvicultural Res. Conf. 2006:591–594. [Google Scholar]
- Damptey F.G., Adofo E., Duah-Gyamfi A., Adusu D., Opuni-Frimpong E. Logging effects on seedling regeneration and diversity in a tropical moist semi-deciduous forest in Ghana. Geol. Ecol. Landsc. 2021;1–12 [Google Scholar]
- de Avila A.L., Ruschel A.R., de Carvalho J.O.P., Mazzei L., Silva J.N.M., Lopes J. do C., Araujo M.M., Dormann C.F., Bauhus J. Medium-term dynamics of tree species composition in response to silvicultural intervention intensities in a tropical rain forest. Biol. Conserv. 2015;191 [Google Scholar]
- Ding Y., Zang R., Lu X., Huang J. The impacts of selective logging and clear-cutting on woody plant diversity after 40 years of natural recovery in a tropical montane rain forest, south China. Sci. Total Environ. 2017;579 doi: 10.1016/j.scitotenv.2016.11.185. [DOI] [PubMed] [Google Scholar]
- Dupuy J.M., Chazdon R.L. Long-term effects of forest regrowth and selective logging on the seed bank of tropical forests in NE Costa Rica. Biotropica. 1998;30:223–237. [Google Scholar]
- Dyderski M.K., Jagodziński A.M. Impact of invasive tree species on natural regeneration species composition, diversity, and density. Forests. 2020;11:1–20. [Google Scholar]
- Fan K., Xu Y., Liu P., Zang R. Recovery of logged tropical montane rainforests as potential habitats for hainan gibbon. Forests. 2021;12 [Google Scholar]
- FAO, UNEP . 2020. The State of the World’s Forests 2020, the State of the World’s Forests 2020. [Google Scholar]
- Gallery R.E. In: Ecology and the Environment. Monson R., editor. 2014. Ecology of tropical rain forests; pp. 247–272. [Google Scholar]
- Ghana Statistical Service . Asunafo North Municipality; 2014. 2010 Population and Housing Census. District Analytical Report. [Google Scholar]
- Gioria M., Osborne B.A. Resource competition in plant invasions: emerging patterns and research needs. Front. Plant Sci. 2014;5:1–21. doi: 10.3389/fpls.2014.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.S., Harris D.J., Medjibe V., Ashton P.M.S. The effects of selective logging on forest structure and tree species composition in a Central African forest: implications for management of conservation areas. For. Ecol. Manag. 2003;183:249–264. [Google Scholar]
- Hawthorne W. Natural Resources Institute, for the Overseas Development Administration; Catham, Kent: 1990. Field Guide to the forest Trees of Ghana. Ghana Forestry Series 1. [Google Scholar]
- Hawthorne W., Jongkind C. Royal Botanic Gardens; Kew, Richmond, Surrey UK: 2006. Woody Plants of Western African Forests: a Guide to the forest Trees, Shrubs and Lianes from Senegal to Ghana. [Google Scholar]
- Hayward R.M., Banin L.F., Burslem D.F.R.P., Chapman D.S., Philipson C.D., Cutler M.E.J., Reynolds G., Nilus R., Dent D.H. Three decades of post-logging tree community recovery in naturally regenerating and actively restored dipterocarp forest in Borneo. For. Ecol. Manag. 2021;488 [Google Scholar]
- Hsieh T.C., Ma K.H., Chao A. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers) Methods Ecol. Evol. 2016;7:1451–1456. [Google Scholar]
- Huth A., Ditzer T. Long-term impacts of logging in a tropical rain forest - a simulation study. For. Ecol. Manag. 2001;142 [Google Scholar]
- Kawaletz H., Mölder I., Zerbe S., Annighöfer P., Terwei A., Ammer C. Exotic tree seedlings are much more competitive than natives but show underyielding when growing together. J. Plant Ecol. 2013;6 [Google Scholar]
- Lábusová J., Morrissey R.C., Trotsiuk V., Janda P., Bače R., Cada V., Mikoláš M., Mrhalová H., Schurman J.S., Svobodová K., Mateju L., Synek M., Svoboda M. Patterns of forest dynamics in a secondary old-growth beech-dominated forest in the jizera mountains beech forest reserve, Czech republic. IForest. 2019;12:17–26. [Google Scholar]
- Lamb D., Gilmour D. IUCN and WWF; Gland, Switzerland and Cambridge, UK: 2003. Rehabilitation and Restoration of Degraded Forests: Issues in forest Conservation, Restoration Ecology. [Google Scholar]
- Lima T.A., Beuchle R., Griess V.C., Verhegghen A., Vogt P. Spatial patterns of logging-related disturbance events: a multi-scale analysis on forest management units located in the Brazilian Amazon. Landsc. Ecol. 2020;35:2083–2100. [Google Scholar]
- Lutz J.A., Larson A.J., Freund J.A., Swanson M.E., Bible K.J. The importance of large-diameter trees to forest structural heterogeneity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrach A., Senior R.A., Rogers A., Nurdin D., Benedick S., Laurance W.F., Santamaria L., Edwards D.P. Selective logging in tropical forests decreases the robustness of liana–tree interaction networks to the loss of host tree species. Proc. Biol. Sci. 2016;283:2–8. doi: 10.1098/rspb.2015.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.J. Philosophical Transactions of the Royal Society B: Biological Sciences; 2010. Anthropogenic Impacts on Tropical forest Biodiversity: A Network Structure and Ecosystem Functioning Perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrón-Juárez R., Holm J., Faybishenko B., Magnabosco-Marra D., Fisher R., Shuman J., de Araujo A., Riley W., Chambers J. Landsat NIR band and ELM-FATES sensitivity to forest disturbances and regrowth in the Central Amazon. Biogeosci. 2019;17:6185–6205. [Google Scholar]
- Nias R.C. In: Encyclopedia of Biodiversity. second ed. Levin Simon A., editor. Elsevier; Amsterdam, The Netherlands: 2013. Endangered ecosystems; pp. 169–175. [Google Scholar]
- Osazuwa-Peters O.L., Chapman C.A., Zanne A.E. Selective logging: does the imprint remain on tree structure and composition after 45 years? Conserv. Physiol. 2015;3 doi: 10.1093/conphys/cov012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpar M.N. In: Tropical Forests - New Edition. Sudarshana P., Nageswara-Rao M., Soneji J., editors. Intech; London: 2018. Tropical forests are an ideal habitat for wide array of wildlife species. [Google Scholar]
- Saiful I., Latiff A. Effects of selective logging on tree species composition, richness and diversity in a hilldipterocarp forest in Malaysia. J. Trop. For. Sci. 2014;26:188–202. [Google Scholar]
- Seidler R., Bawa K.S. Logged forests. Encyclop. Biodivers. 2001;3:747–760. [Google Scholar]
- Shvidenko A. In: Encyclopedia of Ecology, Five-Volume Set. Jørgensen S.E., Fath B.D., editors. Elsevier; Amsterdam, The Netherlands: 2008. Deforestation; pp. 853–859. [Google Scholar]
- Simard S.W., Roach W.J., Beauregard J., Burkart J., Cook D., Law D., Murphy-Steed A., Schacter T., Zickmantel A., Armstrong G., Fraser K.M., Hart L., Heath O.R.J., Jones L., Sachs N.S., Sachs H.R., Snyder E.N., Tien M., Timmermans J. Partial retention of legacy trees protect mycorrhizal inoculum potential, biodiversity, and soil resources while promoting natural regeneration of interior douglas-fir. Front. For. Glob. Change. 2021;3:1–18. [Google Scholar]
- Tálamo A., Lopez de Casenave J., Garibaldi L.A., Núñez-Regueiro M. Direct and indirect relationships between logging intensity and regeneration of two timber species in the Dry Chaco of Argentina. For. Ecol. Manag. 2020;474:1–7. [Google Scholar]
- Torras O., Saura S. Effects of silvicultural treatments on forest biodiversity indicators in the Mediterranean. For. Ecol. Manag. 2008;255:3322–3330. [Google Scholar]
- Xu H., Li Y., Liu S., Zang R., He F., Spence J.R. Partial recovery of a tropical rain forest a half-century after clear-cut and selective logging. J. Appl. Ecol. 2015;52 [Google Scholar]
- Yalley M.K., Adusu D., Bunyamin A.R., Okyere I., Asare A. Natural regeneration of indigenous tree species in Broussonetia papyrifera invaded sites in Pra-Anum Forest Reserve. Int. J. Financ. Res. 2020;2020:1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.