Abstract
Background
Larval source management (LSM) may help reduce Plasmodium parasite transmission in malaria‐endemic areas. LSM approaches include habitat modification (permanently or temporarily reducing mosquito breeding aquatic habitats); habitat manipulation (temporary or recurrent change to environment); or use of chemical (e.g. larviciding) or biological agents (e.g. natural predators) to breeding sites. We examined the effectiveness of habitat modification or manipulation (or both), with and without larviciding.
This is an update of a review published in 2013.
Objectives
1. To describe and summarize the interventions on mosquito aquatic habitat modification or mosquito aquatic habitat manipulation, or both, on malaria control.
2. To evaluate the beneficial and harmful effects of mosquito aquatic habitat modification or mosquito aquatic habitat manipulation, or both, on malaria control.
Search methods
We used standard, extensive Cochrane search methods. The latest search was from January 2012 to 30 November 2021.
Selection criteria
Randomized controlled trials (RCT) and non‐randomized intervention studies comparing mosquito aquatic habitat modification or manipulation (or both) to no treatment or another active intervention. We also included uncontrolled before‐after (BA) studies, but only described and summarized the interventions from studies with these designs. Primary outcomes were clinical malaria incidence, malaria parasite prevalence, and malaria parasitaemia incidence.
Data collection and analysis
We used standard Cochrane methods. We assessed risk of bias using the Cochrane RoB 2 tool for RCTs and the ROBINS‐I tool for non‐randomized intervention studies. We used a narrative synthesis approach to systematically describe and summarize all the interventions included within the review, categorized by the type of intervention (habitat modification, habitat manipulation, combination of habitat modification and manipulation). Our primary outcomes were 1. clinical malaria incidence; 2. malaria parasite prevalence; and 3. malaria parasitaemia incidence. Our secondary outcomes were 1. incidence of severe malaria; 2. anaemia prevalence; 3. mean haemoglobin levels; 4. mortality rate due to malaria; 5. hospital admissions for malaria; 6. density of immature mosquitoes; 7. density of adult mosquitoes; 8. sporozoite rate; 9. entomological inoculation rate; and 10. harms. We used the GRADE approach to assess the certainty of the evidence for each type of intervention.
Main results
Sixteen studies met the inclusion criteria. Six used an RCT design, six used a controlled before‐after (CBA) study design, three used a non‐randomized controlled design, and one used an uncontrolled BA study design. Eleven studies were conducted in Africa and five in Asia. Five studies reported epidemiological outcomes and 15 studies reported entomological outcomes. None of the included studies reported on the environmental impacts associated with the intervention. For risk of bias, all trials had some concerns and other designs ranging from moderate to critical.
Ten studies assessed habitat manipulation (temporary change to the environment). This included water management (spillways across streams; floodgates; intermittent flooding; different drawdown rates of water; different flooding and draining regimens), shading management (shading of drainage channels with different plants), other/combined management approaches (minimal tillage; disturbance of aquatic habitats with grass clearing and water replenishment), which showed mixed results for entomological outcomes. Spillways across streams, faster drawdown rates of water, shading drainage canals with Napier grass, and using minimal tillage may reduce the density of immature mosquitoes (range of effects from 95% reduction to 1.7 times increase; low‐certainty evidence), and spillways across streams may reduce densities of adult mosquitoes compared to no intervention (low‐certainty evidence). However, the effect of habitat manipulation on malaria parasite prevalence and clinical malaria incidence is uncertain (very low‐certainty evidence).
Two studies assessed habitat manipulation with larviciding. This included reducing or removal of habitat sites; and drain cleaning, grass cutting, and minor repairs. It is uncertain whether drain cleaning, grass cutting, and minor repairs reduces malaria parasite prevalence compared to no intervention (odds ratio 0.59, 95% confidence interval (CI) 0.42 to 0.83; very low‐certainty evidence).
Two studies assessed combination of habitat manipulation and permanent change (habitat modification). This included drainage canals, filling, and planting of papyrus and other reeds for shading near dams; and drainage of canals, removal of debris, land levelling, and filling ditches. Studies did not report on epidemiological outcomes, but entomological outcomes suggest that such activities may reduce the density of adult mosquitoes compared to no intervention (relative risk reduction 0.49, 95% CI 0.47 to 0.50; low‐certainty evidence), and preventing water stagnating using drainage of canals, removal of debris, land levelling, and filling ditches may reduce the density of immature mosquitoes compared to no intervention (ranged from 10% to 55% reductions; low‐certainty evidence).
Three studies assessed combining manipulation and modification with larviciding. This included filling or drainage of water bodies; filling, draining, or elimination of rain pools and puddles at water supply points and stream bed pools; and shoreline work, improvement and maintenance to drainage, clearing vegetation and undergrowth, and filling pools. There were mixed effect sizes for the reduction of entomological outcomes (moderate‐certainty evidence). However, filling or draining water bodies probably makes little or no difference to malaria parasite prevalence, haemoglobin levels, or entomological inoculation rate when delivered with larviciding compared to no intervention (moderate‐certainty evidence).
Authors' conclusions
Habitat modification and manipulation interventions for preventing malaria has some indication of benefit in both epidemiological and entomological outcomes. While the data are quite mixed and further studies could help improve the knowledge base, these varied approaches may be useful in some circumstances.
Keywords: Adult, Animals, Humans, Culicidae, Ecosystem, Hemoglobins, Larva, Malaria, Malaria/epidemiology, Malaria/prevention & control, Mosquito Control, Mosquito Control/methods, Water
Plain language summary
Which permanent and temporary changes to the water environments of immature mosquitoes work better to reduce malaria in people?
Why is it important to reduce malaria in people?
Malaria has a very high impact on the health of the public, mostly in people in Africa and Asia. Strategies to reduce malaria have been studied for many years. Most strategies focus on reducing the number of immature mosquitoes (larvae and pupae) to prevent them from becoming adult mosquitoes, since it is the adult female mosquito that can spread malaria through biting people.
What are permanent and temporary changes to the environment of immature mosquitoes?
The water environments where immature mosquitoes live can be disturbed using permanent (modification) and temporary (manipulation) changes. Examples of permanent changes include construction of drainage canals, land levelling, and filling ditches. Examples of temporary changes include altering the flow of water in streams, draining canals, cutting grass, shading of water using plants. These interventions may be used on their own or together with other standard treatments, such as the regular application of insecticides to water bodies (larviciding).
What did we want to find out?
We wanted to find out which permanent and temporary changes to the environment of immature mosquitoes reduce malaria in people (clinical outcomes), and the quantity of immature and adult mosquitoes (entomological outcomes).
What did we do?
We searched for studies that looked at permanent and temporary changes to the environment of immature mosquitoes compared to no intervention or a different permanent or temporary change. We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods.
What did we find?
The review included 16 studies that used a range of different randomized and non‐randomized study designs. Eleven studies were conducted in Africa and five in Asia. Only a few studies reported clinical outcomes, with most focussing on the number of immature mosquitoes, or adult mosquitoes, or both (entomological outcomes). We found there was some evidence to support the use of permanent (modification) and temporary (manipulation) changes to the water environments to reduce the number of immature mosquitoes in specific settings. However, when looking at clinical outcomes, 1. the effect of habitat manipulation on malaria parasite prevalence and clinical malaria incidence was unclear; 2. malaria parasite prevalence may be reduced when using habitat manipulation with larviciding; 3. combining manipulation and modification with larviciding probably makes little or no difference to malaria parasite prevalence and haemoglobin levels.
What are the limitations of the evidence?
The review included a wide range of different changes to the water environment of immature mosquitoes, with some combining them with water treatments (larviciding), which meant that very few studies looked at the same intervention. Many of the included studies had issues regarding how well they were conducted.
How up to date is the evidence?
This review updates a 2013 Cochrane Review. The evidence is up to date to 30 November 2021.
Summary of findings
Summary of findings 1. Habitat manipulation versus no intervention for control of malaria.
| Habitat manipulation versus no intervention for control of malaria | ||||||
|
Patient or population: people at risk of malaria Setting: various (India, Philippines, Ethiopia, Benin, Tanzania) Co‐intervention: mixed (case management, indoor residual spraying with DDT, insecticide‐treated nets) Intervention: habitat manipulation (including floodgates; spillways; water drawdown rate; intermittent and different water regimens; shading with Napier grass, unweeded rice, arrowroot, water ferns; frequent and intermediate cleating grass and replenishing water in aquatic habitats; minimal tillage) Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Habitat manipulation | No intervention | |||||
| Clinical malaria incidence | 181.1 events per 1000 | 1000 events per 1000 | Not estimable, P < 0.01 | (1 CBA) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain whether or not habitat manipulation has an effect on clinical malaria incidence compared to no intervention. |
| Malaria parasite prevalence | — | — | RR 0.01 (95% CI 0.00 to 0.16) | (2 CBA) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain whether or not habitat manipulation has an effect on malaria parasite prevalence compared to no intervention. |
| EIR | 3.6% | 0 | RR 0.05 (0.00 to 1.03) | (1 CBA) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain whether or not habitat manipulation has an effect on the EIR compared to no intervention. |
| Density of adult mosquitoes | Reduced from 0.4 to 0.0 | No change, 0.3 to 0.3 | Not estimable | (2 CBA) | ⊕⊕⊝⊝ Lowa | Habitat manipulation may reduce the density of adult mosquitoes compared to no intervention. |
| Density of immature mosquitoes | — | — | Varied estimates, ranging from 95% reduction through to 1.7 times increase | (3 cRCT, 1 RCT, 2 non‐RCT, 3 CBA studies) | ⊕⊕⊝⊝ Lowa | Habitat manipulation may reduce the density of immature mosquitoes compared to no intervention. |
| *The risk in the intervention arm (and its 95% CI) is based on the assumed risk in the comparison arm and the relative effect of the intervention (and its 95% CI). The assumed risk of the comparison arm is calculated from the data contributing to the control arms of the studies. CBA: controlled before‐after; CI: confidence interval; cRCT: cluster‐randomized controlled trial; DDT: dichlorodiphenyltrichloroethane; EIR: entomological inoculation rate; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to risk of bias. Risk of bias domain was 'serious' where the overall risk of bias was classified as high for RCT designed studies or where non‐randomized intervention designed studies had moderate or low risk of bias rating for confounding and a maximum of one serious rating for other domains. The risk of bias was rated as 'very serious' for non‐randomized intervention designed studies with a serious risk of bias rating for the confounding domain. bDowngraded one level due to imprecision. Imprecision was 'serious' due to small size of sample and wide CIs/ranges, or both; or 'very serious' due to extremely small size of sample and wide CIs/ranges.
Summary of findings 2. Habitat manipulation with larviciding versus no intervention for control of malaria.
| Habitat manipulation with larviciding versus no intervention for control of malaria | ||||||
|
Patient or population: people at risk of malaria
Setting: various (Tanzania, India) Co‐intervention: indoor residual spraying with DDT Intervention: habitat manipulation with larviciding (repairing and clearing drains, cutting grasses, and making minor repairs; encouraging community to eliminate domestic mosquito aquatic habitat sites) Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects (95% CI)* | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Habitat manipulation with larviciding | No intervention | |||||
| Malaria parasite prevalence | — | — | OR 0.59 (0.42 to 0.83) | (1 CBA) | ⊕⊝⊝⊝ Very lowa,b,c | It is uncertain whether habitat manipulation with larviciding has an effect on malaria parasite prevalence compared to no intervention. |
| *The risk in the intervention arm (and its 95% CI) is based on the assumed risk in the comparison arm and the relative effect of the intervention (and its 95% CI). The assumed risk of the comparison arm is calculated from the data contributing to the control arms of the studies. CBA: controlled before‐after study; CI: confidence interval; DDT: dichlorodiphenyltrichloroethane; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to risk of bias. Risk of bias domain was rated as 'serious' where the overall risk of bias was classified as high for RCTs or moderate or low risk of bias rating for confounding and a maximum of one serious rating for other domains. The risk of bias was rated as 'very serious' for non‐randomized intervention designed studies with a serious risk of bias rating for the confounding domain. bDowngraded one level due to imprecision. Imprecision was rated as 'serious' due to small size of sample or wide CIs/ranges (or both), or 'very serious' due to extremely small size of sample and wide CIs/ranges. cDowngraded one level due to indirectness. Indirectness was rated as 'serious' due to directness of intervention where the independent effect of the eligible intervention could not be assessed due to use of larviciding in the intervention group only.
Summary of findings 3. Habitat manipulation and modification versus no intervention for control of malaria.
| Habitat manipulation and modification versus no intervention for control of malaria | ||||||
|
Patient or population: people at risk of malaria
Setting: various (Ethiopia, Kenya) Co‐intervention: mixed (case management, indoor residual spraying with DDT used during the pre‐intervention phase only, insecticide treated nets) Intervention: habitat manipulation and modification (drainage canals, filling, and planting of papyrus and other reeds for shading near dams; and drainage of canals, removal of debris, land levelling, and filling ditches) Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Habitat manipulation and modification | No intervention | |||||
| Density of adult mosquitoes | 0.66 | 0.20 | RRR 0.49 (0.47 to 0.50) | (1 CBA) | ⊕⊕⊝⊝ Lowa | Habitat manipulation and modification may reduce the density of adult mosquitoes compared to no intervention. |
| Density of immature mosquitoes | — | — | Varied estimates, ranging from 10% reduction to 55% reduction |
(2 CBA) | ⊕⊕⊝⊝ Lowa | Habitat manipulation and modification may reduce the density of immature mosquitoes compared to no intervention. |
| *The absolute mean in the intervention arm (and its 95% CI) is based on the assumed geometric mean in the comparison arm and the absolute effect of the intervention (and its 95% CI). The assumed mean of the comparison arm is calculated from the geometric mean data contributing to the control arms of the studies. CBA: controlled before‐after study; CI: confidence interval; DDT: dichlorodiphenyltrichloroethane; RRR: relative risk reduction. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to risk of bias. Risk of bias was rated as 'serious' due to an overall risk of bias of high for RCTs or serious for non‐randomized intervention studies, or 'very serious' due to multiple domains receiving a 'serious' rating for non‐randomized intervention studies.
Summary of findings 4. Habitat manipulation and modification with larviciding versus no intervention for control of malaria.
| Habitat manipulation and modification with larviciding versus no intervention for control of malaria | ||||||
|
Patient or population: people at risk of malaria
Setting: various (Malawi, Eritrea) Co‐intervention: national malaria programme Intervention: habitat manipulation and modification with larviciding (filling, drainage, or elimination of rain pools and puddles at water supply points and stream pools bedded with sediment; filling or draining water bodies) Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|
Habitat manipulation and modification with larviciding |
No intervention | |||||
| Malaria parasite prevalence | 14.9 | 10.4 | Women: OR 0.80 (95% CI 0.41 to 1.55) | (1 cRCT) | ⊕⊕⊕⊝ Moderatea | Habitat manipulation and modification with larviciding probably has little or no effect on malaria parasite prevalence compared to no intervention. |
| Children: OR 1.80 (95% CI 0.90 to 3.60) | ||||||
| Haemoglobin levels | 10.14 | 10.61 | Women: MD −0.11 g/dL (95% CI −0.37 to 0.15) | (1 cRCT) | ⊕⊕⊕⊝ Moderatea | Habitat manipulation and modification with larviciding probably has little or no effect on malaria parasite prevalence compared to no intervention. |
| Children: MD −0.02 g/dL (95% CI −0.35 to 0.31) | ||||||
| Entomological inoculation rate | 0 | 0 | Not estimable due to no events in either group | (1 cRCT) | — | — |
| Density of adult mosquitoes | — | — | Indoors: RaR 2.18 (95% CI 0.44 to 10.9) | (2 cRCT) | ⊕⊕⊕⊝ Moderatea | Habitat manipulation and modification with larviciding probably reduces the density of adult mosquitoes compared to no intervention. |
| Outdoors: RaR 1.95 (95% CI 0.45 to 8.41) | ||||||
| Density of immature mosquitoes | 0.87 | 3.17 | Not estimable, P < 0.001 | (1 cRCT) | ⊕⊕⊕⊝ Moderatea | Habitat manipulation and modification with larviciding probably reduces the density of immature mosquitoes compared to no intervention. |
| *The risk in the intervention arm (and its 95% CI) is based on the assumed risk in the comparison arm for adult women and the relative effect of the intervention (and its 95% CI). The assumed risk of the comparison arm is calculated from the data from adult women contributing to the control arms of the studies. CI: confidence interval; cRCT: cluster randomized controlled trial; MD: mean difference; OR: odds ratio; RaR: rate ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to indirectness. Indirectness is rated as 'serious' due to directness of intervention where the independent effect of the eligible intervention cannot be assessed due to larviciding only being used in the intervention group.
Background
Description of the condition
Malaria is a global public health priority. In 2019, there were an estimated 229 million cases in 87 malaria‐endemic countries (WHO 2020). Worldwide, malaria‐related deaths have been reduced by 44% over the 2000 to 2019 period, from 736,000 in 2000 to 409,000 in 2019 (WHO 2020). While there has been substantial progress against malaria between 2010 and 2019, a recent resurgence of malaria has been observed in certain geographies (e.g. Venezuela, Yemen, Democratic Republic of the Congo, Sudan, Rwanda, Burundi, and Tanzania) (WHO 2019a; WHO 2020).
Malaria is caused by Plasmodium parasites (primarily Plasmodium falciparum and Plasmodium vivax) and is transmitted to humans by adult female mosquitoes of the genus Anopheles. The Global Technical Strategy for Malaria 2016–2030 calls for malaria programmes to "ensure universal access to malaria prevention, diagnosis and treatment" (WHO 2015a). To do so, any malaria control programme requires an integrated rather than a siloed approach, combining prevention with early diagnosis, prompt treatment, and surveillance. Main programmatic approaches under prevention include chemoprevention and vector control. Long‐lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) of households with insecticide are core vector control interventions to reduce malaria transmission by targeting the adult mosquito population (WHO 2019b). In some specific settings, these core interventions can be supplemented with larval source management (LSM) techniques and be delivered as part of an integrated vector management (IVM) approach (WHO 2012; WHO 2017).
Description of the intervention
LSM is a method for reducing malaria transmission by targeting the mosquitoes' immature forms (i.e. larvae and pupae), which thrive in aquatic habitats. There are four main types of LSM: 1. larviciding: the regular application of biological or chemical insecticides to water bodies; 2. biological control: the introduction of natural mosquito predators into water; 3. habitat modification: a permanent alteration to the environment (e.g. land reclamation and filling); and 4. habitat manipulation: a recurrent environmental management activity (e.g. flushing of streams and drain clearance) (WHO 2013; WHO 2019b). Similar to LLINs and IRS, LSM is a context‐specific intervention and should be adapted to the local setting, depending on factors such as vector species, immature habitats, vector behaviour, seasonality, feasibility, and community acceptability (WHO 2019b).
In the past, LSM was very much part of successful malaria prevention and control programming. One recent review highlighted that substantial reductions in malaria (e.g. in Cuba, Panama, Indonesia, Zambia, the USA) and even its elimination (Italy, southeast USA) were observed following habitat modification and manipulation interventions alone or in combination with other interventions (Wilson 2020). In addition, two systematic reviews assessed the effectiveness of permanent or temporary environment modifications and the use of larviciding (Keiser 2005; Tusting 2013). The two reviews found that there were very high protective effects on clinical malaria irrespective of the type of habitat modification or manipulation (or both) used. The authors concluded that LSM is a programmatic option that can be used alongside LLINs and IRS for reducing malaria morbidity where a sufficient proportion of mosquito aquatic habitats can be targeted. However, the 2019 World Health Organization (WHO) Guidelines for Vector Control contained no recommendations on the use of habitat manipulation and modification, with the guidelines specifically stating that an additional systematic review to assess the evidence of their effectiveness was needed (WHO 2019b).
LSM approaches are made with the express purpose of reducing larvae, particularly where a permanent alteration to the environment is made using habitat modification. However, habitat modification and habitat manipulation can also be used for other purposes, such as for irrigation for agriculture or power generation, with the added effect on immature forms of mosquitoes.
The effectiveness of both larviciding and larvivorous fish (biological control) has been systematically reviewed (Choi 2019; Walshe 2017; WHO 2019b). For larviciding, it was concluded that this intervention is conditionally recommended for use in specific areas and in particular circumstances as a supplementary measure alongside the core interventions (Choi 2019; WHO 2019a). Regarding biological control with larvivorous fish, the evidence was insufficient (Walshe 2017; WHO 2019a).
How the intervention might work
Vector control interventions, such as LSM, aim to reduce the vectorial capacity of a vector population. For example, interventions that target the aquatic stages of mosquitoes typically work through an entomological mode of action to reduce vector capacity by reducing or destroying aquatic habitats of immature stages of the Anopheles vectors in the short and long term and by disrupting breeding (Vontas 2014), thus impacting on malaria transmission (Muema 2017). In the past years, a number of methods that create permanent or temporary unfavourable conditions for malaria vectors have been implemented in different settings and with variable results on entomological and epidemiological outcomes. Based on the literature, several types of habitat manipulation and modification interventions have been assessed. For habitat manipulation, these include the following: floodgates on a dam, spillways across streams, shading using local plants, and repairing and cleaning drains. For habitat modification, these include: construction of drainage canals, levelling of land, and permanent filling of ditches. In addition, there has recently been renewed interest in the use of LSM as it currently represents the only available, WHO‐recommended tool to control outdoor transmission, as well as represents an additional tool to manage the insecticide resistance that developed following the large‐scale use of LLINs and IRS (WHO 2019b). A logic model describes the main entomological and epidemiological outcomes of habitat modification and habitat manipulation interventions (Figure 1).
1.
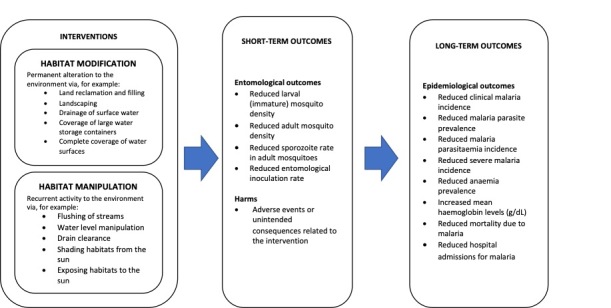
Logic model of the anticipated effects of habitat modification and habitat manipulation intervention.
Why it is important to do this review
As stated in the 2019 WHO Guidelines for Vector Control, an updated systematic review is required to determine whether there is sufficient evidence available to inform the development of policy recommendations for mosquito aquatic habitat modification or manipulation (or both) for the reduction of malaria (WHO 2019b). This determination would ensure that the future iterations of the WHO guidelines will be based on the most up‐to‐date information. LSM activities have generally been shown to be cost‐effective as, compared to other more conventional malaria programming, they do not require a large workforce or intensive resources (van den Berg 2018). Therefore, using LSM alone, or as a complement to existing interventions as part of an IVM approach, could lead to further reduction of malaria transmission and burden (McCann 2017). However, given the diversity of potential habitat modification or habitat manipulation (or both) interventions, an updated systematic review is required to document which interventions have been evaluated and, where possible, to assess the effectiveness of such interventions.
Objectives
1. To describe and summarize the interventions on mosquito aquatic habitat modification or mosquito aquatic habitat manipulation, or both, on malaria control.
2. To evaluate the beneficial and harmful effects of mosquito aquatic habitat modification or mosquito aquatic habitat manipulation, or both, on malaria control.
Methods
Criteria for considering studies for this review
Types of studies
We included the following study designs for the evaluation of the effectiveness of the interventions.
Randomized controlled trials (RCTs; parallel and cluster designs).
Randomized cross‐over trials.
Stepped wedge cluster randomized trials (SW‐CRT).
Non‐randomized intervention studies, including but not limited to, controlled before‐after (CBA) studies and interrupted time series (ITS) studies.
We included cluster‐randomized controlled trials (cRCT) that had at least two intervention and two comparator sites, and CBA studies that had at least two intervention and two comparator sites. However, due to very limited numbers of cRCTs or CBA studies identified for each type of interventions, we relaxed the number of sites restriction. We included ITS studies that had at least three data points before and three data points after the intervention, and where there was a clearly defined point in time when the intervention occurred.
We also included the following lower form of evidence in addition to those detailed above for describing and summarizing all types of eligible interventions.
Uncontrolled before‐after (BA) studies.
We included studies irrespective of their publication status and language of publication.
Types of participants
We included all participants, irrespective of age, gender, and ethnicity, residing in countries/regions with any level of malaria endemicity.
Types of interventions
Eligible interventions included any that aimed to either modify or manipulate the habitat of the aquatic stages of Anopheles to reduce or completely avoid its presence.
Habitat modification
We defined habitat modification as any permanent alteration to the environment such as land reclamation and filling, landscaping, drainage of surface water, coverage of large water storage containers (e.g. wells) with mosquito‐proof lids and permanent slabs or complete coverage of water surfaces with a material that is impenetrable to mosquitoes (e.g. expanded polystyrene beads).
Habitat manipulation
We defined habitat manipulation as any recurrent activity applied to the environment, such as flushing of streams, water level manipulation, drain clearance, shading or exposing habitats to the sun.
Habitat modification or manipulation may have been used alone or in combination with other interventions, including other LSM interventions (e.g. biological control of anopheline mosquitoes) or co‐interventions (e.g. larvicidal treatments, LLINs). Where habitat modification or manipulation was combined with co‐interventions, we included studies where the same co‐intervention was given to both the intervention and control groups or in one of the treatment groups. Regarding other LSM interventions, we included studies that evaluated mosquito aquatic habitat modification or manipulation in combination with biological control of anopheline mosquitoes or larvicidal treatments when compared to the use of the biological control or larvicidal or to no intervention.
Types of outcome measures
Primary outcomes
Epidemiological
Clinical malaria incidence, defined as new malaria cases occurring in a specific population during a finite period of time, who have clinical symptoms (including fever greater than 37.5 °C) or a history of fever during the preceding three days as well as parasitaemia diagnostically confirmed by microscopy, rapid diagnostic test (RDT), or another method.
Malaria parasite prevalence, defined as the proportion of the human population with malaria parasites circulating in the participant's blood (diagnostically confirmed by microscopy, RDT, or another method).
Malaria parasitaemia incidence, defined as new malaria infections occurring in a specific population during a finite period of time, with parasitaemia diagnostically confirmed by microscopy, RDT, or another method.
We included all malaria parasite species (P falciparum, P vivax, P ovale, and P malariae).
Secondary outcomes
Epidemiological
Incidence of severe malaria, characterized by 1. and either 2. or 3. (WHO 2015b): 1. demonstration of parasitaemia by blood smear, 2. symptoms of cerebral malaria including coma or prostration or multiple seizures, 3. severe life‐threatening anaemia.
Anaemia prevalence (WHO 2011).
Mean haemoglobin levels (g/dL).
Mortality rate due to malaria.
Hospital admissions for malaria.
Entomological
Density of immature mosquitoes, immature mosquitoes collected with a standard dipping method.
-
Density of adult mosquitoes measured by:
human biting rate: number of mosquitoes per person per time period, measured directly using human baits, or indirectly using light traps, baited huts, or other methods of biting rate determination;
other density measures: number of mosquitoes per person or catch, measured using light traps, knock‐down catches, baited huts, or other methods of adult vector density determination.
Sporozoite rate, defined as the number of caught adult mosquitoes positive for malaria sporozoites in their salivary glands observed by dissection or detected by molecular or immunological methods.
Entomological inoculation rate (EIR), defined as the estimated number of bites by infectious mosquitoes per person per unit time (measured directly using human baits or indirectly using light traps, baited huts, human‐landing catch, and infectivity determined as defined under the 'sporozoite rate' listed above).
Harms
We defined harms as adverse events or unintended consequences related to the interventions.
Search methods for identification of studies
Electronic searches
We identified relevant studies through comprehensive electronic searches using the following databases, from January 2012 (the previous review version, Tusting 2013, searched to 24 October 2012) to 30 November 2021.
Cochrane Infectious Diseases Group Specialized Register (CIDG SR) (30 November 2021)
Cochrane Central Register of Controlled Trials (CENTRAL) (30 November 2021)
MEDLINE (30 November 2021)
Embase (30 November 2021)
Global Health (30 November 2021)
CAB Abstracts (30 November 2021)
LILACS (30 November 2021)
Using search terms from Tusting 2013 as initial terms, we further developed the search strategies for each database using comprehensive search terms for the intervention and outcomes. We reported the full search strategy for each database in Appendix 1.
Searching other resources
We identified further studies through other relevant databases and handsearching of grey literature sources.
ProQuest Natural Science Collection
ZETOC
Tropical Diseases Bulletin
Archives of the WHO
Literature Database of the Armed Forces Pest Management Board
US National Institute of Health Ongoing Trials Register (www.ClinicalTrials.gov/)
ISRCTN registry (www.isrctn.com/)
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int.ictrp)
Where required, we contacted experts within the field of habitat modification and manipulation as vector control methods for malaria to provide information about ongoing and further completed studies. We conducted forward and backwards citation tracking of all studies screened at the full‐text screening stage. |We screened the reference lists of included studies to identify any further eligible studies. There were no language restrictions applied, and we sought translations where necessary. In cases of dual publication of a study, we used the most informative study publication.
We also scanned the list of studies excluded at full‐text stage from Tusting 2013; this allowed us to reconsider any relevant studies, which met our amended inclusion criteria.
Data collection and analysis
Selection of studies
We imported all search hits identified into a bibliographic database, Mendeley Desktop (London, UK). Following deduplication, two review authors (EM and GY) independently screened titles and abstracts according to the inclusion/exclusion criteria and calculated an inter‐rater agreement measure. We sought full‐text papers for all studies that were included at the title and abstract stage. Where there was insufficient information available in the title and abstract, we retrieved the full‐text article for further inspection. Two review authors (EM and GY) independently screened the full‐text papers, and calculated an inter‐rater agreement measure. We resolved any disagreements by consensus or by a consulting a third review authors (JLB). We reported studies excluded at the full‐text stage with their reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (EM and GY) independently extracted data using a previously piloted data extraction form within a spreadsheet database. Initially two review authors (EM and GY) independently tested piloted the data extraction form on a random sample of three included studies to enable an assessment of consistency in data extraction and to identify where amendments needed to be made to the template. We discussed any disagreements or, if necessary, consulted a third review author (JLB).
Assessment of risk of bias in included studies
Two review authors (EM and GY) independently assessed the risk of bias of the results for each outcome measure at the end of the intervention of the included studies using an assessment of risk of bias tool appropriate to the design of the study. We discussed any disagreements, or where necessary, resolved by consulting a third review author (JLB).
We used the Cochrane RoB 2 tool for RCTs and cRCTs (with signalling questions relating to the following domains: randomization process, timing of identification and recruitment of individual participants in relation to timing of randomization process (cRCTs only), deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result), with judgements reported as low, some concerns or high (Sterne 2019). The effect of interest that was assessed within the RoB 2 tool was the effect of assignment.
For non‐RCTs, we used the ROBINS‐I risk of bias assessment (within domains for confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of reported results) (Sterne 2016). We compared the domains for each non‐randomized controlled study against a theoretical target RCT designed study, with judgements reported as low, moderate, serious, or critical. Potential confounders included demographics, socioeconomic, entomological, and environmental factors at the individual, household, and village levels.
We assigned uncontrolled BA studies a critical overall risk of bias due to the inherent biases associated with the study design.
We generated risk of bias plots using robvis (mcguinlu.shinyapps.io/robvis/).
Measures of treatment effect
The results from studies with eligible designs for assessing the effectiveness of the interventions were extracted as adjusted effect measures, crude effect measures or as raw data. Where possible, we have reported dichotomous outcomes using risk ratios (RR), count data using rate ratios (RaR), and continuous data using mean difference (MD) based on either arithmetic or geometric means, together with 95% confidence intervals (CI). Where there were insufficient quantitative results in the publication to aid re‐analysis, we extracted quantitative results or P values (or both) from statistical testing from the publications.
Unit of analysis issues
For cRCTs, our intent was to extract adjusted measures of effect where these were available. However, most cRCTs did not account for clustering in their analyses. Therefore, we attempted to contact the study authors to provide estimates of the intraclass correlation coefficient (ICC), but none responded. Therefore, we performed analyses of most cRCTs without adjustment for clustering, which may have resulted in overly precise results.
Dealing with missing data
Where possible, we contacted the authors of the included studies with eligible designs for assessing the effectiveness of interventions to provide missing data relating to results, for example, measures of dispersion. However, no authors responded. Therefore, we analyzed data on an available‐case analysis, ignoring any missing data.
Assessment of heterogeneity
Due to the insufficient number of studies for each intervention, we were unable to conduct meta‐analyses; consequently, we were unable to quantify heterogeneity between the studies using the I2 statistic (Higgins 2003). We would have considered a value greater than 50% to reflect substantial heterogeneity between findings of RCTs. However, due to the inherent biases within other experimental designs, we would have considered a value greater than 75% to reflect substantial heterogeneity for non‐RCTs.
Assessment of reporting biases
Due to not being able to conduct meta‐analyses and also insufficient studies for each intervention (fewer than 10 studies), we were unable to assess evidence of publication bias (small‐study bias) using funnel plots.
Data synthesis
For all study designs, except for the uncontrolled BA studies, we initially used a narrative synthesis approach to systematically describe and summarize all the interventions considered in the studies fulfilling the inclusion criteria. We categorized the studies by type of intervention (i.e. habitat modification alone, habitat manipulation alone, combination of habitat modification and manipulation), the type of modification or manipulation (e.g. water management), and the purpose of the intervention (i.e. LSM, non‐LSM).
Where possible, we analyzed the quantitative findings from all included studies, except those that used an uncontrolled BA design, to assess the effectiveness of the interventions. Due to the insufficient number of studies, we were unable to conduct random‐effects meta‐analysis models to pool data from studies to estimate a weighted treatment effect for each categorization of the type of intervention separately for RCTs and non‐RCTs. A random‐effects model would have been the most appropriate, due to the anticipated clinical and methodological differences in protocols and inherent biases within the study designs, which are likely to impact the magnitude of the effectiveness of the interventions. Findings from meta‐analyses would have been reported using appropriate measures of effect together with 95% CIs.
We categorized uncontrolled BA studies based on the definition and type of the intervention and the purpose of the intervention (as previously defined). We then provided comprehensive narrative descriptions of each study including the nature and scope of the considered interventions and the outcomes assessed. We reported results from the studies in terms of the clinical significance of the effect, but made no statistical inferences.
Subgroup analysis and investigation of heterogeneity
Where data permitted, we planned to investigate sources of heterogeneity in the meta‐analyses using subgroup analyses based on:
different eco‐epidemiological settings, for example: malaria of deep forests, forest fringe, and hills; rural malaria attributable to irrigation and large dams; rural malaria attributable to wetlands, rivers, streams, coasts, and non‐agricultural manufactured water habitats; and urban and peri‐urban malaria (Keiser 2005);
participants (aged less than five years, pregnant woman, adult, mixed age groups);
species of the main vector(s);
responsibility for the delivery of the intervention (trial staff, community, mixed);
WHO region.
Sensitivity analysis
Where data permitted, we planned to perform sensitivity analyses to assess the effect of study design on the primary and secondary outcomes using stratification (e.g. for RCTs, stratifying by cluster and non‐cluster designs; for non‐RCTs, stratifying by study design used). We also planned to assess the effect of excluding studies with a ROBINS‐I rating of serious/critical risk of bias or a RoB 2 rating of high risk of bias, in at least one domain of the risk of bias assessment. However, we did not perform any sensitivity analyses due to insufficient studies to perform meta‐analyses.
Summary of findings and assessment of the certainty of the evidence
Two review authors (EM and JLB) were assessed the certainty of the evidence for each intervention across each critical or important outcome measure using GRADE (Guyatt 2008). Critical and important outcome measures were decided by consensus between the authors. Critical outcome measures were: clinical malaria incidence, malaria parasite prevalence, and malaria parasitaemia incidence. Important outcome measures were: incidence of severe malaria, mortality rate due to malaria, density of immature mosquitoes, and density of adult mosquitoes.
Since all designs included in the review were intervention studies, we initially ranked all studies as high‐certainty evidence (Schünemann 2019). We downgraded the certainty of the evidence if there was evidence of risk of bias, imprecision, inconsistency of evidence, indirectness, or publication bias. We rated risk of bias, imprecision, inconsistency of evidence, and indirectness as 'very serious', 'serious' or 'not serious', and downgraded by one level for a 'serious' rating or by two levels for a 'very serious' rating; there was no downgrading applied for those rated as 'not serious'.
The risk of bias domain was rated as 'serious' where the overall risk of bias was classified as high for RCTs or where non‐randomized intervention designed studies had moderate or low risk of bias rating for confounding and a maximum of one serious rating for other domains. The risk of bias was rated as 'very serious' for non‐randomized intervention designed studies with a serious risk of bias rating for the confounding domain.
The imprecision domain was rated as 'serious' where there were small event rates (fewer than 400) or wide CIs and 'very serious' where the numbers of events were very small (fewer than 100).
The inconsistency domain was rated as 'serious' where there was evidence of inconsistency in the findings of multiple studies.
The indirectness domain was rated as 'serious' where there was evidence of indirectness of the population, intervention, or outcome measure, and 'very serious' where there was evidence of indirectness in at least two of population, intervention, or outcome measure.
Publication bias was rated as either suspected or not suspected; a rating of 'suspected' was given if there was evidence of publication bias from a funnel plot. Due to only intervention studies being considered, upgrading of the certainty of evidence was not considered.
We interpreted the certainty of the evidence as follows:
high: the review authors are very confident that the true effect is similar to the estimated effect;
moderate: the review authors believe that the true effect is probably close to the estimated effect;
low: the true effect might be markedly different from the estimated effect;
very low: the true effect is markedly different from the estimated effect.
Results
Description of studies
Results of the search
We identified 4733 studies through database searching from January 2012 to November 2021. After removal of duplicates, we screened 3392 records by title and abstract. We excluded 3352 records, and assessed 40 full‐text records for eligibility (35 papers from searches, and five full‐text papers identified from previous published review version (Tusting 2013)). After full‐text assessment, we included 16 full‐text articles in this review update, excluded 23 reports and found one ongoing study. The study selection process is shown in Figure 2.
2.
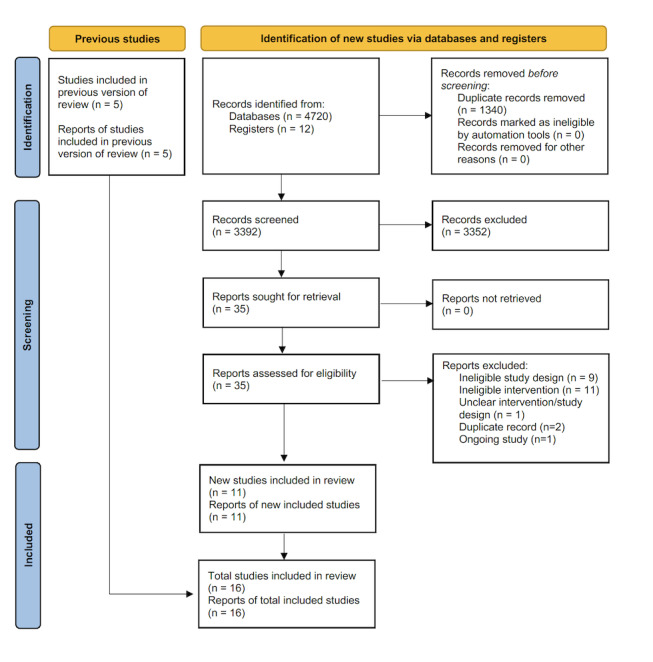
Study flow diagram. n: number.
The previous version of this review identified 13 studies for inclusion, but only six studies evaluated habitat modification and habitat manipulation interventions (Tusting 2013). Of these six studies, we included five in this review update (Castro 2009; Samnotra 1980; Santiago 1960; Sharma 2008; Shililu 2007). We excluded one study because it described a habitat modification intervention that was so poorly described it was unclear when the construction of the modification started or whether it was complete by the end of the study (Balfour 1936). The two additional LSM interventions included in the review (use of larvivorous fish, or larviciding with no habitat modification or manipulation) were recently assessed in two other Cochrane Reviews (Choi 2019; Walshe 2017).
Included studies
We have presented the characteristics of the 16 included studies in the Characteristics of included studies table, and additional information in Appendix 2.
Design
Of the 16 included studies, one was a parallel RCT (Wamae 2010), five were cRCTs (Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007), six were CBA studies (Castro 2009; Sahu 2014; Samnotra 1980; Santiago 1960; Sharma 2008; Yohannes 2005), three were non‐RCTs (Djegbe 2020; Imbahale 2011; Imbahale 2012), and one was an uncontrolled BA study (Lee 2010).
Location
Eleven studies were conducted in Africa (Kenya, Eritrea, Tanzania, Malawi, Benin, and Ethiopia) (Castro 2009; Djegbe 2020; Imbahale 2011; Imbahale 2012; Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007; Wamae 2010; Yohannes 2005), and five studies in Asia (Philippines, India, Singapore, and Sri Lanka) (Lee 2010; Sahu 2014; Samnotra 1980; Santiago 1960; Sharma 2008).
Interventions
The purpose of the intervention was irrigation in two studies (Djegbe 2020; Sharma 2008). The purpose of the intervention was LSM in the remaining 14 studies. The types of interventions were classified into four comparisons.
Comparison 1. habitat manipulation (subcategory: 1.1. water management approaches (six studies); 1.2. shading management approaches (three studies); 1.3. other/combined management approaches (two studies)) versus no intervention.
Comparison 2. habitat manipulation with larviciding versus no intervention (two studies).
Comparison 3. combination of habitat manipulation and modification versus no intervention (two studies).
Comparison 4. combination of habitat manipulation and modification with larviciding versus no intervention (three studies).
Note: two studies assessed different interventions, where one study assessed habitat modification (drainage of canals, land levelling, or filling of ditches with soil) and habitat manipulation (shade management) (Imbahale 2012). The second study assessed two eligible habitat manipulation strategies: intermittent flooding and minimal tillage (land levelling was not an eligible intervention to consider) (Djegbe 2020). The specific interventions of the included studies are described below.
1. Habitat manipulation versus no intervention
Nine studies assessed the effects of habitat manipulation versus no intervention (Imbahale 2011; Imbahale 2012; Kibret 2018; Munga 2013; Mutero 2000; Sahu 2014; Santiago 1960; Sharma 2008; Wamae 2010). The habitat manipulation interventions took either a water management approach, shading management approach or another/combined management approach.
1.1. Water management approaches
Six studies compared the effect of water management as a habitat manipulation approach versus no intervention (Djegbe 2020; Kibret 2018; Mutero 2000; Sahu 2014; Santiago 1960; Sharma 2008). The specific interventions considered were:
intermittent flooding versus continuous flooding of irrigated rice fields (non‐LSM purpose) (Djegbe 2020);
different drawdown rates of water versus no drawdown in ground pools (Kibret 2018);
different flooding and draining regimens versus continuously flooding of irrigated rice fields (Mutero 2000);
spillways (automatic syphons) versus no spillway across streams (Santiago 1960);
floodgates (sluice gates) versus no flood gates on a bed dam (Sahu 2014);
floodgates (sluice gates) versus no flood gates on a dam (non‐LSM purpose) (Sharma 2008).
1.2. Shading management approaches
Three studies compared the effect of shading management as a habitat manipulation approach versus no intervention (Imbahale 2012; Imbahale 2011; Wamae 2010). The specific interventions considered were:
shading with a range of crop and non‐crop plants versus no shading (Imbahale 2011);
shading with arrowroot versus no shading (Imbahale 2012);
shading with Napier grass versus no shading (Wamae 2010).
1.3. Other/combined management approaches
Two studies compared the effect of other/combination management as a habitat manipulation approach versus no intervention (Djegbe 2020; Munga 2013). The specific interventions considered were:
minimal tillage versus deep tillage of irrigated rice fields (non‐LSM purpose) (Djegbe 2020);
disturbance of mosquito aquatic habitat with grass clearing and water replenishment versus no disturbance (Munga 2013).
2. Habitat manipulation with larviciding versus no intervention
Two studies assessed the effects of habitat manipulation with larviciding versus no intervention (Castro 2009; Samnotra 1980). The specific interventions considered were:
reduce or removal of domestic larval habitat sites with larviciding versus no intervention (Samnotra 1980);
drain cleaning, grass cutting, and minor repairs (e.g. slab replacement) with larviciding versus no intervention (Castro 2009).
3. Combination of habitat manipulation and modification versus no intervention
Two studies assessed the combined effects of habitat manipulation and modification versus no intervention (Imbahale 2012; Yohannes 2005). The specific intervention considered was:
construction of drainage canals, prohibition, and filling of crossing points of cattle and humans along riverbed; draining the base of dam embankment; and shading using papyrus and other reeds versus no intervention (Yohannes 2005);
drainage of canals, land levelling, or filling ditches with soil versus no intervention (Imbahale 2012).
4. Combination of habitat manipulation and modification with larviciding versus no intervention
Three studies assessed the combined effect of habitat manipulation and modification with larviciding versus no intervention (Lee 2010; McCann 2021; Shililu 2007). The specific interventions considered were:
filling or drainage or elimination of rain pools, puddles at water supply points, and stream bed pools with larviciding versus no intervention (Shililu 2007);
filling or draining of water bodies with larviciding versus no intervention (McCann 2021);
shoreline work, improvement to drainage, maintenance of drains, clearing of vegetation and undergrowth, filling up pools of water, with larviciding (note: uncontrolled study, thus no comparator group) (Lee 2010).
Larviciding
Five studies combined habitat modification or manipulation with larviciding (Castro 2009; Lee 2010; McCann 2021; Samnotra 1980; Shililu 2007). The larvicides used were Bacillus thuringiensis israelensis (Bti) alone (Lee 2010; McCann 2021); pirimiphos‐methyl alone (Samnotra 1980); Bti, Bacillus sphaericus (Bsph), and temephos in rotation (Shililu 2007); the fifth study did not specify the larvicide used (Castro 2009).
Co‐interventions
Included studies implemented a range of different co‐interventions alongside habitat modification or habitat manipulation. These included case management and treatment for fever cases (Samnotra 1980); IRS with dichlorodiphenyltrichloroethane (DDT) (Castro 2009; Yohannes 2005); ITNs, IRS, and case management (Imbahale 2012); "routine malaria control activities under the primary health care system" (case management) and IRS with DDT (Sharma 2008); or the national malaria control programme interventions (McCann 2021). Two studies provided no information about co‐interventions (Kibret 2018; Santiago 1960), and a further study was unclear, but possibly used IRS with DDT (Sahu 2014). The remaining seven studies reported no co‐interventions; however, for one of these studies, ITNs and IRS were conducted as part of national malaria control programming (coverage not reported) (Shililu 2007).
Outcomes
One study reported the incidence of clinical malaria, which was in participants of all ages (Sharma 2008). Five studies measured malaria parasite prevalence, with studies reporting the outcome in infants and children aged two to 10 years (Santiago 1960), children under the age of 10 years (Yohannes 2005), children aged six to 59 months and women aged 15 to 29 years (McCann 2021), or in participants of all ages (Sharma 2008; Castro 2009). None of the studies reported the primary outcome of malaria parasitaemia incidence. One study reported mean haemoglobin levels (McCann 2021). None of the included studies reported other secondary epidemiological outcomes such as incidence of severe malaria, anaemia prevalence, mortality rate due to malaria, or hospital admissions for malaria.
Most studies reported secondary entomological outcomes. Thirteen studies evaluated the density of immature mosquitoes, with 10 studies reporting density specific to larvae (Djegbe 2020; Imbahale 2011; Imbahale 2012; Kibret 2018; Mutero 2000; Samnotra 1980; Santiago 1960; Shililu 2007; Wamae 2010; Yohannes 2005); two studies to larvae plus pupae (Castro 2009; Munga 2013); one study to larvae or pupae (or both) (Sahu 2014). Six studies reported the density of adult mosquitoes (Lee 2010; McCann 2021; Samnotra 1980; Santiago 1960; Shililu 2007; Yohannes 2005). Two studies reported EIR (McCann 2021; Santiago 1960).
None of the included studies reported on harms as adverse events or unintended consequences associated with the intervention.
Vectors and eco‐epidemiology of study areas
Eleven studies were undertaken in Africa and targeted An gambiae or An arabiensis (or both) as primary vectors (Castro 2009; Djegbe 2020; Imbahale 2011; Imbahale 2012; Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007; Wamae 2010; Yohannes 2005). Other Anopheles spp collected within these studies included: An funestus,An coustani, An cinereus, An rufipes, An marshalli, An maculipalpis, An azaniae, An implexus, An pretoriensis, An d'thali, An squamosus, An adenensis, An demeilloni, and An pharoensis. In the five studies conducted in Asia, the most common vectors reported were An minimus flavirostris, An fluviatilis, An culicifacies, An stephensi, An sundaicus,An maculates, An maculipennis, An vagus, An annularis, and An subpictus (Lee 2010; Sahu 2014; Samnotra 1980; Santiago 1960; Sharma 2008). Most studies that reported entomological outcomes did not analyse the data by Anopheles spp, while other studies only analyzed data on the major vector.
Ten studies were conducted in rural areas (Imbahale 2012; Kibret 2018; Lee 2010; Munga 2013; Mutero 2000; Sahu 2014; Sharma 2008; Shililu 2007; Wamae 2010; Yohannes 2005); the remaining studies were conducted in solely urban areas (Castro 2009; Samnotra 1980), solely semi‐urban areas (Imbahale 2011), or a combination of urban and semi‐urban areas (Santiago 1960). Two studies did not provide sufficient information to ascertain the eco‐epidemiology of the study areas (Djegbe 2020; McCann 2021).
Responsibility of the delivery of the intervention
The interventions within the included studies were co‐ordinated and performed by different institutions or people (or both). The study staff and the local community were involved in the intervention activities in six studies (Castro 2009; Djegbe 2020; McCann 2021; Samnotra 1980; Shililu 2007; Yohannes 2005). One study reported the local community and the Public Health Service were responsible (Santiago 1960). In four studies the institutions responsible for the delivery of the interventions were the Armed Forces (Lee 2010), an irrigation and agricultural development experimental station (Mutero 2000), or the District Rural Development Agency (DRDA) (Sahu 2014; Sharma 2008). The remaining five studies did not clearly report who delivered the interventions (Imbahale 2011; Imbahale 2012; Kibret 2018; Munga 2013; Wamae 2010).
Excluded studies
We excluded 23 studies after full‐text review. Reasons are detailed in the Characteristics of excluded studies table, and below.
Study design did not match inclusion criteria, specifically: a review paper (Laporta 2019), modelling papers (Kibret 2019; Ohta 2014), a cross‐sectional study (Jaleta 2013), and four observational studies (Amerasinghe 1991; Getachew 2020; Gezie 2018; Thapar 2019).
Intervention did not match inclusion criteria, specifically: abstracts from a symposium with wrong or no intervention in place (Clark 2012; Clark 2013; Clark 2014; Cohnstaedt 2016; Cohnstaedt 2017), no intervention described (Kibret 2014; Kiszewski 2014; Nasreen 2016; Saxena 2014; Srivastava 2013), and ineligible intervention described (Frake 2017).
Intervention was too poorly reported to determine when it was initiated and whether the design was observational in nature (Balfour 1936).
Ineligible study design and ineligible outcome measures (Tchoumbou 2020).
Duplicate records of an included study (Phiri 2021; van den Berg 2018) — duplicates of McCann 2021 included in the review.
Ongoing studies
We identified one ongoing open‐label, block‐cluster sequential multiple assignment RCT with variable number of arms (adaptive design), with baseline period with no cross‐over in 36 randomly selected clusters (village or several neighbouring villages) comprising low and high elevation localities in western Kenya (Zhou 2020). The outcome measures to be assessed at the end of the study are clinical malaria incidence, density of adult mosquitoes, and EIRs. The study is conducted in two stages. In stage 1, clusters are equally randomized to one of three groups for 12 months' follow‐up:
LLINs: 2% permethrin with 150 denier yarn or deltamethrin with either 75 denier yarn or 100 denier yarn;
piperonyl butoxide (PBO)‐treated LLINs: 2% permethrin and 1% PBO. One net per two people, with appropriate training for its proper usage;
LLIN with IRS with microencapsulated pirimiphos‐methyl (Actellic 300CS) once per year.
In stage 2, if the stage 1 intervention of PBO LLINs was 'effective' within cluster, then intervention continued; if 'not effective', then clusters were equally randomized to one of two groups for 18 months of follow‐up:
PBO LLIN plus habitat manipulation and modification with larviciding: physical filling or removal of temporary larval habitats and larviciding of semipermanent and permanent habitats, larviciding with Bti (6% by weight) and Bsph (1% by weight), retreatment every four to five months;
intervention determined by an enhanced reinforcement learning method.
In stage 2, if stage 1 intervention of LLINs with IRS was 'effective' within cluster, then intervention continued; if 'not effective', then the cluster was equally randomized to one of two groups for 18 months of follow‐up:
LLIN with IRS plus habitat manipulation and modification with larviciding: physical filling or removal of temporary larval habitats and larviciding of semipermanent and permanent habitats, larviciding with Bti (6% by weight) and Bsph (1% by weight), retreatment every four to five months;
PBO LLINs with IRS.
Risk of bias in included studies
The summary risk of bias assessments at results level for 15 of the included studies is shown in Appendix 3. We classified the remaining included study, which used an uncontrolled design, at critical overall risk of bias due to the lack of a comparator group for the secondary outcome measure, density of adult mosquitoes (Lee 2010).
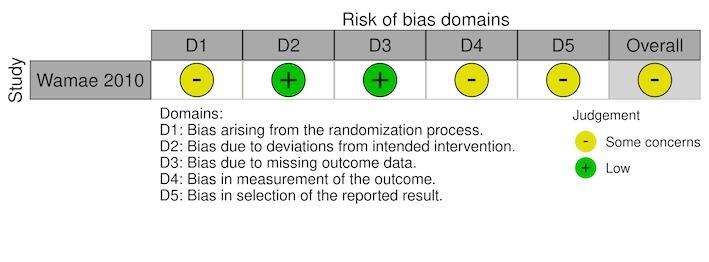
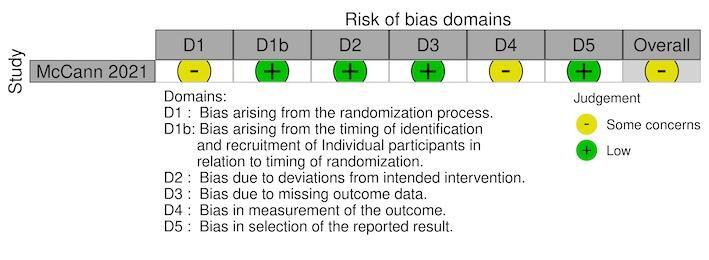
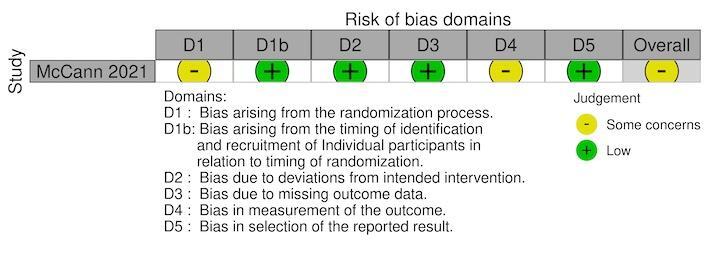
For the RCTs, we assessed the risk of bias using either the standard or cRCT extension to the Cochrane RoB 2 tool (Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007; Wamae 2010). Five RCTs reported the secondary outcome, density of immature mosquitoes; with one RCT also reported the density of adult mosquitoes (Shililu 2007). One cRCT reported parasite prevalence, density of adult mosquitoes, haemoglobin levels, and EIR (McCann 2021). The overall risk of bias was 'some concerns' for all five cRCTs and the individual RCT (Figure 3; Figure 4; Figure 5; Figure 6; Figure 7).
3.
Risk of bias traffic light plot of included studies with cluster‐randomized controlled trial design for primary outcome, parasite prevalence.
4.
Risk of bias traffic light plot of included studies with cluster‐randomized controlled trial designs for secondary outcome, density of immature or adult mosquitoes.
5.
Risk of bias traffic light plot of included studies with randomized controlled trial designs for secondary outcome, density of immature mosquitoes.
6.
Risk of bias traffic light plot of included studies with cluster‐RCT design for secondary outcome, mean haemoglobin levels
7.
Risk of bias traffic light plot of included studies with cluster‐randomized controlled trial design for secondary outcome, entomological inoculation rate.
RCTs assessed using RoB 2 tool
We assessed five cRCTs (Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007) and one RCT (Wamae 2010) using the RoB 2 tool.
Randomization process
We identified some concerns in relation to the bias arising from the randomization process in all five cRCTs and the RCT (Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007; Wamae 2010). Although all six studies reported that the interventions were 'randomly' allocated, methods for generating the randomization sequence were missing from three cRCTs (Kibret 2018; Munga 2013; Shililu 2007) and one RCT (Wamae 2010). One cRCT reported that the randomization sequence was performed using block sizes of four (Mutero 2000), and one cRCT reported the randomization sequence was performed using a two‐stage approach by drawing lots from opaque folded cards (McCann 2021). There were some concerns regarding allocation concealment in five cRCTs (Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007) and one RCT (Wamae 2010) due to none of the studies reporting whether the randomization sequence was blinded (allocation concealment).
Timing of identification and recruitment of individuals in relation to timing of randomization (cRCTs only)
For five cRCTs, the bias arising from the timing of identification and recruitment of individuals in relation to timing of randomization was at low risk of bias (Kibret 2018; McCann 2021; Munga 2013; Mutero 2000; Shililu 2007).
Deviations from the intended interventions
Although the trial personnel were aware of the assigned interventions during the trial, because there were no deviations from the intended intervention and no clusters or individuals were analyzed in a different group to the one which they were randomized, four cRCTs (Kibret 2018; McCann 2021; Munga 2013; Shililu 2007) and one RCT (Wamae 2010) were at low risk of bias for this domain. For one cRCT there was a high risk of bias as there was evidence of contamination of the intervention, due to seepage occurring from continuously flooded plots to adjacent subplots, thereby resulting in an unbalance between groups, which likely affected the outcome (Mutero 2000).
Missing outcome data
For the outcome, density of immature mosquitoes, there was a low risk of bias within four cRCTs (Kibret 2018; Munga 2013; Mutero 2000; Shililu 2007) and one RCT (Wamae 2010), since data were available for all, or nearly all, of individuals randomized and for all clusters randomized. There was a low risk of for the studies that reported density of adult mosquitoes (McCann 2021; Shililu 2007), malaria parasite prevalence (McCann 2021), haemoglobin levels (McCann 2021), and EIR (McCann 2021), for the same reason.
Measurement of the outcome
For the outcome, density of immature mosquitoes, although the outcome assessors were aware of the intervention received by the individuals, the assessment of the outcome was unlikely to be influenced by this knowledge of the intervention received due to it being an objective measures which was assessed using standard methods; therefore, a moderate risk of bias was found within all the RCTs (Kibret 2018; Munga 2013; Mutero 2000; Shililu 2007; Wamae 2010). A moderate risk of bias was given for the studies which reported density of adult mosquitoes (McCann 2021; Shililu 2007), malaria parasite prevalence (McCann 2021), haemoglobin levels (McCann 2021), and EIR (McCann 2021), for the same reason.
Selection of the reported results
For the outcome, density of immature mosquitoes, we deemed three cRCTs at low risk of bias due to the reported outcome data being unlikely to be selected on the basis of the results (Munga 2013; Mutero 2000; Shililu 2007). The fourth cRCT was given some concerns risk of bias due to reporting multiple results based on or wet and dry seasons (Kibret 2018); however, the results were similar therefore not suggesting serious selection. For the outcomes, density of immature mosquitoes and density of adult mosquitoes, we deemed one RCT to have some concerns for risk of bias due to no prespecified analysis plan; although the RCT reported multiple results based on stratification of outcome by village; the results were similar therefore not suggesting serious selection (Wamae 2010). For the remaining outcomes of malaria parasite prevalence, density of adult mosquitoes, haemoglobin levels, and EIR, one cRCT was at low of risk of bias due to the reported outcome data being unlikely to be selected on the basis of the results (McCann 2021).
Non‐randomized controlled studies assessed using ROBINS‐I tool
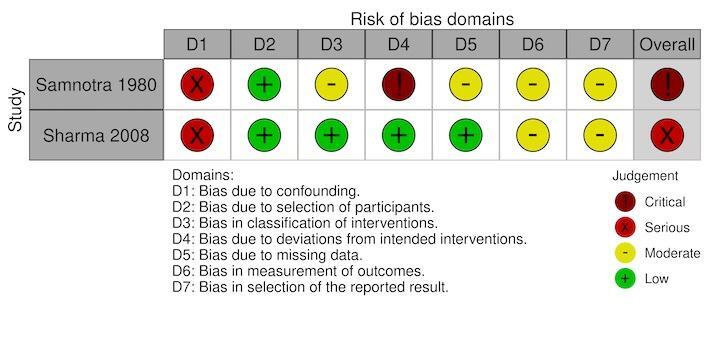
We assessed nine included studies for risk of bias using the ROBINS‐I tool for the primary outcomes, clinical malaria incidence and malaria parasite prevalence; and for the secondary outcome, density of immature mosquitoes, density of adult mosquitoes and EIR (Castro 2009; Djegbe 2020; Imbahale 2011; Imbahale 2012; Sahu 2014; Samnotra 1980; Santiago 1960; Sharma 2008; Yohannes 2005).
Overall risk of bias
For one of the nine studies, Samnotra 1980, there was an overall critical risk of bias for all outcomes assessed, because of critical concerns for bias due to deviations from the intervention. The study was deemed too problematic to provide any useful evidence. The findings from this study are not reported in the results of this review; however, a full description of the intervention conducted in the study is presented in the Characteristics of included studies table. The overall risks of bias by each outcome measure for the remaining eight studies were:
clinical malaria incidence: serious in one study (Sharma 2008; Figure 8);
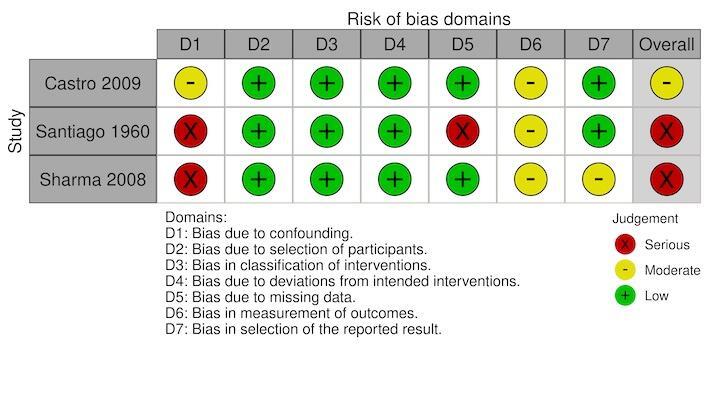
malaria parasite prevalence: serious in two studies (Santiago 1960; Sharma 2008), and moderate in one study (Castro 2009) (Figure 9);
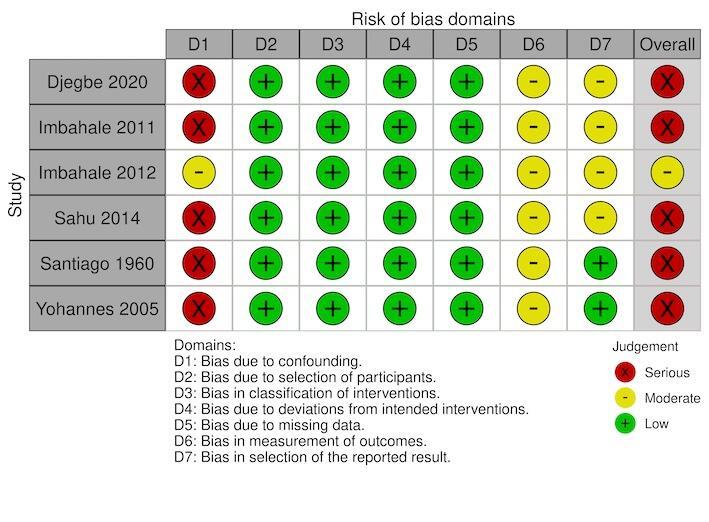
density of immature mosquitoes: serious in five studies (Djegbe 2020; Imbahale 2011; Sahu 2014; Santiago 1960; Yohannes 2005), and moderate in one study (Imbahale 2012) (Figure 10);
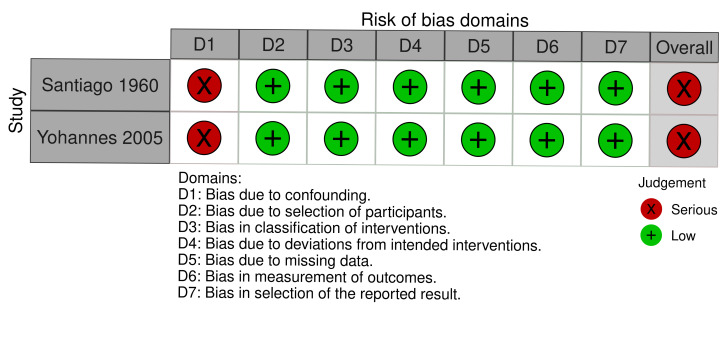
density of adult mosquitoes: serious in two studies (Santiago 1960; Yohannes 2005) (Figure 11);
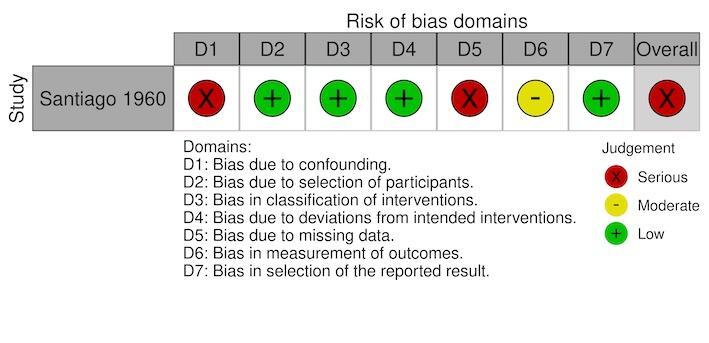
EIR: serious in one study (Santiago 1960; Figure 12).
8.
Risk of bias traffic light plot of included studies with non‐randomised designs (ROBINS‐I) for primary outcome, clinical malaria incidence.
9.
Risk of bias traffic light plot of included studies with non‐randomized designs (ROBINS‐I) for primary outcome, parasite prevalence.
10.
Risk of bias traffic light plot of included studies with non‐randomized designs (ROBINS‐I) for secondary outcome, density of immature mosquitoes.
11.
Risk of Bias traffic light plot of included studies with non‐randomised designs (ROBINS‐I) for secondary outcome, density of adult mosquitoes
12.
Risk of bias traffic light plot of included studies with non‐randomized designs (ROBINS‐I) for secondary outcome, entomological inoculation rate.
Confounding
Two studies had a moderate risk of bias where most confounding would remain between the intervention groups due to the intervention and control either being conducted within the same village with the same timings and the analysis accounting for changes from baseline (Imbahale 2012), or due to the analysis taking into account confounding of several important factors (Castro 2009). The remaining six studies had a serious risk of bias due to the differences at baseline in the outcome measure and no adjustment for important confounders (Djegbe 2020; Imbahale 2011; Sahu 2014; Santiago 1960; Sharma 2008; Yohannes 2005).
Selection of participants
All eight studies had a low risk of selection bias where there was no evidence that individuals had been selected based on their characteristics (Castro 2009; Djegbe 2020; Imbahale 2011; Imbahale 2012; Sahu 2014; Santiago 1960; Sharma 2008; Yohannes 2005).
Classification of interventions
All eight studies had a low risk of bias due to having clear classification of interventions reported (Castro 2009; Djegbe 2020; Imbahale 2011; Imbahale 2012; Sahu 2014; Santiago 1960; Sharma 2008; Yohannes 2005).
Deviations from intended interventions
All eight studies had a low risk of bias due to there being no evidence of deviations from the intended interventions (Castro 2009; Djegbe 2020; Imbahale 2011; Imbahale 2012; Sahu 2014; Santiago 1960; Sharma 2008; Yohannes 2005).
Missing data
For the primary outcome clinical malaria incidence, the single study had a low risk of bias for missing data (Sharma 2008). For the primary outcome parasite prevalence, two studies had a low risk of bias for missing data (Castro 2009; Sharma 2008); the third study had a serious risk of bias due to the differential rates of missing data between the intervention groups (Santiago 1960). For the outcome density of immature mosquitoes, all six studies had a low risk of bias for missing data (Djegbe 2020; Imbahale 2011; Imbahale 2012; Sahu 2014; Santiago 1960; Yohannes 2005). For the outcome density of adult mosquitoes, both studies has a low risk of bias for missing data (Santiago 1960; Yohannes 2005). For the outcome EIR, the single study had a serious risk of bias due to differential rates of missing data between the intervention groups and no analysis to assess the robustness to the presence of missing data (Santiago 1960).
Measurement of outcomes
For the primary outcome clinical malaria incidence, the single study had a moderate risk of bias for the measurement of outcomes due to the outcome assessor being aware of intervention implemented, but the assessment of the outcome was unlikely to be influenced by knowledge of intervention implemented (Sharma 2008). For the primary outcome parasite prevalence, all three studies had a moderate risk of bias for measurement of outcomes for the same reason (Castro 2009; Santiago 1960; Sharma 2008). For the outcome density of immature mosquitoes, all six studies had a moderate risk of bias for measurement of outcomes for the same reason (Djegbe 2020; Imbahale 2011; Imbahale 2012; Sahu 2014; Santiago 1960; Yohannes 2005). For the outcome density of adult mosquitoes, both studies had a moderate risk of bias for measurement of outcomes for the same reason (Santiago 1960; Yohannes 2005). For the outcome EIR, the single study had a moderate risk of bias for measurement of outcomes for the same reason (Santiago 1960).
Selection of the reported result
For the primary outcome clinical malaria incidence, the single study had a moderate risk of bias due to separate analyses being reported for children aged one to five years and all populations (Sharma 2008). For the primary outcome parasite prevalence, two studies had a low risk of bias (Castro 2009; Santiago 1960), but the remaining study had a moderate rating due to separate analyses being reported for children aged one to five years and all populations (Sharma 2008). For the outcome density of immature mosquitoes, two studies had a low risk of bias (Santiago 1960; Yohannes 2005); the remaining four studies had a moderate risk of bias due to presenting separate analyses for either upstream and downstream results (Sahu 2014), for different larval stages (Imbahale 2011), for each village (Imbahale 2012), or for each of the three development stages of rice (transplanting, tillering, and maturation) (Djegbe 2020). For the outcome density of adult mosquitoes, both studies had a low risk of bias (Santiago 1960; Yohannes 2005). For the outcome EIR, the single study had a low risk of bias (Santiago 1960).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The results from the studies are categorized into the type of intervention (i.e. habitat modification, habitat manipulation, combination of habitat modification and manipulation), the specific type of modification or manipulation, and the purpose of the intervention (i.e. LSM, non‐LSM).
Comparison 1. Habitat manipulation versus no intervention
Ten studies assessed habitat manipulation (temporary change to the environment) (Djegbe 2020; Imbahale 2011; Imbahale 2012; Kibret 2018; Munga 2013; Mutero 2000; Sahu 2014; Santiago 1960; Sharma 2008; Wamae 2010). This included either water management approaches (spillways across streams; floodgates; intermittent flooding; different drawdown rates of water; different flooding and draining regimens), shading management approaches (shading of drainage channels with different plants), and other/combined management approaches (minimal tillage; disturbance of aquatic habitats with grass clearing and water replenishment), which showed mixed results on entomological outcomes.
Habitat manipulation interventions may reduce the densities of adult and immature mosquitoes compared to no intervention (low‐certainty evidence; Table 1). However, the effect of habitat manipulation on malaria parasite prevalence and clinical malaria incidence is uncertain compared to no intervention because the certainty of evidence was very low (Table 1).
The findings of the individual studies are presented below in alphabetical order based on the first author of the study by water, shading, or a combination as the management approaches.
1.1. Water management approaches
Two cRCTs (Kibret 2018; Mutero 2000), three CBA studies (Sahu 2014; Santiago 1960; Sharma 2008), and one non‐RCT (Djegbe 2020) evaluated the effect of habitat manipulation using water management (using spillways, floodgates, different drawdown rates of water, or different flooding and draining regimens) versus no intervention. Five studies reported entomological outcomes (Djegbe 2020; Kibret 2018; Mutero 2000; Sahu 2014; Santiago 1960), and two studies reported the epidemiological outcomes, malaria parasite prevalence (Santiago 1960; Sharma 2008) and clinical malaria incidence (Sharma 2008).
One non‐RCT, in Benin, assessed the effect of intermittent flooding compared to continuous flooding during rice cultivation (Djegbe 2020). Intermittent flooding significantly reduced the number of Anopheles larvae in all stages of rice development compared to control (continuous flooding), relating to an 80.8% reduction in the densities during transplanting periods (P < 0.001), 30.8% reduction during tillering periods (P < 0.001), and 40.7% reduction during maturation periods (P < 0.001) (CIs not reported).
One cRCT, conducted in Ethiopia, assessed the effectiveness of water level management as a habitat manipulation intervention with the purpose of targeting larvae habiting natural ground pools (Kibret 2018). Compared with control, the change from baseline in density of immature mosquitoes during the main transmission season was generally reduced by 30% with a drawdown rate of 10 mm/day (odds ratio (OR) 0.70; P < 0.05), 70% with a drawdown rate of 15 mm/day (OR 0.29; P < 0.05), and 84% with a drawdown rate of 20 mm/day (OR 0.16; P < 0.05) (CIs not reported).
One cRCT, performed in Kenya, evaluated four different water regimens for irrigating rice fields (Mutero 2000). The absolute number of larvae collected was the greatest in plots using intermittent irrigation (4306 larvae), followed by using 'drained then flooded' and 'flooded then flooded', with continuous flooding (control) resulting in the lowest number of larvae (425 larvae) (insufficient data to allow statistical analysis).
One CBA study, conducted in the Philippines, used automatic syphons to flush water in two main streams to control larvae (Santiago 1960). There was no difference in malaria parasite prevalence in children aged two to 10 years between the intervention and control (no flushing) areas at baseline (31/560 with intervention versus 11/277 with control; RR 1.39, 95% CI 0.71 to 2.73; Analysis 1.1); however, there was a decrease in prevalence during the first year of intervention (0/586 with intervention versus 24/280 with control; RR 0.01, 95% CI 0.00 to 0.16; Analysis 1.1). The monthly mean density of larvae per dip in the intervention area decreased from a mean of 1.40 (SD 0.25) to 0.059 (SD 0.20) and in the control area from a mean of 0.7 (SD 0.30) to 0.49 (SD 0.15), corresponding to a 0.43 reduction in the mean density of larvae per dip (95% CI 0.30 to 0.56; Analysis 1.2). The mean density of adult mosquitoes per month in the intervention area was reduced by 0.4 (from 0.4 to 0.0) but there was no change in the control area (0.3) (insufficient data to statistically compare or estimate effect size). At baseline, there was no difference in EIR in children under one year of age between the intervention and control groups (4/175 with intervention versus 2/54 with control; RR 0.62, 95% CI 0.12 to 3.28; Analysis 1.3), with some evidence of a difference in EIR during the first year of the intervention (0/222 with intervention versus 3/83 with control; RR 0.05, 95% CI 0.00 to 1.03; Analysis 1.3).
1.1. Analysis.

Comparison 1: Spillways across streams versus no intervention, Outcome 1: Malaria parasite prevalence (children aged 2 to 10 years)
1.2. Analysis.

Comparison 1: Spillways across streams versus no intervention, Outcome 2: Mean density of immature mosquitoes
1.3. Analysis.

Comparison 1: Spillways across streams versus no intervention, Outcome 3: Entomological inoculation rate (EIR)
One CBA study, conducted in India, used floodgates (sluice gates) across streams to target larvae (Sahu 2014). Baseline data showed similar densities of immature mosquitoes between the intervention and control groups, both upstream (range in number per dip: intervention 0 to 0.05; control 0.01 to 0.03) and downstream (range in number per dip: intervention 0.02 to 0.04; control 0 to 0.02) of the dams. In the postintervention period, there was a decrease in the density of immature mosquitoes between the intervention and control groups downstream of the dams (P < 0.01); there was no difference upstream.
One CBA study, conducted in India, used floodgates (sluice gates) for irrigation and to discharge excess water (Sharma 2008). There were differences in baseline rates of clinical malaria between the intervention and control groups in all participants (643.9/1000 with intervention versus 274.8/1000 with control) and children aged one to five years (1304.3/1000 with intervention versus 785.7/1000 with control). In the postintervention period, there was a reduction of malaria incidence in the intervention compared to the control group in children aged one to five years (181.8/1000 with intervention versus 1000/1000 with control; P < 0.01; CIs not reported). Malaria parasite prevalence in the baseline period was similar in the intervention and control groups (17.6% with intervention and 18.9% with control; P = 0.75). Compared to the baseline, in the postintervention period, the study reported a decrease in malaria parasite prevalence in the intervention group (P < 0.01), but no decline in the control group (P > 0.05; CIs not reported).
1.2. Shading management approaches
One RCT (Wamae 2010) and two non‐RCTs (Imbahale 2011; Imbahale 2012) assessed the effect of shade management as a habitat manipulation intervention compared to no intervention. The three studies only reported entomological outcomes.
One non‐RCT, conducted in Africa, used shading with local plants to target larvae in irrigated agricultural lands (Imbahale 2011). Four locally grown plant species, Napier grass (Pennisetum purpureum), arrowroot (Maranta arudinacea), papyrus reeds (Cyperus spp), and weeded and unweeded rice (Oryza sativa), were planted as the intervention. Compared to the control (unplanted) habitat, there were postintervention reductions in the density of early instars of anophelines (all species) for shading with Napier grass (OR 0.41, 95% CI 0.24 to 0.72; Analysis 2.1), arrowroot habitats (OR 0.58, 95% CI 0.33 to 1.00; Analysis 2.1), and unweeded rice habitats (OR 0.49, 95% CI 0.25 to 0.96; Analysis 2.1). There were reductions in late‐stage larvae for unweeded rice habitats (OR 0.09, 95% CI 0.01 to 0.76; Analysis 2.1) and arrowroot habitats (OR 0.05, 95% CI 0.01 to 0.38; Analysis 2.1), but not for Napier grass (OR 0.50, 95% CI 0.18 to 1.41; Analysis 2.1). There were no differences between the intervention and control groups postintervention for weeded rice. An gambiaes.l. was found in unweeded rice and control habitats and An coustani was present in all habitats, except for those planted with unweeded rice.
2.1. Analysis.

Comparison 2: Shading using local plants, Outcome 1: Density of immature mosquitoes
One non‐RCT, conducted in Kenya, assessed the effect of shading with arrowroot to target larvae (Imbahale 2012). The intervention was shading by arrowroot crops. The during and postintervention mean densities of early and late instars were zero in the intervention group; therefore no statistical testing could be performed.
One RCT, conducted in Kenya, assessed planting with Napier grass to target larvae (Wamae 2010). The postintervention mean densities of the intervention groups were: village 1: 0.24 (SD 0.08); village 2: 0.45 (SD 0.09), and in the control groups: village 1: 1.61 (SD 0.24); village 2: 3.82 (SD 0.34). This corresponded to a reduction in the mean number of An gambiaes.l. larvae in the intervention compared to the control group within each village (village 1: 78.4% reduction; analysis of variance (ANOVA), P < 0.0001; village 2: 88.0% reduction; ANOVA, P < 0.0001; CIs not reported).
1.3. Other/combination management approaches
One cRCT (Munga 2013) and one non‐RCT (Djegbe 2020) assessed other or combination management approaches as a habitat manipulation intervention. They only reported entomological outcomes.
One cRCT, conducted in Kenya, assessed the effect of disturbance of the mosquito aquatic habitat (Munga 2013). Habitats in the intervention groups were cleared of grass and had water replenishment from the local stream either every 10 days (frequent disturbance), or every 20 days (intermediate disturbance). The habitats in the control group where left undisturbed (no clearing of grass and no water replenishment). Postintervention, compared to non‐disturbed control habitats, there was a 1.7‐fold increase in larval density of frequently disturbed habitats (P < 0.001; CIs not reported) and a 1.3‐fold increase in the larval density of larvae in intermediate disturbed habitats (P < 0.05; CIs not reported).
One non‐RCT, conducted in Benin, assessed the effect of tillage during rice cultivation (Djegbe 2020). Intervention plots used minimal tillage and control plots used deep tillage. Minimal tillage reduced the number of Anopheles larvae in all stages of rice development compared to control (deep tillage) (P < 0.001 for all three comparisons for the stages of rice development) (no estimates of effect reported).
Comparison 2. Habitat manipulation with larviciding versus no intervention
Two CBA studies assessed habitat manipulation with larviciding (Castro 2009; Samnotra 1980). This included reducing or removal of habitat sites; and drain cleaning, grass cutting, and minor repairs. Both studies reported epidemiological outcomes; however, only one study reported entomological outcomes (Samnotra 1980).
The effect of habitat manipulation delivered with larviciding on malaria parasite prevalence compared to no intervention is uncertain as the certainty of evidence is very low (Table 2).
The findings of the individual studies are presented below in alphabetical order based on the first author of the study.
One CBA study, performed in Tanzania, evaluated repairing and cleaning drains, cutting grass, and making minor repairs to the drains (e.g. slab replacement) to target larvae inhabiting drains, with larviciding (Castro 2009). At postintervention, the intervention sites had lower odds of malaria parasite prevalence than the control group (adjusted OR 0.59, 95% CI 0.42 to 0.83; Analysis 3.1).
3.1. Analysis.

Comparison 3: Repairing and clearing of drains, cutting grasses, and making minor repairs (e.g. slab replacement) combined with larviciding, Outcome 1: Malaria parasite prevalence
One CBA study, performed in India, evaluated encouraging households to reduce or remove domestic mosquito aquatic habitat sites, including tanks, pitchers, and cisterns to target larvae, with larviciding (Samnotra 1980). The intervention was not successfully implemented, and as a consequence, the results from this study reflected solely the effect of larviciding compared to no intervention, which is beyond the scope of this review.
Comparison 3. Combination of habitat manipulation and modification versus no intervention
One CBA (Yohannes 2005) and one non‐RCT (Imbahale 2012) assessed a combination of habitat manipulation and permanent change (habitat modification). This included drainage canals, filling, and planting of papyrus and other reeds for shading near dams; and drainage of canals, removal of debris, land levelling, and filling ditches. The studies only reported entomological outcomes.
The combination of habitat manipulation and modification may reduce the density of adult and immature mosquitoes compared to no intervention (low‐certainty evidence; Table 3).
The findings of the individual studies are presented below in alphabetical order based on the first author of the study.
One non‐RCT, conducted in Kenya, assessed the effect of drainage of canals, removal of debris, land levelling, or filling ditches with soil to prevent any water stagnating to target larvae inhabiting a range of temporary and permanent habits (Imbahale 2012). In one village, postintervention abundance of early and late instar Anopheles were less likely to be sampled from drainage compared to control habitats (early instars: OR 0.45; P < 0.05; late instars: OR 0.13; P < 0.05; CIs not reported). However, in the second village, postintervention late instars were less likely to be sampled from drainage, but there was no effect for early instars compared to control habitats (early instars: OR 0.91; P > 0.05; late instars: OR 0.90; P < 0.05; CIs not reported).
One CBA study, conducted in Ethiopia, assessed draining a dam embankment, construction of drainage canals, prohibition and filling of crossing points of cattle and humans along riverbeds to prevent the destruction of plants and the creation of mosquito aquatic habitat sites with hoof‐footprints, planting of papyrus and other reeds to create shading, to target larvae (Yohannes 2005). There was a reduction in the total number (density) of third and fourth instars (119 with intervention versus 673 with control) and all larval stages (163 with intervention versus 720 with control) belonging to An arabiensis in the intervention village compared to the baseline phase; however, no statistical testing was performed to compare the change in the density of larvae between the intervention and control groups. There was a 49% significant relative reduction in the change in mean number (density) of adult mosquitoes in the intervention village, adjusting for the change in the control village (95% CI 46.6% to 50%; intervention village: geometric mean 4.01 to 0.66; control village: geometric mean 0.63 to 0.20).
Comparison 4. Combination of habitat manipulation and modification with larviciding versus no intervention
Two cRCTS (McCann 2021; Shililu 2007) and one uncontrolled BA study (Lee 2010) assessed combining manipulation and modification with larviciding. This included filling or drainage of water bodies; filling, draining, or eliminating rain pools and puddles at water supply points and stream bed pools; and shoreline work, improvement and maintenance to drainage, clearing vegetation and undergrowth, and filling pools.
The combination of habitat manipulation and modification with larviciding probably makes little or no difference to malaria parasite prevalence, haemoglobin levels, or EIR compared to no intervention (moderate‐certainty evidence; Table 4), but probably reduces the density of immature and adult mosquitoes (moderate‐certainty evidence; Table 4).
The findings of the individual studies are presented below in alphabetical order based on the first author of the study.
One uncontrolled BA study, conducted in Singapore, evaluated shoreline works, drainage, maintenance of drains vegetation clearing, and filling up pools of water, with larviciding (Lee 2010). Due to the design of the study, the effect of the intervention could not be determined; however, there was a 94% reduction in the mean number of mosquitoes postintervention.
One cRCT, conducted in Malawi, assessed the effect of filling or draining of water bodies, when feasible and if the community did not use the water for a designated purpose, with larviciding (McCann 2021). Malaria parasite prevalence decreased over the three years of the trial in all age categories; however, two‐year aggregated outcome data found no differences between the intervention and control groups for positivity relating to parasite prevalence (women aged 15 to 49 years: adjusted OR 0.80, 95% CI 0.41 to 1.55; children aged 6 to 59 months: adjusted OR 1.80, 95% CI 0.90 to 3.60; children aged 6 to 23 months: adjusted OR 2.77, 95% CI 0.64 to 11.94; Analysis 4.1). Haemoglobin levels increased over the three years of the trial in all age categories; however, two‐year aggregate outcome data found no differences between the intervention and control groups for absolute haemoglobin levels (women aged 15 to 49 years: adjusted MD −0.11 g/dL (95% CI −0.37 to 0.15); children aged 6 to 59 months: adjusted MD −0.02 g/dL, 95% CI −0.35 to 0.31; children aged 6 to 23 months: adjusted MD −0.40 g/dL, 95% CI −0.90 to 0.10; Analysis 4.2). There were no differences in the density of female adult mosquitoes indoors or outdoors between the intervention and control groups using two‐year aggregated outcome data (indoors: adjusted RaR 2.18, 95% CI 0.44 to 10.9; outdoors: adjusted RaR 1.95, 95% CI 0.45 to 8.41). However, there was an increase in density for An arabiensis females indoors in the intervention group compared to the control group (adjusted RaR 11.30, 95% CI 2.12 to 60.30), but not for outdoors (RaR 0.91, 95% CI 0.28 to 2.94), or for An funestus female mosquitoes (indoor: adjusted RaR 0.41, 955 CI 0.07 to 2.56; outdoors: adjusted RaR 3.49, 95% CI 0.42 to 28.8; Analysis 4.3). The EIR was reported to fluctuate annually but declined over the three years of the trial. The mean nightly EIR at the end of the trial period was zero across the intervention and control group and, therefore, could not be statistically assessed.
4.1. Analysis.

Comparison 4: Filling and draining water bodies with larviciding versus no intervention, Outcome 1: Malaria parasite prevalence
4.2. Analysis.

Comparison 4: Filling and draining water bodies with larviciding versus no intervention, Outcome 2: Haemoglobin levels (g/dL)
4.3. Analysis.

Comparison 4: Filling and draining water bodies with larviciding versus no intervention, Outcome 3: Entomological inoculation rate (EIR)
One cRCT, conducted in Eritrea, evaluated filling, drainage, or elimination of rain pools and puddles at water supply points and stream pools bedded with sediment, together with larviciding (Shililu 2007). Postintervention, there was a reduction in the mean larval density in the intervention compared to control villages (ANOVA, P < 0.001). Quantitative data were only reported for one zone, where the mean number of larvae was smaller in the intervention compared to the control villages (mean number 0.87 (SD 0.04) with intervention versus 3.17 (SD 0.11) with control). Postintervention, there was a reduction in the total number (density) of adult An arabiensis in the intervention compared to control villages (ANOVA, P < 0.05; data on adult densities not reported).
Discussion
We included 16 studies assessing the impact of a wide range of various habitat modification or habitat manipulation (or both) on malaria transmission. Of the included studies, one was an RCT, five were cRCTs; six were CBA studies, three were non‐RCTs, and one was an uncontrolled BA study. Five studies reported epidemiological outcomes and 15 studies reported entomological outcomes. None of the studies reported on the environmental impacts associated with the intervention. None of the included studies has at low overall risk of bias. The RCT and cRCTs were deemed to have some concerns, and the other designs ranged in their risk of bias from moderate through to critical, with most having a serious risk of bias.
Summary of main results
Habitat manipulation only
Habitat manipulation interventions included in this review were based on water management (spillways across streams; floodgates; intermittent flooding; different drawdown rates of water; different flooding and draining regimens), shading management (shading of drainage channels with different plants), and other/combined management approaches (minimal tillage; disturbance of aquatic habitats with grass clearing and water replenishment). The results from the studies were mixed in relation to entomological outcomes but seemed to demonstrate that habitat manipulation interventions may reduce densities of immature and adult mosquitoes. However, the certainty of the evidence for the use of habitat manipulation interventions on malaria parasite prevalence and incidence of clinical malaria was very low, and it is uncertain if there is an effect on epidemiological outcomes.
Six studies assessed the effect in relation to using water management as a habitat manipulation intervention (Djegbe 2020; Kibret 2018; Mutero 2000; Sahu 2014; Santiago 1960; Sharma 2008), which consistently found reductions in the density of immature mosquitoes in the intervention compared to the control (no intervention) group; however, effect sizes ranged considerably. The interventions ranged considerably and therefore, it is challenging to generalize since, whilst spillways and floodgates appeared to have positive effects on entomological outcomes, one study found the lowest levels of immature mosquitoes in undisturbed habitats compared to those with disturbance through clearing grass and replenishing water from local streams (Munga 2013). The latter finding could be due to stable water having a high effect of predation together with the development of algae. Additionally, there was some evidence of reductions in epidemiological outcomes, including malaria parasite prevalence and clinical malaria incidence, and potentially EIR, associated with using water management as a habitat manipulation intervention.
There was consistent evidence of a beneficial effect in relation to using shading of water sources with specific plants as a habitat manipulation intervention to control malaria vectors (Imbahale 2011; Imbahale 2012; Wamae 2010); however, its effect on epidemiological outcomes has not been assessed. The apparent effect may be due to the reduction in sunlight and water temperature as well as lowering algae production, thereby decreasing larval development and survival.
Combination of habitat manipulation and modification
Five studies assessed the effects of interventions that used a combination of habitat manipulation and permanent change (habitat modification) (Imbahale 2012; Lee 2010; McCann 2021; Shililu 2007; Yohannes 2005). The specific interventions included drainage canals, filling, and planting of papyrus and other reeds for shading near dams; drainage of canals, removal of debris, land levelling, and filling ditches; filling or drainage of water bodies; filling, draining, or eliminating rain pools and puddles at water supply points and stream bed pools; and shoreline work, improvement and maintenance to drainage, clearing vegetation and undergrowth, and filling pools. Most studies only considered entomological outcomes.
The combination of habitat manipulation and modification seems to positively impact on reducing entomological outcomes compared to no intervention. The impact on epidemiological outcomes is less clear since the evidence from the studies found it probably had little or no effect on epidemiological outcomes (clinical malaria incidence, malaria parasite prevalence, and EIR); however, caution is needed in interpreting these findings as the intervention group also used larviciding.
Overall completeness and applicability of evidence
This review demonstrates that there is currently insufficient evidence regarding whether habitat manipulation alone, or in combination with modification, reduces malaria transmission. In some cases, studies reported that the intervention under study may lead to a reduction in adult or immature mosquitoes. For those studies demonstrating marked impact on mosquitoes, data collected were limited in the breadth of settings and geographic areas where specific interventions were performed, and so generalizing those results with positive entomological outcomes to other areas should only be done with extreme caution. Moreover, in these cases, results simply show that the intervention may have a potential benefit worthy of further research. Only five studies reported epidemiological outcomes, and none had a low risk of bias, so it is difficult to use these to draw firm conclusions on the effect of tested interventions. Additionally, it should be recognized that it is largely impossible to blind the habitat manipulation or modification interventions to trial personnel and participants. The lack of blinding may result in bias if it leads to deviations from the intended interventions; however, this was unlikely to have resulted in a serious issue for the interventions considered in this review.
Certainty of the evidence
We assessed studies that used habitat modification and manipulation as single or additional interventions to control malaria vectors and reduce malaria transmission. Our review found variable certainty of evidence of habitat modification and manipulation as interventions to control malaria vectors. Only a limited number of studies reported epidemiological outcomes on malaria. For those studies demonstrating marked impact on mosquitoes, data collected were confined to specific study areas and settings areas where the interventions were performed, and so generalizing those results with positive vector control outcomes to other areas should only be done with extreme caution. Several factors were taken into account when assessing the certainty of the evidence, including: the study design, the type of intervention, the length of the intervention, the type of outcome, the statistical analysis, the setting, the seasonality, the frequency of data collection, the presence of other biotic and abiotic factors influencing the results.
Potential biases in the review process
The strength of this systematic review is that two review authors independently conducted a comprehensive search for selecting studies, extracted data, and assessed risk of bias, which minimizes the risk of eligible studies being missed and inaccuracies in the reported results. Additionally, there were no restrictions in terms of language. However, this systematic review has certain limitations. We were unable to perform meta‐analyses to provide pooled estimates of the effectiveness of the reviewed interventions due to insufficient studies using similar interventions and insufficient reporting of the intervention effects, where most studies relied on solely reporting P values. This also meant that we were unable to formally assess the presence of publication bias and investigate reasons for heterogeneity using planned subgroup and sensitivity analyses. Furthermore, none of the included studies had a low overall risk of bias. The overall risk of bias of the RCT and cRCTs was 'some concerns' for the trials, and most studies with other designs were deemed to have a 'serious' risk of bias, with only two having a 'moderate' risk of bias. We also included two studies that had 'critical' risks of bias; however, results from these studies were not reported in the review. We contacted study authors where information was missing or unclear and where we needed raw data to perform further analysis, but none provided additional information.
Agreements and disagreements with other studies or reviews
Keiser 2005 assessed the use of environmental management measures to reduce malaria. In their systematic review, they only included papers analysing epidemiological outcomes, with studies conducted before the Global Malaria Eradication Campaign (1955 to 1969); therefore, a limitation of this older review in comparison to our review is that it did not consider entomological outcomes. Keiser 2005 concluded that clinical malaria morbidity and mortality were reduced regardless of whether environmental modification or manipulation, or modification or manipulation of human dwellings or behaviour were used. In general, we are in agreement with this previous review in terms of emphasising the relevance of possibly using LSM in addition to other interventions to reduce malaria as part of a comprehensive integrated malaria control programme. However, we judged the quality of the data used in the Keiser 2005 review to be very poor, with the conclusions of the review not being supported by a strong systematic review methodology, including no assessment of the quality of evidence being performed.
The last published version of this Cochrane Review, Tusting 2013, assessed the literature of the four main LSM approaches; as mentioned earlier, inclusion criteria slightly differed from ours. Thirteen studies met the inclusion criteria, but only six studies evaluated habitat modification or manipulation (or both). We included five of these studies in our review. We excluded the sixth study because the effect of the intervention was limited to the application of larviciding, rather than habitat modification plus larviciding as classified by Tusting 2013 (Balfour 1936). Moreover, the date of the construction of the intervention (i.e. dam) was not clearly stated (which seemed to be before the outcome data were collected, thus, the study could be considered as having an observational study design). Although we included Samnotra 1980 in our review, we elected not to present any of the findings since we believe that the results of the intervention should be fully assigned to the larviciding intervention, rather than habitat manipulation plus larviciding as classified by Tusting 2013, because of the failure of the community to perform habitat manipulation. Therefore, we considered it inappropriate to extract data from this study, which is in contrast to Tusting 2013, who reported the results as an integrated intervention of habitat manipulation plus larviciding.
Additionally, Tusting 2013 performed risk of bias assessment using a modified version of the Cochrane Effective Practice and Organisation of Care Risk of Bias guidelines (Cochrane 2009). We elected to use the Cochrane RoB 2 and the ROBINS‐I tools for assessing studies' risk of bias as these tools can be used directly without modification and also enable the risk of bias of non‐randomized studies to be assessed based only on relevant domains (Sterne 2016). We do strongly agree with the conclusions of Tusting 2013 regarding that 1. most included studies were not conducted rigorously, 2. data were not appropriately analyzed, 3. there was a lack of negative or null studies, and 4. generalizing results with positive outcomes to areas different from the original place where they were performed should only be done with extreme caution.
Authors' conclusions
Implications for practice.
By reducing mosquito aquatic habitats, environmental management (i.e. habitat modification and manipulation) could be considered as a potential strategy to control malaria, alongside core vector control interventions such as long‐lasting insecticidal nets (LLINs) or indoor residual spraying (IRS).
As shown in this review, a variety of habitat modification and manipulation techniques have been studied. Some of these techniques appear to be promising in reducing mosquito aquatic habitats within their specific settings (e.g. the use of shading channels with specific local plants, using floodgates or spillways across streams).
Very few studies used epidemiological data to test the effectiveness of interventions and for most of these studies, the certainty of evidence was low to very low; for the intervention with a moderate certainty (combination habitat manipulation and modification with larviciding), the finding was that the intervention probably makes little or no difference to epidemiological outcomes. There is a wealth of historical research on the effect of environmental management of malaria. Unfortunately, much of this literature is insufficiently robust to be included in this systematic review. Therefore, in the absence of studies demonstrating a significant reduction in immature/adult mosquito vector densities as well as incidence of disease, it is difficult to recommend habitat manipulation/modification for reducing malaria incidence or parasite prevalence. Similarly, studies included in this review varied in their protocol, ranging from study design to selection of entomological or epidemiological (or both) outcome indicators. Additionally, the techniques that were evaluated were highly specific to the study sites, and caution in interpreting and extrapolating results to other ecological areas should be taken. Therefore, in the absence of studies demonstrating significant evidence in a reduction of immature/adult mosquito vector densities as well as incidence of disease, it is difficult to make broad recommendations of habitat manipulation/modification for reducing malaria incidence or parasite prevalence.
The WHO's Global Technical Strategy 2016–2030 (WHO 2015b) and many national malaria strategic plans (Atkinson 2011) emphasize the engagement of communities (e.g. using locally selected and trained volunteers) in vector control efforts. Community engagement was reportedly a key element in many of the studies reviewed: it informs communities on the importance of malaria and can promote behaviour change that leads to a healthier environment – a crucial aspect for vector control efforts such as habitat modification and manipulation to succeed.
Should national malaria control programmes decide to use habitat modification or manipulation (or both) to compliment other core vector control interventions such as LLINs and IRS, they should implement their approach based on expert guidance and local knowledge; a thorough understanding of local environment (e.g. type of aquatic habitats), entomology (e.g. vector behaviour, vector aquatic habitat preference), and epidemiology; community engagement; as well as applying a programmatic approach that would allow assessment of the intervention's impact on both entomological and epidemiological outcome measures.
Implications for research.
Several of the included studies were conducted more than 30 years ago and the data collected together with the analyses reported were unclear or missing, so it was difficult to support with certainty for many of the findings. Most of the study designs varied in the degree with which they allowed observed effects to be attributed – with confidence – to the intervention. Statistical comparisons between intervention and control groups were often missing, and it is not clear whether the effect of the intervention was significant as a vector control approach. Where clustered designs were used, these typically did not include two or more intervention and control sites for the older studies; however, it is acknowledged that having sufficient sites for such studies can be difficult and expensive to achieve in practice. Furthermore, most of the studies collected data on entomological (secondary) outcomes rather than epidemiological (primary) outcomes. Based on these observations, further high‐quality studies, preferably using either controlled before‐after (CBA) or randomized controlled trial (RCT) designs, assessing the effect of habitat or habitat modification (or both) should be conducted. Such studies should include:
baseline data of standardized entomological and epidemiological outcomes;
a longer intervention/follow‐up period to assess seasonal impacts;
an evaluation of the short‐ and long‐term effects of the intervention;
an appropriate randomization process in the assignment of intervention and control groups;
two or more intervention and control sites for cluster designed studies;
repetition of the same intervention in different settings and geographic areas;
the use of the appropriate statistical methods to analyse data and compare intervention with control groups.
Primary epidemiological outcomes should include clinical malaria incidence, anaemia prevalence, malaria parasite prevalence, incidence of severe malaria, malaria‐related hospital admissions, mortality rate due to malaria, in addition to secondary entomological outcomes alone.
It is acknowledged that more‐robust study designs and epidemiological outcome measures can incur higher study costs and – in a constrained funding environment – may consequently not be prioritized by research funding agencies. However, their inclusion is critical for a more comprehensive assessment of the impact of habitat manipulation and modification, as well as to guide future policy and programmatic recommendations. Embedding studies into operational programmes, by, for example, using stepped‐wedge designs and using routine health management information systems data, should be explored, as this could offset some higher study costs and allow for a more robust assessment.
What's new
| Date | Event | Description |
|---|---|---|
| 2 November 2022 | New citation required and conclusions have changed | Sixteen studies met the inclusion criteria in this review update. |
| 2 November 2022 | New search has been performed | The title of this review was amended from 'Mosquito larval source management for controlling malaria' to 'Mosquito aquatic habitat modification and manipulation interventions to control malaria'. The prespecified changes to the protocol were approved by the CIDG Editors on 29 January 2020, before the review update commenced. The author team updated the search to 30 November 2021. |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 8, 2013
Acknowledgements
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this review.
Sign‐off Editor (final editorial decision): Professor Paul Garner, Cochrane Infectious Diseases Group (CIDG).
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Dr Deirdre Walshe, CIDG.
Copy Editor (copy editing and production): Anne Lawson, Central Production Service, Cochrane.
Peer‐reviewers (provided comments and recommended an editorial decision): Leslie Choi, LSTM, UK (clinical/context review)a, Dr Marty Chaplin, CIDG Statistical Editor (statistical peer review). One additional peer reviewer provided content peer review, but chose not to be publicly acknowledged.
aLeslie Choi was a CIDG staff member at the time of this peer review, and provided peer‐review comments on this article, but was not otherwise involved in the editorial process or decision‐making for this article.
The CIDG editorial base is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the official policies of the UK Government.
We thank Dr Jan Kolaczinski and Dr Jennifer Stevenson (WHO) for their advice and comments on the revised protocol and review update.
We are grateful to Professor Paul Garner and the CIDG editorial team for their advice and guidance during the protocol revision and review update development.
Appendices
Appendix 1. Detailed search strategy
| CIDG SRa | CENTRAL | LILACS | MEDLINE | Embase | CABS Abstract | |
| 1 | Mosquito* | Mosquito* ti, ab, kw | Mosquito* | Mosquito* mp | Mosquito* mp | Mosquito* mp |
| 2 | Anopheles | Anopheles [Mesh] |
Anopheles | Anopheles mp, Mesh | Anopheles mp, Emtree | Anopheles mp |
| 3 | 1 OR 2 | 1 OR 2 | 1 OR 2 | 1 OR 2 | 1 OR 2 | 1 OR 2 |
| 4 | Malaria | Malaria [Mesh] | Malaria | Malaria mp, Mesh | Malaria mp, Emtree | Malaria mp |
| 5 | 3 AND 4 | 3 AND 4 | 3 AND 4 | 3 AND 4 | 3 AND 4 | 3 AND 4 |
| 6 | Control | Mosquito control [Mesh] | Control | Mosquito control mp, Mesh | Mosquito control mp, Emtree | Mosquito control mp |
| 7 | Manag* | Larv* control ti, ab, kw | Manag* | Larv* control mp | Larv* control mp | Larv* control mp |
| 8 | 6 OR 7 | 6 OR 7 | 6 OR 7 | Environmental management mp | Environmental management mp | Environmental management mp |
| 9 | 5 AND 8 | 5 AND 8 | 5 AND 8 | ((Habitat adj 2 modification*) OR modification* OR (habitat adj 2 alteration*) OR alteration* OR landscaping OR drain* OR land reclamation OR land fill OR reclamation ground OR (coverage adj 2 water storage container) OR (coverage adj 2 water surface) OR deforest*) mp | ((Habitat adj 2 modification*) OR modification* OR (habitat adj 2 alteration*) OR alteration* OR landscaping OR drain* OR land reclamation OR land fill OR reclamation ground OR (coverage adj 2 water storage container) OR (coverage adj 2 water surface) OR deforest*) mp | ((Habitat adj 2 modification*) OR modification* OR (habitat adj 2 alteration*) OR alteration* OR landscaping OR drain* OR land reclamation OR land fill OR reclamation ground OR (coverage adj 2 water storage container) OR (coverage adj 2 water surface) OR deforest*) mp |
| 10 | ((Habitat adj 2 manipulation*) OR manipulation* OR flushing OR water level OR drain clear* OR shading OR (expos* adj 2 sun) OR sprinkler sanitation OR intermittent irrigation OR vegetation management OR water management OR alternate wet dry irrigation) mp | ((Habitat adj 2 manipulation*) OR manipulation* OR flushing OR water level OR drain clear* OR shading OR (expos* adj 2 sun) OR sprinkler sanitation OR intermittent irrigation OR vegetation management OR water management OR alternate wet dry irrigation) mp | ((Habitat adj 2 manipulation*) OR manipulation* OR flushing OR water level OR drain clear* OR shading OR (expos* adj 2 sun) OR sprinkler sanitation OR intermittent irrigation OR vegetation management OR water management OR alternate wet dry irrigation) mp | |||
| 11 | 6 OR 7 OR 8 OR 9 OR 10 | 6 OR 7 OR 8 OR 9 OR 10 | 6 OR 7 OR 8 OR 9 OR 10 | |||
| 12 | 5 AND 11 | 5 AND 11 | 5 AND 11 | |||
| aCochrane Infectious Diseases Group Specialized Register. | ||||||
Appendix 2. Summary of interventions and eco‐epidemiological settings for included studies
| Study ID | Study design | Details of the intervention | Who was responsible for the intervention | Ecosystem | Vectors | Malaria transmission intensity |
| Habitat manipulation | ||||||
| Djegbe 2020 | Non‐RCT | 1. Intermittent irrigation 2. Minimal tillage |
Farmers and technicians | NI | Anopheles spp | High |
| Kibret 2018 | Cluster‐RCT | Experimental dam construction (12) with 3 water drawdown treatments and 1 control (3 replicates each). The intervention or no intervention was randomly assigned to the dams: 1. 10 mm/day 2. 15 mm/day 3. 20 mm/day |
NI | Rural | An arabiensis, An pharoensis | High |
| Mutero 2000 | cRCT | 3 water regimens: 1. flooded before transplanting, drained during transplanting, flooded after transplanting. 2. flooded before transplanting, flooded during transplanting, flooded after transplanting. 3. flooded before transplanting, drained during transplanting, alternately flooded and drained after transplanting (= intermittent irrigation) |
Mwea Irrigation and Agricultural Development experimental station | Rural | An arabiensis | Low |
| Santiago 1960 | CBA study | Automatic syphons were constructed over 2 streams. Water was flushed to control larvae over distances of 1073 m and 2897 m downstream. Existing syphons were repaired. | Local operators within the community and the United States Public Health Service | Urban | An minimus flavirostris | High |
| Sahu 2014 | CBA study | Bed dam construction with 2 sluice gates, opening and closing weekly | Residents of the village – volunteers | Rural | An fluviatilis | High |
| Sharma 2008 | CBA study | A small concrete dam construction fitted with 3 operational gates with sluice iron sheets across the village stream to provide water for irrigation | Government of India | Rural | An fluviatilis, An culicifacies | High |
| Imbahale 2011 | Non‐RCT | 1. Shading with local plants. Mosquito aquatic habitats were created by building a shallow dyke around each habitat. Each of the 4 locally grown plant species Napier grass (Pennisetum purpureum), arrowroot (Maranta arudinacea), papyrus reeds (Cyperus spp) and rice (Oryza sativa) weeded and unweeded 2. Water management with manufactured pools, small water canals, paddies. |
Centre of Global Health Research (CGHR), KEMRI, Kisian |
Peri‐urban | An gambiae s.s., An coustani | High |
| Imbahale 2012 | Non‐RCT | 1. Shading with arrowroot (M arundinacea) 2. Drainage of canal, land levelling, filling ditches with soil |
NI | Rural | An gambiae s.l., An arabiensis, An funestus | NI |
| Wamae 2010 | RCT | Shading drainage channels with Napier Grass planted on both sides of the entire length of the channel. Usual farm activities uninterrupted (occasional cleaning, drainage, land cultivation) | NI | Rural | An gambiae s.l., An funestus, An coustani, An rufipes, An marshalli, An maculipalpis, An azaniae, An implexus | Moderate to high |
| Munga 2013 | cRCT | Habitats were cleared of grass and water replenishment at different frequency from the local streams: 1. every 10 days (frequent disturbance) 2. every 20 days (intermediate disturbance) |
NI | Rural | An gambiae s.l., An funestus, An coustani, An implexus | NI |
| Habitat manipulation + larviciding | ||||||
| Castro 2009 | CBA study | Drains in the city were cleared to increase the water flow and to reduce flooding in the rainy season. Minor repairs such as slab replacement. Larviciding: at the end of the study all sites were treated with larviciding spray | Drain clearance was initially conducted by a contractor with 90% of the workforce local. Intensive education of the local community led to community‐led maintenance of drains. Larviciding was organized by the Urban Malaria Control Program | Urban | An gambiae (not specified if s.s. or s.l.), An funestus | NI |
| Samnotra 1980 | CBA study | Encouraged households to eliminate domestic aquatic habitats alongside larviciding. Control mosquito aquatic habitats like tanks, pitchers, cisterns not treated with larviciding. The attempts were unsuccessful Larviciding: pirimiphos‐methyl (sprayed 12.5 g active ingredient/hectare) |
Study staff applied larviciding. Attempts to involve the community for habitat management were unsuccessful | Urban | An culicifacies, An stephensi | Low |
| Habitat manipulation + modification | ||||||
| Yohannes 2005 | CBA study | Filling, draining, shading mosquito aquatic habitats, prohibiting the entry of humans and livestock and filling crossing points of cattle and humans to prevent destruction of plants and creation of mosquito aquatic habitats with hoof‐footprints | Local community | Rural | An arabiensis and other anophelines | Low |
| Imbahale 2012 | Non‐RCT | 1. Shading with arrowroot (M arundinacea) 2. Drainage of canal, land levelling, filling ditches with soil |
NI | Rural | An gambiae s.l., An arabiensis, An funestus | NI |
| Habitat manipulation + modification + larviciding | ||||||
| McCann 2021 | cRCT | Filling or draining of water bodies to permanently eliminate habitats in cases where this was feasible, and the water was not used by the community for the designated purpose. All other water bodies were targets with larviciding Larviciding: Bti |
NI | NI | An arabiensis, An funestus | High |
| Shililu 2007 | cRCT | Filling or drainage or elimination of rain pools, puddles at water supply points and stream bed pools Larviciding: treatment in rotation with Bti granules, Bsph corn granules and temephos |
Study staff and local community | Rural | An arabiensis, An cinereus, An pretoriensis, An d'thali, An funestus, An squamosus, An adenensis, An demeilloni | NI |
| Lee 2010 | Uncontrolled before‐after study | Prevent importation of malaria: 8 weeks of quarantine on return from malaria endemic countries, screening for foreigners, early detection of human cases, mosquito control programme, shoreline works, drainage, maintenance of drains‐clearance vegetation, filling up pools of water, larvicide and adulticide, IRS, personal protection measures, malaria contingency plan in case of outbreak | Singapore Armed Forces | Rural | An sundaicus, An maculates | Very low |
| Bti: Bacillus thuringiensis israelensis; CBA: controlled before‐after; cRCT: cluster‐randomized controlled trial; IRS: indoor residual spraying; NI: no information; RCT: randomized controlled trial. | ||||||
Appendix 3. Risk of bias assessments for non‐randomized studies of interventions
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: primary outcome: parasite prevalence | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Moderate | 1.1 Potential for confounding? Yes 1.4 Appropriate analysis to control for baseline confounding? Probably yes 1.5 Controlled for confounding domains measured validly and reliably? Probably yes 1.6 Control for postintervention variables? Yes 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Adjusted for important confounders relating to rainfall, bed net use, age adjusted for. Controlled for postintervention variables relating to use of larviciding. But potential for other important confounders not adjusted for. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Yes |
Selection made independent of characteristics and timings coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined, information used to classify groups was recorded at start of intervention, and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no |
Outcome data probably available for all participants, with none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? No 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Low | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? No 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results or multiple outcome measurements, analyses of the data, or multiple subgroups. |
| Overall bias | Moderate | — | Low risk of bias for most domains except for due to confounding and measurement of outcomes, which were moderate. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different fields were used for intervention and control and insufficient information given about the location of the fields to assess similarity. Analyses did not adjust for important confounders or postintervention variables. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Yes |
Selection made independent of characteristics and timings coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? No |
Outcome data probably available for all participants (mosquitoes), with none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Moderate | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? Yes 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures or multiple subgroups. However, multiple analyses were conducted due to considering 3 development stages of rice (transplanting, tillering and maturation) and estimates of effect measures were only presented for some comparisons. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and selection in reported results and measurement of outcomes, which were moderate. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different habitats were used for intervention and control and insufficient information given about the location of the habitats to assess similarity. Analyses did not adjust for important confounders or postintervention variables. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no |
Outcome data probably available for all participants (mosquitoes), with probably none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Moderate | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? Yes 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures or multiple subgroups. However, multiple analyses were conducted due to considering 3 types of plants; however, estimates of effect were presented for each plant type. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and selection in reported results and measurement of outcomes, which were moderate. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Moderate | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
The same villages were used for the intervention and controls groups so unlikely to be village level differences; however, different habitats were used within each village and insufficient information given about these to assess similarity for other important confounders. Analyses did not adjust for important confounders or postintervention variables. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Yes |
Selection made independent of characteristics and timings coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? No |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? No |
Outcome data probably available for all participants (mosquitoes), with none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Moderate | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? Yes 7.3 Different subgroups? Probably no |
Numerical outcome unlikely to be selected based on results of multiple outcome measures or multiple subgroups. However, multiple analyses were conducted due to considering early and late stages of mosquitoes and separate analyses for each village; however, effect estimates were presented for all comparisons. |
| Overall bias | Moderate | — | Low risk of bias for most domains except for due to confounding, selection in reported results, and measurement of outcomes, which were moderate. |
| Risk of bias (RoB 2) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Probably yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably no |
Method of randomization not reported. Allocation sequence not clear if concealed, unlikely to be baseline differences between groups. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Probably yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants (mosquitoes) between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Probably no 2.1b Participants aware of assigned intervention? Probably no 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? Probably no 2.5b Where any participants analyzed in a group different from assigned cluster? Probably no |
Participants (mosquitoes) probably unaware they were in a trial or assigned intervention, but people delivering the intervention were aware of assigned intervention. Probably no evidence of deviations from intended intervention and no clusters or participants (mosquitoes) analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Yes |
Data available for all clusters and probably all participants (mosquitoes) within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants (mosquitoes) but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Some concerns | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses, although separate results were presented for wet and dry seasons, but the results were similar. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process, measurement of outcome, and selection of reported results, which have some concerns. |
| Risk of bias (no tool used) | ||
| Bias | Review authors' judgement | Support for judgement |
| Overall bias | Critical | Study design: uncontrolled before‐after study, no control group. |
| Risk of bias (RoB 2) | |||
| Outcome assessed: primary outcome: malaria parasite prevalence | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably yes |
Method of randomization based on 2‐stage approach by drawing lots from opaque folded cards. Allocation sequence unclear if concealed, evidence of baseline differences between groups. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Yes 2.1b Participants aware of assigned intervention? Yes 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? No 2.5b Where any participants analyzed in a group different from assigned cluster? No |
Participants and people delivering the intervention were aware of assigned intervention, no evidence of deviations from intended intervention, no clusters or participants analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Probably yes |
Data available for all clusters and probably all participants within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Outcome assessed: secondary outcome: density of adult mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably yes |
Method of randomization based on 2‐stage approach by drawing lots from opaque folded cards. Allocation sequence not clear if concealed, evidence of baseline differences between groups. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Probably yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants (mosquitoes) between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Probably no 2.1b Participants aware of assigned intervention? Probably no 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? Probably no 2.5b Where any participants analyzed in a group different from assigned cluster? Probably no |
Participants (mosquitoes) probably unaware of being in a trial. People delivering the intervention were aware of assigned intervention, but no evidence of deviations from intended intervention, and no clusters or participants (mosquitoes) analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Yes |
Data available for all clusters and probably all participants (mosquitoes) within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants (mosquitoes) but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Outcome assessed: secondary outcome: haemoglobin levels | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably yes |
Method of randomization based on 2‐stage approach by drawing lots from opaque folded cards. Allocation sequence unclear if concealed, evidence of baseline differences between groups. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Yes 2.1b Participants aware of assigned intervention? Yes 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? No 2.5b Where any participants analyzed in a group different from assigned cluster? No |
Participants and people delivering the intervention were aware of assigned intervention, no evidence of deviations from intended intervention, no clusters or participants analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Probably yes |
Data available for all clusters and probably all participants within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Outcome assessed: secondary outcome: entomological inoculation rate | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably yes |
Method of randomization based on 2‐stage approach by drawing lots from opaque folded cards. Allocation sequence not clear if concealed, evidence of baseline differences between groups. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Yes 2.1b Participants aware of assigned intervention? Yes 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? No 2.5b Where any participants analyzed in a group different from assigned cluster? No |
Participants and people delivering the intervention were aware of assigned intervention, no evidence of deviations from intended intervention, no clusters or participants analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Probably yes |
Data available for all clusters and probably all participants within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Risk of bias (RoB 2) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Probably yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably no |
Method of randomization not reported. Allocation sequence not clear if concealed, insufficient information to determine if there are baseline differences between groups. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Probably yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Probably no 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? Probably no 2.5b Where any participants analyzed in a group different from assigned cluster? Probably no |
Participants (mosquitoes) probably unaware they were in a trial or assigned intervention, but people delivering the intervention were aware of assigned intervention. Probably no evidence of deviations from intended intervention and no clusters or participants (mosquitoes) analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Yes |
Data available for all clusters and probably all participants (mosquitoes) within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? No |
Outcome assessors aware of intervention received by participants (mosquitoes) but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Risk of bias (RoB 2) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably no |
Randomized block design. Allocation sequence not clear if concealed, unlikely to be baseline difference between groups due to closeness of plots. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants (mosquitoes) between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Probably no 2.1b Participants aware of assigned intervention? Probably yes 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? Probably no 2.5b Where any participants analyzed in a group different from assigned cluster? Probably no |
Participants (mosquitoes) probably unaware they were in a trial or assigned intervention, but people delivering the intervention were aware of assigned intervention. Probably no evidence of deviations from intended intervention and no clusters or participants (mosquitoes) analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Yes |
Data available for all clusters and probably all participants (mosquitoes) within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants (mosquitoes) but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: secondary outcome: density immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different streams were used for intervention and control from different villages. Insufficient information given about the streams to assess similarity other than villages are in same district. Analyses did not adjust for important confounders or postintervention variables. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? Probably no 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection probably made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? No 5.3 Participants excluded due to missing data on other variables? No |
Outcome data probably available for all participants (mosquitoes), with probably none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? Probably no |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Moderate | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? Probably yes 7.3 Different subgroups? Probably no |
Numerical outcome unlikely to be selected based on results of multiple outcome measures or multiple subgroups. However, multiple analyses were conducted due to considering 2 locations (upstream and downstream) but estimates of effect measures were presented for both comparisons. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and selection in reported results and measurement of outcomes, which were moderate. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: primary outcome: clinical malaria incidence (due to critical risk of bias regarding study design, the risk of bias for other outcome measures have not been provided due to same issue) | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different towns were used for intervention and control groups, which had substantially different population sizes (92,000 versus 8000) and were 8 km apart in location. Insufficient information given about the towns to assess similarity. Analyses did not adjust for important confounders or postintervention variables. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? Probably no 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection probably made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Moderate | 3.1 Intervention groups clearly defined? Probably no 3.2 Information to define intervention groups recorded at start of intervention? Probably yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups not clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Critical | 4.1 Deviations from intended intervention? Yes 4.2 Deviations unbalanced between groups and likely to affect the outcome? Yes |
Evidence of substantial deviations from intended intervention and unbalanced deviations between groups, which will substantially affect the outcome. |
| Bias due to missing data | Moderate | 5.1 Outcome data available for all, or nearly all, participants? Probably no 5.2 Participants excluded due to missing data on intervention status? No information 5.3 Participants excluded due to missing data on other variables? No information 5.4 Proportion of missing data similar across interventions? No information 5.5 Evidence that results were robust to missing data? Probably no |
Outcome data probably not available for all participants and no evidence of robust results, with no information available regarding whether participants were excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? Probably no |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Moderate | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? Probably no 7.2 Multiple analyses? No information 7.3 Different subgroups? Probably no |
Numerical outcome unlikely to be selected based on results of multiple outcome measures or multiple subgroups. Insufficient information to determine whether multiple analyses were conducted for each month of the study. |
| Overall bias | Critical | — | Moderate risk of bias for most domains except for due to confounding, which was serious and deviations from intended interventions, which was critical. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: primary outcome: malaria parasite prevalence | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different streams were used for intervention and control groups, although the authors reported no evidence of differences between the stream at baseline in terms of the terrain and living conditions of the inhabitants, the population sizes were reported to differ considerably, and the streams were 9 km apart. Insufficient information given about the towns to assess similarity. Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? Probably no 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection probably made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Serious | 5.1 Outcome data available for all, or nearly all, participants? Probably no 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no 5.4 Proportion of missing data similar across interventions? Probably no 5.5 Evidence that results were robust to missing data? Probably no |
Outcome data probably not available for all participants and no evidence of robust results, but probably no participants excluded due to missing intervention status or missing data on other variables, but missing data not similar across interventions. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? Probably no |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Low | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? No 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures, multiple subgroups, or multiple analyses. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding and missing data, which were serious and measurement of outcomes, which was moderate. |
| Outcome assessed: primary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? Probably no 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different streams were used for intervention and control groups, although the authors reported no evidence of differences between the stream at baseline in terms of the terrain and living conditions of the inhabitants, the population sizes were reported to differ considerably, and the streams were 9 km apart. Insufficient information given about the towns to assess similarity. Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? Probably no 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection probably made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? No 5.3 Participants excluded due to missing data on other variables? No |
Outcome data probably available for all participants (mosquitoes), with none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? Probably no |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Low | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? No 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures, multiple subgroups, or multiple analyses. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and measurement of outcomes, which was moderate. |
| Outcome assessed: primary outcome: density of adult mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? Probably no 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different streams were used for intervention and control groups, although the authors reported no evidence of differences between the stream at baseline in terms of the terrain and living conditions of the inhabitants, the population sizes were reported to differ considerably and the streams were 9 km apart. Insufficient information given about the towns to assess similarity. Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? Probably no 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection probably made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? No 5.3 Participants excluded due to missing data on other variables? No |
Outcome data probably available for all participants (mosquitoes), with none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? Probably no |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Low | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? No 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures, multiple subgroups, or multiple analyses. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and measurement of outcomes, which was moderate. |
| Outcome assessed: secondary outcome: entomological inoculation rate | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different streams were used for intervention and control groups, although the authors reported no evidence of differences between the stream at baseline in terms of the terrain and living conditions of the inhabitants, the population sizes were reported to differ considerably and the streams were 9 km apart. Insufficient information given about the towns to assess similarity. Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? Probably no 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection probably made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Serious | 5.1 Outcome data available for all, or nearly all, participants? Probably no 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no 5.4 Proportion of missing data similar across interventions? Probably no 5.5 Evidence that results were robust to missing data? Probably no |
Outcome data probably not available for all participants and no evidence of robust results, but probably no participants excluded due to missing intervention status or missing data on other variables, but missing data not similar across interventions. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? Probably no 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? Probably no |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Low | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? No 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures, multiple subgroups, or multiple analyses. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding and missing, which were serious and measurement of outcomes, which was moderate. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: primary outcome: clinical malaria incidence | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different villages were used for intervention and control groups, although the authors reported the P value for differences in baseline malaria incidence rates as > 0.05, the numerical values appeared considerably different in children aged 1 to 5 years (685.7 with intervention versus 1304.3 with control). Authors noted significant difference in all age groups (643.9 with intervention versus 274.8 with control). Villages are similar sizes and in close proximity to each other. Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Yes |
Selection made independent of characteristics and timings coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no |
Outcome data probably available for all participants, with probably none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? No 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Moderate | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? Probably yes 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures or multiple subgroups. However, multiple analyses were conducted based on age groups and results for children aged 1 to 5 years only reported in abstract. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and measurement of outcomes and selection of reported result, which were moderate. |
| Outcome assessed: primary outcome: parasite prevalence | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different villages were used for intervention and control groups, although the authors reported the P value for differences in baseline malaria incidence rates as > 0.05, the numerical values appeared considerably different in children aged 1 to 5 years (685.7 with intervention versus 1304.3 with control). Authors noted significant difference in all age groups (643.9 with intervention versus 274.8 with control). Villages are similar sizes and in close proximity to each other. Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Yes |
Selection made independent of characteristics and timings coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? Probably no |
Intervention groups clearly defined and classification of intervention probably unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no |
Outcome data probably available for all participants, with probably none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? No 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Moderate | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? Probably yes 7.3 Different subgroups? No |
Numerical outcome unlikely to be selected based on results of multiple outcome measures or multiple subgroups. However, multiple analyses were conducted based on age groups. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and measurement of outcomes and selection of reported result, which were moderate. |
| Risk of bias (RoB 2) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Probably yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably no |
Method of randomization not reported. Allocation sequence not clear if concealed, unlikely to be baseline difference between groups due to similarities in ecology, human population density, house types, and accessibility. |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Probably yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants (mosquitoes) between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Probably no 2.1b Participants aware of assigned intervention? Probably no 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the 1 which assigned? Probably no 2.5b Where any participants analyzed in a group different from assigned cluster? Probably no |
Participants (mosquitoes) and people delivering the intervention were aware of assigned intervention, no evidence of deviations from intended intervention, no clusters or participants (mosquitoes) analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Yes |
Data available for all clusters and probably all participants (mosquitoes) within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants (mosquitoes) but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Outcome assessed: secondary outcome: density of adult mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1a.1 Allocation sequence random? Probably yes 1a.2 Allocation sequence concealed? Probably no 1a.3 Baseline differences between groups? Probably no |
Method of randomization not reported. Allocation sequence not clear if concealed, unlikely to be baseline difference between groups due to similarities in ecology, human population density, house types, and accessibility |
| Bias arising from the timing and identification and recruitment of individual participants in relation to timing of randomization | Low | 1b.1 All participants identified before randomization of clusters? Probably yes 1b.3 Baseline imbalances that suggest differential identification/recruitment of individual participants between arms? Probably no |
Probably no evidence of baseline imbalances suggesting differential identification/recruitment of individual participants (mosquitoes) between arms. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1a Participants aware they were in a trial? Probably no 2.1b Participants aware if assigned interventions? Probably no 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.5a Were any clusters analyzed in a group different from the one which assigned? Probably no 2.5b Where any participants analyzed in a group different from assigned cluster? Probably no |
Participants (mosquitoes) and people delivering the intervention were aware of assigned intervention, no evidence of deviations from intended intervention, no clusters or participants (mosquitoes) analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1a Data available for all, or nearly all, clusters? Yes 3.1b Data available for all, or nearly all, participants within clusters? Yes |
Data available for all clusters and probably all participants (mosquitoes) within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1a Outcome assessors aware that trial was taking place? Yes 4.1b Outcome assessors aware of the intervention received by participants? Yes 4.2 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants (mosquitoes) but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Low | 5.1 Numerical result likely to be selected based on multiple outcome measurements? Probably no 5.2 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
Numerical results unlikely to be selected based on multiple outcome measurements or analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process and measurement of outcome, which have some concerns. |
| Risk of bias (RoB 2) | |||
| Outcome assessed: secondary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to randomization process | Some concerns | 1.1 Allocation sequence random? Probably yes 1.2 Allocation sequence concealed? Probably no 1.3 Baseline differences between groups? Probably no |
Method of randomization not reported. Allocation sequence not clear if concealed, unlikely to be baseline differences between groups due to similarities in the channels used in terms of slow‐flowing water, permanence of channel, plot owners' consent to plant. |
| Bias due to deviations from the intended interventions (effect of assignment to intervention) | Low | 2.1 Participants aware of their assigned intervention during the trial? Probably no 2.2 People delivering intervention were aware of assignment during the trial? Yes 2.3 Deviations from intended intervention? Probably no 2.6 Appropriate analysis used to estimate the effect of assignment to intervention? Yes |
Participants (mosquitoes) and people delivering the intervention were aware of assigned intervention, no evidence of deviations from intended intervention, no clusters or participants (mosquitoes) analyzed in group different to assigned. |
| Bias due to missing outcome data | Low | 3.1 Data available for all, or nearly all, participants within clusters? Probably yes | Data available for all clusters and probably all participants (mosquitoes) within clusters. |
| Bias due to measurement of the outcome | Some concerns | 4.1 Method of measuring outcome inappropriate? No 4.2 Measurement or ascertainment of outcome have differed between intervention groups? No 4.3 Outcome assessors aware of intervention received? Yes 4.4 Assessment of outcome likely to be influenced by knowledge of intervention received? Probably no |
Outcome assessors aware of intervention received by participants (mosquitoes) but objective assessment unlikely to be influenced by knowledge. |
| Bias due to selection of the reported result | Some concerns | 5.1 Results analyzed in accordance with prespecified analysis plan? No 5.2 Numerical result likely to be selected based on multiple outcome measurements? Probably yes 5.3 Numerical results likely to be selected based on multiple eligible analyses? Probably no |
No statistical analysis plan or protocol published/registered. Numerical results likely to be selected based on multiple outcome measurements due to separate analysis for each village but not multiple analyses. |
| Overall bias | Some concerns | — | Low risk of bias for most domains except for due to randomization process, measurement of outcome, and selection in reported results, which have some concerns. |
| Risk of bias (ROBINS‐I) | |||
| Outcome assessed: secondary outcome: density of adult mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different villages were used for intervention and control groups, differences in baseline density of adult mosquitos between villages (2.3% with intervention versus 0.3% with control). Villages had different population densities (372 with intervention versus 1237 with control) but in close proximity to each other (3 km to 4 km apart) and similar altitudes (1750 m to 1790 m). Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? No |
Intervention groups clearly defined and classification of intervention unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no |
Outcome data probably available for all participants (mosquitoes), with probably none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? No 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Low | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? No 7.3 Different subgroups? No |
Numerical results unlikely to be selected based on multiple outcome measurements, analyses, or subgroups. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and measurement of outcomes, which was moderate. |
| Outcome assessed: primary outcome: density of immature mosquitoes | |||
| Bias | Review authors' judgement | Signalling questions and responses | Support for judgement |
| Bias due to confounding | Serious | 1.1 Potential for confounding? Probably yes 1.4 Appropriate analysis to control for baseline confounding? No 1.6 Control for postintervention variables? No 1.7 Appropriate analysis to control for baseline and time varying confounders? No |
Different villages were used for intervention and control groups, differences in baseline density of adult mosquitos between villages (2.3% with intervention versus 0.3% with control). Villages had different population densities (372 with intervention versus 1237 with control) but in close proximity to each other (3 km to 4 km apart) and similar altitudes (1750 m to 1790 m). Analyses did not adjust for important confounders or postintervention variable. |
| Bias in selection of participants into the study | Low | 2.1 Selection of participants based on their characteristics? No 2.4 Start of follow‐up and intervention coincide? Probably yes |
Selection made independent of characteristics and timings probably coincided. |
| Bias in classification of interventions | Low | 3.1 Intervention groups clearly defined? Yes 3.2 Information to define intervention groups recorded at start of intervention? Yes 3.3 Classification of intervention status affected by knowledge of outcome or risk of outcome? No |
Intervention groups clearly defined and classification of intervention unaffected by knowledge or risk of outcome. |
| Bias due to deviations from intended interventions | Low | 4.1 Deviations from intended intervention? No | No evidence of deviations from intended intervention. |
| Bias due to missing data | Low | 5.1 Outcome data available for all, or nearly all, participants? Probably yes 5.2 Participants excluded due to missing data on intervention status? Probably no 5.3 Participants excluded due to missing data on other variables? Probably no |
Outcome data probably available for all participants (mosquitoes), with probably none excluded due to missing intervention status or missing data on other variables. |
| Bias in measurement of outcomes | Moderate | 6.1 Outcome measures have been influenced by knowledge of intervention? No 6.2 Outcome assessor aware of intervention received? Yes 6.3 Methods of outcome assessment comparable across groups? Yes 6.4 Systematic errors in measurement of outcome related to intervention? No |
Method of measuring the outcome was appropriate, and did not differ between groups. Outcome assessor aware of intervention implemented. Assessment of outcome unlikely to be influenced by knowledge of intervention implemented. |
| Bias in selection of the reported result | Low | Reported effect estimate likely to be selected on 7.1 Multiple outcome measurements? No 7.2 Multiple analyses? No 7.3 Different subgroups? No |
Numerical results unlikely to be selected based on multiple outcome measurements, analyses, or subgroups. |
| Overall bias | Serious | — | Low risk of bias for most domains except for due to confounding, which was serious and measurement of outcomes, which was moderate. |
NA: not applicable, NI: no information.
Appendix 4. Prespecified changes for review update
| Protocol section | Prespecified changes |
| Background and research question |
|
| Inclusion criteria | Secondary outcomes
Types of controls
Type of studies additionally included
Study selection
|
| Methods |
|
| This table was checked and approved by the Cochrane Infectious Diseases Group Editors on 29 January 2020. WHO: World Health Organization. | |
Appendix 5. Glossary of terms
| Term | Description |
| Density per dip | Total number of mosquito larvae divided by the number of dips performed. The dip method consists of the use of a dipper (cup) attached to the end of a pole to scoop the sample from water bodies considered to be putative mosquito aquatic habitat sites. The dipper is inspected for the presence of mosquito larvae. |
| Flushing | Flushing is a method that can be used to increase the flow of water in streams. |
| Instar | The stages between larval moults are called instars. Mosquito larvae moult 4 times; at the fourth instar, the larvae become pupae. |
Data and analyses
Comparison 1. Spillways across streams versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Malaria parasite prevalence (children aged 2 to 10 years) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 Baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.2 Follow‐up during first year of intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Mean density of immature mosquitoes | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Entomological inoculation rate (EIR) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3.1 Baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3.2 Follow‐up during first year of intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Shading using local plants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Density of immature mosquitoes | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.1 Local plant = Napier grass; outcome = early instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.2 Local plant = Napier grass; outcome = late instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.3 Local plant = unweeded rice; outcome = early instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.4 Local plant = unweeded rice; outcome = late instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.5 Local plant = arrowroot; outcome = early instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.6 Local plant = arrowroot; outcome = late instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.7 Local plant = papyrus, outcome = early instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.8 Local plant = papyrus, outcome = late instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.9 Local plant = weeded rice; outcome = early instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1.10 Local plant = weeded rice; outcome = late instars | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Repairing and clearing of drains, cutting grasses, and making minor repairs (e.g. slab replacement) combined with larviciding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Malaria parasite prevalence | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Filling and draining water bodies with larviciding versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Malaria parasite prevalence | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.1.1 Women aged 15 to 49 years | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.1.2 Children aged 6 to 59 months | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.1.3 Children aged 6 to 23 months | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Haemoglobin levels (g/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.1 Women aged 15 to 49 years | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.2 Children aged 6 to 59 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.3 Children aged 6 to 23 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3 Entomological inoculation rate (EIR) | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.3.1 All Anopheles females indoors | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.3.2 All Anopheles females outdoors | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.3.3 A arabiensis females indoors | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.3.4 A funestus females indoors | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.3.5 A arabiensis females outdoors | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.3.6 A funestus females outdoors | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Castro 2009.
| Study characteristics | ||
| Methods |
Study design: controlled before‐after study Type of cluster: city Cluster size: 9070 people Number of clusters in each arm: intervention arm 1: 2 clusters; intervention arm 2: 2 clusters; control arm: 2 cluster Adjusted for clustering? No |
|
| Participants |
Age: any Sex: any Comorbidities or pregnancy (or both): any Primary outcome sample size (parasite prevalence): 9070 individuals Secondary outcome sample size: NA |
|
| Interventions |
Intervention: habitat manipulation plus larviciding.
Control: no intervention. Duration of intervention: 12 months (intervention arm 1: July 2007 to March 2008 habitat manipulation only, April 2008 to July 2008 habitat manipulation plus larviciding; intervention arm 2: July 2007 to July 2008, larviciding; control arm: July 2007 to March 2008 no intervention, April 2008 to July 2008 larviciding. The type and dosage of larviciding used was not specified. Who was responsible for LSM? Drain clearance was initially conducted by a contractor with 90% of the workforce local. Intensive education of the local community led to community‐led maintenance of drains. Larviciding was organized by the Urban Malaria Control Program. Co‐interventions: IRS with DDT In the previous review they stated: ITN in the area. We think this treatment was described in the paper as a future strategy development by The National Malaria Medium Term Strategic Plan for 2008–2013. Co‐interventions equal in each arm? Yes |
|
| Outcomes |
Primary outcome
Secondary outcome: NA. Larval data were used to calculate monthly time series of the percentage of water habitats with immatures in environmental management sites. A 3‐month moving average was used to extract the time trend observed in each drain, and the slope (and CI) of the trend after cleaning was calculated. This outcome does not match with our inclusion criteria since no data on the number (density) of immature mosquitoes were reported. |
|
| Notes |
Continent: Africa Country: Tanzania Ecosystem: urban Transmission intensity: NI Transmission season(s): NI Vectors:An gambiae (not specified if s.s. or s.l.), An funestus Malaria parasite:P falciparum Source of funding: Japan International Cooperation Agency Study included in the previous review: yes It is relevant to underline that larviciding was applied in the last 4 months only in all sites and no data before and after this second intervention were shown. But, the analysis performed by the authors of the paper was able to capture the positive effect of habitat manipulation alone adjusting for age, rainfall, bed net use, and larviciding spray. |
|
Djegbe 2020.
| Study characteristics | ||
| Methods |
Study design: non‐randomized controlled trial Type of cluster: experimental rice field plots Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? NA |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation (2 methods)
Control: habitat manipulation (2 methods)
Duration of intervention: 13 months (3 developmental stages of rice, 1 sampling every stage over 1 year: transplanting, tillering, maturation) Who was responsible for LSM? Farmers and technicians Co‐interventions: no. Avoidance of agrochemicals (herbicides, pesticides, and insecticides) by all farmers Co‐interventions equal in each arm? NA |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Benin Urban or rural: urban Transmission intensity: high Transmission season(s): rainy season (July) and dry season (October), when intensive rice production was ongoing. Vectors:Anopheles spp Malaria parasite: NI Source of funding: WHO/TDR re‐entry grant and Fp5‐A4NH programme of the CGIAR Study included in the previous review: no |
|
Imbahale 2011.
| Study characteristics | ||
| Methods |
Study design: non‐randomized controlled trial Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? NA |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation
Control: no intervention, habitats left unplanted. Duration of intervention 1. 13 weeks (March 2007 to June 2007) 2. 13 weeks (February 2008 to May 2008) Who was responsible for LSM? Centre of Global Health Research, KEMRI, Kisian, Kenya Co‐interventions: no Co‐interventions equal in each arm? NA |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Kenya Ecosystem: peri‐urban Transmission intensity: high Transmission season(s): NI Vectors:An gambiae s.s., An coustani Malaria parasite: NI Source of funding: Dioraphte Foundation, the Netherlands Study included in the previous review: no |
|
Imbahale 2012.
| Study characteristics | ||
| Methods |
Study design: non‐randomized controlled trial Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? NA |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation with/without modification
Control: no intervention Duration of intervention
Who was responsible for LSM? NI Co‐interventions: Roll Back Malaria initiative: ITNs, IRS, and antimalarial drugs Co‐interventions equal in each arm? Yes |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Kenya Ecosystem: rural Transmission intensity: NI Transmission season(s): NI Vectors:An gambiae s.l., An arabiensis, An funestus Malaria parasite: NI Source of funding: NI Study included in the previous review: no |
|
Kibret 2018.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomized controlled trial Type of cluster: dam site Cluster size: NA Number of clusters in each arm: 3 Adjusted for clustering? No |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation
Control
Duration of intervention: 12 weeks (October 2013 to November 2013 main season, February 2014 to March 2014 dry season) Who was responsible for LSM? NA Co‐interventions: NI Co‐interventions equal in each arm? NI |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Ethiopia Ecosystem: rural Transmission intensity: high Transmission season(s): October to November Vectors:An arabiensis, Anpharoensis Malaria parasite:P falciparum, P vivax Source of funding: International Foundation for Science and University of New England Study included in the previous review: no |
|
Lee 2010.
| Study characteristics | ||
| Methods |
Study design: uncontrolled before‐after study Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? NA |
|
| Participants |
Age: any Sex: any Comorbidities or pregnancy (or both): NI Primary outcome sample size: NA Secondary outcome sample size (density of adult mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation + modification with larviciding
Control: no control group Duration of intervention: 24 months (December 2006 to December 2008) Who was responsible for LSM? Singapore Armed Forces Co‐interventions: prevent importation of malaria: 8 weeks of quarantine on return from malaria‐endemic countries, screening for non‐nationals, early detection of human cases, larvicide and adulticide, IRS, personal protection measures, malaria contingency plan in case of outbreak. Co‐interventions equal in each arm? NA |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Asia Country: Singapore Ecosystem: rural Transmission intensity: very low Transmission season(s): NI Vectors:An sundaicus, An maculatus Malaria parasite: NI Source of funding: Singapore Armed Forces Study included in the previous review: no |
|
McCann 2021.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomized controlled trial Type of cluster: village Cluster size: approximately 1000 participants per cluster Number of clusters in each arm: 3 to 5 clusters per arm Adjusted for clustering? Yes |
|
| Participants |
Age: children aged 6 to 59 months, women aged 15 to 49 years Sex: any Comorbidities or pregnancy (or both): NI Primary outcome sample size (parasite prevalence): 20,013 individuals Secondary outcome sample size
|
|
| Interventions |
Intervention: habitat manipulation + modification + larviciding
Control: no intervention Duration of intervention: 2 years (May 2016 to May 2018) Who was responsible for LSM? NI Co‐interventions: yes Co‐interventions equal in each arm? Yes |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Continent: Africa Country: Malawi Urban or rural: NI Transmission intensity: high Transmission season(s): NI Vectors:An arabiensis, An funtestus Malaria parasite:P falciparum Source of funding: Stichting Dioraphite Study included in the previous review: no |
|
Munga 2013.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomized controlled trial Type of cluster: habitat area Cluster size: NI Number of clusters in each arm: 10 Adjusted for clustering? No |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation (frequent and intermediate disturbance). 30 mosquito aquatic habitats were randomly assigned to 2 types of treatments or no treatment (10 habitat replicates per group). Habitats were cleared of grass and water replenishment at different frequencies.
Control: no intervention (no disturbance for 30 days). Duration of intervention: 6 months (September 2005 to February 2006). This experiment was conducted in 1 month (30 days) after which the no‐disturbance habitats were also cleared of water and grass and the experiment repeated. Who was responsible for LSM? NI Co‐interventions: no Co‐interventions equal in each arm? NA |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Kenya Ecosystem: rural Transmission intensity: NI Transmission season(s): NI Vectors:An gambiae s.l., An funestus, An coustani, An implexus Malaria parasite:P falciparum Source of funding: WHO/United Nations Development Programme/World Bank Special Programme for Research and Training in Tropical Diseases (TDR) Study included in the previous review: no |
|
Mutero 2000.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomized controlled trial Type of cluster: experimental plot Cluster size: NI Number of clusters in each arm: 4 Adjusted for clustering? No |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation. 3 experimental plots divided into 12 subplots, 3 subplots randomly allocated to 3 different water regimens.
Control: 1 experimental plot divided into 3 subplots that were continuously flooded without rice cultivation. Duration of intervention: 12 weeks (7 September 1998 to 24 November 1998) Who was responsible for LSM? Mwea Irrigation and Agricultural Development experimental station Co‐interventions: no Co‐interventions equal in each arm? NA |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Kenya Ecosystem: rural Transmission intensity: low Transmission season(s): NI Vectors:An arabiensis Malaria parasite:P falciparum Source of funding: NI Study included in the previous review: no |
|
Sahu 2014.
| Study characteristics | ||
| Methods |
Study design: controlled before‐after study Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? NA |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation
Control: no sluice gates on bed dam Duration of intervention: 4 months (June 2010 to September 2010) Who was responsible for LSM? Residents of the village – volunteers Co‐interventions: unclear (probably IRS using DDT) Co‐interventions equal in each arm? Unclear |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Asia Country: India Ecosystem: rural Transmission intensity: high Transmission season(s): winter and early summer Vectors:An fluviatilis Malaria parasite:P falciparum Source of funding: unclear (District administration, Koraput) Study included in the previous review: no |
|
Samnotra 1980.
| Study characteristics | ||
| Methods |
Study design: controlled before‐after study Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? NA |
|
| Participants |
Age: any Sex: any Comorbidities or pregnancy (or both): any Primary outcome sample size
Secondary outcome sample size
|
|
| Interventions |
Intervention: habitat manipulation with larviciding
Control: no intervention Duration of intervention: 16 months (August 1976 to November 1977) Who was responsible for LSM? Study staff applied larviciding. Attempts to involve the community for habitat management were unsuccessful. Co‐interventions: case management and treatment for fever cases Co‐interventions equal in each arm? Yes |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Continent: Asia Country: India Ecosystem: urban Transmission intensity: low Transmission season(s): NI Vectors:An culicifacies, An stephensi Malaria parasite:P falciparum Source of funding: Haryana State Health Authorities, Alkali and Chemical Corporation of India Ltd, ICI Plant Protection Division Study included in the previous review: yes Given the information provided in the text on the failure in the attempt of performing habitat manipulation by the community, we consider the results of the intervention should be totally addressed to larviciding. Therefore, we considered it appropriate not to extract data in this review. The previous review reported the results as an integrated intervention instead. |
|
Santiago 1960.
| Study characteristics | ||
| Methods |
Study design: controlled before‐after study Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? NA |
|
| Participants |
Age: children aged 2 to 10 years, infants aged < 1 year Sex: any Comorbidities or pregnancy (or both): any Primary outcome sample size (malaria parasite prevalence): children aged 2 to 10 years only. Intervention area: pre‐intervention 646, postintervention: 566. Control area: pre‐intervention 210 (flushing over 1073 m), 277 (flushing over 2897 m); postintervention 280 Secondary outcome sample size
|
|
| Interventions |
Intervention: habitat manipulation
Control: no flushing Duration of intervention: 16 months (July 1953 to October 1954) Who was responsible for LSM? Local operators within the community and the United States Public Health Service Co‐interventions: NI Co‐interventions equal in each arm? NA |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Continent: Asia Country: Philippines Ecosystem: urban Transmission intensity: high Transmission season(s): NI Vectors:An minimus flavirostris Malaria parasite:P falciparum Source of funding: Malaria Eradication Project, San Pablo City Study included in the previous review: yes Duration of study was 16 months and not 12 as reported in the previous review (Tusting 2013), being the duration of the continuous flushing equal to 16 months. |
|
Sharma 2008.
| Study characteristics | ||
| Methods |
Study design: controlled before‐after study Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? No |
|
| Participants |
Age: any Sex: any Comorbidities or pregnancy (or both): any Primary outcome sample size
Secondary outcome sample size: NA |
|
| Interventions |
Intervention: habitat manipulation
Control: no intervention Duration of intervention: 16 months (September 2002 to December 2003) Who was responsible for LSM? Government of India Co‐interventions: routine malaria control activities under the primary healthcare system included IRS with DDT from 2001 to 2005 and a single round of IRS with a synthetic pyrethroid in 2001, 2003, and 2005. The mean house coverage with residual spraying in all the study villages was 60% to 80% during 2001 to 2005. Co‐interventions equal in each arm? Yes |
|
| Outcomes |
Primary outcomes
Secondary outcome: NA |
|
| Notes |
Continent: Asia Country: India Ecosystem: rural Transmission intensity: high Transmission season(s): perennial transmission throughout the year but peaks during the postmonsoon months of October to December. Malaria transmission season: March (intermediate), June (low), and November (high). Vectors:An fluviatilis, An culicifacies Malaria parasite:P falciparum Source of funding: Integrated Disease Vector Control Project funded by the Indian Council of Medical Research and Ministry of Health and Family Welfare, Government of India. Study included in the previous review: yes Duration of intervention in previous review was calculated as 23 months (Tusting 2013). |
|
Shililu 2007.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomized controlled trial Type of cluster: villages within zones Cluster size: NI Number of clusters in each arm: 4 Adjusted for clustering? No |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size
|
|
| Interventions |
Intervention: Details of the intervention: integrated vector control (habitat manipulation and habitat modification with larviciding)
Control: no intervention Duration of intervention: 24 months (no dates specified) Who was responsible for LSM? Study staff and local community Co‐interventions: none. However, ITNs and IRS were conducted as part of the national malaria control programme (coverage not reported). Co‐interventions equal in each arm? Not reported |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Eritrea Ecosystem: rural Transmission intensity: NI Transmission season(s): short period of transmission coinciding with short rainy season Vectors:An arabiensis, An cinereus, An pretoriensis, An d'thali, An funestus, An squamosus, An adenensis, An demeilloni Malaria parasite:P falciparum Source of funding: United States Agency for International Development, Environmental Health Project, International Center of Insect Physiology and Ecology, National Institutes of Health Study included in the previous review: yes |
|
Wamae 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? No |
|
| Participants |
Age: NA Sex: NA Comorbidities or pregnancy (or both): NA Primary outcome sample size: NA Secondary outcome sample size (density of immature mosquitoes): not reported |
|
| Interventions |
Intervention: habitat manipulation
Control: no shaded channels (11; 6 in Lunyerere and 5 in Emutete village). Duration of intervention: Lunyerere 10 months (November 2006 to August 2007), Emutete 8 months (January 2007 to August 2007) Who was responsible for LSM? NA Co‐interventions: no Co‐interventions equal in each arm? NA |
|
| Outcomes |
Primary outcome: NA Secondary outcome
|
|
| Notes |
Continent: Africa Country: Kenya Ecosystem: rural Transmission intensity: moderate to high (in study area: 20% to 44.3% school‐aged children) Transmission season(s): NI Vectors:An gambiae s.l., An funestus, An coustani, An rufipes, An marshalli, An maculipalpis, An azaniae, An implexus Malaria parasite: NI Source of funding: Dioraphte Foundation, the Netherlands Study included in the previous review: no |
|
Yohannes 2005.
| Study characteristics | ||
| Methods |
Study design: controlled before‐after study Type of cluster: NA Cluster size: NA Number of clusters in each arm: NA Adjusted for clustering? No |
|
| Participants |
Age: children aged < 10 years Sex: any Comorbidities or pregnancy (or both): any Primary outcome sample size
Secondary outcome sample size
|
|
| Interventions |
Intervention: habitat manipulation + habitat modification
Control: no intervention Duration of intervention: 11 months (February 2000 to December 2000) Who was responsible for LSM? Local community Co‐interventions: IRS with DDT used during the pre‐intervention only Co‐interventions equal in each arm? Yes |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes |
Continent: Africa Country: Ethiopia Ecosystem: rural Transmission intensity: low Transmission season(s): rainy season Vectors:An arabiensis and other anophelines Malaria parasite:P falciparum Source of funding: NA Study included in the previous review: no Tusting 2013 excluded the paper from the review due to reported differences in habitats between intervention and control at baseline. |
|
CDC: Centers for Disease Control and Prevention; CI: confidence interval; DDT: dichlorodiphenyltrichloroethane; IRS: indoor residual spraying; ITN: insecticide‐treated net; LSM: larval source management; NA: not applicable; NI: no information; WHO: World Health Organization.
See Appendix 5 for a glossary of terms.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amerasinghe 1991 | Study design did not match inclusion criteria, observational study. |
| Balfour 1936 | Intervention was too poorly reported to determine when it was initiated and thus whether the design was observational in nature. |
| Clark 2012 | Intervention did not match inclusion criteria. Abstracts from symposium. |
| Clark 2013 | Intervention did not match inclusion criteria. Abstracts from symposium. |
| Clark 2014 | Intervention did not match inclusion criteria. Abstracts from symposium. |
| Cohnstaedt 2016 | Intervention did not match inclusion criteria. Abstracts from symposium. |
| Cohnstaedt 2017 | Intervention did not match inclusion criteria. Abstracts from symposium. |
| Frake 2017 | Intervention did not match inclusion criteria, Master's thesis. |
| Getachew 2020 | Study design did not match inclusion criteria, observational study. |
| Gezie 2018 | Study design did not match inclusion criteria, observational study. |
| Jaleta 2013 | Study design did not match inclusion criteria, cross‐sectional study. |
| Kibret 2014 | Intervention did not match inclusion criteria, no intervention described. |
| Kibret 2019 | Study design did not match inclusion criteria, modelling paper. |
| Kiszewski 2014 | Intervention did not match inclusion criteria, no intervention described. |
| Laporta 2019 | Study design did not match inclusion criteria, review. |
| Nasreen 2016 | Intervention did not match inclusion criteria, no intervention described. |
| Ohta 2014 | Study design did not match inclusion criteria, modelling paper. |
| Phiri 2021 | Duplicate of included study. |
| Saxena 2014 | Intervention did not match inclusion criteria, no intervention described. |
| Srivastava 2013 | Intervention did not match inclusion criteria. |
| Tchoumbou 2020 | Study design did not match inclusion criteria, ineligible outcome measures. |
| Thapar 2019 | Study design did not match inclusion criteria, observational study. |
| van den Berg 2018 | Duplicate of included study. |
Characteristics of ongoing studies [ordered by study ID]
Zhou 2020.
| Study name | Adaptive interventions for optimizing malaria control: an implement study protocol for a block‐cluster randomized, sequential multiple assignment trial |
| Methods | Open‐label, block‐cluster sequential multiple assignment randomized controlled trial with variable number of arms (adaptive design), with baseline period with no cross‐over |
| Participants | 36 randomly selected clusters (village or several neighbouring villages) comprising low‐ and high‐elevation localities in western Kenya. |
| Interventions | Stage 1: equal randomization to 1 of 3 groups for 12 months' follow‐up
Stage 2: if stage 1 intervention of PBO‐treated LLINs was 'effective' within cluster, then intervention will continue; if 'not effective', then equally randomized to 1 of 2 groups for 18 months' follow‐up
Stage 2: if stage 1 intervention of LLINs with IRS was 'effective' within cluster, then intervention will continue; if 'not effective', then equally randomized to 1 of 2 groups for 18 months' follow‐up
|
| Outcomes | Clinical malaria incidence Density of adult mosquitoes Entomological inoculation rates |
| Starting date | July 2019 |
| Contact information | Guiyun Yan: guiyuny@uci.edu |
| Notes | ClinicalTrials.gov study ID NCT04182126 |
Bsph: Bacillus sphaericus; Bti: Bacillus thuringiensis israelensis; IRS: indoor residual spraying; LLIN: long‐lasting insecticide‐treated net; PBO: piperonyl butoxide.
Differences between protocol and review
Prespecified changes for review update
We have provided a table in Appendix 4 with the prespecified changes to the protocol before we performed this update to the published Cochrane Review (Tusting 2013). We amended the title from 'Mosquito larval source management for controlling malaria' (Tusting 2013) to 'Mosquito aquatic habitat modification and manipulation interventions to control malaria'.
Differences between revised protocol (prespecified changes in Appendix 4) and review update
As described in the Methods section, initially we planned to include cluster‐randomized trials (cRCT) that had at least two intervention and two comparator sites, and controlled before‐after (CBA) studies that had at least two intervention and two comparator sites. However, we relaxed the number of sites condition as there were insufficient cRCT and CBA studies identified for each type of intervention.
We anticipated being able to summarize the effects of interventions on adverse events as an outcome measure. However, this became impractical, and no studies were identified that reported environmental and health impacts affecting either human or animal populations, such as changes to biodiversity and ecosystem due to active intervention of the habitat.
For non‐randomized controlled studies, we stated we would use either the Effective Practice and Organisation of Care (EPOC) risk of bias assessment (with domains for selection bias, performance bias, detection bias, attrition bias, reporting bias, recruitment bias, baseline characteristics, contamination of intervention, appropriateness of statistical analysis, and adjustment for confounding) or the ROBINS‐I risk of bias assessment (within domains for confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results). During the risk of bias assessment, it became apparent that the domains in the ROBINS‐I risk of bias assessment aligned substantially better to strengths and weaknesses of non‐randomized controlled studies; therefore, we used the ROBINS‐I tool throughout the review for such study designs.
Contributions of authors
JLB ran the searches.
EM and GY selected studies.
EM, GY, and JLB extracted characteristics and study data, and assessed risk of bias.
JLB analyzed the data.
EM and JLB assessed the certainty of the evidence.
EM, GY, JLB, and RR drafted the review.
All authors contributed to the review update design and approved the final version for publication.
Sources of support
Internal sources
-
University of Nottingham, UK
Internal support for University of Nottingham authors
Liverpool School of Tropical Medicine, UK
External sources
-
Global Malaria Programme, WHO (WHO Global Malaria Programme Agreement for Performance of Work (APW) Grant 2019, Switzerland
WHO registration 2019/940667‐0
-
Foreign, Commonwealth, and Development Office (FCDO), UK
Project number: 300342‐104
Declarations of interest
EM: none.
GY: none.
RR is a Vice President of Global Health for RTI International, a non‐profit research institute based in North Carolina, USA.
JLB: consultancy fees from undertaking independent statistical review for Danone Nutricia Research, and from providing statistical expertise to the Food Standards Agency, which are both outside the subject of this review. JLB is a Content Editor for the Cochrane Diagnostic Accuracy Reviews Editorial Team.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Castro 2009 {published data only}
- Castro MC, Tsuruta A, Kanamori S, Kannady K, Mkude S. Community-based environmental management for malaria control: evidence from a small-scale intervention in Dar es Salaam, Tanzania. Malaria Journal 2009;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Djegbe 2020 {published data only}
- Djegbe I, Zinsou M, Flavien Dovonou E, Tchigossou G, Soglo M, Adeoti R, et al. Minimal tillage and intermittent flooding farming systems show a potential reduction in the proliferation of Anopheles mosquito larvae in a rice field in Malanville, Northern Benin. Malaria Journal 2020;19:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imbahale 2011 {published data only}
- Imbahale SS, Mweresa CK, Takken W, Mukabana WR. Development of environmental tools for anopheline larval control. Parasites and Vectors 2011;4:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imbahale 2012 {published data only}
- Imbahale SS, Githeko A, Mukabana WR, Takken W. Integrated mosquito larval source management reduces larval numbers in two highland villages in western Kenya. BMC Public Health 2012;12:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kibret 2018 {published data only}
- Kibret S, Wilson G, Ryder D, Tekie H, Petros B. Can water-level management reduce malaria mosquito abundance around large dams in sub-Saharan Africa? PLOS One 2018;13(4):e0196064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee VJ, Ow S, Heah H, Tan MY, Lam P, Ng L-C, et al. Elimination of malaria risk through integrated combination strategies in a tropical military training island. American Journal of Tropical Medicine and Hygiene 2010;82(6):1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCann 2021 {published data only}
- McCann RS, Kabaghe AN, Moraga P, Gowelo S, Mburu MM, Tizifa T, et al. The effect of community-driven larval source management and house improvement on malaria transmission when added to the standard malaria control strategies in Malawi: a cluster-randomized controlled trial. Malaria Journal 2021;20:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munga 2013 {published data only}
- Munga S, Vulule J, Kweka E. Response of Anopheles gambiae s.l. (Diptera: Culicidae) to larval habitat age in western Kenya highlands. Parasites and Vectors 2013;6(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutero 2000 {published data only}
- Mutero CM, Blank H, Konradsen F, Hoek W. Water management for controlling the breeding of Anopheles mosquitoes in rice irrigation schemes in Kenya. Acta Tropica 2000;76(3):253-63. [DOI] [PubMed] [Google Scholar]
Sahu 2014 {published data only}
- Sahu SS, Gunasekaran K, Jambulingam P. Environmental management through sluice gated bed-dam: a revived strategy for the control of Anopheles fluviatilis breeding in streams. Indian Journal of Medical Research 2014;140(2):296-301. [PMC free article] [PubMed] [Google Scholar]
Samnotra 1980 {published data only}
- Samnotra K, Kumar P. Field evaluation of pirimiphos-methyl as a mosquito larvicide in an urban area of India as part of the national malaria eradication programme. Mosquito News 1980;40:257-63. [Google Scholar]
Santiago 1960 {published data only}
- Santiago D. Malaria control by automatic flushing of streams. Philippine Journal Science 1960;8:373-95. [Google Scholar]
Sharma 2008 {published data only}
- Sharma SK, Tyagi PK, Upadhyay AK, Haque MA, Adak T, Dash AP. Building small dams can decrease malaria: a comparative study from Sundargarh District, Orissa, India. Acta Tropica 2008;107(2):174-8. [DOI] [PubMed] [Google Scholar]
Shililu 2007 {published data only}
- Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R. Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. American Journal of Tropical Medicine and Hygiene 2007;76(1):103-10. [PubMed] [Google Scholar]
Wamae 2010 {published data only}
- Wamae PM, Githeko AK, Menya DM, Takken W. Shading by napier grass reduces malaria vector larvae in natural habitats in Western Kenya highlands. EcoHealth 2010;7(4):485-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yohannes 2005 {published data only}
- Yohannes M, Haile M, Ghebreyesus TA, Witten KH, Getachew A, Byass P, et al. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Tropical Medicine and International Health 2005;10(12):1274-85. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amerasinghe 1991 {published data only}
- Amerasinghe FP, Amerasinghe PH, Peiris JS, Wirtz RA. Anopheline ecology and malaria infection during the irrigation development of an area of the Mahaweli Project, Sri Lanka. American Journal of Tropical Medicine and Hygiene 1991;45(2):226-35. [DOI] [PubMed] [Google Scholar]
Balfour 1936 {published data only}
- Balfour MC. Some features of malaria in Greece and experience with its control. Rivista di Malariologia 1936;15:114-31. [Google Scholar]
Clark 2012 {published data only}
- Clark GG, Rubio-Palis Y. Mosquito vector biology and control in Latin America 22nd symposium. Journal of the American Mosquito Control Association 2012;28(2):102-10. [DOI] [PubMed] [Google Scholar]
Clark 2013 {published data only}
- Clark GG, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 23rd symposium. Journal of the American Mosquito Control Association 2013;29(3):251-69. [DOI] [PubMed] [Google Scholar]
Clark 2014 {published data only}
- Clark GG, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 24th symposium. Journal of the American Mosquito Control Association 2014;30(3):204-14. [DOI] [PubMed] [Google Scholar]
Cohnstaedt 2016 {published data only}
- Cohnstaedt LW, Alfonso-Para C, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 26th Symposium. Journal of American Mosquito Control Association 2016;32(4):315-22. [DOI] [PubMed] [Google Scholar]
Cohnstaedt 2017 {published data only}
- Cohnstaedt LW, Alfonso-Parra C, Fernandez-Salas I. Mosquito vector biology and control in Latin America – a 27th symposium. Journal of the American Mosquito Control Association 2017;33(3):215-24. [DOI] [PubMed] [Google Scholar]
Frake 2017 {published data only}
- Frake A, Messina J, Walker ED, Zulu L, Mangani C, Mkwaila W, et al. Scaling irrigation and malaria risk in Malawi. American Journal of Tropical Medicine and Hygiene 2017;97(5):509. [Google Scholar]
Getachew 2020 {published data only}
- Getachew D, Blakew M, Tekie H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malaria Journal 2020;19:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gezie 2018 {published data only}
- Gezie A, Assefa WW, Getnet B, Anteneh W, Dejen E, Mereta ST. Potential impacts of water hyacinth invasion and management on water quality and human health in Lake Tana watershed, Northwest Ethiopia. Biological Invasions 2018;20(9):2517-34. [Google Scholar]
Jaleta 2013 {published data only}
- Jaleta KT, Hill SR, Seyoum E, Balkew M, Gebre-Michael T, Ignell R, et al. Agro-ecosystems impact malaria prevalence: large-scale irrigation drives vector population in western Ethiopia. Malaria Journal 2013;12:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kibret 2014 {published data only}
- Kibret S, Wilson GG, Tekie H, Petros B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malaria Journal 2014;13(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kibret 2019 {published data only}
- Kibret S, Ryder D, Wilson GG, Lalit K. Modeling reservoir management for malaria control in Ethiopia. Scientific Reports 2019;9(1):18075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiszewski 2014 {published data only}
- Kiszewski AE, Teffera Z, Wondafrash M, Ravesi M, Pollack RJ. Ecological succession and its impact on malaria vectors and their predators in borrow pits in western Ethiopia. Journal of Vector Ecology 2014;39(2):414-23. [DOI] [PubMed] [Google Scholar]
Laporta 2019 {published data only}
- Laporta GZ. Amazonian rainforest loss and declining malaria burden in Brazil. Lancet Planetary Health 2019;3(1):e4-5. [DOI] [PubMed] [Google Scholar]
Nasreen 2016 {published data only}
- Nasreen A, Nagpal BN, Kapoor N, Srivastava A, Gupta HP, Saxena R, et al. Impact of ecological and climatic changes on vectors of malaria in four North-Eastern states of India. Indian Journal of Ecology 2016;43(1):1-15. [Google Scholar]
Ohta 2014 {published data only}
- Ohta S, Kaga T. Effect of irrigation systems on temporal distribution of malaria vectors in semi-arid regions. International Journal of Biometeorology 2014;58(3):349-59. [DOI] [PubMed] [Google Scholar]
Phiri 2021 {published data only}
- Phiri MD, McCann RS, Kabaghe AN, den Berg H, Malenga T, Gowelo S, et al. Cost of community‑led larval source management and house improvement for malaria control: a cost analysis within a cluster‑randomized trial in a rural district in Malawi. Malaria Journal 2021;20:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saxena 2014 {published data only}
- Saxena R, Nagpal BN, Singh VP, Srivastava A, Dev V, Sharma MC, et al. Impact of deforestation on known malaria vectors in Sonitpur district of Assam, India. Journal of Vector Borne Diseases 2014;51(3):211-5. [PubMed] [Google Scholar]
Srivastava 2013 {published data only}
- Srivastava AK, Kharbuli B, Shira DS, Sood A. Effect of land use and land cover modification on distribution of anopheline larval habitats in Meghalaya, India. Journal of Vector Borne Diseases 2013;50(2):121-6. [PubMed] [Google Scholar]
Tchoumbou 2020 {published data only}
- Tchoumbou MA, Mayi MP, Malange EN, Foncha FD, Kowo C, Fru-cho J, et al. Effect of deforestation on prevalence of avian haemosporidian parasites and mosquito abundance in a tropical rainforest of Cameroon. International Journal for Parasitology 2020;50(1):63-73. [DOI] [PubMed] [Google Scholar]
Thapar 2019 {published data only}
- Thapar R, Singh S, Srivastava P. The study on impact of Ujina irrigation canal on malaria transmission in District Nuh (Erstwhile Mewat), Haryana. Journal of Communicable Diseases 2019;51(3):46-54. [Google Scholar]
van den Berg 2018 {published data only}
- den Berg H, Vugt M, Kabaghe A, Nkalapa M, Kaotcha R, Truwah Z, et al. Community-based malaria control in southern Malawi: a description of experimental interventions of community workshops, house improvement and larval source management. Malaria Journal 2018;17(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Zhou 2020 {published data only}
- Zhou G, Lee M-C, Atieli HE, Githure JI, Githeko AK, Kazura JW, et al. Adaptive interventions for optimizing malaria control: an implement study protocol for a block-cluster randomized, sequential multiple assignment trial. Trials 2020;21:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Atkinson 2011
- Atkinson JA, Vallely A, Fitzgerald L, Whittaker M, Tanner M. The architecture and effect of participation: a systematic review of community participation for communicable disease control and elimination. Implications for malaria elimination. Malaria Journal 2011;10:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2019
- Choi L, Majambere S, Wilson AL. Larviciding to prevent malaria transmission. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD012736. [DOI: 10.1002/14651858.CD012736.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 2009
- Cochrane Effective Practice and Organisation of Care Group (EPOC). Risk of bias guidelines. EPOC Author Resources. epoc.cochrane.org/ (accessed 30 September 2020).
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keiser 2005
- Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Infectious Diseases 2005;5(11):695-708. [DOI] [PubMed] [Google Scholar]
McCann 2017
- McCann RS, den Berg H, Diggle PJ, Vugt M, Terlouw DJ, Phiri KS, et al. Assessment of the effect of larval source management and house improvement on malaria transmission when added to standard malaria control strategies in southern Malawi: study protocol for a cluster-randomised controlled trial. BMC Infectious Diseases 2017;17:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muema 2017
- Muema JM, Bargul JL, Njeru SN, Onyango JO, Imbahale SS. Prospects for malaria control through manipulation of mosquito larval habitats and olfactory-mediated behavioural responses using plant-derived compounds. Parasites and Vectors 2017;10(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2019;111:105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán Mi, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Vontas 2014
- Vontas J, Moore S, Kleinschmidt I, Ranson H, Lindsay S, Lengeler C, et al. Framework for rapid assessment and adoption of new vector control tools. Trends in Parasitology 2014;30(4):191-204. [DOI] [PubMed] [Google Scholar]
Walshe 2017
- Walshe DP, Garner P, Adeel AA, Pyke GH, Burkot TR. Larvivorous fish for preventing malaria transmission. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD008090. [DOI: 10.1002/14651858.CD008090.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity, 2011. apps.who.int/iris/handle/10665/85839 (accessed 29 September 2020).
WHO 2012
- World Health Organization. Handbook for integrated vector management, June 2012. www.who.int/publications/i/item/9789241502801 (accessed 19 May 2021).
WHO 2013
- World Health Organization. Larval source management: a supplementary measure for malaria vector control, July 2013. www.who.int/publications/i/item/9789241505604 (accessed 29 September 2020).
WHO 2015a
- World Health Organization. Global technical strategy for malaria 2016–2030, June 2015. www.who.int/publications/i/item/9789240031357 (accessed 19 May 2021).
WHO 2015b
- World Health Organization. Guidelines for the treatment of malaria. Third edition, April 2015. www.afro.who.int/publications/guidelines-treatment-malaria-third-edition (accessed 29 September 2020).
WHO 2017
- World Health Organization. Global vector control response 2017–2030, October 2017. www.who.int/publications/i/item/9789241512978 (accessed 19 May 2021).
WHO 2019a
- World Health Organization (WHO). World malaria report 2019. www.who.int/publications/i/item/9789241565721 (accessed 29 September 2020).
WHO 2019b
- World Health Organization (WHO). Guidelines for malaria vector control, February 2019. apps.who.int/iris/bitstream/handle/10665/310862/9789241550499-eng.pdf (accessed 29 September 2020).
WHO 2020
- World Health Organization. World malaria report 2020 – 20 years of global progress & challenges. www.who.int/publications/i/item/9789240015791 (accessed 19 May 2021).
Wilson 2020
- Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLOS Neglected Tropical Diseases 2020;14(1):e0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Thwing 2011
- Thwing J, Fillinger U, Gimnig J, Newman R, Lindsay S. Mosquito larval source management for controlling malaria. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: CD008923. [DOI: 10.1002/14651858.CD008923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tusting 2013
- Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD008923. [DOI: 10.1002/14651858.CD008923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


