Abstract
Background and Objectives
Exposure to socioeconomic disadvantage is associated with early-onset cognitive aging. Biological aging, the progressive loss of system integrity that occurs as we age, is proposed as a modifiable process mediating this health inequality. We examined whether socioeconomic disparities in cognitive aging in mid-to late-life adults is explained by accelerated biological aging similarly across race, ethnicity, and sex/gender.
Methods
Data were from a prospective cohort study of the US Health and Retirement Study DNA methylation substudy. Socioeconomic status (SES) was measured from years of education and household wealth at baseline. The extent and pace of biological aging were quantified using 3 DNA methylation measures: PhenoAge, GrimAge, and DunedinPoAm. Cognitive aging was measured from repeated longitudinal assessments of immediate and delayed word recall. Latent growth curve modeling estimated participants' level of memory performance and rate of decline over 2–11 follow-up assessments spanning 2–20 years. Multiple-group models were estimated to assess whether the relationship between SES and memory trajectories was mediated by biological aging across racial-ethnic by sex/gender subgroups.
Results
Data from a total of 3,997 adults aged 50–100 years were analyzed. Participants with lower SES had a lower memory performance, had a faster decline, and exhibited accelerated biological aging (SES effect size associations [β] ranged from 0.08 to 0.41). Accelerated biological aging was associated with decreased memory performance and faster memory decline (effect size range 0.03–0.23). SES-biological aging associations were the strongest for White men and women and weakest for Latinx women. The relationship between biological aging measures and memory was weaker for Black participants compared with that for White and Latinx people. In mediation analysis, biological aging accounted for 4%–27% of the SES-memory gradient in White participants. There was little evidence of mediation in Black or Latinx participants.
Discussion
Among a national sample of mid-to late-life adults, DNA methylation measures of biological aging were variably associated with memory trajectories and SES across White, Black, and Latinx mid-to late-life adults. These results challenge the assumption that DNA methylation biomarkers of aging that were developed in primarily White people can equivalently quantify aging processes affecting cognition in Black and Latinx mid-to late-life adults.
Aging and socioeconomic status (SES) are 2 important determinants of late-life cognitive health. Physicians often regard age and SES as immutable factors rather than quantities that could be modified to improve patient health. However, both may represent promising targets for intervention. Moreover, they may be connected.
The biological process of aging involves the progressive loss of system integrity, causing decreased resilience of cellular networks and organ systems, ultimately leading to disease and death.1 The process of biological aging begins in early life2 and manifests in variable rates of decline in organ-system integrity already in young adulthood.3 In midlife adults, faster-paced decline in organ-system integrity is associated with signs of brain aging and cognitive decline.4 Aging-related changes in brain integrity (e.g., cortical thinning and hippocampal atrophy) are accompanied by decline in cognitive performance.5
Low SES, commonly measured from wealth, income, occupational status, and/or educational experience, is associated with an earlier onset and faster pace of aging-related changes in the brain.6,7 The mechanism by which SES inequalities affect health may be through acceleration of the aging process through repeated adaptation to stressors that cause cumulative wear and tear on the body's system and increase vulnerability to multiple disease processes.8,9 Until recently, we have not had a way of comprehensively measuring these processes. DNA methylation (DNAm) biomarkers that quantify molecular alterations that occur with aging provide promising tools for measuring SES inequalities in aging. Several proposed measures indicate more advanced biological aging in individuals with greater socioeconomic disadvantage.10-13 Some of these measures have also been associated with cognitive performance in small samples.10,13,14 However, it is unclear whether DNAm patterns mediate the relationship between SES and late-life cognitive health.
There is no gold standard measure of biological aging; several methods have been proposed based on different biological measurements and analytic strategies.15 Three recently developed measures of biological aging have gained popularity in the literature and have been consistently associated with SES and cognitive aging: 2 DNAm clocks, PhenoAge16 and GrimAge,17 and the DunedinPoAm10 pace of aging measure. DNAm clocks measure methylation levels at different age-related CpG sites to quantify an individual's biological age. PhenoAge was developed using the Infinium Methylation EPIC array to capture clinical measures to increase the accuracy of morbidity predictions.16 GrimAge uses plasma protein predictors to identify 1,030 CpG sites that forecast time-to-death due to all-cause mortality.17 A more recent aging index, DunedinPoAm, uses DNAm signatures to capture within-individual variation in the pace of aging of health-relevant systems. Unlike the other 2 methylation clocks, this index records how much time has passed; it is designed to function as a speedometer, recording how fast the individual is aging.10
Most research on DNAm biomarkers of aging has focused non-Latinx White samples and for whom the spectrum of socioeconomic variation may be limited relative to what exists in the United States. Furthermore, population subgroup differences in the pathways that link SES to cognition may be present at the intersection of race, ethnicity, and sex/gender groups, which is critical to consider for potential intervention on SES or biological aging. To address these gaps in the literature, this study was conducted to examine associations between SES, 3 DNAm biomarkers, and memory trajectories in a representative national sample of mid-to late-life adults. We hypothesize that these measures of biological aging will mediate the relationship between SES and late-life memory trajectories similarly across racial-ethnic-sex/gender subgroups.
Methods
Participants
The Health and Retirement Study (HRS) is a nationally representative longitudinal study of Americans older than 50 years, designed to examine the health, social, and economic factors associated with aging.18 Data collection began in 1992, with the initial cohort born between 1931 and 1941 (aged 51–61 years in 1992). In 1993, a second cohort was added consisting of individuals born before 1924 (aged 70 years or older in 1993). Two additional cohorts were added in 1998 to address age gaps and create a representative sample of those older than 50 years. These additional cohorts consist of individuals born between 1924 and 1930 and 1942–1947, respectively. Since then, a new cohort of individuals aged 51–56 years has been continually added to the HRS sample every 6 years (2004, 2010, and 2016). The HRS oversamples Black and Hispanic/Latino/a/x/e (Latinx, hereafter) participants to improve the reliability of estimates. Additional details of the HRS sample may be found elsewhere.19
Participants are followed up every 2 years to complete core interviews. These core interviews consist of content including (but not limited to) the following: demographics, assets and income, physical conditions and treatment, health behaviors, cognitive function, among others.18 In 2016, alive HRS participants were asked to consent to a venous blood draw, of which samples from 9,934 participants were collected (65% completion rate among eligible cases).20 DNAm assays were performed on a subsample of these participants (n = 4,104); approximately 98% passed quality control checks (n = 4,018). This DNAm sample is representative of the entire HRS sample. Participants were excluded from the current analyses if they were missing data on race and ethnicity (n = 2) and years of education (n = 19). The remaining sample (n = 3,997) included 1,165 non-Latinx White men, 1,504 non-Latinx White women, 224 non-Latinx Black men, 433 non-Latinx Black women, 223 Latinx men, 328 Latinx women, and 47 men and 73 women who identified their ethnicity as non-Latinx and race as other.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all participants in the HRS study. Ethical approval for the HRS was obtained from the University of Michigan institutional review board.
Measures
Measures of Biological Aging
The 3 aging measures are described in detail in the eMethods, links.lww.com/WNL/C296.
In short, the PhenoAge and GrimAge clocks estimate the age at which a person's mortality risk would be approximately normal in their respective reference samples: PhenoAge was developed in the US National Health and Nutrition Examination Survey III and Invecchiare in Chianti studies,16 GrimAge was developed in the Framingham Heart Study Offspring cohort.17 Both clocks were developed using 2-step approaches that involved modeling physiologic parameters (clinical chemistries and complete blood count data for PhenoAge, blood proteins for GrimAge) and mortality risk. Clock ages that are older than the chronological age of the person being measured indicate an advanced state of biological aging; younger clock ages indicate delayed aging.21 For analysis, HRS participants' DNAm clock ages were regressed on their chronological ages, and residual values were computed. These residual values, referred to as “age acceleration residuals,” were standardized to mean = 0, SD = 1. Age acceleration residual values greater than 0 indicate higher than expected biological aging based on chronological age; values less than 0 indicate lower than expected biological age.
DunedinPoAm estimates a person's pace of aging, the rate of decline in system integrity that occurs with advancing chronological age. DunedinPoAm was developed by analyzing longitudinal change in 18 biomarkers tracking multiorgan-system integrity in a birth cohort followed up from ages 26–38 years.10 Values are interpretable as years of physiologic change occurring per 12-month calendar interval in healthy adults. DunedinPoAm values > 1 indicate a faster than normal pace of aging; values <1 indicate a slower pace of aging. For analysis, values of DunedinPoAm were standardized to mean = 0, SD = 1.
Race, Ethnicity, and Sex/Gender
HRS collected information about self-reported primary race and ethnicity by (1) asking participants whether they were Hispanic or Latino; and (2) asking participants to classify themselves racially as White, Black, Asian, American Indian, Alaska Native, or Pacific Islander. Participants were only asked to self-report whether they were male or female. We use the term sex/gender because it is unknown whether individuals actually reported their biological sex or their gender identity.22
Socioeconomic Status
An SES composite was created based on measures collected during the first study visit. Years of education was measured by the highest self-reported completed grade of school. We calculated birth cohort–based education standardized scores (z scores) by subtracting each participant's years of education by the mean education for their birth cohort and dividing by its SD. Wealth was measured by total wealth available in the RAND corporation HRS dataset income and wealth imputation dataset.23 Total wealth was adjusted for inflation using the consumer price index24 to calculate inflation relative to 2012 and converted all other waves to 2012 dollars.25 We then transformed these scores using the inverse hyperbolic sine.26 Z scores were created for wealth based on 5-year age groups (i.e., 50–54, 55–59, 60–64, etc.) for each study wave to adjust for age and period effects. Education and wealth z scores were averaged to represent the SES composite.
Memory Performance
Memory was assessed through the immediate and delayed recall scores of the Consortium to Establish a Registry for Alzheimer's Disease27 10-item word list memory test. Only data from Wave 3 (1996) or later were used because this was the first wave the 10-item memory test was administered. HRS RAND data include imputed cognitive scores for respondents who participated in a given wave but were missing cognitive data. We restricted longitudinal data to visits that respondents were aged older than 50 years. Raw scores on immediate and delayed recall trials were converted into z scores using the mean values and SDs at Wave 13 (2016). Composite scores were computed by averaging these z scores at each occasion because this method has been shown to improve reliability28 and contains much of the same information as a common memory factor score.29
Statistical Analyses
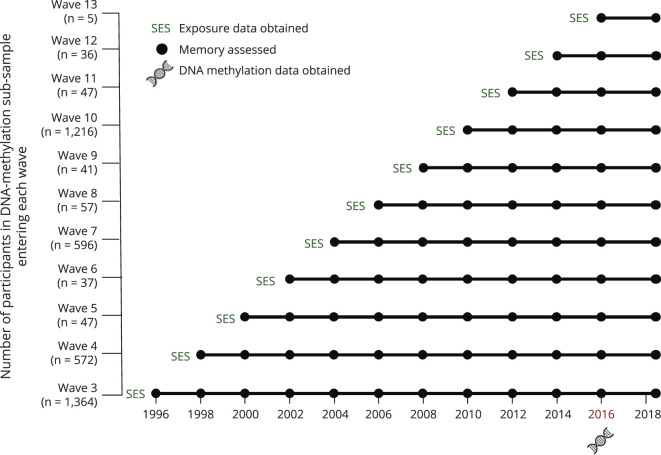
Figure 1 depicts the timing of measurements included in analyses described further. Data from 1996 to 2018 were used to model memory trajectories through a latent growth curve model that included age and study wave at first memory assessment as covariates. To derive estimates of memory functioning when DNA samples were collected, performance at the 2016 visit was used as the growth curve model intercept. Time was also centered at the 2016 visit, indicating the amount of time each visit occurred in years from 2016. Thus, intercepts indicate memory performance when DNA samples were collected, and slopes indicate the average rate of decline throughout the study. Models allowing only linear change fit better than those allowing both linear and quadratic slopes. Intercept and slope estimates were saved for each participant and used as outcomes in subsequent models to avoid multicollinearity issues.30
Figure 1. Timing of Measurements Included in the Study.
We estimated a series of models to evaluate independent relationships between each DNAm indicator, SES, and memory intercept and slope. Next, mediation analyses were conducted, separately for each DNAm indicator. For these models, we specified paths between (1) SES and the DNAm indicator, (2) the DNAm indicator and memory outcomes, and (3) SES to the memory outcomes. The indirect effect was calculated as the product of the coefficients corresponding to the path between SES and DNAm indicator and the path between DNAm indicator and memory.31 Mediation effects were examined using the products of coefficient approach with bootstrapping.32 Standardized parameter estimates and proportion mediated (PM; proportion of the effect of SES on memory trajectories mediated through the biological aging measure) were reported. Sampling weights for the 2016 Venous Blood Study were used to obtain population-based estimates.
Each model was first estimated across the entire sample. To examine racial-ethnic-sex/gender subgroup differences, we used known-class mixture models with racial-ethnic-sex/gender subgroups as known classes. This known grouping variable is incorporated as a moderator variable, allowing model parameters to vary as a function of membership in the identified groups.33 Subgroup differences were examined using the “Model Constraint” option in Mplus. Multiple-group models were estimated only for White, Black, and Latinx men and women (N = 3,877) because sample sizes of the other racial-ethnic-sex/gender subgroups were too small for this type of analysis.
Additional analyses were conducted to examine relationships between individual components of the SES composite with memory and the biological aging indicators. Given that the memory decline outcome in our analyses contains information from measurements before measurement of the biological aging indicators, sensitivity analyses were conducted, restricting the sample to the subset with available 2018 data. Latent difference score analyses were used to model change in memory performance between the 2016 and 2018 visits. The derived change score was regressed on SES, and the biological aging indicators and mediation models were conducted according to the procedures described earlier.
All analyses were performed using Mplus version 8.5.34 Both p values and CIs were used to determine statistical significance.35 Bootstrapping (1,000 bootstrapped samples) was used to obtain 95% CIs of all direct and indirect effects. A p value of 0.01 (i.e., 99% CI) was used for multiple group comparisons to decrease the likelihood of type I error.
Data Availability
The datasets used for analyses are available from the HRS website (hrs.isr.umich.edu/dataproducts/access-to-public-data).
Results
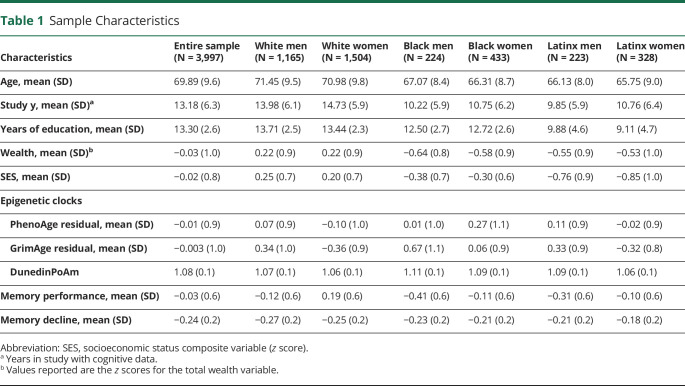
Characteristics of the full sample and the 6 racial-ethnic-sex/gender subgroups are summarized in Table 1. Participants had an average of 4.2 (SD = 3.1) visits with cognitive data. Black men demonstrated the lowest 2016 memory performance and White women had the highest. White men and women were older in 2016, completed more study visits, and demonstrated steeper rates of memory decline compared with Black and Latinx participants (p values < 0.001).
Table 1.
Sample Characteristics
SES and Memory Trajectories
Overall, a 1 SD increase in SES increases 2016 memory performance by 0.41 (0.37–0.44) SDs and lowers the rate of memory decline by 0.16 (0.12–0.20) SDs. Higher SES was associated with higher memory level and a slower rate of decline across race-ethnicity-sex/gender (Figure 2). The relationship between SES and 2016 memory was stronger for Black women compared with that for White women (βdiff = −0.11 [−0.18 to −0.02]). For Latinx women, SES had a stronger relationship with memory decline when compared with that for White men (βdiff = −0.04 [−0.07 to −0.02]).
Figure 2. Socioeconomic Gradients in Memory Level and Decline Across Racial-Ethnic-Sex/Gender Subgroups.
Figure 2 plots effect sizes for associations between SES and memory trajectories estimated across race/ethnicity-sex/gender subgroups. The x-axis represents the effect size estimate. The figure shows that each SD increase in SES was associated with 0.34–0.47 SD higher scores on 2016 memory performance (top panel) and 0.07–0.22 SD less decline in memory scores per 10-year follow-up period in the study. SES = socioeconomic status.
SES and Biological Aging
Participants' DNAm clock ages were correlated with their chronological ages (PhenoAge r = 0.73; GrimAge r = 0.83). DunedinPoAm was not correlated with chronological age (r = 0.02). PhenoAge indicated that Latinx and White men had the most-advanced aging and Latinx and White women had the most delayed aging (Table 1). GrimAge and DunedinPoAm indicated that Black men had the fastest biological aging and White and Latinx women had the slowest aging (p-values < 0.001).
An SES gradient in biological aging was noted in the entire sample, where higher SES was associated with slower aging for each DNAm measure (PhenoAge β = −0.08 [−0.12 to −0.04]; GrimAge β = −0.27 [−0.31 to −0.23]; DunedinPoAm β = −0.21 [−0.25 to −0.17]). The SES-PhenoAge gradient was present among White women (Figure 3) and was stronger than the gradient among Latinx men (βdiff = −1.09 [−2.39 to −0.33]) and Latinx women (βdiff = −0.83 [−2.01 to −0.28]). For GrimAge, the SES-biological aging gradient was present for all groups except Latinx women, and estimates were stronger for White men (βdiff = −1.61 [−2.48 to −0.95]), White women (βdiff = −1.30 [−1.97 to −0.67]), and Black women (βdiff = −1.02 [−2.0 to −0.16]) compared with those for Latinx women. A similar pattern of group differences was noted for DunedinPoAm, where the relationship with SES was stronger for White men (βdiff = −0.20 [−0.04 to −0.01]), White women (βdiff = −0.02 [−0.03 to −0.01]), and Black women (βdiff = −0.02 [−0.04 to −0.01]) compared with that for Latinx women. An examination of education and wealth as separate predictors of the biological aging measures revealed weaker associations than using the composite measure, with a few exceptions: relationships between wealth and GrimAge were stronger for Latinx men (β = −0.18 [−0.33 to −0.02]) and women (β = −0.11 [−0.24 to 0.02]) and between wealth and PhenoAge for Black men (β = −0.08 [−0.25 to 0.11]) compared with estimates for the overall SES composite.
Figure 3. Socioeconomic Gradients in DNA Methylation Measures of Aging Across Racial-Ethnic-Sex/Gender Subgroups.
Scatterplots showing associations between SES (x-axis) and each DNAm measure (y-axis) across racial-ethnic-sex/gender subgroups. Higher values on PhenoAge, GrimAge, and Dunedin indicate accelerated biological aging. SES = socioeconomic status.
Biological Aging and Memory Trajectories
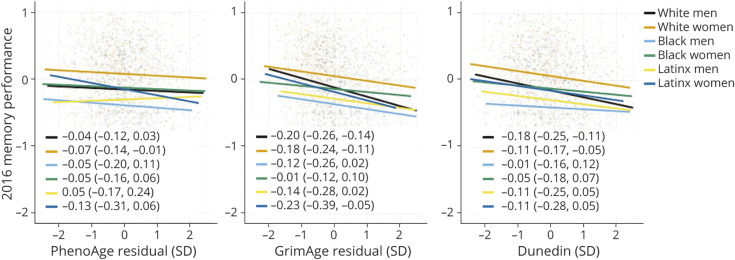
Overall, accelerated biological aging was associated with a lower 2016 memory performance (PhenoAge β = −0.08 [−0.12 to −0.04]; GrimAge β = −0.23 [−0.27 to −0.20]; DunedinPoAm β = −0.15 [−0.19 to −0.11]). More-advanced PhenoAge (β = −0.04 [−0.09 to −0.001]) and GrimAge (β = −0.10 [−0.13 to −0.06]) and faster DunedinPoAm (β = −0.07 [−0.12 to −0.03]) were associated with faster memory decline. Associations of DNAm measures of aging with 2016 memory performance (Figure 4) and memory decline (Figure 5) varied across race/ethnic-sex/gender groups. PhenoAge was only reliably associated with 2016 memory performance for White women. Older GrimAge and Higher DunedinPoAm values were associated with a lower 2016 memory performance and faster memory decline for White men and women. While many of the relationships between biological aging indicators and memory performance were the strongest for Latinx women, estimated CIs were wide, suggesting lack of precision of the estimated effects. Black women consistently demonstrated the weakest associations between DNAm measures and memory trajectories. Reliable group differences were noted for the relationship between GrimAge and memory trajectories between Black women and White men (2016 Memory βdiff = −0.030 [−0.05 to −0.01]; Memory Decline βdiff = −0.006 [−0.011 to −0.001]), White women (2016 Memory βdiff = −0.022 [−0.039 to −0.005]; Memory Decline βdiff = −0.008 [−0.014 to −0.002]), and Latinx women (2016 Memory βdiff = −0.030 [−0.052 to −0.007]; Memory Decline βdiff = −0.009 [−0.017 to −0.002]) and for DunedinPoAm and 2016 memory between White men and Black men (βdiff = −1.17 [−2.32 to −0.118]) and women (βdiff = −0.918 [−1.61 to −0.235]).
Figure 4. Relationship Between DNA Methylation Measures of Aging and Memory Level Across Racial-Ethnic-Sex/Gender Subgroups.
Scatterplots showing associations between each DNAm measure (x-axis) and 2016 memory performance (y-axis) across racial-ethnic-sex/gender subgroups.
Figure 5. Relationship Between DNA Methylation Measures of Aging and Memory Decline Across Racial-Ethnic-Sex/Gender Subgroups.
Scatterplots showing associations between each DNAm measure (x-axis) and memory decline (y-axis) across racial-ethnic-sex/gender subgroups.
Mediation Analyses
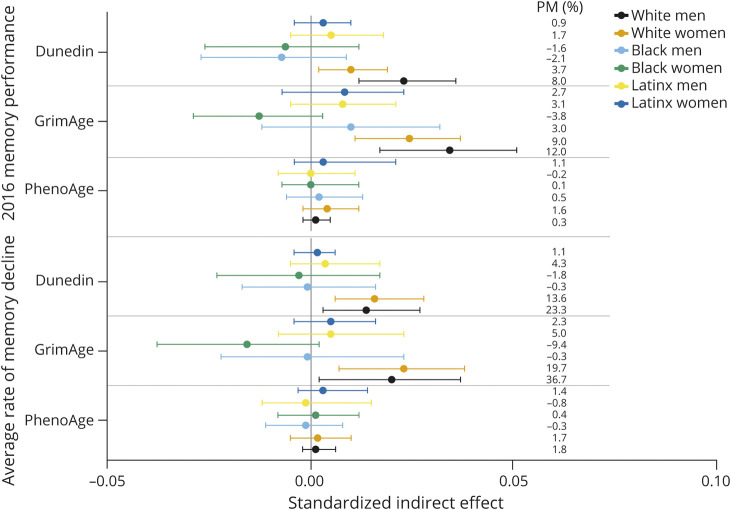
Mediation models were estimated for each DNAm measure separately. Each DNAm measure partially mediated the SES gradient on 2016 memory performance (PhenoAge indirect effect = 0.003 [0.001–0.006]; GrimAge indirect effect = 0.031 [0.025–0.039]; DunedinPoAm indirect effect = 0.014 [0.009–0.019]). PM estimates indicated that GrimAge (PM = 11%) conveyed a larger portion of the SES gradient than DunedinPoAm (PM = 5%) or PhenoAge (PM = 1%). GrimAge (indirect effect = 0.013 [0.006–0.020]; PM = 24%) and DunedinPoAm (indirect effect = 0.010 [0.005–0.016]; PM = 18%) partially mediated the SES-memory decline gradient.
Standardized indirect effect estimates for the PhenoAge models were small across race-ethnicity-sex/gender groups (Figure 6). Compared with the other groups, GrimAge and DunedinPoAm mediated larger proportions of the SES gradient in memory level and decline for White men and women.
Figure 6. Indirect Effect Estimates From Mediation Analyses Across Racial-Ethnic-Sex/Gender Subgroups.
Sensitivity Analyses
Sensitivity analyses examining change in memory performance from 2016 to 2018 revealed a similar pattern of associations compared with the main analyses. Specifically, higher SES (β = 0.16 [0.12–0.19]), less-advanced PhenoAge (β = −0.03 [−0.06 to 0.004]) and GrimAge (β = −0.08 [−0.11 to −0.05]), and slower DunedinPoAm (β = −0.05 [−0.09 to −0.02]) were associated with a smaller decrease in change from 2016 to 2018. Indirect effect estimates were similar to those reported for the SES-memory decline gradient (PhenoAge: β = 0.001 [−0.001 to −0.004]; GrimAge: β = 0.014 [0.01–0.02]; DunedinPoAm β = 0.006 [0.001–0.013]).
Discussion
DNAm biomarkers of aging are emerging as a powerful approach for understanding how social exposures, such as SES, are embodied to shape health in aging. We tested how 3 DNAm measures representing the extent and pace of biological aging related to the socioeconomic gradient in memory trajectories in a nationally representative sample of mid-to late-life adults. There were 4 main findings: (1) socioeconomic gradients in biological aging were present–those with more wealth and more years of school exhibited less-advanced biological aging; (2) accelerated biological aging was associated with a lower 2016 memory performance and faster memory decline; (3) biological aging mediated a portion of the socioeconomic gradient in memory level and decline; and (4) magnitudes of associations between SES, biological aging, and memory trajectories varied across race-ethnicity-sex/gender.
Our finding that higher SES was associated with less-advanced/slower biological aging among people in the United States extends observations from studies of White and European samples.10-13 They also highlight potential heterogeneity in these associations in populations with different social lifecourse exposures. We found that, consistent with previous studies in non-White US populations,36 GrimAge and DunedinPoAm demonstrated the expected SES gradient among some of the non-White groups in our study. Only White women showed the expected SES gradient in PhenoAge. Our findings were less consistent among Latinx women and men, although sample sizes for these analyses were small.
DNAm clocks and related measures, such as the DunedinPoAm pace of aging, represent a cutting-edge approach to quantifying biological aging in epidemiologic and clinical studies.37 So far, few studies have described how these measurements relate to the process of cognitive aging. In the large US representative sample we analyzed, mid-to late-life adults with more advanced biological aging demonstrated a lower memory performance and faster memory decline than those of the same chronological age with less advanced/slower biological aging. This finding expands on observations from smaller, primarily White European and New Zealand samples,10,13,14 which established a link between the extent and pace of biological aging and later-life memory decline.
Effect sizes were small for associations of DNAm measures of aging with cognitive trajectories and varied between the different measures of aging and across racial-ethnic-sex/gender strata. Both biological aging and memory are measured imprecisely. It is notable that, among the 3 measures we tested, effect sizes were consistently the largest for the GrimAge clock, which shows substantially higher measurement reliability when compared with those for the PhenoAge Clock and DunedinPoAm.38,39 New methods to develop more reliable DNAm measures of aging38,39 may reveal stronger relationships. Nevertheless, our findings encourage a cautious approach to integrating these novel measures of biological aging into research on cognitive aging, especially in Black Americans.
Comparisons of associations across race/ethnic-sex/gender groups revealed stronger relationships between the biological aging measures and memory trajectories among White men and women when compared with Black men and women and among Latinx women compared with Black women. Racially patterned lifecourse social experiences that influence cognitive trajectories may shape epigenetic processes differently across racial and ethnic groups, and such profiles may not be adequately represented with these current DNAm measures. Racism is associated with exposure to a myriad of acute and chronic stressors that accumulate over the lifecourse and accelerates physiologic deterioration.40 These stress pathways have been linked to age-related memory decline among Black Americans, independent of SES.41 More research is needed to determine whether the cumulative stress exposure experienced by Black people is associated with distinct epigenetic pathways (e.g., associated with different CpG sites or differentially methylation regions).
Even among White men and women, the DNAm measures mediated only a small fraction of the SES gradient in aging-related memory decline. This small mediation fraction could reflect limitations of the simple mediation models tested in this study.42 For example, it is possible that the primary effect of biological aging is on other intermediary factors (e.g., cardiovascular disease) on the pathway linking SES to memory trajectories. In addition, molecular pathways43 not captured in DNAm measures of aging may be more salient biological links between SES and late-life memory.
Previous work has documented sex/gender differences in biological aging processes,21,44 with women having lower epigenetically predicted biological ages compared with men. However, findings for sex/gender differences in associations of SES with biological aging are inconsistent.12,45 In our study, we used an intersectional approach46 comparing racial-ethnic-sex/gender subgroups that revealed a more complex pattern of differences, highlighting contrasts between groups at the extremes of social power. White men showed stronger SES-biological aging associations compared with Latinx women and stronger biological aging-memory trajectory associations compared with Black women. While White and Latinx women demonstrated delayed/slower aging compared with men, Black women tended to show biological aging that was similar to that of men. Taking an intersectional approach may provide a more nuanced understanding of the links between SES, biological aging, and cognitive health outcomes.
This study provides preliminary evidence on the utility of 3 DNAm measures in quantifying biological aging pathways that link SES to cognition among Black and Latinx women and men. We cannot be sure that our findings generalize because of the relatively small number of Black and Latinx HRS participants with DNAm measures. Larger samples are needed to generate more precise estimates (i.e., smaller CIs).47
SES does not capture all upstream structural disadvantages faced by minoritized populations. Societies foster racial discrimination through systems that affect housing, education, employment, earnings, and health care.48 Future studies should include measures of upstream structural determinants, such as structural racism48 and sexism,49 to better understand how social disadvantages influence biological aging processes among minoritized communities.
Another study limitation is that each DNAm measure was obtained at a single point in time. While steeper decline in memory throughout the study was associated with more advanced biological aging in 2016, analyses cannot rule out reverse causation (i.e., poor memory performance contributing to advanced biological aging). Results from sensitivity analyses with change from 2016 to 2018 as the outcome were similar to those demonstrated for the rate of memory decline, providing some evidence against reverse causation. Among those who consented to the 2016 Venous Blood Study, Latinx and Non-Latinx Black participants were less likely than Non-Latinx White Participants to complete the study.20 While the sampling weights used in the current analyses account for differential participation by age, sex/gender, race, and ethnicity, it is unclear whether SES drove differential study participation.
Among a nationally representative sample of mid-to late-life adults in the United States, more advanced/faster biological aging, as measured by the GrimAge DNAm clock and DunedinPoAm pace of aging, was associated with lower scores on memory tests and faster decline in memory functioning over time. GrimAge and DunedinPoAm also indicated more advanced and faster aging in mid-to late-life adults with lower SES. Biological aging measures mediated only small fractions of the SES gradient in aging-related memory decline. Moreover, associations of biological aging measures with memory trajectories were consistent only in Whites but not in Black or Latinx people. DNAm biomarkers of aging have the potential to provide surrogate endpoints for interventions that aim to prevent aging-related functional decline, including cognitive decline, but lose that potential if their utility is limited to White people.50
Acknowledgment
The authors acknowledge the Health and Retirement Study (HRS) study participants and the HRS research and support staff for their contributions to this study. Author J. Manly had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
- DNAm
DNA methylation
- HRS
Health and Retirement Study
- PM
proportion mediated
- SES
socioeconomic status
Appendix. Authors

Study Funding
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Avila-Rieger is in part supported by P30AG059303.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183-192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladyshev VN. The ground zero of organismal life and aging. Trends Mol Med. 2021;27(1):11-19. doi: 10.1016/j.molmed.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci. 2015;112(30):E4104-E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott ML, Belsky DW, Knodt AR, et al. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol Psychiatry. 2021;26(8):3829-3838. doi: 10.1038/s41380-019-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murman DL. The impact of age on cognition. Semin Hear. 2015;36(3):111-121. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steptoe A, Zaninotto P. Lower socioeconomic status and the acceleration of aging: an outcome-wide analysis. Proc Natl Acad Sci. 2020;117(26):14911-14917. doi: 10.1073/pnas.1915741117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenfield EA, Moorman SM. Childhood socioeconomic status and later life cognition: evidence from the Wisconsin longitudinal study. J Aging Health. 2019;31(9):1589-1615. doi: 10.1177/0898264318783489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger N. Embodying inequality: a review of concepts, measures, and methods for studying health consequences of discrimination. Int J Health Serv. 1999;29(2):295-352. doi: 10.2190/M11W-VWXE-KQM9-G97Q. [DOI] [PubMed] [Google Scholar]
- 9.Geronimus AT, Hicken M, Keene D, Bound J. Weathering and age patterns of allostatic load scores among blacks and Whites in the United States. Am J Public Health. 2006;96(5):826-833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. ELife. 2020;9:e54870. doi: 10.7554/eLife.54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillary RF, Stevenson AJ, McCartney DL, et al. Epigenetic measures of ageing predict the prevalence and incidence of leading causes of death and disease burden. Clin Epigenetics. 2020;12(1):115. doi: 10.1186/s13148-020-00905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorito G, McCrory C, Robinson O, et al. Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: a multi-cohort analysis. Aging (Albany NY). 2019;11(7):2045-2070. doi: 10.18632/aging.101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrory C, Fiorito G, Hernandez B, et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J Gerontol Ser A. 2021;76(5):741-749. doi: 10.1093/gerona/glaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillary RF, Stevenson AJ, Cox SR, et al. An epigenetic predictor of death captures multi-modal measures of brain health. Mol Psychiatry. 2021;26(8):3806-3816. doi: 10.1038/s41380-019-0616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrucci L, Gonzalez-Freire M, Fabbri E, et al. Measuring biological aging in humans: a quest. Aging Cell. 2020;19(2):e13080. doi: 10.1111/acel.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnega A, Weir D. The health and retirement study: a public data resource for research on aging. Open Health Data. 2014;2(1):e7. doi: 10.5334/ohd.am. [DOI] [Google Scholar]
- 19.Fisher GG, Ryan LH. Overview of the health and retirement study and introduction to the special issue. Work Aging Retire. 2018;4(1):1-9. doi: 10.1093/workar/wax032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crimmins E, Faul J, Kim JK, Thyagarajan B, Weir D. HRS 2016 VBS–innovative sub sample assays: homocysteine, clusterin, brain-derived neurotrophic factor (BDNF), and mtDNA copy number. 7. [Google Scholar]
- 21.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371-384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 22.Tannenbaum C, Greaves L, Graham ID. Why sex and gender matter in implementation research. BMC Med Res Methodol. 2016;16(1):145. doi: 10.1186/s12874-016-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurd MD, Meijer E, Moldoff M, Rohwedder S. Improved Wealth Measures in the Health and Retirement Study: Asset Reconciliation and Cross-Wave Imputation. RAND Corporation. 2016;79. [Google Scholar]
- 24.U.S. Bureau of Labor Statistics. CPI Home. Accessed March 4, 2022. bls.gov/cpi/. [Google Scholar]
- 25.CPI inflation calculator. Accessed March 4, 2022. data.bls.gov/cgi-bin/cpicalc.pl.
- 26.Friedline T, Masa RD, Chowa GAN. Transforming wealth: using the inverse hyperbolic sine (IHS) and splines to predict youth's math achievement. Soc Sci Res. 2015;49:264-287. doi: 10.1016/j.ssresearch.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159-1165. [DOI] [PubMed] [Google Scholar]
- 28.Song MK, Lin FC, Ward SE, Fine JP. Composite variables: when and how. Nurs Res. 2013;62(1):45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends of cognition in the Health and Retirement Study, 1992-2004. Psychol Aging. 1992-20042007;22(3):525-545. [DOI] [PubMed] [Google Scholar]
- 30.Ten Have TR, Localio AR. Empirical Bayes estimation of random effects parameters in mixed effects logistic regression models. Biometrics. 1999;55(4):1022-1029. doi: 10.1111/j.0006-341x.1999.01022.x. [DOI] [PubMed] [Google Scholar]
- 31.Mascha EJ, Dalton JE, Kurz A, Saager L. Statistical grand rounds: understanding the mechanism: mediation analysis in randomized and nonrandomized studies. Anesth Analg. 2013;117(4):980-994. doi: 10.1213/ANE.0b013e3182a44cb9. [DOI] [PubMed] [Google Scholar]
- 32.MacKinnon DP. Introduction to Statistical Mediation Analysis. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008;10:477. [Google Scholar]
- 33.Ryu E, Cheong J. Comparing indirect effects in different groups in single-group and multi-group structural equation models. Front Psychol. 2017;8:747. doi: 10.3389/fpsyg.2017.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthen LK, Muthen BO. Mplus User's Guide: Statistical Analysis with Latent Variables, 3rd ed. Muthen & Muthen; 2004. [Google Scholar]
- 35.du Prel JB, Hommel G, Röhrig B, Blettner M. Confidence interval or p-value? part 4 of a series on evaluation of scientific publications. Dtsch Arzteblatt Int. 2009;106(19):335-339. doi: 10.3238/arztebl.2009.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence KG, Kresovich JK, O'Brien KM, et al. Association of neighborhood deprivation with epigenetic aging using 4 clock metrics. JAMA Netw Open. 2020;3(11):e2024329. doi: 10.1001/jamanetworkopen.2020.24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kritchevsky SB, Justice JN. Testing the Geroscience Hypothesis: Early Days. Oxford University Press US; 2020;75:99-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife. 2022;11:e73420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins-Chen AT, Thrush KL, Wang Y, et al. A computational solution for bolstering reliability of epigenetic clocks: implications for clinical trials and longitudinal tracking. bioRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boen C. Death by a thousand cuts: stress exposure and black–white disparities in physiological functioning in late life. J Gerontol B Psychol Sci Soc Sci. 2020;75(9):1937-1950. doi: 10.1093/geronb/gbz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuelsdorff M, Okonkwo OC, Norton D, et al. Stressful life events and racial disparities in cognition among middle-aged and older adults. J Alzheimers Dis. 2020;73(2):671-682. doi: 10.3233/JAD-190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deckers K, Cadar D, van Boxtel MPJ, Verhey FRJ, Steptoe A, Köhler S. Modifiable risk factors explain socioeconomic inequalities in dementia risk: evidence from a population-based prospective cohort study. J Alzheimers Dis. 2019;71(2):549-557. doi: 10.3233/JAD-190541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu-Rumeileh S, Steinacker P, Polischi B, et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res Ther. 2020;12(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):171. doi: 10.1186/s13059-016-1030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Normando P, Bezerra FF, Santana BA, et al. Association between socioeconomic markers and adult telomere length differs according to sex: pro-Saúde study. Braz J Med Biol Res. 2020;53(11):e10223. doi: 10.1590/1414-431x202010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowleg L. The problem with the phrase women and minorities: intersectionality-an important theoretical framework for public health. Am J Public Health. 2012;102(7):1267-1273. doi: 10.2105/AJPH.2012.300750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233-239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 49.Homan P. Structural sexism and health in the United States: a new perspective on health inequality and the gender system. Am Sociol Rev. 2019;84(3):486-516. doi: 10.1177/0003122419848723. [DOI] [Google Scholar]
- 50.Justice JN, Kritchevsky SB. Putting epigenetic biomarkers to the test for clinical trials. Elife. 2020;9:e58592. doi: 10.7554/eLife.58592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for analyses are available from the HRS website (hrs.isr.umich.edu/dataproducts/access-to-public-data).