Abstract
Immunological intervention, in addition to vector control and malaria chemotherapy, will be needed to stop the resurgence of malaria, a disease with a devastating impact on the health of 300 to 500 million people annually. We have pursued a vaccination strategy, based on DNA immunization in mice with genes encoding two antigens present on the sexual stages of Plasmodium falciparum, Pfs25 and Pfg27, to induce biologically important antibodies that can block development of the parasite in the Anopheles mosquito and thus transmission of the disease. DNA encoding Pfs25 when administered by the intramuscular route, either alone or with DNA encoding Pfg27, had the most potent transmission-blocking effects, resulting in up to a 97% decrease in oocyst numbers in mosquito midguts and a 75% decrease in rate of infection. Immunization with DNA encoding a Pfg27-Pfs25 fusion protein was less effective and DNA encoding Pfg27 elicited antibodies in sera that had only modest effects on the infectivity of the parasite. These results show for the first time that DNA vaccination can result in potent transmission-blocking antibodies in mice and suggest that the Pfs25 gene should be included as part of a multicomponent DNA vaccine.
Malaria continues to exact a heavy toll on human life despite intensive chemotherapeutic intervention and vector control campaigns. It is transmitted from humans to mosquitoes through the sexual stages of the parasite, the gametocytes, that develop in the blood of the infected person. Following a blood meal, gametogenesis in the mosquito midgut liberates the male and female gametes from the erythrocyte and these gametes undergo fertilization, followed by the formation of oocysts which further develop into sporozoites. Several midgut stages of Plasmodium have been shown to be susceptible to immune factors like antibodies and complement ingested with the blood meal. This could result in the reduction or even elimination of parasite infectivity in the mosquito vector and forms a rational basis for the development of malaria transmission-blocking (TrB) vaccines (1, 4, 15). Such a vaccine, based on antigens expressed in the sexual stages of Plasmodium falciparum, would block the passage of parasites from humans to mosquitoes and is thus an attractive strategy to limit the transmission of malaria. In addition, when combined with vaccines targeting other life cycle stages of Plasmodium or chemotherapy, TrB vaccines could also help to limit the spread of mutant parasites. Long-term control of this widespread disease may thus become possible.
Several proteins have been identified in P. falciparum as candidate antigens for the development of malaria TrB vaccines (15, 16, 24, 35). Some of these, like Pfs230, Pfs48/45, and Pfg27, are synthesized predominantly in the gametocytes (vertebrate host) with some residual expression seen after gametogenesis and fertilization (19, 32), while others, like Pfs25 and Pfs28 (9, 12, 13), are expressed only after initiation of gametogenesis and fertilization in the vector host. Studies on purified Pfs25 recombinant proteins expressed in Escherichia coli or yeast have demonstrated a need for proper conformational folding of target epitopes, administration of adjuvants, and multiple immunizations (3, 9, 14). In view of the fact that DNA immunization can overcome some of these immunogenicity requirements, we combined the genes coding for two target antigens found on different sexual stages, Pfg27 in gametocytes and Pfs25 in zygotes, and evaluated their potential as experimental DNA-based TrB vaccines as single immunogens, coimmunogens, and a hybrid gene fusion.
DNA-based vaccines have been shown to generate cellular and humoral immune responses against various pathogens in diverse animal species. In fact, experimental nucleic acid vaccines against a wide variety of infectious diseases, including leishmaniasis (36), human immunodeficiency virus (2), tuberculosis (20), malaria (10, 28, 29, 33), hepatitis B (16), and influenza (30), are all under development (7). Polynucleotide vaccines based on Plasmodium yoelii sporozoite and hepatocyte stage proteins have resulted in up to 90% protection in mice (8, 28). More recently, it has been shown that immunization with DNA encoding two preerythrocytic malaria antigens followed by boosting with a vaccinia virus expressing the same antigen conferred complete protection in mice (27, 29). Thus, DNA vaccines may offer the best prospect for success and possess a significant number of advantages over conventional methods of immunization.
This study demonstrates for the first time an induction of high-titer antibodies in mice immunized with DNA-based malaria TrB immunogens. These antibodies, when tested in membrane feeding transmission assays, proved to be highly effective inhibitors of parasite development in the mosquito. This provides strong support for the development of a DNA-based P. falciparum TrB vaccine and its inclusion in global strategies to control malaria.
MATERIALS AND METHODS
DNA constructs used for immunizations.
DNA vectors VR1012 and VR1020 (Vical Inc.) were obtained from S. L. Hoffman (Naval Medical Research Institute, Rockville, Md.). The plasmids contain a polyadenylation termination sequence downstream of the multiple-cloning region, a prokaryotic origin of replication, and the kanamycin resistance gene as a selectable marker. Expression of the foreign gene is driven by a strong eukaryotic cytomegalovirus promoter. VR1020 has a tissue plasminogen activator (TPA) signal sequence just upstream of the cloning sites which facilitates secretion of the expressed protein when the foreign gene is cloned in frame with it.
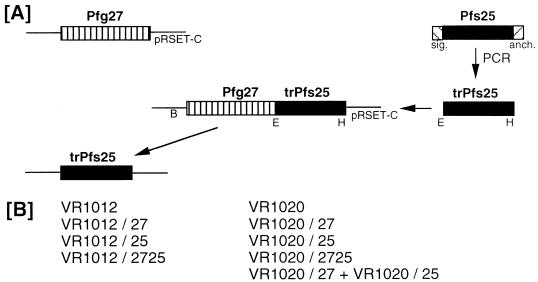
Recombinant plasmids VR1012/27, VR1012/25, VR1012/2725, VR1020/27, VR1020/25, and VR1020/2725 were constructed as detailed in Fig. 1. The entire coding sequence of Pfg27 was used in all DNA constructs containing Pfg27 sequence, whereas a truncated version of Pfs25 lacking putative signal (amino acids 1 to 17) and anchor sequences (amino acids 203 to 217) was used to create the truncated Pfs25 (trPfs25) constructs. The plasmids were initially constructed by cloning a PCR-generated fragment of DNA encoding Pfg27 into the HincII site of pRSETC (22). The trPfs25 gene (obtained by PCR using primer 5′GTG TAT GAA TTC GAA ATA TAA TAA TGC G 3′ containing an EcoRI site [underlined] and bp 52 to 66 of Pfs25 and primer 5′ GTG TAT AAG CTT TTA ATT TAA AAT ATT 3′ containing a HindIII site [underlined] and bp 595 to 606 of Pfs25) was then introduced downstream of, and in frame with, the Pfg27 sequence to create the hybrid Pfg27-trPfs25 (2725) construct in pRSETC. Finally, the Pfg27 sequence was deleted from the hybrid construct by using restriction enzymes BamHI and EcoRI followed by treatment with T4 DNA polymerase to flush ends to create the trPfs25 plasmid. The three inserts Pfg27, Pfg27-trPfs25, and trPfs25 were then cloned into VR1012 or VR1020. The various pRSETC plasmids were digested with NdeI and HindIII, and inserts were treated with T4 DNA polymerase and cloned into blunt-ended BamHI/BglII sites of VR1012/1020 to generate the final plasmids that were used for immunizations. All constructs were sequenced to verify the fidelity of the sequence. Plasmid DNA was purified by use of Qiagen columns, sterilized by ethanol precipitation, and dissolved in sterile phosphate-buffered saline (PBS) for injection.
FIG. 1.
Schematic outline (A) showing construction of the various plasmids (B) used for immunization. sig., signal; anch, anchor; B, BamHI; E, EcoRI; H, HindIII.
Animals and immunizations.
Six- to 8-week-old female BALB/c mice were used for all the immunizations (five mice per immunogen). For the intramuscular (i.m.) immunizations, two DNA inoculations (50 μg each in sterile PBS) were given 4 weeks apart. DNA was administered into the right and left tibialis cranialis muscles of the mouse. Intradermal (i.d.) immunizations employed the same amount of DNA and the same immunization schedule as those used for the i.m. immunizations and were administered along the tail. Blood was collected preimmunization and 4 weeks after the first and booster immunizations, and sera were stored at −20°C.
Immunological analysis.
The sera obtained were assayed for end point titers, isotype of specific antibodies elicited, and reactivity to native parasite protein.
(i) Enzyme-linked immunosorbent assay (ELISA).
Immulon-2 microtiter plates were coated (100 μl/well) with 2 μg of either rPfg27 or rPfs25 per ml in bicarbonate buffer (4 mM Na2CO3, 8 mM NaHCO3 [pH 9.6]) overnight at 4°C. For end point titer determination, sera from all five mice in a group were pooled and diluted 1:100 to 1:800,000 in 1% bovine serum albumin in PBS–0.05% Tween. Plates were incubated for 2 h, washed extensively, and incubated in the presence of a 1:1,000 dilution of goat anti-mouse immunoglobulin G [IgG(heavy plus light chains)] conjugated with horseradish peroxidase (Gibco-BRL). After washes, the plates were developed with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] single-reagent substrate (Kirkegaard & Perry Labs, Gaithersburg, Md.) and absorbance was read at 405 nm. End point titers were defined as the highest serial dilutions of sera yielding absorbance values greater than 0.05, a value corresponding to an average absorbance value for pools of preimmune serum ± 2 standard deviations. For the determination of antibody isotype, all conditions used were as described above except that a single serum dilution of 1:1,000 was employed and the secondary antibody was rabbit anti-mouse immunoglobulin (heavy chain) specific for IgG1, IgG2a, IgG2b, IgG3, or IgM.
For the estimation of antibody avidity, a modification of the method of Pullen et al. (5, 23) was followed. Various concentrations (0 to 6 M) of NaSCN were used (15 min, room temperature) to allow for the disruption of the antigen-antibody binding. This was followed by the standard ELISA protocol detailed above.
(ii) Analysis of antibody recognition of parasite-derived proteins.
Mature P. falciparum gametocytes of the NF54 isolate were obtained by in vitro culture as previously described (11). After induction of gametogenesis and exflagellation (21), gametes and zygotes were purified by using a discontinuous Nycodenz gradient (31). The extracts of purified parasites (a mixture of gametocytes, gametes, and zygotes) were run on a nonreducing sodium dodecyl sulfate–5 to 20% polyacrylamide (SDS–5 to 20%) PAGE gel and blotted onto nitrocellulose filters and probed with pooled mouse sera, used at a dilution of 1:200 (22).
Membrane feeding TrB assays.
The infectivity of P. falciparum gametocytes to Anopheles mosquitoes in the presence of the different sera was tested by membrane feeding assays (24, 35). Freshly drawn and washed human erythrocytes, normal human serum, and the sera to be tested (final dilution, 1:5 to 1:20) were mixed with mature gametocyte cultures (3D7 clone; 14 to 18 days old). Twenty to 30 Anopheles stephensi mosquitoes in a cage (starved for 5 to 6 h) were fed each suspension through a Parafilm membrane warmed to 39°C by a glass water jacket (membrane feeder). Mosquitoes were allowed to engorge for 15 min. Blood-fed mosquitoes were maintained at 26°C and 60 to 80% relative humidity and dissected 8 days after feeding. Midguts were examined for the presence of oocysts. Infection rates (number of mosquitoes infected/total number dissected) and number of oocysts (geometric means) in mosquitoes fed test sera were compared to those obtained from feeds using sera from VR1020-immunized animals. Statistical significance was assessed by the Mann-Whitney test.
RESULTS
Antibody titers.
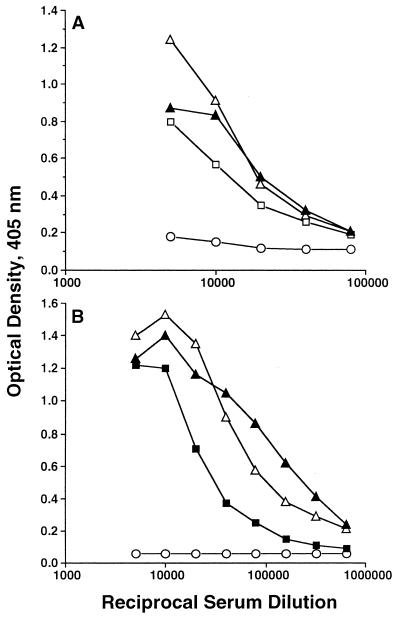
In the initial series of immunizations, mice were immunized with the VR1012 series of constructs (lacking TPA secretion sequence), and antibody responses against recombinant Pfg27 (rPfg27) and rPfs25 were measured by ELISA. All animals developed antibody responses to the respective immunogen; however, ELISA end point titers revealed significant differences in antibody levels between the two routes of immunization evaluated in this study. Higher titers of antibodies were elicited for the various VR1012 constructs when the i.m. route was employed (Table 1). Antibody titers against Pfg27 were 30-fold higher by the i.m. route of immunization than by the i.d. route. In view of this difference, the second series of constructs, the VR1020 series (containing a TPA secretion signal sequence) was administered only via the i.m. route. Mice in groups of five were immunized with plasmids encoding Pfg27 (VR1020/27) or trPfs25 (VR1020/25) alone, a combination of both plasmids (VR1020/27+25), or a chimeric Pfg27-Pfs25 hybrid (VR1020/2725). Antibody titers against rPfs25 averaged 160,000 when VR1020/25 or VR1020/27+25 was used to immunize mice, and a titer of 80,000 was obtained with the hybrid VR1020/2725 (Fig. 2B). Likewise, the antibody end point titers against rPfg27 were in excess of 80,000 in mice immunized with VR1020/27, VR1020/2725, or VR1020/27+25 (Fig. 2A). These results thus demonstrated that the i.m. route of immunization and the VR1020 series of plasmids allowing expression of proteins fused with a putative TPA leader sequence elicited high-titer antibody responses against the various antigens tested. Additionally, the antibodies elicited were specific to the immunogen used for immunization (data not shown).
TABLE 1.
Antibody titers in mice immunized i.d. and i.m. with the VR1012 series of constructs
| DNA immunogen | End point Titera
|
|||
|---|---|---|---|---|
| rPfg27
|
rPfs25
|
|||
| i.d. | i.m. | i.d. | i.m. | |
| VR1012/27 | 600 | 20,000 | NTb | NT |
| VR1012/25 | NT | NT | 600 | 2,400 |
| VR1012/2725 | 600 | 1,200 | 2,400 | 2,400 |
Determined relative to VR1012 vector immunizations.
NT, not tested.
FIG. 2.
Antibody titers measured by ELISA to rPfg27 (A) and rPfs25 (B). (A) Symbols: ○, VR1020; □, VR1020/27; ▴, VR1020/27+25; ▵, VR1020/2725. (B) Symbols: ○, VR1020; ■, VR1020/25; ▴, VR1020/27+25; ▵, VR1020/2725. ELISA end points were defined as the highest serial dilutions yielding absorbance readings at 405 nm of greater than 0.05 (see Materials and Methods).
Avidity assays.
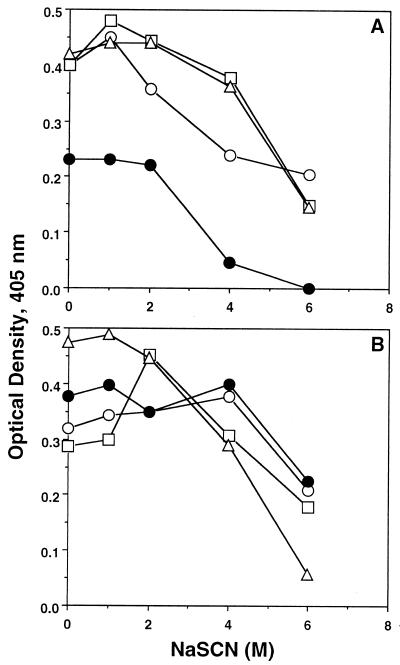
An ELISA in the presence of various concentrations of NaSCN (Fig. 3) suggested that the antibodies elicited by the various DNA constructs containing the gene for Pfs25 bound to the yeast-derived rPfs25 with higher avidity than did the anti-Pfs25 monoclonal antibodies (MAbs) 1C1 and 1D2 (13). No significant difference in the avidities for rPfg27 was found between the various anti-Pfg27 sera.
FIG. 3.
Estimation of antibody avidity in sera against rPfs25 (A) and rPfg27 (B). (A) Mice were immunized with VR1020/25 (○), VR1020/2725 (□), VR1020/27+25 (▵), and MAbs against Pfs25 (●). (B) Mice were immunized with VR1020/27 (○), VR1020/2725 (□), VR1020/2725 (□), VR1020/27+25 (▵), and MAbs against Pfg27 (●).
Recognition of parasite antigen.
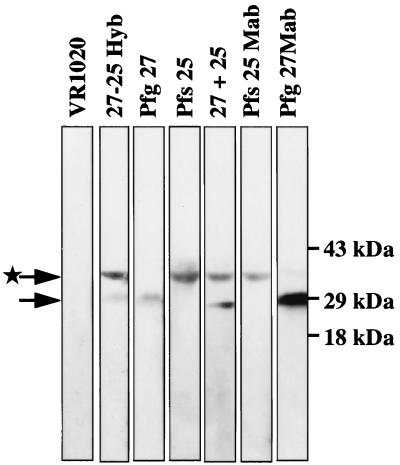
Pooled sera from the immunized mice were tested in a Western blot analysis on nonreduced extracts of gametocytes, gametes, and zygotes of P. falciparum. Figure 4 shows that all the antibodies recognized the parasite antigen specific to the construct used for immunization. Constructs containing the gene encoding Pfg27 resulted in antisera reactive with the 27-kDa protein, whereas constructs encoding Pfs25 elicited antibodies that recognized the 25-kDa protein. Pfs25 shows anomalous migration on an SDS-PAGE gel due to the conformation imposed by the presence of multiple disulfide bonds in the protein. Sera from animals immunized with VR1020 alone did not react with any parasite protein. Appropriate MAbs recognizing Pfg27 and Pfs25 were used as positive controls.
FIG. 4.
Western blot analysis of the various pooled sera (1:200) using nonreduced gametocyte and gamete extracts. Lanes are as indicated. The arrow marked with a star identifies Pfs25 running anomalously under nonreducing conditions.
TrB assays.
The ability of the various sera to block the formation of oocysts in P. falciparum gametocyte-fed mosquitoes was evaluated, and these results are summarized in Table 2. Antisera from VR1020/25- or VR1020/27+25-immunized mice dramatically reduced the infectivity of the parasite. The average number of oocysts per mosquito midgut in both these groups was 0.17 to 0.39, in contrast to 4.9 to 9.1 for VR1020-immunized mice. Additionally, the rate of infection in these mosquitoes was also significantly reduced relative to that of the control group. However, mice immunized with the hybrid VR1020/2725 construct elicited antibodies in sera that appeared to result in a less potent inhibition of parasite development. In this group, the oocyst burden was reduced by 78 to 95%. Only a modest reduction in oocyst numbers per midgut was seen when VR1020/27 was used as the immunogen (see Table 2).
TABLE 2.
TrB activity of sera in membrane feeding assays
| DNA immunogen | Dilutiona | Mean no. of oocysts | Infectivity (% of control)b | Rate of infectionc |
|---|---|---|---|---|
| Expt 1 | ||||
| None | 6.5 | 18/19 | ||
| VR1020 | 1:5 | 4.95 | 100 | 8/9 |
| 1:10 | 7.4 | 100 | 10/11 | |
| VR1020/2725 | 1:5 | 1.1d | 22.4 | 8/12 |
| 1:10 | 0.8d | 10.3 | 5/12 | |
| VR1020/27 | 1:5 | 3.5 | 70.6 | 10/10 |
| 1:10 | 4.3 | 58.3 | 7/10 | |
| VR1020/25 | 1:5 | 0.17d | 3.4 | 2/9 |
| 1:10 | 0.19d | 2.6 | 3/12 | |
| VR1020/27+25 | 1:5 | 0.18d | 3.6 | 2/11 |
| 1:10 | 0.27d | 3.7 | 3/12 | |
| Expt 2 | ||||
| None | 9.1 | 15/16 | ||
| MAb | 1:10 | 0.2 | 2.2 | 3/17 |
| VR1020 | 1:10 | 6.84 | 100 | 16/17 |
| 1:20 | 9.1 | 100 | 11/11 | |
| VR1020/2725 | 1:10 | 0.36d | 5.2 | 5/15 |
| 1:20 | 0.38d | 4.2 | 7/15 | |
| VR1020/25 | 1:10 | 0.26d | 3.8 | 4/12 |
| 1:20 | 0.39d | 4.3 | 6/16 |
Final dilution of test sera used for assay.
Values are expressed as percentages of those obtained with corresponding dilutions of sera from VR1020-immunized mice.
Number of mosquitoes infected/total number of mosquitoes dissected.
Significant at P < 0.01 by Mann-Whitney test.
Analysis of antibody isotype.
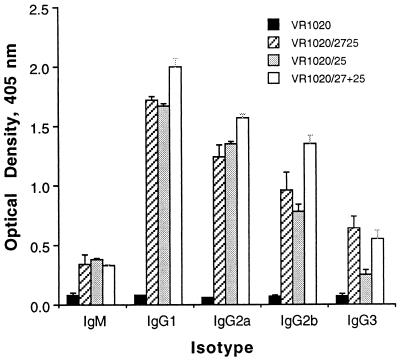
Previous studies have suggested that TrB activity of MAbs against Pfs230 was influenced by the antibody isotype (26). To evaluate whether the TrB activity of the sera from mice immunized with the DNA immunogens correlated with a certain antibody isotype elicited, we looked for the representation of the various isotypes of the antigen-specific immunoglobulins. Figure 5 depicts the distribution of the isotypes of antibodies when tested in an ELISA using rPfs25 as the capture antigen. DNA immunization resulted in the production of all IgG family subclasses, with IgG1 and IgG2a representing the major antibody isotypes. Substantial amounts of IgG2b and lower levels of IgG3 and IgM were also detectable in the sera of the immunized mice. ELISA analysis of antibody isotype using rPfg27 as a target antigen revealed very similar isotype profiles for sera from mice immunized with the different immunogens (data not shown).
FIG. 5.
Isotype analysis of Pfs25-specific antibodies.
DISCUSSION
Our hypothesis was that a malaria TrB vaccine based on two sexual stage antigens expressed at different stages of the parasite would induce a more potent immune response than that generated by either when used alone. Additionally, we delivered these antigens in the form of DNA-based immunogens, taking into account the various advantages conferred by the use of DNA. Low cost and ease of production, heat stability (lack of need of refrigeration), and amenability to genetic manipulation are just some of the areas where a nucleic acid immunogen outranks a conventional protein antigen. Additionally, DNA immunizations have been shown to result in the in vivo synthesis of proteins whose conformation and posttranslational modifications are similar to those of the native protein (7). This was especially important for the use of Pfs25 as an immunogen since previous studies have clearly established that disulfide-bonded conformational epitopes on Pfs25 are targets of TrB antibodies (3). In fact, E. coli-derived recombinant Pfs25 protein could not elicit complete TrB antibodies in mice since it did not recreate the known conformational epitope(s) that is the target of TrB MAbs (14). The only forms of rPfs25 shown to be effective have been those expressed in vaccinia virus or in yeast (3, 9, 14).
In contrast to some studies which have indicated that the i.d. route of immunization results in higher-titer antibodies than the i.m. route does, our results indicate that the converse is true for the immunogens evaluated in this study. We obtained up to 30-fold-higher titers (Table 1) 8 weeks postimmunization when the same immunogen was delivered i.m. rather than i.d. Additionally, these titers increased significantly when the DNA was encoded in a vector (VR1020) which facilitated expression of the proteins containing a TPA signal sequence. In fact, the antibody titers obtained by DNA immunization with Pfg27 in VR1020 were in the same range as those obtained by immunization with E. coli-derived recombinant Pfg27 protein (17, 18). Antibodies induced by the immunization of the VR1020 series of constructs recognized epitopes in parasite-derived Pfs25. This suggests that those proteins are synthesized in the appropriate conformations in the DNA vector. Previous studies have shown this to be a critical factor in eliciting TrB antibodies (14).
A key finding of this study was that sera from mice vaccinated with the DNA-based immunogens were highly effective in reducing the infectivity of P. falciparum in mosquitoes. In these assays, the sera reduced both the percentage of infected mosquitoes and the average number of developing oocysts, thus demonstrating significant TrB activity. This TrB activity was observed even at a 1:20 dilution of the various sera. This difference is not a reflection of antibody titers (as measured by ELISA). Thus, the immune sera from mice vaccinated with the DNA immunogens not only appear to be strong blockers in TrB assays but also seem to contain antibodies having a higher avidity for the yeast-derived rPfs25, as suggested by thiocyanate elution studies.
Another unexpected observation was revealed by the comparative evaluation of sera from mice immunized with VR1020/25 or VR1020/2725 plasmids. TrB effectiveness was generally lower in the sera of the animals immunized with the molecular hybrid (Pfg27-Pfs25) than with VR1020/25 alone, although the Pfs25-specific titers were not different. It is possible that expression of the Pfg27-Pfs25 hybrid protein may affect the conformation or presentation of immunologically important epitopes in Pfs25, resulting in induction of antibodies less effective in reducing parasite infectivity in the mosquito. Sera from VR1020/25-immunized mice were found to be more effective than sera from VR1020/2725-immunized mice in five independent TrB assays. These results could have important implications in the design of future multicomponent DNA vaccines. In these studies, antibodies elicited in response to immunization with vaccines encoding Pfg27 were not effective as TrB mediators, although MAbs recognizing a linear epitope in Pfg27 have been shown previously to be highly effective in blocking parasite transmission (35).
The inhibitory activity obtained by immunization with the VR1020/25 plasmid is comparable to the blocking obtained when yeast-derived rPfs25 was used as the immunogen (3). However, the various features of DNA vaccines outlined above confer an advantage in their use over more conventional approaches based on immunizations with recombinant protein. Additionally, plasmids used for DNA immunization have been shown to contain immunostimulatory sequences (34), and, thus, such vaccines could be effective without the need for adjuvant formulation. It has previously been shown that the immune response to some of the malarial TrB vaccine candidates was adjuvant dependent (14, 25). DNA immunization could thus circumvent the problem of the paucity of adjuvants available for use in humans. Further work is in progress to demonstrate comparable immunogenicity and effectiveness of these DNA immunogens in nonhuman primates prior to contemplating human vaccine trials.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (AI38403 and AI41879) and from the United Nations Development Program/World Bank/World Health Organization.
We thank M. Kent for technical assistance, D. Kaslow and I. Ploton for providing us with rPfs25 and rPfg27 for use in the ELISA analysis, I. Quakyi for MAbs 1C1 and 1D2, S. Hoffman for the gift of the Vical vectors VR1012 and VR1020, and D. Griffin and N. Rose for critical reading of the manuscript.
REFERENCES
- 1.Alano P, Carter R. Sexual differentiation in malaria parasites. Ann Rev Microbiol. 1990;44:429–449. doi: 10.1146/annurev.mi.44.100190.002241. [DOI] [PubMed] [Google Scholar]
- 2.Bagarazzi M, Boyer J, Ayyavoo V, Weiner D. Nucleic acid-based vaccines as an approach to immunization against the human immunodeficiency virus 1. Curr Top Microbiol Immunol. 1998;226:107–143. doi: 10.1007/978-3-642-80475-5_8. [DOI] [PubMed] [Google Scholar]
- 3.Barr P J, Green K M, Gibson H L, Bathrust I C, Quakyi I A, Kaslow D C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter R, Kumar N, Quakyi I, Good M F, Mendis K, Graves P M, Miller L. Immunity to sexual stages of malaria parasites. Prog Allergy. 1986;41:193–214. [PubMed] [Google Scholar]
- 5.Charoenvit Y, Mellouk S, Cole C, Bechara R, Leef M F, Sedegah M, Yuan L, Robey F, Beaudoin R, Hoffman S L. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelli sporozoites. J Immunol. 1991;146:1020–1025. [PubMed] [Google Scholar]
- 6.Davis H L, Mancini M, Michel M, Whalen R. DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost. Vaccine. 1996;14:910–915. doi: 10.1016/0264-410x(95)00255-y. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly J, Ulmer J, Shiver J, Liu M. DNA vaccines. Annu Rev Immunol. 1997;16:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 8.Doolan D, Sedegah M, Hedstrom R, Hobart P, Charoenvit Y, Hoffman S. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy P E, Kaslow D C. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman S L, Sedegah M, Hedstrom R C. Protection against malaria by immunization with a Plasmodium yoelii circumsporozoite protein nucleic acid vaccine. Vaccine. 1994;12:1529–1533. doi: 10.1016/0264-410x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 11.Ifediba T, Vanderberg J. Complete in vitro maturation of P. falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 12.Kaslow D C, Quakyi I, Syin C, Raum M, Keister D, Coligan J, McCutchan T, Miller L. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaslow D C, Isaacs I, Quakyi I, Gwadz R, Moss B, Keister D. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science. 1991;252:1310–1313. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow D C, Bathurst I, Lensen T, Ponnudurai T, Barr P, Keister D. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994;62:5576–5580. doi: 10.1128/iai.62.12.5576-5580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaslow D C. Transmission-blocking immunity against malaria and other vector-borne diseases. Curr Opin Immunol. 1993;5:557–565. doi: 10.1016/0952-7915(93)90037-s. [DOI] [PubMed] [Google Scholar]
- 16.Kocken C, Jansen J, Kaan A, Beckers P, Ponnudurai T, Kaslow D, Konings R, Schoenmakers J. Cloning and expression of a gene coding for transmission-blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol. 1993;61:59–68. doi: 10.1016/0166-6851(93)90158-t. [DOI] [PubMed] [Google Scholar]
- 17.Koski G, Ploton I, Viscidi R, Kumar N. Tandem use of PCR and synthetic peptides to map helper T-cell epitopes on the 27 kDa sexual stage antigen of Plasmodium falciparum. Peptide Res. 1996;9:127–135. [PubMed] [Google Scholar]
- 18.Kumar N, Ploton I, Koski G, Lobo C, Contreras C. Malaria transmission-blocking immunity—identification of epitopes and evaluation of immunogenicity. Adv Exp Med Biol. 1995;383:65–72. [PubMed] [Google Scholar]
- 19.Lobo C A, Konings R, Kumar N. Expression of early gametocyte-stage antigens Pfg27 and Pfs16 in synchronized gametocytes and non-gametocyte producing clones of Plasmodium falciparum. Mol Biochem Parasitol. 1994;68:151–154. doi: 10.1016/0166-6851(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 20.Lowrie D B, Tascon R E, Colston M J, Silva C L. Towards a DNA vaccine against tuberculosis. Vaccine. 1994;12:1537–1540. doi: 10.1016/0264-410x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 21.Nijhout M. Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Exp Parasitol. 1979;48:75–80. doi: 10.1016/0014-4894(79)90056-0. [DOI] [PubMed] [Google Scholar]
- 22.Ploton I, Wizel B, Viscidi R, Kumar N. Mapping of two overlapping linear epitopes in Pfg27 recognized by Plasmodium falciparum transmission-blocking monoclonal antibodies. Vaccine. 1995;13:1161–1169. doi: 10.1016/0264-410x(95)00033-w. [DOI] [PubMed] [Google Scholar]
- 23.Pullen G R, Fitzgerald M G, Hosking C S. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 24.Quakyi I A, Carter R, Rener J, Kumar N, Good M F, Miller L. The 230-kD gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- 25.Rawlings D J, Kaslow D C. A novel 40-kDa membrane-associated, EFhand calcium binding protein in Plasmodium falciparum. J Exp Med. 1992;176:1483–1487. [PubMed] [Google Scholar]
- 26.Roeffen W, Geeraedts F, Eling W, Beckers P, Kumar N, Lensen T, Sauerwein R. Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230 specific antibodies is isotype dependent. Infect Immun. 1995;63:467–471. doi: 10.1128/iai.63.2.467-471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider J, Gilbert S, Blanchard T, Hanke T, Robson K, Hannan C, Becker M, Sinden R, Smith G, Hill A. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 28.Sedegah M, Hedstrom R, Hobart P, Hoffman S. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia virus increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulmer J, Donnelly J, Parker S, Rhodes G, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen A, Ponnudurai T, Beckers P, Verhave J, Smits M, Meuwissen J. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission blocking antibodies in the mosquito. J Exp Med. 1986;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeulen A N, Ven Deursen J, Brakenhoff R H, Lensen A H, Ponnudurai T, Meuwissen J H. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Mol Biochem Parasitol. 1986;20:155–163. doi: 10.1016/0166-6851(86)90027-7. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Doolan D L, Thong P, Hedstrom R C, Coonan K, Charoenvit Y, Jones T, Hobart P, Margalith M, Ng J, Weiss W, Sedegah M, de Taisne C, Norman J, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 34.Weiner G J, Liu H, Woolridge J E, Dahle C, Krieg A M. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wizel B, Kumar N. Identification of a continuous and cross-reacting epitope for Plasmodium falciparum transmission-blocking immunity. Proc Natl Acad Sci USA. 1991;88:9533–9537. doi: 10.1073/pnas.88.21.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu D, Liew F Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein gp63 of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]