ABSTRACT.
The effects of azithromycin mass drug administration (MDA) on trachoma and yaws have been addressed. However, the secondary effects of azithromycin MDA remain unclear. This study aimed to explore the secondary effects of azithromycin MDA. PubMed, Embase, Cochrane Library, Web of Science, and ClinicalTrials.gov were searched from conception to January 5, 2022. Studies on secondary effects of azithromycin MDA were included. A total of 34 studies were included. Six of them reported on child mortality, 10 on malaria, and 20 on general morbidity and condition. Azithromycin MDA reduced child mortality, and quarterly MDA may be most beneficial for reducing child mortality. The effect of azithromycin MDA on malaria was weak. No association was observed between azithromycin MDA and malaria parasitemia (rate ratio: 0.71, 95% confidence interval: 0.43–1.15). Azithromycin MDA was associated with a lower risk of respiratory tract infections and diarrhea. Additionally, it was associated with a lower risk of fever, vomiting, and headache. The carriage of pathogenic organisms such as Streptococcus pneumoniae and gut Campylobacter species was reduced. However, these secondary effects of azithromycin MDA appeared to last only a few weeks. Moreover, no association was observed between azithromycin MDA and nutritional improvement in children. In conclusion, azithromycin MDA had favorable secondary effects on child mortality and morbidity. However, the effects were short term.
INTRODUCTION
Azithromycin mass drug administration (MDA) is an important strategy for controlling tropical diseases such as trachoma and yaws. A single dose of oral azithromycin can be effective for treatment because of the long biological half-life properties.1 Trachoma, caused by Chlamydia trachomatis, is one of the leading neglected tropical diseases requiring azithromycin MDA.2,3 For trachoma-prevalent districts, an annual MDA with a single dose of oral azithromycin for at least 3 years was recommended in the trachoma program.1 Because trachoma has been prevalent in many districts, the administration of azithromycin is large scale. To date, partners in trachoma programs have distributed more than 900 million doses of azithromycin. However, more than 100 million people still require intervention.4,5
The use of azithromycin MDA for trachoma and other conditions may have secondary effects on people’s health, especially in districts without universal healthcare. Considering that azithromycin has broad-spectrum antibacterial properties, several studies have demonstrated that azithromycin MDA may have important effects in other infectious diseases, such as malaria, diarrhea, and respiratory infections.6–8 Importantly, the mortality rate may be reduced due to the decrease in the incidence of infectious diseases.9 Given that azithromycin MDA is mainly used in tropical areas where there is a high burden of infectious diseases, it is important to elucidate the effects of azithromycin MDA on the disease spectrum and mortality for promoting people’s health. Previous studies have discussed the secondary effects of azithromycin MDA on drug resistance and adverse events10,11; this study aimed to evaluate the secondary effects of azithromycin MDA with the exception of drug resistance and adverse events.
METHODS
Search strategy.
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. PubMed, Embase, Cochrane Central Register of Controlled Trials, Web of Science, and ClinicalTrials.gov were searched for eligible studies from conception to January 5, 2022. No language restrictions were applied. Free terms and Medical Subject Headings terms were used in the search. The keywords “azithromycin,” “Zithromax,” “Vinzam,” “AZM,” “Vinzam AZM,” and “AZT” were used to identify studies on azithromycin. The keywords “mass drug administration,” “mass administration,” “mass treatment,” “mass distribution,” “preventative chemotherapy,” and “MDA” were used to search for studies involving MDA. The above steps were combined to identify studies related to azithromycin MDA. The reference lists of the included studies were manually reviewed to identify potential studies.
Study selection.
Study selection was independently performed by two authors. The inclusion criteria were as follows: 1) studies that used azithromycin MDA. Strictly, MDA was defined as the administration of drugs to the whole population in a community at the same time. Some studies used household- or age-level randomization, which was considered a broader definition of MDA and included in the systematic review. 2) Studies that included outcomes other than controlling for targeted tropical diseases. After removing duplicate searches, the titles and abstracts were screened to identify relevant articles. We conducted a full-text review of studies that passed the screening to assess their eligibility. Case reports, conference abstracts, reviews, editorials, and animal studies were excluded.
Data extraction.
Two authors independently extracted the data and assessed discrepancies. Discrepancies were resolved through a discussion with a third author. Baseline information about the author, year of publication, country, sample size, study design, and participant information were extracted. In addition, the details of azithromycin MDA, such as the frequency, duration, and main outcome, including disease prevalence and effect size, were extracted.
Quality assessment.
With regard to the risk of bias assessment, the Cochrane Collaboration’s risk of bias tool was used for randomized studies, and the ROBINSI tool was used for nonrandomized studies. These two tools were slightly modified to ensure that they contained similar items.12 The included studies were rated as having a high, moderate, low, or unclear risk of bias in the following seven categories: confounding bias, selection bias, misclassification bias, performance bias, detection bias, attrition bias, and reporting bias. Two authors independently assessed the risk of bias.
Data synthesis.
We conducted a series of analyses of the included studies with different outcomes involving child mortality, malaria, and general morbidity or condition (general morbidity or condition is the incidence of common diseases, such as respiratory tract infection, diarrhea, and general symptoms, such as fever, diarrhea, and vomiting). For child mortality, a meta-analysis was not performed because of the substantial heterogeneity in the study design across studies. For the other outcomes, a meta-analysis was conducted when two or more studies were included without substantial heterogeneity. Heterogeneity was determined using the I2 statistic and Q tests. A random-effects model was used when a significant heterogeneity was observed. Meta-analysis was conducted using Stata version 12.0 (StataCorp, College Station, TX). Statistical significance was set at P < 0.05.
RESULTS
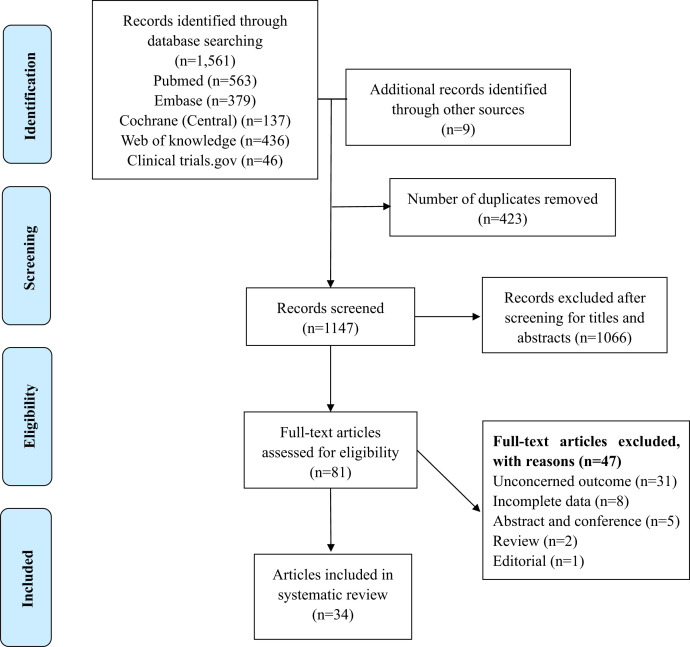
A total of 1,570 studies were identified. After screening the titles and abstracts, 81 studies were considered relevant and reviewed for eligibility. Finally, 34 studies (22 randomized controlled trials [RCTs] and 12 non-RCTs) that investigated the secondary effects of azithromycin MDA were included after reviewing the full texts (Figure 1). A total of six studies reported child mortality (Supplemental Table 1).9,13–17 Ten studies were related to malaria (Supplemental Table 2).6,13,18–25 Twenty studies reported on the patients’ general morbidity or condition (Supplemental Table 3),7,8,13,26–42 of which six investigated general morbidity,7,8,13,26,33,42 10 examined the carriage of pathogenic organisms,27–36 and five assessed children’s nutrition.37–41
Figure 1.
Flowchart of selection of studies for inclusion in the systematic review. This figure appears in color at www.ajtmh.org.
Risk of bias of the included studies.
The risk of bias of the included studies is presented in Supplemental Tables 4 through 6. For RCTs, most of the included studies had a low or moderate risk of bias from random sequence generation, whereas a few studies had an unclear risk of bias in this category. The risk of bias from allocation concealment was low in 12 of the 22 RCTs and unclear in 10 RCTs. The risk of bias from masking of participants was low in seven of the 22 RCTs, high in seven studies, and unclear in eight studies. The risk of bias from masking of outcome assessors was low in 16 studies and high in six studies. In addition, the risk of bias from incomplete outcome data and selective reporting was low in all RCTs. For nonrandomized studies, the risk of bias from confounding was low or moderate in seven of the 12 studies, whereas it was unclear in five studies. The risk of bias from the selection of participants for the study was low or moderate in eight studies and unclear in four. The risk of bias from intervention classification was low in 10 studies, moderate in one study, and unclear in one study. The risks of bias from intervention deviations were low in most studies and unclear in four studies. Moreover, the risk of bias from masking of outcome assessors was low in three studies and unclear in nine studies. All studies had a low or moderate risk of bias owing to incomplete outcome data and reporting bias.
Child mortality.
Association between azithromycin MDA and child mortality.
Four studies assessed the association between azithromycin MDA and child mortality (Supplemental Table 1). Three of these studies (two RCTs9,17 and one cohort study15) showed that azithromycin MDA reduced child mortality. One RCT reported a rate ratio (RR) of 0.53 [95% confidence interval [CI]: 0.26–0.84). The cohort study reported an RR of 0.35 (95% CI: 0.17–0.74].17 In addition, the cohort study reported a significant reduction in infectious mortality (RR: 0.20; 95% CI: 0.07–0.58).15 Notably, the Macrolides Oraux pour Réduire les Décès avec un Oeil sur la Résistance (MORDOR-I) trial, including more than 1,500 communities in three countries (Malawi, Niger, and Tanzania), also reported a significant decrease in child mortality (RR: 0.86; 95% CI: 0.80–0.93).9 Moreover, further analysis revealed that significantly lower mortality was observed only in Niger, which has a high child mortality rate.9 Additionally, it was seen that children aged less than 6 months had the greatest benefit. This study provided reliable data regarding the reduction in mortality after azithromycin MDA.9 On the contrary, one RCT using different MDA strategies containing antimalaria agents in either the azithromycin group or placebo group reported a nonsignificant effect size in mortality between these two groups (RR: 1.1; 95% CI: 0.88–1.3).13 After the mortality decrease findings of the MORDOR-I study, a subsequent study (MORDOR-II trial) was performed.14 In this study, two more doses (biannual for 1 year) of azithromycin were administered to the study population in MORDOR-I (received biannual azithromycin or placebo for 2 years). The results showed that child mortality still decreased after subsequent treatments.14 The findings of the MORDOR-II trial suggest that azithromycin MDA remains effective in reducing child mortality, even in communities that had previously received azithromycin.
Frequencies of azithromycin MDA and child mortality.
Two RCTs investigated the effects of different frequencies of azithromycin MDA on child mortality (Supplemental Table 1).16,17 One RCT was conducted in Ethiopia and consisted of 48 communities with 18,415 children aged 1 to 9 years. This study showed that the mortality rate was lower in the annual (3.2/1,000 person-years), biannual (4.9/1,000 person-years), and quarterly groups (4.7/1,000 person-years) compared with the untreated group (8.3/1,000 person-years). However, no significant difference was observed among these three frequencies (Supplemental Table 1).17 The other study was conducted in Niger, which included 48 communities with 5,304 children aged 6 months to 12 years. The results showed that biannual MDA (29.0/1,000 person-years) had a lower mortality than annual MDA (35.6/1,000 person-years), but without significant differences (RR: 0.81, 95% CI: 0.66–1.00, P = 0.07).16 However, this study had limitations because it lacked an untreated group to assess the efficacy of azithromycin MDA on mortality.
Malaria.
Association between azithromycin MDA and malaria prevalence.
Ten studies (eight RCTs6,13,19,20,22–25 and two cohort studies18,21) investigated the secondary effects of azithromycin MDA on malaria (Supplemental Table 2). These studies were conducted in districts with a high incidence of malaria.
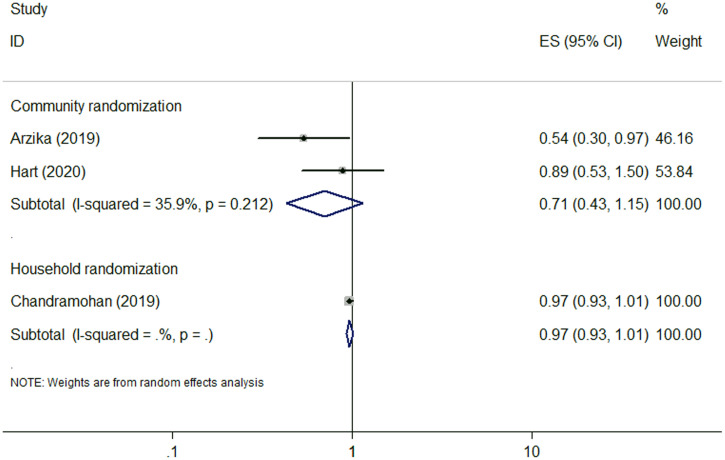
Seven of the 10 studies compared malaria prevalence between the azithromycin-treated and non–azithromycin-treated groups.6,13,18–21,25 Three RCTs of the seven studies investigated the incidence of malaria parasitemia.6,13,20 Two of which were community randomized but one was household randomized. One RCT (community randomized) showed a lower incidence of malaria parasitemia in the azithromycin group,6 whereas the other two RCTs (one community and one household randomized) reported a nonsignificant association.13,20 The pooled results for RCTs used community randomization showed no association between azithromycin MDA and the prevalence of malaria parasitemia (RR: 0.71, 95% CI: 0.43–1.15), with moderate heterogeneity (I2 = 35.9%, P = 0.212) (Figure 2 and Table 1), indicating that azithromycin MDA did not reduced the prevalence of malaria parasitemia. Other outcomes were investigated by the other four studies.18,19,21,25 Two RCTs reported that azithromycin MDA resulted in a reduced spleen size.19,25 One cohort study showed no significant difference in parasitemia test positivity rate or incidence of clinical malaria between the azithromycin and placebo groups.18 Two studies found that azithromycin MDA was associated with lower incidences of Plasmodium falciparum infection21,25 and clinical malaria,25 but the effects were transient with only a 1-month effect.21,25
Figure 2.
Forest plot for azithromycin mass drug administration and malaria parasitemia. ES = effect size. This figure appears in color at www.ajtmh.org.
Table 1.
The meta-analyses for azithromycin mass drug administration and outcomes
| Outcome | Study design | N | Pooled RR (95% CI) | I2 %, P for heterogeneity |
|---|---|---|---|---|
| Malaria parasitemia | Community RCT | 2 | 0.71 (0.43–1.15) | 35.9%, 0.212 |
| Household RCT | 1 | 0.97 (0.93–1.01) | – | |
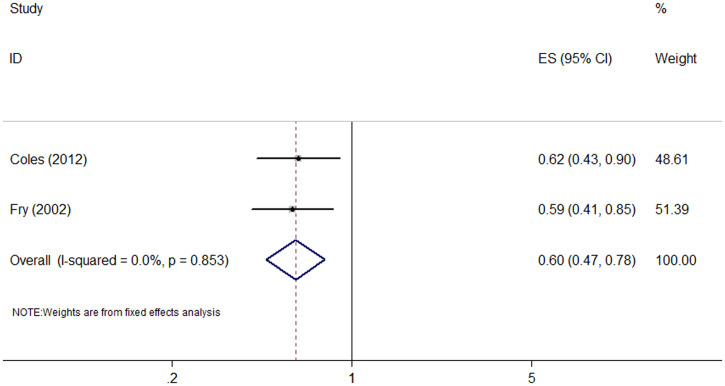
| Respiratory tract infection | Cohort | 2 | 0.60 (0.47–0.78) | 0.0%, 0.853 |
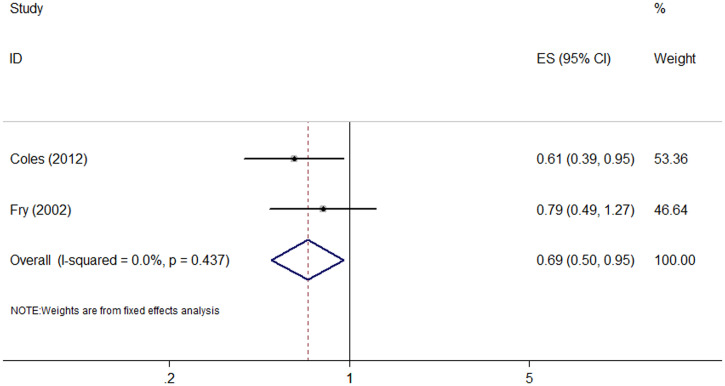
| Diarrhea | Cohort | 2 | 0.69 (0.50–0.95) | 0.0%, 0.437 |
| Abdominal pain | RCT | 2 | 0.81 (0.64–1.03) | 0.0%, 0.752 |
| Cohort | 1 | 0.91 (0.59–1.40) | – | |
| Vomiting | RCT | 2 | 0.67 (0.52–0.85) | 31.7%, 0.226 |
| Cohort | 1 | 0.62 (0.33–1.16) | – | |
| Nutrition | ||||
| Wasting | RCT | 4 | 0.95 (0.84–1.07) | 0%, 0.638 |
| Stunting | RCT | 4 | 0.90 (0.75–1.09) | 42.9%, 0.154 |
| Underweight | RCT | 4 | 0.94 (0.86–1.02) | 2.1%, 0.382 |
| Low MUAC | RCT | 3 | 0.98 (0.84–1.14) | 8.8%, 0.334 |
CI = confidence interval; MUAC = mid-upper arm circumference; RCT = randomized controlled trial; RR = rate ratio. Bold text indicates significant effect size.
Moreover, three of the 10 studies investigated malaria prevalence between annual (at peak transmission season) and biannual (at peak and low transmission season) azithromycin MDA groups (Supplemental Table 2).22–24 Two of them involving 3-year treatment showed no statistical significance in reducing parasitemia prevalence, parasite density, clinical malaria prevalence, level of hemoglobin, and gametocytemia between annual and biannual groups.23,24 In contrast, one study involving 1-year treatment reported that biannual MDA was better than annual MDA in reducing parasitemia and parasite density.22
General morbidity or condition.
Respiratory tract infection.
Four studies (two RCTs13,26 and two cohort studies7,33) investigated the association between azithromycin MDA and the incidence of respiratory tract infection (Supplemental Table 3). One RCT (community randomized)26 showed a nonsignificant association, but the other RCT (household randomized)13 and two cohort studies7,33 reported a lower incidence of respiratory tract infection in the azithromycin MDA group. The meta-analysis of the cohort studies showed that azithromycin MDA was associated with reduced incidence of respiratory tract infection (RR: 0.60, 95% CI: 0.47–0.78; I2 = 0%, P = 0.853) (Figure 3 and Table 1). Notably, a reduction in the prevalence of respiratory tract infections was only observed in the first month after MDA.7
Figure 3.
Forest plot for azithromycin mass drug administration and respiratory tract infection. ES = effect size. This figure appears in color at www.ajtmh.org.
Diarrhea.
Five studies (three RCTs and two cohort studies) assessed the risk of diarrhea after azithromycin MDA (Supplemental Table 3).8,13,26,33,42 Four of them (three RCTs and one cohort) found that azithromycin MDA was associated with a lower risk of diarrhea.8,13,26,42 The other cohort study did not show a significant association.33 The meta-analysis of the cohort studies showed that azithromycin MDA was associated with a lower incidence of diarrhea (RR: 0.69, 95% CI: 0.50–0.95; I2 = 0%, P = 0.437) (Figure 4 and Table 1). Meta-analysis of the RCTs was not adopted because of great heterogeneity (I2 = 89%). Interestingly, a reduction in diarrhea was only observed in the first month after MDA.8
Figure 4.
Forest plot for azithromycin mass drug administration and diarrhea. ES = effect size. This figure appears in color at www.ajtmh.org.
Clinical symptoms (fever, abdominal pain, vomiting, and headache).
Four studies (three RCTs and one cohort study) assessed the risk of clinical symptoms, including fever, abdominal pain, vomiting, and headache (Supplemental Table 3).13,26,33,42 Three studies (two RCTs13,26 and one cohort study33) reported that azithromycin MDA was associated with a lower incidence of fever. Meta-analysis of the two RCTs was not performed because one RCT was community randomized but the other one was household randomized. Three studies (two RCTs26,42 and one cohort study33) reported no significant association between azithromycin MDA and abdominal pain. The meta-analysis of the two RCTs still showed nonsignificant association (RR: 0.81, 95% CI: 0.64–1.03; I2 =0%, P = 0.752) (Supplemental Figure 1 and Table 1). Three studies (two RCTs26,42 and one cohort study33) investigated the association between azithromycin MDA and the incidence of vomiting. The meta-analysis of the two RCTs showed a reduced incidence of vomiting (RR: 0.67; 95% CI: 0.52–0.85; I2 = 31.7%, P = 0.226). However, the cohort study showed a nonsignificant association (Supplemental Figure 2 and Table 1). Moreover, two studies (one RCT26 and one cohort study33) found that azithromycin MDA was associated with a lower incidence of headache.
Carriage of pathogenic organisms.
Ten studies investigated the association between azithromycin MDA and the carriage of pathogenic organisms (Supplemental Table 3).27–36 Seven of these studies assessed the carriage of Streptococcus pneumoniae.29–35 Among them, four studies did not show a significant reduction in the Streptococcus pneumoniae carriage rate.30,32,34,35 However, another three studies reported that the carriage rate was transiently reduced for a period of 1 month.29,31,33 One study found a reduction in the viral load of coronavirus with azithromycin MDA, but the prevalence of coronavirus colonization remained unchanged.36 Two studies found that azithromycin MDA altered the gut microbiome structure and reduced the number of harmful bacteria, which was thought to be associated with an improvement in children’s health.27,28 One study revealed that azithromycin MDA reduced the number of gut pathogenic bacteria, especially the abundance of two Campylobacter species, which are commonly detected in children with diarrhea.28
Nutrition.
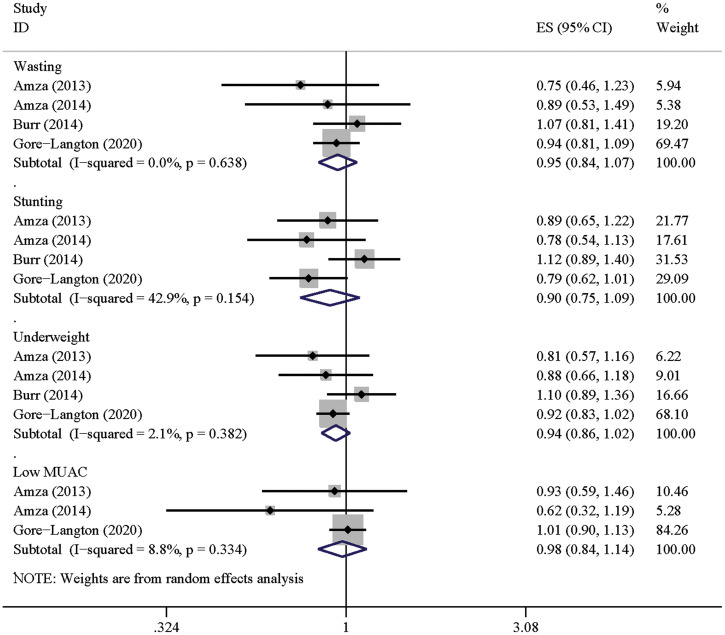
Five RCTs calculated anthropometric indices among children (Supplemental Table 3).37–41 Four studies compared the prevalence of wasting, stunting, underweight, and low mid-upper arm circumference.37,39–41 None of them reported a significant association between azithromycin MDA and better nutritional outcomes. The pooled effect sizes were not significant in each index: wasting (RR: 0.95, 95% CI: 0.84–1.07), stunting (RR: 0.90, 95% CI: 0.75–1.09), underweight (RR: 0.94, 95% CI: 0.86–1.02), and mid-upper arm circumference (RR: 0.98, 95% CI: 0.84–1.14), respectively (Figure 5 and Table 1). In addition, one study that compared the height and weight between the azithromycin and untreated groups also showed no significant differences.38
Figure 5.
Forest plot for azithromycin mass drug administration and anthropometric indices. MUAC = mid-upper arm circumference. ES = effect size. This figure appears in color at www.ajtmh.org.
DISCUSSION
This study provided a comprehensive overview of the secondary effects of azithromycin MDA. Azithromycin MDA was associated with a lower prevalence of child mortality, respiratory tract infection, diarrhea, and clinical symptoms including fever, vomiting, and headache. Moreover, azithromycin MDA reduced the carriage rate of Streptococcus pneumoniae, gut pathogenic bacteria, and coronaviruses.
Three studies, including the large-scale MORDOR-I study, showed that azithromycin MDA was associated with lower child mortality.9,15,17 One RCT by Chandramohan et al. showed a nonsignificant association.13 This difference may be due to the co-administration of seasonal malaria chemoprevention drugs in the Chandramohan et al.’s study.13 Therefore, the potential effect of azithromycin on malaria-related mortality could be attenuated because malaria is one of the predominant causes of death in children living in the tropical areas.43,44 In addition, most of the children had received a pneumococcal conjugate vaccine in the study by Chandramohan et al., which may diminish the potential effects of azithromycin in reducing pneumonia-related mortality. Moreover, unlike community randomization used in the MORDOR-I study, household randomization was used in the study by Chandramohan et al., which could lead to the loss of benefits from community MDA.
Interestingly, the MORDOR-I study showed that the effect on child mortality was only significant in Niger, which has a high baseline mortality rate, but not in Malawi and Tanzania. These findings suggest that the effects on child mortality may differ across different geographic settings. Recently, however, two secondary analyses were conducted that found a nonsignificant relationship between the effect size of mortality reduction and baseline mortality.45,46 This means that azithromycin MDA in high-mortality areas is highly beneficial, but the benefit in low-mortality areas cannot be excluded. Nonetheless, some studies have suggested that targeting azithromycin MDA only in areas with high mortality could generate maximal efficacy in reducing the absolute number of deaths.45,46
The meta-analysis for child mortality was not conducted in our study because of high heterogeneity (I2 =80.5%), which may be due to differences in study areas, study designs, MDA strategies, and sample sizes. Oldenburg et al.47 reported a significant pooled effect size between azithromycin MDA and reduced child mortality (RR 0.86, 95% CI: 0.78–0.94; I2 = 22.6%). The low heterogeneity found in the study by Oldenburg et al. may be due to the different inclusion of the original studies. Three RCTs, including one comparing child mortality between annual and biannual MDA, were pooled by Oldenburg et al. Although the study inclusions were different, our finding that azithromycin MDA is related to reduced child mortality is consistent with those reported by Oldenburg et al.
Regarding the effect of different frequencies on child mortality, researchers have reported a reduction in the mortality rate with a higher MDA frequency, but the difference was not significant (P = 0.07). Usually, it is difficult to generate a statistical difference when it comes to rare events such as child death. Therefore, the current findings could not exclude the effect of higher MDA frequency in reducing mortality. Interestingly, mortality reduction was reported in the first 3 months after MDA.48 These findings support the hypothesis that quarterly MDA is most effective in reducing child mortality.
Similar to the effect of azithromycin MDA on child mortality, the effect of azithromycin MDA on the prevalence of some diseases was short term. The prevalence of Plasmodium falciparum infection, diarrhea, and respiratory tract infection and the carriage rate of Streptococcus pneumoniae were reduced in a few weeks post-MDA, but these effects wore off over time.7,8,21,29,31 Therefore, a higher frequency of MDA (e.g., quarterly) may yield greater efficacy in reducing child mortality and morbidity than annual or biannual MDA. Further studies are required to confirm this hypothesis.
Among the infectious diseases, malaria is life-threatening and the leading cause of death among children younger than five years who live in the tropical areas.49 Malaria-endemic districts are expected to benefit from azithromycin MDA because this drug has antimalarial properties.6 However, the present study indicated that the effect of azithromycin MDA in the incidence of malaria was weak and transient. Therefore, intermittent preventive treatment in infants or seasonal malaria chemoprophylaxis is required to reduce malaria-related morbidity and mortality in epidemic areas.
Besides malaria, diarrhea and respiratory tract infection are also the leading causes of infection-related death in tropical areas. This study showed that azithromycin MDA could significantly reduce the prevalence of these conditions, although the effects only lasted for 1 month after MDA. Furthermore, azithromycin MDA reduced the carriage of pathogens, suggesting that azithromycin may play a role in preventing the development of invasive infections.
Moreover, the effects of azithromycin MDA on health gains are balanced by antibiotic resistance, which occurs after the implementation of azithromycin MDA. The level of antibiotic resistance could decline after cessation of the intervention.31,34,50 If repeated azithromycin MDA is planned to reduce child mortality and disease prevalence, more studies are needed for continuous monitoring of drug resistance.
Although this study systemically provides evidence regarding the secondary effects of MDA, a few limitations must be mentioned. First, the data of the mortality studies were not pooled to perform a meta-analysis because of great heterogeneity. Second, original studies on Streptococcus pneumoniae carriage reported inconsistent findings. However, a meta-analysis could not be conducted because of effect sizes were not available for every study.
CONCLUSION
Several secondary effects of MDA have been identified. Azithromycin MDA has been shown to reduce child mortality rate, prevalence of diarrhea and respiratory tract infection, as well as the carriage of Streptococcus pneumoniae and gut Campylobacter species.
Supplemental files
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1. Solomon A Zondervan M Kuper H Buchan J Mabey DAF , 2006. Trachoma Control: A Guide for Programme Managers. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2. Resnikoff S Pascolini D Etya’ale D Kocur I Pararajasegaram R Pokharel GP Mariotti SP , 2004. Global data on visual impairment in the year 2002. Bull World Health Organ 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- 3. Francis V Turner V , Blindness WHOPftPo , 1995. Achieving Community Support for Trachoma Control: A Guide for District Health Work. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4. International Trachoma Initiative home page Available at: http://www.trachoma.org/. Accessed February 12, 2022.
- 5. Tao X et al. 2021. The effectiveness of azithromycin mass drug administration on trachoma: a systematic review. Chin Med J (Engl) 134: 2944–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arzika AM Maliki R Boubacar N Kane S Cotter SY Lebas E , 2019. Biannual mass azithromycin distributions and malaria parasitemia in pre-school children in Niger: a cluster-randomized, placebo-controlled trial. PLoS Med 16: e1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coles CL Levens J Seidman JC Mkocha H Munoz B West S , 2012. Mass distribution of azithromycin for trachoma control is associated with short-term reduction in risk of acute lower respiratory infection in young children. Pediatr Infect Dis J 31: 341–346. [DOI] [PubMed] [Google Scholar]
- 8. Coles CL Seidman JC Levens J Mkocha H Munoz B West S , 2011. Association of mass treatment with azithromycin in trachoma-endemic communities with short-term reduced risk of diarrhea in young children. Am J Trop Med Hyg 85: 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keenan JD et al. 2018. Azithromycin to reduce childhood mortality in Sub-Saharan Africa. Engl J Med 378: 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng L et al. 2020. Safety of azithromycin in pediatrics: a systematic review and meta-analysis. Eur J Clin Pharmacol 76: 1709–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Brien KS Emerson P Hooper PJ Reingold AL Dennis EG Keenan JD Lietman TM Oldenburg CE , 2019. Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review. Lancet Infect Dis 19: e14–e25. [DOI] [PubMed] [Google Scholar]
- 12. O’Brien KS Emerson P Hooper PJ Reingold AL Dennis EG Keenan JD Lietman TM Oldenburg CE , 2018. Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review. Lancet Infect Dis 19: e14–e25. [DOI] [PubMed] [Google Scholar]
- 13. Chandramohan D et al. 2019. Effect of adding azithromycin to seasonal malaria chemoprevention. N Engl J Med 380: 2197–2206. [DOI] [PubMed] [Google Scholar]
- 14. Keenan JD et al. 2019. Longer-term assessment of azithromycin for reducing childhood mortality in Africa. N Engl J Med 380: 2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keenan JD Ayele B Gebre T Zerihun M Zhou Z House JI Gaynor BD Porco TC Emerson PM Lietman TM , 2011. Childhood mortality in a cohort treated with mass azithromycin for trachoma. Clin Infect Dis 52: 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Brien KS et al. 2018. Childhood mortality after mass distribution of azithromycin: a secondary analysis of the PRET cluster-randomized trial in Niger. PLoS Negl Trop Dis 37: 1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Porco TC et al. 2009. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA 302: 962–968. [DOI] [PubMed] [Google Scholar]
- 18. Bloch EM Munoz B Mrango Z Weaver J Mboera LEG Lietman TM Sullivan DJ Jr West SK , 2019. The impact on malaria of biannual treatment with azithromycin in children age less than 5 years: a prospective study. Malar J 18: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hart JD Edwards T Burr SE Harding-Esch EM Takaoka K Holland MJ Sillah A Mabey DC Bailey RL , 2014. Effect of azithromycin mass drug administration for trachoma on spleen rates in Gambian children. Trop Med Int Health 19: 207–211. [DOI] [PubMed] [Google Scholar]
- 20. Hart JD Samikwa L Sikina F Kalua K Keenan JD Lietman TM Burr SE Bailey RL , 2020. Effects of biannual azithromycin mass drug administration on malaria in malawian children: a cluster-randomized trial. Am J Trop Med Hyg 103: 1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schachterle SE Mtove G Levens JP Clemens E Shi L Raj A Dumler JS Munoz B West S Sullivan DJ , 2014. Short-term malaria reduction by single-dose azithromycin during mass drug administration for trachoma, Tanzania. Emerg Infect Dis 20: 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaynor BD et al. 2014. Impact of mass azithromycin distribution on malaria parasitemia during the low-transmission season in Niger: a cluster-randomized trial. Am J Trop Med Hyg 90: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Brien KS et al. 2017. Mass azithromycin and malaria parasitemia in Niger: results from a community-randomized trial. Am J Trop Med Hyg 97: 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oldenburg CE et al. 2018. Annual versus biannual mass azithromycin distribution and malaria parasitemia during the peak transmission season among children in Niger. Pediatr Infect Dis J 37: 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sadiq ST Glasgow KW Drakeley CJ Muller O Greenwood BM Mabey DC Bailey RL , 1995. Effects of azithromycin on malariometric indices in The Gambia. Lancet 346: 881–882. [DOI] [PubMed] [Google Scholar]
- 26. Whitty CJ Glasgow KW Sadiq ST Mabey DC Bailey R , 1999. Impact of community-based mass treatment for trachoma with oral azithromycin on general morbidity in Gambian children. Pediatr Infect Dis J 18: 955–958. [DOI] [PubMed] [Google Scholar]
- 27. Doan T et al. 2018. Mass azithromycin distribution and community microbiome: a cluster-randomized trial. Open Forum Infect Dis 5: ofy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doan T et al. 2019. Gut microbiome alteration in MORDOR I: a community-randomized trial of mass azithromycin distribution. Nat Med 25: 1370–1376. [DOI] [PubMed] [Google Scholar]
- 29. Leach AJ Shelby-James TM Mayo M Gratten M Laming AC Currie BJ Mathews JD , 1997. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis 24: 356–362. [DOI] [PubMed] [Google Scholar]
- 30. Batt SL Charalambous BM Solomon AW Knirsch C Massae PA Safari S Sam NE Everett D Mabey DC Gillespie SH , 2003. Impact of azithromycin administration for trachoma control on the carriage of antibiotic-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 47: 2765–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burr SE et al. 2014. Mass administration of azithromycin and Streptococcus pneumoniae carriage: cross-sectional surveys in The Gambia. Bull World Health Organ 92: 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coles CL Mabula K Seidman JC Levens J Mkocha H Munoz B Mfinanga SG West S , 2013. Mass distribution of azithromycin for trachoma control is associated with increased risk of azithromycin-resistant Streptococcus pneumoniae carriage in young children 6 months after treatment. Clin Infect Dis 56: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 33. Fry AM Jha HC Lietman TM Chaudhary JS Bhatta RC Elliott J Hyde T Schuchat A Gaynor B Dowell SF , 2002. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis 35: 395–402. [DOI] [PubMed] [Google Scholar]
- 34. Haug S et al. 2010. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis 51: 571–574. [DOI] [PubMed] [Google Scholar]
- 35. Skalet AH et al. 2010. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med 7: e1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doan T Hinterwirth A Arzika AM Worden L Chen C Zhong L Oldenburg CE Keenan JD Lietman TM , 2020. Reduction of coronavirus burden with mass azithromycin distribution. Clin Infect Dis 71: 2282–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amza A et al. 2013. A cluster-randomized controlled trial evaluating the effects of mass azithromycin treatment on growth and nutrition in Niger. Am J Trop Med Hyg 88: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keenan JD et al. 2019. Linear growth in preschool children treated with mass azithromycin distributions for trachoma: a cluster-randomized trial. PLoS Negl Trop Dis 13: e0007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amza A et al. 2014. Does mass azithromycin distribution impact child growth and nutrition in Niger? A cluster-randomized trial. PLoS Negl Trop Dis 8: e3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burr SE Hart J Edwards T Harding-Esch EM Holland MJ Mabey DC Sillah A Bailey RL , 2014. Anthropometric indices of Gambian children after one or three annual rounds of mass drug administration with azithromycin for trachoma control. BMC Public Health 14: 1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gore-Langton GR et al. 2020. Effect of adding azithromycin to the antimalarials used for seasonal malaria chemoprevention on the nutritional status of African children. Trop Med Int Health 25: 740–750. [DOI] [PubMed] [Google Scholar]
- 42. Oldenburg CE et al. 2018. Safety of azithromycin in infants under six months of age in Niger: a community randomized trial. PLoS Negl Trop Dis 12: e0006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalter HD Roubanatou AM Koffi A Black RE , 2015. Direct estimates of national neonatal and child cause-specific mortality proportions in Niger by expert algorithm and physician-coded analysis of verbal autopsy interviews. J Glob Health 5: 010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keenan JD et al. 2020. Cause-specific mortality of children younger than 5 years in communities receiving biannual mass azithromycin treatment in Niger: verbal autopsy results from a cluster-randomised controlled trial. Lancet Glob Health 8: e288–e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Porco TC et al. 2019. Efficacy of mass azithromycin distribution for reducing childhood mortality across geographic regions. Am J Trop Med Hyg 103: 1291–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oron AP Burstein R Mercer LD Arzika AM Kalua K Mrango Z West SK Bailey RL Porco TC Lietman TM , 2020. Effect modification by baseline mortality in the MORDOR azithromycin trial. Am J Trop Med Hyg 103: 1295–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oldenburg CE et al. 2019. Mass azithromycin distribution to prevent childhood mortality: a pooled analysis of cluster-randomized trials. Am J Trop Med Hyg 100: 691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Porco TC et al. 2018. Mass oral azithromycin for childhood mortality: timing of death after distribution in the MORDOR trial. Clin Infect Dis 68: 2114–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Varo R Chaccour C Bassat Q , 2020. Update on malaria. Med Clin (Barc) 155: 395–402. [DOI] [PubMed] [Google Scholar]
- 50. Bojang E et al. 2017. Short-term increase in prevalence of nasopharyngeal carriage of macrolide-resistant Staphylococcus aureus following mass drug administration with azithromycin for trachoma control. BMC Microbiol 17: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.