Abstract
The development of more-effective antituberculosis vaccines would assist in the control of the global problem of infection with Mycobacterium tuberculosis. One recently devised vaccination strategy is immunization with DNA plasmids encoding individual microbial genes. Using the genes for the M. tuberculosis secreted proteins MPT64 (23 kDa), Ag85B (30 kDa), and ESAT-6 (6 kDa) as candidate antigens, DNA vaccines were prepared and tested for immunogenicity and protective efficacy in a murine model of aerosolized tuberculosis (TB). Intramuscular immunization with DNA-64 or DNA-85B resulted in the activation of CD4+ T cells, which produce gamma interferon (IFN-γ), and high titers of specific immunoglobulin G antibodies. Further, DNA-64 induced major histocompatibility complex class I-restricted CD8+ cytotoxic T cells. The addition of a eukaryotic leader sequence to mpt64 did not significantly increase the T-cell or antibody response. Each of the three DNA vectors stimulated a significant reduction in the level of M. tuberculosis infection in the lungs of mice challenged 4 weeks after immunization, but not to the levels resulting after immunization with Mycobacterium bovis BCG. The vaccines showed a consistent hierarchy of protection, with the most effective being Ag85B, followed by ESAT-6 and then MPT64. Coimmunization with the three vectors resulted in a greater degree of protection than that induced by any single vector. This protective efficacy was associated with the emergence of IFN-γ-secreting T cells earlier than in infected animals immunized with a control vector. The efficacy of these DNA vaccines suggests that multisubunit vaccination may contribute to future vaccine strategies against TB.
Infection with Mycobacterium tuberculosis continues to be a major cause of morbidity and mortality throughout the world, resulting in 3 million deaths and over 8 million new cases of tuberculosis (TB) each year (4). The current vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG), has variable protective efficacy, ranging from 0 to 85% in different studies (9), and second-generation anti-TB vaccines are urgently needed. The development of new vaccines requires an understanding of the protective immune response against M. tuberculosis and of the construction of delivery vectors with the ability to elicit this protective response. The critical component of protective immunity against TB is a T-cell-mediated response characterized by the secretion of gamma interferon (IFN-γ) and other cytokines (26). Although subunit vaccines were previously considered ineffective against mycobacteria, vaccines based on culture filtrate proteins of M. tuberculosis and an adjuvant have induced protective immunity in mice (1, 27) and guinea pigs (17). Genetic immunization may be a successful alternate method of delivery of these secreted proteins. This form of immunization induces antibody and cell-mediated immune responses involving the CD4+- and CD8+-T-cell compartments. DNA vaccines have induced protective immunity against a number of pathogens and tumors, most recently against mycobacteria (11, 18, 35, 37).
Proteins secreted by mycobacteria are recognized early in the course of experimental TB infection (3, 27) and by lymphocytes of TB patients (5). The antigen 85 complex is exhibited widely by mycobacterial species, and both 85A and 85B elicit T-cell responses in TB patients (21, 29, 30). The 23-kDa protein MPT64, which is restricted to M. tuberculosis, virulent M. bovis strains, and a small number of strains of BCG, is recognized by the immune systems of the majority of TB patients and their contacts (28, 30). The smaller, 6-kDa protein ESAT-6 is expressed only in virulent M. bovis strains and M. tuberculosis. ESAT-6 stimulated the early release of IFN-γ in mice reinfected with M. tuberculosis (2). We have prepared DNA vaccines expressing the M. tuberculosis antigens 85B and MPT64 and demonstrated that they stimulated both CD4+- and CD8+-T-cell responses. The protective efficacies of these vectors, as well as a third one expressing ESAT-6, in a mouse model of aerosolized TB were assessed. The vaccine containing antigen 85B was the most effective of the individual vaccines at stimulating protective immunity, and combined vaccination with the three DNA vaccines was more effective than vaccination with a single vector. Protection was associated with the early emergence of IFN-γ-secreting CD4+ T cells.
MATERIALS AND METHODS
Bacteria.
For aerosol challenge, M. tuberculosis H37Rv (ATCC 27294) was grown in Proskauer and Beck liquid medium for 14 days at 37°C. M. bovis BCG CSL (CSL Bioscience, Melbourne, Australia) was grown in Middlebrook 7H9 broth with ADC supplement (Difco Laboratories, Detroit, Mich.) for 14 days at 37°C. The bacteria were washed with 30% glycerol in phosphate-buffered saline (PBS) and then enumerated on OADC-supplemented Middlebrook 7H11 agar (Difco). The cells were dispensed and then stored at −70°C. For manipulation of plasmids, Escherichia coli MC1061 was grown in Luria-Bertani broth or agar (32) supplemented with ampicillin (100 μg/ml) as required. For large-scale plasmid preparations, the transformed bacteria were grown in Circlegrow broth (BIO 101, Vista, Calif.) with ampicillin.
Production of DNA vaccines.
The vector pJW4303, which was kindly provided by J. I. Mullins, Stanford University, contains the cytomegalovirus immediate-early promoter with intron A upstream and a bovine growth hormone polyadenylation sequence downstream of the gene of interest. The foreign gene can be inserted in frame with the tissue plasminogen activator (tPA) signal sequence. The gene for the MPT64 protein was amplified from the plasmid pTJ1 (31), while the genes for Ag85B and ESAT-6 were amplified from M. tuberculosis genomic DNA. The ag85b-specific primers were 5′ GTC CGA AGC TTA TGA CAG ACG TGA CGG GA and 3′ TAA TAG GAT CCT CAG CCG GCG CCT AAC GA, and the primers for esat6 were 5′ ATA TAA GCT TGC TAG CAT GAC AGA GCA GC and 3′ CGC GCG GAT CCC TAT GCG AAC ATC. The genes for MPT64, Ag85B, and ESAT-6 were cloned into pJW4303 by standard molecular biology techniques (32) to yield plasmids pJI23 (DNA-64), pJI30 (DNA-85B), and pJIE6 (DNA-E6), respectively. The mpt64 gene was also cloned in frame with the tPA signal sequence of pJW4303. This clone, pJS23 (DNA-64sec), permitted secretion of the mycobacterial protein from eukaryotic cells. The genes were checked by double-stranded sequencing (Sequenase; United States Biochemical Corporation, Cleveland, Ohio). The parental vector, pJW4303, was used as the negative control. DNA for immunization was purified by CsCl centrifugation, adjusted to a concentration of 1 mg/ml in PBS, and stored at −20°C until required.
Immunization and animals.
C57Bl/6 female mice were supplied as specific-pathogen-free mice by the Animal Resource Centre (Perth, Australia) and were maintained under specific-pathogen-free conditions. Mice were immunized at between 8 and 12 weeks of age. Fifty micrograms of plasmid was injected intramuscularly into the tibialis anterior muscle of each hindleg. Control mice were immunized with PBS or the parental vector, pJW4303. Mice were immunized either once only or three times, at 3-week intervals. In combination experiments, control vector DNA was added to preparations so that all mice received the same amount of total DNA.
Protein antigens.
The M. tuberculosis MPT64 and M. bovis Ag85B proteins were expressed in E. coli as fusion proteins with glutathione S-transferase and prepared as described previously (28, 29).
Mycobacterial challenge.
Four weeks after the last boost with the DNA vaccine, mice were challenged with M. tuberculosis H37Rv via aerosol. The mice were exposed in a Middlebrook airborne infection apparatus (Glas-Col, Terre Haute, Ind.) to an infective dose of approximately 100 viable bacilli, as confirmed by culture of lung homogenates. After 4 weeks, the number of bacteria in one lung lobe was enumerated by homogenizing the tissue and plating 10-fold dilutions, prepared in water, on supplemented Middlebrook 7H11 Bacto Agar. The colonies were counted visually after 21 days. In challenge experiments, control mice were vaccinated with 5 × 104 CFU of BCG (CSL) by subcutaneous injection 100 days or more before infection with M. tuberculosis.
Antibody determination.
Antigen-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (28, 29), using recombinant mycobacterial proteins (at 10 μg/ml) and alkaline phosphatase-conjugated goat anti-murine immunoglobulin G (IgG) (Sigma, St. Louis, Mo.). To determine the titer of the antigen-specific antibody, the mean absorbance plus three standard deviations of the mean for normal mouse serum, at 1:100, was adopted as the cutoff absorbance.
Lymphocyte proliferation.
The inguinal, axillary, and para-aortic lymph nodes or the spleens of five immunized mice were pooled, and single-cell suspensions were prepared in complete RPMI medium supplemented with 2 mM glutamate, 50 μM β-mercaptoethanol, and 10% fetal calf serum. From spleen or lymph node tissue, T lymphocytes were enriched by passing leukocytes through a nylon wool column. Syngeneic antigen-presenting cells were γ-irradiated (2,300 rads) splenocytes. The semipurified T lymphocytes and antigen-presenting cells (2 × 105 cells each) were added to 96-well plates and were incubated in triplicate with various concentrations of antigen, the mitogen concanavalin A (ConA; Sigma), or medium alone. The plates were incubated at 37°C in an atmosphere of 5% CO2 for 3 days and then pulsed with 1 μCi of [3H]thymidine (NEN Life Sciences, Boston, Mass.) per well for 6 h before incorporated [3H]thymidine was determined.
For depletion of T-cell subsets, nylon wool-purified lymphocytes were incubated with the monoclonal antibodies GK1.5 (anti-CD4) and 53.6.72 (anti-CD8) followed by incubation with anti-rat IgG-covered magnetic beads (Dynal, Oslo, Norway). Depletion was >95% as determined by flow cytometry. Cells were washed and cultured as described above. Proliferative results were calculated as the mean counts per minute for triplicate wells with antigen minus the mean counts per minute for triplicate wells with medium alone. In these experiments, the latter was <500 cpm. The proliferative response to ConA was 100,000 to 150,000 cpm.
Cytokine assays.
Cells were cultured as described above. After 48 h, culture supernatants were collected and stored at −20°C with later analysis of cytokines by capture ELISA. IFN-γ was detected with monoclonal antibodies R46A2 and biotinylated XMG 1.2 (Endogen, Woburn, Mass.) and a recombinant murine IFN-γ (rmIFN-γ) standard (5.08 × 106 U/mg; Genzyme, Cambridge, Mass.); the limit of detection was 0.4 U/ml. Interleukin-4 (IL-4) was detected with monoclonal antibodies 11B11 and biotinylated BVDG-24G2 (kindly donated by P. Hodgkin, Centenary Institute) and an mrIL-4 standard (PeproTech, Rocky Hill, N.J.); the limit of detection was 0.3 U/ml.
Recombinant vaccinia virus.
Recombinant vaccinia virus expressing MPT64 (VV-64) was generated by inserting the MPT64 gene into the plasmid pBCB06 followed by homologous recombination into wild-type vaccinia virus. The recombinant virus was purified through three rounds of plaque purification under selective conditions in the human osteosarcoma cell line 143. Expression of the mycobacterial gene was confirmed by Western blot analysis (11a). Plasmid pBCB06 and a control recombinant vaccinia virus expressing influenza virus A/PR8/34 hemagglutinin (VV-HA) were kind gifts of David Boyle, CSIRO, Geelong, Australia.
Cytotoxic T-cell (CTL) response.
Following DNA immunization, splenocytes were restimulated with VV-64-infected syngeneic splenocytes for 6 days. The targets, EL-4 (H-2b) and P815 (H-2d), were infected with VV-64 at a multiplicity of infection of 10:1 for 1 hour, after which they were cultured overnight in complete RPMI. They were then labelled with 100 μCi of 51Cr (NEN Life Sciences) for 1.5 h at 37°C and subsequently incubated with effectors at various ratios. After 4 h of incubation, the supernatant was collected and the radioactivity was counted. The total release was determined by Triton X-100 lysis of labelled target cells. The percentage of specific lysis was calculated as follows: (experimental − spontaneous release)/(total − spontaneous release) × 100. Spontaneous release was less than 10%.
ELIspot for cytokine-producing cells.
To quantify IFN-γ-secreting cells, as described previously (20), spleens were removed from DNA-85B-immunized and M. tuberculosis-infected mice. Splenic mononuclear cells were purified by centrifugation on Histopaque-1083 (ρ = 1.083; Sigma). The cells were added to 96-well plates (4 × 105/well) and incubated with Ag85B (5 μg/ml), purified protein derivative (PPD; 1 μg/ml; Statens Serum Institut, Copenhagen, Denmark), ConA (1 μg/ml), or medium alone. The plates were incubated for 48 h at 37°C in an atmosphere of 5% CO2. The cells were then collected and counted. The nitrocellulose wells of an Immobilon-P plate (Millipore, Bedford, Mass.) were individually coated with 100 μl of the anti-IFN-γ monoclonal antibody R46A2 (10 μg/ml; Endogen). After the coated wells were washed and then blocked with PBS containing 2% fetal calf serum, 2.4 × 104 cells were added per well, and then the plates were incubated at 37°C in an atmosphere of 5% CO2 overnight. The cells were removed, and the plate wells were washed with 0.5% Tween 20 in PBS prior to addition of 100 μl of biotinylated anti-IFN-γ monoclonal antibody XMG 1.2 (1 μg/ml; Endogen) to each well for 2 h. After being washed, the plate was incubated with the avidin-alkaline phosphatase conjugate (Sigma) diluted 1:1,000 in PBS containing 1% bovine serum albumin for 1 h. The presence of IFN-γ-producing cells were visualized by using an AP Conjugate Substrate Kit (Bio-Rad, Hercules, Calif.). Spot-forming cells were counted under 5× magnification.
RESULTS
DNA vaccines induced production of antibodies.
Antigen-specific antibodies were detected 2 weeks after the first immunization (data not shown). Increasing titers of specific IgG were generated over the course of immunization with either DNA-64 or DNA-85B, with titers of log10 (4.98 ± 0.20) and log10 (4.81 ± 0.21), respectively, at 10 weeks. There was no response in mice immunized with the control vector.
DNA vaccines generated T-helper response and cytokine production.
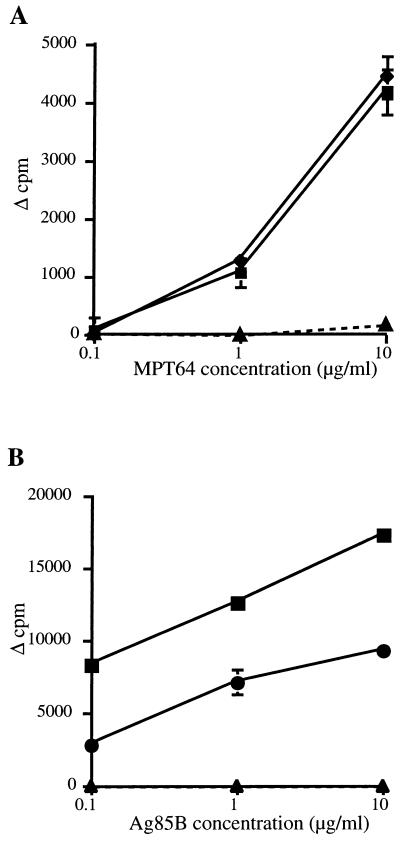
The proliferative and cytokine responses of nylon wool-purified T cells from spleens and lymph nodes were investigated after DNA immunization. Splenocytes from mice immunized with DNA-64 proliferated in response to MPT64, as did cells from DNA-85B-immunized mice in response to Ag85B (Fig. 1). Similar results were observed for the lymph nodes (data not shown). IFN-γ was detected in the culture supernatants of lymphocytes from mice immunized with DNA-64 or DNA-85B but not the control vector (Table 1). No IL-4 was detected in the supernatants of T cells derived from DNA-64-, DNA-85B-, or control vector-immunized mice (data not shown). Immunization with DNA-64 and DNA-85B together did not affect the recall proliferative response to either antigen when compared with splenocytes from mice immunized with either DNA-64 or DNA-85B (Fig. 1). There was no response to Ag85B by lymphocytes from DNA-64-immunized mice, and there was no response to MTP64 by lymphocytes derived from DNA-85B mice (data not shown). In addition, lymphocytes from coimmunized mice secreted levels of IFN-γ similar to those secreted by mice immunized with DNA-64 or DNA-85B (Table 1). Therefore, coimmunization with two mycobacterial-protein-expressing vectors generated an immune response to the individual antigen similar to that elicited by a single DNA vaccine.
FIG. 1.
Proliferative responses of pooled spleen-derived lymphocytes from five mice immunized intramuscularly three times, at 3-week intervals, with 100 μg of DNA-64 (⧫), DNA-85B (●), both DNA-64 and DNA-85B (■), or the control vector (▴). Two weeks after the final immunization, the incorporation of [3H]thymidine in response to MPT64 (A) or Ag85B (B) was measured as described in Materials and Methods. The error bars indicate the standard errors of the means.
TABLE 1.
IFN-γ production by lymphocytes of mice immunized with vector DNA-64 and/or DNA-85B
| Immunogena | Mean IFN-γ production ± SEMb (U/ml) after immunization with antigen:
|
|
|---|---|---|
| MPT64 | Ag85B | |
| DNA-64 | 53.3 ± 3.1 | NDc |
| DNA-85B | ND | 34.0 ± 2.0 |
| DNA-64 plus DNA-85B | 36.1 ± 4.0 | 43.1 ± 4.8 |
| Control vector | 3.1 ± 2.2 | 2.8 ± 1.3 |
Mice were immunized with 100 μg of DNA vaccine intramuscularly three times at 3-week intervals. Two weeks later, cytokine production was analyzed.
Production of IFN-γ by spleen-derived lymphocytes from mice immunized i.m. with 10 μg of antigen/ml was measured in triplicate by ELISA.
ND, not done.
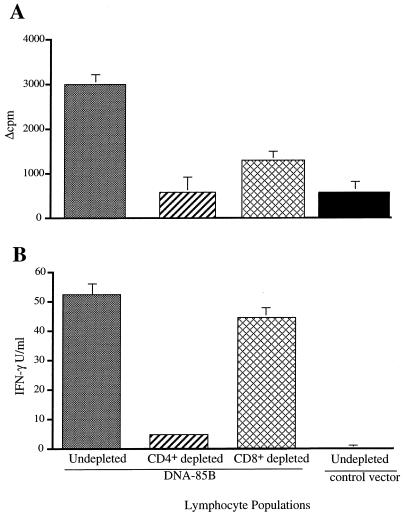
To characterize the contributions of CD4+ and CD8+ T cells to the immune response, these subsets were depleted from the purified T-cell populations by magnetic-bead depletion prior to stimulation. Removal of CD4+ T cells completely abrogated the lymphocyte proliferative response to the antigen (Fig. 2A), while there was a partial reduction in the proliferative response of lymphocytes depleted of CD8+ T cells. Depletion of the CD4+ T cells, but not the CD8+ T cells, blocked the antigen-specific production of IFN-γ (Fig. 2B).
FIG. 2.
The contribution of T-cell subsets to the DNA-induced immune response. Pooled spleen-derived lymphocytes from five mice immunized with DNA-85B (100 μg, intramuscularly) were depleted of CD4+ and CD8+ cells by using magnetic beads as described in Materials and Methods. The results indicate the proliferative response (A) and IFN-γ production (B) of undepleted cells and subsets and of undepleted cells from mice immunized with the control vector in a recall response to Ag85B (10 μg/ml). Error bars indicate standard errors of the means.
DNA vaccines activated CTL.
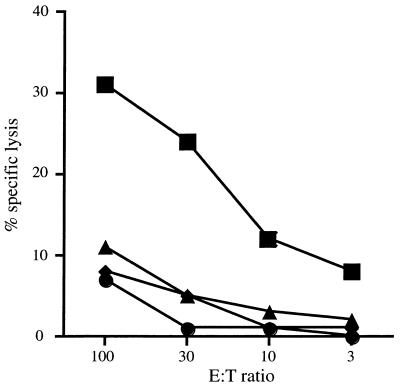
To investigate the generation of CTL, splenocytes from DNA-64-immunized mice were restimulated with syngeneic splenocytes infected with a vaccinia virus expressing MPT64. An antigen-specific cytotoxic effect was observed, since the effector cells from immunized mice lysed only EL-4 cells expressing MPT64 (Fig. 3) and not EL-4 cells alone or EL-4 cells infected with a vaccinia virus expressing an irrelevant protein, influenza virus hemagglutinin. Since EL-4 cells do not express major histocompatibility complex (MHC) class II antigens (23), and VV-64-infected P815 cells (H-2d) were not susceptible to lysis, cytotoxicity was MHC class I restricted.
FIG. 3.
Generation of CTL by DNA vaccination. Two weeks after the final immunization, splenocytes from mice immunized with 100 μg of DNA-64 twice, 3 weeks apart, were stimulated for 6 days with EL-4 cells infected with VV-64. A standard 51Cr release assay was used to measure target lysis. These effector cells were incubated with EL-4 (H-2b) cells alone (●), EL-4 cells infected with VV-64 (■) or VV-HA (⧫), or VV-64-infected P815 (H-2d) cells (▴). E, effector, T, target.
DNA vaccines encoding secreted and nonsecreted forms of MPT64 generated similar lymphocyte responses.
To test the effect of secretion of the mycobacterial protein on antigen expression and presentation, two versions of the DNA vaccine encoding the gene for MPT64 were constructed. Immunization with DNA-64sec, in which mpt64 is in frame with the tPA signal sequence, stimulated a mean MPT-64-specific proliferative response ± standard error of the mean of 4,410 ± 883 cpm, while DNA-64 stimulated a response of 4,365 ± 915 cpm. There was also no difference in the titers of specific antibodies (data not shown), indicating that the presence of the signal sequence had no effect on the immunogenicity of the antigen.
Immune response to DNA vaccines during M. tuberculosis infection.
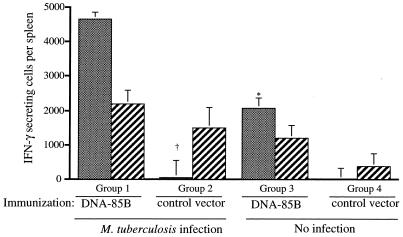
The effect of immunization on the immune response during M. tuberculosis infection was examined. After a single immunization with DNA-85B, mice were infected by aerosol with approximately 100 CFU of M. tuberculosis H37Rv. Following inoculation of this dose, infection develops in both the lung and the spleen 2.5 weeks postchallenge and then reaches its height at week 4 (11a). Therefore, the systemic immune response was analyzed early in the course of infection (at 17 days) and just after the peak of infection (at 5 weeks). Following in vitro stimulation of splenic lymphocytes with Ag85B, IFN-γ-secreting cells were observed in immunized mice (Fig. 4). The combination of DNA immunization and M. tuberculosis infection resulted in over a twofold increase in cytokine-producing cells at day 17 compared to the number resulting in uninfected mice which had been immunized with DNA-85B at the same time. In mice infected following immunization with the control vector, there were 83-fold fewer IFN-γ-producing cells early in infection. By week 5, there were similar numbers of IFN-γ-producing cells present in all infected mice, regardless of their immunization status. Infection with M. tuberculosis also induced IFN-γ-producing cells in response to PPD (data not shown). Therefore, DNA immunization had primed for a higher frequency of antigen-specific IFN-γ-secreting cells earlier in the course of M. tuberculosis infection.
FIG. 4.
Immunization with DNA vaccines increases the frequency of antigen-specific IFN-γ-secreting cells early in infection. Ten mice were immunized with DNA-85B or the control vector (100 μg, intramuscularly). Four weeks later, half of each treatment group (five/group) was then challenged with aerosol M. tuberculosis H37Rv (100 CFU). All groups were analyzed at the same time point. Early in infection (day 17 [gray bars]) and just after the peak of infection (week 5 [hatched bars]), the numbers of splenocytes secreting IFN-γ in response to Ag85B were measured by ELIspot as described in Materials and Methods. The levels of significance of the differences between groups at day 17 were calculated by Student’s t test. The level of significance of the difference between group 1 and group 2 was P < 0.001 (†), and that between group 1 and group 3 was P < 0.005 (∗). Error bars indicate the standard errors of the means.
Protection against M. tuberculosis challenge delivered by aerosol.
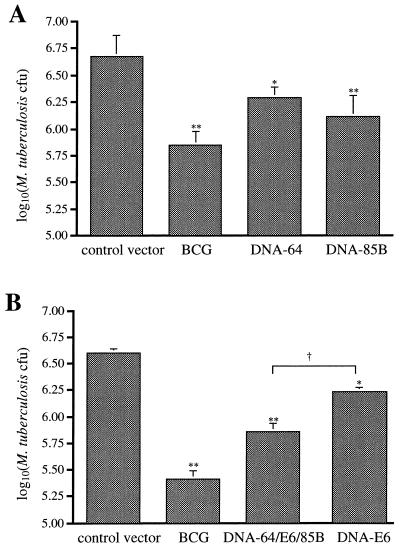
The ability of DNA vaccines to protect against M. tuberculosis H37Rv was analyzed by challenging with an aerosol containing the bacterium. Mice were immunized with DNA-64, DNA-85B, DNA-E6, combinations of these vectors, or the control vector and then challenged with M. tuberculosis 4 weeks later. Control mice were immunized with BCG subcutaneously 3 months prior to challenge. Four weeks after infection via aerosol, the extent of bacterial growth was determined. Immunization with DNA-85B (Fig. 5A) or DNA-E6 (Fig. 5B) achieved a consistent and significant level of protection against M. tuberculosis infection in the lung. DNA-64 immunization resulted in a more variable level of protection, the maximum level achieved being 0.38 log10 CFU. To investigate the effect of combining antimycobacterial DNA vaccines, mice were immunized with all three vectors. Combined immunization resulted in strong protection against M. tuberculosis challenge, significantly more than that stimulated with DNA-E6 alone. Mice immunized with BCG demonstrated a greater level of protection than that achieved with DNA vaccination, singly or combined. However, immunization with more than one antigen had significantly increased the protective effect compared to that provided by a single antigen.
FIG. 5.
Protection provided by DNA vaccines. Mice (five/group) were immunized with DNA-85B or DNA-64 (A) or with DNA-E6 or DNA-64 plus DNA-85B plus DNA E6 (B) three times, at 3-week intervals. As a positive control, some mice were given a subcutaneous immunization of BCG (104 CFU), and the empty vector was used as a negative control. Four weeks after the last immunization, mice were aerogenically challenged with M. tuberculosis H37Rv (100 CFU). Four weeks postinfection, the bacteria in the lung were enumerated as described in Materials and Methods. The levels of statistical significance for differences between test groups and the control vector were determined by the Mann-Whitney test (∗, P < 0.05 [A] or P < 0.02 [B]; ∗∗, P < 0.01 [A] or P < 0.01 [B]). The level of significance of the difference between the combination vaccination and DNA-E6 was P < 0.01 (†). Error bars indicate standard errors of the means.
DISCUSSION
The recognition that immunization with M. bovis BCG has a variable impact on the transmission of M. tuberculosis has renewed interest in developing more-effective vaccines against TB. This study demonstrates that DNA vaccines against M. tuberculosis generate antimycobacterial immunity and reduce the level of pulmonary infection following challenge with M. tuberculosis via aerosol 4 weeks postimmunization. We showed that there was a hierarchy in efficacy, with the vaccine expressing the 30-kDa protein DNA-85B being more effective than those expressing ESAT-6 or MPT64. A combination of the three vaccines achieved a reduction in the pulmonary bacterial load of 0.75 logs, which was greater than that observed with DNA-85B or DNA-E6 alone but less than that observed with BCG.
Protective immunity against M. tuberculosis is dependent on the recruitment of antigen-specific T cells, principally CD4+, to the lung and the release of cytokines, particularly IFN-γ, for the activation of macrophage-killing mechanisms (12). The DNA vaccines stimulated strong antigen-specific T-cell responses, with a preferential release of IFN-γ and not IL-4 (Fig. 1 and Table 1). Cell depletion studies confirmed that CD4+ T cells were the major source of IFN-γ (Fig. 2). Using DNA-64 as a model, we demonstrated that the DNA vaccine immunization stimulated MHC class I-restricted CD8+ T cells (Fig. 3). Although mycobacterium-specific CD8+ T cells are stimulated in humans (20) and mice (25) by infection and by DNA immunization (18, 35, 37), their contribution to protective immunity remains unclear.
There were differences in the levels of protection stimulated by the DNA vaccines expressing the three different secreted proteins, and this has important implications with regard to choosing antigens for the next generation of vaccines. DNA-85B consistently induced the highest level of protection, while immunization with DNA-E6 was more effective than immunization with DNA-64. Huygen and coworkers (18) demonstrated that immunization with a DNA vaccine containing the 32-kDa 85A protein, another member of the antigen 85 complex, stimulated protective immunity against BCG and M. tuberculosis infections. These proteins exhibit 72.8% amino acid identity (6), and about 60% of TB patients have T-cell responses to antigen 85B or 85A (21, 29). Further, immunization with the 85B protein in an adjuvant gave partial protection against TB in guinea pigs (17). Therefore, at least one member of the antigen 85 complex should be included in any future subunit vaccine against TB.
DNA vaccines expressing other mycobacterial proteins have also been effective. Either a DNA vaccine or transfected macrophage cell lines expressing the Mycobacterium leprae 65-kDa heat shock protein (HSP65) stimulated partial protection against systemic M. tuberculosis infection (35). Plasmid vectors expressing the 38-kDa phosphate transport protein induced a comparable level of protection (37). However, not all anti-TB DNA vaccines have been effective; a vaccine containing the gene for the 19-kDa lipoprotein stimulated a nonprotective antibody response rather than a T-cell response against the protein (11).
The protective effect of DNA-85B was associated with a systemic expansion of Ag85B-specific IFN-γ-secreting cells early in the course of infection, at day 17, which exceeded that in immunized but noninfected mice (Fig. 4). By contrast, mice immunized with the control vector failed to mount an Ag85B IFN-γ response until later in the course of the infection. This prompt recall response to a protein secreted early in the course of infection may have contributed to the protective effect. A similar early expansion of IFN-γ-secreting cells was observed in DNA-85B-immunized mice infected intravenously with M. bovis BCG (data not shown).
The addition of the tPA signal sequence upstream of the mpt64 gene did not enhance the T-cell proliferative or antibody response following immunization. A similar pattern was seen with DNA plasmids encoding the immunodominant 35-kDa protein of M. leprae (21a). There has been conflicting evidence of the value of including secretory signal sequences in DNA vaccines. This approach did not enhance the response to DNA vectors encoding some antigens (13–15), or any beneficial effect was lost after more than one immunization (22). By comparison, expression of ovalbumin as a secreted protein enhanced the immunogenicity of a DNA vector (8). These differences may be due to the inherent immunogenicity of the foreign protein or to differences in the backbone of the DNA vaccine.
A significant level of protection was observed with these DNA vaccines when mice were challenged with an aerosol of M. tuberculosis 4 weeks after the last immunization. With the identification of effective candidate mycobacterial antigens, the induction of long-lived memory T cells by combined DNA vaccines and the impact on bacillary dissemination to other organs should be assessed in the future; however, protection was still apparent in the lung when challenge by aerosol occurred 10 weeks after immunization with the DNA vaccine expressing Ag85A (18). In the case of these and other DNA vaccines, the level of protection was less than that induced by BCG immunization. Therefore, strategies for increasing the effectiveness of DNA vaccines are required. It is unlikely that vaccines based on single antigens will confer protection against M. tuberculosis in a human population. One concern with the codelivery of multiple DNA plasmids is the possible effect of antigenic competition. In fact, this did not occur in mice coimmunized with DNA-64 and DNA-85B, which developed strong T-cell responses to each protein (Fig. 1). Studies of malaria in rodent models have also indicated that combining DNA vaccines does not affect the immune response to individual antigens (14), allowing subunit vaccines to overcome genetic restriction of single proteins (10, 16). The combination of the three vectors had a greater protective efficacy than DNA-85B or DNA-ESAT6 alone (Fig. 5B).
Other approaches to increasing the efficacy of DNA vaccines include coimmunization with vectors expressing cytokines, such as granulocyte-macrophage colony-stimulating factor IFN-γ, or IL-12 (24, 36), or the use of costimulatory molecules (7, 19). Alternatively, it may be possible to manipulate the DNA backbone, to increase its adjuvant effects, by the addition of multiple immunostimulatory sequences (33). Finally, a combination of DNA and oral vaccines expressing the same candidate antigen may increase the protective response to complex parasitic organisms (34). A combination of these approaches may be necessary to obtain clinically significant long-lived protective efficacy for DNA vaccines in humans.
ACKNOWLEDGMENTS
This work was supported financially by the National Health and Medical Research Council of Australia and the Immunology of Mycobacteria (IMMMYC) Program of the World Health Organization. Arun Kamath is a recipient of an Australian Post-Graduate Research Award.
We are grateful to A. Bean and J. Triccas for helpful discussions.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Gottschau A, Bennedsen J, Nagai S, Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol. 1992;36:823–831. doi: 10.1111/j.1365-3083.1992.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 4.Bloom B R, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 5.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borremans M, De Wit L, Volckaert G, Ooms J, De Bruyn J, Huygen K, Van Vooren J-P, Stelandre M, Verhofstadt R, Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989;57:3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle J S, Brady J L, Lew A M. Enhanced responses to a DNA vaccine encoding a fusion antigen that is directed to sites of immune induction. Nature. 1998;392:408–411. doi: 10.1038/32932. [DOI] [PubMed] [Google Scholar]
- 8.Boyle J S, Silva A, Brady J L, Lew A M. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc Natl Acad Sci USA. 1997;94:14626–14631. doi: 10.1073/pnas.94.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colditz G A, Brewer T F, Berkley C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 10.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erb K J, Kirman J, Woodfield L, Wilson T, Collins D M, Watson J D, Legros G. Identification of potential CD8+ T-cell epitopes of the 19 kDa and ahpc proteins from Mycobacterium tuberculosis—no evidence for CD8+ T-cell priming against the identified peptides after DNA-vaccination of mice. Vaccine. 1998;16:692–697. doi: 10.1016/s0264-410x(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 11a.Feng, C. G. Unpublished data.
- 12.Flynn J-A L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad D, Liljeqvist S, Stahl S, Andersson I, Perlmann P, Berzins K, Ahlborg N. Comparative study of DNA-based immunization vectors: effect of secretion signals on the antibody responses in mice. FEMS Immunol Med Microbiol. 1997;18:193–202. doi: 10.1111/j.1574-695X.1997.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 14.Hedstrom R C, Doolan D L, Wang R, Gardner M J, Kumar A, Sedegah M, Gramzinski R A, Sacci J B, Jr, Charoenvit Y, Weiss W R, Margalith M, Norman J A, Hobart P, Hoffman S L. The development of a multivalent DNA vaccine for malaria. Springer Semin Immunopathol. 1997;19:147–159. doi: 10.1007/BF00870265. [DOI] [PubMed] [Google Scholar]
- 15.Hedstrom R C, Doolan D L, Wang R B, Kumar A, Sacci J B, Gardner M J, Aguiar J C, Charoenvit Y, Sedegah M, Tine J A, Margalith M, Hobart P, Hoffman S L. In vitro expression and in vivo immunogenicity of Plasmodium falciparum pre-erythrocytic stage DNA vaccines. Int J Mol Med. 1998;2:29–38. doi: 10.3892/ijmm.2.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman S L, Doolan D L, Sedegah M, Aguiar J C, Wang R, Malik A, Gramzinski R A, Weiss W R, Hobart P, Norman J A, Margalith M, Hedstrom R C. Strategy for development of a pre-erythrocytic Plasmodium falciparum DNA vaccine for human use. Vaccine. 1997;15:842–845. doi: 10.1016/s0264-410x(96)00273-3. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz M A, Lee B-W E, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki A, Stiernholm B J, Chan A K, Berinstein N L, Barber B H. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 20.Lalvani A, Brookes R, Wilkinson R J, Malin A S, Pathan A A, Andersen P, Dockrell H, Pasvol G, Hill A V. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Launois P, DeLeys R, Niang M N, Drowart A, Andrien M, Dierckx P, Cartel J-L, Sarthou J-L, Van Vooren J-P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Martin, E. Unpublished data.
- 22.Montgomery D L, Huygen K, Yawman A M, Deck R R, DeWitt C M, Content J, Liu M A, Ulmer J B. Induction of humoral and cellular immune responses by vaccination with M. tuberculosis antigen 85 DNA. Cell Mol Biol. 1997;43:285–292. [PubMed] [Google Scholar]
- 23.Moore M W, Carbone F R, Bevan M J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 24.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 25.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 26.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 27.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 28.Roche P W, Feng C G, Britton W J. Human T-cell epitopes on the Mycobacterium tuberculosis secreted protein MPT64. Scand J Immunol. 1996;43:662–670. doi: 10.1046/j.1365-3083.1996.d01-260.x. [DOI] [PubMed] [Google Scholar]
- 29.Roche P W, Peake P W, Billman-Jacobe H, Doran T, Britton W J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche P W, Triccas J A, Avery D T, Fifis T, Billman-Jacobe H, Britton W J. Differential T-cell responses to mycobacterial secreted proteins distinguish between vaccination with bacille Calmette Guerin (BCG) and infection with Mycobacterium tuberculosis. J Infect Dis. 1994;170:1326–1330. doi: 10.1093/infdis/170.5.1326. [DOI] [PubMed] [Google Scholar]
- 31.Roche P W, Winter N, Triccas J A, Feng C G, Britton W J. Expression of Mycobacterium tuberculosis MPT64 in recombinant Myco. smegmatis: purification, immunogenicity and application to skin tests for tuberculosis. Clin Exp Immunol. 1996;103:226–232. doi: 10.1046/j.1365-2249.1996.d01-613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 34.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V S. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 35.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 36.Xiang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]