Abstract
Aim
To explore whether combined interventions i.e. psychotherapeutic plus psychosocial interventions are more effective than monotherapies in the treatment of alcohol use disorders.
Methods
Systematic review of the results of randomized controlled trials that compared combined therapies with monotherapies (either pharmacotherapy or psychotherapy).
Results
The search resulted in 28 eligible studies. Data from these RCTs showed that 10 out of 19 RCTs (53%) demonstrated an added value of combined therapy (psychotherapy + pharmacotherapy) compared to psychotherapy only, whereas only three out of nine RCTs (33%) comparing combined therapy with pharmacotherapy showed a possible added value for combined therapy.
Conclusions
Pharmacotherapy is effective to treat AUD with or without psychotherapy and that psychotherapy can best be offered in combination with pharmacotherapy.
Short Summary: This systematic review shows that 10 out of 19 RCTs (53%) demonstrated an added value of combined therapy for alcohol use disorders (psychotherapy + pharmacotherapy) compared to psychotherapy only, whereas only three out of nine RCTs (33%) showed a possible added value for combined therapy compared to pharmacotherapy.
INTRODUCTION
Worldwide, the estimated last year prevalence of alcohol use disorders (AUDs) is 8.5%, with an estimated lifetime prevalence of 20% (Slade et al., 2016). In the US, the estimated last year and life-time prevalence of AUDs was 13.9 and 29.1% (Grant et al., 2015), and is increasing (Grant et al., 2017). AUDs are associated with high societal and health care costs and considerable social and financial burden (Bouchery et al., 2011; Sacks et al., 2015). AUDs are a significant cause of morbidity and mortality (Kendler et al., 2017), but remain underdiagnosed and undertreated (Mark et al., 2009; Grant et al., 2015). For instance, in 2018 in the USA, only 4.6% of people aged 12 or older with an AUD received specialized AUD treatment (SAMHSA, 2019). In the Netherlands, 10.3% of people aged 18 years or older received specialized AUD treatment (Tuithof et al., 2016). In 2015, 45% of the patients in specialized addiction treatment services has a primary AUD diagnosis (LADIS, 2016).
Psychotherapeutic or psychosocial AUD treatments, including cognitive-behavioral therapy (CTB), motivational enhancement therapy, and twelve-step programs (e.g. Alcoholics Anonymous) (Anton et al., 2006; Martin and Rehm, 2012; 1998) are effective and constitute the mainstay of AUD treatment worldwide (MATCH, 1998; Anton et al., 2006; Martin and Rehm, 2012; SAMHSA, 2014). Like psychotherapy, pharmacotherapy of AUD (e.g. disulfiram, acamprosate, nalmefene, naltrexone and topiramate) is known to be effective in improving alcohol consumption outcomes (prevention of relapse to any or heavy drinking) (Magill et al., 2019) and recommended by most clinical guidelines (Jonas et al., 2014; Donoghue et al., 2015). The American Psychiatric Association (APA) recommended to offer these medications to patients with moderate to severe alcohol use disorder (Reus et al., 2019). In meta-analyses, as well as other studies (Anton et al., 2006; Rösner et al., 2010a; Mann et al., 2013; Jonas et al., 2014; Akbar et al., 2018; Kranzler and Soyka, 2018), both acamprosate and naltrexone showed effectiveness in terms of return to any or heavy drinking or reduction in drinking days. Other effective agents to treat AUD were briefly reviewed recently (Kim et al., 2018). A more recent meta-analysis on interventions in primary care showed that only acamprosate was effective in maintaining abstinence among primary care patients with AUD for up to 12 months (Cheng et al., 2020).
The aim of psychotherapeutic interventions is to create and strengthen the mindset to reduce or to stop drinking, whereas pharmacotherapeutic agents are designed to inhibit the pathways of drinking induced pleasure and stimuli induced cue-reactivity and craving. As such, the combination of these two therapeutic approaches is believed to retain higher efficacy in the treatment of AUD than each approach separately. Despite this expectation, the added value of one therapeutic approach to the other has not been systematically evaluated. The objective of this review was not to assess how these two clinical interventions compare with each other, but whether the addition of a psychosocial intervention to medically prescribed pharmaceuticals improves treatment outcome in AUD and vice versa, i.e. whether prescribed pharmaceuticals have an added value to psychosocial interventions.
METHODS
Using the PRISMA-protocol, a systematic review was performed on 2 April 2022, to retrieve eligible ‘Clinical Trials’ or ‘Randomized Controlled Trials’, including published studies and studies ahead of print using PubMed, PsychInfo and EMBASE about the combined treatment (pharmacotherapy plus psychotherapy) of alcohol use disorder (AUD). For detailed search string and PRISMA checklist see Supplement.
After removal of duplicates, two authors (PB and RS) independently processed 454 publications to determine eligibility in two steps: (a) by screening the title and abstract and (b) by applying the inclusion- and exclusion criteria. Inclusion criteria: (a) clinical trials or randomized clinical trials testing the efficacy of psychotherapy as an add-on to pharmacotherapy for the treatment of AUD, and (b) clinical trials or randomized clinical trials testing the efficacy of pharmacotherapy as an add-on to psychotherapy for the treatment of AUD. Exclusion criteria: studies performed (a) in patients with a psychiatric diagnosis beyond alcohol dependence (i.e. double diagnosis), like depression, PTSD, bipolar disorder or cocaine dependence, (b) with samples smaller than 40 patients, (c) non-double blinded, (d) use of historical matched controls, (e) use of brief interventions, coping therapy, supportive therapy and (f) use of recommended by not-obligatory counselling i.e. no overall standardization of the psychotherapeutic intervention.
RESULTS
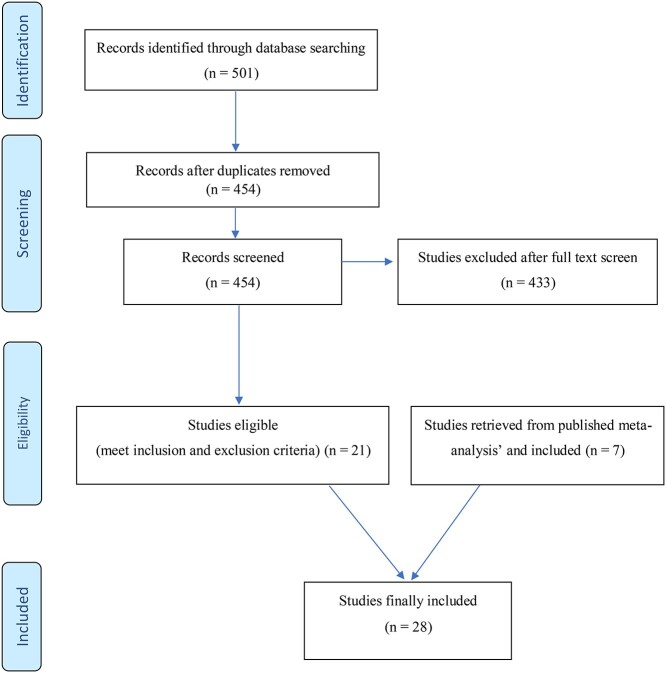
Based on the inclusion- and exclusion criteria, 21 studies were eligible for analysis. However, four publications reported on the same sample (Anton et al., 2006 and Donovan et al., 2008, as well as, Anton et al., 1999 and Anton et al., 2001) leaving 19 studies for analysis. Using the reference lists of five published meta-analyses and systematic reviews (Srisurapanont and Jarusuraisin, 2005; Roozen et al., 2006; Agosti et al., 2012; Jarosz et al., 2013; Gao et al., 2018; Ray et al., 2020), seven additional eligible studies were identified and included resulting in 28 studies for analysis. Fig. 1 shows the PRISMA flow chart for the identification, screening and inclusion of the studies.
Fig. 1.
PRISMA flow diagram.
Table 1 presents the results of the eligible studies on the added value of psychotherapy to pharmacotherapy: 9 studies with 590 AUD patients receiving monotherapy (medication) and 595 AUD patients receiving combined therapy (medication + psychotherapy). Three out of these nine studies (Balldin et al., 2003; Schaumberg et al., 2013; Berner et al., 2014) showed that the combination of psychotherapy and pharmacotherapy was more effective to prevent lapse to drinking than pharmacotherapy (monotherapy), whereas the other six studies showed no significant difference between the two treatment options. It should be noted, however, that the drop-out rate in the positive study of Berner et al. (2014) was very high (63% in both groups) raising doubt about the internal validity of this study. The other two studies showing a difference between the two treatments were performed in a specific group of AUD patients i.e. men having sex with men, which may not be representative for the AUD patients in general.
Table 1.
Added value of psychotherapy to pharmacotherapy in the treatment of alcohol-dependent patients (dose of naltrexone (NTX) was 50 mg per day, unless otherwise stated)
| No. | Monotherapy (pharmacotherapy) | Combined therapy (psychotherapy + pharmacotherapy) | Specifications of psychotherapy | Results | Ref. |
|---|---|---|---|---|---|
| 1 | Medical management (MM) + medication (NTX: n = 24; acamprosate 2 g/d, n = 13; placebo: n = 11). Ntot = 48 | CBT + medical management + medication (NTX: n = 22; acamprosate: 2 g/d, n = 18; placebo: n = 8). Ntot = 48 | CBT, 20 sessions over 4 month | NTX + CBT + MM more effective than NTX + MM: longer abstinence (hazard ratio: 0.54; 95% CI: 0.34–0.86, P = 0.01) | (Berner et al., 2014) |
| 2 | NTX (n = 31) | NTX + CBT (n = 25) | 9 sessions supportive psychotherapy (40–60 min) over 12 weeks. | NTX + CBT more effective than NTX. First time to relapse (F = 7.37, df = 3, P = 0.007 | (Balldin et al., 2003) |
| 3a | NTX (100 mg/d) + medical management (n = 51) | NTX (100 mg/d) + CBT + MI (n = 51) | 12 one-hour. sessions over 12 weeks. Self-identification as being sexually active with other men |
NTX + CBT + MI more effective than NTX + MM to induce confidence in continuing changes without further medication (rating 6.9 vs. 6.2) | (Schaumberg et al., 2013) |
| 4a | NTX (100 mg/d) (n = 51) | NTX (100 mg/d) + MI + CBT (n = 51) | 12 one-hour MI + CBT sessions over 12 weeks. Self-identification as being sexually active with other men | NTX + CBT + MI not more effective than NTX on number of heavy drinking days | (Morgenstern et al., 2012) |
| 5 | NTX (100 mg/d) (n = 39) | NTX (100 mg/d) + CBT (n = 40) | CBT 18 sessions over 24 weeks. | NTX + CBT not more effective than NTX to improve drinking outcomes (% abstinence, days of heavy drinking) | (Oslin et al., 2008) |
| 6 | NTX + supportive therapy (n = 26) | NTX + CBT (n = 30) | Supportive therapy to remain abstinent without specific coping skills. CBT: 10 sessions of 50 min. Weekly over 10 weeks. | NTX + CBT not more effective than NTX + supportive therapy to prolong abstinence | (O'Malley et al., 2003) |
| 7 | NTX (100 mg/d; n = 154) or acamprosate (3 g/d; n = 152) | NTX (100 mg/d) + CBI (n = 155) or acamprosate (3 g/d) + CBI (n = 151) | 20 CBI 50 min. Sessions over 16 weeks. | NTX + CBI not more effective than NTX to increase % days abstinent; acamprosate was not effective | (Anton et al., 2006; Donovan et al., 2008) |
| 8 | Acamprosate (20–30 mg/kg) (n = 78) | Acamprosate (20–30 mg/kg) + CBT (n = 82) | CBT (7 weekly sessions of 60 min) in week. 2–8. | Acamprosate + CBT not more effective than acamprosate on relapse or abstinence | (De Wildt et al., 2002) |
| 9 | Nefazodone + group counselling (GC, n = 50) | Nefazodone + CBT (n = 53) | 24 GC (nonspecific support without psychotherapeutic issues). CBT: 18 90-min. Sessions over 12 weeks. | Nefazodone + CBT not more effective than nefazodone + GC to prolong abstinence or to reduce relapse rate or number of relapses | (Wetzel et al., 2004) |
aProblem drinking in men who have sex with men; CBT: cognitive behavior therapy; MI: motivational interviewing; MET: motivational enhancement therapy; CBI: Combined Behavioral Intervention.
Table 2 presents the results of the eligible studies on the added value of pharmacotherapy to psychotherapy: 19 studies with 1241 AUD patients receiving monotherapy (psychotherapy) and 1653 AUD patients receiving combined therapy (psychotherapy + medication). The added value of combined therapy (psychotherapy + pharmacotherapy) to prevent relapse to alcohol use was demonstrated in 10 studies, whereas the remaining 9 RCTs failed to detect a significant difference between the two treatments of AUD.
Table 2.
Added value of pharmacotherapy to psychotherapy in the treatment of alcohol-dependent patients (dose of naltrexone (NTX) was 50 mg/day, unless otherwise stated)
| No. | Monotherapy (psychotherapy) | Combined therapy (psychotherapy + pharmacotherapy) | Specifications of psychotherapy | Results | Reference |
|---|---|---|---|---|---|
| 1 | Placebo + CBT (n = 63) | NTX + CBT (n = 68) | CBT: 12 weekly manual-guided sessions | NTX + CBT more effective than CBT to decrease rate to first relapse: Kaplan–Meier log-rank analysis, 3.90; df = 1; P = 0.048) (12% difference in final relapse rate) | (Anton et al., 1999) (Anton et al., 2001) |
| 2 | Placebo (25 μg/kg midazolam) + MET (n = 23) | Ketamine (0.71 mg/kg) + MET (n = 17) | MET: five weeks. Twice weekly spaced by 3–4 days; on 3 consecutive days in week. 2 | NTX + MET across the 21 days follow-up more effective than MET + active control on alcohol abstinent days: F = 8.21, df = 1, 797, P = 0.004 | (Dakwar et al., 2020) |
| 3 | CBT (n = 43) | NTX (50 mg/d) + CBT (n = 43) | 8 one-hour sessions over the 12 weeks. | NTX + CBT more effective than CBT: longer abstinence (P = 0.002) and more abstinent (P = 0.051) | (Feeney et al., 2004) |
| 4 | CBT (n = 59) | All groups: n = 59. CBT + acamprosate (1332–1998 mg/d); CBT + NTX (50 mg/d); CBT + combined (NTX + acamprosate in dose as above) | CBT: one hour weekly for the first four weeks. Followed by fortnightly for the subsequent eight weeks. | NTX + acamprosate + CBT more effective than CBT alone with a mean difference of 19.7, P = 0.034. Cumulative abstinence duration (days): acamprosate + CBT: 45.1; NTX + CBT: 50.0; NTX + acamprosate + CBT: 53.6 and CBT: 33.9 | (Feeney et al., 2006) |
| 5 | Placebo + CBT (n = 35) | Nalmefene (NAL; 20 or 80 mg/d for 12 weeks) + CTB (n = 70) | CBT: 45 min sessions, weekly for 12 weeks. | Nalmefene + CBT more effective than CBT to prevent relapse to heavy drinking: OR = 2.4; 95% CI: 10.5–5.59, P < 0.02 | (Mason et al., 1999) |
| 6 | Placebo + support (n = 99) | NTX (50 mg/d) + support (n = 93) | Support: weekly group support for relapse prevention and individual counselling | NTX + CBT more effective than support to prevent relapse: 18.8 vs. 7.9% (χ2 = 5.89, df = 2, P = 0.050) | (Guardia et al., 2002) |
| 7 | Placebo + CBT (n = 40) | All groups (n = 40) received CBT. Acamprosate (1998 mg/d), NTX (50 mg/d) or both | CBT: 90-min group sessions, weekly for 12 weeks. | NTX + CBT or acamprosate + CBT or NTX + acamprosate + CBT more effective than CBT to reduce relapse rate than individual support (P = 0.02) | (Kiefer et al., 2003) |
| 8 | Placebo + group support (n = 56) | NTX (50 mg/d) + Group support (n = 55) | 12 weekly 1.5 hour group sessions of psychological education and social support | NTX + CBT more effective to reduce relapse: 50 vs. 79% (P = 0.001), but no effect on the number of drinking days per week | (Morris et al., 2001) |
| 9 | Placebo + group counselling (n = 54) | NTX (50 mg/d) + group counselling (n = 45) | Group counselling related to alcohol dependence (2x weekly for 11 month) | NTX + CBT more effective than group counselling to prevent relapse: 23 vs. 54%, P < 0.01) | (Volpicelli et al., 1992) |
| 10 | Placebo + individual counselling (n = 48) | NTX (50 mg/d) + individual counselling for 12 weeks. (n = 49) | Individual relapse prevention counselling (first month: 2x weekly, then once weekly for 12 weeks. | NTX + individual counselling more effective than individual counselling to reduce drinking days (2.8 vs. 11.0, P = 0.01) and to prevent relapse (14 vs. 52%, P = 0.002) | (Volpicelli et al., 1997) |
| 11 | Placebo + intensive counselling (CBI) (n = 156) | NTX (100 mg/d) + CBI (n = 155) or acamprosate (3 g/d) + CBI (n = 151) | CBI: 20 50 min. Sessions over 16 weeks. | NTX + CBI not more effective than CBI to prolong abstinence (Cohen d: 0.07; 95% CI: −0.11-0.25 vs. Cohen d: 0.17; 95% CI: −0.02 to 0.35, respectively, P = 0.009) | (Anton et al., 2006) |
| 12 | Placebo (i.m.) + HaRT-A2 (n = 78) + behavioral treatment | HaRT-A + NTX (380 mg i.m. extended-release) (n = 74) | Five sessions at baseline (week 0) and in weeks. 1, 4, 8 and 12 | NTX + HaRT-A + behavioral treatment not more effective than HaRT-A + behavioral treatment on self-reported drinking during 24 weeks. Follow-up (quantity and frequency) | (Collins et al., 2021) |
| 13 | Placebo + CET + CST (n = 128) | NTX + CET + CST (50 mg/d; n = 165) for 12 weeks. | CET + CST: two weeks training in coping and communication skills | NTX + CET + CST at 12-month follow-up not more effective than CET + CST to reduce % heavy drinking days, nor to increase % relapse | (Monti et al., 2001) |
| 14 | Placebo + psychosocial treatment n = 87) |
NTX (50 mg/d) + psychosocial treatment (Psy-Tr; n = 84) | Weekly one-hour sessions of psychosocial alcohol treatment program | NTX + Psy-Tr not more effective than Psy-Tr to increase abstinence rate at week 12: NTX + Psy-Tr (54%) vs. Psy-Tr (51%) | (Gastpar et al., 2002) |
| 15 | Placebo + individual support (n = 20) | NTX (50 mg/d) + individual support (n = 20) | Weekly 30-min sessions individual psychotherapy for abstinence and compliance enhancement for 12 weeks. | NTX + individual support not more effective than individual support to reduce relapse rates (P = 0.67) | (Huang et al., 2005) |
| 16 | Placebo + CBT (n = 32) | NTX + CBT (n = 31) | CBT: 21 days of dependency treatment | NTX + CBT not more effective than CBT to reduce craving nor recidivism after treatment | (Knox and Donovan, 1999) |
| 17 | Placebo + CBT (n = 63) | NTX + CBT (50 mg/d; n = 61) or nefazodone + CBT (400 mg/d; n = 59) | CBT: 12 weekly sessions | NTX + CBT not more effective than CBT to prevent relapse to heavy drinking or to reduce drinking days | (Kranzler et al., 2000) |
| 18 | Placebo + CBT-based counselling (n = 32) | Baclofen (50 mg/d) for 12 weeks. n = 32 | Weekly support CBT-based counselling; motivational interviewing, education and therapy | Baclofen + CBT-based counselling not more effective than CBT-based counselling to prevent relapse to heavy drinking nor to increase abstinent days at 52 weeks. Follow-up | (Ponizovsky et al., 2015) |
| 19 | Placebo + IBT (n = 125) | Acamprosate (2 g/d) + IBT (n = 124) | 24 IBT (integrative behavior therapy) 30-min sessions for 6 month | Acamprosate + IBT not more effective than IBT: rate of abstinence at 6-month follow-up (47.6 and 48.0%, respectively) | (Wölwer et al., 2011) |
aCounselling sessions providing information about alcohol use and abuse, and the consequences of alcohol dependence; 2 HaRT-A: behavioral treatment, consisting of low-intensity not requiring abstinence; CBT: Cognitive Behavior Therapy; CBI: Combined Behavioral Intervention; IBT: integrative behavior therapy (relapse prevention, social skill trainings, and motivational and cognitive methods).
DISCUSSION
The main result of the present systematic review is that 10 out of 19 RCTs (52.6%) about treatment of patients with AUD demonstrated an added value of combined therapy (psychotherapy + pharmacotherapy) compared to psychotherapy alone, whereas only three out of nine (33.3%) studies showed added value of combined therapy compared to pharmacotherapy alone.
The use of combinations of behavioral and pharmacological approaches in the treatment of alcohol dependence may theoretically have significant advantages over monotherapies, because they can allow dose-reduction and provide additive (or even synergistic) effects on efficacy (Hosking et al., 2005). The aim of the present study was therefore to assess the added value of pharmacotherapy to cognitive behavior therapy and vice versa in the treatment of alcohol use disorders. Typically, meta-analytic reviews in the AUD-literature have been conducted on groups of different pharmacotherapies, on some specific pharmacotherapy or on (specific) behavioral interventions. However, much less is known about the empirical added value of combined therapies over single interventions. In a systematic review and meta-analysis with 30 RCTs in SUD patients, Ray et al. (2020) concluded that in SUD patients the efficacy of combined treatment i.e. pharmacotherapy plus CBT (or other specific psychotherapies), is superior compared to pharmacotherapy (plus TAU i.e. plus treatment as usual). However, for several reasons, the results obtained by Ray et al. (2020) are not comparable with the currently presented results: first, only 15 of the 30 RCTs were conducted in AUD patients and second, only eight studies in their meta-analysis, evaluating the added value of combined therapy (CBT + Pharmacotherapy) vs. Pharmacotherapy (+ TAU) referred to AUD patients (O'Malley et al., 1992; Carroll et al., 1994; Carroll et al., 1998; Schmitz et al., 2001; Balldin et al., 2003; O'Malley et al., 2003; Schmitz et al., 2004; Wetzel et al., 2004). Except for the quantity outcome in the study by Schmitz et al. (2004), none of these studies showed a significant added value of CBT to pharmacotherapy. However, this study was excluded from our systematic review because the subjects included in this study were dependent on both cocaine and alcohol. The meta-analysis, however showed a small, but significant added effect of CBT (g = 0.18 on frequency outcomes and g = 0.28 on quantity outcomes). Interestingly, a meta-analysis comparing CBT + TAU + Pharmacotherapy vs. Pharmacotherapy + TAU (13 studies) showed no significant added value of combined therapy vs. pharmacotherapy. We, therefore, conclude that the conclusion drawn by Ray et al. (2020) that adding CBT to pharmacotherapy (combined therapy) has an added benefit compared with pharmacotherapy (alone or with TAU) is rather questionable for AUD patients. This complies with our finding that only three of the nine studies showed some indication for an added value of combined therapy compared to pharmacotherapy alone. Due to large differences in study design with respect to medications (doses), psychotherapies (types and number of sessions), motivation to stop drinking, and the number of patients per study, we concluded that it was not feasible to perform a valid meta-analysis. This renders our findings and conclusions somewhat less robust, but the overall conclusion that pharmacotherapy is effective to treat AUD with or without psychotherapy and that psychotherapy can best be offered in combination with pharmacotherapy is not jeopardized.
The current findings collectively suggest that best practices in addiction treatment should include pharmacotherapy with TAU and if not effective or requested by the patient pharmacotherapy plus CBT or another evidence-based psychotherapy, rather than TAU or nonspecific counselling services. These findings corroborate with previous data showing a lower relapse rate following psychosocial intervention in combination with pharmacotherapy as compared to psychosocial intervention alone (Irvin et al., 1999; Anton et al., 2006). The efficacy of the different evidence-based psychosocial interventions, such as social behavior, network therapy, CBT and MET appears to be grossly similar (Srisurapanont and Jarusuraisin, 2005; Assanangkornchai and Srisurapanont, 2007).
Obviously, the sample characteristics and methodological features of AUD studies show a large variety, and all of these may moderate the treatment outcome and explain the differences found among the studies. Using the dataset of the COMBINE Study, one of the largest studies using combined treatment of AUD, various clinical and demographic factors i.e. moderators of treatment success could be identified. For instance, treatment-seeking predicted beneficial treatment outcomes (Ray et al., 2017). Behavioral markers of alcohol-induced stimulation, sedation and craving may also affect clinical trial outcomes (Ray et al., 2021) which may explain—at least for those highly susceptible to craving—the clinical benefit of naltrexone for AUD (Rösner et al., 2010b; Maisel et al., 2013) as it reliably blunts the reinforcing effects of alcohol (Hendershot et al., 2017; Ray et al., 2019).
The clinical treatment success may also depend on the length of the follow-up. For instance, a combination of naltrexone and psychotherapy results in high clinical efficacy in the treatment of alcohol dependent patients following short 12–16 weeks of treatment, (Jarosz et al., 2013). Finally, a known confounder of clinical studies using pharmacotherapy, in general, is patient compliance with taking the medication and the context in which the medication is administered on which the effectiveness of pharmacotherapy depends (Starosta et al., 2006). Reduced medication compliance may also be related to the suggested decrease of the suppressant effect of naloxone on drinking behavior, which would be limited to the first 3 months of treatment (Volpicelli et al., 1997; Davidson et al., 2007). As such, enhancing compliance during treatment is crucial for treatment success with a two-fold higher treatment effect size in the most compliant individuals (Baros et al., 2008).
In conclusion, the current results suggest that pharmacotherapy is effective to treat patients with AUD either without or with psychotherapy and that psychotherapy can best be offered in combination with pharmacotherapy.
STATEMENT OF ETHICS
The paper is exempt from ethical committee approval.
AUTHOR CONTRIBUTIONS
Jv.A. and T.N. performed the systematic search and T.N., V.H., Jv.A. and Wvd.B. drafted the paper.
FUNDING
The authors received no financial support for the research, authorship and/or publication of this article.
CONFLICT OF INTEREST STATEMENT
Jv.A., P.B., R.S. and V.H. have no potential conflict of interest with respect to the research, authorship and/or publication of this article. Wvd.B. has a potential conflict of interest as a consultant for Lundbeck, D&A Pharma and Kinnov Therapeutics.
Supplementary Material
Contributor Information
Jan van Amsterdam, Amsterdam UMC, University of Amsterdam, Department of Psychiatry, Amsterdam Neuroscience, Research Program Compulsivity, Impulsivity & Attention, P.O. Box 22660, 1100 DD, Amsterdam, The Netherlands.
Peter Blanken, Parnassia Addiction Research Centre (PARC), Parnassia Psychiatric Institute, Zoutkeetsingel 40, 2512 HN, The Hague, The Netherlands.
Renske Spijkerman, Parnassia Addiction Research Centre (PARC), Parnassia Psychiatric Institute, Zoutkeetsingel 40, 2512 HN, The Hague, The Netherlands.
Wim van den Brink, Amsterdam UMC, University of Amsterdam, Department of Psychiatry, Amsterdam Neuroscience, Research Program Compulsivity, Impulsivity & Attention, P.O. Box 22660, 1100 DD, Amsterdam, The Netherlands.
Vincent Hendriks, Parnassia Addiction Research Centre (PARC), Parnassia Psychiatric Institute, Zoutkeetsingel 40, 2512 HN, The Hague, The Netherlands; Leiden University Medical Center, Albinusdreef 2, 2333 ZA, Leiden, The Netherlands.
References
- Agosti V, Nunes EV, O'Shea D. (2012) Do manualized psychosocial interventions help reduce relapse among alcohol-dependent adults treated with naltrexone or placebo? A meta-analysis. Am J Addict 21:501–7. [DOI] [PubMed] [Google Scholar]
- Akbar M, Egli M, Cho YE et al. (2018) Medications for alcohol use disorders: An overview. Pharmacol Ther 185:64–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR et al. (1999) Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 156:1758–64. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham PK et al. (2001) Posttreatment results of combining naltrexone with cognitive-behavior therapy for the treatment of alcoholism. J Clin Psychopharmacol 21:72–7. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA et al. (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–17. [DOI] [PubMed] [Google Scholar]
- Assanangkornchai S, Srisurapanont M. (2007) The treatment of alcohol dependence. Curr Opin Psychiatry 20:222–7. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berglund M, Borg S et al. (2003) A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res 27:1142–9. [DOI] [PubMed] [Google Scholar]
- Baros AM, Latham PK, Anton RF. (2008) Naltrexone and cognitive behavioral therapy for the treatment of alcohol dependence: Do sex differences exist? Alcohol Clin Exp Res 32:771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner MM, Wahl S, Brueck R et al. (2014) The place of additional individual psychotherapy in the treatment of alcoholism: a randomized controlled study in nonresponders to anticraving medication-results of the PREDICT study. Alcohol Clin Exp Res 38:1118–25. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ et al. (2011) Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med 41:516–24. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT et al. (1994) Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry 51:177–87. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA et al. (1998) Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 93:713–27. [DOI] [PubMed] [Google Scholar]
- Cheng HY, McGuinness LA, Elbers RG et al. (2020) Treatment interventions to maintain abstinence from alcohol in primary care: systematic review and network meta-analysis. BMJ 371:m3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SE, Duncan MH, Saxon AJ et al. (2021) Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry 8:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E, Levin F, Hart CL et al. (2020) A single ketamine infusion combined with motivational enhancement therapy for alcohol use disorder: A randomized midazolam-controlled pilot trial. Am J Psychiatry 177:125–33. [DOI] [PubMed] [Google Scholar]
- Davidson D, Wirtz PW, Gulliver SB et al. (2007) Naltrexone's suppressant effects on drinking are limited to the first 3 months of treatment. Psychopharmacology (Berl) 194:1–10. [DOI] [PubMed] [Google Scholar]
- De Wildt WA, Schippers GM, Van Den Brink W et al. (2002) Does psychosocial treatment enhance the efficacy of acamprosate in patients with alcohol problems? Alcohol Alcohol 37:375–82. [DOI] [PubMed] [Google Scholar]
- Donoghue K, Elzerbi C, Saunders R et al. (2015) The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction 110:920–30. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Anton RF, Miller WR et al. (2008) Combined pharmacotherapies and behavioral interventions for alcohol dependence (The COMBINE Study): examination of posttreatment drinking outcomes. J Stud Alcohol Drugs 69:5–13. [DOI] [PubMed] [Google Scholar]
- Feeney GF, Connor JP, Young RM et al. (2004) Alcohol dependence: the impact of cognitive behaviour therapy with or without naltrexone on subjective health status. Aust N Z J Psychiatry 38:842–8. [DOI] [PubMed] [Google Scholar]
- Feeney GF, Connor JP, Young RM et al. (2006) Combined acamprosate and naltrexone, with cognitive behavioural therapy is superior to either medication alone for alcohol abstinence: a single centres' experience with pharmacotherapy. Alcohol Alcohol 41:321–7. [DOI] [PubMed] [Google Scholar]
- Gao J, Cao J, Guo T et al. (2018) Association between alcoholic interventions and abstinence rates for alcohol use disorders: A meta-analysis. Medicine (Baltimore) 97:e13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastpar M, Bonnet U, Böning J et al. (2002) Lack of efficacy of naltrexone in the prevention of alcohol relapse: results from a German multicenter study. J Clin Psychopharmacol 22:592–8. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD et al. (2015) Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiat 72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD et al. (2017) Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiat 74:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia J, Caso C, Arias F et al. (2002) A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res 26:1381–7. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Samokhvalov AV et al. (2017) Effects of naltrexone on alcohol self-administration and craving: meta-analysis of human laboratory studies. Addict Biol 22:1515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JD, Cisler RA, Couper DJ et al. (2005) Design and analysis of trials of combination therapies. J Stud Alcohol Suppl 34-42:discussion 33. [DOI] [PubMed] [Google Scholar]
- Huang MC, Chen CH, Yu JM et al. (2005) A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol dependence in Taiwan. Addict Biol 10:289–92. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Bowers CA, Dunn ME et al. (1999) Efficacy of relapse prevention: a meta-analytic review. J Consult Clin Psychol 67:563–70. [DOI] [PubMed] [Google Scholar]
- Jarosz J, Miernik K, Wąchal M et al. (2013) Naltrexone (50 mg) plus psychotherapy in alcohol-dependent patients: a meta-analysis of randomized controlled trials. Am J Drug Alcohol Abuse 39:144–60. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C et al. (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311:1889–900. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K et al. (2017) Drug abuse-associated mortality across the lifespan: a population-based longitudinal cohort and co-relative analysis. Soc Psychiatry Psychiatr Epidemiol 52:877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Tarnaske T et al. (2003) Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry 60:92–9. [DOI] [PubMed] [Google Scholar]
- Kim Y, Hack LM, Ahn ES et al. (2018) Practical outpatient pharmacotherapy for alcohol use disorder. Drugs Context 7:212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox PC, Donovan DM. (1999) Using naltrexone in inpatient alcoholism treatment. J Psychoactive Drugs 31:373–88. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Soyka M. (2018) Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 320:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Van Kirk J. (2000) Naltrexone vs. nefazodone for treatment of alcohol dependence. A Placebo-Controlled Trial Neuropsychopharmacology 22:493–503. [DOI] [PubMed] [Google Scholar]
- LADIS . (2016). Landelijk Drugs en Alcohol Informatie Systeem (LADIS). Hulpvraag verslavingszorg 2015 [National Drug and Alcohol Information System (LADIS). Requests for help addiction care 2015]. Alcohol. Available at: http://www.ladis.eu/nl/middelen/alcohol (26 April 2022, date last accessed).
- Magill M, Ray L, Kiluk B et al. (2019) A meta-analysis of cognitive-behavioral therapy for alcohol or other drug use disorders: Treatment efficacy by contrast condition. J Consult Clin Psychol 87:1093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL et al. (2013) Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108:275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Lemenager T, Hoffmann S et al. (2013) Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in G ermany and comparison with the US COMBINE study. Addict Biol 18:937–46. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kassed CA, Vandivort-Warren R et al. (2009) Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug Alcohol Depend 99:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GW, Rehm J. (2012) The effectiveness of psychosocial modalities in the treatment of alcohol problems in adults: a review of the evidence. Can J Psychiatry 57:350–8. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD et al. (1999) A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry 56:719–24. [DOI] [PubMed] [Google Scholar]
- MATCH . (1998) Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Project MATCH Research Group. J Stud Alcohol 59:631–9. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM et al. (2001) Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res 25:1634–47. [PubMed] [Google Scholar]
- Morgenstern J, Kuerbis AN, Chen AC et al. (2012) A randomized clinical trial of naltrexone and behavioral therapy for problem drinking men who have sex with men. J Consult Clin Psychol 80:863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PL, Hopwood M, Whelan G et al. (2001) Naltrexone for alcohol dependence: a randomized controlled trial. Addiction 96:1565–73. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G et al. (1992) Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49:881–7. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Rounsaville BJ, Farren C et al. (2003) Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch Intern Med 163:1695–704. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Lynch KG, Pettinati HM et al. (2008) A placebo-controlled randomized clinical trial of naltrexone in the context of different levels of psychosocial intervention. Alcohol Clin Exp Res 32:1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponizovsky AM, Rosca P, Aronovich E et al. (2015) Baclofen as add-on to standard psychosocial treatment for alcohol dependence: A randomized, double-blind, placebo-controlled trial with 1year follow-up. J Subst Abuse Treat 52:24–30. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM et al. (2017) Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse 43:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Green R, Roche DJO et al. (2019) Naltrexone effects on subjective responses to alcohol in the human laboratory: A systematic review and meta-analysis. Addict Biol 24:1138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Meredith LR, Kiluk BD et al. (2020) Combined pharmacotherapy and cognitive behavioral therapy for adults with alcohol or substance use disorders: a systematic review and meta-analysis. JAMA Netw Open 3:e208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Du H, Green R et al. (2021) Do behavioral pharmacology findings predict clinical trial outcomes? A proof-of-concept in medication development for alcohol use disorder. Neuropsychopharmacology 46:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus VI, Fochtmann LJ, Bukstein O et al. (2019) The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Focus (Am Psychiatr Publ) 17:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen HG, de Waart R, van der Windt DA et al. (2006) A systematic review of the effectiveness of naltrexone in the maintenance treatment of opioid and alcohol dependence. Eur Neuropsychopharmacol 16:311–23. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S et al. (2010a) Acamprosate for alcohol dependence. Cochrane Database Syst Rev 8:Cd004332. doi: 10.1002/14651858.CD004332.pub2. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S et al. (2010b) Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev 8:Cd001867. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE et al. (2015) 2010 national and state costs of excessive alcohol consumption. Am J Prev Med 49:e73–9. [DOI] [PubMed] [Google Scholar]
- SAMHSA . (2014). Substance Abuse and Mental Health Services Administration (SAMHSA). National Survey On Drug Use And Health 2014. Population data. Available at: https://www.datafiles.samhsa.gov/dataset/national-survey-drug-use-and-health-2014-nsduh-2014-ds0001 (29 April 2022, date last accessed). [PubMed] [Google Scholar]
- SAMHSA . (2019). Substance Abuse and Mental Health Services Administration (SAMHSA). Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. Rockville, USA. Substance Abuse and Mental Health Services Administration; Available at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf (29 April 2022, date last accessed). [Google Scholar]
- Schaumberg K, Kuerbis A, Morgenstern J et al. (2013) Attributions of change and self-efficacy in a randomized controlled trial of medication and psychotherapy for problem drinking. Behav Ther 44:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM et al. (2001) Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav 26:167–80. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Sayre SL et al. (2004) Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. Am J Addict 13:333–41. [DOI] [PubMed] [Google Scholar]
- Slade T, Chiu WT, Glantz M et al. (2016) A cross-national examination of differences in classification of lifetime alcohol use disorder between DSM-IV and DSM-5: Findings from the World Mental Health Survey. Alcohol Clin Exp Res 40:1728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. (2005) Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol 8:267–80. [DOI] [PubMed] [Google Scholar]
- Starosta AN, Leeman RF, Volpicelli JR. (2006) The BRENDA model: Integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. J Psychiatr Pract 12:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuithof M, Ten Have M, van den Brink W et al. (2016) Treatment seeking for alcohol use disorders: Treatment gap or adequate self-selection? Eur Addict Res 22:277–85. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M et al. (1992) Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49:876–80. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS et al. (1997) Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry 54:737–42. [DOI] [PubMed] [Google Scholar]
- Wetzel H, Szegedi A, Scheurich A et al. (2004) Combination treatment with nefazodone and cognitive-behavioral therapy for relapse prevention in alcohol-dependent men: a randomized controlled study. J Clin Psychiatry 65:1406–13. [DOI] [PubMed] [Google Scholar]
- Wölwer W, Frommann N, Jänner M et al. (2011) The effects of combined acamprosate and integrative behaviour therapy in the outpatient treatment of alcohol dependence: a randomized controlled trial. Drug Alcohol Depend 118:417–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.