Abstract
Biologics have emerged as an effective treatment of rheumatoid arthritis (RA). However, there is a significant proportion of patients who fail to respond to biologics. Identifying the predictors that affect the response to biologics remains challenging. A comprehensive literature search of PubMed, Embase, and Web of Science databases was conducted through May 1, 2022. We included all studies that used a multivariate model to assess for the predictors of remission in RA patients treated with biologics. We calculated pooled odds ratios (OR) with 95% confidence intervals (CI) for risk factors reported in ≥ 3 studies using a random-effects model. A total of 16,934 patients with RA who were treated with biologics were included in twenty-one studies. Our study showed that old age (OR 0.98 (0.97, 0.99), P < 0.00001), female gender (OR 0.66 (0.56, 0.77), P < 0.00001), smoking history (OR 0.86 (0.75, 0.99), P 0.04), obesity (OR 0.95 (0.91, 0.99), P 0.02), poor functional status (OR 0.62 (0.48, 1.27), P < 0.00001), high disease activity (OR 0.90 (0.85, 0.96), P 0.0005), and elevated erythrocyte sedimentation rate (OR 0.99 (0.98, 1.00), P 0.009) were poor predictors of remission. On the other hand, positive anti-citrullinated protein antibodies (OR 2.52 (1.53, 4.12), P 0.0003) was associated with high remission rate. Old age, female gender, obesity, smoking history, poor functional status, high disease activity, and elevated ESR at the time of diagnosis have been associated with poor response to biologics. Our findings could help establish a risk stratification model for predicting the remission rate in RA patients receiving biologics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10067-022-06307-8.
Keywords: Biologic therapy, Rheumatoid arthritis, Disease activity score, Remission
Introduction
Rheumatoid arthritis is a chronic autoimmune disease characterized by inflammatory polyarthritis that mainly affects the small joints [1]. Biological disease-modifying antirheumatic drugs (bDMARDs) have emerged as an important advancement in the treatment of rheumatoid arthritis [2]. There are several types of biologics, each of which targets a specific type of molecule involved in the pathogenesis of the disease. These include tumor necrosis factor alpha (TNF-α) inhibitors, such as etanercept, adalimumab, infliximab, certolizumab pegol, and golimumab. Other biologics that target other molecules include abatacept (a selective co-stimulation modulator that inhibits T-cells), rituximab (B-cell inhibitor), tocilizumab (IL-6 receptor antagonist), and anakinra (IL-1 receptor antagonist).
Despite the increasing number of biologics, the ability to achieve complete remission in certain RA patients remains challenging. Approximately 66% of RA patients failed treatment with TNF inhibitors in 6 months of follow-up [3], and a minimum of 10% who tried a second bDMARD had their medication stopped due to lack of response [2]. This suggests that there is a significant proportion of patients who do not respond to bDMARDs.
Several observational studies have identified different predictors of remission in RA patients receiving biologics [4–24]. However, many of these predictors remain inconsistent. Some studies showed that old age, female gender, smoking history, obesity, presence of comorbidities, increased disease activity at the time of diagnosis, increased disease duration, and poor functional status at baseline have been associated with a lower response rate to biologics [4–6, 8, 9, 14, 15, 17]. While other studies showed no significant association between age, gender, and remission rate [15, 18, 23]. Patients with elevated ESR at the time of diagnosis have also shown poor response to biologics in some studies [9, 14], but there was no significant association in other studies [8, 17]. A meta-analysis was also conducted in 2018 to assess for the predictors of remission in RA patients regardless of the treatment that the patients received [4]. In this study, we conducted a systematic review and meta-analysis to assess the strength of association between these predictors and the rate of remission in RA patients treated with bDMARDs.
Methods
We conducted this systematic review and meta-analysis based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis [5], and Meta-analysis of Observational Studies in Epidemiology [6].
Data sources and search strategy
We performed a comprehensive search for published studies indexed in PubMed, Embase, and Web of Science databases from inception through May 1, 2022. We also performed a manual search for additional relevant studies using references of the included articles. The following search terms were used: “biologics OR Etanercept OR Infliximab OR Adalimumab OR Certolizumab OR Golimumab OR Anakinra OR Tocilizumab OR Sarilumab OR Abatacept OR Rituximab” AND “relapse OR remission” AND “arthritis OR rheumatoid OR rheumatoid arthritis” AND “risk factors OR predictors.” The search was not limited by language, study design, or country of origin. Two investigators (YK and AB) independently performed the literature search, screened using a priori criteria, and shortlisted the studies for final review. The bibliographic software EndNote was used for screening. Any discrepancies were resolved by a third reviewer (SG).
Inclusion and exclusion criteria
Studies meeting the following inclusion criteria were included: (1) full-text peer-reviewed publications of retrospective or prospective, cohort or case–control studies, (2) assessed for predictors to response to different types of biologics in RA patients, and (4) reported odds ratio (OR) for this association after multivariate analysis and adjustment of potential confounding factors. We excluded conference abstracts. We also excluded studies reported data based on hazard ratio or univariate analysis rather than multivariate analysis.
Data extraction
The following data were extracted from the studies: study characteristics (author, publication year, study design, country of origin, and study population), patients’ baseline characteristics, the follow-up duration, and variables that were adjusted in a multivariable analysis. Risk factors that were assessed in at least three studies were included in the meta-analyses. Two investigators (YK and AB) independently extracted the data from the articles, and discrepancies were resolved by a third reviewer (SG).
Statistical analysis
We performed a meta-analysis of the included studies using Review Manager 5.3 (Cochrane Collaboration, Copenhagen) and Comprehensive Meta-Analysis 3.3 software (Biostat, Englewood, USA). Multivariate adjusted odds ratios (OR) for individual studies were pooled using a random-effects model and reported using a 95% confidence interval (CI) for each risk factor where applicable. Pooling was undertaken if at least three studies reported an odds ratio for a given risk factor. A P value < 0.05 was considered statistically significant. Heterogeneity was assessed using the Higgins I2 index, where I2 values > 50% implied the presence of significant heterogeneity [7].
Sensitivity analysis
To evaluate the robustness of results, leave-one-out analysis was attempted for risk factors reported by ten or more studies.
Bias assessment
We assessed the quality of the included studies using the Newcastle–Ottawa Scale [8]. Two authors (YK and AB) independently assessed each study for bias. For risk factors reported by ten or more studies, publication bias assessment across studies was performed qualitatively by visualization of the funnel plot [9] and quantitatively, using Egger’s regression analysis [10]. A P value was generated using Egger’s analysis, and a value of < 0.05 was associated with significant publication bias. If bias was present on Egger’s test, further statistics using the Fail-Safe N test and Duval and Tweedie’s “Trim and Fill” test were used to ascertain the impact of the bias.
Results
Study selection
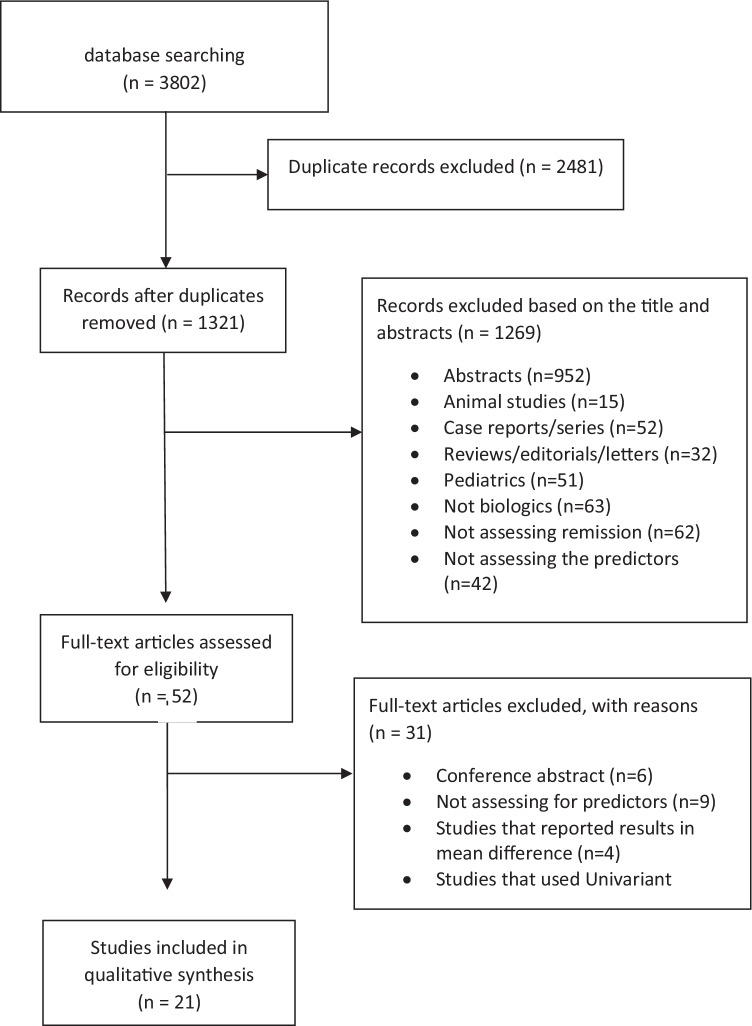
We included a total of 3802 studies in our analysis (647 studies from PubMed, 2076 studies from Embase, 347 studies from Cochrane, and 732 studies from Web of Science). A total of 2481 duplicated studies were excluded, and a total of 1321 studies were reviewed based on the abstracts. Out of these, 1269 studies were excluded after reviewing the title and the abstract. Then, 52 studies were reviewed based on the full text. Thirty-one studies were excluded (nine studies did not assess predictors of remission, four studies reported results in mean difference, twelve studies did not report risk factors that underwent multivariate analysis, and six studies were conference abstracts). Finally, a total of 21 studies [11–31] met our inclusion criteria and were included in our analysis. A PRISMA flowchart that demonstrates how the included studies were selected is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram for the selection of studies
Characteristics and quality of included studies
Table 1 shows the characteristics of the studies included in the meta-analysis. All the included studies were published between April 2006 and August 2021. Based on country of origin, six studies originated from Japan [22, 25–29], two studies originated from the USA [14, 24], two studies originated from Italy [15, 20], two studies originated from United Kingdom [11, 21], two studies from France [18, 19], one study from Canada [16], one study from Greece [17], one study from Germany [12], one study from Ireland [30], one study from Australia [31], and one study from Taiwan [23]. Regarding the study design, all the included studies were either retrospective or prospective cohort except of Listing et al. [12] that was a randomized control trial.
Table 1.
Characteristics of studies included in the meta-analysis
| Study/year | Country | Study design | Sample size, (female), n | Age, mean (SD), years | Biologics used | Follow-up period (months) | Remission criteria | Variables adjusted in meta-analysis |
|---|---|---|---|---|---|---|---|---|
| Atzeni 2014 | Italy | Prospective cohort | 1300 (1064) | 54.6 (13.7) | INF, ETA, ADA | 12 | DAS28 ≤ 2.6 | Age, gender |
| Barnabe 2014 | Canada | Prospective cohort | 1116 (826) | 54.4 (13.6) | INF, ETA, ADA, CTZ, GOM | 30 | DAS28 ≤ 2.6 | Gender, BMI, RF smoking history |
| Biggioggero 2019 | Italy | Retrospective cohort | 346 (282) | 53.4 (13) | ETA, ADA | 12 | DAS28 ≤ 2.6 | Age, gender, smoking, disease duration |
| Canhao 2012 | USA | Retrospective cohort | 617 (544) | 42.2 (12.4) | INF, ETA, ADA | 12 | DAS28 ≤ 2.6 | Age, gender, smoking, disease duration |
| Collins 2020 | USA | Prospective cohort | 380 (316) | 53.0 (12) | Tocilizumab | 6 | SDAI ≤ 2.8 | Age, gender, disease activity, disease duration |
| Flouri 2014 | Greece | Prospective cohort | 1208 (1007) | 58 (17) | INF, ETA, ADA | 12 | DAS28 ≤ 2.6 | Gender |
| Hamann 2019 | UK | Retrospective cohort | 14,436 (10,971) | 56.0 (12.3) | INF, ETA, ADA, CTZ | 6 | DAS28 ≤ 2.6 | Age, gender, smoking, disease activity |
| Hyrich 2006 | UK | Prospective cohort | 1,267 (1100) | 56 (12) | ETA | 6 | DAS28 ≤ 2.6 | Age, gender, smoking, disease activity, disease duration |
| Hyrich 2006 | UK | Prospective cohort | 1612 (1387) | 55 (12) | INF | 6 | DAS28 ≤ 2.6 | Age, gender, smoking, disease activity, disease duration |
| Kawashiri 2021 | Japan | Retrospective cohort | 125 (90) | 59 (18) | INF, ETA, ADA | 12 | SDAI ≤ 3.3 | Use of steroids, ACPA, swollen joint count |
| Kida 2020 | Japan | Prospective cohort | 554 (441) | 67.8 (12.4) | ABC | 13 | SDAI ≤ 3.3 | Age, gender, ACPA, disease activity, disease duration |
| Listing 2006 | Germany | RCT | 818 (627) | 55 (12.3) | INF, ETA, ADA, Anakinra | 12 | DAS28 ≤ 2.6 | Age, disease activity |
| Marie Pers 2013 | France | Retrospective cohort | 204 (166) | 55.2 (13.8) | Tocilizumab | 6 | DAS28 ≤ 2.6 | Age, smoking, disease activity |
| Murakami 2019 | Japan | Prospective cohort | 118 (97) | 65 (12) | ABC | 12 | DAS28 ≤ 2.6 | Age, gender |
| Murray 2021 | Ireland | Prospective cohort | 274 (207) | 55 (11.9) | INF, ETA, ADA, RIX | 144 | DAS28 ≤ 2.6 | Age, gender |
| Nakashima 2020 | Japan | Prospective cohort | 110 (97) | 58.6 (12.8) | Tocilizumab | 13 | DAS28 ≤ 2.6 | MTX use, disease activity |
| Nourisson 2017 | France | Prospective cohort | 990 (784) | 58.7 (11.6) | ABC, Tocilizumab | 12 | DAS28 ≤ 2.6 | Gender |
| Rubbert 2021 | Austria | Prospective cohort | 5462 (4420) | 53.3 (12.3) | Tocilizumab | 6 | SDAI ≤ 3.3 | Age, gender, disease activity, disease duration |
| Tanaka1 2020 | Japan | Prospective cohort | 159 (NR) | NR | CTZ | 13 | SDAI ≤ 3.3 | Gender, BMI, RF, disease activity |
| Wang 2019 | Taiwan | Prospective cohort | 70 (67) | 54.1 (10.6) | RIX | 24 | DAS28 ≤ 2.6 | Age, gender, RF, ACPA, disease activity and duration |
| Yamaguchi 2020 | Japan | Prospective cohort | 75 (68) | 59.7 (10.7) | ADA | 6 | DAS28 ≤ 2.6 | Age, MTX use, disease duration |
INF infliximab, ETA etanercept, ADA adalimumab, CTZ certolizumab, GOM golimumab, ABC abatacept, RIX rituximab, DAS28 disease activity score, SDAI simple disease activity index, BMI body mass index, RF rheumatoid factor, ACPA anticitrullinated peptide antibody, MTX methotrexate
A total of 16,934 patients were included in the 21 studies. Remission criteria was defined as disease activity score (DAS28) of less than or equal to 2.6 [11–23, 26, 28, 30]. Other studies used the simplified disease activity index (SDAI) score of less than or equal to 3.3 to assess for remission [24, 25, 27, 29, 31]. The average follow-up period after staring biologics was around 18 months. Across the 21 studies, the rate of remission was about 53%. Most of the studies reported age, female gender, smoking history, presence of comorbidities, disease duration, and disease activity at the time of diagnosis as predictors of remission. Other studies reported different predictors such as body mass index (BMI), erythrocyte sedimentation rate (ESR), anti-citrullinated protein antibodies (ACPA), and rheumatoid factor (RF). The characteristics of the included studies are described in detail in Table 1. The predictors of remission in RA treated with biologics are summarized in Table 2. We then performed a subgroup meta-analysis for predictors of remission in RA patients treated with TNF-α inhibitors alone as shown in Supplementary Table 3.
Table 2.
Predictors of all biologics included in the meta-analysis
| Risk factor (number of studies) | Effect size (95% CI) | P value | I2 | I2heterogeneity | Egger’s test |
|---|---|---|---|---|---|
| Sociodemographic-related risk factors | |||||
| Age ≥ 50 (15) | OR 0.98 (0.97, 0.99) | < 0.00001 | 46% | 0.03 | 0.89 |
| Female gender (16) | OR 0.66 (0.56, 0.77) | < 0.00001 | 61% | 0.0009 | 0.63 |
| BMI ≥ 30 kg/m2 (4) | OR 0.95 (0.91, 0.99) | 0.02 | 65% | 0.03 | NR |
| Presence of comorbidities (3) | OR 0.77 (0.51, 1.15) | 0.2 | 79% | 0.008 | NR |
| Current of ex-smoker (7) | OR 0.86 (0.75, 0.99) | 0.04 | 67% | 0.006 | NR |
| Baseline HAQ score ≥ 2 (9) | OR 0.62 (0.48, 1.27) | < 0.00001 | 42% | 0.09 | 0.68 |
| Disease-related risk factors | |||||
| Disease duration ≥ 10 years (11) | OR 0.99 (0.98, 1.00) | 0.18 | 59% | 0.007 | 0.34 |
| DAS28 at diagnosis ≥ 3.2 (13) | OR 0.90 (0.85, 0.96) | 0.0005 | 88% | < 0.00001 | 0.65 |
| TJC ≥ 10 (5) | OR 0.99 (0.97, 1.01) | 0.33 | 76% | 0.002 | NR |
| SJC ≥ 7 (6) | OR 1.00 (0.95, 1.06) | 0.94 | 79% | 0.0002 | NR |
| RF positive (8) | OR 0.99 (0.97, 1.01) | 0.24 | 18% | 0.29 | NR |
| ACPA positive (3) | OR 2.52 (1.53, 4.12) | 0.0003 | 0% | 0.44 | NR |
| ESR > 20 mm/h (4) | OR 0.99 (0.98, 1.00) | 0.009 | 0% | 0.69 | NR |
| Treatment-related risk factors | |||||
| Prior or concurrent use of MTX (11) | OR 1.16 (0.9, 1.5) | 0.25 | 85% | < 0.00001 | 0.33 |
| Prior or concurrent use of steroids (8) | OR 0.97 (0.89, 1.06) | 0.48 | 39% | 0.12 | NR |
Predictors of remission of RA in patients treated with biologics
A total of fifteen predictors were reported in ≥ 3 studies and included in the systematic review and meta-analysis. The predictors were classified as sociodemographic-related, disease-related, and treatment-related predictors.
Sociodemographic-related predictors
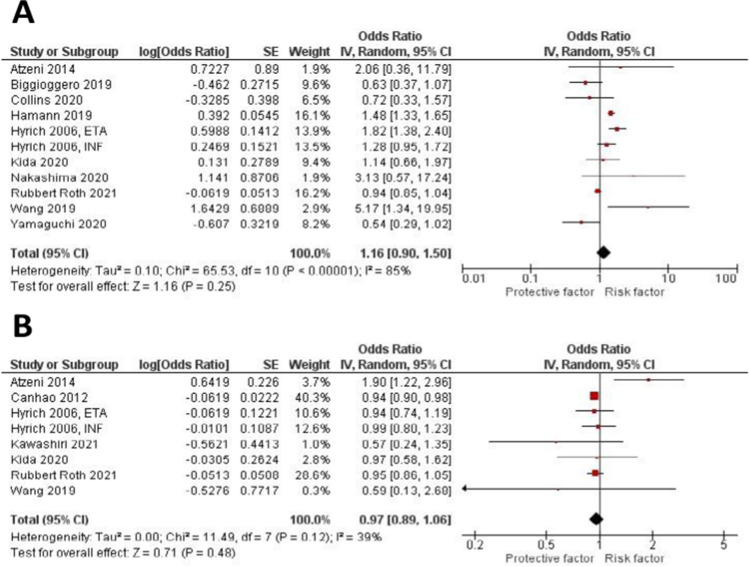
The effect estimate and forest plot of each predictor are shown in Table 2 and Fig. 2, respectively. We performed meta-analyses for six sociodemographic-related factors including age older than 55 year old (fifteen studies [11, 12, 14, 15, 18, 20–23, 25, 28–31]), female gender (sixteen studies [11, 14–17, 19–25, 27, 30, 31]), obesity defined as BMI ≥ 30 kg/m2 (four studies [16, 21, 27, 31]), smoking status defined as current or ex-smoker (seven studies [11, 14–16, 18, 21]), poor baseline functional status defined as Health Assessment Questionnaire (HAQ) of more than two (nine studies [11, 14, 16, 20, 21, 24, 25, 27]), and presence of comorbidities (three studies [11, 15]). Our analysis showed that old age (OR 0.98 (0.97, 0.99), P < 0.00001), female gender (OR 0.66 (0.56, 0.77), P < 0.00001), BMI > 30 (OR 0.95 (0.91, 0.99), P 0.02), smoking history (OR 0.86 (0.75, 0.99), P 0.04), and baseline HAQ > 2 (OR 0.62 (0.48, 1.27), P < 0.00001) are significantly associated with low rate of remission. Presence of comorbidities, on the other hand, was not associated with significant decrease in remission rate (OR 0.77 (0.51, 1.15), P 0.20). Leave-one-out sensitivity analysis showed consistent results for age, and female gender as shown in Supplementary Fig. 6A and 6B, respectively.
Fig. 2.
Forest plots of sociodemographic-related predictors of remission of RA in patients treated with biologics: age > 50 years old, female gender, BMI > 30 kg/m.2, smoking history, and HAQ score > 2
Disease-related risk factors
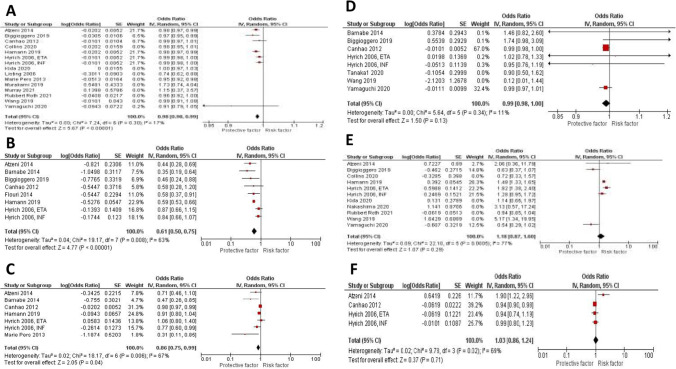
The effect estimate and forest plot of each predictor are shown in Table 2 and Fig. 3, respectively. We performed meta-analyses for seven disease-related factors including disease duration of more than 10 years (eleven studies [11, 14–16, 20, 21, 23–25, 28, 31]), disease activity score (DAS28) ≥ 3.2 (thirteen studies [11, 12, 14, 18, 20, 21, 23–27, 31]), tender joint count (TJC28) ≥ 10 (five studies [12, 15, 16, 18, 21]), swollen joint count (SJC28) ≥ 7 (six studies [15–18, 21, 29]), positive rheumatoid factor (RF) (eight studies [11, 14, 16, 20, 23, 27, 28]), positive anti-citrullinated protein Antibody (ACPA) (three studies [12, 23, 25]), and elevated erythrocyte sedimentation rate (ESR) > 20 mm/h (four studies [15, 16, 21, 24]). Our analysis showed that high disease activity at the time of diagnosis (OR 0.90 (0.85, 0.96), P 0.0005), and elevated ESR (OR 0.99 (0.98, 1.00), P 0.009) are significantly associated with lower remission rate. While disease duration (OR 0.99 (0.98, 1.00), P 0.18), high TJC (OR 0.99 (0.97, 1.01), P 0.33), high SJC (OR 1.00 (0.95, 1.06), P 0.94), and positive RF (OR 0.99 (0.97, 1.01), P 0.24) were all associated with decrease rate of remission, but that was not statistically significant. While positive ACPA was associated with significant increase in remission rate (OR 2.52 (1.53, 4.12), P 0.0003). Leave-one-out sensitivity analysis showed consistent results for disease duration, and disease activity as shown in Supplementary Fig. 6C, and 6D respectively.
Fig. 3.
Forest plots of disease-related predictors of remission in RA patients treated with biologics: disease duration ≥ 10 years, DAS28 at time of diagnosis ≥ 3.2, TJC28 ≥ 10, SJC28 ≥ 7, positive RF, positive ACPA, and ESR > 20 mm/h
Treatment-related risk factors
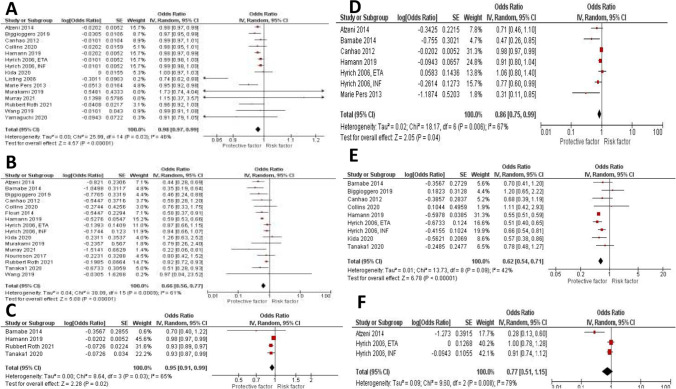
The effect estimate and forest plot of each predictor are shown in Table 2 and Fig. 4, respectively. We performed meta-analyses for two treatment-related factors including prior or concurrent use of methotrexate (eleven studies [11, 15, 20, 21, 23–26, 28, 31]), and prior or concurrent use of steroids (eight studies [11, 14, 15, 23, 25, 29, 31]). Our analysis showed that prior or concurrent use of MTX (OR 1.16 (0.9, 1.5), P 0.25), and prior or concurrent use of steroids were not associated with significant increase in remission rate (OR 0.97 (0.89, 1.06), P 0.48). Consistent results were obtained on leave-one-out sensitivity analysis for MTX use as shown in Supplementary Fig. 6E.
Fig. 4.
Forest plots of treatment-related predictors of remission in RA patients treated with biologics: prior or concurrent use of MTX, and prior or concurrent use of steroids
Subgroup analysis
We performed a subgroup analysis to assess for predictors of remission in RA patients receiving tumor necrosis factor alpha inhibitors (TNF-inhibitors). A total of eight studies that included only TNF-inhibitors were used in the subgroup analysis. The effect estimate and forest plot of each predictor are shown in Table 3 and Fig. 5, respectively. Age (OR 0.98 (0.97, 0.99), P < 0.00001), Female gender (OR 0.61 (0.50, 0.75), P < 0.00001), and smoking history (OR 0.86 (0.75, 0.99), P 0.04) were significantly associated with lower remission rate. On the other hand, prior use of MTX (OR 1.18 (0.87, 1.6), P 0.29), positive RF (OR 0.99 (0.98, 1.00), P 0.13), and prior use of steroids (OR 1.03 (0.86, 1.24), P 0.71) were not significantly associated with increasing or decreasing the remission rate.
Table 3.
Predictors of TNF inhibitors included in the meta-analysis
| Risk factor (number of studies) | Effect size (95% CI) | P value | I2 | I2heterogeneity | Egger’s test |
|---|---|---|---|---|---|
| Sociodemographic-related risk factors | |||||
| Age > = 50 (7) | OR 0.98 (0.97, 0.99) | < 0.00001 | 17% | 0.3 | 0.01 |
| Female gender (8) | OR 0.61 (0.50, 0.75) | < 0.00001 | 63% | 0.008 | NR |
| Current of ex-smoker (7) | OR 0.86 (0.75, 0.99) | 0.04 | 67% | 0.006 | NR |
| Disease-related risk factors | |||||
| RF positive (6) | OR 0.99 (0.98, 1.00) | 0.13 | 11% | 0.34 | NR |
| Treatment-related risk factors | |||||
| Prior or concurrent use of MTX (6) | OR 1.18 (0.87, 1.6) | 0.29 | 77% | < 0.0005 | NR |
| Prior or concurrent use of steroids (4) | OR 1.03 (0.86, 1.24) | 0.71 | 69% | 0.02 | NR |
Fig. 5.
Forest plots for predictors of remission in RA patients treated with TNF-inhibitors alone: age > 50 years old, female gender, smoking history, positive RF, prior or concurrent use of MTX., and prior or concurrent use of steroids
Evaluation of publication bias
We used both visual inspection and statistical analysis to assess for publication bias. The funnel plot revealed no publication bias (symmetric) for risk factors reported by ten or more studies, further confirmed by significant Egger’s regression test defined as P ≤ 0.01. Funnel plots for predictors of remission in RA patients receiving biologics are shown in Supplementary Fig. 6. Funnel plots were symmetric for age, female gender, disease activity, disease duration, and prior use of MTX, suggesting no publication bias. Moreover, Egger’s test was not statistically significant for these predictors which supports the absence of publication bias. Publication biases of the remaining risk factors could not be performed due to the small number of included studies.
Discussion
Biologic therapies have successfully revolutionized the management of RA. However, there is a significant proportion of patients who do not respond to the treatment. Identifying the predictors that will affect the treatment response before starting medications with known serious side effects remains challenging. We preformed this systematic review and meta-analysis to investigate the strength of association between different predictors and remission rate in RA patients treated with biologics. In this analysis, 67% of patients achieved complete remission of disease after a follow-up period of 6–12 months. Remission criteria was defined as DAS28 score of less than or equal to 2.6 or SDAI score of less than or equal to 3.3. Old age, female gender, smoking history, obesity, high disease activity at the time of diagnosis, poor functional status, and elevated ESR were associated with lower remission rate. On the other hand, positive ACPA at the time of diagnosis has been associated with higher remission rate. While disease duration, positive RF, prior or concurrent use of steroid, prior or concurrent use of MTX, high TJC, and high SJC score at the time of diagnosis were not significantly associated with lower remission rate. These results were consistent with those treated with TNF-α inhibitors alone.
Many studies supported our findings that women with RA had worse progression of the disease as compared to men despite being on similar treatment [32]. Similar findings have been reported by other studies [33–35]. It has been demonstrated that men and women respond differently to the same treatment due to physiologic differences. Another explanation to our finding is that we used the DAS28 score, which is highly dependent on pain perception, to assess for disease remission. Men may have a higher threshold for reporting joint tenderness which lowers their score. However, we cannot exclude the possibility that men may have a form of the disease that remits more often in comparison with women. Regarding age, our study showed that patient aged > 55 years old were responding poorly to biologics which contradicts the results of other registries that showed no effect of age on response to biologics [11, 36]. Older patients are more likely to have long disease duration which may negatively affect the therapeutic efficacy of biologics. Moreover, elderly patients usually have multiple comorbidities at baseline that make biologic agents potentially more dangerous which results in early discontinuation of these medications.
Obesity, defined as BMI > 30, was found to be a poor predictor of remission in patients receiving biologics. Studies showed that the adipose tissue produces pro-inflammatory cytokines such as TNF-α, and IL-6. The higher fat mass, the higher concentrations of these cytokines which may affect the therapeutic response [37]. Moreover, being a current or former smoker decreases the chances of response to biologics. Smoke acts on both cellular and humoral immunity that leads to a systemic proinflammatory state [38, 39]. Chronic cigarette smoking appears to trigger various morphological, physiological, and enzymatic changes that impairs inflammatory responses [38–40].
Regarding MTX, only 15–20% of our included patients received biologic drugs without prior or concurrent use of MTX. Our analysis showed that MTX prescription at baseline has no significant association with remission. Results were consistent among patients who received TNF- α inhibitors in combination with MTX. Our findings contradict the outcomes of a randomized controlled trial that was conducted in 1998 to investigate the impact of concurrent use of MTX with infliximab in 101 patients with RA [41]. That study showed that MTX has been associated with reduced immunogenicity of infliximab after repeated infusions which helped improve the clinical response. Our results also contradict the outcomes of a network meta-analysis that was conducted in 2019 that also showed that combination therapy of MTX with biologics improved clinical response as compared with biologic monotherapy [42]. Although many studies show that biologic use with MTX improves the clinical outcomes, this should not be considered as a standard of care for different reasons. First, many prescribers require MTX failure before starting biologics. Second, many patients prefer starting MTX prior to biologics because of the cost, and potential side effects. So far, we do not know whether starting biologic treatment rather than MTX improves long-term prognosis given that most of the patients included in the studies were started on MTX prior to biologics. On the other hand, our results should be further investigated by looking at the clinical background of the patients who were started on MTX and those who tried biologics without prior use of MTX. Studies showed that positive RF, younger age at symptom onset, and higher baseline disease activity are associated with higher rates of MTX failure [43]. Further subgroup analysis should be conducted to eliminate the effect of these confounders before making a conclusion.
Currently, there is no biomarker that is known to predict response to biologics in RA patients. Our analysis showed that RF was not significantly associated with poor response to biologics. However, elevated ESR of more than or equal to 20 mm per hour was found to be a significant poor predictor of remission. While patients with positive ACPA showed high remission rate in response to biologics. Several studies reported no relationship between RF or ACPA positivity and the clinical response to tocilizumab treatment [18, 44, 45]. In fact, ACPA positivity has emerged as an important predictor of response to biologics. A post hoc analysis of the AMPLE trial in 2016 initially showed that baseline ACPA positivity was associated with a better response to abatacept and adalimumab [46]. Such association can be explained by the fact that ACPA exert their biological functions by binding to the Fc receptors, expressed particularly by immune cells of the myeloid lineage, and activating the complement system via the classical and alternative pathways [47]. Given that most of the biologics work on inhibiting T-cells, B-cells, and their products of antibodies and inflammatory cytokines, partially explains their relative effectiveness in patients with positive ACPA [48].
Several limitations to our meta-analysis should be mentioned. First, our included studies had inherent bias given their observational nature. Second, there was a significant heterogeneity among the studies that investigated several risk factors such as age, female gender, obesity, smoking, prior use of MTX, baseline functional status, positive RF, and elevated ESR. This heterogeneity could be due to difference in remission criteria, variation in patient demographics, and absence of consistent follow-up period among the studies. Despite the use of the random-effects model to assess for heterogeneity, our results should be interpreted carefully. Third, our study included some methodological limitations that need to be considered while interpreting the results. In our included studies, the patients treated with biologics had long-standing disease and had failed several previous DMARDs. The evaluation of disease remission in these patients using the DAS28 scoring system is tricky given that joint pain and swelling could result from structural and permanent damage due to prolonged disease course. In addition to that, we used ESR value of more than 20 mm/h as a poor predictor of biologics. However, ESR level significantly increases with age, so higher cutoff values should have been considered positive given that most of our patients are older than 40 years old. Moreover, patients were followed-up for an average of 6 months in most of the included studies, and only six out of twenty-one studies had a follow-up period of more than one year which may have affected the response rate to biologics [4]. Finally, some risk factors were excluded given that they were reported in less than three studies such as family history and elevated CRP.
Despite these limitations, our study has several strengths. Up to our knowledge, this is the first meta-analysis that summarizes the available literature and provides a quantitative assessment of different risk factors associated with remission. Moreover, our analysis reported a large cohort of 16,934 patients from twenty-one studies. We also performed sensitivity analysis to the risk factors reported by ten or more studies, and no publication bias was detected in any of them. Finally, our results remained consistent when we preformed subgroup analysis for TNF inhibitors.
In conclusion, RA patients who are females with advanced age, obesity, smoking history, poor functional status, high disease activity, and elevated ESR at the time of diagnosis showed significantly decreased rate of disease remission after receiving biologics. On the other hand, positive ACPA, and prior use of MTX can increase remission rate in these patients. These predictors should be taken into consideration before starting medications with known serious side effects like biologics. Our findings might help develop a clinical prediction model to estimate the rate of remission in RA patients treated with biologics.
Supplementary Fig. 6 Sensitivity analysis for: A, age. B, female gender. C, disease activity. D, disease duration. E, prior use of MTX.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Conception and design: Khader. Analysis and interpretation of the data: all authors. Drafting of the article: Khader, Beran, Ghazaleh. Critical revision of the article for important intellectual content: all authors. Statistical analysis: Khader. Study supervision: Beran, Smith, Altorok. Final approval of the article: all authors..
Declarations
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare no competing interests.
Data registration
Data is registered and publicly available at OSF registries. Data including the Review Manager file used for analysis, tables and figures summarizing the included studies, and the predictors that were extracted are all available at OSF registries.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yasmin Khader, Email: Yasmin.Khader@Utoledo.EduYasmin.Khader.
Azizullah Beran, Email: Azizullah.Beran@Utoledo.Edu.
Sami Ghazaleh, Email: Sami.Ghazaleh@Utoledo.Edu.
Wade Lee-Smith, Email: Wade.Lee@Utoledo.Edu.
Nezam Altorok, Email: Nezam.Altorok@Utoledo.Edu.
References
- 1.Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poór G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O'Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 3.Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, Kollerup G, Linde L, Lindegaard HM, Poulsen UE, Schlemmer A, Jensen DV, Jensen S, Hostenkamp G, Østergaard M. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22–32. doi: 10.1002/art.27227. [DOI] [PubMed] [Google Scholar]
- 4.Yu C, Jin S, Wang Y, Jiang N, Wu C, Wang Q, Tian X, Li M, Zeng X. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-to-target strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol. 2019;38:727–738. doi: 10.1007/s10067-018-4340-7. [DOI] [PubMed] [Google Scholar]
- 5.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 9.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45:1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 12.Listing J, Strangfeld A, Rau R, Kekow J, Gromnica-Ihle E, Klopsch T, Demary W, Burmester GR, Zink A. Clinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low–results from RABBIT, the German biologics register. Arthritis Res Ther. 2006;8:R66. doi: 10.1186/ar1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atzeni F, Antivalle M, Pallavicini FB, Caporali R, Bazzani C, Gorla R, Favalli EG, Marchesoni A, Sarzi-Puttini P. Predicting response to anti-TNF treatment in rheumatoid arthritis patients. Autoimmun Rev. 2009;8:431–437. doi: 10.1016/j.autrev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Canhão H, Rodrigues AM, Mourão AF, Martins F, Santos MJ, Canas-Silva J, Polido-Pereira J, Pereira Silva JA, Costa JA, Araújo D, Silva C, Santos H, Duarte C, da Silva JA, Pimentel-Santos FM, Branco JC, Karlson EW, Fonseca JE, Solomon DH. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford) 2012;51:2020–2026. doi: 10.1093/rheumatology/kes184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atzeni F, Bongiovanni S, Marchesoni A, Filippini M, Caporali R, Gorla R, Cavagna L, Favalli EG, Saccardo F, Sarzi-Puttini P. Predictors of response to anti-TNF therapy in RA patients with moderate or high DAS28 scores. Joint Bone Spine. 2014;81:37–40. doi: 10.1016/j.jbspin.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Barnabe C, Homik J, Barr SG, Martin L, Maksymowych WP. The effect of different remission definitions on identification of predictors of both point and sustained remission in rheumatoid arthritis treated with anti-TNF therapy. J Rheumatol. 2014;41:1607–1613. doi: 10.3899/jrheum.131451. [DOI] [PubMed] [Google Scholar]
- 17.Flouri I, Markatseli TE, Voulgari PV, Boki KA, Papadopoulos I, Settas L, Zisopoulos D, Skopouli FN, Iliopoulos A, Bertsias GK, Geborek P, Drosos AA, Boumpas DT, Sidiropoulos P. Comparative effectiveness and survival of infliximab, adalimumab, and etanercept for rheumatoid arthritis patients in the Hellenic Registry of Biologics: Low rates of remission and 5-year drug survival. Semin Arthritis Rheum. 2014;43:447–457. doi: 10.1016/j.semarthrit.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Pers YM, Fortunet C, Constant E, Lambert J, Godfrin-Valnet M, De Jong A, Mercier G, Pallot Prades B, Wendling D, Gaudin P, Jorgensen C, Marotte H, Maillefert JF. Predictors of response and remission in a large cohort of rheumatoid arthritis patients treated with tocilizumab in clinical practice. Rheumatology (Oxford) 2014;53:76–84. doi: 10.1093/rheumatology/ket301. [DOI] [PubMed] [Google Scholar]
- 19.Nourisson C, Soubrier M, Mulliez A, Baillet A, Bardin T, Cantagrel A, Combe B, Dougados M, Flipo RM, Schaeverbeke T, Sibilia J, Vittecoq O, Ravaud P, Gottenberg JE, Mariette X, Tournadre A. Impact of gender on the response and tolerance to abatacept in patients with rheumatoid arthritis: results from the 'ORA' registry. RMD Open. 2017;3:e000515. doi: 10.1136/rmdopen-2017-000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggioggero M, Mesina F, Favalli EG. The use of rheumatic disease comorbidity index for predicting clinical response and retention rate in a cohort of rheumatoid arthritis patients receiving tumor necrosis factor alpha inhibitors. Biomed Res Int. 2019;2019:6107217. doi: 10.1155/2019/6107217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamann PDH, Pauling JD, McHugh N, Shaddick G, Hyrich K. Predictors, demographics and frequency of sustained remission and low disease activity in anti-tumour necrosis factor-treated rheumatoid arthritis patients. Rheumatology (Oxford) 2019;58:2162–2169. doi: 10.1093/rheumatology/kez188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami K, Sekiguchi M, Hirata S, Fujii T, Matsui K, Morita S, Ohmura K, Kawahito Y, Nishimoto N, Mimori T, Sano H. Predictive factors for structural remission using abatacept: Results from the ABROAD study. Mod Rheumatol. 2019;29:406–412. doi: 10.1080/14397595.2018.1482609. [DOI] [PubMed] [Google Scholar]
- 23.Wang KC, Liao HT, Chen WS, Lai CC, Chou CT, Chen MH, Tsai CY. Real-world effectiveness and safety of rituximab in the treatment of rheumatoid arthritis: a single-center experience in Taiwan. Int J Rheum Dis. 2019;22:860–868. doi: 10.1111/1756-185x.13511. [DOI] [PubMed] [Google Scholar]
- 24.Collins JE, Johansson FD, Gale S, Kim S, Shrestha S, Sontag D, Stratton J, Trinh H, Xu C, Losina E, Solomon DH. Predicting remission among patients with rheumatoid arthritis starting tocilizumab monotherapy: model derivation and remission score development. ACR Open Rheumatol. 2020;2:65–73. doi: 10.1002/acr2.11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kida D, Takahashi N, Kaneko A, Hirano Y, Fujibayashi T, Kanayama Y, Hanabayashi M, Yabe Y, Takagi H, Oguchi T, Kato T, Funahashi K, Matsumoto T, Ando M, Kuwatsuka Y, Tanaka E, Yasuoka H, Kaneko Y, Hirata S, Murakami K, Sobue Y, Nishiume T, Suzuki M, Yokota Y, Terabe K, Asai S, Ishiguro N, Kojima T. A retrospective analysis of the relationship between anti-cyclic citrullinated peptide antibody and the effectiveness of abatacept in rheumatoid arthritis patients. Sci Rep. 2020;10:19717. doi: 10.1038/s41598-020-76842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima Y, Kondo M, Shono E, Ishinishi T, Tsukamoto H, Kuroda K, Maeyama A, Harada H, Maekawa M, Shimauchi T, Nagamine R, Jojima H, Yoshizawa S, Tsuru T, Otsuka T, Miyahara H, Suematsu E, Wada K, Yoshizawa S, Inoue Y, Fukuda T, Ikemura S, Haraguchi A. Suppression of joint destruction with subcutaneous tocilizumab for Japanese patients with rheumatoid arthritis in clinical practice. Mod Rheumatol. 2020;30:807–815. doi: 10.1080/14397595.2019.1676369. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y, Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, Watanabe A, Origasa H, Shoji T, Miyasaka N, Koike T. Factors associated with successful discontinuation of certolizumab pegol in early rheumatoid arthritis. Int J Rheum Dis. 2020;23:316–324. doi: 10.1111/1756-185x.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi A, Hirata S, Kubo S, Fukuyo S, Hanami K, Nakano K, Nakayamada S, Saito K, Tanaka Y. 5-year remission rate after the discontinuation of adalimumab in patients with rheumatoid arthritis: Long-term follow-up results of the HONOR study. Mod Rheumatol. 2020;30:799–806. doi: 10.1080/14397595.2019.1702141. [DOI] [PubMed] [Google Scholar]
- 29.Kawashiri SY, Endo Y, Okamoto M, Tsuji S, Shimizu T, Sumiyoshi R, Koga T, Iwamoto N, Ichinose K, Tamai M, Nakamura H, Origuchi T, Kawakami A. Contributing factors of clinical outcomes at 1 year post-diagnosis in early rheumatoid arthritis patients with tightly controlled disease activity in clinical practice: a retrospective study. Mod Rheumatol. 2021;31:343–349. doi: 10.1080/14397595.2020.1795392. [DOI] [PubMed] [Google Scholar]
- 30.Murray K, Turk M, Alammari Y, Young F, Gallagher P, Saber T, Fearon U, Veale DJ. Long-term remission and biologic persistence rates: 12-year real-world data. Arthritis Res Ther. 2021;23:25. doi: 10.1186/s13075-020-02380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubbert-Roth A, Aletaha D, Devenport J, Sidiropoulos PN, Luder Y, Edwardes MD, Jacobs JWG. Effect of disease duration and other characteristics on efficacy outcomes in clinical trials of tocilizumab for rheumatoid arthritis. Rheumatology (Oxford) 2021;60:682–691. doi: 10.1093/rheumatology/keaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jawaheer D, Maranian P, Park G, Lahiff M, Amjadi SS, Paulus HE. Disease progression and treatment responses in a prospective DMARD-naive seropositive early rheumatoid arthritis cohort: does gender matter? J Rheumatol. 2010;37:2475–2485. doi: 10.3899/jrheum.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiper S, van Gestel AM, Swinkels HL, de Boo TM, da Silva JA, van Riel PL. Influence of sex, age, and menopausal state on the course of early rheumatoid arthritis. J Rheumatol. 2001;28:1809–1816. [PubMed] [Google Scholar]
- 34.Forslind K, Hafström I, Ahlmén M, Svensson B. Sex: a major predictor of remission in early rheumatoid arthritis? Ann Rheum Dis. 2007;66:46–52. doi: 10.1136/ard.2006.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iikuni N, Sato E, Hoshi M, Inoue E, Taniguchi A, Hara M, Tomatsu T, Kamatani N, Yamanaka H. The influence of sex on patients with rheumatoid arthritis in a large observational cohort. J Rheumatol. 2009;36:508–511. doi: 10.3899/jrheum.080724. [DOI] [PubMed] [Google Scholar]
- 36.Mueller RB, Kaegi T, Finckh A, Haile SR, Schulze-Koops H, von Kempis J. Is radiographic progression of late-onset rheumatoid arthritis different from young-onset rheumatoid arthritis? Results from the Swiss prospective observational cohort. Rheumatology (Oxford) 2014;53:671–677. doi: 10.1093/rheumatology/ket399. [DOI] [PubMed] [Google Scholar]
- 37.Francisco V, Pino J, Gonzalez-Gay MA, Mera A, Lago F, Gómez R, Mobasheri A, Gualillo O. Adipokines and inflammation: is it a question of weight? Br J Pharmacol. 2018;175:1569–1579. doi: 10.1111/bph.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holt PG, Keast D. Environmentally induced changes in immunological function: acute and chronic effects of inhalation of tobacco smoke and other atmospheric contaminants in man and experimental animals. Bacteriol Rev. 1977;41:205–216. doi: 10.1128/br.41.1.205-216.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 40.Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:707–715. doi: 10.1038/ncprheum0655. [DOI] [PubMed] [Google Scholar]
- 41.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::Aid-art5>3.0.Co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Donahue KE, Schulman ER, Gartlehner G, Jonas BL, Coker-Schwimmer E, Patel SV, Weber RP, Bann CM, Viswanathan M. Comparative effectiveness of combining MTX with biologic drug therapy versus either MTX or biologics alone for early rheumatoid arthritis in adults: a systematic review and network meta-analysis. J Gen Intern Med. 2019;34:2232–2245. doi: 10.1007/s11606-019-05230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bluett J, Sergeant JC, MacGregor AJ, Chipping JR, Marshall T, Symmons DPM, Verstappen SMM. Risk factors for oral methotrexate failure in patients with inflammatory polyarthritis: results from a UK prospective cohort study. Arthritis Res Ther. 2018;20:50. doi: 10.1186/s13075-018-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burmester GR, Feist E, Kellner H, Braun J, Iking-Konert C, Rubbert-Roth A. Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA) Ann Rheum Dis. 2011;70:755–759. doi: 10.1136/ard.2010.139725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narváez J, Magallares B, Díaz Torné C, Hernández MV, Reina D, Corominas H, Sanmartí R, JM LL, Rodriguez de la Serna A, Nolla JM (2016) Predictive factors for induction of remission in patients with active rheumatoid arthritis treated with tocilizumab in clinical practice. Semin Arthritis Rheum 45:386-390. 10.1016/j.semarthrit.2015.07.001 [DOI] [PubMed]
- 46.Sokolove J, Schiff M, Fleischmann R, Weinblatt ME, Connolly SE, Johnsen A, Zhu J, Maldonado MA, Patel S, Robinson WH. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis. 2016;75:709–714. doi: 10.1136/annrheumdis-2015-207942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pongratz G, Fleck M. Anti citrullinated protein antibodies and mechanism of action of common disease modifying drugs–insights in pathomechanisms of autoimmunity. Curr Pharm Des. 2012;18:4526–4536. doi: 10.2174/138161212802502161. [DOI] [PubMed] [Google Scholar]
- 48.McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. doi: 10.1016/s0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.