Abstract
Cardiometabolic disease risk factors, including metabolic syndrome and physical inactivity, are prevalent among young adults. However, few young adults are aware of their risk status. The risk perception attitude (RPA) framework was used to categorize participants (n = 456) enrolled in a three-arm randomized controlled weight management trial by their baseline values of cardiometabolic risk perceptions and physical activity self-efficacy. Trial recruitment occurred at two universities from 2015 to 2018 and participants were randomly assigned to one of three weight management interventions: Tailored, Targeted, Control. Cross-sectional and longitudinal analyses were conducted to examine associations between RPA category (i.e., Responsive, Indifferent, Avoidant, Proactive) and physical activity behavior. At baseline, the Responsive group had the highest amount of physical activity (mean [95% CI]: 379.2 [332.6 to 425.8] min/week), the Indifferent group had the lowest (296.7 [261.98 to 331.32] min/week), and the Avoidant/Proactive groups showed intermediate values. Over 6 months, there was a significant interaction between RPA group and intervention arm on change in physical activity adjusted for age, sex, race/ethnicity, baseline body mass index, and baseline moderate-to-vigorous physical activity (p = .017). Among Tailored intervention participants only, the Proactive participants were the only group to have an increase in physical activity (19.97 min/week) and the Indifferent participants had the most significant decrease in physical activity (127.62 min/week). Results suggest the importance of early screening for young adults to help raise awareness of cardiometabolic risk and ultimately support them in health promotion efforts.
Keywords: Risk perception attitude framework, Cardiometabolic risk, Physical activity, Self-efficacy
Young adults in a tailored weight management intervention with low perceived risk of cardiometabolic disease and high physical activity self-efficacy had the greatest 6-month increase in device-measured physical activity.
Implications.
Practice: Future health promotion efforts for young adults should incorporate tailoring on cardiometabolic risk perceptions and self-efficacy to impact physical activity behavior and health outcomes.
Policy: Currently, there are no clinical preventive risk or physical activity guidelines specific to young adults, which would assist in ensuring appropriate care and health promotion recommendations.
Research: Future research is needed regarding risk screening and health communication interventions targeted to young adults ages 18–35 years.
BACKGROUND
Cardiometabolic disease risk factors among young adults include dysregulated high-density lipoprotein (HDL-C; 27%–40%), hypertension (7%–27%) [1–3], abdominal obesity (7%–24%), elevated triglycerides (9%–16%), and hyperglycemia (3%–15%) [3]. Approximately 5% of young adults aged 18–30 meet criteria for metabolic syndrome, a clustering of three or more of the five interrelated factors cited above [4]. When the age range is extended to include up to 44 years, nearly 22% of young adults meet criteria for metabolic syndrome [5], indicating young adulthood is a critical public health risk period for the development of cardiometabolic disease. The presence of cardiometabolic risk factors early in life portends increased risk throughout adulthood. For example, those with an elevated low-density lipoprotein, systolic blood pressure (SBP), or diastolic blood pressure (DBP) had increased odds of developing heart disease later in life or experiencing cardiovascular events [6, 7].
Physical activity has a number of health benefits specific to cardiometabolic risk and outcomes, including decreased cardiovascular-related mortality; prevention of hypertension and cardiovascular disease; and improvements in blood pressure, lipids, and glycemic control [8, 9]. Current USA [10] and global recommendations [11] for adults are to achieve at least 150 min of moderate-to-vigorous intensity physical activity per week. Achieving the physical activity guidelines was associated with a 66% reduction in odds of cardiometabolic dysregulation (CD) among young adults with overweight and obesity, indicating the importance of physical activity in mitigating cardiometabolic risk in high-risk groups [12].
Despite this importance, young adults may not be aware of the link between physical activity and health. Indeed, only 30% of physically inactive university students and about 60% of those meeting physical activity guidelines had knowledge that physical activity was an important factor in overall cardiovascular health [13]. Further, awareness of cardiovascular risk indicators among young adults is low and varies by risk factor and age. Overall awareness of hypertension in those aged 18–39 years was lower than for those aged ≥40 years [2], indicating that younger adults may possess an “optimism bias” [14] or may believe they are not at risk due to their age and lack of overt disease. College students perceive the severity of risk factors for cardiometabolic disease to be low [15]. The lack of awareness is especially deleterious as data suggest an increased incidence of cardiometabolic disease throughout the transition to early adulthood, especially when coupled with weight gain [16].
Frameworks and models can assist in the understanding of behavioral and cognitive responses to risk, and several have included risk appraisal as a key element [17]. Experimental evidence and meta-analyses have shown that heighted risk appraisals were associated with subsequent changes in behavior, with largest effect sizes being present when response and self-efficacy were also enhanced [17]. Further, some have proposed that risk and self-efficacy are activating mechanisms (or motivators) that relate to information-seeking behavior and message processing [18, 19]. The risk perception attitude (RPA) framework [20] categorizes individuals by their risk perceptions and self-efficacy, or confidence to make behavior change. Awareness of risk can be the gateway to making health behavior changes, with efficacy being an essential component of behavior change [20].
The RPA framework [20] conceptualizes risk perceptions as the motivators and efficacy beliefs as the facilitators of behaviors. Those who believe they are at risk for disease and also believe that they can take preventive actions to avert the threat are classified as being in the Responsive group. This group is hypothesized to take behavioral action the most. Those whose risk perceptions and efficacy beliefs are both low are thought to have neither the motivation nor the ability to act; they are classified as being in the Indifferent group, and they are hypothesized to take the least amount of preventive action. Those with high-risk perceptions and low efficacy beliefs are classified as being in the Avoidant group. Those with low-risk perceptions and high efficacy beliefs are classified as being in the Proactive group. These four groups have been shown to distinguish differences in attitudes and behavioral intentions for diabetes prevention [21], as well as meeting physical activity guidelines during pregnancy [22]. Studies have reported similar findings in other domains: breast cancer [23], HIV prevention [24], nutrition [25], climate change [26], workplace safety [27], and general information seeking [28]. Most studies using the RPA framework, however, have been cross-sectional in design.
We examined baseline risk and efficacy as they relate to both self-reported and device-measured physical activity at baseline and at 6 months. We hypothesized that: (a) the four RPA framework groups will differ from each other in physical activity at baseline regardless of assessment modality, (b) RPA group will determine level of physical activity at 6 months; however, this relation will vary by intervention group, specifically: (a) the greatest positive improvement in physical activity will occur in the Responsive group exposed to the tailored weight management intervention, (b) the least improvement in physical activity will occur in the Indifferent group exposed to the non-tailored interventions, and (c) the other combinations will occupy intermediate values.
METHODS
Study population
This paper uses data from the [29] trial in which young adults were recruited from two sites, between 2015 and 2018, to take part in a randomized controlled trial examining digital interventions for weight management. Eligibility criteria for study entry included: (a) being 18–35 years of age; (b) enrolled as a current college or university student in the District of Columbia or Boston area; (c) having a body mass index (BMI) of 25 to 45 kg/m²; (d) actively using Facebook (i.e., accessed at least one time within the last month); (e) having regular text message access; and (f) being generally healthy enough to participate in physical activity and weight loss. For more details regarding inclusion and exclusion criteria, see [29]. Participants with completed questionnaires (self-reported physical activity) and/or fasting blood samples (abdominal circumference, blood pressure, fasting glucose, HDL-C, and triglycerides) (n = 456) baseline were included in these analyses. A subset of participants (n = 405) with validated accelerometry at baseline is included in the device-measured physical activity analyses. See Table 1. The study was approved by the Institutional Review Boards of The George Washington University and the University of Massachusetts Boston. Written informed consent was obtained from study participants. Primary study outcomes are reported elsewhere [30].
Table 1.
Characteristics of the sample and by RPA framework group
| All N = 456 |
Indifferent N = 124 |
Avoidant N = 119 |
Proactive N = 152 |
Responsive N = 61 |
p | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 23.3 ± 4.4 | 22.1 ± 3.9 | 24.9 ± 4.3 | 22.3 ± 4.3 | 25.0 ± 4.2 | < .001 |
| Sex | .762 | |||||
| % Male | 98 (21.5%) | 23 (18.5%) | 25 (21.0%) | 36 (23.7%) | 14 (23.0%) | |
| % Female | 358 (78.5%) | 101 (81.5%) | 94 (79.0%) | 116 (76.3%) | 47 (77.0%) | |
| Race/ethnicity | .052 | |||||
| % Non-Hispanic Black | 91 (20.0%) | 26 (21.0%) | 34 (28.6%) | 24 (15.8%) | 7 (11.5%) | |
| % Non-Hispanic White | 225 (49.3%) | 55 (44.4%) | 51 (42.9%) | 83 (54.6%) | 36 (59.0%) | |
| % Asian/Haw Pacific Isl | 43 (9.4%) | 19 (15.3%) | 9 (7.6%) | 11 (7.2%) | 4 (6.6%) | |
| % Hispanic (any race) | 60 (13.2%) | 18 (14.5%) | 14 (11.8%) | 19 (12.5%) | 9 (14.8%) | |
| % Multiracial/Unk/Ref | 37 (8.1%) | 6 (4.8%) | 11 (9.2%) | 15 (9.9%) | 5 (8.2%) | |
| Intervention group | .430 | |||||
| % Tailored | 148 (32.5%) | 43 (34.7%) | 43 (36.1%) | 41 (27.0%) | 21 (34.4%) | |
| % Targeted | 152 (33.3%) | 38 (30.6%) | 43 (36.1%) | 50 (32.9%) | 21 (34.4%) | |
| % Control | 156 (34.2%) | 43 (34.7%) | 33 (27.7%) | 61 (40.1%) | 19 (31.1%) | |
| Other characteristics | ||||||
| Perceived Risk Index | 2.1 ± 1.2 | 1.1 ± 0.8 | 3.4 ± 0.5 | 1.3 ± 0.7 | 3.3 ± 0.5 | < .001 |
| PA Self-Efficacy | 13.3 ± 4.2 | 9.7 ± 2.2 | 10.7 ± 2.5 | 16.1 ± 2.5 | 18.6 ± 2.8 | < .001 |
Data are mean ± SD or n (%). Unadjusted four group comparisons using ANOVA for continuous variables and Pearson χ 2 for categorical variables.
Interventions
Participants were randomly assigned to receive one of three interventions administered via digital channels: (a) Targeted (or generic) treatment (n = 152), (b) Tailored (or personalized) treatment (n = 148), and (c) Contact Control (n = 156). Program content was delivered across 18 months through Facebook, text messaging, and weekly reports, with weekly contact for the first 6 months and tapering thereafter.
Targeted treatment
Participants in this intervention condition received weight loss content adapted from the Diabetes Prevention Program [31]. Weekly lesson topics (e.g., “Ways to increase motivation” “Physical Activity: Getting Started,” “Tip the Calorie Balance”) were released twice weekly on Facebook in the form of short didactic and peer-led videos modeling the key concepts for the week. Lesson topics (n = 38) focused on diet (n = 11; e.g., “Tip the Calorie Balance”), physical activity (n = 6; “Jumpstart Your Activity Plan”), behavioral/psychosocial factors (n = 19; e.g., “Ways to increase motivation”), or general greeting/conclusion (n = 2). Handouts, including goal-setting worksheets, were provided. Participants received a text message on each day of the week: reminder texts to check for new program content (n = 2); prompts for general self-monitoring (n = 1; i.e., whether weight, calorie, and/or physical activity were monitored); tips focusing on the importance of self-monitoring (n = 2); tips related to addressing high-risk weight behaviors (n = 2; e.g., late-night snacking, portion control, physical activity). Sample high-risk weight behavior messages include: “Buy single-serve bags of chips or sweets to help u practice portion control while snacking”, “Be sure to schedule exercising in ur planner!” “If time is an issue, check out what u can let go of – there is always something!” Finally, a weekly report summarizing the key lesson topic for the week was provided.
Tailored treatment
Participants in the Tailored treatment received the same content via Facebook and text messaging. The key differences in the Tailored treatment, compared with Targeted, were: (a) the tips related to addressing high-risk weight behaviors were personalized based on participant’s endorsement of their own high-risk behaviors; (b) one additional day of general monitoring; and (c) specific monitoring of weight, physical activity, and daily calories data with a text message at the end of the week providing feedback on number of days successfully monitored. For the specific monitoring, prompts were sent in the morning on 3 days per week for the participant to provide self-monitoring data on weight, physical activity, and diet (daily calories) that evening, which were used to derive a weekly personalized report. The report included both narrative and graphical feedback on weekly and week-to-date progress regarding weight, physical activity, and calories. Narrative feedback was motivational in nature (e.g., “Based on what you sent us, you exercised an average of 20 minutes per day this week. That is lower than the goal this week of 30 minutes each day on at least 5 days. This week we talked about using your thoughts to help you take charge and meet your goals. Maybe you can use this strategy in this upcoming week to see how you might change some thoughts around meeting your activity goals. For example, reflect on your thoughts. If you said something like, ‘I skipped my walk today—I’ll never succeed in this program’ use the strategies we gave you to replace that negative thought with a positive one. Next week, try to get 30 minutes of physical activity each day, for at least 5 days a week. We know you can do it!”)
Contact control treatment
Participants in the contact control treatment received healthy body weight content on the same schedule and via the same channels as the Targeted group. Text messages focused on tips related to this topic and prompt to report self-monitoring of key behaviors one time per week. The content was not weight loss focused, rather centering around the study-branded Three Pillars of Health: Mind, Body, and Energy. Sample topics include: “Time Management,” “Society and Body Attitude,” and “What Disrupts Sleep?” Text messages included the following example: “Improving sleep can help with daytime alertness and concentration, and will help u manage stress.”
Measures
Anthropometrics
Height and weight were measured in duplicate and averaged according to standard protocols. For details, see [29]. BMI was calculated using average weight (kg) and height (m2).
Cardiometabolic outcomes
Blood pressure.
Blood pressure was measured in triplicate using a digital monitor, the OMRON HEM-907XL following standard procedures [32]. Averages for each timepoint were used for analysis.
Abdominal circumference.
To obtain waist circumference measures, participants were asked to remove their shirts or expose their abdomen. The research assistant placed a measurement tape at the umbilicus verifying the tape was parallel to the floor and not twisted. This site was selected for measurement for this trial as the umbilicus is an easily identifiable and reproducible landmark [33]. Measures were taken in triplicate to the nearest 0.1 cm and averaged.
Blood samples.
Following an overnight fast of at least 8 hr, both venous and capillary blood samples were taken. HbA1c and glucose were measured at point-of-care. HDL-C and triglycerides were analyzed from serum samples.
Cardiometabolic clustering score.
A cardiometabolic clustering score (CCS) was created [12] based on whether a standard risk cutpoint was exceeded (0 = no; 1 = yes) for abdominal circumference (>102 cm [men] or >88 cm [women]), HbA1c (≥5.7%), HDL-C (<40 mg/dL [men] or <50 mg/dL [women]), SBP (≥130 mmHg), and DBP (≥85 mmHg) [4, 34]. Scores for each individual risk factor were summed to create the CCS, with scores ranging from 0 to 5. CD was defined as CCS ≥3.
Physical activity
Device-measured.
Participants were instructed to wear an ActiGraph accelerometer (wGT3X-BT) for seven days prior to randomization. Valid wear time was counted as 4 days of 10 hr per day [35], which was validated using the ActiLife software. Established thresholds of accelerometer counts were used to define moderate-to-vigorous physical activity (MVPA) with MVPA defined by ≥1,952 counts per minute [36]. To adjust for variability in number of days the ActiGraph was worn, the average daily total was multiplied by seven to obtain an average weekly total of MVPA.
Self-reported.
Participants self-reported weekly physical activity using the seven-item International Physical Activity Questionnaire (IPAQ) [37]. Questions ask about the number of days per week and the number of hours/minutes per day during which participants engage in vigorous physical activity, moderate physical activity, and walking, respectively. Responses were processed and summed using the IPAQ guidelines [38].
Self-efficacy.
Self-efficacy for physical activity was assessed across five situations (i.e., fatigue, weather, time, negative mood, vacation) [39]. Responses were summed into an index (α = 0.77). The variable for physical activity self-efficacy has a total score that ranges from 5 to 25.
Metabolic risk awareness index
Four items assess metabolic risk awareness [40, 41]: (a) Have you ever heard of the term metabolic risk; (b) Do you know what your blood pressure is; (c) Have you ever had blood work to check for cholesterol; (d) Have you ever had blood work to check for high glucose? We created an index score of Risk Awareness based on responses to all four questions, giving one point for each “Yes” for a continuous measure of 0-4 points. Responses were summed into an index (α = 0.54).
Analyses
RPA framework groups
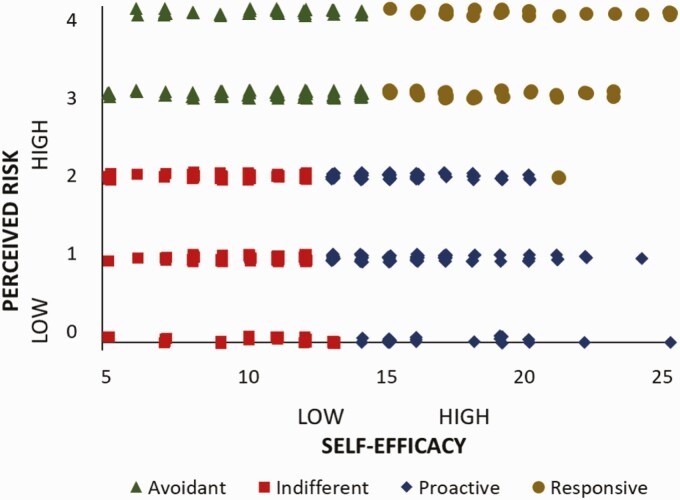
The RPA framework categorizes individuals by their risk and efficacy. See Fig. 1. To derive four RPA framework groups based on the self-efficacy and the metabolic risk awareness index (RAI) variables, we standardized the variables to a mean of 0 and SD of 1 because the two variables are not measured in the same units and could not be assumed to have equal variance. Then, we performed a cluster analysis on the standardized data to create four groupings based on the two continuous variables. The procedure used to form clusters for the four RPA framework groups was based on an algorithm that maximizes the differences across clusters (we used the SAS software’s FASTCLUS procedure). This is iteratively done by first calculating the cluster mean and then minimizing the sum of the squared distances from the cluster means [42]. A similar process of creating the four groups through a cluster analysis has been adopted in other RPA framework studies, including in the domains of HIV prevention behaviors [43, 44], diabetes control [45], and tourism safety behaviors [46]. See Table 1 and Fig. 1.
Fig. 1.
Quadrants of risk knowledge and physical activity self-efficacy.
Symbols represent individual’s baseline risk perception attitude (RPA) classification based upon self-report of perceived risk (y axis) and physical activity self-efficacy (x-axis).
Statistical analyses
Descriptive statistics are presented as percentages for categorical variables or mean ± SD for continuous variables. Comparisons between groups were computed using ANOVA for continuous variables and chi-squared tests for categorical variables. p-values < .05 were considered nominally statistically significant, with no adjustments made for multiple tests. Cross-sectional analyses at baseline included linear regression models to explore the relationship between RPA framework group and age as well as RPA framework group and physical activity. Models were adjusted for age, sex, and race/ethnicity. For longitudinal analyses over 6 months of follow-up, the outcomes of interest were change in physical activity minutes per week (M6 minus baseline) as measured by self-report using the IPAQ and by the ActiGraph accelerometer device. Covariates included age, sex, race/ethnicity, baseline BMI, and the baseline MVPA. General linear models were fit to first assess the presence of interactions between each predictor and treatment group. Next, models were fit to assess the main effect of each predictor on the two outcome variables, adjusting for treatment group in addition to the covariates specified above. Finally, the same models were employed stratified by treatment group to assess the effect of each predictor on MVPA (measured by self-report and accelerometer) adjusting for covariates within treatment group. For models with RPA framework group as the predictor, least-square means for change in activity and 95% CIs for each RPA framework group adjusted for the specified covariates were calculated.
RESULTS
Cross-sectional results
Characteristics by RPA framework group
At baseline, there were significant differences in age by RPA framework group. The Indifferent and Proactive participants were significantly younger than the Responsive and Avoidant participants (p < .001). The Avoidant participants had the highest adiposity at baseline (highest BMI, waist, and weight). There were no significant differences by sex, race/ethnicity, or intervention group (Tables 1 and 2).
Table 2.
Baseline cardiometabolic characteristics
| All | Indifferent | Avoidant | Proactive | Responsive | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | N | N | N | |||||||
| BMI (kg/m2) | 456 | 31.2 ± 4.4 | 124 | 31.2 ± 4.0 | 119 | 32.5 ± 5.0 | 152 | 30.1 ± 3.8 | 61 | 31.6 ± 5.0 | < .001 |
| Abdominal circum (cm) | 456 | 99.6 ± 11.6 | 124 | 99.5 ± 10.8 | 119 | 102.4 ± 12.5 | 152 | 97.3 ± 10.7 | 61 | 100.4 ± 12.4 | .004 |
| HbA1c (%) | 413 | 5.3 ± 0.4 | 111 | 5.3 ± 0.4 | 110 | 5.4 ± 0.4 | 137 | 5.3 ± 0.4 | 55 | 5.3 ± 0.5 | .265 |
| Systolic BP (mmHg) | 456 | 114.1 ± 11.3 | 124 | 112.0 ± 12.1 | 119 | 114.4 ± 11.8 | 152 | 114.6 ± 10.5 | 61 | 116.9 ± 10.1 | .037 |
| Diastolic BP (mmHg) | 456 | 72.6 ± 8.8 | 124 | 71.0 ± 8.9 | 119 | 74.6 ± 9.1 | 152 | 71.5 ± 8.6 | 61 | 74.7 ± 7.7 | < .001 |
| HDL cholesterol (mg/dL) | 426 | 48.6 ± 11.2 | 114 | 47.9 ± 9.2 | 107 | 47.0 ± 12.4 | 144 | 49.5 ± 11.3 | 61 | 50.7 ± 11.6 | .124 |
| Cardiometabolic clustering score | 456 | 1.65 ± 0.92 | 124 | 1.72 ± 0.85 | 119 | 1.85 ± 0.94 | 152 | 1.49 ± 0.88 | 61 | 1.56 ± 1.04 | .009 |
Data are mean ± SD or n (%). Unadjusted four group comparisons using ANOVA for continuous variables and Pearson χ 2 for categorical variables.
Differences in baseline MVPA (device-measured) among the four RPA framework groups
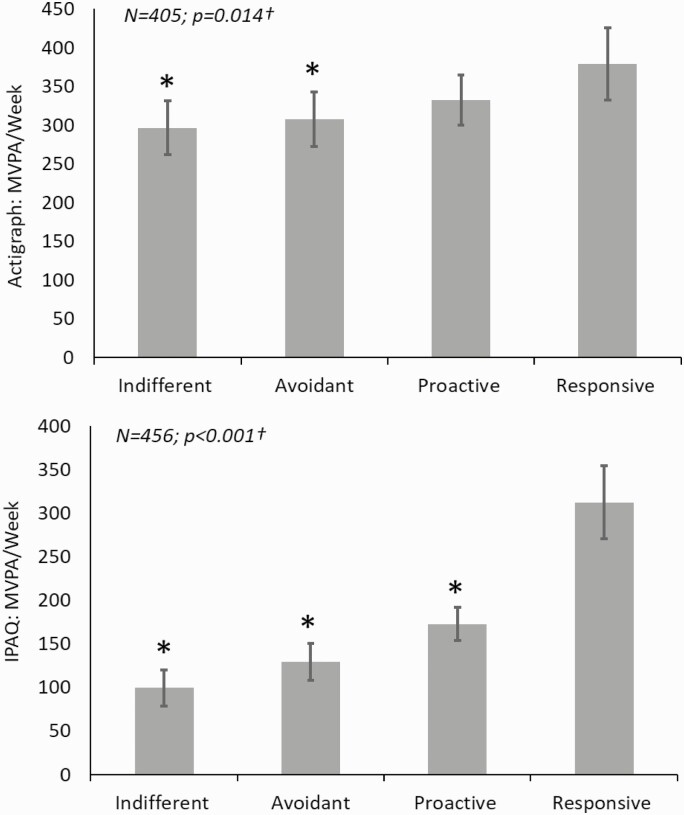
Differences were found in baseline actigraphy MVPA among the four RPA framework groups after adjusting for age, sex, and race/ethnicity. At baseline, as would be expected, the Responsive group had the highest level of physical activity (Adj mean [95% CI]; 379.2 [332.6 to 425.8] min/week), the Indifferent group had the lowest (Adj mean [95% CI]; 296.7 [261.98 to 331.32] min/week), and the other two occupied intermediate values (Fig. 2).
Fig. 2.
Differences in baseline moderate-to-vigorous physical activity (MVPA) among the four RPA framework groups.
Adjusted mean baseline physical activity by baseline RPA framework group adjusted for age, sex, race/ethnicity, and baseline BMI; †Global p-value for 4-group ANCOVA. *p < .05 for comparison of RPA framework group to the reference group (Responsive participants).
Differences in baseline MVPA (self-report) among the four RPA framework groups
Similar differences (as with baseline device-measured) were also found in baseline self-reported MVPA among the four RPA framework groups after adjusting for age, sex, and race/ethnicity. At baseline, the Responsive group reported the most physical activity (Adj mean [95% CI]; 312.51 [270.58 to 354.45] min/week), the Indifferent group the least (Adj mean [95% CI]; 99.52 [78.92 to 120.11] min/week), with the other two groups occupying intermediate values (Fig. 2).
Longitudinal results
Change in MVPA (device-measured) from baseline to month 6
We identified a significant interaction between RPA Framework group and Intervention group on change in device-measured physical activity (MVPA) over 6 months adjusted for age, sex, race/ethnicity, baseline BMI, and baseline MVPA (p = .017). Change in MVPA was significantly associated with RPA framework group only among the Tailored treatment group (p = 0.040). Among the Tailored participants, the Proactive participants were the only group to have an increase in MVPA (approximately 19.97 minutes/week) and the Indifferent participants had the most significant decrease in MVPA (approximately 127.62 min/week; Table 3).
Table 3.
Adjusted mean change in physical activity from baseline to month 6
| Adjusted mean change in MVPA per week (measured by accelerometer) p-value for treatment group by RPA framework group interaction = .0169 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alla | Tailoredb | Targetedb | Controlb | |||||||||
| p = .448 | p = .040 | p = .134 | p = .071 | |||||||||
| Adjusted mean | 95% CI | Adjusted mean | 95% CI | Adjusted mean | 95% CI | Adjusted mean | 95% CI | |||||
| Indifferent | −92.477 | −134.394 | −50.560 | −127.616 | −210.810 | −44.421 | −68.448 | −138.210 | 1.314 | −76.772 | −145.985 | −7.559 |
| Avoidant | −50.417 | −93.081 | −7.752 | −48.528 | −125.631 | 28.576 | −94.609 | −168.588 | −20.630 | 9.966 | −67.094 | 87.025 |
| Proactive | −75.842 | −115.267 | −36.417 | 19.969 | −67.436 | 107.375 | −161.697 | −223.151 | −100.244 | −81.371 | −145.126 | −17.617 |
| Responsive | −86.659 | −141.261 | −32.058 | −59.950 | −162.894 | 42.993 | −108.767 | −196.798 | −20.736 | −140.337 | −234.884 | −45.791 |
|
Adjusted mean change in MVPA per week (measured by self-report/IPAQ)
p-value for treatment group by RPA framework group interaction = .7920 |
||||||||||||
|
All
a
N = 270 |
Tailored b | Targeted b | Control b | |||||||||
| p = .420 | p = .555 | p = .915 | p = .857 | |||||||||
| Adjusted mean | 95% CI | Adjusted mean | 95% CI | Adjusted mean | 95% CI | Adjusted mean | 95% CI | |||||
| Indifferent | 43.500 | 16.459 | 70.542 | 37.451 | −9.279 | 84.180 | 56.731 | −2.740 | 116.202 | 42.442 | −8.959 | 93.843 |
| Avoidant | 45.028 | 19.801 | 70.256 | 26.234 | −16.832 | 69.299 | 63.559 | 10.160 | 116.958 | 63.960 | 12.016 | 115.904 |
| Proactive | 64.839 | 41.751 | 87.928 | 67.066 | 20.105 | 114.028 | 70.828 | 20.984 | 120.671 | 59.354 | 22.153 | 96.556 |
| Responsive | 66.367 | 32.860 | 99.874 | 48.160 | −5.416 | 101.736 | 85.035 | 12.722 | 157.349 | 78.401 | 14.829 | 141.973 |
aEffect of baseline RPA framework group on change in physical activity over 6 months adjusted for age, sex, race/ethnicity, baseline BMI, baseline MVPA, and study group.
bEffect of baseline RPA framework group on change in physical activity over 6 months adjusted for age, sex, race/ethnicity, baseline BMI, and baseline MVPA within study group.
Change in physical activity (self-report) from baseline to month 6
When assessing change in self-reported physical activity, the interaction between RPA Framework Group and Intervention group was not significant (p = .595). The main effect of RPA framework group on change in self-reported physical activity (self-report MVPA) over 6 months adjusted for age, sex, race/ethnicity, baseline BMI, baseline MVPA, and Intervention group also was not significant (p = .420). The Indifferent participants reported the smallest increase in self-reported MVPA of approximately 43 min per week; in comparison, the Responsive participants reported the largest increase in MVPA of approximately 66 min per week (Table 3).
Discussion
Study results indicate that those in the Indifferent group (low perceptions of both risk and efficacy) and Proactive group (low-risk perceptions and high efficacy beliefs) were likely to be younger than those classified as Responsive (high risk and high efficacy) or Avoidant (high risk and low efficacy). This is consistent with other data demonstrating perceptions of cardiovascular risk are higher among older college students compared with younger [47]. Those who are Responsive (or having both high risk and high efficacy) may have had experience in making behavior changes and may have had more opportunities to learn their own risk status. Young adults in earlier life stages may require education to enhance their awareness of cardiometabolic risk [48]. Currently, there are no specific guidelines for physical activity or health communication approaches focused on young adults ages 18–35 years. As informed by these results, guidelines tailored to this vulnerable demographic should be a target of future efforts.
At baseline, both device-measured and self-reported activity patterns followed expected patterns based on RPA framework groupings with the Responsive participants having the highest physical activity and Indifferent the lowest. Both Avoidant and Indifferent groups’ activity were significantly different from Responsive for both outcomes. Similar findings were noted among pregnant women with Responsive and Proactive groups being the most likely to meet physical activity guidelines [22]. Similarly, in a study of diabetes self-management, behavioral intentions were highest among Responsive and Proactive groups [21].
When examining change in physical activity from baseline to month 6, there was an RPA framework group by Intervention group interaction such that the Tailored Intervention mitigated the decline in device-measured physical activity for Proactive participants only. A similar pattern was seen when examining the self-reported physical activity outcome with Proactive participants who were part of the Tailored Intervention had the greatest increase in physical activity, although this model was not statistically significant. The intervention was designed to address weight loss and not specifically cardiometabolic risk; however, the physical activity messages were structured to be motivational and instill continued confidence in a participant’s ability to become and remain physically activity as well as participate in other healthy weight-related behaviors. It is possible that for the Proactive participants, who reported high efficacy but low-risk awareness, the intervention was a good match for their level of motivation [49]. Matching appears to be important relative to RPA Framework (or attitudinal) group, as well: those receiving a motivational message that was matched to their RPA framework group reported more positive diabetes screening attitudes than those receiving an unmatched or control message [21]. Future studies should explore tailoring on risk perception and actual risk as this may increase the salience and personal relevance.
Additionally, the intervention might have increased the salience of physical activity such that participants became more aware of their activity. It is also possible that participants were only reporting “purposeful” activity and did not quantify lifestyle activity, such as walking to class or for transport, which may have resulted in the different values reported on the ActiGraph device and via self-report. Previous work indicates that greater specificity in physical activity questionnaires is needed for measuring intervention-related change in self-reported physical activity [50]. Understanding both perceived and device-measured physical activity may be important for developing intervention materials that are salient and motivating across RPA framework groups. For example, increases in perceived (or self-reported) physical activity might better indicate intervention saliency.
We should also note that the two groups found to be affected by the intervention—the Proactive and Indifferent—share in common their low levels of risk perception and they differ in their efficacy beliefs, with the Proactive group having higher levels of self-efficacy. Our finding would thus indicate that, when risk perceptions are similar, efficacy beliefs should be the focus of intervention messaging. Those with higher efficacy levels were not only more active in the absence of any intervention (a finding from baseline), but they were also more amenable to follow the intervention’s central message. This implies that interventions developed around efficacy-based tailoring may be more potent; this is worthy of future research.
There are strengths to this investigation, including both the self-report of physical activity, combined with device-measured assessments in the form of accelerometry monitoring. This combination, along with an examination of how different groups receive and react to intervention materials based on their risk and efficacy profile, is a novel approach. We selected physical activity as the outcome of interest given the relationship between physical activity and risk mitigation, and the measure of self-efficacy was domain-specific to physical activity.
Despite these strengths, there are a few limitations. First, we did not have a measure of self-efficacy for cardiometabolic behaviors. Second, based on the assessment battery, the RAI did not include questions regarding one’s own perceived risk of metabolic syndrome. Previous work using the RPA framework has classified individuals into groups based on their perceptions of risk, but the current analysis had only questions about awareness of risk available. Physical activity was high at baseline. Although participants did not receive feedback from the ActiGraph monitor, reactivity was possible. Additionally, given the timing of the assessment battery, the days captured by the ActiGraph monitor and those reported via self-report may not have overlapped. Also, while there are benefits to using a CCS, including it being a more accurate assessment of the risk factors demonstrated by young adults [51], this limits comparison to other recognized standards such as metabolic syndrome. Further, the sample was recruited from universities and was relatively diverse, but the findings may not apply to the general population. It is also unclear the extent to which participants had knowledge of the links between physical activity and reduction in cardiometabolic risk [48], as knowledge was not measured. Studies suggest this is an important factor as those who believed in the health benefits of physical activity were three times more likely to be active than those who did not [13].
These results suggest the importance of early screening for young adults as they transition to college or the workforce to help raise awareness of cardiometabolic risk and ultimately support them in health promotion. Supporting young adults’ engagement with care for cardiometabolic disease and preventive health behaviors is essential as many fall out of care during the transition from pediatric to adult care [2, 52]. With lack of knowledge and access to care, young adults may dismiss results of screening tests thinking they are young and “invincible” [53] and have time later in life to change behavior. This is deleterious especially in light of data indicating the harmful sequelae of early exposure to CVD risk, independent of exposure later in life [6]. Young adulthood is a critical time to empower individuals with sufficient knowledge, awareness, and efficacy to engage in preventive health behaviors [54].
Acknowledgments
The authors recognize the project team members who contributed to the data collection especially Erika Blankenship, Catherine Cameron, Jamie Faro, Rachel Ingersoll, Meghan Maverdes, Benjamin Shambon, and Timothy Tsung. The authors also would like to acknowledge Jeanne Jordan, PhD, and her laboratory team. Research reported in this manuscript was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK100916 to MA Napolitano. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Melissa A Napolitano, Department of Prevention and Community Health, The George Washington University Milken Institute School of Public Health, Washington, DC, USA; Department of Exercise and Nutrition Sciences, The George Washington University Milken Institute School of Public Health, Washington, DC, USA.
Ashley Hogan Tjaden, Department of Epidemiology, The George Washington University Milken Institute School of Public Health, Washington, DC, USA.
Caitlin P Bailey, Department of Prevention and Community Health, The George Washington University Milken Institute School of Public Health, Washington, DC, USA.
Loretta DiPietro, Department of Exercise and Nutrition Sciences, The George Washington University Milken Institute School of Public Health, Washington, DC, USA.
Rajiv Rimal, Department of Prevention and Community Health, The George Washington University Milken Institute School of Public Health, Washington, DC, USA; Department of Health, Behavior, and Society, Johns Hopkins Blumberg school of Public Health, Baltimore, MD, USA.
Compliance With Ethical Standards
Conflict of Interest: The authors declare that they have no conflicts of interest to disclose.
Human Rights: This article was approved by the Institutions Review Boards of The George Washington University (#121325) and the University of Massachusetts Boston (#2014046).
Informed Consent: Participants provided informed consent before taking part in this study.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
Transparency Statements
Study registration. The study was registered at Clinical Trials.gov https://clinicaltrials.gov/ct2/show/NCT02342912.
Analytic plan pre-registration. While the analysis plan was prespecified by the Investigative team, the analysis plan was not formally pre-registered in a central repository.
Data Availability
De-identified data from this study are not available in a public archive. Deidentified data that support the findings of this study will be made available (as allowable according to institutional IRB standards) 6 months following publication for 5 years to researchers submitting a specific request and data sharing agreement to the corresponding author.
Analytic code availability. Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author.
Materials availability. Some of the materials used to conduct the study including intervention protocols, and examples of intervention content are available in [23].
References
- 1. Bucholz EM, Gooding HC, de Ferranti SD. Awareness of cardiovascular risk factors in U.S. young adults aged 18-39 years. Am J Prev Med. 2018;e67–e77. doi: 10.1016/j.amepre.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension. 2017;70(4):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nolan PB, Carrick-Ranson G, Stinear, JW, et al. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev Med Rep. 2017; 7: 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323(24): 2526–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;4(3): 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pletcher MJ, Vittinghoff E, Thanataveerat A, et al. Young adult exposure to cardiovascular risk factors and risk of events later in life: The Framingham Offspring Study. PLoS One. 2016; 11(5): e0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U. S. Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: Office of Disease Prevention and Health Promotion; 2018. [Google Scholar]
- 9. Bull F, Goenka S, Lambert V, et al. Chapter 5. Physical activity for the prevention of cardiometabolic disease. In: Prabhakaran, D., Anand, S., Gaziano, T. A., et al. , eds. Cardiovascular, Respiratory, and Related Disorders. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/ The World Bank; 2017. [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed. Washington, DC: Department of Health and Human Services, 2018. [Google Scholar]
- 11. World Health Organization. Global Recommendations on Physical Activity and Health; 2020. https://www.who.int/publications/i/item/9789241599979. Accessed December 10, 2020.
- 12. DiPietro L, Zhang Y, Mavredes M, et al. Physical activity and cardiometabolic risk factor clustering in young adults with obesity. Med Sci Sports Exerc, 2020;52(5):1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haase A, Steptoe A, Sallis JF, Wardle J. Leisure-time physical activity in university students from 23 countries: associations with health beliefs, risk awareness, and national economic development. Prev Med. 2004;39(1):182–190. [DOI] [PubMed] [Google Scholar]
- 14. Green JS, Grant M, Hill KL, et al. Heart disease risk perception in college men and women. J Am Coll Health. 2003;51(5):207–211. [DOI] [PubMed] [Google Scholar]
- 15. Antwi J, Lavin R, Sullivan S, Bellavia M. Perception of and risk factors for type 2 diabetes among students attending an upstate New York college: a pilot study. Diabetol Metab Syndr. 2020;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. J Am Med Assoc. 2017;318(3):255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychol Bull. 2014;140(2):511–543. [DOI] [PubMed] [Google Scholar]
- 18. Wilson TD. Models in information behaviour research. J Doc. 1999;55(3):249–270. [Google Scholar]
- 19. Case DO, Andrews JE, Johnson JD, Allard SL. Avoiding versus seeking: the relationship of information seeking to avoidance, blunting, coping, dissonance, and related concepts. J Med Libr Assoc. 2005;93(3):353–362. [PMC free article] [PubMed] [Google Scholar]
- 20. Rimal R. Perceived risk and efficacy beliefs as motivators of change: Use of the Risk Perception Attitude (RPA) Framework to understand health behaviors. Hum Comm Res. 2003;29:370–399. [Google Scholar]
- 21. Rains SA, Hingle MD, Surdeanu M, et al. A test of the risk perception attitude framework as a message tailoring strategy to promote diabetes screening. Health Commun. 2019;34(6):672–679. [DOI] [PubMed] [Google Scholar]
- 22. Connolly CP, Pivarnik JM, Mudd LM, et al. The influence of risk perceptions and efficacy beliefs on leisure-time physical activity during pregnancy. J Phys Act Health. 2016;13(5):494–503. [DOI] [PubMed] [Google Scholar]
- 23. Rimal RN, Juon H-S. Use of the risk perception attitude framework for promoting breast cancer prevention. J Appl Soc Psychol 2010;40(2): 287–310. [Google Scholar]
- 24. Rimal RN, Bose K, Brown J, et al. Extending the purview of the risk perception attitude framework: findings from HIV/AIDS prevention research in Malawi. Health Commun. 2009;24(3):210–218. [DOI] [PubMed] [Google Scholar]
- 25. Sullivan HW, Beckjord EB, Rutten LJF, Hesse BW. Nutrition-related cancer prevention cognitions and behavioral intentions: testing the risk perception attitude framework. Health Educ Behav, 2008;35(6):866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mead E, Roser-Renouf C, Rimal RN, et al. Information seeking about global climate change among adolescents: the role of risk perceptions, efficacy beliefs and parental influences. Atlantic Journal of Communication 2012;20(1):31–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Real K. Information seeking and workplace safety: A field application of the risk perception attitude framework. J Appl Commun Res. 2008;36:39–359. [Google Scholar]
- 28. Grasso KL, Bell RA. Understanding Health Information Seeking: A Test of the Risk Perception Attitude Framework. J Health Commun. 2015;20(12):1406–1414. [DOI] [PubMed] [Google Scholar]
- 29. Napolitano MA, Whiteley JA, Mavredes MN, et al. Using social media to deliver weight loss programming to young adults: Design and rationale for the Healthy Body Healthy U (HBHU) trial. Contemp Clin Trials. 2017;60:1–13. doi: 10.1016/j.cct.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Napolitano MA, Whiteley JA, Mavredes M, et al. Effect of tailoring on weight loss among young adults receiving digital interventions: an 18 month randomized controlled trial. Transl Behav Med. 2021. doi: 10.1093/tbm/ibab017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diabetes Prevention Program Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Examination Protocol. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 33. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: A consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85(5):1197–1202. [DOI] [PubMed] [Google Scholar]
- 34. Norberg M, Eriksson JW, Lindahl B, et al. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. J Intern Med. 2006;260(3):263–271. [DOI] [PubMed] [Google Scholar]
- 35. Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008; 40(1): 181–188. [DOI] [PubMed] [Google Scholar]
- 36. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. [DOI] [PubMed] [Google Scholar]
- 37. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 38. IPAQ. (2005). Guidelines for the Data Processing and Analysis of the International Physical Activity Questionnaire.https://www.researchgate.net/file.PostFileLoader.html?id=5641f4c36143250eac8b45b7&assetKey=AS%3A294237418606593%401447163075131
- 39. Marcus BH, Selby VC, Niaura RD, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1993;63(1):60–66. [DOI] [PubMed] [Google Scholar]
- 40. Seo Y, Kim J-S, Park E-S, Ryu E.. Assessment of the awareness and knowledge of cancer survivors regarding the components of metabolic syndrome. PLoS One. 2018;13(6):e0199142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarpong DF, Curry IY, Williams M. Assessment of knowledge of critical cardiovascular risk indicators among college students: Does stage of education matter? Int J Environ Res Public Health. 2017;14(3):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. SAS. SAS/STAT 13.2 User’s Guide, the FASTCLUS procedure. Cary, NC: SAS Institute Inc.; 2014. [Google Scholar]
- 43. Rimal RN, Brown J, Mkandawire G, et al. Audience segmentation as a social-marketing tool in health promotion: Use of the risk perception attitude framework in HIV prevention in Malawi. Am J Public Health. 2009;99:2224–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sewell WC, Patel RP, Blankeship S, et al. Associations among HIV risk perception, sexual health efficacy, and intent to use PrEP among women: An application of the risk perception attitude framework. AIDS Educ Prev. 2020;32:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Turner MM, Rimal RN, Morrison D, Kim H. The role of anxiety in seeking and retaining risk information: Testing the risk perception attitude framework in two studies. Hum Commun Res. 2006;32(2):130–156. [Google Scholar]
- 46. Wang J, Liu-Lastres B, Ritchie BW, et al. Risk reduction and adventure tourism safety: An extension of the risk perception attitude framework. Tour Manag. 2019;74:247–257. [Google Scholar]
- 47. Holt EW, Cass AL, Park H, et al. Perceived versus actual risk of cardiovascular disease in college students. Am J Health Educ. 2020;51(1):59–68. [Google Scholar]
- 48. Yahia N, Brown C, Ralpley M, Chung M. Assessment of college students’ awareness and knowledge about conditions relevant to metabolic syndrome. Diabetol Metab Syndr. 2014;6(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lippke S, Schwarzer R, Ziegelmann JP, et al. Testing stage-specific effects of a stage-matched intervention: A randomized controlled trial targeting physical exercise and its predictors. Health Educ Behav. 2010;37(4):533–546. [DOI] [PubMed] [Google Scholar]
- 50. Limb ES, Ahman S, Cook DG, et al. Measuring change in trials of physical activity interventions: A comparison of self-report questionnaire and accelerometry within the PACE-UP trial. Int J Behav Nutr Phys Act. 2019;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Magge SN, Goodman E, Armstrong SC. The metabolic syndrome in children and adolescents: Shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140(2):e20171603. [DOI] [PubMed] [Google Scholar]
- 52. Chung RJ, Mackie AS, Baker A, de Ferranti SD. Cardiovascular risk and cardiovascular health behaviours in the transition from childhood to adulthood. Can J Cardiol. 2020;36(9):1448–1457. [DOI] [PubMed] [Google Scholar]
- 53. Gooding HC, Sheldrick RC, Leslie LK, et al. Adolescent perceptions of cholesterol screening results: “Young invincibles” or developing adults? J Adolesc Health. 2016;59(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gooding H, Johnson HM. The unchartered frontier: Preventive cardiology between the ages of 15 and 35 years. Curr Cardiovasc Risk Rep. 2016;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data from this study are not available in a public archive. Deidentified data that support the findings of this study will be made available (as allowable according to institutional IRB standards) 6 months following publication for 5 years to researchers submitting a specific request and data sharing agreement to the corresponding author.
Analytic code availability. Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author.
Materials availability. Some of the materials used to conduct the study including intervention protocols, and examples of intervention content are available in [23].