Abstract
(1) Background: Carbohydrate combined with dietary fiber (DF) applied as a surrogate marker of overall carbohydrate quality is a more essential determinant of cardiometabolic health. However, to date, no studies have applied this metric to analyze its associations with poor blood pressure control in hypertensive patients. (2) Methods: A cross-sectional design was implemented in one tertiary hospital and one community hospital in China. Using Feihua Nutrition Software to analyze participants’ two-day dietary log, the quantity of carbohydrate and fiber was obtained and the carbohydrate to fiber ratio (CFR) was calculated. The participants were divided into Q1, Q2, Q3, and Q4 groups by quartile method, from low to high according to CFR. The poor systolic and diastolic blood pressure (SBP and DBP) controls were defined as ≥140 mmHg and ≥90 mmHg, respectively. (3) Results: A convenience sample of 459 participants was included and the mean CFR was 29.6. Taking Q1 as reference, after adjusting for covariates, the CFR in Q4 was associated with higher poor SBP-controlled rate (OR, 4.374; 95% CI, 2.236–8.559). Taking Q2 as reference, after adjusting for covariates, the CFRs in Q3 and Q4 were associated with higher poor DBP-controlled rates [(OR = 1.964, 95% CI: 1.016–3.795) and (OR = 4.219, 95% CI: 2.132–8.637), respectively]. The CFR was the stronger protective determinant of SBP and DBP than DF or carbohydrate alone. (4) Conclusions: A higher CFR is a stronger risk factor for blood pressure (BP) control, and low CFR foods or a combination of corresponding food components, should be recommended in the dietary management of hypertensive patients.
Keywords: dietary carbohydrate to fiber ratio, blood pressure control, essential hypertension
1. Introduction
Lifestyle changes are the cornerstone of cardiovascular disease (CVD) prevention and diet is one of the most effective strategies for blood pressure (BP) control [1]. In the past decade or so, researchers have focused their attention on low salt intake, which is the most effective lifestyle for lowering BP [2]. However, the European Society of Cardiology/European Society of Hypertension guidelines report that salt hidden in processed foods accounts for 80% of total salt intake, so it is not always easy to limit salt [3]. Therefore, there is growing interest in the potential for nutrients other than sodium to improve BP control.
Carbohydrate, as an important element of diet, is important source of energy and accounts for more than half of daily caloric intake [4,5]. Compared to the lowest quintile group, the highest quintile of carbohydrate intake (g/day) had a 34% lower risk of elevated BP [6]. However carbohydrate is a readily used source of energy and is also a risk factor of obesity [7], which further affects BP [8,9]. Thus, it is not enough to consider the effect of carbohydrate quantity alone on BP or cardiovascular health [10,11,12,13,14,15]. Studies show that some attributes of carbohydrate (such as whole grain, which is a carbohydrate rich in fiber) or total fiber have been applied as surrogate markers of overall carbohydrate quality [16], and a large amount of evidence shows this is a more important determinant of health than carbohydrate quantity alone [10,11,12,13,14,15].
Dietary fiber (DF) is the fraction of the edible part of plants that are resistant to digestion and absorption in the human small intestine [17] and can regulate the gut microbiota [18], thus potentially contributing to the modulation of the renin–angiotensin–aldosterone system and autonomic nerves [19]. It is an important component of the recommended vaso-protective dietary approaches [20]. A recent meta-analysis suggests that increasing dietary fiber intake reduces both systolic blood pressure (SBP) and diastolic blood pressure (DBP) [21]. A national cohort study from China, which included 12,177 adults without hypertension at baseline to assess the relationship between carbohydrate quality and new-onset hypertension, showed that a lower intake of high-quality carbohydrates (whole grains, legumes, or fruits) and a higher intake of low-quality carbohydrates (refined rice or noodles) increased the risk of hypertension [22]. However, compared to individual measures of carbohydrate or fiber, the dietary carbohydrate to fiber ratio (CFR) provides a more holistic picture of a patient’s diet [23]. A higher dietary CFR means a diet having a lower intake of whole foods, while a lower dietary CFR ratio may indicate an increased consumption of whole foods [23]. Fontanelli et al. took grain foods having a dietary CFR of ≤10:1 to identify healthy foods and determined their association with cardiometabolic protective factors [24]. However, to date, despite evidence implicating carbohydrate and fiber intake in the shared pathogenesis of hypertension [25,26], no studies have combined both indicators into one metric to investigate its relationship with BP control in patients with essential hypertension.
The hypothesis of this study is that, the poor BP-controlled rate increases in the higher CFR group, compared with that in the low CFR group, and this increase is greater than the individual contribution of carbohydrate or DF.
2. Materials and Methods
2.1. Design and Study Participants
This was a cross-sectional study to investigate the association between the dietary CFR and the BP control in patient with essential hypertension (EH). We enrolled patients by convenience sampling who met the inclusion criteria from Jinchang Community and No. 1 Affiliated Hospital of Soochow University in Suzhou, China, between September 2019 and August 2021. The study protocol was approved by the ethics committee of the First Affiliated Hospital of Soochow University on 27 September 2019 (ECSU-2019000148) and implemented in accordance with the Declaration of Helsinki.
The inclusion criteria were adults over 18 years old with proven diagnosis of EH according to the latest guideline of hypertension [27] and obtained informed consent. Patients (1) having secondary hypertension; (2) having severe comorbidities or complications, cognitive dysfunction, diarrhea, or other gastrointestinal diseases within the past one month [28]; (3) receiving psychotherapy within the past one month, or being pregnant or lactating women; or (4) participating in other research were excluded.
2.2. Blood Pressure
After resting for ten minutes, upper arm BP measurements were performed twice with a five-minute break between 8:30 am and 10:30 am, in a seated position with a calibrated electronic sphygmomanometer (HEM-8102A, Dalian, China) in a community service room or hospital demonstration room before taking anti-hypertensive drugs. We took the average of the two values as the final BP value.
In this study, SBP ≥ 140 mmHg was defined as poorly controlled SBP and DBP ≥ 90 mmHg was defined as poorly controlled DBP according to the Chinese definition of hypertension [27].
2.3. Dietary Fiber and Carbohydrate Intake
Participants were instructed by three well-trained researchers to record their two-day dietary log [29], which included the names and estimated quality of all foods consumed on one day of the weekend and one working day. The first diet log was collected by reviewing the previous day’s detailed diet after participants completed filling out their demographics. The second diet log was collected on a day of the following weekend by phone or WeChat. The amount of nutrient intake, including carbohydrate, DF, energy, protein, fat, cholesterol, calcium, potassium, and sodium, was calculated according to dietary records using Feihua Nutrition Software (V2.7.6.10, Beijing, China). The average of the two daily nutrient intakes was taken as the final intake. CFR = dietary carbohydrate (g/day)/fiber (g/day). Participants were divided into the four groups from low to high according to CFR: Q1, Q2, Q3, and Q4, using the quartile method.
2.4. Sociodemographic and Clinical Data
Sociodemographic data (age, sex, marital status, education, occupational status, medical payment, duration of sleep, smoking status, and alcohol drinking) and clinical data (duration of hypertension, taking anti-hypertensive drugs) were assessed by general information questionnaire. Exercise ≤3 times a week, ≤20 min each time, and continuous time ≤3 months, was considered irregular exercise; otherwise, it was considered exercise [30]. A 10-point VAS was used to assess sleep quality, and scores ≤3, 4–6, and ≥7 were considered poor, fair, and good, respectively [31]. Body height and weight were assessed by trained and quality-monitored researchers, and participants wore only light underwear and no shoes [32]. BMI is the ratio of weight (kilograms) to height (meters) squared. Constipation symptoms include infrequent bowel movements, hard stools, sensation of incomplete evacuation or blockage and, in some instances, the use of manual maneuvers to facilitate evacuation [33]. The complications of hypertension include cerebral hemorrhage, left ventricular hypertrophy, and myocardial infarction [34]. Comorbidities are diseases not related to hypertension including malignant tumors, and liver and kidney failure. The researchers helped if participants could not distinguish between complications and comorbidities. V1.0—Depression 8b and Anxiety 8a short form were used to assess depression and anxious symptoms. Both scales contain 8 items, with each item ranked from 1 to 5, and the total score for each scale is a cumulative score of 8 items [35]. The T-score was converted to the total score according to the scoring manual, with a T-score range of 37.1 to 81.1 [36]. A T score > 55 is considered depression or anxiety [35].
2.5. Statistical Analysis
Statistical analysis was performed using SPSS statistical software (version 25.0; SPSS, Inc, Chicago, IL, USA) and R programming language (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria). All p-values were 2-tailed, and a significance level of 0.05 was used.
-
(1)
Description of demographic, clinical data, and nutrients: The categorical variables were expressed as frequencies (percentages); the continuous variables were expressed as mean ± standard deviation (SD) if they conformed to normality, or quartiles M (P25, P75) if they were skewed.
-
(2)
Comparisons of demographic, clinical data, and nutrients: For continuous variables, the independent samples T test (normal), the Mann–Whitney U test (skewed), or the analysis of variance (ANOVA) were applied. For categorical variables, Pearson’s chi-square test, Yates’ correction chi-square, or Fisher’s exact test was used.
-
(3)
Restricted cubic splines (RCS) were plotted by the ggplot2 and rms packages of R software 4.0.2, with nodes assigned at the 5th, 35th, 65th, and 95th percentiles to assess the shape of the relationship between the dietary CFR (continuous data) and BP control.
-
(4)
Binary logistic regression was used to investigate the associations between the dietary CFR, carbohydrate, or DF alone, and poorly controlled rates of SBP and DBP.
“Crude” represents the use of univariate binary logistic regression. The variables with p < 0.1 in Table 1 were included in the multivariate binary logistic regression. Model 1, model 2, and model 3 were adjusted for nutrients, clinical data, and demographic data, respectively.
Table 1.
Socio-demographic, clinical characteristics, and nutrients (n = 459).
| Characteristics | SBP Control ± s/M (P25,P75)/n(%) |
DBP Control ± s/M (P25,P75)/n(%) |
|||||
|---|---|---|---|---|---|---|---|
| Poor (n = 187) | t/χ 2 /z | p | Poor (n = 179) | t/χ 2 /z | p | ||
| Demographic Information | |||||||
| Age (year) | 50.09 ± 13.90 | 1.712 a | 0.088 | 46.15 ± 11.56 | 7.393 a | <0.001 *** | |
| Sex | Male | 115 (61.5) | 0.210 b | 0.647 | 124 (69.3) | 5.350 b | 0.021 * |
| Marital status | Single | 1 (0.5) | 11.327 c | 0.001 ** | 5 (2.8) | 1.221 c | 0.625 |
| Married | 185 (98.9) | 173 (96.6) | |||||
| Others | 1 (0.5) | 1 (0.6) | |||||
| Education degree | High school and above | 109 (58.3) | 1.859 b | 0.173 | 121 (67.6) | 20.402 b | <0.001 *** |
| Occupational status | On the job | 121 (64.7) | 1.922 c | 0.393 | 143 (79.9) | 40.333 c | <0.001 *** |
| Retired | 62 (33.2) | 34 (19.0) | |||||
| No job | 4 (2.1) | 2 (1.1) | |||||
| Medical payment method | Medical Insurance | 12 (6.4) | 1.097 b | 0.578 | 11 (6.1) | 2.135 b | 0.344 |
| Agricultural insurance | 14 (7.5) | 11 (6.1) | |||||
| Self-paying | 161 (86.1) | 157 (87.7) | |||||
| Regular exercise | No | 137 (73.3) | 0.058 b | 0.810 | 131 (73.2) | 0.069 b | 0.793 |
| Duration of sleep (h/day) | 6.82 ± 1.07 | 0.445 a | 0.657 | 6.90 ± 1.05 | −0.923 a | 0.357 | |
| Quality of sleep | Good | 16 (8.6) | 2.099 b | 0.350 | 14 (7.8) | 0.916 b | 0.633 |
| Fair | 113 (60.4) | 111 (62.0) | |||||
| Poor | 58 (31.0) | 54 (30.2) | |||||
| Smoking status | Yes | 49 (26.2) | 0.041 b | 0.840 | 60 (33.5) | 9.375 b | 0.002 ** |
| Alcohol drinking | Yes | 47 (25.1) | 0.236 b | 0.627 | 59 (33.0) | 13.031 b | <0.001 *** |
| Clinical Information | |||||||
| BMI (kg/m2) | 25.15 (23.35, 27.43) |
−1.757 d | 0.079 | 25.35 (23.84, 27.83) |
−3.572 d | <0.001 * | |
| Constipation | Yes | 25 (13.4) | 5.949 b | 0.015 * | 21 (11.7) | 1.931 b | 0.165 |
| Duration of HTN (year) | 2.00 (0.50, 7.00) |
−3.852 d | <0.001 *** | 2.00 (0.50, 7.00) |
−3.593 d | <0.001 *** | |
| Taking drugs | Yes | 130 (69.5) | 11.021 b | 0.001 ** | 118 (65.9) | 21.839 b | <0.001 *** |
| Complication | Yes | 4 (2.1) | 1.793 e | 0.181 | 1 (0.6) | 0.172 e | 0.678 |
| Comorbidity | Yes | 27 (14.4) | 0.234 b | 0.629 | 21 (11.7) | 0.792 b | 0.373 |
| Anxiety | Yes | 45 (24.1) | 6.432 b | 0.011 * | 44 (24.6) | 7.147 b | 0.008 ** |
| Depression | Yes | 52 (27.8) | 13.460 b | <0.001 *** | 54 (30.2) | 20.757 b | <0.001 *** |
| Nutrition intake (d) | |||||||
| Energy (kcal) | 1966.12 ± 329.44 | 1.597 a | 0.111 | 2005.84 ± 348.66 | −0.303 a | 0.762 | |
| Protein (g) | 72.28 ± 22.02 | 1.582 a | 0.114 | 76.71 ± 22.64 | −1.890 a | 0.059 | |
| Fat (g) | 69.29 ± 19.37 | −0.395 a | 0.693 | 70.75 ± 19.49 | −1.631 a | 0.104 | |
| Cholesterol (mg) | 377.29 ± 226.50 | −1.932 a | 0.054 | 379.70 ± 238.81 | −2.056 a | 0.040 * | |
| Calcium (mg) | 456.66 ± 281.03 | 3.866 a | <0.001 *** | 620.03 ± 595.27 | −1.416 a | 0.157 | |
| Potassium (mg) | 1656.10 ± 483.42 | 1.466 a | 0.143 | 1722.64 ± 445.44 | −1.138 a | 0.256 | |
| Sodium (mg) | 2126.86 ± 631.48 | −2.135 a | 0.033 * | 2008.35 ± 843.01 | 0.418 a | 0.676 | |
Notes: ***: p < 0.001; **: p < 0.01; *: p < 0.05. SBP, systolic blood pressure; DBP, diastolic blood pressure; HTN: hypertension; Taking drugs means taking antihypertensive drugs; BMI, body mass index. a Independent-samples T test; b Pearson chi-square; c Fisher’s exact test; d Mann–Whitney U; M (P25, P75), median (25th and 75th percentiles); e Yates’ correction chi-square.
3. Results
3.1. General Characteristics of Participants
A total of 459 EH participants were included in the analysis. The shorter duration of hypertension, not taking anti-hypertensive drugs, and having anxiety and depression were related with the poor control of both SBP and DBP. Participants with poor SBP control were more likely to be married and have constipation, lower calcium intake, and higher sodium intake, while those with poor DBP control were younger, more likely to be male, had a higher education level, worked, were more likely to smoke and drink, and had higher BMI and cholesterol intake (Table 1).
3.2. The Status of Carbohydrate, Dietary Fiber Intake and CFR
The average values of dietary carbohydrate and DF of total participants were 286.12 and 11.24 g/day, respectively. The average quantities of carbohydrate intake in Q1, Q2, Q3, and Q4 were 252.49 ± 91.92, 274.29 ± 66.61, 309.41 ± 77.71, and 308.49 ± 63.18 g/day, respectively; whereas those of DF were 16.02 ± 5.48, 11.91 ± 3.10, 10.18 ± 2.72, and 6.82 ± 2.21 g/day, respectively. The average value of CFR was 29.56. The dietary CFRs in Q1, Q2, Q3, and Q4 were <20.66, 20.66 to < 26.69, 26.69 to < 36.05, and ≥36.05, respectively. Regarding the increase in the dietary CFR, the carbohydrate intake increased (F = 15.438, p < 0.001), while the DF decreased (F = 129.740, p < 0.001) (Table 2 and Table 3).
Table 2.
The status of carbohydrate and dietary fiber intake.
| Group | Carbohydrate (g/day) | Fiber (g/day) | ||||||
|---|---|---|---|---|---|---|---|---|
| Range | ± S | F | p | Range | ± S | F | p | |
| Q1 | (77.00, 559.00) | 252.49 ± 91.92 | 15.438 | <0.001 *** | (7.30, 28.85) | 16.02 ± 5.48 | 129.740 | <0.001 *** |
| Q2 | (135.00, 454.00) | 274.29 ± 66.61 | (5.40, 21.60) | 11.91 ± 3.10 | ||||
| Q3 | (149.00, 515.00) | 309.41 ± 77.71 | (4.50, 18.50) | 10.18 ± 2.72 | ||||
| Q4 | (191.00, 503.00) | 308.49 ± 63.18 | (2.70, 11.40) | 6.82 ± 2.21 | ||||
Notes: Q: Quintile; Q1, P0−P25; Q2, P25−P50; Q3, P50–P75; Q4, P75–P100. ***: p < 0.001.
Table 3.
The rates of poor blood pressure control in different dietary carbohydrate to fiber ratios.
| Carbohydrate to Fiber Ratio | SBP Control, n(%) | DBP Control, n(%) | ||||
|---|---|---|---|---|---|---|
| Poor (n = 187) | χ 2 | p | Poor (n = 179) | χ 2 | p | |
| Q1 (<20.66) | 36 (31.8) | 25.265 | <0.001 *** | 43 (38.0) | 3.473 | 0.324 |
| Q2 (20.66 to <26.69) | 42 (36.2) | 39 (33.6) | ||||
| Q3 (26.69 to <36.05) | 40 (34.7) | 45 (39.1) | ||||
| Q4 (≥36.05) | 69 (60.0) | 52 (45.2) | ||||
Notes: Q1, P0−P25; Q2, P25−P50; Q3, P50–P75; Q4, P75–P100. ***: p < 0.001. SBP, systolic blood pressure; DBP, diastolic blood pressure.
3.3. The Dietary Carbohydrate to Fiber Ratio and the Poor BP-Controlled Rate
In this study, 187 (40.7%) patients had poor SBP control and 179 (39.0%) had poor DBP control. The highest poorly controlled SBP and DBP rates were in Q4 (60.0% and 45.2%), while the lowest poor SBP-controlled rate and poor DBP-controlled rate were found in Q1 (31.8%) and Q2 (33.6%), respectively (Table 3).
3.4. The Association between the Dietary Carbohydrate to Fiber Ratio and SBP Control
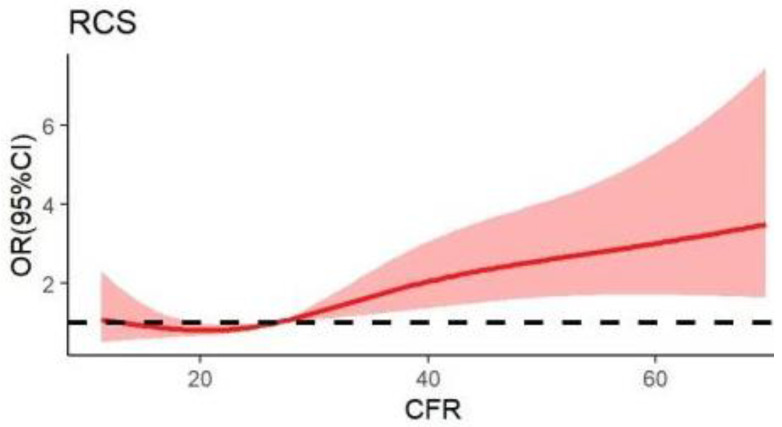
Unadjusted multivariate RCS analysis showed a non-linear relationship between the continuous variable CFR and the poor SBP control (Figure 1). A CFR of 26.29 was the lowest rate of poor SBP control.
Figure 1.
Correlation curve between the carbohydrate to dietary fiber ratio (CFR) and systolic blood pressure (SBP) control. The solid line indicates the estimated SBP control risk and the shaded area indicates the 95% confidence interval (CI).
First of all, the continuous variable CFR was positively correlated with poor control of SBP after adjusting for variables (OR = 1.037, 95% CI: 1.019–1.055). Secondly, in univariate logistic regression, taking Q1 as the reference group, the dietary CFR in Q4 was significantly associated with the poorly controlled SBP rate. After adjusting for nutrient intake, demographics, and clinical indicators, the dietary CFR in Q4 was still significantly associated with the poorly controlled SBP rate (OR = 4.374, 95% CI: 2.236–8.559). In addition, the association between DF or carbohydrate alone and the poor BP-controlled rate was analyzed separately. The results indicated that after adjusting for the same covariates, the higher the DF intake, the better the control of SBP (OR= 0.868, 95% CI: 0.814–0.927); carbohydrate intake was not associated with SBP control (OR = 1.001, 95% CI: 0.998–1.005) (Table 4).
Table 4.
Odds ratios and 95% confidence intervals for SBP according to different CFRs.
| Variable | Crude | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Dietary CFR | 1.029 (1.015, 1.044) *** | 1.027 (1.011, 1.043) ** | 1.037 (1.020, 1.055) *** | 1.037 (1.019, 1.055) *** | |
| Group by quartile of CFR | Q1 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Q2 | 1.263 (0.730, 2.182) | 1.208 (0.686, 2.128) | 1.212 (0.662, 2.216) | 1.118 (0.602, 2.078) | |
| Q3 | 1.170 (0.675, 2.029) | 1.119 (0.620, 2.021) | 1.323 (0.706, 2.480) | 1.224 (0.645, 2.324) | |
| Q4 | 3.365 (1.952, 5.800) *** | 3.200 (1.752, 5.847) *** | 4.522 (2.356, 8.679) *** | 4.374 (2.236, 8.559) *** | |
| Carbohydrate | Alone | 0.998 (0.995, 1.001) | 0.999 (0.996, 1.002) | 1.001 (0.998, 1.004) | 1.001 (0.998, 1.005) |
| Fiber | Alone | 0.879 (0.835, 0.924) *** | 0.887 (0.839, 0.939) *** | 0.873 (0.822, 0.927) *** | 0.868 (0.814, 0.927) *** |
| Covariates | Cholesterol (mg/day) | - | 1.001 (1.000, 1.002) ** | - | - |
| Calcium (mg/day) |
- | 0.999 (0.999, 1.000) * | 0.999 (0.999, 1.000) ** | 0.999 (0.999, 1.000) * | |
| Duration of HTN (year) | - | - | 0.962 (0.932, 0.992) * | 0.967 (0.936, 0.999) * | |
| Taking drugs | - | - | 0.380 (0.219, 0.660) ** | 0.384 (0.221, 0.669) ** | |
| Depression | - | - | 1.969 (1.105, 3.510) * | 1.861 (1.040, 3.328) * | |
Notes: SBP, systolic blood pressure; CFR, dietary carbohydrate to fiber ratio; ***: p < 0.001; **: p < 0.01; *: p < 0.05. Model 1, Adjusted for cholesterol, calcium, sodium. Model 2, Adjusted for variables in model 1 + body mass index (BMI), constipation, duration of hypertension (HTN), taking antihypertensive drugs, depression, anxiety. Model 3, Adjusted for variables in model 1 + model 2 + age, marital status.
3.5. The Association between the Dietary Carbohydrate to Fiber Ratio and DBP Control
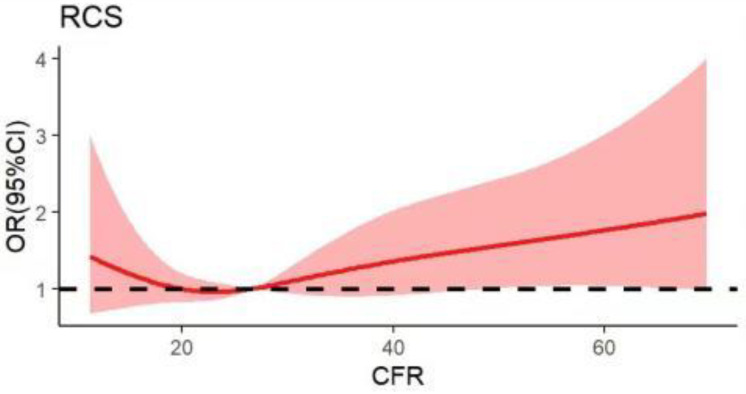
Unadjusted multivariate RCS analysis showed a non-linear relationship between continuous variable CFR and DBP control (Figure 2). A CFR of 26.69 was the lowest rate of poor DBP control.
Figure 2.
Correlation curve between the carbohydrate to dietary fiber ratio (CFR) and diastolic blood pressure (DBP) control. The solid line indicates the estimated SBP control risk and the shaded area indicates the 95% confidence interval.
First, the continuous variable CFR was positively correlated with poor control of DBP after adjusting for variables (OR = 1.033, 95% CI: 1.015–1.051). Secondly, in univariate logistic regression, taking Q2 as the reference group, the dietary CFRs in remaining groups were not significantly associated with the poorly controlled DBP rate. However, after adjusting for nutrient intake, demographics, and clinical indicators, the CFRs in Q3 (OR = 1.964, 95% CI: 1.016–3.795) and Q4 (OR = 4.291, 95% CI: 2.132–8.637) were significantly associated with the poorly controlled DBP rates. In addition, the association between DF or carbohydrate alone and the poor DBP-controlled rate was analyzed separately. The results indicated that after adjusting for the same covariates, the higher the DF intake, the better the control of DBP (OR = 0.920, 95% CI: 0.869–0.974); carbohydrate intake was not associated with SBP control (OR = 1.004, 95% CI: 1.000–1.007) (Table 5).
Table 5.
Odds ratios and 95% confidence intervals for DBP according to different CFRs.
| Variable | Crude | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Dietary CFR | 1.011 (0.998, 1.024) | 1.016 (1.002, 1.031) * | 1.027 (1.012, 1.043) ** | 1.033 (1.015, 1.051) *** | |
| Group by quartile of CFR | Q2 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Q1 | 1.164 (0.678, 1.997) | 1.133 (0.655, 1.960) | 1.246 (0.695, 2.232) | 1.213 (0.644, 2.283) | |
| Q3 | 1.253 (0.732, 2.145) | 1.466 (0.843, 2.550) | 1.962 (1.072, 3.591) * | 1.964 (1.016, 3.795) * | |
| Q4 | 1.634 (0.958, 2.787) | 2.070 (1.179, 3.635) * | 3.179 (1.693, 5.968) *** | 4.291 (2.132, 8.637) *** | |
| Carbohydrate | Alone | 0.999 (0.997, 1.002) | 0.999 (0.996, 1.002) | 1.001 (0.998, 1.005) | 1.004 (1.000, 1.007) |
| Fiber | Alone | 0.966 (0.925, 1.008) | 0.933 (0.888, 0.980) ** | 0.923 (0.876, 0.973) ** | 0.920 (0.869, 0.974) ** |
| Covariates | Duration of HTN (year) | - | - | 0.961 (0.931, 0.991) * | - |
| Taking drugs | - | - | 0.408 (0.243, 0.686) ** | 0.493 (0.281, 0.868) * | |
| Depression | - | - | 2.547 (1.447, 4.484) ** | 2.292 (1.252, 4.194) ** | |
| Age | - | - | - | 0.964 (0.942, 0.986) ** | |
| Education degree | - | - | - | 0.561 (0.335, 0.939) * | |
| Retired | - | - | - | 0.528 (0.297, 0.939) * | |
| Alcohol drinking | - | - | - | 2.225 (1.332, 3.716) ** | |
Notes: DBP, diastolic blood pressure; CFR, dietary carbohydrate to fiber ratio; ***: p < 0.001; **: p < 0.01; *: p < 0.05. Model 1, Adjusted for protein, cholesterol. Model 2, Adjusted for variables in model 1 + body mass index (BMI), duration of hypertension (HTN), taking antihypertensive drugs, depression, anxiety. Model 3, Adjusted for variables in model 1 + model 2 + age, sex, education degree, occupational status, smoking status, alcohol drinking.
4. Discussion
The dietary CFR is an indicator used to define carbohydrate quality. Higher ratios, reflective of a poorer carbohydrate quality diet, have been associated with higher risk of T2DM [12] and coronary heart disease (CHD) [13]. A previous study identified grain foods having a dietary CFR ≤ 10:1 as being healthy foods, and found that a higher intake of these foods was associated with less atherogenic dyslipidemia and insulin resistance [24]. However, the Chinese population is accustomed to treating refined rice and noodles as staple foods, resulting in less intake of whole grain foods, so their dietary whole grain fiber intake is significantly reduced; furthermore, they are used to eating more fiber-rich vegetables, which to some extent compensates for the deficiencies of insufficient grain DF intake [37]. Thus, this study took the ratio of total carbohydrates to total DF intake as a pragmatic metric, and analyzed its relationship with the rate of poor BP control in patients with hypertension. The results showed that, compared with the group having low dietary CFR, the high dietary CFR group had a significantly higher rate of poor SBP and DBP control, which is generally in line with our research hypothesis.
4.1. General Characteristics of Participants
The average age of the participants in this study was 51.31 ± 12.62 years and the majority were male (62.7%), which is consistent with the epidemiological characteristics of hypertension in China [27,38]. Compared to participants with good control of BP, those with poorly controlled BP were more likely to be married, smoke, drink alcohol, be constipated, have a higher BMI, and to not take anti-hypertensive drugs, which is consistent with the study by Tuoyire and Wu et al. [39,40]. Participants were also younger and employed [41], which are the risk factors for increased BP [42]. The BP control was rather poor for the highly educated, which was inconsistent with Sabine’s study [43]. The possible reason for this is that, compared to participants having a lower level of education, the more educated patients were more likely to be working (50.2% vs. 72.4%). Participants with shorter disease duration often have less awareness of hypertension, which may lead to poorer adherence to treatment [44], and further result in poor BP control.
It has been found that the anxiety and depression in hypertensive patients can lead to poor adherence to anti-hypertensive medication and, consequently, poor BP control [45], which is consistent with this study. The results of this study showed that higher dietary cholesterol was associated with poor BP control; this may be due to higher dietary cholesterol raising serum cholesterol levels [46], which are positively correlated with BP [47]. Convincing evidence shows that high dietary sodium intake and low potassium intake are associated with increased BP [48], and this study also supported this view.
4.2. The Status of Carbohydrate and Dietary Fiber Intake
Staple foods are mainly coarse grains in the traditional Chinese diet; therefore, DF intake increases as carbohydrate intake increases [49]. However, recently, China has experienced a shift from traditional to Western dietary patterns, with a decrease in cereal and vegetable consumption, and an increase in meat and packaged food consumption [37], which leads to significant changes in the macronutrient composition, including fiber in the diet [50]. In this study, the mean values of dietary carbohydrate, fiber, and CFR were 286.12, 11.24, and 29.56, respectively. The mean dietary carbohydrate was basically in the normal range [51], while fiber intake was significantly reduced (far lower than 25–30 g/day recommended by 2022 dietary guidelines for Chinese residents). The statuses of dietary carbohydrate and fiber intake (Table 2) indicate that, with the increase in the dietary CFR, the carbohydrate intake increased, while the DF intake decreased; this means the factors contributing to the change in dietary CFR include the changes in both carbohydrate and DF intake. At the same time, the proportion of the population having a dietary CFR < 20.66 was only 25%, which is far more than 10:1 C/F ratio [24] identified as being healthy grain foods. This indicates that low-quality carbohydrate was consumed by patients with essential hypertension.
4.3. Association between Dietary CFR and SBP
There has been an increased focus on carbohydrate quality over quantity in determining the risk of chronic disease [16]. However, carbohydrate quality is a multilayered concept [24]. First, both carbohydrate and fiber are mainly contained in plant-based foods, so the designation of carbohydrates and dietary fiber by food group (e.g., fruit, vegetables, and cereals) may be a useful indicator for assessing carbohydrate quality [52]. Second, dietary recommendations for carbohydrate quality are usually to increase dietary fiber and whole grains, and limit added sugars. Such recommendations ignore refined grain intake and the fact that products containing more fiber also contain more starch and sugar [24]. It has been found that dietary CFR is one of the simplest and most effective ratios used to establish carbohydrate quality [52], because it combines the relative contributions of starch and sugar with DF, and is more easily understood by the public.
Lower dietary CFR as a protective factor for T2DM and CHD has been proven by relevant studies. However, there is currently little evidence on the association between CFR and poor BP control. SBP is known to be an important independent risk factor for cardiovascular disease (CVD) in hypertensive patients over 50 years of age [53], and is the primary contributor to the global burden of disease [54]. In this study, lower CFR as a protective factor reduced the risk of poorly controlled SBP rate by 3.374 multiples (OR = 4.374; 95% CI: 2.236–8.559), compared with that in higher CFR. In addition, we found a stronger association between dietary CFR and the poor SBP-control rate than with DF (OR = 0.868; 95% CI: 0.814–0.927) alone or carbohydrate (OR, 1.001; 95% CI, 0.998–1.005) alone, after adjusting for covariates. This indicates a potential biological interaction between these nutrients could explain these findings.
4.4. Association between Dietary CFR and DBP
Due to the concept that “SBP is the most important”, the awareness and treatment of DBP in hypertensive patients is very low [55]; for example, according to the data of the PEACE Million People Project, in China the awareness rate is 10.3% and the untreated rate is 86.1% [56]. Concordant elevations in both SBP and DBP pose the greatest risk for cardiovascular disease-related mortality [57].
In this study, we found that compared with Q2, CFRs in Q3 (OR = 1.964; 95% CI: 1.106–3.795) and Q4 (OR = 4.291; 95% CI: 2.132–8.637) had higher poor DBP-controlled rates, after adjusting for covariates. At the same time, we found that CFRs had a stronger association than carbohydrate (OR = 1.004; 95% CI:1.000–1.007) or DF (OR = 0.920; 95% CI: 0.869–0.974) alone.
Regarding the mechanism of CFR lowering blood pressure, one area of emerging interest is intestinal dysbiosis induced by a deficit in high-glycemic/high-carbohydrate food in DF [58], which contributes to the development of hypertension. DF is fermented by the intestinal microbiota to produce short-chain fatty acids (SCFAs). Adequate DF regulates the gut microbiota ecosystem, which increases the number of bacteria that produce SCFAs and further enhances the production of SCFAs [59,60]. SCFAs activate G protein-coupled receptors (GPR43, GPR41) on kidney cells to inhibit renin secretion, resulting in lower blood pressure [61,62]. SCFAs also act on GCPR expressed in the vagal nerve ganglion, which activates the vagus nerve to lower BP [63,64]. Another mechanism may be that low carbohydrate decreases insulin resistance, which in turn reduces stimulation of endothelial function and inflammation, thus lowering BP. In addition, low carbohydrate also reduces insulin levels and further weakens sympathetic nervous system activity, thereby reducing vascular resistance and cardiac output, and promoting sodium excretion, ultimately lowering BP.
5. Conclusions
This study showed a lower dietary carbohydrate to fiber ratio had a greater protection against poor blood pressure control in patients with essential hypertension. Thus, low CFR foods or a combination of corresponding food components can be recommended in the dietary management of hypertensive patients.
6. Strengths and Limitations
To our knowledge, this is the first study to investigate the relationship between the dietary CFR and blood pressure control. We found that a low quality of carbohydrate was consumed by most participants with essential hypertension; a lower dietary CFR showed a greater protective effect against poor BP control in patients with essential hypertension. However, there are several limitations to this study that need to be considered. First, this study was a cross-sectional design and a causal relationship could not be established. Therefore, further studies are needed to assess the relationship between the CFR and BP control. Second, we used 24 h dietary recall for dietary intake assessment. Therefore, some participants may have misreported their dietary intake due to memory-related issues. Third, the effect of other micronutrients such as vitamin D and minerals on BP control was not considered, although the intakes of sodium and potassium were adjusted for in the multivariate model. Finally, this study did not consider participants’ adherence to anti-hypertensive medication, which may limit the interpretation of the effect of CFR on BP control.
Acknowledgments
We thank the patients with hypertension who volunteered to participate in this study. We also thank all of the staff in the outpatient department of the First Affiliated Hospital of Soochow University and Jinchang Community, who provided us with assistance so as to ensure that the study was conducted.
Author Contributions
Q.D.: data collecting, analyzing, and initial draft writing; L.W.: study design, methodology, data collecting; H.H., L.C. and A.L.: data collecting; C.Q.: funding; X.W.: study design and methodology; X.D.: study design, methodology, source, and supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the ethics committee of The First Affiliated Hospital of Soochow University on 27 September 2019 (ECSU-2019000148).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data in this study involves privacy issues, data should not be shared.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by hospital-level scientific research project of the First Affiliated Hospital of Soochow University (Grant number HLYJ-2022-03).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Strilchuk L., Cincione R.I., Fogacci F., Cicero A. Dietary interventions in blood pressure lowering: Current evidence in 2020. Kardiol. Pol. 2020;78:659–666. doi: 10.33963/KP.15468. [DOI] [PubMed] [Google Scholar]
- 2.Cicero A., Veronesi M., Fogacci F. Dietary Intervention to Improve Blood Pressure Control: Beyond Salt Restriction. High Blood Press. Cardiovasc. Prev. 2021;28:547–553. doi: 10.1007/s40292-021-00474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B., Mancia G., Spiering W., Agabiti R.E., Azizi M., Burnier M., Clement D.L., Coca A., de Simone G., Dominiczak A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 4.Chandel N.S. Carbohydrate Metabolism. Cold Spring Harb. Perspect. Biol. 2021;13:a040568. doi: 10.1101/cshperspect.a040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L., Wang Z., Wang H., Zhao L., Jiang H., Zhang B., Ding G. Nutrition transition and related health challenges over decades in China. Eur. J. Clin. Nutr. 2021;75:247–252. doi: 10.1038/s41430-020-0674-8. [DOI] [PubMed] [Google Scholar]
- 6.Song S., Song Y. Three types of a high-carbohydrate diet are differently associated with cardiometabolic risk factors in Korean adults. Eur. J. Nutr. 2019;58:3279–3289. doi: 10.1007/s00394-018-1871-2. [DOI] [PubMed] [Google Scholar]
- 7.Shon J., Han Y., Park Y.J. Effects of Dietary Fat to Carbohydrate Ratio on Obesity Risk Depending on Genotypes of Circadian Genes. Nutrients. 2022;14:478. doi: 10.3390/nu14030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall J.E., Mouton A.J., Da S.A., Omoto A., Wang Z., Li X., Do C.J. Obesity, kidney dysfunction, and inflammation: Interactions in hypertension. Cardiovasc. Res. 2021;117:1859–1876. doi: 10.1093/cvr/cvaa336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn S.K., Lee J.M., Ji S.M., Kim K.H., Park J.H., Hyun M.K. Incidence Hypertension and Fasting Blood Glucose from Real-World Data: Retrospective Cohort for 7-Years Follow-Up. Int. J. Environ. Res. Public Health. 2021;18:2085. doi: 10.3390/ijerph18042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te M.L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 11.Buyken A.E., Goletzke J., Joslowski G., Felbick A., Cheng G., Herder C., Brand-Miller J.C. Association between carbohydrate quality and inflammatory markers: Systematic review of observational and interventional studies. Am. J. Clin. Nutr. 2014;99:813–833. doi: 10.3945/ajcn.113.074252. [DOI] [PubMed] [Google Scholar]
- 12.Alessa H.B., Bhupathiraju S.N., Malik V.S., Wedick N.M., Campos H., Rosner B., Willett W.C., Hu F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015;102:1543–1553. doi: 10.3945/ajcn.115.116558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alessa H.B., Cohen R., Malik V.S., Adebamowo S.N., Rimm E.B., Manson J.E., Willett W.C., Hu F.B. Carbohydrate quality and quantity and risk of coronary heart disease among US women and men. Am. J. Clin. Nutr. 2018;107:257–267. doi: 10.1093/ajcn/nqx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopinath B., Flood V.M., Kifley A., Louie J.C., Mitchell P. Association Between Carbohydrate Nutrition and Successful Aging Over 10 Years. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:1335–1340. doi: 10.1093/gerona/glw091. [DOI] [PubMed] [Google Scholar]
- 15.Willett W.C., Liu S. Carbohydrate quality and health: Distilling simple truths from complexity. Am. J. Clin. Nutr. 2019;110:803–804. doi: 10.1093/ajcn/nqz215. [DOI] [PubMed] [Google Scholar]
- 16.Sawicki C.M., Lichtenstein A.H., Rogers G.T., Jacques P.F., Ma J., Saltzman E., Mckeown N.M. Comparison of Indices of Carbohydrate Quality and Food Sources of Dietary Fiber on Longitudinal Changes in Waist Circumference in the Framingham Offspring Cohort. Nutrients. 2021;13:997. doi: 10.3390/nu13030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makki K., Deehan E.C., Walter J., Backhed F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Xue Y., Cui L., Qi J., Ojo O., Du X., Liu Y., Wang X. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021;31:2458–2470. doi: 10.1016/j.numecd.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Catapano G., Pedone C., Nunziata E., Zizzo A., Passantino A., Incalzi R.A. Nutrient intake and serum cytokine pattern in elderly people with heart failure. Eur. J. Heart Fail. 2008;10:428–434. doi: 10.1016/j.ejheart.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds A.N., Akerman A., Kumar S., Diep P.H., Coffey S., Mann J. Dietary fibre in hypertension and cardiovascular disease management: Systematic review and meta-analyses. BMC Med. 2022;20:139. doi: 10.1186/s12916-022-02328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q., Liu C., Zhang S., Li R., Zhang Y., He P., Zhang Z., Liu M., Zhou C., Ye Z., et al. Dietary Carbohydrate Intake and New-Onset Hypertension: A Nationwide Cohort Study in China. Hypertension. 2021;78:422–430. doi: 10.1161/HYPERTENSIONAHA.120.16751. [DOI] [PubMed] [Google Scholar]
- 23.Makhani S.S., Davies C., George K.A., Castro G., Rodriguez D.L.V.P., Barengo N.C. Carbohydrate-to-Fiber Ratio, a Marker of Dietary Intake, as an Indicator of Depressive Symptoms. Cureus. 2021;13:e17996. doi: 10.7759/cureus.17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontanelli M.M., Micha R., Sales C.H., Liu J., Mozaffarian D., Fisberg R.M. Application of the </= 10:1 carbohydrate to fiber ratio to identify healthy grain foods and its association with cardiometabolic risk factors. Eur. J. Nutr. 2020;59:3269–3279. doi: 10.1007/s00394-019-02165-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaye D.M., Shihata W.A., Jama H.A., Tsyganov K., Ziemann M., Kiriazis H., Horlock D., Vijay A., Giam B., Vinh A., et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation. 2020;141:1393–1403. doi: 10.1161/CIRCULATIONAHA.119.043081. [DOI] [PubMed] [Google Scholar]
- 26.Kopp W. Pathogenesis and etiology of essential hypertension: Role of dietary carbohydrate. Med. Hypotheses. 2005;64:782–787. doi: 10.1016/j.mehy.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Guidelines for the Prevention and Control of Hypertension in China (2018 Revised Edition) Chin. Cardiovasc. J. 2019;24:24–56. [Google Scholar]
- 28.Bajinka O., Tan Y., Abdelhalim K.A., Ozdemir G., Qiu X. Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express. 2020;10:130. doi: 10.1186/s13568-020-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu A., Huang J., Jiang N. The effectiveness of a 3-day dietary diary intervention in peritoneal dialysis patients. Chin. J. Nurs. 2014;49:157–160. [Google Scholar]
- 30.Sharman J.E., La Gerche A., Coombes J.S. Exercise and Cardiovascular Risk in Patients with Hypertension. Am. J. Hypertens. 2015;28:147–158. doi: 10.1093/ajh/hpu191. [DOI] [PubMed] [Google Scholar]
- 31.Wang F., Luo X., Zhang J., Chen F. The use of visual analogue rating scales in the consultation of influenza patients. Chin. Gen. Pract. 2019;22:2472–2475. [Google Scholar]
- 32.Menzel J., Abraham K., Stangl G.I., Ueland P.M., Obeid R., Schulze M.B., Herter-Aeberli I., Schwerdtle T., Weikert C. Vegan Diet and Bone Health-Results from the Cross-Sectional RBVD Study. Nutrients. 2021;13:685. doi: 10.3390/nu13020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz I., Whitehead W.E., Palsson O.S., Törnblom H., Simrén M., Sahlgrenska A., Institute of Medicine D.O.I.M., Göteborgs U., Gothenburg U., Institutionen För Medicin A.F.I.O., et al. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev. Gastroent. 2020;14:39–46. doi: 10.1080/17474124.2020.1708718. [DOI] [PubMed] [Google Scholar]
- 34.Oparil S., Acelajado M.C., Bakris G.L., Berlowitz D.R., Cifkova R., Dominiczak A.F., Grassi G., Jordan J., Poulter N.R., Rodgers A., et al. Hypertension. Nat. Rev. Dis. Primers. 2018;4:18014. doi: 10.1038/nrdp.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schalet B.D., Pilkonis P.A., Yu L., Dodds N., Johnston K.L., Yount S., Riley W., Cella D. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J. Clin. Epidemiol. 2016;73:119–127. doi: 10.1016/j.jclinepi.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunderland M., Batterham P., Calear A., Carragher N. Validity of the PROMIS depression and anxiety common metrics in an online sample of Australian adults. Qual. Life Res. 2018;27:2453–2458. doi: 10.1007/s11136-018-1905-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Wang Z., Du W., Huang F., Jiang H., Bai J., Zhang X., Zhang B., Wang H. Twenty-Five-Year Trends in Dietary Patterns among Chinese Adults from 1991 to 2015. Nutrients. 2021;13:1327. doi: 10.3390/nu13041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J., Lu Y., Wang X., Li X., Linderman G.C., Wu C., Cheng X., Mu L., Zhang H., Liu J., et al. Prevalence, awareness, treatment, and control of hypertension in China: Data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 39.Tuoyire D.A., Ayetey H. Gender differences in the association between marital status and hypertension in Ghana. J. Biosoc. Sci. 2019;51:313–334. doi: 10.1017/S0021932018000147. [DOI] [PubMed] [Google Scholar]
- 40.Wu J., Li T., Song X., Sun W., Zhang Y., Liu Y., Li L., Yu Y., Liu Y., Qi C., et al. Prevalence and distribution of hypertension and related risk factors in Jilin Province, China 2015: A cross-sectional study. BMJ Open. 2018;8:e20126. doi: 10.1136/bmjopen-2017-020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu H. Age Differences in Work Stress, Exhaustion, Well-Being, and Related Factors from an Ecological Perspective. Int. J. Env. Res. Public Health. 2018;16:50. doi: 10.3390/ijerph16010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landsbergis P.A., Dobson M., Koutsouras G., Schnall P. Job strain and ambulatory blood pressure: A meta-analysis and systematic review. Am. J. Public Health. 2013;103:e61–e71. doi: 10.2105/AJPH.2012.301153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Oort S., Beulens J., van Ballegooijen A.J., Grobbee D.E., Larsson S.C. Association of Cardiovascular Risk Factors and Lifestyle Behaviors with Hypertension: A Mendelian Randomization Study. Hypertension. 2020;76:1971–1979. doi: 10.1161/HYPERTENSIONAHA.120.15761. [DOI] [PubMed] [Google Scholar]
- 44.Pan J., Wu L., Wang H., Lei T., Hu B., Xue X., Li Q. Determinants of hypertension treatment adherence among a Chinese population using the therapeutic adherence scale for hypertensive patients. Medicine. 2019;98:e16116. doi: 10.1097/MD.0000000000016116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamam M.S., Kunjummen E., Hussain M.S., Nasereldin M., Bennett S., Miller J. Anxiety, Depression, and Pain: Considerations in the Treatment of Patients with Uncontrolled Hypertension. Curr. Hypertens. Rep. 2020;22:106. doi: 10.1007/s11906-020-01117-2. [DOI] [PubMed] [Google Scholar]
- 46.Vincent M.J., Allen B., Palacios O.M., Haber L.T., Maki K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019;109:7–16. doi: 10.1093/ajcn/nqy273. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald C., Madika A., Bonnet F., Fagherazzi G., Lajous M., Boutron-Ruault M. Cholesterol and Egg Intakes, and Risk of Hypertension in a Large Prospective Cohort of French Women. Nutrients. 2020;12:1350. doi: 10.3390/nu12051350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang L., Wang H., Wang Z., Wang Y., Zhang B., Ding G. Associations of Dietary Sodium, Potassium, and Sodium to Potassium Ratio with Blood Pressure- Regional Disparities in China. Nutrients. 2020;12:366. doi: 10.3390/nu12020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhai F.Y., Du S.F., Wang Z.H., Zhang J.G., Du W.W., Popkin B.M. Dynamics of the Chinese diet and the role of urbanicity, 1991-2011. Obes. Rev. 2014;15:16–26. doi: 10.1111/obr.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bu T., Tang D., Liu Y., Chen D. Trends in Dietary Patterns and Diet-related Behaviors in China. Am. J. Health Behav. 2021;45:371–383. doi: 10.5993/AJHB.45.2.15. [DOI] [PubMed] [Google Scholar]
- 51.Yu D., He Y., Guo Q., Fang H., Xu X., Fang Y., Li J., Zhao L. Trends of energy and nutrients intake among Chinese population in 2002–2012. Wei Sheng Yan Jiu. 2016;45:527–533. [PubMed] [Google Scholar]
- 52.Comerford K.B., Papanikolaou Y., Jones J.M., Rodriguez J., Slavin J., Angadi S., Drewnowski A. Toward an Evidence-Based Definition and Classification of Carbohydrate Food Quality: An Expert Panel Report. Nutrients. 2021;13:2667. doi: 10.3390/nu13082667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaignon M.M., Mourad J.J., Guedon J. Comparative effects of antihypertensive drugs on systolic blood pressure. J. Hypertens. Suppl. 1993;11:S27–S31. doi: 10.1097/00004872-199303001-00005. [DOI] [PubMed] [Google Scholar]
- 54.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franklin S.S., Jacobs M.J., Wong N.D., L′Italien G.J., Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: Analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.HYP.37.3.869. [DOI] [PubMed] [Google Scholar]
- 56.Mahajan S., Zhang D., He S., Lu Y., Gupta A., Spatz E.S., Lu J., Huang C., Herrin J., Liu S., et al. Prevalence, Awareness, and Treatment of Isolated Diastolic Hypertension: Insights from the China PEACE Million Persons Project. J. Am. Heart Assoc. 2019;8:e12954. doi: 10.1161/JAHA.119.012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Wei F.F., Wang S., Cheng Y.B., Wang J.G. Cardiovascular risks associated with diastolic blood pressure and isolated diastolic hypertension. Curr. Hypertens. Rep. 2014;16:489. doi: 10.1007/s11906-014-0489-x. [DOI] [PubMed] [Google Scholar]
- 58.Spreadbury I. Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity. Diabetes Metab. Syndr. Obes. 2012;5:175–189. doi: 10.2147/DMSO.S33473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kristek A., Wiese M., Heuer P., Kosik O., Schär M.Y., Soycan G., Alsharif S., Kuhnle G.G.C., Walton G., Spencer J.P.E. Oat bran, but not its isolated bioactive β-glucans or polyphenols, have a bifidogenic effect in an in vitro fermentation model of the gut microbiota. Brit. J. Nutr. 2019;121:549–559. doi: 10.1017/S0007114518003501. [DOI] [PubMed] [Google Scholar]
- 60.Robles Vera I., Toral M., la Visitación N., Sánchez M., Gómez Guzmán M., Romero M., Yang T., Izquierdo Garcia J.L., Jiménez R., Ruiz Cabello J., et al. Probiotics Prevent Dysbiosis and the Rise in Blood Pressure in Genetic Hypertension: Role of Short-Chain Fatty Acids. Mol. Nutr. Food Res. 2020;64:e1900616. doi: 10.1002/mnfr.201900616. [DOI] [PubMed] [Google Scholar]
- 61.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L., Rey F., Wang T., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Natarajan N., Hori D., Flavahan S., Steppan J., Flavahan N.A., Berkowitz D.E., Pluznick J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nøhr M.K., Egerod K.L., Christiansen S.H., Gille A., Offermanns S., Schwartz T.W., Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 64.Moreira T.S., Antunes V.R., Falquetto B., Marina N. Long-term stimulation of cardiac vagal preganglionic neurons reduces blood pressure in the spontaneously hypertensive rat. J. Hypertens. 2018;36:2444–2452. doi: 10.1097/HJH.0000000000001871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study involves privacy issues, data should not be shared.