Abstract
Background: Recent studies suggest the positive role of flavonols on blood pressure (BP) values, although there are not many conducted on humans. The aim of this study was to examine the relationship between flavonol intake and their main sources of consumption, and systolic (SBP) and diastolic (DBP) BP values in coronary artery disease (CAD) patients. Methods and results: forty CAD patients completed a food-frequency questionnaire dedicated to flavonol-intake assessment. The analysis revealed significant correlation between isorhamnetin intake and SBP values—absolute (R: −0.36; 95% CI: −0.602 to −0.052; p = 0.02), and related to body mass (R: −0.38; 95% CI: −0.617 to −0.076; p = 0.02. This effect was observed in male participants (R: −0.65; 95% CI: −0.844 to −0.302; p = 0.001 and R: −0.63; 95% CI: −0.837 to −0.280; p = 0.002 respectively), but not in female patients. The main contributors were onions, tomatoes, blueberries, apples, tea, coffee and wine. White onion (R: −0.39; 95% CI: −0.624 to −0.088; p = 0.01) consumption was inversely correlated with SBP, and tomato consumption (R: −0.33; 95% CI: −0.581 to −0.020; p = 0.04) with DBP. The comparison between patients with BP < 140 mmHg and ≥140 mmHg revealed significant differences in white onion (p = 0.01) and blueberry (p = 0.04) intake. Conclusions: This study revealed the relationship between long-term dietary isorhamnetin intake and SBP values. The analysis of specific food intake showed that onion, tomato and blueberry consumption could impact BP values. This may suggest that a dietary approach which includes a higher intake of isorhamnetin-rich products could possibly result in BP lowering in CAD patients.
Keywords: flavonols, quercetin, hypertension, blood pressure, isorhamnetin
1. Introduction
Elevated blood pressure (BP) is the leading cardiovascular disease (CVD) in Poland and it is present in 9.94 million adults, which is about 26% of total Polish population [1]. The overall prevalence of hypertension in adults is around 30–45% [2]. It is also a recognized cardiovascular risk factor, which is why finding a pattern leading to this condition is the main target of primary and secondary prevention. Apart from pharmacological treatment, which is introduced after a hypertension diagnosis, lifestyle changes including dietary approach are essential to prevent this condition, and as the first-line treatment [3]. The European Society of Cardiology recommendations for hypertension management include a diet rich in vegetables and fruit, although they are not very precise [3]. Vegetables and fruits are the sources of flavonoids which are investigated in varying contexts of human health, due to their antioxidative properties. Flavonols are the group of flavonoids distinguished by their chemical structure including a 3-hydroxyflavone backbone. They differ in the presence and position of hydroxyl and methyl groups. The main flavonols are quercetin, kaempferol, isorhamnetin and myricetin, although there is a large group of flavonols which are less abundant in the everyday diet, e.g., morin, galangin, fisetin, kaempferide, azaleatin, natsudaidain, pachypodol and rhamnazin [4,5,6]. The bioactivity of each compound depends on the number and type of functional groups. Quercetin, kaempferol and myricetin differ in the number of hydroxyl groups, while isorhamnetin is O-methylated in the R3 position, compared to quercetin.
The products particularly rich in flavonols are onions, tea, and apples, although kale, lettuce, tomatoes, broccoli, grapes, berries and red wine are also known to be flavonol-rich [6,7,8]. The main dietary contributors for quercetin intake are tomatoes, kale, apples and tea; for an intake of kaempferol, kale, beans, tea, spinach, and broccoli; for isorhamnetin, pears, olive oil, wine, and tomato sauce; for myricetin intake, tea, wine, kale, oranges, and tomatoes [9].
The most investigated flavonol is quercetin. The interventional studies in humans suggest the impact of its supplementation in BP regulation [10,11,12,13]. The other flavonols have not been the subject of interventional studies in humans yet, although the studies on animal models also suggest a positive role for isorhamnetin supplementation in hypertension management [14]. What is more, its potential role as a cardioprotective, neuroprotective, anti-tumor and anti-obesity agent was also suggested in in vitro and animal model studies [15,16,17,18]. Nonetheless, the results from the only observational human study which investigated the relationship between dietary-antioxidant habitual intake and hypertension are not consistent with this, as there was no observed correlation between flavonol (quercetin, kaempferol, isorhamnetin and myricetin) intake and hypertension incidence [7]. Nonetheless, it is worth noting that general hypertension diagnosis is based on crossing the limit of 140 mmHg for systolic BP (SBP) and/or 90 mmHg for diastolic BP (DBP) [3]. There has not yet been any study investigating the linear relationship between flavonol intake and BP values in coronary artery disease (CAD).
The consumption of apples, which are a main source of flavonols, is generally advised in terms of health benefits (“an apple a day keeps a doctor away”); however, there have not been any studies which have analyzed the impact of apple consumption on BP values [19]. On the other hand, patients are often discouraged from drinking coffee, which is also a good source of flavonols, due to its potential negative impact on BP values, even though the recent studies do not confirm this [20].
The aim of this study was to analyze the impact of long-term dietary intake of the selected flavonols (quercetin, kaempferol, isorhamnetin and myricetin) and their main dietary sources, on levels of SBP and DBP among patients with CAD. Additionally, the impact of long-term consumption of apples and coffee, which are sources of flavonols, on BP values, was also investigated.
2. Materials and Methods
Forty adult patients hospitalized between March and July 2022 due to CAD were enrolled in this study. Inclusion criteria were: (1) CAD diagnosis (2) age ≥ 18 years (3) written consent (4) mental condition that enabled a one-year retrospective dietary interview. The food-frequency questionnaire dedicated to specific flavonol one-year-intake assessment was administered to the patients [21]. On the basis of this, the mean daily intake for quercetin, kaempferol, isorhamnetin, myricetin and total flavonols was calculated for each patient. The information about mean daily intake of flavonol sources was also derived from the questionnaire.
Patient weight was measured to 0.05 kg accuracy using the WTL-150A scale (Lubelskie Fabryki Wag), by a trained professional. The patient was allowed to wear only underwear for this measurement. BP was measured by a trained professional to 1 mmHg accuracy with the Omron M3 monitor (Omron Healthcare). The measurement was performed according to the European Society of Cardiology recommendations [22].
The study was approved by the local Bioethics Committee of the Medical University of Lublin (consent no. KE-0254/9/01/2022). The study was conducted in line with the directives of the Declaration of Helsinki on Ethical Principles for Medical Research. All participants signed a written consent agreement.
Statistical analyses were performed with the RStudio software v. 4.2.0. The normality of the distribution of each parameter was checked by the Shapiro–Wilk test. The variables were presented as means (SD). Pearson correlation was used to analyze the association between selected flavonol mean daily-intake and SBP or DBP, and between selected products mean daily-intake and SBP or DBP. The cut-off points used for correlation coefficient were as follows: <0.20 as low, 0.20–0.49 as moderate and ≥0.50 as high correlation. A p value below 0.05 was considered significant.
The patients were also divided into two groups due to SBP value—below 140 mmHg and 140 mmHg or higher. The differences in selected flavonol mean daily-intake and selected mean daily products between the groups were investigated by the Mann–Whitney test. A p value below 0.05 was considered significant.
3. Results
A total of 40 patients (21 men and 19 women) were enrolled in the study. The mean age was 68 (±9) years and mean weight was 80.40 (±10.30) kg. The mean daily total flavonol intake was 62.64 (±33.98) mg/day, and for specific flavonols: 29.77 (±22.18) mg/day for quercetin, 14.86 (±8.56) mg/day for kaempferol, 2.46 (±2.02) mg/day for isorhamnetin and 5.55 (±4.16) mg/day for myricetin. When the values were referred to body mass, the mean daily intake was 0.80 (±0.45) mg/kg for total flavonols, 0.51 (±0.29) mg/kg for quercetin, 0.19 (±0.11) mg/kg for kaempferol, 0.03 (±0.02) mg/kg for isorhamnetin and 0.07 (±0.05) mg/kg for myricetin. The mean measured SBP was 134.23 (±25.49) mmHg and DBP was 73.50 (±11.29) mmHg. The main contributors to flavonol intake were onions (white and red), tomatoes, blueberries, apples, tea, coffee and wine.
The results revealed a significant moderate correlation between daily isorhamnetin intake and SBP values. The relation was present in both absolute daily intake (R: −0.36; 95% CI: −0.602 to −0.052; p = 0.02) and daily intake related to body mass (R: −0.38; 95% CI −0.617 to −0.076; p = 0.02). The detailed results for all analyzed flavonols are presented in Table 1.
Table 1.
The correlation between selected flavonol daily intake and systolic and diastolic blood pressure.
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| R | 95% CI | p | R | 95% CI | p | |
| Quercetin daily intake | −0.23 | −0.506; 0.087 | 0.15 | 0.05 | −0.264; 0.357 | 0.75 |
| Kaempferol daily intake | 0.02 | −0.292; 0.331 | 0.89 | 0.19 | −0.125; 0.477 | 0.23 |
| Isorhamnetin daily intake | −0.36 | −0.602; −0.052 | 0.02 | 0.05 | −0.263; 0.359 | 0.74 |
| Myricetin daily intake | −0.08 | −0.379; 0.241 | 0.64 | 0.09 | −0.230; 0.388 | 0.59 |
| Total flavonol daily intake | −0.18 | −0.462; 0.143 | 0.28 | 0.10 | −0.222; 0.396 | 0.55 |
| Quercetin daily intake/body mass | −0.28 | −0.542; 0.037 | 0.08 | 0.04 | −0.272; 0.350 | 0.79 |
| Kaempferol daily intake/body mass | −0.05 | −0.354; 0.268 | 0.77 | 0.18 | −0.138; 0.466 | 0.26 |
| Isorhamnetin daily intake/body mass | −0.38 | −0.617; −0.076 | 0.02 | 0.07 | −0.250; 0.370 | 0.68 |
| Myricetin daily intake/body mass | −0.13 | −0.426; 0.187 | 0.41 | 0.07 | −0.247; 0.374 | 0.67 |
| Total flavonol daily intake/body mass | −0.23 | −0.503; 0.091 | 0.16 | 0.09 | −0.232; 0.387 | 0.60 |
The analysis of the male and female subgroup revealed that this effect was observed only in male participants (R: −0.65; 95% CI: −0.844 to −0.302; p = 0.001 for absolute isorhamnetin intake and R: −0.63; 95% CI: −0.837 to −0.280; p = 0.002 for related-to-body-mass isorhamnetin intake). The detailed results are presented in Table 2 and Table 3.
Table 2.
The correlation between selected flavonols daily intake and systolic and diastolic blood pressure in male participants.
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| R | 95% CI | p | R | 95% CI | p | |
| Quercetin daily intake | −0.32 | −0.661; 0.128 | 0.16 | −0.14 | −0.542; 0.307 | 0.53 |
| Kaempferol daily intake | 0.11 | −0.340; 0.515 | 0.64 | 0.01 | −0.422; 0.441 | 0.96 |
| Isorhamnetin daily intake | −0.65 | −0.844; −0.302 | 0.001 | −0.12 | −0.515; 0.340 | 0.64 |
| Myricetin daily intake | −0.06 | −0.478; 0.383 | 0.80 | −0.11 | −0.518; 0.336 | 0.63 |
| Total flavonol daily intake | −0.27 | −0.626; 0.187 | 0.24 | −0.13 | −0.530; 0.323 | 0.58 |
| Quercetin daily intake/body mass | −0.27 | −0.628; 0.183 | 0.24 | −0.08 | −0.497; 0.361 | 0.72 |
| Kaempferol daily intake/body mass | 0.16 | −0.292; 0.554 | 0.49 | 0.11 | −0.338; 0.517 | 0.63 |
| Isorhamnetin daily intake/body mass | −0.63 | −0.837; −0.280 | 0.002 | −0.07 | −0.491; 0.369 | 0.75 |
| Myricetin daily intake/body mass | −0.03 | −0.457; 0.405 | 0.89 | −0.08 | −0.491; 0.369 | 0.75 |
| Total flavonol daily intake/body mass | −0.21 | −0.585; 0.248 | 0.37 | −0.05 | −0.470; 0.392 | 0.84 |
Table 3.
The correlation between selected flavonol daily intake and systolic and diastolic blood pressure in female participants.
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| R | 95% CI | p | R | 95% CI | p | |
| Quercetin daily intake | −0.17 | −0.580; 0.308 | 0.49 | 0.30 | −0.180; 0.663 | 0.21 |
| Kaempferol daily intake | −0.09 | −0.521; 0.381 | 0.72 | 0.34 | −0.136; 0.687 | 0.16 |
| Isorhamnetin daily intake | 0.01 | −0.447; 0.461 | 0.97 | 0.34 | −0.130; 0.691 | 0.15 |
| Myricetin daily intake | −0.14 | −0.556; 0.339 | 0.58 | 0.37 | −0.101; 0.706 | 0.12 |
| Total flavonol daily intake | −0.14 | −0.556; 0.339 | 0.58 | 0.32 | −0.157; 0676 | 0.18 |
| Quercetin daily intake / body mass | −0.27 | −0.643; 0.213 | 0.27 | 0.27 | −0.206; 0.647 | 0.26 |
| Kaempferol daily intake / body mass | −0.19 | −0.591; 0.292 | 0.44 | 0.30 | −0.179; 0.664 | 0.21 |
| Isorhamnetin daily intake / body mass | −0.06 | −0.502; −0.404 | 0.80 | 0.37 | −0.105; 0.703 | 0.12 |
| Myricetin daily intake / body mass | −0.23 | −0.618; 0.252 | 0.35 | 0.32 | −0.161; 0.674 | 0.19 |
| Total flavonol daily intake / body mass | −0.24 | −0.625; 0.242 | 0.33 | 0.29 | −0.192; 0.656 | 0.23 |
The analysis of the main flavonol sources mean daily intake showed that onion (R: −0.38; 95% CI −0.616 to −0.074; p = 0.02) and white onion (R: −0.39; 95% CI: −0.624 to −0.088; p = 0.01) intake is correlated with SBP values, while tomato intake (R: −0.33; 95% CI: −0.581 to −0.020; p = 0.04) is correlated with DBP values. The detailed results for all analyzed products are presented in the Table 4.
Table 4.
The correlation between selected product daily intake and systolic and diastolic blood pressure.
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| p | 95% CI | R | p | 95% CI | R | |
| White onion | 0.01 | −0.624; −0.088 | −0.39 | 0.87 | −0.335; 0.288 | −0.03 |
| Red onion | 0.15 | 0.508; 0.084 | −0.23 | 0.34 | −0.166; 0.444 | 0.15 |
| Onion (total) | 0.02 | −0.616; −0.073 | −0.38 | 0.76 | −0.265; 0.357 | 0.05 |
| Tomatoes | 0.31 | −0.454; 0.153 | −0.17 | 0.04 | −0.581; −0.020 | −0.33 |
| Blueberry | 0.21 | −0.483; 0.116 | −0.20 | 0.79 | −0.349; 0.273 | −0.04 |
| Apples | 0.39 | −0.431; 0.181 | −0.14 | 0.68 | −0.371; 0.249 | −0.07 |
| Black tea | 0.58 | −0.228; 0.391 | 0.09 | 0.22 | −0.121; 0.480 | 0.20 |
| Green tea | 0.57 | −0.393; 0.225 | −0.09 | 0.25 | −0.132; 0.472 | 0.19 |
| Coffee | 0.97 | −0.306; 0.317 | 0.01 | 0.96 | −0.318; 0.305 | −0.01 |
| Wine | 0.89 | −0.337; 0.294 | −0.02 | 0.52 | −0.215; 0.409 | 0.11 |
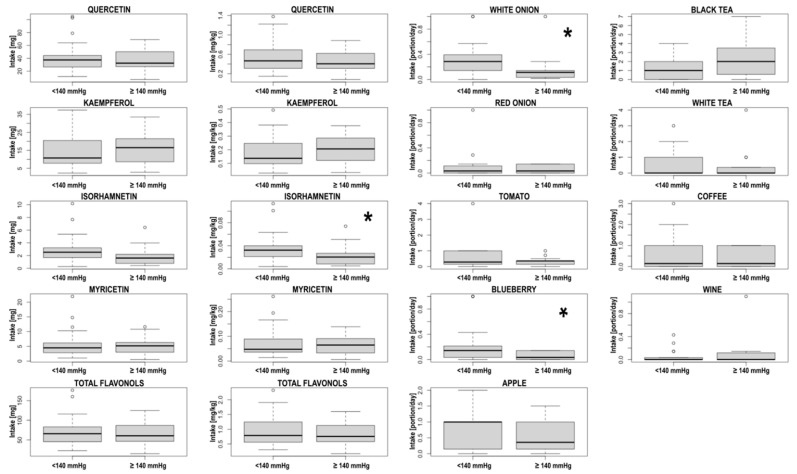
The subgroup analysis revealed significant differences in isorhamnetin intake related to body mass (p = 0.048), white onions (p = 0.01) and blueberries (p = 0.04) among the patients with normal BP (<140 mmHg) and elevated BP (≥140 mmHg). The detailed results for all compounds and food are presented in the Table 5 and Figure 1. The additional analysis between the patients consuming less than 1 apple a day and ≥1 apple daily did not show any significant differences in terms of SBP (p = 0.55) or DBP (p = 0.95). A similar observation was made for coffee consumption (p = 0.64 for SBP and p = 0.43 for DBP).
Table 5.
Differences in flavonol and selected product daily intake in patients with normal systolic blood pressure (<140 mmHg) and elevated systolic blood pressure (≥140 mmHg).
| Systolic Blood Pressure | |||||
|---|---|---|---|---|---|
| <140 mmHg | ≥140 mmHg | ||||
| Mean | SD | Mean | SD | p | |
| Quercetin [mg/day] | 42.02 | ±24.81 | 36.73 | ±17.24 | 0.61 |
| Kaempferol [mg/day] | 13.91 | ±8.66 | 16.14 | ±8.24 | 0.39 |
| Isorhamnetin [mg/day] | 2.88 | ±2.20 | 1.90 | ±1.53 | 0.08 |
| Myricetin [mg/day] | 5.66 | ±4.80 | 5.39 | ±3.02 | 0.59 |
| Total flavonols [mg/day] | 72.09 | ±39.77 | 67.57 | ±30.94 | 0.94 |
| Quercetin [mg/kg*day] | 0.55 | ±0.33 | 0.45 | ±0.22 | 0.55 |
| Kaempferol [mg/kg*day] | 0.18 | ±0.12 | 0.20 | ±0.10 | 0.52 |
| Isorhamnetin [mg/kg*day] | 0.04 | ±0.03 | 0.02 | ±0.02 | 0.048 |
| Myricetin [mg/kg*day] | 0.07 | ±0.06 | 0.07 | ±0.04 | 0.94 |
| Total flavonols [mg/kg*day] | 0.94 | ± 0.54 | 0.83 | ±0.40 | 0.68 |
| White onion [portion/day] | 0.31 | ±0.25 | 0.17 | ±0.25 | 0.01 |
| Red onion [portion/day] | 0.09 | ±0.21 | 0.07 | ±0.06 | 0.51 |
| Tomatoes [portion/day] | 0.58 | ±0.82 | 0.34 | ±0.27 | 0.64 |
| Blueberries [portion/day] | 0.24 | ±0.31 | 0.06 | ±0.06 | 0.04 |
| Apples [portion/day] | 0.68 | ±0.51 | 0.56 | ±0.47 | 0.65 |
| Black tea [portion/day] | 1.26 | ±1.22 | 2.27 | ±2.03 | 0.10 |
| Green tea [portion/day] | 0.49 | ±0.77 | 0.45 | ±1.08 | 0.44 |
| Coffee [portion/day] | 0.76 | ±0.88 | 0.39 | ±0.47 | 0.37 |
| Wine [portion/day] | 0.05 | ±0.10 | 0.11 | ±0.29 | 0.82 |
Figure 1.
Box-plots presenting differences in flavonol intake and their main dietary source consumption among patients with systolic blood pressure <140 mmHg and ≥140 mmHg (significant differences are marked with *).
4. Discussion
Flavonols are the subgroup of flavonoids which share a 3-hydroxyflavone backbone. Individual flavonols differ in their chemical structure (i.e., presence and position of hydroxyl and methyl groups) which impacts their bioactivity [23,24]. The most investigated flavonol is quercetin, and the studies in humans suggest the positive role of its supplementation in BP regulation [10,11,12,13]. The other flavonols are not as widely analyzed in this context. Most of the interventional studies about isorhamnetin impact on BP come from animal models, where isorhamnetin restored vasodilatation in hypertensive rats [14]. The mechanisms of this effect are still under investigation. However, the role of modification of protein kinases (C and Rho) activity, cytosolic H2O2 production or angiotensin-converting enzyme inhibition potential are suggested by other authors [14,25,26,27]. On the other hand, in the only observational human study which analyzed the impact of dietary antioxidant intake on hypertension, there was no correlation between general habitual dietary flavonoid intake and a reduction in incidents of hypertension [7]. A similar observation was also made in this study for specific flavonols (quercetin, kaempferol, isorhamnetin and myricetin) [7]. It is worth noting that the study was conducted on a very large population (a total of 156 957 participants from the Nurses’ Health Study and Health Professionals follow-up study); nonetheless, it was based on patients’ self-assessment and self-reporting, and analyzed the impact of the above-mentioned dietary agents only on hypertension incidence, without taking exact BP-value measurements into consideration [7]. The flavonol intake in that study was also calculated on the basis of a general semiquantitative food-frequency questionnaire, and not by the dedicated tool [7]. That is why it could possibly omit some of the subtle relationships between isorhamnetin intake and BP values shown in this study. The products particularly rich in isorhamnetin are onions (white and red), kale, asparagus, elderberry, dill and parsley [6,8].
The presented results revealed also significant differences between male and female participants in terms of the size of the effect of isorhamnetin consumption. These effects were observed only in the male subgroup, while the correlation was not significant in the female subgroup. This is an interesting observation, as in the study by Knekt et al. the effect of flavonoid intake on general CAD mortality was observed in women, but not in men [28]. It is worth noting that hypertension is a CAD risk factor, but in the study by Knekt et al. the direct BP values were not taken into consideration. However, in the studies which analyzed the impact of other flavonol intake (quercetin) directly on hypertension, it was shown that quercetin supplementation results in antihypertensive effects in men [29]. Nonetheless, the present study is the first study which has investigated the antihypertensive potential of dietary isorhamnetin in humans.
The results showed that the main contributors to flavonol intake were onions, tomatoes among the vegetables; blueberries and apples among the fruit; and tea, coffee and wine among the beverages, which matches the observations made in the Zutphen Elderly study [30]. The analysis showed that among these selected products, only onions and tomato mean daily-intakes were significantly correlated with SBP and DBP, respectively. The subgroup analysis confirmed this observation, as the patients with elevated SBP were characterized by significantly lower white onion consumption. Onion extract was already proven to have potential in BP-lowering, although the doses used in the study by Brull et al. and Kalus et al. were higher than reachable in an everyday diet [31,32]. The impact of onion and apple intake on cardiovascular mortality was shown in the study by Knekt et al. and Hertog et al. [28,30]. The subgroup analysis showed that patients with elevated and normal BP also significantly differed in terms of blueberry consumption. This relationship was not presented for berries in the above-mentioned study; however, it could be explained that in the presented study berries were divided into species, and only blueberry intake was taken into consideration, in contrast with the other studies [28]. Even though the studies did not analyze the direct values of BP, it is worth noting that hypertension is a cardiovascular risk factor. The relation between the intake of food rich in flavonoids such as onions, apples or tea, and cardiovascular risk factors (including BP) was also shown in French women, although the authors of the SU.VI.MAX study did not reveal the exact correlations [33]. The important factor could be also the preparation of the meals, including the products mentioned, as boiling could decrease the antihypertensive potential in onions [34].
Apples consumption is popular, due to the beneficial role for health. The term “an apple a day keeps a doctor away” was examined in the course of this study, and it revealed that patients who consume one apple a day or more do not have significantly lower SBP or DBP, compared to the patients with lower apple-consumption. This observation might be caused by the lower border for the minimal apple-consumption impact, as the study investigating the impact of fresh fruit intake and acute coronary syndrome proved that the level of consumption of 25 g/day reduces the risk of acute coronary syndromes [19]. What is more, coffee consumption, which is suggested to elevate BP, did not present such properties in CAD patients. The patients who drink one coffee a day or more did not have significantly higher SBP or DBP compared to the patients who drink less than one coffee a day. This observation matches the results from other studies regarding CVD risk and coffee consumption [20].
On the basis of the presented results, we can suggest that incorporating products such as onion, tomatoes and blueberries into the everyday diet could be possibly beneficial in terms of BP values. Nonetheless, a longer observation on a larger population, or ideally, a controlled prospective study is needed to support this.
In our observational study, the mean daily intake of quercetin was much lower (29.77 [±22.18] mg/day) than the supplementation doses used in randomized controlled studies in humans (50 mg to 730 mg/day), so this may explain why the results from this observation do not match the results from the meta-analysis, which showed that quercetin supplementation could decrease BP values [12]. It is also worth noting that bioavailability from an artificial supplement can differ from that from a dietary source.
Apart from the mentioned study, there are no other available observational or interventional studies focused on the impact of other flavonols (kaempferol and myricetin) on BP level.
5. Conclusions
This study revealed the relationship between long-term dietary isorhamnetin consumption and SBP values in male patients. The correlation was not proved for other flavonols or for DBP. The analysis of specific foods showed that onion, tomato and blueberry intake could impact BP values. This may suggest that a dietary approach which includes a higher intake of products rich in this compound could possibly result in BP lowering.
Acknowledgments
The authors would like to thank Grzegorz Kalisz for the support in the statistical analysis.
Author Contributions
Conceptualization, J.P.-K.; data curation, J.P.-K.; funding acquisition, E.F.; investigation, J.P.-K.; methodology, J.P.-K.; resources, J.P.-K. and P.B.; visualization, J.P.-K.; writing—original draft, J.P.-K.; writing—review and editing, J.P.-K., P.B. and E.F. All authors edited and approved the final version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of the Medical University of Lublin (consent no. KE-0254/9/01/2022).
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the Ministry of Education and Science in Poland within the statutory activity of the Medical University of Lublin (DS 472/2022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Narodowy Fundusz Zdrowia . NFZ o Zdrowiu. Nadciśnienie Tętnicze. Narodowy Fundusz Zdrowia; Warsaw, Poland: 2019. [Google Scholar]
- 2.Chow C.K., Teo K.K., Rangarajan S., Islam S., Gupta R., Avezum A., Bahonar A., Chifamba J., Dagenais G., Diaz R., et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 3.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D., Coca A., De Simone G., Dominiczak A., et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) Blood Press. 2018;27:314–340. doi: 10.1080/08037051.2018.1527177. [DOI] [PubMed] [Google Scholar]
- 4.Zamora-Ros R., Andres-Lacueva C., Lamuela-Raventós R.M., Berenguer T., Jakszyn P., Barricarte A., Ardanaz E., Amiano P., Dorronsoro M., Larrañaga N., et al. Estimation of Dietary Sources and Flavonoid Intake in a Spanish Adult Population (EPIC-Spain) J. Am. Diet. Assoc. 2010;110:390–398. doi: 10.1016/j.jada.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Sampson L., Rimm E., Hollma P.C.H., de Vries J.H.M., Katan M.B. Flavonol and Flavone Intakes in US Health Professionals. J. Am. Diet. Assoc. 2002;102:1414–1420. doi: 10.1016/S0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 6.Zamora-Ros R., Knaze V., Luján-Barroso L., Slimani N., Romieu I., Fedirko V., Santucci de Magistris M., Ericson U., Amiano P., Trichopoulou A., et al. Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24 hour dietary recall cohort. Br. J. Nutr. 2011;106:1915–1925. doi: 10.1017/S000711451100239X. [DOI] [PubMed] [Google Scholar]
- 7.Cassidy A., O’Reilly É.J., Kay C., Sampson L., Franz M., Forman J., Curhan G., Rimm E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011;93:338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland T.M., Agarwal P., Wang Y., Leurgans S.E., Bennett D.A., Booth S.L., Morris M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology. 2020;94:e1749–e1756. doi: 10.1212/WNL.0000000000008981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conquer J.A., Maiani G., Azzini E., Raguzzini A., Holub B.J. Supplementation with Quercetin Markedly Increases Plasma Quercetin Concentration without Effect on Selected Risk Factors for Heart Disease in Healthy Subjects. J. Nutr. 1998;128:593–597. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- 11.Edwards R.L., Lyon T., Litwin S.E., Rabovsky A., Symons J.D., Jalili T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007;137:2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 12.Popiolek-Kalisz J., Fornal E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022;47:101350. doi: 10.1016/j.cpcardiol.2022.101350. [DOI] [PubMed] [Google Scholar]
- 13.Popiolek-Kalisz J., Fornal E. The Impact of Flavonols on Cardiovascular Risk. Nutrients. 2022;14:1973. doi: 10.3390/nu14091973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibarra M., Moreno L., Vera R., Cogolludo A., Duarte J., Tamargo J., Perez-Vizcaino F. Effects of the Flavonoid Quercetin and its Methylated Metabolite Isorhamnetin in Isolated Arteries from Spontaneously Hypertensive Rats. Planta Med. 2003;69:995–1000. doi: 10.1055/s-2003-45144. [DOI] [PubMed] [Google Scholar]
- 15.Gao L., Yao R., Liu Y., Wang Z., Huang Z., Du B., Zhang D., Wu L., Xiao L., Zhang Y. Isorhamnetin protects against cardiac hypertrophy through blocking PI3K–AKT pathway. Mol. Cell. Biochem. 2017;429:167–177. doi: 10.1007/s11010-017-2944-x. [DOI] [PubMed] [Google Scholar]
- 16.Gong G., Guan Y.Y., Zhang Z.L., Rahman K., Wang S.J., Zhou S., Luan X., Zhang H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020;128:110301. doi: 10.1016/j.biopha.2020.110301. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Rodríguez C., Torres N., Gutiérrez-Uribe J.A., Noriega L.G., Torre-Villalvazo I., Leal-Díaz A.M., Antunes-Ricardo M., Márquez-Mota C., Ordaz G., Chavez-Santoscoy R.A., et al. The effect of isorhamnetin glycosides extracted from Opuntia ficus-indica in a mouse model of diet induced obesity. Food Funct. 2015;6:805–815. doi: 10.1039/C4FO01092B. [DOI] [PubMed] [Google Scholar]
- 18.Antunes-Ricardo M., Guardado-Félix D., Rocha-Pizaña M.R., Garza-Martínez J., Acevedo-Pacheco L., Gutiérrez-Uribe J.A., Villela-Castrejón J., López-Pacheco F., Serna-Saldívar S.O. Opuntia ficus-indica Extract and Isorhamnetin-3-O-Glucosyl-Rhamnoside Diminish Tumor Growth of Colon Cancer Cells Xenografted in Immune-Suppressed Mice through the Activation of Apoptosis Intrinsic Pathway. Plant Foods Hum. Nutr. 2021;76:434–441. doi: 10.1007/s11130-021-00934-3. [DOI] [PubMed] [Google Scholar]
- 19.Hansen L., Dragsted L.O., Olsen A., Christensen J., Tjønneland A., Schmidt E.B., Overvad K. Fruit and vegetable intake and risk of acute coronary syndrome. Br. J. Nutr. 2010;104:248–255. doi: 10.1017/S0007114510000462. [DOI] [PubMed] [Google Scholar]
- 20.Ding M., Bhupathiraju S.N., Satija A., Van Dam R.M., Hu F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popiolek-Kalisz J., Fornal E. Dietary Isorhamnetin Intake Is Inversely Associated with Coronary Artery Disease Occurrence in Polish Adults. Int. J. Environ. Res. Public Health. 2022;19:12546. doi: 10.3390/ijerph191912546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams B., Mancia G., Spiering W., Rosei E.A., Azizi M., Burnier M., Clement D.L., Coca A., De Simone G., Dominiczak A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Kardiol. Pol. 2019;77:71–159. doi: 10.5603/KP.2019.0018. [DOI] [PubMed] [Google Scholar]
- 23.Dabeek W.M., Marra M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. 2019;11:2288. doi: 10.3390/nu11102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J., Muzashvili T.S., Georgiev M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014;32:1145–1156. doi: 10.1016/j.biotechadv.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Galindo P., Rodriguez-Gómez I., González-Manzano S., Dueñas M., Jiménez R., Menéndez C., Vargas F., Tamargo J., Santos-Buelga C., Pérez-Vizcaíno F., et al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS ONE. 2012;7:e32673. doi: 10.1371/journal.pone.0032673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cogolludo A., Frazziano G., Briones A.M., Cobeño L., Moreno L., Lodi F., Salaices M., Tamargo J., Perez-Vizcaino F. The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc. Res. 2007;73:424–431. doi: 10.1016/j.cardiores.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Hussain F., Jahan N., Rahman K.-U., Sultana B., Jamil S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their Angiotensin-Converting Enzyme (ACE) inhibition potential. Oxid. Med. Cell. Longev. 2018;2018:4643736. doi: 10.1155/2018/4643736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knekt P., Jarvinen R., Reunanen A., Maatela J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondratiuk V.E., Synytsia Y.P. Effect of quercetin on the echocardiographic parameters of left ventricular diastolic function in patients with gout and essential hypertension. Wiad. Lek. 2018;71:1554–1559. [PubMed] [Google Scholar]
- 30.Hertog M.G., Feskens E.J., Kromhout D., Hertog M.G., Hollman P.C., Hertog M.G., Katan M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 31.Brüll V., Burak C., Stoffel-Wagner B., Wolffram S., Nickenig G., Müller C., Langguth P., Alteheld B., Fimmers R., Naaf S., et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015;114:1263–1277. doi: 10.1017/S0007114515002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalus U., Pindur G., Jung F., Mayer B., Radtke H., Bachmann K., Mrowietz C., Koscielny J., Kiesewetter H. Influence of the onion as an essential ingredient of the mediterranean diet on arterial blood pressure and blood fluidity. Arzneimittelforschung. 2000;50:795–801. doi: 10.1055/s-0031-1300291. [DOI] [PubMed] [Google Scholar]
- 33.Mennen L.I., Sapinho D., De Bree A., Arnault N., Bertrais S., Galan P., Hercberg S. Consumption of Foods Rich in Flavonoids Is Related to A Decreased Cardiovascular Risk in Apparently Healthy French Women. J. Nutr. 2004;134:923–926. doi: 10.1093/jn/134.4.923. [DOI] [PubMed] [Google Scholar]
- 34.Kawamoto E., Sakai Y., Okamura Y., Yamamoto Y. Effects of boiling on the antihypertensive and antioxidant activities of onion. J. Nutr. Sci. Vitaminol. 2004;50:171–176. doi: 10.3177/jnsv.50.171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.