Abstract
Diazinon is an organophosphate pesticide that has a history of wide use. Developmental exposures to organophosphates lead to neurobehavioral changes that emerge early in life and can persist into adulthood. However, preclinical studies have generally evaluated changes through young adulthood, whereas the persistence or progression of deficits into middle age remain poorly understood. The current study evaluated the effects of maternal diazinon exposure on behavior and neurochemistry in middle age, at 1 year postpartum, comparing the results to our previous studies of outcomes at adolescence and in young adulthood (4 months of age) (Hawkey 2020). Female rats received 0, 0.5 or 1.0 mg/kg/day of diazinon via osmotic minipump throughout gestation and into the postpartum period. The offspring were tested on a battery of locomotor, affective, and cognitive tests at young adulthood and during middle age. Some of the neurobehavioral consequences of developmental DZN seen during adolescence and young adulthood faded with continued aging, whereas other neurobehavioral effects emerged with aging. At middle age, the rats showed few locomotor effects, in contrast to the locomotor hyperactivity that had been observed in adolescence. Notably, though, DZN exposure during development impaired reference memory performance in middle-aged males, an effect that had not been seen in the younger animals. Likewise, middle-aged females exposed to DZN showed deficient attentional accuracy, an effect not seen in young adults. Across adulthood, the continued potential for behavioral defects was associated with altered dopaminergic function, characterized by enhanced dopamine utilization that was regionally-selective (striatum but not frontal/parietal cortex). This study shows that the neurobehavioral impairments from maternal low dose exposure to diazinon not only persist, but may continue to evolve as animals enter middle age.
Keywords: Diazinon, Neurobehavioral Toxicity, Aging, rats
1. Introduction
Organophosphate (OP) insecticides are a group of widely available and widely used pest control products in agriculture that have been a popular replacement for older, problematic organochlorine insecticides since the 1970’s. Despite the increasingly diverse array of replacement insecticides introduced over the years, OPs currently account for the second largest share of the insecticide market (Phillips 2020), behind neonicotinoid insecticides, and ahead of pyrethroid insecticides. However, certain OPs, such as diazinon (DZN), have faced increasing regulation over the past two decades, including a restriction from all indoor uses and restrictions in use in lawncare and agriculture (EPA 2004), leading to an overall reduction in usage since its peak in 2005 (EPA 2019a). Environmental and human health risks associated with continued OP use remain a major concern, and regulatory review of select OPs is ongoing (EPA 2019b). In humans, early life exposures to OPs are associated with a range of behavioral and psychiatric effects in children, including changes to IQ, attention, mood regulation, hyperactivity, and executive functions, as well as increased risk for neurodevelopmental disorders like attention-deficit disorder and autism (Muñoz-Quezada 2013). Unfortunately, the slow development of humans means that investigations of effects later in life are sparse and less well characterized, so many important questions remain as to how long the effects of a developmental OP exposure may last, or how those effects may differ across the lifespan.
Animal models such as rodents have been critical for predicting the longer-term effects of OPs, with a number of studies investigating the effects of DZN, chlorpyrifos (CPF) and similar compounds across adolescence and into early adulthood. Rodent studies have shown that DZN exposure during early development can lead to impactful changes in brain function and widespread changes in behavior across early development. Prenatal treatment studies have reported impairments in glutamatergic (Win-Shwe 2013), serotonergic and cholinergic function (Slotkin 2019) in the brains of exposed offspring. These neurochemical changes are associated with a variety of behavioral deficits, including adolescent hyperactivity and reduced risk-avoidance (Hawkey 2020), impaired passive avoidance learning (Vatanparast 2013) and alterations in novel object recognition (Win-Shwe 2013, Hawkey 2020). A variety of similar risks have been observed in neonatal treatment models (Roegge 2008, Slotkin 2008a, Timofeeva 2008a, Slotkin 2008b, Timofeeva 2008b), which overlap stages of brain development occurring in the human 3rd trimester (Dobbing J. 1979, Semple 2013). While these effects are pervasive and broadly impactful on neural and behavioral function, there are notable gaps in the current understanding of these effects across development. The most glaring gap is in the persistence of behavioral and neurochemical effects across the lifespan. While prior studies have carried testing forward into adulthood, these generally cover no more than 5 months of development, whereas the lifespan of rats in captivity is generally 2–3 years.
Although it has often been assumed that any effects apparent in adulthood should be stable beyond this point, a number of studies suggest this is not the case. As adulthood goes on, the brain undergoes deterioration associated with aging. For example, between 12–24 months of age, Miguez and colleagues (1999) noted reductions in dopamine and/or DOPAC levels in the striatum, hippocampus, amygdala and brain stem of rats, as well as reductions in norepinephrine in the brain stem and serotonin in the frontal cortex during this same time-period. Likewise, behavioral functions in a number of tests have been shown to shift with age, including locomotor activity, novel object recognition, and tests of learning and memory (Altun 2007, Gallagher 2015, Hamezah 2017). These findings raise the prospect that early life DZN exposure could lead to a different spectrum of deficits as animals age from adolescence through young adulthood and into middle age.
The current study was conducted to determine the long-term neurochemical and behavioral effects of maternal DZN exposure in offspring as they enter middle age. Rat offspring who had been previously evaluated in adolescence and young adulthood (Hawkey 2020) were then reexamined at 1 year of age. The middle-aged rats completed the same behavioral battery so as to assess whether behavioral impairments remain fixed, or continue to change as the animals age. In addition to effects on behavioral performance, we assessed the impact on dopamine (DA) levels and utilization (turnover) in the striatum and frontal/parietal cortex, the latter as a measure of presynaptic neuronal activity. We chose this neurotransmitter because developmental DZN exposure has been shown to affect development of the dopaminergic phenotype (Slotkin, MacKillop et al. 2007, Slotkin and Seidler 2007, Slotkin and Seidler 2009); further, organophosphate exposure in humans is associated with increased risk of Parkinson’s Disease (Sanchez-Santed, Colomina et al. 2016), which is characterized by loss of striatal dopamine projections, and DZN targets the expression of genes associated with that disorder (Slotkin and Seidler 2011). Dopaminergic systems also are critical for a broader array of behavioral functions. They play key roles in cognition and emotional processing (Abraham, Neve et al. 2014, Puig, Rose et al. 2014).
2. Methods
2.1. Subjects and housing
Male and female Sprague Dawley rats were obtained from Charles River Labs (Raleigh, NC, USA) and mated to produce offspring for behavioral testing. Animals were housed with 1–2 same-sex rats from the time of weaning (21 days of age) until the end of the study. Housing conditions were maintained on a 12:12 reversed day-night cycle with ad libitum access to food and water, except during testing of food-motivated behaviors, which required food restriction. All testing was conducted under low, ambient light conditions and during the dark phase of the rats’ reversed light cycle (800 h - 1700 h). The Institutional Animal Care and Use Committee at Duke University approved all methods and procedures.
2.2. Experimental Design and Maternal Exposures
The initial design and exposures are identical to those presented by Hawkey et al (2020). Briefly, female rats were anaesthetized using ketamine (60 mg/kg) and dexdormitor (15 mg/kg) and a single Alzet osmotic infusion pump (2ML4, Durect Inc, Cupertino, CA, USA) was implanted subcutaneously on the animal’s back. These pumps contained a DMSO vehicle or DZN solution (purity 98.8%, Chem Service Inc. West Chester, PA, USA). Solutions were prepared adjusted for body weight as follows: the DMSO vehicle control (100%), 0.5 mg/kg/day or 1.0 mg/kg/day of DZN. The pumps, although marketed as a four-week infusion device, actually run for five weeks (information supplied by the manufacturer), thus ensuring DZN exposure throughout gestation and into the early neonatal period. Animals recovered from surgery for 72 h and were subsequently mated with untreated males for 5 days. Because the dams gain weight throughout pregnancy, the per kg dose fell by parturition (0.5mg/kg/day to 0.3mg/kg/day; 1.0mg/kg/day to 0.74mg/kg/day). Maternal exposure to this low dose range of DZN was not found to cause significant changes in maternal weight gain, litter size, sex-distribution, anogenital distance of the offspring, birth weight or later weight gain of the offspring (Hawkey, Pippen et al. 2020).
One male and one female pup were randomly selected from each litter at weaning for inclusion in the behavioral study. Siblings were retained and used to provide tissue for neurochemical analysis. Offspring were initially tested in a battery of seven behavioral and cognitive tests which began at 4-weeks of age and continued into early adulthood. The results of this initial round of testing were presented in Hawkey et al (2020). These offspring were then maintained until one year of age and tested on the same battery used in the prior analysis at this older stage of development. All tests were performed sequentially, with only one test being conducted in any given week. Elevated plus maze was excluded from the second battery, as this test is typically run on 4-week old rats, and the maze arms were too narrow to allow 1-year old rats to easily turn around when in the enclosed arms of the maze.
2.3. Behavioral Battery
2.3.1. Figure-8 maze
The first test conducted after rats reached one-year of age was the figure-8 maze locomotor activity test. For this test, rats were placed in an enclosed maze in the shape of a figure-8 (alley width = 10 cm, total maze layout = 70cm × 42 cm) and allowed to freely explore for 60 min. Locomotor activity was measured by eight photobeams located at equal points throughout the maze, which registered the frequency of transitions between different portions of the maze. Photobeam breaks across the 60 min session were subdivided in 5 min blocks across to track the habituation, or systematic decrease over time, of locomotion.
2.3.2. Novelty suppressed feeding
The novelty-suppressed feeding test is an indication of stress or fear-like response in rodents. For this test, rats were deprived of food for 24 h and then placed in a novel environment for 10 min with food provided. As fear-like stress responses suppress appetite, changes in the interest in food in this environment are interpreted as changes in the fear-like response. The novel environment consisted of a plastic rectangular cage, without a cage top or bedding, placed in the middle of a brightly lit testing room. Twelve standard rat-chow pellets were spread across the floor of the cage in 4 rows of 3 pellets and an experimenter recorded eating behaviors in real time. Several outcomes were recorded, including the latency for the rat to begin eating, the time spent eating and the frequency bouts of eating. Food pellets were also weighted prior to and after the test to determine the amount of food eaten. Eating was defined as chewing the food and not merely sniffing, holding, varying the food pellets.
2.3.3. Novel object recognition
The novel object recognition task is an indication of attention and memory in a low-motivational state, meaning interest in the objects involved was spontaneous and not based supported by deprivation or the delivery of rewarding consequences for any behavior. This test consisted of two, 10 min sessions where two objects were provided for each rat to explore. The testing enclosure was an opaque plastic box measuring 70 cm × 41 cm × 33 cm, with objects placed in two adjacent corners of the box. Each rat was acclimated to this empty enclosure through two, 10 min sessions across the two days before testing. Objects presented together were matched based on material (plastic, glass, or ceramic), size and coloration, and the assignment of objects was randomized for each animal. The first session (AA, or familiarization) provided two identical objects, which the rat could become familiar with through investigations (e.g. sniffing, touching) of the objects. The second session (AB, or recognition memory) was conducted 60 min after the AA session, and provided one of the two objects presented in the AA session (A), along with a novel object the rat had never investigated before (B). Between sessions, the enclosure and all objects were wiped with 10% acetic acid to remove odor cues. This is the same procedure that was sensitive to developmental neurobehavioral toxicity in the offspring of the dams given 1 mg/kg/day prenatal DZN dose when they were young adults (Hawkey, Pippen et al. 2020). Experimenters collected videos of these two sessions and, while blinded to treatment condition, scored the number and length of investigations for each object within each session. Rats generally show a novel-object preference in the AB session, with greater numbers and length of investigation devoted to the novel object. As the novel object becomes more familiar over the course of that 10 min AB session, scores were subdivided into two, 5 min session-blocks to allow measurement of habituation to the novel object over time and successive investigations.
2.3.4. 16-Arm Radial Maze
The radial arm maze is an indication of spatial learning and memory. The maze was made of painted wood with a central platform (50 cm in diameter), and had 16 rectangular arms (10 cm across × 60 cm long) radiating from that central platform. Before training began, rats completed two, 10 min acclimation sessions, where the rat was confined to the central hub and provided with sugar-coated cereal pieces (halves of Froot Loops®; Kellogg’s Inc, Battle Creek MI, USA) which would later be used as food reinforcers within radial arm maze training. Testing consisted of allowing rats to explore a 16-arm radial arm maze over 12, 10 min sessions (one per day) to retrieve cereal pieces placed in food cups located 2 cm from distal end of the arm. Twelve of the arms were consistently baited with food across the 12 sessions, while the other 4 arms were never baited. The object of training was to see a reduction in entries into the arms which were never baited, indicating long-term or reference memory for these locations. Within each session, the trays were not re-baited once the food had been removed, so avoidance of re-entering arms that had been previously entered would be supported by short-term or working memory. Visual cues were placed around the walls of the room to facilitate spatial orientation. Each session ended after 10 min or when all twelve baited arms had been visited, whichever came first. Working memory errors were tallied as re-entries into a baited arm, and reference memory errors were tallied as entries into any of the consistently non-baited arms. To assess motivation to explore the maze, latency to respond was calculated as the session duration divided by the number of arm entries.
2.3.5. Operant visual signal detection task
The operant visual signal detection task is an indication of attention. Testing was conducted in a standard operant chamber, equipped with two retractable levers, and a single visual cue-light. Each rat completed a sequential training program which trained the rats to press a lever to receive a 2- mg food pellet reward, then to press either the left or right lever, depending on whether the visual cue-light was illuminated (500 ms) prior to the presentation of the levers. If the cue-light was illuminated (“signal” trial), a press on one specific lever would result in food delivery. If the cue-light was not illuminated (“blank” trial), a press on the opposite lever would result in food delivery. The position of the cue-associated lever (left, right) was randomized across rats. Initially, the cue light was placed immediately above the associated lever, and once rats performed “signal” and “blank” trials reliably, the light was shifted to a high, central position between the two levers. The lever presses on each lever were then used to determine whether each rat successfully detected the brief cue presentations, and to generate choice accuracy scores for “signal” and “blank” trial types. Correct lever presses following a cue presentation were labelled as “hits” and correct lever presses following no-cue presentation were labelled “correct rejections”. A failure to respond within 5 s of the lever presentations resulted in an “omission error”, where the trial was ended and the levers were retracted without food delivery. Primary analysis for DZN-induced deficits was conducted using the tertiary testing protocol contained 7 sessions with a multiple schedule of “signal” and :blank” trials, with each test session consisting of 240 trials.
2.4. Methods for Neurochemical Assays
Animals were euthanized via decapitation at two different ages, postnatal day PN150 and following behavioral testing (~15 months or PN450, “Middle aged”), and the striatum and frontal/parietal cortex were dissected and frozen in liquid nitrogen, and then stored at −80 °C until utilized. Tissues were deproteinized by homogenization in 0.1 N perchloric acid containing 3,4-dihydroxybenzylamine (Sigma Chemical Co., St. Louis MO, USA) as an internal standard. Homogenates were sedimented at 26,000 × g for 10 min and supernatants were trace-enriched by alumina adsorption. DA and dihydroxyphenylacetic acid (DOPAC) were separated by reverse-phase high performance liquid chromatography, and quantitated by electrochemical detection (Seidler and Slotkin 1981), standardized against external standards containing 3,4-dihydroxybenzylamine and known quantities of DA (Sigma) and DOPAC (Sigma); values were corrected for recovery of the internal standard. Transmitter utilization was then calculated by the DOPAC/DA ratio, so as to assess the proportion of DA released into the synapse (Slotkin, Wrench et al. 2009).
2.5. Statistical methods
All behavioral data was analyzed using the following method. All tests were analyzed using mixed factors Analysis of Variance (ANOVA), with treatment group, sex and litter as the primary between-subjects variables, and time block (to detect changes in behavior across sessions) as the primary within-subjects variables. Post hoc comparisons between treatment groups were performed using Tukey’s correction for multiple testing. The cutoff for statistical significance for all main effects and post hoc tests was p = 0.05.
Hypothesis testing consisted of the following. An initial analysis was conducted on behavioral and neurochemical measures using the middle aged rats, with the aim being to detect differences based on treatment within the latter stages of development. A secondary analysis concerned the hypothesis that DZN-treatment may alter aging-induced changes in behavioral performance. For this, data from adolescence or early adulthood and late-adulthood were included in one analysis and used to determine whether age significantly interacted with treatment.
3. Results
3.1. Behavioral Analyses
3.1.1. Locomotor Activity and Habituation in the Figure-8 Apparatus
Young adult rats with perinatal 1.0mg/kg/day exposure showed reduced activity levels on the final time block (p < 0.05) relative to controls, but not the 0.5mg/kg/day group (Fig. 1a). In contrast, for middle-aged rats (Fig. 1b), there was a significant time block (5 min) by treatment interaction (p < 0.05), reflecting that the 1.0mg/kg/day DZN group was hyperactive relative to controls in time block 4 (min 20–25) (p < 0.05); no such effect was seen for the 0.5mg/kg/day group. A main effect of time block was also detected (p < 0.0005), indicating significant habituation across the session, as was a main effect of sex (p <0.05), whereby females had higher activity relative to males. Aging-based analyses detected no main effect of age on locomotor activity, but did detect a main effect of sex (females more active than males) (p < 0.0005), and age by time block interaction, and an age by treatment by session block interaction (p < 0.05). Overall, the age by time block by treatment interaction was expressed by differences in linear trends (linear interaction, p< 0.05) (Fig. 1c/d). Among young adults, rats with 0.5mg/kg/day exposure showed higher y-intercepts and steeper slopes in the habituation function relative to controls (p < 0.05), but not the higher 1.0 dose condition. No linear trend differences were observed in the middle-aged rats.
Figure 1.
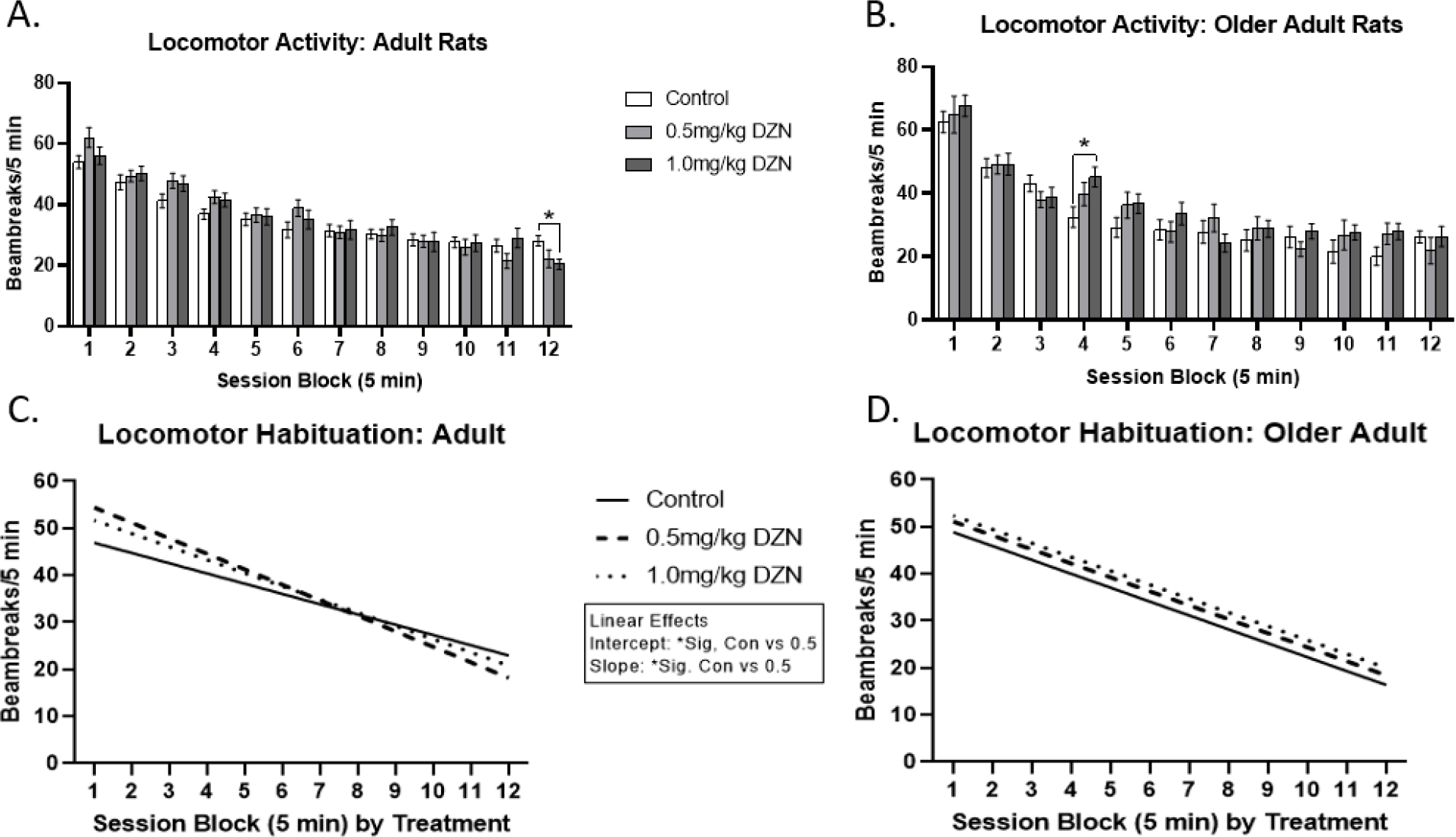
Diazinon effects on locomotor activity and its habituation in the Figure-8 apparatus (mean ± sem). (A) Locomotor activity at 5 months of age. (B) Locomotor activity at 1 year of age. (C) Linear habituation function for 5 month old rats. (D) Linear habituation function for 1 year old rats. The higher 1.0 mg/kg/day DZN dose caused significant hypoactivity relative to controls during the blocks 12 (p < 0.05) at 5 months of age and hyperactivity in block 4 (p < 0.05) at 1 year of age. 5 month old rats with 1.0mg/kg/day DZN exposure showed higher intercepts and steeper habituation slopes than controls in the linear analysis. No linear differences were detected in middle-aged adults.
Calculated difference scores across aging (middle-aged – younger) did not differ between treatments, although they did differ between ages, as evidenced by a significant age by time block effect (Suppl. Fig S1). Middle-aged rats showed higher initial activity scores in block 1 (p < 0.05) and trends towards reduced activity levels in the following time blocks which only reached significance in block 3 (p < 0.05).
3.1.2. Novelty Suppressed Feeding
Among middle-aged adult rats, no main effects of treatment were observed on measures of novel environment suppressed feeding (Suppl. Table S1). Main effects of sex were observed for body weight (p < 0.0005) (males higher than females), amount eaten (p < 0.0005) (males higher than females), latency to eat (p < 0.0005) (males less than females) and time spent eating (p < 0.0005) (males higher than females) (Suppl. Table S2). Aging-based analyses detected age by sex interactions on body weight, amount of food eaten, latency to begin feeding and total amount of time spent eating (p < 0.05), but not the number of eating episodes (Suppl. Table S3). Males showed increased patterns of weight gain and increases in food consumption relative to females (p < 0.05), although both sex showed positive change scores (middle-aged – younger). For time spent eating, males and females also differed (p < 0.05), whereby only middle-aged males showed an increase in time spent eating due to age. For latency to begin eating, males and females differed in their change due to aging (p < 0.05), whereby middle-aged males showed reduced latency to eat, and middle-aged females showed increased latency to eat.
3.1.3. Novel Object Recognition
Among middle-aged adults, no significant effects of treatment or relevant interactions were found on object investigation during the familiarization trial or on object preference during the novel object recognition trial (Suppl. Fig S2). When comparing these scores across ages, there was a significant overall lowering of investigation with aging during the familiarization (p < 0.0005) (Suppl. Fig. S3a) and the novel object (p = 0.001) trials (Suppl. Fig. S3b). No treatment effects or interactions were detected. Main effects of object familiarity (p < 0.0005), time block (p < 0.0005) and an object by time block interaction (p < 0.0005) were detected, indicating object preference, discrimination and familiarization were adequate on this test regardless of age.
3.1.4. Radial-arm Maze Spatial memory
Among middle-aged adults, maternal DZN exposure was not found to significantly impair choice accuracy or latency in the radial-arm maze for working memory errors. However, aging-based analyses detected interactions of age by sex by treatment by session block on reference memory error rates (Fig 2a/b). No differences were detected among late adolescent rats. Among middle-aged males, rats with 1.0mg/kg/day exposure showed impaired improvement over training, as evidenced by elevated numbers of reference memory errors during session block 2 (sessions 4–6) relative to controls (p < 0.05) but not the lower dose (0.5 mg/kg/day) group. No differences were observed among middle-aged females. No differences were observed for working memory errors (Suppl. Fig. S4)
Figure 2.
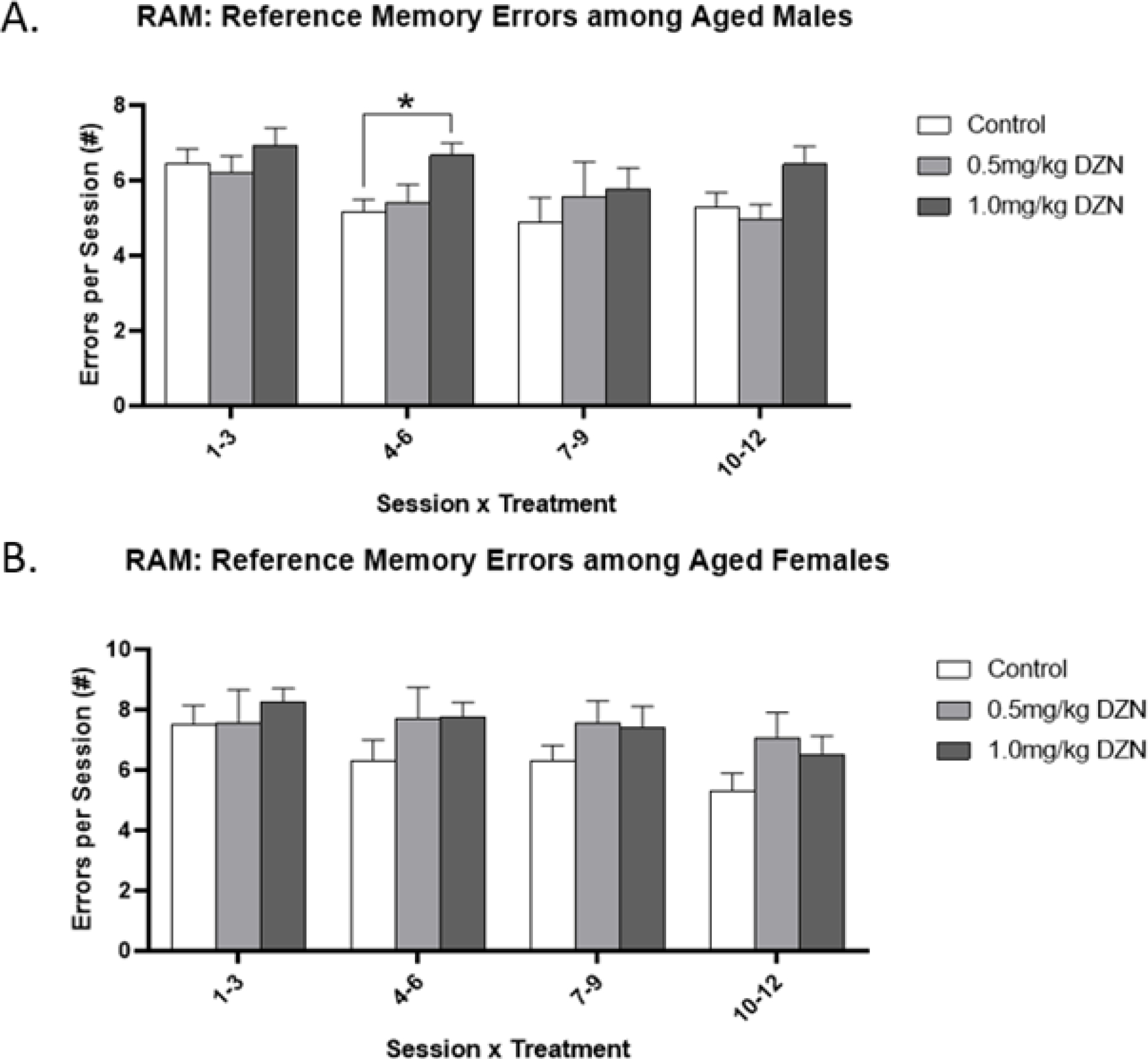
Diazinon effects on reference memory errors in the radial arm maze (mean ± sem). (A) Males with DZN exposure showed higher numbers of errors relative to controls in session block 2 (sessions 4–6) of the test phase (p < 0.05). (B) No treatment effects were detected in female rats.
Additionally, aging analyses detected age effects or interactions for each of the main outcomes on this test (Supplemental Fig. S5). For reference memory errors (Supplemental Fig. S5a), an age by sex interaction was detected (p < 0.05) whereby error rates increased due to aging among females (p < 0.05) but not males. For working memory errors (Supplemental Fig. S5b, Left), scores improved from younger to middle-aged adult testing. Overall, an age by session block interaction was observed (p < 0.05), whereby middle-aged rats committed fewer errors during session block 3 (Sessions 7–9) compared to late adolescents. An age by sex interaction was also observed (p < 0.05) whereby males showed improved error rates due to aging (p < 0.05), but females did not (Suppl. Fig. S5b, Right). For response latency (Suppl. Fig. S5c), an age by sex interaction (p < 0.05) was detected, and both males and females showed reduced latencies when tested at later ages. Overall, an age by session-block interaction (p < 0.05) indicated that middle-aged rats responded significantly faster than late adolescents during the first 3 session blocks (sessions 1–9).
3.1.5. Operant Visual Signal Detection Attention Task
Among middle-aged rats, a sex by treatment interaction was observed for choice accuracy (p < 0.05), regardless of trial type (cue present vs absent). Among males, no treatment differences were observed (Fig 3). Among females, rats with 1.0mg/kg/day exposure showed reduced choice accuracy (p< 0.05) relative to controls, but not the 0.5mg/kg/day group (Fig. 3a). Additionally, a sex by treatment by session by trial type (cue present vs absent = hit vs correct rejection). No within session differences were detected for males on either hits or correct rejections. Female rats showed session-specific treatment effects (Fig. 3b). For hits (cue present), females with 1.0mg/kg/day DZN exposure were less accurate on session 2 of the testing series (p< 0.05) compared to controls, but the DZN groups did not differ from one another. For correct rejections (cue absent), both DZN-treated groups of females differed from controls (p < 0.05), but not from one another. In the aging analysis, a sex by treatment by trial type (cue present vs absent) interaction was detected (p < 0.05) which was not dependent on age. However, no post hoc comparisons reached significance. Overall, an age by trial type (cue present vs absent) interaction was detected (p < 0.01), whereby middle-aged rats were more accurate than younger rats on “cue present (hit) trials (p < 0.05), but not “cue absent” trials (correct rejections) (Supplemental Fig. 6).
Figure 3.
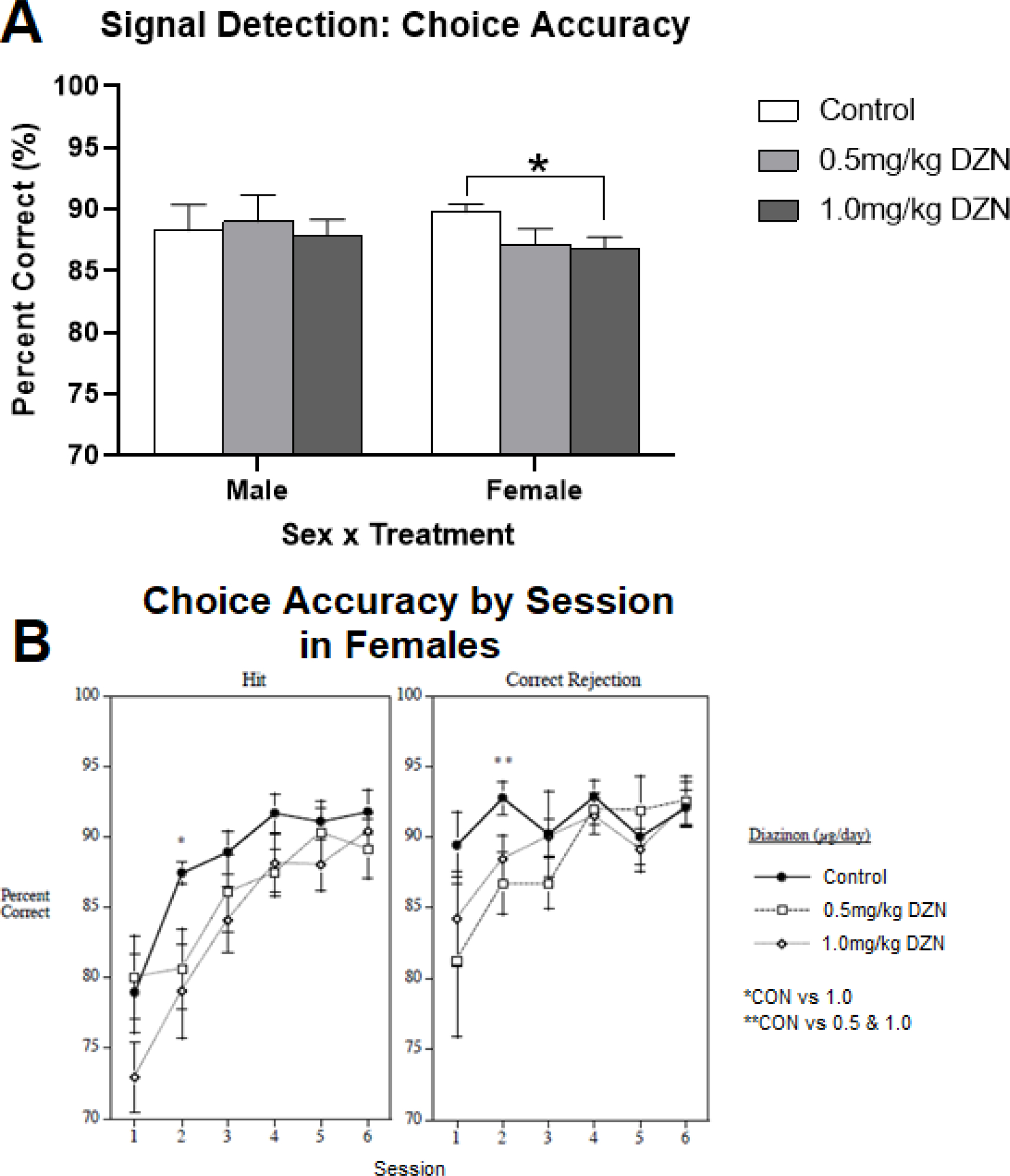
Diazinon effects on the visual signal detection test of attention (mean ± sem). Females with DZN exposure showed impaired overall choice accuracy relative to controls (p < 0.05). No treatment effects were detected in male rats.
3.2. Neurochemical Assays
The initial statistical analysis of neurochemical results were carried out with a five-factor repeated measures ANOVA: treatment, sex, age, region and the two primary measures (DA, DOPAC). The latter were considered as repeated measures since both values were obtained from the same sample, and data were log transformed because of heterogeneous variance between regions and between DA and DOPAC. In this initial test, we did not find any significant main effect of sex or interaction of sex with the other variables, so values were combined for males and females, resulting in a four-factor repeated measures ANOVA. For this test, we identified significant interactions of treatment × [DA vs. DOPAC] (p < 0.02) and treatment × region × [DA vs. DOPAC] (p < 0.05). Accordingly, we evaluated the effects on DA and DOPAC separately for the two regions, and then calculated the turnover (DOPAC/DA ratio).
In the striatum, there was a differential effect of treatment on DOPAC as compared to DA (treatment × [DA vs. DOPAC], p < 0.002. Whereas there was no significant effect on striatal DA levels (Figure 4B), DOPAC was significantly elevated in the group exposed to the higher DZN dose (Figure 4A). Consequently, turnover was also higher in that group (Figure 4C). For both these effects, the high dose group was statistically distinguishable from the lower DZN dose as well as control. Notably, the effect seen at PN150 remained present in the middle-aged animals (absence of a significant interaction of treatment × age or of treatment × age × other variables).
Figure 4.
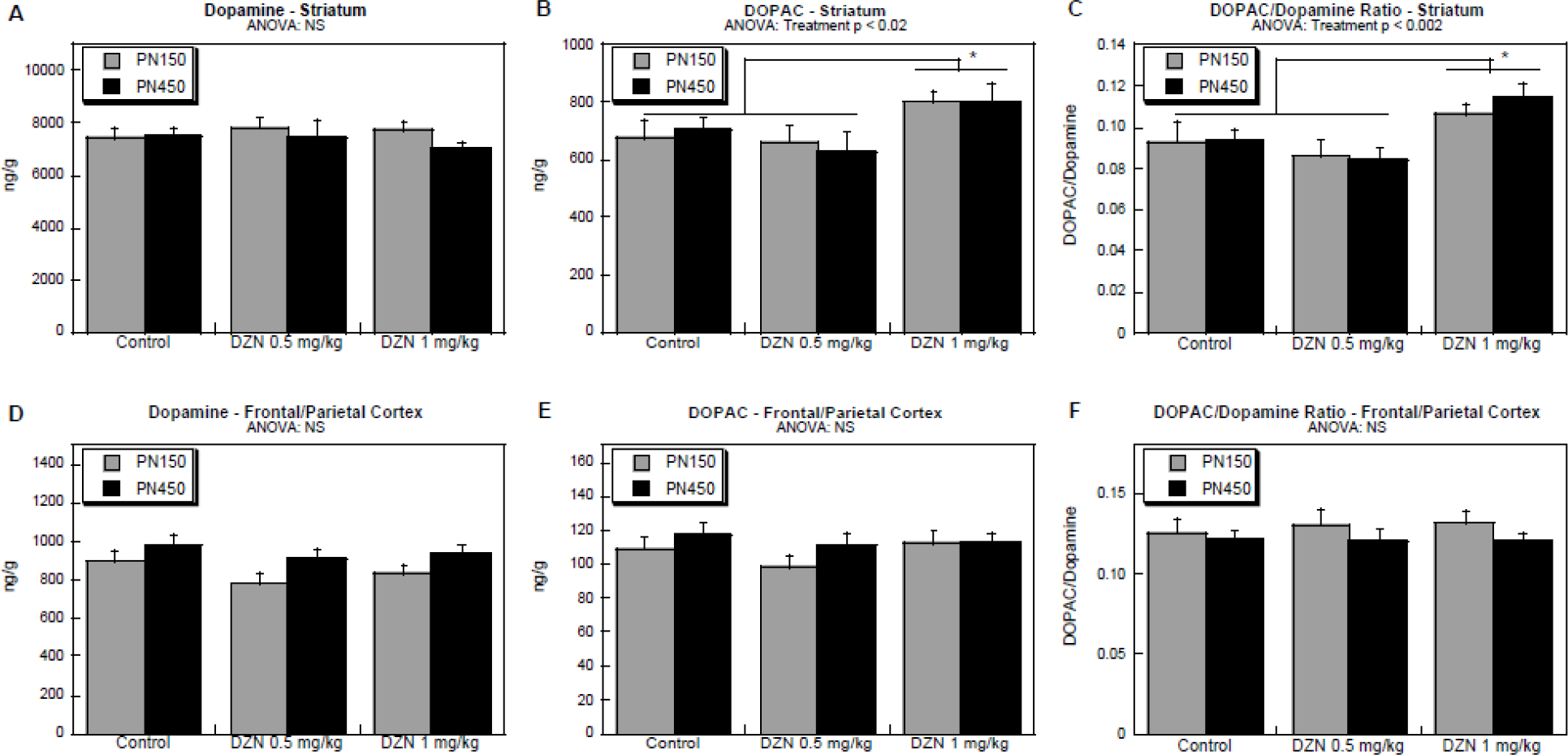
Effects of DZN exposure on DA and DOPAC levels and DOPAC/DA ratio (turnover): (A) DA in striatum, (B) DOPAC in striatum, (C) DOPAC/DA in striatum, (D) DA in frontal/parietal cortex, (E) DOPAC in frontal/parietal cortex, (F) DOPAC/DA in frontal/parietal cortex. Assessments were made on at PN150 (5 months of age) and at ~PN450 (15 months age). Data represent mean ± SE obtained from 7–17 animals in each treatment group at each age. Where significant differences were found, posthoc Tukey tests were used to establish specific intergroup differences (brackets and asterisks). NS = not significant.
In contrast, in the frontal/parietal cortex, we did not observe any significant effects on dopamine (Figure 4D), DOPAC (Figure 4E) or the DOPAC/DA ratio (Figure 4F); the lack of effect in this region was statistically distinguishable from the significant effects in the striatum as noted from the treatment × region × [DA vs. DOPAC] interaction, as noted above.
4. Discussion
The present study was conducted to evaluate the persistence or further progression of developmental DZN-induced neurobehavioral and neurochemical impacts from early adulthood into middle age. This work extended previous studies (Slotkin 2019, Hawkey 2020) which evaluated related endpoints from four weeks to five months of age, covering a developmental period of adolescence to early adulthood. Overall, utilizing a wide array of behavioral endpoints, we found both types of outcomes: persistence of earlier defects, but also further changes that emerged between young adulthood and middle-age. Accompanying aging analyses and age by treatment interactions showed that while significant treatment effects at this age differ from the prior Hawkey et al findings (2020), only some of the behavioral deficits found in middle age reflected an aging-related event. Corresponding neurochemical analyses confirmed the persistence of DZN-induced synaptic abnormalities into middle age, characterized by elevated striatal dopamine utilization.
Within the behavioral analysis, two age × treatment interactions were observed. The first of these reflected a DZN-induced increase in the rate of habituation in the figure-8 maze in early adulthood which attenuated by middle age. The second was a change in the rate of learning in the radial-arm maze, whereby DZN induced a male-specific slowing of improvements in reference memory errors among middle-age rats, but not late adolescents. Taken together, these data indicate that aging across adulthood can lead to significant modulation of DZN-induced impairments, allowing these alterations to either attenuate or emerge. In each case, the overall difference only met significance vs. control in one of the two DZN-treated groups, but the two DZN dosage groups also did not significantly differ from one another. Therefore, these changes cannot be interpreted as being dose-specific.
While the aforementioned behavioral effects were significantly modulated by aging, no other tests or outcomes showed differing treatment effects based on age: for these tests, behaviors that were significantly altered by DZN in young animals remained so in middle age, whereas those that were not affected in young animals were also unchanged in the older animals. In the signal detection test, it was observed that females with 1.0 mg/kg/day exposure showed reduced choice accuracy in middle-age relative to control females, and either dose led to impaired improvement across sessions within the testing series. Although this was not predicted based on the results of Hawkey et al. (2020), no direct interaction of age and deficient performance was observed, so the differences between young adult and middle-age cannot be reliably interpreted. Likewise, the prior study observed impaired object discrimination in the novel object recognition test in late adolescence, whereas the present analysis observed no significant performance detriments in middle-age, but the lack of a significant treatment × age interaction indicates that these two outcomes cannot be distinguished from each other. These apparent disparities may suggest one of two interpretations. First, it may be the case that treatment effects were truly indistinguishable at each time point, preventing an age by treatment interaction, but that minor differences in effect size from one assessment to the next were sufficient to cause that effect to differentially reach or fail to reach significance. An alternative interpretation may stem from the fact that the present analysis included only 3 of the initial 5 cohorts used to generate the Hawkey et al (2020) data. This reduced the sample size and may have impaired the likelihood of detecting higher order interactions which require greater statistical power. This interpretation is more plausible than performance equivalence across aging for novel object recognition, as preference patterns for the 1.0mg/kg/day group are visibly trending in opposite directions at the different ages (reduced novel object preference among adolescents, enhanced in middle-age).
By contrast, changes in dopaminergic function remained consistent from young adulthood through middle age. Overall, there were increases in DOPAC, the primary metabolite of dopamine, with no corresponding change in dopamine levels. This led to an increase in the DOPAC/DA ratio, indicating that dopamine is utilized more quickly in the DZN-exposed animals than in controls. This hyperdopaminergic state may then contribute to persistent potential for behavioral impairments over time. However, as this effect is stable, it alone cannot be the driver of the age × treatment interactions observed in the behavioral data; likewise, the neurochemical changes did not share the sex-selectivity seen in the behavioral outcomes. It is notable, however, that the effects were specific to dopamine projections to the striatum, as the effects were not shared by projections to the frontal/parietal cortex. These effects could contribute to the known association between DZN exposure and Parkinson’s Disease (Sánchez-Santed 2016), a malady that results from loss of striatal dopamine synapses. Increased dopamine turnover is one of the early signs of the onset of Parkinson’s Disease, as remaining dopamine neurons upregulate their activity to compensate for the loss of overall synaptic function (Sossi 2004). Further, these data extend previously established neurochemical effects of DZN in younger animals, including regionally-specific abnormalities in cell size and packing density (Slotkin 2008a), as well as disruption of cholinergic and serotonergic synaptic function (Slotkin 2006, Slotkin 2008a, Slotkin 2008b, Slotkin 2019).
Prior studies have reported that young animals exposed to organophosphates can show changes in locomotor activity(Levin 2002, N’Go 2013, Baldwin 2017, Mohammadzadeh 2018), including DZN (Hawkey 2020). In our previously published data from this study (Hawkey 2020), it was found that these effects markedly attenuated from 5 weeks to ~5 months of age, and this extended analysis indicates that this attenuation continues through middle age. This mirrors a known pattern in the clinical literature where hyperactivity effects observed in young patients become less prominent as patients mature (Willoughby 2003, Martel 2016). We have also previously associated early DZN exposures with learning deficits, although prenatal DZN exposure appears somewhat unique from neonatal exposures. Whereas neonatal DZN exposure was associated with slowed radial arm maze learning in adulthood (Timofeeva 2008a), prenatal DZN exposure produces comparable effects which onset much later in adulthood. Additionally, this effect was specific to male offspring in this prenatal exposure series and specific to reference memory errors. These differences suggest that the timing of DZN exposure may influence the age of onset and specificity of learning difficulties, although additional studies will be needed to directly address this hypothesis. The learning rate in the radial-arm maze over twelve sessions of training in the current study was not substantial. Further studies should examine potential effects with more extended training. Effects on executive-like functions, including attention, are relatively understudied, and no known studies of developmental organophosphate exposures have used the current signal detection task, apart from the preceding analysis of these animals at younger ages (Hawkey 2020). The present findings suggest that such functions may indeed be sensitive to organophosphate-induced dysfunction, and that further investigation is warranted. Mild cognitive impairment emerging in pre-senescence is increasingly recognized as a predictor of later cognitive decline, including incidence of dementia and neurodegenerative disorders like Parkinson’s disease (Etgen 2011, Chen 2018).
Although no comparable studies of DZN-induced effects in aging are available in rats, a study on postnatal exposure to another OP insecticide, parathion (PRT) has been conducted. Levin et al (2010) exposed neonatal rat pups to PRT and conducted radial arm maze training and testing at 14 and 19 months of age. Increased error rates were seen overall due to PRT exposure among aged male rats. As in the present study, PRT-induced increases in error rates were specific to middle-aged rats, as none were found in these rats in younger adulthood (Timofeeva 2008b). The age-related changes in behavioral effects of prenatal PRT were paralleled by emergent synaptic functional defects. Specifically, in aging, animals exposed to PRT showed loss of acetylcholine innervation with compensatory upregulation of presynaptic activity of remaining synapses. Together, these data suggest that while OPs may induce aging-related cognitive issues rather than persistent deficits across adulthood, and that males may be particularly susceptible to those effects. These data support associations between developmental OP exposures and cognitive health in human populations (Sánchez Lizardi 2008, Bouchard 2011, Doherty 2019), and suggest that the risk for cognitive symptoms and neurochemical dysfunction could contribute to cognitive decline and neurological disorders of aging, such as dementia and Parkinson’s disease.
The persisting neurobehavioral toxicity of gestational exposure to low doses of DZN seen in the current study was not dramatic. Some of the behavioral impairment seen during younger adulthood such as with the novel object recognition test were not seen during later adulthood. However, there were both behavioral and neural effects that persisted into later adulthood after gestational DZN exposure point to lifelong consequences of this developmental exposure.
In addition to being able to assess the persistence and emergence of DZN effects on neurobehavioral performance, our findings also address the changes that accompany aging itself. As this study utilized a longitudinal design (i.e. the same animals were evaluated at the various ages), data from younger (adolescent-early adult) and middle-aged can be compared on a repeated measures basis, and reveal that certain tests are sensitive to differences in performance across these repeated testing windows. One apparent difference due to age was a shift in locomotor activation and habituation in the figure-8 maze. Middle-aged rats showed higher peak activity in the first time block when compared to young adults, followed by reduced activity in select time blocks during the first half of the session. This indicates that middle-aged rats are hyperstimulated initially, but habituate more quickly than younger rats. Additional changes were noted in novelty-suppressed feeding. Middle-aged rats weighed more and ate more food during the test, in fewer episodes, when compared to younger rats. In the first two cases, these effects were significantly higher among males than females. With respect to time spent eating, only males showed increases due to age. For the latency to eat, a primary indicator of novelty-suppression of consumption, middle-aged males ate sooner than younger males, and middle-aged females waited longer to eat than younger females. With respect to cognitive outcomes, aging-effects were noted on novel object recognition task, the radial arm maze task and signal detection task. Middle-aged rats spent less time investigating objects overall in the NOR. This did not prevent adequate object discrimination, but does indicate reduced engagement in the test. In the radial arm maze test, males showed improvements in the acquisition of working memory errors across the middle-aged training sequence, relative to the younger animals. Females showed an aging-based increase in reference memory errors. In the signal detection task, middle-aged rats also showed enhanced accuracy for “hit” trials (cue present), the trials which generally show lower accuracy overall in both younger and middle-aged rats. These aging effects indicate that performance meaningfully changes across a longitudinal testing sequence, even when those phases of testing are quite far apart. Detrimental performance differences, such as those in initial hyperactivity in the figure-8 maze, NOR task engagement and reference memory errors, may indicate aging-related impairments in cognitive function. Enhanced performance, as seen on working memory errors and hit choice accuracy, may indicate long-term retention of strategies or cue-relations which support performance on these tests. However, as age is conflated with experience in this study, these changes across time cannot be definitively interpreted as specific effects of aging. However, previous studies investigating behavioral changes across adult development often observe comparable changes, and the present study adds new support to this literature.
Aging in rats is often associated with a decrease in locomotor activity, although the age of onset for these effects is mixed across studies activity (Willig 1987, Hebert 1998, Altun 2007, Salvatore 2016, Hamezah 2017). These studies generally agree that the most pronounced changes occur after 24 months of age, although less pronounced effects have been reported as young as 12–14 months of age (Hebert 1998, Salvatore 2016). These prior studies generally used open field rather than figure-8 style mazes and shorter testing sessions. The present study highlights that when tracking habituation within a fully enclosed maze, with no conflict between locomotor stimulation and avoidance of the open center, aging effects are present and time dependent across the session. Initial activity, rather than activity overall, appears to be the most sensitive aspect of locomotor activity for changes from 5 months to 1 year of life.
Age-related changes in affective function were also evident in our dataset. Aging accounted for an overall increase in eating during the novelty-suppressed eating test. Some of these changes may be attributable to increased body weight at middle-age, such as the increased total amount consumed, although the overall pattern of eating in this mildly-stressful environment is altered more generally. Both males and females engaged in fewer episodes of eating at the aged time point, while consuming more food (both males and female) over a longer period of time (males only). Although all rats gained weight and ate more food in fewer sessions, only males began eating sooner within the session. To the contrary, females waited longer to begin eating, suggesting that the appetite-suppressing effect of the novel environment is enhanced with age for females, and reduced with age for males. Prior studies have noted age-related enhancements in anxiety- or fear-like behaviors through aging (Darwish 2001, Pietrelli 2012, Hiew 2020), although the rat studies have used alternative procedures rather than the novelty-suppressed feeding test. In a study with mice, Joeyen-Waldorf and colleagues (2009) reported that both males and females showed increased latencies to eat in the novelty-suppressed feeding test from 3 to 12 months of age. Overall, these results support the assertion that anxiety-like stress effects can grow with age. The unique finding in the current study is that in rats of a less advanced age (12 months, vs 15–24 months in other anxiety/fear-like studies), females show an enhancement of eating latency, while males do not. This may suggest that males and females have different ages of onset for these aging effects. Alternatively, these may reflect a difference between males and females in any potential carryover effects from undergoing the same test twice, albeit 10 months apart.
Cognitive changes across adult development have also been reported. As in our study, Marshall and colleagues (2019) reported reduced interest and investigation of objects in the novel object recognition task across aging, indicating reduced engagement in the task. Also as in our study, this reduction did not interfere with object discrimination. With respect to the radial arm maze, aging-related impairments in working memory and reference memory errors have been reported in middle-aged aged rats (18–24 months) (Chrobak 1995, Noda 1997, Shukitt-Hale 2004, Pietrelli 2012) but not at intermediate ages comparable to the current study (Shukitt-Hale 2004), although results have been mixed (Ward 1999). The present study suggests that as increases in reference memory errors due to age were restricted to female rats, so inclusion of females and analysis of sex differences may be critical to detecting memory deficits in earlier periods of the aging process.
In summary, the present study demonstrated that certain effects of prenatal DZN exposure persist into middle age and some emerge with more advanced age. A number of neurobehavioral anomalies continue to change as the animals transition from adolescence to young adulthood and thence to middle age. This reinforces the concern over the long-term effects of early-life organophosphate exposures, specifically the worry that further deterioration may occur with aging.
Supplementary Material
Acknowledgments
Research was supported from the Duke University Superfund Research Center ES010356.
TAS and FJS were responsible for the neurochemical measurements.
Abbreviations:
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
Footnotes
COI statement
TAS has received consultant income in the past three years from Gjording Fouser (Boise, ID), Thorsnes Bartolotta McGuire (San Diego, CA), Cracken Law (Dallas, TX), Matthews Law (Houston, TX), Segal Law (Charleston, WV), Armstrong Teasdale (St. Louis, MO) and the State of Arizona.
References
- Abraham AD, Neve KA and Lattal KM (2014). “Dopamine and extinction: a convergence of theory with fear and reward circuitry.” Neurobiol Learn Mem 108: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun M, Bergman E, Edström E, Johnson H, & Ulfhake B (2007). “Behavioral impairments of the aging rat.” Physiology & Behavior 92(5): 911–923. [DOI] [PubMed] [Google Scholar]
- Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, & Patisaul HB (2017). “Sex specific placental accumulation and behavioral effects of developmental Firemaster 550 exposure in Wistar rats.” Scientific Reports 7(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB & Eskenazi B (2011). “Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children.” Environmental health perspectives 119(8): 1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, Cheng SJ, Lin HC, Lee CY, & Chou CH (2018). “Risk factors for the progression of mild cognitive impairment in different types of neurodegenerative disorders.” Behavioral Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Hanin I, Lorens SA, & Napier TC (1995). “Within-subject decline in delayed-non-match-to-sample radial arm maze performance in aging Sprague-Dawley rats.” Behavioral neuroscience 109(2). [DOI] [PubMed] [Google Scholar]
- Darwish M, Korányi L, Nyakas C, & Almeida OFX (2001). “Exposure to a novel stimulus reduces anxiety level in adult and aging rats.” Physiology & behavior 72(3): 403–407. [DOI] [PubMed] [Google Scholar]
- Dobbing J, S. J. (1979). “Comparative aspects of the brain growth spurt.” Early Human Development 3(79–83). [DOI] [PubMed] [Google Scholar]
- Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, Olshan AF and Daniels JL (2019). “Prenatal exposure to organophosphate esters and cognitive development in young children in the Pregnancy, Infection, and Nutrition Study.” Environmental research 169: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (2004). “Interim Reregistration Eligibility Decision Diazinon.”
- EPA (2019a). “Diazinon National and State Use and Usage Summary.”.
- EPA (2019b). “EPA Reinitiation of Formal Consultation with NMFS on Chlorpyrifos, Diazinon, and Malathion.”.
- Etgen T, Sander D, Bickel H, & Förstl H (2011). “Mild cognitive impairment and dementia: the importance of modifiable risk factors.” Deutsches Ärzteblatt International 108(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, & Burchinal M (2015). “Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze.” Behavioral Neuroscience 129(4): 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamezah HS, Durani LW, Ibrahim NF, Yanagisawa D, Kato T, Shiino A, Tanaka S, Damanhuri HA, Ngah WZW and Tooyama I (2017). “Volumetric changes in the aging rat brain and its impact on cognitive and locomotor functions.” Experimental gerontology 99: 69–79. [DOI] [PubMed] [Google Scholar]
- Hawkey A, Pippen E, White H, Kim J, Greengrove E, Kenou B, Holloway Z and Levin ED (2020). “Gestational and perinatal exposure to diazinon causes long-lasting neurobehavioral consequences in the rat.” Toxicology 429: 152327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey A, Pippen E, White H, Kim J, Greengrove E, Kenou B, Holloway Z and Levin ED (2020). “Gestational and perinatal exposure to diazinon causes long-lasting neurobehavioral consequences in the rat.” Toxicology 429: 152327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, & Gerhardt GA (1998). “Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats.” Brain research bulletin 797(1): 42–54. [DOI] [PubMed] [Google Scholar]
- Hiew LF, Khairuddin S, Aquili L, Koh J, Fung ML, Lim WL, & Lim LW (2020). “Behavioural responses of anxiety in aversive and non-aversive conditions between young and aged Sprague-Dawley rats.” Behavioural brain research 385. [DOI] [PubMed] [Google Scholar]
- Joeyen-Waldorf J, Edgar N, & Sibille E. (2009). “The roles of sex and serotonin transporter levels in age-and stress-related emotionality in mice.” Brain research 1286: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, & Slotkin TA (2002). “Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations.” Neurotoxicology & Teratology 24(6): 733–741. [DOI] [PubMed] [Google Scholar]
- Levin ED, Timofeeva OA, Yang L, Petro A, Ryde IT, Wrench N, Seidler FJ and Slotkin TA (2010). “Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging.” Behavioural brain research 208(2): 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HJ, Pezze MA, Fone KC, & Cassaday HJ (2019). “Age-related differences in appetitive trace conditioning and novel object recognition procedures.” Neurobiology of Learning and Memory 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Levinson CA, Langer JK, & Nigg JT (2016). “A network analysis of developmental change in ADHD symptom structure from preschool to adulthood.” Clinical Psychological Science 4(6): 988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguez JM, Aldegunde M, Paz-Valinas L, Recio J, & Sanchez-Barcelo E (1999). “Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging.” Journal of Neural Transmission 106(11): 1089–1098. [DOI] [PubMed] [Google Scholar]
- Mohammadzadeh L, Hosseinzadeh H, Abnous K, & Razavi BM (2018). “Neuroprotective potential of crocin against malathion-induced motor deficit and neurochemical alterations in rats.” Environmental Science and Pollution Research 25(5): 4904–4914. [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, Iglesias V, Alvarado S, Concha C, Rojas E & Vega C (2013). “Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review.” Neurotoxicology 39: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Go PK, Azzaoui FZ, Ahami AOT, Soro PR, Najimi M, & Chigr F (2013). “Developmental effects of Malathion exposure on locomotor activity and anxiety-like behavior in Wistar rat.” Health 5(3): 603–611. [Google Scholar]
- Noda Y, Yamada K, & Nabeshima T (1997). “Role of nitric oxide in the effect of aging on spatial memory in rats.” Behavioural brain research 83: 153–158. [DOI] [PubMed] [Google Scholar]
- Phillips MWA (2020). “Agrochemical industry development, trends in R&D and the impact of regulation.” Pest Management Science 76(10): 3348–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrelli A, Lopez-Costa J, Goñi R, Brusco A, & Basso N (2012). “Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle-aged and old rats.” Neuroscience 202: 252–266. [DOI] [PubMed] [Google Scholar]
- Puig MV, Rose J, Schmidt R and Freund N (2014). “Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds.” Front Neural Circuits 8: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, & Levin ED (2008). “Developmental diazinon neurotoxicity in rats: later effects on emotional response.” Brain research bulletin 75(1): 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Terrebonne J, Fields V, Nodurft D, Runfalo C, Latimer B, & Ingram DK (2016). “Initiation of calorie restriction in middle-aged male rats attenuates aging-related motoric decline and bradykinesia without increased striatal dopamine.” Neurobiology of aging 37: 192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Lizardi P, O’Rourke MK, & Morris RJ (2008). “The effects of organophosphate pesticide exposure on Hispanic children’s cognitive and behavioral functioning.” Journal of pediatric psychology 33(1): 91–101. [DOI] [PubMed] [Google Scholar]
- Sanchez-Santed F, Colomina MT and Herrero Hernandez E (2016). “Organophosphate pesticide exposure and neurodegeneration.” Cortex 74: 417–426. [DOI] [PubMed] [Google Scholar]
- Sánchez-Santed F, Colomina MT, & Hernández EH (2016). “Organophosphate pesticide exposure and neurodegeneration.” Cortex 74: 417–426. [DOI] [PubMed] [Google Scholar]
- Seidler FJ and Slotkin TA (1981). “Development of central control of norepinephrine turnover and release in the rat heart: responses to tyramine, 2-deoxyglucose and hydralazine.” Neuroscience 6: 2081–2086. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, & Noble-Haeusslein LJ (2013). “Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species.” Progress in neurobiology 106: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, McEwen JJ, Szprengiel A, & Joseph JA (2004). “Effect of age on the radial arm water maze—a test of spatial learning and memory.” Neurobiology of aging 25(2): 223–229. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, & Seidler FJ (2008a). “Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems.” Environmental health perspectives 116(3): 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA and Seidler FJ (2007). “Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel.” Environ. Health Perspect. 115: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, & Seidler FJ (2008b). “Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood.” Brain research bulletin 75(5): 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2007). “Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems.” Brain Res. Bull. 72: 232–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2009). “Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells.” Brain Res. Bull. 78: 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2011). “Developmental exposure to organophosphates triggers transcriptional changes in genes associated with Parkinson’s Disease in vitro and in vivo.” Brain Res. Bull. 876: 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Ko A, Levin ED, & Seidler FJ (2019). “Perinatal diazinon exposure compromises the development of acetylcholine and serotonin systems.” Toxicology 424: 152240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, & Seidler FJ (2006). “Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition.” environmental health perspectives 114(10): 1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Wrench N, Ryde IT, Lassiter TL, Levin ED and Seidler FJ (2009). “Neonatal parathion exposure disrupts serotonin and dopamine synaptic function in rat brain regions: modulation by a high-fat diet in adulthood.” Neurotoxicol. Teratol. 31: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi V, de la Fuente-Fernández R, Holden JE, Schulzer M, Ruth TJ, & Stoessl J (2004). “Changes of dopamine turnover in the progression of Parkinson’s disease as measured by positron emission tomography: their relation to disease-compensatory mechanisms.” Journal of Cerebral Blood Flow & Metabolism 24(8): 869–876. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, & Levin ED (2008a). “Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon.” Neurotoxicology & Teratology 30(1): 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D & Wells C (2008b). “Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion.” Brain research bulletin 77(6): 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanparast J, Naseh M, Baniasadi M, & Haghdoost-Yazdi H (2013). “Developmental exposure to chlorpyrifos and diazinon differentially affect passive avoidance performance and nitric oxide synthase-containing neurons in the basolateral complex of the amygdala.” Brain research 1494: 17–27. [DOI] [PubMed] [Google Scholar]
- Ward MT, Stoelzel CR, & Markus EJ (1999). “Hippocampal dysfunction during aging II: Deficits on the radial-arm maze.” Neurobiology of aging 20(4): 373–380. [DOI] [PubMed] [Google Scholar]
- Willig F, Palacios A, Monmaur P, M’Harzi M, Laurent J, & Delacour J (1987). “Short-term memory, exploration and locomotor activity in aged rats.” Neurobiology of aging 8(5): 393–402. [DOI] [PubMed] [Google Scholar]
- Willoughby MT (2003). “Developmental course of ADHD symptomatology during the transition from childhood to adolescence: a review with recommendations.” Journal of Child Psychology and Psychiatry 44(1): 88–106. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Nakajima D, Ahmed S, & Fujimaki H (2013). “Impairment of novel object recognition in adulthood after neonatal exposure to diazinon.” Archives of toxicology 87(4): 753–762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


