Abstract
Garlic (Allium sativum) is a functional food, having hydrogen sulfide (H2S)-releasing capacity, which exhibits considerable effects on hypertension and gut microbiota. H2S is strongly associated with hypertension and chronic kidney disease (CKD). Maternal CKD leads to hypertension in adult rat progeny, which was linked to disruption of the gut microbiota. This study validated the benefits of perinatal garlic oil supplementation against offspring hypertension induced by maternal CKD via modulation of H2S signaling, nitric oxide (NO), and the gut microbiota. Before pregnancy, female rats received a 0.5% adenine diet for 3 weeks to develop an animal model to mimic human CKD. Garlic oil (100 mg/kg/day) or vehicle was administered to pregnant rats by oral gavage during gestation and lactation. Perinatal garlic oil supplementation protected against maternal CKD-induced hypertension in offspring at 12 weeks of age. The beneficial effects of garlic oil are associated with enhanced H2S signaling, increased NO bioavailability, and shifts in gut microbiota. Perinatal garlic oil supplementation reduces abundance of genera Variovorax, Nocardia, Sphingomonas, and Rhodococcus. Our findings provide insight into the role of early H2S-targeted intervention as a preventive strategy in hypertension for further translational research.
Keywords: garlic, hypertension, hydrogen sulfide, developmental origins of health and disease (DOHaD), gut microbiota, renin-angiotensin system, nitric oxide
1. Introduction
Hydrogen sulfide (H2S) is a gaseous molecule with a biological impact on human health and disease [1,2]. Even though H2S is recognized as a toxic gas, it has an essential biofunctional role at the physiological level [2]. Importantly, H2S signaling participates in the regulation of renal physiology and blood pressure (BP) [1,3]. The formation of H2S can occur through the enzymatic pathway, non-enzymatic pathway, and gut microbial origins [1,4]. H2S is enzymatically synthesized from L-cysteine via three enzymes: 3-mercaptopyruvate sulfurtransferase (3MST), cystathionine γ-lyase (CSE), and cystathionine β-synthase (CBS) [1]. In addition, non-enzymatic synthesis of H2S can occur in organic thiol. Thiosulfate can be reduced and regenerate H2S [5]. Rather, thiosulfate is a major oxidation product formed during H2S metabolism. As a result, thiosulfate may reflect H2S recycling because it is not only a metabolite of H2S, but also an index of the sulfide pool [6]. Of note, half of fecal H2S is generated from gut microbiota [4]. The sulfate-reducing bacteria (SRB) represent a non-enzymatic source of fecal H2S. Additionally, gut bacteria can enzymatically produce H2S by sulfite reduction. Increasing evidence suggests the key role of H2S in hypertension and kidney disease [3,6,7]. Nevertheless, limited information is available on whether the dysregulated H2S signaling pathway is involved in the gut–kidney axis, leading to the development of kidney disease and hypertension.
Adult disease can originate from an adverse intrauterine environment, which is referred to as the developmental origins of health and disease (DOHaD) [8]. About 3–4% of childbearing-aged women have chronic kidney disease (CKD) [9]. Maternal CKD is closely connected to adverse maternal and offspring outcomes [10]. Recent evidence suggests the interactions between gut microbiota and the kidney through the gut–kidney axis contribute to hypertension [11]. We previously found that maternal adenine-induced CKD induced hypertension in adult rat offspring, which was accompanied by disruption of the gut microbiota [12]. Conversely, maternal CKD-primed offspring hypertension can be protected by maternal L-cysteine supplementation, suggesting the protective role of H2S [13].
Garlic (Allium sativum), a popular functional food, is rich in natural polysulfides as a dietary source of H2S donors [14], which confer a health benefit due to its antihypertensive effect [15]. The beneficial effects of H2S in hypertension are associated with reducing oxidative stress, increasing nitric oxide (NO) bioavailability, rebalancing of the renin-angiotensin aldosterone system (RAAS), and altering the gut microbiota [1,2,3,16]. Our prior work indicates that maternal garlic oil supplementation protects hypertension in offspring born to high-fat diet-fed dams, and its protective effects are associated with enhancing the H2S signaling system and shaping gut microbiota [17]. The aim of this study was to assess whether perinatal garlic oil supplementation has the potential to avert maternal CKD-induced offspring hypertension and whether its protective effects are associated with enhancement of H2S signaling, increases in NO bioavailability, and shifts in gut microbiota composition.
2. Materials and Methods
2.1. Animal Care and Experimental Design
The procedures carried out on the animals were approved by our Institutional Animal Ethics Committee (Permit # 2020110202) and according to the rules of the Care and Use of Laboratory Animals of the National Institutes of Health. We purchased virgin Sprague Dawley (SD) rats from BioLASCO Taiwan Co. Ltd. (Taipei, Taiwan) for breeding. Rats were housed in our AAALAC-accredited animal facility.
To conduct a CKD model, eight-week-old female rats were fed with chow containing 0.5% adenine for 3 weeks before pregnancy [12]. Female rats were successfully mated with a male rat at 11 weeks of age, as confirmed by the occurrence of a copulatory plug. We randomly divided the dams into four groups: CN (control), CKD (adenine-treated rats), CN+GO (control rats received garlic oil), and CKD+GO (adenine-treated rats received garlic oil). Daily administration of garlic oil (GO, Sigma-Aldrich, St. Louis, MO, USA) or vehicle by oral gavage at the dose of 100 mg/kg/day was carried out during gestation and lactation according to prior work in rats [16]. All litters were standardized to eight pups at one day of age. As males have a higher likelihood of hypertension and have hypertension earlier than females [18], we only included male progeny from each litter for the experiment.
BP was measured every four weeks using the CODA rat tail-cuff system (Kent Scientific Corporation, Torrington, CT, USA). One week prior to the actual recording sessions, rat offspring were allowed to adapt to the restraint chamber. A total of 32 rats belonging to four experimental groups (n = 8 per group) were sacrificed at 12 weeks old. Prior to sacrifice, fecal samples were collected in the morning and kept at −80 °C until analyses. The renal cortex and medulla were separated and subsequently snap-frozen at −80 °C. Blood samples were collected using heparin tubes.
2.2. Analysis of NO Parameters by HPLC
As L-arginine is the substrate for NO synthase (NOS), and symmetric and asymmetric dimethylarginine (SDMA and ADMA) are NOS inhibitors, their levels were determined using HPLC (HP series 1100, Agilent Technologies, Inc., Santa Clara, CA, USA) with the OPA-3MPA derivatization reagent based on our previously described method [12].
2.3. Analysis of Plasma H2S and Thiosulfate Levels by HPLC-MS
Plasma H2S and thiosulfate concentrations were measured by an HPLC-Mass Spectrometry (MS) protocol previously validated in our lab [19] using an Agilent Technologies 1290 HPLC system together with an Agilent 6470 Triple Quadrupole LC/MS (Agilent Technologies, Wilmington, DE, USA) and an electrospray ionization (ESI) source [18]. Chromatographic separation was carried out using a Supelco C18 column (3 µm, 50 × 2.1 mm; Sigma–Aldrich) protected by an Ascentis C18 column (3 µm, 20 × 2.1 mm, Merck KGaA, Darmstadt, Germany). Solvents used in the elution step were composed of 0.1% formic acid (v/v) in acetonitrile, at a flow rate of 300 µL/min. We measured thiosulfate derivative pentafluorobenzyl (PFB)-S2O3H and H2S derivative sulfide dibimane (SDB). Selected reaction monitoring mode was utilized to detect target compounds with a targeted 415→223 m/z and 292.99→81 m/z, for SDB and PFB-S2O3H, respectively. Phenyl 4-hydroxybenzoate (PHB) was used as an internal standard and detection was at 212.99→93 m/z. The percentage of coefficient of variation for the intra-assay variability was 4% and 6% for H2S and thiosulfate, respectively.
2.4. Analysis of RAAS Components by qPCR
RNA was extracted from the renal cortical tissues. RAAS components’ mRNA levels were analyzed by quantitative polymerase chain reaction (qPCR) in duplicate using SYBR Green; results were normalized to the 18S ribosomal RNA (R18S), as previously described [12,13]. The primers for renin, angiotensinogen (AGT), angiotensin converting enzyme-1 (ACE1) and -2 (ACE2), angiotensin II type 1 receptor (AT1R), and angiotensin-(1–7)/Mas receptor (MAS) are provided in Table 1. The relative mRNA expression levels of genes were calculated using the comparative CT method. The fold change for gene expression relative to the control was calculated using the formula 2−ΔΔCT.
Table 1.
PCR primer sequences.
| Gene | Forward | Reverse |
|---|---|---|
| Renin | 5 aacattaccagggcaactttcact 3 | 5 acccccttcatggtgatctg 3 |
| AGT | 5 gcccaggtcgcgatgat 3 | 5 tgtacaagatgctgagtgaggcaa 3 |
| ACE1 | 5 caccggcaaggtctgctt 3 | 5 cttggcatagtttcgtgaggaa 3 |
| ACE2 | 5 acccttcttacatcagccctactg 3 | 5 tgtccaaaacctaccccacatat 3 |
| AT1R | 5 gctgggcaacgagtttgtct 3 | 5 cagtccttcagctggatcttca 3 |
| MAS | 5 catctctcctctcggctttgtg 3 | 5 cctcatccggaagcaaagg 3 |
| R18S | 5 gccgcggtaattccagctcca 3 | 5 cccgcccgctcccaagatc 3 |
AGT = angiotensinogen; ACE = angiotensin converting enzyme; ACE2 = angiotensin converting enzyme-2; AT1R = angiotensin II type 1 receptor; MAS = angiotensin-(1–7)/Mas receptor, R18S = 18S ribosomal RNA.
2.5. Analysis of H2S-Producing Enzymes by Western Blot
As stated previously [19], renal cortex homogenates were prepared for Western blotting with antibody incubation. A total of 200 µg protein was loaded and electrophoresed through a 10% polyacrylamide gel. Electrophoretically separated proteins were electrotransferred onto a nitrocellulose membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). In this procedure, Ponceau S staining (PonS, Sigma-Aldrich) was utilized to correct for variations in the total protein loading. For the detection of H2S-producing enzymes, the membranes were incubated with a mouse monoclonal antibody against rat CBS (1:1000, overnight incubation; Abnova Corporation, Taipei, Taiwan), a rabbit monoclonal antibody against rat 3MST (1:500, overnight incubation; Novus Biologicals, Littleton, CO, USA), or a rabbit polyclonal antibody against rat CSE (1:1000, overnight incubation; Proteintech Group, Inc. Chicago, IL, USA). The protein abundance of the samples was quantified using Quantity One Analysis software (Bio-Rad) following enhanced chemiluminescence reagent detection (PerkinElmer, Waltham, MA, USA). Band density was represented as the integrated optical density (IOD)/PonS to correct the variations in total protein loading.
2.6. Metagenomics Analysis of Gut Microbiota
Fecal genomic DNA was extracted and analyzed by 16S rRNA gene-based metagenomics analysis at Biotools Co., Ltd. (New Taipei City, Taiwan), as we described previously [20]. The full-length 16S genes were amplified with barcoded multiplexed primers for SMRTbell library preparation and sequencing (PacBio, Menlo Park, CA, USA). The QIIME2 was utilized to process data from a high-throughput 16S rRNA sequencing. A phylogenetic tree was derived from the amplicon sequence variants (ASVs) via FastTree (QIIME2) [21]. We computed two α-diversity metrics to measure the richness and evenness of the communities by Faith’s phylogenetic diversity (PD) index and the Shannon index. The similarities between communities across groups (i.e. β-diversity) were examined using the analysis of similarities (ANOSIM) and principal coordinate analysis (PCoA) based on the unweighted UniFrac distance.
2.7. Statistics
All data are presented as means ± the standard error of the mean (SEM), and p < 0.05 was considered statistically significant. Statistical analyses were carried out by one-way ANOVA or two-way ANOVA where appropriate. To produce post hoc multiple comparison tests, Tukey’s post hoc test was utilized. Statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Offspring Blood Pressure and Weight
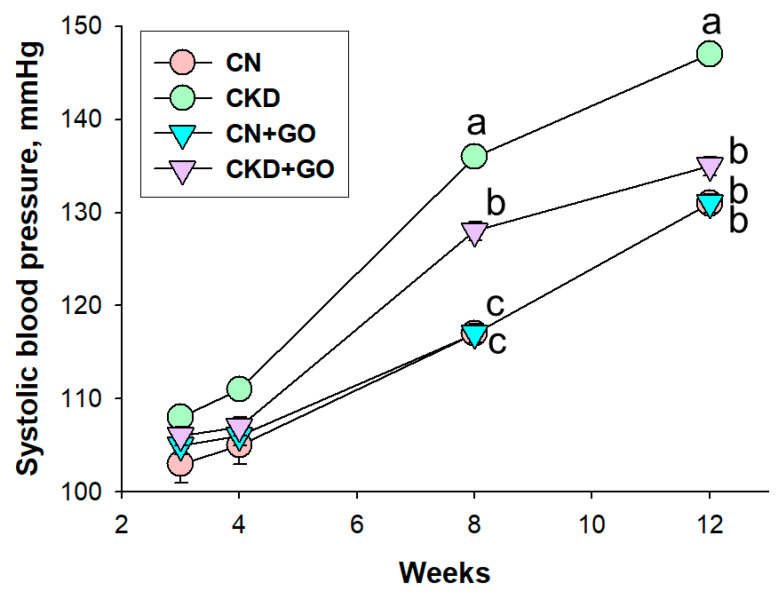
Table 2 shows the pup mortality rate was zero. The body weight (BW) was not affected by maternal CKD or perinatal garlic oil supplementation. The kidney weight (KW) and the KW-to-BW ratio were not different among the four groups. However, at 12 weeks of age, male offspring of dams with CKD had higher systolic and diastolic BPs, and mean arterial pressure, than male offspring of dams without CKD. Conversely, perinatal garlic oil supplementation averted the elevation of BP. Figure 1 illustrates that longitudinal measurement of BP between week 3 and week 12. Similarly, systolic BP elevations started at week 8 in CKD offspring, which was averted by garlic oil treatment. Overall, these findings indicate that maternal CKD induced offspring hypertension, which was averted by perinatal garlic oil supplementation.
Table 2.
Weights and blood pressure.
| Groups | CN | CKD | CN+GO | CKD+GO |
|---|---|---|---|---|
| Mortality | 0% | 0% | 0% | 0% |
| Body weight (BW), g | 277 ± 14 | 286 ± 11 | 285 ± 12 | 287 ± 11 |
| Left kidney weight (KW), g | 1.3 ± 0.06 | 1.29 ± 0.04 | 1.39 ± 0.110 | 1.27 ± 0.062 |
| Left KW/100 g BW | 0.47 ± 0.01 | 0.45 ± 0.01 | 0.49 ± 0.029 | 0.44 ± 0.015 |
| Systolic blood pressure, mmHg | 131 ±1 b | 147 ± 1 a | 131 ±1 b | 135 ±1 b |
| Diastolic blood pressure, mmHg | 87 ± 1 b | 101 ± 2 a | 87 ± 2 b | 87 ± 3 b |
| Mean arterial pressure, mmHg | 102 ± 1 b | 117 ± 1 a | 102 ± 1 b | 103 ± 2 b |
N = 8/group; the letters a and b indicate the differences between the groups (p < 0.05, one-way ANOVA).
Figure 1.
Effect of perinatal garlic oil supplementation on systolic blood pressure in offspring at 3–12 weeks of age. The letters a, b and c indicate the differences between the groups (p < 0.05, two-way ANOVA); N = 8/group.
3.2. H2S Pathway
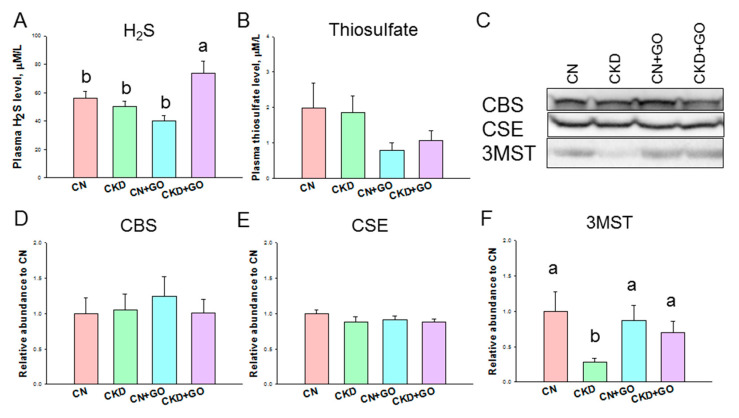
Figure 2 illustrates the H2S pathway, which includes plasma H2S and thiosulfate levels and protein levels of H2S-producing enzymes in offspring kidneys. The plasma H2S level was higher in the CKD+GO group compared to the three other groups (Figure 2A), whereas the plasma thiosulfate level was comparable among the four groups (Figure 2B). Renal protein abundance of H2S-producing enzymes CBS, CSE, and 3MST is compared in Figure 2C. Garlic oil supplementation has a negligible effect on renal CBE and CSE abundance (Figure 2D,E). Maternal CKD led to a reduction in the renal 3MST protein level, which garlic oil supplementation prevented (Figure 2F). Together, these findings show that garlic oil treatment increases plasma H2S and the renal 3MST protein level.
Figure 2.
Effect of perinatal garlic oil supplementation on (A) plasma level of H2S and (B) thiosulfate, and H2S-producing enzymes in 12-week-old offspring kidneys. (C) Representative Western blots demonstrate cystathionine β-synthase (CBS, ~61 kDa), cystathionine γ-lyase (CSE, ~45 kDa), and 3-mercaptopyruvate sulfurtransferase (3MST, ~52 kDa) bands. The relative protein levels of renal cortical (D) CBS, (E) CSE, and (F) 3MST were calculated. The letters a and b indicate the differences between the groups (p < 0.005, one-way ANOVA); N = 8/group.
3.3. NO Pathway
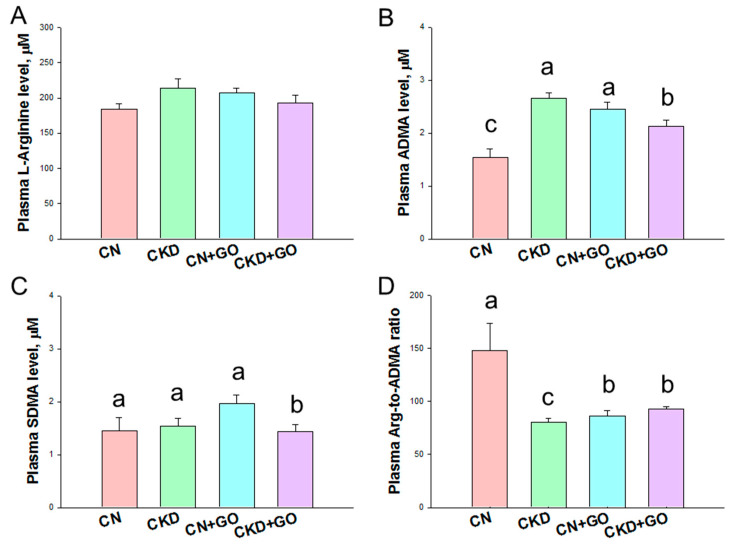
Plasma NO-related parameters are compared in Figure 3. The plasma level of L-arginine, the substrate for NOS to generate NO, was not different among the four groups (Figure 3A). Maternal CKD caused an increase in the plasma ADMA level, which was prevented by perinatal garlic oil supplementation (Figure 3B).
Figure 3.
Effect of perinatal garlic oil supplementation on NO pathway in 12-week-old offspring. These NO parameters include (A) L-arginine, (B) asymmetric dimethylarginine (ADMA), (C) symmetric dimethylarginine (SDMA), and (D) the ratio of L-arginine-to-ADMA. The letters a, b and c indicate the differences between the groups (p < 0.05, one-way ANOVA); N = 8/group.
Compared to the CN+GO group, the plasma SDMA level was lower in the CKD+GO group (Figure 3C). Additionally, CKD reduced the L-arginine-to-ADMA ratio, an index of NO bioavailability [22], in the CKD group (Figure 3D). In contrast, the decreased L-arginine-to-ADMA ratio was improved by garlic oil supplementation.
3.4. The RAAS
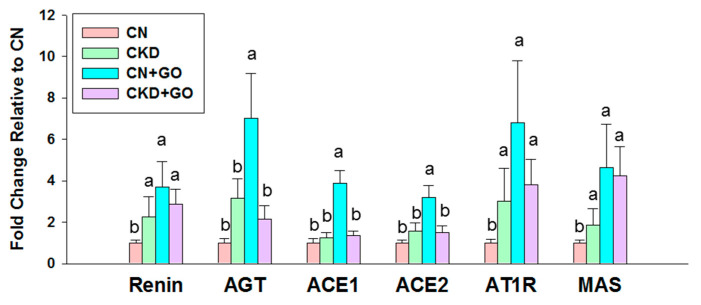
As aberrant activation of the RAAS participates in hypertension of developmental origins [23], we then looked at the renal mRNA expression of RAAS components. Compared to the CN group, renal mRNA expression of renin, AGT, ACE1, ACE2, AT1R, and MAS was higher in the CN+GO group (Figure 4). That is, two axes of RAAS—classical axis (ACE1/Angiotensin II) and nonclassical axis (ACE2/Ang-(1–7))—are both activated by perinatal garlic oil treatment. Additionally, garlic oil supplementation enhanced AT1R and MAS expression in the offspring kidneys of the CKD+GO group vs. the CKD group. It is known that activating AT1R promotes vasoconstriction, while agonizing MAS in favor of vasodilatation. As the two RAAS axes act in an opposite manner, our data suggest that the RAAS may not be the primary mechanism behind the protective effect of garlic oil on maternal CKD-induced hypertension.
Figure 4.
Effect of perinatal garlic oil supplementation on the renin-angiotensin aldosterone system (RAAS) in offspring kidneys at 12 weeks of age. The letters a and b indicate the differences between the groups (p < 0.05, one-way ANOVA); N = 8/group.
3.5. Alterations in Gut Microbiota
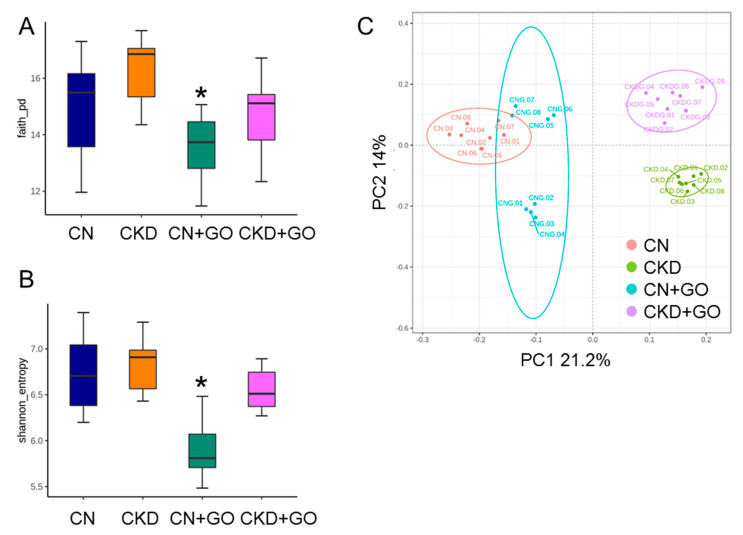
We first analyzed α- and β-diversity metrics to elucidate how maternal CKD and perinatal garlic oil supplementation influence the establishment of gut microbiota in adult offspring. Community richness and evenness were estimated by Faith’s PD index and the Shannon index, respectively. We found maternal CKD has a negligible effect on both α-diversity metrics, while these metrics were lower in the CN+GO group than those in the CN group (Figure 5A,B). PCoA plots of β-diversity based on the unweighted UniFrac metric were utilized to illuminate the samples clustered according to study groups, as shown in Figure 5C. We observed from ANOSIM that there were significant differences in the groups (all p < 0.05). Our data reveal that perinatal garlic oil supplementation caused offspring gut microbiota shifts in diversity. Regarding the composition, the predominant phyla are Firmicutes and Bacteroidetes, followed by Actinobacteria, Deferribacteres, and Proteobacteria; these results are consistent with prior animal studies that showed gut microbiota are dominated by these bacteria [11,12,13].
Figure 5.
Comparison of α-diversity among four groups of 12-week-old offspring in (A) Faith’s phylogenetic diversity (PD) index and (B) Shannon index; (C) principal coordinate analysis (PCoA) plots of β-diversity calculated by the unweighted UniFrac metric across the four groups; each point represents the microbiota of a single sample, and colors reflect metadata for that sample. * p < 0.05 vs. CN.
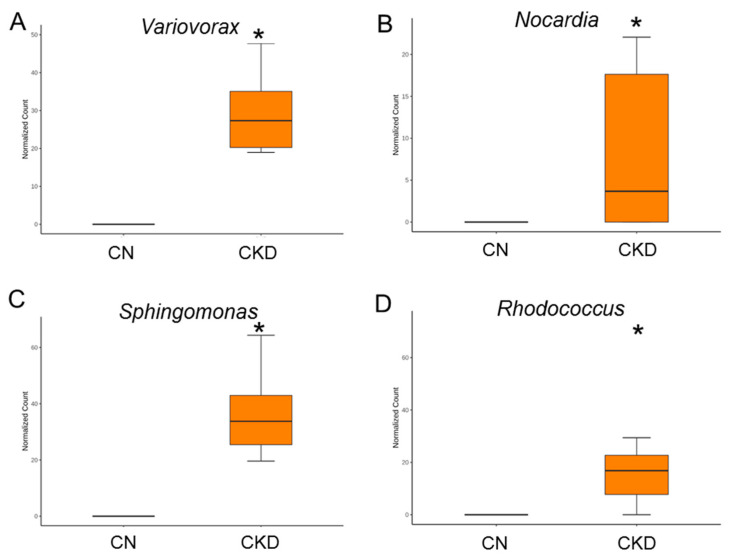
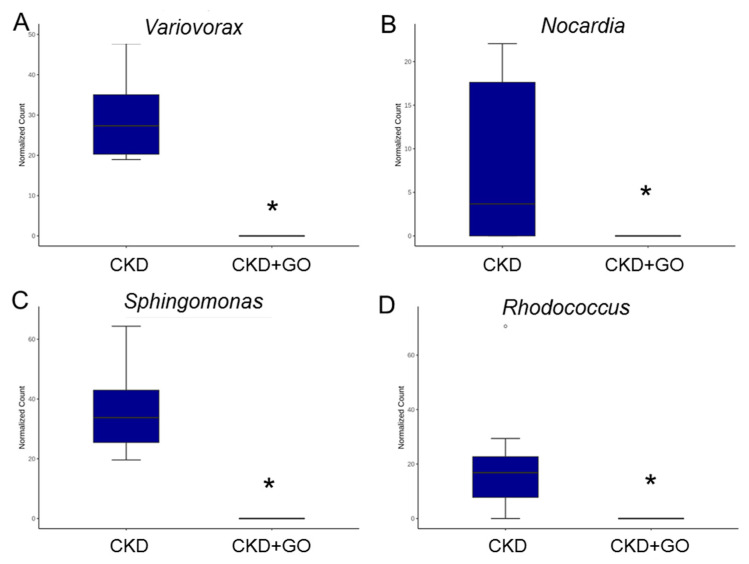
Maternal CKD caused a greater proportion of genera Variovorax Nocardia, Sphingomonas, and Rhodococcus in the CKD group vs. the CN group (Figure 6A–D). On the contrary, the proportion of these enriched genera was lowered by perinatal garlic acid supplementation (Figure 7A–D).
Figure 6.
Effect of maternal CKD on the gut microbiota in 12-week-old male offspring. Genus-level relative abundance of (A) Variovorax, (B) Nocardia, (C) Sphingomonas, and (D) Rhodococcus. * p < 0.05.
Figure 7.
Effect of perinatal garlic oil on the gut microbiota of CKD+GO group compared to the CKD group. Genus-level relative abundance of (A) Variovorax, (B) Nocardia, (C) Sphingomonas, and (D) Rhodococcus. * p < 0.05.
4. Discussion
Our results provide novel insights into the protective roles of perinatal garlic oil supplementation on maternal CKD-induced offspring hypertension through regulation of the H2S signaling, shifts in gut microbiota, and restoration of NO. The major findings are described as follows: (1) perinatal supplementation with garlic oil prevented the increase in BP in male offspring born to dams with CKD at 12 weeks of age; (2) perinatal garlic oil supplementation increased renal 3MST protein levels and the plasma H2S level; (3) the benefits of garlic oil for offspring hypertension were linked to increases in NO bioavailability, characterized by decreases in ADMA and increases in the L-arginine-to-ADMA ratio; (4) perinatal garlic oil supplementation led to gut microbiota shifts in diversity and composition; and (5) the advantageous effect of garlic oil against offspring hypertension coincided with decreases in the genera Variovorax, Nocardia, Sphingomonas, and Rhodococcus.
Although prior research suggests the BP-lowering effect of garlic [15,17], our report goes beyond previous reports and demonstrates that perinatal garlic oil supplementation averts maternal CKD-induced offspring hypertension. Considering sulfur-containing compounds derived from garlic are natural precursors of H2S [14], the findings of the current research agree with past findings, suggesting that H2S plays a role in the development of hypertension [7,24].
The protective effect of garlic oil against maternal CKD-induced hypertension may be relevant to its ability to increase renal 3MST protein levels and the plasma H2S level. The findings of this study are consistent with prior research, which showed that the uses of different H2S-based interventions in early life to increase H2S bioavailability may be a reprogramming approach to avert offspring hypertension in several models of developmental origin [17,19,25].
Another protective mechanism of garlic oil may also be associated with increased NO bioavailability. Increasing evidence has indicated that early-life interventions targeting the NO pathway can be a preventive strategy to avert the development of hypertension [26]. This concept is corroborated by our present study, which showed that the beneficial effects of garlic oil coincided with increased NO bioavailability, represented by a decrease in ADMA and an increase in the ratio of L-arginine-to-ADMA. A previous study revealed sodium hydrosulfide, a H2S donor, can rescue NO bioavailability and prevent hypertension in a NO deficiency rat model [27]. As such, there may be a crosstalk between H2S and NO in the control of BP. Accordingly, maternal CKD-induced hypertension may be counterbalanced by the garlic oil-mediating NO signaling pathway and shifted toward vasodilation.
Our findings support results of previous human studies showing the pathophysiological importance of H2S and NO bioavailability during cardiovascular disease [28,29]. Although H2S and its metabolites have been utilized as biomarkers for several human diseases [30], different analytical methods have obvious limitations and provide contradictory results, especially in the clinical setting [28,29,30,31]. A previous study reported that elevation of plasma-free H2S found in patients with peripheral arterial disease was due to a compensatory response to endothelial dysfunction and dysregulation of NO bioavailability [28]. Using the animal model, we not only detected plasma H2S and thiosulfate levels, but also tissue H2S-producing enzymes. Our data suggest that protection by garlic oil supplementation against maternal CKD-primed offspring hypertension can be attributed to increased renal 3MST protein levels, the plasma H2S level, and NO bioavailability. Thus, future work in developing an ideal methodology to reduce the gap between human and animal research is required to better assess H2S detection in clinical practice.
Additionally, the beneficial effect of garlic oil is possibly due to alterations of the gut microbiota. Consistent with findings in hypertensive humans and animals [32,33,34], a high abundance of the genera Variovorax, Sphingomonas, and Rhodococcus was identified as a microbial marker for hypertension in the maternal CKD-induced hypertension model. Although Nocardia spp. are opportunistic bacteria related to kidney disease and hypertension in humans [35], the association between high BP and a high Nocardia abundance was addressed, for the first time, by our study.
Since the gut is another major source of H2S [4], we also determined the composition of gut microbiota with a focus on sulfate- or sulfite-reducing bacteria. We observed almost all SRB (e.g., Desulfobacter and Desulfovibrio) were unnoticeable in both groups receiving garlic oil. Sulfite reductase exists in several species, such as E coli, Salmonella, Klebsiella, Corynebacterium, Rhodococcus, and Baccilus [36]. Our current data showed the abundance of most sulfite-reducing bacteria was unaltered in response to perinatal garlic oil supplementation, with the exception that the abundance of genus Rhodococcus was reduced by garlic oil. Hence, whether the protective role of garlic oil is related to gut microbiota-derived H2S and alterations of sulfate- or sulfite-reducing bacteria awaits further clarification. Surprisingly, we observed that perinatal garlic oil supplementation reduced indices of richness and evenness in offspring gut microbiota. As the decrease in α-diversity has been linked to disease risk [37], further studies of the long-term outcomes or moderating role of garlic oil in normal controls is therefore needed.
In addition to H2S and the NO system, accumulated evidence suggests that early blockade of the RAAS affords protection against the offspring hypertension programmed by various maternal insults [23]. However, our data suggested that the RAAS is less likely to be a primary protective mechanism of garlic oil against maternal CKD-programmed hypertension as RAAS components were not different between the CKD and CKD+GO groups.
This study has some limitations. One limitation is that gut microbiota analysis was only carried out in adult offspring, but not in mothers and young progeny. Whether perinatal garlic oil supplementation can regulate gut microbes involved in H2S metabolism in both dams and neonate offspring, and whether gut microbiota-derived fecal H2S is associated with offspring outcomes later in life, both deserve further evaluation. Secondly, we only investigated male offspring. Further work is required to distinguish whether garlic oil has a sex-specific effect and whether sex differences exist behind maternal CKD-induced programmed hypertension. Finally, in addition to organosulfur, garlic contains important biological compounds such as polyphenols [38]. Given that the major active compounds of garlic oil were not determined, the extent of its protective effect throughout the H2S signaling pathway deserves additional research.
5. Conclusions
In conclusion, our study demonstrated that perinatal garlic oil supplementation averted offspring hypertension programmed by maternal CKD. There are several protective mechanisms by which garlic oil therapy protects adult offspring against hypertension, including enhancement of the renal H2S-generating system, increases in NO bioavailability, and shifts in gut microbiota. Our pre-clinical investigation provides an in-depth understanding of the perinatal use of functional foods targeting H2S signaling and impacting offspring hypertension. This may help us develop effective reprogramming strategies to avert hypertension in children born to mothers with CKD.
Acknowledgments
We would like to thank the Super Micro Mass Research and Technology Center, the Institute of Environmental Toxin and Emerging Contaminant, and the Center for Environmental Toxin and Emerging Contaminant Research, Cheng Shiu University, Kaohsiung, for technical support.
Author Contributions
Y.-L.T. contributed to funding application, data collection, concept generation, methodology, drafting of the manuscript, data interpretation, and approval of the article; C.-Y.H. contributed to methodology and data analysis; G.-P.C.-C. contributed to data analysis and methodology; S.L. contributed to data analysis; C.-N.H. contributed to methodology, drafting of the manuscript, concept generation, data collection, critical revision of the manuscript, and approval of the article. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animal studies were approved by the Institutional Animal Ethics Committee (IACUC) of Chang Gung Memorial Hospital (Permit # 2020110202).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grant MOST 110-2314-B-182-020-MY3 (Y.-L.T.) from the Ministry of Science and Technology, Taiwan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4–10. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Kajimura M., Fukuda R., Bateman R.M., Yamamoto T., Suematsu M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 2010;13:157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dugbartey G.J. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharmacol. Rep. 2018;70:196–205. doi: 10.1016/j.pharep.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Linden D.R. Hydrogen Sulfide Signaling in the Gastrointestinal Tract. Antioxid. Redox Signal. 2014;20:818–830. doi: 10.1089/ars.2013.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson K.R., Deleon E.R., Gao Y., Hurley K., Sadauskas V., Batz C., Stoy G.F. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R592–R603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- 6.Koning A.M., Frenay A.R., Leuvenink H.G., van Goor H. Hydrogen sulfide in renal physiology, disease and transplantation—The smell of renal protection. Nitric Oxide. 2015;46:37–49. doi: 10.1016/j.niox.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Van Goor H., van den Born J.C., Hillebrands J.L., Joles J.A. Hydrogen sulfide in hypertension. Curr. Opin. Nephrol. Hypertens. 2016;25:107–113. doi: 10.1097/MNH.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 8.Haugen A.C., Schug T.T., Collman G., Heindel J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2015;6:55–64. doi: 10.1017/S2040174414000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munkhaugen J., Lydersen S., Romundstad P.R., Widerøe T.-E., Vikse B.E., Hallan S. Kidney function and future risk for adverse pregnancy outcomes: A population-based study from HUNT II, Norway. Nephrol. Dial. Transplant. 2009;24:3744–3750. doi: 10.1093/ndt/gfp320. [DOI] [PubMed] [Google Scholar]
- 10.Piccoli G.B., Alrukhaimi M., Liu Z.H., Zakharova E., Levin A., World Kidney Day Steering Committee What we do and do not know about women and kidney diseases; Questions unanswered and answers unquestioned: Reflection on World Kidney Day and International Woman’s Day. Physiol. Int. 2018;105:1–18. doi: 10.1556/2060.105.2018.1.6. [DOI] [PubMed] [Google Scholar]
- 11.Yang T., Richards E.M., Pepine C.J., Raizada M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018;14:442–456. doi: 10.1038/s41581-018-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu C.N., Yang H.W., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020;21:7237. doi: 10.3390/ijms21197237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants. 2022;11:483. doi: 10.3390/antiox11030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iciek M., Kwiecien I., Wlodek L. Biological properties of garlic and garlic-derived Organosulfur compounds. Environ. Mol. Mutagen. 2009;50:247. doi: 10.1002/em.20474. [DOI] [PubMed] [Google Scholar]
- 15.Ried K., Fakler P. Potential of garlic (Allium sativum) in lowering high blood pressure: Mechanisms of action and clinical relevance. Integr. Blood Press. Control. 2014;7:71. doi: 10.2147/IBPC.S51434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu C.N., Tain Y.L. Hydrogen Sulfide in Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2018;19:1438. doi: 10.3390/ijms19051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Maternal Garlic Oil Supplementation Prevents High-Fat Diet-Induced Hypertension in Adult Rat Offspring: Implications of H2S-Generating Pathway in the Gut and Kidneys. Mol. Nutr. Food Res. 2021;65:e2001116. doi: 10.1002/mnfr.202001116. [DOI] [PubMed] [Google Scholar]
- 18.Reckelhoff J.F. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.HYP.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 19.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants. 2020;9:856. doi: 10.3390/antiox9090856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tain Y.L., Hou C.Y., Chang-Chien G.P., Lin S.F., Hsu C.N. Perinatal Propionate Supplementation Protects Adult Male Offspring from Maternal Chronic Kidney Disease-Induced Hypertension. Nutrients. 2022;14:3435. doi: 10.3390/nu14163435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bode-Böger S.M., Scalera F., Ignarro L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007;114:295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Hsu C.N., Tain Y.L. Targeting the Renin–Angiotensin–Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021;22:2298. doi: 10.3390/ijms22052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu C.N., Tain Y.L. Preventing Developmental Origins of Cardiovascular Disease: Hydrogen Sulfide as a Potential Target? Antioxidants. 2021;10:247. doi: 10.3390/antiox10020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tain Y.L., Lee C.T., Chan J.Y., Hsu C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016;215:636. doi: 10.1016/j.ajog.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Hsu C.N., Tain Y.L. Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease. Int. J. Mol. Sci. 2019;20:681. doi: 10.3390/ijms20030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong G., Chen F., Cheng Y., Tang C., Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Peter E.A., Shen X., Shah S.H., Pardue S., Glawe J.D., Zhang W.W., Reddy P., Akkus N.I., Varma J., Kevil C.G. Plasma free H2S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2013;2:e000387. doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan Y., Wang S., Ren X., Zhang C., Xu F. The prognostic implications of perioperative endogenous hydrogen sulfide and nitric oxide levels in children with congenital heart disease complicated by pulmonary arterial hypertension. Eur. J. Pediatr. 2021;180:1915–1922. doi: 10.1007/s00431-020-03897-w. [DOI] [PubMed] [Google Scholar]
- 30.Kolluru G.K., Shen X., Kevil C.G. Reactive Sulfur Species: A New Redox Player in Cardiovascular Pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2020;40:874–884. doi: 10.1161/ATVBAHA.120.314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang G., Wu L. Trend in H2S Biology and Medicine Research—A Bibliometric Analysis. Molecules. 2017;22:2087. doi: 10.3390/molecules22122087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmu J., Lahti L., Niiranen T. Targeting Gut Microbiota to Treat Hypertension: A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18:1248. doi: 10.3390/ijerph18031248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waghulde H., Cheng X., Galla S., Mell B., Cai J., Pruett-Miller S.M., Vazquez G., Patterson A., Vijay Kumar M., Joe B. Attenuation of Microbiotal Dysbiosis and Hypertension in a CRISPR/Cas9 Gene Ablation Rat Model of GPER1. Hypertension. 2018;72:1125–1132. doi: 10.1161/HYPERTENSIONAHA.118.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jing Y., Zhou H., Lu H., Chen X., Zhou L., Zhang J., Wu J., Dong C. Associations Between Peripheral Blood Microbiome and the Risk of Hypertension. Am. J. Hypertens. 2021;34:1064–1070. doi: 10.1093/ajh/hpab084. [DOI] [PubMed] [Google Scholar]
- 35.Han Y., Huang Z., Zhang H., He L., Sun L., Liu Y., Liu F., Xiao L. Nocardiosis in glomerular disease patients with immunosuppressive therapy. BMC Nephrol. 2020;21:516. doi: 10.1186/s12882-020-02179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blachier F., Davila A.M., Mimoun S., Benetti P.H., Atanasiu C., Andriamihaja M., Benamouzig R., Bouillaud F., Tomé D. Luminal sulfide and large intestine mucosa: Friend or foe? Amino Acids. 2010;39:335–347. doi: 10.1007/s00726-009-0445-2. [DOI] [PubMed] [Google Scholar]
- 37.Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang A., Cao S.Y., Xu X.Y., Gan R.Y., Tang G.Y., Corke H., Mavumengwana V., Li H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.) Foods. 2019;8:246. doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.