Abstract
Autism spectrum disorder (ASD) is characterized by cognitive inflexibility and social deficits. Probiotics have been demonstrated to play a promising role in managing the severity of ASD. However, there are no effective probiotics for clinical use. Identifying new probiotic strains for ameliorating ASD is therefore essential. Using the maternal immune activation (MIA)-based offspring ASD-like mouse model, a probiotic-based intervention strategy was examined in female mice. The gut commensal microbe Parabacteroides goldsteinii MTS01, which was previously demonstrated to exert multiple beneficial effects on chronic inflammation-related-diseases, was evaluated. Prenatal lipopolysaccharide (LPS) exposure induced leaky gut-related inflammatory phenotypes in the colon, increased LPS activity in sera, and induced autistic-like behaviors in offspring mice. By contrast, P. goldsteinii MTS01 treatment significantly reduced intestinal and systemic inflammation and ameliorated disease development. Transcriptomic analyses of MIA offspring indicated that in the intestine, P. goldsteinii MTS01 enhanced neuropeptide-related signaling and suppressed aberrant cell proliferation and inflammatory responses. In the hippocampus, P. goldsteinii MTS01 increased ribosomal/mitochondrial and antioxidant activities and decreased glutamate receptor signaling. Together, significant ameliorative effects of P. goldsteinii MTS01 on ASD relevant behaviors in MIA offspring were identified. Therefore, P. goldsteinii MTS01 could be developed as a next-generation probiotic for ameliorating ASD.
Keywords: autism spectrum disorders, lipopolysaccharides, maternal immune activation, Parabacteroides goldsteinii, next-generation probiotic
1. Introduction
Autism (autism spectrum disorder [ASD]) is a neurodevelopmental disorder characterized by social deficits, speech and nonverbal communication abnormalities, cognitive inflexibility, and repetitive/restricted sensory–motor behaviors in combination with various degrees (mild to severe) of hyperactivity, intellectual disability, and anxiety [1,2]. The reported incidence of ASD is high, occurring at an estimated rate of 1 in 88 births in the United States as of 2008 [3] and 1 in every 100 in the global population [4], indicating that the condition represents a significant medical and social problem worldwide. Despite years of studies, there are still no satisfactory medications approved for managing the core symptoms of ASD [5].
The etiology of ASD is complex, comprising multiple host genetic defects and environmental interferences characterized by maternal factors, gender differences, autoimmunity, prenatal infection, and inflammation [6,7,8,9,10]. Among these, maternal immune activation (MIA) was highlighted as an important causal factor in the later life of offspring [11,12,13]. Specifically, prenatal exposure to pro-inflammatory agents such as lipopolysaccharide (LPS) has been used as an MIA model to observe deficits in social interaction and anxiety-like behaviors in offspring [14]. Although preventive treatment with anti-inflammatory agents reduced inflammation, this did not restore structural and behavioral alterations in MIA offspring (MIAO) mice [15]. Additionally, although analyses of brain disorders including the hippocampal region of MIAO mice revealed abnormalities, the underlying molecular mechanisms were not completely characterized [16,17]. Therefore, ASD development might not be solely attributable to increased inflammatory activity, and the role of the hippocampus in the development of MIA-induced ASD in offspring also needs to be further clarified.
Recently, the gut microbiota–brain axis has been recognized as a key modulator of neuropsychiatric health, and aberrant compositional and structural shifts of microbes and their related metabolites might be causal factors of ASD [18,19,20]. Under this framework, the administration of probiotics, which are live beneficial bacteria, to reverse gut microbiota dysbiosis is expected to improve gastrointestinal dysfunction, optimize neuronal function, and consequently improve brain activities. Probiotic administration might therefore represent an effective approach to improve the abnormal behavioral profiles of children with ASD [21]. However, treatment with traditional probiotics (consortium) such as Lactobacillus reuteri [22], L. acidophilus [23], and DSF, a mixture of eight probiotic strains [24], only displayed potential ameliorative effects. Based on these findings, it is important to develop novel next-generation probiotics (NGPs) for ASD interventions as pharmaceutical applications [25,26].
In autistic subjects, a significant increase in the Firmicutes/Bacteroidetes ratio because of a reduction of the relative abundance in Bacteroidetes together with a decrease in that of Parabacteroides and other bacteria was reported [27,28]. In addition, the abundance of Bacteroides and Parabacteroides was also significantly decreased in germ-free mice harboring human ASD microbiomes and exhibiting ASD-like behaviors [29]. These results therefore suggested that Bacteroides and Parabacteroides species can slow the development of ASD and highlighted the importance of developing Bacteroidales-associated NGPs for ameliorating ASD-related abnormalities. In concordance, oral treatment of MIAO mice with the gut commensal bacterium Bacteroides fragilis corrected gut permeability, altered the microbial composition, and ameliorated ASD-related defects in communicative, stereotypic, anxiety-like, and sensorimotor behaviors [27].
The commensal bacterial strain Parabacteroides goldsteinii MTS01 was previously reported to ameliorate inflammation-related diseases such as obesity/metabolic syndrome and chronic obstructive pulmonary disease (COPD), and its effects were mediated through reducing LPS-related inflammation in the intestine and peripheral target organs such as adipose tissue, the liver, and the lungs [30,31,32]. In addition, P. goldsteinii MTS01 also restored aberrant cellular mitochondrial and ribosomal metabolic activities and maintained tissue integrity and host hemostasis [32]. Subsequent studies indicated that anti-inflammatory hypoacylated LPS derived from P. goldsteinii MTS01 (Pg-LPS) might act as a functional element [32]. Therefore, P. goldsteinii MTS01 might have the potential for development as an NGP for ameliorating LPS-induced, MIA-associated ASD relevant behaviors.
In this study, following the growth process of female MIAO mice, we longitudinally evaluated the ameliorative effects of P. goldsteinii MTS01 on anxiety-like behaviors and social behavioral deficits. In addition, the effects of P. goldsteinii MTS01 on transcriptomic patterns in both the intestine and hippocampus of MIAO mice were assessed.
2. Results
2.1. P. goldsteinii MTS01 Ameliorates Anxiety-like Behaviors
The experimental design on the evaluation of the effects of P. goldsteinii MTS01 on LPS-induced MIAO mice is presented in Figure 1. Female mice were selected and grouped as CTL group, MIAO group, and MIAO + Pg group. The abundance of P. goldsteinii was confirmed to be significantly increased in the MIAO + Pg group as compared to the MIAO group (Supplementary Figure S1).
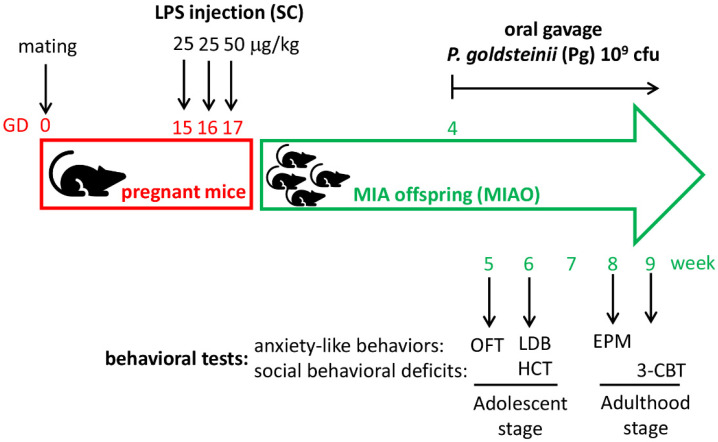
Figure 1.
Experimental design for evaluating the ameliorative effects of Parabacteroides goldsteinii (Pg) MTS01 on the aberrant behaviors of offspring with lipopolysaccharide (LPS)-induced maternal immune activation (MIA). On gestation days (GD) 15, 16, and 17, pregnant mice were subcutaneously (SC) injected with 25, 25, and 50 μg/kg LPS, respectively. In the control group, pregnant mice were injected with phosphate-buffered saline. Female mice offspring were selected and grouped. Mouse offspring were orally gavaged with live Pg MTS01 at 1 × 109 colony-forming units (cfu) per day for 5 days per week from week 4 through to the end of the experiment. For anxiety-like behavior assessments, the open-field test (OFT), light–dark box (LDB) test, and elevated plus maze (EPM) test were conducted at the ages of 5, 6, and 8 weeks, respectively. Regarding the social behavioral deficits evaluation, the home cage test (HCT) and three-chamber behavioral test (3-CBT) were performed at 6 and 9 weeks of age, respectively.
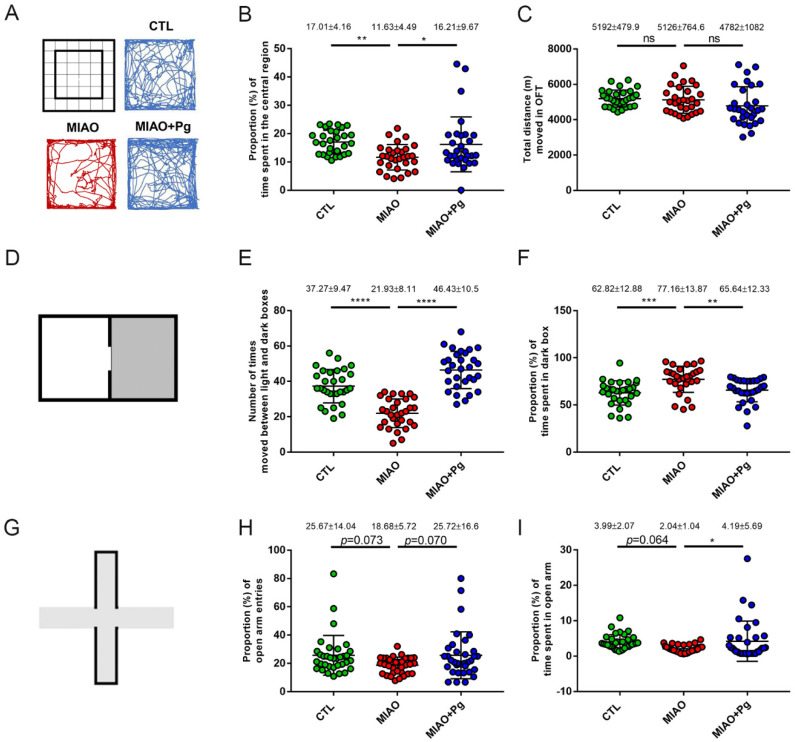
Five weeks after birth, the OFT [33], which measures general locomotor activity levels and the willingness to explore, was performed. The results of representative path tracings are presented in Figure 2A. The proportion of time spent in the central region was 17.01% ± 4.16% in the control group. By contrast, that in the MIAO group was reduced to 11.63% ± 4.49% (p < 0.01; Figure 2B). Oral administration of P. goldsteinii MTS01 significantly increased the proportion of time spent in the central region (16.21% ± 9.67%, p < 0.05; Figure 2B). In comparison, no difference in locomotive activity (total distance moved in the OFT) was observed among the three groups (Figure 2C).
Figure 2.
Parabacteroides goldsteinii (Pg) MTS01 ameliorates the anxiety-like behaviors of MIAO. Offspring from phosphate-buffered sale control group (CTL, n = 30), LPS-induced MIA group (MIAO, n = 30), and MIAO orally gavaged with Pg MTS01 (MIAO + Pg, n = 30) were evaluated for anxiety-like behaviors using the open-field test (OFT; A–C), light–dark box (D–F), and elevated plus maze (G–I). The central and peripheral regions in 36 squares as well as the representative path tracing during the OFT are presented (A). The proportion of time spent in the central region (B) and the total distances moved in the test period (C) are presented. A schematic representation of the LDB is presented (D). The number of times the mice moved between the light and dark boxes within 10 min were recorded (E), and the proportion of time spent in the dark box (F) is presented. A schematic representation of the EPM test is presented (G). The proportion of open arm entries (H) and the time spent in the open arm (I) are presented. Data are presented as the mean ± standard deviation. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns, not significant (one-way ANOVA, Tukey’s post hoc test).
To evaluate whether the natural aversion of offspring mice to brightly illuminated areas and their spontaneous exploratory behavior in response to mild stress such as novel environments and light were affected by treatment, the LDB test [34] (Figure 2D) was performed at the age of 6 weeks old. As presented in Figure 2E, the time needed to move between the open and dark boxes was shorter in the MIAO group than in the control group (21.93 ± 8.11 vs. 37.27 ± 9.47, p < 0.0001). In comparison, P. goldsteinii MTS01-treated MIAO mice had a significantly higher time in moving between the boxes (46.43 ± 10.5 vs. 21.93 ± 8.11, p < 0.0001; Figure 2E). Specifically, the proportions of time spent in the dark box were 62.82% ± 12.88%, 77.16% ± 13.87%, and 65.64% ± 12.33% in control, MIAO, and P. goldsteinii MTS01-treated MIAO mice, respectively (control vs. MIAO, p < 0.001; MIAO vs. MIAO + P. goldsteinii MTS01, p < 0.01; Figure 2F). Together, the results of the OFT and LDB tests indicated that in adolescence, the developed anxiety-like behaviors in MIAO mice were improved by P. goldsteinii MTS01 treatment.
The anxiety-like behaviors of mice were further evaluated in adulthood. Eight weeks after birth, the EPM test [35] (Figure 2G), which is frequently used in neurobiological anxiety research, was performed. Although there was no statistical difference (p = 0.073 and 0.070, respectively), the proportion (%) of open arm entries tended to be lower in the MIAO group than in the control group, whereas P. goldsteinii MTS01 treatment in MIAO increased open arm entry (Figure 2H). The proportion of time spent in the open arms also tended to be lower in the MIAO group than in the control group (p = 0.064; Figure 2I). By contrast, oral treatment with P. goldsteinii MTS01 in MIAO significantly increased the time spent in the open arms (2.04% ± 1.04% vs. 4.19% ± 5.69%, p < 0.05; Figure 2I).
2.2. Social Behavioral Deficits Are Restored by P. goldsteinii MTS01
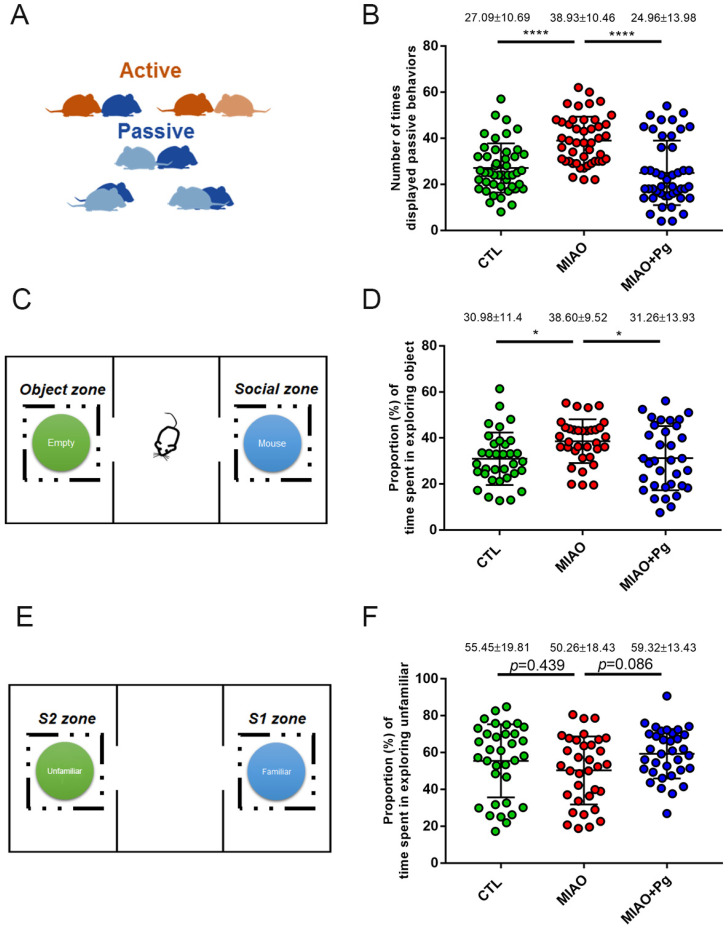
To determine whether P. goldsteinii MTS01 administration also restored the social behaviors and mutual interactions of mice, MIAO mice were subjected to the HCT [36] (Figure 3A) at 6 weeks old. MIAO mice displayed more passive interactive behaviors with other mice in the home cage than with control mice (38.93 ± 10.46 times vs. 27.09 ± 10.69 times, p < 0.0001; Figure 3B). Oral treatment with P. goldsteinii MTS01 significantly reduced the number of passive behaviors (24.96 ± 13.98 times, p < 0.0001).
Figure 3.
Parabacteroides goldsteinii (Pg) MTS01 ameliorates the social deficit behaviors of MIAO. Offspring from the phosphate-buffered saline control group (CTL), lipopolysaccharide-induced MIA group (MIAO), and MIAO orally gavaged with Pg MTS01 (MIAO + Pg) were evaluated for social deficit behaviors using the home cage test (n = 46; A,B) and three-chamber behavioral test (3-CBT, n = 34; C–F). A schematic representation of the phenotypic expression of active (nose-to-nose or nose-to-body) and passive (body-to-body) behaviors is presented (A). The number of times that passive behaviors were displayed was recorded (B). Schematic representation of the 3-CBT is presented (C,E). The proportion of time spent in the empty chamber (Object zone) in contrast to that in the Social zone was recorded (D). The proportion of time spent exploring unfamiliar (S2 zone) in contrast to familiar mice (S1 zone) is presented (F). Data are presented as the mean ± standard deviation. * p < 0.05; **** p < 0.0001 (one-way ANOVA, Tukey’s post hoc test).
As mice grew to adulthood (9 weeks old), the behaviors of “sociability” and “social novelty” related to the propensity of mice to spend time with a previously unmet mouse compared to time spent alone in an identical but empty chamber were subsequently tested using the 3-CBT [37] (Figure 3C,E). Regarding sociability, the proportion of time spent exploring objects was significantly higher in MIAO mice than in control mice (38.60% ± 9.52% vs. 30.98% ± 11.4%, p < 0.05; Figure 3D). P. goldsteinii MTS01 administration significantly decreased the proportion of time spent exploring objects (31.26% ± 13.93%, p < 0.05; Figure 3D). In terms of social novelty, the proportion of time spent exploring the unfamiliar tended to be lower in MIAO mice (50.26% ± 18.43%) than in normal control offspring (55.45% ± 19.81%, p = 0.439; Figure 3F). The proportion of time spent exploring the unfamiliar became higher by P. goldsteinii MTS01 treatment (59.32% ± 13.43%), although significance was not reached (p = 0.086; Figure 3F). Together, the results highlighted the potential to develop P. goldsteinii MTS01 as an intervention for ASD.
2.3. P. goldsteinii MTS01 Alleviates MIA-Induced Intestinal Inflammation and Endotoxemia in Offspring
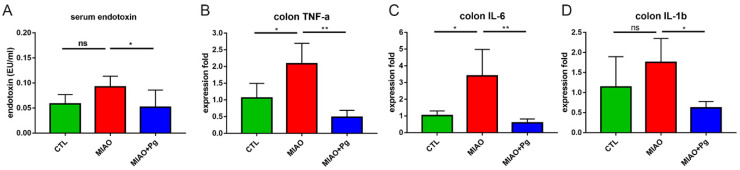
Previous studies indicated that prenatal MIA could increase the levels of interleukin-17A (IL-17A), which initiated immune-primed phenotypes in offspring that exhibited autism-like phenotypes and increased susceptibility to develop intestinal inflammation later in life [38,39]. Concordantly, intestinal inflammation was identified in MIAO mice, and P. goldsteinii MTS01 reduced the intestinal levels of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α (Figure 4B–D). MIAO mice can also develop low-grade endotoxemia in sera [40]. Thus, the effects of P. goldsteinii MTS01 on endotoxemia in MIAO mice were evaluated. Whereas serum LPS activity was increased in MIAO mice, it was significantly reduced by P. goldsteinii MTS01 treatment (Figure 4A). Thus, P. goldsteinii MTS01 treatment reduced both local intestinal and systematic inflammation.
Figure 4.
Parabacteroides goldsteinii (Pg) MTS01 ameliorates the gut inflammation of lipopolysaccharide (LPS)-induced MIAO. The serum endotoxin level (A) and colon inflammatory cytokine level (B–D) of offspring from the phosphate-buffered saline control group (CTL), LPS-induced MIA group (MIAO), and MIAO orally gavaged with Pg MTS01 (MIAO + Pg) were determined. Data are presented as the mean ± standard deviation. * p < 0.05; ** p < 0.01; ns, not significant (one-way ANOVA, Tukey’s post hoc test).
2.4. P. goldsteinii MTS01 Restores Aberrant Transcriptomes in the Intestine of MIAO Mice
The effects of P. goldsteinii MTS01 on the mRNA transcriptomic patterns in the intestine were next examined. To evaluate whether an a priori defined set of genes exhibits statistically significant and concordant differences among the groups, GSEA was performed. Several pathways significantly differed between the control and MIAO groups (Supplementary Figure S2A). In the MIAO group, increased immune cell responses and DNA replication, enhanced tRNA aminoacylation/ribosomal protein expression, and activated cell mitosis were identified. By contrast, sensory perception (olfactory, taste) and G-protein coupled receptor (GPCR), GABA receptor, pheromone receptor, and ion channel signaling were inhibited (Supplementary Figure S2B).
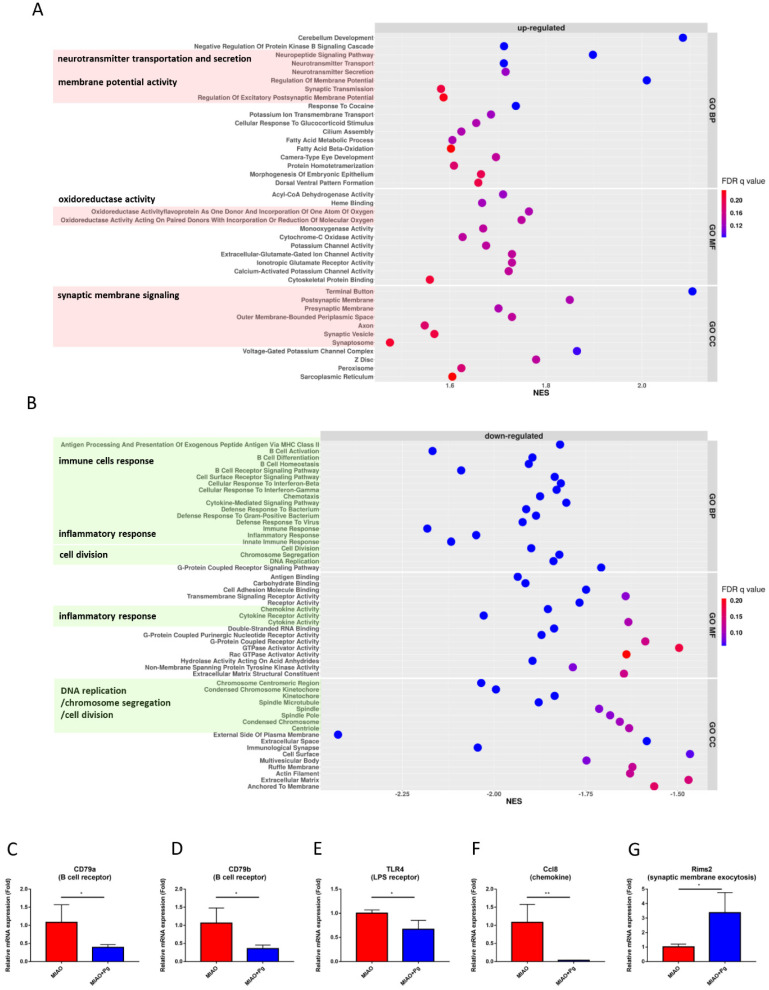
P. goldsteinii MTS01 administration enhanced neurotransmitter transportation and secretion, membrane potential, oxidoreductase activity, and voltage-gated potassium/calcium ion channel and synaptic membrane signaling in MIAO mice (Figure 5A). In addition, P. goldsteinii MTS01 suppressed immune cell responses, inflammatory responses, DNA replication, chromosome segregation and cell division, and GPCR purinergic nucleotide receptor activity (Figure 5B). qPCR was used to clarify the relative expression of CD79a and CD79b (B cell receptors), TLR4 (LPS receptor), Ccl8 (chemokine), and RimS2 (synaptic membrane exocytosis). In brief, P. goldsteinii MTS01 reversed the aberrant transcriptomic patterns in the intestine of MIAO mice (Figure 5C–G).
Figure 5.
Transcriptome alterations in colon of MIAO orally gavaged with Parabacteroides goldsteinii MTS01. Dot plots of the significantly upregulated (normalized enrichment score [NES] > 1 and false-discovery rate [FDR] q value < 0.25) and downregulated ([NES] < −1 and FDR q value < 0.25) gene sets from gene set enrichment analysis of the MIAO + Pg group compared to the MIAO group (top 20 gene sets in GO_BP, GO_MF and GO_CC) are presented in (A,B), respectively. Gene sets with similar functions were labeled in color, and the functions were also added. Five genes contributing to the leading-edge subset within the gene sets (immune responses such as B cell receptor, lipopolysaccharide receptor, and chemokine responses; synapse responses such as synaptic membrane exocytosis) as validated by quantitative RT-PCR are presented in (C–G). Data are presented as the mean ± standard deviation. * p < 0.05; ** p < 0.01(unpaired Student’s t-test). MIAO + Pg, MIAO treated with P. goldsteinii MTS01.
2.5. Molecular Characterization of the Effects of P. goldsteinii MTS01 Effects in the Hippocampus of MIAO Mice
Previous studies highlighted the important role of gut commensal bacteria in the development of hippocampal neurogenesis [41,42] and identified aberrant physiological phenomena in the hippocampus in MIAO mice [43]. Whether P. goldsteinii MTS01 administration altered the expression of ASD-related genes in the hippocampus was analyzed. Transcriptomic analyses of hippocampus tissue-derived cells from MIAO mice with or without P. goldsteinii MTS01 treatment were conducted, followed by GSEA. In MIAO mice, increased expression of genes related to protein localization and targeting, positive regulation of leukocyte apoptotic processes, MHC protein complex binding, large and small ribosomal proteins, mitochondrial and respirasome protein complex components, and spliceosomal complex components were observed (Supplementary Figure S3A). By contrast, decreased expression in genes related to neuropeptide signaling pathways/endocrine system development, ion-gated channel activity, serotonin production, and GPCR and GABA receptor activity (Supplementary Figure S3B) were observed in MIAO mice in comparison to the findings in control mice.
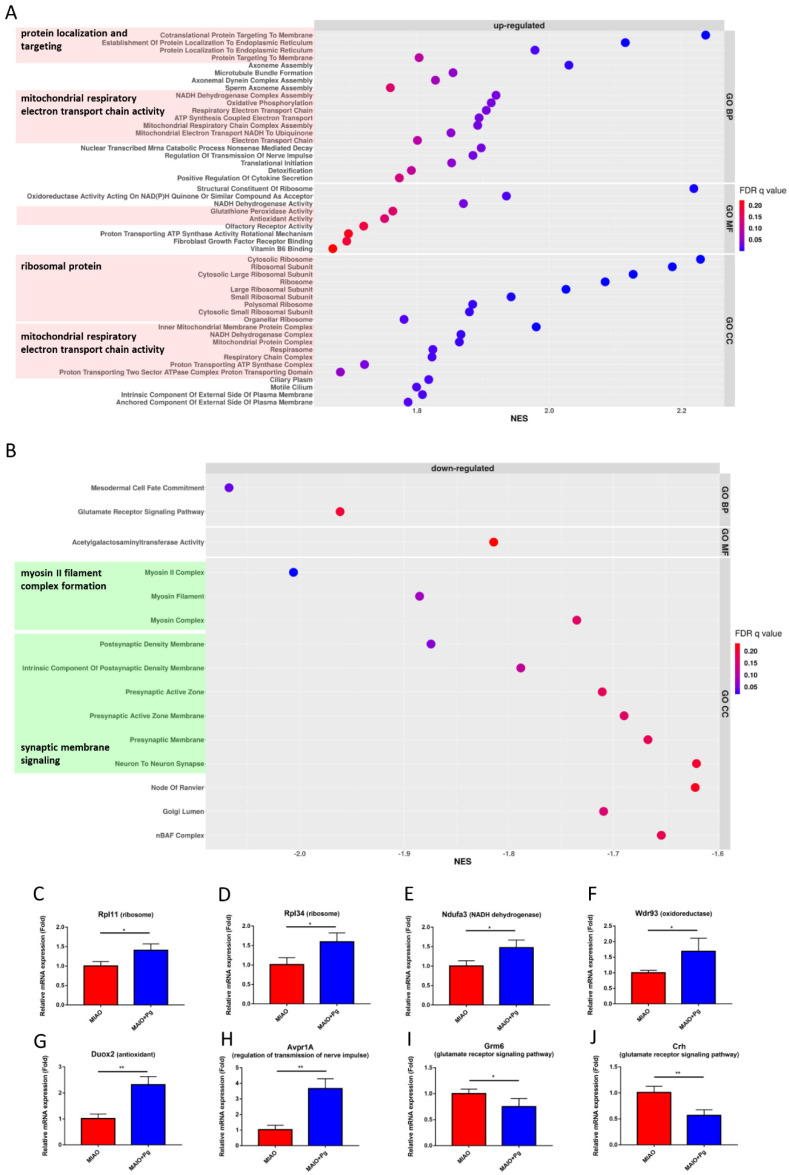
P. goldsteinii MTS01 administration in MIAO mice affected multiple pathways (Figure 6). Increased expression of genes involved in protein localization and targeting, mitochondrial respiratory electron transport chain activity and ATP synthesis, the regulation of nerve impulse transmission, detoxification, glutathione peroxidase and antioxidant/vitamin B6-binding activities, and olfactory receptor activity, in addition to genes encoding ribosomal proteins, were observed (Figure 6A). Conversely, reduced expression of genes involved in the glutamate receptor signaling pathway, myosin II filament complex formation, and synaptic membrane signaling were detected (Figure 6B).
Figure 6.
Transcriptome alterations in hippocampus of MIAO orally gavaged with Parabacteroides goldsteinii MTS01. Dot plots of the significantly upregulated (normalized enrichment score [NES] > 1 and false-discovery rate [FDR] q value < 0.25) and downregulated ([NES] < −1 and FDR q value < 0.25) gene sets from gene set enrichment analysis of the MIAO + Pg group compared to the MIAO group (top 20 gene sets in GO_BP, GO_MF and GO_CC) are presented in (A,B), respectively. Gene sets with similar functions were labeled in color, and the functions were also added. Eight genes contributing to the leading-edge subset within the gene sets (ribosome, oxidoreductase, NADH dehydrogenase, antioxidant, regulation of transmission of nerve impulse, and glutamate receptor signaling pathway) as validated by quantitative RT-PCR are presented in (C–J). Data are presented as the mean ± standard deviation. * p < 0.05; ** p < 0.01 (unpaired Student’s t-test). MIAO + Pg, MIAO treated with P. goldsteinii MTS01.
The expression of P. goldsteinii MTS01-mediated genes was further validated by qPCR. Eight genes contributed to the leading-edge subset within the gene sets measured (Figure 6C–J). Of these, six genes were upregulated by P. goldsteinii MTS01 treatment, including Rpl34 and Rpl11 (involved in ribosome biogenesis), Wdr93 (located in mitochondrial respiratory chain complex I and involved in oxidoreductase response), Ndufa3 (related to NADH dehydrogenase activity), Duox2 (associated with antioxidant function), and Avpr1A (arginine vasopressin receptor 1A involved in regulation of transmission of nerve impulse; Figure 6C–H). In addition, the downregulation of Grm6 (Figure 6I) and Crh (Figure 6J), which are related to the glutamate receptor signaling pathway, by P. goldsteinii MTS01 was also validated.
2.6. P. goldsteinii MTS01 Modulates Immune and Neuronal Signaling in the Colon of Germ-Free Mice
As P. goldsteinii MTS01 was administered orally, whether P. goldsteinii MTS01 alone regulated the expression of genes related to neuronal development in intestinal tissue was next addressed. P. goldsteinii MTS01 influenced multiple pathways in germ-free mice (Supplementary Figure S4). Among these, the expression of genes related to innate immune responses and anti-microbial humoral responses were upregulated. By contrast, decreased expression of genes related to nervous system development and synaptic signaling was observed (Supplementary Figure S4). Previous results revealed a functional microbiota–neurohumoral relationship during conventionalization and suggested a delayed neuronal response that is elicited only after the microbiota accommodating homeostasis has been established [41]. In this study, the administration of P. goldsteinii MTS01 significantly decreased the neuronal response in the colon, implying the involvement of P. goldsteinii in the development of the gut–brain circuit.
3. Discussion
The gut microbiota was recently recognized as a key modulator of neuropsychiatric health, in which gut microbiota dysbiosis, which is closely associated with the development of gastrointestinal symptoms, might be a causal factor in altered brain/hippocampal neurogenesis and ASD development [29,44,45,46]. To ameliorate ASD, the correction of gut microbiota dysbiosis was highlighted as a treatment goal [47]. As probiotics can correct dysbiosis and improve gastrointestinal dysfunction through multiple mode of actions (MOAs), it is plausible to speculate that they can also improve the behavioral profiles of patients with ASD [24,48,49]. In addition to influencing microbiota composition, reducing gut inflammation, strengthening intestinal barrier functions, and altering mucosal immune responses [50], probiotics can also involve control of the production of metabolites, hormones, and neurotransmitters in the host [21]. However, though traditional probiotics displayed evidence of clinical efficacy against irritable bowel syndrome or ulcerative colitis, the results of previous clinical studies on ASD were inconsistent. For example, the clinical treatment of children with ASD using L. plantarum was reported to have a positive ameliorative effect [51]. In addition, abnormal brain plasticity and neurogenesis can be prevented by pretreatment with the Probio’Stick® formulation, which modulates neuroregulatory factors and signaling pathways in the central nervous system-related stress response [52]. Furthermore, treatment with L. reuteri restored social deficits in several ASD mouse models by rescuing social interaction-induced synaptic plasticity via the oxytocinergic system in a vagus nerve-dependent manner [22]. Moreover, mice treated with L. rhamnosus JB-1 exhibited stimulation of the transcription of GABA receptors in the vagus nerve, which regulates emotional behavior [53]. Conversely, supplementation with a cocktail comprising eight probiotic strains only displayed potentially positive effects in a subset of children with ASD [24]. Furthermore, the results of a clinical meta-analysis also revealed no significant difference is patients’ anxiety scores between the probiotics and placebo groups [54].
The identification of NGPs as therapeutic agents (or more specifically “psychobiotics”) for improving the clinical treatment of aberrant neuroactivity is currently under intensive study [55]. In contrast to traditional probiotics, NGPs are beneficial bacteria basically identified from the results of recently conducted microbiota-related research. Alterations of the gut microbiota composition during ASD development, in contrast to the findings in normal controls, might reveal novel NGPs involved in the regulation of ASD pathogenesis [28]. Under strict safety regulatory control, NGPs can be used as live biotherapeutic drugs targeting psychotic diseases [26,28,49]. In one example, the administration of a non-toxigenic Bacteroides fragilis strain reduced bacteria-driven chronic colitis [56], protected against antibiotic-associated diarrhea [57], and also restored aberrant host metabolism and ameliorated ASD-related defects in MIAO [27]. B. fragilis lacking the enterotoxin gene might therefore be a good candidate for development as a potential NGP for ASD treatment [26,58].
In this study, we reported that a beneficial bacterial strain P. goldsteinii MTS01 reversed aberrant anxiety-like and social deficit behaviors. These changes were accompanied by reductions of intestinal and systemic (serum) inflammation, reversing intestinal abnormalities and optimizing hippocampus functions in MIAO SPF mice. MIAO mice avoided elevated areas (EPM test) and preferred darker areas over lighter areas (LDB test), indicating higher anxiety and stress in novel environments. By contrast, mice treated with P. goldsteinii MTS01 behaved more positively, and they tended to explore areas. In addition, MIAO mice exhibited abnormal sociability activities, i.e., reduced activity and increased passive interactions with other mice (HCT), less time spent with other rodents, and less time investigating a novel unfamiliar than a familiar one (social novelty) compared to the controls (3-CBT). By contrast, P. goldsteinii MTS01 treatment also significantly restored the social behavioral deficits observed in MIAO mice. Conversely, our research and previous studies revealed intestinal inflammation together with increased pro-inflammatory LPS activity in the blood of hosts with ASD [59,60]. Treatment with P. goldsteinii MTS01 effectively reduced this inflammation and the related abnormalities. Concordantly, similar phenomena of reducing inflammation were also observed in our previous studies on the amelioration of high-fat diet-induced obesity and metabolic syndrome [31], and smoking initiated COPD by this bacterium [32]. Although the underlying molecular mechanisms of the effects of oral P. goldsteinii MTS01 on inflammation were not completely clarified, Pg-LPS, an anti-inflammatory LPS derived from P. goldsteinii MTS01 might play an important role [32]. Pg-LPS characterized by hypoacylated lipid A moieties can alleviate the inflammation caused by pro-inflammatory E. coli LPS in the intestine and sera [32]. These findings might, at least partially, explain why P. goldsteinii MTS01 ameliorated pro-inflammatory E. coli LPS-induced ASD development in MIAO mice. Briefly, P. goldsteinii MTS01 administration reduced inflammation and ameliorated ASD-related abnormal behaviors, further highlighting its potential as an NGP for treating ASD-related diseases.
ASD is a sex-biased neurodevelopmental disorder, which is more commonly diagnosed in males than females. Differences in behavioral phenotypes between males and females were reported; therefore, their behavior should be analyzed separately. In this study, the ameliorative effect on ASD-relevant behavior of P. goldsteinii in female mice was prioritized to report that which was less studied in previous reports. Meanwhile, the same effect was also observed in male mice (our unpublished data) and needs further investigations.
P. goldsteinii MTS01 exhibited multiple MOAs in MIOA mice (Supplementary Figure S5). In addition to anti-inflammation, P. goldsteinii MTS01 also affected mRNA transcriptomic patterns in both the intestine and hippocampus. In the intestine of MIAO mice, inflammatory activities, cell division, and ribosomal protein expression were aberrantly increased, in addition to sensory perception, GPCR receptor signaling and ion channels were inhibited. By contrast, P. goldsteinii MTS01 administration reduced aberrant inflammatory activities and cell division, together with GPCR signaling, whereas it increased neurotransmitter, synaptic, oxidoreductase, and ion channel activities. In the hippocampus of MIAO mice, aberrant increases in ribosomal protein expression and mitochondrial activities were observed, whereas reduced neuropeptide signaling, ion channel activity, and GPCR and GABA/serotonin receptor activities were identified. P. goldsteinii MTS01 administration modulated these activities, specifically increasing ribosomal protein expression and mitochondrial, anti-oxidative/glutathione peroxidase, and olfactory receptor activities and decreasing glutamate receptor and synaptic membrane signaling. In brief, MIAO mice developed chronic inflammation-related deficits in neurotransmission and behavioral abnormalities, which were reversed by P. goldsteinii MTS01.
A previous study revealed beneficial effects of palmitoylethanolamide/luteolin, a combination of anti-inflammatory and antioxidant, in a mouse model of autism and in an autism case [61]. A recent study reported an anthocyanin-rich extract obtained from fruits alleviated autism-like symptoms in a mouse model of ASD and modulated the gut microbiota composition [62]. These results and our study suggested the possible linkage among antioxidant, gut microbiota, and brain.
Imbalance of the excitation and inhibition of sensory processing in the brain may lead to alterations of neural signaling, information processing, and responding behavior, which are closely associated with ASD development. Either hyper- or hyporesponsiveness in the aspect of input, cognitive, and behavioral reactivity is considered abnormal [63]. Several GPCR heteromers containing receptors sensing neurotransmitters were reported to be related to autism development. These included GABAergic, glutamatergic, dopamine, oxytocin, and 5-hydroxytryptamine receptors [5]. GPCRs are associated with different subsets of G-proteins that in turn regulate specific ion channels and trigger cAMP cascades, leading to the activation of distinctive cellular signaling pathways under differential stimulatory conditions [64]. Dysfunction in the formation and/or function of GPCR heteromers in the GPCR interactome, including olfactory receptors [65], could potentially contribute to ASD. Currently, the majority of ameliorative strategies under development mainly aim to restore the brain excitatory/inhibitory imbalance described in autism by optimizing the expression and signaling of these GPCR heteromers [5]. GPCRs might therefore represent new pharmacotherapeutic targets for autism [5].
Several neurotransmitters and specific neuropeptides are critically involved in the regulation of social behaviors. Alterations in some neurotransmitter systems that modulate brain network activities occur and potentially underlie the etiology/pathophysiology of ASD [20,66]. Among these, the imbalance between excitatory glutamatergic and inhibitory GABAergic tones has been most studied. Reduced GABA levels or the impairment of GABAergic transmission in the higher-order motor areas integrating multiple sensory modalities or aberrant increases in glutamate signaling might underlie the sensory hyperresponsiveness in patients with ASD [67,68]. Hence, GABA agonists or agents that antagonize glutamate receptors have been developed for optimizing this imbalance in the treatment of ASD [69]. Conversely, to fine-tune the excitatory/inhibitory imbalance described in autism, dopaminergic, serotonergic, oxytocinergic, and cannabinoid systems have also been addressed [5]. P. goldsteinii MTS01 fine-tuned many of the neurosensory systems, which may underlie the important mechanism of amelioration. In addition, although the gut microbiota is involved in the production of neuroactive compounds such as GABA and glutamate [20], whether P. goldsteinii MTS01 produces some of these neurotransmitters remains to be further studied.
Multiple other abnormal physiological activities are involved in ASD development. Increased enterocyte and Paneth cell counts in the intestinal epithelium and lamina propia, together with increased crypt cell proliferation, have been found in autistic children [70]. In parallel, increased brain growth and mutations in autism were also reported [71]. Meanwhile, a growing body of evidence from whole-genome and whole-exome sequencing has suggested a linkage of several ASD susceptibility genes for potassium channels in subjects with ASD. Genetic dysfunction of potassium channels may be involved in the altered neuronal excitability and abnormal brain function in the pathogenesis of ASD [72,73]. In addition, the roles of ribosomal and mitochondria-related activities were also highlighted. Abnormal (either increased or decreased) ribosomal protein gene expression and RNA spliceosome activity in the brain [72,74], together with aberrant mitochondrion-related electron transportation chain and oxidative phosphorylation/respiratory function activities [75], was reported to be closely associated with the development of neurodevelopmental pathologies in hosts with ASD [76]. In this study, GSEA revealed the upregulation of genes involved in ribosomal biogenesis and mitochondrial activities in MIAO mice, and their expression was further enhanced by P. goldsteinii MTS01. Such phenomena warrant further detailed studies. Another important factor involved in ASD is the neuronal activities responsible for regulating localized protein synthesis within dendrites and the post-translational modification of synaptic molecules. These were associated with regulating synaptic function and allowing neuronal circuits to respond dynamically to stimuli [66]. Dysregulation of such signaling pathways was ameliorated by P. goldsteinii MTS01, which may also play a key role in ameliorating ASD. P. goldsteinii MTS01 optimally delayed the expression of neuron development-related genes in the intestine of germ-free mice, indicating the important effects of P. goldsteinii MTS01 on the homeostatic development of neuroma system in the intestine.
Taken together, P. goldsteinii MTS01 administration appeared to optimize multiple activities in both the intestine and hippocampus of MIAO mice, simultaneously leading to the maintenance of cellular homeostasis, optimal turnover of biochemical macromolecules, increased cellular antioxidant activities, and optimization of the signaling pathways related to the control of neurobehaviors. Therefore, P. goldsteinii MTS01 has the potential for development as an NGP or live biotherapeutic product for the potential prevention or treatment of ASD. In addition, the active component(s) of P. goldsteinii MTS01, as well as the underlying molecular mechanisms that control multiple regulatory activities and restore aberrant functions and phenotypes in ASD models, remain to be further characterized.
4. Materials and Methods
4.1. Animals
The animal experiments performed in this study were approved by the Institutional Review Board of National Kaohsiung Normal University, Taiwan and performed according to the instructions of the Animal Protection Act (Kaohsiung, Taiwan). In the study 8- and 10-week-old female C57BL/6J mice were purchased from the National Laboratory Animal Center (NLAC, Taipei, Taiwan) and bred under specific pathogen-free (SPF) conditions with a strict 12-h light (8–19 o’clock)/dark (20–7 o’clock) cycle (22–24 °C; 55–65% humidity). The experiments were performed from 10:00 AM to 12:00 PM. All mice were individually acclimated at cage for 3–5 days. For mating, the female acclimated at another cage that harbored the odor of male mouse for 3 days followed by 2 female mice being mated with 1 male mouse. On the day that vaginal plugs appeared, females were housed separately, and this day was designated as gestational day 0 (GD0). The offspring were routinely housed in separate cages with 2–3 females per cage and fed a standard food diet (Oriental Yeast Co., Chiba, Japan). Germ-free C57BL/6JNarl mice were purchased from NLAC. Mice were maintained in a vinyl isolator and confirmed their germ-free status by culturing feces, bedding, and drinking water in thioglycollate medium (DIFCO, Camarillo, CA, USA).
4.2. Experimental Design
Escherichia coli LPS is commonly used for inducing maternal immune activation of C57BL/6J mice at prenatal infection [11]. To evaluate the appropriate doses, E. coli O55:B5 LPS ranging from 25 to 80 μg /kg were tested before formal experiments. Eventually, pregnant mice injecting subcutaneously (sc) E. coli O55:B5 LPS three times (GD15, 25 μg/kg; GD16, 25 μg/kg; and GD17, 50 μg/kg; Figure 1), with optimal offspring survival rate >92% and maternal abortion rate <10%, were used for MIA induction in this study.
Female MIAO mice were selected and grouped, followed by behavioral experiments. Offspring mice derived from pregnant mice subcutaneously injected with phosphate-buffered saline were used as the normal control. For MIAO mice treated with P. goldsteinii MTS01, each offspring was orally gavaged with 1 × 109 colony-forming units (cfu) of live bacteria once per day for five days per week (from week 4 to the end of the experiment). Control and MIAO that served as control were gavaged with PBS.
Germ-free mice were orally administrated with P. goldsteinii MTS01 (5 × 108 cfu) once at 8 weeks old. After 2 weeks of mono-colonization, mice were sacrificed, and their colons were collected.
4.3. P. goldsteinii Cultivation
P. goldsteinii MTS01 was isolated from the feces of a healthy adult who received P. goldsteinii MTS01 isolated from a healthy mouse [32] for 6 months. Bacteria were grown at 37 °C in a Whitley DG250 anaerobic chamber (Don Whitley, Bingley, UK) with mixed anaerobic gas (5% carbon dioxide, 5% hydrogen, 90% nitrogen). The anaerobic condition was confirmed using an anaerobic indicator (Oxoid, Basingstoke, UK). P. goldsteinii MTS01 was cultivated on anaerobic blood agar (Creative, New Taipei City, Taiwan) and liquid thioglycollate medium (BD, Franklin Lakes, NJ, USA).
4.4. Behavioral Tests
In this study, a total of 17 (MIAO), 18 (MIAO + Pg), and 13 (CTL) littermates were used for behavioral tests (details in Supplementary Table S1). The mice from different littermates were maintained at body weights (ranging from 16 to 20 g, aged 5 to 8 weeks old), locomotor activities (ranging from 49 to 52 m observed by OFT at 5 weeks of age), and muscle strength (scores ≥ 4 points, measured by standard wire-hanging evaluation at 8 weeks of age). All tested mice had similar physiological conditions throughout the study.
Individually housed female offspring completed the open-field test (OFT), light–dark box (LDB) test, and home cage test (HCT) during adolescence (5 weeks old) as well as the three-chamber behavioral test (3-CBT) and elevated plus maze (EPM) test in adulthood (8 weeks old) [77]. Each behavioral experiment was separated by 2 days. All tests were automatically video-recorded (iCATCH, DVR-413DH-J; Panasonic, Taipei, Taiwan) and imaged using software (iWATCH DVR, v2; Panasonic) per standard protocols [77]. Before testing, individual mice were allowed to move freely for 10 min at individual equipment. Briefly, the OFT was performed at 60 × 60 cm of testing area including 36 squares (10 × 10 cm per square; outer, 20 squares; inner, 16 squares) illuminated at 65 lux for 10 min. For LDB, the light–dark box (45 × 27 × 454 cm) was set to 45 and <1 lux, respectively. The mice faced the open door (7.5 × 7.5 cm) at the light area to start the test for 10 min. The EPM apparatus was set to open arms (30 × 5 cm, 100 lux, without wall) and closed arms (30 × 5 cm, 90 lux), surrounded by 16-cm high whitewalls, no shadow) at 50 cm above the floor. The central platform, which was located at a space between the open and closed arms, was a 5 × 5 cm square with illumination of 100 lux. The test was started with mice facing the open arm and performed for 10 min. For HCT, the interactions of 2 tested mice (sibling) were recorded every 30 s for 30 min. Totally, 122 measurements were recorded. For the 3-CBT, the mice were placed at one of the wire-mesh cages (20 × 20 × 45 cm, every cage) illuminated by 70 lux at the start. Another empty cage recognized as an object or the mouse from a different home cage with a similar body weight, age, and sex, was designated as unfamiliar.
4.5. Endotoxin Detection
Serum LPS levels were measured using a murine HEK-Blue™ LPS Detection Kit (InvivoGen, San Diego, CA, USA) based on the manufacturer’s instructions.
4.6. Quantification of Gene Expression Levels and Abundance of P. goldsteinii
At sacrifice, mice were anesthetized with isoflurane and blood was collected via intracardiac puncture. The colon and hippocampus tissues were collected in RNAlater solution (Sigma-Aldrich, St. Louis, MO, USA). Total RNA was extracted using a Genezol TriRNA pure kit (Geneaid, New Taipei City, Taiwan) per manufacturer’s protocol. RNA was reverse-transcribed into cDNA using a Quant II fast reverse transcriptase kit (BioTools, New Taipei City, Taiwan). The resulting cDNA was used as a template for quantitative PCR (qPCR) using the primers listed in Supplementary Table S2. The PCR conditions were as follows: initial pre-incubation-step at 95 °C for 3 min; 50 cycles at 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 5 s; and one melting curve cycle. GAPDH was used as the internal control for qPCR. Relative gene expression was calculated using the 2−ΔΔCT method. Feces samples were snap-frozen in liquid nitrogen and stored at −80 °C. DNA was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). Determination of P. goldsteinii abundance in fecal DNA was performed by real-time qPCR using P. goldsteinii 16S rRNA gene specific primers listed in Supplementary Table S2. The 16S rRNA gene V3−V4 primers were used as the internal control for qPCR assay.
4.7. RNA Sequencing and Bioinformatics Analyses
Total RNA was prepared from the intestine and hippocampus tissues of mice. A total of 11 colon (CTL, n = 3; MIAO, n = 4; MIAO + Pg, n = 4) and 10 hippocampus (CTL, n = 4; MIAO, n = 3; MIAO + Pg, n = 3) tissues were subjected for transcriptomic analysis. RNA sequencing was conducted on an Illumina HiSeq4000 using a paired-end run (2 × 150 bases). We used the STAR (v2.7.3a) two-pass mapping strategy to align the raw FASTQ reads against the mouse reference genome (GENCODE Mouse M24) downloaded from the GENCODE database. DESeq2 (v1.26) was used to normalize the raw read counts quantified by STAR with GENCODE Mouse M24 gene annotation. To perform gene set enrichment analysis (GSEA), we first converted gene expression data into the pre-ranked format by calculating the log2 fold change of different conditions. Next, we used GSEA software (v4.1.0) and MSigDB (v7.2) (http://www.gsea-msigdb.org/gsea/msigdb/) (accessed on March 2022) with the pre-rank mode to calculate the normalized enrichment scores and false-discovery rate (FDR). In particular, we used GO gene sets (MSigDB C5). Gene sets were considered significantly enriched at FDR < 0.25 when using Signal2Noise as a metric and 1000 permutations of gene sets. The dot plots displaying the most significantly upregulated (normalized enrichment score [NES] > 1) and downregulated (NES < −1) gene sets were generated using the ggplot function of the ggplot2 R package.
4.8. Statistical Analysis
Data are presented as the mean ± standard deviation. Because each group in the behavioral tests had at least 30 observations, data sets were assessed by parametric one-way ANOVA with Tukey’s post hoc multiple comparisons. The normality of other data in this study was confirmed by using the Shapiro–Wilk test. Differences between two groups were assessed using an unpaired two-tailed Student’s t-test. Data sets involving more than two groups were assessed by one-way ANOVA with Tukey’s post hoc multiple comparisons.
Acknowledgments
We sincerely appreciate the assistance in analyzing transcriptome and bioinformatic analysis from Next Generating Sequencing Core, Bioinformatics Core, and Molecular Medicine Research Center, Chang Gung University, Taoyuan, Taiwan. We also sincerely appreciate the assistance in performing germ-free mice experiments from the National Laboratory Animal Canter, Taipei, Taiwan.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232113070/s1.
Author Contributions
T.-L.L., C.-C.L., H.-C.L. and Y.-L.C. designed this work. T.-L.L., C.-C.L. and Y.-L.C. performed the experimental study. T.-L.L., T.-W.C., C.-W.H. and W.-F.L. performed data analysis and interpretation. T.-L.L., C.-C.L., H.-C.L. and Y.-L.C. wrote the manuscript. J.-J.L., T.-S.W. and C.-H.L. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of National Kaohsiung Normal University, Taiwan.
Informed Consent Statement
This study does not involve the use of human subjects. The ethical statements regarding animal experiments are included in the “Materials and Methods” section.
Data Availability Statement
The sequence reads of transcriptomic analysis were deposited under NCBI BioProject numbers PRJNA871204 and PRJNA871205. All relevant data is contained within the article. The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no competing interest.
Funding Statement
We would like to express our thanks for the funding provided from CORPD1J0053, CMRPD1J0333, and CMRPD1M0081 from Chang Gung Memorial Hospital, 109-2320-B-182-034-MY3, 109-2320-B-030-010, 110-2327-B-182-001 from the Ministry of Science and Technology (MOST), Microbiota Research Center from Chang Gung University, and the Research Center for Emerging Viral Infections from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and the Ministry of Science and Technology (MOST), Taiwan (MOST 111-2634-F-182-001).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matson J.L., Rieske R.D., Williams L.W. The relationship between autism spectrum disorders and attention-deficit/hyperactivity disorder: An overview. Res. Dev. Disabil. 2013;34:2475–2484. doi: 10.1016/j.ridd.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Kerns C.M., Kendall P.C., Berry L., Souders M.C., Franklin M.E., Schultz R.T., Miller J., Herrington J. Traditional and atypical presentations of anxiety in youth with autism spectrum disorder. J. Autism Dev. Disord. 2014;44:2851–2861. doi: 10.1007/s10803-014-2141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maenner M.J., Shaw K.A., Bakian A.V., Bilder D.A., Durkin M.S., Esler A., Furnier S.M., Hallas L., Hall-Lande J., Hudson A., et al. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill. Summ. 2012;61:1. [PubMed] [Google Scholar]
- 4.Fernell E., Eriksson M.A., Gillberg C. Early diagnosis of autism and impact on prognosis: A narrative review. Clin. Epidemiol. 2013;5:33–43. doi: 10.2147/CLEP.S41714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DelaCuesta-Barrutia J., Penagarikano O., Erdozain A.M. G Protein-Coupled Receptor Heteromers as Putative Pharmacotherapeutic Targets in Autism. Front. Cell Neurosci. 2020;14:588662. doi: 10.3389/fncel.2020.588662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theoharides T.C., Kempuraj D., Redwood L. Autism: An emerging ‘neuroimmune disorder’ in search of therapy. Expert. Opin. Pharmacother. 2009;10:2127–2143. doi: 10.1517/14656560903107789. [DOI] [PubMed] [Google Scholar]
- 7.Herbert M.R. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr. Opin. Neurol. 2010;23:103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- 8.Ornoy A., Weinstein-Fudim L., Ergaz Z. Genetic Syndromes, Maternal Diseases and Antenatal Factors Associated with Autism Spectrum Disorders (ASD) Front. Neurosci. 2016;10:316. doi: 10.3389/fnins.2016.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikshak N.N., Swarup V., Belgard T.G., Irimia M., Ramaswami G., Gandal M.J., Hartl C., Leppa V., Ubieta L.T., Huang J., et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature. 2016;540:423–427. doi: 10.1038/nature20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prosperi M., Turi M., Guerrera S., Napoli E., Tancredi R., Igliozzi R., Apicella F., Valeri G., Lattarulo C., Gemma A., et al. Sex Differences in Autism Spectrum Disorder: An Investigation on Core Symptoms and Psychiatric Comorbidity in Preschoolers. Front. Integr. Neurosci. 2020;14:594082. doi: 10.3389/fnint.2020.594082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boksa P. Effects of prenatal infection on brain development and behavior: A review of findings from animal models. Brain Behav. Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Sotgiu S., Manca S., Gagliano A., Minutolo A., Melis M.C., Pisuttu G., Scoppola C., Bolognesi E., Clerici M., Guerini F.R., et al. Immune regulation of neurodevelopment at the mother-foetus interface: The case of autism. Clin. Transl. Immunol. 2020;9:e1211. doi: 10.1002/cti2.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han V.X., Patel S., Jones H.F., Nielsen T.C., Mohammad S.S., Hofer M.J., Gold W., Brilot F., Lain S.J., Nassar N., et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry. 2021;11:71. doi: 10.1038/s41398-021-01198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Depino A.M. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56–65. doi: 10.1016/j.neuroscience.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 15.Aria F., Bonini S.A., Cattaneo V., Premoli M., Mastinu A., Maccarinelli G., Memo M. Brain Structural and Functional Alterations in Mice Prenatally Exposed to LPS Are Only Partially Rescued by Anti-Inflammatory Treatment. Brain Sci. 2020;10:620. doi: 10.3390/brainsci10090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergdolt L., Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 2019;175:1–19. doi: 10.1016/j.pneurobio.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalish B.T., Kim E., Finander B., Duffy E.E., Kim H., Gilman C.K., Yim Y.S., Tong L., Kaufman R.J., Griffith E.C., et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat. Neurosci. 2021;24:204–213. doi: 10.1038/s41593-020-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q., Han Y., Dy A.B.C., Hagerman R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell Neurosci. 2017;11:120. doi: 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster J.A., McVey Neufeld K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Eisenstein M. Microbiome: Bacterial broadband. Nature. 2016;533:S104-6. doi: 10.1038/533S104a. [DOI] [PubMed] [Google Scholar]
- 21.Abdellatif B., McVeigh C., Bendriss G., Chaari A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020;21:4159. doi: 10.3390/ijms21114159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sgritta M., Dooling S.W., Buffington S.A., Momin E.N., Francis M.B., Britton R.A., Costa-Mattioli M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019;101:246–259.e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaluzna-Czaplinska J., Blaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition. 2012;28:124–126. doi: 10.1016/j.nut.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Santocchi E., Guiducci L., Prosperi M., Calderoni S., Gaggini M., Apicella F., Tancredi R., Billeci L., Mastromarino P., Grossi E., et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry. 2020;11:550593. doi: 10.3389/fpsyt.2020.550593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Toole P.W., Marchesi J.R., Hill C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 26.Chang C.J., Lin T.L., Tsai Y.L., Wu T.R., Lai W.F., Lu C.C., Lai H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019;27:615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu M., Xu X., Li J., Li F. Association Between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry. 2019;10:473. doi: 10.3389/fpsyt.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharon G., Cruz N.J., Kang D.W., Gandal M.J., Wang B., Kim Y.M., Zink E.M., Casey C.P., Taylor B.C., Lane C.J., et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell. 2019;177:1600–1618.e17. doi: 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C.J., Lin C.S., Lu C.C., Martel J., Ko Y.F., Ojcius D.M., Tseng S.F., Wu T.R., Chen Y.Y., Young J.D., et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu T.R., Lin C.S., Chang C.J., Lin T.L., Martel J., Ko Y.F., Ojcius D.M., Lu C.C., Young J.D., Lai H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68:248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 32.Lai H.C., Lin T.L., Chen T.W., Kuo Y.L., Chang C.J., Wu T.R., Shu C.C., Tsai Y.H., Swift S., Lu C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 2022;71:309–321. doi: 10.1136/gutjnl-2020-322599. [DOI] [PubMed] [Google Scholar]
- 33.Prut L., Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 34.Bourin M., Hascoet M. The mouse light/dark box test. Eur. J. Pharmacol. 2003;463:55–65. doi: 10.1016/S0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 35.Pellow S., Chopin P., File S.E., Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 36.Kim D.G., Gonzales E.L., Kim S., Kim Y., Adil K.J., Jeon S.J., Cho K.S., Kwon K.J., Shin C.Y. Social Interaction Test in Home Cage as a Novel and Ethological Measure of Social Behavior in Mice. Exp. Neurobiol. 2019;28:247–260. doi: 10.5607/en.2019.28.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M., Silverman J.L., Crawley J.N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. 2011;8:Unit 8.26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W., Chen M., Feng X., Song M., Shao M., Yang Y., Zhang L., Liu Q., Lv L., Su X. Maternal immune activation alters adult behavior, intestinal integrity, gut microbiota and the gut inflammation. Brain Behav. 2021;11:e02133. doi: 10.1002/brb3.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi G.B., Yim Y.S., Wong H., Kim S., Kim H., Kim S.V., Hoeffer C.A., Littman D.R., Huh J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fattorusso A., Di Genova L., Dell’Isola G.B., Mencaroni E., Esposito S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients. 2019;11:521. doi: 10.3390/nu11030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller P.A., Schneeberger M., Matheis F., Wang P., Kerner Z., Ilanges A., Pellegrino K., Del Marmol J., Castro T.B.R., Furuichi M., et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583:441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott G.A., Terstege D.J., Vu A.P., Law S., Evans A., Epp J.R. Disrupted Neurogenesis in Germ-Free Mice: Effects of Age and Sex. Front Cell Dev. Biol. 2020;8:407. doi: 10.3389/fcell.2020.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaddad A., Desrosiers C., Hassan L., Tanougast C. Hippocampus and amygdala radiomic biomarkers for the study of autism spectrum disorder. BMC Neurosci. 2017;18:52. doi: 10.1186/s12868-017-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasilewska J., Klukowski M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks—a possible new overlap syndrome. Pediatric. Health Med. Ther. 2015;6:153–166. doi: 10.2147/PHMT.S85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinan T.G., Cryan J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly J.R., Minuto C., Cryan J.F., Clarke G., Dinan T.G. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front. Neurosci. 2017;11:490. doi: 10.3389/fnins.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Q., Xing C., Long W., Wang H.Y., Liu Q., Wang R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation. 2019;16:53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Critchfield J.W., van Hemert S., Ash M., Mulder L., Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol. Res. Pract. 2011;2011:161358. doi: 10.1155/2011/161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin T.L., Shu C.C., Lai W.F., Tzeng C.M., Lai H.C., Lu C.C. Investiture of next generation probiotics on amelioration of diseases—Strains do matter. Med. Microecol. 2019;1–2:100002. doi: 10.1016/j.medmic.2019.100002. [DOI] [Google Scholar]
- 50.Tsai Y.L., Lin T.L., Chang C.J., Wu T.R., Lai W.F., Lu C.C., Lai H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019;26:3. doi: 10.1186/s12929-018-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y.W., Liong M.T., Chung Y.E., Huang H.Y., Peng W.S., Cheng Y.F., Lin Y.S., Wu Y.Y., Tsai Y.C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019;11:820. doi: 10.3390/nu11040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ait-Belgnaoui A., Colom A., Braniste V., Ramalho L., Marrot A., Cartier C., Houdeau E., Theodorou V., Tompkins T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014;26:510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- 53.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao L., Liu C., Sutthawongwadee S., Li Y., Lv W., Chen W., Yu L., Zhou J., Guo A., Li Z., et al. Effects of Probiotics on Depressive or Anxiety Variables in Healthy Participants Under Stress Conditions or With a Depressive or Anxiety Diagnosis: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2020;11:421. doi: 10.3389/fneur.2020.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng L.H., Liu Y.W., Wu C.C., Wang S., Tsai Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019;27:632–648. doi: 10.1016/j.jfda.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan J.L., Wu S., Geis A.L., Chan G.V., Gomes T.A.M., Beck S.E., Wu X., Fan H., Tam A.J., Chung L., et al. Non-toxigenic Bacteroides fragilis (NTBF) administration reduces bacteria-driven chronic colitis and tumor development independent of polysaccharide A. Mucosal Immunol. 2019;12:164–177. doi: 10.1038/s41385-018-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W., Zhu B., Xu J., Liu Y., Qiu E., Li Z., Li Z., He Y., Zhou H., Bai Y., et al. Bacteroides fragilis Protects Against Antibiotic-Associated Diarrhea in Rats by Modulating Intestinal Defenses. Front Immunol. 2018;9:1040. doi: 10.3389/fimmu.2018.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun F., Zhang Q., Zhao J., Zhang H., Zhai Q., Chen W. A potential species of next-generation probiotics? The dark and light sides of Bacteroides fragilis in health. Food Res. Int. 2019;126:108590. doi: 10.1016/j.foodres.2019.108590. [DOI] [PubMed] [Google Scholar]
- 59.Jyonouchi H., Geng L., Ruby A., Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: Their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51:77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- 60.Lee M., Krishnamurthy J., Susi A., Sullivan C., Gorman G.H., Hisle-Gorman E., Erdie-Lalena C.R., Nylund C.M. Association of Autism Spectrum Disorders and Inflammatory Bowel Disease. J. Autism Dev. Disord. 2018;48:1523–1529. doi: 10.1007/s10803-017-3409-5. [DOI] [PubMed] [Google Scholar]
- 61.Bertolino B., Crupi R., Impellizzeri D., Bruschetta G., Cordaro M., Siracusa R., Esposito E., Cuzzocrea S. Beneficial Effects of Co-Ultramicronized Palmitoylethanolamide/Luteolin in a Mouse Model of Autism and in a Case Report of Autism. CNS Neurosci. Ther. 2017;23:87–98. doi: 10.1111/cns.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serra D., Henriques J.F., Sousa F.J., Laranjo M., Resende R., Ferreira-Marques M., de Freitas V., Silva G., Peca J., Dinis T.C.P., et al. Attenuation of Autism-like Behaviors by an Anthocyanin-Rich Extract from Portuguese Blueberries via Microbiota-Gut-Brain Axis Modulation in a Valproic Acid Mouse Model. Int. J. Mol. Sci. 2022;23:9259. doi: 10.3390/ijms23169259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thye M.D., Bednarz H.M., Herringshaw A.J., Sartin E.B., Kana R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2018;29:151–167. doi: 10.1016/j.dcn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padgett C.L., Slesinger P.A. GABAB receptor coupling to G-proteins and ion channels. Adv. Pharmacol. 2010;58:123–147. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- 65.Wicker B., Monfardini E., Royet J.P. Olfactory processing in adults with autism spectrum disorders. Mol. Autism. 2016;7:4. doi: 10.1186/s13229-016-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebert D.H., Greenberg M.E. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493:327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rojas D.C. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. 2014;121:891–905. doi: 10.1007/s00702-014-1216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foss-Feig J.H., Adkinson B.D., Ji J.L., Yang G., Srihari V.H., McPartland J.C., Krystal J.H., Murray J.D., Anticevic A. Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biol. Psychiatry. 2017;81:848–861. doi: 10.1016/j.biopsych.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez M., Mollinedo-Gajate I., Penagarikano O. Neural Circuits for Social Cognition: Implications for Autism. Neuroscience. 2018;370:148–162. doi: 10.1016/j.neuroscience.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 70.Torrente F., Ashwood P., Day R., Machado N., Furlano R.I., Anthony A., Davies S.E., Wakefield A.J., Thomson M.A., Walker-Smith J.A., et al. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol. Psychiatry. 2002;7 doi: 10.1038/sj.mp.4001077. [DOI] [PubMed] [Google Scholar]
- 71.Wang M., Wei P.C., Lim C.K., Gallina I.S., Marshall S., Marchetto M.C., Alt F.W., Gage F.H. Increased Neural Progenitor Proliferation in a hiPSC Model of Autism Induces Replication Stress-Associated Genome Instability. Cell Stem Cell. 2020;26:221–233.e6. doi: 10.1016/j.stem.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang J.Y., Tian Y., Wang H.J., Shen H., Wang H., Long S., Liao M.H., Liu Z.R., Wang Z.M., Li D., et al. Functional Genomic Analyses Identify Pathways Dysregulated in Animal Model of Autism. CNS Neurosci. Ther. 2016;22:845–853. doi: 10.1111/cns.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng P., Qiu Z., Du Y. Potassium channels and autism spectrum disorder: An overview. Int. J. Dev. Neurosci. 2021;81:479–491. doi: 10.1002/jdn.10123. [DOI] [PubMed] [Google Scholar]
- 74.Hetman M., Slomnicki L.P. Ribosomal biogenesis as an emerging target of neurodevelopmental pathologies. J. Neurochem. 2019;148:325–347. doi: 10.1111/jnc.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varga N.A., Pentelenyi K., Balicza P., Gezsi A., Remenyi V., Harsfalvi V., Bencsik R., Illes A., Prekop C., Molnar M.J. Mitochondrial dysfunction and autism: Comprehensive genetic analyses of children with autism and mtDNA deletion. Behav. Brain Funct. 2018;14:4. doi: 10.1186/s12993-018-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balachandar V., Rajagopalan K., Jayaramayya K., Jeevanandam M., Iyer M. Mitochondrial dysfunction: A hidden trigger of autism? Genes Dis. 2021;8:629–639. doi: 10.1016/j.gendis.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsueh P.T., Lin H.H., Wang H.H., Liu C.L., Ni W.F., Liu J.K., Chang H.H., Sun D.S., Chen Y.S., Chen Y.L. Immune imbalance of global gene expression, and cytokine, chemokine and selectin levels in the brains of offspring with social deficits via maternal immune activation. Genes Brain Behav. 2018;17:e12479. doi: 10.1111/gbb.12479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence reads of transcriptomic analysis were deposited under NCBI BioProject numbers PRJNA871204 and PRJNA871205. All relevant data is contained within the article. The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.