Abstract
Bone-marrow-derived mesenchymal stromal cells (BMSCs) showed therapeutic potential in the treatment of musculoskeletal diseases, including osteoarthritis (OA). Their soluble mediators and extracellular vesicles (EVs), which make up the secretome, suppress immune response, attenuate inflammation and promote cartilage repair. EVs, as well as the whole secretome, have been investigated as cell free approaches for OA although, to date, a disease-tailored molecular fingerprint is missing. In this study, soluble mediators and miRNAs were sifted in the BMSCs’ secretome and EVs, respectively, and analyzed in the frame of cell types and factors involved in OA. The majority of identified molecules repress the activation of immune cells and the production of OA-related inflammatory mediators, as well as promote cartilage protection by acting on both chondrocytes homeostasis and extracellular matrix-degrading enzymes. These data provide the molecular ground for the therapeutic potential of BMSCs for regenerative applications for OA and support the use of secretome or EVs as cell-free applications in joint diseases.
Keywords: bone marrow, mesenchymal stromal cells, secretome, extracellular vesicles, miRNA, osteoarthritis
1. Introduction
Osteoarthritis (OA) is the most common degenerative joint disorder affecting more than 500 million people worldwide, with particular prevalence in those >65 years of age [1]. OA is characterized by changes across all joint tissues, in particular cartilage and synovial membrane [2]. Although often underestimated, synovitis is associated with cartilage damage [3] and fibroblast-like synoviocytes (FLS) proliferation [4], M1 inflammatory macrophage recruitment [4] and T cell activation [5]. No effective therapies are available to halt or delay OA progression, and joint replacement with an artificial prosthesis is still the most effective measure to improve patient quality of life [6]. For this reason, current clinical trials are mainly focused to restore a suitable microenvironment for cartilage regeneration/repair and targeting of pro-inflammatory cells/mediators by means of intra-articular injection of chemicals or biologics. Among orthobiologics, mesenchymal stromal cells (MSCs) gained interest due to their regenerative and anti-inflammatory properties, with particular attention on bone marrow as an MSC source (BMSCs) [7] due to its ease of harvest and lack of ethical issues, although sometimes it has lower availability with respect to other sources such as fat or placenta. For these reasons, to date, more than 30 clinical trials using BMSCs-based products have been registered under https://www.clinicaltrials.gov/ (accessed on 30 September 2022) for OA.
In the last year, it has become evident that the regenerative and immunomodulatory properties of MSCs relies on their capacity to secrete bioactive molecules [8]. The secreted molecules, either free or conveyed within extracellular vesicles (EVs), are collectively termed the “secretome”. With respect to the resolution of inflammation and promotion of cartilage regeneration, both the whole secretome and purified EVs have showed promising results in in vivo OA models [9]. Accordingly, in March 2020 and September 2021, the first clinical studies which explored the possibility of using clinical-grade MSC secretome or EVs for the treatment of OA were registered (https://www.clinicaltrials.gov/, NCT04314661 and NCT05060107, accessed on 30 September 2022). Nevertheless, the promising results of both BMSCs and their secretome/Evs in OA field [10,11] have not yet been associated to a thorough disease-related fingerprint of soluble molecules, including cytokine/chemokines or EV-miRNAs. This would be of particular relevance since it would help to better understand the therapeutic potential of BMSCs and their released factors/miRNAs in the frame of those reported to influence OA-related cell types and disease progression or resolution [12,13].
The aim of this work was to characterize the presence of soluble factors In the whole secretome and miRNAs in purified eVs from BMSCs. Identified molecules were analyzed using a disease-related approach. OA molecular determinants and OA-affected cell types and tissues, such as cartilage, FLS, macrophages and T cells, were all covered. The data obtained will provide a disease-focused molecular perspective of the therapeutic properties of BMSCs and their secretomes/EVs when used in the treatment of OA.
2. Materials and Methods
2.1. Bone Marrow Retrieval and BMSC Isolation and Expansion
Total bone marrow was collected from the iliac crest of 3 female OA patients (mean age: 50 ± 2, Kellgren and Lawrence II–III) and seeded at 50,000 total nucleated cells/cm2 in αMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS at 37 °C, 5% CO2 and 95% humidity. After 3 days, the supernatant was discarded and replaced by fresh complete medium. BMSCs were selected due to their plastic adhesion and, after 2 weeks, colonies were detached and BMSCs seeded at 4000 cells/cm2. For secretome collection, BMSCs at passage 3 and 90% confluence were washed 3 times with PBS, and serum-free αMEM was added at a ratio of 0.07 mL/cm2. After 48 h, conditioned medium was collected and centrifuged (376× g for 5 min at 4 °C, 1000× g for 15 min at 4 °C, 2000× g for 15 min at 4 °C and twice at 4000× g for 15 min at 4 °C), recovering the supernatant at each run to be further processed with the following centrifugation. Eventually, clarified secretome was split and used for analysis of EVs as well as enzyme-linked immunosorbent assay (ELISA). After secretome removal, BMSCs were detached and counted before being assessed for viability using a NucleoCounter NC-3000 (ChemoMetec, Allerod, Denmark).
2.2. BMSCs Characterization by Flow Cytometry
After 30 min of staining at 4 °C in the dark and antibody wash with FACS buffer (PBS, 5% FBS, 0.1% sodium azide), MSC (CD44-PE Vio770 clone REA690, CD73-PE clone REA804, CD90-FITC clone REA897, CD105-PerCP Vio700 clone REA794 and CD271-PE clone REA844) or hemato/endothelial (CD31-PerCP Vio700 clone REA730, CD34-FITC clone AC136 and CD45-PE Vio770 clone REA747) markers (Miltenyi Biotec, Bergisch Gladbach, Germany) were detected by flow cytometry using a CytoFLEX flow cytometer (Beckman Coulter, Fullerton, CA, USA). A minimum of 30,000 events were collected. The following antibody combinations were used: CD73/90/105/44 and CD34/271/31/45.
2.3. Multiplex ELISA Assay
Two-hundred soluble receptors, chemokines, cytokines, growth and inflammatory factors were quantified by Quantibody® Human Cytokine Array 4000 Kit (https://www.raybiotech.com/quantibody-human-cytokine-array-4000/, accessed on 30 September 2022) in the cleared BMSCs secretome according to the manufacturers’ protocol (RayBiotech, Norcross, GA, USA). To allow the absorbance readings within the standard curve values, a 1:1 dilution was performed. For each presented value, the mean of 4 technical replicates is shown. The amount of each factor in pg/mL was converted into pg/million cells by multiplying the original value for the total collected volume in ml and finally dividing by the total number of cells. Values are shown as mean ± SD.
2.4. Protein-Protein Interaction Networks
The online tool STRING (http://www.string-db.org, accessed on 13 May 2022) was used to build interactome maps of ELISA-identified proteins (database v11.5) with the following properties: (i) organism, Homo Sapiens; (ii) meaning of network edges, evidence; (iii) active interaction sources, experiments; and (iv) minimum required interaction scores, low confidence (0.150).
2.5. EVs Characterization
Flow cytometry: Cleared secretome was 1:1 diluted with PBS and divided into 3 aliquots: (i) unstained, (ii) CFSE (1 µM final concentration) stained for 30 min at 37 °C and (iii) after CFSE supplementation, further staining for 30 min at 4 °C with one of the following antibodies: CD9-APC clone HI9A, CD63-APC clone H5C6, CD81-APC clone 5A6, CD44-APC clone BJ18, CD73-APC clone AD2 or CD90-APC clone 5E10) (Biolegend, San Die-go, CA, USA). After a further 1:3 dilution with PBS, samples were analyzed with a CytoFlex flow cytometer. FITC-fluorescent beads of 160, 200, 240 and 500 nm (Biocytex, Mar-seille, France) were used as an internal control. At least 30,000 events were collected.
Nanoparticle tracking analysis (NTA): Cleared secretome was 1:1 diluted in PBS and visualized by Nanosight LM10-HS system (NanoSight Ltd., Amesbury, UK). Each sample was run with 5 recordings of 60 s. NTA software provided both concentration measurements and high-resolution distribution profiles of particle size.
2.6. Total RNA Isolation and miRNA Quantification
Five ml of cleared secretome was 1:1 diluted in PBS before ultracentrifugation at 100,000× g for 9 h at 4 °C. Resulting pellets were processed with miRNeasy and RNeasy Cleanup Kits (Qiagen, Hilden, Germany) after addition of 30 pg of exogenous Arabidopsis thaliana ath-miR-159a synthetic miRNA spike. This was used to evaluate RNA recovery and cDNA synthesis performed as previously reported [14]. The OpenArray system (Life Technologies, Foster City, CA, USA) was used to determine miRNA expression in 384-well OpenArray plates according to the manufacturer’s protocol. Each single miRNA was considered as present when amplification resulted in all three samples. Eventually, ath-miR-159 spike-in CRT was used to equalize technical differences, and the global mean method [15] allowed normalization between samples.
2.7. miRNA Target Identification
miRTarBase v8.0 (https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2022/php/index.php, accessed on 14 March 2022) was used to analyze miRNAs under analysis to identify mRNA targets [16]. Only miRNA–mRNA interactions supported by strong experimental evidence were considered.
2.8. Statistical Analyses
GraphPad Prism Software version 5 (GraphPad, San Diego, CA, USA) was used to perform statistical analyses. Linear association between samples was determined by Pearson’s correlation coefficient (R2) and the outcome results were interpreted according to the degree of association [17].
ClustVis package (https://biit.cs.ut.ee/clustvis/, accessed on 13 May 2022) [18] was used to perform principal component analysis (PCA) and hierarchical clustering on normalized CRT values. After row centering, maps were generated using the following settings for both rows’ and columns’ clustering method and distance: average and correlation, respectively.
3. Results
3.1. BMSCs Phenotypic Characterization
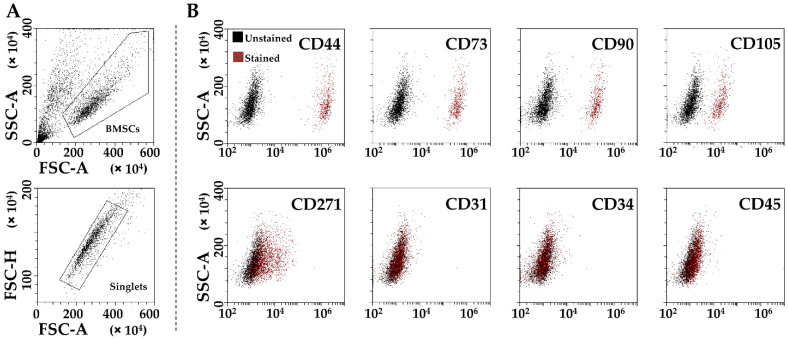
BMSCs were positive for mesenchymal (CD44/73/90/105) markers and negative for hemato-endothelial (CD31/34/45) ones (Figure 1A,B). CD271, considered a marker present in adult MSCs, was also detected at 17% ± 9, in agreement with previous findings [19].
Figure 1.
Flow cytometry analysis of BMSCs. (A), Identification of single cells by exclusion of debris (upper panel) and aggregates (lower panel). (B), staining for general mesenchymal (CD44, CD73, CD90 and CD105, positive), BMSC-specific (CD271, positive) and hemato-endothelial markers (CD31, CD34 and CD45, negative), confirming BMSCs identity. Representative plots are shown.
3.2. BMSCs Secreted Factors
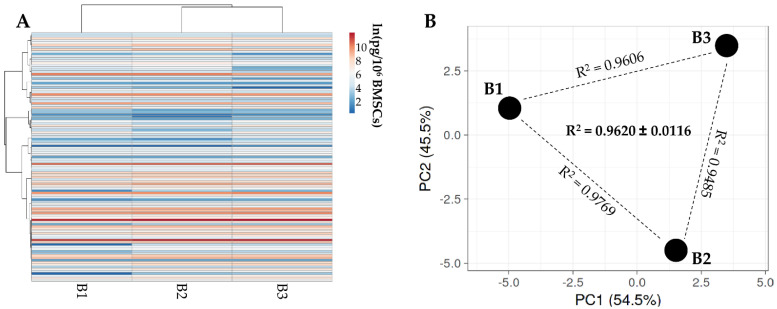
Out of 200 molecules, including inflammatory mediators and growth factors, chemokines, receptors and cytokines, 111 were present in all donors (Table 1). Hierarchical clustering showed higher similarity for BMSC 1 and 2 (Figure 2A), although within a pattern of overall conserved distance between donors in both PCA (Figure 2B) and correlation analysis (mean R2 of 0.96 ± 0.01). Thus, the average value was calculated to provide a guide to the level of each factor (Table 1). In 48 h, only insulin-like growth-factor-binding proteins-4 and -3 were secreted with an average amount superior to 100 ng per million BMSCs (IGFBP4, mean 132 ± 26; IGFBP3, mean 105 ± 16). Six other factors were detected between 10 and 100 ng: TIMP2 (41 ± 4), TGFB1 (20 ± 7), IFNL1 (18 ± 1), SERPINE1 (18 ± 2), TIMP1 (17 ± 3) and IGFBP6 (11 ± 1). It was also found that a further 24 factors had values ranging between 1 ng and 10 ng; the remaining 79 factors were below 1 ng.
Table 1.
BMSCs secreted factors detected in all donors and ordered by abundance of the mean values.
| pg/Million BMSCs per 48 h | |||||||
|---|---|---|---|---|---|---|---|
| Role | Factor | B1 | B2 | B3 | Mean | SD | Function |
| GF | IGFBP4 | 96,122 | 154,302 | 146,144 | 132,189 | 25,720 | Insulin-like growth factor-binding protein 4 |
| GF | IGFBP3 | 83,291 | 108,773 | 121,569 | 104,545 | 15,910 | Insulin-like growth factor-binding protein 3 |
| INF | TIMP2 | 36,744 | 39,361 | 45,708 | 40,604 | 3764 | Metalloproteinase inhibitor 2 |
| GF | TGFB1 | 24,241 | 25,312 | 10,951 | 20,168 | 6532 | Transforming growth factor beta-1 |
| CHE | IFNL1 | 18,892 | 18,815 | 15,755 | 17,821 | 1461 | Interferon lambda-1 |
| CYT | SERPINE1 | 14,532 | 17,906 | 20,270 | 17,570 | 2354 | Plasminogen activator inhibitor 1 |
| INF | TIMP1 | 14,137 | 16,618 | 20,310 | 17,022 | 2536 | Metalloproteinase inhibitor 1 |
| GF | IGFBP6 | 9478 | 11,134 | 13,129 | 11,247 | 1493 | Insulin-like growth factor-binding protein 6 |
| GF | BMP4 | 12,217 | 9427 | 6622 | 9422 | 2284 | Bone morphogenetic protein 4 |
| GF | IGFBP2 | 4926 | 11,110 | 7451 | 7829 | 2539 | Insulin-like growth factor-binding protein 2 |
| GF | VEGFA | 4498 | 6802 | 6811 | 6037 | 1088 | Vascular endothelial growth factor A |
| REC | VCAM1 | 5052 | 6333 | 6116 | 5834 | 560 | Vascular cell adhesion protein 1 |
| CHE | MIF | 4137 | 4918 | 5835 | 4963 | 694 | Macrophage migration inhibitory factor |
| INF | TNFRSF1A | 3763 | 4516 | 4897 | 4392 | 471 | Tumor necrosis factor receptor superfamily member 1A |
| CHE | XCL1 | 3176 | 5103 | 3256 | 3845 | 890 | Lymphotactin |
| CYT | INHBA | 3536 | 4186 | 3812 | 3845 | 266 | Inhibin beta A chain |
| CYT | ICAM2 | 3013 | 4715 | 3609 | 3779 | 705 | Intercellular adhesion molecule 2 |
| CHE | CCL27 | 2460 | 6090 | 2445 | 3665 | 1715 | C-C motif chemokine 27 |
| CHE | CXCL16 | 2945 | 3952 | 3942 | 3613 | 473 | C-X-C motif chemokine 16 |
| CYT | FST | 1925 | 3169 | 3700 | 2931 | 744 | Follistatin |
| CHE | MST1 | 2232 | 2598 | 2135 | 2322 | 200 | Hepatocyte growth-factor-like protein |
| CHE | CCL21 | 934 | 2655 | 3282 | 2290 | 992 | C-C motif chemokine 2 |
| CYT | ANGPT1 | 1542 | 2114 | 2277 | 1978 | 315 | Angiopoietin-1 |
| REC | PLAUR | 1301 | 2257 | 1872 | 1810 | 393 | Urokinase plasminogen activator surface receptor |
| CYT | IL6ST | 1135 | 2164 | 1532 | 1610 | 424 | Interleukin-6 receptor subunit beta |
| CHE | PF4 | 3191 | 387 | 1071 | 1550 | 1194 | Platelet factor 4 |
| INF | CCL2 | 841 | 1368 | 1855 | 1355 | 414 | C-C motif chemokine 2 |
| CYT | CTSS | 1051 | 1535 | 1461 | 1349 | 213 | Cathepsin S |
| CYT | ANG | 1204 | 1397 | 1364 | 1322 | 85 | Angiogenin |
| REC | ALCAM | 1034 | 1342 | 1390 | 1255 | 158 | CD166 antigen |
| CHE | CXCL11 | 1443 | 1370 | 828 | 1214 | 274 | C-X-C motif chemokine 11 |
| INF | IL11 | 1611 | 711 | 1261 | 1194 | 371 | Interleukin-11 |
| CYT | IL23A | 530 | 1172 | 1263 | 989 | 326 | Interleukin-23 subunit alpha |
| CHE | CCL25 | 257 | 965 | 1495 | 906 | 507 | C-C motif chemokine 25 |
| CHE | SPP1 | 742 | 954 | 848 | 848 | 86 | Osteopontin |
| CYT | IL13RA2 | 683 | 873 | 557 | 704 | 130 | Interleukin-13 receptor subunit alpha-2 |
| CHE | LIF | 960 | 251 | 844 | 685 | 311 | Leukemia inhibitory factor |
| INF | IL6 | 530 | 641 | 795 | 655 | 109 | Interleukin-6 |
| INF | IL1RN | 439 | 807 | 695 | 647 | 154 | Interleukin-1 receptor antagonist protein |
| GF | HGF | 383 | 537 | 741 | 554 | 147 | Hepatocyte growth factor |
| CYT | CED | 386 | 587 | 663 | 545 | 117 | Diaphyseal Dysplasia 1 |
| REC | PDGFRB | 162 | 647 | 805 | 538 | 274 | Platelet-derived growth factor receptor beta |
| REC | CD14 | 361 | 385 | 457 | 401 | 41 | Monocyte differentiation antigen CD14 |
| GF | KDR | 167 | 575 | 350 | 364 | 167 | Vascular endothelial growth factor receptor 2 |
| CHE | CXCL10 | 307 | 456 | 290 | 351 | 75 | C-X-C motif chemokine 10 |
| CHE | IFNL2 | 371 | 182 | 466 | 340 | 118 | Interferon lambda-2 |
| GF | GDF15 | 255 | 334 | 345 | 311 | 40 | Growth/differentiation factor 15 |
| INF | TNFRSF1B | 263 | 328 | 330 | 307 | 31 | Tumor necrosis factor receptor superfamily member 1B |
| CHE | TNFSF14 | 402 | 245 | 213 | 287 | 83 | Tumor necrosis factor ligand superfamily member 14 |
| CHE | CXCL12 | 248 | 244 | 360 | 284 | 54 | C-X-C motif chemokine 12, splicing variant alpha |
| INF | IL6R | 324 | 294 | 197 | 272 | 55 | Interleukin-6 receptor subunit alpha |
| CYT | IL17B | 189 | 513 | 56 | 253 | 192 | Interleukin-17B |
| REC | IL21R | 210 | 194 | 353 | 252 | 71 | Interleukin-21 receptor |
| INF | ICAM1 | 200 | 265 | 268 | 244 | 31 | Intercellular adhesion molecule 1 |
| CHE | AXL | 177 | 427 | 80 | 228 | 146 | Tyrosine-protein kinase receptor UFO |
| INF | IL16 | 172 | 162 | 349 | 228 | 86 | Pro-interleukin-16 |
| INF | IL1A | 107 | 261 | 285 | 218 | 79 | Interleukin-1 alpha |
| INF | TNF | 122 | 158 | 363 | 215 | 106 | Tumor necrosis factor |
| CYT | DKK1 | 182 | 299 | 156 | 212 | 62 | Dickkopf-related protein 1 |
| INF | PDGFB | 150 | 89 | 288 | 176 | 83 | Platelet-derived growth factor subunit B |
| GF | NTF4 | 112 | 170 | 233 | 172 | 49 | Neurotrophin-4 |
| GF | EGFR | 141 | 170 | 203 | 171 | 26 | Epidermal growth factor receptor |
| CYT | CXCL12 | 146 | 219 | 146 | 170 | 34 | C-X-C motif chemokine 12, splicing variant beta |
| INF | CXCL9 | 161 | 194 | 144 | 166 | 21 | C-X-C motif chemokine 9 |
| INF | IL7 | 147 | 144 | 139 | 144 | 3 | Interleukin-7 |
| GF | TNFRSF11B | 127 | 121 | 178 | 142 | 26 | Tumor necrosis factor receptor superfamily member 11B |
| CYT | THPO | 106 | 269 | 9 | 128 | 107 | Thrombopoietin |
| INF | CXCL8 | 86 | 99 | 180 | 122 | 42 | Interleukin-8 |
| INF | CCL5 | 87 | 120 | 141 | 116 | 22 | C-C motif chemokine 5 |
| REC | CNTN2 | 49 | 193 | 90 | 111 | 61 | Contactin-2 |
| INF | IL15 | 87 | 88 | 150 | 108 | 30 | Interleukin-15 |
| CHE | CCL7 | 137 | 42 | 112 | 97 | 40 | C-C motif chemokine 7 |
| CHE | BTC | 85 | 89 | 109 | 94 | 11 | Probetacellulin |
| CYT | VEGFC | 105 | 110 | 61 | 92 | 22 | Vascular endothelial growth factor C |
| INF | IL2 | 46 | 135 | 66 | 83 | 38 | Interleukin-2 |
| INF | IFNG | 45 | 148 | 44 | 79 | 49 | Interferon gamma |
| INF | CSF2 | 95 | 81 | 53 | 76 | 18 | Granulocyte-macrophage colony-stimulating factor |
| GF | KIT | 88 | 50 | 83 | 74 | 17 | Mast/stem cell growth factor receptor Kit |
| INF | CCL1 | 88 | 78 | 49 | 72 | 17 | C-C motif chemokine 1 |
| CYT | TNFRSF10D | 56 | 108 | 13 | 59 | 39 | Tumor necrosis factor receptor superfamily member 10D |
| REC | ENG | 52 | 80 | 45 | 59 | 15 | Endoglin |
| INF | IL4 | 60 | 30 | 85 | 59 | 22 | Interleukin-4 |
| CYT | SHH | 47 | 58 | 71 | 58 | 10 | Sonic hedgehog protein |
| CYT | CD40 | 66 | 77 | 32 | 58 | 19 | Tumor necrosis factor receptor superfamily member 5 |
| GF | IGFBP1 | 54 | 80 | 35 | 56 | 19 | Insulin-like growth-factor-binding protein 1 |
| REC | FAS | 51 | 63 | 47 | 54 | 7 | Tumor necrosis factor receptor superfamily member 6 |
| CHE | CCL20 | 22 | 66 | 64 | 51 | 20 | C-C motif chemokine 20 |
| CHE | CCL8 | 55 | 50 | 24 | 43 | 14 | C-C motif chemokine 8 |
| INF | CSF3 | 57 | 41 | 32 | 43 | 10 | Granulocyte colony-stimulating factor |
| INF | CSF1 | 31 | 32 | 54 | 39 | 10 | Macrophage colony-stimulating factor 1 |
| CYT | EPCAM | 30 | 35 | 36 | 34 | 3 | Epithelial cell adhesion molecule |
| GF | KITLG | 40 | 11 | 37 | 30 | 13 | Kit ligand |
| GF | PGF | 13 | 33 | 34 | 27 | 10 | Placenta growth factor |
| REC | EDA2R | 1 | 41 | 33 | 25 | 17 | Tumor necrosis factor receptor superfamily member 27 |
| INF | IL1B | 8 | 26 | 21 | 19 | 8 | Interleukin-1 beta |
| GF | PROK1 | 19 | 13 | 16 | 16 | 2 | Prokineticin-1 |
| CYT | IL1RL1 | 3 | 30 | 11 | 15 | 11 | Interleukin-1 receptor-like 1 |
| GF | FLT4 | 22 | 7 | 12 | 14 | 6 | Vascular endothelial growth factor receptor 3 |
| REC | TNFRSF21 | 16 | 18 | 5 | 13 | 6 | Tumor necrosis factor receptor superfamily member 21 |
| REC | MOK | 1 | 16 | 21 | 13 | 9 | MAPK/MAK/MRK overlapping kinase |
| INF | CCL24 | 15 | 2 | 19 | 12 | 7 | C-C motif chemokine 24 |
| REC | ERBB3 | 23 | 10 | 1 | 12 | 9 | Receptor tyrosine-protein kinase erbB-3 |
| INF | CCL11 | 8 | 4 | 19 | 11 | 6 | Eotaxin |
| REC | TNFRSF10C | 9 | 16 | 4 | 10 | 5 | Tumor necrosis factor receptor superfamily member 10C |
| CHE | CCL13 | 10 | 6 | 11 | 9 | 2 | C-C motif chemokine 13 |
| CHE | CCL17 | 6 | 7 | 9 | 7 | 1 | C-C motif chemokine 17 |
| REC | FLT3LG | 6 | 7 | 7 | 7 | 0 | Fms-related tyrosine kinase 3 ligand |
| INF | CCL4 | 4 | 6 | 8 | 6 | 2 | C-C motif chemokine 4 |
| CHE | PPBP | 7 | 5 | 5 | 6 | 1 | Platelet basic protein |
| INF | CXCL13 | 4 | 2 | 3 | 3 | 1 | C-X-C motif chemokine 13 |
| INF | IL12A/B | 1 | 1 | 3 | 2 | 1 | Interleukin-12 subunit alpha |
CHE = chemokine; CYT = cytokine; GF = growth factor; ING = inflammation; REC = receptor.
Figure 2.
Comparison of secreted factor profiles between BMSCs under study. (A), heat map of hierarchical clustering analysis of the ln(x)-transformed pg/million BMSCs values of detected factors with sample clustering tree at the top. Absolute expression levels are indicated by the color scale: blue shades = low expression levels and red shades = high expression levels. (B) principal component analysis of the ln(x)-transformed pg/million BMSCs values of detected factors. X and Y axis show principal component 1 and principal component 2 that explain 54.5% and 45.5% of the total variance.
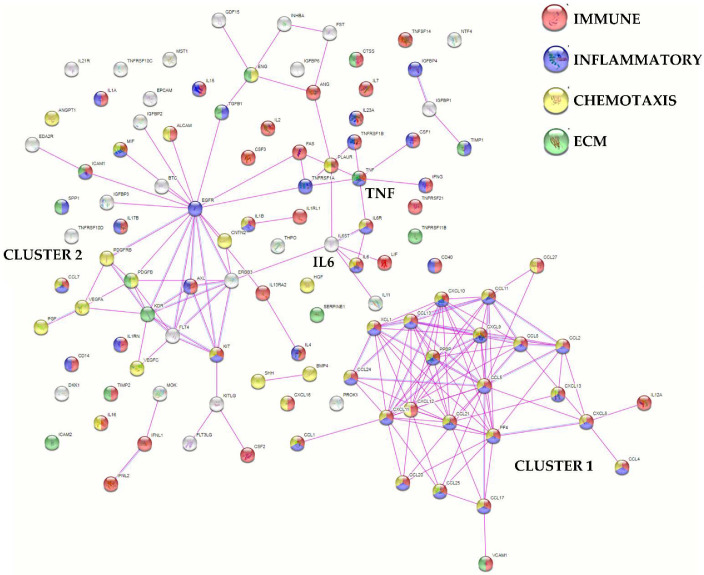
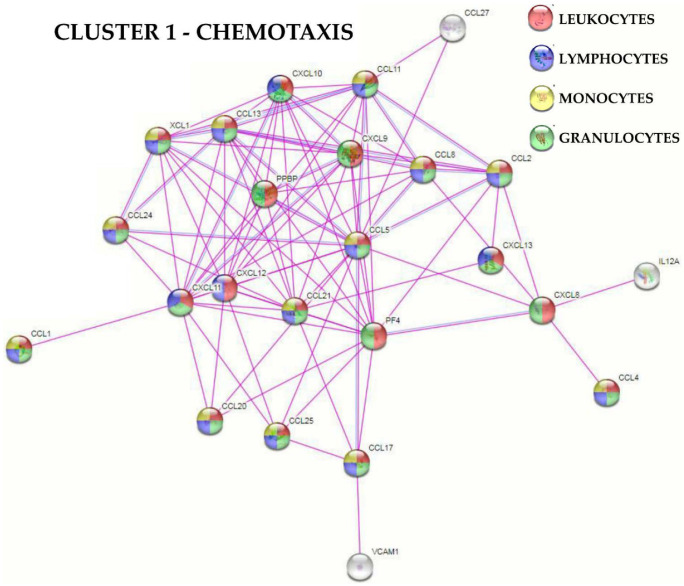
Functional protein association network analysis based on experimental and database-annotated interactions allowed for the definition of two main clusters (Figure 3). The first cluster was tighter, composed of 24 factors, mainly involved in immune (gene ontology GO:0006955, Supplementary Table S1A for all analyzed factors) and inflammatory (GO:0006954) responses and chemotaxis (GO:0006935). The second cluster was looser, centered on EGFR with seven players defined by the GO term chemotaxis. Connected to this group, two smaller clusters emerged, one centered on IL6 and its receptor subunits (IL6R and IL6ST), while the other centered on TNF and its receptors (TNFRSF1A and B). Eventually, 14 molecules were related to extracellular matrix organization (GO:0030198), without the definition of a specific cluster (Figure 3). Dissecting further the GO term chemotaxis, several terms related to immune cells appeared. These were mainly associated with cluster 1 (Figure 4), which included leukocytes (30 overall factors, GO:0030595) and their subtypes: granulocytes (23, GO:0071621), lymphocytes (19, GO:0048247) and monocytes (17, GO:0002548) (Supplementary Figure S1 and Supplementary Table S1B for all analyzed factors). Interestingly, the leukocyte activation term was defined by several players (31, GO:0045321) without the identification of a specific cluster (Supplementary Figure S2 and Supplementary Table S1C for all analyzed factors). In this frame, lymphocytes (18, GO:0046649) and the subcategory T cells (12, GO:0042110) were the most present terms, followed by neutrophils (10, GO:0042119) and macrophages (4, GO:0042116).
Figure 3.
Functional association network for identified secreted factors. Protein–protein interaction levels for 111 proteins of the BMSCs secretome were mined using the online tool STRING. The blue connections are for proteins with known interactions based on curated databases; violet connections for proteins with experimentally determined interactions. Colorless nodes for proteins not related to the GO terms: immune, inflammatory, chemotaxis and ECM in the STRING database v 11.5. Empty nodes, proteins of unknown 3D structure; filled nodes, known or predicted 3D structure. Immune, inflammatory, chemotaxis and ECM-related GO terms are shown.
Figure 4.
Functional association network for Cluster 1 secreted factors related to the GO term “chemotaxis”. Using the online tool STRING, protein–protein interaction levels for 24 proteins of the BMSCs Cluster 1 related to the GO term “chemotaxis” for leukocytes, lymphocytes, monocytes and granulocytes were mined. The different colors represent the immune cell type the “chemotaxis” term is associated with. The blue connections are for proteins with known interactions based on curated databases; violet connections for proteins with experimentally determined interactions. Colorless nodes for proteins not related to the GO terms: leukocytes chemotaxis, lymphocytes chemotaxis, monocytes chemotaxis and granulocytes chemotaxis in the STRING database v 11.5. Empty nodes, proteins of unknown 3D structure; filled nodes, known or predicted 3D structure.
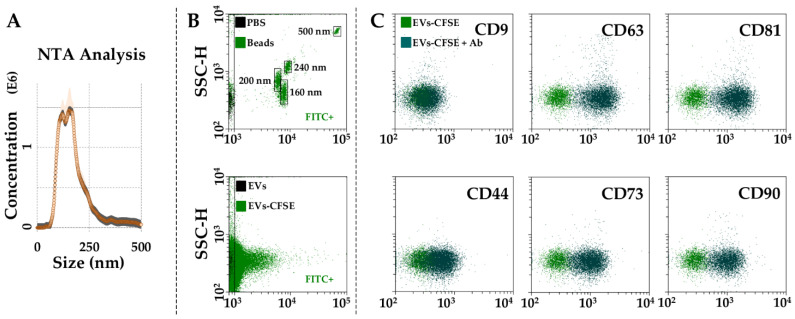
3.3. Characterization of BMSC-EVs
BMSCs released around 650 EVs per cell in 48 h. The mean size calculated using NTA technology resulted to be 138 nm ± 7 with 73% ± 1 of particles being below 200 nm (Figure 5A). Dimensional analysis was confirmed by flow cytometry through direct comparison, with FITC-fluorescent nanobeads showing 77% ± 1 of EVs below 200 nm (Figure 5B). Particles resulted positive for the EV markers CD63 (93% ± 1) and CD81 (91% ± 2). The particles were almost negative for CD9 (5% ± 1, Figure 5C) as already demonstrated for BMSC-EVs [19] and EVs from other MSC types, adipose- [20] or amniotic-membrane-derived [21]. With respect to MSC markers, CD44 staining gave a 46% ± 5 positivity, although the complete shift of the population suggests a homogeneous staining of all EVs. CD73 and CD90 were strongly positive, too (81% ± 3 and 83% ± 1, respectively, Figure 5C).
Figure 5.
Characterization of BMSC-EVs. (A), EVs size analysis from NTA data. (B), flow cytometer was first calibrated to score FITC-fluorescent particles of nanometer scale (upper panel, starting from 160 nm). EVs were then CFSE stained to allow their identification and gating in the FITC channel (lower panel). (C), after gating, CFSE+ EVs showed positive staining for CD63 and CD81 extracellular vesicle defining molecules and CD44, CD73 and CD90 MSC markers. CD9, another EV postulated marker, was barely detectable. Representative cytograms are presented.
3.4. Identification of BMSC EV-miRNAs
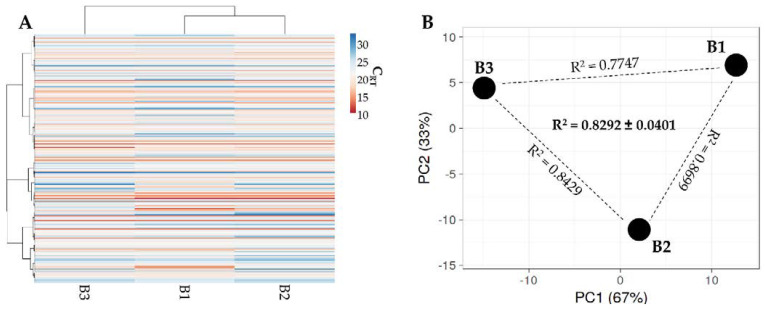
Out of 754 molecules, 201 miRNAs were detected in all samples (Supplementary Table S2). Hierarchical clustering was able to show higher similarity for BMSC 1 and 2 (Figure 6A), although the same pattern of overall conservation already observed for released factors emerged, characterized by a preserved distance between donors in both PCA (Figure 6B) and correlation analysis (mean R2 of 0.83 ± 0.04). Thus, as for released factors, an average miRNA CRT value was calculated (Supplementary Table S2). Furthermore, since in MSC-EVs no more than one miRNA copy per EV is present [22], and no fewer than 100 EVs are needed to transfer one miRNA copy to a target cell [23], only those within the first quartile of expression were selected. This resulted in a list of 53 miRNAs, covering 97.2% of the detected genetic message (Table 2). The results show that the most represented miRNAs were hsa-mir-518f-3p (25.3% of the total weight), followed by hsa-miR-24-3p (11.5%) and hsa-miR-222-3p (7.7%). The miRNAs hsa-miR-720, hsa-miR-520e-3p and hsa-mir-193a-5p (all around 0.14%) were found at the bottom of the quartile. The miRNAs hsa-miR-720, as well as hsa-miR-1274A/B, were not considered further, as these are likely to be a fragment of a tRNA [24]. By sifting experimentally validated miRNA–mRNA interactions (Supplementary Table S3), 1152 univocal targets were identified (Supplementary Table S4). Gene ontology analysis of identified targets vs. the whole genome showed that the first ten enriched processes were related to biological, cellular and metabolic processes without a clear definition of regulated pathways (Table 3), preventing the definition of a disease-tailored prediction of efficacy. This is also emphasized by the transcriptional pattern in pathological tissues or cell types that may greatly diverge from the whole genome, further reducing the weight of broad bioinformatics analysis “vs. the whole genome”.
Figure 6.
Comparison of EV-miRNA expression profiles between BMSCs under study. (A) heat map of hierarchical clustering analysis of the normalized CRT values of detected miRNAs with sample clustering tree at the top. Absolute expression levels are indicated by the color scale: blue shades = low expression levels (high CRT values) and red shades = high expression levels (low CRT values). (B) Principal component analysis of the normalized CRT values of detected miRNAs. X and Y axes show principal component 1 and principal component 2 that explain 67% and 33% of the total variance.
Table 2.
First quartile EV-miRNAs.
| CRT | CRT | CRT | CRT | CRT | CRT | CRT | CRT | CRT | CRT | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| miRBase ID | B1 | B2 | B3 | Mean | SD | Weight % | miRBase ID | B1 | B2 | B3 | Mean | SD | Weight % |
| hsa-miR-518f-3p | 10.20 | 10.12 | 13.23 | 11.18 | 1.45 | 25.25552 | hsa-miR-409-3p | 16.97 | 17.12 | 16.73 | 16.94 | 0.16 | 0.46626 |
| hsa-miR-24-3p | 12.66 | 12.19 | 12.09 | 12.31 | 0.25 | 11.52898 | hsa-miR-618 | 11.91 | 15.11 | 23.98 | 17.00 | 5.10 | 0.44726 |
| hsa-miR-222-3p | 13.16 | 13.01 | 12.51 | 12.89 | 0.28 | 7.70535 | hsa-miR-106a-5p | 17.15 | 16.86 | 17.43 | 17.15 | 0.23 | 0.40375 |
| hsa-miR-574-3p | 13.11 | 13.22 | 12.77 | 13.03 | 0.19 | 6.99275 | hsa-miR-657 | 18.32 | 16.08 | 17.18 | 17.19 | 0.91 | 0.39180 |
| hsa-miR-193b-3p | 13.08 | 13.23 | 12.87 | 13.06 | 0.15 | 6.87580 | hsa-miR-221-3p | 18.56 | 17.13 | 16.95 | 17.55 | 0.72 | 0.30620 |
| hsa-miR-191-5p | 13.20 | 13.30 | 13.00 | 13.16 | 0.13 | 6.39020 | hsa-miR-34a-5p | 15.92 | 17.18 | 19.68 | 17.59 | 1.56 | 0.29686 |
| hsa-miR-484 | 13.91 | 14.04 | 13.83 | 13.93 | 0.09 | 3.76555 | hsa-miR-627-5p | 16.27 | 16.58 | 20.00 | 17.62 | 1.69 | 0.29156 |
| hsa-miR-1274B | 13.99 | 13.97 | 14.23 | 14.06 | 0.12 | 3.42521 | hsa-miR-302c-3p | 14.25 | 20.79 | 17.81 | 17.62 | 2.67 | 0.29149 |
| hsa-miR-197-3p | 14.17 | 14.20 | 14.56 | 14.31 | 0.18 | 2.89092 | hsa-miR-92a-3p | 18.04 | 17.63 | 17.67 | 17.78 | 0.19 | 0.25999 |
| hsa-miR-320a-3p | 14.27 | 14.17 | 14.73 | 14.39 | 0.24 | 2.74067 | hsa-miR-132-3p | 18.16 | 17.62 | 17.87 | 17.88 | 0.22 | 0.24247 |
| hsa-miR-662 | 17.80 | 16.69 | 12.09 | 15.53 | 2.47 | 1.24360 | hsa-miR-205-5p | 14.88 | 20.81 | 18.07 | 17.92 | 2.42 | 0.23693 |
| hsa-miR-523-3p | 14.57 | 14.89 | 17.56 | 15.67 | 1.34 | 1.12287 | hsa-miR-483-5 | 17.43 | 18.43 | 18.00 | 17.95 | 0.41 | 0.23125 |
| hsa-miR-214-3p | 15.85 | 15.62 | 15.77 | 15.75 | 0.10 | 1.06599 | hsa-miR-382-5p | 17.24 | 18.16 | 18.65 | 18.02 | 0.58 | 0.22137 |
| hsa-miR-125b-5p | 16.24 | 15.54 | 15.69 | 15.82 | 0.30 | 1.01269 | hsa-miR-199a-3p | 18.76 | 17.68 | 17.65 | 18.03 | 0.52 | 0.21903 |
| hsa-miR-145-5p | 16.24 | 15.98 | 15.45 | 15.89 | 0.33 | 0.96495 | hsa-miR-31-5p | 18.23 | 18.15 | 17.96 | 18.12 | 0.11 | 0.20659 |
| hsa-miR-19b-3p | 15.93 | 16.01 | 15.94 | 15.96 | 0.04 | 0.92244 | hsa-miR-138-5p | 18.27 | 17.82 | 18.36 | 18.15 | 0.24 | 0.20206 |
| hsa-miR-342-3p | 16.10 | 16.05 | 15.77 | 15.98 | 0.15 | 0.91121 | hsa-miR-20a-5p | 18.45 | 18.08 | 18.03 | 18.19 | 0.19 | 0.19663 |
| hsa-miR-99a-5p | 16.16 | 15.85 | 16.19 | 16.07 | 0.15 | 0.85571 | hsa-miR-376c-3p | 18.24 | 18.27 | 18.44 | 18.31 | 0.09 | 0.18027 |
| hsa-miR-16-5p | 16.46 | 15.99 | 15.94 | 16.13 | 0.23 | 0.81990 | hsa-miR-146b-5p | 18.68 | 18.58 | 17.93 | 18.39 | 0.33 | 0.17046 |
| hsa-miR-30c-5p | 16.37 | 16.19 | 16.06 | 16.20 | 0.13 | 0.77693 | hsa-miR-28-3p | 18.77 | 18.23 | 18.20 | 18.40 | 0.26 | 0.16979 |
| hsa-miR-21-5p | 16.68 | 15.99 | 15.96 | 16.21 | 0.33 | 0.77407 | hsa-miR-194-5p | 17.13 | 20.51 | 18.07 | 18.57 | 1.42 | 0.15075 |
| hsa-miR-29a-3p | 16.27 | 16.14 | 16.33 | 16.25 | 0.08 | 0.75516 | hsa-miR-186-5p | 18.83 | 18.72 | 18.18 | 18.58 | 0.28 | 0.14995 |
| hsa-miR-30b-5p | 16.58 | 16.21 | 16.35 | 16.38 | 0.15 | 0.68770 | hsa-miR-720 | 18.81 | 18.48 | 18.66 | 18.65 | 0.14 | 0.14235 |
| hsa-let-7b-5p | 17.41 | 16.26 | 16.00 | 16.56 | 0.61 | 0.60872 | hsa-miR-520e-3p | 15.35 | 17.71 | 23.08 | 18.72 | 3.23 | 0.13621 |
| hsa-miR-17-5p | 17.00 | 16.75 | 16.94 | 16.90 | 0.10 | 0.48147 | hsa-miR-193a-5p | 19.25 | 18.40 | 18.54 | 18.73 | 0.37 | 0.13502 |
| hsa-miR-1274A | 16.88 | 16.85 | 16.99 | 16.91 | 0.06 | 0.47782 | Total | 97.2 |
Table 3.
Gene ontology analysis of miRNA targets vs. the whole genome; first ten enriched biological processes.
| GO | Biological Process | Count in Network | FDR |
|---|---|---|---|
| GO:0048518 | Positive regulation of biological process | 826 of 6112 | 2.85 × 10−165 |
| GO:0048522 | Positive regulation of cellular process | 791 of 5579 | 4.50 × 10−165 |
| GO:0048519 | Negative regulation of biological process | 762 of 5389 | 6.31 × 10−154 |
| GO:0048523 | Negative regulation of cellular process | 720 of 4874 | 7.11 × 10−150 |
| GO:0009893 | Positive regulation of metabolic process | 642 of 3893 | 3.33 × 10−147 |
| GO:0010604 | Positive regulation of macromolecule metabolic process | 611 of 3600 | 1.93 × 10−142 |
| GO:0031325 | Positive regulation of cellular metabolic process | 587 of 3413 | 5.69 × 10−137 |
| GO:0051173 | Positive regulation of nitrogen compound metabolic process | 567 of 3239 | 1.25 × 10−133 |
| GO:0031323 | Regulation of cellular metabolic process | 781 of 6239 | 1.77 × 10−130 |
| GO:0019222 | Regulation of metabolic process | 823 of 6948 | 1.27 × 10−129 |
FDR = false discovery rate; GO = gene ontology classification.
3.5. Target and Effect Prediction of BMSC EV-miRNAs on OA-Related Cell Types
To obtain a disease-framed influence of first quartile EV-miRNAs, initially identified univocal targets were compared with factors reported to be involved in OA at different levels, as well as factors expressed by at least 1% of resident chondrocytes, type B synoviocytes and immune cells [25]. EV-miRNAs in the first quartile targeted 32 molecules (Table 4), including 7 cytokines, 13 growth factors and 12 players related to proteolytic activities involved in extracellular matrix (ECM) homeostasis. Of note, all targeted cytokines are involved with inflammation and cartilage erosion, including the two most studied OA-driving molecules interleukin 1β (IL1B) and tumor necrosis factor α (TNF). Moreover, if also including cytokines expressed by a very limited percentage of synoviocytes and macrophages (<1%), interferon γ (IFNG) also emerged as mainly targeted by hsa-miR-24-3p (11.53%). For growth factors, several (10 out of 13) are endorsed with an OA-promoting capacity, especially related to ECM degradation and inflammation. In addition, hepatocyte growth factor (HGF) and connective tissue growth factor (CTGF) promote osteophyte formation, while transforming growth factor β (TGFB1) and KIT ligand (KITLG) promote synovial fibrosis and hyperplasia, making EV-miRNAs possible regulators of all OA-affected tissues. Eventually, 10 out of 13 protease-related factors are direct ECM-degrading enzymes. This includes the most studied matrix metalloproteinase (MMP)1, 9 and 13, with activated protein C (APC) being an MMP activity promoter. Altogether, cartilage-destructive and pro-inflammatory targets largely overcome protective molecules. With respect to single miRNA contribution, hsa-miR-24-3p was found to be the most abundant player (11.53% of the total weight) targeting TGFB1 and MMP14. The next most abundant player was hsa-miR-222-3p (7.71%), which regulates the expression of two molecules with opposite roles, such as MMP1 and the tissue inhibitor of metalloproteinases (TIMP)3. Two other miRNAs with almost identical weight, hsa-193b-3p (6.88%) and hsa-miR-191-5p (6.39%), target OA-inducing mRNAs’ plasminogen activator urokinase (PLAU) and interleukin 1 α (IL1A), respectively. Notably, hsa-miR-125b-5p (1.01%) is the molecule regulating the larger number of targets (six), followed by hsa-miR-145-5p (0.96%, three), hsa-miR-16-5p (0.82%, three) and hsa-miR-29a-3p (0.76%, 3). Lastly, type B synoviocytes appeared as the preferential EV-miRNAs interactors, since almost all identified targets are expressed by these cells (32 out of 33), followed by HLA-DR+ cells (19), chondrocytes (17) and T cells (2).
Table 4.
OA-related factors targeted by EV-miRNAs.
| Expressing Cell (>1%) * | Weight% | Main EV-miRNA (%) | Function | ||||
|---|---|---|---|---|---|---|---|
| C | S | H | T | ||||
| CYTOKINES | |||||||
| TNF | X | X | 1.49 | hsa-miR-125b-5p (1.01) | Pro-inflammatory | ||
| IL6 | X | X | 0.17 | hsa-miR-146b-5p (0.17) | Pro-inflammatory | ||
| IL1B | X | X | 0.77 | hsa-miR-21-5p (0.77) | Pro-inflammatory | ||
| IL1A | X | X | 6.39 | hsa-miR-191-5p (6.39) | Pro-inflammatory | ||
| CXCL12 | X | X | 0.52 | hsa-miR-221-3p (0.31) | Articular cartilage matrix degeneration | ||
| CCL5 | X | X | X | 1.07 | hsa-miR-214-3p (1.07) | Cartilage erosion | |
| IL11 | X | X | X | 0.78 | hsa-miR-30c-5p (0.78) | Pro-inflammatory | |
| GROWTH FACTORS | |||||||
| TGFB1 | X | X | X | X | 18.52 | hsa-miR-24-3p (11.53) | Cartilage homeostasis, chondrocytes hypertrophy |
| IGF1 | X | X | 0.98 | hsa-miR-29a-3p (0.76) | Promotes chondrocyte anabolic activity | ||
| FGF2 | X | X | 0.97 | hsa-miR-16-5p (0.82) | Promotes catabolic and anti-anabolic effects in OA joints | ||
| BMP2 | X | X | X | 0.88 | hsa-miR-17-5p (0.48) | Promotes cartilage regeneration | |
| VEGFA | X | X | X | 8.04 | hsa-miR-320a-3p (2.74) | Chondrocyte catabolism | |
| HGF | X | X | 1.04 | hsa-miR-16-5p (0.82) | Cartilage homeostasis, osteophyte formation | ||
| ANGPT2 | X | X | 1.97 | hsa-miR-125b-5p (1.01) | Abnormal angiogenesis in OA | ||
| CTGF | X | X | X | 1.98 | hsa-miR-145-5p (0.96) | Promotes osteophyte formation and ECM degradation | |
| KITLG | X | X | X | 2.74 | hsa-miR-320a-3p (2.74) | Promotes synovial mast cell hyperplasia and inflammation | |
| TGFB2 | X | X | X | 1.73 | hsa-miR-145-5p (0.96) | Cartilage homeostasis, high levels during OA development | |
| INHBB | X | 0.30 | hsa-miR-34a-5p (0.30) | TGFB superfamily, upregulated in OA | |||
| IGF2 | X | X | 1.01 | hsa-miR-125b-5p (1.01) | Promotes cartilage matrix levels | ||
| BDNF | X | 1.06 | hsa-miR-16-5p (0.82) | Promotes joint pain and inflammation | |||
| PROTEASES | |||||||
| ADAM12 | X | X | 0.76 | hsa-miR-29a-3p (0.76) | Metalloproteinase involved in ECM degradation | ||
| ADAM17 | X | X | X | 0.96 | hsa-miR-145-5p (0.96) | Metalloproteinase involved in ECM degradation | |
| ADAMTS9 | X | 0.76 | hsa-miR-29a-3p (0.76) | Metalloproteinase involved in ECM degradation | |||
| MMP1 | X | 8.67 | hsa-miR-222-3p (7.71) | Metalloproteinase involved in ECM degradation | |||
| MMP2 | X | X | 2.56 | hsa-miR-125b-5p (1.01) | Metalloproteinase involved in ECM degradation | ||
| MMP9 | X | X | 0.24 | hsa-miR-132-3p (0.24) | Metalloproteinase involved in ECM degradation | ||
| MMP14 | X | X | 12.49 | hsa-miR-24-3p (11.53) | Metalloproteinase involved in ECM degradation | ||
| PLAU | X | X | 6.88 | hsa-miR-193b-3p (6.88) | ECM-degrading enzyme | ||
| PLAT | X | X | 0.77 | hsa-miR-21-5p (0.77) | ECM-degrading enzyme | ||
| APC | X | X | 1.41 | hsa-miR-125b-5p (1.01) | Promotes MMP activity | ||
| TIMP2 | X | X | 0.60 | hsa-miR-106a-5p (0.40) | MMP inhibitor | ||
| TIMP3 | X | X | 9.27 | hsa-miR-222-3p (7.71) | MMP inhibitor | ||
C = chondrocytes; S = synoviocytes; H = HLA–DR+ cells; T = T cells * [25].
The second step was to compare identified first-quartile miRNAs with those reported to be directly involved with OA at cartilage [26] and synovia [27] levels or macrophage polarization [28] and T cell activation [29] (Table 5). Regarding cartilage, nine protective and six destructive miRNAs were identified, with hsa-miR-145-5p having a dual role. In the first group, five miRNAs were found to have a weight > 1%, driven by hsa-miR-24-3p (11.53%), hsa-miR-222-3p (7.71%) and hsa-miR-193b-3p (6.88%), for a total weight of 31.13%. In the second group, no miRNAs with a weight > 1% emerged, for a total weight of 3.98%. Therefore, the protective vs. destructive ratio was 10.3-fold. Concerning synovia, the definition of OA-related miRNAs is still in its infancy. Our data revealed two players, hsa-miR-29a-3p (0.76%), which reduces OA-induced synovia remodeling, and hsa-miR-34a-5p (0.30%), which upregulates synovial inflammation. Regarding immune cells, four miRNAs were related to anti-inflammatory M2 and two to pro-inflammatory M1 macrophage polarization. In particular, M2 polarizing features were driven by hsa-miR-24-3p (11.53%) and hsa-miR-222-3p (7.71%), for an M2 vs. M1 ratio of 10.2 fold. Eventually, 8 miRNAs have an activating and 3 miRNAs a repressing function on T cells, for a repression vs. activation ratio of 2.8 fold. Again, hsa-miR-24-3p (11.53%) led the anti-activation properties of EVs together with hsa-miR-125b-5p (1.01%), while only-activating hsa-miR-214-3p have a weight > 1%. Thus, overall, protective and anti-inflammatory signals largely overcame OA-driving inputs.
Table 5.
EV-miRNAs involved in OA-related cell types and mechanisms.
| Cartilage | Weight% | Role |
|---|---|---|
| Protective | ||
| hsa-miR-24-3p | 11.52898 | Regulates chondrocyte senescence |
| hsa-miR-222-3p | 7.70535 | Reduces cartilage degradation |
| hsa-miR-193b-3p | 6.87580 | Reduces inflammation |
| hsa-miR-320a-3p | 2.74067 | Increases chondrocyte viability |
| hsa-miR-125b-5p | 1.01269 | Prevents aggrecan loss |
| hsa-miR-17-5p | 0.48147 | Induces autophagy |
| hsa-miR-221-3p | 0.30620 | Prevents ECM degradation |
| hsa-miR-92a-3p | 0.25999 | Increases collagen deposition |
| hsa-miR-199a-3p | 0.21903 | Anti-catabolic |
| TOTAL | 31.13017 | |
| Destructive | ||
| hsa-miR-16-5p | 0.81990 | Cartilage degradation |
| hsa-miR-21-5p | 0.77407 | Negatively regulates chondrogenesis |
| hsa-miR-30b-5p | 0.68770 | Pro-apoptotic, ECM degradation |
| hsa-miR-34a-5p | 0.29686 | Pro-apoptotic |
| hsa-miR-483-5p | 0.23125 | Chondrocyte hypertrophy, ECM degradation and cartilage angiogenesis |
| hsa-miR-138-5p | 0.20206 | Cartilage degradation |
| TOTAL | 3.01185 | |
| Dual | ||
| hsa-miR-145-5p | 0.96495 | Regulates chondrocyte proliferation and fibrosis |
| SYNOVIA | ||
| Protective | ||
| hsa-miR-29a-3p | 0.75516 | Protects synovial remodeling |
| Destructive | ||
| hsa-miR-34a-5p | 0.29686 | Synovial inflammation |
| MACROPHAGE | ||
| M1 | ||
| hsa-miR-125b-5p | 1.01269 | Pro-M1 |
| hsa-miR-145-5p | 0.96495 | Pro-M1 |
| TOTAL | 1.97764 | |
| M2 | ||
| hsa-miR-24-3p | 11.52898 | Pro-M2, anti-M1 |
| hsa-miR-222-3p | 7.70535 | Pro-M2 |
| hsa-miR-34a-5p | 0.29686 | Pro-M2 |
| hsa-let-7b-5p | 0.60872 | Pro-M2 |
| TOTAL | 20.13992 | |
| T CELL | ||
| Pro-activation | ||
| hsa-miR-214-3p | 1.06599 | Reduces PTEN repressor |
| hsa-miR-19b-3p | 0.92244 | Reduces PTEN repressor |
| hsa-miR-21-5p | 0.77407 | Reduces PTEN repressor |
| hsa-let-7b-5p | 0.60872 | Targets IL10 |
| hsa-miR-17-5p | 0.48147 | Reduces PTEN repressor and promotes IFNG |
| hsa-miR-106a-5p | 0.40375 | Targets IL10 |
| hsa-miR-221-3p | 0.3062 | Downregulates PIK3R1 |
| hsa-miR-132-3p | 0.24247 | Downregulates PIK3R1 |
| TOTAL | 4.80510 | |
| Anti-activation | ||
| hsa-miR-24-3p | 11.52898 | Represses IFNG |
| hsa-miR-125b-5p | 1.01269 | Targets key molecules for T cell activation |
| hsa-miR-342-3p | 0.91121 | Downregulated during activation |
| TOTAL | 13.45289 | |
| Dual | ||
| hsa-miR-31-5p | 0.20659 | Upregulates IL2, downregulated with activation |
| hsa-miR-146b-5p | 0.17046 | Reduces TREF6 repressor, downregulated with activation |
| TOTAL | 0.37706 |
4. Discussion
In this manuscript, soluble factors and EV-miRNAs’ fingerprint gave the molecular basis for the observed regenerative and anti-inflammatory properties of BMSCs in the frame of OA [30,31,32] and, as a consequence, paved the way for the use of purified secretome or EVs as a cell-free therapeutic approach in the treatment of osteoarthritis.
Soluble factors analysis confirmed that BMSCs secrete several leukocyte chemokines able to attract a wide array of immune cells, such as lymphocytes, monocytes and granulocytes (Supplementary Figure S1). This mechanism is part of the immunosuppressive BMSCs activity that, after chemoattraction, relies on the immune-inhibitory effects of an array of soluble mediators such as indoleamine 2, 3-dioxygenase (IDO) and nitric oxide (NO) that are most active on cells in close proximity. As an example, NO produced by BMSCs suppresses responsiveness [33] and inhibits proliferation in T cells [34]. Similar results are observed for IDO [35]. This is of relevance in OA, since pro-inflammatory T cells are among the major constituents of both synovial membranes [36,37] and synovial fluids infiltrates [37,38]. Moreover, IDO triggers monocyte differentiation into anti-inflammatory M2 macrophages [39] that in turn suppress T cell proliferation [40], thus amplifying the immunosuppressive effect generated by MSCs. This is again crucial, since in both synovial membranes and fluids the M1/M2 ratio is tipped towards M1 polarization, and these pro-inflammatory macrophages are among the most abundant immune cell types contributing to cartilage damage and bone alterations through the release of cytokines such as IL1B and TNF. Eventually, BMSCs may also act on neutrophils, the most abundant type of granulocytes found at high levels in OA synovial membranes and fluids [41]. BMSCs may suppress hydrogen peroxide production in activated neutrophils [42,43], thus limiting the intensity of a respiratory burst upon inflammatory stimulation. To date, the role of neutrophils in OA is still underestimated. Thus, BMSCs chemoattraction towards the different subsets of leukocytes is a crucial mechanism to directly interact with activated immune cells, abundant in OA tissues, and reduce their pro-inflammatory status. Consistently, among the most abundant (>1 ng/million cells) factors related to chemotaxis, we found vascular endothelial growth factor α (VEGFA, granulocytes), X-C motif chemokine ligand 1 (XCL1, lymphocytes/monocytes/granulocytes), C-X-C motif chemokine ligand 16 (CXCL16, lymphocytes), C-C Motif Chemokine Ligand 21 (CCL21, lymphocytes/monocytes/granulocytes), platelet factor 4 (PF4, granulocytes), CCL2 (lymphocytes/monocytes/granulocytes) and CXCL11 (lymphocytes/granulocytes).
Another important feature framed by soluble factors is their involvement with ECM, which, during the progression of OA, is actively remodeled under inflammatory conditions due to increased action of the matrix metalloproteinases (MMPs) in combination with a reduction in their inhibitors (TIMPs). In mice, TIMP2 reduction leads to accelerated OA [44], while in dogs, TIMP2 expression is decreased in OA synovial fluids [45] and cartilage [46]. Intriguingly, in BMSCs secretome, TIMP1 and 2 resulted among the most released factors (>10,000 pg/mL) (Table 1). In a similar range, serpin family E member 1 (SERPINE1) was found. This protein, found at reduced levels in OA cartilage, counteracts the activity of elevated urokinase/tissue-type plasminogen activators [47] and positively correlates with cartilage synthesis during pathophysiologic processes [48]. Importantly, although not directly related to ECM, the urokinase plasminogen activator surface receptor (PLAUR) found at >1000 pg/mL may contribute to reducing plasminogen activation in plasmin, which in turn activates MMPs [49]. The other ECM-related secreted factor with an average level >10,000 is TGFB1. TGFBs play critical roles in regulating chondrocyte differentiation from early to terminal stages, including condensation, proliferation, terminal differentiation and maintenance of articular chondrocytes [50]. TGFB supplementation can enhance cartilage repair and is therefore a potential therapeutic tool [51], considering also its pleiotropic effects on T cells by inhibiting TH1, TH2 and CTL differentiation and in concert with other factors promoting TH17 or pTreg cell differentiation [52]. Eventually, other players found at high levels (>10,000 pg/mL), such as insulin-like growth factor (IGF) binding proteins IGFBP3/4/6 and IGFBP2 (nearly 8000 pg/mL), are indirectly related to ECM. IGF-1 regulates cartilage repair by promoting cartilage anabolism and inhibiting cartilage catabolism and IGFBPs, by altering the bioavailability and function of IGFs, may deliver IGFs-independent signals for chondrocyte survival [53]. Intriguingly, increased IGFBP levels were reported in both the synovial fluid and articular cartilage from OA patients [54], although this mechanism is still to be clarified. Therefore, BMSCs secretome may orchestrate ECM homeostasis at different levels regulating both catabolic and anabolic pathways.
EV-miRNAs resulted in targeting both the majority of OA-driving factors and OA-related cell types. Several pro-inflammatory molecules, mainly secreted by synoviocytes and HLA-DR+ (including macrophages) cells, emerged. In this group fell IL1B, IL6 and TNF, together with IL1A, the cytokine with the strongest regulation, being a target of hsa-miR-191-5p (6.39% of the total EV genetic weight). This is consistent with the reported capacity of MSCs to both suppress immune response, by inhibiting production of inflammatory cytokines in immune cells, and attenuate inflammation in osteoarthritic joints [55]. With respect to growth factors, many identified molecules were related with cartilage homeostasis and OA progression, with TGFB1 and (VEGFA) being the two most regulated (18.52% and 8.04%, respectively). In particular, TGFB1 is targeted by one of the most abundant miRNAs, hsa-miR-24-3p (11.53%). Therefore, TGFB1 levels are regulated in a twofold manner after BMSCs or secretome administration. On one side, the molecule is directly added as soluble mediator, and on the other side, EV-miRNAs reduce its de novo production. This might be of crucial importance, since excess of TGFB1 can enhance cartilage repair but may also result in synovial fibrosis and osteophyte formation [51]. Interestingly, its downregulation at the cellular level has been proposed as a therapeutic option, in association with exogenous supplementation of TGFB1, to locally inhibit TGF-beta production, as EV-miRNAs can do. Regarding VEGFA, its targeting may be crucial in counteracting possible detrimental effects due to its presence as a soluble factor in the secretome. In fact, VEGFA excess plays a key role in controlling chondrocyte catabolism and angiogenesis as a crucial step for endochondral ossification of cartilage progenitors, which ultimately leads to progressive ECM breakdown [56]. This regulation on ECM homeostasis is also emphasized by the numerous metalloproteinases targeted by EV-miRNAs, such as MMPs and a disintegrin and metalloprotease/a disintegrin and metalloproteinase with thrombospondin motifs (ADAM/ADAMTS). In particular, MMP1 and 14 resulted to be highly regulated (8.67% and 12.49%) with the main contribution of hsa-miR-222-3p (7.71%) and hsa-miR-24-3p, respectively. Moreover, MMP2 was a preferential target (2.56%). In addition, two other ECM-related molecules as PLAU and plasminogen activator tissue type (PLAT) emerged. In particular, PLAU is strongly targeted due to hsa-miR-193b-3p (6.88%). This mechanism may act in combination with the presence of a PLAU receptor (PLAUR) as a free molecule in the secretome, therefore reducing both PLAU synthesis at the cellular level and PLAU bioavailability as a soluble mediator. In fact, it has been proposed that both PLAU/PLAUR-mediated cell surface proteolysis and/or PLAUR-mediated signaling may promote inflammatory joint disease, indicating that disruption of this key proteolytic/signaling system could provide a novel therapeutic strategy to limit OA [57].
EV-miRNAs, together with the direct targeting of OA-related molecules, may also act at a more general level on the different cell types usually involved in the pathology (Table 5). Intriguingly, with the exception of the synovial membrane that needs further studies to define miRNA’s role in the definition of its pathological state, the most abundant EV-miRNAs resulted to have protective and anti-inflammatory features. In detail, EV-miRNAs had a preponderance towards protection for cartilage (ratio of 10.3), anti-inflammatory phenotype for macrophages (M2 vs. M1 ratio of 10.2) and anti-activation for T cells (ratio of 3.0). This is again consistent with the amelioration of cartilage structure and reduction in inflammation observed after MSCs or MSC-based products in OA patients [58,59,60]. In particular, two miRNAs tipped the balance towards healing capacity, hsa-miR-24-3p and hsa-miR-222-3p. hsa-miR-24-3p levels were lower in OA patients, and IL1B decreased its abundance in chondrocytes in vitro [61]. Consistently, its overexpression led to an increase in cell viability, reduction in pro-inflammatory cytokines and ECM degradation. Moreover, hsa-miR-24-3p plays an important role in macrophage polarization [62,63]. Its overexpression decreased the production of M1 phenotype markers and increased the production of M2 markers. Conversely, its knockdown promoted M1 and diminished M2 macrophage polarization. Eventually, regarding T cells, hsa-miR-24-3p represses IFNG production in human activated CD4+ [64] and CD8+ [65] T cells. Of note, hsa-miR-24-3p found in tumor EVs inhibited T-cell proliferation and differentiation [66]. Regarding hsa-miR-222-3p, it resulted in reduced OA in patients [67] and OA chondrocytes [68], and its overexpression led to the suppression of apoptotic death and ECM degradation. In addition, hsa-miR-222-3p was reported to be an M2 macrophage inducer [69], since its overexpression in macrophages induced polarization of the M2 phenotype. Consistently, tumor EVs miR-222-3p was shown to be an effective regulator in the polarization of M2 macrophages [70]. Finally, although found at lower levels with respect to hsa-miR-24-3p and hsa-miR-222-3p, hsa-miR-193b-3p was also reported to have a protective effect on cartilage. Its expression was elevated in chondrogenic MSCs, while being significantly reduced in OA cartilage [71]. Moreover, overexpression of hsa-miR-193b-3p suppressed MMP19 mRNA and inhibited the IL1B-induced expression of MMP19 in vitro [72] and strongly enhanced in vivo cartilage formation in mice [71]. Thus, these three miRNAs may be envisioned as therapeutic molecules, and strategies to increase their amounts in EVs would greatly enhance the protective and anti-inflammatory potential of BMSCs and their secretome. In this perspective, future studies aimed at increasing the knowledge of miRNA roles in synovia could further sharpen the understanding of these therapeutic features, this compartment being the most responsive within the joint for the inflammatory response [71,73].
We are aware that this report has some limitations. First, the number of donors is small. Nevertheless, the high correlation between single donors for both secreted factors and miRNAs suggests that the analyzed patterns are shared and consistent. This is further demonstrated by 84% of proteins >1000 pg/mL and 78% of miRNAs within the first quartile of expression having all three single values falling into the selected expression range, respectively. Moreover, the comparison of miRNAs falling in the first quartile of this work with those recently published for BMSC-EVs obtained from a different bone marrow source (femoral channel) [74] showed a 64% overlap, with 7 of the top 10 most-expressed miRNAs of our work being found in the other list, including those we observed as potential disease modifiers such as has-miR-24-3p, has-miR-222-3p and has-miR-193b-3p, which lay in the top 10 positions of both lists. Due to this high homogeneity of molecular patterns, we preferred to describe the shared signals able to interact with diseased cell types at pleiotropic and molecular levels, being aware that differences in a few factors may differently modulate the overall message at the patient level. For such analysis, a higher number of donors will have to be screened in the future to identify specific modulated pathways in the frame of the “personalized medicine” concept that relies on the selection of appropriate and optimal therapies based on the context of a patient’s molecular or cellular analysis; in this case, the secretome composition for OA. Second, the array of soluble factors and miRNAs is also limited. We preferred to score molecules with a deep characterization and several data available, including many reports related to their role in OA. Future studies sifting a wider portfolio of factors and miRNAs will be needed to better frame secretome’s and EVs’ fingerprints. Furthermore, data here-reported are obtained from culturing cells in standard conditions, thus relying on a MEM-based medium and fetal bovine serum (FBS) as supplement, collecting the secretome in absence of serum to minimize its interference in protein and EVs detection and because the presence of animal contaminants would not allow the secretome to be used as therapeutics by regulatory entities. Moreover, there is growing evidence that cells may respond differently depending on the in vitro culturing conditions, e.g., inflammatory [75] or mechanical [76] stimuli that may enhance MSCs anti-inflammatory behavior, or in vivo environment, e.g., proximity with other tissues and cell types (chondrocytes, synoviocytes and immune cells) [77] or the synovial fluid [78,79] for OA. Therefore, the molecular data of secretome or EVs that were presented in this manuscript might be the first milestone to decipher their potential as cell-free approaches, while for the use of MSCs as therapeutics, the effect of an OA environment will be crucial to better frame shuttled signals. Eventually, to reduce effects due to gender, only female donors were selected. We opted for this choice since the majority of OA patients are women [80]; albeit, we are aware that male donors might result in slight differences to be unraveled in future research works.
5. Conclusions
EV-miRNAs and secreted factors account for the immunomodulatory and healing potential of BMSCs in the musculoskeletal field. Several molecules target both specific cytokines/chemokines and several cell types shaping OA initiation and progression. This molecular fingerprint gives ground for the use of BMSCs, and especially their secretome/EVs, as therapeutics and supports the promising results obtained in the first trials and therapeutic procedures using these innovative and cutting-edge regenerative products for joint diseases.
Acknowledgments
The authors want to thank all healthcare workers of the IRCCS Istituto Ortopedico Galeazzi for their exceptional work during this unexpected world challenge. The authors would like to thank Joshua Harliono for his help as a native language proofreader.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/cells11213501/s1, Figure S1. Functional association network for secreted factors associated to GO term “chemotaxis”. Colorless nodes are for proteins that are not related to the GO terms: leukocytes chemotaxis, lymphocytes chemotaxis, monocytes chemotaxis and granulocytes chemotaxis in the STRING database v 11.5; Figure S2. Functional association network for secreted factors associated to the GO term “activation”. Colorless nodes are for proteins that are not related to the GO terms: leukocytes activation, lymphocytes activation, macrophages activation and neutrophils activation in the STRING database v 11.5; Table S1. Protein lists for the most representative GO terms identified within scored secreted factors; Table S2. EV-miRNAs detected in BMSCs secretomes; Table S3. Experimentally validated targets for first-quartile EV-miRNAs; Table S4. Comprehensive list of targets of all first quartile EV-miRNAs.
Author Contributions
Conceptualization, E.R., C.P.O. and L.d.G.; methodology, E.R. and C.P.O.; software, C.P.O.; validation, C.P.O.; formal analysis, E.R.; investigation, E.R.; resources, L.d.G.; data curation, E.R.; writing—original draft preparation, E.R.; writing—review and editing, L.d.G.; visualization, C.P.O.; supervision, L.d.G.; project administration, L.d.G.; funding acquisition, L.d.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was performed at IRCCS Istituto Ortopedico Galeazzi. Institutional Review Board approval (San Raffaele Hospital Ethics Committee approval on date 16 December 2020, registered under number 214/int/2020) was granted before the beginning of the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data presented in this study are openly available in OSF at https://osf.io/e4uzj/?view_only=3cf3946cb97841cd8684ae5baea12cd6, accessed on 8 April 2022.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The work reported in this publication was funded by the Italian Ministry of Health, RCR-2021-23671217 project under the Italian Musculoskeletal Apparatus Network RAMS and Ricerca Corrente. The APC was funded by the Italian Ministry of Health, RCR-2021-23671217 project under the Italian Musculoskeletal Apparatus Network RAMS and Ricerca Corrente.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12:412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry T., Parkes M., Hodgson R., Felson D., Arden N., O′Neill T. Association between Bone marrow lesions&synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2019;28:316–323. doi: 10.1016/j.joca.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez-Lopez E., Coras R., Torres A., Lane N.E., Guma M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022;18:258–275. doi: 10.1038/s41584-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes M.K., Hume E.L., Smith J.B. Phenotypic Characterization of Inflammatory Cells from Osteoarthritic Synovium and Synovial Fluids. Clin. Immunol. 2002;105:315–325. doi: 10.1006/clim.2002.5283. [DOI] [PubMed] [Google Scholar]
- 6.Grässel S., Muschter D. Recent advances in the treatment of osteoarthritis. F1000Research. 2020;9:325. doi: 10.12688/f1000research.22115.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasiliadis A.V., Galanis N. Human bone marrow-derived mesenchymal stem cells from different bone sources: A panorama. Stem Cell Investig. 2020;7:15. doi: 10.21037/sci-2020-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan A.I. Medicinal signalling cells: They work, so use them. Nature. 2019;566:39. doi: 10.1038/d41586-019-00490-6. [DOI] [PubMed] [Google Scholar]
- 9.Bousnaki M., Bakopoulou A., Kritis A., Koidis P. The Efficacy of Stem Cells Secretome Application in Osteoarthritis: A Systematic Review of In Vivo Studies. Stem Cell Rev. Rep. 2020;16:1222–1241. doi: 10.1007/s12015-020-09980-x. [DOI] [PubMed] [Google Scholar]
- 10.Loo S.J.Q., Wong N.K. Advantages and challenges of stem cell therapy for osteoarthritis (Review) Biomed. Rep. 2021;15:67. doi: 10.3892/br.2021.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni Z., Zhou S., Li S., Kuang L., Chen H., Luo X., Ouyang J., He M., Du X., Chen L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molnar V., Matišić V., Kodvanj I., Bjelica R., Jeleč Ž., Hudetz D., Rod E., Čukelj F., Vrdoljak T., Vidović D., et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021;22:9208. doi: 10.3390/ijms22179208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliviero A., Della Porta G., Peretti G., Maffulli N. MicroRNA in osteoarthritis: Physiopathology, diagnosis and therapeutic challenge. Br. Med. Bull. 2019;130:137–147. doi: 10.1093/bmb/ldz015. [DOI] [PubMed] [Google Scholar]
- 14.Ragni E., Perucca Orfei C., De Luca P., Lugano G., Viganò M., Colombini A., Valli F., Zacchetti D., Bollati V., De Girolamo L. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 2019;10:109. doi: 10.1186/s13287-019-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Haene B., Mestdagh P., Hellemans J., Vandesompele J. Next-Generation MicroRNA Expression Profiling Technology. Humana Press; Totowa, NJ, USA: 2012. miRNA Expression Profiling: From Reference Genes to Global Mean Normalization; pp. 261–272. [DOI] [PubMed] [Google Scholar]
- 16.Huang H.-Y., Lin Y.-C.-D., Li J., Huang K.-Y., Shrestha S., Hong H.-C., Tang Y., Chen Y.-G., Jin C.-N., Yu Y., et al. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020;48:D148–D154. doi: 10.1093/nar/gkz896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akoglu H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018;18:91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metsalu T., Vilo J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barilani M., Peli V., Cherubini A., Dossena M., Dolo V., Lazzari L. NG2 as an Identity and Quality Marker of Mesenchymal Stem Cell Extracellular Vesicles. Cells. 2019;8:1524. doi: 10.3390/cells8121524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortati L., De Girolamo L., Orfei C.P., Viganò M., Brayda-Bruno M., Ragni E., Colombini A. In Vitro Study of Extracellular Vesicles Migration in Cartilage-Derived Osteoarthritis Samples Using Real-Time Quantitative Multimodal Nonlinear Optics Imaging. Pharmaceutics. 2020;12:734. doi: 10.3390/pharmaceutics12080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papait A., Ragni E., Cargnoni A., Vertua E., Romele P., Masserdotti A., Orfei C.P., Signoroni P.B., Magatti M., Silini A.R., et al. Comparison of EV-free fraction, EVs, and total secretome of amniotic mesenchymal stromal cells for their immunomodulatory potential: A translational perspective. Front. Immunol. 2022;13:960909. doi: 10.3389/fimmu.2022.960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Chevillet J.R., Kang Q., Ruf I.K., Briggs H.A., Vojtech L.N., Hughes S.M., Cheng H.H., Arroyo J.D., Meredith E.K., Gallichotte E.N., et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA. 2014;111:14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schopman N.C.T., Heynen S., Haasnoot J., Berkhout B. A miRNA-tRNA mix-up: tRNA origin of proposed miRNA. RNA Biol. 2010;7:573–576. doi: 10.4161/rna.7.5.13141. [DOI] [PubMed] [Google Scholar]
- 25.Chou C.-H., Jain V., Gibson J., Attarian D.E., Haraden C.A., Yohn C.B., Laberge R.-M., Gregory S., Kraus V.B. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 2020;10:10868. doi: 10.1038/s41598-020-67730-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endisha H., Rockel J., Jurisica I., Kapoor M. The complex landscape of microRNAs in articular cartilage: Biology, pathology, and therapeutic targets. JCI Insight. 2018;3:e121630. doi: 10.1172/jci.insight.121630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavallaee G., Rockel J.S., Lively S., Kapoor M. MicroRNAs in Synovial Pathology Associated With Osteoarthritis. Front. Med. 2020;7:376. doi: 10.3389/fmed.2020.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu S.J., Hu H.T., Li H.L., Chang S. The Role of miRNAs in Immune Cell Development, Immune Cell Activation, and Tumor Immunity: With a Focus on Macrophages and Natural Killer Cells. Cells. 2019;8:1140. doi: 10.3390/cells8101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galan A.R., Fernández-Messina L., Sánchez-Madrid F. Control of Immunoregulatory Molecules by miRNAs in T Cell Activation. Front. Immunol. 2018;9:2148. doi: 10.3389/fimmu.2018.02148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han X., Yang B., Zou F., Sun J. Clinical therapeutic efficacy of mesenchymal stem cells derived from adipose or bone marrow for knee osteoarthritis: A meta-analysis of randomized controlled trials. J. Comp. Eff. Res. 2020;9:361–374. doi: 10.2217/cer-2019-0187. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Zhou L., Zhang Y., Huang L., Shi Q. Mesenchymal stem cells—A promising strategy for treating knee osteoarthritis. Bone Jt. Res. 2020;9:719–728. doi: 10.1302/2046-3758.910.BJR-2020-0031.R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Yi H., Song Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021;12:545. doi: 10.1186/s13287-021-02609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2006;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 35.Meesuk L., Tantrawatpan C., Kheolamai P., Manochantr S. The immunosuppressive capacity of human mesenchymal stromal cells derived from amnion and bone marrow. Biochem. Biophys. Rep. 2016;8:34–40. doi: 10.1016/j.bbrep.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakkas L.I., Scanzello C., Johanson N., Burkholder J., Mitra A., Salgame P., Katsetos C.D., Platsoucas C.D. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin Diagn Lab Immunol. 1998;5:430–437. doi: 10.1128/CDLI.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosshirt N., Trauth R., Platzer H., Tripel E., Nees T.A., Lorenz H.-M., Tretter T., Moradi B. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. Ther. 2021;23:37. doi: 10.1186/s13075-020-02410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y.-S., Luo W., Zhu S.-A., Lei G.-H. T Cells in Osteoarthritis: Alterations and Beyond. Front. Immunol. 2017;8:356. doi: 10.3389/fimmu.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viola A., Munari F., Sánchez-Rodríguez R., Scolaro T., Castegna A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019;10:1462. doi: 10.3389/fimmu.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.François M., Romieu-Mourez R., Li M., Galipeau J. Human MSC Suppression Correlates With Cytokine Induction of Indoleamine 2,3-Dioxygenase and Bystander M2 Macrophage Differentiation. Mol. Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 41.Hsueh M., Zhang X., Wellman S.S., Bolognesi M., Kraus V.B. Synergistic Roles of Macrophages and Neutrophils in Osteoarthritis Progression. Arthritis Rheumatol. 2020;73:89–99. doi: 10.1002/art.41486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue Y., Iriyama A., Ueno S., Takahashi H., Kondo M., Tamaki Y., Araie M., Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp. Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Joel M.D.M., Yuan J., Wang J., Yan Y., Qian H., Zhang X., Xu W., Mao F. MSC: Immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am. J. Transl. Res. 2019;11:3890–3904. [PMC free article] [PubMed] [Google Scholar]
- 44.Mi M., Shi S., Li T., Holz J., Lee Y.-J., Sheu T.-J., Liao Q., Xiao T. TIMP2 deficient mice develop accelerated osteoarthritis via promotion of angiogenesis upon destabilization of the medial meniscus. Biochem. Biophys. Res. Commun. 2012;423:366–372. doi: 10.1016/j.bbrc.2012.05.132. [DOI] [PubMed] [Google Scholar]
- 45.Alam R., Ji J.R., Kim M.S., Kim N.S. Biomarkers for identifying the early phases of osteoarthritis secondary to medial patellar luxation in dogs. J. Vet. Sci. 2011;12:273–280. doi: 10.4142/jvs.2011.12.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clements D.N., Fitzpatrick N., Carter S.D., Day P.J. Cartilage gene expression correlates with radiographic severity of canine elbow osteoarthritis. Vet. J. 2009;179:211–218. doi: 10.1016/j.tvjl.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Garcia F.J.B. Catabolic events in osteoarthritic cartilage. Osteoarthr. Cartil. 1999;7:308–309. doi: 10.1053/joca.1998.0174. [DOI] [PubMed] [Google Scholar]
- 48.Li J., Ny A., Leonardsson G., Nandakumar K.S., Holmdahl R., Ny T. The Plasminogen Activator/Plasmin System Is Essential for Development of the Joint Inflammatory Phase of Collagen Type II-Induced Arthritis. Am. J. Pathol. 2005;166:783–792. doi: 10.1016/S0002-9440(10)62299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dreier R., Wallace S., Fuchs S., Bruckner P., Grassel S. Paracrine interactions of chondrocytes and macrophages in cartilage degradation: Articular chondrocytes provide factors that activate macrophage-derived pro-gelatinase B (pro-MMP-9) J. Cell Sci. 2001;114:3813–3822. doi: 10.1242/jcs.114.21.3813. [DOI] [PubMed] [Google Scholar]
- 50.Wang W., Rigueur D., Lyons K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014;102:37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaney Davidson E.N., van der Kraan P.M., van den Berg W.B. TGF-β and osteoarthritis. Osteoarthr. Cartil. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Oh S.A., Li M.O. TGF-β: Guardian of T Cell Function. J. Immunol. 2013;191:3973–3979. doi: 10.4049/jimmunol.1301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galasso O., De Gori M., Nocera A., Brunetti A., Gasparini G. Regulatory Functions of Insulin-like Growth Factor Binding Proteins in Osteoarthritis. Int. J. Immunopathol. Pharmacol. 2011;24:55–59. doi: 10.1177/03946320110241S211. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka N., Tsuno H., Ohashi S., Iwasawa M., Furukawa H., Kato T., Fukui N. The attenuation of insulin-like growth factor signaling may be responsible for relative reduction in matrix synthesis in degenerated areas of osteoarthritic cartilage. BMC Musculoskelet. Disord. 2021;22:231. doi: 10.1186/s12891-021-04096-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrell C.R., Markovic B.S., Fellabaum C., Arsenijevic A., Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2018;109:2318–2326. doi: 10.1016/j.biopha.2018.11.099. [DOI] [PubMed] [Google Scholar]
- 56.Vadalà G., Russo F., Musumeci M., Giacalone A., Papalia R., Denaro V. Targeting VEGF-A in cartilage repair and regeneration: State of the art and perspectives. J. Biol. Regul. Homeost. Agents. 2019;32:217–224. [PubMed] [Google Scholar]
- 57.Thornton S., Raghu H., Cruz C., Frederick M.D., Palumbo J.S., Mullins E.S., Almholt K., Usher P.A., Flick M.J. Urokinase plasminogen activator and receptor promote collagen-induced arthritis through expression in hematopoietic cells. Blood Adv. 2017;1:545–556. doi: 10.1182/bloodadvances.2016004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiang X.-N., Zhu S.-Y., He H.-C., Yu X., Xu Y., He C.-Q. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 2022;13:14. doi: 10.1186/s13287-021-02689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang J.J., Rim Y.A., Nam Y., Ju J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021;12:631291. doi: 10.3389/fimmu.2021.631291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song Y., Jorgensen C. Mesenchymal Stromal Cells in Osteoarthritis: Evidence for Structural Benefit and Cartilage Repair. Biomedicines. 2022;10:1278. doi: 10.3390/biomedicines10061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J., Qian X., Ding R. MiR-24-3p attenuates IL-1β-induced chondrocyte injury associated with osteoarthritis by targeting BCL2L12. J. Orthop. Surg. Res. 2021;16:371. doi: 10.1186/s13018-021-02378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naqvi J.B.F.A.R., Nares S., Fordham J.B. miR-24 Regulates Macrophage Polarization and Plasticity. J. Clin. Cell. Immunol. 2015;6:362. doi: 10.4172/2155-9899.1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jingjing Z., Nan Z., Wei W., Qinghe G., Weijuan W., Peng W., Xiangpeng W. MicroRNA-24 Modulates Staphylococcus aureus-Induced Macrophage Polarization by Suppressing CHI3L1. Inflammation. 2017;40:995–1005. doi: 10.1007/s10753-017-0543-3. [DOI] [PubMed] [Google Scholar]
- 64.Fayyad-Kazan H., Hamade E., Rouas R., Najar M., Fayyad-Kazan M., El Zein N., El Dirani R., Hussein N., Fakhry M., Al-Akoum C., et al. Downregulation of microRNA-24 and -181 parallels the upregulation of IFN-γ secreted by activated human CD4 lymphocytes. Hum. Immunol. 2014;75:677–685. doi: 10.1016/j.humimm.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Chandran P.A., Keller A., Weinmann L., Seida A.A., Braun M., Andreev K., Fischer B., Horn E., Schwinn S., Junker M., et al. The TGF-β-inducible miR-23a cluster attenuates IFN-γ levels and antigen-specific cytotoxicity in human CD8+ T cells. J. Leukoc. Biol. 2014;96:633–645. doi: 10.1189/jlb.3A0114-025R. [DOI] [PubMed] [Google Scholar]
- 66.Ye S.-B., Zhang H., Cai T.-T., Liu Y.-N., Ni J.-J., He J., Peng J.-Y., Chen Q.-Y., Mo H.-Y., Cui J., et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016;240:329–340. doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 67.Kopańska M., Szala D., Czech J., Gabło N., Gargasz K., Trzeciak M., Zawlik I., Snela S. MiRNA expression in the cartilage of patients with osteoarthritis. J. Orthop. Surg. Res. 2017;12:51. doi: 10.1186/s13018-017-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song J., Jin E.-H., Kim D., Kim K.Y., Chun C.-H. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. 2015;3:79–89. doi: 10.1016/j.bbacli.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graff J.W., Dickson A.M., Clay G., McCaffrey A.P., Wilson M.E. Identifying Functional MicroRNAs in Macrophages with Polarized Phenotypes. J. Biol. Chem. 2012;287:21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ying X., Wu Q., Wu X., Zhu Q., Wang X., Jiang L., Chen X., Wang X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–43087. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meng F., Li Z., Zhang Z., Yang Z., Kang Y., Zhao X., Long D., Hu S., Gu M., He S., et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics. 2018;8:2862–2883. doi: 10.7150/thno.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang Z., Meng F., Zhang Z., Mao G., Huang Z., Liao W., He A. MicroRNA-193b-3p regulates matrix metalloproteinase 19 expression in interleukin-1β-induced human chondrocytes. J. Cell. Biochem. 2018;119:4775–4782. doi: 10.1002/jcb.26669. [DOI] [PubMed] [Google Scholar]
- 73.Mathiessen A., Conaghan P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017;19:18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colombini A., Libonati F., Lopa S., Ragni E., De Luca P., Zagra L., Sinigaglia F., Moretti M., de Girolamo L. Immunomodulatory potential of secretome from cartilage cells and mesenchymal stromal cells in an arthritic context: From predictive fiction toward reality. Front. Med. 2022;9:992386. doi: 10.3389/fmed.2022.992386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ragni E., Orfei C.P., De Luca P., Mondadori C., Viganò M., Colombini A., De Girolamo L. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: The example of joint disease. Stem Cell Res. Ther. 2020;11:165. doi: 10.1186/s13287-020-01677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skibber M.A., Olson S.D., Prabhakara K.S., Gill B.S., Cox C.S.J. Enhancing Mesenchymal Stromal Cell Potency: Inflammatory Licensing via Mechanotransduction. Front. Immunol. 2022;13:3327. doi: 10.3389/fimmu.2022.874698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao X., Zhao Y., Sun X., Xing Y., Wang X., Yang Q. Immunomodulation of MSCs and MSC-Derived Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2020;8:575057. doi: 10.3389/fbioe.2020.575057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cifù A., Domenis R., Pozzi-Mucelli M., Di Benedetto P., Causero A., Moretti M., Stevanato M., Pistis C., Parodi P.C., Fabris M., et al. The Exposure to Osteoarthritic Synovial Fluid Enhances the Immunomodulatory Profile of Adipose Mesenchymal Stem Cell Secretome. Stem Cells Int. 2020;2020:4058760. doi: 10.1155/2020/4058760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ragni E., Colombini A., Viganò M., Libonati F., Orfei C.P., Zagra L., de Girolamo L. Cartilage Protective and Immunomodulatory Features of Osteoarthritis Synovial Fluid-Treated Adipose-Derived Mesenchymal Stem Cells Secreted Factors and Extracellular Vesicles-Embedded miRNAs. Cells. 2021;10:1072. doi: 10.3390/cells10051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Long H., Liu Q., Yin H., Wang K., Diao N., Zhang Y., Lin J., Guo A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022;74:1172–1183. doi: 10.1002/art.42089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data presented in this study are openly available in OSF at https://osf.io/e4uzj/?view_only=3cf3946cb97841cd8684ae5baea12cd6, accessed on 8 April 2022.